Abstract
Background
At birth, infants' lungs are fluid‐filled. For newborns to have a successful transition, this fluid must be replaced by air to enable gas exchange. Some infants are judged to have inadequate breathing at birth and are resuscitated with positive pressure ventilation (PPV). Giving prolonged (sustained) inflations at the start of PPV may help clear lung fluid and establish gas volume within the lungs.
Objectives
To assess the benefits and harms of an initial sustained lung inflation (SLI) (> 1 second duration) versus standard inflations (≤ 1 second) in newborn infants receiving resuscitation with intermittent PPV.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 3), MEDLINE via PubMed (1966 to 1 April 2019), Embase (1980 to 1 April 2019), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 1 April 2019). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles to identify randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs comparing initial sustained lung inflation (SLI) versus standard inflations given to infants receiving resuscitation with PPV at birth.
Data collection and analysis
We assessed the methodological quality of included trials using Cochrane Effective Practice and Organisation of Care Group (EPOC) criteria (assessing randomisation, blinding, loss to follow‐up, and handling of outcome data). We evaluated treatment effects using a fixed‐effect model with risk ratio (RR) for categorical data; and mean standard deviation (SD), and weighted mean difference (WMD) for continuous data. We used the GRADE approach to assess the quality of evidence.
Main results
Ten trials enrolling 1467 infants met our inclusion criteria. Investigators in nine trials (1458 infants) administered sustained inflation with no chest compressions. Use of sustained inflation had no impact on the primary outcomes of this review: mortality in the delivery room (typical RR 2.66, 95% confidence interval (CI) 0.11 to 63.40 (I² not applicable); typical RD 0.00, 95% CI −0.02 to 0.02; I² = 0%; 5 studies, 479 participants); and mortality during hospitalisation (typical RR 1.09, 95% CI 0.83 to 1.43; I² = 42%; typical RD 0.01, 95% CI −0.02 to 0.04; I² = 24%; 9 studies, 1458 participants). The quality of the evidence was low for death in the delivery room because of limitations in study design and imprecision of estimates (only one death was recorded across studies). For death before discharge the quality was moderate: with longer follow‐up there were more deaths (n = 143) but limitations in study design remained. Among secondary outcomes, duration of mechanical ventilation was shorter in the SLI group (mean difference (MD) −5.37 days, 95% CI −6.31 to −4.43; I² = 95%; 5 studies, 524 participants; low‐quality evidence). Heterogeneity, statistical significance, and magnitude of effects of this outcome are largely influenced by a single study at high risk of bias: when this study was removed from the analysis, the size of the effect was reduced (MD −1.71 days, 95% CI −3.04 to −0.39; I² = 0%). Results revealed no differences in any of the other secondary outcomes (e.g. risk of endotracheal intubation outside the delivery room by 72 hours of age (typical RR 0.91, 95% CI 0.79 to 1.04; I² = 65%; 5 studies, 811 participants); risk of surfactant administration during hospital admission (typical RR 0.99, 95% CI 0.91 to 1.08; I² = 0%; 9 studies, 1458 participants); risk of chronic lung disease (typical RR 0.99, 95% CI 0.83 to 1.18; I² = 0%; 4 studies, 735 participants); pneumothorax (typical RR 0.89, 95% CI 0.57 to 1.40; I² = 34%; 8 studies, 1377 infants); or risk of patent ductus arteriosus requiring pharmacological treatment (typical RR 0.99, 95% CI 0.87 to 1.12; I² = 48%; 7 studies, 1127 infants). The quality of evidence for these secondary outcomes was moderate (limitations in study design ‒ GRADE) except for pneumothorax (low quality: limitations in study design and imprecision of estimates ‒ GRADE). We could not perform any meta‐analysis in the comparison of the use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with chest compressions because we identified only one trial for inclusion (a pilot study of nine preterm infants).
Authors' conclusions
Our meta‐analysis of nine studies shows that sustained lung inflation without chest compression was not better than intermittent ventilation for reducing mortality in the delivery room (low‐quality evidence ‒ GRADE) or during hospitalisation (moderate‐quality evidence ‒ GRADE), which were the primary outcomes of this review. However, the single largest study, which was well conducted and had the greatest number of enrolled infants, was stopped early for higher mortality rate in the sustained inflation group. When considering secondary outcomes, such as rate of intubation, rate or duration of respiratory support, or bronchopulmonary dysplasia, we found no benefit of sustained inflation over intermittent ventilation (moderate‐quality evidence ‒ GRADE). Duration of mechanical ventilation was shortened in the SLI group (low‐quality evidence ‒ GRADE); this result should be interpreted cautiously, however, as it might have been influenced by study characteristics other than the intervention. There is no evidence to support the use of sustained inflation based on evidence from our review.
Plain language summary
Prolonged lung inflation for resuscitation of babies at birth
Review question
Does the use of prolonged (or sustained) lung inflation (> 1 second duration) rather than standard inflations (≤ 1 second) improve survival and other important outcomes among newly born babies receiving resuscitation at birth?
Background
At birth, the lungs are filled with fluid which must be replaced by air for babies to breathe properly. Some babies have difficulty establishing effective breathing at birth, and one in every 20 to 30 babies receives help to do so. A variety of devices are used to help babies begin normal breathing. Some of these devices allow caregivers to give long (or sustained) inflations. These sustained inflations may help inflate the lungs and may keep the lungs inflated better than if they are not used.
Study characteristics
We collected and analysed all relevant studies to answer the review question and found 10 studies enrolling 1467 infants. In all studies, babies were born before the due date (from 23 to 36 weeks of gestational age). The sustained inflation lasted between 15 and 20 seconds at pressure between 20 and 30 cmH₂O. Most studies provided one or more additional sustained inflations in cases of poor clinical response, for example persistent low heart rate. We analysed one study (which included only nine babies) separately because researchers combined use of sustained or standard inflations with chest compressions, an additional intervention that might help babies begin normal breathing.
Key results
The included studies showed no important differences among babies who received sustained versus standard inflations in terms of mortality, rate of intubation during the first three days of life, or chronic lung disease. Babies receiving sustained inflation at birth may spend fewer days on mechanical ventilation. The results of several ongoing studies might help us to determine whether sustained inflations are beneficial or harmful. At present we cannot exclude small to moderate differences between the two treatments.
Quality of evidence
The quality of evidence is low to moderate because only a small number of studies have looked at this intervention, few babies were included in these studies and some studies could have been better designed.
How up to date is this review?
We searched for studies that had been published up to April 2019.
Summary of findings
Background
Description of the condition
At birth, infants' lungs are filled with fluid which must be cleared for effective respiration to occur. Most newly born infants achieve this spontaneously and may use considerable negative pressure (up to −50 cmH₂O) for initial inspirations (Karlberg 1962; Milner 1977). However, it is estimated that 3% to 5% of newly born infants receive some help to breathe at delivery (Saugstad 1998). Adequate ventilation is the key to successful neonatal resuscitation and stabilisation (Wyckoff 2015). Positive pressure ventilation (PPV) is recommended for infants who have absent or inadequate respiratory efforts or bradycardia (or both) at birth (Wyckoff 2015). Use of manual ventilation devices — self‐inflating bags, flow‐inflating (or anaesthetic) bags, and T‐piece devices — with a face mask or endotracheal tube (ETT) is advised. Although it is not included in the International Liaison Committee on Resuscitation (ILCOR) guidelines, respiratory support of infants in the delivery room with a mechanical ventilator and a nasopharyngeal tube has been described (Lindner 1999).
Description of the intervention
Devices recommended for PPV in the delivery room differ in terms of physical characteristics and ability to deliver sustained lung inflation (SLI). The most commonly used self‐inflating bag may be of insufficient size to support sustained inflation (> 1 second) (O'Donnell 2004a; O'Donnell 2004b). Both flow‐inflating bags and T‐pieces may be used to consistently deliver inflations of more than one second. In addition, many of the self‐inflating bags are unsatisfactory at delivering an appropriate volume mainly because of serious leaks in the valves of the bags (Tracy 2019). Although target inflation pressures and long inspiratory times are achieved more consistently in mechanical models when T‐piece devices rather than bags are used, no recommendation can be made as to which device is preferable (Wyckoff 2015; Wyllie 2015). Positive end‐expiratory pressure (PEEP) is very important for aerating the lungs and improving oxygenation; SLI consists of prolonged high‐level PEEP or, more precisely, a prolonged peak inflation pressure.
How the intervention might work
When airways are filled with liquid, it might be unnecessary to interrupt inflation pressures to allow the lung to deflate and exhale CO₂ (Hooper 2016). Boon 1979 described a study of 20 term infants delivered by Caesarean section under general anaesthesia who were resuscitated with a T‐piece via an ETT. Study authors reported that gas continued to flow through the flow sensor placed between the T‐piece and the ETT toward the infant at the end of a standard inflation of one second on respiratory traces obtained (Boon 1979). On the basis of this observation, this group performed a non‐randomised trial of sustained inflations given via a T‐piece and an ETT to nine term infants during delivery room resuscitation. Investigators reported that initial inflation with a T‐piece lasting five seconds produced a two‐fold increase in inflation volume compared with standard resuscitation techniques (Vyas 1981). Citing these findings, a retrospective cohort study described the effects of a change in management strategy for extremely low birth weight infants in the delivery room (Lindner 1999). The new management strategy included the introduction of an initial sustained inflation of 15 seconds obtained with a mechanical ventilator via a nasopharyngeal tube. This change in strategy was associated with a reduction in the proportion of infants intubated for ongoing respiratory support without an apparent increase in adverse outcomes. Pulmonary morbidity in very low birth weight infants was reported to be related directly to mortality in 50% of cases of death (Drew 1982). Moreover, multiple SLIs in very preterm infants improved both heart rate and cerebral tissue oxygen saturation, in the absence of any detrimental effects (Fuchs 2011). An observational study showed that sustained inflation of 10 seconds at 25 cmH₂O in 70 very preterm infants at birth was not effective for infants who were not breathing, possibly owing to active glottic adduction (van Vonderen 2014). Newly born infants frequently take a breath and then prolong expiration via glottic closure and diaphragmatic braking, giving themselves prolonged end‐expiratory pressure.
Why it is important to do this review
Recommendations regarding use of sustained inflation at birth have varied between international bodies. Although European Resuscitation Council guidelines suggest giving five inflation breaths if the newborn is gasping or is not breathing (Wyllie 2015), the American Heart Association states that evidence is insufficient to recommend an optimum inflation time (Wyckoff 2015). Differences between these guidelines and their algorithms are intriguing (Klingenberg 2016). A narrative review reported that sustained inflation may reduce the need for mechanical ventilation among preterm infants at risk for respiratory distress syndrome (RDS) (Lista 2010). The same review showed that respiratory outcomes among infants receiving sustained inflation (25 cmH₂O for 15 seconds) were improved over those reported for an historical group (Lista 2011).
Our previous review 'Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes' included eight trials enrolling 941 (Bruschettini 2017). Sustained inflation was not better than intermittent ventilation for reducing mortality, need for intubation, need for or duration of respiratory support, or bronchopulmonary dysplasia. The quality of evidence for these outcomes was low to moderate. This version updated the previous review which was published in the Cochrane Database of Systematic Reviews in 2017 (Bruschettini 2017).
Objectives
To assess the benefits and harms of an initial sustained lung inflation (SLI) (> 1 second duration) versus standard inflations (≤ 1 second) in newborn infants receiving resuscitation with intermittent PPV.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs. We excluded observational studies (case‐control studies, case series) and cluster‐RCTs.
Types of participants
Term and preterm infants resuscitated via PPV at birth.
Types of interventions
Interventions included resuscitation with initial sustained (> 1 second) inflation versus resuscitation with regular (≤ 1 second) inflations:
with no chest compressions as part of the initial resuscitation; or
with chest compressions as part of the initial resuscitation.
Types of outcome measures
Primary outcomes
Death in the delivery room
Death during hospitalisation
Death to latest follow‐up
Secondary outcomes
Heart rate at 5 minutes
Endotracheal intubation in the delivery room
Endotracheal intubation in the first 72 hours of age
Surfactant administration in the delivery room or during hospital admission
Mechanical ventilation (yes/no)
Duration in hours of respiratory support (i.e. nasal continuous airway pressure and ventilation via an ETT considered separately and in total)
Duration in days of supplemental oxygen requirement
Chronic lung disease: rate of supplemental oxygen at 28 days of age; rate of supplemental oxygen at 36 weeks' postmenstrual age for infants born at or before 32 weeks of gestation
Air leaks (pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema) reported individually or as a composite outcome
Cranial ultrasound abnormalities: any intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978), and cystic periventricular leukomalacia
Seizures including clinical and electroencephalographic
Hypoxic‐ischaemic encephalopathy for term and late preterm infants (grade 1 to 3 (Sarnat 1976))
Long‐term neurodevelopmental outcomes (rates of cerebral palsy on physician assessment, developmental delay (i.e. intelligence quotient (IQ) 2 standard deviations (SDs) < mean on validated assessment tool (e.g. Bayley's Mental Developmental Index))
Retinopathy of prematurity (ROP) (all stages and ≥ stage 3)
Patent ductus arteriosus (PDA) (pharmacological treatment and surgical ligation)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register).
Electronic searches
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 3) in the Cochrane Library; MEDLINE via PubMed (1966 to 1 April 2019); Embase (1980 to 1 April 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 1 April 2019). See Appendix 1 for full search strategy for each database. We did not apply language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (ClinicalTrials.gov; the World Health Organization International Trials Registry and Platform ‒ www.whoint/ictrp/search/en; and the ISRCTN Registry).
Searching other resources
We also searched abstracts of the Pediatric Academic Society (PAS) from 2000 to 2019, electronically through the PAS website, using the following key words: "sustained inflation" AND "clinical trial".
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
For this update, two review authors (MB, MGC) independently screened all titles and abstracts to determine which trials met the inclusion criteria. We retrieved full‐text copies of all papers that were potentially relevant. We resolved disagreements by discussion between review authors.
Data extraction and management
Two review authors (MB, MGC) independently undertook data abstraction using a data extraction form developed ad hoc and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group (EPOC) data collection checklist (EPOC 2015).
We extracted the following characteristics from each included trial.
Administrative details: study author(s); published or unpublished; year of publication; year in which trial was conducted; details of other relevant papers cited.
Trial details: study design; type, duration, and completeness of follow‐up; country and location of study; informed consent; ethics approval.
Details of participants: birth weight; gestational age; number of participants.
Details of intervention: type of ventilation device used; type of interface; duration and level of pressure of sustained lung inflation (SLI).
Details of outcomes: death during hospitalisation or to latest follow‐up; heart rate at 5 minutes; duration in hours of respiratory support; duration in days of supplemental oxygen requirement; long‐term neurodevelopmental outcomes; any adverse events.
We resolved disagreements by discussion between review authors. When available, we described ongoing trials identified by detailing primary trial author, research question(s) posed, and methods and outcome measures applied, together with an estimate of the reporting date.
When queries arose or additional data were required, we contacted trial authors.
Assessment of risk of bias in included studies
Two review authors (MB, MGC) independently assessed risk of bias (low, high, or unclear) of all included trials for the following domains using the Cochrane ‘Risk of bias’ tool (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or via consultation with a third assessor. See Appendix 2 for a detailed description of risk of bias for each domain.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included trial, we categorised risk of bias regarding random sequence generation as follows.
Low risk ‒ adequate (any truly random process, e.g. random number table; computer random number generator).
High risk ‒ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number).
Unclear risk ‒ no or unclear information provided.
Allocation concealment
For each included trial, we categorised risk of bias regarding allocation concealment as follows.
Low risk ‒ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes).
High risk ‒ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth).
Unclear risk ‒ no or unclear information provided.
Performance bias
Owing to the nature of the intervention, all trials were unblinded, leading to high risk of performance bias.
Detection bias
For each included trial, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or different classes of outcomes.
Attrition bias
For each included trial and for each outcome, we described completeness of data including attrition and exclusions from analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes.
Reporting bias
For each included trial, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed methods as follows.
Low risk ‒ adequate (when it is clear that all of a trial's prespecified outcomes and all expected outcomes of interest to the review have been reported).
High risk ‒ inadequate (when not all of a trial's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; or the trial failed to include results of a key outcome that would have been expected to be reported).
Unclear risk ‒ no or unclear information provided (study protocol was not available).
Other bias
For each included trial, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific trial design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each trial was free of other problems that could put it at risk of bias as follows.
Low risk ‒ no concerns of other bias raised.
High risk ‒ concerns raised about multiple looks at data with results made known to investigators, differences in numbers of participants enrolled in abstract, and final publications of the paper.
Unclear ‒ concerns raised about potential sources of bias that could not be verified by contacting trial authors.
We did not score blinding of the intervention because this was not applicable.
One review author entered data into RevMan Web, and a second review author checked entered data for accuracy.
Measures of treatment effect
We conducted measures of treatment effect data analysis using RevMan Web. We determined outcome measures for dichotomous data (e.g. death, endotracheal intubation in the delivery room, frequency of retinopathy) as risk ratios (RRs) with 95% confidence intervals (CIs). We calculated continuous data (e.g. duration of respiratory support, Apgar score) using mean differences (MDs) and SDs.
Unit of analysis issues
The unit of randomisation was the intended unit of analysis (individual neonate).
Dealing with missing data
We contacted trial authors to request missing data when needed.
Assessment of heterogeneity
As a measure of consistency, we used the I² statistic and the Q (Chi²) test (Deeks 2011). We judged statistical significance of the Q (Chi²) statistic by P < 0.10 because of the low statistical power of the test. We used the following cut‐offs for heterogeneity: less than 25% no heterogeneity; 25% to 49% low heterogeneity; 50% to 74% moderate heterogeneity; and ≥ 75% high heterogeneity (Higgins 2003). We combined trial results using the fixed‐effect model, regardless of statistical evidence of heterogeneity effect sizes.
Assessment of reporting biases
See Appendix 2.
Data synthesis
We performed statistical analyses using RevMan Web. We used the standard methods of the Cochrane Neonatal Review Group. For categorical data, we used RRs, relative risk reductions, and absolute risk difference (RDs). We obtained means and SDs for continuous data and performed analyses using MDs and WMDs when appropriate. We calculated 95% CIs. We presented the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), as appropriate. For each comparison reviewed, meta‐analysis could be feasible if we identified more than one eligible trial, and if homogeneity among trials was sufficient with respect to participants and interventions. We combined trials using the fixed‐effect model, regardless of statistical evidence of heterogeneity effect sizes. For estimates of RR and RD, we used the Mantel‐Haenszel method.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses of the safety and efficacy of sustained inflation during resuscitation in subgroups.
Term (≥ 37 weeks of gestation) and preterm (< 37 weeks of gestation) infants.
Type of ventilation device used (self‐inflating bag, flow‐inflating bag, T‐piece, mechanical ventilator).
Interface used (i.e. face mask, ETT, nasopharyngeal tube).
Duration of sustained lung inflation (i.e. > 1 second to 5 seconds, > 5 seconds).
Sensitivity analysis
We planned to conduct sensitivity analyses to explore effects of the methodological quality of trials and check to ascertain whether studies with high risk of bias overestimated treatment effects.
Summary of findings and assessment of the certainty of the evidence
We used GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: death in the delivery room or during hospitalisation; endotracheal intubation in the delivery room or outside the delivery room during hospitalisation; surfactant administration in the delivery room or during hospital admission; rate of mechanical ventilation; chronic lung disease; air leaks; and cranial ultrasound abnormalities.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1).
1.
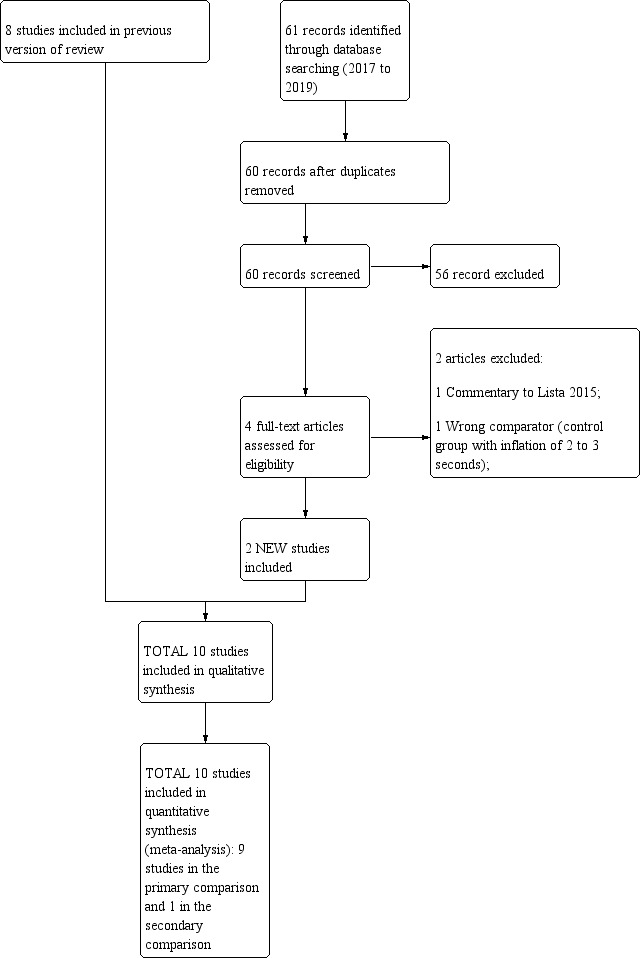
Study flow diagram: review update.
Results of the search
See Table 1, Table 2, Characteristics of included studies,Characteristics of excluded studies, and Characteristics of ongoing studies sections for details.
Summary of findings for the main comparison. Use of initial sustained inflation compared to standard inflations in newborns receiving resuscitation with no chest compressions for.
| Use of initial sustained inflation compared to standard inflations in newborns receiving resuscitation with no chest compressions during resuscitation | ||||||
| Population: preterm infants resuscitated by PPV at birth Settings: delivery room in Europe (Austria, Germany, Italy, the Netherlands), Canada, Egypt, Thailand, USA, Australia, South Korea, and Singapore Intervention: use of initial sustained inflation in newborns receiving resuscitation with no chest compressions Comparison: standard inflations in newborns receiving resuscitation with no chest compressions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard inflations in newborns receiving resuscitation with no chest compressions | Use of initial sustained inflation | |||||
| Death ‒in the delivery room | Study population | RR 2.66 (0.11 to 63.4) | 479 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Death ‒before discharge | Study population | RR 1.09 (0.83 to 1.43) | 1458 (9 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 112 per 1000 | 122 per 1000 (93 to 160) | |||||
| Medium risk population | ||||||
| 58 per 1000 | 63 per 1000 (48 to 83) | |||||
| Rate of mechanical ventilation | Study population | RR 0.89 (0.77 to 1.02) | 910 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 461 per 1000 | 410 per 1000 (355 to 470) | |||||
| Medium risk population | ||||||
| 439 per 1000 | 391 per 1000 (338 to 448) | |||||
| Chronic lung disease ‒any grade | Study population | RR 0.98 (0.84 to 1.13) | 1418 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 340 per 1000 | 333 per 1000 (286 to 384) | |||||
| Medium risk population | ||||||
| 211 per 1000 | 207 per 1000 (177 to 238) | |||||
| Chronic lung disease ‒moderate to severe BPD | Study population | RR 0.95 (0.74 to 1.22) | 683 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 257 per 1000 | 244 per 1000 (190 to 314) | |||||
| Medium risk population | ||||||
| 211 per 1000 | 200 per 1000 (156 to 257) | |||||
| Pneumothorax | Study population | RR 0.89 (0.57 to 1.39) | 1458 (9 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 52 per 1000 | 46 per 1000 (30 to 72) | |||||
| Medium risk population | ||||||
| 50 per 1000 | 44 per 1000 (28 to 69) | |||||
| Cranial ultrasound abnormalities ‒Intraventricular haemorrhage grade 3 to 4 | Study population | RR 0.85 (0.56 to 1.28) | 735 (6 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 116 per 1000 | 99 per 1000 (65 to 148) | |||||
| Medium risk population | ||||||
| 54 per 1000 | 46 per 1000 (30 to 69) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 all studies at high or unclear risk of bias in at least one domain (lack of blinding) and 1 study stopped early 2 few events
Summary of findings 2. Use of initial sustained inflation for.
| Use of initial sustained inflation for | ||||||
| Population: infants below 33 weeks of postmenstrual age who required resuscitation in the delivery room Settings: delivery room in Canada Intervention: use of initial sustained inflation in newborns receiving resuscitation with chest compressions Comparison: standard inflations in newborns receiving resuscitation with chest compressions | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Use of initial sustained inflation | |||||
| Death ‒in the delivery room | See comment | See comment | not reported | 9 (1 study) | The included study did not report on this outcome | |
| Death ‒before discharge | Study population | RR 4.17 (0.25 to 68.16) | 9 (1 study) | ⊝⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Rate of mechanical ventilation | See comment | See comment | not reported | 9 (1 study) | The included study did not report on this outcome | |
| Chronic lung disease ‒any grade | See comment | See comment | not reported | 9 (1 study) | The included study did not report on this outcome | |
| Chronic lung disease ‒moderate to severe BPD | Study population | RR 0.89 (0.33 to 2.37) | 7 (1 study) | ⊝⊝⊝⊝ very low1,2 | ||
| 750 per 1000 | 668 per 1000 (248 to 1000) | |||||
| Medium risk population | ||||||
| 750 per 1000 | 668 per 1000 (248 to 1000) | |||||
| Pneumothorax ‒at any time | See comment | See comment | Not estimable | 9 (1 study) | ⊝⊝⊝⊝ very low1,2 | No events |
| Cranial ultrasound abnormalities ‒intraventricular haemorrhage grade 3 to 4 | Study population | RR 0.4 (0.05 to 2.98) | 9 (1 study) | ⊝⊝⊝⊝ very low1,2 | ||
| 500 per 1000 | 200 per 1000 (25 to 1000) | |||||
| Medium risk population | ||||||
| 500 per 1000 | 200 per 1000 (25 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Limitations in study design: downgraded by 1 level due to included study at high or unclear risk of bias in 4 domains 2 Imprecision: downgraded by 2 levels due to extremely low sample size, few events
Included studies
Ten trials that recruited 1467 infants (768 in SLI groups, 699 in control groups) met the inclusion criteria (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2018; Schwaberger 2015). We pooled nine trials (with 1458 infants) in the comparison of the use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with no chest compressions (Comparison 1) (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015; see Table 1). In contrast to other trials, Schwaberger 2015 sought to use near‐infrared spectroscopy (NIRS) to investigate whether SLI affected physiological changes in cerebral blood volume and oxygenation.
We could not perform any meta‐analysis in the comparison of the use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with chest compressions because we identified only one trial for inclusion (a pilot study of 9 preterm infants) (Schmölzer 2018, see Table 2).
We have listed characteristics of populations and interventions and comparisons of the 10 trials under Characteristics of included studies and in Table 3.
1. Populations and interventions in included trials.
|
Trial (no. infants) |
Antenatal steroids | Gestational age, weeks | Birth weight, grams | Device/Interface | Interventions/Controls | ||||
| SLI | Control | SLI | Control | SLI | Control | SLI and control | SLI | Control | |
| Abd 2017 (100) | 70% to 80% | 55% | 29.3 to 29.7 | 29.4 (SD 2.1) | 1363 to 1367 | mean 1249 (SD 363) | T‐piece | 4 different arms: PIP of either 15 or 20 cmH₂O for either 10 or 20 seconds | PEEP 5 cmH₂O, oxygen 30% |
| El‐Chimi 2017 (112) | 39% | 34.5% | mean 31.1 (SD 1.7) | mean 31.3 (SD 1.7) | mean 1561 (SD 326) | mean 1510 (SD 319) | Mask and T‐piece in SLI group Mask and self‐inflating bag with an oxygen reservoir in control group |
PIP of 20 cmH₂O for 15 seconds, followed by PEEP of 5 cmH₂O If needed: a second SLI of 15 seconds of 25 cmH₂O for 15 seconds, followed by PEEP of 6 cmH₂O; then a third SLI of 15 seconds of 30 cmH₂O for 15 seconds, followed by PEEP of 7 cmH₂O If still not satisfactory: intubated in delivery room |
PIP maximum 40 cmH₂O, rate of 40 to 60 breaths/min for 30 seconds |
| Jiravisitkul 2017 (81) | 63% | 74% | 25 to 28 weeks:
n = 17; 29 to 32 weeks: n = 26 |
25 to 28 weeks:
n = 16; 29 to 32 weeks: n = 22 |
mean 1206 (SD 367) | mean 1160 (SD 411) | Mask and T‐piece | PIP of 25 cmH₂O for 15 seconds If HR 60 to 100 beats/min and/or poor respiratory effort: a second SLI (25 cmH₂O, 15 seconds) |
PIP 15 to 20 cmH₂O, PEEP 5 cmH₂O for 30 seconds, followed by resuscitation according to AHA guidelines |
| Kirpalani 2019 (426) | 97% | 97% | 23 to 24 weeks: n = 76; 25 to 26 weeks: n = 139 |
23 to 24 weeks: n = 75; 25 to 26 weeks: n = 136 |
median 725 (IQR 620 to 855) | median 731 (IQR 630 to 854) | Either mask or a nasopharyngeal tube (as unit protocol dictated) and T‐piece resuscitator | PIP of 20 cmH₂O for 15 seconds. If needed: a second SLI of 15 seconds of 25 cmH₂O |
PIP with PEEP |
| Lindner 2005 (61) | 81% | 80% | median 27.0 (IQR 25.0 to 28.9) | median 26.7 (IQR 25.0 to 28.9) | median 870 (IQR 410 to 1320) | median 830 (IQR 370 to 1370) | Nasopharyngeal tube (fixed at 4 to 5 cm) and mechanical ventilator | PIP of 20 cmH₂O for 15 seconds If response was not satisfactory: 2 further SLIs of 15 seconds (25 and 30 cmH₂O). Then PEEP at 4 to 6 cmH₂O |
PIP 20 cmH₂O, PEEP 4 to 6 cmH₂O; inflation time 0.5 seconds; inflation rate 60 per min. Then, PEEP at 4 to 6 cmH₂O |
| Lista 2015 (301) | 87% | 91% | mean 26.8 (SD 1.2); 25 to 26 weeks: n = 55 27 to 28 weeks: n = 88 |
mean 26.8 (SD 1.1); 25 to 26 weeks: n = 52; 27 to 28 weeks: n = 96 |
mean 894 (SD 247) | mean 893 (SD 241) | Mask and T‐piece | PIP 25 cmH₂O for 15 seconds. Then reduced to PEEP of 5 cmH₂O | PEEP 5 cmH₂O, followed by resuscitation according to AHA guidelines |
| Mercadante 2016 (185) | 40% | 32% | mean 35.2 (SD 0.8) | mean 35.2 (SD 0.8) | mean 2345 (SD 397) | mean 2346 (SD 359) | Mask and T‐piece | PIP 25 cmH₂O for 15 seconds, followed by PEEP of 5 cmH₂O. In case of persistent heart failure (HR < 100 bpm): SLI repeated | PEEP 5 cmH₂O, followed by resuscitation according to AAP guidelines |
| Ngan 2017 (162) | 78% | 70% | mean 28 (SD 2.5) | mean 28 (SD 2.5) | mean 1154 (SD 426) | mean 1140 (SD 406) | Mask and T‐piece | Two PIPs of 24 cmH₂O. Duration of first SLI was 20 seconds. Duration of second SLI was 20 or 10 seconds, guided by ECO₂ values. After SLIs, CPAP if breathing spontaneously or, if found to have apnoea or laboured breathing, mask IPPV at a rate of 40 to 60 bpm | IPPV, rate of 40 to 60 inflations/min until spontaneous breathing, at which time CPAP will be provided |
| Schmölzer 2018 (9) | 80%a | 100%a | mean 24.6 (SD 1.3)a | mean 25.6 (SD 2.3)a | mean 707 (SD 208)a | mean 808 (SD 192)a | Mask and T‐piecea | PIP for 20 + 20 secondsa during chest compressions | 3:1 compression:ventilation ratio according to resuscitation guidelines |
| Schwaberger 2015 (40) | not reported | not reported | mean 32.1 (SD 1.4) | mean 32.1 (SD 1.6) | mean 1692 (SD 297) | mean 1722 (SD 604) | Mask and T‐piece | PIP 30 cmH₂O for 15 seconds, to be repeated once or twice with HR remaining < 100 bpm. Infants with HR > 100 bpm: PPV at 30 cmH₂O PIP or CPAP at PEEP level of 5 cmH₂O depending on respiratory rate | Resuscitation according to AHA guidelines PEEP 5 cmH₂O if respiratory rate > 30 and signs of respiratory distress PPV at 30 cmH₂O PIP if insufficient breathing efforts |
aInformation provided by study authors
Settings and populations
Researchers conducted the included studies on five different continents: two in Italy (Lista 2015; Mercadante 2016); two in Canada by the same contact author (Ngan 2017; Schmölzer 2018); two in Egypt (Abd 2017; El‐Chimi 2017); one in Germany (Lindner 2005); one in Austria (Schwaberger 2015); one in Thailand (Jiravisitkul 2017); and one international multicentre (Kirpalani 2019) conducted in 18 neonatal intensive care units in nine countries (USA, Australia, the Netherlands, Canada, Germany, Italy, Austria, South Korea, and Singapore). Two studies were conducted at multiple centres (Kirpalani 2019; Lista 2015). Six of the 10 trials included infants with mean birth weight of more than 1 kg (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Mercadante 2016; Ngan 2017; Schwaberger 2015), whereas three included studies enrolled extremely low birth weight infants (Kirpalani 2019; Lindner 2005; Lista 2015), as did the pilot trial (Schmölzer 2018). Mercadante 2016 was the only trial conducted in late preterm infants. No trials enrolled full‐term infants. Table 3 shows additional information on populations.
Interventions
Trials pooled in Comparison one (i.e. without chest compressions) reported that peak inspiratory pressure (PIP) was sustained for 15 seconds in seven trials (El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Schwaberger 2015); and for 20 seconds in Ngan 2017. However, levels of PIP ranged from 20 cmH₂O (El‐Chimi 2017; Kirpalani 2019; Lindner 2005) to 24 (Ngan 2017), 25 (Jiravisitkul 2017; Lista 2015; Mercadante 2016), and 30 cmH₂O (Schwaberger 2015). Investigators provided additional SLIs in cases of poor response, with the same (Jiravisitkul 2017; Mercadante 2016; Schwaberger 2015) or higher PIP (El‐Chimi 2017; Kirpalani 2019; Lindner 2005); researchers in Ngan 2017 based the duration of the second SLI on exhaled CO₂ values. One study consisted of five groups: PIP of either 15 or 20 cmH₂O for either 10 or 20 seconds; control arm with PEEP 5 cmH₂O, oxygen 30% (Abd 2017). As regards interface and ventilation devices, most included trials used mask and T‐piece. Lindner 2005 used nasopharyngeal tube and ventilator, however, and El‐Chimi 2017 introduced a relevant bias into the study design by using a T‐piece ventilator in the SLI group and a self‐inflating bag in the control group (mask in both SLI and control groups). No trials reported whether prespecified levels of pressure for the SLI were actually delivered according to the protocol. Study authors did not monitor leaks at the mask and lung volumes during the manoeuvre. Whether the infant breathed before or during the SLI was not recorded: apnoeic newborns at birth are known to show less gain in lung volume during an SLI than actively breathing infants (Lista 2017).
For Comparison 2, in which infants in both SLI and control groups were resuscitated with chest compressions, duration of SLI was 20 + 20 seconds (Schmölzer 2018).
Table 3 shows additional information on interventions.
Excluded studies
We have summarised the reasons for exclusion of potentially eligible trials in the Characteristics of excluded studies table (Bouziri 2011; Gupta 2017; Harling 2005; Hunt 2019; te Pas 2007).
In particular, we excluded te Pas 2007 because sustained inflation was only one element of the intervention, and because it is not possible to determine the relative contributions of various elements of this intervention to differences observed between groups. We excluded Harling 2005, as investigators randomised infants in this trial to receive inflation for 2 seconds or 5 seconds at initiation of PPV. Similarly, in Hunt 2019 the duration of inflation in the control group was 2 to 3 seconds. All infants thus received sustained (> 1 second) inflations as defined in our protocol (O'Donnell 2004).
Risk of bias in included studies
We have presented a summary of the 'Risk of bias' assessment in Figure 2 and Figure 3. We have provided details of the methodological quality of included trials in the Characteristics of included studies section.
2.
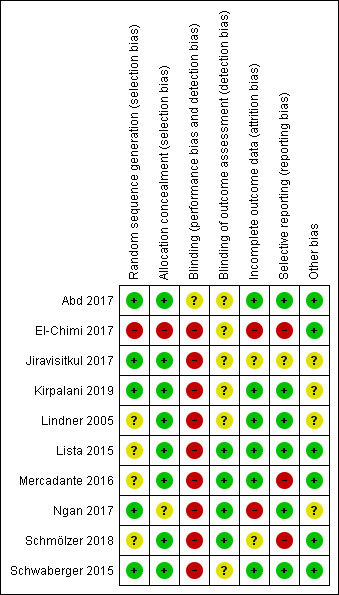
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
3.
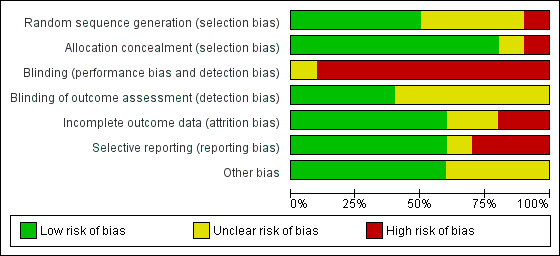
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Allocation
One trial had high risk of selection bias: This quasi‐randomised trial (odd‐numbered sheets indicated allocation to the SLI group, and even‐numbered sheets to the control group) did not use opaque envelopes (information provided by study authors) (El‐Chimi 2017). In Abd 2017, Kirpalani 2019, Jiravisitkul 2017 and Schwaberger 2015, risk of selection bias was low as regards random sequence generation and allocation concealment (opaque, numbered envelopes). In Ngan 2017, risk of selection bias was low as regards random sequence generation and was unclear for allocation concealment: timing of randomisation resulted in many post‐randomisation exclusions, as results showed more post‐randomisation exclusions in the SLI group than in the control group. In the other four trials, risk of selection bias was unclear as regards random sequence generation and was low as regards allocation concealment (opaque, numbered envelopes) (Lindner 2005; Lista 2015; Mercadante 2016; Schmölzer 2018).
Blinding
Owing to the nature of the intervention, all trials were unblinded, leading to high risk of performance bias. However, five trials blinded researchers assessing trial endpoints to the nature of study treatments (Kirpalani 2019; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2018). Furthermore it should be considered that blinding does not affect mortality. This outcome was considered by all included primary studies, limiting the risk of dealing with spurious or biased findings.
Incomplete outcome data
El‐Chimi 2017 transferred almost half of enrolled infants to other NICUs; we excluded this study from analysis owing to the high rate of follow‐up, although the primary outcome of the study (treatment failure/success within 72 hours) could have been determined and reported for all randomised infants. In Ngan 2017, post‐randomisation exclusion (27%) resulted in fewer included infants in the SLI group. Most trials accounted for all outcomes (Abd 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Schwaberger 2015).
Selective reporting
Six trials provided complete results for all planned outcomes (Abd 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015).
Other potential sources of bias
El‐Chimi 2017 and Schwaberger 2015 did not report sample size calculations. For Schwaberger 2015, investigators registered the protocol after study initiation. Jiravisitkul 2017 planned sample sizes of 40 infants for each group but allocated only 38 to the control group. Lindner 2005 was stopped after the interim analysis. It was unclear why study authors made this decision. In Kirpalani 2019 the DSMB halted the trial for harm when 426 infants had been enrolled (of a planned sample size of 600). Ngan 2017 did not achieve the planned sample size; in addition, the incidence of the primary outcome in the control group was less than that assumed for the sample size calculation, leading to lack of power to detect the chosen effect size. The other trials appear free of other bias.
We did not explore possible bias through generation of funnel plots because fewer than 10 trials met the inclusion criteria of this Cochrane Review.
Effects of interventions
Comparison 1: use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with no chest compressions
Nine trials (with 1458 infants) are included in the comparison of the use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with no chest compressions (Comparison 1) (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015) (see Table 1).
Primary outcomes
Death (Outcome 1.1)
Death in the delivery room (Outcome 1.1.1)
Five trials (N = 479) reported this outcome (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Schwaberger 2015); only one event occurred across both arms (death caused by severe birth asphyxia as the result of a prolapsed cord in the SLI group in Jiravisitkul 2017) (typical RR 2.66, 95% CI 0.11 to 63.40; typical RD 0.00, 95% CI −0.02 to 0.02; I² not applicable for RR and I² = 0% for RD; 5 studies, 479 participants) (Analysis 1.1 and Figure 4). The quality of the evidence (GRADE) for this outcome was low due to limitations in study design and imprecision (see Table 1). For two trials we obtained mortality data from trial authors (Jiravisitkul 2017; Lindner 2005).
1.1. Analysis.
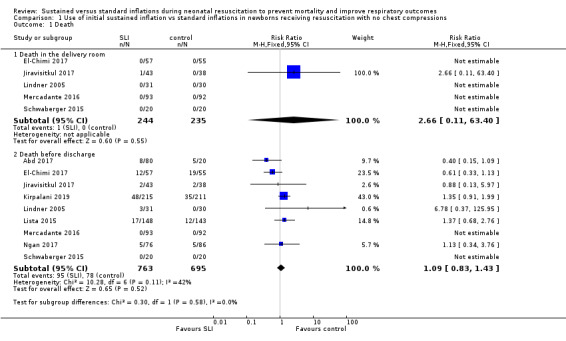
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 1 Death.
4.
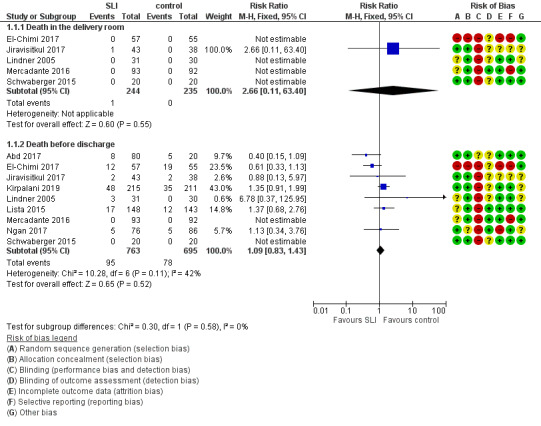
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions ‒ Outcome: 1.1 Death.
Death during hospitalisation (Outcome 1.1.2)
The observed death rate increased with longer follow‐up. All trials reported mortality during hospitalisation (typical RR 1.09, 95% 0.83 to 1.43; typical RD 0.01, 95% CI −0.02 to 0.04; I² = 42% for RR and I² = 24% for RD; 9 studies, 1458 participants; observed deaths = 143) (Analysis 1.1 and Figure 4) (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015). The quality of the evidence (GRADE) for this outcome was moderate due to limitations in study design (see Table 1). For three trials we obtained data from trial authors (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005).
In El‐Chimi 2017, 12 and 19 infants died in SLI and control groups, respectively. In Jiravisitkul 2017, two infants in each group died: in the SLI group, one died of severe birth asphyxia as the result of a prolapsed cord, and the other died at three hours of life of suspected umbilical catheter migration with haemothorax; in the control group, one died of severe respiratory distress syndrome at two hours of life, and the other of septic shock at 168 days of life. In Lindner 2005, three deaths occurred in the sustained inflation group: at day 1 (respiratory failure), at day 36 (necrotising enterocolitis), and at day 107 (liver fibrosis of unknown origin). In Lista 2015, 12 infants in the control group and 17 in the sustained inflations group died during the trial. Mercadante 2016 and Schwaberger 2015 reported no events. Kirpalani 2019 was stopped earlier than planned because of increased early mortality (before 48 hours) in the SLI group. Of the 19 early deaths, 13 were in the lower gestational age stratum, with the predominant cause being assigned as cardiorespiratory failure (respiratory failure, 5; asphyxia or failed transition, 4; pulmonary hypertension, 2; haemorrhagic shock, 1; and pneumothorax, 1). Only 8 of 19 (42.1%) of those who had an early death survived long enough for a head ultrasound. Of these three (37.5%) had an intraventricular haemorrhage, one of whom also had a catastrophic gastrointestinal perforation. There were three cases of sepsis.
Death to latest follow‐up
No data were provided in addition to those already presented for death during hospitalisation (Analysis 1.1).
Secondary outcomes
Heart rate at 5 minutes (Outcome 1.1)
One trial (N = 426) reported on heart rate after the first resuscitation manoeuvre (Kirpalani 2019). Heart rate was more frequently low in the SLI group, with heart rate of less than 60 beats per minute (BPM) occurring in 23% and 11% of the infants in the SLI and control group, respectively, whereas heart rate was greater than 100 BPM in 25% and 41% of the infants in the SLI and control group, respectively (P < 0.001).
Endotracheal intubation (Outcome 1.2)
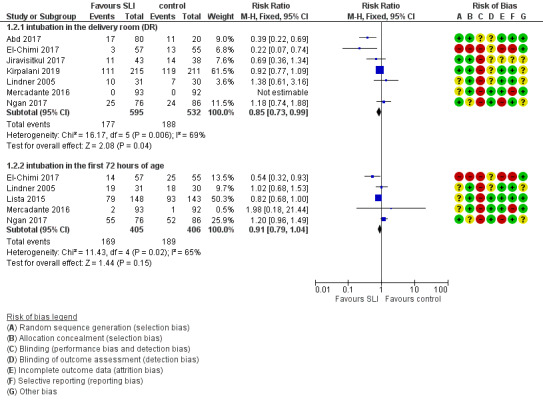
Endotracheal intubation in the delivery room (Outcome 1.2.1)
Seven trials (N = 1127) reported this outcome (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Mercadante 2016; Ngan 2017). Rate of endotracheal intubation in the delivery room was lower in SLI group (typical RR 0.85, 95% CI 0.73 to 0.99; typical RD −0.05, 95% CI −0.10 to −0.00; I² = 69% for RR and I² = 84% for RD; 7 studies, 1127 participants) (Analysis 1.2; Figure 5). Heterogeneity was moderate (I² = 69%) for RR and high for RD (I² = 84%). We obtained data for this outcome directly from trial authors (Mercadante 2016).
1.2. Analysis.
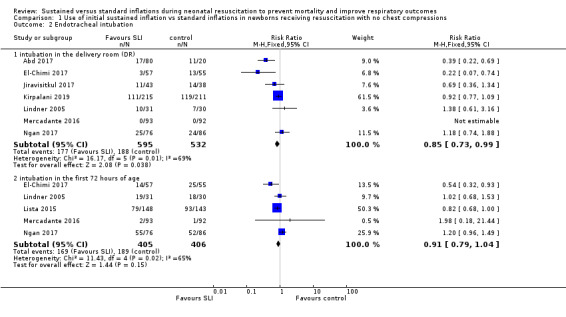
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 2 Endotracheal intubation.
5.
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions ‒ Outcome: 1.4 Endotracheal intubation.
Endotracheal intubation in the first 72 hours of life (Outcome 1.2.2)
Five included trials (N = 811) reported this outcome (typical RR 0.91, 95% CI 0.79 to 1.04; typical RD −0.04, 95% CI −0.10 to 0.01; I² = 65% for RR and I² = 79% for RD; 5 studies, 811 participants) (Analysis 1.2; Figure 5) (El‐Chimi 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017). We obtained data for this outcome directly from trial authors (Lindner 2005; Mercadante 2016).
Surfactant administration (Outcome 1.3)
Surfactant administration in the delivery room (Outcome 1.3.1)
Four trials (N = 761) reported this outcome (typical RR 1.06, 95% CI 0.88 to 1.27; typical RD 0.02, 95% CI −0.04 to 0.08; I² = 0 % for RR and RD; 4 studies, 761 participants) (Analysis 1.3; Figure 6) (El‐Chimi 2017; Kirpalani 2019; Lindner 2005; Ngan 2017). We obtained data for this outcome directly from trial authors (El‐Chimi 2017).
1.3. Analysis.
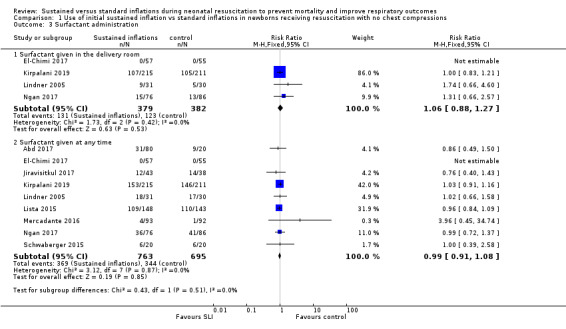
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 3 Surfactant administration.
6.
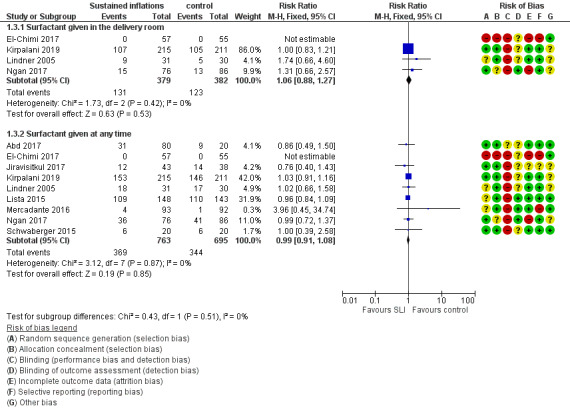
Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions ‒ Outcome: 1.5 Surfactant administration.
Surfactant administration during hospital admission (Outcome 1.3.2)
All trials included in Comparison 1 (N = 1458) reported this outcome (typical RR 0.99, 95% CI 0.91 to 1.08; typical RD −0.00, 95% CI −0.05 to 0.04; I² = 0% for RR and RD; 9 studies, 1458 participants) (Analysis 1.3; Figure 6) (Abd 2017; El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015). We obtained data for this outcome directly from trial authors (El‐Chimi 2017; Lindner 2005; Mercadante 2016).
Rate of mechanical ventilation (Outcome 1.4)
Four trials (N = 910) reported this outcome (typical RR 0.89, 95% CI 0.77 to 1.02; typical RD −0.05, 95% CI −0.11 to 0.01; I² = 0% for RR and I² = 69% for RD; 4 studies, 910 participants) (Analysis 1.4) (El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lista 2015). The quality of the evidence (GRADE) for this outcome was moderate due to limitations in study design (see Table 1). We obtained data for this outcome directly from trial authors (El‐Chimi 2017).
1.4. Analysis.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 4 Need for mechanical ventilation.
Duration of nasal continuous airway pressure (Outcome 1.5)
Three trials (N = 355) reported this outcome (MD 0.26 days, 95% CI −0.19 to 0.72; I² = 59%; 3 studies, 355 participants) (Analysis 1.5) (El‐Chimi 2017; Lindner 2005; Mercadante 2016). We obtained data for this outcome directly from trial authors; data for this outcome refer to survivors at time of assessment (El‐Chimi 2017; Lindner 2005; Mercadante 2016).
1.5. Analysis.
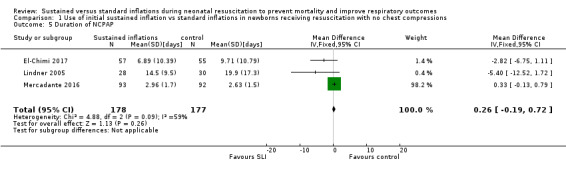
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 5 Duration of NCPAP.
Duration of ventilation via an ETT (Outcome 1.6)
Five trials (N = 524) reported this outcome (MD −5.37 days, 95% CI −6.31 to −4.43; I² = 95%; 5 studies, 524 participants) (Analysis 1.6) (Jiravisitkul 2017; Lindner 2005; Mercadante 2016; Ngan 2017; Schwaberger 2015). Data for this outcome refer to survivors at time of assessment (Jiravisitkul 2017; Lindner 2005; Mercadante 2016). We obtained data for this outcome directly from trial authors (Jiravisitkul 2017; Mercadante 2016; Ngan 2017; Schwaberger 2015). Heterogeneity, statistical significance, and magnitude of effects of this outcome are largely influenced by a single study (Ngan 2017): when this study was removed from the analysis, the size of the effect was reduced (MD −1.71 days, 95% CI −3.04 to −0.39; I² = 0%). In Ngan 2017, a second SLI was delivered to 84% of the infants in the SLI group and was guided by the amount of ECO₂.
1.6. Analysis.
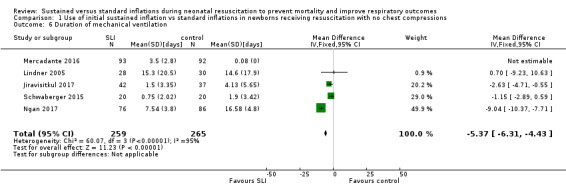
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 6 Duration of mechanical ventilation.
Duration of respiratory support (nasal continuous airway pressure and ventilation via an ETT, considered in total) (Outcome 1.7)
Two trials (N = 243) reported this outcome (MD 0.69 days, 95% CI 0.23 to 1.16; 2 studies, 243 participants; I² = 0%) (Analysis 1.7) (Lindner 2005; Mercadante 2016). We obtained data for this outcome directly from trial authors; data refer to survivors at time of assessment (Lindner 2005; Mercadante 2016). Abd 2017 provided medians and interquartile range and observed significantly shorter times in the conventional group: 7.5 days (4 to 13.75 days) in SLI groups vs 2 (1 to 4.25 Days) in control group (P < 0.01).
1.7. Analysis.
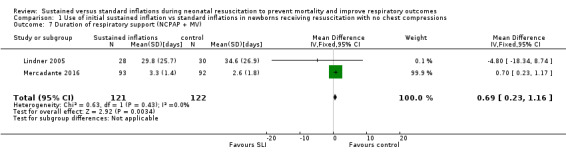
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 7 Duration of respiratory support (NCPAP + MV).
Duration of supplemental oxygen requirement (days) (Outcome 1.8)
One trial (N = 81) reported this outcome (MD −9.73, 95% CI −25.06 to 5.60; 1 study, 81 participants) (Analysis 1.8) (Jiravisitkul 2017). The test for heterogeneity was not applicable. We obtained data for this outcome directly from trial authors (Jiravisitkul 2017). Abd 2017 provided medians and interquartile range: 6 days (2 to 15 days) in SLI groups vs 4 (0 to 6.5 Days) in control group.
1.8. Analysis.
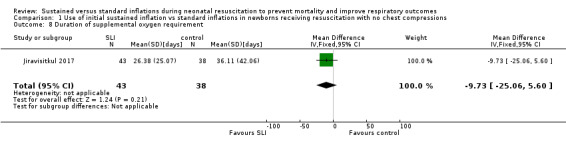
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 8 Duration of supplemental oxygen requirement.
Chronic lung disease (i.e. rate of supplemental oxygen at 36 weeks of gestational age for infants born at or before 32 weeks of gestation) (Outcome 1.9)
Bronchopulmonary dysplasia (BPD) any grade (Outcome 1.9.1)
Four trials (N = 735) reported this outcome (typical RR 0.99, 95% CI 0.83 to 1.18; typical RD −0.00, 95% CI −0.07 to 0.07; I² = 0% for RR and I² = 0% for RD; 4 studies, 735 participants) (Abd 2017; Kirpalani 2019; Lindner 2005; Ngan 2017). The quality of the evidence (GRADE) for this outcome was moderate due to limitations in study design (see Table 1). We obtained data for this outcome directly from trial authors; data refer to survivors at time of assessment (Lindner 2005)(Analysis 1.9).
1.9. Analysis.
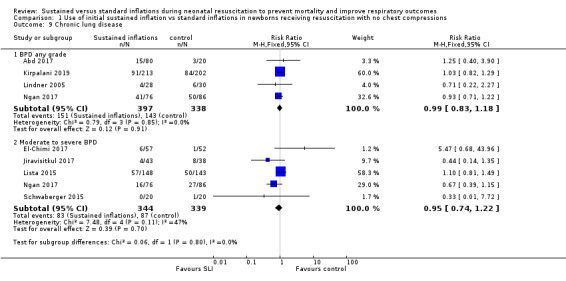
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 9 Chronic lung disease.
Moderate to severe BPD (Outcome 1.9.2)
Five included trials (N = 683) reported this outcome (typical RR 0.95, 95% CI 0.74 to 1.22; typical RD −0.01, 95% CI −0.07 to 0.05; I² = 47% for RR and I² = 57% for RD; 5 studies, 683 participants) (Analysis 1.9) (El‐Chimi 2017; Jiravisitkul 2017; Lista 2015; Ngan 2017; Schwaberger 2015). The quality of the evidence (GRADE) for this outcome was moderate due to limitations in study design (see Table 1).
Air leaks (pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema) reported individually or as a composite outcome (Outcome 1.10)
Pneumothorax in first 48 hours of life (Outcome 1.10.1)
One trial (N = 81) reported this outcome (RR 0.88, 95% CI 0.06 to 13.65; RD −0.00, 95% CI −0.07 to 0.06) (Jiravisitkul 2017). The test for heterogeneity was not applicable (Analysis 1.10).
1.10. Analysis.
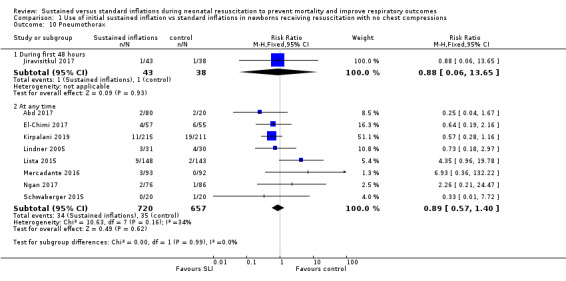
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 10 Pneumothorax.
Pneumothorax at any time (Outcome 1.10.2)
Eight included studies (N = 1377) reported this outcome (typical (RR 0.89, 95% CI 0.57 to 1.40; typical RD −0.01, 95% CI −0.03 to 0.02; I² = 34% for RR and I² = 49% for RD; 8 studies, 1377 participants) (Analysis 1.10) (Abd 2017; El‐Chimi 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schwaberger 2015). The quality of the evidence (GRADE) for this outcome was low due to limitations in study design and imprecision (see Table 1).
Pulmonary interstitial emphysema (Outcome 1.11)
One trial (N = 426) reported this outcome (RR 1.14, 95% CI 0.39 to 3.35; RD 0.00, 95% CI −0.03 to 0.04). The test for heterogeneity was not applicable (Analysis 1.11) (Kirpalani 2019).
1.11. Analysis.
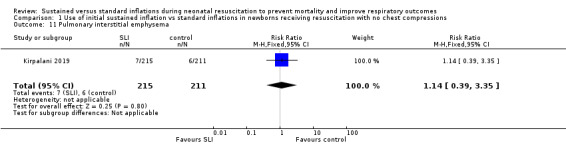
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 11 Pulmonary interstitial emphysema.
Pneumopericardium (Outcome 1.12)
One trial (N = 426) reported this outcome. No events were observed (Kirpalani 2019).
Cranial ultrasound abnormalities (Outcome 1.13)
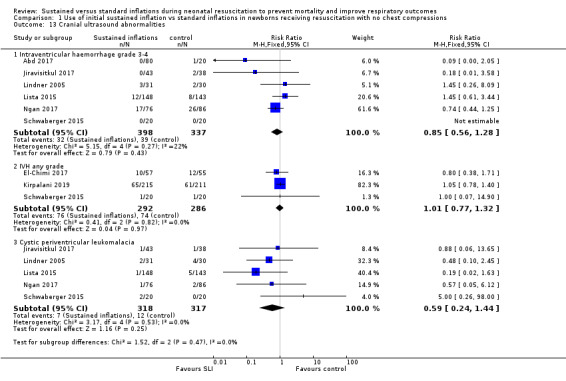
Intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978) (Outcome 1.13.1)
Six included trials (N = 735) reported this outcome (typical RR 0.85, 95% CI 0.56 to 1.28; typical RD −0.02, 95% CI −0.06 to 0.03; I² = 22% for RR and I² = 0% for RD; 6 studies, 735 participants) (Analysis 1.13) (Abd 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015). The quality of the evidence (GRADE) for this outcome was low due to limitations in study design and imprecision (see Table 1).
1.13. Analysis.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 13 Cranial ultrasound abnormalities.
IVH any grade (Outcome 1.13.2)
Three included trials (N = 578) reported this outcome (typical RR 1.01, 95% CI 0.77 to 1.32; typical RD 0.00, 95% CI −0.07 to 0.07; I² = 0% for RR and I² = 0% for RD; 3 studies, 578 participants) (Analysis 1.13) (El‐Chimi 2017; Kirpalani 2019; Schwaberger 2015).
Cystic periventricular leukomalacia (Outcome 1.13.3)
Five included trials (N = 635) reported this outcome (typical RR 0.59, 95% CI 0.24 to 1.44; typical RD −0.04, 95% CI −0.04 to 0.01; I² = 0% for RR and I² = 0% for RD; 5 studies, 635 infants) (Analysis 1.13) (Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015).
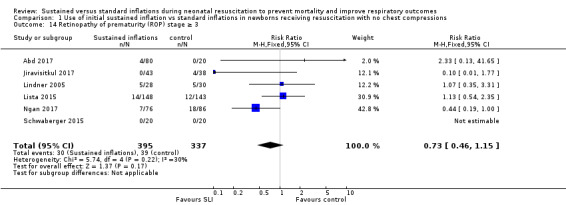
Retinopathy of prematurity (ROP) ≥ stage 3 (Outcome 1.14)
Six trials (N = 732) reported this outcome (typical RR 0.73, 95% CI 0.46 to 1.15; typical RD −0.03, 95% CI −0.07 to 0.01; I² = 30% for RR and I² = 51% for RD; 6 studies, 732 participants; Analysis 1.14) (Abd 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015). For Lindner 2005, data refer to survivors at time of assessment; and for Abd 2017, data refer to pre‐threshold values. Kirpalani 2019 reports data ROP of any grade: 99/196 (50.5%) in SLI group vs 97/182 (53.3%) in conventional group (Analysis 1.14).
1.14. Analysis.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 14 Retinopathy of prematurity (ROP) stage ≥ 3.
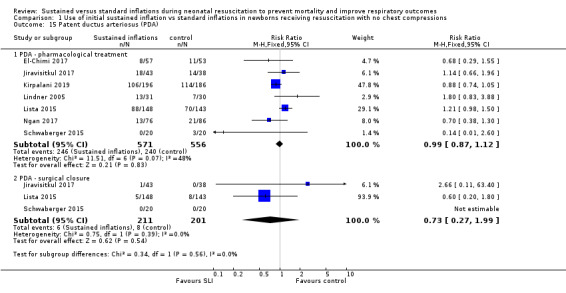
Patent ductus arteriosus (PDA) (Outcome 1.15)
Rate of PDA ‒ pharmacological treatment (Outcome 1.15.1)
Seven included trials (N = 1127) reported this outcome (typical RR 0.99, 95% CI 0.87 to 1.12; typical RD −0.01, 95% CI −0.06 to 0.05; I² = 48% for RR and I² = 53% for RD; 7 studies, 1127 participants; Analysis 1.15) (El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Lista 2015; Ngan 2017; Schwaberger 2015). We obtained data for this outcome directly from trial authors (Schwaberger 2015).
1.15. Analysis.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 15 Patent ductus arteriosus (PDA).
One trial reported PDA rates (2/80 vs 2/20) without specifying whether requiring pharmacological or surgical treatment, and therefore they could not be added to the meta‐analysis (Abd 2017).
Rate of PDA ‒ surgical closure (Outcome 1.15.2)
Three trials (N = 412) reported this outcome (typical RR 0.73, 95% CI 0.27 to 1.99; typical RD −0.01, 95% CI −0.05 to 0.03; I² = 0% for RR and I² = 26% for RD; 3 studies, 412 infants; Analysis 1.15) (Jiravisitkul 2017; Lista 2015; Schwaberger 2015). We obtained data for this outcome directly from trial authors (Schwaberger 2015).
The data refer to all randomised infants, unless otherwise specified.
No data were reported for the following outcomes: heart rate; rate of supplemental oxygen at 28 days of life; seizures including clinical and electroencephalographic; hypoxic‐ischaemic encephalopathy in term and late preterm infants (grade 1 to 3) (Sarnat 1976); and long‐term neurodevelopmental outcomes.
Subgroup analysis for Comparison 1
For Comparison 1, we were unable to conduct any of the four prespecified subgroup analyses for the following reasons.
No term infants were included.
For ventilation devices, all trials used a T‐piece except Lindner 2005 (mechanical ventilator). We did not perform a separate analysis because of the very small sample size and the presence of high or unclear risk of bias in most GRADE domains. Moreover, El‐Chimi 2017 used a T‐piece ventilator in the SLI group and a self‐inflating bag in the control group, thus we could not include this as a subgroup.
For interface, all trials used a face mask, except Lindner 2005 (nasopharyngeal tube). As for ventilation devices, we did not perform a separate analysis for Lindner 2005.
No trials used SLI < 5 seconds.
Comparison 2: use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with chest compressions
We could not perform any meta‐analysis in the comparison of the use of initial sustained inflation versus standard inflations in newborns receiving resuscitation with chest compressions because we identified only one trial for inclusion (a pilot study of nine preterm infants) (Schmölzer 2018) (see Table 2).
Primary outcomes
Death (Outcome 2.1)
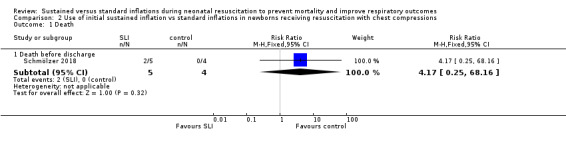
Death in the delivery room (Outcome 2.1.1)
The included trial (N = 9) did not report this outcome (Schmölzer 2018).
Death during hospitalisation (Outcome 2.1.2)
One trial (N = 9) reported this outcome (RR 4.17, 95% CI 0.25 to 68.16; RD 0.40, 95% CI −0.07 to 0.87); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 1 Death.
Secondary outcomes
Endotracheal intubation in the delivery room (Outcome 2.2)
One trial (N = 9) reported this outcome (RR 1.00, 95% CI 0.68 to 1.46; RD 0.00, 95% CI −0.34 to 0.34); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.2). We obtained data for this outcome directly from trial authors (Schmölzer 2018).
2.2. Analysis.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 2 Endotracheal intubation.
Surfactant administration in the delivery room (Outcome 2.3)
One trial (N = 9) reported this outcome (RR 0.65, 95% CI 0.31 to 1.35; RD −0.40, 95% CI −0.87 to 0.07); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.3).
2.3. Analysis.
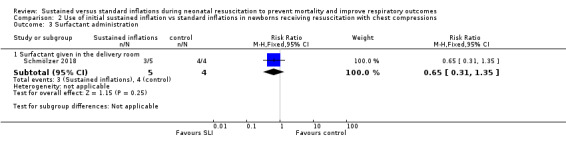
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 3 Surfactant administration.
Chronic lung disease (2.4)
Moderate to severe BDP (Outcome 2.4.1)
One trial (N = 9) reported this outcome (RR 0.89, 95% CI 0.33 to 2.37; RD −0.08, 95% CI −0.76 to 0.60); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.4).
2.4. Analysis.
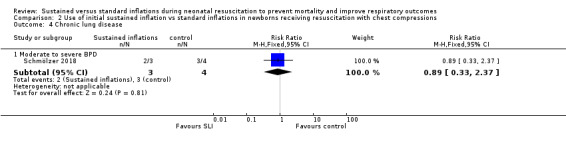
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 4 Chronic lung disease.
Pneumothorax at any time (Outcome 2.5)
One trial (N = 9) reported this outcome: no events occurred (Analysis 2.5).
2.5. Analysis.
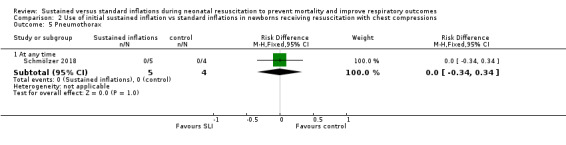
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 5 Pneumothorax.
Cranial ultrasound abnormalities (Outcome 2.6)
Intraventricular haemorrhage (IVH), grade 3 or 4 according to the Papile classification (Papile 1978) (Outcome 2.6.1)
One trial (N = 9) reported this outcome (RR 0.40, 95% CI 0.05 to 2.98; RD −0.30, 95% CI −0.90 to 0.30); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.6).
2.6. Analysis.
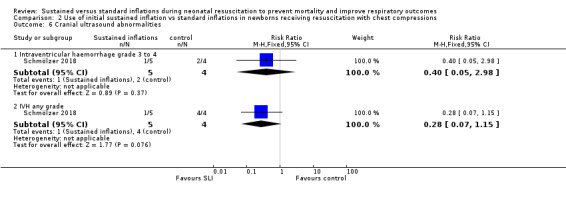
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 6 Cranial ultrasound abnormalities.
IVH any grade (Outcome 2.6.2)
One trial (N = 9) reported this outcome (RR 0.28, 95% CI 0.07 to 1.15; RD −0.80, 95% CI −1.23 to −0.37); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.6).
Retinopathy of prematurity (ROP) ≥ stage 3 (Outcome 2.7)
One trial (N = 9) reported this outcome (RR 0.27, 95% CI 0.04 to 1.68; RD −0.55, 95% CI −1.10 to 0.00); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.7).
2.7. Analysis.
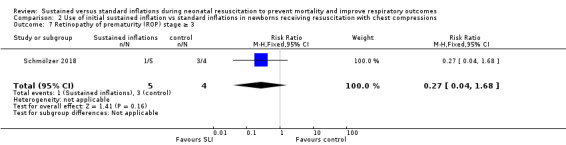
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 7 Retinopathy of prematurity (ROP) stage ≥ 3.
Rate of PDA ‒ pharmacological treatment (Outcome 2.8)
One trial (N = 9) reported this outcome (RR 0.46, 95% CI 0.17 to 1.25; RD −0.60, 95% CI −1.07 to −0.13); thus, the test for heterogeneity was not applicable for this outcome (Schmölzer 2018) (Analysis 2.8).
2.8. Analysis.
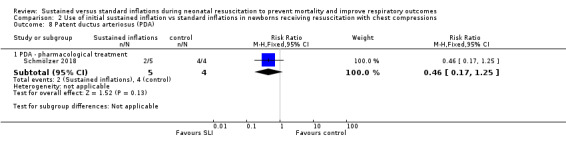
Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 8 Patent ductus arteriosus (PDA).
For Comparison 2, investigators provided no data on other prespecified outcomes.
Subgroup analysis for the Comparison two
For Comparison 2, we were unable to conduct any subgroup analysis as we included only one trial.
Discussion
Summary of main results
We evaluated the benefits and harms of sustained lung inflation (SLI) versus intermittent ventilation in infants requiring resuscitation and stabilisation at birth. Ten trials enrolling 1467 preterm infants compared the interventions in which we were interested (Abd 2017El‐Chimi 2017; Jiravisitkul 2017; Kirpalani 2019Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2018; Schwaberger 2015), with one exception. Schmölzer 2018 was not meta‐analysed with the other studies because investigators considered as experimental intervention chest compressions in combination with SLI.
Sustained lung inflation was not better than intermittent ventilation for reducing mortality — the primary outcome of this review. We rated the quality of evidence as moderate (GRADE) for death before discharge (limitations in study design of most included trials) and as low (GRADE) for death in the delivery room (limitations in study design and imprecision of estimates). When considering secondary outcomes, such as intubation, rate or duration of respiratory support, bronchopulmonary dysplasia, or pneumothorax, we found no benefit of SLI over intermittent ventilation. The quality of evidence for secondary outcomes was moderate (limitations in study design of most included trials ‒ GRADE), except for pneumothorax (low quality: limitations in study design and imprecision of estimates ‒ GRADE). Duration of mechanical ventilation was shorter in the SLI group (low quality: limitations in study design and imprecision of estimates ‒ GRADE).
We identified eight ongoing trials.
Overall completeness and applicability of evidence
To date, 10 trials comparing sustained versus standard inflations for initial resuscitation have enrolled 1467 newborns. Available data were insufficient for assessment of clinically important outcomes, which were identified a priori. Study authors did not report outcomes such as duration of supplemental oxygen requirement and long‐term neurodevelopmental outcomes and did not enrol term infants. We could not perform a priori subgroup analyses (gestational age, ventilation device, interface, duration of sustained inflation) to detect differential effects because of the paucity of included trials. Relevant questions such as the following remain unanswered: What is the optimal duration for an SLI? Which level of positive end‐expiratory pressure (PEEP) should follow? Which is the optimal interface/device? (McCall 2016). We were able to summarise available evidence in a comprehensive way, as we obtained additional information about study design and outcome data from most of the included trials (El‐Chimi 2017; Jiravisitkul 2017; Lindner 2005; Lista 2015; Mercadante 2016; Ngan 2017; Schmölzer 2018; Schwaberger 2015) and from two excluded trials (Harling 2005; te Pas 2007). The eight ongoing trials that we identified reported important differences in choice of gestational age (NCT01255826; NCT01440868; NCT02493920; NCT02858583; NCT02887924; NCT03165305; NCT03437499; NCT03518762). NCT02493920 enrols infants at 25 to 36 weeks, NCT01440868 25 to 28 weeks, NCT01255826 26 to 34 weeks, NCT03518762 27 to 32 weeks, NCT03437499 28 to 30, NCT02887924 26 to 29 weeks, NCT02858583 enrols term and preterm infants, whereas NCT03165305 only term infants. These differences among study populations might prove to be important, as trials have reported that sustained inflation was more effective in infants at 28 to 30 weeks than at less than 28 or more than 30 weeks of gestation (te Pas 2007).
Quality of the evidence
According to the GRADE approach, we rated the overall quality of evidence for clinically relevant outcomes as low to moderate (see Table 1 for Comparison 1. We downgraded the overall quality of evidence for critical outcomes because of limitations in study design (i.e. selection bias due to lack of allocation concealment) and imprecision of results (few events for death in the delivery room and wide confidence intervals for pneumothorax). In addition, three trials did not report sample size calculations (Abd 2017; El‐Chimi 2017; Schwaberger 2015), and four did not achieve them (Jiravisitkul 2017; Kirpalani 2019; Lindner 2005; Ngan 2017). Results of smaller studies are subject to greater sampling variation, and hence are less precise. Indeed, imprecision is reflected in the confidence interval around the intervention effect estimate from each study and in the weight given to the results of each study included in the meta‐analysis (Higgins 2011).
Potential biases in the review process
A major limitation of this Cochrane Review is the definition of sustained lung inflation, as trials used different pressures, which may have impacted study results. We excluded Harling 2005 and Hunt 2019 because the control group received two and two to three seconds of inflation, respectively, whereas we defined 'sustained' as more than one second. An additional limitation consists of the high number of outcomes we specified, leading to low statistical power for most of the analyses. As one review author (PD) is also the author of one of the trials that was included (Kirpalani 2019), the other review authors conducted quality assessments of this trial.
No trials were blinded owing to the nature of the intervention. We excluded a potentially relevant trial because sustained inflation was only one element of the intervention (te Pas 2007), and it is not possible to determine the relative contributions of various elements of this intervention to differences observed between groups. For the 2017 update of this review, we made a post hoc decision to add a comparison based on the presence of chest compressions during resuscitation.
Agreements and disagreements with other studies or reviews
Several systematic reviews of SLI have been recently published. Schmölzer 2014 conducted a systematic review of randomised clinical trials comparing SLI versus intermittent positive‐pressure ventilation (IPPV) as the primary respiratory intervention during respiratory support in preterm individuals at less than 33 weeks of gestational age in the delivery room. This review included four trials, including two that we excluded from our systematic review (Harling 2005; te Pas 2007). Schmölzer 2014 reported a significant reduction in the rate of mechanical ventilation within 72 hours after birth (typical risk ratio (RR) 0.87, 95% confidence interval (CI) 0.74 to 1.03) and an increased rate of treatment for PDA in the SLI group (RR 1.27, 95% CI 1.05 to 1.54). Results showed no differences in bronchopulmonary dysplasia (BPD), death at latest follow‐up, or the combined outcome of death or BPD among survivors between groups. The findings of Schmölzer 2014 differ from the findings of this Cochrane Review because of differences in the definition of duration of the intervention, and therefore in determination of included trials. Foglia 2016, a narrative review including five trials (Harling 2005; Lindner 2005; Lista 2015; Mercadante 2016; te Pas 2007), concluded that data are at present insufficient to support the use of SLI in clinical practice. An observational analytical case‐control study of 78 preterm infants showed that SLI resulted in lower rates of intubation in the delivery room, lower rates of invasive mechanical ventilation, and higher rates of intraventricular haemorrhage (Grasso 2015).
Authors' conclusions
Implications for practice.
Our meta‐analysis of nine studies shows that sustained lung inflation without chest compression was not better than intermittent ventilation for reducing mortality in the delivery room (low‐quality evidence – GRADE) or during hospitalisation (moderate‐quality evidence – GRADE), which were the primary outcomes of this review. However, the single largest study, which was well‐conducted and had the greatest number of enrolled infants, was stopped early for higher mortality rate in the sustained inflation group. When considering secondary outcomes, such as rate of intubation, rate or duration of respiratory support, or bronchopulmonary dysplasia, we found no benefit of sustained inflation over intermittent ventilation (moderate‐quality evidence – GRADE). Duration of mechanical ventilation was shortened in the SLI group (low‐quality evidence – GRADE); however, this result should be interpreted cautiously, as it might have been influenced by study characteristics other than the intervention. There is no evidence to support the use of sustained inflation based on evidence from our review.
Implications for research.
Additional studies of SLI for infants receiving respiratory support at birth should provide more detailed monitoring of the procedure, such as measurements of lung volume and presence of apnoea before or during SLI. Future randomised controlled trials should aim to enrol infants who are at higher risk of morbidity and mortality, and should stratify participants by gestational age. Researchers should also measure long‐term neurodevelopmental outcomes (e.g. Bayley Scales of Infant Development administered at two years of corrected age).
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2019 | New citation required and conclusions have changed | We updated searches in 2019 and found two new eligible studies for inclusion |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 21 July 2017 | Amended | Typo corrected: Schwaberger 2015 used near‐infrared spectroscopy (NIRS) not a numerical rating scale (NRS). |
| 13 June 2017 | New search has been performed | We updated searches in 2017 and found six new eligible studies for inclusion |
| 13 June 2017 | New citation required but conclusions have not changed | We included six new studies but made no changes to the main conclusions |
| 6 July 2015 | Amended | We updated review author affiliation |
| 10 July 2008 | Amended | We converted the review to new review format |
Acknowledgements
We thank Drs. Lindner, te Pas, Harling, El‐Farghali (for El‐Chimi 2017), Mercadante, Nuntnarumit (Jiravisitkul 2017), Schmolzer (for Ngan 2017 and Schmölzer 2018), and Schwaberger for their gracious assistance in providing extra data.
We thank Simona Zappettini for her contribution to the previous versions of this review.
We thank Matthias Bank (Library and ICT services, Lund University) for designing and running the search strategy of this update.
We thank Colleen Ovelman, Caitlin O'Connell and Roger Soll for the editorial support.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Standard search method
PubMed, 20190401
#1 (infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB]) 1,108,534
#2 (sustained inflation) OR (sustained AND (inflat* AND (lung OR pulmonary))) 542
#3 #1 AND #2 140
#4 Limit to publication date 2017/01/01 to 2019/12/31 32
Embase, 20190401
#1. 'sustained inflation'/exp OR 'sustained inflation’ OR sustained NEAR/3 inflation 292
#2. sustained AND inflat* AND (lung OR pulmonary) 488
#3. #1 OR #2 531
#4. 'prematurity'/exp OR 'infant'/exp 1,106,519
#5. newborn*:ti,ab OR 'new born':ti,ab OR 'new borns':ti,ab OR 'newly born':ti,ab OR baby*:ti,ab OR babies:ti,ab OR premature:ti,ab OR prematurity:ti,ab OR preterm:ti,ab OR 'pre term':ti,ab OR 'low birth weight':ti,ab OR 'low birthweight':ti,ab OR vlbw:ti,ab OR lbw:ti,ab OR infant:ti,ab OR infants:ti,ab OR infantile:ti,ab OR infancy:ti,ab OR neonat*:ti,ab 1,007,985
#6. #4 OR #5 1,554,664
#7. #3 AND #6 202
#8. #3 AND #6 AND [1‐1‐2017]/sd 50
Cochrane Library, 20190401
#1. MESH DESCRIPTOR Infant, Newborn EXPLODE ALL 14,968
#2. (infan* or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm* or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU):ti,ab,kw 79,808
#3. #2 OR #1 79,808
#4 (sustained inflation):ti,ab,kw 151
#5 (sustained AND inflat* AND (lung OR pulmonary)):ti,ab,kw 70
#6 #4 OR #5 151
#7 #3 AND #6 55
#8 Limit to 2017 to 2019 18 trials
1 review
CINAHL, 20190401
| S4 | S1 AND S2 | Published Date: 20170101‐20191231 | 17 |
| S3 | S1 AND S2 | 60 | |
| S2 | sustained inflation OR ( sustained AND inflat* AND (lung OR pulmonary) ) | 125 | |
| S1 | (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) | 424,755 |
Appendix 2. Risk of bias tool
We planned to use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological certainty of the trials. For each trial, we planned to seek information regarding the method of randomisation, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We planned to assess each criterion as being at either low, high, or unclear risk of bias. Two review authors separately planned to assess each study and resolve any disagreements through discussion. We planned to add this information to the ‘Characteristics of included studies' table. We planned to evaluate the following issues and enter the findings into the ‘Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we planned to categorise the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we planned to categorise the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
or unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we planned to categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we planned to categorise the methods used to blind outcome assessment. Blinding was to be assessed separately for different outcomes or class of outcomes. We planned to categorise the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we planned to describe the completeness of data including attrition and exclusions from the analysis. We planned to note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We planned to categorise the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
or unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we planned to describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we planned to compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we planned to contact study authors to gain access to the study protocol. We planned to assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported: one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we planned to describe any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We planned to assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in the delivery room | 5 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.11, 63.40] |
| 1.2 Death before discharge | 9 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.83, 1.43] |
| 2 Endotracheal intubation | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 intubation in the delivery room (DR) | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 2.2 intubation in the first 72 hours of age | 5 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 3 Surfactant administration | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Surfactant given in the delivery room | 4 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.88, 1.27] |
| 3.2 Surfactant given at any time | 9 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 4 Need for mechanical ventilation | 4 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 5 Duration of NCPAP | 3 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.19, 0.72] |
| 6 Duration of mechanical ventilation | 5 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐5.37 [‐6.31, ‐4.43] |
| 7 Duration of respiratory support (NCPAP + MV) | 2 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.23, 1.16] |
| 8 Duration of supplemental oxygen requirement | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐9.73 [‐25.06, 5.60] |
| 9 Chronic lung disease | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 BPD any grade | 4 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 9.2 Moderate to severe BPD | 5 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.22] |
| 10 Pneumothorax | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 During first 48 hours | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.65] |
| 10.2 At any time | 8 | 1377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.40] |
| 11 Pulmonary interstitial emphysema | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.35] |
| 12 Pneumopericardium | 1 | 426 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 13 Cranial ultrasound abnormalities | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Intraventricular haemorrhage grade 3‐4 | 6 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.28] |
| 13.2 IVH any grade | 3 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.32] |
| 13.3 Cystic periventricular leukomalacia | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.44] |
| 14 Retinopathy of prematurity (ROP) stage ≥ 3 | 6 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 15 Patent ductus arteriosus (PDA) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 PDA ‐ pharmacological treatment | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 15.2 PDA ‐ surgical closure | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.99] |
1.12. Analysis.
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 12 Pneumopericardium.
Comparison 2. Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death before discharge | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.25, 68.16] |
| 2 Endotracheal intubation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endotracheal intubation in the delivery room | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.68, 1.46] |
| 3 Surfactant administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Surfactant given in the delivery room | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.31, 1.35] |
| 4 Chronic lung disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Moderate to severe BPD | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.33, 2.37] |
| 5 Pneumothorax | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At any time | 1 | 9 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.34, 0.34] |
| 6 Cranial ultrasound abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Intraventricular haemorrhage grade 3 to 4 | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.05, 2.98] |
| 6.2 IVH any grade | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.07, 1.15] |
| 7 Retinopathy of prematurity (ROP) stage ≥ 3 | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 1.68] |
| 8 Patent ductus arteriosus (PDA) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 PDA ‐ pharmacological treatment | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd 2017.
| Methods | Prospective randomised parallel controlled trial Setting: delivery room of Maternity Hospital, Mansoura University Children's Hospital, Egypt Conducted: March 2013 to June 2016 |
|
| Participants | Inclusion criteria: preterm infants born ≤ 32 weeks' gestation with RDS. Exclusion criteria: major congenital anomalies. |
|
| Interventions |
|
|
| Outcomes | Primary outcome: rate of endotracheal intubation in the delivery room. Secondary outcomes: rate of MV (within 72 hours), duration of MV, duration of CPAP support, rate of surfactant (within 72 hours), death before hospital discharge, BPD (within 90 days), IVH (within 14 days), ROP (within 50 days), NEC (within 40 days), hospital stay, air‐leak syndrome (within 14 days), pneumothorax or pneumomediastinum. The following outcomes were reported in the manuscript but not in the protocol: mortality within 90 days, requirement for oxygen therapy more than 30% by 36 weeks' corrected gestational age, length of NICU stay. |
|
| Notes | Study was registered at ClinicalTrials.gov (Identifier: NCT02846597) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "using internet based random table technique" |
| Allocation concealment (selection bias) | Low risk | Quote "opaque sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote "The clinical pathologist who performed the laboratory measures and the nursing staff responsible for the care of preterm infants in the NICU were blinded to groups of intervention". Not specified for the attending neonatologists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the protocol were reported in the manuscript |
| Other bias | Low risk | Appears free of other bias |
El‐Chimi 2017.
| Methods | Prospective quasi‐randomised parallel controlled trial Setting: delivery room of Maternity Hospital, Ain Shams University, Cairo, Egypt Conducted: April 2012 to March 2014 |
|
| Participants | Inclusion criteria (as specified in the protocol): gestational age 26 to 33 weeks, birth weight > 750 grams Exclusion criteria (as specified in the protocol): major congenital anomalies; meconium aspiration syndrome, congenital diaphragmatic hernia, anterior abdominal wall defect, maternal chorioamnionitis |
|
| Interventions |
|
|
| Outcomes | Primary outcome was either "success" (defined as no rate of further ventilatory support, rate of exclusive NCPAP, or rate of intubation beyond the first 72 hours after delivery) or "failure" (defined as rate of intubation within first 72 hours of life, including DR intubation) Secondary outcomes were blood IL‐1b and TNF‐a levels, air leaks, BPD, IVH, PDA, and NEC |
|
| Notes | Study was registered at ClinicalTrials.gov (Identifier: NCT01255826) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | For randomisation, sequentially numbered sheets were used to assign eligible infants to resuscitation: odd‐numbered sheets indicated those allocated to the SLI group, and even‐numbered to the control group |
| Allocation concealment (selection bias) | High risk | No opaque envelopes were used (information provided by study authors) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After enrolment (n = 202), infants referred to other NICUs were excluded from analysis owing to failure of follow‐up. At study end, SLI group comprised 57 babies and CBMI group comprised 55 babies |
| Selective reporting (reporting bias) | High risk | Some outcomes were specified at clinicaltrials.gov/ct2/show/NCT01255826 but were not reported in the manuscript (e.g. duration of oxygen therapy, length of NICU stay) |
| Other bias | Low risk | Appears free of other bias |
Jiravisitkul 2017.
| Methods | Prospective randomised parallel controlled trial Setting: delivery room of Ramathibodi Hospital, Mahidol University, Bangkok, Thailand Conducted: November 2013 to March 2015 |
|
| Participants | Included: 81 preterm infants (25 to 32 weeks of gestational age) requiring positive‐pressure ventilation or continuous positive airway pressure Exclusion criteria: major congenital anomalies, hydrops foetalis, prenatal diagnosis of upper airway obstruction, meconium‐stained amniotic fluid |
|
| Interventions |
All enrolled infants were resuscitated with an initial fraction of inspired oxygen (FiO₂) of 0.3, which was adjusted by 0.1 every 30 seconds to achieve the target SpO₂. Criteria for intubation included 1 of the following: remaining apnoeic after PPV; HR of 30 seconds before the start of chest compressions; or SpO₂ < 80% despite CPAP via mask with FiO₂ of 1.0 for 5 to 10 minutes Infants of multiple gestations were enrolled in the same intervention group |
|
| Outcomes | Primary outcomes: change in oxygen requirements, HR, and SpO₂ during resuscitation; proportion of infants on room air during first 10 minutes after birth; rate of intubation in the delivery room Secondary outcomes: survival at discharge, duration of hospitalisation, proportion of infants on MV within first 72 hours of life, duration of MV, duration of oxygen supplementation, rate of surfactant, rate of postnatal steroids, pneumothorax within first 48 hours after NICU admission, moderate to severe BPD as defined by Jobe and Bancalari, Apgar score at 5 minutes, PDA and rate of surgical closure, grade 3 to 4 IVH, cystic periventricular leukomalacia, stage > 2 ROP, ROP requiring treatment |
|
| Notes | Study was registered in the Thai Clinical Trials Registry (TCTR20140418001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block of 4 randomisation stratified by GA: 25 to 28 weeks and 29 to 32 weeks. Random sequence was generated by computer random number generator (information provided by study authors) |
| Allocation concealment (selection bias) | Low risk | Sequence numbers were kept in opaque sealed envelopes that were opened just before birth in the delivery room by a person not involved in resuscitation of infants |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear why 43 infants in SLI group and 38 in control group |
| Selective reporting (reporting bias) | Unclear risk | According to the Thai Clinical Trials Registry (TCTR20140418001), the only primary outcome was intubation in DR; only a key secondary outcome was specified: BPD |
| Other bias | Unclear risk | Planned sample size: 40 infants in each group; however, only 38 in control group |
Kirpalani 2019.
| Methods | International, multicenter, prospective, unblinded, RCT in 17 hospitals recruiting between 2014 to 2017 Infants were randomised using 1:1 allocation, variable block sizes and stratification by site and GA. A sealed opaque envelope was opened after delivery. A sample size of 592 was sufficient to detect a reduction in the rate of BPD/death from 65% to 52.5% with 80% power, adjusting for interim analyses and multiple births. Stopping rules for efficacy, and signals for harm (including death and early death at < 48 hours of life), were both prespecified. An independent DSMB including a neonatal ethicist reviewed all data |
|
| Participants | Infants from 23 to 26 weeks' gestational age were eligible if they required resuscitation for inadequate respiratory effort or heart rate < 100 bpm Exclusion criteria: resuscitation not provided; refusal of informed consent; clinically suspected pulmonary hypoplasia Consent was sought, (approved by local IRB boards) either antenatally (all sites) or by a deferred process (6 sites) A total of 460 infants were recruited; 34 families refused post‐waiver consent, and 1 infant had a missing primary outcome. Thus 425 were analysed |
|
| Interventions | Treatment with SI (Up to 2 SLI; first at 20 cmH₂O for 15 seconds, followed if needed by a second SI of 25 cmH₂O for 15 seconds) ‐ as compared to standard care | |
| Outcomes | Primary outcome of BPD or death at 36 weeks' postmenstrual age The DSMB upon review (the 2nd for efficacy and the 4th for safety), halted the trial for harm. Demographics of infants did not significantly differ by group (Table 1). Rates of the primary outcome, or its 2 components, were not statistically different (Table 2). (RR 1.10, 95% CI 0.9 to 1.3). Rates of pneumothorax and IVH were similar. An excess of early deaths (< 48 hours of age) was seen in the SI arm (7.5% vs 1.4%, P = 0.002). Furthermore, in a blinded adjudication, 12/19 early deaths were considered as possibly attributable to resuscitation (SI N = 11 vs NRP 1), but no cause of death predominated |
|
| Notes | Study was registered at ClinicalTrials.gov (Identifier: NCT02139800). The DSMB halted the trial for harm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computer‐generated permuted block randomisation, with variable block sizes of 2, 4, or 6, stratified by site and gestational age |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used, colour coded by gestational age strata, and opened on confirming eligibility |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The primary outcome was analysed blinded to allocation. Unclear for the secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the protocol were reported in the manuscript |
| Other bias | Unclear risk | Trial lacks power because only 426 infants were enrolled (instead of 600) |
Lindner 2005.
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Ulm, Germany Conducted: August 1999 to February 2002 |
|
| Participants | Inclusion criteria: newly born infants at 25 to 28 weeks of gestation inclusive Exclusion criteria: severe malformations, oligohydramnios before 20 weeks of gestation, foeto‐foetal transfusion syndrome A total of 61 infants were enrolled (31 in sustained inflation group and 30 in control group) |
|
| Interventions |
Infants received support from a mechanical ventilator via a nasopharyngeal tube Infants in both groups who had apnoea on NCPAP could be treated with NIMV (PIP 20 cmH₂O; inflation time 0.3 seconds; inflation rate 60/min) for up to 4 minutes Treatment was deemed to have failed if infants had shown persistently poor respiratory effort, bradycardia, or cyanosis/low SpO₂ in the delivery room; or if criteria combining clinical assessments of respiratory distress and evidence of impaired oxygenation, impaired ventilation (high CO₂), or apnoea were met within 48 hours of birth |
|
| Outcomes | Primary outcome: rate of infants reaching criteria for intubation and mechanical ventilation at < 48 hours of life Secondary outcomes: mortality, Apgar score, endotracheal intubation, surfactant administration, duration of respiratory support, chronic lung disease, air leak, intraventricular haemorrhage, cystic periventricular leukomalacia, retinopathy of prematurity, PDA |
|
| Notes | Trial was stopped before target sample was recruited owing to slow enrolment. Clinical outcomes were reported for all randomised infants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomised, stratified for gestational age (25 to 26 weeks, 27 to 28 weeks) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for |
| Selective reporting (reporting bias) | Low risk | All reported outcomes provided with complete results |
| Other bias | Unclear risk | Trial lacks power because only 61 infants were enrolled (instead of 110) |
Lista 2015.
| Methods | Multi‐centre prospective randomised parallel controlled trial Setting: Delivery Room, Italy Conducted: October 2011 to January 2013 Infants were assigned immediately after birth before the first breath to receive SLI manoeuvres and NCPAP or NCPAP alone in a 1:1 ratio in permuted blocks of variable size. Randomisation was stratified according to centre and gestational age (25 or 26 weeks and 27 or 28 weeks). Group assignment was contained in sequentially numbered, sealed, opaque envelopes that were prepared by an independent statistician. The trial was not blinded |
|
| Participants | Newly born infants at 25 to 28 weeks of gestation inclusive without major congenital malformations (i.e. congenital heart, cerebral, lung, abdominal malformations), foetal hydrops, and lack of parental consent. A total of 294 infants were enrolled (150 in the sustained lung inflation group and 144 in the control group) | |
| Interventions |
Infants in both groups who were not intubated in the delivery room were transferred to the NICU on NCPAP at 5 cmH₂O with a fraction of inspired oxygen (FiO₂) of 0.21 to 0.40 (in agreement with local protocols) |
|
| Outcomes | Primary outcome: rate of infants reaching mechanical ventilation within the first 72 hours of life Secondary outcomes: MV in the first 3 hours of life, highest FiO₂, duration of NCPAP, rate and duration of bi‐level NCPAP, nasal IMV, conventional or high‐frequency ventilation, duration of hospitalisation, rate and number of doses of surfactant, occurrence of RDS, BPD, and mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomised (1:1 ratio), stratified for gestational age (25 to 26 weeks, and 27 to 28 weeks) |
| Allocation concealment (selection bias) | Low risk | Group assignment was contained in sequentially numbered, automatically generated, sealed, opaque envelopes that were prepared by an independent statistician and distributed to participating centres |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff performing the study also cared for infants later on. However, the decision to start MV was made by clinicians other than investigators involved in the study according to specific guidelines, and researchers assessing study endpoints were blinded to the nature of study treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 0.7% (control group) and 1.3% (SLI group) of participants were lost |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Appears free of other bias |
Mercadante 2016.
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, NICU in Milan, Italy Conducted: September 2013 to June 2014 |
|
| Participants | Inclusion criteria: inborn infants with a gestational age of 34 to 36 weeks after parental consent is obtained Exclusion criteria: major congenital anomalies |
|
| Interventions |
In both groups, mask and T‐piece system were used |
|
| Outcomes | Primary outcome: rate of respiratory support Secondary outcomes: air leak syndromes, NICU admission, NICU admission for respiratory disease, length of stay, exclusive breastfeeding at discharge |
|
| Notes | Sample size described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The decision to start respiratory support was made by clinicians other than investigators involved in the study according to specific guidelines, and researchers assessing study endpoints were blinded to the nature of study treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for |
| Selective reporting (reporting bias) | High risk | We could not ascertain whether deviations from the original protocol were evident in the final publication |
| Other bias | Low risk | |
Ngan 2017.
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, NICU, Royal Alexandra Hospital (RAH), Edmonton, Canada Conducted: June 2013 to August 2014 |
|
| Participants | Inclusion criteria: infants between 23+0 and 32+6 weeks of gestation who require respiratory support for resuscitation in the delivery room Exclusion criteria: congenital abnormality or condition that might have an adverse effect on breathing or ventilation; absence of parents' consent for inclusion in the study |
|
| Interventions |
|
|
| Outcomes | Primary outcome: BPD (rate of respiratory support or supplemental oxygen at corrected gestational age of 36 weeks) Secondary outcomes: rate of endotracheal intubation in the DR or the NICU, duration of MV and non‐invasive ventilation, neonatal death, air leak, PDA (medical or surgical), NEC, ROP, periventricular leukomalacia, abnormal cranial ultrasound (including IVH, parenchymal injury, and ventriculomegaly), surfactant administration, postnatal steroids, respiratory support or oxygen requirements at 28 days, neonatal death before discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme (1:1 ratio). Randomisation stratified according to gestational age (to infants 23+0 to 27+6 and 28+0 to 32+6 weeks). Twins and/or triplets were randomised as individuals |
| Allocation concealment (selection bias) | Unclear risk | A sequentially numbered, brown, sealed envelope contained a folded card box with treatment allocation opened by the clinical team immediately before delivery. Timing of randomisation resulted in many post‐randomisation exclusions with the potential of inadequate allocation concealment, as more post‐randomisation exclusions occurred in the SLI group than in the control group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After admission into the NICU, the clinical team was not made aware of treatment allocation. In addition, both data collector and outcome assessor were unaware of group allocation. The research team was not involved in clinical care of the infants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Post‐randomisation exclusion (27%) resulted in fewer included infants in the SLI group; this discrepancy might have yielded different results |
| Selective reporting (reporting bias) | Low risk | Protocol was registered at ClinicalTrials.gov (NCT01739114) |
| Other bias | Unclear risk | Planned sample size of 93 infants in each group was not achieved. Moreover, incidence of the primary outcome in the control group was lower than assumed for the sample size calculation, further underpowering the trial to detect the desired effect size |
Schmölzer 2018.
| Methods | Prospective randomised parallel controlled trial Pilot (5 infants randomised to each group) Setting: Royal Alexandra Hospital, Edmonton, Alberta, Canada |
|
| Participants | Inclusion criteria: inborn infants between 23+0 and 32+6 weeks of postmenstrual age who required chest compressions in the delivery room Exclusion criteria: congenital abnormality or condition that might have an adverse effect on breathing or ventilation (e.g. congenital pulmonary or airway anomalies, congenital diaphragmatic hernia, congenital heart disease requiring intervention in neonatal period) |
|
| Interventions |
Default settings for airway pressures: PIP of 24 cmH₂O and PEEP of 6 cmH₂O |
|
| Outcomes | Primary outcome: return of spontaneous circulation Secondary outcomes (we obtained the following information directly from trial authors): all mortality before discharge from hospital, delivery room interventions (rate of intubation, use of epinephrine), mechanical ventilation, use of inotropic agents, NEC, moderate to severe BPD, ROP, brain injury as indicated by abnormal neuroimaging |
|
| Notes | Trial was registered at ClinicalTrials.gov: NCT02083705 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | A sequentially numbered, brown, sealed envelope contained a folded card box with treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both data collector and outcome assessor were unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Trial was registered at ClinicalTrials.gov: NCT02083705. However, secondary outcomes were not specified |
| Other bias | Low risk | Appears free of other bias |
Schwaberger 2015.
| Methods | Prospective randomised parallel controlled trial Setting: Delivery Room, Graz, Austria Conducted: April 2012 to December 2013 |
|
| Participants | Inclusion criteria: preterm infants (28 weeks 0 days to 33 weeks 6 days) delivered by elective Caesarean section with HR < 100 or irregular breathing and/or pronounced signs of respiratory distress (grunting, tachypnoea, and increased work of breathing) Exclusion criteria: major congenital malformations, inherited disorders of metabolism and necessity of primary intubation within first 15 minutes after birth. In cases of multiple birth, only 1 of the infants was included |
|
| Interventions | Cord clamping within 30 seconds after delivery. Respiratory support with a T‐piece system in the delivery room
Initial fraction of inspired oxygen (FiO₂) of 0.3 was adapted to achieve defined oxygen saturation targets (3 min: > 60%; 5 min: > 75%; 10 min: > 85%) |
|
| Outcomes | Primary outcome: changes in cerebral blood volume and cerebral tissue oxygenation index during immediate postnatal transition Secondary outcomes: SpO₂, HR, VT, face mask leak, FiO₂ within first 15 minutes after birth |
|
| Notes | Trial was registered at the German Clinical Trials Register (DRKS00005161) in July 2013, after study initiation (April 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation, 1:1 ratio, with a block size of 8 (www.randomizer.at) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. We obtained the following information directly from trial authors: Envelopes were opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assigned intervention could not be blinded to the resuscitation team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cerebral ultrasound pictures were evaluated by a neonatologist blinded to participants. No information was provided for the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes accounted for |
| Selective reporting (reporting bias) | Low risk | Protocol for this trial is available as supporting information. Reporting of the study conforms to Consolidated Standards of Reporting Trials (CONSORT) 2010 statement |
| Other bias | Low risk | Appears free of other bias |
AAP: American Academy of Pediatrics AHA: American Heart Association BPD: bronchopulmonary dysplasia C:V: compression:ventilation CBMI: conventional bag/mask inflation CPAP: continuous positive airway pressure DR: delivery room ECO₂: enzymatic carbonate (measure of carbon dioxide in the blood) FiO₂: fraction of inspired oxygen HR: heart rate IL‐1b: interleukin‐1beta IMV: intermittent mandatory ventilation IPPV: intermittent positive pressure ventilation IVH: intraventricular haemorrhage MV: mechanical ventilation NCPAP: nasal continuous positive airway pressure NEC: necrotising enterocolitis NICU: neonatal intensive care unit NIMV: nasal intermittent mandatory ventilation PDA: patent ductus arteriosus PEEP: positive end‐expiratory pressure PIP: peak inspiratory pressure PPV: positive pressure ventilation RDS: respiratory distress syndrome ROP: retinopathy of prematurity SLI: sustained lung inflation SpO₂: blood oxygen saturation level TNF‐a: tumour necrosis factor‐alpha VT: ventricular tachycardia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bouziri 2011 | Not a clinical trial. Does not investigate sustained lung inflation |
| Gupta 2017 | Commentary to Lista 2015 |
| Harling 2005 | Control group consisted of inflation for 2 seconds (5 seconds for intervention): as we defined sustained if > 1 second, this trial could not be included. Infants in the SLI group were born more preterm and had lower median birth weight than those in the conventional group, although the P value was not provided. Median birth weight (range) was 885 (518 to 1460) grams in the SLI group and 1095 (560 to 1562) grams in the conventional group. Median gestational age (range) was 27 (23 to 30) weeks in the SLI group and 28 (23 to 31) weeks in the conventional group |
| Hunt 2019 | Control group consisted of inflation for 2 to 3 seconds (15 seconds for intervention): as we defined sustained if > 1 second, this trial could not be included |
| te Pas 2007 | This RCT enrolled newly born infants born at < 33 weeks' gestation free of known major congenital anomalies with respiratory distress Infants were randomised to inflation of 10 seconds at 20 cmH₂O with a T‐piece via a nasal tube, or to intermittent PPV with a self‐inflating bag via a face mask. Infants randomised to the T‐piece received inflation for 10 seconds at 20 cmH₂O followed by NCPAP at 5 to 6 cmH₂O. If the infant's clinical response was unsatisfactory, another inflation of 10 seconds at 25 cmH₂O and NIMV (PIP 20 to 25 cmH₂O, inflation rate 60 per minute) could be given. If the infants' condition improved (satisfactory heart rate and colour) but they had irregular breathing, they could receive NIMV for several minutes. Infants who were judged to have inadequate breathing, remained bradycardic, or remained cyanosed in the delivery room after these interventions were intubated and mechanically ventilated. Infants randomised to the self‐inflating bag received initial inflations of 30 to 40 cmH₂O, followed by inflations not > 20 cmH₂O (inflation time was not specified or recorded) for 30 seconds. Infants judged to have inadequate breathing, remained bradycardic, or remained cyanosed in the delivery room after this intervention were intubated and mechanically ventilated. Infants in the sustained lung inflation group who were not intubated were transferred to the NICU on NCPAP at 5 to 6 cmH₂O; non‐intubated infants in the control group were transferred to the NICU with supplemental oxygen and were monitored with pulse oximetry The intervention in this trial was multi‐faceted. In addition to a sustained inflation, many other aspects of respiratory care provided at birth differed between groups (ventilation device used; interface used; whether PEEP was used; whether NIMV was used; time allowed for stabilisation before intubation was considered; time of starting NCPAP). It is not possible to determine the relative contribution (if any) of each element of this intervention to differences in outcomes observed between groups |
NCPAP: nasal continuous positive airway pressure NICU: neonatal intensive care unit NIMV: nasal intermittent mandatory ventilation PEEP: positive end‐expiratory pressure PIP: peak inspiratory pressure PPV: positive pressure ventilation RCT: randomised controlled trial SLI: sustained lung inflation
Characteristics of ongoing studies [ordered by study ID]
NCT01255826.
| Trial name or title | Ventilatory management of the preterm neonate in the delivery room |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Inclusion criteria: preterm infants (gestational age 26 to 34 weeks) with birth weight > 750 grams Exclusion criteria: neonates with major congenital anomalies. Meconium aspiration syndrome, congenital diaphragmatic hernia and anterior abdominal wall defect. Maternal chorioamnionitis |
| Interventions | SLI group: SLI was given using a peak pressure of 20 cmH₂O sustained for 15 seconds, using a T‐piece resuscitator, Neopuff device Control group: CPAP through an appropriate mask using a pressure 5 cmH₂O, using a T‐piece resuscitator, Neopuff device. |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | January 2012 |
| Contact information | Dina Mohamed Mohamed Shinkar, MD, Ain Shams University, Cairo, Egypt |
| Notes | Original estimated enrolment: 50 Actual enrolment: 112 Estimated primary completion date: December 2013 |
NCT01440868.
| Trial name or title | Sustained lung inflation in the delivery room in preterm infants at high risk of respiratory distress syndrome. A RCT study |
| Methods | Multicentre prospective randomised controlled trial |
| Participants | Preterm infants of 25 to 28 weeks of gestational age Exclusion criteria: fetal hydrops, major congenital malformation, inherited metabolic diseases |
| Interventions | SLI group: in this group the preterm infants will receive SLI with mask in the delivery room SLI will be performed with mask using a pressure control system (Neopuff, Fisher & Paykel, Inc). PIP of 25 cmH₂O will be delivered for 15 seconds and then reduced to a PEEP of 5 cmH₂O. A second SLI manoeuvre will be repeated in case of persistent hearth failure (HR < 100 bpm ) Control group: preterm infants will be assisted in the delivery room without sustained lung inflation |
| Outcomes | Primary outcome: rate of mechanical ventilation(MV) within the first 72 hrs of life Secondary outcome: occurrence of MV > 3 hrs of life, length of MV and other non‐invasive respiratory supports, rate of surfactant, mortality, the occurrence of the main prematurity complication such as BPD, IVH , PVL, ROP and NEC, sepsis, and length of NICU and hospital stay. |
| Starting date | October 2011 |
| Contact information | Carlo Dani, MD University of Florence, Italy |
| Notes | Original estimated enrolment: 276 Estimated primary completion date: September 2012 |
NCT02493920.
| Trial name or title | Evaluation of pulmonary mechanics in preterm infant treated with sustained lung inflation at birth |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants at 25 to 36 weeks |
| Interventions | SLI group: PIP of 25 cmH₂O for 15 seconds followed by PEEP of 5 cmH₂O; second SLI in case of poor response Control group: CPAP of 5 cmH₂O with mask |
| Outcomes | Primary outcomes: change in reactance values measured by forced oscillation technique Secondary outcomes: rate of intubation within first 72 hours of life; duration of respiratory support; death in hospital; number of surfactant doses; ROP stage 3 or greater requiring treatment; PDA requiring treatment; BPD; IVH |
| Starting date | July 2015 |
| Contact information | Mariarosa Colnaghi, MD; mariarosa.colnaghi@mangiagalli.it Domenica Mercadante, MD; domenica.mrc@hotmail.it |
| Notes | Estimated enrolment: 48 Estimated primary completion date: December 2015 |
NCT02858583.
| Trial name or title | SURV1VE‐Trial ‐ Sustained inflation and chest compression versus 3:1 chest compression to ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: a randomised controlled trial |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Infants (term or preterm infants) requiring chest compressions in the delivery room |
| Interventions | SLI group: PIP of 25 to 30 cmH₂O for 45 seconds while receiving chest compression. This will be followed by PEEP of 5 to 8 cmH₂O. If heart rate < 60/min, continue with chest compression + SLI for another 45 seconds. If heart rate remains < 60/min, continue with CC + SI Control group: chest compression at a rate of 90/min and 30 ventilations/min in a 3:1 C:V ratio |
| Outcomes | Primary outcomes: return of spontaneous circulation; duration of chest compression heart rate is > 60/min for 15 seconds |
| Starting date | January 2017 |
| Contact information | Georg Schmolzer, MD, PhD; georg.schmoelzer@me.com University of Alberta |
| Notes | Estimated enrolment: 218 Estimated primary completion date: May 2021 |
NCT02887924.
| Trial name or title | The effect of sustained lung inflation maneuver applied through nasal prong on early and late respiratory morbidities in preterm infants |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants of 26 weeks 0 days and 29 weeks 6 days |
| Interventions | SLI group: PIP 25 cmH₂O for 15 seconds with T‐piece and bi‐nasal prongs; second SLI in case of poor response Control group: CPAP |
| Outcomes | Primary outcome: rate of surfactant, intubation and mechanical ventilation needs within first 72 hours of life Secondary outcomes: heart rate, fractional inspiratory oxygen, CPAP pressure and oxygen saturation within first 72 hours of life in preterm infants; total non‐invasive, invasive respiratory support time; BPD; PDA; IVH, NEC; ROP; feeding intolerance, reaching birth weight; transition to full oral feeding time |
| Starting date | August 2016 |
| Contact information | Zekai Tahir Burak Women's Health Research and Education Hospital, Ankara, Turkey |
| Notes | Estimated enrolment: 250 Estimated primary completion date: September 2017 |
NCT03165305.
| Trial name or title | The role of sustained inflation on short term respiratory outcomes in term infants |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Term newborns Exclusion criteria: major congenital/chromosomal abnormalities, lack of informed consent, outborn infants |
| Interventions | SLI group: administering a pressure of 30 cmH₂O by a T‐piece resuscitator for 5 seconds immediately after birth Control group: includes routine neonatal care in the delivery room |
| Outcomes | Primary outcome measures: respiratory morbidity (time frame: 2 hours), RDS, TTN, requirement for supplemental oxygen, intubation or mechanical ventilation support |
| Starting date | January 2017 |
| Contact information | Merih Cetinkaya, Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey drmerih@yahoo.com |
| Notes | Estimated enrolment: 200 Estimated primary completion date: March 2018 |
NCT03437499.
| Trial name or title | Effects of sustained inflation or positive pressure ventilation on release of adrenomedullin in preterm infants with respiratory failure at birth |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Inclusion criteria: gestational age between 28 + 0 and 30 + 6; need for respiratory support in the delivery room. Exclusion criteria: major malformations (i.e. congenital heart disease, cerebral, lung and abdominal malformations), fetal hydrops, lack of parental consent. Need for endotracheal intubation at birth. |
| Interventions | SLI group: application of positive pressure by face mask with T‐piece resuscitator for a prolonged period of 15 seconds at a peak pressure of 25 cmH₂O followed by PEEP set at 5 cmH₂O Control group: Application of positive pressure by face mask with T‐piece resuscitator at a peak pressure of 25 cmH₂O, PEEP set at 5 cmH₂O, for 40 inflations/minute |
| Outcomes | Primary outcome measures: adrenomedullin levels in plasma and urine in preterm infants with respiratory failure within 24 hours |
| Starting date | March 2013 |
| Contact information | Gianluca Lista, MD PhD Vittore Buzzi Children's Hospital, Milan Italy |
| Notes | Actual enrolment: 45 Estimated primary completion date: October 2014 |
NCT03518762.
| Trial name or title | Sustained lung inflation in preterm infants |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Preterm infants of 27 weeks 0 days and 32 weeks 6 days; appropriate for gestational age; weight > 800 grams |
| Interventions | SLI group: PIP 20 cmH₂O for 15 seconds, using a T‐piece resuscitator, Neopuff device Control group: CPAP with mask, pressure 5 cmH₂O, using a T‐piece resuscitator, Neopuff device |
| Outcomes | Primary outcome: rate of invasive mechanical ventilation at 72 hours of life Secondary outcomes: duration of invasive mechanical ventilation; duration of intubation and invasive mechanical ventilation; pneumothorax; BPD |
| Starting date | December 2014 |
| Contact information | Douaa El Saied El Sherbiny, Doctor, Kasr El Aini Hospital |
| Notes | Estimated enrolment: 160 Estimated primary completion date: April 2017 |
BPD: bronchopulmonary dysplasia CC: chest compression CPAP: continuous positive airway pressure C:V: compression: ventilation DR: delivery room FiO₂: fraction of inspired oxygen IVH: intraventricular haemorrhage NEC: necrotising enterocolitis NICU: neonatal intensive care unit PDA: patent ductus arteriosus PEEP: positive end‐expiratory pressure PIE: pulmonary interstitial emphysema PIP: peak inspiratory pressure PPV: positive pressure ventilation PVL: periventricular leukomalacia RDS: respiratory distress syndrome ROP: retinopathy of prematurity SI: sustained inflation SLI: sustained lung inflation TNN: transient tachypnoea of the newborn
Differences between protocol and review
We added clinically relevant outcomes (surfactant administration, rate of mechanical ventilation, retinopathy of prematurity, and PDA).
We planned subgroup analyses according to gestational age (< 37 weeks, ≥ 37 weeks), ventilation device used (self‐inflating bag, flow‐inflating bag, T‐piece, mechanical ventilator), patient interface used (face mask, ETT, nasopharyngeal tube), and duration of sustained inflation (> 1 second to 5 seconds, > 5 seconds). We were unable to conduct any subgroup analyses as few trials met the inclusion criteria.
For the 2017 update, we made the post hoc decision to add a comparison based on use of chest compression during resuscitation. Moreover, we specified Unit of analysis issues and Sensitivity analysis.
For the 2019 update, the search strategy has been modified (see Appendix 1); the outcome "endotracheal intubation outside the delivery room during hospitalization" has been modified into "endotracheal intubation in the first 72 hours of age"; Apgar score was removed as an outcome.
Contributions of authors
Dr. Bruschettini and Dr. O'Donnell performed the literature search, extracted and analysed data, and wrote the manuscript. Prof. Davis performed the literature search, extracted data, checked the analysis, and reviewed the manuscript. Prof. Morley and Dr. Moja reviewed the manuscript. Dr. Calevo analysed data, checked the analysis, and reviewed the manuscript.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University; Research & Development, Skåne University Hospital, Lund, Sweden.
MB is employed by this organization
-
Royal Women's Hospital, Melbourne, Australia.
PD is employed by this organization
-
University of Melbourne, Australia.
PD is employed by this organization
-
Istituto Giannina Gaslini, Genoa, Italy.
MGC is employed by this organization
External sources
-
Murdoch Children's Research Institute, Australia.
PD is employed by this organization
-
National Health and Medical Research Council, Australia.
PD is employed by this organization
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
MB, COD, PD, CM, LM, and MGC have no known conflicts of interest to declare.
Edited (conclusions changed)
References
References to studies included in this review
Abd 2017 {published data only}
- Abd El‐Fattah N, Nasef N, Al‐Harrass MF, Khashaba M. Sustained lung inflation at birth for preterm infants at risk of respiratory distress syndrome: The proper pressure and duration. Journal of Neonatal‐perinatal Medicine 2017;10(4):409‐17. [DOI: 10.3233/NPM-171760; PUBMED: 29286940] [DOI] [PubMed] [Google Scholar]
El‐Chimi 2017 {published data only}
- El‐Chimi MS, Awad HA, El‐Gammasy TM, El‐Farghali OG, Sallam MT, Shinkar DM. Sustained versus intermittent lung inflation for resuscitation of preterm infants: a randomized controlled trial. Journal of Maternal‐fetal & Neonatal Medicine 2017;30(11):1273‐8. [DOI: 10.1080/14767058.2016.1210598; PUBMED: 27384245] [DOI] [PubMed] [Google Scholar]
Jiravisitkul 2017 {published data only}
- Jiravisitkul P, Rattanasiri S, Nuntnarumit P. Randomised controlled trial of sustained lung inflation for resuscitation of preterm infants in the delivery room. Resuscitation 2017;111:68‐73. [DOI: 10.1016/j.resuscitation.2016.12.003; PUBMED: 27987395] [DOI] [PubMed] [Google Scholar]
Kirpalani 2019 {published data only}
- Foglia EE, Owen LS, Thio M, Ratcliffe SJ, Lista G, Pas A, et al. Sustained aeration of infant lungs (SAIL) trial: study protocol for a randomized controlled trial. Trials 2015;16:95. [DOI: 10.1186/s13063-015-0601-9; PUBMED: 25872563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpalani H, Ratcliffe SJ, Keszler M, Davis PG, Foglia EE, Pas A, et al. Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: the SAIL randomized clinical trial. JAMA 2019;321(12):1165‐75. [DOI: 10.1001/jama.2019.1660; PUBMED: 30912836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindner 2005 {published data only}
- Lindner W, Högel J, Pohlandt F. Sustained pressure‐controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? A randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatrica 2005;94(3):303‐9. [PUBMED: 16028648] [DOI] [PubMed] [Google Scholar]
Lista 2015 {published data only}
- Lista G, Boni L, Scopesi F, Mosca F, Trevisanuto D, Messner H, et al. SLI Trial Investigators. Sustained lung inflation at birth for preterm infants: a randomized clinical trial. Pediatrics 2015;135(2):e457‐64. [DOI: 10.1542/peds.2014-1692; PUBMED: 25624390] [DOI] [PubMed] [Google Scholar]
Mercadante 2016 {published data only}
- Mercadante D, Colnaghi M, Polimeni V, Ghezzi E, Fumagalli M, Consonni D, et al. Sustained lung inflation in late preterm infants: a randomized controlled trial. Journal of Perinatology 2016;36(6):443‐7. [DOI: 10.1038/jp.2015.222; PUBMED: 26820220] [DOI] [PubMed] [Google Scholar]
Ngan 2017 {published data only}
- Ngan AY, Cheung PY, Mason AH, O’Reilly M, Os S, Kumar M, et al. Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial. Archives of Disease in Childhood 2017;102(6):F525‐31. [DOI: 10.1136/archdischild-2016-312286] [DOI] [PubMed] [Google Scholar]
Schmölzer 2018 {published data only}
- Schmölzer G, O’Reilly M, Kushniruk K, Aziz K, Cheung PY. Sustained inflation and chest compression versus 3:1 chest compression: ventilation ratio during neonatal CPR – a randomized controlled trial. Paediatrics and Child Health 2015;20(5):e31. [Google Scholar]
- Schmölzer GM, O Reilly M, Fray C, Os S, Cheung PY. Chest compression during sustained inflation versus 3:1 chest compression:ventilation ratio during neonatal cardiopulmonary resuscitation: a randomised feasibility trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(5):F455‐60. [DOI: 10.1136/archdischild-2017-313037; PUBMED: 28988159] [DOI] [PubMed] [Google Scholar]
Schwaberger 2015 {published data only}
- Schwaberger B, Pichler G, Avian A, Binder‐Heschl C, Baik N, Urlesberger B. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? A randomized controlled pilot study. PloS One 2015;10(9):e0138964. [DOI: 10.1371/journal.pone.0138964; PUBMED: 26406467] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bouziri 2011 {published data only}
- Bouziri A, Hamdi A, Khaldi A, Bel Hadj S, Menif K, Ben Jaballah N. Management of meconium aspiration syndrome with high frequency oscillatory ventilation. Tunisie Medicale 2011;89(7):632‐7. [PUBMED: 21780039] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Gupta N, Agarwal R. Prophylactic sustained lung inflation followed by early CPAP vs. early CPAP at birth in extreme preterm neonates. Acta Paediatrica 2017;106(3):517. [DOI: 10.1111/apa.13692; PUBMED: 28120525] [DOI] [PubMed] [Google Scholar]
Harling 2005 {published data only}
- Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does sustained lung inflation at resuscitation reduce lung injury in the preterm infant?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(5):F406‐10. [DOI: 10.1136/adc.2004.059303; PUBMED: 15863490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2019 {published data only}
- Hunt KA, Ling R, White M, Ali KK, Dassios T, Milner AD, et al. Sustained inflations during delivery suite stabilisation in prematurely‐born infants ‐ A randomised trial. Early Human Development 2019;130:17‐21. [DOI: 10.1016/j.earlhumdev.2019.01.005; PUBMED: 30641326] [DOI] [PubMed] [Google Scholar]
te Pas 2007 {published data only}
- Pas AB, Walther FJ. A randomized, controlled trial of delivery‐room respiratory management in very preterm infants. Pediatrics 2007;120(2):322‐9. [DOI: 10.1542/peds.2007-0114; PUBMED: 17671058] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01255826 {published data only}
- NCT01255826. Ventilatory management of the preterm neonate in the delivery room. clinicaltrials.gov/ct2/show/record/NCT01255826 (first received 8 December 2010).
NCT01440868 {published data only}
- NCT01440868. SLI STUDY: sustained lung inflation in the delivery room in preterm infants at high risk of respiratory distress syndrome. A RCT study. clinicaltrials.gov/ct2/show/record/NCT01440868 (first received 27 September 2011).
NCT02493920 {published data only}
- NCT02493920. Sustained lung inflation and pulmonary mechanics in preterm infant [Evaluation of pulmonary mechanics in preterm infant treated with sustained lung inflation at birth]. clinicaltrials.gov/show/NCT02493920 (first received 23 June 2015).
NCT02858583 {published data only}
- NCT02858583. SI + CC Versus 3:1 C:V Ratio During Neonatal CPR [SURV1VE‐Trial ‐ Sustained inflation and chest compression versus 3:1 chest compression to ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: a randomized controlled trial]. clinicaltrials.gov/show/NCT02858583 (first received 8 August 2016).
- Schmölzer GM, Pichler G, Solevåg AL, Fray C, Os S, Cheung PY. The SURV1VE trial‐sustained inflation and chest compression versus 3:1 chest compression‐to‐ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: study protocol for a cluster randomized controlled trial. Trials 2019;20(1):139. [DOI: 10.1186/s13063-019-3240-8; PUBMED: 30782199] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02887924 {published data only}
- NCT02887924. SLI maneuver and respiratory morbidities [The effect of sustained lung inflation maneuver applied through nasal prong on early and late respiratory morbidities in preterm infants]. clinicaltrials.gov/show/NCT02887924 (first received 2 September 2016).
NCT03165305 {published data only}
- Merih Cetinkaya, Kanuni Sultan Suleyman Training and Research Hospital. The role of sustained inflation on short term respiratory outcomes in term infants. clinicaltrials.gov/ct2/show/NCT03165305 January 2017.
NCT03437499 {published data only}
- Gianluca Lista, Vittore Buzzi Children's Hospital, Milan Italy. Effects of sustained inflation or positive pressure ventilation on release of adrenomedullin in preterm infants with respiratory failure at birth. clinicaltrials.gov/ct2/show/record/NCT03437499 February 2018.
NCT03518762 {published data only}
- NCT03518762. Sustained lung inflation in preterm infants. clinicaltrials.gov/show/NCT03518762 (first received 8 May 2018).
Additional references
Boon 1979
- Boon AW, Milner AD, Hopkin IE. Lung expansion, tidal exchange, and formation of the functional residual capacity during resuscitation of asphyxiated neonates. Journal of Pediatrics 1979;95(6):1031‐6. [DOI: 10.1016/s0022-3476(79)80304-2; PUBMED: 387935] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Drew 1982
- Drew JH. Immediate intubation at birth of the very‐low‐birth‐weight infant. Effect on survival. American Journal of Diseases of Children 1982;136(3):207‐10. [DOI: 10.1001/archpedi.1982.03970390021006; PUBMED: 7039303] [DOI] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). Cochrane EPOC review group data collection checklist. epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf (accessed prior to 25 February 2020).
Foglia 2016
- Foglia EE, Pas AB. Sustained lung Inflation: physiology and practice. Clinics in Perinatology 2016;43(4):633‐46. [DOI: 10.1016/j.clp.2016.07.002; PUBMED: 27837749] [DOI] [PubMed] [Google Scholar]
Fuchs 2011
- Fuchs H, Lindner W, Buschko A, Trischberger T, Schmid M, Hummler HD. Cerebral oxygenation in very low birth weight infants supported with sustained lung inflations after birth. Pediatric Research 2011;70(2):176–80. [DOI: 10.1203/PDR.0b013e318220c1e0; PUBMED: 21522035] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 August 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grasso 2015
- Grasso C, Sciacca P, Giacchi V, Carpinato C, Mattia C, Palano GM, et al. Effects of sustained lung inflation, a lung recruitment maneuver in primary acute respiratory distress syndrome, in respiratory and cerebral outcomes in preterm infants. Early Human Development 2015;91(1):71‐5. [DOI: 10.1016/j.earlhumdev.2014.12.002; PUBMED: 25549915] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hooper 2016
- Hooper SB, Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three‐phase process. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(3):F266‐71. [DOI: 10.1136/archdischild-2013-305704; PUBMED: 26542877] [DOI] [PubMed] [Google Scholar]
Karlberg 1962
- Karlberg P, Cherry RB, Escardó FE, Koc G. Respiratory studies in newborn infants. II: Pulmonary ventilation and mechanics of breathing in the first minutes of life, including the onset of respiration. Acta Paediatrica 1962;51(2):121‐36. [DOI: 10.1111/j.1651-2227.1962.tb06521.x] [DOI] [Google Scholar]
Klingenberg 2016
Lindner 1999
- Lindner W, Vossbeck S, Hummler H, Pohlandt F. Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation?. Pediatrics 1999;103(5 Pt 1):961‐7. [DOI: 10.1542/peds.103.5.961; PUBMED: 10224173] [DOI] [PubMed] [Google Scholar]
Lista 2010
- Lista G, Castoldi F. Alveolar recruitment in the delivery room: sustained lung inflation [Reclutamento alveolare in sala parto: la sustained lung inflation]. Minerva Pediatrica 2010;62(3 Suppl 1):17‐8. [PUBMED: 21089711] [PubMed] [Google Scholar]
Lista 2011
- Lista G, Fontana P, Castoldi F, Cavigioli F, Dani C. Does sustained lung inflation at birth improve outcome of preterm infants at risk for respiratory distress syndrome?. Neonatology 2011;99(1):45‐50. [DOI: 10.1159/000298312; PUBMED: 20616570] [DOI] [PubMed] [Google Scholar]
Lista 2017
- Lista G, Cavigioli F, Verde PA, Castoldi F, Bresesti I, Morley CJ. Effects of breathing and apnoea during sustained inflations in resuscitation of preterm infants. Neonatology 2017;111(4):360‐6. [DOI: 10.1159/000454799; PUBMED: 28118641] [DOI] [PubMed] [Google Scholar]
McCall 2016
- McCall KE, Davis PG, Owen LS, Tingay DG. Sustained lung inflation at birth: what do we know, and what do we need to know?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(2):F175‐80. [DOI: 10.1136/archdischild-2015-309611; PUBMED: 26527635] [DOI] [PubMed] [Google Scholar]
Milner 1977
- Milner AD, Saunders RA. Pressure and volume changes during the first breath of human neonates. Archives of Disease in Childhood 1977;52(12):918‐24. [DOI: 10.1136/adc.52.12.918; PUBMED: 606168] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2004a
- O'Donnell CPF, Davis PG, Morley CJ. Neonatal resuscitation: review of ventilation equipment and survey of practice in Australia and New Zealand. Journal of Paediatrics and Child Health 2004;40(4):208‐12. [PUBMED: 15009551] [DOI] [PubMed] [Google Scholar]
O'Donnell 2004b
- O'Donnell CP, Davis PG, Morley CJ. Positive pressure ventilation at neonatal resuscitation: review of equipment and international survey of practice. Acta Paediatrica 2004;93(5):583‐8. [PUBMED: 15174776] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI: 10.1016/s0022-3476(78)80282-0; PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696–705. [DOI: 10.1001/archneur.1976.00500100030012; PUBMED: 987769] [DOI] [PubMed] [Google Scholar]
Saugstad 1998
- Saugstad OD. Practical aspects of resuscitating asphyxiated newborn infants. European Journal of Pediatrics 1998;157 Suppl 1:S11‐5. [PUBMED: 9462900] [DOI] [PubMed] [Google Scholar]
Schmölzer 2014
- Schmölzer GM, Kumar M, Aziz K, Pichler G, O'Reilly M, Lista G, et al. Sustained inflation versus positive pressure ventilation at birth: a systematic review and meta‐analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015; Vol. 100, issue 4:F361‐8. [DOI: 10.1136/archdischild-2014-306836; PUBMED: 25550472] [DOI] [PubMed]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Tracy 2019
- Tracy MB, Halliday R, Tracy SK, Hinder MK. Newborn self‐inflating manual resuscitators: precision robotic testing of safety and reliability. Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(4):F403‐8. [DOI: 10.1136/archdischild-2018-315391; PUBMED: 30337333] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Vonderen 2014
- Vonderen JJ, Hooper SB, Hummler HD, Lopriore E, Pas AB. Effects of a sustained inflation in preterm infants at birth. Journal of Pediatrics 2014;165(5):903‐8.e1. [DOI: 10.1016/j.jpeds.2014.06.007; PUBMED: 25039041] [DOI] [PubMed] [Google Scholar]
Vyas 1981
- Vyas H, Milner AD, Hopkin IE, Boon AW. Physiologic responses to prolonged and slow‐rise inflation in the resuscitation of the asphyxiated newborn infant. Journal of Pediatrics 1981;99(4):635‐9. [DOI: 10.1016/s0022-3476(81)80279-x; PUBMED: 7277110] [DOI] [PubMed] [Google Scholar]
Wyckoff 2015
- Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 Suppl 2):S543‐60. [DOI: 10.1161/CIR.0000000000000267; PUBMED: 26473001] [DOI] [PubMed] [Google Scholar]
Wyllie 2015
- Wyllie J, Bruinenberg J, Roehr CC, Rudiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation 2015;95:249‐63. [DOI: 10.1016/j.resuscitation.2015.07.029; PUBMED: 26477415] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bruschettini 2017
- Bruschettini M, O'Donnell CP, Davis PG, Morley CJ, Moja L, Zappettini S, et al. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD004953.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2004
- O'Donnell CPF, Davis PG, Morley CJ. Sustained inflations for neonatal resuscitation. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004953] [DOI] [Google Scholar]
O'Donnell 2015
- O'Donnell CP, Bruschettini M, Davis PG, Morley CJ, Moja L, Calevo MG, et al. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004953.pub2] [DOI] [PubMed] [Google Scholar]


