Abstract
The discovery of aberrant crypt foci (ACF) more than three decades ago not only enhanced our understanding of how colorectal tumors form, but provided new opportunities to detect lesions prior to adenoma development and intervene in the colorectal carcinogenesis process even earlier. Since not all ACF progress to neoplasia, it is important to stratify these lesions based on the presence of dysplasia and establish early detection methods and interventions that specifically target dysplastic ACF (microadenomas). Significant progress has been made in characterizing the morphology and genetics of dysplastic ACF in both preclinical models and humans. Image-based methods have been established and new techniques that utilize bioactivatable probes and capture histological abnormalities in vivo are emerging for lesion detection. Successful identification of agents that target dysplastic ACF holds great promise for intervening even earlier in the carcinogenesis process to maximize tumor inhibition. Future preclinical and clinical prevention studies should give significant attention to assessing the utility of dysplastic ACF as the earliest identifiable biomarker of colorectal neoplasia and response to therapy.
Keywords: colorectal, microadenomas, dysplastic, aberrant crypt foci
Significant progress has been made in identifying both natural and synthetic agents that are efficacious in preventing the formation of colorectal tumors in preclinical animal models. Unfortunately, translation of these data to a clinical setting has been challenging due in part to the lack of an early intestinal event, either morphological or genetic, that can serve as a robust and reliable biomarker of response to chemopreventive intervention. In theory, an ideal biomarker would arise very early during inception of the neoplastic process and prior to any evidence of grossly-identifiable disease. Since the genetic alterations associated with colorectal tumor formation are highly heterogeneous, the search for such predictive biomarkers has focused on the earliest histological alterations arising in a background of non-neoplastic colonic mucosa. The following review focuses on the ability of a small foci of dysplastic crypts (microadenoma) to serve as a biomarker of the earliest sign of colorectal neoplasia and response to preventive intervention.
Aberrant crypt foci (ACF) are the earliest histological alterations to arise during the multi-step formation of colorectal neoplasia. These lesions were first reported in 1987 by Bird (1) who, with the use of a dissecting scope, observed clusters of differentially stained crypts with an abnormally thick epithelial lining in the colonic mucosa of C57BL/6 and CF1 mice treated with the colon carcinogen azoxymethane (AOM). The clinical significance of this finding was confirmed in 1991, when similar atypical crypts were identified in whole mounts of normal-appearing human colon stained with methylene blue (2, 3). The grossly observed aberrant foci (1 to more than 30 crypts) were 3-fold larger in diameter than normal human colon crypts and exhibited atypical oval to slit-shaped luminal openings (2).
Histology of ACF
ACFs can be classified as hyperplastic (enlarged and elongated) or dysplastic lesions (4). Dysplastic ACF (microadenomas) exhibit epithelial changes that are neoplastic in nature and identical to those seen in tubular and villous adenomas, including hypercellularity with enlarged hyperchromatic nuclei, varying degrees of nuclear stratification, loss of polarity, high nuclear/cytoplasmic ratio, nuclear crowding and increased mitotic index (3). In contrast to hyperplastic ACF and normal mucosa, proliferation (Ki67 and PCNA positivity) in dysplastic ACF extends to the epithelial surface. By definition, dysplastic ACF are microadenomas and precursors of grossly visible neoplastic lesions/tubular adenomas.
Dysplastic ACF (microadenomas) often exhibit accumulation of nuclear β-catenin and/or mucin depletion. β-catenin accumulated crypts were first identified in AOM-treated rats (5) and are found frequently in other carcinogen-induced models of colorectal tumorigenesis (6). Dysplastic ACF are also present in the human colon and show nuclear localization of β-catenin. While these lesions exhibit the histological features of dysplasia, most cannot be identified grossly on the mucosal surface without magnification or staining with methylene blue (7). Based on an increased frequency of β-catenin mutations and enhanced proliferative activity as compared to classic ACF, β-catenin accumulated crypts are more likely to progress to neoplasia (6). Mucin-depleted crypts exist as focal lesions and contain scant if any mucin on topography and positive staining with Alcian blue-periodic acid Schiff on histological analysis (8). The presence of mucin-depleted crypts in humans was first observed by Femia et al. (9) in colon specimens surgically resected from individuals at high risk for colorectal cancer. Detection was accomplished by staining the tissues first with methylene blue and then with high-iron diamine Alcian blue. All mucin-depleted crypts identified harbored some degree of dysplasia. Mucin-depleted crypts were heterogeneous in size (some only 3-6 crypts) and present at a density 30-fold less than that of ACF in patients with familial adenomatous polyposis (FAP), a heritable syndrome where individuals develop hundreds to thousands of polyps and ultimately colorectal cancer due to a mutation in the adenomatous polyposis coli (APC) gene. Mucin-depleted crypts were located much less frequently in colorectal cancer patients with sporadic disease as compared to patients with FAP. Interestingly, mucin-depleted crypts were found more often adjacent to colorectal cancers in the normal colonic mucosa of fresh colectomy specimens (8). While these data imply a temporal association between β-catenin-accumulated crypts, mucin-depleted crypts and adenoma formation, additional studies are needed to establish this relationship and eliminate the impact of a potential field effect.
Biomarkers of Colorectal Cancer Risk
Several studies have documented an association between the presence of dysplastic ACF and risk for colorectal cancer. In the case of FAP, the vast majority of patients (93.6%) had dysplastic ACF, while these lesions were detected in only 7.0% of patients who lacked a germline APC mutation (10). The relative risk ratio (RR) for developing dysplastic ACF was higher (RR 18.14) than that for developing nondysplastic ACF (RR 1.29) in patients with colorectal cancer (11). The average number of ACF per cm2 was highest among FAP patients (19.9 per cm2), as compared to colon cancer patients (0.37 per cm2) and normal individuals (0.18 per cm2) (3). In contrast, Cho and colleagues (12) did not observe any dysplasia among 655 ACF from 45 subjects with sporadic adenomas; 78% of the subjects possessed non-dysplastic ACF. In an independent study, only 2.0% of patients with adenocarcinomas also had dysplastic ACF in the rectum, while 38% of the rectal ACF identified by chromoendoscopy were histologically confirmed to be hyperplastic (13).
Although the role of dysplastic ACF in predicating colon cancer risk and/or recurrence remains controversial, several groups report an increase in the prevalence of ACF that parallels the stepwise progression of disease (normal colon to adenoma/dysplasia to cancer). This has been observed in both patients with colon and rectal cancer (14-16) and in individuals with colitis-associated dysplasia (17). Anderson et al. (18) reported a direct relationship between a high number (>6) of ACF in the distal colorectum and the development of advanced neoplasia over the next 5-years (adjusted odds ratio 12.27). To our knowledge, no association has been established between environmental and demographic factors and the risk of developing dysplastic ACF in humans. Irrespective of dysplasia, the total number of ACF increases with age; single ACF are found predominantly in younger patients (below the age of 40) while the highest numbers are observed in subjects 50 −70 years of age (19). An association has been observed between smoking long-term and number of ACF (18, 20). Individuals with a smoking history of ≥ 20 pack years had a significantly higher number of ACF detected during endoscopy than never-smokers (age adjusted odds ratio 3.16) (20).
Spontaneous Regression
Despite their recognition as very early lesions, ACF are known to regress over time as part of their natural history (21-24). Use of confocal endomicroscopy in mice with conditional deletion of the Apc and/or K-ras gene revealed the appearance of colonic dysplastic ACF (microadenomas) within 3 weeks of AdenoCre delivery. However, most of these small lesions regressed spontaneously by 10 weeks of age, while a few progressed to larger adenomas (21).
Although adult ApcMin/+ mice have approximately 20 dysplastic ACF (microadenomas) per colon, only 1-3 gross adenomas develop, indicating that most dysplastic ACF do not progress (22). These dysplastic ACF are less than 300 μm in diameter and lack mucin. Oyama et al. (23) reported that the size of dysplastic ACF was stable in ApcMin/+ mice, with no change in diameter observed between 5 and 35 weeks of age. These self-limiting features appear to mimic those of human ACF. In a clinical study to monitor ACF over time, 60% of the population had at least 1 rectal ACF at baseline colonoscopy. Less than 50% of the ACF identified originally could be re-identified one year later (24). However, more than 50% of the subjects developed new ACF. While these data clearly demonstrate the dynamic growth characteristics of ACF within the colon, the potential ability of even a single small dysplastic lesion to progress to colorectal cancer is of clinical significance and cannot be overemphasized.
Early Detection in Humans
Establishment of a standardized and reliable method for the endoscopic detection of human ACF in vivo remains challenging, primarily due to their small size (< 5 mm in diameter). Numerous attempts to deviate from routine macroscopic surveillance via white-light endoscopy have been met with limited enthusiasm, primarily due to the additional time needed to accurately detect ACF during colonoscopy. Despite this challenge, ACF, including β-catenin-accumulated crypts and mucin-depleted crypts, can be identified in humans using magnifying chromoendoscopes and narrow-band imaging techniques (25). High-definition magnifying colonoscopy, when used in combination with indigo carmine or methylene blue spray, is providing new insight into the growth characteristics of ACF and has facilitated the detection of ACF within the proximal colon of 39% of healthy adults during routine surveillance (26). The percentage of dysplastic ACF was 4-fold higher in the proximal colon (52%) than in the distal region (13%). Dysplasia was 7 times more likely to occur in ACF in the proximal vs. distal colorectum. Although it is recognized that not all studies have yielded a strong correlation between ACF and cancer development (12, 27), the present data provide support for continued investigation of ACF, in particular dysplastic ACF, as an early surrogate biomarker of risk for colorectal cancer.
Despite demonstrated successes in identifying human ACF using high definition/magnification chromoendoscopy, a need exists to develop methods that are less specialized and more compatible with routine clinical care. A few endoscopic techniques in particular show great promise for detecting dysplastic ACF in humans in the future. First, by placing a high magnification, flexible endocytoscope in direct contact with the stained bowel wall, Cipolletta and colleagues (28) were able to distinguish the histological features of colonic crypts with low grade dysplasia from those without dysplasia in real-time with high sensitivity (91.4%). The resulting in vivo images of the gastrointestinal mucosa were of high quality and comparable to those of conventional histology. Dysplastic ACF displayed polygonal crypt contours and irregular and elongated cell nuclei with pseudostratification toward the lumen of the crypt. Such histological approaches, while promising, are compromised by significant barriers that will need to be overcome prior to routine clinical use. Consistent with strategies developed by others, endocytoscopy was time-consuming (average - 44 min) and interrogated only a small area of the colon. Clinical implementation of such technology would be paradigm shifting for gastrointestinal endoscopists, as the operator would be required to obtain specialized training in histology.
Results from recent studies indicate that ACF can be detected in the human colon without using dye spray or methylene blue. In a prospective study, patients with colorectal neoplasms were examined using either narrow-band imaging or blue-laser imaging in combination with magnifying endoscopy (29). This so-called image-enhanced endoscopy accentuates the microvasculature and surface features of colorectal lesions, leading to improvements in detection (30, 31). Detection rates for dysplastic and nondysplastic ACF in the prospective study were 84.4% and 80.3%, respectively. Use of image-enhanced endoscopy led to reductions in both the time required for bowel preparation and ACF detection. Muguruma and colleagues (32) successfully identified ACF within the normal-appearing colorectum of patients with colorectal cancer post-operatively using a novel fluorogenic probe (DNAT-Me) activated by glutathione S-transferase P1-1. Direct comparison of DNAT-Me with conventional methylene blue staining revealed DNAT-Me was superior, as it exhibited a significantly higher signal to noise ratio, was faster to use, and did not require high magnification endoscopy for ACF detection due to its strong fluorescence intensity. Unfortunately, no attempts were made in this study to distinguish between dysplastic and nondysplastic ACF. Clearly, the approaches outlined above hold great promise for the future and warrant further investigation.
Detection of Dysplastic ACF in Mice
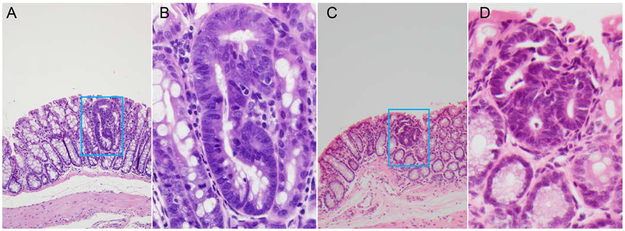
As in humans, dysplastic ACF arise early in a unique strain of multiple intestinal neoplasia (Apc+/Min-FCCC) mice established by this group (Figure 1). Apc+/Min-FCCC mice develop colorectal adenomas at a higher multiplicity (3.8 ± 0.3, mean ± SEM) than that reported by others for the C57BL/6J strain (1.1 ± 0.1) (33). At 7 weeks of age, the incidence of dysplastic ACF is 45% and increases to 65% by 21 weeks of age (34). The average multiplicity of dysplastic ACF is 0.63 per mouse. Of note, based on our experience, full-length colons that have been “bread loafed” are best suited for the histological identification of dysplastic ACF, as compared to “jelly rolls”. Unfortunately, few mouse models of colorectal carcinogenesis have been evaluated for the presence of dysplastic ACF.
Figure 1.
Dysplastic ACF (microadenoma) within the colonic mucosa of Apc+/Min - FCCC mice. Panels show a representative single crypt (A) and a three-crypt (C) microadenoma (100X). Panels B and D include a high-power view (400X) of the same microadenoma, respectively.
While stains can be used to accentuate the irregular topography of ACF (enlarged crypts, thickened epithelial lining, elliptical luminal openings), detection of dysplastic regions is much more challenging. Decolorization of carcinogen-treated rat colons with 70% methanol following incubation with 0.2% methylene blue led to a differential staining pattern and facilitated the detection of aberrant crypts harboring dysplasia (35). Dysplastic regions were more likely to retain methylene blue after destaining than normal or nondysplastic crypts, most likely due to a higher DNA content and cell density. This demonstrated success dictates further investigation of this method for identifying small dysplastic lesions without the need for histology.
Identification of areas within the colonic mucosa that harbor aberrations in RNA or protein expression, prior to gross evidence of tumor formation, remains a promising strategy for the earliest detection of dysplasia. However, selection of an optimal cellular alteration that can be monitored in real-time with great specificity and sensitivity has been challenging. This group (36) and others (37) have focused on the overexpression of matrix metalloproteinases (MMPs) as an early event in colorectal tumorigenesis. In ApcMin mice, matrilysin (MMP-7) mRNA was detected in 88% of adenomas (not present in the normal colonic mucosa) and localized to the luminal surface of dysplastic glands instead of in the extracellular matrix as expected (38). Knockout of MMP-7 in ApcMin mice caused a 58% reduction in the multiplicity of adenomas, as well as a decrease in tumor size. The ability of MMP-7 to impact the rate of tumor growth by activating luminal or membrane-bound cytokines and growth factors or as the result of tumor-induced changes in its secretion and access to potential substrates has been postulated. In humans, MMP-7 was upregulated very early in dysplastic colonic epithelial cells, including low grade dysplastic adenomas, and its concentration increased with grade of dysplasia (39). When combined, these data suggest that MMP-7 plays an important role in the earliest stages of colon tumor development.
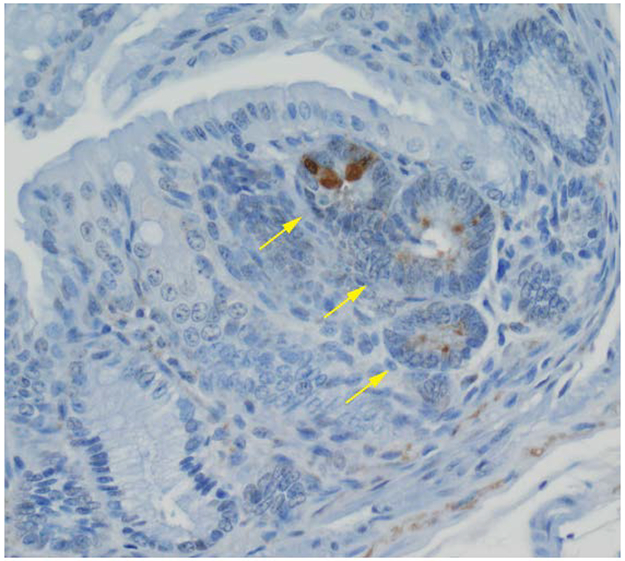
Dysplastic ACF have been detected in tumor-prone Apc+/Min-FCCC mice by this group using bioactivatable MMP probes that employ near-infrared fluorophores as optical sensors (Visen Medical/PerkinElmer) (36). In these mice, transcript levels of MMP-7, a downstream target of TCF signaling, are 100-fold higher in colorectal adenomas as compared to those of the non-neoplastic colonic mucosa (40). Injection of Apc+/Min-FCCC mice with MMPSense 680, a near-infrared bioactivatable probe, resulted in its proteolytic cleavage in discrete areas of the colon by a wide array of MMP isoforms and the detection of fluorescent signal. In addition to detecting all grossly visible polypoid lesions, the probe identified 50% of the earliest nonpolypoid lesions (1-2.4 mm) and 25% of the dysplastic ACF (0.05 mm), as confirmed by histology (36) (Figure 2). All dysplastic ACF (≤ 4 crypts) exhibited cytoplasmic localization of MMP-7 in the epithelial compartment (Figure 3). These data demonstrate the feasibility of using molecular image-based probes to detect dysplastic ACF in vivo within the murine colonic mucosa. The current need for intravenous delivery of the probe and the unknown long-term safety of the agent remain barriers to the clinical translation of this approach.
Figure 2.
Fluorescent image of a colon excised from an Apc+/Min-FCCC mouse, generated using the IVIS Spectrum. The upper colon lesion (purple arrow) was not visible grossly, while the lower lesion (green arrow) was detected at the time of necropsy.
Figure 3.
Immunohistochemical staining of MMP-7 of a colonic microadenoma in an Apc+/Min-FCCC mouse. The microadenoma (arrows) exhibits expression of MMP-7, while the non-neoplastic crypts are negative for MMP-7 (400X).
Genetic Aberrations in ACF
The genetic alterations that arise in ACF vary depending upon the transgenic (e.g. ApcMin) or carcinogen-induced model being studied. For example, AOM induces mutations in both β-catenin and K-ras (41). Thus, the resulting mutational spectra may not mimic the natural course of ACF formation and progression. For this reason, the following section focuses on only human ACF.
Dysplastic and hyperplastic ACF from subjects with heritable (FAP) or sporadic colorectal cancer possess distinct profiles of APC and K-RAS mutations (Table 1). K-RAS mutations are found in the majority of hyperplastic ACF (67-100%) irrespective of the population, while FAP patients harbor dysplastic ACF with K-RAS mutations less frequently than sporadic colorectal cancer patients (0-13% vs. 40-67%, respectively) (10, 42-45). APC mutations are rarely found in hyperplastic ACF (0-11%) from patients with either FAP or sporadic colorectal cancer, and are completely absent in dysplastic ACF from those with sporadic disease. As expected, all dysplastic ACF from FAP patients possess APC mutations. Similar to K-RAS, mutation of BRAF is not a common event in ACF (0-12.5%), irrespective of the lesion subtype or patient population (46, 47).
Table 1.
Profile of K-RAS and APC Mutations in Human ACFa
| Mutation | ||||
|---|---|---|---|---|
| ACF | K-RAS | APC | ||
| Dysplastic | FAP |
Sporadic CRC |
FAP |
Sporadic CRC |
| 0-13% | 40-67% | 100% | 0% | |
| Hyperplastic | Abundant (67-100%) |
Rare (0-11%) |
||
Even subjects who are not at high risk for colorectal cancer develop ACF that bear mutations in key tumor suppressor genes and oncogenes. Mutations in APC, BRAF and K-RAS were found in dysplastic ACF from “healthy” adults at a frequency of 75%, 12.5% and 12.5%, respectively. While these subjects did not have a history of colorectal cancer or meet the Amsterdam criteria for FAP or HNPCC, 25% had a history of colorectal polyps (26). Synchronous polyps were identified in approximately half (54.7%) by high-definition chromoendoscopy. Analysis of ACF for a large panel of driver mutations revealed mutations in BRAF, K-RAS, N-RAS and ERBB2 (8.3%, 16.7%, 8.3% and 4.2% respectively), but not APC in hyperplastic ACF. In summary, APC mutations are common in dysplastic ACF from healthy adults, while K-RAS mutations are the more frequent mutation in hyperplastic ACF. Collectively, these data indicate that APC and K-RAS are the key genetic drivers of ACF formation.
With the advent of genomic technologies has emerged new opportunities to characterize ACF with respect to methylation status, microsatellite instability (MSI) and loss of heterozygosity (LOH). Methylation of p16, MINT1, MINT2, MINT31, MGMT and MLH1 was found more frequently in dysplastic ACF from sporadic colorectal cancer patients than in FAP patients (45). Methylation of PTCH1 was present in 64.8% of dysplastic ACF and 19.8% of non-dysplastic ACF from healthy subjects and subjects with colon polyps, adenomas or cancer (48). Although the frequency of MSI and LOH of APC, PTPRJ, TP53 and DCC were similar in dysplastic and non-dysplastic ACF (49), MSI analysis using five mononucleotide repeat targets (BAT-25, BAT-26, NR-21, NR-24 and NR-27) showed a larger percentage of hyperplastic vs. dysplastic ACF were MSI-high (35% vs. 13.8%, respectively) (26).
Biomarkers of Therapeutic Response
Preclinical Models:
Numerous preclinical studies have used ACF as a surrogate endpoint when evaluating the efficacy of a chemopreventive agent. However, most studies report the total number of ACF without confirming the presence (or degree) of dysplasia, either by histology or using stains specific for mucins. Such evaluations are critical for comprehensive interpretation of the data, as most ACF do not progress to colorectal adenomas.
The ability of various dietary interventions to modulate the growth of dysplastic ACF has been documented in several studies. Administration of a high fat diet (20%), a known risk factor for colorectal cancer, to AOM-treated rats increased the number of animals with dysplastic ACF by 53%, as compared to controls maintained on a standard AIN93M diet (50). As anticipated, a corresponding increase in tumor incidence was also observed in the same animals. In contrast, dietary supplementation of a high fat diet with polyphenol polyphenon E (PPE), a purified extract of green tea catechins, for 8 weeks caused a dose-dependent reduction in the number of histologically-confirmed dysplastic ACF within the colon of AOM-treated rats (51). Most impressive was the ability of PPE to inhibit ACF with high-grade dysplasia by more than 50% (p < 0.05). These data are consistent with clinical, epidemiological and preclinical studies that have established an association between consumption of green tea and a decreased risk of colorectal cancer (52-54). The number of mucin-negative ACF was 65% lower in DMH-treated animals fed a diet containing lycopene (300 ppm), a carotenoid found in tomatoes, as compared to those on a diet without lycopene (55). Recently, the effect of apiaceous and cruciferous vegetables on sialomucin-expressing and mucin-depleted ACF was examined in DMH-treated rats (56). Rats fed apiaceous vegetables (celery and parsnips) for 10 weeks developed approximately 20% fewer total dysplastic (sialomucin-expressing plus mucin-depleted) ACF, as compared to those fed a basal diet (p <0.05). Likewise, the density of dysplastic ACF (number per cm2) was reduced, although not significantly, in animals consuming the cruciferous vegetable diet, as compared to untreated controls (P=0.075). These data provide additional support for the use of dysplastic ACF as surrogate biomarkers of the preventive or promotional properties of dietary interventions in vivo.
Preventive agents have been identified that specifically target dysplastic ACF (microadenomas) in mice. This discovery is most exciting, and holds great promise for: 1) intervening very early in the carcinogenesis process, and 2) using these lesions as biomarkers of early drug efficacy in preclinical tumor models. However, it should be noted that the ability of an agent to inhibit the formation of dysplastic ACF is predicated, in part, by the presence or absence of adenomas at the time of treatment initiation. Chronic exposure of Apc+/Min-FCCC mice to atorvastatin (100 ppm for 14 weeks) completely inhibited the development of dysplastic ACF in mice that did not have gross colon tumors at the time of study enrollment, as confirmed by colonoscopy. As expected, colon tumor incidence was also reduced significantly (32%) in mice treated with atorvastatin as compared to untreated controls (34). Interestingly, a similar reduction was not observed in Apc+/Min-FCCC mice that received the same regimen of atorvastatin (100 ppm for 14 weeks) but possessed colorectal adenomas at baseline colonoscopy. Thus, the chemopreventive activity of an agent can vary depending on the tumor status of the animal at treatment initiation. This finding not only stresses the underlying importance of knowing the tumor status of all animals at baseline, but suggests that the inhibitory activity of specific agents may have been missed in the past due to the lack of attention given to dysplastic ACF as an endpoint and/or the characteristics of the population being treated. These data underscore the importance of tailoring preventive interventions based on the risk profile of the target population to achieve maximal protection from cancer and ensure safety.
Administration of a vaccine against MASH2, the murine ortholog of the basic helix-loop-helix transcription factor Human achaete scute homolog 2 (HASH2), to tumor-prone Apc+/Min-FCCC mice led to significant inhibition of dysplastic colorectal ACF (57). HASH2 plays a critical role in controlling stem cell fate in the non-neoplastic intestine and is overexpressed in the majority of colorectal cancers (58, 59). Similar to the atorvastatin intervention described above, tumor inhibition was observed only when the immunotherapy was introduced prior to the formation of gross adenomas. Prophylactic use of recombinant MASH2 protein combined with AS15 immunostimulant caused an approximate 3-fold reduction in the multiplicity of dysplastic ACF in Apc+/Min-FCCC mice, as compared to controls injected with only buffer (57). No significant effect was apparent when the immunotherapy was delivered to animals with established colorectal adenomas. Thus, while dysplastic ACF most likely continue to develop within the colorectum throughout the life of Apc+/Min-FCCC mice, it appears a small window of opportunity exists during which their growth can be interrupted successfully.
Clinical Chemoprevention Trials:
While several therapeutic trials have been conducted in humans using ACF as an endpoint, very few have specifically evaluated the response of dysplastic ACF to the intervention. Shpitz and colleagues (60) assessed the effect of chronic administration of aspirin on the histological characteristics and distribution of ACF in patients with colorectal cancer. Samples of normal colon, at least 2 cm from the tumor margin, were collected from 59 patients who had been treated with aspirin (56 subjects - 100 mg/day; 3 subjects - 325 mg/day) on a regular basis for at least one year (median exposure 48 months) and 135 patients who were not taking aspirin or any other nonsteroidal anti-inflammatory drug (control group). The overall incidence of histopathologically-confirmed ACF was reduced 47% in patients treated with aspirin as compared to controls. In the left colon, aspirin not only reduced the percentage of samples with ACF by 52.5% (p < 0.0001), but significantly decreased the density of ACF by 82% (p < 0.01) as compared to those in the control group. Most importantly, the percentage of ACF that were dysplastic was reduced 48% from that of the control group. This result did not achieve statistical significance most likely due to the small sample size. A similar trend was observed in the right colon.
Our inability to readily identify which ACF are dysplastic in vivo has severely compromised their use as a biomarker of colorectal cancer risk in humans. Likewise, few studies have assessed the impact of therapy on the development of dysplastic ACF retrospectively in banked specimens. In light of this technical challenge, numerous preclinical and clinical studies have employed total ACF as a surrogate biomarker of cancer risk and therapeutic response. As summarized in Table 2, the impact of preventive agents on total ACF and colon tumors in animal studies is highly consistent. Such a correlation is less clear when comparing the effect of the same agent/drug on: 1) total ACF in preclinical vs. clinical studies, or 2) total ACF vs. colorectal adenomas following a clinical intervention. Although patient characteristics, drug dose, treatment length and agent bioavailability could contribute to these inconsistent findings, the total ACF, irrespective of presence of dysplasia, may not be sufficiently robust to serve as a biomarker of tumor response, especially in humans.
Table 2.
Effect of Select Agents/Drugs on Total ACF and Colon Tumors in Preclinical and Clinical Studiesa
| Agent/Drug | Preclinical Studies | Clinical Studies | ||
|---|---|---|---|---|
| Total ACF (model) | Adenoma/Cancer (model) | Total rectal ACF (subject population) |
Adenoma (subject population) |
|
| Aspirin | ↓ (AOM) (61-65)
↓ (DMH) (66) |
↓ (DMH) (67) ↓ (AOM) (65, 68, 69) ↓ (AOM-DSS) (70) No effect (AOM) (71) |
↓ (CRC) (60) | ↓ (CRC) (72) ↓ (adenomas) (73) ↓ (nonsmokers with adenoma or adenocarcinomas) (74) |
| Celecoxib | ↓ (DMH) (66, 75) | ↓ (AOM) (68) ↓ (ApcMin) (76, 77) |
No effect (adenomas) (12) | ↓ (adenomas) (78) |
| DFMO | ↓ (AOM) (79, 80) | ↓ (AOM) (62, 71, 80, 81) ↓ (ApcMin) (77) |
||
| Sulindac | ↓ (AOM) (82) | ↓ (ApcMin) (77) ↓ (Pirc) (83) ↓ size only (ApcMin-FCCC) (34) |
↓ (no history of polyps) (84) No effect (CRC or advanced adenomas) (13) |
↓ (FAP) (85-88) ↓ (no history of polyps) (84) |
| DFMO + Aspirin | ↓ (AOM) (62) | ↓ (CRC or advanced adenomas) (89) | No effect (CRC or advanced adenomas) (89) | |
| DFMO + Sulindac | ↓ (ApcMin) (77) | ↓ (adenomas) (90) Ongoing (FAP) (91) |
||
| DFMO + Celecoxib | ↓ (ApcMin) (77) | No better than celecoxib alone (FAP) (92) | ||
| Etodolac (COX-2 inhibitor) |
↓ (AOM) (82) | ↓ (AOM-DSS) (93) |
No effect (no history of polyps) (84) | No effect (no history of polyps) (84) |
| Atorvastatin | ↓ (dysplastic ACF) (baseline tumor-free ApcMin-FCCC) (34) | ↓ (AOM) (68) ↓ (ApcMin) (76) ↓ Incidence (baseline tumor-free ApcMin-FCCC) (34) |
No effect (CRC or advanced adenomas) (13) | |
| Curcumin | ↓ (AOM) (94, 95) ↓ (DMH) (75, 96) |
↓ (AOM) (97, 98) ↓ (AOM-DSS) (99) |
↓ (smokers, no history of polyps) (100) | No effect (FAP) (101) |
| Metformin | ↓ (DMH & diabetic) (102) ↓ (DMH) (103) ↓ (AOM) (104) |
↓ (DMH & diabetic) (102) ↓ (AOM) (104) |
↓ (nondiabetic, no CRC) (105) ↓ (impaired glucose tolerance, no polyps) (106) |
↓ (nondiabetic adults with adenomas) (107) |
DFMO: difluoromethylornithine; CRC: colorectal cancer; FAP: familial adenomatous polyposis; DMH: dimethylhydrazine; AOM: azoxymethane; DSS: dextran sulfate sodium; Apc: adenomatous polyposis coli; ApcMin-FCCC: Fox Chase Cancer Center strain of ApcMin mice; COX-2: cyclooxygenase-2
In summary, few preclinical efficacy studies have utilized dysplastic ACF as a primary experimental endpoint. Emerging data from this group and others suggest that dysplastic ACF/microadenomas represent a promising biomarker of very early response to preventive intervention. Successful identification of agents that target dysplastic ACF holds promise for intervening even earlier in the carcinogenesis process, prior to the formation of gross lesions. The feasibility of using dysplastic ACF as an intermediate endpoint of drug efficacy in a clinical setting will rely on the development of new image-based strategies with which to detect their presence in the non-neoplastic colonic mucosa.
Future Perspectives
Recent advances in next generation sequencing have provided new insight into the genetic basis of colorectal tumors. Application of this technology to colonic epithelial cells microdissected from dysplastic ACF will reveal the earliest events associated with tumor initiation and the preneoplastic state. Future studies should also focus on identifying those genes that are differentially expressed in dysplastic ACF vs. adjacent non-neoplastic colonic mucosa using whole transcriptome profiling (RNASeq). Such alterations are anticipated to inform the: 1) development of chemopreventive strategies with which to disrupt the earliest molecular alterations that lead to tumor formation; and 2) selection of potential biomarkers of the responsiveness of early lesions to therapeutic regimens. Our success in translating these data to a clinical setting relies heavily on the generation of a noninvasive molecular imaging approach for the accurate identification of early dysplastic lesions in situ. While chromoendoscopy has become an effective method for detecting ACF during routine endoscopy, further development of an image-based method to detect dysplastic ACF without a tissue biopsy is anticipated to revolutionize colorectal cancer screening and significantly reduce the morbidity and mortality associated with this prevalent disease.
Acknowledgements
We wish to thank Darlene Curran for her excellent assistance in preparing this article for publication. This publication was supported by grant number P30 CA006927 from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
REFERENCES
- 1.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 1987;37(2):147–51. [DOI] [PubMed] [Google Scholar]
- 2.Pretlow TP, Barrow BJ, Ashton WS, O’Riordan MA, Pretlow TG, Jurcisek JA, et al. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res 1991;51(5):1564–7. [PubMed] [Google Scholar]
- 3.Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol 1991;22(3):287–94. [DOI] [PubMed] [Google Scholar]
- 4.Perše Martina and Cerar Anton (March 12th 2014). The role, significance and applicability of aberrant crypt foci in clinical practice, colorectal cancer - surgery, diagnostics and treatment, Khan Jim S, IntechOpen, DOI: 10.5772/57474. Available from: https://www.intechopen.com/books/colorectal-cancer-surgery-diagnostics-and-treatment/the-role-significance-and-applicability-of-aberrant-crypt-foci-in-clinical-practice [DOI] [Google Scholar]
- 5.Yamada Y, Yoshimi N, Hirose Y, Kawabata K, Matsunaga K, Shimizu M, et al. Frequent beta-catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res 2000;60(13):3323–7. [PubMed] [Google Scholar]
- 6.Mori H, Yamada Y, Kuno T, Hirose Y. Aberrant crypt foci and beta-catenin accumulated crypts; significance and roles for colorectal carcinogenesis. Mutat Res 2004;566(3):191–208. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Mori H. Pre-cancerous lesions for colorectal cancers in rodents: a new concept. Carcinogenesis 2003;24(6):1015–9. [DOI] [PubMed] [Google Scholar]
- 8.Gupta B, Das P, Ghosh S, Manhas J, Sen S, Pal S, et al. Identification of high-risk aberrant crypt foci and mucin-depleted foci in the human colon with study of colon cancer stem cell markers. Clin Colorectal Cancer 2017;16(3):204–13. [DOI] [PubMed] [Google Scholar]
- 9.Femia AP, Giannini A, Fazi M, Tarquini E, Salvadori M, Roncucci L, et al. Identification of mucin depleted foci in the human colon. Cancer Prev Res (Phila) 2008;1(7):562–7. [DOI] [PubMed] [Google Scholar]
- 10.Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology 2001;121(3):599–611. [DOI] [PubMed] [Google Scholar]
- 11.Alrawi SJ, Schiff M, Carroll RE, Dayton M, Gibbs JF, Kulavlat M, et al. Aberrant crypt foci. Anticancer Res 2006;26(1A):107–19. [PubMed] [Google Scholar]
- 12.Cho NL, Redston M, Zauber AG, Carothers AM, Hornick J, Wilton A, et al. Aberrant crypt foci in the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila) 2008;1(1):21–31. [DOI] [PubMed] [Google Scholar]
- 13.Limburg PJ, Mahoney MR, Ziegler KL, Sontag SJ, Schoen RE, Benya R, et al. Randomized phase II trial of sulindac, atorvastatin, and prebiotic dietary fiber for colorectal cancer chemoprevention. Cancer Prev Res (Phila) 2011;4(2):259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seike K, Koda K, Oda K, Kosugi C, Shimizu K, Nishimura M, et al. Assessment of rectal aberrant crypt foci by standard chromoscopy and its predictive value for colonic advanced neoplasms. Am J Gastroenterol 2006;101(6):1362–9. [DOI] [PubMed] [Google Scholar]
- 15.Hurlstone DP, Karajeh M, Sanders DS, Drew SK, Cross SS. Rectal aberrant crypt foci identified using high-magnification-chromoscopic colonoscopy: biomarkers for flat and depressed neoplasia. Am J Gastroenterol 2005;100(6):1283–9. [DOI] [PubMed] [Google Scholar]
- 16.Adler DG, Gostout CJ, Sorbi D, Burgart LJ, Wang L, Harmsen WS. Endoscopic identification and quantification of aberrant crypt foci in the human colon. Gastrointest Endosc 2002;56(5):657–62. [DOI] [PubMed] [Google Scholar]
- 17.Kukitsu T, Takayama T, Miyanishi K, Nobuoka A, Katsuki S, Sato Y, et al. Aberrant crypt foci as precursors of the dysplasia-carcinoma sequence in patients with ulcerative colitis. Clin Cancer Res 2008;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JC, Swede H, Rustagi T, Protiva P, Pleau D, Brenner BM, et al. Aberrant crypt foci as predictors of colorectal neoplasia on repeat colonoscopy. Cancer Causes Control 2012;23(2):355–61. [DOI] [PubMed] [Google Scholar]
- 19.Kowalczyk M, Orlowski M, Siermontowski P, Mucha D, Zinkiewicz K, Kurpiewski W, et al. Occurrence of colorectal aberrant crypt foci depending on age and dietary patterns of patients. BMC Cancer 2018;18(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JC, Pleau DC, Rajan TV, Protiva P, Swede H, Brenner B, et al. Increased frequency of serrated aberrant crypt foci among smokers. Am J Gastroenterol 2010;105(7):1648–54. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Kim P, Kim JK, Kim YR, Fukumura D, Yun SH. Longitudinal tracing of spontaneous regression and anti-angiogenic response of individual microadenomas during colon tumorigenesis. Theranostics 2015;5(7):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada Y, Hata K, Hirose Y, Hara A, Sugie S, Kuno T, et al. Microadenomatous lesions involving loss of Apc heterozygosity in the colon of adult Apc(Min/+) mice. Cancer Res 2002;62(22):6367–70. [PubMed] [Google Scholar]
- 23.Oyama T, Yamada Y, Hata K, Tomita H, Hirata A, Sheng H, et al. Further upregulation of beta-catenin/Tcf transcription is involved in the development of macroscopic tumors in the colon of ApcMin/+ mice. Carcinogenesis 2008;29(3):666–72. [DOI] [PubMed] [Google Scholar]
- 24.Schoen RE, Mutch M, Rall C, Dry SM, Seligson D, Umar A, et al. The natural history of aberrant crypt foci. Gastrointest Endosc 2008;67(7):1097–102. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczyk M, Siermontowski P, Mucha D, Ambrozy T, Orlowski M, Zinkiewicz K, et al. Chromoendoscopy with a standard-resolution colonoscope for evaluation of rectal aberrant crypt foci. PLoS One 2016;11(2):e0148286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drew DA, Mo A, Grady JJ, Stevens RG, Levine JB, Brenner BM, et al. Proximal aberrant crypt foci associate with synchronous neoplasia and are primed for neoplastic progression. Mol Cancer Res 2018;16(3):486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutch MG, Schoen RE, Fleshman JW, Rall CJ, Dry S, Seligson D, et al. A multicenter study of prevalence and risk factors for aberrant crypt foci. Clin Gastroenterol Hepatol 2009;7(5):568–74. [DOI] [PubMed] [Google Scholar]
- 28.Cipolletta L, Bianco MA, Rotondano G, Piscopo R, Meucci C, Prisco A, et al. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: a prospective in vivo study. Endoscopy 2009;41(2):129–32. [DOI] [PubMed] [Google Scholar]
- 29.Kagemoto K, Okamoto K, Takaoka T, Sato Y, Kitamura S, Kimura T, et al. Detection of aberrant crypt foci with image-enhanced endoscopy. Endosc Int Open 2018;6(8):E924–E933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida N, Yagi N, Inada Y, Kugai M, Okayama T, Kamada K, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc 2014;26(2):250–8. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida N, Hisabe T, Hirose R, Ogiso K, Inada Y, Konishi H, et al. Improvement in the visibility of colorectal polyps by using blue laser imaging (with video). Gastrointest Endosc 2015;82(3):542–9. [DOI] [PubMed] [Google Scholar]
- 32.Muguruma N, Okamoto K, Nakagawa T, Sannomiya K, Fujimoto S, Mitsui Y, et al. Molecular imaging of aberrant crypt foci in the human colon targeting glutathione S-transferase P1-1. Sci Rep 2017;7(1):6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper HS, Chang W-CL, Coudry R, Gary MA, Everley L, Spittle CS, et al. Generation of a unique strain of multiple intestinal neoplasia (Apc+/Min-FCCC) mice with significantly increased numbers of colorectal adenomas. Mol Carcinog 2005;44(1):31–41. [DOI] [PubMed] [Google Scholar]
- 34.Chang WL, Jackson C, Riel S, Cooper HS, Devarajan K, Hensley HH, et al. Differential preventive activity of sulindac and atorvastatin in Apc(+/Min-FCCC)mice with or without colorectal adenomas. Gut 2018;67(7):1290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochiai M, Watanabe M, Nakanishi M, Taguchi A, Sugimura T, Nakagama H. Differential staining of dysplastic aberrant crypt foci in the colon facilitates prediction of carcinogenic potentials of chemicals in rats. Cancer Lett 2005;220(1):67–74. [DOI] [PubMed] [Google Scholar]
- 36.Clapper ML, Hensley HH, Chang WC, Devarajan K, Nguyen MT, Cooper HS. Detection of colorectal adenomas using a bioactivatable probe specific for matrix metalloproteinase activity. Neoplasia 2011;13(8):685–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newell KJ, Witty JP, Rodgers WH, Matrisian LM. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog 1994;10(4):199–206. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A 1997;94(4):1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polistena A, Cucina A, Dinicola S, Stene C, Cavallaro G, Ciardi A, et al. MMP7 expression in colorectal tumours of different stages. In Vivo 2014;28(1):105–10. [PubMed] [Google Scholar]
- 40.Coudry RA SJ, Wilde JA, Cooper HS, Clapper ML, and Spittle CS. Altered expression of TCF-4 target genes in Apc+/Min-FCCC mouse adenomas. Proc Am Assoc Cancer Res 2007;67(9). [Google Scholar]
- 41.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci 2004;95(6):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994;54(21):5523–6. [PubMed] [Google Scholar]
- 43.Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol 1997;28(12):1396–407. [DOI] [PubMed] [Google Scholar]
- 44.Otori K, Konishi M, Sugiyama K, Hasebe T, Shimoda T, Kikuchi-Yanoshita R, et al. Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer 1998;83(5):896–900. [DOI] [PubMed] [Google Scholar]
- 45.Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol 2002;160(5):1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg DW, Yang S, Pleau DC, Greenspan EJ, Stevens RG, Rajan TV, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res 2007;67(8):3551–4. [DOI] [PubMed] [Google Scholar]
- 47.Beach R, Chan AO, Wu TT, White JA, Morris JS, Lunagomez S, et al. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol 2005;166(4):1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng L, Hu J, Li S, Wang Z, Xia B, Jiang B, et al. Aberrant methylation of the PTCH1 gene promoter region in aberrant crypt foci. Int J Cancer 2013;132(2):E18–25. [DOI] [PubMed] [Google Scholar]
- 49.Luo L, Shen GQ, Stiffler KA, Wang QK, Pretlow TG, Pretlow TP. Loss of heterozygosity in human aberrant crypt foci (ACF), a putative precursor of colon cancer. Carcinogenesis 2006;27(6):1153–9. [DOI] [PubMed] [Google Scholar]
- 50.Baijal PK, Fitzpatrick DW, Bird RP. Comparative effects of secondary bile acids, deoxycholic and lithocholic acids, on aberrant crypt foci growth in the postinitiation phases of colon carcinogenesis. Nutr Cancer 1998;31(2):81–9. [DOI] [PubMed] [Google Scholar]
- 51.Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis 2008;29(1):113–9. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev 2008;17(11):3020–5. [DOI] [PubMed] [Google Scholar]
- 53.Yang G, Shu XO, Li H, Chow WH, Ji BT, Zhang X, et al. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev 2007;16(6):1219–23. [DOI] [PubMed] [Google Scholar]
- 54.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res 2005;65(22):10623–31. [DOI] [PubMed] [Google Scholar]
- 55.Dias MC, Vieiralves NF, Gomes MI, Salvadori DM, Rodrigues MA, Barbisan LF. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem Toxicol 2010;48(3):772–80. [DOI] [PubMed] [Google Scholar]
- 56.Kim S, Trudo SP, Gallaher DD. Apiaceous and cruciferous vegetables fed during the post-initiation stage reduce colon cancer risk markers in rats. J Nutr 2019;149(2):249–57. [DOI] [PubMed] [Google Scholar]
- 57.Rioux CR, Clapper ML, Cooper HS, Michaud J, St Amant N, Koohsari H, et al. Self-antigen MASH2 combined with the AS15 immunostimulant induces tumor protection in colorectal cancer mouse models. PLoS One 2019;14(1):e0210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jubb AM, Chalasani S, Frantz GD, Smits R, Grabsch HI, Kavi V, et al. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene 2006;25(24):3445–57. [DOI] [PubMed] [Google Scholar]
- 59.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, et al. The intestinal Wnt/TCF signature. Gastroenterology 2007;132(2):628–32. [DOI] [PubMed] [Google Scholar]
- 60.Shpitz B, Klein E, Buklan G, Neufeld D, Nissan A, Freund HR, et al. Suppressive effect of aspirin on aberrant crypt foci in patients with colorectal cancer. Gut 2003;52(11):1598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shpitz B, Bomstein Y, Kariv N, Shalev M, Buklan G, Bernheim J. Chemopreventive effect of aspirin on growth of aberrant crypt foci in rats. Int J Colorectal Dis. 1998;13(4):169–72. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Schut HA, Conran P, Kramer PM, Lubet RA, Steele VE, Hawk EE, Kelloff GJ, Pereira MA. Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999;20(3):425–30. [DOI] [PubMed] [Google Scholar]
- 63.Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, Price R, Gray K, Kelloff GJ. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21(6):1149–55. [PubMed] [Google Scholar]
- 64.Liu Y, Ju J, Xiao H, Simi B, Hao X, Reddy BS, Huang MT, Newmark H, Yang CS. Effects of combination of calcium and aspirin on azoxymethane-induced aberrant crypt foci formation in the colons of mice and rats. Nutr Cancer. 2008;60(5):660–5. [DOI] [PubMed] [Google Scholar]
- 65.Bousserouel S, Gosse F, Bouhadjar M, Soler L, Marescaux J, Raul F. Long-term administration of aspirin inhibits tumour formation and triggers anti-neoplastic molecular changes in a pre-clinical model of colon carcinogenesis. Oncol Rep. 2010;23(2):511–7. [PubMed] [Google Scholar]
- 66.Kanwar SS, Vaiphei K, Nehru B, Sanyal SN. Chemopreventive effects of nonsteroidal anti-inflammatory drugs on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Toxicol Mech Methods. 2007;17(4):197–204. [DOI] [PubMed] [Google Scholar]
- 67.Barnes CJ, Lee M. Determination of an optimal dosing regimen for aspirin chemoprevention of 1,2-dimethylhydrazine-induced colon tumours in rats. Br J Cancer. 1999;79(11-12):1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66(8):4542–6. [DOI] [PubMed] [Google Scholar]
- 69.Mohammed A, Janakiram NB, Madka V, Zhang Y, Singh A, Biddick L, Li Q, Lightfoot S, Steele VE, Lubet RA, Suen CS, Miller MS, Sei S, Rao CV. Intermittent dosing regimens of aspirin and naproxen inhibit azoxymethane-induced colon adenoma progression to adenocarcinoma and invasive carcinoma. Cancer Prev Res (Phila). 2019. Epub 2019/September/17. doi: 10.1158/1940-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Y, Liu Y, Zhang C, Su ZY, Li W, Huang MT, Kong AN. The epigenetic effects of aspirin: the modification of histone H3 lysine 27 acetylation in the prevention of colon carcinogenesis in azoxymethane- and dextran sulfate sodium-treated CF-1 mice. Carcinogenesis. 2016;37(6):616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tao L, Kramer PM, Wang W, Yang S, Lubet RA, Steele VE, Pereira MA. Altered expression of c-myc, p16 and p27 in rat colon tumors and its reversal by short-term treatment with chemopreventive agents. Carcinogenesis. 2002;23(9):1447–54. [DOI] [PubMed] [Google Scholar]
- 72.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–90. Erratum in: N Engl J Med. 2003;348(19):1939. [DOI] [PubMed] [Google Scholar]
- 73.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–9. [DOI] [PubMed] [Google Scholar]
- 74.Ishikawa H, Mutoh M, Suzuki S, Tokudome S, Saida Y, Abe T, Okamura S, Tajika M, Joh T, Tanaka S, Kudo SE, Matsuda T, Iimuro M, Yukawa T, Takayama T, Sato Y, Lee K, Kitamura S, Mizuno M, Sano Y, Gondo N, Sugimoto K, Kusunoki M, Goto C, Matsuura N, Sakai T, Wakabayashi K. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63(11):1755–9. [DOI] [PubMed] [Google Scholar]
- 75.Shpitz B, Giladi N, Sagiv E, Lev-Ari S, Liberman E, Kazanov D, Arber N. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion. 2006;74(3-4):140–4. [DOI] [PubMed] [Google Scholar]
- 76.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66(14):7370–7. [DOI] [PubMed] [Google Scholar]
- 77.Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr Cancer. 2008;60 Suppl 1:30–5. [DOI] [PubMed] [Google Scholar]
- 78.Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH, Chow SH, Ahnen DJ, Boland CR, Heigh RI, Fay DE, Hamilton SR, Jacobs ET, Martinez EM, Alberts DS, Lance P. Celecoxib for the prevention of colorectal adenomas: results of a suspended randomized controlled trial. J Natl Cancer Inst. 2016;108(12): djw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira MA, Khoury MD. Prevention by chemopreventive agents of azoxymethane-induced foci of aberrant crypts in rat colon. Cancer Lett. 1991;61(1):27–33. [DOI] [PubMed] [Google Scholar]
- 80.Wargovich MJ, Harris C, Chen CD, Palmer C, Steele VE, Kelloff GJ. Growth kinetics and chemoprevention of aberrant crypts in the rat colon. J Cell Biochem Suppl. 1992;16G:51–4. Review. [DOI] [PubMed] [Google Scholar]
- 81.Luk GD, Zhang SZ, Hamilton SR. Effects of timing of administration and dose of difluoromethylornithine on rat colonic carcinogenesis. J Natl Cancer Inst. 1989;81(6):421–7. [DOI] [PubMed] [Google Scholar]
- 82.Kishimoto Y, Takata N, Jinnai T, Morisawa T, Shiota G, Kawasaki H, Hasegawa J. Sulindac and a cyclooxygenase-2 inhibitor, etodolac, increase APC mRNA in the colon of rats treated with azoxymethane. Gut. 2000;47(6):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Femia AP, Soares PV, Luceri C, Lodovici M, Giannini A, Caderni G. Sulindac, 3,3’-diindolylmethane and curcumin reduce carcinogenesis in the Pirc rat, an Apc-driven model of colon carcinogenesis. BMC Cancer. 2015; 15:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takayama T, Nagashima H, Maeda M, Nojiri S, Hirayama M, Nakano Y, Takahashi Y, Sato Y, Sekikawa H, Mori M, Sonoda T, Kimura T, Kato J, Niitsu Y. Randomized double-blind trial of sulindac and etodolac to eradicate aberrant crypt foci and to prevent sporadic colorectal polyps. Clin Cancer Res. 2011;17(11):3803–11. [DOI] [PubMed] [Google Scholar]
- 85.Winde G, Schmid KW, Schlegel W, Fischer R, Osswald H, Bünte H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Advantages of a low-dose nonsteroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis Colon Rectum. 1995;38(8):813–30. [DOI] [PubMed] [Google Scholar]
- 86.Giardiello FM, Offerhaus JA, Tersmette AC, Hylind LM, Krush AJ, Brensinger JD, Booker SV, Hamilton SR. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996;38(4):578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122(3):641–5. [DOI] [PubMed] [Google Scholar]
- 88.Matsumoto T, Nakamura S, Esaki M, Yao T, Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. J Gastroenterol Hepatol. 2006;21(1 Pt 2):251–7. [DOI] [PubMed] [Google Scholar]
- 89.Sinicrope FA, Velamala PR, Wong Kee Song LM, Viggiano TR, Bruining DH, Rajan E, Gostout C, Kriachley RE, Buttar NS, Schroeder KW, Kisiel JB, Larson MV, Sweetser SR, Sedlack RR, Sinicrope SN, Richmond E, Umar A, Della’Zanna G, Noaeill JS, Meyers J, Foster NR. Efficacy of difluoromethylornithine and aspirin for treatment of adenomas and aberrant crypt foci in patients with prior advanced colorectal neoplasms. Cancer Prev Res (Phila). 2019. Epub 2019/September/06. doi: 10.1158/1940-6207. [DOI] [PubMed] [Google Scholar]
- 90.Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, Meyskens FL. Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas. Br J Cancer. 2013;108(3):512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burke CA, Dekker E, Samadder NJ, Stoffel E, Cohen A. Efficacy and safety of eflornithine (CPP-1X)/sulindac combination therapy versus each as monotherapy in patients with familial adenomatous polyposis (FAP): design and rationale of a randomized, double-blind, Phase III trial. BMC Gastroenterol. 2016;16(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lynch PM, Burke CA, Phillips R, Morris JS, Slack R, Wang X, Liu J, Patterson S, Sinicrope FA, Rodriguez-Bigas MA, Half E, Bulow S, Latchford A, Clark S, Ross WA, Malone B, Hasson H, Richmond E, Hawk E. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65(2):286–95. [DOI] [PubMed] [Google Scholar]
- 93.Inoue T, Murano M, Yoda Y, Kuramoto T, Kakimoto K, Ishida K, Kawakami K, Abe Y, Morita E, Murano N, Tokioka S, Maemura K, Umegaki E, Higuchi K. R-etodolac induces E-cadherin and suppresses colitis-related mouse colon tumorigenesis. Oncol Rep. 2010;24(6):1487–92. [DOI] [PubMed] [Google Scholar]
- 94.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14(11):2219–25. [DOI] [PubMed] [Google Scholar]
- 95.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis. 2005;26(8):1450–6. [DOI] [PubMed] [Google Scholar]
- 96.Bounaama A, Djerdjouri B, Laroche-Clary A, Le Morvan V, Robert J. Short curcumin treatment modulates oxidative stress, arginase activity, aberrant crypt foci, and TGF-beta1 and HES-1 transcripts in 1,2-dimethylhydrazine-colon carcinogenesis in mice. Toxicology. 2012;302(2-3):308–17. [DOI] [PubMed] [Google Scholar]
- 97.Pereira MA, Grubbs CJ, Barnes LH, Li H, Olson GR, Eto I, Juliana M, Whitaker LM, Kelloff GJ, Steele VE, Lubet RA. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis. 1996;17(6):1305–11. [DOI] [PubMed] [Google Scholar]
- 98.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59(3):597–601. [PubMed] [Google Scholar]
- 99.Guo Y, Wu R, Gaspar JM, Sargsyan D, Su ZY, Zhang C, Gao L, Cheng D, Li W, Wang C, Yin R, Fang M, Verzi MP, Hart RP, Kong AN. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis. 2018;39(5):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL Jr., Brenner DE. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila). 2011;4(3):354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr., Montgomery EA, Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, Umar A, Rodriguez LM, Giardiello FM. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155(3):668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia Y, Ma Z, Liu X, Zhou W, He S, Xu X, Ren G, Xu G, Tian K. Metformin prevents DMH-induced colorectal cancer in diabetic rats by reversing the warburg effect. Cancer Med. 2015;4(11):1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bordini HP, Kremer JL, Fagundes TR, Melo GP, Conchon-Costa I, da Silva SS, Cecchini AL, Panis C, Luiz RC. Protective effect of metformin in an aberrant crypt foci model induced by 1,2-dimethylhydrazine: Modulation of oxidative stress and inflammatory process. Mol Carcinog. 2017;56(3):913–922. [DOI] [PubMed] [Google Scholar]
- 104.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, Nozaki Y, Yoneda K, Fujita K, Yoneda M, Inamori M, Tomatsu A, Chihara T, Shimpo K, Nakagama H, Nakajima A. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49(7):662–71. [DOI] [PubMed] [Google Scholar]
- 105.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila). 2010;3(9):1077–83. [DOI] [PubMed] [Google Scholar]
- 106.Zhao X, Li Y, Chen M, Chen Y, Dai Y, Wang Y, Xie H. Effects of different doses of metformin treatment for 6 months on aberrant crypt foci in Chinese patients with impaired glucose tolerance. Eur J Cancer Prev. 2015;24(1):27–36. [DOI] [PubMed] [Google Scholar]
- 107.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, Hattori A, Nagase H, Kessoku T, Arimoto J, Matsuhashi N, Inayama Y, Yamanaka S, Taguri M, Nakajima A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–483. [DOI] [PubMed] [Google Scholar]