Abstract
Objective
To assess the impact of provider incentive policy on smoking status documentation.
Data Sources
Primary data were extracted from structured electronic medical records (EMRs) from 15 community health centers (CHCs).
Study Design
This was an observational study of data from 2006 to 2013, assessing changes in documentation of smoking status over time.
Data Extraction Methods
We extracted structured EMR data for patients age 18 and older with at least one primary care visit.
Principal Findings
Rates of documented smoking status rose from 30 percent in 2006 to 90 percent in 2013; the largest increase occurred from 2011 to 2012 following policy changes (21.3% [95% CI, 8.2%, 34.4%] from the overall trend). Rates varied by clinic and across patient subgroups.
Conclusions
Documentation of smoking status improved markedly after introduction of new federal standards. Further improvement in documentation is still needed, especially for males, nonwhite patients, those using opioids, and HIV + patients. More research is needed to study whether changes in documentation lead to improvements in counseling, cessation, and patient outcomes.
Keywords: community health centers, electronic health records, meaningful use criteria, tobacco use, uniform data system standards
What this study adds.
What is currently known?
Asking patients about their smoking status and documenting the result is a critical first step toward implementing evidence‐based smoking cessation interventions.
The federal government implemented two incentive programs in 2011 to encourage documentation of smoking status, particularly in community health centers (CHCs).
Data suggest that rates of documentation are rising over time, but no prior study has examined changes in documentation rates following the 2011 policy changes.
What this study adds.
Rates of smoking status documentation among 15 US CHCs increased from 30 to 90 percent from 2006 to 2013.
The percentage of patients with no smoking status documented fell from 50 to 20 percent in the year following the 2011 policy changes, suggesting that these changes may have encouraged smoking status documentation.
Rates of documentation remained highly variable in 2013, varying both by clinic and by individual patient characteristics (age, sex, race/ethnicity, and comorbidities).
1. INTRODUCTION
Some 40 million individuals in the United States are cigarette smokers1 and over 400 000 Americans die from smoking‐related illnesses each year, making smoking a primary cause of preventable death in the United States.2 Although evidence‐based guidelines identify delivering smoking cessation treatments in primary care settings as standard care,3, 4, 5 rates of delivery of appropriate cessation services remain low.6, 7, 8, 9, 10, 11, 12
Documenting current smoking status is critical for implementing smoking cessation interventions and is highlighted as the first step in the “5 A’s” of smoking cessation interventions (Ask, Advise, Assess, Assist, and Arrange).13 The US Preventive Services Task Force (USPTF) recommends that clinicians ask all adult patients about tobacco use and advise and offer cessation interventions to those who smoke.14, 15 Smoking status fields in the electronic medical record (EMR) can remind clinicians to ask about tobacco use and provide a way for health systems, public health agencies, and researchers to assess whether this asking is taking place.16 Given the number of smokers served in primary care settings,3 the impacts of smoking‐related illnesses,17 and the availability of cost‐effective interventions,18 documenting tobacco use should be a priority.
In 2011, the federal government created “meaningful use” (MU) criteria that allowed providers to earn incentive payments by recording smoking status and offering cessation assistance, among other metrics (the complexity of the system makes it impossible to estimate the size of incentives for smoking documentation per se). Also in 2011, the Health Resources and Services Administration (HRSA), using the Uniform Data System (UDS), updated its standards for documenting smoking and cessation counseling; these standards apply to all community health centers (CHCs) certified as Federally Qualified Community Health Centers and meeting all reporting requirements is a condition of funding. However, the effects of these policies on documentation of smoking status have not been systematically examined.
Using EMR data from 15 CHCs in nine states,19, 20 we assessed documentation of smoking status between 2006 and 2013. These data afford an opportunity to examine the early impact of federal policy changes on CHC policies and provider behavior, as well as the role of individual demographic and clinical characteristics.
2. METHODS
2.1. Study design and data sources
We conducted an observational study of EHR data from 15 CHCs from 2006 to 2013 to assess changes in documentation of smoking status over that time period. Data were extracted from the Community Health Applied Research Network (CHARN) data warehouse (CDW). The CDW identifies and defines the same clinical variables across the three EMRs used by 17 CHCs, and is limited to adult patients (≥18 years of age) with at least one primary care visit. Between 2006 and 2014, CHARN's 17 CHCs served over one million patients in nine states across the United States (Figure S1). Eleven CHCs collected smoking data in structured fields in their EMR for all years in the study period; one more began collecting smoking data in each of the years 2007‐2010; these data were included as they became available. Two CHCs were excluded because they had not incorporated smoking data fields into their EMRs by 2013. After these exclusions, we included data from 706 840 patients seen in one of the 15 clinics at least once during the study period.
The 15 included CHCs ranged in size from 4700 to 67 000 patients. A total of 531 000 patients were seen in these centers in 2013. Only one was located in a rural area. Descriptive data on the patient population at each CHC are shown in the Table S1; 72 percent of patients at these CHCs were members of ethnic and racial minority groups and 73 percent reported incomes below the Federal Poverty Level (FPL).
We included structured EMR data (not free text) on smoking status and patient characteristics from the 15 CHCs with smoking data beginning either in 2006 or in the earliest year in which data were recorded. No clinics dropped out, and the cumulative number of clinics included in each year is shown in Figure 1. Overall rates of documentation were assessed for each year from 2006 to 2013 at the clinic level (the denominator increased as clinics were added to the database); analyses of how documentation related to patient characteristics used data from the final two years of the study period, 2012‐2013, which included 436 652 individuals.
Figure 1.
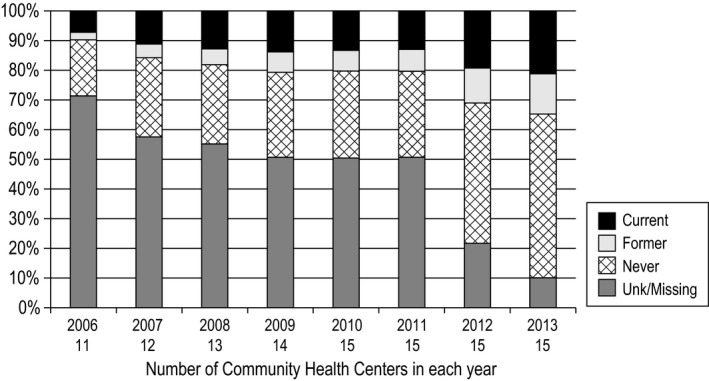
Smoking status documentation for all CHCs combined, 2006‐2013
2.2. Variables
2.2.1. Documentation over time
All data were drawn from the EMR as reported to the CDW. For the longitudinal analyses shown in the figures, we included all encounters for all individuals age 18 or older. Data were aggregated across all clinical encounter types (eg, clinic visits, telephone calls) by individual patient for each year of the study using a CHARN‐specific patient ID number. Smoking status was recorded as current, former, never, or unknown/missing. The EMRs in this study carried forward smoking status from previous visits to inform clinical staff of prior smoking status, which could then be reviewed and changed if necessary. If the smoking status was unchanged, this was often not specifically noted. Since the CDW can link individual data over time, if no smoking status was recorded in a given year, status was set as that of the last recorded value. Thus, missing/unknown smoking status indicated that providers had never recorded smoking status for a given individual.
2.2.2. Association between documentation and patient characteristics
To assess whether documentation varied by patient characteristics, we performed a cross‐sectional analysis combining data from 2012 to 2013. Demographic and smoking variables were defined using the most recent data available as of 2013. Gender, race, ethnicity, and household income were entered into the EMR by clinic staff using patient self‐report. Household income was classified in reference to the FPL: ≤100 percent, 101‐150 percent, 151‐200 percent, and >200 percent. Age as of 2013 was calculated from birthdates.
Insurance status was recorded at each visit for all patients. Changes in insurance coverage were frequent, so we defined a measure of insurance stability across the observation period for each patient, consisting of five categories: continuously insured, continuously uninsured, insurance gaps present, single encounter, or missing. We did not include insurance type in our analyses as most patients served by CHCs are insured by Medicare or Medicaid or are uninsured. Finally, we included the number of in‐person primary care visits from 2012 to 2013 in our multivariable analyses to adjust for differences in the opportunity for smoking status to be assessed.
Medical and psychiatric comorbidities commonly associated with smoking—including depression, anxiety disorders, serious mental illness (schizophrenia spectrum, bipolar spectrum, other psychosis), alcohol, and opioid use disorders—were identified and included in the dataset if the corresponding diagnosis code appeared at least once either in the problem list or a visit encounter at any time during 2012‐2013.21 We used the Charlson Comorbidity Index (CCI) to assess overall health, using the total score as a continuous variable.22
2.3. Statistical analysis
We calculated the relative frequency of each smoking status category in the EMR, including “unknown/missing,” for each year across all study clinics. We also compared the frequency of each smoking status category across individual CHCs in both 2006 and 2013. We used an interrupted time series (ITS) regression to test whether the observed trend in documentation rate changed in 2012 (ie, test of whether there was an immediate effect in 2012 compared to predicted trajectory).23, 24, 25
Using 2012‐2013 data, we fit a hierarchical generalized linear model (HGLM)26, 27, 28, 29 to assess the association between having a valid smoking status (vs unknown/missing) and the demographic and clinical variables described above. HGLM allows for the simultaneous analysis across multiple levels (eg, patient and CHC level) and accounts for the clustering of patients nested within CHCs. Because the outcome was binary, we used a model with a logit link and binomial distribution (ie, random effects binomial logistic regression). Because all the independent variables were patient‐level characteristics, they were included in the first level of each model as fixed effects. Clinic was included in the second level of each model as a random effect. We report the odds ratios and associated 95% confidence intervals, as well as the predictive margins (ie, average adjusted predicted probability), for each variable from the multivariable model. All inferential tests were conducted with a two‐tailed alpha of 0.05.
3. RESULTS
Table 1 shows the characteristics of the study sample. About 60 percent were female, only 10 percent were over age 65, and the majority were people of color. Hypertension, diabetes, anxiety, and depression diagnoses were common. Rates of alcohol and opioid use disorders were likely undercounted as they are based on diagnosis codes, not self‐report.
Table 1.
Characteristics of the study sample in 2012‐2013
| Characteristic | N | Col % |
|---|---|---|
| Total | 436 652 | 100.0 |
| Gender | ||
| Male | 170 494 | 39.0 |
| Female | 264 145 | 60.5 |
| Transgender | 1945 | 0.4 |
| Unknown or missing | 68 | — |
| Age (2013) | ||
| 18‐34 | 173 959 | 39.8 |
| 35‐64 | 220 486 | 50.5 |
| 65+ | 42 207 | 9.7 |
| Race | ||
| White | 195 930 | 44.9 |
| African American | 75 233 | 17.2 |
| American Indian | 9955 | 2.3 |
| Asian/Pacific Islander | 92 133 | 21.1 |
| Other | 63 401 | 14.5 |
| Ethnicity all races | ||
| Hispanic or Latino | 92 914 | 21.3 |
| Not Hispanic or Latino | 282 547 | 64.7 |
| Missing (reported unknown) | 18 009 | 4.1 |
| Missing (left blank) | 43 182 | 9.9 |
| Insurance status | ||
| Continuously insured | 178 904 | 41.0 |
| Continuously uninsured | 68 669 | 15.7 |
| Insurance gaps present | 67 428 | 15.4 |
| Single encounter | 71 103 | 16.3 |
| No insurance information | 50 548 | 11.6 |
| N | Prevalence % | |
|---|---|---|
| Comorbidities | ||
| Hypertension | 65 944 | 15.1 |
| Diabetes, type 2 | 30 789 | 7.1 |
| COPD | 15 671 | 3.6 |
| HIV diagnosis | 6168 | 1.4 |
| Depression | 47 983 | 11.0 |
| Anxiety disorder | 32 604 | 7.5 |
| Serious mental illness | 11 469 | 2.6 |
| Alcohol use disorder diagnosis | 7395 | 1.7 |
| Opioid use disorder diagnosis | 2667 | 0.6 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus (HIV [042], AIDS [079.53, v08]).
Figure 1 shows the crude rate of smoking status documentation between 2006 and 2013 for all included CHCs (the number of CHCs contributing data each year is noted). In 2006, only 30 percent of patients had documented smoking status. This number rose to about 50 percent in 2009 and held steady until 2011, after which there was a fairly abrupt increase to 80 percent in 2012 and 90 percent in 2013.
Documentation rates were highly variable across the CHCs. Figure 2 (top) shows the rates at each CHC in 2006. Among the 11 CHCs with data, the percentage of patients with documented smoking status rate ranged from 70 percent to nearly zero; all but two of the CHCs were missing smoking status on at least 50 percent of patients. Figure 2 (bottom) shows the same data for 2013: Although the improvement is apparent, there remain large differences between CHCs, with documented smoking status ranging from 98 to 50 percent across centers. The ITS showed that the increase in documentation rate between 2011 and 2012 of 21.3 percent (95% CI [8.2%, 34.4%]) from the current trend was significant, P = .011.
Figure 2.
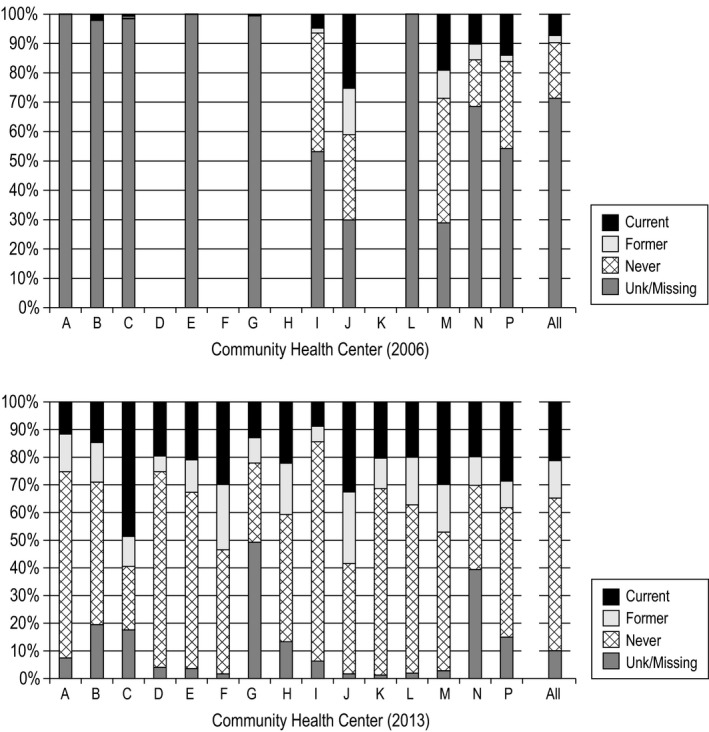
Smoking documentation and status in 2006 and 2013, by CHC and combined
Table 2 shows the results of the HGLM analysis assessing predictors of smoking documentation in 2012‐2013 among the 292 127 patients with data in those years. Table 2 presents both the odds of having documented status and the adjusted proportion of patients with documented status from the same multivariable model. Rates of smoking status documentation varied across subgroups by 3‐4 percentage points, controlling for all other predictors. Significantly higher odds of having documented smoking status were seen for patients age 35 + compared to younger than 35 and for cisgender women and transgender patients compared to cisgender men. Compared to white, non‐Hispanic patients, all other racial and ethnic minority groups except Hispanic patients had significantly lower odds of documented smoking status. Financial difficulties—both lack of health insurance and having low income—were associated with significantly higher odds of having documented smoking status. Patients with medical or psychiatric comorbidities, or with more comorbidities in general (higher CCI score), had significantly higher odds of documented smoking status, with the exception of HIV + patients. Documented alcohol use disorder was associated with higher odds of having documented smoking status, while patients with an opioid use disorder had lower odds of having smoking status documented. Finally, having more primary care visits was associated with slightly but significantly higher odds of smoking status documentation. Because primary care visit frequency was included in the multivariable model, the other associations observed were independent of this effect.
Table 2.
Predictors of smoking status documentation in all study CHCs (n = 15) for 2012‐2013 (n = 292 127) in a single multivariable model
| Independent variables | Adjusted predicted probability documented (%) | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|
| Age | ||||
| 18‐34 | 86.1 | 0.62 | 0.60 | 0.64 |
| 35‐64 (reference) | 90.8 | — | — | — |
| 65+ | 89.4 | 0.84 | 0.80 | 0.89 |
| Gender | ||||
| Female | 90.0 | |||
| Male | 86.3 | |||
| Transgender | 90.7 | |||
| Female vs male | 1.46 | 1.41 | 1.50 | |
| Transgender vs male | 1.58 | 1.38 | 1.80 | |
| Race | ||||
| Non‐Hispanic white (reference) | 89.5 | — | — | — |
| Hispanic | 89.5 | 1.00 | 0.96 | 1.05 |
| African American | 88.5 | 0.90 | 0.86 | 0.95 |
| American Indian | 87.5 | 0.82 | 0.74 | 0.90 |
| Asian/Pacific Islander | 86.5 | 0.75 | 0.70 | 0.79 |
| Multiracial | 88.3 | 0.88 | 0.80 | 0.97 |
| Insurance status | ||||
| Always insured (reference) | 88.5 | — | — | — |
| Always uninsured | 91.1 | 1.35 | 1.28 | 1.41 |
| Insurance gaps | 91.4 | 1.40 | 1.34 | 1.46 |
| Single encounter (vs multiple encounters) | 84.3 | 0.69 | 0.67 | 0.72 |
| Household income <100%FPL: yes/no | 89.1/88.3 | 1.09 | 1.06 | 1.12 |
| Comorbidities | ||||
| Hypertension: yes/no | 92.4/87.9 | 1.68 | 1.60 | 1.76 |
| Diabetes, type 2: yes/no | 90.6/88.6 | 1.25 | 1.15 | 1.37 |
| COPD: yes/no | 91.1/88.6 | 1.33 | 1.22 | 1.45 |
| Charlson Index | ||||
| High | 90.6 | |||
| Mean | 88.9 | |||
| Per 1‐unit increase | 1.21 | 1.15 | 1.26 | |
| HIV diagnosis: yes/no | 87.9/88.7 | 0.92 | 0.69 | 1.23 |
| Depression: yes/no | 91.6/88.3 | 1.45 | 1.38 | 1.53 |
| Anxiety disorder: yes/no | 91.1/88.5 | 1.34 | 1.26 | 1.42 |
| Serious mental illness: yes/no | 91.5/88.6 | 1.40 | 1.28 | 1.54 |
| Alcohol use disorder diagnosis: yes/no | 90.1/88.6 | 1.18 | 1.07 | 1.30 |
| Opioid use disorder diagnosis: yes/no | 85.9/88.7 | 0.77 | 0.66 | 0.90 |
| Number of primary care encounters | ||||
| High (7.9 visits/year) | 89.7 | |||
| Mean (3.9 visits/year) | 88.8 | |||
| Per each additional visit per year | 1.024 | 1.019 | 1.029 | |
Hierarchical generalized linear model. Figures in the second column are the average adjusted predicted probability of documentation, controlling for all other variables and for clinic (ie, random effect = 0). For continuous variables, the “high” value is the mean +1 SD. The odds ratios are for the contrasts shown in the first column; significant ORs (P < .05) in boldface.
Abbreviations: COPD, chronic obstructive pulmonary disease; FPL, Federal Poverty Level; HIV, human immunodeficiency virus (HIV [042], AIDS [079.53, v08]).
4. DISCUSSION
Our data show considerable progress in documenting patients' smoking status among 15 US CHCs from 2006 to 2013. The overall rate of smoking documentation increased from 30 percent in 2006 to 90 percent in 2013. Consistent with the hypothesis that federal policy changes led to more complete documentation, the greatest increase occurred the year the changes went into effect (between 2011 and 2012), with rates of missing data dropping from about 50 percent to about 20 percent. However, there are no comparison clinics that did not face new requirements, so causal inference is limited.
Despite large increases in documentation overall, there was still considerable variation in missing data rates across CHCs in 2013, from about 2 percent to nearly 50 percent. In addition to variability by clinic, odds of having documentation varied significantly depending on individual‐level factors: The odds were lower for younger patients, men, nonwhite subgroups, and patients with opioid use disorders. Most comorbidities were associated with higher odds of documented smoking status, but documentation among people with HIV was not significantly different from non‐HIV + patients. This is concerning, since 40 percent of patients with HIV smoke and smoking has a strong effect on morbidity and mortality in this group.30, 31 Reasons for this disparity are unclear, but it has been observed elsewhere.32
This study is the first to examine rates of smoking documentation in EMRs from a multistate sample of CHCs before and after the 2011 changes. In an earlier study of 26 CHCs in Oregon, Bailey et al also found an increase in smoking assessment comparing 2014 to 2010 (adjusted OR 2.52 [95% CI 2.37, 2.69]) although in these CHCs the smoking status documentation rate was high even in 2010 (93.9 percent).33
Our findings are also consistent with earlier reports that 60 percent of patients in CHCs in New York City were missing smoking documentation between 2009 and 2012,34 and that 85 percent of patients in a nationally representative sample reported that providers asked them about tobacco use in 2013‐2014.35 Like our study, both of these studies found that smoking documentation was significantly more common for patients with comorbidities than for those without. Additionally, both our study and the New York study found that smoking status documentation was more common for older patients.
Documentation of smoking status is an important beginning. However, the CHARN dataset did not allow assessment of counseling rates or quitting. Past studies of provider reminders have shown consistent improvements in documentation, but inconsistent effects on advice or cessation. Ahluwalia et al36 and Milch et al37 found improvements in advice rates but Boyle et al38 and Maizlish et al39 did not. A systematic review found that provider reminders to assess smoking increased documentation and cessation advice, but coupling reminders with training led to better outcomes.16
We were fortunate to have consistently reported structured EMR data from 15 geographically dispersed CHCs spanning seven years, allowing us to study a large patient population in a diverse set of safety net clinics across the United States. However, this sample is neither representative of all CHC patients nor of all indigent and minority populations in the United States. This study is also limited by the use of data from structured EMR fields, as extracting chart notes was beyond our scope. In earlier study years, providers may have documented smoking status in their notes but not in structured fields.
We could not examine whether specific CHC‐level characteristics were associated with differences in documentation rates. Given the variability in documentation we observed, future research should examine clinic‐level factors that may facilitate or impede documentation.
Another challenge was our inability to track repeated documentation across visits. Tobacco use documentation is most useful when regularly updated, but when status is unchanged, clinicians rarely re‐enter the same data value. Better understanding the frequency of smoking status documentation should be the focus of future research.
Finally, documentation should be followed by cessation advice and support. Bailey et al33 found improvement in smoking cessation treatment following MU implementation, but research is needed on whether changes in documentation lead to improvements in counseling, cessation, and patient outcomes.
CONFLICT OF INTEREST
None of the authors have any conflict of interest.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: We acknowledge the significant contributions to this research effort of all our CHARN partners across the following organizations: AAPCHO, San Leandro, CA; Asian Health Services, Oakland, CA; Charles B. Wang Community Health Center, NY,; Waianae Coast Comprehensive Health Center, Waianae, HI; Waimānalo Health Center, Waimānalo, HI; University of California, Los Angeles, CA. Alliance of Chicago Community Health Services, Chicago, IL; Erie Family Health Center, Chicago, IL; Heartland Health Outreach, Chicago, IL; Howard Brown Health, Chicago, IL; Near North Health Service Corporation, Chicago, IL; North Country HealthCare, Flagstaff, AZ; PCC Community Wellness, Chicago, IL; Northwestern University, Chicago, IL. Fenway Health, Boston, MA; Chase Brexton Health Care, Baltimore, MD; Beaufort‐Jasper‐Hampton Comprehensive Health Services, Ridgeland, SC; University of Washington, Seattle, WA. OCHIN, Inc, Portland, OR; Open Door Community Health Centers, Arcata, CA; Virginia Garcia Memorial Health Centers, Hillsboro, OR; Multnomah County Health Department, Portland, OR; Oregon Health & Science University, Portland, OR; Richmond Family Medicine Clinic, Portland, OR. Kaiser Permanente, Center for Health Research, Portland, OR; Data Coordinating Center.
This work was supported by the Health Resources and Services Administration, contract HHSH250201400001C and by grant UB3HA20236. The research presented in this paper is that of the authors and does not reflect official policy of Department of Health and Human Services.
Fortmann SP, Bailey SR, Brooks NB, et al. Trends in smoking documentation rates in safety net clinics. Health Serv Res. 2020;55:170–177. 10.1111/1475-6773.13259
REFERENCES
- 1. Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults ‐ United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 2. U. S. Department of Health and Human Services . The Health Consequences of Smoking ‐ 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. Rockville, MD: US Dept of Health and Human Services; Public Health Service; 2008. [Google Scholar]
- 4. Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollis JF, Bills R, Whitlock E, Stevens VJ, Mullooly J, Lichtenstein E. Implementing tobacco interventions in the real world of managed care. Tob Control. 2000;9(Suppl 1):I18‐l24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chase EC, McMenamin SB, Halpin HA. Medicaid provider delivery of the 5A's for smoking cessation counseling. Nicotine Tobacco Res. 2007;9(11):1095‐1101. [DOI] [PubMed] [Google Scholar]
- 7. Thorndike AN, Regan S, Rigotti NA. The treatment of smoking by US physicians during ambulatory visits: 1994 2003. Am J Public Health. 2007;97(10):1878‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001–2004 national ambulatory medical care survey (NAMCS). Prev Med. 2006;43(6):472‐476. [DOI] [PubMed] [Google Scholar]
- 9. Jamal A, Dube SR, Malarcher AM, et al. Tobacco use screening and counseling during physician office visits among adults–National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005–2009. MMWR ‐ Morbidity Mortality Weekly Rep. 2012;61(Suppl):38‐45. [PubMed] [Google Scholar]
- 10. Mahoney MC, Masucci Twarozek A, Saad‐Harfouche F, et al. Assessing the delivery of cessation services to smokers in urban, safety‐net clinics. J Commun Health. 2014;39(5):879‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kruger J, Shaw L, Kahende J, Frank E. Health care providers' advice to quit smoking, national health interview survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park ER, Gareen IF, Japuntich S, et al. Primary care provider‐delivered smoking cessation interventions and smoking cessation among participants in the national lung screening trial. JAMA Intern Med. 2015;175:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel‐Liaisons‐and Staff . A clinical practice guideline for treating tobacco use and dependence: 2008 Update: A U.S. Public Health Service Report. Am J Prev Med. 2008;35(2):158‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siu AL, Force USPST . Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. preventive services task force recommendation Statement. Ann Intern Med. 2015;163(8):622‐634. [DOI] [PubMed] [Google Scholar]
- 15. Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. preventive services task force. Ann Intern Med. 2015;163:608‐621. [DOI] [PubMed] [Google Scholar]
- 16. Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20(2 Suppl):16‐66. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Smoking‐attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226‐1228. [PubMed] [Google Scholar]
- 18. Solberg LI, Maciosek MV, Edwards NM, Khanchandani HS, Goodman MJ. Repeated tobacco‐use screening and intervention in clinical practice: health impact and cost effectiveness. Am J Prev Med. 2006;31(1):62‐71. [DOI] [PubMed] [Google Scholar]
- 19. Laws R, Gillespie S, Puro J, et al. The Community Health Applied Research Network (CHARN) data warehouse: a resource for patient‐centered outcomes research and quality improvement in underserved, safety net populations. EGEMS (Wash DC). 2014;2(3):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Likumahuwa S, Song H, Singal R, et al. Building research infrastructure in community health centers: a Community Health Applied Research Network (CHARN) report. J Am Board Fam Med. 2013;26(5):579‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cummings JR, Lynch FL, Rust KC, et al. Health services utilization among children with and without autism spectrum disorders. J Autism Dev Disord. 2016;46(3):910‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 23. Gottman JM. Time‐Series Analysis: A Comprehensive Introduction for Social Scientists. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- 24. Simonton DK. Cross‐sectional time‐series experiments: Some suggested statistical analyses. Psychol Bull. 1977;84(3):489‐502. [Google Scholar]
- 25. Huitema BE, McKean JW. Design specification issues in time‐series intervention models. Educ Psychol Meas. 2000;60(1):38‐58. [Google Scholar]
- 26. Hox JJ, Moerbeek M, van de Schoot R. Multilevel Analysis: Techniques and Applications. Florence, KY: Routledge; 2010. [Google Scholar]
- 27. Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- 28. Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications, Inc; 1999. [Google Scholar]
- 29. Skrondal A‐HS. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Boca Raton, FL: Chapman & Hall; 2004. [Google Scholar]
- 30. Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV‐1‐infected individuals: a nationwide, population‐based cohort study. Clin Infect Dis. 2013;56(5):727‐734. [DOI] [PubMed] [Google Scholar]
- 31. Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US‐Based modeling study. J Infect Dis. 2016;214(11):1672‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vijayaraghavan M, Yuan P, Gregorich S, et al. Disparities in receipt of 5As for smoking cessation in diverse primary care and HIV clinics. Prev Med Rep. 2017;6:80‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bailey SR, Heintzman JD, Marino M, et al. Smoking‐cessation assistance: before and after stage 1 meaningful use implementation. Am J Prev Med. 2017;53:192‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silfen SL, Cha J, Wang JJ, Land TG, Shih SC. Patient characteristics associated with smoking cessation interventions and quit attempt rates across 10 community health centers with electronic health records. Am J Public Health. 2015;105(10):2143‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keith DR, Stanton CA, Gaalema DE, et al. Disparities in US healthcare provider screening and advice for cessation across chronic medical conditions and tobacco products. J Gen Intern Med. 2017;32:974‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahluwalia JS, Gibson CA, Kenney RE, Wallace DD, Resnicow K. Smoking status as a vital sign. J Gen Intern Med. 1999;14(7):402‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Milch CE, Edmunson JM, Beshansky JR, Griffith JL, Selker HP. Smoking cessation in primary care: a clinical effectiveness trial of two simple interventions. Prev Med. 2004;38(3):284‐294. [DOI] [PubMed] [Google Scholar]
- 38. Boyle R, Solberg LI. Is making smoking status a vital sign sufficient to increase cessation support actions in clinical practice? Ann Fam Med. 2004;2(1):22‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maizlish NA, Ruland J, Rosinski ME, Hendry K. A systems‐based intervention to promote smoking as a vital sign in patients served by community health centers. Am J Med Qual. 2006;21(3):169‐177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


