Abstract
Objective
To test the hypotheses that insufficient duration, high fragmentation, and poor sleep quality are temporally associated with migraine onset on the day immediately following the sleep period (day 0) and the following day (day 1).
Methods
In this prospective cohort study of 98 adults with episodic migraine, participants completed twice-daily electronic diaries on sleep, headaches, and other health habits, and wore wrist actigraphs for 6 weeks. We estimated the incidence of migraine following nights with short sleep duration, high fragmentation, or low quality compared to nights with adequate sleep with conditional logistic regression models stratified by participant and adjusted for caffeine intake, alcohol intake, physical activity, stress, and day of week.
Results
Participants were a mean age of 35.1 ± 12.1 years. We collected 4,406 days of data, with 870 headaches reported. Sleep duration ≤6.5 hours and poor sleep quality were not associated with migraine on day 0 or day 1. Diary-reported low efficiency was associated with 39% higher odds of headache on day 1 (odds ratio [OR] 1.39, 95% confidence interval [CI] 1.06–1.81). Actigraphic-assessed high fragmentation was associated with lower odds of migraine on day 0 (wake after sleep onset >53 minutes, OR 0.64, 95% CI 0.48–0.86; efficiency ≤88%, OR 0.74, 95% CI 0.56–0.99).
Conclusion
Short sleep duration and low sleep quality were not temporally associated with migraine. Sleep fragmentation, defined by low sleep efficiency, was associated with higher odds of migraine on day 1. Further research is needed to understand the clinical and neurobiologic implications of sleep fragmentation and risk of migraine.
Migraine affects about 12% of adults and is the second leading cause of disability worldwide.1,2 Migraine is characterized by paroxysms of severe headaches associated with sensory, autonomic, and cognitive perturbations. Ninety-five percent of patients with migraine attribute their headaches to at least one migraine trigger,3 with sleep disturbance as one of the most common triggers, reported by nearly half of patients with migraine.3–6 Recent neurophysiologic research suggests that migraine may be mediated by the same neurotransmitters that control the states of sleep and wake,7–9 supporting a plausible mechanism of the association. However, few studies have prospectively examined the precise temporal relationships between nightly sleep and subsequent daily risk of migraine.
Most of the prior studies investigating sleep as a trigger of migraine evaluated cross-sectional associations between sleep disturbance and migraine and relied on retrospective reports of sleep.4,5,10–12 A few studies prospectively collected data on sleep in adults with chronic migraine in the naturalistic setting,13–15 though only one examined the temporal association between nightly sleep and headache onset.16 Other short-term laboratory-based studies used overnight polysomnographic assessments to examine nightly sleep and migraine onset in small samples.17–19 However, there is a gap in knowledge about the independent role of sleep characteristics as a temporal precedent of migraine in the naturalistic setting.
Therefore, we conducted a prospective cohort study of patients with episodic migraine who provided detailed daily information on sleep, headaches, and behavioral and psychological characteristics every morning and evening, and wore actigraphs for objective assessment of sleep for 6 weeks. We used a repeated-measures analysis to test the hypotheses that insufficient duration, high fragmentation, and self-rated poor sleep quality are associated with migraine onset on the day immediately following the sleep period (day 0) and the following day (day 1).
Methods
Study setting
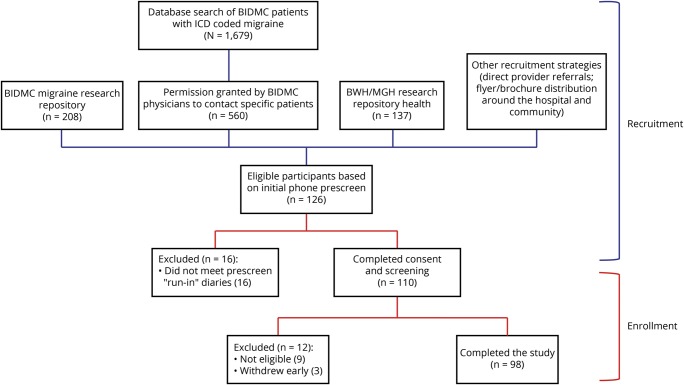
Between March 2016 and August 2017, we recruited participants from multiple sources. The majority were patients of the headache, neurology, and primary care practices at Beth Israel Deaconess Medical Center (BIDMC) who were identified (1) by a physician diagnosis of migraine in the hospital online medical record; (2) by use of a research repository at BIDMC of patients with migraine who had previously participated in or expressed interest in future migraine research; and (3) by direct referrals from neurologists and primary care providers at BIDMC. We initially contacted these patients by phone or letter. In addition, we posted paper flyers advertising the study around the BIDMC campus and surrounding areas, and posted an electronic advertisement on a Brigham and Women's Hospital (BWH)/Massachusetts General Hospital research repository for patients with migraine who have expressed interest in receiving information about research studies (figure 1).
Figure 1. Diagram of recruitment and enrollment.
BIDMC = Beth Israel Deaconess Medical Center; BWH = Brigham and Women's Hospital; ICD = International Classification of Diseases; MGH = Massachusetts General Hospital.
Interested individuals initially underwent phone screening to see if they met preliminary criteria for inclusion, followed by a 1-week diary run-in. For patients in the BIDMC system, online medical records were reviewed for information to confirm a diagnosis of migraine. These individuals were scheduled for a screening visit based on the discretion of the study physician. All potential participants were then interviewed by 1 of 2 physicians, who were trained in internal medicine and had experience in diagnosing migraine. An in-depth migraine and treatment history was obtained and in-depth health history reviewed, and only those patients who met criteria for migraine with or without aura based on International Classification of Headache Disorders criteria were enrolled.
Following enrollment, participants completed baseline questionnaires on demographics, medical history, and habits. They completed twice-daily web-based electronic diaries and wrist actigraphy for 6 weeks, for which they received verbal and written instructions at the baseline visit. Study data were collected using REDCap (Research Electronic Data Capture)20 hosted at BIDMC. All visits were conducted at BIDMC. The BIDMC Committee on Clinical Investigations approved the study. All participants provided written informed consent.
Study population
Participants with episodic migraine were enrolled if they were ≥18 years of age, reported history of migraine for at least 3 years and at least 2 migraine headaches per month during the last 3 months, met the criteria for International Classification of Headache Disorders for migraine with or without aura21–23 based on physician diagnosis, were able to communicate in English, and gave informed consent. Exclusion criteria included ≥15 headache days per month for the previous 3 months, chronic pain condition, current opioid use, high risk of obstructive sleep apnea24 or known untreated obstructive sleep apnea, pregnancy, uncontrolled medical problems that precluded participation, and failure to complete 4 days out of 7 days of run-in diaries. Among 126 individuals interested in participation, 101 met inclusion criteria and agreed to participate. Three withdrew with <21 days of data, resulting in a study population of 98 participants who completed the study and provided 4,406 days of data. We estimated that our final sample size would provide 80% power to detect an incidence rate ratio of 1.50 between the exposed and unexposed periods, using a 2-sided test, though this estimation did not account for within-person variability.
Sleep assessments
Every morning participants completed the Consensus Sleep Diary.25 It includes questions on sleep timings, quality (very poor, poor, fair, good, very good), awakenings, and medications. Participants wore an actigraph (Actiwatch Spectrum; Philips Respironics, Murrysville, PA) on their nondominant wrist for 24 hours a day for 42 consecutive days.26 Actigraphs collected data on movement and environmental light in 30-second intervals, and indicated any off-wrist time. Trained research coordinators provided instructions on use. Upon return, data were transmitted to the BWH Sleep Reading Center for scoring.26,27
A trained technician blinded to information on headache status scored actigraphy data using a standardized protocol. The technician manually identified the start and stop of the rest period using a hierarchical approach.28 Once the rest interval was designated, sleep/wake status for each 30-second epoch was determined using the Actiware 6.0 algorithm,29 which weights the activity counts in relationship to activity levels in the surrounding 2-minute periods, using a wake threshold activity count of 40. Sleep onset was defined as 5 minutes of immobile time and sleep offset as the last epoch in the rest interval.30
From these data, we calculated total sleep time, wake after sleep onset (WASO, minutes awake after sleep onset), and sleep efficiency (proportion of total sleep duration/duration of rest period). We defined cutpoints for duration as ≤6.5 hours, >6.5 hours to <8.5 hours, and ≥8.5 hours, consistent with clinical guidelines.31 This also approximated to the 25th and 75th percentiles of the distribution in our sample. Cutpoints for WASO correspond to the 75th percentile of their distribution and were categorized as high WASO, >12 minutes on diary and >53 minutes on actigraphy; cutpoints for sleep efficiency correspond to the 25th percentile of their distribution and we categorized low efficiency as ≤90% on diary and ≤88% on actigraphy. Low sleep quality was categorized as poor or very poor.
Migraines
Each morning and evening, participants reported presence of headache, time of onset, and whether the headache was ongoing. When the participant reported headache resolution, he or she provided additional information about time of resolution, duration, pain intensity, use of headache medications, and associated symptoms (e.g., photophobia), as well as if their headache was “similar to their usual migraines” (yes/no). When headache onset was reported within 1 calendar day of a prior headache's resolution, it was considered a relapse. Two headaches were excluded due to their reported short duration (1 and 10 minutes, respectively).
Covariates and sample characteristics
Each morning, participants reported the number of alcoholic servings they drank the previous day. Premenopausal women reported their menstrual cycle day. Each evening, patients reported the number of caffeinated beverages consumed and minutes of moderate (e.g., fast walking, average bicycling) and vigorous (e.g., fast bicycling, running) physical activity that day. Every evening, participants rated their current level of stress—“How stressed do you feel?”—using a visual analog scale (0, not at all, to 100, as much as possible). In addition, we collected information on sociodemographics, medical history, medication use, sleep,32,33 and migraine-related quality of life34 at the baseline visit.
Statistical analysis
In this prospective cohort study, we estimated incidence of migraine following nights with short sleep duration, high fragmentation, or low quality (exposed) compared to nights with adequate sleep (unexposed) using fixed-effects repeated-measures analyses.35 The fixed-effects repeated-measures analyses effectively eliminates confounding by risk factors that are constant within individuals over the observation period but often differ between participants, such as age and other long-term risk factors for migraine. To minimize the potential for reverse causality, the calendar day following migraine resolution was not included in analyses. The start of at-risk periods was defined as the day following headache termination. We excluded headaches reported as starting during nighttime sleep (n = 110).
Given limited missing data, we employed complete-case analyses. We constructed conditional logistic regression models stratifying by participant to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between sleep duration, fragmentation, and quality and headache onset on the day immediately following the main sleep period (day 0) and on the following day (day 1). While the fixed-effects repeated-measures analysis eliminates confounding by fixed or slowly-varying characteristics (e.g., sex, age, other chronic risk factors), there can be confounding by time-varying characteristics that are related to sleep and headache. Therefore, our analyses adjusted for daily servings of alcohol and caffeine intake (categorical), self-reported physical activity (continuous), stress (continuous), and day of week. In secondary analyses, we further adjusted for menstrual cycle day and nightly use of a sedative/hypnotic, separately. Since previous research suggests that the association between sleep and headache may be related to cumulative sleep insufficiency over several days,16 we also examined the odds of headache following 2 consecutive nights of sleeping ≤6.5 hours. To test the robustness of our results, we conducted sensitivity analyses excluding headaches classified as unlikely to be migraine (n = 18). In secondary post hoc analyses, we explored potential effect modification by migraine with aura status. Two-sided p values of <0.05 were considered statistically significant. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Data availability
Deidentified data not published within this article will be made available by request from any qualified investigator. To gain access, data requestors will need to sign a data access and use agreement. Data will be shared via secured portal.
Results
Participant characteristics
Tables 1 and 2 list the characteristics of the 98 participants who completed the study. At baseline, over 2-thirds (68.4%) of the sample attributed too little sleep as a trigger to their migraines. Twenty-six percent of participants used daily migraine prophylactic medication. On average, baseline assessments indicated low levels of sleep disturbance, depressive symptoms, or perceived stress.
Table 1.
Baseline characteristics among 98 participants with episodic migraine followed for 6 weeksa
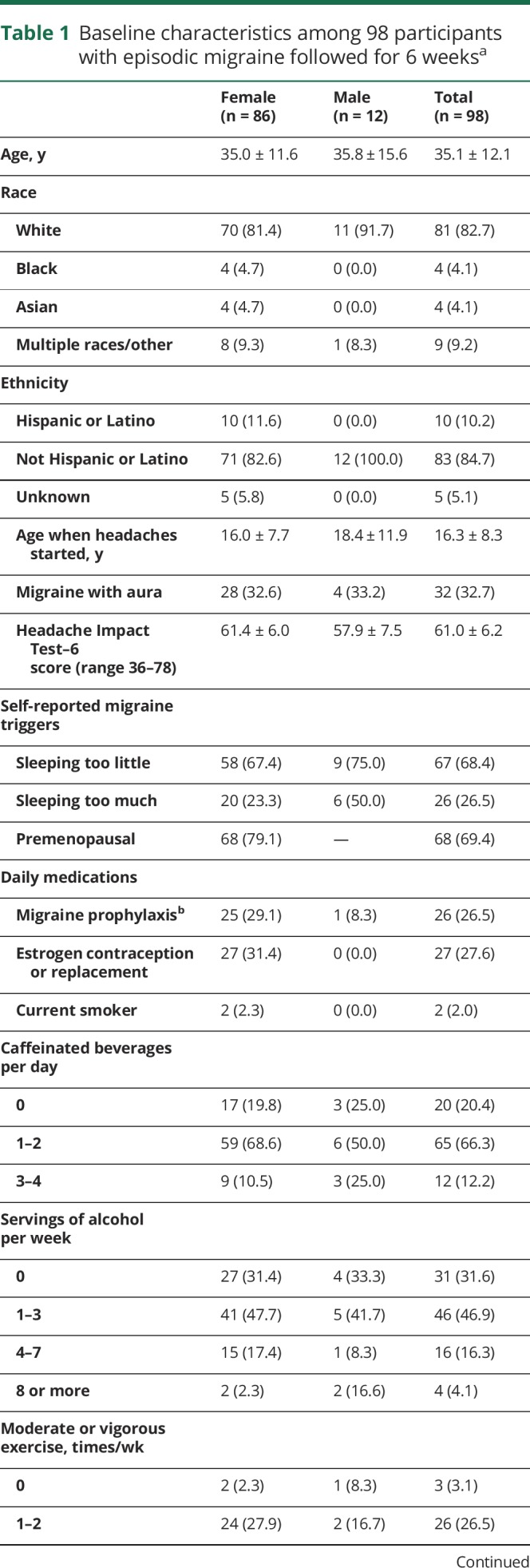
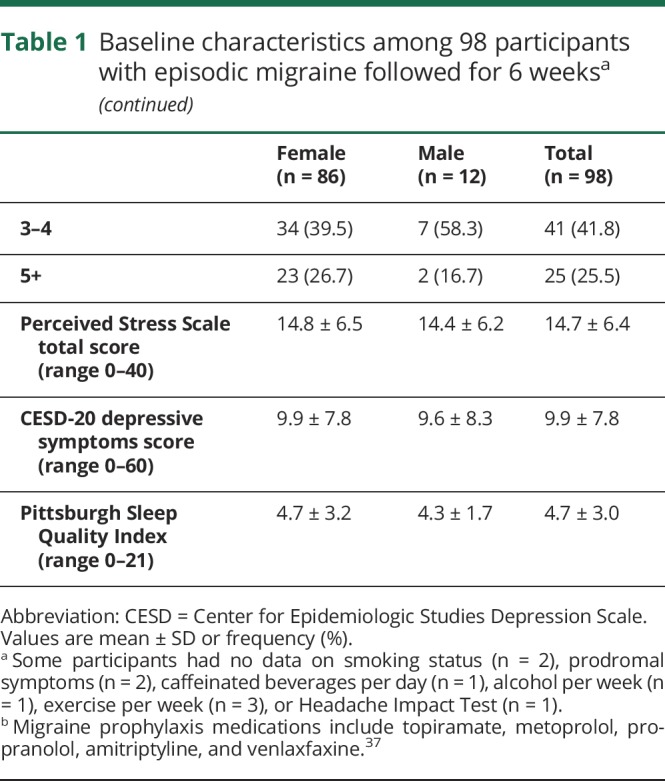
Table 2.
Daily characteristics among 98 participants with episodic migraine during the 6-week observation period (n = 4,406 days)
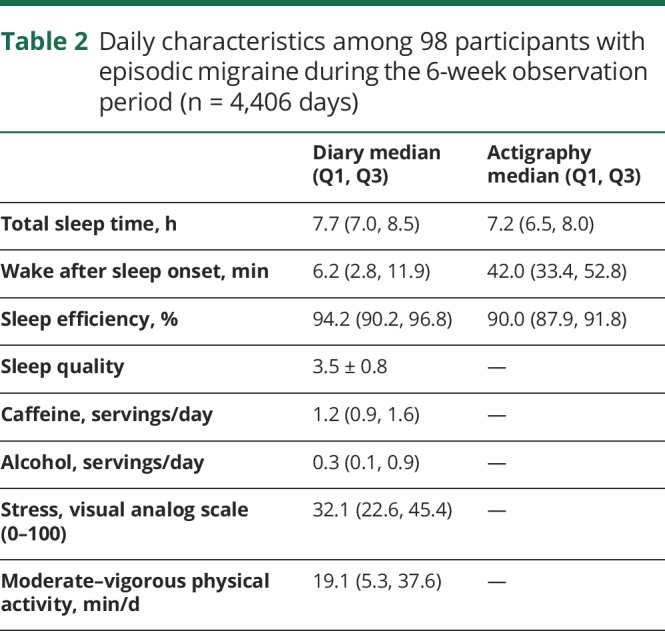
During the 6-week study, participants reported a total of 870 headaches with an average of 8.4 headaches per participant (range 1–20). During this period, average diary-reported total sleep time was 7.7 hours per night, with low levels of reported sleep disturbance as indicated by low WASO (11.9 ± 18.0 minutes) and high sleep efficiency (94.2, interquartile range 90.2%–96.8%). The average sleep duration estimated by actigraphy was 7.3 hours (SD 1.2), with higher sleep fragmentation as indicated by an average WASO of 44.8 minutes.
Day immediately following the sleep period (day 0)
We did not observe any associations between diary-based sleep characteristics and headache on day 0 (figure 2A). There was a 36% lower odds of headache following a night of actigraphy-based high WASO (OR 0.64, 95% CI 0.48–0.86) and low sleep efficiency (OR 0.74, 95% CI 0.56–0.99) (figure 2B). We did not observe any associations between actigraphic-estimated shorter or longer sleep duration and headache on day 0.
Figure 2. Odds of headache, day immediately following the sleep period (day 0).

(A) Diary. (B) Actigraphy. WASO = wake after sleep onset.
Following day (day 1)
Diary-based low sleep efficiency was associated with 39% higher odds of headache on day 1 (OR 1.39, 95% CI 1.07–1.81). No other statistically significant associations were observed (figure 3A). However, we observed higher odds of headache on day 1 following a night of actigraphic-assessed low sleep efficiency (OR 1.17, 95% CI 0.88–1.55), with CIs that overlapped 1.0 (figure 3B).
Figure 3. Odds of headache, following day (day 1).

(A) Diary. (B) Actigraphy. WASO = wake after sleep onset.
Our results were not substantively altered when we further adjusted for menstrual cycle day or nightly sedative/hypnotic use, or excluded headaches classified as unlikely to be migraine, or when we used cutpoints for sleep duration based on each individual's distributions. Two consecutive nights of sleep duration ≤6.5 hours as assessed by diary or actigraphy were also not significantly associated with headache (diary OR 1.14, 95% CI 0.85–1.51; actigraphy OR 0.97, 95% CI 0.71–1.32). In secondary post hoc exploratory analyses, for which we had limited power, we observed higher odds of headache on day 1 following a night of self-reported low sleep efficiency (OR 1.87, 95% CI 1.31–2.68) and poor/very poor sleep quality (OR 1.79, 95% CI 1.14–2.82) only among participants reporting a history of migraine without aura at baseline.
Discussion
In this prospective cohort study of 98 adults with episodic migraine, we did not observe associations between nightly diary-reported short sleep duration, high sleep fragmentation, or low sleep quality. However, nightly lower sleep efficiency was associated with subsequent headache onset in patients with episodic migraine not on the day immediately following sleep (day 0), but on day 1 (the following day). Specifically, nightly diary-assessed low sleep efficiency was associated with 39% higher odds of headache on day 1 (the following day), even after accounting for daily caffeine, alcohol, physical activity, and stress. Similarly, nightly actigraphic-assessed low sleep efficiency had an apparent 16% higher odds of headache onset on day 1. We also observed that nightly actigraphy-assessed high fragmentation was associated with a 64%–74% lower odds of migraine on the day immediately following sleep (day 0). These data provide preliminary evidence that nightly low sleep efficiency temporally precedes headache onset in patients with episodic migraine on day 1 (but not day 0), but that nightly high actigraphy-assessed fragmentation may relate to lower odds of headache onset on day 0.
Unexpectedly, we did not observe a temporal relationship between nightly sleep duration of ≤6.5 hours and odds of headache. To our knowledge, only 2 previous studies reported the temporal relationship between nightly self-reported sleep duration and headaches in adults with migraine in the naturalistic setting. Park et al.15 prospectively studied 62 adults with episodic migraine for 3 months. Headaches occurred on 55.1% of days with reported sleep deprivation, but was not more likely to be reported on migraine vs nonmigraine headache days. In another sample of 55 adults including 33 with chronic migraine and 22 with chronic tension-type headache, Houle et al.16 observed that 2 consecutive nights of insufficient sleep (<4 hours each night) was predictive of a headache, while 2 nights of adequate sleep (∼8 hours) was protective, after accounting for daily stress. Though our data suggest that ≤6.5 hours of sleep a night does not influence migraine onset, it is plausible that more severe sleep deprivation, such as 2 nights of sleep <4 hours, may affect migraine onset. We could not address this hypothesis in this sample due to the infrequency of severe sleep restriction across multiple nights. Future work in larger populations is needed to investigate which potential thresholds of cumulative nights of sleep of <4 hours may place patients with episodic migraine at higher risk of headache, as these periods may represent critical windows for intervention as well as potentially elucidate shared underlying neurobiologic processes.
Our study is among the first to examine the relationship between nightly sleep fragmentation and headache on day 1 (the following day), and represents the largest study collecting objective sleep data in adults with episodic migraine to date. While our findings generally support patient attribution of sleep disturbance as a trigger of migraine,3–5 our results indicate that nightly sleep fragmentation precedes headache onset not on the day immediately after the sleep period (day 0), but on the following day (day 1). Thus, it is plausible that fragmented sleep may represent changes in hypothalamic control or homeostasis that may portend higher risk of migraine more than 24 hours prior to headache onset. Another potential explanation may be that low sleep fragmentation, as assessed by actigraphy, may be an early marker of headache onset. These findings support the need for future research investigating distinct signatures of sleep that precede headache onset by a few days.
Most of the prior literature17,18 examining the role of nightly sleep fragmentation (vs duration or quality) and migraine evaluated EEG measures of sleep fragmentation during 1- to 4-night stays in a laboratory and presence of migraine on the following day (day 0), with results showing no associations between sleep fragmentation and headache. Similarly, one study examining nightly actigraphic-assessed sleep in 18 children with migraine for 2 weeks reported no association between sleep fragmentation and headache on day 0.13 This is in contrast to our findings that suggest an association between actigraphic-assessed higher sleep fragmentation, defined as WASO >53 minutes and efficiency ≤88%, and lower risk of headache onset on day 0. These differences may reflect measurements of sleep (polysomnography vs actigraphy), in-laboratory vs habitual assessments of sleep, or differences in study samples (adults vs children). Nonetheless, these findings may be consistent with emerging data indicating that decreased cortical activation during sleep may indicate a higher risk of migraine on the day immediately following the sleep period.18,36 These data also highlight the importance of utilizing prospective and objective assessments of nightly sleep in the naturalistic setting as part of future investigations seeking to elucidate patterns of habitual sleep characteristics and headache risk among patients with migraine.
To our knowledge, this study represents the first prospective cohort study to evaluate the temporal relationships between various dimensions of nightly subjectively and objectively assessed sleep in adults with episodic migraine in the naturalistic setting studied over a prolonged period. Participants in this prospective cohort completed morning and evening electronic daily diaries and wore wrist actigraphs for at least 6 weeks, which are thought to represent information about habitual sleep patterns and are distinct from information collected from diaries.26,27 Prospective and objectively collected data minimize recall bias, which is a risk when data collection occurs after headache onset, while the fixed-effects repeated-measures analysis accounts for many individual characteristics that remain stable or vary slowly over time (e.g., demographics, chronic health conditions, habitual health behaviors, daily medications). In addition, we used a standardized protocol for actigraphy scoring, with all scoring performed by a single-blinded technician. We also had high rates of adherence to diary completion.
Our study has some limitations. Headache timings and sleep assessments are potentially prone to misclassification. Though polysomnography is the gold standard for assessing sleep, its costs and burden preclude its widespread use in longer-term epidemiologic and clinical studies. We could not assess sleep architecture, but we used actigraphy, which represents a validated approach to noninvasive objective sleep assessments. It should be noted that previous research indicates that actigraphy tends to overestimate polysomnographic WASO by about 5 minutes for WASO <30 minutes and underestimate polysomnographic WASO for WASO ≥30 minutes.29 To minimize the influence of the systematic measurement error, we had participants wear the same device throughout the observation period and we used fixed-effects repeated-measures analyses to compare each individual to himself or herself. Thus the absolute cutoffs for actigraphy may differ from polysomnographic measures of WASO, but our results should be internally valid with respect to actigraphically assessed WASO. We were not able to capture information on other daily exposures that may relate to sleep and migraine onset, and some of our instruments (e.g., stress assessment) might not have fully captured daily fluctuations. This is another potential explanation for our day 0 findings. Finally, our sample had low levels of sleep disturbance, so our results may not be generalizable to patient populations with more disrupted sleep, such as patients with chronic insomnia comorbid with migraine.
Our findings provide new data showing that while nightly short sleep duration does not appear to trigger migraine, variability in sleep fragmentation temporally precedes migraine onset. Future studies are needed to evaluate the role of sleep fragmentation as a potential target for reducing risk of migraine attacks as well as a potential marker for more frequent headaches in patients with episodic migraine.
Glossary
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women's Hospital
- CI
confidence interval
- OR
odds ratio
- WASO
wake after sleep onset
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Suzanne Bertisch: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Wenyuan Li: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Catherine Buettner: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. Elizabeth Mostofsky: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Michael Rueschman: data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Emily Kaplan: analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Jacqueline Fung: data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data. Shaelah Huntington: data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. Tess Murphy: data acquisition, accepts responsibility for conduct of research and final approval. Courtney Stead: data acquisition, study concept or design, accepts responsibility for conduct of research and final approval, study supervision. Rami Burstein: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval. Susan Redline: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Murray Mittleman: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis.
Study funding
No industry sponsored this work. This work was funded by grants from the National Institute of Neurologic Disorders and Stroke (R21-NS091627) and the American Sleep Medicine Foundation. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the NIH, or the funding organizations.
Disclosure
S. Bertisch reports receiving research support from Merck, Sharpe & Dohme and Lockheed Martin (DARPA primary funding source) and has served as a consultant for Verily. W. Li reports no disclosures relevant to the manuscript. C. Buettner has received honorarium for consulting for Dr. Reddy Pharmaceutical. E. Mostofsky, M. Rueschman, E. Kaplan, J. Fung, S. Huntington, T. Murphy, and C. Stead report no disclosures relevant to the manuscript. R. Burstein has received research grants from Depomed, Teva, Trigemina, Allergan, and Strategic Science and Technologies, and has received honoraria for consulting with Dr. Reddy Pharmaceutical, Allergan, Teva, Trigemina, and Pernix. S. Redline and M. Mittleman report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 3.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394–402. [DOI] [PubMed] [Google Scholar]
- 4.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache 2010;50:1366–1370. [DOI] [PubMed] [Google Scholar]
- 5.Chabriat H, Danchot J, Michel P, et al. Precipitating factors of headache: a prospective study in a national control-matched survey in migraineurs and nonmigraineurs. Headache 1999;39:335–338. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino ABW, Davis-Martin RE, Houle TT, et al. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia 2018;38:1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vgontzas A, Pavlović JM. Sleep disorders and migraine: review of literature and potential pathophysiology mechanisms. Headache 2018;58:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437:1257. [DOI] [PubMed] [Google Scholar]
- 9.Noseda R, Borsook D, Burstein R. Neuropeptides and neurotransmitters that modulate thalamo-cortical pathways relevant to migraine headache. Headache 2017;57:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lateef T, Swanson S, Cui L, et al. Headaches and sleep problems among adults in the United States: findings from the National Comorbidity Survey-Replication study. Cephalalgia 2011;31:648–653. [DOI] [PubMed] [Google Scholar]
- 11.Walters AB, Hamer JD, Smitherman TA. Sleep disturbance and affective comorbidity among episodic migraineurs. Headache 2014;54:116–124. [DOI] [PubMed] [Google Scholar]
- 12.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache 2005;45:904–910. [DOI] [PubMed] [Google Scholar]
- 13.Bruni O, Russo PM, Violani C, et al. Sleep and migraine: an actigraphic study. Cephalalgia 2004;24:134–139. [DOI] [PubMed] [Google Scholar]
- 14.Ong JC, Taylor HL, Park M, et al. Can circadian dysregulation exacerbate migraines? Headache 2018;58:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JW, Chu MK, Kim JM, et al. Analysis of trigger factors in episodic migraineurs using a smartphone headache diary applications. PLoS One 2016;11:e0149577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houle TT, Butschek RA, Turner DP, et al. Stress and sleep duration predict headache severity in chronic headache sufferers. Pain 2012;153:2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engstrom M, Hagen K, Bjork M, et al. Sleep-related and non-sleep-related migraine: interictal sleep quality, arousals and pain thresholds. J Headache Pain 2013;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goder R, Fritzer G, Kapsokalyvas A, et al. Polysomnographic findings in nights preceding a migraine attack. Cephalalgia 2001;21:31–37. [DOI] [PubMed] [Google Scholar]
- 19.Lovati C, D'Amico D, Bertora P, et al. Correlation between presence of allodynia and sleep quality in migraineurs. Neurol Sci 2010;31(suppl 1):S155–S158. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olesen J. International Classification of Headache Disorders. Lancet Neurol 2018;17:396–397. [DOI] [PubMed] [Google Scholar]
- 22.Olesen J, Steiner T. The International Classification of Headache Disorders (ICDH-II). 2nd ed. London: BMJ Publishing Group; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Kong Q, Chen J, et al. International Classification of Headache Disorders 3rd edition beta-based field testing of vestibular migraine in China: demographic, clinical characteristics, audiometric findings and diagnosis statues. Cephalalgia 2016;36:240–248. [DOI] [PubMed] [Google Scholar]
- 24.Senaratna CV, Perret JL, Matheson MC, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2017;36:116–124. [DOI] [PubMed] [Google Scholar]
- 25.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2018;14:1209–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss AR, Johnson NL, Berger NA, et al. Validity of activity-based devices to estimate sleep. J Clin Sleep Med 2010;6:336–342. [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SR, Weng J, Rueschman M, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep 2015;38:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013;36:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakley N. Validation With Polysomnography of the Sleepwatch Sleep/Wake Scoring Algorithm Used by the Actiwatch Activity Monitoring System. Bend: Mini Mitter, Cambridge Neurotechnology; 1997. [Google Scholar]
- 31.Mukherjee S, Patel SR, Kales SN, et al. An official American Thoracic Society statement: the importance of healthy sleep: recommendations and future priorities. Am J Respir Crit Care Med 2015;191:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2012;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Rendas-Baum R, Varon SF, et al. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011;31:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken: John Wiley & Sons; 2012. [Google Scholar]
- 36.Strenge H, Fritzer G, Goder R, et al. Non-linear electroencephalogram dynamics in patients with spontaneous nocturnal migraine attacks. Neurosci Lett 2001;309:105–108. [DOI] [PubMed] [Google Scholar]
- 37.Silberstein SD. Preventive migraine treatment. Continuum 2015;21:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data not published within this article will be made available by request from any qualified investigator. To gain access, data requestors will need to sign a data access and use agreement. Data will be shared via secured portal.