Abstract
Neuroendocrine neoplasms constitute a diverse group of tumors that derive from the sensory and secretory neuroendocrine cells and predominantly arise within the pulmonary and gastrointestinal tracts. The majority of these neoplasms have a well-differentiated grade and are termed neuroendocrine tumors (NETs). This subgroup is characterized by limited proliferation and patients affected by these tumors carry a good to moderate prognosis. A substantial subset of patients presenting with a NET suffer from the consequences of endocrine syndromes as a result of the excessive secretion of amines or peptide hormones, which can impair their quality of life and prognosis. Over the past 15 years, critical developments in tumor grading, diagnostic biomarkers, radionuclide imaging, randomized controlled drug trials, evidence-based guidelines, and superior prognostic outcomes have substantially altered the field of NET care. Here, we review the relevant advances to clinical practice that have significantly upgraded our approach to NET patients, both in diagnostic and in therapeutic options.
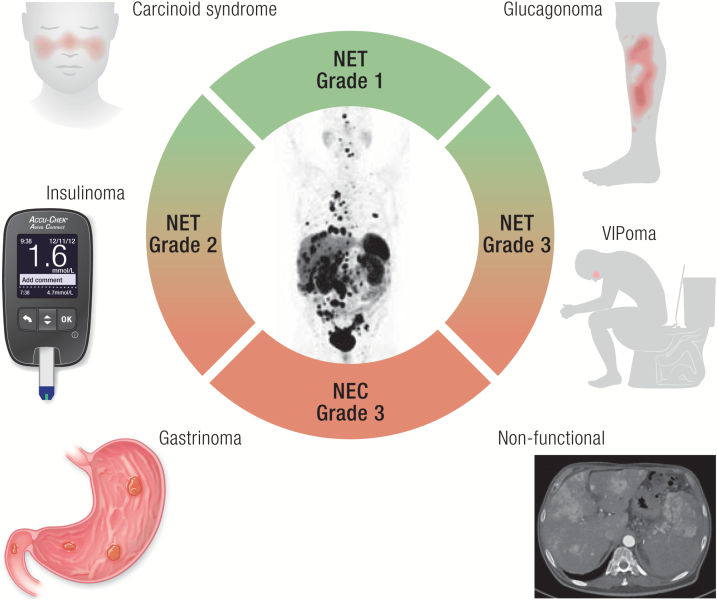
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Clinicians are increasingly confronted with neuroendocrine neoplasms as their incidence and prevalence are rising across all primary sites
Patients presenting with a neuroendocrine neoplasm should be scrutinized for the presence of a functional hormonal syndrome as this can impair survival, offers the possibility of sensitive biomarkers, and requires dedicated therapy
Obtaining histology of a suspected neuroendocrine neoplasm is crucial for confirmation of the diagnosis as well as for classification into well-differentiated neuroendocrine tumor or poorly differentiated neuroendocrine carcinoma
Functional imaging with 68Gallium-labelled somatostatin analog and 18F-FDG PET tracers ensures superior staging and prognostication of neuroendocrine neoplasms
Long-acting somatostatin analogs constitute the preferred first-line option for several hormonal syndromes associated with neuroendocrine neoplasms as well as for growth control in well-differentiated irresectable or metastatic gastroenteropancreatic tumors, while several novel treatment options for hormonal and/or antiproliferative control in neuroendocrine neoplasms have shown efficacy in randomized controlled trials, expanding the clinical repertoire and allowing for improved management based on individual patient and tumor characteristics
Background on Neuroendocrine Neoplasms
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of epithelial neoplastic lesions that irrespective of their primary site of origin share features of neural and endocrine differentiation including the presence of secretory granules, synaptic-like vesicles, and the ability to produce amines and/or peptide hormones (1). Previously used terms for NENs include APUDomas or carcinoid tumors. NENs express general markers of neuroendocrine differentiation, organ-specific bioactive substances, and tissue-specific transcription factors and predominately arise from the bronchopulmonary (BP) and gastrointestinal (GI) system including the pancreas (2). NENs encompass a wide spectrum of neoplasms defined by conventional morphology from well-differentiated and relatively slowly growing but potentially malignant tumors, to highly aggressive poorly differentiated neuroendocrine carcinomas (1).
Location and epidemiology
Although neuroendocrine differentiation can occur in many epithelial carcinomas, including breast and prostate cancer, NENs are considered a separate entity because of their explicit origin from neuroendocrine cells of the diffuse endocrine system. Although NENs are mainly encountered in the BP and GI tracts, other organs can also give rise to these tumors. Key examples from endocrine organs are parathyroid adenoma, medullary thyroid carcinoma, pheochromocytoma, and paraganglioma (3), whereas a reclassification of pituitary adenoma as a neuroendocrine tumor has also been proposed recently (4). Other NENs are rarely encountered in endocrine practice and include among others Merkel cell carcinoma of the skin (5) and the neuroendocrine adenoma or the middle ear (NAME) (6). Recently, a uniform classification was proposed for NENs of all sites for consistent reporting, intertumoral comparisons, and management (7).
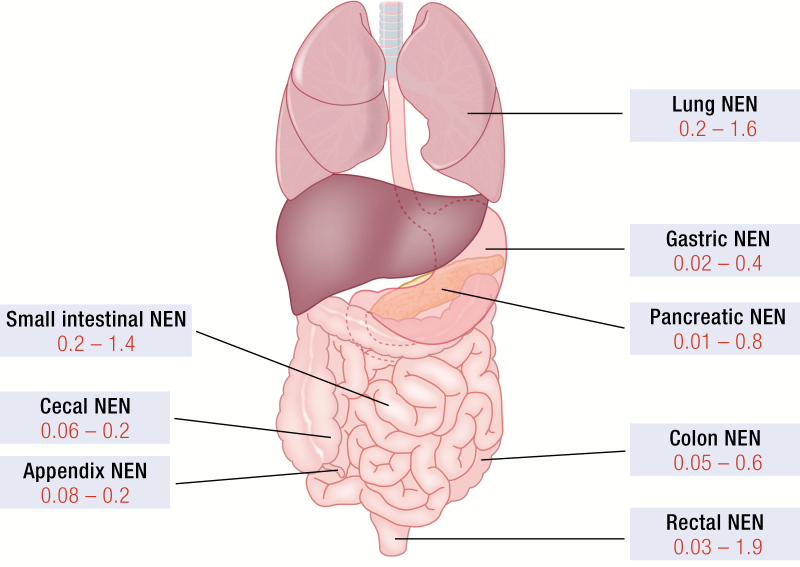
Fig. 1 depicts the most common NEN sites of the bronchial and gastroenteropancreatic (GEP) systems and their reported incidence rates. The most common primary GEP NEN sites are the rectum and small intestine (8, 9). Up to 20% of patients present with metastases at the time of diagnosis (9). However, there is a clear distinction in metastatic potential across sites such as appendix and gastric NENs predominantly present with localized stages of disease while a majority of patients with pancreatic or small intestinal NENs is diagnosed in metastasized setting (10). Despite major improvements in modern imaging techniques still approximately 5% of metastasized NENs have an unknown primary tumor (11).
Figure 1.
Neuroendocrine neoplasms (NEN) locations and incidence rates. The most common primary NEN sites of the pulmonary and gastroenteropancreatic systems are depicted. Incidence rates were collected from Fraenkel et al. (9). and Dasari et al. (8). and are shown in red as the incident number of cases per 100 000 per year.
As NENs predominantly derive from the embryonic gut, historically tumor sites are subdivided into foregut, midgut, and hindgut NENs (12). Foregut NENs include BP and thymic NENs and esophageal, gastric, duodenal, and pancreatic NENs. There is a specific classification for gastric NENs as these have different pathophysiologic mechanisms. Type 1 NENs develop multifocally in enterochromaffin-like cells of the stomach as a consequence of chronic hypergastrinemia resulting from atrophic gastritis (13). Similarly, type 2 gastric NENs arise in these cells due to endocrine stimulation by a gastrin-secreting NEN (gastrinoma) in the context of the MEN-1 syndrome (14). Type 3 gastric NENs are sporadic, solitary NENs, which develop in the absence of elevated gastrin levels and display an aggressive biologic behavior despite their well-differentiated morphology. Type 4 gastric NENs are poorly differentiated carcinomas with limited prognosis (15). Midgut NENs arise in the GI section vascularized by the superior mesenteric artery with a predilection for the ileocecal region. Appendix NENs are also categorized as midgut NENs, but these tumors are generally considered a distinct entity because of the peak incidence in children and young adults and its relative benign behavior (16). Incidence rates of hindgut NENs show a preference of rectal NENs over colonic NENs, both of which are increasingly recognized on colonoscopy (17). Other primary tumor sites that are encountered on rare occasions include the trachea, esophagus, ovaries, testis, prostate, kidney, and breast.
The major sources of NEN epidemiology data are national cancer registries in Western Europe and the US National Cancer Institute Surveillance, Epidemiology and End Results. The incidence of all NENs in all primary sites has been steadily increasing 3.6- to 4.8-fold over the previous 4 decades in the western world (8, 18, 19). The biggest increase in incidence was found for the gastric and rectal NENs and the smallest increase was found for the small intestinal and cecal NENs (8, 9). The overall estimated annual incidence of GEP NENs is between 3.6 and 3.9 per 100 000 population. Studies have identified gender and racial differences, which differed site by site. In Asian patients, small intestinal NENs seem to be rarer, whereas gastric and rectal NENs seem more prevalent (20).
Pathophysiology of NEN
Despite their variety in biologic behavior, there are commonalities in underlying pathophysiologic mechanisms and associated genetic aberrations in NENs across sites. Although still much is unknown about NEN pathogenesis, several key molecular pathways have been shown to contribute to tumor formation in either indolent or more aggressive NENs. Below some causative markers which possess diagnostic potential are discussed, but the reader is referred to a recent publication on an in-depth discussion of underlying (epi-)genetic factors in NENs (21).
Pancreatic, gastric, duodenal, thymic, and bronchial NENs can be found in the spectrum of the multiple endocrine neoplasia type 1 syndrome (MEN1, MIM 131100) (22). Pancreatic NENs (PanNENs) can also be found in the spectrum of von Hippel Lindau disease (MIM 193300) (23). Periampullary somatostatinomas can be diagnosed in patients with neurofibromatosis 1 (MIM 162200) (24). In the Pacak–Zhuang syndrome, HIF2A mutations lead to the development of somatostatinomas, next to paragangliomas/pheochromocytomas and polycythemia (25). In Mahvash disease caused by a mutant P86S glucagon receptor (GCGR), there is an increased incidence of PanNENs and in patients with a MAFA mutation (MIM 147630) insulinomatosis of the pancreas has been found (26, 27).
Endocrine-related symptoms and syndromes caused by NENs
Isolated or metastatic NENs can present with a spectrum of hormone-related symptoms and syndromes which result from the hypersecretion of one or more amines and/or peptides by these tumors. The production of bioactive compounds can be characteristic of the specific tissue of origin leading to a secretory syndrome (eutopic secretion) or rarely compounds that are typically originating from other anatomical sites (ectopic secretion) (28). The representative endocrine syndromes encountered in NEN patients are shortly described below.
Carcinoid syndrome
The carcinoid syndrome (CS) is the result of multiple secreted tumor products. Predominantly midgut, followed by thymic and bronchial and very rarely pancreatic, or other gastrointestinal NENs are the main primary sources of this syndrome (29). It occurs in approximately 20% to 30% of patients with liver and/or bone metastases from these tumors. The secretory products which are potentially involved in the CS are serotonin (5-hydroxytryptamine, 5-HT), histamine, brady- and tachykinins, kallikrein and prostaglandins (30). As these hormones are effectively metabolized by the liver, symptoms of the CS generally only occur when tumor localizations are outside of or bypass the portal vein drainage system (31). Examples of these bypasses include ovarian, rectal of extensive peritoneal sites.
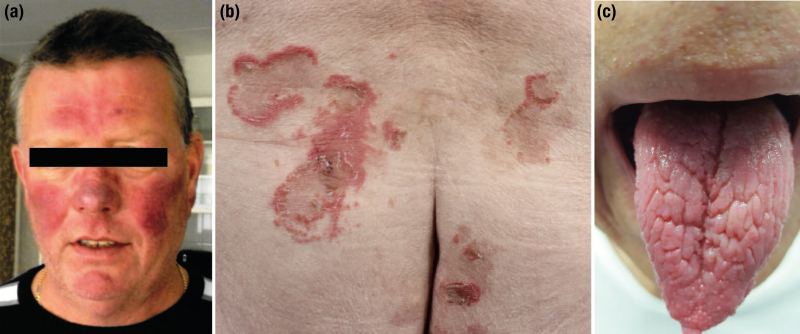
The breakdown metabolite of serotonin is 5-hydroxyindoleacetic acid (5-HIAA) which is excreted in the urine. Serotonin acts via seven types of G protein–coupled receptors and among various other functions regulates motility of and fluid secretion into the intestinal tract next to the inhibition of absorption. Serotonin also has a role in fibrosis. In the CS, diarrhea and—predominantly right sided—heart failure resulting from endocardial and heart valve fibrosis are dominant symptoms attributed to systemic serotonin excess. The increased conversion of tryptophan to serotonin may lead to tryptophan deficiency with subsequent decreased protein synthesis, hypoalbuminemia and nicotinic acid deficiency. Another dominant symptom in the CS is the flushing of the face and upper trunk, which cannot be directly associated to serotonin, but which most probably is mediated by vasoactive substances (bradykinins, prostaglandins, tachykinins, substance P, histamine) released by the tumor and its metastases (Fig. 2A) (32, 33).
Figure 2.
Clinical signs of hormonal excess in neuroendocrine neoplasms (NENs). (A) Facial flushing in the context of carcinoid syndrome in a patient with a metastasized midgut neuroendocrine tumor. (B) Necrolytic migratory erythema at the sacral region and (C) glossitis in a patient with a metastasized glucagonoma.
Insulinoma
Insulinomas are PanNENs that through inappropriate secretion of insulin or insulin precursors can cause severe hypoglycemias. Usually, the so-called Whipple’s triad consisting of (1) symptoms of hypoglycemia, (2) plasma glucose levels <2.2 mmol/L (<40 mg/dL), and (3) relief of symptoms with the administration of glucose remains fundamentally sound. Approximately 10% of insulinomas are multiple and less than 10% can be metastatic at presentation.
Insulinomas have an estimated incidence of 1 to 3 per million population per year. Hypoglycemic symptoms can be grouped into those resulting from neuroglycopenia (commonly including headache, diplopia, blurred vision, confusion, dizziness, abnormal behavior, lethargy, amnesia, whereas, rarely, hypoglycemia may result in seizures and coma) and those resulting from activation of the autonomic nervous system (including sweating, weakness, hunger, tremor, nausea, feelings of warmth, anxiety, and palpitations). Symptoms usually resolve with food. Weight gain is nonspecific (34, 35).
Gastrinoma
Gastrin is a peptide hormone that stimulates the secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. The precursor molecule preprogastrin can be enzymatically cleaved into progastrin, which can be further processed into various forms of gastrin. The most important forms of gastrin are gastrin-34 (“big gastrin”), gastrin-17 (“little gastrin”), and gastrin-14 (“minigastrin”), which contain 34, 17, and 14 amino acids, respectively (36). These gastrin isoforms bind to a specific G protein–coupled gastrin receptor.
Gastrinomas are NENs which secrete gastrin. The incidence of gastrinomas is 0.5 to 3 per million population per year. These tumors can be located in the duodenum (50% to 88%) and pancreas. Gastric acid hypersecretion can result in (recurrent) Helicobacter pylori-negative severe peptic disease (peptic ulcer disease and/or gastroesophageal reflux disease) which can be resistant to regular treatments and diarrhea (37, 38). The first description of gastrinoma by Robert Zollinger and Edwin Ellison dates from 1955. Therefore, the gastrinoma syndrome has also been named Zollinger–Ellison syndrome (39).
VIPoma
Vasoactive intestinal polypeptide (VIP) is a 28 amino acid peptide and a ligand to a specific G protein–coupled receptor. It has a multitude of actions on many tissues, organ systems, and functions including neuronal, digestive, cardiovascular, respiratory, reproductive, exocrine, endocrine, neuroendocrine, immune, and renal functions.
VIPomas are NENs which secrete VIP. Their annual incidence is 1 to 2 per 10 million population. These tumors can be localized in the pancreas (75%), or in the sympathetic ganglia (25%). The first cases of VIPoma were reported in 1958 by John V. Verner Jr. and Ashton B. Morrison. VIPoma syndrome has also been named Verner–Morrison syndrome (39).
VIPoma patients suffer from profuse large volumes of watery (secretory) diarrhea. This will eventually lead to severe electrolyte disturbances, such as loss of bicarbonate and potassium in the stools. Other symptoms include facial flushing and inhibition of gastric acid secretion (40). VIPoma syndrome has been termed watery diarrhea hypokalemia achlorhydria syndrome. About 50% of patients also present with hypercalcemia. The mechanism of action for this effect is unknown, but it might be related to cosecretion of parathyroid hormone-related peptide (41).
Glucagonoma
Glucagon is a 29 amino acid peptide and a ligand to a specific G protein–coupled receptor. It is the most important catabolic hormone of the body causing a rise of the concentrations of glucose and fatty acids. Glucagonomas are PanNENs which secrete glucagon.
In the majority of glucagonoma patients, there is either a new onset or worsening of diabetes mellitus. The catabolic effect of glucagon leads to significant weight loss in 70% to 80% of patients. Also, cheilosis, glossitis, and stomatitis is reported in 30% to 40% of patients. Thromboembolic events and anemia frequently occur in these patients. But the most distinct feature of glucagonomas remains a typical skin manifestation named necrolytic migratory erythema (Fig. 2B, C) (42).
Somatostatinoma
Somatostatin is present in the human body in two major subforms: somatostatin-14 (consisting of 14 amino acids) and somatostatin-28 (28 amino acids). The 5 different somatostatin receptor (SSTR) subtypes are G protein–coupled receptors through which hormone release by various endocrine organs can be inhibited. Furthermore somatostatin plays a role in neurotransmission.
Somatostatinomas are somatostatin-secreting NENs which can be localized in the pancreas (60%) or in the duodenum (40%). Their annual incidence is extremely rare at 1 per 40 million population. The somatostatinoma syndrome is characterized by somatostatin hypersecretion resulting in diabetes mellitus of recent onset, decreased gastric acid secretion, cholelithiasis, steatorrhea, anemia, and weight loss (43).
Other hormonal syndromes in NENs
Apart from the secretion of a single hormone, multiple and secondary hormone secretion can be found in 3% to 10% patients with metastatic PanNENs. Also, in time, PanNENs may secrete another hormone, or nonfunctioning PanNENs may start secreting a biologically active hormone. This is named “metachronous” secretion. Secondary hormone secretion is usually associated with disease progression and is also associated with increased morbidity and mortality, particularly in patients with insulin hypersecretion (44, 45).
Paraneoplastic humoral syndromes can be caused by adrenocorticotropic hormone or corticotropin-releasing hormone secretion causing Cushing’s syndrome (46), parathyroid hormone-related peptide secretion causing hypercalcemia (41), antidiuretic hormone secretion causing hyponatremia, and growth hormone-releasing hormone secretion causing acromegaly (47).
In 2013, the first pancreatic cholecystokinimoma secreting cholecystokinins was described by Rehfeld and colleagues (48). Cholecystokinins are a group of polypeptides composed of varying numbers of amino acids which all are ligands to a specific G protein–coupled receptor. Cholecystokinins have many structural similarities with gastrin. This syndrome is characterized by nonwatery diarrhea, cholelithiasis, peptic ulcer disease, and significant weight loss (48).
Nonhormonal symptoms
Given the expanded use of cross-sectional imaging and endoscopy procedures, an increasing number of patients will present with a NEN without related symptoms, so-called incidentalomas. However, depending on the location of the primary tumor and its metastases, complaints can occur due to compression, ingrowth, or obstruction of vital structures. Because of their gastrointestinal location some primary tumors of the gut or pancreatic tumors growing into the bowel might give rise to blood loss and iron-deficiency anemia. Abdominal pain complaints are often encountered in patients with gastroenteropancreatic NENs (49). A pathognomonic feature of mesenteric metastases of midgut NENs is fibrosis, leading to intermittent (postprandial) pain due to venous ischemia and possible perforation (50). Mechanical bowel obstruction because of a NEN is a rare complication. Given the extensive liver metastases in a considerable subset of stage IV NENs, patients can show complications of hepatomegaly, including pain and jaundice. Bone pain because of skeletal metastases is occasionally a presenting feature, but more often develops during the disease course. Respiratory symptoms of recurring infections, cough, dyspnea, chest pain, and wheezing can be seen in patients with lung NENs or lung metastases, especially when tumors are located near the central airways. Systemic symptoms of malignancy such as cachexia, fever, and night sweats are seldomly observed in patients with well-differentiated NENs, but appear more frequently in high-grade NENs.
Diagnosis
Histology
The diagnosis of a NEN is based on its distinctive histologic and immunohistopathologic profile such as expression of the general markers of neuroendocrine differentiation chromogranin A and synaptophysin. Consequently, histology should be obtained by biopsy or resection in all patients suspected of having a NEN. In addition, immunohistochemistry is also useful for identifying prognostic and theranostic markers (51).
In the past, a major problem in the management of patients with NENs was the lack of universally accepted standards regarding their nomenclature, prognostic stratification, and staging (52). Although the World Health Organization (WHO) classification of 2000 attempted to address some of these issues, the clinicopathologic classification that was introduced was rather complicated, whereas terminologies such as lesions of “uncertain behavior” were confusing and not widely accepted (53). It subsequently became apparent that NENs constitute a heterogeneous group of lesions with ubiquitous malignant potential. Their ability to metastasize or invade adjacent structures depended on tumor site, type, and biologic behavior (54).
However, it was not until the European Neuroendocrine Tumor Society (ENETS) introduced a grade classification and a site-specific staging system that some of these issues were addressed (52, 55). The proposed classification by ENETS attempted to combine tumor heterogeneity according to the tissue of origin, along with tumor differentiation and malignant potential (52). Based on this classification, it became evident that tumors originating from specific anatomical sites such as gastric NENs related to hypergastrinemia, duodenal, appendiceal, and rectal NENs follow a less aggressive course than those derived from other parts of the GI tract and the pancreas (56).
As per other malignancies, the TNM staging system was also incorporated in this classification system to denote the anatomical extent of the disease. Tumors localized to the organ of origin are staged as I or II depending on their size and extent, tumors with spread to regional nodes are staged as III, and those with distant metastases as stage IV. This classification is adopted with the intention that categories within each group are more or less homogeneous in respect of survival, and that the survival rates are distinctive between groups (52, 55). As the potential for tumor spread is directly related to the tissue of origin, tumor size incorporated in the TNM staging differs among tumors originating from different anatomical sites (52, 55). Subsequently, in the 2010 WHO classification the term “neuroendocrine” was fully adopted to highlight the expression of neural markers in tumors exhibiting endocrine properties and phenotype (56).
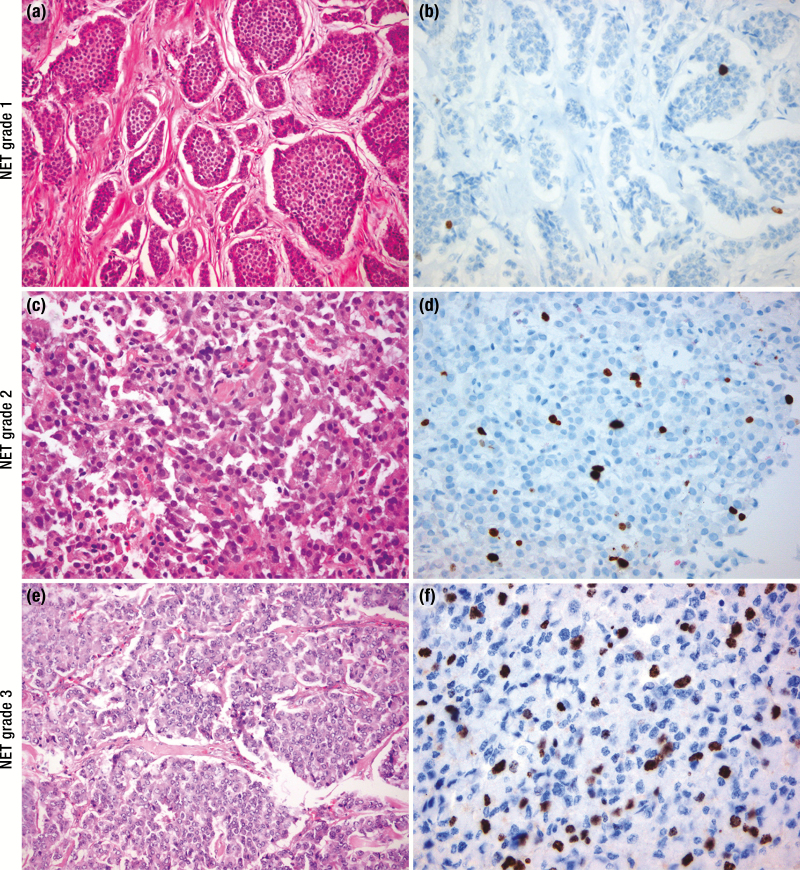
In addition, grading, to denote tumoral biologic behavior, was based on the proliferation rate according to that introduced by ENETS along with traditional morphologic features (Fig. 3). The proposed grading based on proliferation rates defines three grades (G1, G2, G3) that utilize specific numerical ranges of the mitotic count and Ki67 proliferation index (PI) (Table 1a). For bronchial NEN, grading incorporates the presence of necrosis rather than the Ki67 PI. Well-differentiated G1 and G2 bronchial NENs are also termed typical and atypical carcinoids, respectively (Table 1b). The grading based on mitotic count requires to be performed in at least 50 high-power fields (1 HPF = mm2) and Ki67 PI using the MIB1 antibody as a percentage of 500 to 2000 cells counted in areas of the strongest nuclear labelling (so called “hot spots”). There is substantial evidence that grading based on Ki67 PI has a strong prognostic value (56). However, existing classification systems varied widely in terminology and criteria among different authorities with robust data on biologic behavior based on Ki67 PI in GI NENs and number of mitoses in bronchial NENs (1).
Figure 3.
Histopathology of neuroendocrine neoplasms (NENs). Hematoxylin and eosin (H&E, A) and Ki67 (B) staining of a grade 1 NET showing nests of neuroendocrine cells with oval nuclei, “salt and paper” chromatin and moderate eosinophilic cytoplasm. The nests are separated by a fibrous stroma. Nuclear staining of Ki67 is only visible in a few neoplastic cells (Ki67 <3%). (C) Histology of a grade 2 neuroendocrine tumor (NET) reveals a homogeneous population of neuroendocrine cells with slight atypia, round to oval nuclei, dense chromatin and moderate eosinophilic/ amphophilic cytoplasm. (D) Ki67 staining in the same tumor revealed 5% positivity in a hotspot. (E) A grade 3 NET displays a well-differentiated histology of neuroendocrine cells with vesicular nuclei without nucleoli and moderate amphophilic cytoplasm arranged in a nested pattern, whereas the Ki67 proliferation index is above 20% (F). H&E and Ki67 images are amplified ×200 and ×400, respectively.
Table 1.
World Health Organization classification of neuroendocrine neoplasms (NENs) of the gastrointestinal and bronchopulmonary tracts.
| Gastroenteropancreatic NENs (2010) | |||
| Classification/Grade | Ki67 Proliferation Index | Mitotic Index | |
| Well-differentiated NENs | |||
| NET G1 | <3% | <2 | |
| NET G2 | 3–20% | 2–20 | |
| High-grade or poorly differentiated NENs | |||
| NEC G3 | >20% | >20 | |
| Mixed adenoneuroendocrine carcinoma or MANEC | |||
| Hyperplastic and preneoplastic lesions | |||
| Thoracic NENs (2010) | |||
| Classification/grade | Mitotic index | Necrosis | Ki67 PI* |
| Well-differentiated NENs | |||
| Typical carcinoid G1 | 0–1 | No | Up to 5% |
| Atypical carcinoid G2 | 2–10 | Focal if present | Up to 20% |
| High-grade or poorly differentiated NENs | |||
| Large cell NEC G3 | >10 (median 70) | Yes | 40–80% |
| Small cell NEC G3 | >10 (median 80) | Yes | 50–100% |
| PanNENs (2017) | |||
| Classification/grade | Ki67 proliferation index | Mitotic index | Genetic aberrations |
| Well-differentiated PanNENs | |||
| PanNET G1 | <2% | <2 | MEN1, ATRX, DAXX, BRAC2, CHECK2, mTOR |
| PanNET G2 | 2–20% | 2–20 | |
| PanNET G3 | >20% | >20 | |
| Poorly differentiated PanNENs | |||
| PanNEC G3 | >20% | >20 | TP53, RB1 |
| Small cell type | |||
| Large cell type | |||
| Mixed Neuroendocrine non-Neuroendocrine Neoplasm (MiNEN) |
* Ki67 PI is not used for classification of lung NENs
The WHO 2010 classification encompassed the previously named carcinoid tumors and defined a neuroendocrine tumor (NET) as a well-differentiated neuroendocrine neoplasm resembling the normal gut–pancreas endocrine cells that expresses general markers of neuroendocrine differentiation (chromogranin A and synaptophysin) and hormones according to the site of origin. However, the majority of NENs are nonfunctioning and general neuroendocrine markers lack specificity for the lineage or site of the tumor. Caudal type homeobox 2 (CDX-2) has showed high sensitivity and specificity for small intestinal NENs, whereas PAX-8 and Islet-1 (ISL-1) are used to identify primary and metastatic PanNENs (57).
Well-differentiated NETs exhibit mild to moderate nuclear atypia and belong to grades 1 and 2. In contrast, a neuroendocrine carcinoma (NEC) is defined as a poorly differentiated, high-grade malignant neoplasm composed of small or large to intermediate cells, sometimes with organoid features resembling a NET. Such neoplasms express diffusely the general markers of neuroendocrine differentiation (mainly synaptophysin and only occasionally focal staining for chromogranin A), showing marked nuclear atypia, multifocal necrosis and a high number of mitoses (>20 per 10 HPF). They are designated as high-grade (G3) neoplasms according to the PI and histology. This definition applies to neoplasms previously classified as small cell carcinoma, large cell (neuro)endocrine carcinoma (SCNC and LCNEC respectively), or poorly differentiated (neuro)endocrine carcinoma (1) (Table 1b).
In the same WHO 2010 classification a separate group of neoplasm was described and was termed as mixed adenoneuroendocrine carcinoma (MANEC). MANECs have a phenotype that is morphologically recognizable as both gland-forming epithelial and neuroendocrine cells and are defined as carcinomas since both components are malignant and should be graded. A component of squamous cell carcinoma is rare. Arbitrarily, at least 30% of either component should be identified to qualify for this definition. The identification in adenocarcinoma of scattered neuroendocrine cells by immunohistochemistry does not qualify for this definition (1, 58).
Currently, the term NEN is used to denote both well- and poorly differentiated neoplasms (NETs and NECs respectively) that can arise at almost any anatomical site and share common histologic, immune-phenotypic and ultrastructural neuroendocrine features although their natural history and prognosis vary significantly (2). The expression of neuroendocrine features can vary according to the tissue of origin along with their differentiation, as NETs predominate in the small bowel and pancreas whereas NECs are much more common in the lung and colon (58). Recent genetic evidence has highlighted not only the diversity of genetic defects according to the tissue of origin but also supported the morphologic subdivision that distinguishes well- from poorly differentiated neoplasms that share different clinical, epidemiologic, histology, and prognostic properties (2). This notion is particularly relevant for neoplasms originating from the pulmonary and GI system (58). Although the value of documenting the hormonal secretion profile in PanNENs is not fully adopted there is preliminary evidence to suggest that the immunohistochemical expression of insulin, even if not bioactive and/or followed by a secretory syndrome, may identify a more indolent tumor phenotype (59).
It subsequently became apparent that although grading could distinguish between neoplasms of different grades, there was considerable heterogeneity in the response to applied therapies particularly in G3 tumors (60). This notion led to the subclassification of G3 tumors according to their differentiation into well-differentiated G3 neoplasms, that were named G3-NETs, and into poorly differentiated G3 neoplasms that were named G3-NECs. The recently proposed WHO 2017 classification is mainly referring to PanNENs and identifies well-differentiated NETs (PanNETs) and poorly differentiated NECs (PanNECs) (2) (Table 1c). Among well-differentiated NENs, aggressiveness increases according to grade but such tumors are still less aggressive than PanNECs (58). PanNECs are rare, mostly large cell type, and may contain components of adenocarcinoma and exhibit an overall worse prognosis with poor response to treatment and overall survival (OS). Progression from G1 to G3 PanNETs may occur as tumors evolve, although very rarely are they transformed to PanNECs. Although there is no clear distinction based on Ki67 PI, G3-NETs have lower Ki67 PI (mean values around 40%) than G3-NECs (mean values >70%). However, Ki67 PI cannot reliably distinguish between G3 PanNETs and PanNECs, necessitating occasionally the use of genetic markers particularly in cases when morphology is not diagnostic (61). A number of recent studies have identified several somatic genetic alterations in MEN1, death associated protein 6 (DAXX), α-thalassemia/mental retardation X-linked (ATRX), phosphatase and tensin homolog (PTEN), and members of the mammalian target of rapamycin (mTOR) signaling pathway along with mutations in the DNA repair genes MUTYH, CHEK2, and BRCA2 (62, 63). These mutations are not encountered in PanNECs that harbor mutations in TP53 and RB1 and may share mutations in KRAS and SMAD4 genes (59). Molecular profiling may help correctly classify the tumor in cases with ambiguous histology (61). The 2017 WHO classification of PanNENs has adopted a change in cut-off Ki67 PI between grade 1 and 2 tumors. Given the pivotal role of Ki67 for grading and subsequent selection of therapy, it is imperative that Ki67 immunohistochemistry in performed according to a standardized protocol using a monoclonal antibody against MIB-1.
Although a similar classification of G3-NETs versus G3-NECs in the remaining GI system has not recently been published, it appears that these two subgroups do exist and behave in a similar manner to that of PanNENs but are less common. However, the majority of G3-NENs of the GI tract are NECs harboring TP53 and RB1 mutations, whereas in the colon APC mutations are also found (58). In contrast to PanNENs relatively few mutations in specific genes have been identified in GI NENs that appear to harbor mostly epigenetic changes (64). In the latest WHO classification the term MANEC was replaced by the term MiNEN (mixed neuroendocrine non-neuroendocrine neoplasm).
Circulating markers including hormones
NENs constitute a heterogeneous group of cancers both in terms of tumor biology and the variety of products that they synthesize and secrete. Some of the produced hormones can be bioactive and are consequently associated with a secretory syndrome (functioning NENs) (54, 65) (Table 2). However, NENs are still diagnosed relatively late when at an advanced stage, as the majority secrete compounds that are nonbioactive (nonfunctioning NENs). The availability of reliable circulating markers is critical for improving diagnostics, prognostic stratification, follow-up, and definition of treatment strategy. Over the years, a number of general and specific circulatory biomarkers have been developed for the diagnosis and follow-up of patients with NENs (66, 67). The relatively late diagnosis of NENs affects the application of early and possibly curative treatment and may be related to the absence of sensitive and specific biomarkers (66).
Table 2.
Biomarkers for neuroendocrine neoplasms (NENs).
| Clinical Setting | Additional Information | |
|---|---|---|
| General biomarkers | ||
| Chromogranin A (CgA) | All NENs (follow-up rather than diagnosis) | Many assays, isoforms. Affected by PPIs, medical conditions. |
| Neuron-specific enolase | High grade neoplasms | Prognostic significance |
| Specific biomarkers | ||
| 5-HIAA | Carcinoid syndrome | Dietary instructions. Spot urinary and blood samples |
| (Pro-)Insulin, C-peptide | Insulinoma | 72 hour supervised fast |
| Gastrin | Gastrinoma, Type 1–2 gastric NENs | 25% of cases have MEN1 mutation. Secretin/Ca2 stimulation test for equivocal levels |
| Glucagon, VIP, Somatostatin | Functioning PanNEN | |
| Ectopically secreted biomarkers | ||
| Parathyroid hormone-related peptid | Hypercalcemia | Can be life threatening necessitating effective management of secretory syndrome |
| Adrenocorticotropic hormone | Cushing’s syndrome | |
| Corticotropin-releasing hormone | ||
| Growth hormone-releasing hormone | Acromegaly | |
| Novel biomarkers | ||
| Circulating tumor cells | Gastrointestinal and PanNENs | Further validation required |
| Circulating tumor DNA | PanNENs >> gastrointestinal NENs | Genomic alterations in PanNENs |
| MicroRNAs | Gastrointestinal and PanNENs | MiR-21 mostly evaluated. No validation or standardization |
| NETest | All NENs | High sensitivity and specificity, informative irrespective of PPIs/SSAs, grade, stage. Monitoring of disease. |
Initially the majority of circulating biomarkers have been the monoanalytes that were measured via enzyme-linked immunosorbent assays. However, their limitations in terms of dimensionality, coupled with a modest specificity, have diminished the enthusiasm with regard to their clinical utility (68). More recently, relatively novel biochemical tumor markers based on tumor biology and their molecular profile have emerged. These signals or signatures in peripheral blood define the activity of the neoplasm or the local tumor microenvironment. This concept is captured between the terms biomarker and more recently “liquid biopsy” (69). Compared with traditional tissue biopsies, liquid biopsies are less invasive and can be easily repeated during the course of the disease, providing longitudinal prognostic and predictive information. Such biomarkers include circulating tumor cells, tumor-derived DNA, mRNAs, and recently micro-RNAs (miRNAs) that are shed into the circulation during cancer progression (68). All have been proposed to provide information pertinent to defining the evolution of cancer in a particular individual, and each appears to provide information that might be of considerable utility. Analysis of these biomarkers offers the prospect of a liquid biopsy to predict/monitor therapeutic responses, assess drug resistance, and quantify residual disease. Compared with single-site biopsies, these markers have the potential to inform intratumor heterogeneity and tumor evolution in a reproducible and less invasive way (64).
Circulating peptide biomarkers
The best known and most used circulating general biomarker in NENs is chromogranin A (CgA). This protein is produced and processed as a component of the neuroendocrine cellular secretory apparatus and exists in the blood stream as a heterogeneous antigen composition ranging from a complete protein to a series of cleavage products in all NENs (70). Increased CgA is considered to be sensitive, and 60% to 90% accurate once a NEN has been identified, but is an inappropriate first-line diagnostic tool (71). Measurements are usually nonspecific (10–35% specificity) since CgA is elevated in other conditions, including other neoplasms, cardiac and inflammatory diseases, renal failure, atrophic gastritis, and proton pump inhibitor (PPI), or H2-blocker administration (68). In addition, CgA assays still lack standardization that affects diagnostic and therapeutic decision-making approaches along with the ability to perform comparative studies (71, 72).
Furthermore, it appears that there is no direct relationship with the amount of circulating CgA and tumoral load, as 30% to 50% of NENs show normal, nonelevated CgA levels, which impairs sensitivity further (71). Consistently high CgA levels were found only in gastrinomas, which is due to gastrin-induced enterochromaffin-like cell hyperplasia (73, 74). Regarding its prognostic value, there is evidence demonstrating that advanced NENs secreting CgA have poorer outcome than those showing nonelevated levels (71). The identification of cut-offs allowing a proper risk stratification of CgA-secreting tumors has not been performed, whereas the trend of elevated circulating CgA does not represent a valid indicator of morphologic evolution as a 25% CgA increase exhibited a concordance with morphologic changes in only 40% of cases (72). A recent meta-analysis of 8 highly selected studies showed that CgA exhibits a sensitivity of 46% to 100% and specificity of 68% to 90%, respectively, when used to monitor disease progression and response to treatment. It exerts a better overall accuracy (84%) during follow-up for the early detection of recurrence rather than in the diagnostic setting. It can thus be used to diagnose recurrence or progression, rather than to rule it out (72).
Bioactive compounds related to a secretory syndrome are used to confirm its presence and along with relevant symptoms to monitor response to treatment. Among these markers the urinary breakdown metabolite of serotonin, 5-HIAA is used for the diagnosis and follow-up of patients with mainly small bowel NENs who experience the symptoms of the CS (70). Serotonin is synthesized and stored in enterochromaffin cells of the GI tract, and when produced in excess 24-hour urinary 5-HIAA excretion exhibits an overall sensitivity and specificity of 70% and 90% respectively (68, 70). Patients with nonmetastatic disease have normal levels whereas tumor burden is related to 5-HIAA levels (68). It appears that a low cut-off of 5-HIAA levels is necessary to exclude a small intestinal NEN, or others derived from the former midgut, whereas high cut-off levels are more predictive of its presence (75). However, a number of commonly prescribed medications, several diseases, and foods may produce falsely high levels (70). There is some evidence to suggest that urinary 5-HIAA levels may be related to overall prognosis and survival in patients with CS, but further studies are required to verify this finding (75, 76). However, there seems to be a correlation with 5-HIAA levels and the development of carcinoid heart disease (CHD) (77), but to a lesser degree with mesenteric fibrosis (78). Recent studies have shown that there is good correlation between plasma and urinary 5-HIAA levels and this tool can be used for diagnosis and follow-up, although it is not widely available (79).
The increase or inappropriate presence of relevant biomarkers (mainly peptide hormones) can confirm the diagnosis of a specific endocrine syndrome (Table 2). Occasionally, when levels of a biomarker related to a specific clinical setting are nondiagnostic a stimulation test may be required. This mostly applies to gastrinoma patients with nondiagnostic basal gastrin levels when the secretin test is performed (80). Also, patients suspected for insulinoma should undergo a 72-hour supervised fast to detect inadequately elevated (pro-)insulin and C-peptide levels during hypoglycemia (35). Neuron-specific enolase is also commonly used and is mostly found to be elevated in patients with high-grade neoplasms also exhibiting a prognostic role (70). Less commonly used markers include pancreastatin, a CgA derivative that is less affected by PPI administration; it is found to be elevated in 58% to 81% of NENs. However, pancreastatin does not correlate with tumor burden and/or disease aggressiveness and its measurement it is not widely available (81). Other less commonly used monoanalytes are neurokinin A and progastrin releasing peptide, whereas N-terminal pro-brain natriuretic protein is a valuable nonspecific tool for evaluating patients with CHD (68, 70).
Circulating tumor cells
Circulating tumor cells (CTCs) are secreted either by the primary tumor or metastatic deposits and are initially found in the circulation in 43% of midgut, 21% of pancreatic, and 31% of bronchopulmonary metastatic NENs (82). CTCs are associated with increased tumor load and grade and are also found to be predictors of a worse progression-free survival (PFS) and OS; this finding is in contrast to elevated CgA levels that failed to reveal such a relation (83). Subsequently, CTCs are measured before and at different time intervals during the application of different therapeutic modalities. Patients with undetectable of substantially reduced (>50%) compared to baseline CTCs are shown to exhibit a radiologic response and an overall better OS (84). However, not many studies have evaluated their role in NENs whereas some technical limitations exist, particularly in respect to validating epithelial cell adhesion molecules (EpCAM) expression by immunohistochemistry in NENs (68). A recent consensus concluded that CTCs are not sensitive and specific for all NENs, could not distinguish between the different subtypes of NENs, and could not provide information regarding tumor burden and grade (67).
Circulating tumor DNA
These nucleic fragments from tumor cells may reveal existing genomic alterations that could be of prognostic significance and could also be druggable. This is more applicable for PanNENs that harbor specific mutations (63), whereas it is less helpful in small bowel NENs that harbor cyclin-dependent kinase inhibitor (CDKN1B) mutations in only 8% of cases (64). However, currently there is a paucity of data regarding the use of circulating tumor DNA for personalized medicine in NENs (85).
micro-RNAs
miRNAs, comprise a family of short (<30 nucleotides) noncoding RNAs designated to regulate a diverse array of biologic processes, including carcinogenesis, where they can act as either oncogenes or tumor suppressor genes (86, 87). It is estimated that miRNAs can regulate approximately 60% of all coding genes targeting many mRNAs, which in turn can be regulated by multiple miRNAs (86). Studies of miRNAs in NENs have been relatively few, including a small number of mainly heterogenous populations, utilizing different methodologies, and lacking control groups (86). Tissue-specific expression of miRNAs has been investigated in NENs, predominantly in bronchial, small intestinal, and PanNENs, whereas data on circulating miRNAs are scarce (87). The most consistently altered miRNA in small bowel and PanNENs was MiR-21 but this epithelial biomarker requires further validation (86).
NETest
Given the limited accuracy of the currently available biomarkers and the known limitations of single analyte measurements in clinical science along with the existing limitations of evolving biomarkers, a blood-based multianalyte NET-specific gene transcript analysis was recently developed and termed NETest (69). This appears as an alternative to the measurement of single analytes, and presents a robust, reproducible polymerase chain reaction-based multianalyte test for the detection of NENs. The multianalyte algorithm is based on the simultaneous measurement of 51 neuroendocrine-specific marker genes in peripheral blood. This approach is superior to single analyte tumor biomarkers as it may concomitantly evaluate different cellular processes such as apoptosis and glucose metabolism. It has a high sensitivity (85–98%) and specificity (93–97%) for the detection of GI NENs and outperforms other monoanalytes such as CgA (69, 81). Furthermore, it is not affected by concomitant treatment with PPIs and/or SSAs, and its performance is not related to stage and grade (88). A prospective study evaluating the performance of the NETest in identifying PanNENs and small bowel NENs showed a diagnostic accuracy of 93% without being affected by other pancreatic cancers or pancreatitis and with only few cases of colon and rectal cancers giving false positive results (69). In addition, the NETest was capable of identifying patients’ response to systemic therapies and detecting early disease relapse, as alterations in the NETest predated those of imaging (89). Although the NETest appears to be an ideal biomarker for establishing the diagnosis, monitoring response, and overall prognosis, it is not yet widely available and needs further validation by different groups as the first independent cohort showed less favorable biomarker metrics (90).
Pancreatic NEN molecular markers
Exome sequencing (of 18 000 coding genes) was initially performed in 10 nonfamilial PanNENs and then checked in 58 other PanNENs. MEN1 mutations were identified in 43%, whereas mutually exclusive mutations in the ATRX and DAXX genes were identified in 43% of cases (18% and 23% of cases respectively in 68 cases studied) (62). A further 14% mutation rate in the mTOR pathway was also found, but these tumors exhibited a 13% overlap with MEN1 (62). ATRX and DAXX are chromatin remodelers but their loss leads to alternative lengthening of telomeres (ALT) and chromosomal instability (CIN) (91). Although it was initially reported that ATRX/DAXX mutant tumors had superior 10-year survival and outcome (62), a larger study of 243 tumors has demonstrated that ATRX and DAXX loss and associated ALT in PanNETs correlates with CIN, advanced tumor stage, development of metastases, poorer progression, and OS (92). Subsequently, whole-genome sequence of 102 PanNETs identified previously unknown germline mutations in DNA repair genes MUTYH (encodes DNA glycosylase), BRCA2, and CHEK2 (63). These previously unidentified mutations in patients without a positive family history indicated that individuals carrying such mutations have an increased albeit unquantifiable risk of disease. Furthermore, it was noted that along with MEN1 and von Hippel Lindau disease these mutations accounted for 17% of germline mutations. In addition, somatic mutations were found to occur in 4 domains: DNA damage repair, chromaffin modification, mTOR signaling, and ALT. New mTOR mutations were also identified that could be utilized as biomarkers to predict therapeutic response, whereas currently known mutational status (DAXX, ATRX, mTOR) can be used to stratify prognosis of G2-NETs (subgroup with the least predictable risk) and in well-differentiated G3-NETs (63). This is particularly important, as TP53 and RB1 genetic alterations are mostly found in patients with PanNECs and are harbingers of a worse outcome.
Small intestinal NEN molecular markers
Loss of chromosome 18 has been reported in 60% to 90% of small intestinal NENs, but no mutated genes on this chromosome have been detected. CDKN1B has recently been revealed as the only recurrently mutated gene in small intestinal NENs but with a relatively low frequency of 8%, suggesting that its role as a driver in NEN development is uncertain (64). Genomic profiling studies have suggested that two distinct groups of small intestinal NENs exist: a more prevalent subset with loss of chromosome 18 as the primary event, with additional losses on other chromosomes, and a further smaller group often with intact chromosome 18 but clustered gains on chromosomes 4, 5, 7, 14, and 20 (93). However, when whole-exome sequencing was performed on 48 small intestinal NENs, an average of only 0.1 somatic single-nucleotide variants (SNVs) per 106 nucleotides in contrast to other epithelial cancers of the colon and rectum were detected, indicating that small intestinal NENs are genetically stable (64). It appears that in small intestinal NENs epigenetic dysregulation is more common, as DNA methylation analysis has shown dysregulation in 70% to 80% of tumors (64). Global hypomethylation was more prevalent in GI than PanNENs and correlated with poor prognosis, lymph node metastases, and loss of chromosome 18 (94). Recently, a putative tumor suppressor role has been suggested for TCEB3C occurring at 18q21 (encoding elongin A3), which may undergo epigenetic repression (95). Integrated genome-wide analysis including exome and whole-genome sequencing, gene expression, DNA methylation, and copy number analysis has identified three novel molecular subtypes of small intestinal NENs with differing clinical outcome (96). The largest subgroup, found at older ages and exhibiting the longest PFS, harbored chromosome 18 LOH along with CDKN1B mutations and lack of DNA methylation whereas a group with multiple copy number changes had a poorer PFS and was encountered in younger patients. A further group with intermediate PFS showed DNA methylation but absence of copy number changes (96).
Imaging markers
The localization and staging of NENs relies on both morphologic (provided by conventional radiology) and functional (provided by nuclear or molecular imaging) techniques as they are considered to exert a complementary role (97) (Fig. 4). Conventional imaging is performed either with computed tomography (CT) scanning or magnetic resonance imaging (MRI) according to the specific tissue of interest and local availability. However, differences in the performance characteristics of these modalities do exist (73). In addition, ultrasonography-related techniques are utilized when additional information regarding primary tumor localization and extent of invasion and histologic confirmation is required. Functional imaging uses hybrid imaging approaches with either single photon emission CT (SPECT) or, more recently, positron emission tomography (PET) as SPECT/CT or PET/CT and can also provide prognostic information and guide treatment decisions. PET/MRI is also available (97).
Figure 4.
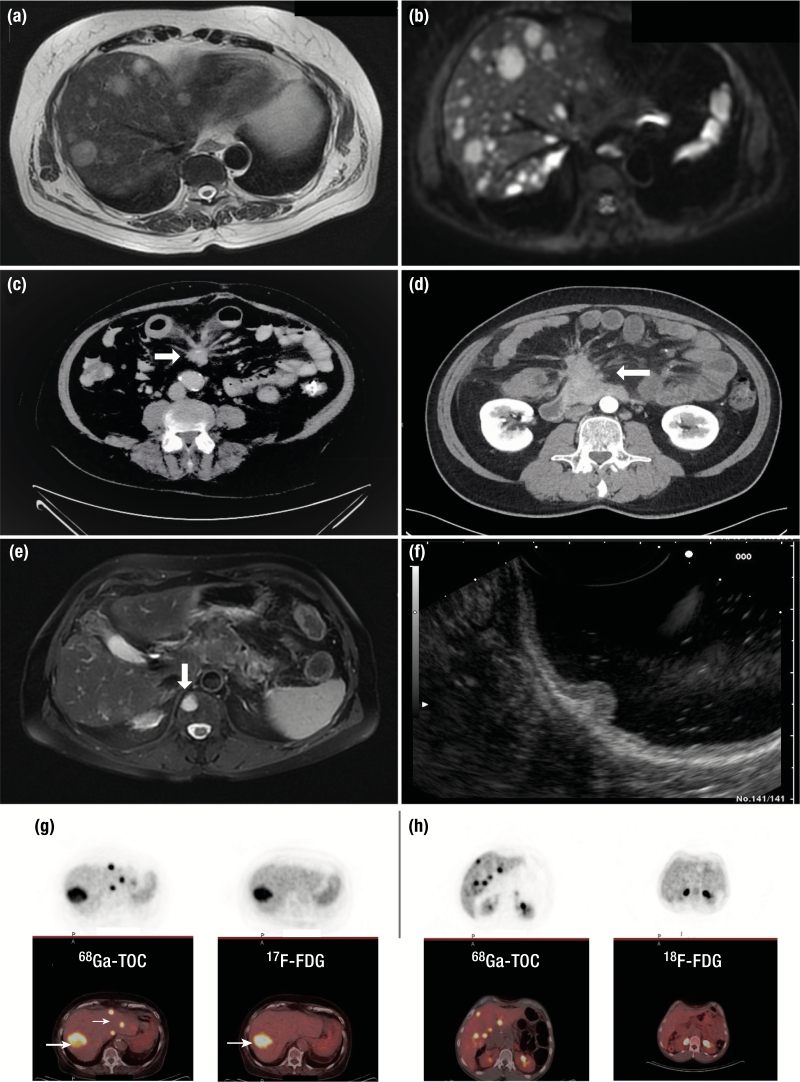
Imaging procedures used in neuroendocrine neoplasm (NEN) diagnostics. (A) Axial T2-weighted magnetic resonance imaging (MRI) showing metastatic deposits in both hepatic lobes from a pancreatic Grade 2 NET. (B) Axial T1 diffusion MRI image of the same patient showing further lesions not detected with the previous MRI sequence. (C) Computed tomography (CT) of the abdomen demonstrating a desmoplastic reaction (white arrow) in the mesentery of a patient with a Grade 1 small bowel NET. (D) Fibrotic strands radiating from a central mesenteric metastatic mass in a patient with multiple small bowel NETs. There is thickening of the bowel wall and fluid retention due to venous ischemia in this patient, causing postprandial abdominal pain. (E) MRI T2-weighted image with fat saturation demonstrating an oval shaped high signal bone lesion from a Grade 2 small bowel NET at the level of Th11 (white arrow). (F) Polypoid lesion arising from the body of the stomach detected by endoscopic ultrasonography infiltrating the mucosa and submucosa. (G) Positive right hepatic lobe 18F-FDG PET uptake (white arrow) in a patient with a small bowel Grade 2 NET. In the same patient positive 68Gallium-DOTATOC positron emission tomography (PET) in the same area of 18F-FDG PET uptake (thick white arrow) and additional uptake in different areas of the left hepatic lobe (thin white arrow). (H) Positive uptake in multiple hepatic areas in a patient with a Grade 2 pancreatic NET following a 68Gallium-DOTATOC PET. Negative 18F-FDG PET in tumor lesions within the same patient.
Computerized tomography
CT has long been the main imaging modality used for localization, staging, decision-making, and monitoring response to treatment in NENs (66, 97). Currently available high-resolution multidetector CT imaging provides whole-body imaging of the thorax, abdomen, and pelvis before and after intravenous (IV) iodine-based contrast administration; late arterial phases are used to identify hepatic and pancreatic lesions whereas venous phase images are used for the remaining structures (97). Potential pitfalls with this form of imaging are the low detection rate of small (<1 cm) infiltrated lymph nodes and bone metastases. A mean 82% and 86% sensitivity and specificity respectively for overall NEN detection, with higher rates for pancreatic and hepatic disease, has been described (73, 97, 98). However, the mean sensitivity for detecting extrahepatic abdominal soft tissue and bone metastases ranges from 61% to 70%, albeit with a higher specificity (99). For the demonstration of hepatic disease, which represents the most common area of NEN metastases, a CT triple-phase examination is required that includes imaging before (nonenhanced) and following IV contrast enhancement in the late arterial (portal venous inflow) and venous phase (97). This approach is sufficient to direct towards NEN-related lesions that are usually hypervascular, but this does not apply for hypovascular lesions (73). For the identification of small intestinal neoplasms that can be of subcentimeter size and multiple in number, CT enteroclysis has shown relatively low, but with a wide range, sensitivity and specificity of 50% to 85% and 25% to 97% respectively (100). Additional information may also be obtained with capsule endoscopy that may identify lesions in approximately 50% of cases (101). In the presence of mesenteric metastases secondary to small intestinal NENs, an intense desmoplastic reaction may develop that appears as a soft tissue mass with areas of calcification surrounded by radiating fibrotic streaks to the mesentery (78).
To homogenize the reporting approach and develop reference standards used to evaluate treatment response, the Response Evaluation Criteria In Solid Tumors (RECIST) have been implemented (102). A potential limitation of CT imaging for patients undergoing prolonged follow-up with imaging surveillance is the radiation dose administered, which varies according to the examination protocol and type of CT scanner.
Magnetic resonance imaging
MRI appears to be superior to CT for imaging of the liver and pancreas and for the detection of metastatic disease in the bones and brain (54, 66, 97). Current 1.5 to 3 Tesla scanners provide conventional T1- and T2-weighted images that can be enriched with contrast administration, obtaining an overall 79% sensitivity and almost 100% specificity in identifying PanNENs (103, 104). A 75% sensitivity and 98% specificity respectively in identifying hepatic metastases has been described with an overall mean detection rate of NEN-related lesions of approximately 76% (range 61–95%) (105). In general, the image contrast is better with MRI than CT, and the use of several MRI sequences provides further diagnostic enhancement (97). Diffusion-weighted imaging, which is based on the restriction of water molecule movement across cell membranes, produces high lesion-to-background resolution without the administration of any contrast media (97). In addition, hepatocyte-specific MRI contrast media (such as Gd-DTPA) are accumulated by the normal hepatocytes and help to identify previously unnoticed metastases (104). Recently, the extent of hepatic involvement, expressed as the percentage of hepatic tissue replaced by tumoral tissue, appears to be of significant prognostic importance directing specific therapeutic decisions, particularly in the form of cytoreduction either surgically or through ablative procedures (66).
Typical NEN lesions exhibit a low signal intensity in T1- and intermediate-to-high signal intensity in T2-weighted images. MRI is particularly helpful for the detection of small (<2 cm) PanNENs that are mostly well-vascularized neoplasms without exerting a compressive effect on the main pancreatic duct (106). Such lesions show higher apparent diffusion coefficient values than more aggressive tumors. MRI represents a valuable tool to monitor patients harboring such lesions and especially patients with MEN1, who are subjected to screening regularly from the age of 5 years (107). Diffusion-weighted MRI sections and/or the administration of IV contrast represent the best means to identify small hepatic metastases from NENs (105). NEN-related metastases exhibit high signal intensity on T2-weighted images and are mostly hypervascular in the hepatic arterial phase (105).
Ultrasonography and related applications
Conventional abdominal ultrasonography is an operator-dependent imaging technique that exhibits an overall low detection rate for PanNENs of approximately 40%. Its performance in identifying hepatic metastases is higher (97). In contrast, endoscopic ultrasound (EUS), which is also operator dependent, presents a sensitive tool in detecting PanNENs with a mean detection rate of 92% (range 74–96%) (108). Detection rates are lower when lesions are located at the pancreatic tail, whereas the detection rates for duodenal neoplasms and adjacent lymph nodes is approximately 63% (108). In addition, EUS allows access to tissue sampling, which facilitates confirmation of the diagnosis while obtaining grading information (1). Evaluation of PanNEN grade by EUS-guided fine needle aspirate (FNA) has revealed low complication rates and reasonable diagnostic concordance compared with surgical specimens (109). However, underestimation of grade in FNA samples has also been reported, especially for small tumors and hypocellular specimens, providing rationale for EUS-guided histologic biopsy in selected cases (110, 111). EUS is particularly useful in establishing the depth of extension in gastrin-related gastric type 1 and 2 NENs, and duodenal and rectal NENs directing further therapeutic decisions and in the follow-up of PanNENs in incidentally discovered lesions and in patients with MEN1 (107). Color Doppler EUS is used to evaluate vascular lesions (97).
Somatostatin receptor imaging
The rationale of performing somatostatin receptor imaging (SRI) is based on the wide expression of SSTRs by NENs and provides information for their presence throughout the body, revealing additional metastases compared with conventional imaging with CT/MRI (66). In addition, it has a prognostic role as SSTR expression is more commonly found in well-differentiated neoplasms whereas the quantification of radionuclide uptake in tumor lesions may provide additional prognostic information (66). This modality can also identify patients suitable for treatment with PRRT based on the intensity of SSTR expression (112). SRI with 111In-pentetreotide along with SPECT (OctreoScan) has been used extensively but lately imaging with 68Gallium-DOTA-somatostatin analogs (68Ga-SSA) along with PET/CT is increasingly utilized (98).
This modality has a better diagnostic performance, and exposes the patient to less radiation as imaging is completed within hours compared with days with OctreoScan (113). In addition, PET has a better spatial resolution to SPECT (0.5 vs 1–1.5 cm) and a better tumor to normal tissue contrast (114). Although several preparations of 68Ga-SSA exist, namely TOC (tyrosine octreotide), TATE (octreotate), and NOC (1-NaI3-octreotide), there is no particular advantage in selecting one of them, for NENs preferentially express SSTR subtype 2 (SSTR2) to which all these compounds bind avidly (115). Overall, the mean sensitivity and specificity of 68Ga-SSA imaging ranges from 88% to 93% and 88% to 95% respectively (114). In a recent meta-analysis the application of this modality has led to a change of management in 44% of individuals who underwent imaging, whereas in four studies in which previous imaging with OctreoScan had also been performed this was 39% (116). Recent evidence has revealed that imaging using SSTR antagonists has a better resolution than agonist-receptor formulations, as it is not internalized and remains bound to the cell surface of the tumor (117). There is also evidence that these compounds may be superior to imaging in treatment with PRRT than those currently used (116).
In areas with limited availability of SRI, immunohistochemistry of SSTR2 in tissue constitutes a suitable alternative with above 90% concordance to imaging for the selection of cases eligible for SSA treatment (118).
[18F]fluorodeoxyglucose
In general, SSTR expression diminishes when proliferation rate increases and 68Ga-SSA imaging becomes negative when grading increases, particularly in NECs (119). Imaging with [18F]fluorodeoxyglucose (18FDG)-PET/CT is widely used in oncology to reveal previously unnoticed cancer lesions and for staging reasons based on the Warburg effect (66, 120). For high-grade NENs and especially NECs, 18FDG-PET/CT is the nuclear medicine modality of choice but can also be positive in G2 to G3 NETs where there can be overlap with 68Ga-SSA imaging. Although no specific Ki67 cut-off value predictive of a positive 18FDG-PET/CT has reliably been found, neoplasms with Ki67 PI values >15% are more likely to exhibit a positive 18FDG-PET/CT (66, 119). Furthermore, a positive 18FDG-PET/CT is a harbinger of a more aggressive course and a negative predictor of response to PRRT (119, 121). There is currently a trend for both modalities to be performed, particularly in non-G1 NENs, as they appear to exert a complementary role (122).
Other imaging tracers
Insulinomas express SSTR2 in approximately 50%, and therefore imaging with 68Ga-SSA may be negative. In such cases, imaging with 68Ga-labelled tracers using as a chelator the glucagon-like peptide receptor-1 has been shown to be superior (123). In a recent prospective study of 52 patients with suspected insulinomas 68Ga-DOTA-exendin-4 PET/CT outperformed 111In-DOTA-exendin-4 SPECT/CT and CT/MRI in the localization of benign insulinomas (124). Imaging with 18F-DOPA-PET-CT has also been utilized and shown to be superior to conventional OctreoScan, particularly for small intestinal NENs (125), but appears to identify fewer lesions than 68Ga-SSA (126). There are also some data using the serotonin precursor 11C-5-hydroxy-tryptophan, but this modality is less widely available (97). Radioisotopes with 64Cu have different properties and despite similar patient-based sensitivity 64Cu-based SSTR PET imaging identified more lesions than 68Ga-PET (114). C-X-C motif chemokine receptor 4 (CXCR4) is expressed in NENs and seems to play a limited role in detecting well-differentiated NETs, whereas increasing receptor expression could be noninvasively observed with increasing tumor grade. 68Ga-CXCR4(pentixafor) PET/CT might serve as a noninvasive means for evaluating the possibility of CXCR-directed PRRT in advanced dedifferentiated SSTR-negative tumors (127).
Integrating diagnostics in NENs
It has recently become apparent that a number of different biomarkers (including advances in histopathologic, functional nuclear imaging, and molecular diagnostics) need to be utilized in order to be able to formulate a patient-orientated diagnosis that would provide prognostic stratification and dictate therapeutic decisions. Although such an approach is related to local expertise and availability, it aims to provide more personalized patient care (Fig. 5).
Figure 5.
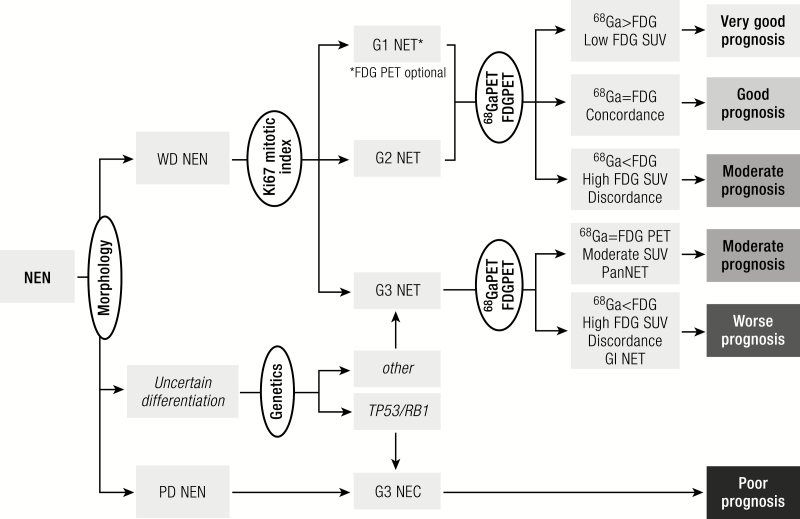
Diagnostic algorithm. Histology should be obtained from tumors suspected of NEN to confirm the diagnosis of a neuroendocrine origin. Morphological examination will subsequently divide neoplasms into well-differentiated tumors or poorly differentiated carcinomas. Uncertain cases can be categorized through the use of genetic analysis or p53 staining. Within the NETs mitotic and Ki-67 indices will classify the tumor into grade 1 to 3. Further prognostic and therapeutic information can be obtained by performing 68Ga-labelled somatostatin receptor imaging and for higher grade or clinically aggressive tumors an 18F-FDG PET. FDG, fluorodeoxyglucose NEN, neuroendocrine neoplasm; WD, well-differentiated; PD, poorly differentiated; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; SUV, standardized uptake value; PET, positron emission tomography; Pan, pancreas; GI, gastrointestinal.
Management
The origin of NETs as sensory and secretory cells provides a unique background in the oncologic field of treatment. Besides the management of morbidity and mortality due to tumor growth, clinicians dealing with NET patients should also be skilled in recognition and treatment of hormonal symptoms. The complications of proliferation and hormonal activity should both be considered in planning the therapeutic strategy within the individual patient. An overview of treatment targets in NEN is provided in Fig. 6.
Figure 6.
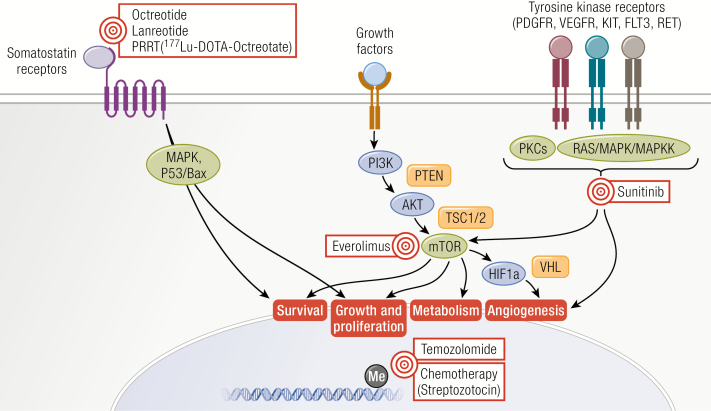
Therapeutic targets for neuroendocrine neoplasms (NENs). Overview of the different therapeutic modalities for proliferative control in NENs and their respective targets within the NEN cell.
Locoregional disease
Patients with stage I to III disease should undergo evaluation for the possibility of a curative surgical resection. The majority of new NET cases still present with locoregional disease, which is consistently accompanied by a better prognosis than stage IV disease (8, 10).
Intraluminal pulmonary or GI NENs (T1-T2) without the presence of lymph node metastases can be candidates for curative endoscopic resection. As lymph node dissection plays a vital role in the risk of and time to recurrence, this should be limited to selected cases. Laser-guided resection has been employed in a series of central pulmonary carcinoids (128–131), but size and purely intraluminal growth on CT were found to be relevant predictors of treatment success. Lesions below 20 mm were successfully resected in 72% of cases in one series (132). Long-term success of endobronchial resection is limited at 58% and often necessitates rescue surgery due to extraluminal extension, but prognosis is very good with a disease-specific 10-year survival rate of 97% (133).
Well-differentiated gastroduodenal or rectal NETs smaller than 2 cm (T1-2) are candidates for endoscopic resection. Endoscopic snare polypectomy constitutes insufficient treatment as the lesions arise submucosally and high rates of recurrence after polypectomy have been described (134). Although endoscopic mucosal resection (EMR) has been advocated for lesions below 0.5 to 1.0 cm (135), there is general consensus that endoscopic submucosal dissection (ESD) or transanal endoscopic microsurgery lead to the greatest chances of obtaining a complete resection for low-grade T1-2 rectal NETs up to 2.0 cm (136–138). Modified EMR techniques have recently shown promise as an alternative to ESD with potentially greater availability and lower risk of perforation (139, 140).
Well-differentiated pulmonary or thymic NETs without distant metastases can be cured by resection, also in the presence of lymph node metastases. Strategies include a segmentectomy, wedge resection, (bi-)lobectomy, or pulmonectomy with lymph node dissection (141). A national surgical series of 661 patients with pulmonary carcinoids displayed excellent long-term prognosis with 92% 10-year survival. Negative prognostic indicators included advanced T stage, nodal involvement, and atypical carcinoids (142–144).
Subcentimeter gastric type 1 NETs confer an excellent prognosis without disease-related mortality, and annual endoscopic surveillance has been proven to be a safe strategy despite a lack of high-quality studies (13, 145). The risk of lymph node and distant metastases was associated with lesion size (146), providing rationale for endoscopic resection by EMR or ESD when tumor size increases beyond 1.0 cm. Treatment of Zollinger-Ellison–associated gastric NENs should take into account the gastrin-producing pancreaticoduodenal NEN or in the case of MEN1 multiple NENs. Given the intermediate malignant potential of type 2 gastric NENs in small series (14, 147), endoscopic or surgical resection should be considered in sporadic cases. For gastric type 3 and 4 NENs a surgical partial or complete gastrectomy with lymphadenectomy is the treatment of choice (148). Duodenal NETs should be radically removed by either endoscopic techniques (≤1.0 cm) or surgical duodenectomy (149–151).
The optimal strategic approach to sporadic nonfunctional small pancreatic NETs is controversial. The risk of lymph node and distant metastases increases with the size of the primary tumor, with 2.0 cm taken as the most applied cut-off for intervention (152). In one retrospective series, the tumor growth rate of asymptomatic lesions below 2.0 cm appeared limited at 0.12 mm per year and the risk of metastases was nil during a median follow-up of 34 months (153). A systematic review including five retrospective series with 540 patients revealed that active surveillance was safe in asymptomatic, sporadic, small, nonfunctioning PanNETs (154). Only 14% underwent surgical resection during follow-up and no disease-related mortality was detected. Surgical exploration with enucleation or resection should be considered in locoregional functional, T2 to T3, or N1 PanNETs. Several reports have described positive outcomes of primary tumor resection in stage IV disease in selected cases (155–157), but further confirmation is needed.
Surgery for midgut NETs is often palliative as most patients present with stage IV disease (10). In the metastasized setting, resection of the primary lesions and affected mesenteric nodes was previously advocated because of reports of survival benefits attributed to this strategy (158–160), but recent studies, including one incorporating propensity-scored matching, have failed to replicate this (161, 162). Given the characteristic desmoplastic reaction in mesenteric metastases with risk of venous ischemia, ileus, and bowel perforation (163), palliative surgery should be considered for symptomatic patients with advanced disease. In the case of a locoregionally confined midgut NET, a small bowel resection or right-sized hemicolectomy should be accompanied by mesenteric lymphadenectomy for accurate staging and cure (164). Recurrence rates of microscopically radical resections are in the range of 11% to 23% (165, 166), giving rise to excellent 10-year disease-related survival for stage I to II and stage III midgut NETs of 100% and 86%, respectively (167). Small colorectal NETs not amenable for endoscopic treatment or those above 2.0 cm or with nodal involvement should undergo oncologic resection similar to adenocarcinoma with hemicolectomy plus lymphadenectomy or anterior resection plus total mesorectal excision.
Liver-directed therapy
GEP-NETs preferentially metastasize to the liver, making this the sole distant metastatic site in a considerable subset of patients. As such, attempts at curative intervention have been investigated. Extensive surgery including resection of the primary tumor, lymphadenopathy, and liver metastasectomy can be accompanied by long-term PFS (168–171). Alternative therapies include radiofrequency ablation (RFA) or microwave ablation for oligometastatic lesions limited in size. Importantly, hepatic micrometastases are common in GEP-NETs and can easily be missed on current imaging modalities (172). Histopathologic evaluation of liver resections has revealed that less than 50% of metastases were detected by CT, MRI, or OctreoScan (173), questioning whether a liver resection can truly be curative.
A variety of liver-directed therapies can also be employed in the palliative setting (174). The decision to pursue this strategy should take into account the liver tumor burden and localizations, growth rate, and hepatic function as well as extrahepatic metastases and availability of systemic treatment options. Segment resection, hemihepatectomy, or thermal ablation can be attempted in individual cases, especially when confronted with accelerated growth of one or a few liver lesions. However, a survival benefit of such strategies has not been definitely proven to date, although some retrospective series might suggest an advantage in selected cases (156, 175).
Hepatic NEN metastases predominantly derive their blood supply from the hepatic artery above that from the portal vein. Consequently, embolization techniques through the hepatic artery have been extensively used as a treatment for NEN liver metastases (176). Transarterial options include bland particle embolization (TAE) producing ischemia, chemoembolization (TACE), and radioembolization (TARE). Historical, retrospective series have reported success rates for both hormonal and proliferative control following TAE or TACE (177–180). Head-to-head comparison between TAE and TACE revealed equal efficacy for both techniques (181, 182). However, higher rates of toxicity have been observed in patients treated with TACE (183). The advent of TARE with 90Yttrium-labelled microspheres provides a valuable alternative with potentially improved tolerability compared with TA(C)E (184, 185). Instead of ischemia, the radiospheres cause tumor response through radiation-induced DNA damage. Individual embolization options should be discussed in an experienced multidisciplinary team, weighing that survival benefit has not been proven for these techniques. In very select cases without extrahepatic disease, several dedicated centers have performed liver transplantation for metastatic NEN patients (186).
Hormonal syndromes
The management of hormonal complaints in patients requires an approach tailored to individual needs. The possibility of a surgical radical resection or debulking should be contemplated as tumor bulk often correlates with the severity of an endocrine syndrome. Pre- and or perioperative antihormonal treatment should be started in patients with uncontrolled complaints. If surgical cure or cytoreduction is not feasible, patients are restricted to palliative care for their symptoms. The tumor mass, location, and growth rate are important factors to consider when deciding for treatment with purely antihormonal effects or those with both antihormonal and antiproliferative effects.
Carcinoid syndrome
Symptoms of diarrhea, flushing, and bronchospasm within the clinical spectrum of the carcinoid syndrome (CS) require dedicated therapy. An incompletely appreciated spectrum of peptides and amines is responsible for increased gut motility, vasodilation, and fibrotic complications observed in CS patients (30). Supportive measures such as avoiding food or stress that evokes complaints and antidiarrheals such as loperamide or codeine phosphate can offer alleviation of symptoms. Chronic diarrhea in CS patients can be multifactorial and include causes such as exocrine pancreatic insufficiency, bile acid sequestration, bacterial overgrowth, or short bowel, often seen after abdominal surgery. Clinicians should treat these conditions accordingly with either dietary modifications, pancreatic enzymes, antibiotics, or cholestyramine.
Targeted medical treatment has been available for decades since the advent of somatostatin analogues (SSAs). Besides their role in NET diagnostics, SSA-induced SSTR activation inhibits the secretion of a variety of humoral factors by NETs. Two systematic reviews and meta-analyses have described that SSAs can induce a symptomatic response for CS in 65% to 74% of patients (32, 187). This efficacy appears to be equal across all relevant SSA formulations. Biochemical response after SSA use measured by a reduction in urinary 5-HIAA levels is also considerable at 39% to 51%. Long-acting formulations such as octreotide LAR and lanreotide autogel should be commenced once the diagnosis of CS is confirmed. Caution should be applied to patients with nearly obstructed bowel as initiation of the SSA can induce an obstructive ileus. In these cases a trial of short-acting SSAs may be considered. SSAs are generally well tolerated by patients, but adverse effects include a change in bowel movements and stool consistency, nausea, steatorrhea, myalgia, injection reactions, diabetes mellitus, and in the long term cholelithiasis (188, 189). Most of the adverse effects are low grade and self-limiting, requiring little need for dose reduction in patients. In cases of symptoms refractory to a standard dose, clinicians may consider increasing the SSA dose further (32, 190). Strategies include reducing the injection interval or increasing the injection dose of long-acting formulations or adding subcutaneous “rescue” octreotide. There is no benefit of switching to the multi-SSTR-targeting pasireotide in CS patients (191).
Given the pathophysiologic role of serotonin in CS-related diarrhea, several serotonin pathway inhibitors have been studied as treatment for CS. Anecdotal evidence has suggested efficacy of the serotonin receptor 3 antagonist ondansetron in CS (192–194). Telotristat ethyl, a serotonin synthesis inhibitor that does not cross the blood–brain barrier, has recently been proven to alleviate CS-induced, SSA-refractory diarrhea in 40% of CS patients in a series of clinical trials (195–198). In the TELESTAR phase 3 trial patients with carcinoid syndrome and 4 or more stools despite SSA use were randomized between telotristat ethyl and placebo (195). The use of telotristat ethyl decreased daily bowel frequency by 1.7 (250 mg 3 times a day) and 2.1 times (500 mg 3 times a day), compared to 0.9 times in placebo-treated patients. Flushing was not significantly affected. Telotristat ethyl at a dose of 250 mg 3 times a day represents a relevant novel possibility for those patients with diarrhea refractory to standard SSA treatment and a 3-month trial is generally advised.
Several options are available to patients with continued CS complaints despite the above-mentioned strategies. Interferon-alpha injections can alleviate CS symptoms in CS patients (199), although their efficacy on top of SSA appears limited (200). Liver-directed therapy is potentially very effective in cases of liver-dominant disease (201). Alternatively, peptide receptor radionuclide therapy (PRRT) with radiolabeled SSAs can induce an improvement of CS symptoms, both in patients with radiologic stable or progressive disease (32, 202).
Importantly, patients with severe CS can suffer from niacin deficiency or pellagra due to a shift of tryptophan metabolism towards serotonin production (203). These patients should be treated with niacin or nicotinamide 200 to 250 mg once daily supplementation. In addition, SSA use is accompanied with an increased risk for deficiencies of fat-soluble vitamins. Their levels should be monitored in patients with steatorrhea and supplemented accordingly (204). Attention should also be given to the possibility of CHD and annual echocardiography should be performed in patients with CS (205).
Insulinoma
The prevention of hypoglycemic events is the primary treatment target of insulinoma management. Patients should receive a glucose monitor and be instructed to regularly check their blood glucose until normoglycemia is maintained throughout day and night. Continuous glucose monitoring can be considered in cases with hypoglycemia unawareness (206). Radical surgical resection of insulinomas is key to treat locoregional disease and occasionally distant metastases (207). Several therapeutic options are available to reduce hypoglycemia risk in patients before surgery or in cases of irresectable disease.
A dietician should be consulted in all patients presenting with insulinoma. Hypoglycemic events can be prevented in a subset of patients by dietary counselling alone. Advice includes eating frequent meals, introduction of long-acting carbohydrates and, if needed, nightly intake. In cases refractory to pharmacologic interventions, enteral feeding through a nasogastric or nasoduodenal tube might be needed. Refractory cases that do not tolerate tube feeding should be switched to parenteral glucose infusion through a central venous indwelling catheter.
Diazoxide is often prescribed for insulinomas because of its insulin-suppressive effect with swift improvement in glucose levels (208). The drug is found to be effective in controlling hypoglycemia in 50% to 59% of insulinoma patients (209, 210). The mechanism of action is thought to be the inhibition of insulin secretion via activation of adenosine triphosphate (ATP)-sensitive potassium channels in insulin-producing cells (211). Doses should be titrated upwards from 100 mg in divided daily doses and clinicians should monitor for signs of volume overload, thrombocytopenia, and hirsutism (209, 210).
Given the overall hormone-suppressive effects of somatostatin, SSA can be utilized for the prevention of hypoglycemia in 58% to 67% of patients with insulinoma (212, 213). As SSAs also potentially decrease the levels of counterregulatory hormones resulting in aggravation of hypoglycemic events (214), the initiation of SSAs in insulinoma patients should preferably be performed in an in-patient setting with short-acting formulations. Glucose response can be immediate with the occurrence of hyperglycemia after the first subcutaneous injection. If short-acting injections are efficacious, patients can be switched to long-acting SSA formulations in an outpatient setting. There is a theoretical advantage for the use of pasireotide above octreotide or lanreotide given its hyperglycemic adverse effects, which is supported by some anecdotal clinical experiences (215, 216).
Glucocorticoids like prednisone and dexamethasone are another class of hyperglycemic agent that possess efficacy in uncontrolled insulinoma patients (217, 218). Doses needed to control blood glucose levels can be accompanied by significant adverse events, such as susceptibility to infection, aberrant wound healing, or weight gain. Moreover, glucocorticoids have been shown to reduce SSTR expression on NETs, which could impair the diagnostic accuracy of SRI or the therapeutic efficacy of (radiolabeled) SSAs (219).
The second-line antiproliferative treatment options everolimus and PRRT (see sections Radionuclide therapy and Targeted therapy) have been shown to alleviate hypoglycemic events in several patients with irresectable or metastasized insulinoma (220, 221). In cases of insufficient hormonal control, especially when combined with radiologic disease progression, these options can be considered. Caution should be applied to the use of sunitinib in these patients, as this drug might increase insulin secretion (222, 223).
Gastrinoma
Given the morbidity due to gastric acid production, the primary treatment goal in patients with Zollinger–Ellison syndrome is to prevent pyrosis, reflux, and complications such as ulcers, perforation, and esophageal stricture. If not already started before diagnosis, patients should be started on high-dose of any proton pump inhibitor, for instance 40 to 120 mg of omeprazole or pantoprazole twice a day (224–228). If the patient continues to suffer from acid-related complaints, treatment can be intensified with histamine type 2 receptor blockers and antacids. Most patients will use a combination of several drugs, often at above-label dosages.
Surgery of the duodenal or pancreatic NET is the only curative option and consequently radical resection should be explored. Given the multiplicity of duodenal gastrinomas there are doubts whether surgical resection is useful in MEN1 patients (229). SSAs decrease tumoral gastrin production and acid production (230). They constitute a valuable addition to patients with complaints resistant to standard antisuppressive therapy and have an additive antiproliferative effect for patients with irresectable or metastasized disease (189). In the metastasized setting, tumor cytoreduction or antiproliferative therapy can secure alleviation of gastrin-related complications.
Rare hormonal syndromes
The clinical sequelae of VIPoma are often severe, necessitating hospital admission for intravenous fluid and potassium administration. Treatment should encompass a combination of high-dose antidiarrheals with SSAs (231, 232). Owing to the severity of complaints, step-up therapy should be quickly applied with cytoreductive or antiproliferative measures, if possible (233, 234). There is anecdotal evidence of steroid treatment leading to improvement of refractory diarrhea (235).
Patients with a glucagonoma can present with the pathognomonic skin lesions, wasting, glossitis, or diabetes mellitus. The disease and particularly debilitating necrolytic migratory erythema is susceptible to treatment with SSAs, making this the first-line choice of therapy (236, 237). Evidence for other therapeutic strategies is limited in the literature. Particular focus should be on dietary counselling, given that cachexia can accompany the disease, and optimization of antidiabetic treatment (42, 238, 239).
Nonsurgical treatment of somatostatinoma has only been described in case reports and small series (240). Measures are mostly supportive with glucose management, cholecystectomy, and the administration of pancreatic enzymes. Intriguingly, SSAs have on occasion been shown to improve clinical symptoms in patients with somatostatinoma (241).
Antiproliferative therapy
Watchful waiting
Given the indolent nature of a subset of well-differentiated NET not all patients with irresectable or metastasized disease require antiproliferative therapy. Especially in grade 1 NET with limited tumor sites a watchful waiting approach can be considered. In the placebo arms of the PROMID and CLARINET trials a median time to progression or PFS of 6 to 18 months was detected, providing some rationale for this approach (189, 242).
Somatostatin analogs
Clinical experience that SSAs can have a growth-stabilizing effect in NET was finally confirmed in two pivotal randomized placebo-controlled phase 3 trials. In the multicenter PROMID trial in Germany, 85 treatment-naive patients with locally inoperable or metastatic grade 1 midgut NET were randomized between placebo or monthly octreotide LAR 30-mg injections (242). Median time since diagnosis was 4.3 months, 66% of patients had undergone resection of the primary tumor, and carcinoid syndrome was present in 39% of patients. Patients treated with octreotide LAR had significantly prolonged time to progression (median 14.3 months) compared with placebo-treated patients (median 6.0 months). The OS was equal in both groups and an objective response was only seen in 2% of patients treated with octreotide. In the international CLARINET trial, 204 patients with nonfunctioning, SSTR-positive GEP NETs and a PI below 10% were randomized between monthly lanreotide autogel 120-mg injections versus placebo (189, 243). Only 4% of patients had documented progression at baseline, 16% had undergone prior treatment, and 39% had had resection of the primary tumor; median time since diagnosis was over 1 year. Again, this study mostly contained indolent neoplasms, as 69% of tumors were grade 1. Lanreotide autogel also increased PFS (median 32.8 months) compared with placebo treatment (median 18.0 months). In this study, patients progressing on placebo were allowed to switch to lanreotide, which resulted in a PFS of 14.0 months. This strategy of placebo followed by lanreotide approached the PFS obtained with upfront lanreotide treatment, strengthening the view that watchful waiting is a viable option in selected cases.
Despite the registration trials being conducted in GEP NETs with a PI below 10%, SSAs are widely prescribed to NETs with a PI of 10% to 20% or of other primary locations, such as the foregut. For patients with metastasized and/or unresectable lung NETs a randomized trial (SPINET, NCT02683941) is currently ongoing. Given their efficacy and tolerability octreotide and lanreotide are generally considered as first-line systemic therapy in grade 1 and 2 NETs. SSAs should not be the primary antiproliferative treatment for fast-growing NETs, massive tumor bulk with local compression of vital structures or grade 3 disease.
Despite the theoretical advantages of using a multi-SSTR agonist for NET, which expresses multiple receptor subtypes, pasireotide has failed to show an additional benefit to other SSAs in patients with GEP NET in monotherapy or combination therapy (244, 245). Lung NETs have been shown to express lower levels of SSTR2A and higher levels of SSTR1 and SSTR5 (246, 247). In the phase 2 open-label LUNA trial in patients with progressive thoracic NETs, pasireotide treatment led to a median PFS of 8.5 months (248). Further studies are needed to confirm an antiproliferative effect of pasireotide in this subgroup of patients.
Radionuclide therapy
The success of SRI in improving diagnostics in patients with NET led to a concept of using radiolabeled SSAs for treatment. This strategy is termed peptide receptor radionuclide therapy (PRRT) and has predominantly been developed for two beta-emitting analogs: yttrium-90 coupled to Tyr3-octreotide (90Y-DOTATOC) and lutetium-177 coupled to octreotate (177Lu-DOTATATE). DOTA (or tetraxetan) is a chelator connecting the radionuclide to the N-terminal end of the SSA. Several large institutional phase 2 studies have shown the efficacy of PRRT using either 90Y-DOTATOC or 177Lu-DOTATATE in metastasized or unresectable SSTR-positive NETs of varying origin (249–252). The objective response rate, a combination of responses or stable disease, after PRRT cycles is estimated at around 80% (250).
The efficacy of PRRT was ultimately proven in a multicenter randomized controlled trial (RCT) (NETTER-1 trial) in 229 patients with metastasized midgut NETs progressive on a standard dose of octreotide LAR (253). The majority of patients included in this study had undergone resection of the primary tumor (80%) and had grade 1 tumors (69%). Approximately half of the patients had been treated with another line of therapy besides SSA before inclusion in this study. Patients randomized to treatment with 4 cycles of 7.4 GBq 177Lu-DOTATATE displayed a significantly improved PFS compared with monthly octreotide LAR at 60 mg. Although the final survival data are pending, there was a trend toward improved OS. The risk of progression or death was 79% lower in patients treated with PRRT than those treated with high-dose octreotide LAR. Importantly, PRRT also provided a clinically significant improvement in quality of life (202, 254). Relevant adverse effects include nausea surrounding the infusion of kidney-protective amino acids, renal toxicity, transient bone marrow suppression, and the development of myelodysplastic syndrome or acute myeloid leukemia in 1% to 2% of cases (255–258).
Given its potent and persistent effects with a median PFS in terms of years, salvage treatment with additional cycles of PRRT is a relevant option after renewed progression of disease (259). In a selected group of patients with a time to progression of more than 18 months after initial infusion of 177Lu-DOTATATE, salvage PRRT can once again secure disease control (260). Novel radionuclides and combinations with chemo- or targeted therapy are currently under investigation, but toxicity, particularly that of the kidneys and the bone marrow, remains a relevant issue.
Targeted therapy
Genetic analysis of NEN tissues has revealed that proliferation of cells is underpinned by several key molecular pathways (21). Translation of the genetic mutations to preclinical research has been hampered by the overall lack of experimental models for this rare disease (261). However, two pivotal signaling cascades have been successfully targeted with proven efficacy in NENs.
First, the hypervascularized aspect of NENs has provided rationale for investigating the role of vascular endothelial growth factor (VEGF). As found in other malignancies, local production and effects of VEGF have been proven in NEN tissue (262). Inhibition of VEGF signaling through the oral multikinase receptor inhibitor sunitinib was subsequently shown to induce tumor control in progressive PanNETs, but not in midgut or pulmonary NETs (263). In a phase 3 randomized clinical trial in 171 PanNET patients with documented progressive disease at baseline, the median PFS on sunitinib 37.5 mg 4 times a day was 11.4 months compared with 5.5 months for placebo-treated patients (264). Approximately half of the patients had a functioning tumor and more than 80% had a grade 2 tumor, while 43% had used or were still using SSAs during the study and 81% of patients had been treated with chemotherapy in the past. Of note, objective tumor response was achieved in 9% to 25% of sunitinib-treated patients (263, 265) and quality of life did not improve (266). Monitoring of adverse effects should include diarrhea, nausea, fatigue, neutropenia, thyroid dysfunction, palmar–plantar erythrodysesthesia, and hypertension.
The second targetable pathway altered in NETs is that of mTOR. mTOR pathway aberrations suggesting increased signaling potential have been detected in lung NETs, PanNETs, and small intestinal NETs (21). Everolimus, an oral MTOR inhibitor, has been shown to exert antiproliferative effects in NETs. Efficacy in PanNET was shown in the RADIANT-3 trial, where 410 patients with progressive tumors were randomized between everolimus 10 mg 4 times a day and placebo (267). About 83% of patients had a grade 1 tumor, 24% were functioning and most patients had received a diagnosis more than 2 years before enrollment. Previous treatment with SSA or chemotherapy was seen in 50% each. The outcome of this study showed that the median PFS in patients treated with everolimus was increased to 11.0 months compared with 4.6 months in placebo-treated patients. In patients with carcinoid syndrome, the RADIANT-2 trial revealed that everolimus plus octreotide LAR prolonged PFS to 16.4 months compared with 11.3 months for placebo plus octreotide LAR treatment (268). Again, about 80% of tumors in this study was grade 1 and 79% of patients had a history of SSA use, while 42% had been treated with other systemic antitumor therapy. A subsequent trial, the RADIANT-4, was performed in 302 patients with nonfunctional lung and gastrointestinal NETs (269). All patients had documented progression at baseline, 54% had been treated with SSA, 25% with chemotherapy, and 21% with radiotherapy; 65% of tumors were grade 1. Treatment with everolimus revealed a comparable potency in these tumor subtypes, with a median PFS of 11.0 months versus 3.9 months in placebo-treated patients. Objective response rates following everolimus therapy across the trials were limited at 2% to 5%. Frequent adverse effects included stomatitis, rash, diarrhea, fatigue, infections, diabetes mellitus, and pneumonitis, which are likely contributors to the lack of improvement in quality of life following treatment with everolimus (270).
Together, sunitinib and everolimus provide relevant additions to the clinical repertoire for treatment of NET patients. A single retrospective, multicenter study of 31 patients revealed equal PFS for sequencing treatment with everolimus after sunitinib versus the alternate regimen (271). In the absence of high-quality data, physicians should consider individual traits for treatment with either drug in PanNETs.
Immunotherapy
Interferon-α was introduced in the 1980s as a treatment for metastasized intestinal neuroendocrine tumors or malignant carcinoid syndrome (272). Subcutaneous injections of interferon-α were reported to induce control of CS symptoms and proliferation with a reported PFS of 34 months in the first series (199). In a meta-analysis on the effects of interferon-α in NETs tumor response rates were found to be limited at 11% (273). Its potency was similar to that of SSAs in a small randomized RCT in patients with GEP NETs, whereas combination therapy did not confer a clear additional benefit (274). Median PFS after lanreotide or interferon-α was less than 12 months in this randomized clinical trial. In a series of 105 patients with metastasized GEP-NET the addition of interferon-α to subcutaneous octreotide showed no potentiating effect and a disappointing median PFS of 6 months in both groups was found (200). Importantly, the adverse effects of interferon-α treatment can be considerable with fatigue, fever, autoimmune disorders, thyroid dysfunction, and liver enzyme abnormalities (275). The limited efficacy combined with the poor tolerability of this drug has restricted its use to selected patients with refractory functional midgut NETs with doses ranging from 3 to 5 MU 3 times weekly subcutaneously. Pegylated interferon-α at 50 to 100 µg per week might constitute a better tolerated alternative (276).
Therapy with immune checkpoint inhibitors has been successfully introduced for multiple cancer subtypes, including melanoma and lung cancer (277, 278). T cell–mediated cancer immunotherapy by drugs targeting Programmed cell death protein 1 (PD-1) or Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) depends on the presence of tumor-infiltrating lymphocytes and tumor cell expression of Programmed death-ligand1 (PD-L1). Recent studies have revealed that well-differentiated NENs displayed immune infiltration or PD-L1 expression in a minority of cases, which is concordant with their low mutational burden (279, 280). Consequently, efficacy of immune checkpoint inhibition might be restricted to a subset of higher grade NENs with immune infiltration. Clinical evidence in well-differentiated tumors is currently restricted to anecdotal cases and subanalysis of a phase 1b basket trial (281, 282), while the outcomes of the first dedicated trials in NEN patients are still awaited.
Chemotherapy
Given their proliferative rate, trials with chemotherapy for grade 1 or 2 NETs have seen overall poor responses (283). Grade 3 well-differentiated NETs also appear to be less susceptible to conventional chemotherapy (284). This is in strong contrast with the highly aggressive grade 3 neuroendocrine carcinomas, where chemotherapy with platinum-based regimens constitutes the first line of choice (285–287).
There is some evidence for chemotherapy in intermediate to high-grade PanNENs with rapid progression or bulky disease. Early studies have described favorable effects of streptozocin and fluorouracil (5-FU) combination therapy in PanNENs (288, 289), but this was before grading was introduced and response was measured by abdominal palpation. These reports have stimulated widespread use of this regimen in grade 2 to 3 PanNENs and retrospective series in recent years have reproduced its efficacy with a PFS of 19 to 23 months and objective response rates of 21% to 43% in mixed NET/NEC groups (290–292). Evidence on other intravenous cytotoxic regimens, like oxaliplatin (293) or doxorubicin (294, 295), has been published, but significant benefit for patients with well-differentiated NETs in terms of efficacy and safety of these regimens has not been proven in prospective clinical trials.
Based on an increasing number of retrospective series (296–301), interest has focused on temozolomide or the combination of temozolomide with capecitabine for the treatment of PanNENs. Preliminary results of a phase 2 trial in which PanNET patients were randomized between temozolomide or temozolomide plus capecitabine were recently presented. Combination therapy induced objective response and disease control rates of 33% and 82% respectively, whereas temozolomide monotherapy elicited a response in 28% and disease control in 68% of subjects (302). Response duration was 9.7 months in the monotherapy group and 12.1 months in the combined treatment group, possibly providing a role for temozolomide-capecitabine treatment in patients with advanced progressive PanNENs.
Prognosis
As NENs display a wide variety of biologic behavior the prognosis differs immensely between indolent limited disease grade 1 tumors and widely spread grade 3 carcinomas. The introduction of the WHO classifications serves to differentiate between patient subcategories with different prognosis. Registration databases reveal median OS rates of 16.2, 8.3 and 0.8 years for grade 1, 2, and 3 NENs, respectively (8). The difference in median OS of 99 months for grade 3 well-differentiated versus 17 months for grade 3 poorly differentiated neoplasms illustrates the need for a further classification (284). Prognosis of patients with metastasized disease immensely differed between primary sites, with poor OS outcomes for colon and lung NETs (median 14 and 24 months) and good outcomes for midgut NETs (median 98–103 months) (8). Survival of patients has substantially improved over the last decades. Proposed mechanisms include the improved and early recognition of NENs, superior therapeutic options, and centralization of care for this rare disease. Several dedicated NET centers have presented their median OS numbers for stage IV disease in well-differentiated NETs, which exceeded 100 months (303, 304), making this a chronic but unfortunately still deadly disease.
Future Directions
Despite significant strides being taken in NEN care over the past decade, much is still to be improved regarding a timely and accurate diagnosis, prognosis, and quality of life. The rarity and heterogeneity of the disease has hampered both preclinical and clinical research efforts. Nevertheless, the formation of dedicated NET societies with the advent of guidelines and standards of care, the successful execution of multicenter phase 3 RCTs, the clarification of the (epi-)genetic background of disease and the introduction of novel preclinical models are all signs of enhanced countering of this disease. This positive scientific evolution should be continued in the coming years in order to advance clinical care for NEN patients to the next level.
Knowledge on biologic processes driving NENs should be further investigated in specific preclinical models. The most commonly used cell lines have an aggressive phenotype and consequently do not always represent well-differentiated NET biology (261). More investigations in well-differentiated human models, such as the GOT-1 and P-STS cell lines or tumor-derived organoids, are urgently needed. Furthermore, the knowledge on (epi-)genetic drivers should be studied to discover novel drug targets. A contemporary approach has been the elucidation of tumor-specific master regulator proteins, which could be utilized for personalized medicine in individual NEN patients (305). On the other hand, insights into neuroendocrine cell physiology has recently flourished due to single cell RNA sequencing (306) and the development of gut organoids (307).
Histologic diagnosis and prognostication can be strengthened through the use of advanced molecular markers. These markers should ideally also be used for prediction of therapeutic efficacy. Molecular biochemistry and imaging could aid treatment selection beyond the presence of SSTR or RB/KRAS mutations (287, 308). Further validation of the use of recently introduced circulating biomarkers is urgently needed to improve noninvasive means for a diagnosis of NEN. With respect to imaging the incorporation of big data analysis might provide new ways of interpreting data from cross-sectional imaging by CT or MRI (309). More importantly, novel nuclear imaging techniques can potentially improve the sensitivity and specificity of functional imaging in NENs. This involves the use of novel radionuclides, such as zirkonium-89 or copper-64 (310), as well as the use of improved radioligands, such as somatostatin receptor antagonists (311), bombesin receptor agonists (312) and CXCR agonists (127).
Despite the recent introduction of telotristat ethyl more efforts should be put into reducing the hormonal production of functional NENs. Further understanding into the regulatory mechanisms for hormone expression and secretion is likely to provide novel targetable pathways. This remains a niche area and effects of currently available therapies on hormonal syndrome control should be further explored.
Currently, much effort is put into optimization of oncologic therapy. Multiple clinical trials are ongoing studying the effects of locoregional therapy (NCT03197012, NCT02067988), SSAs (NCT02651987), targeted therapy (NCT02588170), immune therapy (NCT02939651, NCT02955069), chemotherapy (NCT02246127), PRRT (NCT03049189, NCT02465112), or combinations thereof (NCT02230176, NCT02358356, NCT02248012). As there are currently several options for treatment, sequencing of systemic therapies and the role of locoregional therapy should be studied in more detail within dedicated clinical trials. An interesting observation has been a retrospective comparison of multiple therapeutic modalities after progression of grade 1 to 2 NENs on SSA. The groups undergoing next line therapy with SSA high dose, PRRT, everolimus, and chemotherapy all showed a comparable PFS, but an increased incidences of adverse events and need for dose reduction was observed in the last two treatment groups (313). Besides the optimization of current therapies, novel targets should follow a thorough understanding of NET cell biology and metastatic behavior.
Conclusions
Care for NEN patients has improved considerably due to a beginning of understanding the underlying key molecular pathways in individual tumors, superior classification of tumor subtypes, the advent of PET imaging techniques, and the registration of radionuclide and targeted therapies. Clinicians dealing with NEN patients should be aware of the heterogeneity of this disease and be able to provide a tailored diagnostic and therapeutic strategy aimed at hormonal and proliferative control. Given its rarity, these patients should be discussed in a multidisciplinary team with experienced representatives from endocrinology, oncology, gastroenterology, pulmonology, radiology, nuclear medicine, and pathology. Only through collaboration between these involved key disciplines and between expert centers can excellent care be accomplished for current and future NEN patients.
Acknowledgments
Dr. Bas Prens is gratefully acknowledged for the images of the glucagonoma patient. We are grateful to Drs. George Kyriakopoulos, Denise Kolomodi, and Sofie Chatzioannou for providing the histopathologic and imaging images.
Disclosure Summary: J.H. has received speaker and/or travel fees from Novartis and Ipsen and has served on the Advisory Board for Novartis. G.K. has received honoraria from Novartis and institutional research funding from Ipsen, Novartis, Pfizer, Sanofi and Shire. W.W. has received speaker and/or travel fees from Novartis, Pfizer and Ipsen, and has served on Advisory Boards for Novartis and Ipsen.
Glossary
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- BP
bronchopulmonary
- CDKN1B
cyclin-dependent kinase inhibitor
- CgA
chromogranin A
- CHD
carcinoid heart disease
- CS
carcinoid syndrome
- CT
computed tomography
- CTC
circulating tumor cell
- EMR
endoscopic mucosal resection
- ENETS
European Neuroendocrine Tumor Society
- ESD
endoscopic submucosal dissection
- EUS
endoscopic, ultrasound
- 18FDG
[18F]fluorodeoxyglucose; fine needle aspirate
- GEP
gastroenteropancreatic
- GI
gastrointestinal
- IV
intravenous
- LCNEC
large cell (neuro)endocrine carcinoma
- MANEC
mixed adenoneuroendocrine carcinoma
- MiNEN
mixed neuroendocrine non-neuroendocrine neoplasm
- miRNA
micro-RNA
- MRI
magnetic resonance imaging
- NAME
neuroendocrine adenoma or the middle ear
- NEC
neuroendocrine carcinoma
- NEN
neuroendocrine neoplasm
- NET
neuroendocrine tumor
- OS
overall survival
- PanNEN
pancreatic NEN
- PET
positron emission tomography
- PFS
progression-free survival
- PI
proliferation index
- PRRT
peptide receptor radionuclide therapy
- RCT
randomized controlled trial
- SCNC
small cell carcinoma
- SPECT
single photon emission CT
- SRI
somatostatin receptor imaging
- SSA
somatostatin analog
- SSTR
somatostatin receptor
- TACE
transarterial chemoembolization
- TAE
transarterial embolization
- TARE
transarterial radioembolization
- VIP
vasoactive intestinal polypeptide
References
- 1. Perren A, Couvelard A, Scoazec JY, et al. ; Antibes Consensus Conference participants . ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pathology: diagnosis and prognostic stratification. Neuroendocrinology. 2017;105(3):196–200. [DOI] [PubMed] [Google Scholar]
- 2. Inzani F, Petrone G, Rindi G. The New World Health Organization classification for pancreatic neuroendocrine neoplasia. Endocrinol Metab Clin North Am. 2018;47(3):463–470. [DOI] [PubMed] [Google Scholar]
- 3. Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25(3):458–511. [DOI] [PubMed] [Google Scholar]
- 4. Asa SL, Casar-Borota O, Chanson P, et al. ; attendees of 14th Meeting of the International Pituitary Pathology Club, Annecy, France, November 2016. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocr Relat Cancer. 2017;24(4):C5–C8. [DOI] [PubMed] [Google Scholar]
- 5. Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. [DOI] [PubMed] [Google Scholar]
- 6. Cardoso FA, Monteiro EMR, Lopes LB, Avila MNDC, Scarioli BO. Adenomatous tumors of the middle ear: a literature review. Int Arch Otorhinolaryngol. 2017;21(3):308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork . Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21(3):R153–R163. [DOI] [PubMed] [Google Scholar]
- 10. Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17(4):909–918. [DOI] [PubMed] [Google Scholar]
- 11. Sadowski SM, Neychev V, Millo C, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34(6):588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–712. [DOI] [PubMed] [Google Scholar]
- 13. Thomas D, Tsolakis AV, Grozinsky-Glasberg S, et al. Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumors: data from a multicenter study. Eur J Endocrinol. 2013;168(2):185–193. [DOI] [PubMed] [Google Scholar]
- 14. Norton JA, Melcher ML, Gibril F, Jensen RT. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgical treatment. Surgery. 2004;136(6):1267–1274. [DOI] [PubMed] [Google Scholar]
- 15. Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20(2):168–172. [DOI] [PubMed] [Google Scholar]
- 16. Pape UF, Niederle B, Costa F, et al. ; Vienna Consensus Conference participants . ENETS consensus guidelines for neuroendocrine neoplasms of the appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103(2):144–152. [DOI] [PubMed] [Google Scholar]
- 17. Ramage JK, De Herder WW, Delle Fave G, et al. ; Vienna Consensus Conference participants . ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):139–143. [DOI] [PubMed] [Google Scholar]
- 18. Lee MR, Harris C, Baeg KJ, Aronson A, Wisnivesky JP, Kim MK. Incidence trends of gastroenteropancreatic neuroendocrine tumors in the United States. Clin Gastroenterol Hepatol. 2019;17(11):2212–2217.e1. [DOI] [PubMed] [Google Scholar]
- 19. Leoncini E, Boffetta P, Shafir M, Aleksovska K, Boccia S, Rindi G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine. 2017;58(2):368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45(2):234–243. [DOI] [PubMed] [Google Scholar]
- 21. Mafficini A, Scarpa A. Genetics and epigenetics of gastroenteropancreatic neuroendocrine neoplasms. Endocr Rev. 2019;40(2):506–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thakker RV, Newey PJ, Walls GV, et al. ; Endocrine Society . Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990–3011. [DOI] [PubMed] [Google Scholar]
- 23. Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. [DOI] [PubMed] [Google Scholar]
- 24. Relles D, Baek J, Witkiewicz A, Yeo CJ. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg. 2010;14(6):1052–1061. [DOI] [PubMed] [Google Scholar]
- 25. Därr R, Nambuba J, Del Rivero J, et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer. 2016;23(12):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu R. Mahvash disease: 10 years after discovery. Pancreas. 2018;47(5):511–515. [DOI] [PubMed] [Google Scholar]
- 27. Iacovazzo D, Flanagan SE, Walker E, et al. MAFA missense mutation causes familial insulinomatosis and diabetes mellitus. Proc Natl Acad Sci U S A. 2018;115(5):1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dimitriadis GK, Angelousi A, Weickert MO, Randeva HS, Kaltsas G, Grossman A. Paraneoplastic endocrine syndromes. Endocr Relat Cancer. 2017;24(6):R173–R190. [DOI] [PubMed] [Google Scholar]
- 29. Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18(4):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Herder WW. Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21(1):33–41. [DOI] [PubMed] [Google Scholar]
- 31. Grahame-Smith DG. Progress report: the carcinoid syndrome. Gut. 1970;11(2):189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofland J, Herrera Martinez AD, Zandee WT, de Herder WW. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer. 2019;26(3):R145–R156. [DOI] [PubMed] [Google Scholar]
- 33. Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Effect of hormone secretory syndromes on neuroendocrine tumor prognosis. Endocr Relat Cancer. 2017;24(7):R261–R274. [DOI] [PubMed] [Google Scholar]
- 34. de Herder WW, Niederle B, Scoazec JY, et al. ; Frascati Consensus Conference; European Neuroendocrine Tumor Society . Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84(3):183–188. [DOI] [PubMed] [Google Scholar]
- 35. Cryer PE, Axelrod L, Grossman AB, et al. ; Endocrine Society . Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. [DOI] [PubMed] [Google Scholar]
- 36. Rehfeld JF, Bardram L, Hilsted L, Poitras P, Goetze JP. Pitfalls in diagnostic gastrin measurements. Clin Chem. 2012;58(5):831–836. [DOI] [PubMed] [Google Scholar]
- 37. Jensen RT, Niederle B, Mitry E, et al. ; Frascati Consensus Conference; European Neuroendocrine Tumor Society . Gastrinoma (duodenal and pancreatic). Neuroendocrinology. 2006;84(3):173–182. [DOI] [PubMed] [Google Scholar]
- 38. Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: recent advances and controversies. Curr Opin Gastroenterol. 2013;29(6):650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Herder WW, Rehfeld JF, Kidd M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Pract Res Clin Endocrinol Metab. 2016;30(1):3–17. [DOI] [PubMed] [Google Scholar]
- 40. Peng SY, Li JT, Liu YB, et al. Diagnosis and treatment of VIPoma in China: (case report and 31 cases review) diagnosis and treatment of VIPoma. Pancreas. 2004;28(1):93–97. [DOI] [PubMed] [Google Scholar]
- 41. Kamp K, Feelders RA, van Adrichem RC, et al. Parathyroid hormone-related peptide (PTHrP) secretion by gastroenteropancreatic neuroendocrine tumors (GEP-NETs): clinical features, diagnosis, management, and follow-up. J Clin Endocrinol Metab. 2014;99(9):3060–3069. [DOI] [PubMed] [Google Scholar]
- 42. Wermers RA, Fatourechi V, Wynne AG, Kvols LK, Lloyd RV. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;75(2):53–63. [DOI] [PubMed] [Google Scholar]
- 43. Larsson LI, Hirsch MA, Holst JJ, et al. Pancreatic somatostatinoma. Clinical features and physiological implications. Lancet. 1977;1(8013):666–668. [DOI] [PubMed] [Google Scholar]
- 44. Crona J, Norlén O, Antonodimitrakis P, Welin S, Stålberg P, Eriksson B. Multiple and secondary hormone secretion in patients with metastatic pancreatic neuroendocrine tumours. J Clin Endocrinol Metab. 2016;101(2):445–452. [DOI] [PubMed] [Google Scholar]
- 45. de Mestier L, Hentic O, Cros J, et al. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med. 2015;162(10):682–689. [DOI] [PubMed] [Google Scholar]
- 46. Kamp K, Alwani RA, Korpershoek E, Franssen GJ, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocrinol. 2016;174(3):271–280. [DOI] [PubMed] [Google Scholar]
- 47. van Hoek M, Hofland LJ, de Rijke YB, et al. Effects of somatostatin analogs on a growth hormone-releasing hormone secreting bronchial carcinoid, in vivo and in vitro studies. J Clin Endocrinol Metab. 2009;94(2):428–433. [DOI] [PubMed] [Google Scholar]
- 48. Rehfeld JF, Federspiel B, Bardram L. A neuroendocrine tumor syndrome from cholecystokinin secretion. N Engl J Med. 2013;368(12):1165–1166. [DOI] [PubMed] [Google Scholar]
- 49. Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41(3):461–466. [DOI] [PubMed] [Google Scholar]
- 50. Blažević A, Hofland J, Hofland LJ, Feelders RA, de Herder WW. Small intestinal neuroendocrine tumours and fibrosis: an entangled conundrum. Endocr Relat Cancer. 2018;25(3):R115–R130. [DOI] [PubMed] [Google Scholar]
- 51. Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472(3):341–349. [DOI] [PubMed] [Google Scholar]
- 52. Rindi G, Klöppel G, Alhman H, et al. ; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS) . TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klöppel G, Couvelard A, Perren A, et al. ; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society . ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90(2):162–166. [DOI] [PubMed] [Google Scholar]
- 54. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61–72. [DOI] [PubMed] [Google Scholar]
- 55. Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451(4):757–762. [DOI] [PubMed] [Google Scholar]
- 56. Klöppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456(6):595–597. [DOI] [PubMed] [Google Scholar]
- 57. Yang MX, Coates RF, Ambaye A, et al. NKX2.2, PDX-1 and CDX-2 as potential biomarkers to differentiate well-differentiated neuroendocrine tumors. Biomark Res. 2018;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rindi G, Klersy C, Albarello L, et al. Competitive testing of the WHO 2010 versus the WHO 2017 grading of pancreatic neuroendocrine neoplasms: data from a large international cohort study. Neuroendocrinology. 2018;107(4):375–386. [DOI] [PubMed] [Google Scholar]
- 59. Sadanandam A, Wullschleger S, Lyssiotis CA, et al. A cross-species analysis in pancreatic neuroendocrine tumors reveals molecular subtypes with distinctive clinical, metastatic, developmental, and metabolic characteristics. Cancer Discov. 2015;5(12):1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. [DOI] [PubMed] [Google Scholar]
- 61. Tang LH, Untch BR, Reidy DL, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22(4):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scarpa A, Chang DK, Nones K, et al. ; Australian Pancreatic Cancer Genome Initiative . Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71. [DOI] [PubMed] [Google Scholar]
- 64. Stålberg P, Westin G, Thirlwell C. Genetics and epigenetics in small intestinal neuroendocrine tumours. J Intern Med. 2016;280(6):584–594. [DOI] [PubMed] [Google Scholar]
- 65. Hofland J, Zandee WT, de Herder WW. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat Rev Endocrinol. 2018;14(11):656–669. [DOI] [PubMed] [Google Scholar]
- 66. Oberg K, Krenning E, Sundin A, et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect. 2016;5(5):174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oberg K, Modlin IM, De Herder W, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16(9):e435–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oberg K, Couvelard A, Delle Fave G, et al. ; Antibes Consensus Conference participants . ENETS consensus guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology. 2017;105(3):201–211. [DOI] [PubMed] [Google Scholar]
- 69. Modlin IM, Kidd M, Bodei L, Drozdov I, Aslanian H. The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am J Gastroenterol. 2015;110(8):1223–1232. [DOI] [PubMed] [Google Scholar]
- 70. Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26(6):791–802. [DOI] [PubMed] [Google Scholar]
- 71. Marotta V, Zatelli MC, Sciammarella C, et al. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer. 2018;25(1):R11–R29. [DOI] [PubMed] [Google Scholar]
- 72. Rossi RE, Ciafardini C, Sciola V, Conte D, Massironi S. Chromogranin A in the follow-up of gastroenteropancreatic neuroendocrine neoplasms: is it really game over? A systematic review and meta-analysis. Pancreas. 2018;47(10):1249–1255. [DOI] [PubMed] [Google Scholar]
- 73. Ito T, Jensen RT. Molecular imaging in neuroendocrine tumors: recent advances, controversies, unresolved issues, and roles in management. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stabile BE, Howard TJ, Passaro E Jr, O’Connor DT. Source of plasma chromogranin A elevation in gastrinoma patients. Arch Surg. 1990;125(4):451–453. [DOI] [PubMed] [Google Scholar]
- 75. Turner GB, Johnston BT, McCance DR, McGinty A, Watson RG, Patterson CC, Ardill JE. Circulating markers of prognosis and response to treatment in patients with midgut carcinoid tumours. Gut. 2006;55(11):1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. Eur J Endocrinol. 2016;175(5):361–366. [DOI] [PubMed] [Google Scholar]
- 77. Davar J, Connolly HM, Caplin ME, et al. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. J Am Coll Cardiol. 2017;69(10):1288–1304. [DOI] [PubMed] [Google Scholar]
- 78. Laskaratos FM, Walker M, Wilkins D, et al. Evaluation of clinical prognostic factors and further delineation of the effect of mesenteric fibrosis on survival in advanced midgut neuroendocrine tumours. Neuroendocrinology. 2018;107(3):292–304. [DOI] [PubMed] [Google Scholar]
- 79. Adaway JE, Dobson R, Walsh J, et al. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann Clin Biochem. 2016;53(Pt 5):554–560. [DOI] [PubMed] [Google Scholar]
- 80. Ito T, Hijioka S, Masui T, et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol. 2017;52(1):9–18. [DOI] [PubMed] [Google Scholar]
- 81. Modlin IM, Oberg K, Taylor A, Drozdov I, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: current status and perspectives. Neuroendocrinology. 2014;100(4):265–277. [DOI] [PubMed] [Google Scholar]
- 82. Khan MS, Tsigani T, Rashid M, et al. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res. 2011;17(2):337–345. [DOI] [PubMed] [Google Scholar]
- 83. Khan MS, Kirkwood A, Tsigani T, Garcia-Hernandez J, Hartley JA, Caplin ME, Meyer T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol. 2013;31(3):365–372. [DOI] [PubMed] [Google Scholar]
- 84. Khan MS, Kirkwood AA, Tsigani T, et al. Early changes in circulating tumor cells are associated with response and survival following treatment of metastatic neuroendocrine neoplasms. Clin Cancer Res. 2016;22(1):79–85. [DOI] [PubMed] [Google Scholar]
- 85. Boons G, Vandamme T, Peeters M, et al. Cell-free DNA from metastatic pancreatic neuroendocrine tumor patients contains tumor-specific mutations and copy number variations. Front Oncol. 2018;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malczewska A, Kidd M, Matar S, Kos-Kudla B, Modlin IM. A comprehensive assessment of the role of miRNAs as biomarkers in gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology. 2018;107(1):73–90. [DOI] [PubMed] [Google Scholar]
- 87. Rizzo FM, Meyer T. Liquid biopsies for neuroendocrine tumors: circulating tumor cells, DNA, and MicroRNAs. Endocrinol Metab Clin North Am. 2018;47(3):471–483. [DOI] [PubMed] [Google Scholar]
- 88. Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer. 2015;22(4):561–575. [DOI] [PubMed] [Google Scholar]
- 89. Bodei L, Kidd M, Modlin IM, et al. Gene transcript analysis blood values correlate with 68Ga-DOTA-somatostatin analog (SSA) PET/CT imaging in neuroendocrine tumors and can define disease status. Eur J Nucl Med Mol Imaging. 2015;42(9):1341–1352. [DOI] [PubMed] [Google Scholar]
- 90. van Treijen MJC, Korse CM, van Leeuwaarde RS, et al. Blood transcript profiling for the detection of neuroendocrine tumors: results of a large independent validation study. Front Endocrinol (Lausanne). 2018;9:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146(2):453–460.e5. [DOI] [PubMed] [Google Scholar]
- 93. Cunningham JL, Díaz de Ståhl T, Sjöblom T, Westin G, Dumanski JP, Janson ET. Common pathogenetic mechanism involving human chromosome 18 in familial and sporadic ileal carcinoid tumors. Genes Chromosomes Cancer. 2011;50(2):82–94. [DOI] [PubMed] [Google Scholar]
- 94. Choi IS, Estecio MR, Nagano Y, et al. Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors). Mod Pathol. 2007;20(7):802–810. [DOI] [PubMed] [Google Scholar]
- 95. Edfeldt K, Ahmad T, Åkerström G, et al. TCEB3C a putative tumor suppressor gene of small intestinal neuroendocrine tumors. Endocr Relat Cancer. 2014;21(2):275–284. [DOI] [PubMed] [Google Scholar]
- 96. Karpathakis A, Dibra H, Pipinikas C, et al. Prognostic impact of novel molecular subtypes of small intestinal neuroendocrine tumor. Clin Cancer Res. 2016;22(1):250–258. [DOI] [PubMed] [Google Scholar]
- 97. Sundin A, Arnold R, Baudin E, et al. ; Antibes Consensus Conference participants . ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology. 2017;105(3):212–244. [DOI] [PubMed] [Google Scholar]
- 98. Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48(4):508–518. [DOI] [PubMed] [Google Scholar]
- 99. Putzer D, Gabriel M, Henninger B, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50(8):1214–1221. [DOI] [PubMed] [Google Scholar]
- 100. Pilleul F, Penigaud M, Milot L, Saurin JC, Chayvialle JA, Valette PJ. Possible small-bowel neoplasms: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology. 2006;241(3):796–801. [DOI] [PubMed] [Google Scholar]
- 101. Johanssen S, Boivin M, Lochs H, Voderholzer W. The yield of wireless capsule endoscopy in the detection of neuroendocrine tumors in comparison with CT enteroclysis. Gastrointest Endosc. 2006;63(4):660–665. [DOI] [PubMed] [Google Scholar]
- 102. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 103. Caramella C, Dromain C, De Baere T, Boulet B, Schlumberger M, Ducreux M, Baudin E. Endocrine pancreatic tumours: which are the most useful MRI sequences? Eur Radiol. 2010;20(11):2618–2627. [DOI] [PubMed] [Google Scholar]
- 104. Brenner R, Metens T, Bali M, Demetter P, Matos C. Pancreatic neuroendocrine tumor: added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur J Radiol. 2012;81(5):e746–e749. [DOI] [PubMed] [Google Scholar]
- 105. d’Assignies G, Fina P, Bruno O, et al. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology. 2013;268(2):390–399. [DOI] [PubMed] [Google Scholar]
- 106. Couvelard A, O’Toole D, Turley H, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol. 2014;386(1-2):2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Puli SR, Kalva N, Bechtold ML, et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: a systematic review and meta analysis. World J Gastroenterol. 2013;19(23):3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25(6):389–395. [DOI] [PubMed] [Google Scholar]
- 110. Boutsen L, Jouret-Mourin A, Borbath I, van Maanen A, Weynand B. Accuracy of pancreatic neuroendocrine tumour grading by endoscopic ultrasound-guided fine needle aspiration: analysis of a large cohort and perspectives for improvement. Neuroendocrinology. 2018;106(2):158–166. [DOI] [PubMed] [Google Scholar]
- 111. Hwang HS, Kim Y, An S, et al. Grading by the Ki-67 labeling index of endoscopic ultrasound-guided fine needle aspiration biopsy specimens of pancreatic neuroendocrine tumors can be underestimated. Pancreas. 2018;47(10):1296–1303. [DOI] [PubMed] [Google Scholar]
- 112. Panagiotidis E, Alshammari A, Michopoulou S, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med. 2017;58(1):91–96. [DOI] [PubMed] [Google Scholar]
- 113. Van Binnebeek S, Vanbilloen B, Baete K, et al. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol. 2016;26(3):900–909. [DOI] [PubMed] [Google Scholar]
- 114. Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. [DOI] [PubMed] [Google Scholar]
- 115. Velikyan I, Sundin A, Sörensen J, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55(2):204–210. [DOI] [PubMed] [Google Scholar]
- 116. Barrio M, Czernin J, Fanti S, et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. 2017;58(5):756–761. [DOI] [PubMed] [Google Scholar]
- 117. Cescato R, Waser B, Fani M, Reubi JC. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med. 2011;52(12):1886–1890. [DOI] [PubMed] [Google Scholar]
- 118. van Adrichem RC, Kamp K, van Deurzen CH, et al. Is there an additional value of using somatostatin receptor subtype 2a immunohistochemistry compared to somatostatin receptor scintigraphy uptake in predicting gastroenteropancreatic neuroendocrine tumor response? Neuroendocrinology. 2016;103(5):560–566. [DOI] [PubMed] [Google Scholar]
- 119. Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16(3):978–985. [DOI] [PubMed] [Google Scholar]
- 120. Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur J Nucl Med Mol Imaging. 2017;44(3):490–499. [DOI] [PubMed] [Google Scholar]
- 121. Bahri H, Laurence L, Edeline J, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55(11):1786–1790. [DOI] [PubMed] [Google Scholar]
- 122. Has Simsek D, Kuyumcu S, Turkmen C, et al. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J Nucl Med. 2014;55(11):1811–1817. [DOI] [PubMed] [Google Scholar]
- 123. Luo Y, Pan Q, Yao S, et al. Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-Exendin-4 for detecting localized insulinoma: a prospective cohort study. J Nucl Med. 2016;57(5):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Antwi K, Fani M, Heye T, et al. Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging. 2018;45(13):2318–2327. [DOI] [PubMed] [Google Scholar]
- 125. Balogova S, Talbot JN, Nataf V, et al. 18F-fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur J Nucl Med Mol Imaging. 2013;40(6):943–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Putzer D, Gabriel M, Kendler D, et al. Comparison of (68)Ga-DOTA-Tyr(3)-octreotide and (18)F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q J Nucl Med Mol Imaging. 2010;54(1):68–75. [PubMed] [Google Scholar]
- 127. Werner RA, Weich A, Higuchi T, et al. Imaging of chemokine receptor 4 expression in neuroendocrine tumors - a triple tracer comparative approach. Theranostics. 2017;7(6):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Neyman K, Sundset A, Naalsund A, et al. Endoscopic treatment of bronchial carcinoids in comparison to surgical resection: a retrospective study. J Bronchol Interv Pulmonol. 2012;19(1):29–34. [DOI] [PubMed] [Google Scholar]
- 129. Luckraz H, Amer K, Thomas L, Gibbs A, Butchart EG. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg. 2006;132(1):113–115. [DOI] [PubMed] [Google Scholar]
- 130. Brokx HA, Risse EK, Paul MA, et al. Initial bronchoscopic treatment for patients with intraluminal bronchial carcinoids. J Thorac Cardiovasc Surg. 2007;133(4):973–978. [DOI] [PubMed] [Google Scholar]
- 131. Dalar L, Ozdemir C, Abul Y, et al. Endobronchial treatment of carcinoid tumors of the lung. Thorac Cardiovasc Surg. 2016;64(2):166–171. [DOI] [PubMed] [Google Scholar]
- 132. Reuling EMBP, Dickhoff C, Plaisier PW, et al. Endobronchial treatment for bronchial carcinoid: patient selection and predictors of outcome. Respiration. 2018;95(4):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brokx HA, Paul MA, Postmus PE, Sutedja TG. Long-term follow-up after first-line bronchoscopic therapy in patients with bronchial carcinoids. Thorax. 2015;70(5):468–472. [DOI] [PubMed] [Google Scholar]
- 134. Jeon SM, Cheon JH. Rectal carcinoid tumors: pitfalls of conventional polypectomy. Clin Endosc. 2012;45(1):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44(4):332–345. [DOI] [PubMed] [Google Scholar]
- 136. Jeon JH, Cheung DY, Lee SJ, et al. Endoscopic resection yields reliable outcomes for small rectal neuroendocrine tumors. Dig Endosc. 2014;26(4):556–563. [DOI] [PubMed] [Google Scholar]
- 137. Nakamura K, Osada M, Goto A, et al. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol. 2016;51(4):448–455. [DOI] [PubMed] [Google Scholar]
- 138. Chen WJ, Wu N, Zhou JL, Lin GL, Qiu HZ. Full-thickness excision using transanal endoscopic microsurgery for treatment of rectal neuroendocrine tumors. World J Gastroenterol. 2015;21(30):9142–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pan J, Zhang X, Shi Y, Pei Q. Endoscopic mucosal resection with suction vs. endoscopic submucosal dissection for small rectal neuroendocrine tumors: a meta-analysis. Scand J Gastroenterol. 2018;53(9):1139–1145. [DOI] [PubMed] [Google Scholar]
- 140. Zhang HP, Wu W, Yang S, Lin J. Endoscopic treatments for rectal neuroendocrine tumors smaller than 16 mm: a meta-analysis. Scand J Gastroenterol. 2016;51(11):1345–1353. [DOI] [PubMed] [Google Scholar]
- 141. Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89(3):998–1005. [DOI] [PubMed] [Google Scholar]
- 142. García-Yuste M, Matilla JM, Cueto A, et al. ; Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung for the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR) . Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg. 2007;31(2):192–197. [DOI] [PubMed] [Google Scholar]
- 143. Lee PC, Osakwe NC, Narula N, et al. Predictors of disease-free survival and recurrence in patients with resected bronchial carcinoid tumors. Thorac Cardiovasc Surg. 2016;64(2):159–165. [DOI] [PubMed] [Google Scholar]
- 144. Cusumano G, Fournel L, Strano S, Damotte D, Charpentier MC, Galia A, Terminella A, Nicolosi M, Regnard JF, Alifano M. Surgical resection for pulmonary carcinoid: long-term results of multicentric study-the importance of pathological N status, more than we thought. Lung. 2017;195(6):789–798. [DOI] [PubMed] [Google Scholar]
- 145. Campana D, Ravizza D, Ferolla P, et al. Clinical management of patients with gastric neuroendocrine neoplasms associated with chronic atrophic gastritis: a retrospective, multicentre study. Endocrine. 2016;51(1):131–139. [DOI] [PubMed] [Google Scholar]
- 146. Grozinsky-Glasberg S, Thomas D, Strosberg JR, et al. Metastatic type 1 gastric carcinoid: a real threat or just a myth? World J Gastroenterol. 2013;19(46):8687–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bordi C, Falchetti A, Azzoni C, et al. Aggressive forms of gastric neuroendocrine tumors in multiple endocrine neoplasia type I. Am J Surg Pathol. 1997;21(9):1075–1082. [DOI] [PubMed] [Google Scholar]
- 148. Kim BS, Oh ST, Yook JH, Kim KC, Kim mg, Jeong JW, Kim BS. Typical carcinoids and neuroendocrine carcinomas of the stomach: differing clinical courses and prognoses. Am J Surg. 2010;200(3):328–333. [DOI] [PubMed] [Google Scholar]
- 149. Kim GH, Kim JI, Jeon SW, et al. ; Korean College of Helicobacter and Upper Gastrointestinal Research . Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29(2):318–324. [DOI] [PubMed] [Google Scholar]
- 150. Mahmud N, Tomizawa Y, Stashek K, Katona BW, Ginsberg GG, Metz DC. Endoscopic resection of duodenal carcinoid tumors: a single-center comparison between simple polypectomy and endoscopic mucosal resection. Pancreas. 2019;48(1):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bartel MJ, Puri R, Brahmbhatt B, et al. Endoscopic and surgical management of nonampullary duodenal neoplasms. Surg Endosc. 2018;32(6):2859–2869. [DOI] [PubMed] [Google Scholar]
- 152. Falconi M, Eriksson B, Kaltsas G, et al. ; Vienna Consensus Conference participants . ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103(2):153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98(12):4784–4789. [DOI] [PubMed] [Google Scholar]
- 154. Partelli S, Cirocchi R, Crippa S, et al. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br J Surg. 2017;104(1):34–41. [DOI] [PubMed] [Google Scholar]
- 155. Gaujoux S, Gonen M, Tang L, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. 2012;19(13):4270–4277. [DOI] [PubMed] [Google Scholar]
- 156. Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol. 2015;22(3):1000–1007. [DOI] [PubMed] [Google Scholar]
- 157. Lewis A, Raoof M, Ituarte PHG, et al. Resection of the primary gastrointestinal neuroendocrine tumor improves survival with or without liver treatment. Ann Surg. 2018;270(6):1131–1137. [DOI] [PubMed] [Google Scholar]
- 158. Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99(11):1480–1486. [DOI] [PubMed] [Google Scholar]
- 159. Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26(8):991–997. [DOI] [PubMed] [Google Scholar]
- 160. Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140(6):891–897; discussion 897. [DOI] [PubMed] [Google Scholar]
- 161. Daskalakis K, Karakatsanis A, Hessman O, et al. Association of a prophylactic surgical approach to stage IV small intestinal neuroendocrine tumors with survival. JAMA Oncol. 2018;4(2):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Blažević A, Zandee WT, Franssen GJH, et al. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr Relat Cancer. 2018;25(3):245–254. [DOI] [PubMed] [Google Scholar]
- 163. Daskalakis K, Karakatsanis A, Stålberg P, Norlén O, Hellman P. Clinical signs of fibrosis in small intestinal neuroendocrine tumours. Br J Surg. 2017;104(1):69–75. [DOI] [PubMed] [Google Scholar]
- 164. Landry CS, Lin HY, Phan A, et al. Resection of at-risk mesenteric lymph nodes is associated with improved survival in patients with small bowel neuroendocrine tumors. World J Surg. 2013;37(7):1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Habbe N, Fendrich V, Heverhagen A, Ramaswamy A, Bartsch DK. Outcome of surgery for ileojejunal neuroendocrine tumors. Surg Today. 2013;43(10):1168–1174. [DOI] [PubMed] [Google Scholar]
- 166. Landerholm K, Zar N, Andersson RE, Falkmer SE, Järhult J. Survival and prognostic factors in patients with small bowel carcinoid tumour. Br J Surg. 2011;98(11):1617–1624. [DOI] [PubMed] [Google Scholar]
- 167. Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117(15):3332–3341. [DOI] [PubMed] [Google Scholar]
- 168. Saxena A, Chua TC, Perera M, Chu F, Morris DL. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol. 2012;21(3):e131–e141. [DOI] [PubMed] [Google Scholar]
- 169. Wängberg B, Westberg G, Tylén U, et al. Survival of patients with disseminated midgut carcinoid tumors after aggressive tumor reduction. World J Surg. 1996;20(7):892–899; discussion 899. [DOI] [PubMed] [Google Scholar]
- 170. Norton JA, Warren RS, Kelly mg, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134(6):1057–1063; discussion 1063. [DOI] [PubMed] [Google Scholar]
- 171. Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12(6):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gibson WE, Gonzalez RS, Cates JMM, Liu E, Shi C. Hepatic micrometastases are associated with poor prognosis in patients with liver metastases from neuroendocrine tumors of the digestive tract. Hum Pathol. 2018;79:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Elias D, Lefevre JH, Duvillard P, et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251(2):307–310. [DOI] [PubMed] [Google Scholar]
- 174. Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72(3):517–528. [DOI] [PubMed] [Google Scholar]
- 175. Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187(1):88–92; discussion 92. [DOI] [PubMed] [Google Scholar]
- 176. Nazario J, Gupta S. Transarterial liver-directed therapies of neuroendocrine hepatic metastases. Semin Oncol. 2010;37(2):118–126. [DOI] [PubMed] [Google Scholar]
- 177. Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9(4):261–267. [DOI] [PubMed] [Google Scholar]
- 178. Swärd C, Johanson V, Nieveen van Dijkum E, et al. Prolonged survival after hepatic artery embolization in patients with midgut carcinoid syndrome. Br J Surg. 2009;96(5):517–521. [DOI] [PubMed] [Google Scholar]
- 179. Carrasco CH, Charnsangavej C, Ajani J, Samaan NA, Richli W, Wallace S. The carcinoid syndrome: palliation by hepatic artery embolization. AJR Am J Roentgenol. 1986;147(1):149–154. [DOI] [PubMed] [Google Scholar]
- 180. Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13(1):72–78. [DOI] [PubMed] [Google Scholar]
- 181. Maire F, Lombard-Bohas C, O’Toole D, et al. Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology. 2012;96(4):294–300. [DOI] [PubMed] [Google Scholar]
- 182. Pericleous M, Caplin ME, Tsochatzis E, Yu D, Morgan-Rowe L, Toumpanakis C. Hepatic artery embolization in advanced neuroendocrine tumors: efficacy and long-term outcomes. Asia Pac J Clin Oncol. 2016;12(1):61–69. [DOI] [PubMed] [Google Scholar]
- 183. Fiore F, Del Prete M, Franco R, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47(1):177–182. [DOI] [PubMed] [Google Scholar]
- 184. Jia Z, Paz-Fumagalli R, Frey G, Sella DM, McKinney JM, Wang W. Single-institution experience of radioembolization with yttrium-90 microspheres for unresectable metastatic neuroendocrine liver tumors. J Gastroenterol Hepatol. 2017;32(9):1617–1623. [DOI] [PubMed] [Google Scholar]
- 185. Rajekar H, Bogammana K, Stubbs RS. Selective internal radiation therapy for gastrointestinal neuroendocrine tumour liver metastases: a new and effective modality for treatment. Int J Hepatol. 2011;2011:404916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47(4):460–466. [DOI] [PubMed] [Google Scholar]
- 187. Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31(2):169–188. [DOI] [PubMed] [Google Scholar]
- 188. Dowling RH, Hussaini SH, Murphy GM, Besser GM, Wass JA. Gallstones during octreotide therapy. Metabolism. 1992;41(9 Suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 189. Caplin ME, Pavel M, Ćwikła JB, et al. ; CLARINET Investigators . Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. [DOI] [PubMed] [Google Scholar]
- 190. Strosberg JR, Benson AB, Huynh L, et al. Clinical benefits of above-standard dose of octreotide LAR in patients with neuroendocrine tumors for control of carcinoid syndrome symptoms: a multicenter retrospective chart review study. Oncologist. 2014;19(9):930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wolin EM, Jarzab B, Eriksson B, et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kiesewetter B, Duan H, Lamm W, et al. Oral ondansetron offers effective antidiarrheal activity for carcinoid syndrome refractory to somatostatin analogs. Oncologist. 2019;24(2):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wymenga AN, de Vries EG, Leijsma MK, Kema IP, Kleibeuker JH. Effects of ondansetron on gastrointestinal symptoms in carcinoid syndrome. Eur J Cancer. 1998;34(8):1293–1294. [DOI] [PubMed] [Google Scholar]
- 194. Platt AJ, Heddle RM, Rake MO, Smedley H. Ondansetron in carcinoid syndrome. Lancet. 1992;339(8806):1416. [DOI] [PubMed] [Google Scholar]
- 195. Kulke MH, Hörsch D, Caplin ME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35(1):14–23. [DOI] [PubMed] [Google Scholar]
- 196. Kulke MH, O’Dorisio T, Phan A, et al. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2014;21(5):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Pavel M, Gross DJ, Benavent M, et al. Telotristat ethyl in carcinoid syndrome: safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Pavel M, Hörsch D, Caplin M, et al. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015;100(4):1511–1519. [DOI] [PubMed] [Google Scholar]
- 199. Oberg K, Norheim I, Lind E, et al. Treatment of malignant carcinoid tumors with human leukocyte interferon: long-term results. Cancer Treat Rep. 1986;70(11):1297–1304. [PubMed] [Google Scholar]
- 200. Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3(8):761–771. [DOI] [PubMed] [Google Scholar]
- 201. Maton PN, Camilleri M, Griffin G, Allison DJ, Hodgson HJ, Chadwick VS. Role of hepatic arterial embolisation in the carcinoid syndrome. Br Med J (Clin Res Ed). 1983;287(6397):932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Strosberg J, Wolin E, Chasen B, et al. ; NETTER-1 Study Group . Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J Clin Oncol. 2018;36(25):2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RR. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005;100(10):2307–2314. [DOI] [PubMed] [Google Scholar]
- 204. Fiebrich HB, Van Den Berg G, Kema IP, et al. Deficiencies in fat-soluble vitamins in long-term users of somatostatin analogue. Aliment Pharmacol Ther. 2010;32(11-12):1398–1404. [DOI] [PubMed] [Google Scholar]
- 205. Møller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348(11):1005–1015. [DOI] [PubMed] [Google Scholar]
- 206. Munir A, Choudhary P, Harrison B, Heller S, Newell-Price J. Continuous glucose monitoring in patients with insulinoma. Clin Endocrinol (Oxf). 2008;68(6):912–918. [DOI] [PubMed] [Google Scholar]
- 207. Zhao YP, Zhan HX, Zhang TP, et al. Surgical management of patients with insulinomas: result of 292 cases in a single institution. J Surg Oncol. 2011;103(2):169–174. [DOI] [PubMed] [Google Scholar]
- 208. Drash A, Wolff F. Drug therapy in leucine-sensitive hypoglycemia. Metabolism. 1964;13:487–492. [DOI] [PubMed] [Google Scholar]
- 209. Niitsu Y, Minami I, Izumiyama H, et al. Clinical outcomes of 20 Japanese patients with insulinoma treated with diazoxide. Endocr J. 2019;66(2):149–155. [DOI] [PubMed] [Google Scholar]
- 210. Gill GV, Rauf O, MacFarlane IA. Diazoxide treatment for insulinoma: a national UK survey. Postgrad Med J. 1997;73(864):640–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sturgess NC, Kozlowski RZ, Carrington CA, Hales CN, Ashford ML. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988;95(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Verschoor L, Uitterlinden P, Lamberts SW, Del Pozo E. On the use of a new somatostatin analogue in the treatment of hypoglycaemia in patients with insulinoma. Clin Endocrinol (Oxf). 1986;25(5):555–560. [DOI] [PubMed] [Google Scholar]
- 213. Vezzosi D, Bennet A, Rochaix P, et al. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152(5):757–767. [DOI] [PubMed] [Google Scholar]
- 214. Stehouwer CD, Lems WF, Fischer HR, Hackeng WH, Naafs MA. Aggravation of hypoglycemia in insulinoma patients by the long-acting somatostatin analogue octreotide (Sandostatin). Acta Endocrinol (Copenh). 1989;121(1):34–40. [DOI] [PubMed] [Google Scholar]
- 215. Hendren NS, Panach K, Brown TJ, et al. Pasireotide for the treatment of refractory hypoglycaemia from malignant insulinoma. Clin Endocrinol (Oxf). 2018;88(2):341–343. [DOI] [PubMed] [Google Scholar]
- 216. Tirosh A, Stemmer SM, Solomonov E, et al. Pasireotide for malignant insulinoma. Hormones (Athens). 2016;15(2):271–276. [DOI] [PubMed] [Google Scholar]
- 217. Novotny J, Janku F, Mares P, Petruzelka L. Symptomatic control of hypoglycaemia with prednisone in refractory metastatic pancreatic insulinoma. Support Care Cancer. 2005;13(9):760–762. [DOI] [PubMed] [Google Scholar]
- 218. Sadoff L, Gordon J, Goldman S. Amelioration of hypoglycemia in a patient with malignant insulinoma during the development of the ectopic ACTH syndrome. Diabetes. 1975;24(6):600–603. [DOI] [PubMed] [Google Scholar]
- 219. de Bruin C, Feelders RA, Waaijers AM, et al. Differential regulation of human dopamine D2 and somatostatin receptor subtype expression by glucocorticoids in vitro. J Mol Endocrinol. 2009;42(1):47–56. [DOI] [PubMed] [Google Scholar]
- 220. Ferrer-García JC, Tolosa-Torréns M, Hernando-Meliá C, Arribas-Palomar L, Sánchez-Juan C. Everolimus resolving hypoglycemia, producing hyperglycemia, and necessitating insulin use in a patient with diabetes and nonresectable malignant insulinoma. Endocr Pract. 2011;17(2):e17–e20. [DOI] [PubMed] [Google Scholar]
- 221. van Schaik E, van Vliet EI, Feelders RA, et al. Improved control of severe hypoglycemia in patients with malignant insulinomas by peptide receptor radionuclide therapy. J Clin Endocrinol Metab. 2011;96(11):3381–3389. [DOI] [PubMed] [Google Scholar]
- 222. Lutz SZ, Ullrich A, Häring HU, Ullrich S, Gerst F. Sunitinib specifically augments glucose-induced insulin secretion. Cell Signal. 2017;36:91–97. [DOI] [PubMed] [Google Scholar]
- 223. Chen J, Wang C, Han J, et al. Therapeutic effect of sunitinib malate and its influence on blood glucose concentrations in a patient with metastatic insulinoma. Expert Rev Anticancer Ther. 2013;13(6):737–743. [DOI] [PubMed] [Google Scholar]
- 224. Maton PN, Vinayek R, Frucht H, et al. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology. 1989;97(4):827–836. [DOI] [PubMed] [Google Scholar]
- 225. Metz DC, Comer GM, Soffer E, et al. Three-year oral pantoprazole administration is effective for patients with Zollinger-Ellison syndrome and other hypersecretory conditions. Aliment Pharmacol Ther. 2006;23(3):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Morocutti A, Merrouche M, Bjaaland T, Humphries T, Mignon M. An open-label study of rabeprazole in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Aliment Pharmacol Ther. 2006;24(10):1439–1444. [DOI] [PubMed] [Google Scholar]
- 227. Metz DC, Sostek MB, Ruszniewski P, Forsmark CE, Monyak J, Pisegna JR. Effects of esomeprazole on acid output in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Am J Gastroenterol. 2007;102(12):2648–2654. [DOI] [PubMed] [Google Scholar]
- 228. Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of omeprazole in Zollinger-Ellison syndrome: a prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7(6):597–610. [DOI] [PubMed] [Google Scholar]
- 229. Frost M, Lines KE, Thakker RV. Current and emerging therapies for PNETs in patients with or without MEN1. Nat Rev Endocrinol. 2018;14(4):216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Vinik AI, Tsai S, Moattari AR, Cheung P. Somatostatin analogue (SMS 201-995) in patients with gastrinomas. Surgery. 1988;104(5):834–842. [PubMed] [Google Scholar]
- 231. Nikou GC, Toubanakis C, Nikolaou P, et al. VIPomas: an update in diagnosis and management in a series of 11 patients. Hepatogastroenterology. 2005;52(64):1259–1265. [PubMed] [Google Scholar]
- 232. Vinik AI, Tsai ST, Moattari AR, Cheung P, Eckhauser FE, Cho K. Somatostatin analogue (SMS 201-995) in the management of gastroenteropancreatic tumors and diarrhea syndromes. Am J Med. 1986;81(6B):23–40. [DOI] [PubMed] [Google Scholar]
- 233. de Mestier L, Walter T, Brixi H, Lombard-Bohas C, Cadiot G. Sunitinib achieved fast and sustained control of VIPoma symptoms. Eur J Endocrinol. 2015;172(1):K1–K3. [DOI] [PubMed] [Google Scholar]
- 234. Zandee WT, Brabander T, Blažević A, et al. Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104(4):1336–1344. [DOI] [PubMed] [Google Scholar]
- 235. Nguyen HN, Backes B, Lammert F, et al. Long-term survival after diagnosis of hepatic metastatic VIPoma: report of two cases with disparate courses and review of therapeutic options. Dig Dis Sci. 1999;44(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 236. Santangelo WC, Unger RH, Orci L, Dueno MI, Popma JJ, Krejs GJ. Somatostatin analog-induced remission of necrolytic migratory erythema without changes in plasma glucagon concentration. Pancreas. 1986;1(5):464–469. [DOI] [PubMed] [Google Scholar]
- 237. Altimari AF, Bhoopalam N, O’Dorsio T, Lange CL, Sandberg L, Prinz RA. Use of a somatostatin analog (SMS 201-995) in the glucagonoma syndrome. Surgery. 1986;100(6):989–996. [PubMed] [Google Scholar]
- 238. Wei J, Song X, Liu X, Ji Z, Ranasinha N, Wu J, Miao Y. Glucagonoma and glucagonoma syndrome: one center’s experience of six cases. J Pancreat Cancer. 2018;4(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol (Oxf). 2011;74(5):593–598. [DOI] [PubMed] [Google Scholar]
- 240. Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. J Gastroenterol Hepatol. 2008;23(4):521–526. [DOI] [PubMed] [Google Scholar]
- 241. Angeletti S, Corleto VD, Schillaci O, et al. Use of the somatostatin analogue octreotide to localise and manage somatostatin-producing tumours. Gut. 1998;42(6):792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Rinke A, Müller HH, Schade-Brittinger C, et al. ; PROMID Study Group . Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. [DOI] [PubMed] [Google Scholar]
- 243. Caplin ME, Pavel M, Ćwikła JB, et al. ; CLARINET Investigators . Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23(3):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Kulke MH, Ruszniewski P, Van Cutsem E, et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol. 2017;28(6):1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Cives M, Kunz PL, Morse B, et al. Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer. 2015;22(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kaemmerer D, Specht E, Sänger J, et al. Somatostatin receptors in bronchopulmonary neuroendocrine neoplasms: new diagnostic, prognostic, and therapeutic markers. J Clin Endocrinol Metab. 2015;100(3):831–840. [DOI] [PubMed] [Google Scholar]
- 247. Herrera-Martínez AD, Gahete MD, Sánchez-Sánchez R, et al. The components of somatostatin and ghrelin systems are altered in neuroendocrine lung carcinoids and associated to clinical-histological features. Lung Cancer. 2017;109:128–136. [DOI] [PubMed] [Google Scholar]
- 248. Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017;18(12):1652–1664. [DOI] [PubMed] [Google Scholar]
- 249. Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–2423. [DOI] [PubMed] [Google Scholar]
- 250. Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617–4624. [DOI] [PubMed] [Google Scholar]
- 251. Baum RP, Kulkarni HR, Singh A, et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9(24):16932–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Garske-Román U, Sandström M, Fröss Baron K, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45(6):970–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators . Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Clin Oncol. 2004;22(13):2724–2729. [DOI] [PubMed] [Google Scholar]
- 255. Bergsma H, Konijnenberg MW, van der Zwan WA, et al. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43(10):1802–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Bergsma H, Konijnenberg MW, Kam BL, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43(3):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Bergsma H, van Lom K, Raaijmakers MHGP, et al. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with 177Lu-DOTATATE: incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2018;59(3):452–458. [DOI] [PubMed] [Google Scholar]
- 258. Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5–19. [DOI] [PubMed] [Google Scholar]
- 259. Severi S, Sansovini M, Ianniello A, et al. Feasibility and utility of re-treatment with (177)Lu-DOTATATE in GEP-NENs relapsed after treatment with (90)Y-DOTATOC. Eur J Nucl Med Mol Imaging. 2015;42(13):1955–1963. [DOI] [PubMed] [Google Scholar]
- 260. van der Zwan WA, Brabander T, Kam BLR, et al. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46(3):704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Hofving T, Arvidsson Y, Almobarak B, et al. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocr Relat Cancer. 2018;25(4):X1–X2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32(2):133–138. [DOI] [PubMed] [Google Scholar]
- 263. Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26(20):3403–3410. [DOI] [PubMed] [Google Scholar]
- 264. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 265. Raymond E, Kulke MH, Qin S, et al. Efficacy and safety of sunitinib in patients with well-differentiated pancreatic neuroendocrine tumours. Neuroendocrinology. 2018;107(3):237–245. [DOI] [PubMed] [Google Scholar]
- 266. Vinik A, Bottomley A, Korytowsky B, et al. Patient-reported outcomes and quality of life with sunitinib versus placebo for pancreatic neuroendocrine tumors: results from an international phase III trial. Target Oncol. 2016;11(6):815–824. [DOI] [PubMed] [Google Scholar]
- 267. Yao JC, Shah MH, Ito T, et al. ; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Pavel ME, Hainsworth JD, Baudin E, et al. ; RADIANT-2 Study Group . Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. [DOI] [PubMed] [Google Scholar]
- 269. Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol. 2016;34(32):3906–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Pavel M, Unger N, Borbath I, et al. Safety and QOL in patients with advanced NET in a phase 3b expanded access study of everolimus. Target Oncol. 2016;11(5):667–675. [DOI] [PubMed] [Google Scholar]
- 271. Angelousi A, Kamp K, Kaltsatou M, O’Toole D, Kaltsas G, de Herder W. Sequential everolimus and sunitinib treatment in pancreatic metastatic well-differentiated neuroendocrine tumours resistant to prior treatments. Neuroendocrinology. 2017;105(4):394–402. [DOI] [PubMed] [Google Scholar]
- 272. Oberg K, Funa K, Alm G. Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N Engl J Med. 1983;309(3):129–133. [DOI] [PubMed] [Google Scholar]
- 273. Oberg K. Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion. 2000;62(Suppl 1):92–97. [DOI] [PubMed] [Google Scholar]
- 274. Faiss S, Pape UF, Böhmig M, et al. ; International Lanreotide and Interferon Alfa Study Group . Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors–the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21(14):2689–2696. [DOI] [PubMed] [Google Scholar]
- 275. Moertel CG, Rubin J, Kvols LK. Therapy of metastatic carcinoid tumor and the malignant carcinoid syndrome with recombinant leukocyte A interferon. J Clin Oncol. 1989;7(7):865–868. [DOI] [PubMed] [Google Scholar]
- 276. Pavel ME, Baum U, Hahn EG, Schuppan D, Lohmann T. Efficacy and tolerability of pegylated IFN-alpha in patients with neuroendocrine gastroenteropancreatic carcinomas. J Interferon Cytokine Res. 2006;26(1):8–13. [DOI] [PubMed] [Google Scholar]
- 277. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Cives M, Strosberg J, Al Diffalha S, Coppola D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr Relat Cancer. 2019;26(1):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Bösch F, Brüwer K, Altendorf-Hofmann A, et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr Relat Cancer. 2019;26(3):293–301. [DOI] [PubMed] [Google Scholar]
- 281. Chauhan A, Horn M, Magee G, et al. Immune checkpoint inhibitors in neuroendocrine tumors: a single institution experience with review of literature. Oncotarget. 2018;9(10):8801–8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Mehnert JM, Rugo HS, O’Neil BH, et al. Pembrolizumab for patients with PD-L1-positive advanced carcinoids or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. J Clin Oncol. 2017;28(Suppl 5):abstr 4270. [Google Scholar]
- 283. Wong MH, Chan DL, Lee A, et al. Systematic review and meta-analysis on the role of chemotherapy in advanced and metastatic neuroendocrine tumor (NET). Plos One. 2016;11(6):e0158140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Heetfeld M, Chougnet CN, Olsen IH, et al. ; other Knowledge Network members . Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22(4):657–664. [DOI] [PubMed] [Google Scholar]
- 285. Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001;92(5):1101–1107. [DOI] [PubMed] [Google Scholar]
- 286. Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. [DOI] [PubMed] [Google Scholar]
- 287. Hijioka S, Hosoda W, Matsuo K, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: a Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res. 2017;23(16):4625–4632. [DOI] [PubMed] [Google Scholar]
- 288. Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303(21):1189–1194. [DOI] [PubMed] [Google Scholar]
- 289. Moertel CG, Douglas HO Jr, Hanley J, Carbone PP. Treatment of advanced adenocarcinoma of the pancreas with combinations of streptozotocin plus 5-fluorouracil and streptozotocin plus cyclophosphamide. Cancer. 1977;40(2):605–608. [DOI] [PubMed] [Google Scholar]
- 290. Clewemar Antonodimitrakis P, Sundin A, Wassberg C, Granberg D, Skogseid B, Eriksson B. Streptozocin and 5-fluorouracil for the treatment of pancreatic neuroendocrine tumors: efficacy, prognostic factors and toxicity. Neuroendocrinology. 2016;103(3-4):345–353. [DOI] [PubMed] [Google Scholar]
- 291. Shibuya H, Hijioka S, Sakamoto Y, et al. Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: focus on weekly regimens and monotherapy. Cancer Chemother Pharmacol. 2018;82(4):661–668. [DOI] [PubMed] [Google Scholar]
- 292. Dilz LM, Denecke T, Steffen IG, et al. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur J Cancer. 2015;51(10):1253–1262. [DOI] [PubMed] [Google Scholar]
- 293. Faure M, Niccoli P, Autret A, Cavaglione G, Mineur L, Raoul JL. Systemic chemotherapy with FOLFOX in metastatic grade ½ neuroendocrine cancer. Mol Clin Oncol. 2017;6(1):44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG; Eastern Cooperative Oncology Group . Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23(22):4897–4904. [DOI] [PubMed] [Google Scholar]
- 295. Krug S, Boch M, Daniel H, et al. Streptozocin-based chemotherapy in patients with advanced neuroendocrine neoplasms–predictive and prognostic markers for treatment stratification. Plos One. 2015;10(12):e0143822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15(1):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Crespo G, Jiménez-Fonseca P, Custodio A, et al. Capecitabine and temozolomide in grade ½ neuroendocrine tumors: a Spanish multicenter experience. Future Oncol. 2017;13(7):615–624. [DOI] [PubMed] [Google Scholar]
- 299. Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist. 2016;21(6):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Abbasi S, Kashashna A, Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas. 2014;43(8):1303–1305. [DOI] [PubMed] [Google Scholar]
- 301. Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. [DOI] [PubMed] [Google Scholar]
- 302. Kunz PL, Catalano PJ, Nimeiri H, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;36(15 suppl):4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89(4):471–476. [DOI] [PubMed] [Google Scholar]
- 304. van Adrichem RC, Kamp K, Vandamme T, Peeters M, Feelders RA, de Herder WW. Serum neuron-specific enolase level is an independent predictor of overall survival in patients with gastroenteropancreatic neuroendocrine tumors. Ann Oncol. 2016;27(4):746–747. [DOI] [PubMed] [Google Scholar]
- 305. Alvarez MJ, Subramaniam PS, Tang LH, et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat Genet. 2018;50(7):979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551(7680):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Grün D, Lyubimova A, Kester L, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525(7568):251–255. [DOI] [PubMed] [Google Scholar]
- 308. Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23(12):2754–2762. [DOI] [PubMed] [Google Scholar]
- 309. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. [DOI] [PubMed] [Google Scholar]
- 310. Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev. 2010;110(5):2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Nicolas GP, Schreiter N, Kaul F, et al. Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase II imaging study. J Nucl Med. 2018;59(6):915–921. [DOI] [PubMed] [Google Scholar]
- 312. Dalm SU, Bakker IL, de Blois E, et al. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J Nucl Med. 2017;58(2):293–299. [DOI] [PubMed] [Google Scholar]
- 313. Faggiano A, Di Maio S, Mocerino C, et al. ; Elios . Therapeutic sequences in patients with grade 1–2 neuroendocrine tumors (NET): an observational multicenter study from the ELIOS group. Endocrine. 2019; doi: 10.1007/s12020-12019-01894-12020. [DOI] [PubMed] [Google Scholar]