Abstract
Pain management is a challenging and unmet medical need. Despite their demonstrated efficacy, currently used opioid drugs and nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently associated with several adverse events. The identification of new and safe analgesics is therefore needed. MP1104, an analogue of 3’-iodobenzoyl naltrexamine, is a potent non-selective full agonist at mu (MOR), kappa (KOR) and delta (DOR) opioid receptors, respectively. It was shown to possess potent antinociceptive effects in acute thermal pain assays without aversion in mice. In this study, we investigated MP1104 in the formalin test, a model of tonic pain. MP1104 (0.05, 0.1 and 1.0 mg/kg) reduced pain-like behaviors in phases I and II of the formalin test in male and female ICR mice. Pretreatment with KOR antagonist (norbinaltorphimine 10 mg/kg) and DOR antagonist (naltrindole 10mg/kg) abolished the antinociceptive effects of MP1104 in the formalin test. These findings support the development of MP1104 for further testing in other pain models.
Keywords: Pain, opioid, MP1104, formalin, mice
INTRODUCTION
Opioids are the most commonly used analgesics for acute and chronic pain conditions. However, opioid prescription for the treatment of persistent and severe pain puts patients at risk for addiction and dependence, along with other severe side effects. With opioid addiction being classified as an epidemic (Rudd et al. 2016), the need to develop new analgesics for the treatment of acute and chronic pain is necessary. We recently reported the synthesis and pharmacological evaluation of an analogue of 3’-Iodobenzoyl naltrexamine (IBNtxA) MP1104. This compound was found to be a non-selective high-affinity agonist to mu (MOR), kappa (KOR), and delta (DOR) opioid receptors with binding affinities of 0.021±0.0034 nM, 0.0064±0.002 nM, and 0.08±0.019 nM, respectively (Váradi et al. 2015). Owing to its super high affinity at KOR it was used to crystallize the active form of KOR (Che et al. 2018).
We hypothesized that by targeting multiple opioid receptors simultaneously, the undesirable effects of each of the individual receptors could be reduced. MP1104 exhibited approximately 15-fold greater antinociceptive potency compared with morphine in the tail-flick test, and its effect was mediated through the activation of KOR and DOR receptors (Váradi et al. 2015). Interestingly, MP1104 did not cause place aversion or preference in mice as well as rats in a place-conditioning assay (Atigari et al. 2019), suggesting little abuse liability.
The present study was designed to characterize the antinociceptive effect of MP1104 in the formalin test after acute administration in mice. The formalin test is commonly used as a model of tonic pain. It has been demonstrated subcutaneous (s.c.) formalin injection into one hind paw in the conscious mouse produces biphasic nociceptive behaviors characterized by a brief initial phase (first phase) and a prolonged later phase (second phase), each consisting of elevation, licking, flinching and even biting of the injected hind paw. Traditionally, the first phase of formalin test has been viewed as being due to acute activation of nociceptors in the periphery while the second phase is due to the ensuing inflammatory response or to central sensitization (Tjølsen et al. 1992; Abbott et al. 1995; Watson et al. 1997). However, recent studies suggest that formalin injection may lead to a chronic pain state with persistent neuronal hyperactivity in mice and rats weeks after the injection (Leitl et al. 2014; Chen et al. 2019). We first studied MP1104 activity and potency in the formalin test, then examined the role of the different opiates receptors subtypes in mediating its antinociceptive effects.
MATERIALS AND METHODS
Animals
Male and female adult (8–10 weeks of age) ICR mice obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–approved animal care facility. They were housed in groups of four and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7:00 AM). Observers of the behavioral tests were blind to the treatment group of all subjects. Only one trained observer was used for each behavioral assay. Mice were randomly selected to be in treatment or control groups. All experiments were performed during the light cycle (between 7:00 AM and 7:00 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Drugs
17-Cyclopropylmethyl-3-hydroxy-4,5α-epoxy-7,8-en-6-β-[(3′-iodo)benzamido]-morphinan (MP1104) and naltrindole (NTI) (with >98% purity) (Váradi et al. 2015) were provided by Dr. Rajendra Uprety (Memorial Sloan Kettering Cancer Centre, New York, USA). Norbinaltorphimine dihydrochloride (norBNI) was a generous gift from the NIDA Drug Supply Program (Research Triangle Park, NC) and naloxone HCl was purchased from Sigma-Aldrich (St. Louis, MO). MP1104 was dissolved in a mixture of 2:2:16 [2 volume ethanol/2 volume Emulphor-620 (Sanofi-Aventis, Bridgewater, NJ) and 16 volumes saline] and administered subcutaneously to mice. NTI, norBNI, and naloxone were dissolved in physiologic saline (0.9% sodium chloride) and injected subcutaneously at a total volume of 1 ml/100 g body weight unless noted otherwise. Control groups received injection MP1104’s vehicle, 2:2:16. All doses are expressed as the free base of the drug.
Formalin Test
The formalin test was carried out in an open Plexiglas cage (29 × 19 × 13 cm each), with a mirror placed at a 45-degree angle behind the cage to allow an unobstructed view of the paws. Mice were allowed to acclimate for 15 min in the test cage prior to injection. Each animal was injected with 20 μL of (2.5%) formalin to the right hindpaw intraplantarly. Each mouse was then immediately placed in a Plexiglas box. Up to two mice at one time were observed from 0 to 5 min (phase I) and 20 to 45 min (phase II) post-formalin injection. The period between the two phases of nociceptive responding is generally considered to be a phase of weak activity. The amount of time spent licking the injected paw was recorded with a digital stopwatch.
MP1104 (0.05, 0.1 and 1 mg/kg) or vehicle (2:2:16) were injected s.c. 30 min before formalin injection. For the antagonist studies, naloxone (2 and 4 mg/kg, s.c. 10 min pretreatment), NTI (10 mg/kg, s.c. 15 min pretreatment), norBNI (10 mg/kg, s.c. 16 hr pretreatment) or vehicle (saline) were injected before MP1104 (1 mg/kg; s.c.) or vehicle injection. Each antagonist was administered to a different group independent from each other except for the cotreatment of naltrindole and norBNI. In the cotreatment group, naltrindole was administered 15 min before norBNI administration. The dose of 1 mg/kg of MP1104 was selected for the antagonists’ studies since it produced the highest antinociceptive effect in the first experiment. These doses of antagonists used were reported to fully block the behavioral effects of various opioid receptor agonists (Takemori et al. 1988; Gupta et al. 2013; Taylor et al. 2015).
Statistical Analysis
The data obtained were analyzed using the GraphPad software, version 8.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the 1-way analysis of variance test (ANOVA), followed by the post hoc Tukey’s test. The P values < 0.05 were considered significant.
RESULTS
Effects of MP1104 on Formalin Test
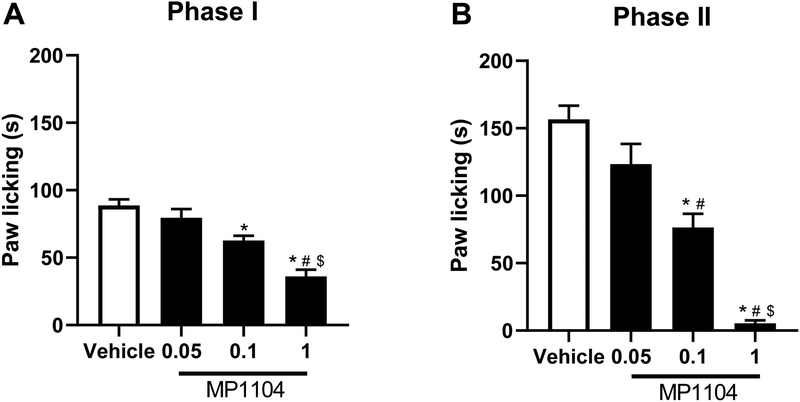
We studied if MP1104 would show antinociceptive effects in both phases of the formalin test. One-way ANOVA revealed MP1104 significantly reduced formalin-induced paw licking in a dose-related manner when compared to the vehicle-treated group [F (3, 32) = 21.25, P < 0.0001, Fig. 1A]. Post hoc analysis revealed the dose of 1.0 mg/kg had significantly more antinociceptive effects than the vehicle group (p < 0.0001) and both doses of 0.05 mg/kg (p<0.0001) and 0.1 mg/kg (p<0.001). The dose of 0.1 mg/kg reduced formalin-induced paw licking (p<0.01 vs vehicle) but there was no difference between doses 0.05 mg/kg and 0.1 mg/kg doses (p>0.05). The dose of 0.05 mg/kg had no effect on formalin-induced paw licking (p>0.05 vs vehicle). The ED50 (value ± confidence limit) of MP1104 in reducing phase I was determined to be 0.55 (0.37–0.81) mg/kg.
Figure 1. MP1104’s antinociceptive effects in both phases of the formalin test.
MP1104 decreases paw licking in formalin-injected mice in a dose-dependent fashion. Mice were treated with s.c. administration of MP1104 (0.05, 0.1, and 1 mg/kg s.c.) 15 min prior to formalin (2.5%, 20 μl) injection into the plantar region of the right hind paw. Data reflect the mean ± S.E.M. of 9 animals (5 male and 4 female) for each group. * p<0.05, significantly different from vehicle group; # p<0.05, significantly different from 0.1 mg/kg MP1104 group; $ p<0.05, significantly different from 0.0.5 mg/kg MP1104 group.
Similarly, in phase II MP1104 significantly reduced formalin-induced paw licking in a dose-related manner when compared to the vehicle-treated group [F (3, 32) = 39.32, P < 0.0001, Fig. 1B]. Post hoc analysis revealed the dose of 1.0 mg/kg had significantly more antinociceptive effects than the vehicle group (p<0.0001) and both doses of 0.05 mg/kg (p<0.0001) and 0.1 mg/kg (p<0.001). The dose of 0.1 mg/kg reduced formalin-induced paw licking (p<0.01 vs vehicle) and had significantly more antinociceptive effects than the dose of 0.05 mg/kg (p<0.05). The dose of 0.05 mg/kg had no effect on formalin-induced paw licking (p>0.05 vs vehicle). The ED50 of MP1104 in phase II was 0.12 (0.07– 0.81) mg/kg.
Effects of Opioid Antagonists with MP1104 on Formalin Test
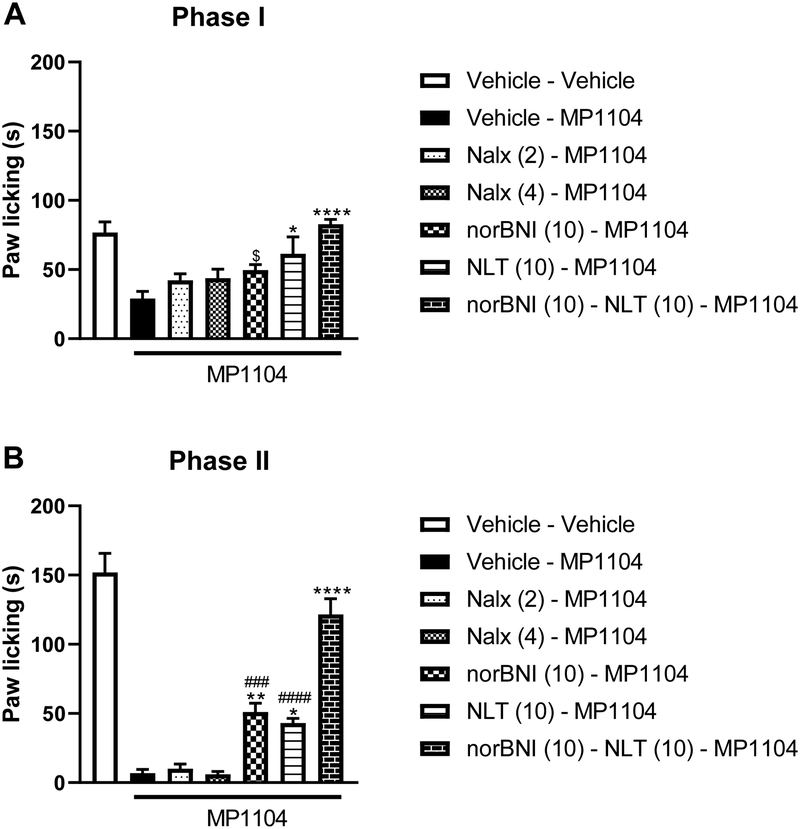
We investigated the effects of opioid antagonists on MP1104 in the formalin test. All the antagonists were administered with 1 mg/kg (s.c.) MP1104. One-way ANOVA revealed significant effects of treatment at phase I [F (6, 49) = 8.052, P < 0.0001, Fig. 2A]. Post hoc analysis of phase I revealed that naltrindole (10 mg/kg), a selective delta antagonist, significantly blocked MP1104’s antinociceptive effect on paw licking and there was no difference when compared to the control group (p>0.05 vs vehicle-vehicle). norBNI (10 mg/kg), a selective kappa antagonist, did not significantly block MP1104’s antinociceptive effects on paw licking (p>0.05 vs vehicle-MP1104). However, cotreatment of norBNI and naltrindole completely blocked MP1104’s antinociceptive effects on paw licking and there was no significant difference when compared to the control group (p>0.05 vs vehicle-vehicle). Neither doses of naloxone (2 mg/kg and 4 mg/kg) did not reverse MP1104 antinociceptive effects when compared to the group only received MP1104 (p>0.05 vs vehicle-MP1104, p>0.05 vs vehicle-MP1104, respectively). There was no difference between doses of 2 mg/kg and 4 mg/kg (p>0.05).
Figure 2. Complete block of MP1104’s antinociceptive effects with the cotreatment of DOR and KOR antagonists.
Naltrindole significantly blocked MP1104’s effects in phase I (Figure 2A) and it exhibited a partial block in phase II (Figure 2B). norBNI partially blocked MP1104’s effects only in phase II (Figure 2B). Cotreatment with naltrindole (10 mg/kg s.c.) and norBNI (10 mg/kg s.c.) completely blocked the MP1104’s antinociceptive effects in phase I and phase II of formalin test (p>0.05 vs vehicle-vehicle group). Data reflect the mean ± S.E.M. of 8 animals (4 male and 4 female) for each group. $ p<0.05, significantly different from norBNI (10)-NLT (10)-MP1104 group; * p<0.05, significantly different from vehicle-MP1104 group; ** p<0.01, significantly different from vehicle-MP1104 group; **** p<0.0001, significantly different from vehicle-MP1104 group; ### p<0.001, significantly different from norBNI (10)-NLT (10)-MP1104 group; #### p<0.0001, significantly different from from norBNI (10)-NLT (10)-MP1104 group.
Similarly, one-way ANOVA revealed significant effects of treatment at phase II of the formalin test [F (6, 49) = 51, P < 0.0001, Fig. 2B]. Post hoc analysis of phase II revealed that naltrindole (10 mg/kg) partially blocked MP1104’s antinociceptive on paw licking when compared to the group treated MP1104 alone (p<0.05 vs vehicle-MP1104) but its effect was significantly less than the control group (p<0.0001 vs vehicle-vehicle). Similarly, norBNI (10 mg/kg) partially blocked MP1104’s antinociceptive effects on paw licking when compared to group received MP1104 alone (p<0.001 vs vehicle-MP1104) but its effect was significantly less than the control group (p<0.0001 vs vehicle-vehicle). However, cotreatment with the combination of norBNI and naltrindole completely blocked MP1104’s antinociceptive effect on paw licking and there was no difference when compared to the control group (p>0.05 vs vehicle-vehicle).
Neither doses of naloxone (2 mg/kg and 4 mg/kg) did not reverse MP1104 antinociceptive effects when compared to the group only received MP1104 (p>0.05 vs vehicle-MP1104, p>0.05 vs vehicle-MP1104, respectively). There was no difference between naloxone doses of 2 mg/kg and 4 mg/kg (p>0.05).
DISCUSSION
MP1104 is a novel mixed opioid agonist that binds to MOR, DOR and KOR receptors, showing 3-fold and 13-fold the affinity towards KOR over MOR and DOR respectively (Váradi et al. 2015). MP1104 lacks many of the side effects associated with KOR, MOR and DOR agonism in rodents (Váradi et al. 2015; Atigari et al. 2019). In addition, MP1104 was recently reported to exhibit antinociceptive effects in the tail-flick test, an acute thermal pain assay (Váradi et al. 2015). However, no studies have evaluated the effect of this mixed opioid agonist in tonic and persistent pain models. In this study, we investigated the MP1104 antinociceptive effects on the formalin test, a model of tonic pain. MP1104 showed dose-dependent antinociception in both phases of the formalin test. However, MP1104 was less 4.5-fold less potent in blocking phase I than phase II behaviors.
Our study is the first to report MP1104’s antinociceptive effects in the formalin test. We used a pharmacological approach to determine the relative contributions of opioid receptor families to MP1104’s anticociceptive effects in the formalin test. Our data suggest that MP1104 produces its antinociceptive effects through agonism at KOR and DOR receptors and not MOR. The formalin test results are similar to those reported with MP1104 in the acute tail-flick test (Váradi et al. 2015) in terms of potency and opioid receptors involvement but formalin test might give more insight to chronic pain states as suggested by recent studies (Leitl et al. 2014; Chen et al. 2019).
Studies involving chronic pain models are needed to fully understand MP1104 analgesic profile as chronic pain has different pathophysiological changes from acute pain (Feizerfan and Sheh 2015); the KOR system may be engaged under prolonged or chronic tissue injury and it is also more involved in the affective component of chronic pain (Liu et al. 2019) but not acute pain (Bagdas et al. 2016).
Our findings suggest that dual kappa-delta agonists could be promising novel analgesics.
Acknowledgements
The authors would like to thank Tie Han for his technical assistance with the formalin studies.
Funding Sources
This research was supported by National Institute on Drug Abuse [grant DA045884] to SJ, National Cancer Institute [grant CA008748] to CBT, and funds from VCU School of Medicine to MID.
Footnotes
Declaration of conflict
None declared.
REFERENCES
- Abbott FV, Franklin KB, Westbrook RF (1995) The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 60:91–102 [DOI] [PubMed] [Google Scholar]
- Atigari DV, Uprety R, Pasternak GW, et al. (2019) MP1104, a mixed kappa-delta opioid receptor agonist has anti-cocaine properties with reduced side-effects in rats. Neuropharmacology. 10.1016/J.NEUROPHARM.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, Alsharari S, et al. (2016) Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 102:236–243. 10.1016/j.neuropharm.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T, Majumdar S, Zaidi SA, et al. (2018) Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 172:55–67.e15. 10.1016/j.cell.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhang J, Sun L, et al. (2019) Long-term imaging of dorsal root ganglia in awake behaving mice. Nat Commun 10:3087 10.1038/s41467-019-11158-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizerfan A, Sheh G (2015) Transition from acute to chronic pain. Contin Educ Anaesth Crit Care Pain 15:98–102. 10.1093/bjaceaccp/mku044 [DOI] [Google Scholar]
- Gupta R, Gupta LK, Bhattacharya SK (2013) Naloxone blocks the beneficial effects of aqueous extract of Murraya koenigii (L.) Spreng leaves in models of pain. Eur Rev Med Pharmacol Sci 17:1748–51 [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, et al. (2014) Sustained pain-related depression of behavior: Effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain 10:1–11. 10.1186/1744-8069-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, et al. (2019) Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci 39:4162–4178. 10.1523/JNEUROSCI.0274-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R (2016) Increases in Drug and Opioid Overdose Deaths-United States, 2000–2014. Am J Transplant 16:1323–1327. 10.1111/ajt.13776 [DOI] [PubMed] [Google Scholar]
- Takemori AE, Schwartz MM, Portoghese PS (1988) Suppression by nor-binaltorphimine of kappa opioid-mediated diuresis in rats. J Pharmacol Exp Ther 247:971–974 [PubMed] [Google Scholar]
- Taylor AMW, Roberts KW, Pradhan AA, et al. (2015) Anti-nociception mediated by a κ opioid receptor agonist is blocked by a δ receptor agonist. Br J Pharmacol 172:691–703. 10.1111/bph.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, et al. (1992) The formalin test: an evaluation of the method. Pain 51:5–17 [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Eans SO, et al. (2015) Synthesis and Characterization of a Dual Kappa-Delta Opioid Receptor Agonist Analgesic Blocking Cocaine Reward Behavior. ACS Chem Neurosci 6:1813–1824. 10.1021/acschemneuro.5b00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Sufka KJ, Coderre TJ (1997) Optimal scoring strategies and weights for the formalin test in rats. Pain 70:53–8 [DOI] [PubMed] [Google Scholar]