Abstract
Calcineurin inhibitors (CNI) are both the savior and Achilles heel of kidney transplantation. Though CNI have significantly reduce rates of acute rejection, their numerous toxicities can plague kidney transplant recipients. By 10 years, virtually all allografts will have evidence of CNI nephrotoxicity. CNI have been strongly associated with hypertension, dyslipidemia, and new onset of diabetes after transplantation – significantly contributing to cardiovascular risk in the kidney transplant recipient. Multiple electrolyte derangements including hyperkalemia, hypomagnesemia, hypercalciuria, metabolic acidosis, and hyperuricemia may be challenging to manage for the clinician. Finally, CNI-associated tremor, gingival hyperplasia, and defects in hair growth can have a significant impact on the transplant recipient’s quality of life. In this review, the authors briefly discuss the pharmacokinetics of CNI and discuss the numerous clinically relevant toxicities of commonly used CNIs, cyclosporine and tacrolimus.
Keywords: calcineurin inhibitors, tacrolimus, cyclosporine, drug toxicity, kidney transplantation
Introduction
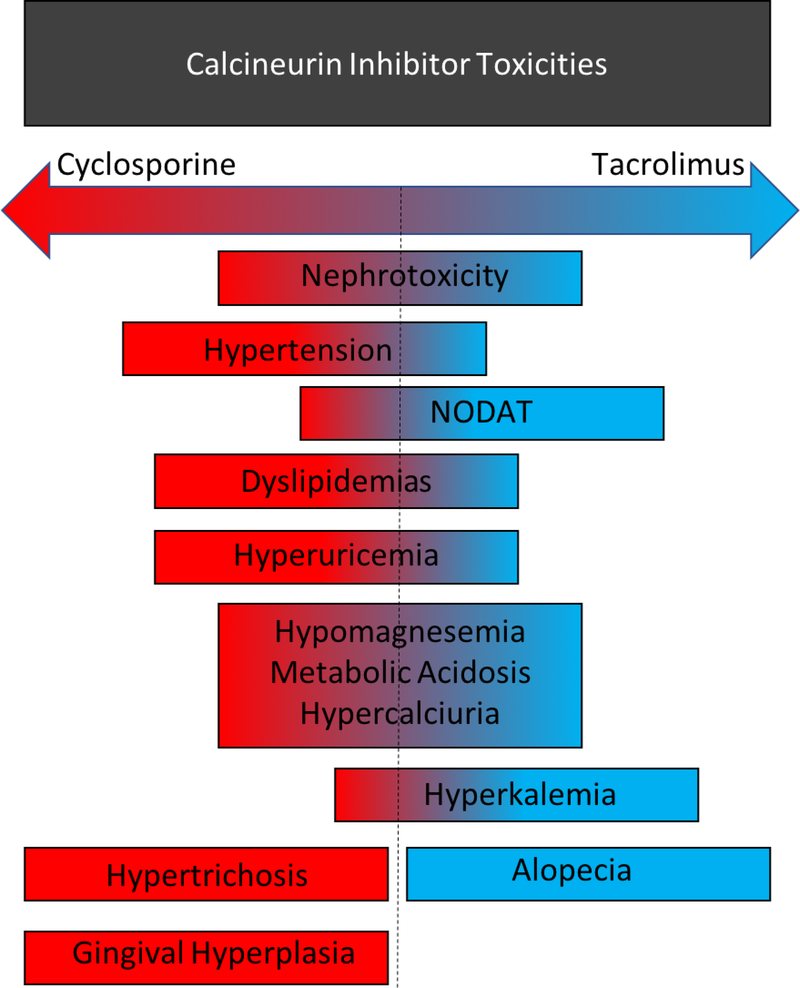
Calcineurin inhibitors (CNIs) have been the backbone of solid organ transplant immunosuppression for several decades, with over 90% of kidney transplant recipients maintained on CNI containing immunosuppression regimens in 2017.1 Outside of transplantation, CNIs are now also being studied and utilized in the treatment of a variety of immune mediated glomerular diseases.2–5 Though CNIs have been successful in preventing acute kidney transplant rejection, their use has been described as a significant contributing factor in acute and chronic allograft injury and ultimately allograft loss – with virtually universal presence of CNI nephrotoxicity on allograft biopsy by 10 years after kidney transplant.6 Here, we review the pharmacokinetics and pharmacodynamics of CNIs, specifically cyclosporine A (CsA) and tacrolimus (FK506 or FK), and discuss the diverse side effect profile including deleterious effects on the kidney and electrolyte homeostasis as well as effects on the cardiovascular, endocrine, nervous, and integumentary systems (Figure 1).
Figure 1. Toxicities of Cyclosporine and Tacrolimus.
The calcineurin inhibitors, cyclosporine and tacrolimus, share many toxicities. Some toxicities are more common with or specific to a particular drug.
CsA was isolated in 1971, from the fungus Tolypocladium inflatum. Its immunosuppressive properties were first described in animals in 1976,7 with demonstration of clinical benefits among transplant recipients by Sir Roy Calne at the University of Cambridge in 1979.8 CsA was ultimately approved for use in kidney transplant recipients by the United States Food and Drug Administration in 1983. A few years later, FK was used in transplant recipients by Dr. Thomas Starzl at the University of Pittsburgh.9 CsA and FK cause immunosuppression through inhibition of T cell activation by binding to their respective intracellular immunophilins, cyclophilin and FK-binding protein 12. The CNI-immunophilin complex subsequently binds and inhibits the dephosphorylation activity of the phosphatase calcineurin, which impairs nuclear translocation of nuclear factor of activated T cells (NFAT), transcription of interleukin-2, and ultimately T cell activation and proliferation.10–12
Pharmacokinetics
CsA and FK can be administered orally (PO), sublingually (SL), or intravenously (IV). PO CNIs have variable intrapatient oral bioavailability, with area-under-the-curves fluctuating up to 50% based on formulation, timing, and concomitant administration with food.13 However, PO CNIs present the lowest potential risk for systemic toxicity and subsequently should be the preferred route of administration whenever possible. Both CsA and FK come in several PO formulations with varying pharmacokinetics and bioavailability, necessitating close monitoring of trough levels if formulations are changed. Following absorption, CNIs are highly plasma protein bound, lipophilic drugs with a large volume of distribution. Metabolism is predominantly hepatically mediated via the cytochrome P450 enzyme system (CYP3A), and metabolites are then excreted in the bile with an elimination half-life ranging from 10–48 hours.14 The therapeutic effects of FK are prolonged relative to CsA owing to a highly active metabolite with equal immunosuppressive potency to the parent drug, compared to CsA metabolites that have only 10–20% of parent drug activity. Polymorphisms resulting in CYP3A5 loss of function may also significantly influence drug metabolism and exposure, and lead to higher incidence of CNI-related nephrotoxicity.15–19 Similarly, polymorphisms in ABCB1 which encodes the efflux transporter P-glycoprotein present in enterocytes, hepatocytes, and kidney cells may influence oral bioavailability and drug clearance as well as CNI concentrations in kidney tubular epithelial cells.20 Though the current mainstay of pharmacokinetic monitoring is 12 hour trough level measurement (C0) of CsA and FK, studies have shown poor correlation with the 0 – 12 hour area-under-the-curve (AUC) and the optimal pharmacokinetic monitoring of CNIs remains controversial.21
Pharmacodynamics
CNIs have a narrow therapeutic window, with low levels increasing the risk of acute allograft rejection while high levels increase the risk of nephrotoxicity that may lead to acute or chronic kidney allograft injury.22, 23 Unfortunately, real-time biomarkers to assess the pharmacodynamic efficacy of CNIs are relatively limited. Measurement of anti-human leukocyte antigen (HLA) antibodies remains the only clinically available pharmacodynamic assessment of CNI efficacy. More specific pharmacodynamic assays include calcineurin phosphatase activity and cytokine expression and production but these are not yet utilized in routine clinical practice. Instead assessment of CNI pharmacodynamic dosing adequacy generally relies upon routine clinical assessment for signs of medication or immune-related toxicities, which may signify over or under-immunosuppression respectively.
Nephrotoxicity
Acute Nephrotoxicity
Acute nephrotoxicity can occur at any time post-transplantation and at any level of drug exposure, but is most commonly observed in patients with supratherapeutic trough levels > 20 ng/mL.22 Acute toxicity is of particular concern immediately after transplantation as CNI-induced vasoconstriction can cause delayed-graft function (DGF) post kidney transplant or primary nonfunction and can impair recovery from AKI of other etiologies. Acute CNI toxicity often presents with an increase in plasma creatinine concentration due to acute afferent arteriole vasoconstriction. However, vasoconstriction and increased allograft vascular resistance may occur prior to clinically evident nephrotoxicity.24 The mechanism is likely due to significant impairment of endothelial cell function resulting from decreased production of vasodilating prostaglandin E2 and nitric oxide and increased production of thromboxane and endothelin in the afferent arteriole.25, 26 This leads to an acute reduction in renal blood flow (RBF), which is reversible after CNI dose reduction or drug cessation. CNIs are also associated with the acute development of de novo thrombotic microangiopathy (TMA) resulting in AKI, hemolytic anemia, and thrombocytopenia. The exact mechanism has not been fully elucidated but likely involves CNI-induced vascular endothelial cell injury, and the risk increases with concomitant use of mTOR inhibitors.27
CNI toxicity may be more pronounced in the setting of volume depletion and diuretic use, older donor age,28 high doses of CSA29, 30 or FK,31 concomitant use of nephrotoxic drugs especially NSAIDs,32 concomitant use of CYP3A4/5 or P-glycoprotein inhibitors, or patients with genetic polymorphisms leading to altered CYP3A4/5 and P-glycoprotein function.33–35 Accordingly, genetic testing for polymorphisms in the CYP3A4/5 or ABCB1 genes may aid in CNI dosing and prognostication for patients likely to have high CNI peak exposures.36–42 Additionally, there is some evidence in animals and in vitro that decreased P-glycoprotein expression may contribute to increased renal CsA levels, leading to nephrotoxicity.43, 44 The use of CYP3A inhibitors can also increase while inducers can decrease total CNI exposure, and CNI dose adjustments are often required upon the initiation or discontinuation of moderate-severe CYP3A/P-glycoprotein inhibitors or inducers (Table 1). Lastly, diarrhea can produce variable effects on CNI levels depending on the etiology. Infectious diarrhea can increase CNI levels due to impaired activity of intestinal CYP3A and decreased drug efflux by intestinal P-glycoprotein,45 while osmotic diarrhea can decrease intestinal CNI absorption resulting in reduced drug levels.
Table 1.
Commonly Used Inhibitors and Inducers of Cytochrome P450 Isoform CYP3A
| CYP3A Inducers | CYP3A Inhibitors |
|---|---|
| Rifampin | Ketoconazole, fluconazole, clotrimazole |
| Phenobarbital | Erythromycin |
| Efavirenz | Diltiazem, verapamil |
| Carbamazepine | Ritonavir, cobicistat, nelfinavir |
| Dexamethasone | Cannabidiol |
| Phenytoin |
Symptoms of PO CNI overdose are generally mild and may include confusion, hypertension, somnolence, nausea, and headache. In contrast, IV overdose is associated with increased morbidity and led to one death due to cerebral edema in a retrospective analysis of CNI overdoses reported to a Swiss poison center.46 This study also found that enteral decontamination with activated charcoal or nasogastric tube aspiration may reduce drug absorption. Of the 28 reported CNI overdoses, 22 cases involved patients already receiving immunosuppression, 5 involved household contacts of CNI-treated patients, and 1 was unknown. Additionally, 13 were iatrogenic errors, 12 were suicidal intent, and 3 were accidental occurring at home. The majority (70%) of iatrogenic errors involved non-capsule drug formulations (PO liquid and IV).
The management of acute nephrotoxicity in the setting of CNI overdose is generally supportive and resolves with a reduction in CNI dose. Highly protein bound CNIs are not effectively cleared with extracorporeal treatment modalities, but renal replacement therapy may still be indicated for volume overload or electrolyte disturbances in the setting of CNI-induced acute kidney injury (AKI). Inducers of the p450 system have been occasionally used to lower toxic CNI levels. However, evidence is limited to several case reports that describe the use of phenytoin or phenobarbital, while use of rifampin or carbamazepine have not been reported.47 Additionally, enzyme induction is not immediate and is typically delayed until at least 48–72 hours after initiation of the medication inducer raising concerns for timing and the potential efficacy of this strategy. Further clinical studies are required before this approach can be recommended for routine treatment of acute CNI toxicity.
With increasing use of recreational and medicinal cannabis worldwide, including cannabidiol (CBD), potential interactions with CNI metabolism are of interest48 as CBD inhibits CYP3A4.49 A case report of a woman taking FK for interstitial nephritis while enrolled in a high dose purified CBD clinical trial of up to almost 3 grams daily, demonstrated a 3-fold increase in dose normalized FK serum concentration.50 CBD inhibits hepatic CSA metabolism in vitro and in mice, but the impact on CSA levels has not been studied in humans.51 As a result of an unregulated market for CBD, product labeling is inaccurate and intermittent use of different brands and products may contribute to unpredictable CNI levels and the potential for toxicity or underdosing.
Of interest, CNI-induced nephrotoxicity has been explored in a novel 3D bioprinted proximal tubule-on-a-chip model where CsA was shown to disrupt cell morphology and cytoskeleton organization leading to disruption of the epithelial barrier function.52 Cell culture based microphysiological models offer a promising means for studying CNI-induced nephrotoxicity.
Chronic Nephrotoxicity
CKD and ESRD can result from chronic CNI exposure in many solid organ transplant recipients.53 Chronic CNI nephrotoxicity commonly presents as an irreversible, progressive decline in allograft function, which is likely from a combination of chronic hemodynamic effects and direct tubular effects. CNI-induced vascular endothelial injury and arteriolar vasoconstriction leads to repeated episodes of allograft ischemia and chronic kidney hypoperfusion, which is exacerbated by salt depletion.54–57 It is difficult to clinically and histologically distinguish CNI-induced nephrotoxicity from chronic allograft nephropathy. Key kidney biopsy findings in chronic CNI toxicity include obliterative arteriolopathy/hyalinization of the afferent arteriole, ischemic collapse or glomerular scarring, tubule vacuolization, focal and global segmental glomerulosclerosis, focal interstitial fibrosis associated with macrophage influx, and tubular atrophy often referred to as striped fibrosis. 6, 58–61 The severity of biopsy findings correlates with dose and duration of CNI use. As with acute nephrotoxicity, patients with polymorphisms coding for functional CYP3A and P-glycoprotein and patients taking CYP3A or P-glycoprotein inducers, both of which increase overall CNI dose requirements, may be at an increased risk for chronic CNI nephrotoxicity due to higher peak drug exposures and increased circulating CNI metabolites.
Several potential therapies have been considered in an attempt to prevent or reverse the cascade of effects resulting from CNI-induced arteriolar vasoconstriction. Calcium channel blockers (CCBs) are generally considered first-line antihypertensives immediately following kidney transplantation and may be beneficial in combating the vasoconstrictive effects of CNIs, though clinical studies have not shown superiority in blood pressure lowering or preservation of kidney function over other anti-hypertensive agents.62–64 In one study of kidney transplant recipients, CsA induced kidney vasoconstriction and the reduction in GFR and RBF were completely prevented by the addition of a CCB.65 The degree of reduction correlated with dose and peak CsA levels. Choice of the optimal CCB agent remains controversial as anti-proteinuric effects are generally greater with non-dihydropyridine CCBs when compared to dihydropyridine CCB, but use of these agents may be complicated by CYP3A4/P-glycoprotein mediated drug interactions. In some instances, the non-dihydropyridines CCBs, diltiazem and verapamil, can be used in transplant recipients to lower the total CNI dose and therefore, the cost of therapy. Diltiazem also attenuates CNI-induced vasoconstriction although there is no definitive evidence that diltiazem prevents chronic CNI nephrotoxicity. Angiotensin converting enzyme inhibitors (ACE-Is) or angiotensin II receptor antagonists (ARBs) also work to block the downstream effects of CNI-induced vasoconstriction although their use remains controversial after transplantation due to potential overlapping toxicities with CNIs. Despite these concerns, a small study demonstrated equivalent efficacy in blood pressure lowering between ACE-I and CCBs post-transplant.66 The comparison between RAAS blockade and CCBs remains controversial. RAAS blockade attenuates CSA induced interstitial fibrosis and arteriolopathy in rats.56, 67 Among kidney transplant recipients, ARBs decrease circulating plasma levels of TGFβ, a cytokine which plays a central role in causing CNI-induced interstitial fibrosis.68 However, a recent meta-analysis has shown inadequate evidence to determine if RAAS blockade improves clinical outcomes in kidney transplant recipients.69
Urinary biomarkers show promise in detecting CNI-induced nephrotoxicity although their use remains in pre-clinical stages. In rats treated with CSA for 3 weeks, increased urinary KIM-1, TNF-α, fibronectin, and microalbuminuria indicated acute CsA nephrotoxicity while a delayed increase in urinary osteopontin and TGF-β indicated chronic CsA nephrotoxicity.70
Though treatment options for now are limited to minimizing CNI exposure at the risk of suboptimal immunosuppression, conversion to belatacept has been shown to stabilize eGFR in patients with chronic CNI nephrotoxicity.71
Electrolyte disorders
CNIs cause numerous electrolyte disturbances including hyperkalemia, metabolic acidosis, hypercalciuria, and hyperuricemia. The clinical significance and possible mechanisms will be discussed. A comprehensive discussion of all CNI-induced electrolyte and acid/base derangements has been reviewed elsewhere.72
Hyperkalemia
While hypertension may be more common with CsA, hyperkalemia may be more common with FK.73 Hyperkalemia is common early post-transplant due to DGF, trimethoprim use, and higher serum CNI levels. CNI-induced hyperkalemia has been attributed to volume expansion induced pseudohypoaldosteronism, with suppression of the renin-angiotensin-aldosterone system.74 In a clinical study, CsA treated patients demonstrated an impaired ability to excrete a PO potassium load and had lower plasma renin activity when supine and after standing compared to those treated with AZA.74 Another study attributed the hyperkalemia to a tubular insensitivity to aldosterone that was partially overcome by stimulating bicarbonaturia.75 Subsequent studies have also hypothesized that CNIs may impair the ability of the distal convoluted tubule to regulate the activity of the sodium chloride cotransporter NCC in response to changes in extracellular potassium.76 Impaired potassium secretion in the aldosterone sensitive distal nephron (ASDN) may also contribute, as CsA has been shown to inhibit apical potassium channels in principal cells of the rabbit cortical collecting duct.77
CNI-induced hyperkalemia is commonly associated with hypertension, hyperchloremic metabolic acidosis, and hypercalciuria, a similar phenotype to Gordon’s syndrome or familial hyperkalemia and hypertension (FHHt). Case series have reported a prevalence of CNI-induced Gordon’s phenotype in between 10–33% of kidney transplant recipients treated with CNIs. Recent investigations in humans and animals have implicated the with-no-lysine (WNK) kinase pathway in the pathogenesis of CNI-induced hyperkalemia 78–81 which is discussed here and the associated hypertension is discussed in a later section. In distal convoluted tubular epithelial cells, calcineurin functions to activate kelch-like 3 (KLHL3), a component of the E3 ubiquitin ligase complex, which targets WNK1 and WNK4 for degradation. In mice, FK prevented KLHL3 activation and therefore unregulated WNK1 and WNK4-SPS1-related proline/alanine-rick kinase (SPAK) mediated activation of NCC, contributing to salt-sensitive hypertension.82 Loss of KLHL3 in the collecting duct increases paracellular chloride conductance through interactions with claudin-8 to recapitulate Gordon’s syndrome.83 Increased chloride reabsorption in the ASDN prevents the generation of a favorable lumen-negative potential for potassium secretion, that may contribute to hyperkalemia.75 Therefore it is tempting to speculate CNIs cause hypertension and hyperkalemia by increasing chloride reabsorption in the ASDN.
Although clinical evidence is lacking, mechanistic studies suggest that CNI-induced hyperkalemia should be treated with alkali salts and thiazide diuretics. Fludrocortisone may also effectively manage CNI-induced hyperkalemia but at the expense of raising blood pressure. Despite the common use of fludrocortisone to treat hyperkalemia, evidence is scarce in kidney transplant recipients and is mostly limited to case reports/series in adults84–87 and children.88, 89 In a small retrospective study of OLT recipients, fludrocortisone decreased serum potassium without effect on serum creatinine, systolic blood pressure, or diastolic blood pressure over 14 days.90
Metabolic Acidosis
Chronic metabolic acidosis is associated with increased risk of graft loss, death-censored graft failure, and mortality among kidney transplant recipients, and this may potentially be exacerbated by concomitant CNI therapy.91, 92 Acidosis may be due to impaired tubular acid secretion from either a direct effect of hyperkalemia, CNI-induced effects, or low aldosterone levels. Additional contributing mechanisms for acidosis include CsA and FK induced reductions in Na+/K+-ATPase pump activity in the medullary thick ascending limb of the loop of Henle and cortical collecting duct in vitro.93, 94 CsA but not FK may also promote distal renal tubular acidosis by interfering with the adaptation of β-intercalated cells to acidosis.95 CNIs increase the kidney avidity to sodium and chloride predisposing to volume expansion, hyperchloremic acidosis, and hypertension. Hyperchloremia can in turn decrease RBF and worsen kidney hypoperfusion through afferent vasoconstriction.96 As chronic CNI toxicity is also mediated by afferent vasoconstriction, we speculate tubular mediated hyperchloremia may also contribute to accelerated GFR decline. Alkali therapy prolongs survival and kidney outcomes among patients with CKD.97 However, the benefits of sodium bicarbonate among kidney transplant recipients remains unknown. An ongoing multi-center, randomized placebo-controlled trial aims to test if sodium bicarbonate treatment will preserve kidney graft function and decrease kidney function decline following transplantation.98
Hypercalciuria
Hypercalciuria is common among kidney transplant recipients treated with CNIs, and CNIs have in turn been shown to cause hypercalciuria in animal models.99 CNIs also cause hypocitraturia, increasing the risk of developing urolithiasis or nephrocalcinosis,100 which can be detrimental to the kidney allograft. CsA has been shown to impair kidney calcium reabsorption through reduced TRPV5 expression in mice,101 and induce high turnover bone disease.102 Thiazides reverse CNI-induced Gordon’s phenotype and are also commonly used to reduce hypercalciuria and prevent recurrent calcium nephrolithiasis in native kidneys, however, no studies have directly studied the efficacy of alkali therapy or thiazides to reduce the risk of calcium stone formation in kidney transplant recipients.
Hypomagnesemia
Hypomagnesemia is also common among kidney transplant recipients treated with CNIs103–105 and is associated with an increased risk of new onset diabetes after transplantation (NODAT) cardiovascular morbidity.106–108 CNIs cause renal magnesium wasting due to impaired tubular reabsorption 109 through TRPM6.101 Clinical management of CNI-induced hypomagnesemia generally consists of exogenous supplementation to replete deficits and raise the serum magnesium level. Magnesium supplementation was even demonstrated to attenuate CsA-induced kidney interstitial fibrosis and tubular atrophy in a rat model.110
Hyperuricemia
CsA contributes to hyperuricemia and increases the risk of gout through a reduction in urate clearance.111–116 Additional predisposing factors to hyperuricemia include diuretic use and poor allograft function. CsA more than FK may in turn lead to chronic hyperuricemia and the formation of uric acid stones. Elevated serum urate levels can also induce endothelial dysfunction and impair vasodilatory nitric oxide secretion potentially contributing to chronic CNI nephropathy.116 Lowering serum uric acid levels attenuated experimental CsA nephropathy in rats however clinical data in humans is lacking.117
Cardiovascular and Metabolic Toxicity
Cardiovascular disease is the leading cause of death after kidney transplantation.118 CNIs contribute to hypertension, dyslipidemia, and NODAT, all of which are known risk factors for the progression of cardiovascular disease and have adverse effects on kidney transplant survival.
Hypertension
Hypertension is associated with adverse short-term and long-term allograft outcomes and can lead to increased morbidity and mortality post-transplant.119 Hypertension is common after kidney transplant due to a number factors including allograft dysfunction, volume overload, corticosteroid use, acute rejection, transplant renal artery stenosis, recurrent disease, and post-transplant proteinuria. Comprehensive reviews on the management of hypertension in transplant patients120 and CNI-induced hypertension have been previously published. 121, 122 A recent Cochrane review established that CsA increases blood pressure in a dose-dependent manner compared to placebo as well as the risk of stroke, myocardial infarction, heart failure, and other hypertension related adverse cardiovascular events.123 CNIs raise blood pressure and cause hypertension through multiple mechanisms including tubular salt reabsorption, peripheral vasoconstriction, and the sympathetic nervous system. When compared to hypertensive AZA-treated kidney transplant recipients, CSA-treated patients demonstrated salt sensitive hypertension that responded to salt restriction.124 CSA has also been shown to prevent proper suppression of renin release by calcineurin in cultured rat juxtaglomerular cells,125 suggesting that CNIs may dysregulate renin secretion and tubuloglomerular feedback in kidney transplant recipients.
The WNK kinase pathway is involved in mediating CNI-induced hypertension. Kidney transplant recipients with CNI-induced hypertension demonstrate a greater increase in fractional excretion of chloride compared to healthy volunteers treated with a thiazide, suggesting a role for inhibition of the sodium chloride cotransporter NCC. Kidney biopsies from patients treated with a CNI had pronounced increase in kidney cortex NCC and phospho-NCC expression compared with the azathioprine (AZA) treated and healthy control groups.126 Another study demonstrated a 4–5 fold higher abundance of NCC and pNCC in urinary extracellular vesicles from CNI treated kidney transplant recipients compared to CNI-free kidney transplant recipients and healthy volunteers.127 Furthermore, higher levels predicted the antihypertensive response to thiazide diuretics. In a randomized non-inferiority crossover trial among kidney transplant recipients receiving FK, treatment with chlorthalidone or amlodipine resulted in similar blood pressure reductions.128
Animal studies have revealed kidney specific deletion of FK binding protein in mice to attenuate FK induced hypertension and hyperkalemia129 and hydrochlorothiazide to reverse FK induced hypertension, which was dependent on WNK4-SPAK pathway activation of NCC.126 Another study of rats treated with CsA revealed increased abundance of WNK4 in kidney tissue, which was further demonstrated in distal convoluted tubule cell culture.130 Other ion transport regulators have been implicated in contributing to hypertension. CsA and FK increased renin content of mouse collecting duct principal cells associated with increased vascular endothelial growth factor production and worsening of local hypoxia and fibrosis.131 Suggesting a role for NKCC2 activity in mediating salt reabsorption, CsA treated rats demonstrated increased NKCC2132 and phosphorylated NKCC2 kidney abundance, which was dependent on the presence of vasopressin.133, 134
Dyslipidemia
Both CsA and FK are associated with impaired lipid metabolism, with CsA having a more profound impact.135 Abnormalities of the lipid profile include increased total cholesterol, low density lipoprotein cholesterol (LDL-C), non-high density lipoprotein cholesterol (non-HDL-C), triglycerides, apolipoprotein B and apoO-III.136 In vitro studies have shown that CsA inhibits sterol 27-hydroxylase (OYP27A1), which is required for 27-hydroxycholesterol formation and cholesterol metabolism.137 As 27-hydroxycholesterol inhibits 3-hydroxy-2-metyhylglutaryl coenzyme A (HMG-CoA), the rate-limiting enzyme involved in cholesterol biosynthesis, CsA leads to an increase in HMG-CoA activity and a subsequent increase in cholesterol levels.138 CsA also inhibits the 26-hydroxylase, leading to a decrease in bile acid synthesis from cholesterol and increased serum levels.139 Lastly, CsA may contribute to reduced triglyceride breakdown via inhibition of lipoprotein lipase activity.140
Management of immunosuppression induced dyslipidemias is typically similar to that observed in the general population. The 2013 K/DOQI Guidelines consider kidney transplantation a cardiovascular risk equivalent.141 Statins are recommended for all patients with kidney transplants and is considered first-line pharmacotherapeutic options and remain the backbone of dyslipidemia management post-transplant for their proven benefits in reducing major adverse cardiovascular events.142
Care should still be taken when selecting a statin agent and dose due to the potential for CYP3A/P-glycoprotein mediated drug interactions. Fluvastatin, pravastatin, rosuvastatin and pitavastatin may be easiest to manage due to their non-CYP3A mediated metabolism. Simvastatin should be avoided whenever possible due to significant potential for drug interactions and increased rates of myopathy and rhabdomyolysis.143
New Onset Diabetes After Transplantation (NODAT)
Although FK use is associated with lower kidney allograft rejection rates when compared to CsA, FK has been associated with a higher incidence of NODAT.144 In addition to CNI use, risk factors for the development of NODAT include increased body mass index (BMI), planned maintenance corticosteroid use, hepatitis C virus infection, and cytomegalovirus (CMV) infection.145 Interestingly, CNI-induced hypomagnesemia, which is more common with FK than CsA, was shown to be an independent risk factor for the development of NODAT.146 CNIs interfere with NFAT signaling in pancreatic b-cells, as they do in T-cells, and decrease insulin secretion.147 The high levels of FK-binding protein 12 in pancreatic β-cells relative to cyclophilin may explain the higher risk of NODAT with FK use.148 In addition to lifestyle modifications, early insulin initiation to manage hyperglycemia post-transplant has been proposed to decrease oxidative stress on the pancreas caused by an absolute insulin deficiency and reduce the odds of developing NODAT in the future.149
Neurotoxicity
Neurotoxicity is a frequent treatment-limiting concern among patients treated with CNIs. Mild symptoms are more common with FK150 and include tremor, neuralgia, and peripheral neuropathy. Severe symptoms affect up to 5 % of patients and include psychoses, hallucinations, dysarthria, vision loss, seizures, cerebellar ataxia, paresis, and leukoencephalopathy.151 Severe neurologic toxicity is more commonly associated with spikes in CNI exposure and typically demonstrates little correlation with trough levels.152 As a result, intravenous CNI administration and rapid titration post-transplant may be risk factors. The exact mechanism of CNI associated neurotoxicity is not completely understood as CNIs are highly lipophilic medications and do not readily cross the blood brain barrier. However, proposed mechanisms include altered CNS permeability due to increased endothelin production as well as increased production of toxic free radicals resulting from CNI-induced mitochondrial dysfunction.153 CNIs have been associated with hypertensive encephalopathy and posterior reversible encephalopathy syndrome (PRES).154 Patients can develop severe headache, visual disturbances, altered consciousness, and seizures. Most cases of PRES resolve over days to weeks without complications, however, death and permanent neurologic disability can occur from cerebral edema either from intracranial hemorrhage or the disease itself.154, 155 Gradual blood pressure lowering and switching to an alternative immunosuppressant are often associated with clinical improvement. Risk of PRES is increased among patients with significant fluid overload, elevated blood pressure, or impaired kidney function.
Gingival Overgrowth & Hair Growth
The underlying pathophysiology of gingival overgrowth (GO) is related to inhibition of intracellular calcium influx, with possible additional roles for fibroblasts, cytokines, and matrix metalloproteinases. GO, more commonly linked to CsA use, is associated with impaired oral hygiene, mastication, pain, and disfiguration. Not surprisingly given the proposed pathogenesis, a synergistic relationship has been shown between CsA and dihydropyridine CCBs in the development of GO.156, 157
CsA and FK have opposing effects on hair growth. Hypertrichosis associated with CsA may be related to inhibition of NFAT in follicular keratinocytes,158 and PO CsA has even been reported for the treatment of alopecia areata.159 Conversely, FK is associated with the development of alopecia,160 though the mechanism is unknown. In females it is thought to potentially be related to an imbalance in sex hormones and generally responds favorably to topical minoxidil therapy.160
Conclusions
CNI have revolutionized kidney transplantation and continue to have widespread use, though unfortunate and sometimes unavoidable toxicities may significantly affect kidney allograft function, overall survival, and patient quality of life. The mainstay of CNI toxicity management relies on both managing the adverse effect, often with additional medications, and also avoiding high plasma CNI levels while balancing the risk of allograft rejection. Though novel immunosuppressive regimens seek to mitigate the risk of CNI nephrotoxicity and ultimately prolong allograft survival, it seems that CNI and their toxicities are here to stay for now.
Clinical Summary.
Calcineurin inhibitors (CNI) are widely used in solid organ transplant and are also used for immune mediated glomerular diseases.
CNI use can lead to a wide variety of toxicities that may cause both acute and chronic kidney parenchymal injury, electrolyte derangements, and harmful effects on the endocrine, nervous, and cardiovascular systems.
Strategies to overcome CNI-mediated toxicities include decreasing the CNI dose, switching between CNIs or other immunosuppressant classes, and the use of calcium-channel blockers.
Acknowledgements
We would like to acknowledge Andrew Santeusanio, PharmD, for his review of the manuscript. SF and JR are supported by NIH T32DK007757 grant.
Footnotes
The authors have no financial conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant. 2019;19 Suppl 2: 19–123. [DOI] [PubMed] [Google Scholar]
- 2.Laurin LP, Gasim AM, Poulton CJ, et al. Treatment with Glucocorticoids or Calcineurin Inhibitors in Primary FSGS. Clin J Am Soc Nephrol. 2016; 11 (3): 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song YH, Cai GY, Xiao YF, et al. Efficacy and safety of calcineurin inhibitor treatment for IgA nephropathy: a meta-analysis. BMC Nephrol. 2017;18(1): 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovin BH, Solomons N, Pendergraft WF 3rd, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95(1): 219–231. [DOI] [PubMed] [Google Scholar]
- 5.Cattran DC, Appel GB, Hebert LA, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59(4): 1484–1490. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24): 2326–2333. [DOI] [PubMed] [Google Scholar]
- 7.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4): 468–475. [DOI] [PubMed] [Google Scholar]
- 8.Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2(8151): 1033–1036. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2(8670): 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352(6338): 803–807. [DOI] [PubMed] [Google Scholar]
- 11.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357(6380): 695–697. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357(6380): 692–694. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JJ, Jones P, Barbeito R. Large within-day variation in cyclosporine absorption: circadian variation or food effect? Clin J Am Soc Nephrol. 2006;1(3): 462–466. [DOI] [PubMed] [Google Scholar]
- 14.Highlights of Prescribing Information for Prograf. [Google Scholar]
- 15.Mancinelli LM, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69(1): 24–31. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Roca P, Medeiros M, Reyes H, et al. CYP3A5 polymorphism in Mexican renal transplant recipients and its association with tacrolimus dosing. Arch Med Res. 2012;43(4): 283–287. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson PA, Oetting WS, Brearley AM, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation. 2011;91 (3): 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oetting WS, Schladt DP, Guan W, et al. Genomewide Association Study of Tacrolimus Concentrations in African American Kidney Transplant Recipients Identifies Multiple CYP3A5 Alleles. Am J Transplant. 2016;16(2): 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32(4): 394–404. [DOI] [PubMed] [Google Scholar]
- 20.Tavira B, Gomez J, Diaz-Corte C, et al. The donor ABCB1 (MDR-1) C3435T polymorphism is a determinant of the graft glomerular filtration rate among tacrolimus treated kidney transplanted patients. J Hum Genet. 2015;60(5): 273–276. [DOI] [PubMed] [Google Scholar]
- 21.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2(2): 374–384. [DOI] [PubMed] [Google Scholar]
- 22.Bottiger Y, Brattstrom C, Tyden G, Sawe J, Groth CG. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol. 1999;48(3): 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israni AK, Riad SM, Leduc R, et al. Tacrolimus trough levels after month 3 as a predictor of acute rejection following kidney transplantation: a lesson learned from DeKAF Genomics. Transpl Int. 2013;26(10): 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis JJ, Luke RG, Dubovsky E, Diethelm AG, Whelchel JD, Jones P. Cyclosporin in therapeutic doses increases renal allograft vascular resistance. Lancet. 1986;2(8505): 477–479. [DOI] [PubMed] [Google Scholar]
- 25.Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91(5): 2144–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Nicola L, Thomson SC, Wead LM, Brown MR, Gabbai FB. Arginine feeding modifies cyclosporine nephrotoxicity in rats. J Clin Invest. 1993;92(4): 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortin MC, Raymond MA, Madore F, et al. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004;4(6): 946–952. [DOI] [PubMed] [Google Scholar]
- 28.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354(4): 343–352. [DOI] [PubMed] [Google Scholar]
- 29.Klintmalm G, Bohman SO, Sundelin B, Wilczek H. Interstitial fibrosis in renal allografts after 12 to 46 months of cyclosporin treatment: beneficial effect of low doses in early post-transplantation period. Lancet. 1984;2(8409): 950–954. [DOI] [PubMed] [Google Scholar]
- 30.Henny FC, Kleinbloesem CH, Moolenaar AJ, Paul LC, Breimer DD, van Es LA. Pharmacokinetics and nephrotoxicity of cyclosporine in renal transplant recipients. Transplantation. 1985;40(3): 261–265. [DOI] [PubMed] [Google Scholar]
- 31.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62(7): 900–905. [DOI] [PubMed] [Google Scholar]
- 32.Soubhia RM, Mendes GE, Mendonca FZ, Baptista MA, Cipullo JP, Burdmann EA. Tacrolimus and nonsteroidal anti-inflammatory drugs: an association to be avoided. Am J Nephrol. 2005;25(4): 327–334. [DOI] [PubMed] [Google Scholar]
- 33.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4): 383–391. [DOI] [PubMed] [Google Scholar]
- 34.Macphee IA, Fredericks S, Tai T, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74(11): 1486–1489. [DOI] [PubMed] [Google Scholar]
- 35.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74(3): 245–254. [DOI] [PubMed] [Google Scholar]
- 36.Trofe-Clark J, Brennan DC, West-Thielke P, et al. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am J Kidney Dis. 2018;71(3): 315–326. [DOI] [PubMed] [Google Scholar]
- 37.Anglicheau D, Verstuyft C, Laurent-Puig P, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14(7): 1889–1896. [DOI] [PubMed] [Google Scholar]
- 38.Thervet E, Anglicheau D, King B, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76(8): 1233–1235. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya N, Satoh S, Tada H, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78(8): 1182–1187. [DOI] [PubMed] [Google Scholar]
- 40.Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int. 2012;25(4): 471–480. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Srivastava A, Kapoor R, Mittal RD. Do drug transporter (ABCB1) SNPs influence cyclosporine and tacrolimus dose requirements and renal allograft outcome in the posttransplantation period? J Clin Pharmacol. 2011;51 (4): 603–615. [DOI] [PubMed] [Google Scholar]
- 42.Pallet N, Etienne I, Buchler M, et al. Long-Term Clinical Impact of Adaptation of Initial Tacrolimus Dosing to CYP3A5 Genotype. Am J Transplant. 2016;16(9): 2670–2675. [DOI] [PubMed] [Google Scholar]
- 43.Del Moral RG, Olmo A, Osuna A, et al. Role of P-glycoprotein in chronic cyclosporine nephrotoxicity and its relationship to intrarenal angiotensin II deposits. Transplant Proc. 1998;30(5): 2014–2016. [DOI] [PubMed] [Google Scholar]
- 44.Koziolek MJ, Riess R, Geiger H, Thevenod F, Hauser IA. Expression of multidrug resistance P-glycoprotein in kidney allografts from cyclosporine A-treated patients. Kidney Int. 2001;60(1): 156–166. [DOI] [PubMed] [Google Scholar]
- 45.Maezono S, Sugimoto K, Sakamoto K, et al. Elevated blood concentrations of calcineurin inhibitors during diarrheal episode in pediatric liver transplant recipients: involvement of the suppression of intestinal cytochrome P450 3A and P-glycoprotein. Pediatr Transplant. 2005;9(3): 315–323. [DOI] [PubMed] [Google Scholar]
- 46.Ceschi A, Rauber-Luthy C, Kupferschmidt H, et al. Acute calcineurin inhibitor overdose: analysis of cases reported to a national poison center between 1995 and 2011. Am J Transplant. 2013;13(3): 786–795. [DOI] [PubMed] [Google Scholar]
- 47.Lange NW, Salerno DM, Berger K, Tsapepas DS. Using known drug interactions to manage supratherapeutic calcineurin inhibitor concentrations. Clin Transplant. 2017;31(11). [DOI] [PubMed] [Google Scholar]
- 48.Rein JL, Wyatt CM. Marijuana and Cannabinoids in ESRD and Earlier Stages of CKD. Am J Kidney Dis. 2018;71(2): 267–274. [DOI] [PubMed] [Google Scholar]
- 49.Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1): 86–95. [DOI] [PubMed] [Google Scholar]
- 50.Leino AD, Emoto C, Fukuda T, Privitera M, Vinks AA, Alloway RR. Evidence of a Clinically Significant Drug-Drug Interaction between Cannabidiol and Tacrolimus. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 51.Jaeger W, Benet LZ, Bornheim LM. Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica. 1996;26(3): 275–284. [DOI] [PubMed] [Google Scholar]
- 52.Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep. 2016;6: 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10): 931–940. [DOI] [PubMed] [Google Scholar]
- 54.Elzinga LW, Rosen S, Bennett WM. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: role of sodium intake. J Am Soc Nephrol. 1993;4(2): 214–221. [DOI] [PubMed] [Google Scholar]
- 55.Klawitter J, Klawitter J, Schmitz V, et al. Low-salt diet and cyclosporine nephrotoxicity: changes in kidney cell metabolism. J Proteome Res. 2012; 11(11): 5135–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pichler RH, Franceschini N, Young BA, et al. Pathogenesis of cyclosporine nephropathy: roles of angiotensin II and osteopontin. J Am Soc Nephrol. 1995;6(4): 1186–1196. [DOI] [PubMed] [Google Scholar]
- 57.Andoh TF, Burdmann EA, Lindsley J, Houghton DC, Bennett WM. Enhancement of FK506 nephrotoxicity by sodium depletion in an experimental rat model. Transplantation. 1994;57(4): 483–489. [PubMed] [Google Scholar]
- 58.Young BA, Burdmann EA, Johnson RJ, et al. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995;48(2): 439–448. [DOI] [PubMed] [Google Scholar]
- 59.Young BA, Burdmann EA, Johnson RJ, et al. Cyclosporine A induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int. 1995;48(2): 431–438. [DOI] [PubMed] [Google Scholar]
- 60.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. Evolution and pathophysiology of renal-transplant glomerulosclerosis. Transplantation. 2004;78(3): 461–468. [DOI] [PubMed] [Google Scholar]
- 61.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78(4): 557–565. [DOI] [PubMed] [Google Scholar]
- 62.Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int. 1996;50(5): 1641–1650. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Castelao A, Hueso M, Sanz V, Rejas J, Alsina J, Grinyo JM. Treatment of hypertension after renal transplantation: long-term efficacy of verapamil, enalapril, and doxazosin. Kidney Int Suppl. 1998;68: S130–134. [DOI] [PubMed] [Google Scholar]
- 64.Rump LC, Oberhauser V, Schwertfeger E, et al. Dihydropyridine calcium antagonists and renal function in hypertensive kidney transplant recipients. J Hypertens. 2000;18(8): 1115–1119. [DOI] [PubMed] [Google Scholar]
- 65.Ruggenenti P, Perico N, Mosconi L, et al. Calcium channel blockers protect transplant patients from cyclosporine-induced daily renal hypoperfusion. Kidney Int. 1993;43(3): 706–711. [DOI] [PubMed] [Google Scholar]
- 66.Mourad G, Ribstein J, Mimran A. Converting-enzyme inhibitor versus calcium antagonist in cyclosporine-treated renal transplants. Kidney Int. 1993;43(2): 419–425. [DOI] [PubMed] [Google Scholar]
- 67.Burdmann EA, Andoh TF, Nast CC, et al. Prevention of experimental cyclosporin-induced interstitial fibrosis by losartan and enalapril. Am J Physiol. 1995;269(4 Pt 2): F491–499. [DOI] [PubMed] [Google Scholar]
- 68.Campistol JM, Inigo P, Jimenez W, et al. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999;56(2): 714–719. [DOI] [PubMed] [Google Scholar]
- 69.Hiremath S, Fergusson DA, Fergusson N, Bennett A, Knoll GA. Renin-Angiotensin System Blockade and Long-term Clinical Outcomes in Kidney Transplant Recipients: A Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis. 2017;69(1): 78–86. [DOI] [PubMed] [Google Scholar]
- 70.Carlos CP, Sonehara NM, Oliani SM, Burdmann EA. Predictive usefulness of urinary biomarkers for the identification of cyclosporine A-induced nephrotoxicity in a rat model. PLoS One. 2014;9(7): e103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta G, Regmi A, Kumar D, et al. Safe Conversion From Tacrolimus to Belatacept in High Immunologic Risk Kidney Transplant Recipients With Allograft Dysfunction. Am J Transplant. 2015;15(10): 2726–2731. [DOI] [PubMed] [Google Scholar]
- 72.Pochineni V, Rondon-Berrios H. Electrolyte and Acid-Base Disorders in the Renal Transplant Recipient. Front Med (Lausanne). 2018;5: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higgins R, Ramaiyan K, Dasgupta T, et al. Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol Dial Transplant. 2004;19(2): 444–450. [DOI] [PubMed] [Google Scholar]
- 74.Bantle JP, Nath KA, Sutherland DE, Najarian JS, Ferris TF. Effects of cyclosporine on the renin-angiotensin-aldosterone system and potassium excretion in renal transplant recipients. Arch Intern Med. 1985;145(3): 505–508. [PubMed] [Google Scholar]
- 75.Kamel KS, Ethier JH, Quaggin S, et al. Studies to determine the basis for hyperkalemia in recipients of a renal transplant who are treated with cyclosporine. J Am Soc Nephrol. 1992;2(8): 1279–1284. [DOI] [PubMed] [Google Scholar]
- 76.Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21 (1): 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ling BN, Eaton DC. Cyclosporin A inhibits apical secretory K+ channels in rabbit cortical collecting tubule principal cells. Kidney Int. 1993;44(5): 974–984. [DOI] [PubMed] [Google Scholar]
- 78.Adu D, Turney J, Michael J, McMaster P. Hyperkalaemia in cyclosporin-treated renal allograft recipients. Lancet. 1983;2(8346): 370–372. [DOI] [PubMed] [Google Scholar]
- 79.Heering PJ, Kurschat C, Vo DT, Klein-Vehne N, Fehsel K, Ivens K. Aldosterone resistance in kidney transplantation is in part induced by a down-regulation of mineralocorticoid receptor expression. Clin Transplant. 2004;18(2): 186–192. [DOI] [PubMed] [Google Scholar]
- 80.Stahl RA, Kanz L, Maier B, Schollmeyer P. Hyperchloremic metabolic acidosis with high serum potassium in renal transplant recipients: a cyclosporine A associated side effect. Clin Nephrol. 1986;25(5): 245–248. [PubMed] [Google Scholar]
- 81.Foley RJ, Hamner RW, Weinman EJ. Serum potassium concentrations in cyclosporine- and azathioprine-treated renal transplant patients. Nephron. 1985;40(3): 280–285. [DOI] [PubMed] [Google Scholar]
- 82.Ishizawa K, Wang Q, Li J, et al. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A. 2015;112(14): 4340–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marfo K, Glicklich D. Fludrocortisone therapy in renal transplant recipients with persistent hyperkalemia. Case Rep Transplant. 2012;2012: 586859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sivakumar V, Sriramnaveen P, Krishna C, et al. Role of fludrocortisone in the management of tacrolimus-induced hyperkalemia in a renal transplant recipient. Saudi J Kidney Dis Transpl. 2014;25(1): 149–151. [DOI] [PubMed] [Google Scholar]
- 86.Pavleska-Kuzmanovska S, Popov Z, Ivanovski O, et al. Cyclosporine nephrotoxicity and early posttransplant hyperkalemia in living-donor renal recipients: report of 4 cases. Exp Clin Transplant. 2014;12(5): 479–483. [DOI] [PubMed] [Google Scholar]
- 87.Lin W, Mou L, Tu H, et al. Clinical analysis of hyperkalemic renal tubular acidosis caused by calcineurin inhibitors in solid organ transplant recipients. J Clin Pharm Ther. 2017;42(1): 122–124. [DOI] [PubMed] [Google Scholar]
- 88.Bacchetta J, Basmaison O, Leclerc AL, Bertholet-Thomas A, Cochat P, Ranchin B. Fludrocortisone as a new tool for managing tubulopathy after pediatric renal transplantation: a series of cases. PediatrNephrol. 2014;29(10): 2061–2064. [DOI] [PubMed] [Google Scholar]
- 89.Ali SR, Shaheen I, Young D, et al. Fludrocortisone-a treatment for tubulopathy post-paediatric renal transplantation: A national paediatric nephrology unit experience. Pediatr Transplant. 2018;22(2). [DOI] [PubMed] [Google Scholar]
- 90.Dick TB, Raines AA, Stinson JB, Collingridge DS, Harmston GE. Fludrocortisone is effective in the management of tacrolimus-induced hyperkalemia in liver transplant recipients. Transplant Proc. 2011;43(7): 2664–2668. [DOI] [PubMed] [Google Scholar]
- 91.Park S, Kang E, Park S, et al. Metabolic Acidosis and Long-Term Clinical Outcomes in Kidney Transplant Recipients. J Am Soc Nephrol. 2017;28(6): 1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Djamali A, Singh T, Melamed ML, et al. Metabolic Acidosis 1 Year Following Kidney Transplantation and Subsequent Cardiovascular Events and Mortality: An Observational Cohort Study. Am J Kidney Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 93.Lea JP, Sands JM, McMahon SJ, Tumlin JA. Evidence that the inhibition of Na+/K(+)-ATPase activity by FK506 involves calcineurin. Kidney Int. 1994;46(3): 647–652. [DOI] [PubMed] [Google Scholar]
- 94.Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na+/K(+)-ATPase activity by cyclosporin A. Kidney Int. 1993;43(1): 246–251. [DOI] [PubMed] [Google Scholar]
- 95.Watanabe S, Tsuruoka S, Vijayakumar S, et al. Cyclosporin A produces distal renal tubular acidosis by blocking peptidyl prolyl cis-trans isomerase activity of cyclophilin. Am J Physiol Renal Physiol. 2005;288(1): F40–47. [DOI] [PubMed] [Google Scholar]
- 96.Rein JL, Coca SG. “I don’t get no respect”: the role of chloride in acute kidney injury. Am J Physiol Renal Physiol. 2019;316(3): F587–F605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9): 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiegand A, Ritter A, Graf N, et al. Preservation of kidney function in kidney transplant recipients by alkali therapy (Preserve-Transplant Study): rationale and study protocol. BMC Nephrol. 2018;19(1): 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterson JM, Butani L. Determinants of urinary calcium and uric acid excretion in children after renal transplantation. Pediatr Transplant. 2007;11(7): 716–720. [DOI] [PubMed] [Google Scholar]
- 100.Stapenhorst L, Sassen R, Beck B, Laube N, Hesse A, Hoppe B. Hypocitraturia as a risk factor for nephrocalcinosis after kidney transplantation. Pediatr Nephrol. 2005;20(5): 652–656. [DOI] [PubMed] [Google Scholar]
- 101.Nijenhuis T, Hoenderop JG, Bindels RJ. Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol. 2004;15(3): 549–557. [DOI] [PubMed] [Google Scholar]
- 102.Lee CT, Huynh VM, Lai LW, Lien YH. Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout and wild-type mice. Kidney Int. 2002;62(6): 2055–2061. [DOI] [PubMed] [Google Scholar]
- 103.Barton CH, Vaziri ND, Martin DC, Choi S, Alikhani S. Hypomagnesemia and renal magnesium wasting in renal transplant recipients receiving cyclosporine. Am J Med. 1987;83(4): 693–699. [DOI] [PubMed] [Google Scholar]
- 104.al-Khursany I, Thomas TH, Harrison K, Wilkinson R. Reduced erythrocyte and leukocyte magnesium is associated with cyclosporin treatment and hypertension in renal transplant patients. Nephrol Dial Transplant. 1992;7(3): 251–255. [DOI] [PubMed] [Google Scholar]
- 105.Scoble JE, Freestone A, Varghese Z, Fernando ON, Sweny P, Moorhead JF. Cyclosporin-induced renal magnesium leak in renal transplant patients. Nephrol Dial Transplant. 1990;5(9): 812–815. [DOI] [PubMed] [Google Scholar]
- 106.Garnier AS, Duveau A, Planchais M, Subra JF, Sayegh J, Augusto JF. Serum Magnesium after Kidney Transplantation: A Systematic Review. Nutrients. 2018;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheungpasitporn W, Thongprayoon C, Harindhanavudhi T, Edmonds PJ, Erickson SB. Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. EndocrRes. 2016;41(2): 142–147. [DOI] [PubMed] [Google Scholar]
- 108.Rodrigues N, Santana A, Guerra J, et al. Serum Magnesium and Related Factors in Long-Term Renal Transplant Recipients: An Observational Study. Transplant Proc. 2017;49(4): 799–802. [DOI] [PubMed] [Google Scholar]
- 109.Wong NL, Dirks JH. Cyclosporin-induced hypomagnesaemia and renal magnesium wasting in rats. Clin Sci (Lond). 1988;75(5): 509–514. [DOI] [PubMed] [Google Scholar]
- 110.Miura K, Nakatani T, Asai T, et al. Role of hypomagnesemia in chronic cyclosporine nephropathy. Transplantation. 2002;73(3): 340–347. [DOI] [PubMed] [Google Scholar]
- 111.Gores PF, Fryd DS, Sutherland DE, Najarian JS, Simmons RL. Hyperuricemia after renal transplantation. Am J Surg. 1988;156(5): 397–400. [DOI] [PubMed] [Google Scholar]
- 112.West C, Carpenter BJ, Hakala TR. The incidence of gout in renal transplant recipients. Am J Kidney Dis. 1987;10(5): 369–372. [DOI] [PubMed] [Google Scholar]
- 113.Burack DA, Griffith BP, Thompson ME, Kahl LE. Hyperuricemia and gout among heart transplant recipients receiving cyclosporine. Am J Med. 1992;92(2): 141–146. [DOI] [PubMed] [Google Scholar]
- 114.Delaney V, Sumrani N, Daskalakis P, Hong JH, Sommer BG. Hyperuricemia and gout in renal allograft recipients. Transplant Proc. 1992;24(5): 1773–1774. [PubMed] [Google Scholar]
- 115.Lin HY, Rocher LL, McQuillan MA, Schmaltz S, Palella TD, Fox IH. Cyclosporine-induced hyperuricemia and gout. N Engl J Med. 1989;321(5): 287–292. [DOI] [PubMed] [Google Scholar]
- 116.Numakura K, Satoh S, Tsuchiya N, et al. Hyperuricemia at 1 year after renal transplantation, its prevalence, associated factors, and graft survival. Transplantation. 2012;94(2): 145–151. [DOI] [PubMed] [Google Scholar]
- 117.Mazali FC, Johnson RJ, Mazzali M. Use of uric acid-lowering agents limits experimental cyclosporine nephropathy. Nephron Exp Nephrol. 2012;120(1): e12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Awan AA, Niu J, Pan JS, et al. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996–2014). Am J Nephrol. 2018;48(6): 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasiske BL, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43(6): 1071–1081. [DOI] [PubMed] [Google Scholar]
- 120.Weir MR, Burgess ED, Cooper JE, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26(6): 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. 2012;25(3): 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moes AD, Hesselink DA, Zietse R, van Schaik RH, van Gelder T, Hoorn EJ. Calcineurin inhibitors and hypertension: a role for pharmacogenetics? Pharmacogenomics. 2014;15(9): 1243–1251. [DOI] [PubMed] [Google Scholar]
- 123.Robert N, Wong GW, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev. 2010(1): CD007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Curtis JJ, Luke RG, Jones P, Diethelm AG. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med. 1988;85(2): 134–138. [DOI] [PubMed] [Google Scholar]
- 125.Madsen K, Friis UG, Gooch JL, et al. Inhibition of calcineurin phosphatase promotes exocytosis of renin from juxtaglomerular cells. Kidney Int. 2010;77(2): 110–117. [DOI] [PubMed] [Google Scholar]
- 126.Hoorn EJ, Walsh SB, McCormick JA, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011; 17(10): 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tutakhel OAZ, Moes AD, Valdez-Flores MA, et al. NaCl cotransporter abundance in urinary vesicles is increased by calcineurin inhibitors and predicts thiazide sensitivity. PLoS One. 2017;12(4): e0176220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moes AD, Hesselink DA, van den Meiracker AH, Zietse R, Hoorn EJ. Chlorthalidone Versus Amlodipine for Hypertension in Kidney Transplant Recipients Treated With Tacrolimus: A Randomized Crossover Trial. Am J Kidney Dis. 2017;69(6): 796–804. [DOI] [PubMed] [Google Scholar]
- 129.Lazelle RA, McCully BH, Terker AS, et al. Renal Deletion of 12 kDa FK506-Binding Protein Attenuates Tacrolimus-Induced Hypertension. J Am Soc Nephrol. 2016;27(5): 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Melnikov S, Mayan H, Uchida S, Holtzman EJ, Farfel Z. Cyclosporine metabolic side effects: association with the WNK4 system. Eur J Clin Invest. 2011;41(10): 1113–1120. [DOI] [PubMed] [Google Scholar]
- 131.Prokai A, Csohany R, Sziksz E, et al. Calcineurin-inhibition Results in Upregulation of Local Renin and Subsequent Vascular Endothelial Growth Factor Production in Renal Collecting Ducts. Transplantation. 2016;100(2): 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esteva-Font C, Ars E, Guillen-Gomez E, et al. Ciclosporin-induced hypertension is associated with increased sodium transporter of the loop of Henle (NKCC2). Nephrol Dial Transplant. 2007;22(10): 2810–2816. [DOI] [PubMed] [Google Scholar]
- 133.Blankenstein KI, Borschewski A, Labes R, et al. Calcineurin inhibitor cyclosporine A activates renal Na-K-Cl cotransporters via local and systemic mechanisms. Am J Physiol Renal Physiol. 2017;312(3): F489–F501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borschewski A, Himmerkus N, Boldt C, et al. Calcineurin and Sorting-Related Receptor with A-Type Repeats Interact to Regulate the Renal Na(+)-K(+)-2Cl(−) Cotransporter. J Am Soc Nephrol. 2016;27(1): 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Badiou S, Cristol JP, Mourad G. Dyslipidemia following kidney transplantation: diagnosis and treatment. Curr Diab Rep. 2009;9(4): 305–311. [DOI] [PubMed] [Google Scholar]
- 136.Akman B, Uyar M, Afsar B, Sezer S, Ozdemir FN, Haberal M. Lipid profile during azathioprine or mycophenolate mofetil combinations with cyclosporine and steroids. Transplant Proc 2007;39(1): 135–137. [DOI] [PubMed] [Google Scholar]
- 137.Gueguen Y, Ferrari L, Souidi M, et al. Compared effect of immunosuppressive drugs cyclosporine A and rapamycin on cholesterol homeostasis key enzymes CYP27A1 and HMG-CoA reductase. Basic Clin Pharmacol Toxicol. 2007;100(6): 392–397. [DOI] [PubMed] [Google Scholar]
- 138.Ballantyne CM, Podet EJ, Patsch WP, et al. Effects of cyclosporine therapy on plasma lipoprotein levels. JAMA. 1989;262(1): 53–56. [PubMed] [Google Scholar]
- 139.de Groen PC. Cyclosporine, low-density lipoprotein, and cholesterol. Mayo Clin Proc. 1988;63(10): 1012–1021. [DOI] [PubMed] [Google Scholar]
- 140.Tory R, Sachs-Barrable K, Hill JS, Wasan KM. Cyclosporine A and Rapamycin induce in vitro cholesteryl ester transfer protein activity, and suppress lipoprotein lipase activity in human plasma. Int J Pharm. 2008;358(1–2): 219–223. [DOI] [PubMed] [Google Scholar]
- 141.KDIGO KDIGOKLWG. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney inter, Suppl 2013;3: 259–305. [Google Scholar]
- 142.Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant. 2012;12(8): 1975–1982. [DOI] [PubMed] [Google Scholar]
- 143.Smith MEB, Lee NJ, Haney E, Carson S. Drug Class Review: HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin: Final Report Update 5. Portland (OR)2009. [PubMed] [Google Scholar]
- 144.Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005(4): CD003961. [DOI] [PubMed] [Google Scholar]
- 145.Ghisdal L, Van Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D. New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care. 2012;35(1): 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, Vanholder R. predictors of new-onset diabetes after transplantation. Am J Transplant. 2009;9(9): 2140–2149. [DOI] [PubMed] [Google Scholar]
- 147.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109): 345–349. [DOI] [PubMed] [Google Scholar]
- 148.Tamura K, Fujimura T, Tsutsumi T, et al. Transcriptional inhibition of insulin by FK506 and possible involvement of FK506 binding protein-12 in pancreatic beta-cell. Transplantation. 1995;59(11): 1606–1613. [PubMed] [Google Scholar]
- 149.Hecking M, Haidinger M, Doller D, et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23(4): 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63(7): 977–983. [DOI] [PubMed] [Google Scholar]
- 151.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5): 313–326. [DOI] [PubMed] [Google Scholar]
- 152.Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar). 2013;8(2): 170–175. [PMC free article] [PubMed] [Google Scholar]
- 153.Wijdicks EF. Neurotoxicity of immunosuppressive drugs. Liver Transpl. 2001;7(11): 937–942. [DOI] [PubMed] [Google Scholar]
- 154.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8): 494–500. [DOI] [PubMed] [Google Scholar]
- 155.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2): 205–210. [DOI] [PubMed] [Google Scholar]
- 156.James JA, Boomer S, Maxwell AP, et al. Reduction in gingival overgrowth associated with conversion from cyclosporin A to tacrolimus. J Clin Periodontol. 2000;27(2): 144–148. [DOI] [PubMed] [Google Scholar]
- 157.Bharti V, Bansal C. Drug-induced gingival overgrowth: The nemesis of gingiva unravelled. J Indian Soc Periodontol. 2013;17(2): 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003;284(6): C1593–1603. [DOI] [PubMed] [Google Scholar]
- 159.Ferrando J, Grimalt R. Partial response of severe alopecia areata to cyclosporine A. Dermatology. 1999;199(1): 67–69. [DOI] [PubMed] [Google Scholar]
- 160.Tricot L, Lebbe C, Pillebout E, Martinez F, Legendre C, Thervet E. Tacrolimus-induced alopecia in female kidney-pancreas transplant recipients. Transplantation. 2005;80(11): 1546–1549. [DOI] [PubMed] [Google Scholar]