Abstract
Objective
To examine the cardiovascular, cerebrovascular, and peripheral vascular safety of erenumab across migraine prevention studies.
Methods
Vascular adverse events (AEs) and blood pressure data were integrated across 4 double-blind, placebo-controlled studies of erenumab and their open-label extensions in patients with chronic or episodic migraine. Subgroup analyses were conducted by acute migraine-specific medication use and number of vascular risk factors at baseline. Standardized search terms were used to identify vascular AEs (cardiovascular, cerebrovascular, or peripheral). An independent committee adjudicated whether targeted events were vascular in origin.
Results
In placebo-controlled studies, 2,443 patients received placebo (n = 1,043), erenumab 70 mg (n = 893), or erenumab 140 mg (n = 507) subcutaneously once monthly. Regardless of acute migraine-specific medication use or vascular risk factors at baseline, AE incidence was similar across the placebo and erenumab treatment groups. Hypertension AEs were reported for 0.9% (placebo), 0.8% (erenumab 70 mg), and 0.2% (erenumab 140 mg) of patients. Vascular AEs, which were similar across double-blind and open-label treatment, generally were confounded, with plausible alternative etiologies. In 18 patients with events reviewed by the independent committee, 4 events were positively adjudicated as cardiovascular in origin: 2 deaths and 2 vascular events. All 4 positively adjudicated cardiovascular events occurred during open-label erenumab treatment.
Conclusion
Selective blockade of the canonical calcitonin gene-related peptide receptor with erenumab for migraine prevention had a vascular safety profile comparable to that of placebo over 12 weeks, with no increased emergence of events over time. Further study of long-term safety of erenumab in patients with migraine is needed.
Clinicaltrials.gov identifiers
NCT02066415, NCT02456740, NCT01952574, NCT02483585, NCT02174861, and NCT01723514.
Classification of evidence
This analysis provides Class II evidence that for patients with migraine, erenumab does not increase the risk of vascular AEs.
Migraine affects over 1 billion people worldwide1 and 15%–20% of Americans.2 More than 25% of adults with migraine are candidates for preventive therapy,3 but fewer than 30% of candidates receive it.4,5 Over 80% of patients with chronic migraine discontinue oral preventive therapy within the first year.6 There is an unmet need for an effective, well-tolerated therapy to prevent migraine.
Calcitonin gene-related peptide (CGRP) plays a key role in migraine pathophysiology.7,8 Monoclonal antibodies have recently been developed that bind to either CGRP or the canonical CGRP receptor to prevent migraine. Because CGRP can mediate vasodilation,9 inhibition of the CGRP pathway could theoretically attenuate compensatory vasodilation during ischemic conditions, but the relative importance of the CGRP receptor pathway compared with other vasodilatory pathways during ischemia (e.g., myocardial) has not been established.9–11 In addition, patients with migraine have an increased risk of vascular events, including stroke and myocardial ischemia,12,13 and acute migraine-specific medications such as triptans and ergotamine have known vasoconstrictive effects.14,15 Thus, it is important to examine vascular safety, particularly over the longer term, in patients treated with therapies that block the effects of CGRP.
Erenumab (in the United States, erenumab-aooe) is a fully human monoclonal antibody that specifically targets and blocks the canonical CGRP receptor to prevent migraine.16 Administered subcutaneously once monthly, erenumab has been shown to be effective for migraine prevention.17–20 This pooled analysis of vascular safety in clinical studies of erenumab for migraine prevention included vascular (cardiovascular, cerebrovascular, or peripheral) adverse events (AEs) that were reported by investigators, events that were adjudicated across all studies by an independent committee of clinical experts, and a pooled analysis of blood pressure (BP) measurements. This report also includes results of a 24-hour ambulatory BP monitoring study that assessed the potential cardiovascular effects of erenumab in healthy controls.
Methods
The primary research questions for this pooled analysis were to examine if the rates of vascular (cardiovascular or cerebrovascular) AEs were higher in the erenumab group vs the placebo group of controlled clinical studies, both overall and in subgroups of patients at a higher risk of vascular AEs, as well as the effect of erenumab treatment on BP. This analysis provides Class II evidence that for patients with migraine, erenumab does not increase the risk of vascular AEs.
Design
Information about AEs was collected as reported by the patient, either spontaneously or in response to the investigator's nondirected questioning, per standard procedures in clinical trials. Regulatory authorities in the regions where the trials were conducted reviewed study protocols, including AE data collection methods, before implementation. Reported AEs and BP results were integrated for 12 weeks of double-blind treatment across 4 placebo-controlled migraine prevention studies of erenumab administered subcutaneously once monthly. For long-term safety, AE data were integrated for any exposure to erenumab in these 4 studies and their open-label extensions.
The pivotal studies testing the doses of erenumab that are approved for migraine prevention (70 mg and 140 mg once monthly) were NCT02066415 (n = 667), which enrolled patients with chronic migraine,17 and NCT02456740 (Study to Evaluate the Efficacy and Safety of Erenumab [AMG 334] in Migraine Prevention [STRIVE]; n = 955), which enrolled patients with episodic migraine.18 The supportive studies NCT01952574 (n = 483)19 and NCT02483585 (Study to Evaluate the Efficacy and Safety of Erenumab [AMG 334] Compared to Placebo in Migraine Prevention [ARISE]; n = 577)20 also enrolled patients with episodic migraine, but in the placebo-controlled periods of these studies, 70 mg was the highest dose tested. Safety data for erenumab doses less than 70 mg, which were investigated in the placebo-controlled period of supportive study NCT01952574 but are not approved for migraine prevention, were excluded from this integrated safety analysis. All but 1 of the studies had a 12-week placebo-controlled period; the episodic migraine STRIVE study had a 24-week placebo-controlled period. Patients assigned to an erenumab arm received the same dose (70 or 140 mg) throughout double-blind treatment.
Patients completing the double-blind, placebo-controlled periods were continued in an active dose-blinded treatment period (STRIVE study) or open-label erenumab treatment (all other studies). For the episodic migraine studies, following completion of the double-blind placebo-controlled period, the active treatment or open-label treatment period was part of the main study protocol. In NCT01952574, open-label erenumab is being maintained for up to 256 weeks (5 years).21 Patients who completed the chronic migraine study could enter a separate open-label extension study (NCT02174861). During the active treatment or open-label periods, patients received only 70 mg (ARISE study), or 70 mg or 140 mg (other studies).
Ambulatory BP was assessed in a phase 1 study (NCT01723514) that enrolled 32 healthy controls and 16 patients with migraine who received erenumab (70 or 140 mg) or placebo subcutaneously once monthly for 12 weeks.22
Standard protocol approvals, registrations, and patient consents
An institutional review board or independent ethics committee approved each study in this analysis. The studies included in this analysis were registered at clinicaltrials.gov: NCT02066415, NCT02456740, NCT01952574, NCT02483585, NCT02174861, and NCT01723514. Patients provided written informed consent to participate in each study.
Eligibility criteria
Each of the migraine prevention studies had an initial screening period (up to 3 weeks), followed by a 4-week, prospective baseline period to confirm study eligibility before the patient was enrolled and randomized. Key inclusion criteria at screening were age 18 years or older (up to 60 or 65 years) and a history of migraine with or without aura for at least 12 months. The chronic migraine study included patients with at least 15 headache days per month during the baseline period, of which at least 8 were migraine days. The episodic migraine studies included patients with fewer than 15 headache days per month during the baseline period, of which at least 4 were migraine days.
In the episodic migraine studies, use of medications for acute headache before screening was restricted as follows: acute migraine-specific medications (triptan or ergot) were limited to fewer than 10 days per month, analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) to fewer than 15 days per month, and opioids or butalbital-containing medications to fewer than 4 days per month. In the chronic migraine study, there was no restriction for prior use of acute migraine-specific medications, analgesics, or NSAIDs; however, in the prior 3 months, patients could not have used opioids on more than 12 days or butalbital on more than 6 days. In each migraine prevention study, after randomization, patients could use acute migraine-specific medications, analgesics, or NSAIDs as needed to treat acute migraine.
Patients were excluded from the migraine prevention studies if they had myocardial infarction, stroke, TIA, unstable angina, or coronary artery bypass surgery, or another revascularization procedure within 12 months prior to screening. Medically stable patients who had any of these listed conditions more than 12 months prior to screening could participate in the studies. Patients were excluded from studies NCT02066415 and NCT01952574 if they had poorly controlled hypertension (systolic BP [SBP] above 150–160 mm Hg or diastolic BP [DBP] above 90–100 mm Hg), and patients with any unstable medical condition were excluded from each study. Healthy controls in the ambulatory BP study had no history of hypertension, hypotension, or vascular disease.
Safety assessments
AEs that were new or worsened after the first dose of study treatment were recorded through 12 weeks after the last dose in studies with doses up to 70 mg (the ARISE study and study NCT01952574 before protocol amendment to include higher doses), and 16 weeks after the last dose in studies with doses up to 140 mg. For each AE, the Medical Dictionary for Regulatory Activities (MedDRA) was used to determine the preferred term and system organ class. AE severity was graded with Common Terminology Criteria for Adverse Events (CTCAE) criteria, version 4.03. A serious AE was defined as an AE that met at least 1 of the following criteria: fatal, life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity, congenital anomaly/birth defect, or another medically important serious event.
Because of the acknowledged interest in the safety of this new class of medication, an independent committee was established to review potential vascular events. The adjudicators, who were clinical experts in cardiovascular and cerebrovascular disease, adjudicated each potential event based on prespecified definitions. Events that were referred to the adjudicators included the following: death, acute myocardial infarction or hospitalization for unstable angina event, nonfatal stroke or TIA, coronary revascularization procedure, hospitalization for hypertension, hospitalization for peripheral artery disease event, or revascularization procedure for peripheral artery disease. Adjudicators were blinded to treatment assignment throughout the adjudication process.
In each study, resting BP was measured at monthly study visits, before each administration of study treatment. The phase 1 study also monitored BP continuously for 24 hours, starting at 9:00 am, on day −2 (i.e., 2 days before the first dose of study treatment) and on days 8, 36, and 64 (i.e., 7 days after each dose of study treatment).
Statistical analysis
Analyses of AEs were conducted for 2 pools of safety data. The “12-week placebo-controlled” pool was the integrated safety dataset from 12 weeks of double-blind treatment (or in the STRIVE study, the first 12 weeks of double-blind treatment). The “any erenumab exposure” pool included AEs that occurred during exposure to either double-blind or open-label erenumab treatment through the data cutoff for the 5-year open-label extension study (NCT01952574) or completion of the study (all other studies). Integrated analyses of AEs for the 12-week placebo-controlled pool were conducted by assigned treatment (placebo, erenumab 70 mg, or erenumab 140 mg). Integrated analyses for any erenumab exposure were summarized by actual treatment received (erenumab 70 mg or erenumab 140 mg) when the AEs occurred. A single patient in the any erenumab exposure pool could have received both erenumab 70 mg and 140 mg because of the second randomization for the active treatment phase in the STRIVE study, or because of study protocol amendments in NCT02174861 and NCT01952574 that increased the open-label dose of erenumab from 70 mg to 140 mg for all patients. If a patient experienced the same AE when he or she received each dose of erenumab, then this AE was counted for both 70 mg and 140 mg. Exposure-adjusted incidence rates of AEs for any erenumab exposure pool were calculated by adjusting for the length of exposure to erenumab.
For the AE analyses in the 12-week placebo-controlled pool, 1 subgroup analysis examined the incidences of AEs separately by use or nonuse at baseline of acute migraine-specific medications (triptans or ergot). Another subgroup analysis examined the incidences of AEs separately by the number of vascular risk factors at baseline (none, 1, or ≥2) using 7 categories for vascular risk factors (table 1). Each subgroup analysis included incidences of any AE, any AE by severity, any serious AE, any AE leading to discontinuation of study treatment, and death.
Table 1.
Determination of baseline vascular risk factors from reported data
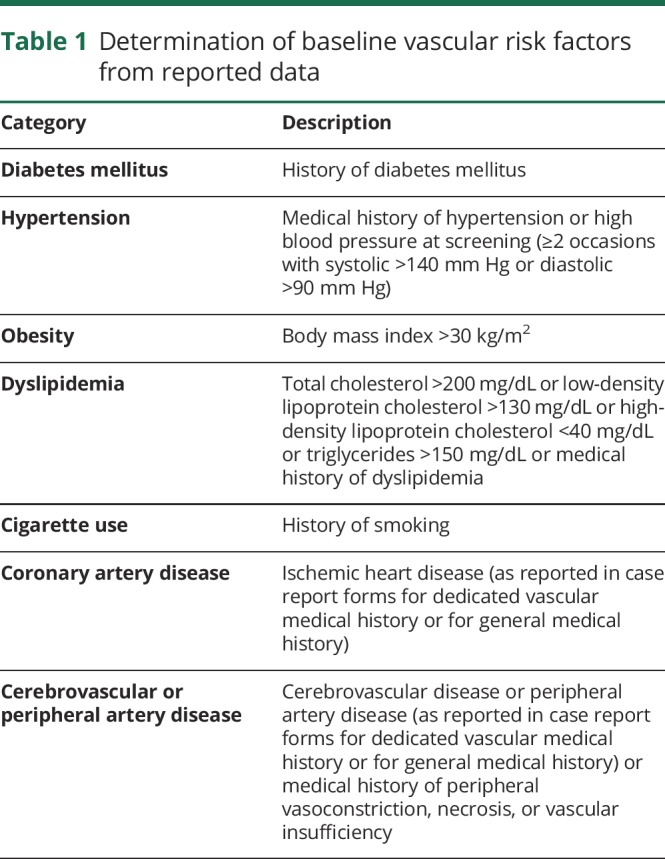
To analyze vascular AEs, standardized MedDRA queries with narrow search terms were used to determine the overall incidences in each treatment group of the 12-week placebo-control pool in the following categories: ischemic CNS vascular conditions, ischemic heart disease, peripheral arterial disease, or hypertension. The same queries were used to analyze vascular AEs for any erenumab exposure. The results of each search were summarized descriptively. Positively adjudicated vascular events were listed.
The integrated data analysis of the 12-week double-blind pool also summarized resting SBP and DBP at monthly study visits. To evaluate BP in a manner that was clinically relevant, the proportion of patients with an increase from baseline of ≥10 mm Hg in DBP or ≥20 mm Hg in SBP was evaluated at the monthly visits. For the phase 1 study of ambulatory BP, hourly SBP and DBP were summarized for each treatment group.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at amgen.com/datasharing.
Results
Patient disposition
During 12 weeks of double-blind treatment across the 4 migraine prevention studies, 2,443 patients with migraine received placebo (n = 1,043), erenumab 70 mg (n = 893), or erenumab 140 mg (n = 507). Most of these patients (94% erenumab, 92% placebo) completed 12 weeks of double-blind treatment. In NCT01952574, an additional 213 patients who received lower doses of erenumab 7 mg (n = 108) or 21 mg (n = 105) were not included in the pooled safety analysis for 12 weeks of double-blind treatment but could be analyzed for AEs during any exposure to erenumab if they switched to 70 mg for the open-label phase.
For the analysis of AEs during any exposure to erenumab, 2,499 patients received at least 1 dose of erenumab 70 mg (n = 2,128) or at least 1 dose of erenumab 140 mg (n = 1,223). A single patient in any study except ARISE could have received both erenumab 70 mg and 140 mg. Thus, considering all erenumab exposure during double-blind or open-label treatment, 852 patients received both doses of erenumab, 1,276 received only 70 mg, and 371 received only 140 mg. The total exposure to either dose of erenumab was 2,639 patient-years.
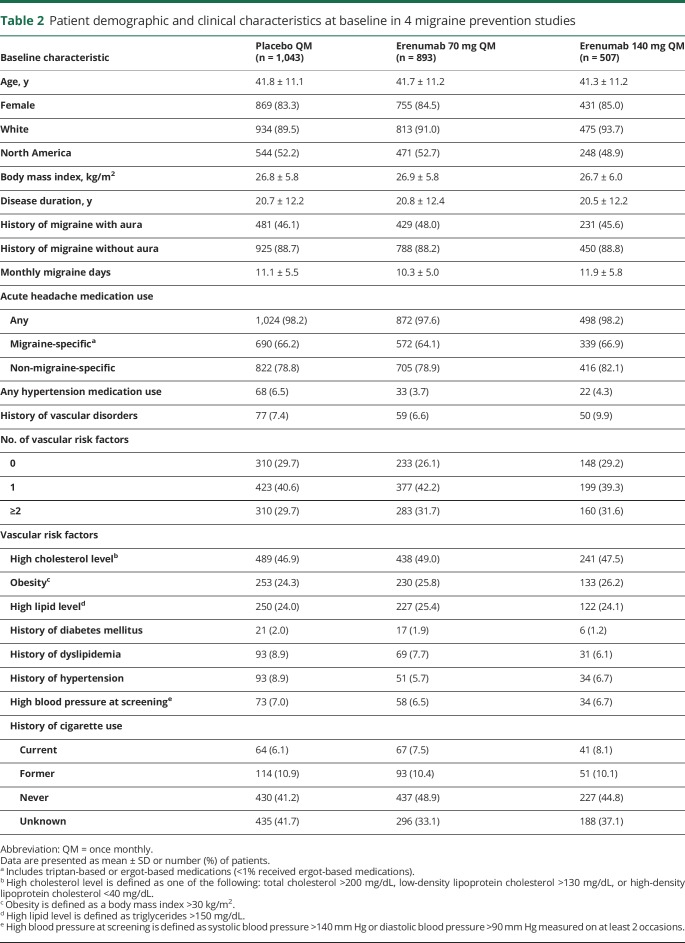
Patient characteristics
Baseline patient characteristics, use of acute migraine-specific medications, and vascular risk factors were balanced across the treatment groups (table 2). Upon entry into the respective study, mean patient age in each treatment group was 41.3–41.8 years, and 83.3%–85.0% of patients were female. Mean disease duration was approximately 21 years in each treatment group, and the mean number of monthly migraine days in each treatment group was 10.3–11.9. At baseline, acute migraine-specific medications were used by 64.1%–66.9% of patients in each treatment group. Almost all of the acute migraine-specific medications used were triptans (<1% were ergot-based). Medications for management of hypertension were used at baseline by 3.7%–6.5% of patients in each treatment group.
Table 2.
Patient demographic and clinical characteristics at baseline in 4 migraine prevention studies
In each treatment group, 6.6%–9.9% of patients had a history of a vascular disorder, most commonly hypertension (3.4%–5.1%). More than 70% of patients in each treatment group had at least 1 vascular risk factor at baseline (table 2). The most common vascular risk factor at baseline was dyslipidemia (based on high cholesterol, high lipids, or history of dyslipidemia). One in 4 patients was obese.
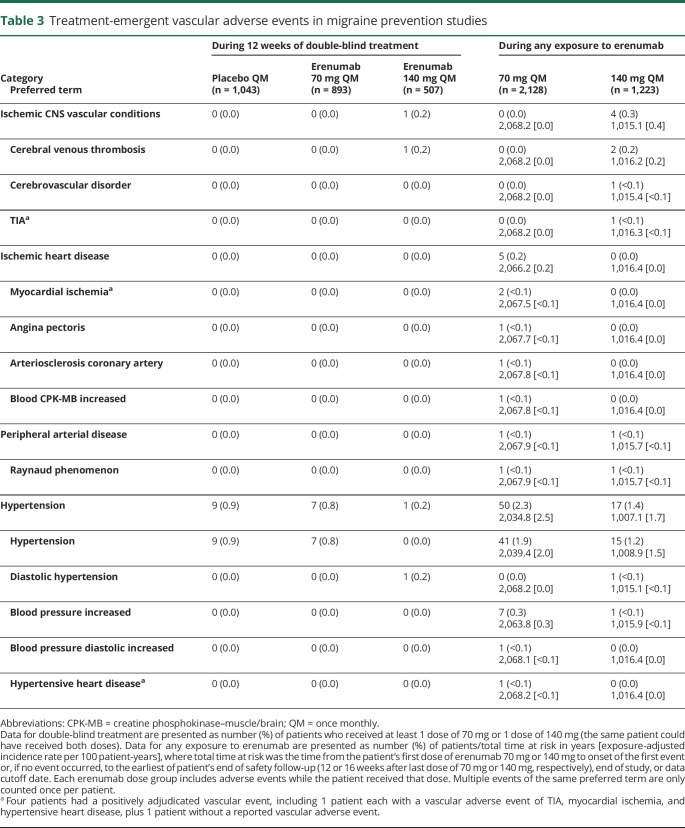
Vascular adverse events in targeted searches
In the targeted searches for vascular AEs during 12 weeks of double-blind treatment, investigators reported AEs of hypertension or diastolic hypertension for 9 (0.9%) patients receiving placebo, 7 (0.8%) receiving erenumab 70 mg, and 1 (0.2%) receiving erenumab 140 mg (table 3). An AE of cerebral venous thrombosis was reported for 1 (0.2%) patient in the erenumab 140 mg group; review of the event did not suggest a causal association for erenumab. This female patient had multiple risk factors for venous thrombosis: age ≥40 years, body mass index >30 kg/m2, a prior history of deep vein thrombosis and pulmonary embolism, recurrent heparin-induced thrombocytopenia, and a recent infection (sepsis, pyelonephritis, and kidney infection about 7 weeks before the event). No other ischemic vascular event was reported in any treatment group in the pooled 12-week placebo-controlled analysis.
Table 3.
Treatment-emergent vascular adverse events in migraine prevention studies
In the targeted searches for vascular AEs during any erenumab exposure (either double-blind or open-label), the exposure-adjusted patient incidence rate for an AE of hypertension was 2.5 per 100 patient-years for erenumab 70 mg and 1.7 per 100 patient-years for erenumab 140 mg (table 3). The exposure-adjusted patient incidence rate for an AE of cerebral venous thrombosis was 0.2 per 100 patient-years for 140 mg, including the AE described above for the double-blind period and another AE during open-label erenumab treatment. The latter AE occurred in a 38-year-old man who experienced subdural hematoma, cerebral venous thrombosis, and facial bone fractures as a result of closed head injury sustained during a fall while hiking. He was found unconscious and under the influence of Ecstasy (3,4-methylenedioxy-methamphetamine). Two patients had an AE of myocardial ischemia (<0.1 per 100 patient-years for 70 mg), both of which occurred during open-label erenumab treatment. One of these patients had demand ischemia that was a positively adjudicated vascular event (see description in the next section). The other patient had exercise-induced ischemia and had taken sumatriptan 4 hours before the exercise test. This patient received a total of 2 doses of erenumab 70 mg, with the second dose 36 days before the event. The dose of sumatriptan on the day of the event was 50 mg, and the patient's previous dose of sumatriptan was administered 68 days before the event. Other AEs in the searches for vascular events occurred in only 1 patient each. Review of the individual events did not suggest a causal association for erenumab.
Adjudicated vascular events
Eighteen patients had AEs that fulfilled criteria for adjudication. The blinded, independent, clinical experts adjudicated the events as cardiovascular in origin for 4 patients. All occurred during open-label erenumab treatment. For each adjudicated event, investigators considered the respective AEs as not related to erenumab treatment; adjudicators did not assess the relationship of positively adjudicated events to treatment. Details on these 4 events are given below.
A 55-year-old woman in the ARISE study had AEs of multifocal pneumococcal bacteremia, life-threatening acute respiratory distress syndrome, atrial fibrillation, myocardial (demand) ischemia, and elevated troponin levels on day 135, during open-label treatment with erenumab 70 mg.
A 54-year-old man in study NCT01952574 with a history of hypertensive cardiovascular disease died of arteriosclerosis and hypertensive heart disease on day 650 of exposure during open-label treatment with erenumab 70 mg. On autopsy, there was evidence of severe coronary atherosclerosis with presence of alcohol and stimulants (phenylpropanolamine and norpseudoephedrine). This case was reported previously.21
A 44-year-old man in the STRIVE study with body mass index of 30 kg/m2, high triglycerides, a history of abnormal ECG findings (first-degree atrioventricular block, intraventricular conduction defect, and anomalies of repolarization), and mild mitral prolapse died after 12 months of exposure to erenumab during open-label treatment with 140 mg. He was asymptomatic for the duration of the study, including during physical exertion. Postmortem genetic assessment revealed a genetic form of arrhythmogenic right ventricular cardiomyopathy/dysplasia, which predisposes to sudden cardiac death.23 Consistent with this diagnosis, postmortem findings included hyperplastic sclerosis of coronary arteries, fat infiltration of the right heart chamber musculature, hypertrophy of the left cardiac musculature, and dilation of both cardiac chambers. The direct cause of death was attributed to heart failure.
A 64-year-old man in study NCT02174861 with a history of hypertension and migraine with aura had monocular visual blurring lasting 2 minutes on day 209 of exposure during open-label treatment with erenumab 140 mg (10 weeks after last dose). The event was reported as an AE of TIA.
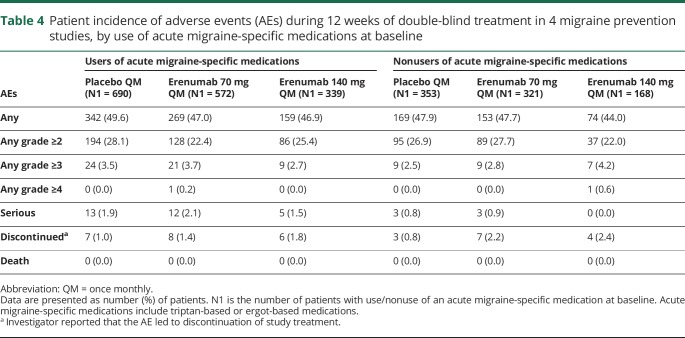
AEs by use of acute migraine-specific medications
Patients who used acute migraine-specific medications at baseline had similar incidences of AEs and serious AEs across the placebo and erenumab treatment groups (table 4). Overall, patients using acute migraine-specific medications had a slightly higher incidence of serious AEs compared with nonusers of acute migraine-specific medications. There was no evidence of an increased incidence of vascular AEs or serious vascular AEs among patients using acute migraine-specific medications.
Table 4.
Patient incidence of adverse events (AEs) during 12 weeks of double-blind treatment in 4 migraine prevention studies, by use of acute migraine-specific medications at baseline
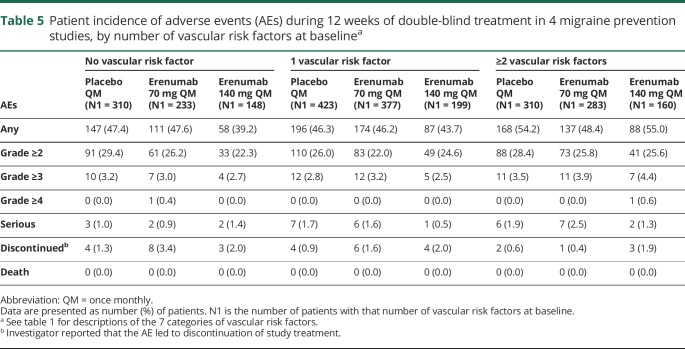
AEs by number of vascular risk factors at baseline
Regardless of the number of vascular risk factors at baseline, incidences of AEs were similar across the placebo and erenumab treatment groups (table 5). In the subgroup of patients with at least 2 vascular risk factors at baseline, there were slightly higher incidences of AEs or serious AEs compared with the subgroups of patients with no risk factor or 1 risk factor at baseline, but incidences were balanced across the placebo and erenumab groups. There was no evidence of an increased incidence of vascular AEs or serious vascular AEs among patients with vascular risk factors at baseline.
Table 5.
Patient incidence of adverse events (AEs) during 12 weeks of double-blind treatment in 4 migraine prevention studies, by number of vascular risk factors at baselinea
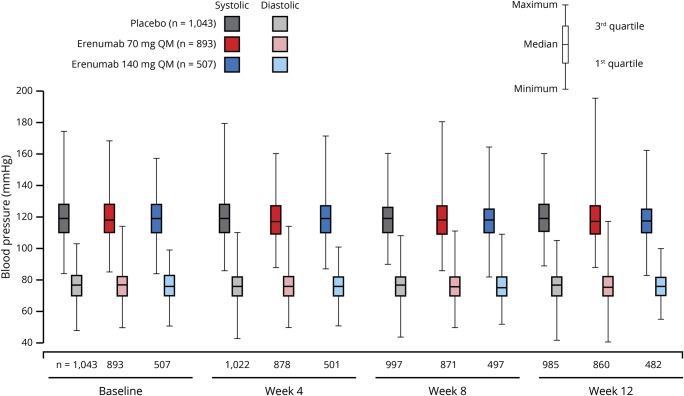
Blood pressure changes
In the integrated analysis of the 4 migraine prevention studies, resting BP readings were similar across the treatment groups and across the study visits (figure 1). Mean (SD) change in SBP from baseline to week 12 was −0.4 (9.6) mm Hg in the placebo group, −0.5 (10.0) mm Hg in the erenumab 70 mg group, and −1.0 (9.8) mm Hg in the erenumab 140 mg group. Mean (SD) change in DBP from baseline to week 12 was −0.6 (7.1) mm Hg in the placebo group, −0.3 (7.7) mm Hg in the erenumab 70 mg group, and −0.4 (7.2) mm Hg in the erenumab 140 mg group.
Figure 1. Blood pressure by visit in patients with migraine during 12 weeks of double-blind treatment in 4 migraine prevention studies.
QM = once monthly.
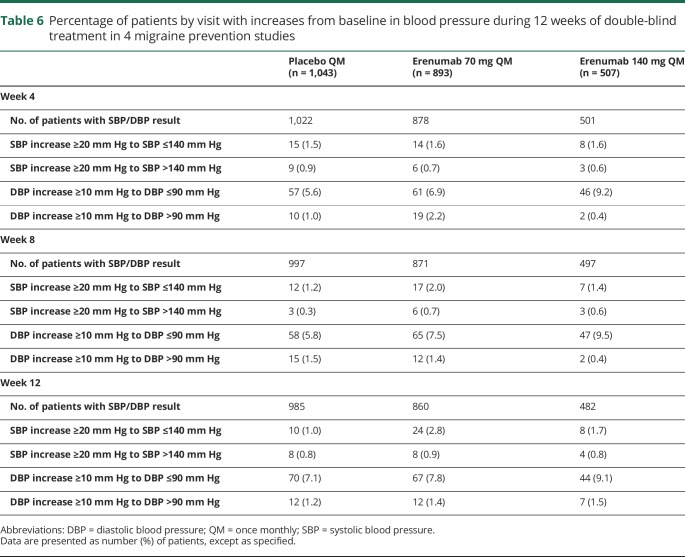
There were no clinically relevant differences across treatment groups for a SBP increase of ≥20 mm Hg or a DBP increase of ≥10 mm Hg from baseline at weeks 4, 8, and 12 (table 6).
Table 6.
Percentage of patients by visit with increases from baseline in blood pressure during 12 weeks of double-blind treatment in 4 migraine prevention studies
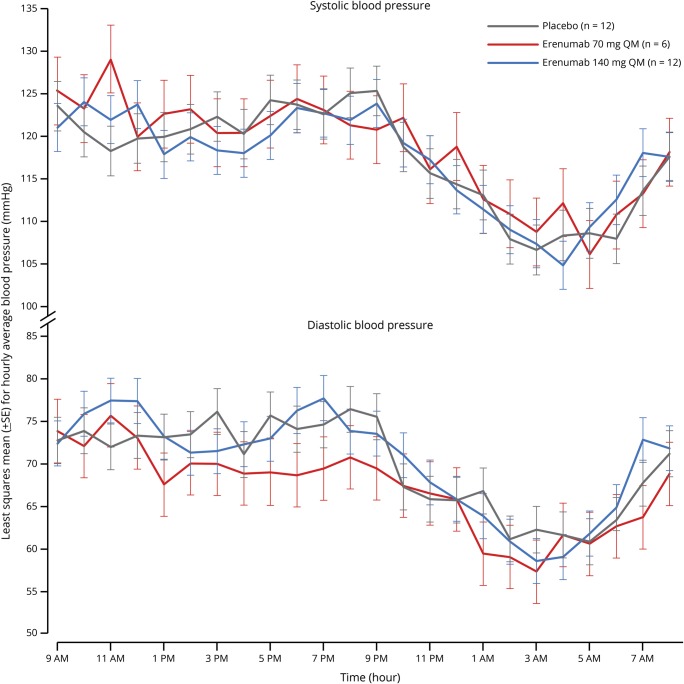
In the 24-hour continuous BP monitoring study, SBP and DBP after 12 weeks of treatment in healthy controls were similar in the erenumab 70 mg group and the erenumab 140 mg group compared with the placebo group, and erenumab had no effect on the diurnal pattern of BP (figure 2). Similar results were observed for 24-hour continuous BP monitoring after the first and second monthly doses of placebo or erenumab in healthy controls, or after each dose of study treatment in patients with migraine (data not shown).
Figure 2. Twenty-four-hour continuous blood pressure in healthy controls after 12 weeks of double-blind treatment in a phase 1 study.
Assessment from day 64; patients received study treatment on days 1, 29, and 57.
Discussion
During 12 weeks of double-blind treatment in 4 clinical studies of migraine prevention, the incidences of AEs were similar across the placebo, erenumab 70 mg, and erenumab 140 mg groups, with no evidence of dose dependency for safety. Review of both individual and aggregate AEs did not find evidence of an association between erenumab treatment and vascular events, including cardiovascular, cerebrovascular, or peripheral vascular events. Vascular ischemic events occurred during open-label erenumab treatment and were confounded with plausible alternative etiologies.
Subgroup analyses were conducted to examine the overall safety of erenumab in patients with migraine who might be at higher risk of vascular events. Approximately two-thirds of the patients were using an acute migraine-specific medication at baseline. Triptans and ergot-based medications have vasoconstrictive effects that may contribute to a higher risk of cardiovascular or cerebrovascular AEs with these acute medications.14,15 Thus, triptans and ergot-based medications have relative contraindications for use in patients at high risk of vascular events, which includes more than 900,000 men and women with migraine in the United States.24 In this analysis, the incidences of AEs were similar between the erenumab and placebo groups, supporting that adding erenumab to an acute migraine-specific medication did not increase risk compared with adding placebo to an acute migraine-specific medication. Overall, users of acute migraine-specific medications had a slightly higher incidence of serious AEs compared with nonusers of acute migraine-specific medications.
Among patients with common vascular risk factors at baseline, the incidences of AEs were similar across the placebo and erenumab treatment groups. Regardless of treatment group, the incidences of AEs and serious AEs were slightly higher in the subgroup of patients with at least 2 vascular risk factors than in those with 0 or 1 risk factor.
Erenumab treatment for 12 weeks also had no relevant effect on BP compared with placebo, either for resting measurements at monthly study visits in patients with episodic or chronic migraine, or for continuous 24-hour ambulatory BP monitoring after 12 weeks of treatment. There were no clinically relevant differences in the number of patients with a ≥10 mm Hg increase in DBP or a ≥20 mm Hg increase in SBP, no difference between erenumab and placebo for mean SBP or DBP, and no imbalances in outliers for BP. This is consistent with the similar incidence of clinically reported AEs of hypertension between the placebo and erenumab treatment groups during the first 12 weeks of the placebo-controlled studies, and a similar incidence of hypertension AEs during long-term erenumab treatment. Collectively, these analyses supported the vascular safety profile for erenumab across different study periods, populations, and methodologies, providing a comprehensive examination of the safety of erenumab treatment for migraine prevention.
Supportive information for these findings was provided by ex vivo studies that showed no vasoactive effect of erenumab beyond its blockade of the vasodilatory effect of CGRP.25,26 In those studies, which measured arterial segments from brain and coronary vessels, exposure to erenumab alone did not affect vascular contractility. Because erenumab is selective for the canonical CGRP receptor, the absence of an effect of erenumab on vascular tone may not be generalizable to other agents that block CGRP from binding to other receptors in the calcitonin-like receptor family or to the CGRP ligand itself.27–29
Recent clinical studies provide additional support for the vascular safety of erenumab. An exercise treadmill safety study in 88 patients with coronary artery disease and stable angina showed no aggravation of myocardial ischemia in the patients who received erenumab compared with those who received placebo.30 Erenumab did not decrease exercise duration as measured by the change from baseline in total exercise time compared to placebo (the primary endpoint of the study). That study enrolled patients with coronary artery disease and stable angina; the study population was older (median age ∼65 years) and contained a higher proportion of men (78%) than this pooled analysis.
A drug–drug interaction study in 34 healthy controls found that concomitant administration of erenumab 140 mg with the acute migraine-specific medication sumatriptan had no clinically meaningful or additive effects on resting BP compared with sumatriptan alone.22 Because the healthy controls in the latter study received sumatriptan, those with elevated BP or heart rate, a prolonged QT interval, or a history of major vascular event or major cardiovascular intervention were excluded from the study.
Strengths of this safety analysis in approximately 2,500 patients with migraine were the size and characteristics of the patient population and the overall extent of exposure to erenumab. Patient demographics (mostly female and mean age of approximately 40 years) were representative of patients with migraine, supporting the generalizability of the results to other patients with chronic or episodic migraine. Safety results from the individual studies were also consistent across patients with either chronic or episodic migraine.17–20 The number of patients was complemented by duration of exposure. The total exposure to erenumab was 2,639 patient-years, and one of the episodic migraine studies includes open-label treatment for up to 5 years. As noted above, the exclusion of patients with major vascular events within 12 months before each study was a limitation of the analysis, but this is typical for most studies of migraine treatment or prevention. In addition, patients with symptomatic vascular disease for more than 12 months before screening were allowed to participate in the erenumab studies of migraine prevention. Hypertension was not an exclusion criterion in the ARISE or STRIVE studies, and patients could participate in the other studies if they did not have poorly controlled hypertension. Thus, patients who might be excluded from many migraine prevention studies were allowed to enroll in the erenumab studies.
This safety analysis used data from the controlled setting of clinical trials. Additional safety data from real-world analyses would be useful to confirm the vascular safety of erenumab with widespread use in a broader patient population. A placebo comparator group was not available for the longer-term safety data in this analysis. A limitation of any open-label study is contextualization of events that occur when there is no comparator group. Assessment and adjudication of potential vascular ischemic events by a panel of clinical experts confirmed only 4 events as being vascular in origin. Each occurred during open-label erenumab treatment, when the adjudicators were not blinded to the patient's study treatment. While the independent adjudicators did not determine treatment relatedness, the investigators did not consider any of these events to be related to treatment. BP also seems to have been unaffected by erenumab treatment. If cardiovascular and cerebrovascular AEs do not occur more often, it might still be speculated that their severity is increased with blockade of the CGRP receptor. In these clinical studies, investigators were not specifically asked to judge whether the study treatment influenced the severity of vascular AEs. Given the rarity of these events (only 4 events in the whole dataset were deemed cardiovascular in origin), only substantial long-term real-world safety data will help address this question. Standardized searches for AE terms of vascular events used narrow search terms to increase specificity of the analyses. Sensitivity analyses using broad search terms identified 2 additional events that were not specific to vascular injury during any exposure to erenumab: T-wave inversion on ECG (n = 1) and increase in blood creatine phosphokinase (n = 17). Review of the individual AEs of increased blood creatine phosphokinase indicated that these were isolated events with no association with cardiac injury (most of them related to intense physical activity).
In this integrated analysis, erenumab had a vascular safety profile comparable to that of placebo over 12 weeks, and the overall safety profiles for erenumab and placebo were similar regardless of whether patients also used an acute migraine-specific medication or had risk factors for vascular events. A vascular safety signal did not emerge with longer-term treatment with dose-blinded or open-label erenumab for up to 256 weeks. Results from ex vivo studies and other clinical studies provided support for the vascular safety of erenumab treatment. Further study of long-term vascular safety of erenumab in patients with migraine is needed.
Glossary
- AE
adverse event
- ARISE
Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) Compared to Placebo in Migraine Prevention
- BP
blood pressure
- CGRP
calcitonin gene-related peptide
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- MedDRA
Medical Dictionary for Regulatory Activities
- NSAID
nonsteroidal anti-inflammatory drug
- STRIVE
Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) in Migraine Prevention
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This study was funded by Amgen Inc. and Novartis.
Disclosure
D. Kudrow has participated in advisory boards for Amgen, Novartis, Alder, Eli Lilly, Biohaven, and Promius; and has received research support from Amgen-Novartis, Alder, Teva, Eli Lilly, Biohaven, Zosano, Allergan, Roche-Genentech, Dr. Reddy's Laboratories, Novartis, and UCB. J. Pascual has received research support from Allergan and personal fees from Allergan, Stendhal, and Amgen-Novartis. P. Winner is an investigator in clinical trials sponsored by Teva, Amgen, Genentech, Novartis, Allergan, AstraZeneca, Biogen Idec, Ipsen, and Lilly; has participated in advisory boards for Teva, Amgen, Avanir, Novartis, Allergan, Supernus, and Lilly; and has been on speakers' bureaus for Allergan, Amgen, Avanir, Lilly, Promius, Novartis, and Supernus. D. Dodick has received personal fees from Amgen, Association of Translational Medicine, University Health Network, Daniel Edelman Inc., Autonomic Technologies, Axsome, Aural Analytics, Allergan, Alder, Biohaven, Charleston Laboratories, Dr. Reddy's Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, University of British Columbia, Zosano, ZP Opco, Foresite Capital, and Oppenheimer; has received CME fees or royalty payments from Healthlogix, Medicom Worldwide, Medlogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, and Wolters Kluwer Health; has stock options for Aural analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, GBS/Nocira, Matterhorn/Ontologics, and King-Devick Technologies; has been a consultant without fee for Aural Analytics, Healint, Second Opinion/Mobile Health, and Epien; has a board of directors position for Epien, Matterhorn/Ontologics, and King-Devick Technologies; has a patent without a fee (Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis); has received professional society fees or reimbursement for travel from American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, International Headache Society, and Canadian Headache Society; and has a use agreement through employer for Myndshft. S. Tepper has received research support (no personal compensation) from Alder, Allergan, Amgen, ATI, Dr. Reddy's, ElectroCore, eNeura, Neurolief, Scion Neurostim, Teva, and Zosano; is a consultant for Acorda, Alder, Alexsa, Allergan, Alphasights, Amgen, ATI, Axsome Therapeutics, Biohaven, Charleston Labs, DeepBench, Dr. Reddy's, ElectroCore, Eli Lilly, eNeura, GLG, Guidepoint Global, GSK, M3 Global Research, Magellan Rx Management, Medicxi, Navigant Consulting, Neurolief, Nordic BioTech, Novartis, Pfizer, Reckner Healthcare, Relevale, Satsuma, Scion Neurostim, Slingshot Insights, Sorrento, Sudler and Hennessey, Supernus, Teva, Theranica, Trinity Partners, XOC, and Zosano; holds stock options from ATI; receives royalties from Springer; and receives a salary from Dartmouth-Hitchcock Medical Center and the American Headache Society. U. Reuter has received personal fees from Amgen, Alder, Allergan, Autonomic Technologies, Eli Lilly, ElectroCore, Medscape, Novartis, StreaMedUp, and Teva. F. Hong is an employee and stockholder of Novartis. J. Klatt is an employee and stockholder of Novartis. F. Zhang is an employee and stockholder of Amgen Inc. S. Cheng is an employee and stockholder of Amgen Inc. H. Picard is an employee and stockholder of Amgen Inc. O. Eisele is an employee and stockholder of Amgen Inc. J. Wang is an employee and stockholder of Amgen Inc. J. Latham received personal fees from Amgen Inc. for medical writing support. D. Mikol is an employee and stockholder of Amgen Inc. Go to Neurology.org/N for full disclosures.
References
- 1.GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Burden of Disease Collaborators, Mokdad AH, Ballestros K, et al. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US States. JAMA 2018;319:1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 4.Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention Study. Headache 2007;47:355–363. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International Burden of Migraine Study (IBMS-II). Headache 2013;53:644–655. [DOI] [PubMed] [Google Scholar]
- 6.Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015;35:478–488. [DOI] [PubMed] [Google Scholar]
- 7.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010;6:573–582. [DOI] [PubMed] [Google Scholar]
- 8.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat Rev Neurol 2018;14:338–350. [DOI] [PubMed] [Google Scholar]
- 9.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MaassenVanDenBrink A, Terwindt GM, van den Maagdenberg AMJM. Calcitonin gene-related peptide (receptor) antibodies: an exciting avenue for migraine treatment. Genome Med 2018;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murinova N, Krashin DL, Lucas S. Vascular risk in migraineurs: interaction of endothelial and cortical excitability factors. Headache 2014;54:583–590. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018;8:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wackenfors A, Jarvius M, Ingemansson R, Edvinsson L, Malmsjö M. Triptans induce vasoconstriction of human arteries and veins from the thoracic wall. J Cardiovasc Pharmacol 2005;45:476–484. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein SD. The pharmacology of ergotamine and dihydroergotamine. Headache 1997;37(suppl 1):S15–S25. [PubMed] [Google Scholar]
- 16.Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther 2016;356:223–231. [DOI] [PubMed] [Google Scholar]
- 17.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425–434. [DOI] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017;377:2123–2132. [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382–390. [DOI] [PubMed] [Google Scholar]
- 20.Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018;38:1026–1037. [DOI] [PubMed] [Google Scholar]
- 21.Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: interim analysis of an ongoing open-label study. Neurology 2017;89:1237–1243. [DOI] [PubMed] [Google Scholar]
- 22.de Hoon J, Van Hecken A, Vandermeulen C, et al. Phase I, randomized, double-blind, placebo-controlled, single-dose, and multiple-dose studies of erenumab in healthy subjects and patients with migraine. Clin Pharmacol Ther 2018;103:815–825. [DOI] [PubMed] [Google Scholar]
- 23.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 24.Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 2017;57:1507–1521. [DOI] [PubMed] [Google Scholar]
- 25.Ohlsson L, Haanes K, Kronvall E, Xu C, Snellman J, Edvinsson L. Erenumab (AMG 334), a monoclonal antagonist antibody against the canonical CGRP receptor, does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries. Epub 2019 Jul 31. [DOI] [PubMed] [Google Scholar]
- 26.Beltrán AER, Ramírez AL, Bogers AJ, et al. Pharmacological selectivity of inhibition of CGRP-induced relaxations by erenumab studied in human isolated internal mammary artery (abstract MTIS2018-041). Cephalalgia 2018;38:30. [Google Scholar]
- 27.Poyner D. Pharmacology of receptors for calcitonin gene-related peptide and amylin. Trends Pharmacol Sci 1995;16:424–428. [DOI] [PubMed] [Google Scholar]
- 28.Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol 2013;170:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2015;2:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache 2018;58:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at amgen.com/datasharing.