Abstract
Objective
To evaluate the long‐term effect of telephone health coaching on health care and long‐term care (LTC) costs in type 2 diabetes (T2D) and coronary artery disease (CAD) patients.
Data Sources/Study Setting
Randomized controlled trial (RCT) data were linked to Finnish national health and social care registries and electronic health records (EHR). Post‐trial eight‐year economic evaluation was conducted.
Study Design
A total of 1,535 patients (≥45 years) were randomized to the intervention (n = 1034) and control groups (n = 501). The intervention group received monthly telephone health coaching for 12 months. Usual health care and LTC were provided for both groups.
Principal Findings
Intention‐to‐treat analysis showed no significant change in total health and long‐term care costs (intervention effect €1248 [3 percent relative reduction], CI −6347 to 2217) in the intervention compared to the control group. There were also no significant changes among subgroups of patients with T2D or CAD.
Conclusions
Health coaching had a nonsignificant effect on health care and long‐term care costs in the 8‐year follow‐up among patients with T2D or CAD. More research is needed to study, which patient groups, at which state of the disease trajectory of T2D and cardiovascular disease, would best benefit from health coaching.
Keywords: coronary artery disease, costs, effectiveness, health coaching, type 2 diabetes
What This Study Adds.
Previous studies have shown mixed results on the cost‐efficiency of telephone‐based health coaching with rather short follow‐ups
In an 8‐year follow‐up of all health care and long‐term care costs, this study found no definitive evidence for cost‐efficiency of health coaching among type 2 diabetes and coronary artery disease patients
Better practices for identifying patients most likely to benefit from health coaching should be developed
1. INTRODUCTION
Chronic diseases contribute to 70‐80 percent of health care costs. Specifically, the care of multimorbidity fails to meet the patients’ complex needs, leading to insufficient care. Often, this leads to acute and unplanned use of health care services, especially in emergency units, and increasing hospitalization in secondary care.
The usual goal of chronic disease management programs is to improve patients’ self‐management skills in increasing treatment adherence, such as keeping appointments with health care professionals and taking prescribed medicines. Improved compliance reduces emergency visits and prevents expensive hospitalization.1, 2 Conventional disease management programs focus on the disease itself, emphasizing coordinated and comprehensive care pathways following evidence‐based clinical guidelines and encouraging patient compliance to treatments, but they focus less on the patient’s individualized needs or behavior.2, 3
Health coaching, a patient‐centered approach aiming to empower patients in comanagement of their disease,4 emphasizes and supports patient autonomy and learning instead of compliance. It is based on shared decision making and collaborative goal setting facilitated by motivational interviewing.5, 6, 7, 8 Based on Hale’s 8 integrative review, health coaching is described throughout the literature as a partnership between the coach and the individual. More specifically, it is “a goal‐oriented, client‐centered partnership that is health‐focused and occurs through a process of client enlightenment and empowerment”.6 Health coaching is usually provided by certified health coaches or health care professionals.6 The role of the coach involves listening, understanding, facilitating, applauding, supporting, motivating, providing feedback, and helping the patient to weigh options, make choices, and identify and overcome challenges in the process of change for better.4 Health coaching guides a learning process for improved disease management; therefore, if successful, it should lead to permanent changes in patient self‐management skills and behavior. These changes in self‐management skills and behavior take time to have an effect on health outcomes,10, 26 and therefore, the impact of health coaching on health care effectiveness and cost‐effectiveness should be assessed in long‐term follow‐ups.
Evidence on the effectiveness of health coaching is conflicting, and it is based on studies with short‐term follow‐up only (up to 24 months).8, 9, 10, 11 Due to heterogeneity of target populations and outcome measures, no systematic reviews with meta‐analyses have been completed.12 Individual studies show either small significant effects or no effects.10 Furthermore, evidence on the cost‐effectiveness of health coaching remains limited: Utilization and cost of health care services has only been evaluated in the short term (usually 12 months), again with mixed outcomes.7, 13, 14, 15, 16, 17, 18 However, due to the nature of the underlying mechanism of change—learning rather than compliance—it might take longer to evidence effects. Therefore, long‐term evaluations of the effectiveness and cost‐effectiveness of health coaching interventions are needed.
The TERVA trial (trial registration: NCT00552903) is a health coaching program that was implemented in the Päijät‐Häme region in Southern Finland and tested as a randomized controlled trial in 2007‐2009. Patients with suboptimally controlled T2D or CAD, including a subgroup of patients with congestive heart failure (CHF), were coached via telephone by trained health coaches during a one‐year intervention period. The aim of the study was to evaluate the total health care and long‐term care (LTC) costs among all participants and in the subgroups (T2D and CAD) for an 8‐year follow‐up of the TERVA trial.
2. METHODS
TERVA was a prospective, longitudinal randomized controlled trial with three disease groups randomized into intervention and control groups. Recruitment of participants from the health care services has been described in detail previously.19 A total of 2,594 patients who fulfilled the eligibility criteria (age 45 years or older, with T2D, CAD or CHF, and unmet treatment targets) were randomized to either the intervention group or the control group with a 2:1 ratio. Of the eligible patients, 1535 (59.2 percent) gave consent: 1,034 in the intervention group and 501 in the control group. There were no significant differences between the groups at baseline.19 Patients with more than one disease were allocated to the highest morbidity disease group using the following hierarchy: 1) CHF, 2) CAD, and 3) T2D. T2D group criteria were medication and serum HbA1c > 7 percent (53 mmol/mol) without clinically evident cardiovascular diseases, for example, MI, stroke, or peripheral vascular disease. In this article, groups 1 and 2 are combined as one, the CAD group.
2.1. Usual care
In Finland, general practitioners and nurses at primary care clinics provide basic medical treatment, follow‐up, and support for compliance. Patients with T2D have 2‐6 planned annual visits to a doctor or nurse, depending on how well the disease is under control. Primary health care wards provide basic care in wards for patient with less severe conditions who are unable to cope at home. Patients with complications are treated for acute needs in secondary care, either at outpatient clinics or as inpatients in hospitals. The CAD patients’ treatment planning is provided in secondary care, in addition to 1‐2 primary care visits per year. Patients in need of LTC receive home‐delivered care, care at service home facilities or nursing homes, or care as inpatients at primary care level. Standards for care are set in the Finnish Current Care Guidelines, which are independent, evidence‐based clinical practice guidelines.20
2.2. Intervention
A detailed description of the health coaching intervention was published earlier.19 In addition to routine care as described above, patients in the intervention group received health coaching by telephone over 12 months.
The intervention included eight key recommendations: 1) know how and when to call for help; 2) learn about the condition and set goals; 3) take medicines correctly; 4) get recommended tests and services; 5) act to keep the condition well controlled; 6) make lifestyle changes and reduce risks; 7) build on strengths and overcome obstacles; and 8) follow‐up with specialists and appointments. Self‐management booklets were sent to patients to support progress toward the key recommendations, and a traffic light system was used to visualize patients’ progress. Health coaches had access to all electronic health records (EHR) in primary and secondary care and could enter patient data into the EHR.
The intervention group was called by the coach 10‐11 times for 12 months. Quality control on the length, frequency, and content of calls was administered. The coaches were tutored individually and in groups throughout the intervention by a psychologist (PA) specializing in lifestyle change and strength‐based behavioral coaching.21 Overall intervention cost per patient was €419 per 12 months.
2.3. Data
Data for the utilization and costs of health care and LTC were collected from the beginning of the intervention (2007) to the eighth year of postintervention follow‐up (2016) from the Finnish national registries maintained by the National Institute for Health and Welfare. In Finland, each citizen has a unique social security code enabling full linkages to the national registries providing comprehensive data about each individual’s use of health care and LTC. Primary care data were collected from the primary health care EHR from 2007 until 2011, after which the EHR were integrated into national registries (AvoHilmo) that provided data for 2012‐2016. Secondary care data included the National Discharge Registry: the use of hospital outpatient care (all types of outpatient visits) and hospital admissions related to diagnosis (diagnosis‐related grouping, DRG). LTC data were collected from Care Registers for Social Welfare, and it includes all types of long‐ and short‐term institutionalized care, housing and residential services, and home care services.
EHR data included structured data for contact types (such as a visit, a phone call, or electronic messaging); the patient’s age; the diagnosis (ICD‐10); the reason for encounter (ICPC‐2); and the employee category of the health care professional in the contact. Extracting the patient‐level data from the patient administration systems (with diagnosis and contact information) made it possible to group each individual encounter type by the Ambulatory and Primary Care Related Patient Groups (APR) grouper, a grouping system equivalent to the DRG used in hospital care.22 The APR groups were supplemented with cost weights indicating the relative consumption of resources. Cost weights were based on large samples of time measurements in primary care contacts and procedures to compile a relative value scale. All costs were deflated using the price index for public health care provided by Statistics Finland.
Hospitalizations and hospital outpatient visit due to any cause were extracted from the Hospital Discharge Register based on the International Classification of Diseases 10th revision (ICD‐10) codes; the Finnish version of the Nordic Classification of Surgical Procedures (NCSP) codes for diagnostic and treatment procedures; and the respective NordDRG patient grouping classifications. The DRG cost weights for hospitalizations and outpatient visits were based on individual‐level cost accounting data from several hospitals. The unit cost estimates for social care encounters and bed‐days were derived from the national price list for unit costs of health care services in Finland.23
2.4. Statistical analysis
Health care and long‐term care costs were assigned to each patient over an 8‐year follow‐up period, and differences in mean costs between research arms were calculated. In the assessment of statistical significance of differences, we used nonparametric bootstrapping. Bootstrapping was used to draw a sample with replacement to calculate 1000 replicates of the mean difference in total costs (difference = mean costs in the intervention group – mean costs in the control group). Stata’s bootstrapped 95% confidence intervals were used to indicate uncertainty in the mean difference estimator. The statistical significance of the difference of mean total costs per patient between the research arms was assessed using bootstrapped t test. Bootstrapping is a common method to account for the non‐normality typical to cost data and for potential dissimilarity in cost distributions of the compared groups.24 Intention‐to‐treat (ITT) strategy was applied, that is, all patients originally allocated to the intervention and control groups were included in the analysis. To assess the effect of the intervention among T2D and CAD patients, subgroup analyses were conducted. In addition to the main ITT analysis, per‐protocol (PP) analyses were conducted excluding those of the randomized patients who did not perform any activities related to the study after giving their consent. The cumulation of cost over time was assessed by drawing cumulative cost curves for each research arm. Statistical analyses were performed using Stata version 15.0.
3. RESULTS
The follow‐up cost data were retrieved for 1033 patients in the intervention and 500 patients in the control group. One patient in each group was missing from the Finnish national registries, probably due to emigration. There were no significant differences in age and gender distribution between the research arms at baseline. The average age of participants was 65 and 65.4 years, and the proportion of females was 406 (39.3 percent) and 207 (41 percent) in the intervention and control groups, respectively. By the end of the eight‐year follow‐up, 26 percent (n = 269) of the patients in the intervention and 28 percent (n = 141) of the patients in the control group had become deceased.
The cumulative cost curves per patient (Figure 1) showed that until a little more than two years after the beginning of the intervention, the cumulative cost was higher in the intervention arm than in the control arm. After this, however, the difference in cumulative cost changed sign, so that the cumulated cost was lower in the intervention arm. The difference grew steadily toward the end of the eight‐year follow‐up. The total costs accumulated per patient were €39 667 in the intervention group and €40 916 in the control group.
Figure 1.
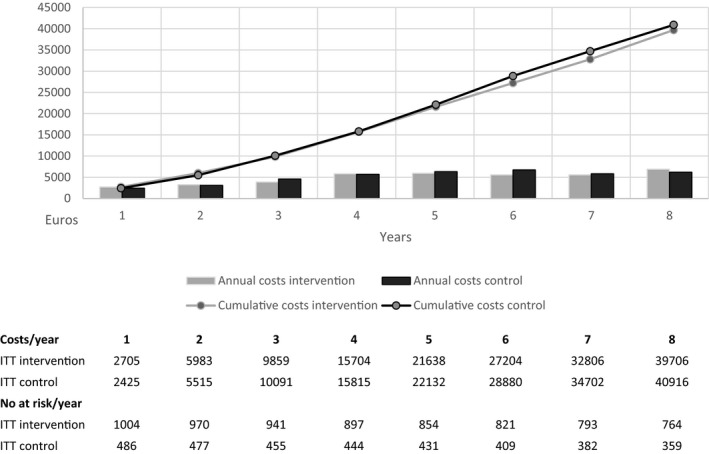
The cumulative and annual health care and LTC (long‐term care) cost per patient over 8 years of follow‐up
Figure 2 shows the mean differences in total cost per patient among all participants and T2D and CAD subgroups. For all participants, the total cost of care was €1248 (3 percent) lower in the intervention group than in the control group. The difference was, however, not statistically significant (95% CI from −6374 to 2217; P = .20). The subgroup analysis among T2D patients showed, in average, 7 percent lower costs (€−3126), while among CAD patients, costs were 10 percent higher (€3543) per patient in the intervention arm. Neither of these effects were, however, statistically significant.
Figure 2.

Mean difference in 8‐year cumulative cost per patient and bootstrapped confidence intervals. Results among all participants and T2D and CAD subgroups [Color figure can be viewed at http://wileyonlinelibrary.com]
To investigate where in the service system the changes in costs accrued, we calculated changes in the eight‐year accumulated cost by different service types: primary care (visits and ward care), secondary care (outpatient and inpatient care), and LTC (home care, service homes, and nursing home). Among both T2D and CAD patients, the analysis revealed lower costs of secondary inpatient care and somewhat higher home care costs in the intervention group. Effects on other service type costs were mixed with mostly savings for T2D patients and increased costs for the CAD patients (Figure 3).
Figure 3.
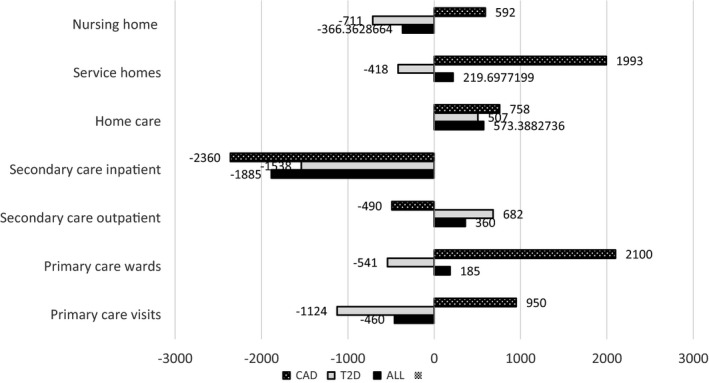
The incremental costs of tele‐based health coaching per patient grouped by service types
In the trial, there were patients in both intervention and control arms, who did not perform any activities related to the study after their consent and allocation to the intervention or control group. These patients were excluded from the PP analysis, resulting in 853 patients in the intervention and 453 patients in the control arm. The proportion of the deceased was 23 percent (=197) and 26 percent (n = 119) in the intervention and control groups, respectively.
In the PP analysis, total costs were €35 863 and €41 816 per patient in the intervention and control groups, respectively. Until a little more than two years after the beginning of the intervention, the cumulative cost was slightly higher in the intervention arm than in the control arm. After this, however, the difference in cumulative cost changed sign to be lower in the intervention arm. The difference grew steadily toward the end of the eight‐year follow‐up (Figure S1). A statistically significant cost saving, €−5953 (14 percent), with a 95 percent bootstrapped confidence interval (CI) from €−9842 to €−1132 and P = .02 was found. PP analysis also showed a statistically significant cost saving of €−7287 (17 percent) per patient due to the intervention in the T2D subgroup (95% CI from €−12 528 to €−1760; P = .02), but no statistically significant effect in the CAD subgroup (Table S2).
4. DISCUSSION
4.1. Principal findings
We studied the eight‐year cumulative health care and LTC costs of patients with T2D and CAD after a randomized controlled trial of a telephone health coaching program. At two years after the beginning of the intervention, the cumulative costs of the control group exceeded those of the intervention group and this difference remained until the end of the eight‐year follow‐up. However, the difference in the total cumulative costs per patient was not statistically significant. The average cost savings were greater in the T2D than in the CAD subgroup, but this result was neither statistically significant. Among both subgroups, cost savings were accrued in the secondary inpatient care, while effects on other health care and LTC costs were mixed.
4.2. Comparison with other studies
To our knowledge, this study has the longest follow‐up of the effects of health coaching on health care and LTC costs reported in the literature. Similarly, to previously reported health coaching interventions,13, 15, 16, 17 this intervention showed no reductions in health care costs in the first 12‐month period.19 As there is a delay from changes in patients’ empowerment, learning, and behavioral changes to changes in physiological outcomes and following use of health services, a long‐term follow‐up of costs over 8 years after the intervention was conducted. In this study, after a little more than 2 years, the cumulative costs in the intervention group were steadily lower than in the control group. However, the difference in the accumulated 8‐year costs was not statistically significant.
Three issues observed in our study may explain why costs in the intervention group were higher during a little more than two years after the beginning of the intervention. First, intervention highlighted the adequate and enough visits to health care for optimizing care and medication. Health coaches prepared patients for visits with health care providers and reflected with patients after the visits—building “a bridge between clinician and patient”.27
This encouragement to collaboration with health care professionals may have at first increased patients’ interest and need to consult their caregiver, and this might explain the increase of primary health care costs in the early stage of the follow‐up. Second, building the health coaching program takes time, and the coaches keep developing their skills over the whole intervention period. In this study, all coaches had worked as nurses before the TERVA health coaching program and then trained to use the coaching methods and other skills required. Adaptation of new skills effectively took at least six months.10 Third, patients were selecting multiple behavioral goals over the entire 12‐month intervention and it must have taken even longer to gradually integrate the changes into their daily lives. With small but sustainable changes, clinical effects are also bound to be delayed.26 This is contrary to studies assigning specific lifestyle goals to participants and implementing strategies for compliance, which may produce large effects at first, but these effects tend to diminish significantly over time.25
4.3. Strengths and limitations
This study has several strengths. RCT design in a real‐life clinical setting allows the strongest evidence of potential effects of an intervention in everyday clinical practice. Use of national registries allowed long‐term follow‐up of all the trial participants. The participants in our study represented two major noncommunicable diseases, T2D and CAD, both among the 10 most frequent causes of mortality in high‐ and middle‐income countries.28 We were able to conduct long‐term follow‐up and include the LTC costs, which to authors’ knowledge have not been reported in any earlier study. LTC, such as residential facilities, cumulates cost over long periods of time and therefore contributes substantially to the total cost of care. There were no simultaneous interventions in the region.
Limitations exist, too. We were not able to blind the participants nor the health care professionals treating the patients. The intervention group was encouraged to be actively engaged in their treatment. This may have influenced the usual care they received. In our experience, some health care personnel perceived the intervention as threatening their areas of expertise, while others found that it added value to their clinical practice.
In this study, we were not able to fully assess patients’ capabilities to participate in the coaching intervention. The inclusion of the participants was solely based on clinical inclusion criteria and EHR review. In both groups, there were patients who gave consent but did not participate in any other activities related to the study, for example, return study questionnaires or participate in the clinical measurements. These patients were found to have deceased earlier than those who performed at least some activities related to the study. Future research should attempt to define inclusion criteria that direct health coaching to those most potential to benefit from such interventions.
The intervention may have been too short for sustained effects to show. In the case studied, the early‐stage observations and analysis on short‐term 1‐year follow‐up showed increased cost in the intervention group, and the regional decision makers terminated the program after the one‐year trial. Finally, the number of recruited patients may have been too small to observe statistically significant differences due to fairly large variation in individual costs. Despite the nonsignificant difference in cost of care, the intervention may still turn out to be preferable if we find marked improvements in long‐term health outcomes.
Despite the steady improvements in diabetes care, approximately 50 percent of patients in Europe and the United States still do not achieve the targets of care.29, 30 While health coaching has been suggested as a feasible means to improve chronic care and avoid expensive complications, evidence of its cost‐efficiency is still lacking. Randomized controlled trials with larger numbers of patients and on interventions more intense or exceeding one year may be needed to show strong evidence for the effect of health coaching. Careful attention must be paid to target the program to suitable patient segments and to execute the health coaching intervention appropriately.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENT
Joint Acknowledgement/Disclosure Statement: Corresponding author (EO) received the special state funding (EVO) grant from Päijät‐Häme Joint Authority for Health and Wellbeing. Finnish Innovation Fund (Sitra) and Pfizer Oy support our study for data acquisition and data analyses fees. Academy of Finland (IMPRO project, 312703) supported the authors (IH and ML) in this study.
Mustonen E, Hörhammer I, Absetz P, et al. Eight‐year post‐trial follow‐up of health care and long‐term care costs of tele‐based health coaching. Health Serv Res. 2020;55:211–217. 10.1111/1475-6773.13251
Trial registration: NCT00552903 (prospectively registered, registration date 1 November 2007, last updated 3 February 2009).
REFERENCES
- 1. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Affairs (Millwood). 2001;20(6):64‐78. [DOI] [PubMed] [Google Scholar]
- 2. Mattke S, Seid M, Ma S. Evidence for the effect of disease management: Is $1 Billion a year a good investment? Am J Managed Care. 2007;13(12):670‐676. [PubMed] [Google Scholar]
- 3. Ellrodt G, Cook DJ, Lee J, et al. Evidence‐based disease management. JAMA. 1997;278(20):1687‐1692 [PubMed] [Google Scholar]
- 4. Hayes E, MacCahon C, Panahi MR, Hamre T, Pohlman K. Alliance not compliance: coaching strategies to improve type 2 diabetes outcomes. J Am Acad Nurse Pract. 2008;20:155‐162. [DOI] [PubMed] [Google Scholar]
- 5. Palmer S, Tubbs I, Whybrow A. Health coaching to facilitate promotion of health behaviour and achievement of health‐related goals. Int J Health Promotion Educ. 2013;41:91‐93. [Google Scholar]
- 6. Olsen JM. Health Coaching: a concept analysis. Nurs Forum. 2014;49(1):18‐29. 10.1111/nuf.12042. [DOI] [PubMed] [Google Scholar]
- 7. Härter M, Dirmaier J, Dwinger S, et al. Effectiveness of telephone‐based health coaching for patients with chronic conditions: A randomized controlled trial. PLoS ONE. 2016;11(9):e0161269 10.1371/journal.pone.0161269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hale R, Giese J. Cost‐effectiveness of health coaching. Prof Case Manag. 2017;22(5):228‐238. [DOI] [PubMed] [Google Scholar]
- 9. Dennis S, Harris M, Lloyd J, et al. Do people with existing chronic conditions benefit from health coaching? A rapid review. Aust Health Rev. 2013;37:381‐388. 10.1071/AH13005. [DOI] [PubMed] [Google Scholar]
- 10. Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Educ Couns. 2014;97:147‐157. [DOI] [PubMed] [Google Scholar]
- 11. Tiede M, Dwinger S, Herbart L, Härter M, Dirmaier J. Long‐term effectiveness of telephone‐based health coaching for heart failure patients: A post‐only randomized controlled trial (Article). J Tele Telecare. 2017;23(8):716‐724. [DOI] [PubMed] [Google Scholar]
- 12. Boehmer KR, Barakat S, Sangwoo A, Prokop LJ, Erwin PJ, Murad HM. Health coaching interventions for persons with chronic conditions: a systematic review and meta‐analysis protocol. Sys Rev. 2016;5:146 10.1186/s13643-016-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steventon A, Tunkel S, Blunt I, Bardsley M. Effect of telephone health coaching (Birmingham OwnHealth) on hospital use and associated costs: cohort study with matched controls. BMJ. 2013;347:f4585 10.1136/bmj.f4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonk Y, Lawson K, O’Connor H, et al. How effective is health coaching in reducing health services expenditure? Med Care. 2015;53(2):133‐140. [DOI] [PubMed] [Google Scholar]
- 15. Billot L, Corcoran K, MacDonald A, Powell‐Davies G, Freyer A‐M. Impact evaluation of system‐wide chronic disease management program on health service. Utilization: A Propensity‐Matched Cohort Study. PLoS Med. 2016; 13(6):e1002035 10.1371/jornal.pmed.1002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morello R, Barker A, Watts J, Bohensky M, Forbes A, Stoelwinder J. A telephone support program to reduce costs and hospital admissions for patients at risk of readmission: Lessons from an evaluation of a complex intervention. Population Health Manage. 2016;19(3):187‐195. [DOI] [PubMed] [Google Scholar]
- 17. Wagner T, Willard‐Grace R, Chen E, Bodenheimer T, Thom D. Costs of a health coaching intervention for chronic care management. Am J Managed Care. 2016;22(4):e141‐e146. [PubMed] [Google Scholar]
- 18. Oksman E, Linna M, Hörhammer I, Lammintakanen J, Talja M. Cost‐effectiveness analysis for a tele‐based health coaching program for chronic disease in primary care. BMC Health Services Res. 2017;17:138 https://doi.org/10.11867s12913-017-2088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patja K, Absetz P, Auvinen A, et al. Health coaching by telephony to support self‐care in chronic diseases: clinical outcomes from The TERVA randomized controlled trial. BMC Health Service Res. 2012;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Finnish Current Care Guidelines . http://www.kaypahoito.fi/web/english/home.
- 21. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008. [Google Scholar]
- 22. Honkasalo M, Linna M, Sane T, et al. A comparative study of two various models of organizing diabetes follow‐up in public primary health care – the model influences the use of services, their quality and costs. BMC Health Services Res. 2014;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapiainen S, Väisänen A, Haula T. Terveyden‐ ja sosiaalihuollon yksikkökustannukset Suomessa. 2011. http://urn.fi/URN:ISBN:978-952-302-079-5
- 24. Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non‐parametric bootstrap. Statist Med. 2000;19:3219‐3236. [DOI] [PubMed] [Google Scholar]
- 25. The Look AHEAD Research Group . Cardiovascular effects of Intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145‐154. 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Absetz P, Oldenburg B, Hankonen N, et al. Type 2 diabetes prevention in the “real world”: Three‐year results of the GOAL implementation trial. Diabetes Care. 2009;32(8):1418‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bennet H, Coleman E, Parry C, Bodenheimer T, Chen E. Health Coaching for Patients. Does your practice “give patients a fish” or “teach patients to fish”? Family Practice Management. 2010. http://www.aafp.org/fpm/20100900/p24. [PubMed]
- 28. World Health Organization . 2015. http://www.who.int/mediacentre/factsheets/fs310/en/index1.html.
- 29. Ali MK, Bullard KM, Saaddine JB, et al. Achievements of goal in U.S. diabetes care 1999–2010. N Engl J Med. 2013;368(17):1613‐1624. [DOI] [PubMed] [Google Scholar]
- 30. Stone M, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European countries. Diabetes Care. 2013;36(9):2628‐2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


