Abstract
Background
Surgical aortic valve replacement (SAVR) is the conventional treatment in patients at low or intermediate surgical risk. Transcatheter aortic valve implantation (TAVI) is a less invasive procedure, originally developed as an alternative for patients at high or prohibitive surgical risk.
Methods
We conducted a health technology assessment of TAVI versus SAVR in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk, which included an evaluation of effectiveness, safety, cost-effectiveness, budget impact, and patient preferences and values. We performed a literature search to retrieve systematic reviews and selected one that was relevant to our research question. We complemented the systematic review with a literature search to identify randomized controlled trials published after the review. Applicable, previously published cost-effectiveness analyses were available, so we did not conduct a primary economic evaluation. We analyzed the net budget impact of publicly funding TAVI in people at intermediate surgical risk in Ontario. To contextualize the potential value of TAVI for people at intermediate surgical risk, we spoke with people who had aortic valve stenosis and their families.
Results
We identified two randomized controlled trials; they found that in patients with severe, symptomatic aortic valve stenosis, TAVI was noninferior to SAVR with respect to the composite endpoint of all-cause mortality or disabling stroke within 2 years of follow-up (GRADE: High). However, compared with SAVR, TAVI had a higher risk of some complications and a lower risk of others. Device-related costs for TAVI (approximately $23,000) are much higher than for SAVR (approximately $6,000). Based on two published cost-effectiveness analyses conducted from the perspective of the Ontario Ministry of Health, TAVI was more expensive and, on average, more effective (i.e., it produced more quality-adjusted life-years) than SAVR. The incremental cost-effectiveness ratios showed that TAVI may be cost-effective, but the probability of TAVI being cost-effective versus SAVR was less than 60% at a willingness-to-pay value of $100,000 per quality-adjusted life-year. The net budget impact of publicly funding TAVI in Ontario would be about $2 million to $3 million each year for the next 5 years. This cost may be reduced if people receiving TAVI have a shorter hospital stay (≤ 3 days). We interviewed 13 people who had lived experience with aortic valve stenosis. People who had undergone TAVI reported reduced physical and psychological effects and a shorter recovery time. Patients and caregivers living in remote or northern regions reported lower out-of-pocket costs with TAVI because the length of hospital stay was reduced. People said that TAVI increased their quality of life in the short-term immediately after the procedure.
Conclusions
In people with severe, symptomatic aortic valve stenosis at intermediate surgical risk, TAVI was similar to SAVR with respect to the composite endpoint of all-cause mortality or disabling stroke. However, the two treatments had different patterns of complications. The study authors also noted that longer follow-up is needed to assess the durability of the TAVI valve. Compared with SAVR, TAVI may provide good value for money, but publicly funding TAVI in Ontario would result in additional costs over the next 5 years. People with aortic valve stenosis who had undergone TAVI appreciated its less invasive nature and reported a substantial reduction in physical and psychological effects after the procedure, improving their quality of life.
OBJECTIVE
This health technology assessment looked at the effectiveness, safety, cost-effectiveness, budget impact, and patient experiences of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk.
BACKGROUND
Health Condition
The aortic valve is located between the aorta and the left ventricle of the heart.1 It opens to allow blood to flow from the left ventricle into the aorta when the heart contracts, and closes to prevent blood from flowing backward into the heart when the heart relaxes.1
Aortic valve stenosis occurs when the valve partially narrows, obstructing blood flow from the heart into the aorta.1 The most common cause in men older than 65 years and women older than 75 years is degenerative calcification2: a buildup of calcium deposits on the valve over time, causing it to narrow.2–4 In younger patients, it is usually due to a congenital bicuspid aortic valve (an inherited condition in which the aortic valve has two leaves instead of the usual three).5 Narrowing of the aortic valve causes the heart to work harder and is usually progressive, leading to left ventricular hypertrophy (thickening of the walls of the left ventricle) and heart failure.2 Symptoms of aortic valve stenosis include chest pain, shortness of breath, and fatigue that decrease people's quality of life and affect their activities of daily living.1
Clinical Need and Target Population
The prevalence of moderate to severe aortic valve stenosis increases with age: it is estimated to affect 0.02% of people 18 to 44 years old and 2% of people over age 65 years.3 One study reported that the prevalence of severe aortic valve stenosis in people over age 75 years was 3.4%, and three-quarters of cases were symptomatic.6 Based on a cohort of patients who underwent surgery in the United States, about 14% of patients with severe aortic valve stenosis are at intermediate surgical risk.7
Severe, symptomatic aortic valve stenosis is associated with a poor prognosis: without aortic valve replacement, a person's estimated life expectancy is less than 5 years,2 and more than half of patients will die within 2 to 3 years of the onset of symptoms.4 Medications may ease the symptoms, but surgical replacement of the valve is the only way to treat aortic valve stenosis.2
Current Treatment Options
The conventional way to correct aortic valve stenosis is SAVR,1,2 except in patients who have inoperable conditions or who are at high surgical risk.8 In SAVR, the damaged aortic valve is removed and replaced with an artificial valve, which can be either mechanical or biological.2 The procedure is an open-heart surgery that requires cardiopulmonary bypass (using a heart– lung machine) and is performed under general anesthesia.2 Patients undergoing SAVR who require revascularization may be considered for SAVR combined with a coronary artery bypass graft (CABG).
In North America, a patient's surgical risk is assessed by a multidisciplinary heart team informed by the Society of Thoracic Surgeons (STS) risk score,9 which considers the presence of comorbidities to predict mortality 30 days after the surgery.10 The STS risk score has been validated in standard surgical-risk populations. In general, a risk score of 8% or more is considered to be high or greater risk,9 and a score of 4% to 8% is considered to be intermediate risk.10 However, other comorbidities that are not represented in the STS score also need to be taken into account when assessing surgical risk,9,10 including frailty, porcelain aorta (an ascending aorta that is heavily calcified), and severe liver disease.11 Because of the complexity of risk assessment, it must be done by a multidisciplinary heart team,10 usually consisting of interventional cardiologists, valve specialists, cardiac surgeons, and anesthetists, among others.8
Health Technology Under Review
Transcatheter aortic valve implantation involves placing a collapsible, bioprosthetic aortic valve inside the existing valve through a catheter, without the need for open-heart surgery.2 When the new valve is expanded, it pushes the narrowed valve outward and takes over regulation of blood flow from the left ventricle to the aorta.5
The TAVI procedure can be done under local or general anesthesia if the catheter is inserted using the transfemoral route, or under general anesthesia if using other routes.9 The transfemoral route is the most common, inserting the catheter via a small incision in the common femoral artery (a large artery in the thigh).5 Other routes — such as the transthoracic route (via an incision in the chest) or the subclavian route (via an artery that sits below the collarbone) — are alternatives for when the femoral artery cannot be used because of size, calcification, or tortuosity.2,12 With the transthoracic route, TAVI can be performed using the transapical route (via a small incision in the chest to enter the aorta through the left ventricle) or the transaortic route (direct access to the aorta through a small cut in the chest).5 The narrowed valve may be expanded ahead of time using a procedure called balloon valvuloplasty.13
Balloon-expandable and self-expanding bioprosthetic valves are currently available in Canada.1 The Sapien valve is a first-generation balloon-expandable valve,14 and since its release, the second-generation Sapien XT and third-generation Sapien 3 balloon-expandable valves have also been developed.14 The Sapien valves consist of a bovine pericardium valve mounted on a stent frame.10 The CoreValve is a first-generation self-expanding valve; its successor is the second-generation Evolut R valve.14 Both the CoreValve and the Evolut R consist of a porcine pericardium valve mounted on a self-expanding stent frame.9 The self-expanding supra-annular Acurate Neo valve is also available: a porcine pericardium valve mounted on a nitinol frame.15 Device-related costs for TAVI (approximately $23,000) are much higher than for SAVR (approximately $6000).16,17
The TAVI procedure is performed by clinicians and teams with specific training or experience in complex endovascular cardiac procedures.2 If revascularization is necessary, percutaneous coronary intervention may be performed, either before or occasionally at the same time as the TAVI procedure.
In November 2016, based on the finding that mortality with TAVI was not higher than with SAVR, and given that both treatments improved patients' quality of life during the first year after the surgery, the Ontario Health Technology Advisory Committee recommended public funding for TAVI in patients with severe, symptomatic, degenerative aortic valve stenosis who were not candidates for SAVR or who had an estimated risk of mortality of 8% or greater within 30 days of surgery.18 The committee also recommended that TAVI be offered in select hospitals, as determined by the Cardiac Care Network of Ontario (now CorHealth Ontario).18 Since that report, studies evaluating TAVI in patients at intermediate surgical risk have been published.10,19
Regulatory Information
Bioprosthetic transcatheter aortic valves have been approved by Health Canada as Class IV devices, either balloon-expandable (Sapien, Sapien XT, and Sapien 3 from Edwards Lifesciences) or self-expanding (CoreValve or Evolut R from Medtronic; Acurate Neo from Boston Scientific).
Health Canada approved the use of these valves in patients with severe, symptomatic, aortic valve stenosis who are at high or greater surgical risk (Health Canada, email communication, December 2017 to May 2018). The Acurate Neo valve is further restricted to patients age 75 years or older and to the transfemoral route of implantation. The Sapien 3 valve has been restricted to the transfemoral route in patients with severe, symptomatic, calcific aortic valve stenosis who are judged by a heart team to be at intermediate risk for open-heart surgery.
Ontario Context
The TAVI procedure is conducted at 11 sites in Ontario (CorHealth Ontario). The annual number of SAVR and TAVI procedures (all cases in which the procedure was started) is provided in Table 1.
Table 1:
Number of SAVR and TAVI Procedures Performed in Ontario, 2011/12 to 2017/18
| Type of Procedure | 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | 2016/17 | 2017/18 |
|---|---|---|---|---|---|---|---|
| SAVR only | 1,720 | 1,691 | 1,843 | 1,764 | 1,864 | 1,887 | 1,978 |
| SAVR + CABG | 1,128 | 1,149 | 1,094 | 1,136 | 1,165 | 1,247 | 1,136 |
| TAVI | 224a | 341a | 486a | 646 | 745 | 863 | 1,022 |
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Data may be incomplete; mandatory TAVI data collection started in November 2013.
Source: CorHealth Ontario Cardiac Registry. Data retrieved May 4, 2018.
Most of the SAVR procedures performed between June 1, 2015, and June 30, 2017, were in patients at low surgical risk (79% for SAVR plus CABG and 89% for SAVR alone); 16% (SAVR plus CABG) and 8% (SAVR alone) were performed in patients at intermediate surgical risk; and 5% (SAVR plus CABG) and 2% (SAVR alone) in patients at high surgical risk. Surgical risk information was not provided for TAVI procedures (CorHealth Ontario Cardiac Registry, data retrieved May 4, 2018).
Currently, in Ontario, there is no formal public funding for TAVI in patients at intermediate surgical risk.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42018093719),20 available at https://www.crd.york.ac.uk/PROSPERO.
CLINICAL EVIDENCE
Research Question
What are the effectiveness and safety of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) in adults with severe, symptomatic aortic valve stenosis who are at intermediate surgical risk?
Methods
We developed the research questions in consultation with health care providers, clinical experts, methodologists, and other health system stakeholders.
Clinical Literature Search
Because systematic reviews have been published on this topic, we selected a relevant systematic review and complemented its literature search. First, we undertook a systematic literature search to identify published systematic reviews that appropriately matched our research question, as well as our population, intervention, comparators, outcomes, timing, and setting (PICOTS). We assessed eligible systematic reviews using the Risk of Bias in Systematic Reviews (ROBIS) tool.21 We then selected the systematic review that matched our research question and PICOTS most closely, and that had the lowest risk of bias. We identified two systematic reviews with a low risk-of-bias profile, and we chose the one with the most recent literature search. Then, we ran a literature search to identify individual studies published since the selected systematic review was conducted.
We performed the initial literature search on March 28, 2018, to retrieve systematic reviews published from inception to the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, Cochrane Database of Systematic Reviews, Health Technology Assessment, and National Health Service Economic Evaluation Database (NHS EED). We used a search filter to restrict search results to systematic reviews, meta-analyses, and health technology assessments.
We then performed a literature search on April 13, 2018, to retrieve randomized controlled trials published from January 1, 2017, to the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, and Cochrane Central Register of Controlled Trials. We used a search filter to restrict search results to randomized controlled trials.
Medical librarians developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategies were peer-reviewed using the PRESS Checklist.22 We created database auto-alerts for the randomized controlled trial search in MEDLINE and Embase and monitored them for the duration of the assessment period.
We also performed targeted grey literature searching of health technology assessment agency websites, PROSPERO, EUnetHTA Assessments, and clinical trial registries. See Appendix 1 for literature search strategies, including all search terms.
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using DistillerSR management software (Evidence Partners, Ottawa, Canada), and then obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. The author then examined the full text articles and selected studies that were eligible for inclusion. The author also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
Search for Systematic Reviews
English-language full-text publications
Reviews published between inception and March 28, 2018
Systematic reviews and health technology assessments that included a systematic review
Reviews that compared TAVI and SAVR
Reviews in adult patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk (determined by the study site multidisciplinary heart team, informed by a Society of Thoracic Surgeons [STS] score [4%–8%] or Logistic EuroScore [10%–20%] and assessment of comorbidities)
Reviews that provided information on the literature search methods, including (at a minimum) the databases searched, search strategy, and start and end search dates
Reviews with prespecified eligibility criteria
Search for Individual Studies
English-language full-text publications
Randomized controlled trials comparing TAVI and SAVR
Studies in adult patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk (determined by the study site multidisciplinary heart team, informed by an STS score [4%–8%] or Logistic EuroScore [10%–20%] and assessment of comorbidities)
Studies identified in the selected systematic review and from our extended systematic literature search (i.e., published between January 1, 2017, and April 13, 2018)
Exclusion Criteria
Search for Systematic Reviews
Nonsystematic reviews, individual studies, editorials, commentaries, conference abstracts, letters
Reviews evaluating TAVI in patients with a pre-existing mechanical or bioprosthetic aortic valve (i.e., valve-in-valve procedures)
Search for Individual Studies
Nonrandomized studies, editorials, commentaries, case reports, conference abstracts, letters
Studies evaluating TAVI in patients with a pre-existing mechanical or bioprosthetic aortic valve (i.e., valve-in-valve procedures)
Studies that included a mixed population with different surgical risks (i.e., low, intermediate, and high) without providing results specific to the intermediate-risk population
Outcomes of Interest
Mortality (2 years)
Stroke/transient ischemic attack (2 years)
Life-threatening or major/disabling bleeding (30 days)
Acute kidney injury (30 days)
Myocardial infarction (2 years)
Atrial fibrillation (30 days)
New permanent pacemaker implantation (30 days)
Major vascular complications (30 days)
Aortic valve hemodynamics (2 years)
Paravalvular aortic regurgitation (2 years)
Valve deterioration (2 years)
Aortic valve reintervention (2 years)
Aortic valve rehospitalization (2 years)
Length of hospital stay (implantation procedure hospitalization)
New York Heart Association (NYHA) symptoms (30 days and 2 years)
Quality of life (30 days and 2 years)
Data Extraction
For systematic reviews, we extracted PICOTS, the literature search date, and eligibility criteria.
For individual studies, we extracted relevant data on study design and characteristics, risk-of-bias items, results, and PICOTS. We extracted the patients' baseline characteristics, including those based on the PROGRESS-Plus categories (place of residence, race/ethnicity, occupation, gender/sex, religion, education, socioeconomic status, social capital), when available.23
We contacted authors of the studies to provide clarification as needed.
Evidence Synthesis and Statistical Analysis
We summarized the results of randomized controlled trials (identified in the selected systematic review and in our systematic literature search for more recently published studies) that compared TAVI and SAVR in adults with symptomatic aortic valve stenosis at intermediate surgical risk. We reported the results at different follow-up points based on information presented in the studies. The PARTNER 2 trial10 reported the results of dichotomous outcomes as risk based on Kaplan–Meier estimates and hazard ratios based on a Cox proportional hazards analysis; mean and standard deviation (SD) were reported for continuous outcomes. The SURTAVI trial19 reported risks and 95% credible intervals for the difference for dichotomous variables and mean (SD) for continuous variables. The SURTAVI trial19 used Bayesian statistical methods, and the PARTNER 2 trial10 used the frequentist approach. When necessary, and if not provided in the study, we calculated the risk difference based on information reported in the studies. We reported the results of intention-to-treat analyses, unless otherwise specified in cases where that information was unavailable.
We presented study results for the full cohort and stratified by implantation access route (transfemoral or transthoracic) and PROGRESS-Plus categories23 when data were available.
For procedure-related outcomes, we based our main results and conclusions on the 30-day follow-up point (life-threatening or major/disabling bleeding, acute kidney injury, atrial fibrillation, new permanent pacemaker implantation, and major vascular complications). For other outcomes, we based our main results and conclusions on the longest follow-up available (i.e., 2 years). For quality of life and NYHA symptoms, we presented the results for both the 30-day and 2-year follow-up points.
When appropriate, we performed meta-analyses using Review Manager version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). One of the studies used Bayesian statistical methods, but because it also used a uniform prior distribution, we assumed that the numerical results would be the same as those obtained using a frequentist approach, and this justified pooling the results of the two included studies. For dichotomous outcomes, we performed meta-analyses using the number of events reported in the studies to calculate absolute risk difference between TAVI and SAVR.
We assessed statistical heterogeneity using the I2 statistic24 and by visually examining forest plots. We used a fixed- or a random-effects model depending on the extent of the heterogeneity in each meta-analysis. If meta-analysis was not appropriate because of clinical, methodological, or statistical heterogeneity, we provided a narrative summary of results.
Critical Appraisal of Evidence
We assessed risk of bias using the ROBIS tool21 for systematic reviews and the Cochrane Risk of Bias tool25 for randomized controlled trials (Appendix 2).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.26 We assessed the body of evidence based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality score reflects our assessment of the reliability of the evidence.
Expert Consultation
We solicited expert feedback on the current and expected use of TAVI among patients with aortic valve stenosis at intermediate surgical risk. The consultation included methodologists and physicians in the specialty areas of the topic being evaluated. The role of the expert advisors was to contextualize the evidence and provide advice on the use of TAVI and SAVR in patients with severe, symptomatic aortic valve stenosis; methodologists provided advice on the analytical methodology used.
Results
Literature Search
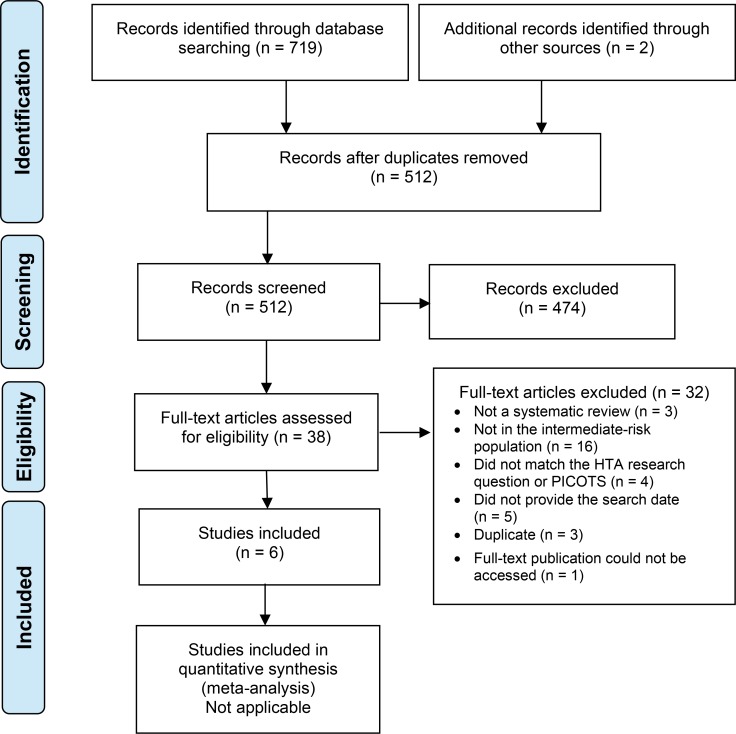
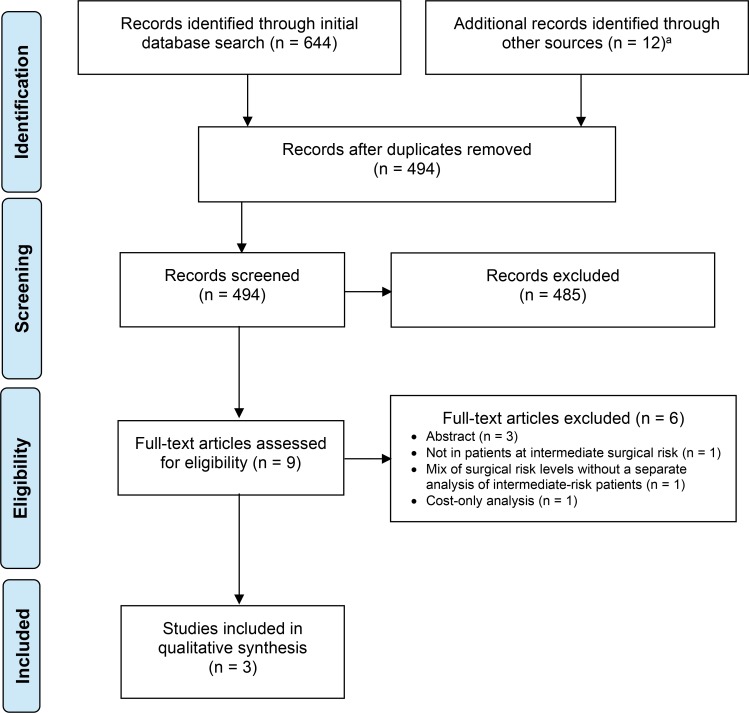
The literature search for systematic reviews yielded 512 citations published from inception to March 28, 2018, after removing duplicates. Six systematic reviews met the inclusion criteria. We identified no eligible HTAs.
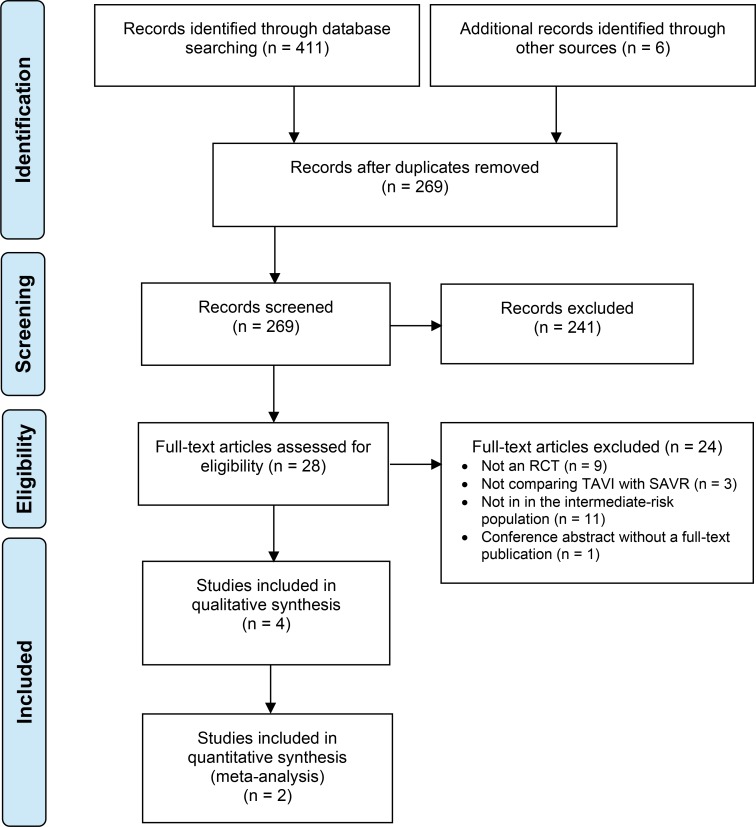
The literature search for randomized controlled trials yielded 269 citations published between January 1, 2017, and April 13, 2018, after removing duplicates. Four publications (two randomized controlled trials) met the inclusion criteria.
Figures 1 and 2 present the flow diagrams for Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) for the systematic review and individual studies searches, respectively.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy for Systematic Reviews.
Source: Adapted from Moher et al.27
Abbreviations: PICOTS, population, intervention, comparators, outcomes, timing, and setting; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 2: PRISMA Flow Diagram—Clinical Search Strategy for Randomized Controlled Trials.
Source: Adapted from Moher et al.27
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
Systematic Reviews
Six systematic reviews met our eligibility criteria.28–33 The reviews were published between 2016 and 2018 and compared TAVI with SAVR in patients at intermediate surgical risk,31–33 or at low and intermediate surgical risk.28–30 Details about the reviews' design and characteristics are provided in Appendix 3.
Four systematic reviews30–33 had a high risk of bias because of one or more of the following and were excluded from our review: insufficient information about the literature search strategy; surgical risk definition differed from our definition; inclusion of studies in patients with a surgical risk level different from the one that was the focus of our review; or missing studies. Appendix 2 provides details of the risk-of-bias assessment.
The two remaining systematic reviews had a low risk of bias.28,29 Both focused on patients at low and intermediate surgical risk, a broader population than the one we had defined. We selected the most recent review, Tam et al,28 for inclusion in this assessment. However, because of the differences in patient population, and because new relevant studies have been published since the selected review's literature search, we were unable to use the analyses in that review; instead, we performed de novo analyses using data from the randomized controlled trials, focusing on our population of interest. Because the literature search for Tam et al28 was conducted on March 21, 2017, we started our literature search from January 1, 2017, to identify individual randomized controlled trials that might have been added to the databases later and because some of the databases did not allow for a specific search date.
Randomized Controlled Trials
The systematic review by Tam et al28 identified four randomized controlled trials that compared TAVI with SAVR, two of which included a population at low surgical risk and were therefore excluded from our review. Two randomized controlled trials met our eligibility criteria: the SURTAVI19 and PARTNER 210 trials. In addition to these two randomized controlled trials, our literature search identified two further publications from the PARTNER 2 trial,10 one reporting results for health-status benefits,34 and one reporting results specific to the SAVR group.35 We included data from these four publications in our analyses.
Study Characteristics
The two randomized controlled trials10,19 compared the effects of TAVI and SAVR in patients with severe, symptomatic (NYHA class ≥ II) aortic valve stenosis in patients at intermediate surgical risk. Surgical risk in both trials was determined by a multidisciplinary heart team and informed by the STS risk score and other comorbidities. The SURTAVI trial19 used a self-expanding TAVI valve, and the PARTNER 2 trial10 used a balloon-expandable valve. The SAVR groups received a bioprosthetic valve, and the choice of operative technique was at the surgeon's discretion.10,19 The main endpoint in both studies was a composite outcome of death from any cause or disabling stroke within 2 years, to test whether TAVI was noninferior to SAVR. Both studies used the 2010 Valve Academic Research Consortium definitions for study outcomes. Both studies have planned a patient follow-up of 5 years, but only the results for the first 2 years had been published at the time of writing this report. Patients with unicuspid or bicuspid aortic valve, or with a pre-existing mechanical or bioprosthetic valve in any position, were excluded from the studies.10,19
The SURTAVI trial19 randomized 1,746 patients to receive either a self-expanding transcatheter aortic valve (n = 879) or a bioprosthetic surgical aortic valve (n = 867) in sites in Europe, the United States, and Canada. Randomization was stratified according to the need for surgical coronary revascularization based on recommendations from the multidisciplinary heart team. Revascularization was recommended in 332 patients (20%). If needed, coronary artery bypass graft (CABG) was performed at the same time as SAVR. In the TAVI group, if needed, percutaneous coronary intervention (PCI) was performed at the same time as TAVI or at least 7 days before. For TAVI, the transfemoral access route was preferred, but if it was unsuitable, either the subclavian or the transaortic approach was used.19
The PARTNER 2 trial10 randomized 2,032 patients to receive a balloon-expandable transcatheter aortic valve (n = 1,011) or a bioprosthetic surgical aortic valve (n = 1,021), stratifying the TAVI group according to implantation access route (transfemoral or transthoracic). The study included sites in the United States and Canada. A total of 1,550 patients (76.3%) were candidates for transfemoral placement, and 482 (23.7%) for the transthoracic route. In the transthoracic cohort, either the transapical or transaortic access route was used. The study was powered for the entire cohort, not for analysis of the prespecified subgroups. Patients who required coronary revascularization were treated with either PCI or CABG according to the judgment of the heart team.10
Additional information on the study characteristics, eligibility criteria, and recommended use of anticoagulant and antiplatelet medications before, during, and after the procedure is provided in Appendix 4.
We have reported the results of intention-to-treat analyses unless otherwise specified.
Risk of Bias
The randomization and allocation concealment were adequately performed. The studies did not blind patients or investigators to the treatment received, but in both trials, an external committee adjudicated the events. The risk of bias was considered low for both randomized controlled trials. Additional information is provided in Appendix 2.
Baseline Patient Characteristics
The mean age of the patients was between 80 and 82 years, and approximately 56% of patients were male.10,19 Approximately 57% of patients in the SURTAVI study19 had NYHA class III or IV symptoms, compared with 77% of patients in the PARTNER 2 study.10 Mean STS scores were 4.5% in the SURTAVI trial and 5.8% in the PARTNER 2 trial.10,19 Previous CABG and PCI had been performed in 16% and 21% of the patients in the SURTAVI trial,19 and 25% and 27% of patients in the PARTNER 2 trial,10 respectively. Additional information is provided in Appendix 5.
Patient Withdrawal
In the SURTAVI trial,19 the assigned procedure was not attempted in 15 patients (1.7%) in the TAVI group and 71 patients (8.2%) in the SAVR group. These patients were excluded from the modified intention-to-treat analyses but were included in the intention-to-treat analyses. In patients in whom the TAVI procedure was attempted, the valve was not implanted in two patients, and one patient crossed over to SAVR. In patients in whom SAVR was attempted, the valve was not implanted in one patient, and two patients crossed over to TAVI. The baseline characteristics of the patients who were withdrawn did not differ from those of the patients who remained.
In the PARTNER 2 trial,10 the assigned procedure was not attempted in 17 patients (1.7%) in the TAVI group and 77 patients (7.5%) in the SAVR group. These patients were excluded from the as-treated analyses but were included in the intention-to-treat analyses. In patients in whom the TAVI procedure was attempted, the valve was not implanted in 20 patients (2%). In the SAVR group, eight (0.8%) did not receive the valve.
Reasons why patients did not receive the assigned treatment are provided in Table 2.
Table 2:
Patient Withdrawal
| Author, Year N (TAVI/SAVR) | Procedure Not Attempted, n (%) | Procedure Initiated but Valve not Implanted, n (%) |
|---|---|---|
| Reardon et al, 201719 SURTAVI 1,746 (879/867) |
Death TAVI: 4 (0.5%) SAVR: 4 (0.5%) |
Valve not implanted TAVI: 2 (0.2%) SAVR: 1 (0.1%) |
| Consent withdrawal TAVI: 6 (0.7%) SAVR: 43 (5.0%) |
Crossover to the other group TAVI: 1 (0.1%) SAVR: 2 (0.3%) |
|
| Physician's decision to withdraw patient TAVI: 5 (0.6%) SAVR: 23 (2.7%) |
||
| Loss to follow-up TAVI: 0 SAVR: 1 (0.1%) |
||
| Leon et al, 201610 PARTNER 2 2,032 (1,011/1,021) |
Death TAVI: 6 (0.6%) SAVR: 5 (0.5%) |
Ineligible based on TEE TAVI: 9 (0.9%) SAVR: 0 |
| Withdrawn before treatment TAVI: 11 (1.1%) SAVR: 68 (6.7%) |
Inability to gain access TAVI: 2 (0.2%) SAVR: 0 |
|
| Ineligible due to aortic calcification or deteriorating condition TAVI: 0 SAVR: 4 (0.4%) |
Device embolizations, annular ruptures, ventricular perforation TAVI: 8 (0.8%) SAVR: 0 |
|
| Aortic calcification TAVI: 0 SAVR: 5 (0.5%) |
||
| Hypotensive event during anesthesia TAVI: 0 SAVR: 1 (0.1%) |
||
| Not treated as assigned TAVI: 1 (0.1%) SAVR: 2 (0.2%) |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement; TEE, transesophageal echocardiogram.
Aortic Valve Implantation Procedure
In the SURTAVI trial,19 for patients in whom the procedure was initiated, the transfemoral implantation route was used in 809 patients (93.6%) in the TAVI group, and the transaortic and subclavian routes were used in 35 (4.1%) and 20 (2.3%) patients, respectively. In the TAVI group, 803 patients (92.9%) received one valve, 54 (6.3%) received two valves, and 4 (0.5%) received three valves. General anesthesia was used in 654 patients (75.7%) in the TAVI group. Balloon valvuloplasty was performed in 250 (29.0%) patients before the TAVI procedure, and 407 (47.2%) patients after. The self-expanding CoreValve was used in 724 patients (83.9%) randomized to TAVI, and the newer, self-expanding Evolut R valve was used in 139 patients (16.1%).
In the PARTNER 2 trial,10 the transfemoral implantation route was used in 775 patients (76.7%) in the TAVI group, and the transthoracic route in 236 patients (23.3%; transapical 174, transaortic 62). In 26 patients (2.6%), a second TAVI valve was placed within the first valve because of moderate or severe aortic regurgitation, or valve embolization. All patients in the TAVI group received the balloon-expandable SAPIEN XT valve. In the SAVR group, among patients in whom a valve was implanted, most had a full sternotomy (n = 797; 85.1%); the remainder had a less invasive incision (n = 140; 14.9%).35
Concomitant Procedures
In the SURTAVI trial, for patients in whom the procedure was initiated,19 CABG was performed in 176 patients (22.1%) in the SAVR group and PCI in 125 patients (14.5%) in the TAVI group. In the PARTNER 2 trial, for patients in whom the procedure was initiated,10 CABG was performed in 137 patients (14.5%) in the SAVR group and PCI in 39 patients (3.9%) in the TAVI group.
Other concomitant procedures were performed in 45 (5.7%) and 86 (9.1%) patients in the SAVR group of the SURTAVI19 and PARTNER 210 trials, respectively. These included aortic endarterectomy, aortic root enlargement or replacement, mitral valve repair, and cardiac ablation, among others.10,19
Composite Endpoint: All-Cause Mortality or Disabling Stroke
The primary objective of both trials was to determine the noninferiority of TAVI versus SAVR in the occurrence of all-cause mortality or disabling stroke within 2 years of follow-up.10,19 Disabling stroke was defined in both trials according to the Valve Academic Research Consortium–2 criteria. All patients were assessed by a trained neurologist or stroke specialist, and neurologic events were adjudicated by a neurologist on the clinical events committee. Additional information is provided in Appendix 6.
Full Cohort
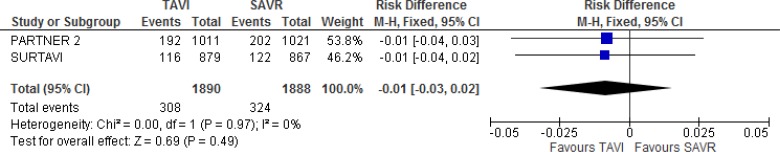
There was no statistically significant difference between TAVI and SAVR for the main endpoint within 2 years of follow-up, and the noninferiority criterion was met in both trials.10,19
In the SURTAVI trial,19 the primary endpoint occurred in 13.2% and 14.1% of the TAVI and SAVR groups, respectively (absolute risk difference −0.9%, 95% credible interval [CrI] −4.7% to 2.7%). In the PARTNER 2 trial,10 19.3% and 21.1% of patients experienced the primary endpoint within 2 years of follow-up, respectively (hazard ratio [HR] 0.89, 95% confidence interval [CI] 0.73–1.09).
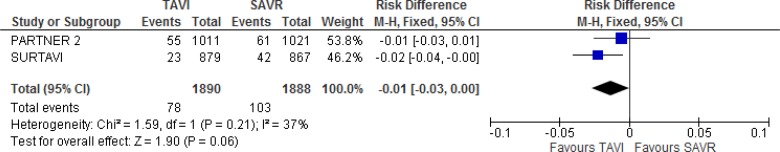
Our meta-analysis did not demonstrate a statistically significant difference between groups for the composite endpoint (absolute risk difference −1% 95% CI −3% to 2%; Figure 3).
Figure 3: All-Cause Mortality or Disabling Stroke, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; MH, Mantel–Haenszel; SAVR, surgical aortic valve replacement; SE, standard error; TAVI, transcatheter aortic valve implantation.
There was no statistically significant difference between TAVI and SAVR for subgroup analyses according to age, sex, body mass index, implantation access, STS score, left ventricular ejection fraction, or revascularization in either study within 2 years of follow-up.10,19
Transfemoral and Transthoracic Cohorts
In the transfemoral cohort of the PARTNER 2 study,10 based on the intention-to-treat analysis, the risk of all-cause mortality or disabling stroke was statistically significantly lower in the TAVI group compared to the SAVR group within 30 days and at 1 year of follow-up. At 2 years, the authors observed no statistically significant difference between groups in the intention-to-treat analysis (HR 0.79, 95% CI 0.62–1.00), but there was a lower risk in the TAVI group in the as-treated analysis (HR 0.78, 95% CI 0.61–0.99).
In the transthoracic cohort, no statistically significant difference between groups was observed throughout follow-up (HR 1.21, 95% CI 0.84–1.74).10
All-Cause Mortality
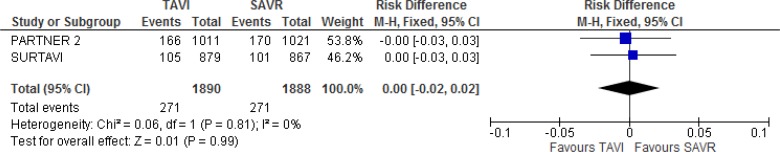
There was no statistically significant difference in mortality between TAVI and SAVR at 2 years of follow-up in either trial, and regardless of implant access route in the PARTNER 2 trial.10,19
In the SURTAVI trial,19 mortality risks in the TAVI group were 2.0%, 7.0%, and 12.0% at 30 days, 1 year, and 2 years of follow-up, respectively, and 1.3%, 6.8%, and 11.6% in the SAVR group (absolute risk difference 0.4%, 95% Crl −3.2 to 3.9 at 2 years). In the PARTNER 2 trial,10 mortality risk in the TAVI group was 3.9%, 12.3%, and 16.7%, at 30 days, 1 year, and 2 years of follow-up, respectively, and 4.1%, 12.9%, and 18.0%, in the SAVR group (HR 0.92, 95% CI 0.74–1.13 at 2 years). Additional information is provided in Appendix 6.
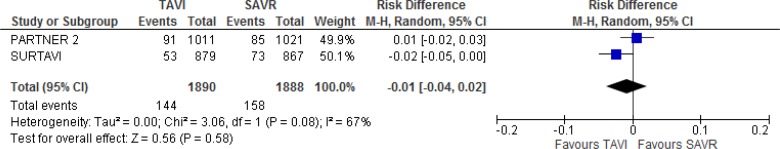
Our meta-analysis did not show a statistically significant difference in all-cause mortality between groups at 2 years of follow-up (absolute risk difference 0%, 95% CI −2% to 2%; Figure 4).
Figure 4: All-Cause Mortality, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; MH, Mantel–Haenszel; SAVR, surgical aortic valve replacement; SE, standard error; TAVI, transcatheter aortic valve implantation.
Stroke
Any Stroke
The SURTAVI trial19 reported a statistically significantly lower risk of stroke with TAVI compared to SAVR at 30 days of follow-up (2.6% vs. 4.8%; absolute risk difference −2.2%, 95% Crl −4.0 to −0.4), but no difference between groups after 30 days. The PARTNER 2 trial10 did not report a statistically significant difference in stroke between groups (risk at 30 days 5.5% in the TAVI group vs. 6.1% in the SAVR group; P = .57) throughout the 2 years of follow-up and regardless of TAVI implantation access route. Additional information is provided in Appendix 6.
When we pooled the results of the 2 studies, we found no statistically significant difference in stroke of any type between groups either at 30 days (absolute risk difference −1%, 95% CI −3% to 0%; Figure 5) or 2 years of follow-up (absolute risk difference −1%, 95% CI −4% to 2%; Figure 6).
Figure 5: Any Stroke, TAVI Versus SAVR, 30 Days of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Figure 6: Any Stroke, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Disabling Stroke
There was no statistically significant difference between TAVI and SAVR in the occurrence of disabling stroke at 2 years of follow-up in either trial, and regardless of TAVI implantation access route in the PARTNER 2 trial.10,19
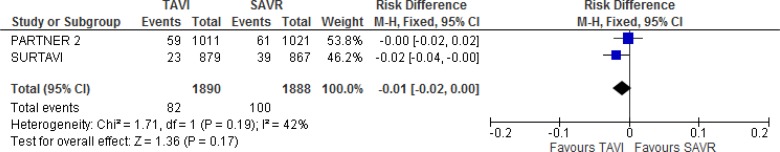
In the SURTAVI trial,19 the risks of disabling stroke in the TAVI group were 1.1%, 2.2%, and 2.6% at 30 days, 1 year, and 2 years of follow-up, respectively, and 2.2%, 3.7%, and 4.6%, in the SAVR group (absolute risk difference −2.0%, 95% Crl −4.0% to 0% at 2 years). In the PARTNER 2 trial,10 the risk of disabling stroke in the TAVI group was 3.2%, 5.0%, and 6.2% at 30 days, 1 year, and 2 years of follow-up, respectively, and 4.3%, 5.8%, and 6.4%, in the SAVR group (HR 0.93, 95% CI 0.65–1.33 at 2 years). Additional information is provided in Appendix 6. Our meta-analysis did not show a statistically significant difference in disabling stroke between groups at 2 years of follow-up (absolute risk difference −1%, 95% CI −2% to 0%; Figure 7).
Figure 7: Disabling Stroke, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; MH, Mantel–Haenszel; SAVR, surgical aortic valve replacement; SE, standard error; TAVI, transcatheter aortic valve implantation.
Transient Ischemic Attack
There was no statistically significant difference in the occurrence of transient ischemic attack between TAVI and SAVR within 2 years of follow-up in both trials and regardless of TAVI implantation access route in the PARTNER 2 trial.10,19
In the SURTAVI trial,19 the risks of transient ischemic attacks in the TAVI group were 0.9%, 3.4%, and 4.4%, at 30 days, 1 year, and 2 years of follow-up, respectively, and 0.7%, 2.0%, and 3.0% in the SAVR group (absolute risk difference 1.4%, 95% Crl −0.6% to 3.5% at 2 years). In the PARTNER 2 trial,10 the risk of transient ischemic attack in the TAVI group was 0.9%, 2.4%, and 3.7%, at 30 days, 1 year, and 2 years of follow-up, respectively, and 0.4%, 1.8%, and 2.3% in the SAVR group. Additional information is provided in in Appendix 6.
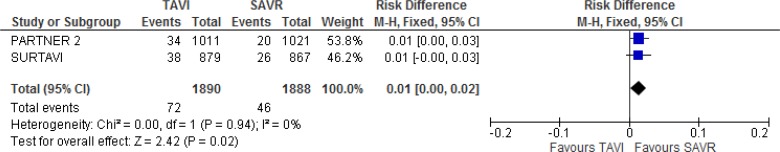
Our meta-analysis did not show a statistically significant difference in transient ischemic attacks between groups at 2 years of follow-up (absolute risk difference 1%, 95% CI 0%–2%; Figure 8).
Figure 8: Transient Ischemic Attack, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; MH, Mantel–Haenszel; SAVR, surgical aortic valve replacement; SE, standard error; TAVI, transcatheter aortic valve implantation.
Life-Threatening or Major/Disabling Bleeding
The SURTAVI trial19 found no statistically significant difference in the risk of life-threatening or major bleeding at 30 days between the TAVI and the SAVR groups. The PARTNER 2 trial10 found a lower risk of life-threatening or disabling bleeding with TAVI compared with SAVR throughout the 2 years of follow-up in the full cohort and when the transfemoral and transthoracic cohorts were analyzed separately.
Results for the 30-day follow-up are shown in Table 3, and long-term follow-up results are presented in Appendix 6.
Table 3:
Life-Threatening or Major/Disabling Bleeding
| Author, Year N (TAVI/SAVR) | Life-Threatening or Major/Disabling Bleeding, 30 Days |
|---|---|
| Full Cohort | |
| Reardon et al, 201719 | Percentageb TAVI: 12.2 |
| SURTAVI, mITTa | TAVI: 12.2 |
| 1,660 (864/796) | SAVR: 9.3 |
| 95% Crl for difference: −0.1 to 5.9 | |
| Leon et al, 201610 | KM estimate,d n (%) TAVI: 105 (10.4) |
| PARTNER 2, ITTc | TAVI: 105 (10.4) |
| 2,032 (1,011/1,021) | SAVR: 442 (43.4) |
| P < .001 | |
| Transfemoral Cohort | |
| Leon et al, 201610 | KM estimate,d n (%) TAVI: 52 (6.7) |
| PARTNER 2, ITTc | TAVI: 52 (6.7) |
| 1,550 (775/775) | SAVR: 320 (41.4) |
| P < .001 | |
| Transthoracic Cohort | |
| Leon et al, 201610 | KM estimate,d n (%) TAVI: 53 (22.6) |
| PARTNER 2, ITTc | TAVI: 53 (22.6) |
| 482 (236/246) | SAVR: 122 (49.8) |
| P < .001 | |
Abbreviations: CrI, credible interval; ITT, intention-to-treat analysis; KM, Kaplan–Meier; mITT, modified intention-to-treat analysis; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
mITT analysis refers to patients in whom the TAVI or SAVR procedure was at least attempted.
Calculated by means of Bayesian analyses.
ITT analysis includes all patients randomized to receive either TAVI or SAVR.
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
We did not perform a meta-analysis because of unexplained substantial heterogeneity in the study results.
Acute Kidney Injury
Both trials reported a statistically significant lower risk of stage 2 to 3 acute kidney injury with TAVI versus SAVR in the full cohort within 30 days of follow-up.10,19 The PARTNER 2 trial10 also reported a lower risk of acute kidney injury with TAVI versus SAVR at 30 days in the transfemoral cohort, but no difference in the transthoracic cohort. Results for the 30-day follow-up are shown in Table 4, and longer-term follow-up results are presented in Appendix 6.
Table 4:
Acute Kidney Injury
| Author, Year N (TAVI/SAVR) | Acute Kidney Injury, 30 Days |
|---|---|
| Full Cohort | |
| Reardon et al, 201719 | Stage 2 or 3, percentageb |
| SURTAVI, mITTa | TAVI: 1.7 |
| 1,660 (864/796) | SAVR: 4.4 |
| 95% CrI for difference: −4.4 to −1.0 | |
| Leon et al, 201610 | KM estimate,d n (%) |
| PARTNER 2, ITTc | TAVI: 13 (1.3) |
| 2,032 (1,011/1,021) | SAVR: 31 (3.1) |
| P = .006 | |
| Transfemoral Cohort | |
| Leon et al, 201610 | Stage 3, KM estimate,d n (%) |
| PARTNER 2, ITTc | TAVI: 4 (0.5) |
| 1,550 (775/775) | SAVR: 23 (3.0) |
| P < .001 | |
| Transthoracic Cohort | |
| Leon et al, 201610 | Stage 3, KM estimate,d n (%) |
| PARTNER 2, ITTc | TAVI: 9 (3.9) |
| 482 (236/246) | SAVR: 8 (3.4) |
| P = .77 | |
Abbreviations: CrI, credible interval; ITT, intention-to-treat analysis; KM, Kaplan–Meier; mITT, modified intention-to-treat analysis; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
mITT analysis refers to patients in whom the TAVI or SAVR procedure was at least attempted.
Calculated by means of Bayesian analyses.
ITT analysis includes all patients randomized to receive either TAVI or SAVR.
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
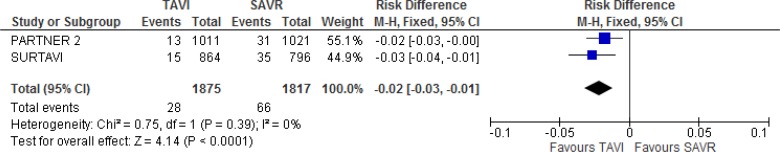
Our meta-analysis of the full cohort showed a statistically significant lower risk of acute kidney injury with TAVI versus SAVR at 30 days of follow-up (absolute risk difference −2%, 95% CI −3.0% to −1.0%; Figure 9).
Figure 9: Acute Kidney Injury, TAVI Versus SAVR, 30 Days of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Myocardial Infarction
There was no statistically significant difference in myocardial infarction between the TAVI and SAVR groups in either trial within 2 years of follow-up.10,19
In the transfemoral cohort of the PARTNER 2 trial,10 there was a lower risk of myocardial infarction with TAVI compared with SAVR at 30 days, but no statistically significant difference at 1 year and 2 years of follow-up. In the transthoracic cohort, there was no statistically significant difference between groups over the 2 years of follow-up. Additional information is shown in Table 5.
Table 5:
Myocardial Infarction
| Author, Year N (TAVI/SAVR) | Myocardial Infarction | ||
|---|---|---|---|
| 30 Days | 1 Year | 2 Years | |
| Full Cohort | |||
| Reardon et al, 201719 | Percentagea | Percentagea | Percentagea |
| SURTAVI | TAVI: 0.9 | TAVI: 2.0 | TAVI: 2.9 |
| 1,746 (879/867) | SAVR: 0.7 | SAVR: 1.7 | SAVR: 2.4 |
| 95% Crl for difference: −0.7 to 1.1 | 95% Crl for difference: −1.0 to 1.7 | 95% Crl for difference: −1.3 to 2.2 | |
| Leon et al, 201610 | KM estimate, n (%)b | KM estimate, n (%)b | KM estimate, n (%)b |
| PARTNER 2 | TAVI: 12 (1.2) | TAVI: 24 (2.5) | TAVI: 33 (3.6) |
| 2,032 (1,011/1,021) | SAVR: 19 (1.9) | SAVR: 29 (3.0) | SAVR: 37 (4.1) |
| P = .22 | P = .47 | P = .56 | |
| Transfemoral Cohort | |||
| Leon et al, 201610 | KM estimate, n (%)b | KM estimate, n (%)b | KM estimate, n (%)b |
| PARTNER 2 | TAVI: 5 (0.6) | TAVI: 14 (1.9) | TAVI: 21 (3.0) |
| 1,550 (775/775) | SAVR: 14 (1.8) | SAVR: 23 (3.2) | SAVR: 29 (4.2) |
| P = .04 | P = .13 | P = .22 | |
| Transthoracic Cohort | |||
| Leon et al, 201610 | KM estimate, n (%)b | KM estimate, n (%)b | KM estimate, n (%)b |
| PARTNER 2 | TAVI: 7 (3.0) | TAVI: 10 (4.5) | TAVI: 12 (5.6) |
| 482 (236/246) | SAVR: 5 (2.1) | SAVR: 6 (2.6) | SAVR: 8 (3.8) |
| P = .53 | P = .29 | P = .40 | |
Abbreviations: CrI, credible interval; KM, Kaplan–Meier; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Calculated by means of Bayesian analyses.
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
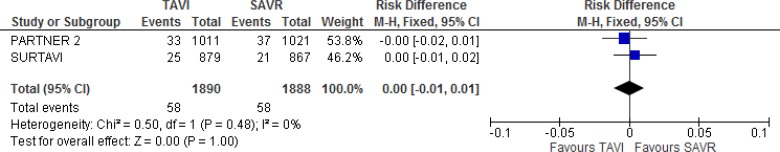
Our meta-analysis did not show a statistically significant difference in myocardial infarction between groups at 2 years of follow-up (absolute risk difference 0%, 95% CI −1% to 1%; Figure 10).
Figure 10: Myocardial Infarction, TAVI Versus SAVR, 2 Years of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Atrial Fibrillation
Both trials reported a statistically significantly lower risk of atrial fibrillation with TAVI at 30 days compared with SAVR.10,19 The outcome was not measured beyond 30 days in the SURTAVI trial.19 In the PARTNER 2 trial,10 there was a lower risk of new atrial fibrillation with TAVI compared with SAVR in the full cohort and in the transfemoral cohort throughout the 2 years of follow-up. There was no difference between groups in the transthoracic cohort. Additional information is shown in Table 6.
Table 6:
Atrial Fibrillation
| Author, Year N (TAVI/SAVR) | Atrial Fibrillation | ||
|---|---|---|---|
| 30 Days | 1 Year | 2 Years | |
| Full Cohort | |||
| Reardon et al, 201719 | Percentageb | Not reported | Not reported |
| SURTAVI, mITTa | TAVI: 12.9 | ||
| 1,660 (864/796) | SAVR: 43.4 | ||
| 95% Crl for difference: | |||
| −34.7 to −26.4 | |||
| Leon et al, 201610 | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d |
| PARTNER 2, ITTc | TAVI: 91 (9.1) | TAVI: 100 (10.1) | TAVI: 110 (11.3) |
| 2,032 (1,011/1,021) | SAVR: 265 (26.4) | SAVR: 272 (27.2) | SAVR: 273 (27.3) |
| P < .001 | P < .001 | P<.001 | |
| Transfemoral Cohort | |||
| Leon et al, 201610 | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d |
| PARTNER 2, ITTc | TAVI: 38 (4.9) | TAVI: 45 (5.9) | TAVI: 55 (7.4) |
| 1,550 (775/775) | SAVR: 204 (26.7) | SAVR: 210 (27.6) | SAVR: 211 (27.8) |
| P < .001 | P < .001 | P<.001 | |
| Transthoracic Cohort | |||
| Leon et al, 201610 | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d | New AF, KM estimate n (%)d |
| PARTNER 2, ITTc | TAVI: 53 (22.8) | TAVI: 55 (23.8) | TAVI: 55 (23.8) |
| 482 (236/246) | SAVR: 61 (25.4) | SAVR: 62 (25.9) | SAVR: 62 (25.9) |
| P = .50 | P = .60 | P = .60 | |
Abbreviations: AF, atrial fibrillation; CrI, credible interval; KM, Kaplan–Meier; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
mITT analysis refers to patients in whom the TAVI or SAVR procedure was at least attempted.
Calculated by means of Bayesian analyses.
ITT analysis includes all patients randomized to receive either TAVI or SAVR
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
We did not perform a meta-analysis because of unexplained substantial heterogeneity in the study results (Figure 11).
Figure 11: Atrial Fibrillation, TAVI Versus SAVR, 30 Days of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
New Permanent Pacemaker Implantation
In the SURTAVI trial,19 the risk of new pacemaker implantation at 30 days in the TAVI group was higher than in the SAVR group (25.9% vs. 6.6%; absolute risk difference 19.3%, 95% Crl 15.9%–22.7%). The risks of new pacemaker implantation were similar for the CoreValve (25.5%) and the newer-generation Evolut R (26.7%).
In the PARTNER 2 trial,10 8.5% of patients in the TAVI group and 6.9% of patients in the SAVR group required a new pacemaker (P = .17). The difference between groups was not statistically significant over the 2 years of follow-up, either when analyzed as the full cohort or subdivided by implantation access route (Appendix 6).10
We did not perform a meta-analysis because of unexplained substantial heterogeneity in the study results (Figure 12).
Figure 12: New Permanent Pacemaker Implantation, TAVI Versus SAVR, 30 Days of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Major Vascular Complications
A major vascular complication was defined as the occurrence of any one of the following events: aortic dissection, aortic rupture, annulus rupture, left ventricular perforation, new apical aneurysm/pseudo-aneurysm, or distal embolization requiring surgery, among others.10,19 Both trials reported a statistically significant higher risk of major vascular complications with TAVI compared with SAVR in the full cohort within 30 days of follow-up.10,19 The PARTNER 2 trial10 also reported a statistically significant higher risk of major vascular complications with TAVI versus SAVR at 30 days in the transfemoral cohort, but not in the transthoracic cohort.
In the SURTAVI trial,19 the risks of major vascular complications at 30 days were 6% in the TAVI group and 1.1% in the SAVR group (absolute risk difference 4.9%; 95% Crl 3.2%–6.7%). This outcome was not measured beyond 30 days.
In the full cohort of the PARTNER 2 trial,10 7.9% of patients in the TAVI group and 5% in the SAVR group experienced a major vascular complication at 30 days (P = .008). In the transfemoral cohort, the risks at 30 days were 8.5% and 3.9%, respectively (P < .001); in the transthoracic cohort, the risks were 5.9% and 8.6%, respectively (P = .26).10 Additional information is provided in Appendix 6.
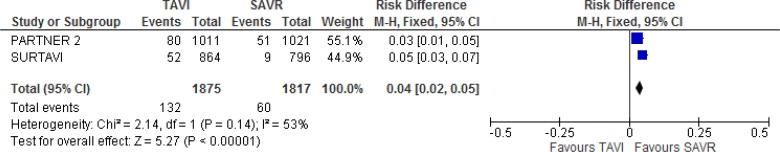
Our meta-analysis showed that he TAVI group had a higher risk of major vascular complications than the SAVR group at 30 days of follow-up (absolute risk difference 4%, 95% CI 2%–5%; Figure 13).
Figure 13: Major Vascular Complications, TAVI Versus SAVR, 30 Days of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Aortic Valve Hemodynamics
The authors of both trials observed an improvement in aortic valve area and a reduction in mean aortic valve gradient in the TAVI and SAVR groups.10,19 Patients in the TAVI group experienced a statistically significant greater improvement in valve area and a greater reduction in mean aortic valve gradient than patients in the SAVR group throughout the 2 years of follow-up (Table 7).10,19 However, it is unclear whether the difference in improvement between groups was clinically important.
Table 7:
Aortic Valve Hemodynamics
| Author, Year N (TAVI/SAVR)a | Mean Aortic Valve Area (SD), cm2 | Mean Aortic Valve Gradient (SD), mm Hg |
|---|---|---|
| Reardon et al, 201719 | Baseline | Baseline |
| SURTAVI | TAVI: 0.8 (0.2) | TAVI: 47.2 (14.3) |
| Baseline | SAVR: 0.8 (0.2) | SAVR: 47.8 (13.8) |
| 1,642 (856/786) | Discharge | Discharge |
| Discharge | TAVI: 2.1 (0.6) | TAVI: 8.9 (4.1) |
| 1,560 (835/725) | SAVR: 1.8 (0.6) | SAVR: 12.4 (5.7) |
| 1 year | 1 year | 1 year |
| 1,090 (590/500) | TAVI: 2.2 (0.6) | TAVI: 8.3 (4.0) |
| 2 years | SAVR: 1.8 (0.6) | SAVR: 11.7 (5.6) |
| 537 (294/243) | 2 years | 2 years |
| TAVI: 2.2 (0.7) | TAVI: 7.8 (3.4) | |
| SAVR: 1.7 (0.5) | SAVR: 11.8 (5.7) | |
| Statistically significant difference between groups at all time points post-procedure | Statistically significant difference between groups at all time points post-procedure | |
| Leon et al, 201610 | Baseline | Baseline |
| PARTNER 2 | TAVI: 0.7 (0.2) | TAVI: 44.9 (13.4) |
| Baseline | SAVR: 0.7 (0.2) | SAVR: 44.6 (12.5) |
| 2,032 (1,011/1,021) | 30 days | 30 days |
| 30 days | TAVI: 1.7 (0.5) | TAVI: 9.7 (3.5) |
| 1,678 (890/788) | SAVR: 1.5 (0.4) | SAVR: 10.9 (4.3) |
| 1 year | 1 year | 1 year |
| 1,384 (751/633) | TAVI: 1.6 (0.4) | TAVI: 10.7 (4.5) |
| 2 years | SAVR: 1.4 (0.4) | SAVR: 11.5 (4.4) |
| 1,162 (626/536) | 2 years | 2 years |
| TAVI: 1.5 (0.4) | TAVI: 10.8 (4.6) | |
| SAVR: 1.4 (0.4) | SAVR: 11.7 (4.7) | |
| Statistically significant difference between groups at all time points post-procedure | Statistically significant difference between groups at all time points post-procedure |
Abbreviations: SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Aortic valve hemodynamics were evaluated in the implanted population.
Moderate to Severe Paravalvular Aortic Regurgitation
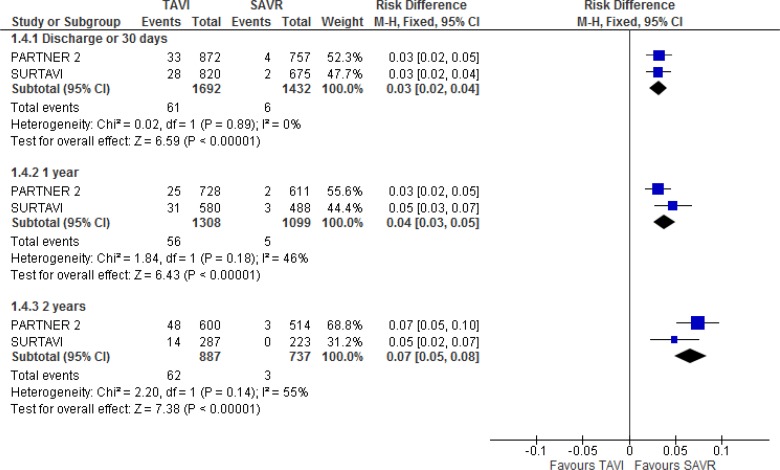
Both trials reported a statistically significant higher risk of moderate to severe paravalvular aortic regurgitation with TAVI compared with SAVR at all time points.10,19 Results from the PARTNER 2 trial were available only for the full cohort.10
In the SURTAVI trial,19 3.4%, 5.3%, and 4.9% of the patients in the TAVI group experienced moderate to severe paravalvular aortic regurgitation at hospital discharge and at 1 year and 2 years of follow-up, respectively, compared with 0.3%, 0.6%, and 0% in the SAVR group.
In the PARTNER 2 trial,10 3.7%, 3.4%, and 8.0% of patients in the TAVI group experienced moderate to severe paravalvular aortic regurgitation at 30 days, 1 year, and 2 years of follow-up, respectively, compared with 0.6%, 0.4%, and 0.6% in the SAVR group.
In our meta-analysis, the TAVI group had a higher risk of moderate to severe paravalvular aortic regurgitation than the SAVR group throughout the follow-up period (absolute risk difference at 2 years of follow-up: 7%; 95% CI 5%–8%). Additional information is provided in Figure 14 and Appendix 6.
Figure 14: Moderate to Severe Paravalvular Aortic Regurgitation, TAVI Versus SAVR, 30 Days, 1 Year, and 2 Years of Follow-up.
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
According to the PARTNER 2 trial,10 patients with moderate to severe paravalvular aortic regurgitation had a higher risk of death at 2 years (HR 2.85, 95% CI 1.57–5.21) than patients who had no or trace paravalvular regurgitation. No difference in mortality was observed for mild versus no or trace paravalvular aortic regurgitation (HR 0.95, 95% CI 0.63–1.45).10
Valve Deterioration
In the SURTAVI trial,19 structural valve deterioration was not observed in either the TAVI or the SAVR group at 2 years. No information on valve deterioration was provided for the PARTNER 2 trial.10
Aortic Valve Reintervention
Aortic valve reinterventions included any intervention to repair, alter, or replace a previously implanted valve, such as balloon dilation, SAVR, valve-in-valve procedures, and interventions to retrieve or reposition the valve.10,19
In the SURTAVI trial,19 the risks of aortic valve reintervention in the TAVI group were 0.7%, 2.0%, and 2.7% at 30 days and 1 and 2 years of follow-up, respectively, and 0.2%, 0.5%, and 0.7% in the SAVR group. The TAVI group had a higher risk of aortic valve reintervention at 1 year (absolute risk difference 1.5%, 95% Crl 0.3%–2.6%) and at 2 years (absolute risk difference 2.0%, 95% Crl 0.6%–3.4%) than the SAVR group.19
In the full cohort of the PARTNER 2 trial,10 0.4%, 1.2%, and 1.4% of patients in the TAVI group underwent aortic valve reintervention within 30 days and 1 and 2 years of follow-up, respectively, versus 0%, 0.5%, and 0.6% in the SAVR group. The difference between groups was not statistically significant for the full cohort or for the transfemoral cohort over the 2 years of follow-up. For the transthoracic cohort, there was a higher risk of aortic valve reintervention with TAVI versus SAVR at 2 years (2% vs. 0%; P = .04). Additional information is provided in Appendix 6.
Aortic Valve Rehospitalization
Neither trial10,19 reported a statistically significant difference in rehospitalizations between the two groups over the 2 years of follow-up in the full cohort, and regardless of implantation cohort in the PARTNER 2 trial.10
In the SURTAVI trial,19 the risks of aortic valve rehospitalization in the TAVI group were 2.4%, 9.0%, and 13.3% at 30 days, and 1 and 2 years of follow-up, respectively, and 2.9%, 8.7%, and 11.0% in the SAVR group.
In the PARTNER 2 trial,10 6.5%, 14.8%, and 19.6% of patients in the TAVI group were rehospitalized at 30 days, and 1 and 2 years of follow-up, respectively, compared with 6.5%, 14.7%, and 17.3% in the SAVR group.
Length of Hospital Stay
The length of hospital stay for the valve replacement procedure was shorter for the TAVI group in the SURTAVI trial,19 (mean [SD] 5.8 [4.9] days vs. 9.8 [8.0] days), and the PARTNER 2 trial10 (median 6 days vs. 9 days; P < .001).
The PARTNER 2 trial10 also reported that patients in the TAVI group had a shorter length of stay in the intensive care unit than patients in the SAVR group (2 days vs. 4 days; P < .001). These results were not broken down by implantation access route cohort.
New York Heart Association Symptoms
Patient symptoms were measured using the NYHA functional classification,36 which assesses how much a patient's symptoms affect their physical activity (Appendix 7).
Both trials reported that NYHA symptoms improved throughout the follow-up period compared to baseline in the TAVI and SAVR groups.10,19 In the SURTAVI trial,19 there was no statistically significant difference between groups for improvement in symptoms from baseline. In the PARTNER 2 trial,10 the TAVI group had fewer cardiac symptoms than the SAVR group at 30 days (P = .001), but there was no difference in symptom improvement between groups at later time points.
Quality of Life
Kansas City Cardiomyopathy Questionnaire
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a 23-item questionnaire that covers specific health domains pertaining to heart failure: physical limitation, symptoms, quality of life, social limitation, symptom stability, and self-efficacy.37 The first four domains are combined into an overall summary score. It is scored from 0 to 100; higher scores indicate a better quality of life.34
Patients' quality of life measured using the KCCQ overall score improved over the 2 years of follow-up in both the TAVI and SAVR groups in the full cohort of the SURTAVI trial19 and regardless of TAVI implantation access route in the PARTNER 2 trial.34 As well, the PARTNER 2 trial34 showed that more than 60% of surviving patients experienced clinically meaningful improvement (> 10 points in the overall score of the KCCQ), regardless of study group or implantation access route.
Because there was a statistically significant interaction between the transfemoral and transthoracic subgroups for several key health-status measurements at 1 month, the PARTNER 2 trial34 provided the results for each subgroup separately, not for the full cohort.
In the SURTAVI trial19 and the transfemoral cohort of the PARTNER 2 trial,34 the TAVI group showed a statistically significant greater improvement in overall score than the SAVR group at 30 days, but there was no difference between groups at 6 months,19 1 year, and 2 years.19,34 In the transthoracic cohort of the PARTNER 2 trial,34 there was no statistically significant difference in improvement between groups throughout the 2 years of follow-up.
The results reported for the KCCQ physical and social limitations, symptoms, and quality of life subscales followed the same pattern as reported for the overall score for the transfemoral and transthoracic cohorts. The results for the physical and social limitations subscales are provided in Appendix 6.
The PARTNER 2 trial34 also reported change in health status, combining survival and health status. This outcome was categorized as an ordinal variable using established thresholds for clinically relevant changes in KCCQ overall score.34 There were six categories, with death as the worst possible outcome, and substantially improved (increase ≥ 20 points) as the best possible outcome.34 Transfemoral TAVI led to a statistically significant substantial improvement compared with SAVR at 30 days.34 At 1 year and 2 years, the differences between TAVI and SAVR were smaller and driven mainly by a trend for lower mortality in the TAVI group, but they were still statistically significant.34 No difference was observed between TAVI and SAVR in the transthoracic cohort throughout the 2 years of follow-up.34
EuroQoL-5D
The EuroQoL 5D (EQ-5D) measures the patient's generic health status by assessing 5 dimensions of general health using a 3-level scale, transformed into preference-based utility weights using validated population-sampling methods.10 The utilities range from 0 (death) to 1 (ideal health).10
In the SURTAVI trial,19 there was no statistically significant difference between TAVI and SAVR for change in generic health status between baseline and 3 months as measured by the EQ-5D; this outcome was not measured beyond 3 months in this trial. In the transfemoral cohort of the PARTNER 2 trial,10 TAVI led to a statistically significantly greater improvement between baseline and 30 days compared with SAVR. No statistically significant difference was observed between groups at 1 year and 2 years in the transfemoral cohort and at any time point in the transthoracic cohort. Additional information is provided in Appendix 6.
Short Form 36
The PARTNER 2 trial34 reported the Short Form 36 (SF-36) physical and mental component summary scales. These components are scored such that the United States population mean is 50 (SD 10), and higher scores represent better health status.34 The minimum clinically important differences for the physical and mental summary scales are approximately 2 points.34
In the transfemoral cohort of the PARTNER 2 trial,34 at 30 days, TAVI patients showed a statistically significant higher score compared with SAVR patients, both in the physical summary scale (adjusted mean difference: 4.6 points, 95% CI 3.7–5.5; P < .01) and the mental summary scale (adjusted mean difference: 5.5 points, 95% CI 4.3–6.8; P < .01). No statistically significant difference was observed between TAVI and SAVR at 1 and 2 years of follow-up in the transfemoral cohort for either scale.34 In the transthoracic cohort, there was no statically significant difference between TAVI and SAVR for either scale throughout the study follow-up.34
The SURTAVI trial19 reported a change in SF-36 score between baseline and 3 months, but it is not clear which scale was represented. Patients who underwent TAVI showed a statistically significantly greater improvement compared with patients who underwent SAVR (difference 1.83, 95% CI 0.74–2.94).
Summary
Tables 8 and 9 summarize the study results for the full and transfemoral/transthoracic cohorts, respectively. A detailed GRADE assessment is provided in Appendix 2.
Table 8:
TAVI Versus SAVR (Full Cohort)
| Outcome | Effect Measure (95% CI) | GRADE | Summary |
|---|---|---|---|
| All-cause mortality or disabling stroke at (composite; 2 years) | Pooled absolute risk difference: −1% (−3% to 2%) | High | TAVI noninferior to SAVR |
| All-cause mortality (2 years) | Pooled absolute risk difference: 0% (−2% to 2%) | Moderate | No statistically significant difference between groups |
| Disabling stroke (2 years) | Pooled absolute risk difference: −1% (−2% to 0%) | Moderate | No statistically significant difference between groups |
| Life-threatening or major/disabling bleeding (30 days) | Absolute risk difference: 2.9% (−0.1% to 5.9%)19 Absolute risk difference: −33% (−36.5% to −29.4%)10a |
Low | One study showed a lower risk of life-threatening or major/disabling bleeding with TAVI, but there was serious inconsistency between the two studies (30 days) |
| Acute kidney injury (30 days) | Pooled absolute risk difference: −0.02% (−0.03% to −0.01%) | High | TAVI had a lower risk of acute kidney injury at 30 days |
| Atrial fibrillation (30 days) | Absolute risk difference: −30.5% (−34.7% to −26.4%)19 Absolute risk difference: −17.3% (−20.5% to −14.1%)10a |
High | TAVI had a lower risk of atrial fibrillation at 30 days |
| New permanent pacemaker implantation (30 days) | Absolute risk difference: 19.3% (15.9% to 22.7%)19 Absolute risk difference: 1.6% (−0.7% to 3.9%)10a |
Low | One study reported an increased risk of need for a new pacemaker with TAVI, but there was serious inconsistency between the two studies |
| Major vascular complications (30 days) | Pooled absolute risk difference: 4% (2% to 5%) | High | TAVI increased the risk of major vascular complications |
| Moderate to severe paravalvular aortic regurgitation (2 years) | Pooled absolute risk difference: 7% (5.0% to 8.0%) | High | TAVI increased the risk of moderate to severe paravalvular aortic regurgitation |
| Aortic valve reintervention (2 years) | Absolute risk difference: 2.0% (0.6% to 3.4%)19 Absolute risk difference: 0.8% (−0.06% to 1.7%)10a |
Low | One study showed an increased risk of aortic valve reinterventions with TAVI, but the other study showed no statistically significant difference |
| Longer-term follow-up is needed to assess the durability of the bioprosthetic TAVI valve | |||
| Length of hospital stay (implantation procedure) | Overall mean difference: −4.0 days19 Overall difference in medians: −3.0 (P < .001)10a ICU difference in medians: −2.0 (P < .001)10a |
High | TAVI resulted in a shorter hospital and ICU stay |
| NYHA symptoms (30 days and 2 years) | Mean difference not provided | High (30 days) Moderate (2 years) |
Improvement from baseline with both TAVI and SAVR TAVI resulted in fewer symptoms at 30 days (1 RCT) but there was no difference in degree of improvement between TAVI and SAVR at 1 year and 2 years (2 RCTs) |
| Quality of life, KCCQ (30 days and 2 years) | Absolute difference: 12.5 (10.1–15) at 30 days19 | High (30 days) Moderate (2 years) |
Both TAVI and SAVR reported improvement from baseline TAVI resulted in greater improvement at 30 days, but there was no difference in degree of improvement between TAVI and SAVR at 1 year and 2 years |
Abbreviations: ICU, intensive care unit; IRR; incidence rate ratio; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
We calculated the absolute risk difference based on the information provided in the study.
Table 9:
TAVI Versus SAVR (Transfemoral and Transthoracic Cohorts)
| Outcomea | Effect Measure (95% CI) | GRADE | Summary |
|---|---|---|---|
| Transfemoral Cohort | |||
| All-cause mortality or disabling stroke (2 years) | As-treated analysis, HR: 0.78 (0.61 to 0.99) Intention-to-treat analysis, HR: 0.79 (0.62 to 1.00) |
Moderate | TAVI may have had a lower risk of the composite endpoint of all-cause mortality and disabling stroke |
| All-cause mortality (2 years) | HR: 0.80 (0.62 to 1.04) | Moderate | No statistically significant difference between groups |
| Disabling stroke (2 years) | HR: 0.77 (0.50 to 1.17) | Moderate | No statistically significant difference between groups |
| Life-threatening or disabling bleeding (30 days) | Absolute risk difference: −34.7% (−38.6% to −30.8%)10b | High | TAVI had a lower risk of life-threatening or disabling bleeding |
| Acute kidney injury (30 days) | Absolute risk difference: −2.5% (−3.8% to −1.2%)10b | High | TAVI had a lower risk of acute kidney injury |
| Atrial fibrillation (30 days) | Absolute risk difference: −21.8% (−25.3% to −18.3%)10b | High | TAVI had a lower risk of atrial fibrillation |
| New permanent pacemaker (30 days) | Absolute risk difference: 1.0% (−1.6% to 3.6%)10b | Moderate | No statistically significant difference between groups |
| Major vascular complications (30 days) | Absolute risk difference: 4.6% (2.2% to 7.0%)10b | High | TAVI increased the risk of major vascular complications |
| Aortic valve reintervention (2 years) | Absolute risk difference: 0.4% (−0.06% to 1.4%)10b | Low | No statistically significant difference between groups |
| Longer-term follow-up is needed to assess the durability of the bioprosthetic TAVI valve | |||
| Quality of life, KCCQ (30 days and 2 years) | Absolute difference: 14.1 (11.7 to 16) at 30 days | High (30 days) Moderate (2 years) |
Both TAVI and SAVR reported improvement from baseline |
| TAVI resulted in greater improvement at 30 days, but no statistically significant differences between groups at 2 years | |||
| Transthoracic Cohort | |||
| All-cause mortality or disabling stroke (2 years) | HR: 1.21 (0.84 to 1.74) | Moderate | No statistically significant difference between groups |
| All-cause mortality (2 years) | HR: 1.26 (0.86 to 1.86) | Moderate | No statistically significant difference between groups |
| Disabling stroke (2 years) | HR: 1.57 (0.78 to 3.16) | Moderate | No statistically significant difference between groups |
| Life-threatening or disabling bleeding (30 days) | Absolute risk difference: −27.2% (−35.4% to −18.9%)10b | High | TAVI had a lower risk of life-threatening or disabling bleeding |
| Acute kidney injury (30 days) | Absolute risk difference: −0.5% (−2.9% to 3.9%)10b | Moderate | No statistically significant difference between groups |
| Atrial fibrillation (30 days) | Absolute risk difference: −2.6% (−10.2% to 5.0%)10b | Moderate | No statistically significant difference between groups |
| New permanent pacemaker (30 days) | Absolute risk difference: 4.0% (−0.8% to 8.8%)10b | Moderate | No statistically significant difference between groups |
| Major vascular complications (30 days) | Absolute risk difference: −2.7% (−7.3% to 1.9%)10b | Moderate | No statistically significant difference between groups |
| Aortic valve reintervention (2 years) | Absolute risk difference: 2.0% (0.2% to 3.8%)10b | Low | TAVI had a higher risk of aortic valve reinterventions, but the very small number of events affected the robustness of results (2 years) |
| Quality of life, KCCQ (30 days and 2 years) | Absolute difference: 3.5 (−1.4 to 8.4) at 30 days | Moderate | Both TAVI and SAVR reported improvement from baseline No statistically significant differences between groups |
Abbreviations: HR, hazard ratio; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
We calculated the absolute risk difference based on the information provided in the study.
The results for the following outcomes were not provided separately for the transfemoral and transthoracic cohorts: length of hospital stay, paravalvular aortic regurgitation, and NYHA symptoms.
Discussion
Surgical aortic valve replacement is the conventional way of treating severe, symptomatic aortic valve stenosis in patients at low and intermediate surgical risk.1,2 Our systematic review identified 2 large randomized controlled trials comparing TAVI and SAVR in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk.10,19 According to the authors of the SURTAVI trial,19 the fact that mean STS scores and 2-year mortality were higher in the PARTNER 2 trial10 (mean STS 5.8% vs. 4.5%; 2-year mortality, TAVI 11.4% vs. 16.7%; 2-year mortality, SAVR: 11.6% vs. 18%) may suggest that the patients in the PARTNER 2 trial, although considered to be at intermediate surgical risk, were at higher surgical risk than the patients in the SURTAVI trial.
Both trials found that TAVI and SAVR showed a similar risk of the combined endpoint of death or disabling stroke at 2 years, and the noninferiority criterion was satisfied in both trials. In the transfemoral cohort of the PARTNER 2 trial,10 no statistically significant difference was observed between groups in the intention-to-treat analysis at 2 years, but there was a statistically significant lower risk of the combined endpoint with TAVI versus SAVR in the as-treated analysis (HR 0.78, 95% CI 0.61–0.99).
There was a greater risk of some complications with TAVI compared with SAVR, including major vascular complications and moderate to severe paravalvular aortic regurgitation. Paravalvular aortic regurgitation is defined as leakage of blood between the TAVI and the native valve,10 usually because of incomplete annular sealing.12 It could also result from prosthesis undersizing, incomplete expansion, or malposition, and may lead to severe hemodynamic consequences.12,38 Paravalvular aortic regurgitation is most often mild to moderate, but if treatment is necessary, it may consist of valve redilation, valve repositioning, or implantation of a second transcatheter valve; any of these procedures may be associated with important risks.12 According to the PARTNER 2 trial,10 patients with moderate to severe paravalvular aortic regurgitation had a higher risk of death at 2 years than those with no or trace paravalvular aortic regurgitation (HR 2.85, 95% CI 1.57–5.21). No difference in mortality was observed in patients with mild versus no or trace paravalvular aortic regurgitation (HR 0.95, 95% CI 0.63–1.45).10
On the other hand, TAVI was associated with a lower risk of some complications compared with SAVR, including acute kidney injury and atrial fibrillation. According to Siemieniuk et al,29 it was difficult to determine whether the increased risk of atrial fibrillation in the SAVR group was a transient effect. There was no statistically significant difference between TAVI and SAVR with respect to disabling stroke, transient ischemic attacks, or rehospitalization.
There were differences between the two studies with respect to the results of some of the outcomes, specifically new pacemaker implantation and life-threatening or major/disabling bleeding. There was a higher risk of new pacemaker implantation with TAVI compared with SAVR in the SURTAVI trial,19 but no difference between groups was observed in the PARTNER 2 trial.10 The need for a new pacemaker is a complication that may be associated with increased rehospitalization and mortality.17 The higher risk of pacemaker implantation in the SURTAVI trial may have been due in part to differences in the design of the valve (which may protrude into the left ventricular outflow tract), the positioning of the valve, or other factors.15
In the PARTNER 2 trial,10 there was a lower risk of life-threatening or disabling bleeding with TAVI compared with SAVR, but this was not observed in the SURTAVI trial.19 The reasons for this inconsistency are unclear.
Overall, quality of life improved from baseline in both trials for both the TAVI and the SAVR groups throughout the 2 years of follow-up. However, patients in the TAVI group had a statistically significant greater improvement in quality of life than the SAVR group early after the procedure (30 days), both in the full cohort (SURTAVI)19 and the transfemoral cohort (PARTNER 2).32 At 6 months,19 1 year and 2 years,19,34 patients in both TAVI and SAVR groups had similar improvements in quality of life. In the transthoracic cohort of the PARTNER 2 trial,32 both groups had similar improvements in quality of life throughout the 2 years of follow-up. According to the PARTNER 2 study authors,32 the greater early improvement seen with TAVI versus SAVR in the transfemoral cohort but not in the transthoracic cohort may have been due to a lower risk of early complications in the former, but not the latter. As well, the manipulation of the chest's musculoskeletal frame required for transthoracic access may cause more postoperative pain,32 affecting quality of life. This was corroborated by the statistically significantly greater improvement observed with TAVI at 30 days in the KCCQ subscales for physical and social limitations, total symptoms, and quality of life in the transfemoral cohort but not the transthoracic cohort.32
Aortic valve hemodynamics improved over baseline in both TAVI and SAVR groups, and both studies showed that the improvement was greater with TAVI compared with SAVR,10,19 although it is not clear whether the difference between groups was clinically important.
Limitations
The current, newer-generation TAVI valves were either not used or used in only a small proportion of patients in the trials identified (16% in the SURTAVI trial19). The noncomparative PARTNER 2 SAPIEN 3 study evaluated the newest-generation SAPIEN 3 TAVI valve in patients with severe, symptomatic aortic valve stenosis at intermediate risk for surgery.39 According to the authors, the favourable results obtained in this noncomparative study may be in part a consequence of increased operator experience, improved patient selection, use of imaging for vascular access, and annulus sizing, but improvements with the newer-generation SAPIEN 3 valve and its delivery system were also contributors.39
As previously mentioned, there may have been differences in surgical risk between the two studies.19 Moreover, the studies evaluated different types of valves: self-expanding19 and balloon-expandable.10 Inconsistencies between the two studies did not allow us to draw firm conclusions or perform a meta-analysis for some of the outcomes. Also, the results by implantation access route (transfemoral/transthoracic) were based on only one of the studies identified.
The studies were powered to assess the noninferiority of TAVI compared with SAVR with respect to the composite outcome of all-cause mortality or disabling stroke within 2 years of follow-up. There may have been inadequate statistical power to evaluate other outcomes.
The authors of the SURTAVI trial19 observed no evidence of structural valve deterioration at 2 years in either group. However, the authors of both studies emphasized that longer follow-up is needed to assess the durability of the bioprosthetic TAVI valve10,19: whether the valve may fail, when it may fail, the mode of failure, and outcomes of reintervention.13 Longer follow-up will also allow for the assessment of the long-term consequences of aortic valve regurgitation.13
Both studies had a relatively high frequency of unplanned withdrawals before the implantation procedure, especially in the SAVR group, the result of either the patient's or the physician's decision (approximately 8% in the SAVR groups and 2% in the TAVI groups of both studies).10,19 However, the baseline characteristics of the patients withdrawn did not differ from those of the patients who remained.19 Also, the fact that the results of the intention-to-treat and as-treated analyses were similar suggests a low risk of selection bias.
The two studies were performed in experienced centres and with experienced operators, and patients with a previous aortic valve or bicuspid valves were excluded,10,19 which may affect the generalizability of the study results.
Conclusions
Overall Analysis (Full Cohort)
TAVI was not inferior to SAVR with respect to the composite endpoint of all-cause mortality or disabling stroke within 2 years of follow-up (GRADE: High), but the two treatments had different patterns of complications:
TAVI had a lower risk of acute kidney injury (GRADE: High) and atrial fibrillation (GRADE: High) compared with SAVR
TAVI had a higher risk of moderate to severe paravalvular aortic regurgitation (GRADE: High) and major vascular complications (GRADE: High) compared with SAVR. One of the included studies also showed a higher risk of new pacemaker implantation (GRADE: Low) and aortic valve reinterventions (GRADE: Low) with TAVI compared with SAVR
Based on the results of one study, TAVI had a lower risk of life-threatening or disabling bleeding (GRADE: Low), but the second study found no statistically significant differences between groups
One study showed a reduction in stroke with TAVI versus SAVR at 30 days, but no statistically significant difference was observed in the other study and in our meta-analysis of both studies. Length of hospital stay — both overall and in the intensive care unit — was reduced with TAVI compared with SAVR (GRADE: High).
Postoperative valve hemodynamics improved with both TAVI and SAVR for the 2 years of follow-up; the degree of improvement was statistically significantly higher with TAVI than with SAVR, but it is unclear whether the difference between groups was clinically important.
Both TAVI and SAVR patients experienced an improvement in NYHA symptoms, but one study showed a statistically significant greater improvement in symptoms with TAVI than with SAVR at 30 days (GRADE: High). The difference was not statistically significant in either study at 2 years (GRADE: Moderate). Similarly, TAVI patients had a greater quality-of-life improvement at 30 days compared with SAVR in the full cohort of the SURTAVI trial and in the transfemoral cohort of the PARTNER 2 trial (GRADE: High), but the difference between groups was not statistically significant at 2 years (GRADE: Moderate). There was no statistically significant difference in quality of life improvement between TAVI and SAVR in the transthoracic cohort of the PARTNER 2 trial throughout study follow-up (GRADE: Moderate).
At the time of writing this report, the 5-year results of the included randomized controlled trials10,19 have not yet been published; according to the study authors, longer-term follow-up is needed to assess the durability of the bioprosthetic TAVI valve.
Subgroup Analyses (Transfemoral and Transthoracic Cohorts)
Patients who underwent TAVI using a transfemoral implantation access route may have had a lower risk of the composite endpoint of all-cause mortality and disabling stroke compared with SAVR (GRADE: Moderate). For the other outcomes, the results followed a direction similar to the full cohort.
Patients who underwent TAVI using a transthoracic implantation access route had a lower risk of life-threatening/disabling bleeding compared with SAVR (GRADE: High), similar to the transfemoral cohort. However, neither the lower risk of acute kidney injury (GRADE: Moderate) and atrial fibrillation (GRADE: Moderate) reported in the transfemoral cohort, nor the increase in major vascular complications (GRADE: Moderate), were observed in this cohort. In contrast to what was observed in the transfemoral cohort, the risk of aortic valve reinterventions was higher with TAVI compared with SAVR in the transthoracic cohort (GRADE: Low).
Although the decision to stratify patients according to implantation access route in the PARTNER 2 trial10 was made a priori, the study was not powered to compare the outcomes of TAVI vs. SAVR in the transfemoral and transthoracic subgroups. According to the study authors, an adequately powered prospective study is needed to show whether transfemoral TAVI is superior to SAVR, and whether transthoracic TAVI is similar or inferior to SAVR.10
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve implementation (SAVR) in adults with severe, symptomatic aortic valve stenosis who are at intermediate surgical risk?
Methods
Economic Literature Search
We performed an economic literature search on March 29, 2018, for studies published from inception to the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, and National Health Service Economic Evaluation Database (NHS EED). To retrieve relevant studies, we applied an economic filter to the clinical search strategy.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We performed targeted grey literature searching of health technology assessment agency websites, PROSPERO, EUnetHTA Assessments, clinical trial registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Evidence literature search, above, for further details on methods used. See Appendix 1 for literature search strategies, including all search terms.
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and performed further assessment for eligibility. Citation flow and reasons for exclusion of full-text articles were reported according to the PRISMA statement.
Inclusion Criteria
English-language full-text publications
Cost-utility, cost-effectiveness, cost-benefit, or cost-consequence analyses, including costs and health outcomes
Studies comparing TAVI and SAVR
Adult patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk (determined by the study site multidisciplinary heart team, informed by a Society of Thoracic Surgeons [STS] score [4%–8%] or EuroScore [10%–20%] and assessment of comorbidities)
Exclusion Criteria
Reviews, letters/editorials, commentaries, abstracts, posters
Studies in people < 18 years old
Studies evaluating TAVI in patients with a pre-existing mechanical or bioprosthetic aortic valve (i.e., valve-in-valve procedures)
Studies that included a mixed population with different surgical risks (i.e., low, intermediate, and high) without providing results specific to the intermediate-risk population
Outcomes of Interest
Cost
Quality-adjusted life-years (QALYs)
Incremental cost and incremental effectiveness
Incremental cost per QALY gained
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, and incremental cost-effectiveness ratios [ICERs])
We contacted authors of the studies to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform development of NICE's clinical guidelines.40 We modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). A summary is presented in Appendix 8. In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Literature Search
The literature search yielded 494 citations published between inception and March 29, 2018, after removing duplicates. We excluded a total of 485 articles based on information in the title and abstract. We then obtained the full texts of nine potentially relevant articles for further assessment. Figure 12 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 12: PRISMA Flow Diagram for the Economic Evidence Review.
Source: Adapted from Moher et al.27
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
aGrey literature (n = 2), literature search updates run for March 29, 2018, to August 17, 2018 (n = 10).
Review of Included Economic Studies
We identified three economic studies that compared the cost-effectiveness of TAVI versus SAVR in adults with severe, symptomatic aortic valve stenosis at intermediate surgical risk. The studies and their results are summarized in Table 10.
Table 10:
Results of Economic Literature Review—Summary
| Name, Year, Location | Analytic Technique, Study Design, Time Horizon, Perspective | Population | Intervention/Comparator | Resultsa | |||
|---|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-effectiveness | Uncertainty | ||||
| Kodera et al, 2018,41 Japan |
|
|
|
|
Reference case Japanese yenc Discounted 2%
|
Reference case Discounted 2% ICER (TF TAVI vs. SAVR): ¥7,523,821/ QALY (∼$89,000 CAD) |
Probabilistic sensitivity analysis TF TAVI probability of cost-effectiveness: 46% at WTP of ¥5,000,000/ QALY (∼$60,000 CAD) TF and TT scenario ICER (TAVI vs. SAVR): ¥56,528,188/QALY (∼$668,000 CAD) |
| Tam et al, 2018,16 Canada |
|
|
|
Reference case (± SD) Discounted 1.5%
|
Reference case (± SD) 2016 Canadian dollars Discounted 1.5%
|
Reference case Discounted 1.5% ICER (TAVI vs. SAVR): $46,083/QALY Undiscounted ICER (TAVI vs. SAVR): $39,661/QALY |
Probabilistic sensitivity analysis TAVI probability of cost-effectiveness:
|
| Tam et al, 2018,17 Canada |
|
|
|
Reference case (± SD) Discounted 1.5%
|
Reference case (± SD) 2016 Canadian dollars Discounted 1.5%
|
Reference case Discounted 1.5% ICER (TAVI vs. SAVR): $76,736/QALY Undiscounted ICER (TAVI vs. SAVR): $71,043/QALY |
Probabilistic sensitivity analysis TAVI probability of cost-effectiveness:
|
Abbreviations: AVS, aortic valve stenosis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TT, transthoracic; WTP, willingness-to-pay.
Numbers may appear inexact due to rounding.
Based on criteria from the randomized controlled trials, which were based on clinical assessment by a multidisciplinary heart team, informed by the Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) score, coexisting conditions, frailty, and disability.
Unclear what year costs were calculated in.
Transfemoral scenario: TAVI vs. SAVR, incremental QALYs 0.40 and incremental costs $9,815.
All three studies conducted cost-utility analyses using Markov models. The clinical inputs were informed by previously published literature. One study16 used the PARTNER 2 randomized controlled trial,10 which evaluated balloon-expandable TAVI; one study17 used the SURTAVI randomized controlled trial,19 which evaluated self-expandable TAVI; and one study41 used both the PARTNER 2 randomized controlled trial42,43 and the OCEAN TAVI registry.16,41 All studies translated clinical inputs, including mortality and complications, into QALYs. The complications considered in each analysis varied. Two studies16,41 conducted analyses in people who had TAVI using the transfemoral access route.
The studies included costs related to the valve, procedure, and complications. When converted to Canadian dollars, the cost of the TAVI valve in the reference case analyses ranged from $22,00017 to ∼$53,000.41 The cost of the TAVI valve was substantially higher than that of the SAVR valve. However, TAVI index hospitalization costs (excluding the valve) were generally lower than SAVR costs and offset some of the valve costs.
Results showed that TAVI was more effective and more expensive than SAVR. The ICER for TAVI varied among the studies. Two studies were conducted using an Ontario perspective and 2016 Canadian dollars. The ICERs in the reference case analyses ranged from $46,083/QALY16 to $76,736/QALY.17 The difference in the results of the two studies was attributed to differences in complication rates between the two trials.17 Moderate to high uncertainty was present in both studies, finding that, at a willingness-to-pay value of $50,000/QALY, TAVI was cost-effective in just over half (53%) of the model simulations. One Ontario study16 conducted an analysis specific to people who underwent transfemoral TAVI, and it found that the ICER decreased substantially ($24,790/QALY) for this population. Similarly, the study conducted in Japan41 found that the ICER was much lower when only transfemoral TAVI was considered.
One-way sensitivity analyses found that valve costs, length of intensive care unit stay after the procedure, periprocedural mortality rate, periprocedural stroke rate, long-term mortality rate and time horizon all influenced the ICER.16,17,41
The authors of the two Ontario studies16,17 concluded that TAVI may be cost-effective in the population at intermediate surgical risk. However, the authors of the study from Japan41 concluded that TAVI was not cost-effective, given that the ICER was >5,000,000 yen per QALY (∼$60,000 CAD per QALY).
Applicability and Limitations of the Included Studies
The results of the methodology checklist applied to the included articles are presented in Appendix 8 (Table A14). Two studies16,17 were deemed directly applicable to the research question, and one41 was considered partially applicable. All three studies were conducted in adults with severe aortic valve stenosis at intermediate surgical risk. Further, the studies included relevant comparators and effectiveness data from recently published randomized controlled trials (i.e., PARTNER 210 and SURTAVI19).
The two directly applicable studies16,17 were conducted using the perspective of the Ontario Ministry of Health. They also used probabilistic analyses and a discount rate of 1.5%, as recommended by the Canadian Agency for Drugs and Technologies in Health.44 The partially applicable study41 was conducted using a Japanese public health care payer perspective and used a discount rate of 2%.
An assessment of the limitations of the directly applicable, Ontario-based studies is presented in Appendix 8 (Table A15). Both studies had only minor limitations. They included relevant costs and used Ontario sources (i.e., Ontario Health Insurance Plan billing codes, Canadian Institute for Health Information Patient Cost Estimator, St. Michael's Hospital) to estimate the costs and resource use associated with TAVI and SAVR. Health outcomes were based primarily on the PARTNER 2 and SURTAVI randomized controlled trials.10,19 These studies had a 2-year follow-up. The cost-utility studies used lifetime horizons, but made the conservative assumption that, after 2 years, mortality and complication rates were the same for people with TAVI and SAVR. Because of a lack of literature on people at intermediate surgical risk at the time of publication, the studies used data from cohorts at high surgical risk (CoreValve US High Risk Pivotal Trial45 and PARTNER 1A46) to inform health-state utility values for TAVI and SAVR.
Discussion
Our economic evidence review identified three studies16,17,41 that assessed the cost-effectiveness of TAVI in people with severe aortic valve stenosis who were at intermediate surgical risk. All three studies found that, on average, TAVI was more effective (i.e., produced more QALYs) than SAVR, but also more expensive.
We identified three additional cost-effectiveness analyses,47–49 but they did not meet our exclusion criteria (i.e., one abstract, one conference presentation, and one study with a mixed surgical risk population). One observational study followed a cohort of people that received TAVI or SAVR in Spain.49 The authors of that study found that TAVI was unlikely to be cost-effective because it was more expensive than SAVR and provided little to no added benefit. The patients in that study had intermediate-risk STS scores, on average. However, the population also included people with high- and low-risk scores. In the absence of a subgroup analysis, it was not clear what the cost-effectiveness would be in the intermediate-risk group alone. In addition, because of the observational nature of the study, the results could have been affected by uncontrolled confounding. The remaining two studies47,48 found that TAVI was more effective than SAVR. One found that TAVI was only marginally more expensive than SAVR and represented good value for money.48 The other found that TAVI was the dominant strategy, because it was more effective and less costly than SAVR.47 However, despite these promising results, we were unable to fully assess the applicability and quality of these studies, given the absence of full-text publications.
Among the studies that did meet our inclusion criteria, two16,17 conducted from an Ontario perspective concluded that TAVI (including all access routes) may be cost-effective compared to SAVR (ICER: ≤ $80,000/QALY). In contrast, the study conducted from a Japanese perspective41 found that TAVI was not cost-effective when considering all access routes (ICER, converted: ≥ $600,000/QALY). Although it used clinical inputs similar to an Ontario analysis16 (i.e., the PARTNER 2 trial10), the study from Japan41 incorporated data from a local trial, different complications, and a device price that was more than double that of the price in Ontario. However, both studies16,41 found that the ICER was greatly reduced when considering only people who received TAVI using the transfemoral access route.
Implanting TAVI using the transfemoral access route can offer a more minimally invasive strategy: general anaesthesia is not needed (only local), transoesophageal echocardiography guidance is not required, and the procedure can be performed in a catheterization lab.50 This minimally invasive approach is associated with a shorter length of hospital stay.50 In general, the transfemoral access route has been shown to have better clinical outcomes10 and fewer costs51 than non-transfemoral TAVI. As minimally invasive techniques are refined and adopted, there is the potential to see improved cost-effectiveness for TAVI compared to SAVR.
The two Ontario studies were directly applicable to our context and research question of interest. However, although the studies were well conducted, their limitations should be considered when interpreting the findings. The authors highlighted uncertainty in the results. When considering parameter uncertainty, the probability that TAVI was cost-effective at a willingness-to-pay value of $100,000/QALY was less than 60%. This finding can be attributed in part to variation in the comparative effectiveness of TAVI and SAVR (i.e., quality of life, clinical effectiveness) and the fact that the clinical evidence came from noninferiority trials. This was consistent with our clinical evidence review, which found no statistically significant difference between TAVI and SAVR for several clinical outcomes, including all-cause mortality and stroke. As discussed by Tam et al,16 additional clinical evidence is needed to reduce uncertainty.
A second limitation highlighted by the authors was the lack of available utility data. At the time of publication, no quality-of-life data had been published from trials in people at intermediate surgical risk. As a result, the authors derived utility data from trials assessing TAVI in people who were at high risk or inoperable.16,17 Since then, 12-month quality-of-life data for people at intermediate surgical risk enrolled in the PARTNER 2 trial have been published.34 The results from the high- and intermediate-risk PARTNER 1A and 2 cohorts were quite similar, finding a significantly higher utility score at 1 month for people who received transfemoral TAVI compared to SAVR.34,46 No significant differences in utility were seen beyond 12 months. Given this similarity, it is unlikely that updated utility data would significantly alter the results. The authors of the cost-effectiveness analyses also varied the utility parameters in sensitivity analyses.
Third, as the authors stated, knowledge about the durability of TAVI is limited, especially in patient at intermediate surgical risk. The durability of the TAVI valve becomes more important as implantations are conducted in lower-risk patients who will live longer. Studies in other risk groups have shown little TAVI valve deterioration at 5 years, but evidence beyond this time frame is limited.52–55 If longer-term evidence specific to the intermediate-risk population becomes available, TAVI durability should be incorporated into future cost-effectiveness models.
Finally, most devices included in the randomized controlled trials that informed the cost-effectiveness analyses were earlier-generation transcatheter aortic valves. The outcomes of newer-generation valves, which are currently in use in Ontario, may not have been fully captured. Ongoing registries have captured some outcomes in patients at intermediate surgical risk using these valves, but no randomized controlled trial evidence is available. If outcomes are better with newer-generation valves, the cost-effectiveness of TAVI may be improved.
The two studies by Tam et al16,17 were both of good quality and applicable to the Ontario context. Based on current randomized controlled trial evidence, they demonstrated that TAVI may be cost-effective in people with severe aortic valve stenosis who are at intermediate surgical risk. Furthermore, one study16 showed that TAVI became more cost-effective among those who undergo the procedure using the transfemoral access route.
Conclusions
Our review of the literature identified three published cost-effectiveness studies that compared TAVI with SAVR in adults with severe, symptomatic aortic valve stenosis who were at intermediate surgical risk. Two directly applicable studies were conducted from the perspective of the Ontario Ministry of Health. The studies showed that TAVI may be cost-effective compared to SAVR. As well, TAVI appears to be more cost-effective among those who can have transfemoral implantation.
PRIMARY ECONOMIC EVALUATION
Two directly applicable studies assessing the cost-effectiveness of TAVI compared to SAVR for the treatment of severe, symptomatic aortic valve stenosis in people at intermediate surgical risk were identified in the economic evidence review.16,17 The studies conducted cost-utility analyses from the perspective of the Ontario Ministry of Health. Both studies used Ontario costing sources, data from recent randomized controlled trials, and had only minor limitations. Given the availability of such analyses, we did not conduct a primary economic evaluation.
BUDGET IMPACT ANALYSIS
Research Question
What is the 5-year budget impact to the Ontario Ministry of Health of publicly funding transcatheter aortic valve implantation (TAVI) in adults with severe, symptomatic aortic valve stenosis who are at intermediate surgical risk?
Methods
Analytic Framework
We estimated the budget impact of TAVI in adults at intermediate surgical risk using the cost difference between two scenarios: current clinical practice without publicly funding TAVI (the current scenario), and the anticipated clinical practice with publicly funding TAVI (the new scenario). The budget impact framework is shown in Figure 13.
Figure 13: Budget Impact Analysis Framework.
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results were affected by varying input parameters and model assumptions.
Key Assumptions
At present in Ontario, there is no formal public funding for TAVI in patients at intermediate surgical risk
Society of Thoracic Surgeons (STS) scores are equivalent to the heart team's judgment
Patients currently receiving combined surgical aortic valve replacement (SAVR) and coronary artery bypass graft for revascularization would be eligible to receive TAVI
Based on the current Health Canada indication in people at intermediate surgical risk, only people eligible for transfemoral access would be eligible for TAVI
Costs obtained from previous analyses (in 2016 CAD) were equivalent to current costs (2018 CAD)
Target Population
The target population for this analysis was adults (≥ 18 years) with severe, symptomatic aortic valve stenosis who were at intermediate surgical risk. Ideally, intermediate surgical risk should be determined by a multidisciplinary heart team, with consideration of the STS risk score (intermediate risk = 4% to 8%, consistent with the PARTNER 2 trial10), EuroScore (intermediate risk = 10% to 20%), risk factors, and/or comorbidities.56
Our method of estimating the target population is described below and in Table 11. We first obtained the number of people undergoing SAVR (isolated or in conjunction with coronary artery bypass graft) in Ontario from the CorHealth Registry from 2009/10 to 2017/18 (retrieved by CorHealth May 4, 2018). We assumed that all patients were over the age of 18 years. Using linear extrapolation, we estimated the number of people who would undergo SAVR over the next 5 years.
Table 11:
Number of People at Intermediate Surgical Risk Eligible to Receive TAVI in Ontario, 2018/19 to 2022/23
| Population | Year | ||||
|---|---|---|---|---|---|
| 2018/19 | 2019/20 | 2020/21 | 2021/22 | 2022/23 | |
| Isolated SAVR,a n | 2001 | 2046 | 2092 | 2137 | 2182 |
| SAVR + CABG,b n | 1171 | 1201 | 1208 | 1215 | 1222 |
| Total SAVR, n | 3172 | 3247 | 3300 | 3352 | 3404 |
| SAVR in patients with aortic valve stenosisc | 65% | 65% | 65% | 65% | 65% |
| Total SAVR, aortic valve stenosis, n | 2062 | 2111 | 2145 | 2179 | 2213 |
| Patients with aortic valve stenosis at intermediate surgical risk | 13.9% | 13.9% | 13.9% | 13.9% | 13.9% |
| SAVR in patients with aortic valve stenosis at intermediate surgical risk, n | 287 | 294 | 299 | 304 | 308 |
| Patients who can have transfemoral TAVI | 92.5% | 92.5% | 92.5% | 92.5% | 92.5% |
| Patients eligible for TAVI, n | 266 | 272 | 276 | 281 | 285 |
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Note: Numbers may appear inexact due to rounding.
Projected from total isolated SAVR volumes from 2009/10 (n = 1,539) to 2017/18 (n = 1,978) in Ontario (Source: CorHealth Ontario Cardiac Registry, data retrieved May 4, 2018).
Projected from SAVR + CABG volumes from 2009/10 (n = 1,158) to 2017/18 (n = 1,136) in Ontario (Source: CorHealth Ontario Cardiac Registry, data retrieved May 4, 2018).
65% of SAVR in patients with aortic stenosis, based on previous analysis in Ontario (2012/13–2017/18) (Harindra Wijeysundera, written communication, August 14, 2019)
Based on a previous analysis in Ontario (2012/13–2017/18; Harindra Wijeysundera, written communication, August 14, 2019), we estimated that approximately 65% of patients receiving SAVR would have aortic valve stenosis. We excluded patients who had SAVR for other indications (e.g., aortic insufficiency and endocarditis), because they would not be eligible for TAVI.
Then, among patients who underwent SAVR for aortic valve stenosis, we estimated the proportion that were at intermediate surgical risk. Based on the STS scores of 141,905 people who underwent SAVR in the United States, we assumed that 13.9% of people would be at intermediate risk.7 We recognize that surgical risk is usually determined by a heart team, and that STS score is only one of the factors that may be considered. However, in the absence of population data on the heart team's risk stratification, we used an STS score of 4% to 8% as a proxy. We examined higher proportions of patients at intermediate surgical risk (including those with an STS score of 3% to 8%) in our sensitivity analyses.
Finally, based on the current Health Canada indication in people at intermediate surgical risk, we assumed that TAVI would be used only when the transfemoral approach was possible. Based on estimates from the manufacturers (Edwards Lifesciences, oral communication, April 23, 2018; Medtronic, oral communication, May 3, 2018) we assumed that 92.5% of TAVI procedures in Ontario would use the transfemoral access route. We examined different rates of transfemoral access in our sensitivity analysis.
Current Intervention Mix, Uptake of the New Intervention, and Future Intervention Mix
We assumed that TAVI is not currently being used in people at intermediate surgical risk. Thus, in our current scenario, we assumed that all patients would receive SAVR (Table 12).
Table 12:
Number of People at Intermediate Surgical Risk Expected to Receive TAVI or SAVR in Ontario, 2018/19 to 2022/23a
| Procedure | Year | ||||
|---|---|---|---|---|---|
| 2018/19 | 2019/20 | 2020/21 | 2021/22 | 2022/23 | |
| Current scenario: No Public Funding for TAVI | |||||
| SAVR, n | 266 | 272 | 276 | 281 | 285 |
| New scenario: Public Funding for TAVI | |||||
| Uptake rate for TAVI | 75% | 80% | 85% | 90% | 95% |
| TAVI, n | 200 | 218 | 235 | 253 | 271 |
| SAVR, n | 67 | 54 | 41 | 28 | 14 |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Note: Numbers may appear inexact due to rounding.
This number is in addition to the current volume of TAVIs being funded in patients who cannot undergo surgery or are at high surgical risk (N = 1,022; see Table 1).
In our new scenario, in which TAVI is publicly funded for people at intermediate surgical risk, we assumed that some would receive TAVI instead of SAVR (Table 12). We assumed that since TAVI has already diffused into the system, there would be a quick uptake to 75% in the first year. After this, we assumed a gradual linear uptake over the next 5 years, levelling out at 95%. We assumed that a small proportion of people (5%) would not receive TAVI because of anatomic incompatibility or personal preference.
Health Care Resources and Costs
We obtained all costs used in our budget impact analyses from two previously published cost-effectiveness analyses of TAVI in people at intermediate surgical risk.16,17 These analyses, which we have described in the Economic Evidence Review, were conducted using an Ontario Ministry of Health perspective. One analysis16 was conducted using device costs and clinical parameters for the balloon-expandable TAVI (using the PARTNER 2 randomized controlled trial10). The other17 was conducted using device costs and clinical parameters for the self-expandable TAVI (using the SURTAVI randomized controlled trial19). Both analyses were conducted based on a cohort of patients with an average age of approximately 80 years. The procedural costs included in the analyses are summarized in Table 13. The authors obtained device costs from the manufacturers, hospitalization costs from St. Michael's Hospital and the published literature, and physician fees from the Ontario Schedule of Benefits.57 Short and long-term complication costs were obtained from the Canadian Institute for Health Information patient cost estimator and the published literature. More detailed descriptions can be found in the original publications.16,17
Table 13:
Procedural Costs Used in the Cost-Effectiveness Models
| Cost-Effectiveness Analysis (RCT) | Resource | Costs (2016 CAD) | |
|---|---|---|---|
| TAVI | SAVR | ||
| Tam et al, 201816 (PARTNER 2) |
Valve | 24,000 | 6,000 |
| Index hospitalization stay | 10,102 | 17,369 | |
| Surgeon, surgical assistant, anesthesiologist fees | 3,737 | 4,253 | |
| Total procedural costsa | 40,274 | 29,856 | |
| Tam et al, 201817 (SURTAVI) |
Valve | 22,000 | 6,000 |
| Index hospitalization stay | 9,866 | 15,357 | |
| Surgeon, surgical assistant, anesthesiologist fees | 2,836 | 3,549 | |
| Total procedural costsa | 39,753 | 27,918 | |
Abbreviations: RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Includes valve, index hospitalization stay, and fees, plus an estimate of costs for periprocedural complications (not provided in the original publications).
From each cost-effectiveness analysis, we derived the undiscounted average annual costs of treatment with SAVR or TAVI (Table 14). For our reference case analysis, we used the costs for transfemoral TAVI as obtained from Tam et al,16 which incorporated a sensitivity analysis in the transfemoral subgroup. In the sensitivity analyses, we looked at the average costs for all TAVI procedures (regardless of access route) obtained from the two Ontario analyses. We assumed that all costs in 2016 CAD were equivalent to 2018 CAD for our reference case analysis. However, we looked at inflation to 2018 CAD in our sensitivity analysis.
Table 14:
Average Annual Per-Patient Costs for TAVI and SAVR
| Cost-Effectiveness Analysis (RCT) | Intervention | Cost per Year Post-Implant,a,b $ | ||||
|---|---|---|---|---|---|---|
| Year 1c | Year 2d | Year 3d | Year 4d | Year 5d | ||
| Transfemoral TAVI Only (Reference Case) | ||||||
| Tam et al, 201816 (PARTNER 2) |
TAVI | 43,424 | 1,826 | 518 | 193 | 113 |
| SAVR | 33,421 | 1,626 | 623 | 320 | 185 | |
| All TAVI (Sensitivity Analysis) | ||||||
| Tam et al, 201816 (PARTNER 2) |
TAVI | 43,957 | 1,940 | 607 | 254 | 148 |
| SAVR | 33,532 | 1,712 | 639 | 319 | 184 | |
| Tam et al, 201817 (SURTAVI) |
TAVI | 42,160 | 1,634 | 349 | 87 | 52 |
| SAVR | 30,892 | 1,310 | 409 | 188 | 111 | |
| Average | TAVI | 43,059 | 1,787 | 478 | 171 | 100 |
| SAVR | 32,212 | 1,511 | 524 | 253 | 148 | |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Costs incorporate mortality.
Includes procedural (valve, index hospitalization, and fees for surgeon, surgical assistant, and anesthesiologist), short-term complication, and long-term complication costs.
Includes long-term complication costs.
Analysis
Reference Case Analysis
In the reference case analysis, we calculated the required budget to publicly fund transfemoral TAVI in adults with severe, symptomatic aortic valve stenosis at intermediate surgical risk in Ontario. We calculated the net budget impact as the cost difference between our new scenario (public funding for TAVI) and the current scenario (no public funding for TAVI). We also presented the budget impact and net budget impact broken down by cost type (i.e., device costs, professional fees, total procedural costs, and complications). Details of our method for calculating the net budget impact are presented in Appendix 9.
Scenario Analyses
We conducted several scenario analyses, summarized in Table 15. The number of people expected to receive TAVI and SAVR in each scenario can be found in Table A17 (Appendix 9).
Table 15:
Scenario Analyses
| Scenario | Description | Reference Case | Sensitivity Analysis |
|---|---|---|---|
| Target Population | |||
| 1 | Proportion of SAVR + CABG patients who are not eligible for TAVI | 0% | 20% |
| 2 | Proportion of SAVR patients who are at intermediate surgical risk | 13.93% | 25% |
| 3 | Proportion of patients who can have transfemoral-access TAVI | 92.5% | 83% |
| 4 | TAVI access route | Transfemoral only (92% of all people eligible for TAVI) | All people eligible for TAVIa |
| Uptake | |||
| 5 | Higher initial uptake | 75% to 95% over 5 years | 90% to 95% over 2 years |
| 6 | Lower initial uptake | 75% to 95% over 5 years | 50% to 95% over 5 years |
| Costing | |||
| 7 | Shorter length of hospital stay and costs for TAVI | Tam et al, 201816: 4 d ICU, 2 d wardb Tam et al, 201817: 2 d ICU, 3.75 d ward |
Kodali et al, 201639 and Attizzani et al, 201550: 3 d total (assume 1.5 d ICU, 1.5 d ward); Table A18 (Appendix 9) |
| 8 | Shorter length of hospital stay and costs for TAVI | Tam et al, 201816: 4 d ICU, 2 d wardb Tam et al, 201817: 2 d ICU, 3.75 d ward |
1d total (assume 0.5 d ICU, 0.5 d ward); Table A18 (Appendix 9) |
| 9 | SAVR valve cost | $6,000 | $3,000 |
| 10 | Inflating costs from 2016 CAD to 2018 CAD | Table 5 | Table A18 (Appendix 9) |
Abbreviations: CABG, coronary artery bypass graft; ICU, intensive care unit; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
In this scenario, we assumed that the costs were equivalent to the average per-person costs from Tam et al, 201816 and Tam et al, 201817 (Table 14).
Used in the reference case analysis.
Expert Consultation
We solicited expert consultation on the current and expected use of TAVI among people with aortic valve stenosis at intermediate surgical risk. Members of the consultation included methodologists and physicians in the specialty areas of the topic being evaluated. The statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Reference Case
The results of our budget impact analysis can be found in Table 16. Funding TAVI in patients at intermediate surgical risk is estimated to cost between $9 million and $12 million per year over the next 5 years. Given the current spending on SAVR, the annual net budget impact of funding TAVI is estimated to be between $2 million and $3 million.
Table 16:
Results of Budget Impact Analysis
| Scenario | Budget Impact, Millionsa | ||||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | ||
| Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | |
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | |
| New scenario | TAVI | 8.66 | 9.81 | 10.69 | 11.56 | 12.40 | 53.13 |
| SAVR | 2.22 | 1.93 | 1.51 | 1.06 | 0.58 | 7.30 | |
| Total | 10.89 | 11.74 | 12.20 | 12.62 | 12.98 | 60.43 | |
| Net budget impact | 2.00 | 2.22 | 2.37 | 2.53 | 2.69 | 11.80 | |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
A summary of cost breakdowns can be found in Appendix 9, Table A19. Although funding TAVI would result in higher valve costs, the costs for procedural hospitalization, professional fees, and complications would likely be reduced.
Sensitivity Analyses
The net budget impact estimated in each scenario analysis can be found in Table 17. Full details can be found in Appendix 9, Table A20. When we assumed that more patients would be at intermediate surgical risk (scenario 2), the net budget impact increased to about $4 million to $5 million per year. We also found that a shorter length of hospital stay in people receiving TAVI led to a lower net budget impact (scenarios 7 and 8).
Table 17:
Net Budget Impact, Scenario Analyses
| Scenario | Net Budget Impact, Millionsa | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference case | 2.00 | 2.22 | 2.37 | 2.53 | 2.69 | 11.80 |
| Scenario 1: 20% of SAVR + CABG patients not eligible for TAVI | 1.85 | 2.05 | 2.20 | 2.34 | 2.50 | 10.94 |
| Scenario 2: 25% of current SAVR patients at intermediate surgical risk | 3.58 | 3.98 | 4.26 | 4.54 | 4.84 | 21.18 |
| Scenario 3: 83% of patients can have transfemoral-access TAVI | 1.79 | 1.99 | 2.13 | 2.27 | 2.42 | 10.59 |
| Scenario 4: all access routes funded | 2.15 | 2.40 | 2.57 | 2.74 | 2.91 | 12.76 |
| Scenario 5: higher initial uptake | 2.39 | 2.63 | 2.65 | 2.67 | 2.68 | 13.03 |
| Scenario 6: lower initial uptake | 1.33 | 1.69 | 2.02 | 2.36 | 2.70 | 10.11 |
| Scenario 7: TAVI 3-day hospital stay | 1.21 | 1.36 | 1.44 | 1.53 | 1.62 | 7.17 |
| Scenario 8: TAVI 1-day hospital stay | 0.39 | 0.47 | 0.48 | 0.49 | 0.51 | 2.34 |
| Scenario 9: SAVR costs $3,000 | 2.59 | 2.87 | 3.07 | 3.29 | 3.50 | 15.33 |
| Scenario 10: costs inflated to 2018 CAD | 2.08 | 2.31 | 2.46 | 2.63 | 2.80 | 12.28 |
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
Discussion
In our current scenario (no public funding for TAVI), we estimated that it would cost between $9 million and $10 million per year to continue funding SAVR in patients at intermediate surgical risk. In our new scenario, if TAVI (and SAVR) were publicly funded, we estimated that the total cost would be between $11 million and $13 million per year ($9 million to $12 million of this would be for TAVI). Therefore, we estimated the net budget impact to be an additional $2 million to $3 million per year. In theory, the net costs should be realized by reducing the number of SAVRs performed, but some factors may affect this. For example, SAVR and TAVI may be performed in different areas of the hospital (operating room vs. catheterization lab) and involve different health care providers (cardiac surgeons vs. interventional cardiologists). Hospital savings obtained by reducing the number of SAVRs may be used to perform other cardiac surgeries, instead of being transferred to catheterization labs for additional TAVI procedures. Such factors should be taken into consideration when interpreting the results from our budget impact analysis.
Consistent with previous analyses, we found that most of the budget impact could be attributed to device-related TAVI costs.58,59 However, additional device-related costs would be offset by savings from a shorter length of hospital stay. The length of stay for TAVI and SAVR we used in our reference case analysis were derived by Tam et al from the PARTNER 2 trial.10,16 Newer evidence from registries and real-world analyses shows that minimally invasive TAVI approaches have led to even shorter lengths of stay.39,50 Our sensitivity analyses demonstrated that with shorter lengths of stay, the net costs of funding TAVI could be reduced even further. At an extreme, if people receiving TAVI had a hospital stay of only 1 day, the additional costs would be reduced to less than $1 million per year. This finding highlights the importance of promoting minimally invasive approaches with shorter hospital stays, where appropriate.
Our analysis had several strengths. We used two Ontario-specific analyses to inform our costing, based our volumes on an Ontario registry, and performed sensitivity analyses to explore our assumptions. However, the analysis should be interpreted with its limitations in mind. The first limitation was the challenge of estimating the number of people at intermediate surgical risk. While the determination of risk considers the STS score, it is ultimately based on a heart team's decision, which incorporates other risk factors and comorbidities.56 The number of people at intermediate surgical risk could be higher if a broader STS score range were used (i.e., as in the SURTAVI trial19) or additional comorbidities were included. We did examine the impact of a larger proportion of people at intermediate surgical risk in our sensitivity analysis and found that the net budget impact would have been about $4 million to $5 million per year. This finding should be taken into account when making implementation decisions. An additional limitation was our ability to accurately predict uptake rates. Several factors may increase the uptake rate of TAVI in this population (e.g., system and infrastructure readiness) or reduce it (e.g., backlogs of people at high surgical risk, in whom TAVI is already funded). In the absence of published evidence, we based the uptake rates in our reference case and scenario analyses on clinical expert judgment.
Conclusions
We estimate that the additional cost to provide public funding for TAVI in people with severe, symptomatic aortic valve stenosis at intermediate surgical risk would range from about $2 million to $3 million per year.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, preferences and priorities of those who have lived experience with aortic valve stenosis. The treatment focus was transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR).
Background
Patient, caregiver and public engagement provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat the health condition. It includes the impact of the condition and its treatment on the patient, the patient's family and other caregivers, and on the patient's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).60–62 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in published literature, we contact and speak directly with people who live with a given health condition, including those who may have experience with the intervention we are exploring.
For this project, we spoke with 10 people with aortic valve stenosis living in Ontario, as well as three family members and caregivers. We spoke with people who had experience with both TAVI and SAVR, and people who had experience with SAVR only. During the interviews, we were unable to ascertain the surgical risk status of the interviewees. Gaining an understanding of the day-to-day functioning of people with aortic valve stenosis and their experiences with available treatments, including TAVI, helped us assess the potential value of TAVI from the perspective of patients and caregivers.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with aortic valve stenosis and those of their families and other caregivers.63 We engaged people via telephone interviews.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with aortic valve stenosis, as well as those of their families and caregivers. Our main task in interviewing is to understand what people tell us and to gain an understanding of the meaning of their experiences.64 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
Participant Outreach
We used an approach called purposive sampling,65–68 which involves actively reaching out to patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations and groups involved in offering the TAVI procedure or who were providing care for people with aortic valve stenosis to spread the word about this engagement activity and to make contact with patients, families, and caregivers, including those with experience of TAVI.
Inclusion Criteria
We sought to speak with people who had their aortic valve stenosis treated by TAVI or SAVR, as well as their caregivers. We were unable to determine the surgical risk status of the participants; therefore, surgical risk status was not an inclusion criterion.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
We interviewed 13 people over the age of 18 who lived in Ontario. Participants differed in terms of their socioeconomic background and place of residence. All participants had lived experience of aortic valve stenosis, but they were not able to comment on their surgical risk level. Of the 13 interviewees, 10 were patients and three were caregivers. Of the 10 patients, nine had undergone the TAVI procedure. All interviewees shared their values, preferences, and perspectives about aortic valve stenosis and its treatment.
Approach
At the beginning of the interview, we explained the role of our organization, the purpose of the health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 10). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 20 to 40 minutes. Interviews were semi-structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.69 Questions focused on the impact of aortic valve stenosis on the quality of life of people with aortic valve stenosis, their experiences with treatments to manage or treat aortic valve stenosis, and their perceptions of the benefits or limitations of TAVI. For family members and caregivers, questions focused on their perceptions of the impact of aortic valve stenosis, as well as the impact of the person's health condition and treatments on the family members and caregivers themselves. See Appendix 11 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.70,71 We used the qualitative data analysis software program NVivo (QSR International, Doncaster, Victoria, Australia) to identify and interpret patterns in interview data. The patterns we identified allowed us to highlight the impact of health conditions and treatments on the patients, family members, and caregivers we interviewed.
Results
During the interviews, people with aortic valve stenosis and their family members emphasized the impact of the condition on their daily functioning. They reported shortness of breath and fatigue as a common issue. People identified SAVR as a commonly available treatment, but they expressed concerns about its invasiveness and recovery period. People who were waiting for treatment reported that they were waiting to qualify for TAVI. Participants reported out-of-pocket costs as a barrier to accessing TAVI.
Impact of Aortic Valve Stenosis
Physical Effects
People with aortic valve stenosis experienced shortness of breath, fatigue, and heart. They said that their condition slowed them down considerably.
I just rested a lot more … when I got tired, I sat. And I found that … when I went out for walks, I couldn't walk very far, and I had to come back home, that kind of thing.
It was a heaviness, or sometimes it felt like a sharp pain, and then it was gone.
It has made me absolutely … slow down as far as doing things … but what I used to do in an hour, now takes me 4 hours, because I have to go slower.
I was not able to do everything that I normally would do, because I would have the shortness of breath on exertion particularly, I would … have to stop what I was doing and get my breathing back to normal and then continue on.
Some interviewees said that they depended on medications to manage their symptoms.
When I am outside and I'm working in the yard, I constantly draw on my nitro, the spray stuff. And I will use that nitro — I think I've used it three, four times already today. And in the wintertime when it was cold … I couldn't even walk 80 feet to the end of my driveway without nitro … because the cold air really affects you.
Participants were dependent on their family or other people to get through their daily activities.
He could not breathe or walk before his surgery. I had to take him to hospital. I had to go with him to help him all the time. Could not leave him alone.
I have a husband, and he is a great help, because he does the shopping, he does the dishwasher, and he does all the washing. And without him, I think I would be in trouble, because it would be too much for me.
Psychological Effects
Participants also noted some psychological distress related to managing their condition:
Well, it was very stressful. You know, hard to breathe, and I didn't have any pain at all, but I couldn't get my breath, and that was the major issue that I had.
I couldn't do much of anything, and I'd barely walk. It sort of isolates you a little bit … because you can't contribute in any way to the everyday life pattern and things to do.
Surgical Aortic Valve Replacement
When asked about the currently available treatments, all patients were aware of the “open-heart” SAVR procedure. They understood that it was an effective way of treating aortic valve stenosis, but considered it to be invasive, involving a great deal of pain and a long recovery period.
Treatment Process
Patients who had received SAVR said that it was effective in treating their condition. Some patients considered SAVR to be the right option for them because of their medical history or other concurrent health conditions.
And he [doctor] felt that … I had low platelet counts and was bleeding easily … So if I started to bleed, he wouldn't be able to stop the bleeding … That's why he said we were going to have to open you up to do this.
Patients noted that hospital clinicians managed their pain levels well after this invasive procedure.
But they [clinicians] manage it [pain] very well when you're down in the ICU [intensive care unit] after surgery. They managed the pain levels very well.
Patients and caregivers mentioned the physical and psychological aftermath of open-heart surgery. They described pain, effects on mood, and a difficult and long recovery period as some of the challenges after surgery.
There was definitely the terrifying aftermath to that open-heart surgery … you have tubes everywhere. You have a large incision. You have a lot of pain. When he was immediately out of surgery, he was frozen like Frosty the Snowman … when the cooling subsided, Grumpy emerged.
Quite a bit of pain in the chest area, having your chest cracked open.
Recovery and Length of Hospital Stay
Patients noted that SAVR involved a long hospital stay and a longer recovery:
It was a difficult recovery … it would be more like a couple of months to maybe even 6 months.
Barriers
Financial Barriers
Patients and caregivers noted that the costs involved with travel were an additional burden and a potential barrier to receiving SAVR. Surgery was associated with a longer hospital stay for patients and higher accommodation costs for the caregivers. People living in Northern Ontario or in remote areas were especially affected by these costs. They noted that the Northern Health Travel Grant did not cover all the travel and associated costs.
Yes, there's cost involved there, for accommodation and meals, and you need family with you at the time.
We're from … the other side of the province. So we had the cost of travel. We had the cost of renting a place to stay, both when he was in the hospital and then also for his post-recovery. He needed to stay 2 more weeks for post-surgical checkups here before we were free to go back … So it was a month in a rental place. Altogether … our estimated our costs were between $5,000 and $6,000, and only $1,000 of that is covered on your travel grant. So we were out of pocket quite a bit.
Psychological Barriers
Because of the invasive nature of the procedure, patients expressed anxiety and worry when describing the possible outcomes of SAVR. A few patients chose to forgo it and were waiting for their condition to get worse, so they could qualify for TAVI.
I didn't want open-heart surgery because I had obvious reasons … like losing my best friend, my skiing buddy, to open-heart surgery. So I … did some research and found out that the TAVI procedure was available.
Patients and caregivers also spoke about the reasons why SAVR was not the right option for them, including age, concurrent health conditions, and others.
She could not stand up with a pillow on her chest. She has trouble just getting out of a chair. We have a stand-up chair for her to get to her feet here at the house. But if they were to do the open-heart procedure, she would be the first year and a half having to hold a pillow to her every time she moved. She can't do it.
Limitations of SAVR
A few patients indicated that they had had a valve rupture several years after their SAVR procedure and had to have another procedure to replace the ruptured valve.
I had valve replacement 11 years before my TAVI. The valve split on the side and was spraying blood on my lungs. I went to the emergency … my valve was split … and I was given TAVI.
Transcatheter Aortic Valve Implantation
Treatment Process
Patients indicated that the TAVI procedure was explained very well to them. They understood that the valve was to be inserted by a catheter, and that the entry point could be either through the chest or near the groin.
And he brought in the actual catheter that they would use … an example. And he had vials containing … examples of the valves. And he went into quite a lot of detail.
He explained to me … [it went] up through the groin, sometimes I guess they go in under your arm … In my case … the doctor… said we're going to do the groin route with you.
Patients perceived that the pain involved with TAVI was much less than it might be with SAVR. A few patients alluded to the invasiveness of SAVR, saying, “they open your ribs wide open.”
Patients who had had the TAVI implantation done through the chest or the groin described little to no pain.
And I didn't have any pain afterwards at all. I didn't even know that I'd had incisions in my groin. I just didn't know it was there. It was amazing.
Definitely less painful, because … they usually go with the catheter in the groin. I couldn't have it … my arteries were too small, so he put it up in the chest, and as I said, you just have one little cut where the catheter goes in.
Recovery and Length of Hospital Stay
Patients noted that the length of hospital stay and overall recovery period after the TAVI procedure were very short. Most patients who had had TAVI inserted via the groin described a shorter length of stay and recovery time than patients who had had it inserted via the chest.
They had the telemetry on me all the time, they were monitoring the heart … And the second day … the surgeon … who did it came in … [and] says, we are watching the telemetry and there's just no problem at all, so there's no point in your staying here. I'm going to send you home tomorrow afternoon, and that was the third day.
Two days later I was discharged.
Your recuperating time is a lot less, because you don't have major surgery. I mean, … it's not having to have your breastbone opened … it's more invasive with open-heart, so it's easier on the body than the open-heart.
Anesthesia
Some patients reported that they were “put out completely,” referring to general anesthesia, while others mentioned that they did not even have “complete anaesthesia.” All patients interviewed who had TAVI done through the chest indicated that they had general anesthesia.
Benefits
Patients thought that the TAVI procedure helped them address the problems they were having with aortic valve stenosis. They thought that it improved their quality of life quickly after the procedure.
I went into the hospital the day before, had the procedure in the afternoon … And in the evening, I was sitting in bed having a sandwich.
Barriers
Cost
Patients described the costs involved with the procedure as personal costs related to travel, accommodation, and parking. Patients who lived further from hospitals that offer TAVI procedures, including in remote and northern parts of Ontario, were most affected by associated travel, accommodation and meal costs. However, given the shorter length of hospital stay, they perceived these costs to be much lower than what they might have incurred with SAVR.
My family wanted to be there when I had the surgery, so there was … overnight accommodation … and meals, and so on. And someone to help with the driving … It was basically … personal expenses.
Access
Patients who lived further from hospitals that offer TAVI reported greater difficulty accessing the procedure.
Limitations of TAVI
We asked patients and caregivers about limitations related to TAVI procedure, but the interviewees reported none.
Discussion
People with aortic valve stenosis shared their experiences of the burden of their health condition on their daily life and relationships. They were able to share their perceptions of the TAVI and SAVR procedures. A limitation of our engagement was that the surgical risk status of the people we spoke to was unknown.
Interviewees identified open-heart SAVR as the currently available treatment for people with aortic valve stenosis. Most people who underwent a SAVR procedure felt that SAVR met their needs by improving their condition after the surgery. However, they mentioned that the pain and slow recovery period resulting from the invasive nature of SAVR made them dependent on their family and reduced their quality of life after the procedure.
People who had experienced TAVI indicated that it improved their medical condition and met their needs by minimizing their pain and their recovery period. It enhanced their quality of life by making it possible for them to get back to their usual activities more quickly than with SAVR. In addition to the above findings, which were mostly consistent with the clinical evidence, people living in northern and remote areas of the province thought they had lower out-of-pocket costs for travel, meal and accommodation with TAVI than they would have had if they had experienced SAVR. This was mostly because of their reduced length of hospital stay and fewer follow-ups with TAVI than with SAVR.
Conclusions
Patients and caregivers perceived that TAVI minimized pain and recovery time involved with the procedure. Most patients felt they returned to their usual activities more quickly than they would have if they had had SAVR. The patient and caregiver consultations indicated a preference for TAVI over SAVR. A limitation of our approach was we did not know the surgical risk status of the people we interviewed.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
The included studies showed that TAVI was similar to SAVR with respect to the composite endpoint of all-cause mortality or disabling stroke within 2 years of follow-up. However, TAVI and SAVR had different patterns of complications. The authors of both studies noted that longer-term follow-up is needed to assess the durability of the bioprosthetic TAVI valve. Two published cost-effectiveness studies found that TAVI may offer good value for money, but there was significant uncertainty in the results. Publicly funding TAVI is estimated to cost an additional $2 million to $3 million per year for the next 5 years. This cost may be reduced if people receiving TAVI have shorter lengths of hospital stay (≤ 3 days). People with aortic valve stenosis who had undergone TAVI appreciated its less invasive nature.
Acknowledgments
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Vania Costa; the primary health economist was Lindsey Falk; the secondary health economists were Hong Anh Tu and Man Wah Yeung; the patient and public partnership analyst was Arshia Ali; and the medical librarian was Melissa Walter.
The medical editor was Jeanne McKane. Others involved in the development and production of this report were Doug Willcocks, Claude Soulodre, Saleemeh Abdolzahraei, Kathryn Schwarz, Kara Cowan, Andrée Mitchell, Sarah McDowell, Vivian Ng, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to the following individuals for lending their expertise to the development of this report: Christopher Feindel (University Health Network, University of Toronto), Gordon Guyatt (Department of Heath Research Methods, Evidence, and Impact, McMaster University), Laurie Lambert (Unité d'Evaluation Cardiovasculaire, Institut National d'Excellence en Santé et en Services Sociaux), Mark Osten (Peter Munk Cardiac Centre, University Health Network), Mark D. Peterson (St. Michael's Hospital, Division of Cardiac Surgery, University of Toronto), Sam Radhakrishnan (Schulich Heart Centre, Sunnybrook Health Sciences Centre), Derrick Y. Tam (Division of Cardiac Surgery, Department of Surgery, University of Toronto), James Velianou (Hamilton Health Sciences, McMaster University), and Harindra Wijeysundera (Sunnybrook Health Sciences Centre, University of Toronto).
We are also grateful to CorHealth Ontario for lending their expertise to the development of this report.
We thank the 13 patients and caregivers who generously gave their time to share their experiences of aortic valve stenosis and the TAVI and SAVR procedures.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- CABG
Coronary artery bypass graft
- CI
Confidence interval
- EQ-5D
EuroQol-5D
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HR
Hazard ratio
- ICER
Incremental cost-effectiveness ratio
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NICE
National Institute for Health and Care Excellence
- NYHA
New York Heart Association
- PCI
Percutaneous coronary intervention
- QALY
Quality-adjusted life-year
- SAVR
Surgical aortic valve replacement
- SD
Standard deviation
- STS
Society of Thoracic Surgeons
- TAVI
Transcatheter aortic valve implantation
GLOSSARY
- Absolute risk difference
The absolute difference in the risk of an outcome occurring between an intervention and an alternative intervention.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cardiopulmonary bypass
Cardiopulmonary bypass is a technique that temporarily takes over the function of the heart and lungs during surgery so that the surgeon can operate on a nonbeating heart with little blood present.
- Coronary artery bypass graft (CABG)
Coronary artery bypass graft (CABG) is a type of surgery that improves blood flow to the heart used to treat people with a blocked coronary artery. The procedure involves grafting (connecting) a healthy artery or vein from another part of the body to the blocked coronary artery. The grafted artery or vein bypasses the blocked portion of the coronary artery, creating a new path for blood to flow into the heart.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost– utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Endovascular surgery
Endovascular surgery is a type of surgery that is less invasive than traditional open surgery; it is used to treat problems with the blood vessels. In this type of surgery, surgeons enter the body through the blood vessels rather than through a large incision in the body.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Percutaneous coronary intervention (PCI)
Percutaneous coronary intervention (PCI) is a nonsurgical technique that uses a catheter (a thin, flexible tube) to place a stent (a metal or plastic tube) into a clogged artery in the heart in order to open up the artery.
- Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost-utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Revascularization
Revascularization is a surgical procedure to improve blood flow to the heart; coronary artery bypass grafting (CABG) is a type of revascularization.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Transfemoral
A transfemoral surgical approach involves entering the body through the femoral artery in the groin area.
- Transthoracic
A transthoracic surgical approach involves entering the body through the thoracic cavity; that is, across the chest wall.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Systematic Reviews Search
Search date: March 28, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to March 21, 2018>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews -NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 13>, Ovid MEDLINE(R) ALL <1946 to March 27, 2018>
Search strategy:
-
1
Aortic valve/ (28551)
-
2
exp Aortic Valve Stenosis/ (38035)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (46306)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (8194)
-
5
or/1–4 (94284)
-
6
Heart Valve Prosthesis Implantation/ (29373)
-
7
Heart Valve Prosthesis/ (52548)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (96763)
-
9
or/6–8 (147747)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (415023)
-
11
9 and 10 (27075)
-
12
Transcatheter aortic valve implantation/ (16968)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (17858)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (184)
-
15
or/11–14 (31433)
-
16
5 and 15 (15603)
-
17
((intermediate or moderate or medium or middle or lower) adj3 risk∗).ti,ab,kf. (153910)
-
18
15 and 17 (1282)
-
19
16 or 18 (15967)
-
20
Meta Analysis.pt. (86255)
-
21
Meta-Analysis/ or Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (288380)
-
22
(((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)) or pooled analysis or published studies or published literature or hand search∗ or handsearch∗ or medline or pubmed or embase or cochrane or cinahl or data synthes∗ or data extraction∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab. (615959)
-
23
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗).mp. (415459)
-
24
or/20–23 (832810)
-
25
19 and 24 (700)
-
26
exp Animals/ not Humans/ (14274594)
-
27
25 not 26 (518)
-
28
Case Reports/ (1871277)
-
29
27 not 28 (515)
-
30
limit 29 to english language [Limit not valid in CDSR; records were retained] (488)
-
31
30 use medall,cleed (280)
-
32
limit 19 to english language [Limit not valid in CDSR; records were retained] (15233)
-
33
32 use coch,clhta (25)
-
34
31 or 33 (305)
-
35
aortic valve/ (28551)
-
36
exp aortic stenosis/ (40209)
-
37
aortic valve disease/ (433)
-
38
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).tw,kw. (46657)
-
39
(aortic valv∗ adj3 disease∗).tw,kw. (8275)
-
40
or/35–39 (96439)
-
41
aorta valve replacement/ (18398)
-
42
exp aortic valve prosthesis/ (2602)
-
43
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).tw,kw,dv. (97015)
-
44
or/41–43 (102582)
-
45
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).tw,kw,dv. (422268)
-
46
44 and 45 (25018)
-
47
transcatheter aortic valve implantation/ (16968)
-
48
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).tw,kw,dv. (19076)
-
49
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).tw,kw,dv. (185)
-
50
or/46–49 (29435)
-
51
40 and 50 (16286)
-
52
intermediate risk patient/ (2498)
-
53
((intermediate or moderate or medium or middle or lower) adj3 risk∗).tw,kw. (156130)
-
54
52 or 53 (156830)
-
55
50 and 54 (1341)
-
56
51 or 55 (16647)
-
57
Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (282678)
-
58
(((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)) or pooled analysis or published studies or published literature or hand search∗ or handsearch∗ or medline or pubmed or embase or cochrane or cinahl or data synthes∗ or data extraction∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab. (615959)
-
59
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗).mp. (415459)
-
60
or/57–59 (831723)
-
61
56 and 60 (749)
-
62
(exp animal/ or nonhuman/) not exp human/ (10345257)
-
63
61 not 62 (749)
-
64
Case Report/ (4090511)
-
65
63 not 64 (743)
-
66
limit 65 to english language [Limit not valid in CDSR; records were retained] (712)
-
67
66 use emez (414)
-
68
34 or 67 (719)
-
69
68 use medall (280)
-
70
68 use coch (3)
-
71
68 use clhta (22)
-
72
68 use cleed (0)
-
73
68 use emez (414)
-
74
remove duplicates from 68 (514)
Randomized Controlled Trials Search
Search date: April 13, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2018>, Embase <1980 to 2018 Week 15>, Ovid MEDLINE(R) ALL <1946 to April 12, 2018>
Search strategy:
-
1
Aortic valve/ (29059)
-
2
exp Aortic Valve Stenosis/ (38708)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (47217)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (8301)
-
5
or/1–4 (95846)
-
6
Heart Valve Prosthesis Implantation/ (30011)
-
7
Heart Valve Prosthesis/ (53149)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (99037)
-
9
or/6–8 (150607)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (431968)
-
11
9 and 10 (27850)
-
12
Transcatheter aortic valve implantation/ (17139)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (18551)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (188)
-
15
or/11–14 (32287)
-
16
5 and 15 (16083)
-
17
((intermediate or moderate or medium or middle or lower) adj3 risk∗).ti,ab,kf. (163281)
-
18
15 and 17 (1377)
-
19
16 or 18 (16475)
-
20
Clinical Trials as Topic/ or Randomized Controlled Trials as Topic/ (476395)
-
21
(randomized controlled trial or controlled clinical trial).pt. (1077996)
-
22
trial.ti. (630454)
-
23
(randomi#ed or randomly or RCT$1 or placebo∗ or sham).tw. (2756677)
-
24
or/20–23 (3502857)
-
25
19 and 24 (1752)
-
26
exp Animals/ not Humans/ (14299495)
-
27
25 not 26 (1324)
-
28
Case Reports/ (1873944)
-
29
27 not 28 (1316)
-
30
limit 29 to yr=“2017 -Current” (327)
-
31
limit 30 to english language (322)
-
32
31 use medall (127)
-
33
limit 19 to yr=“2017 -Current” (2969)
-
34
limit 33 to english language (2917)
-
35
34 use cctr (85)
-
36
32 or 35 (212)
-
37
aortic valve/ (29059)
-
38
exp aortic stenosis/ (40967)
-
39
aortic valve disease/ (454)
-
40
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).tw,kw. (47585)
-
41
(aortic valv∗ adj3 disease∗).tw,kw. (8376)
-
42
or/37–41 (98064)
-
43
aorta valve replacement/ (18414)
-
44
exp aortic valve prosthesis/ (2646)
-
45
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).tw,kw,dv. (99340)
-
46
or/43–45 (104929)
-
47
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).tw,kw,dv. (438631)
-
48
46 and 47 (25762)
-
49
transcatheter aortic valve implantation/ (17139)
-
50
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).tw,kw,dv. (19759)
-
51
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).tw,kw,dv. (190)
-
52
or/48–51 (30252)
-
53
42 and 52 (16801)
-
54
intermediate risk patient/ (2554)
-
55
((intermediate or moderate or medium or middle or lower) adj3 risk∗).tw,kw. (163387)
-
56
54 or 55 (164105)
-
57
52 and 56 (1431)
-
58
53 or 57 (17180)
-
59
exp “controlled clinical trial (topic)”/ (149255)
-
60
randomized controlled trial/ or controlled clinical trial/ (1221886)
-
61
trial.ti. (630454)
-
62
(randomi#ed or randomly or RCT$1 or placebo∗ or sham).tw. (2756677)
-
63
or/59–62 (3396739)
-
64
58 and 63 (1828)
-
65
(exp animal/ or nonhuman/) not exp human/ (10376052)
-
66
64 not 65 (1815)
-
67
case report/ (4102334)
-
68
66 not 67 (1799)
-
69
limit 68 to yr=“2017 -Current” (364)
-
70
limit 69 to english language (357)
-
71
70 use emez (199)
-
72
36 or 71 (411)
-
73
72 use medall (127)
-
74
72 use cctr (85)
-
75
72 use emez (199)
-
76
remove duplicates from 72 (271)
Economic Evidence Search
Search date: March 29, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <February 2018>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to March 28, 2018>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 13>, Ovid MEDLINE(R) ALL <1946 to March 28, 2018>
Search strategy:
-
1
Aortic valve/ (28943)
-
2
exp Aortic Valve Stenosis/ (38595)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (47067)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (8270)
-
5
or/1–4 (95577)
-
6
Heart Valve Prosthesis Implantation/ (29948)
-
7
Heart Valve Prosthesis/ (53048)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (98765)
-
9
or/6–8 (150254)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (430663)
-
11
9 and 10 (27723)
-
12
Transcatheter aortic valve implantation/ (16975)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (18434)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (186)
-
15
or/11–14 (32128)
-
16
5 and 15 (16019)
-
17
((intermediate or moderate or medium or middle or lower) adj3 risk∗).ti,ab,kf. (162299)
-
18
15 and 17 (1363)
-
19
16 or 18 (16407)
-
20
economics/ (256280)
-
21
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (801251)
-
22
economics.fs. (402188)
-
23
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (790291)
-
24
exp “costs and cost analysis”/ (551700)
-
25
(cost or costs or costing or costly).ti. (241935)
-
26
cost effective∗.ti,ab,kf. (284006)
-
27
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (186574)
-
28
models, economic/ (11192)
-
29
markov chains/ or monte carlo method/ (71918)
-
30
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (36554)
-
31
(markov or markow or monte carlo).ti,ab,kf. (114743)
-
32
quality-adjusted life years/ (34894)
-
33
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (60941)
-
34
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (99424)
-
35
or/20–34 (2352052)
-
36
19 and 35 (656)
-
37
exp Animals/ not Humans/ (14274848)
-
38
36 not 37 (423)
-
39
Case Reports/ (1871345)
-
40
38 not 39 (421)
-
41
limit 40 to english language [Limit not valid in CDSR; records were retained] (392)
-
42
41 use medall,coch,cctr,clhta (228)
-
43
limit 19 to english language [Limit not valid in CDSR; records were retained] (15642)
-
44
43 use cleed (17)
-
45
42 or 44 (245)
-
46
aortic valve/ (28943)
-
47
exp aortic stenosis/ (40769)
-
48
aortic valve disease/ (433)
-
49
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).tw,kw. (47484)
-
50
(aortic valv∗ adj3 disease∗).tw,kw. (8357)
-
51
or/46–50 (97797)
-
52
aorta valve replacement/ (18398)
-
53
exp aortic valve prosthesis/ (2602)
-
54
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).tw,kw,dv. (99154)
-
55
or/52–54 (104721)
-
56
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).tw,kw,dv. (438306)
-
57
55 and 56 (25665)
-
58
transcatheter aortic valve implantation/ (16975)
-
59
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).tw,kw,dv. (19653)
-
60
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).tw,kw,dv. (188)
-
61
or/57–60 (30121)
-
62
51 and 61 (16740)
-
63
intermediate risk patient/ (2498)
-
64
((intermediate or moderate or medium or middle or lower) adj3 risk∗).tw,kw. (164605)
-
65
63 or 64 (165305)
-
66
61 and 65 (1421)
-
67
62 or 66 (17120)
-
68
Economics/ (256280)
-
69
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (130590)
-
70
Economic Aspect/ or exp Economic Evaluation/ (427028)
-
71
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (814894)
-
72
exp “Cost”/ (551700)
-
73
(cost or costs or costing or costly).ti. (241935)
-
74
cost effective∗.tw,kw. (294991)
-
75
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (194114)
-
76
Monte Carlo Method/ (57755)
-
77
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (40310)
-
78
(markov or markow or monte carlo).tw,kw. (119724)
-
79
Quality-Adjusted Life Years/ (34894)
-
80
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (64717)
-
81
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (118840)
-
82
or/68–81 (1994797)
-
83
67 and 82 (691)
-
84
(exp animal/ or nonhuman/) not exp human/ (10345515)
-
85
83 not 84 (689)
-
86
Case Report/ (4090581)
-
87
85 not 86 (685)
-
88
limit 87 to english language [Limit not valid in CDSR; records were retained] (652)
-
89
88 use emez (399)
-
90
45 or 89 (644)
-
91
90 use medall (206)
-
92
90 use coch (0)
-
93
90 use cctr (21)
-
94
90 use clhta (1)
-
95
90 use cleed (17)
-
96
90 use emez (399)
-
97
remove duplicates from 90 (489)
Grey Literature Search
Performed: March 8–April 16, 2018
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: TAVI, TAVR, transcatheter aortic, trans-catheter aortic, aortic valve
Results (included in PRISMA): 2
Ongoing trials: 14 (clinicaltrials.gov)
Ongoing HTAs: 6 (PROSPERO/EUnetHTA)
Appendix 2: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Author, Year | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|
| Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review | |
| Singh et al, 201831 | Low | High | Low | High | High |
| Tam et al, 201728 | Low | Low | Low | Low | Low |
| Garg et al, 201730 | Low | Unclear | High | High | High |
| Sardar et al, 201732 | High | High | Low | High | High |
| Siemieniuk et al, 201629 | Low | Low | Low | Low | Low |
| Khan et al, 201633 | High | Low | Low | High | High |
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Table A2:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk of Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Reardon et al, 201719 | Low | Low | Low | Low | Low | None |
| Leon et al, 201610 | Low | Low | Low | Low | Low | None |
Possible risk of bias levels: low, high, and unclear.
Table A3:
GRADE Evidence Profile for Comparison of TAVI and SAVR
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| All-Cause Mortality and Disabling Stroke, Composite Endpoint—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitationsa | Undetected | — | ⊕⊕⊕⊕ High |
| All-Cause Mortality and Disabling Stroke, Composite Endpoint—Transfemoral Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)b | Undetected | — | ⊕⊕⊕ Moderate |
| All-Cause Mortality and Disabling Stroke, Composite Endpoint—Transthoracic Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| All-Cause Mortality—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| All- Cause Mortality—-Transfemoral and Transthoracic Cohorts (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Disabling Stroke—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Disabling Stroke—-Transfemoral and Transthoracic Cohorts (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Transient Ischemic Attack—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Life-Threatening or Major/Disabling Bleeding—Full Cohort (30 Days) | |||||||
| 2 (RCTs)10,19 | No serious limitations | Serious limitations (–1)d | No serious limitations | Serious limitations (–1)e | Undetected | — | ⊕⊕ Low |
| Life-Threatening or Major/Disabling Bleeding—-Transfemoral and Transthoracic Cohorts (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Acute Kidney Injury—Full Cohort (30 Days) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Acute Kidney Injury—Transfemoral Cohort (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Acute Kidney Injury—Transthoracic Cohort (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Myocardial Infarction—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Myocardial Infarction—Transfemoral Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)f | Undetected | — | ⊕⊕⊕ Moderate |
| Myocardial Infarction—Transthoracic Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Atrial Fibrillation—Full Cohort (30 Days–2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Atrial Fibrillation—Transfemoral Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Atrial Fibrillation—Transthoracic Cohort (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| New Permanent Pacemaker Implantation—Full Cohort (30 Days) | |||||||
| 2 (RCTs)10,19 | No serious limitations | Serious limitations (–1)d | No serious limitations | Serious limitations (–1)e | Undetected | — | ⊕⊕ Low |
| New Permanent Pacemaker Implantation—-Transfemoral and Transthoracic Cohorts (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Major Vascular Complications—Full Cohort (30 Days) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Major Vascular Complications—Transfemoral Cohort (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Major Vascular Complications—Transthoracic Cohort (30 Days) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Moderate to Severe Paravalvular Aortic Regurgitation—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Aortic Valve Reintervention—Full Cohort (2 Years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (–2)g | Undetected | — | ⊕⊕ Low |
| Aortic Valve Reintervention—Transfemoral and Transthoracic Cohorts (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Very serious limitations (–2)i | Undetected | — | ⊕⊕ Low |
| Aortic Valve Rehospitalization—Full Cohort (2 years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (–2)g | Undetected | ⊕⊕ Low | |
| Aortic Valve Rehospitalization—Transfemoral and Transthoracic Cohorts (2 Years) | |||||||
| 1 (RCT)10 | No serious limitations | Could not be evaluated | No serious limitations | Very serious limitations (–2)i | Undetected | ⊕⊕ Low | |
| Length of Stay—Full Cohort (Implantation Procedure) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| NYHA Symptoms—Full Cohort (30 Days) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| NYHA Symptoms—Full Cohort (2 years) | |||||||
| 2 (RCTs)10,19 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Quality of Life—Full and Transfemoral Cohorts (30 Days) | |||||||
| 2 (RCTs)19,34 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | — | ⊕⊕⊕⊕ High |
| Quality of Life—Full and Transfemoral Cohorts (1 Year and 2 Years) | |||||||
| 2 (RCTs)19,34 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
| Quality of Life—Transthoracic Cohort (30 Days to 2 Years) | |||||||
| 1 (RCT)34 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (–1)c | Undetected | — | ⊕⊕⊕ Moderate |
Abbreviations: RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI transcatheter aortic valve replacement.
No statistically significant difference was observed between groups; however, because both trials were designed to demonstrate the noninferiority of TAVI versus SAVR and because the P-value for noninferiority was statistically significant in both trials, we decided not to downgrade for imprecision.
A statistically significant difference between TAVI and SAVR was reported in the as-treated analysis, but the difference between groups was not statistically significant in the intention-to-treat analysis at 2 years. Other factors that corroborated the decision to downgrade for imprecision included the following: although power was defined a priori and randomization was stratified according to the subgroups, the study was not powered to assess these subgroups separately; and the upper limit of the 95% confidence interval was very close to the null.
There was insufficient statistical power to detect a difference between groups.
The results were inconsistent between the two trials (i.e., only one study observed a statistically significant difference between groups).
There was insufficient statistical power to detect a difference between groups in one of the studies. Additionally, the number of events was very low, which affected the robustness of the results.
In the transfemoral cohort, there was a lower risk of myocardial infarction in the TAVI group versus SAVR at 30 days, but not at 1 and 2 years of follow-up. We decided to downgrade the evidence for imprecision.
There was insufficient statistical power to detect a difference between groups in one of the studies. As well, the number of events was very low in both studies, which may have seriously affected the robustness of the results.
The number of events was very low in both studies, which may have seriously affected the robustness of the results.
The number of events was very low in the study, which may have seriously affected the robustness of the results.
Appendix 3: Design and Characteristics of the Systematic Reviews
Table A4:
Design and Characteristics of the Systematic Reviews
| Author, Year Literature Search End Date | Population Inclusion Criteria | Outcomes |
|---|---|---|
| Singh et al, 201831 April 29, 2016 |
|
|
| Tam et al, 201728 March 21, 2017 |
|
|
| Garg et al, 201730 March 17, 2017 |
|
|
| Sardar et al, 201732 April 30, 2016 |
|
|
| Siemieniuk, et al, 201629 May 12, 2016 |
|
|
| Khan et al, 201633 February 25, 2015 |
|
|
Abbreviations: NYHA, New York Heart Association; RCT, randomized controlled trial; STS, Society of Thoracic Surgeons.
Appendix 4: Design and Characteristics of the Randomized Controlled Trials
Table A5:
Design and Characteristics of the Randomized Controlled Trials
| Author, Year N (TAVI/SAVR) Follow-up Location TAVI Device | Study Design | Population | TAVI | SAVR | Outcomes |
|---|---|---|---|---|---|
| Reardon et al, 201719 SURTAVI 1,746 (879/867) 2 years (primary outcome); 5 years (unpublished) for secondary outcomes United States, Canada, Europe Self-expanding TAVI bioprosthesis |
|
Inclusion criteria
|
|
|
|
| Leon et al, 201610 PARTNER 2 2,032 (1,011/1,021) 5 years (ongoing) for secondary outcomes United States and Canada Balloon-expandable TAVI prosthesis |
|
Inclusion criteria
|
|
|
|
Abbreviations: ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; EQ, EuroQoL; ITT, intention-to-treat; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; SF, short form; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Appendix 5: Baseline Characteristics
Table A6:
Baseline Characteristics
| Author, Year N (TAVI/SAVR) | Mean Age (SD), y | Male, n (%) | NYHA Class, n (%) | Mean STS Score (SD), % | Mean Logistic EuroScore (SD), % | Previous Revascularizations, n (%) | Atrial Fibrillation, n (%) |
|---|---|---|---|---|---|---|---|
| Reardon et al, 201719 SURTAVI 1,746 (879/867) |
TAVI: 79.9 (6.2) SAVR: 79.8 (6.0) |
TAVI: 508 (57.8) SAVR: 484 (55.8) |
Class II TAVI: 350 (39.8) SAVR: 367 (42.3) Class III TAVI: 480 (54.6) SAVR: 448 (51.7) Class IV TAVI: 49 (5.6) SAVR: 52 (6.0) |
TAVI: 4.4 (1.5) SAVR: 4.5 (1.6) |
TAVI: 11.9 (7.6) SAVR: 11.6 (8.0) |
CABG TAVI: 142 (16.2) SAVR: 145 (16.7) PCI TAVI: 187 (21.3) SAVR: 182 (21.0) |
Atrial fibrillation or flutter TAVI: 247 (28.1) SAVR: 230 (26.5) |
| Leon et al, 201610 PARTNER 2 2,032 (1,011/1,021) |
TAVI: 81.5 (6.7) SAVR: 81.7 (6.7) |
TAVI: 548 (54.2) SAVR: 560 (54.8) |
Class III/IV TAVI: 782 (77.3) SAVR: 776 (76.1) |
TAVI: 5.8 (2.1) SAVR: 5.8 (1.9) |
Not reported | CABG TAVI: 239 (23.6) SAVR: 261 (25.6) PCI TAVI: 274 (27.1) SAVR: 282 (27.6) |
Atrial fibrillation TAVI: 313 (31.0) SAVR: 359 (35.2) |
Abbreviations: CABG, coronary artery bypass graft; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transfemoral aortic valve implantation.
Appendix 6: Study Results
Table A7:
Study Results—Mortality and Stroke
| Author, Year N (TAVI/SAVR) | All-Cause Mortality or Disabling Stroke | All-Cause Mortality | Stroke and TIA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | |
| Full Cohort | |||||||||
| Reardon et al, 201719 SURTAVI 1,746 (879/867) |
Percentagea TAVI: 2.7 SAVR: 3.2 95% Crl for difference: −2.2 to 1.1 |
Percentagea TAVI: 8.3 SAVR: 8.8 95% Crl for difference: −3.3 to 2.2 |
Percentagea TAVI: 13.2 SAVR: 14.1 95% Crl for difference: −4.7 to 2.7 P < .001 for noninferiority |
Percentagea TAVI: 2.0 SAVR: 1.3 95% Crl for difference: −0.5 to 1.9 |
Percentagea TAVI: 7.0 SAVR: 6.8 95% Crl for difference: −2.3 to 2.7 |
Percentagea TAVI: 12.0 SAVR: 11.6 95% Crl for difference: −3.2 to 3.9 |
PercentageaAny stroke TAVI: 2.6 SAVR: 4.8 95% Crl for difference: −4.0 to −0.4 |
PercentageaAny stroke TAVI: 5.5 SAVR: 6.8 95% Crl for difference: −3.7 to 1.1 |
Percentagea Any stroke TAVI: 6.1 SAVR: 8.5 95% Crl for difference: −5.1 to 0.3 |
| Disabling stroke TAVI: 1.1 SAVR: 2.2 95% Crl for difference: −2.3 to 0.2 |
Disabling stroke TAVI: 2.2 SAVR: 3.7 95% Crl for difference: −3.2 to 0.2 |
Disabling stroke TAVI: 2.6 SAVR: 4.6 95% Crl for difference: −4.0 to 0 |
|||||||
| TIA TAVI: 0.9 SAVR: 0.7 95% Crl for difference: −0.7 to 1.1 |
TIA TAVI: 3.4 SAVR: 2.0 95% Crl for difference: −0.2 to 3.0 |
TIA TAVI: 4.4 SAVR: 3.0 95% Crl for difference: −0.6 to 3.5 |
|||||||
| Leon et al, 201610 PARTNER 2 2,032 (1,011/1,021) |
KM estimateb, n (%) TAVI: 62 (6.1) SAVR: 80 (8.0) P = .11 |
KM estimateb, n (%) TAVI: 145 (14.5) SAVR: 160 (16.4) P = .24 |
KM estimateb, n (%) TAVI: 192 (19.3) SAVR: 202 (21.1) P = .33 HR (95% CI) 0.89 (0.73–1.09) P = .25 RR (95% CI) 0.92 (0.77–1.09) P = .001 for noninferiority |
KM estimateb, n (%) TAVI: 39 (3.9) SAVR: 41 (4.1) P = .78 |
KM estimateb, n (%) TAVI: 123 (12.3) SAVR: 124 (12.9) P = .69 |
KM estimateb, n (%) TAVI: 166 (16.7) SAVR: 170 (18.0) P = .45 HR (95% CI) 0.92 (0.74; 1.13) P = .42 |
KM estimateb, n (%) Any stroke TAVI: 55 (5.5) SAVR: 61 (6.1) P = .57 Disabling stroke TAVI: 32 (3.2) SAVR: 43 (4.3) P = .20 TIA TAVI: 9 (0.9) SAVR: 4 (0.4) P = .17 |
KM estimateb, n (%) Any stroke TAVI: 78 (8.0) SAVR: 79 (8.1) P = .88 Disabling stroke TAVI: 49 (5.0) SAVR: 56 (5.8) P = .46 TIA TAVI: 23 (2.4) SAVR: 16 (1.8) P = .38 |
KM estimateb, n (%) Any stroke TAVI: 91 (9.5) SAVR: 85 (8.9) P = .67 Disabling stroke TAVI: 59 (6.2) SAVR: 61 (6.4) P = .83 HR (95% CI) 0.93 (0.65; 1.33) P = .70 TIA TAVI: 34 (3.7) SAVR: 20 (2.3) P = .09 |
| Transfemoral Cohort | |||||||||
| Leon et al, 201610 PARTNER 2 1,550 (775/775) |
KM estimateb, n (%) TAVI: 38 (4.9) SAVR: 59 (7.7) P = .02 |
KM estimateb, n (%) TAVI: 94 (12.3) SAVR: 118 (15.9) P = .04 |
KM estimateb, n (%) TAVI: 128 (16.8) SAVR: 149 (20.4) P = .07 HR (95% CI) 0.79 (0.62–1.00) P = .05 |
KM estimateb, n (%) TAVI: 23 (3.0) SAVR: 31 (4.1) P = .24 |
KM estimateb, n (%) TAVI: 77 (10.0) SAVR: 90 (12.3) P = .17 |
KM estimateb, n (%) TAVI: 108 (14.2) SAVR: 124 (17.2) P = .11 HR (95% CI) 0.80 (0.62–1.04) P = .09 |
KM estimateb, n (%) Any stroke TAVI: 32 (4.2) SAVR: 48 (6.3) P = .06 Disabling stroke TAVI: 18 (2.3) SAVR: 32 (4.2) P = .04 TIA TAVI: 7 (0.9) SAVR: 2 (0.3) P = .10 |
KM estimateb, n (%) Any stroke TAVI: 52 (6.9) SAVR: 63 (8.5) P = .24 Disabling stroke TAVI: 32 (4.3) SAVR: 44 (6.0) P = .13 TIA TAVI: 19 (2.6) SAVR: 12 (1.8) P = .33 |
KM estimateb, n (%) Any stroke TAVI: 62 (8.4) SAVR: 67 (9.2) P = .60 Disabling stroke TAVI: 39 (5.3) SAVR: 48 (6.7) P = .28 HR (95% CI) 0.77 (0.50–1.17) P = .22 TIA TAVI: 27 (3.8) SAVR: 14 (2.1) P = .07 |
| Transthoracic Cohort | |||||||||
| Leon et al, 201610 PARTNER 2 482 (236/246) |
KM estimateb, n (%) TAVI: 24 (10.2) SAVR: 21 (8.7) P = .57 |
KM estimateb, n (%) TAVI: 51 (22.0) SAVR: 42 (18.2) P = .31 |
KM estimateb, n (%) TAVI: 64 (27.7) SAVR: 53 (23.4) P = .29 HR (95% CI) 1.21 (0.84–1.74) P = .31 |
KM estimateb, n (%) TAVI: 16 (6.8) SAVR: 10 (4.2) P = .21 |
KM estimateb, n (%) TAVI: 46 (19.9) SAVR: 34 (15.0) P = .17 |
KM estimateb, n (%) TAVI: 58 (25.2) SAVR: 46 (20.7) P = .26 HR (95% CI): 1.26 (0.86–1.86) P = .24 |
KM estimateb, n (%) Any stroke TAVI: 23 (9.8) SAVR: 13 (5.4) P = .07 Disabling stroke TAVI: 14 (6.0) SAVR: 11 (4.5) P = .35 TIA TAVI: 2 (0.9) SAVR: 2 (0.8) P = .97 |
KM estimateb, n (%) Any stroke TAVI: 26 (11.3) SAVR: 16 (6.8) P = .09 Disabling stroke TAVI: 17 (7.5) SAVR: 12 (5.0) P = .27 TIA TAVI: 4 (1.9) SAVR: 4 (1.9) P = .99 |
KM estimateb, n (%) Any stroke TAVI: 29 (12.9) SAVR: 18 (7.9) P = .09 Disabling stroke TAVI: 20 (9.1) SAVR: 13 (5.6) P = .16 HR (95% CI): 1.57 (0.78–3.16) P = .20 TIA TAVI: 7 (3.5) SAVR: 6 (3.0) P = .79 |
Abbreviations: CI, confidence interval; CrI, credible interval; HR, hazard ratio; KM, Kaplan–Meier; RR, risk ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement; TIA, transient ischemic attack.
Calculated by means of Bayesian analyses.
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
Table A8:
Study Results—Life-Threatening or Major/Disabling Bleeding and Acute Kidney Injury
| Author, Year N (TAVI/SAVR) | Life-Threatening or Major/Disabling Bleeding | Acute Kidney Injury | ||||
|---|---|---|---|---|---|---|
| 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | |
| Full Cohort | ||||||
| Reardon et al, 201719 SURTAVI, mITT 1,660 (864/796) |
Percentagea TAVI: 12.2 SAVR: 9.3 95% Crl for difference: −0.1 to 5.9 |
Not reported | Not reported | Percentagea Stage 2 or 3 TAVI: 1.7 SAVR: 4.4 95% Crl for difference: 4.4 to −1.0 |
Not reported | Not reported |
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2 | TAVI: 105 (10.4) | TAVI: 151 (15.2) | TAVI: 169 (17.3) | TAVI: 13 (1.3) | TAVI: 32 (3.4) | TAVI: 36 (3.8) |
| SAVR: 442 (43.4) | SAVR: 460 (45.5) | SAVR: 471 (47.0) | SAVR: 31 (3.1) | SAVR: 48 (5.0) | SAVR: 57 (6.2) | |
| 2,032 (1,011/1,021) | P < .001 | P < .001 | P < .001 | P = .006 | P = .07 | P = .02 |
| Transfemoral Cohort | ||||||
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2 | TAVI: 52 (6.7) | TAVI: 84 (11.1) | TAVI: 101 (13.6) | Stage 3 | Stage 3 | Stage 3 |
| SAVR: 320 (41.4) | SAVR: 333 (43.4) | SAVR: 341 (44.7) | TAVI: 4 (0.5) | TAVI: 16 (2.2) | TAVI: 18 (2.5) | |
| 1,550 (775/775) | P < .001 | P < .001 | P < .001 | SAVR: 23 (3.0) | SAVR: 38 (5.2) | SAVR: 45 (6.4) |
| P < .001 | P = .002 | P < .001 | ||||
| Transthoracic Cohort | ||||||
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2 | TAVI: 53 (22.6) | TAVI: 67 (29.1) | TAVI: 68 (29.6) | Stage 3 | Stage 3 | Stage 3 |
| SAVR: 122 (49.8) | SAVR: 127 (52.3) | SAVR: 130 (54.1) | TAVI: 9 (3.9) | TAVI: 16 (7.3) | TAVI: 18 (8.4) | |
| 482 (236/246) | P < .001 | P < .001 | P < .001 | SAVR: 8 (3.4) | SAVR: 10 (4.3) | SAVR: 12 (5.5) |
| P = .77 | P = .18 | P = .23 | ||||
Abbireviations: CrI, credible interval; KM, Kaplan-Meier; mITT, modified intention to treat; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
Calculated by means of Bayesian analyses.
The percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
Table A9:
Study Results—New Permanent Pacemaker Implantation and Major Vascular Complications
| Author, Year | New Permanent Pacemaker Implantation | Major Vascular Complications | ||||
|---|---|---|---|---|---|---|
| N (TAVI/SAVR) | 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years |
| Entire Cohort | ||||||
| Reardon et al, 201719 SURTAVI, mITT 1,660 (864/796) |
Percentagea TAVI: 25.9 SAVR: 6.6 95% Crl for difference: 15.9–22.7 |
Not reported | Not reported | Percentagea TAVI: 6.0 SAVR: 1.1 95% Crl for difference: 3.2–6.7 |
Not reported | Not reported |
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2, KM estimates | TAVI: 85 (8.5) | TAVI: 98 (9.9) | TAVI: 114 (11.8) | TAVI: 80 (7.9) | TAVI: 84 (8.4) | TAVI: 86 (8.6) |
| SAVR: 68 (6.9) | SAVR: 85 (8.9) | SAVR: 96 (10.3) | SAVR: 51 (5.0) | SAVR: 54 (5.3) | SAVR: 55 (5.5) | |
| 2,032 (1,011/1,021) | P = .17 | P = .43 | P = .29 | P = .008 | P = .007 | P = .006 |
| Transfemoral Cohort | ||||||
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2, ITT | TAVI: 62 (8.1) | TAVI: 73 (9.6) | TAVI: 85 (11.4) | TAVI: 66 (8.5) | TAVI: 68 (8.8) | TAVI: 69 (9.0) |
| 1,550 (775/775) | SAVR: 54 (7.1) | SAVR: 69 (9.5) | SAVR: 77 (10.8) | SAVR: 30 (3.9) | SAVR: 33 (4.3) | SAVR: 30 (4.5) |
| P = .49 | P = .93 | P = .71 | P < .001 | P < .001 | P < .001 | |
| Transthoracic Cohort | ||||||
| Leon et al, 201610 | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) | KM estimateb, n (%) |
| PARTNER 2, ITT | TAVI: 23 (9.9) | TAVI: 25 (10.9) | TAVI: 29 (13.1) | TAVI: 14 (5.9) | TAVI: 16 (6.9) | TAVI: 17 (7.5) |
| 482 (236/246) | SAVR: 14 (5.9) | SAVR: 16 (6.9) | SAVR: 19 (8.6) | SAVR: 21 (8.6) | SAVR: 21 (8.6) | SAVR: 21 (8.6) |
| P = .11 | P = .13 | P = .13 | P = .26 | P = .49 | P = .65 | |
Abbreviations: CrI, credible interval; KM, Kaplan–Meier; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve replacement.
Calculated by means of Bayesian analyses.
The percentages provided are Kaplan–Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
Table A10:
Study Results—Paravalvular Aortic Regurgitation, Aortic Valve Reintervention, and Aortic Valve Rehospitalization
| Author, Year N (TAVI/SAVR) | Paravalvular Aortic Regurgitation | Aortic Valve Reintervention | Rehospitalization | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Discharge or 30 Days, n (%) | 1 Year, n (%) | 2 Years, n (%) | 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | |
| Full Cohort | |||||||||
| Reardon et al, 201719 SURTAVI Discharge 1,495 (820/675) 1 year 1,068 (580/488) 2 years 510 (287/223) |
DischargeNone/trace TAVI: 516 (63.0) SAVR: 644 (95.4) Mild TAVI: 276 (33.7) SAVR: 29 (4.3) Moderate or severe TAVI: 28 (3.4) SAVR: 2 (0.3) Statistically significant |
None/trace TAVI: 364 (62.8) SAVR: 458 (93.8) Mild TAVI: 185 (31.9) SAVR: 27 (5.5) Moderate or severe TAVI: 31 (5.3) SAVR: 3 (0.6) Difference (95% Crl): 4.5% (2.8%–6.8%) |
None/trace TAVI: 179 (62.3) SAVR: 210 (94.2) Mild TAVI: 94 (32.8) SAVR: 13 (5.8) Moderate or severe TAVI: 14 (4.9) SAVR: 0 Statistically significant |
Percentagea TAVI: 0.7 SAVR: 0.2 95% Crl for difference: −0.2 to 1.1 |
Percentagea TAVI: 2.0 SAVR: 0.5 95% Crl for difference: 0.3–2.6 |
Percentagea TAVI: 2.7 SAVR: 0.7 95% Crl for difference: 0.6–3.4 |
Percentagea TAVI: 2.4 SAVR: 2.9 95% Crl for difference: −2.0 to 1.0 |
Percentagea TAVI: 9.0 SAVR: 8.7 95% Crl for difference: −2.6 to 3.1 |
Percentagea TAVI: 13.3 SAVR: 11.0 95% Crl for difference: −1.1 to 5.8 |
| Leon et al, 201610 PARTNER 2 30 days 1,629 (872/757) 1 year 1,339 (728/611) 2 years 1,114 (600/514) |
30 Days None/traceb TAVI: 643 (73.7) SAVR: 732 (96.7) Mildb TAVI: 196 (22.5) SAVR: 21 (2.8) Moderate or severeb TAVI: 33 (3.7) SAVR: 4 (0.6) P < .001 |
None/traceb TAVI: 534 (73.4) SAVR: 586 (95.9) Mildb TAVI: 169 (23.2) SAVR: 23 (3.8) Moderate to severeb TAVI: 25 (3.4) SAVR: 2 (0.4) P < .001 |
None/traceb TAVI: 391 (65.2) SAVR: 493 (95.9) Mildb TAVI: 161 (26.8) SAVR: 18 (3.5) Moderate to severeb TAVI: 48 (8.0) SAVR: 3 (0.6) P < .001 |
KM estimate, n (%) TAVI: 4 (0.4) SAVR: 0 P = .05 |
KM estimate, n (%) TAVI: 11 (1.2) SAVR: 4 (0.5) P = .10 |
KM estimate, n (%) TAVI: 13 (1.4) SAVR: 5 (0.6) P = .09 |
KM estimate, n (%) TAVI: 64 (6.5) SAVR: 62 (6.5) P = .99 |
KM estimate, n (%) TAVI: 142 (14.8) SAVR: 135 (14.7) P = .92 |
KM estimate, n (%) TAVI: 183 (19.6) SAVR: 156 (17.3) P = .22 |
| Transfemoral Cohort | |||||||||
| Leon et al, 201610 PARTNER 2, ITT 1,550 (775/775) |
Not reported | KM estimate, n (%) TAVI: 3 (0.4) SAVR: 0 P = .08 |
KM estimate, n (%) TAVI: 8 (1.1) SAVR: 4 (0.6) P = .33 |
KM estimate, n (%) TAVI: 9 (1.2) SAVR: 5 (0.8) P = .39 |
KM estimate, n (%) TAVI: 42 (5.5) SAVR: 47 (6.5) P = .44 |
KM estimate, n (%) TAVI: 97 (13.1) SAVR: 104 (14.8) P = .34 |
KM estimate, n (%) TAVI: 131 (18.1) SAVR: 116 (16.8) P = .52 |
||
| Transthoracic Cohort | |||||||||
| Leon et al, 201610 PARTNER 2, ITT 482 (236/246) |
Not reported | KM estimate, n (%) TAVI: 1 (0.4) SAVR: 0 P = .32 |
KM estimate, n (%) TAVI: 3 (1.4) SAVR: 0 P = .08 |
KM estimate, n (%) TAVI: 4 (2.0) SAVR: 0 P = .04 |
KM estimate, n (%) TAVI: 22 (9.9) SAVR: 15 (6.5) P = .20 |
KM estimate, n (%) TAVI: 45 (20.9) SAVR: 31 (14.2) P = .07 |
KM estimate, n (%) TAVI: 52 (24.7) SAVR: 40 (19.2) P = .18 |
||
Abbreviations: AMD, adjusted mean difference; CI, confidence interval; CrI, credible interval; KCCQ, Kansas City Cardiomyopathy Questionnaire; mITT, modified intention to treat; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Considered a substantial or moderate improvement.
Results for the full cohort were not provided because there was a significant interaction between access site and treatment group for several key health status measurements at 1 month.
Table A11:
Study Results—Quality of Life (KCCQ Overall Score)
| Author, Year N (TAVI/SAVR) | KCCQ Overall Score, Mean Change from Baseline (95% CI) AMD (95% CI), TAVI vs. SAVR | KCCQ Overall Score % With ≥ 10 Pointsa Improvement Over Time | |||||
|---|---|---|---|---|---|---|---|
| 30 Days | 6 Months | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | |
| Reardon et al, 201719 SURTAVI, mITT 1,660 (864/796) mITT |
TAVI: 18.4 (SD 22.8) SAVR: 5.9 (SD 27.0) 95% CrI for difference: 10.0–15.1 |
TAVI: 21.8 (SD 22.3) SAVR: 21.3 (SD 22.3) 95% Crl for difference: −1.9 to 2.8 |
TAVI: 20.9 (SD 22.2) SAVR: 20.6 (SD 22.2) 95% CrI for difference: −2.2 to 2.9 |
Not reported | Not reported | Not reported | Not reported |
| Leon et al, 2016b10 PARTNER 2, transfemoral cohort 1,550 (775/775) |
TAVI: 17.5 (15.8–19.3) SAVR: 3.2 (1.3–5.5) AMD: 14.1 (11.7–16.4) P < .001 |
Not reported | TAVI: 22.1 (20.4–23.9) SAVR: 22.1 (20.1–24.1) AMD: −0.1 (−2.2 to 2.1) P = .94 |
TAVI: 20.2 (18.2–22.2) SAVR: 18.4 (16.3–20.6) AMD: 1.0 (−1.5 to 3.5) P = .42 |
TAVI: 64.0 SAVR: 41.2 P < .001 |
TAVI: 71.1 SAVR: 68.9 P = .36 |
TAVI: 67.2 SAVR: 66.2 P = .98 |
| Leon et al, 2016b10 PARTNER 2, transthoracic cohort 482 (236/246) |
TAVI: 6.4 (2.5–10.3) SAVR: 5.6 (1.5–9.6) AMD: 3.5 (−1.4 to 8.4) P = .17 |
Not reported | TAVI: 16.7 (13.0–20.4) SAVR: 18.6 (15.2–21.9) AMD: −0.5 (−5.1 to 4.0) P = .82 |
TAVI: 15.8 (12.0–19.7) SAVR: 17.7 (13.6–21.7) AMD: −1.2 (−6.5 to 4.1) P = .66 |
TAVI: 46.4 SAVR: 42.2 P = .17 |
TAVI: 57.8 SAVR: 65.8 P = .099 |
TAVI: 62.3 SAVR: 68.2 P = .31 |
Abbreviations: AMD, adjusted mean difference; CI, confidence interval; CrI, credible interval; KCCQ, Kansas City Cardiomyopathy Questionnaire; mITT, modified intention to treat; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Considered a substantial or moderate improvement.
Results for the full cohort were not provided because there was a significant interaction between access site and treatment group for several key health status measurements at 1 month.
Table A12:
Study Results—Quality of Life (KCCQ Overall Subscales)
| Author, Year N (TAVI/SAVR) | KCCQ Physical Limitations, Mean Change from Baseline (95% CI) AMD (95% CI), TAVI vs. SAVR | KCCQ Social Limitations, Mean Change from Baseline (95% CI) AMD (95% CI), TAVI vs. SAVR | ||||
|---|---|---|---|---|---|---|
| 30 Days | 1 Year | 2 Years | 30 Days | 1 Year | 2 Years | |
| Reardon et al, 201719 SURTAVI 1,660 (864/796) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Leon et al, 2016b10 PARTNER 2, transfemoral cohort 1,550 (775/775) |
TAVI: 12.5 (10.5–14.5) SAVR: −1.7 (−4.1 to 0.7) AMD: 13.7 (10.9–16.5) P < .001 |
TAVI: 13.2 (11.1–15.4) SAVR: 13.4 (11.1–15.6) AMD: −0.8 (−3.5 to 1.9) P = .57 |
TAVI: 9.7 (7.4–12.0) SAVR: 7.8 (5.1–10.5) AMD: 1.0 (−2.1 to 4.0) P = .55 |
TAVI: 16.5 (13.8–19.3) SAVR: −3.8 (−7.2 to 0.4) AMD: 21.6 (18.0–25.2) P < .001 |
TAVI: 23.8 (21.0–26.5) SAVR: 24.7 (21.4–27.9) AMD: 0.4 (−2.8 to 3.7) P = .79 |
TAVI: 21.3 (18.2–24.5) SAVR: 18.0 (14.4–21.5) AMD: 2.7 (−1.1 to 6.6) P = .16 |
| Leon et al, 2016b10 PARTNER 2, transthoracic cohort 482 (236/246) |
TAVI: 1.5 (−3.1 to 6.0) SAVR: 0.5 (−3.9 to 4.9) AMD: 3.3 (−2.5; 9.1) P = .26 |
TAVI: 10.0 (5.4–14.5) SAVR: 13.0 (9.2–16.8) AMD: −0.2 (−5.9 to 5.5) P = .94 |
TAVI: 3.8 (−0.8 to 8.5) SAVR: 8.3 (3.0–13.5) AMD: −3.4 (−10.2 to 3.4) P = .32 |
TAVI: 1.2 (−4.3 to 6.7) SAVR: −5.0 (−11.9 to 2.0) AMD: 7.0 (−0.5–14.4) P = .07 |
TAVI: 21.2 (15.9–26.5) SAVR: 15.3 (9.8–20.9) AMD: 6.3 (−0.6 to 13.3) P = .08 |
TAVI: 16.0 (9.7–22.3) SAVR: 16.0 (9.8–22.2) AMD: −1.3 (−9.6 to 6.9) P = .75 |
Abbreviations: AMD, adjusted mean difference; CI, confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Considered a substantial or moderate improvement.
Results for the full cohort were not provided as there was a significant interaction between access site and treatment group for several key health status measurements at 1 month.
Table A13:
Study Results—EuroQOL-5D Utilities
| Author, Year N (TAVI/SAVR) | EuroQOL-5D Utilities, Mean Change from Baseline (95% CI) AMD (95% CI), TAVI vs. SAVR | ||
|---|---|---|---|
| 30 Days to 3 Months | 1 Year | 2 Years | |
| Reardon et al, 201719 | 3 months | Not reported | Not reported |
| SURTAVI | TAVI: 0.06 (SD 0.18) | ||
| 1,660 (864/796) | SAVR: 0.05 (SD 0.18) | ||
| 95% Crl of the difference: −0.01 to 0.03 | |||
| Leon et al, 201610 | At 30 days | TAVI: 0.044 (0.029–0.059) | TAVI: 0.027 (0.011–0.043) |
| PARTNER 2, transfemoral cohort | TAVI: 0.058 (0.043–0.072) | SAVR: 0.066 (0.048–0.083) | SAVR: 0.037 (0.018–0.055) |
| SAVR: −0.002 (−0.019 to 0.014) | AMD: −0.011 (−0.031 to 0.008) | AMD: −0.002 (−0.024 to 0.019) | |
| 1,550 (775/775) | AMD: 0.066 (0.047–0.086) | P = .26 | P = .83 |
| P < .001 | |||
| Leon et al, 201610 | At 30 days | TAVI: 0.029 (−0.003 to 0.061) | TAVI: 0.018 (−0.012 to 0.049) |
| PARTNER 2, transthoracic cohort | TAVI: −0.021 (−0.054 to 0.011) | SAVR: 0.032 (0.003–0.060) | SAVR: −0.001 (−0.035 to 0.032) |
| SAVR: −0.022 (−0.055 to 0.010) | AMD: 0 (−0.042 to 0.042) | AMD: 0.018 (−0.029 to 0.065) | |
| 482 (236/246) | AMD: 0.007 (−0.034 to 0.048) | P = .99 | P = .45 |
| P = .72 | |||
Abbreviations: AMD, adjusted mean difference; CI, confidence interval; CrI, credible interval; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
aConsidered a substantial or moderate improvement.
Appendix 7: New York Heart Association Functional Classification
Under the New York Heart Association (NYHA) Functional Classification system, the heart failure class (I–IV) is defined according to the severity of the patient's symptoms and how much the patients is limited during physical activity, as follows36:
I: No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea (shortness of breath)
II: Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, dyspnea (shortness of breath)
III: Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea
IV: Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases
Appendix 8: Results of Applicability and Limitation Checklists for Studies Included in the Economic Literature Review
Table A14:
Assessment of the Applicability of Studies Assessing the Cost-Effectiveness of Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement for the Treatment of Severe Aortic Valve Stenosis in People at Intermediate Surgical Risk
| Objective: To assess the cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement for the treatment of severe aortic valve stenosis in people at intermediate surgical risk | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Were the perspectives clearly stated and what were they? | Are estimates of relative treatment effect from the best available source? |
| Kodera et al, 201841 | Yes (people with severe aortic valve stenosis at intermediate surgical risk) | Yes (TAVI vs. SAVR) | No (perspective of Japanese public health care payer) | Yes (Japanese public health care payer) | Yes (data from PARTNER 2, OCEAN TAVI) |
| Tam et al, 201816 | Yes (people with severe aortic valve stenosis at intermediate surgical risk) | Yes (TAVI vs. SAVR) | Yes (perspective of Ontario Ministry of Health) | Yes (Ontario Ministry of Health) | Yes (data from PARTNER 2) |
| Tam et al, 201817 | Yes (people with severe aortic valve stenosis at intermediate surgical risk) | Yes (TAVI vs. SAVR) | Yes (perspective of Ontario Ministry of Health) | Yes (Ontario Ministry of Health) | Yes (data from SURTAVI) |
| Author, Year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgment (directly applicable/partially applicable/ not applicable) |
|---|---|---|---|---|
| Kodera et al, 201841 | Yes (2%) | Yes | NA | Partially applicable |
| Tam et al, 201816 | Yes (1.5%) | Yes | NA | Directly applicable |
| Tam et al, 201817 | Yes (1.5%) | Yes | NA | Directly applicable |
Abbreviations: TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Table A15:
Assessment of the Limitations of Studies Assessing the Cost-Effectiveness of Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement for the Treatment of Severe Aortic Valve Stenosis in People at Intermediate Surgical Risk
| Objective: To assess the cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement for the treatment of severe aortic valve stenosis in people at intermediate surgical risk | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the estimates of relative treatment effects obtained from best available sources? | Do the estimates of relative treatment effect match the estimates contained in the clinical report? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from best available sources? |
| Tam et al, 201816 | Yes (30 d cycles, short-term and long-term complications captured) | Yes (Conservative assumptions made about outcomes occurring after 2 y) | Yes (captures short- and longer-term [2 y] complications, utility data from high-risk cohort) | Yes (didn't use relative effects, but all efficacy inputs from the PARTNER 2 trial) | Yes | Yes (included both procedural costs and complication costs) | Yes (used Ontario sources) |
| Tam et al, 201817 | Yes (30 d cycles, short-term and long-term complications captured) | Yes (Conservative assumptions made about outcomes occurring after 2 y) | Yes (captures short and longer-term [2 y] complications, utility data from high-risk cohort) | Yes (didn't use relative effects, but all efficacy inputs from the SURTAVI trial) | Yes | Yes (included both procedural costs and complication costs) | Yes (used Ontario sources) |
| Author, Year | Are the unit costs of resources obtained from best available resources? | Is an appropriate incremental analysis presented or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall assessment including applicability to the project (Minor limitations/ potentially serious limitations/very serious limitations) |
|---|---|---|---|---|---|
| Tam et al, 201816 | Yes (used Ontario sources) | Yes | Yes | No | Minor limitations |
| Tam et al, 201817 | Yes (used Ontario sources) | Yes | Yes | No | Minor limitations |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Appendix 9: Budget Impact Analysis
We calculated our net budget impact as the difference in annual total costs between our new scenario (public funding for TAVI, thus a mix of TAVI and SAVR) and our current scenario (no public funding for TAVI, thus SAVR only).
We calculated the costs for each treatment group as follows. We calculated the annual costs for 2018/19 by multiplying the total volume of patients in 2018/19 (see Appendix 4, Table A16) by the relevant first-year treatment costs (see Table 5; Equation 1). We calculated annual costs for subsequent years using the ongoing costs of 2018/19 patients and costs of volumes of patients expected in respective years (Equations 2 to 5).
Note: The costs derived from the Tam and colleagues analyses16,17 consider mortality, so calculations are based on the total number of people in the cohort; however, the number of patients that are expected to be alive over the analysis can be seen in Table A16.
| Equation 1 |
| Equation 2 |
| Equation 3 |
| Equation 4 |
| Equation 5 |
Table A16:
Number of Patients Considered in Budget Impact Analysis, Reference Case
| Scenario | Year | Patients per Year,a,b Total (Alivec), n | Total Patients, n | |||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||||
| Current | TAVI | 2018/19 | 0 | — | — | — | — | 0 |
| scenario | 2019/20 | 0 | 0 | — | — | — | 0 | |
| 2020/21 | 0 | 0 | 0 | — | — | 0 | ||
| 2021/22 | 0 | 0 | 0 | 0 | — | 0 | ||
| 2022/23 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SAVR | 2018/19 | 266 (266) | — | — | — | — | 266 (266) | |
| 2019/20 | 272 (272) | 266 (231) | — | — | — | 538 (503) | ||
| 2020/21 | 276 (276) | 272 (236) | 266 (215) | — | — | 814 (727) | ||
| 2021/22 | 281 (281) | 276 (240) | 272 (220) | 266 (200) | 1,095 (941) | |||
| 2022/23 | 285 (285) | 281 (244) | 276 (223) | 272 (205) | 266 (186) | 1,380 (1,43) | ||
| New scenario | TAVI | 2018/19 | 200 (200) | — | — | — | — | 200 (200) |
| 2019/20 | 218 (218) | 200 (179) | — | — | — | 417 (397) | ||
| 2020/21 | 235 (235) | 218 (195) | 200 (169) | — | — | 652 (599) | ||
| 2021/22 | 253 (253) | 235 (210) | 218 (185) | 200 (158) | — | 905 (806) | ||
| 2022/23 | 271 (271) | 253 (227) | 235 (199) | 218 (173) | 200 (147) | 1,175 (1,017) | ||
| SAVR | 2018/19 | 67 (67) | — | — | — | — | 67 (67) | |
| 2019/20 | 54 (54) | 67 (58) | — | — | — | 121 (112) | ||
| 2020/21 | 41 (41) | 54 (47) | 67 (54) | — | — | 162 (142) | ||
| 2021/22 | 28 (28) | 41 (36) | 54 (44) | 67 (50) | — | 190 (158) | ||
| 2022/23 | 14 (14) | 28 (24) | 41 (33) | 54 (41) | 67 (46) | 205 (159) | ||
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
Year 1 represents all new patients, years 2 to 5 represent patients who have received TAVI or SAVR in a previous year.
Table A17:
Number of Patients Considered in Budget Impact Analysis, Scenario Analyses
| Scenario | Yeara | ||||||
|---|---|---|---|---|---|---|---|
| 2018/19 | 2019/20 | 2020/21 | 2021/22 | 2022/23 | |||
| Reference case | Current scenario | SAVR, n | 266 | 272 | 276 | 281 | 285 |
| Scenario 7: TAVI 3-day hospital stay Scenario 8: TAVI 1-day hospital stay |
New scenario | TAVI uptake rate | 75% | 80% | 85% | 90% | 95% |
| Scenario 9: SAVR costs $3,000 | TAVI, n | 200 | 218 | 235 | 253 | 271 | |
| Scenario 10: costs inflated to 2018 CAD | SAVR, n | 67 | 54 | 41 | 28 | 14 | |
| Scenario 1: 20% of SAVR + CABG patients not eligible for TAVI | Current scenario | SAVR, n | 246 | 252 | 256 | 260 | 265 |
| New scenario | TAVI uptake rate | 75% | 80% | 85% | 90% | 95% | |
| TAVI, n | 185 | 202 | 218 | 234 | 252 | ||
| SAVR, n | 62 | 50 | 38 | 26 | 13 | ||
| Scenario 2: 25% of current SAVR patients at intermediate surgical risk | Current scenario | SAVR, n | 477 | 488 | 496 | 504 | 512 |
| New scenario | TAVI uptake rate | 75% | 80% | 85% | 90% | 95% | |
| TAVI, n | 358 | 390 | 422 | 454 | 486 | ||
| SAVR, n | 119 | 98 | 74 | 50 | 26 | ||
| Scenario 3: 83% of patients can have transfemoral-access TAVI | Current scenario | SAVR, n | 238 | 244 | 248 | 252 | 256 |
| New scenario | TAVI uptake rate | 75% | 80% | 85% | 90% | 95% | |
| TAVI, n | 179 | 195 | 211 | 227 | 243 | ||
| SAVR, n | 60 | 49 | 37 | 25 | 13 | ||
| Scenario 4: all access routes funded | Current scenario | SAVR, n | 287 | 294 | 299 | 304 | 308 |
| New scenario | TAVI uptake rate | 75% | 80% | 85% | 90% | 95% | |
| TAVI, n | 215 | 235 | 254 | 274 | 293 | ||
| SAVR, n | 72 | 59 | 45 | 30 | 15 | ||
| Scenario 5: higher initial uptake | Current scenario | SAVR, n | 266 | 272 | 276 | 281 | 285 |
| New scenario | TAVI uptake rate | 90% | 95% | 95% | 95% | 95% | |
| TAVI, n | 239 | 258 | 262 | 267 | 271 | ||
| SAVR, n | 27 | 14 | 14 | 14 | 14 | ||
| Scenario 6: lower initial uptake | Current scenario | SAVR, n | 266 | 272 | 276 | 281 | 285 |
| New scenario | TAVI uptake rate | 50% | 61% | 73% | 84% | 95% | |
| TAVI, n | 133 | 167 | 200 | 235 | 271 | ||
| SAVR, n | 133 | 105 | 76 | 46 | 14 | ||
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
Table A18:
Average Annual Per-Patient Costs for TAVI and SAVR,a Sensitivity Analysis
| Source | Intervention | Year Post-Implantb | ||||
|---|---|---|---|---|---|---|
| Year 1c | Year 2d | Year 3d | Year 4d | Year 5d | ||
| Transfemoral TAVI Only, 2016 CAD (Reference Case) | ||||||
| Tam et al, 201816 PARTNER 2 |
TAVI | 43,424 | 1,826 | 518 | 193 | 113 |
| SAVR | 33,421 | 1,626 | 623 | 320 | 185 | |
| Transfemoral TAVI Only, 2018 CADe | ||||||
| Tam et al, 201816 PARTNER 2, adjusted |
TAVI | 45,169 | 1,899 | 539 | 201 | 118 |
| SAVR | 34,764 | 1,691 | 648 | 333 | 193 | |
| Transfemoral TAVI Only, 2016 CAD, 3-Day Length of Hospital Stay | ||||||
| Tam et al, 201816 PARTNER 2, adjusted |
TAVI | 39,481 | 1,826 | 518 | 193 | 113 |
| SAVR | 33,421 | 1,626 | 623 | 320 | 185 | |
| Transfemoral TAVI Only, 2018 CAD, 1-Day Length of Hospital Stay | ||||||
| Tam et al, 201816 PARTNER 2, adjusted |
TAVI | 35,376 | 1,826 | 518 | 193 | 113 |
| SAVR | 33,421 | 1,626 | 623 | 320 | 185 | |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Costs incorporate mortality.
Numbers may appear inexact due to rounding.
Includes procedural (valve, index hospitalization, and fees for surgeon, surgical assistant, and anesthesiologist), short-term complication, and long-term complication costs.
Includes long-term complication costs.
Updated from 2016 CAD to 2018 CAD based on a factor of 1.04 (Consumer Price Index, Ontario, Health care goods and services, January 2016 to January 2018).72
Table A19:
Cost Breakdowns in the Budget Impact Analysis Results, Reference Case
| Scenario | Budget Impact, Millionsa | |||||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |||
| Current scenario | TAVI | Valve | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Initial hospitalizationb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Professional feesc | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Complications | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Total | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| SAVR | Valve | 1.60 | 1.63 | 1.66 | 1.69 | 1.71 | 8.28 | |
| Initial hospitalizationb | 4.62 | 4.72 | 4.79 | 4.88 | 4.95 | 23.97 | ||
| Professional feesc | 1.13 | 1.16 | 1.17 | 1.20 | 1.21 | 5.87 | ||
| Complications | 1.54 | 2.01 | 2.21 | 2.33 | 2.42 | 10.51 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | Valve | 4.81 | 5.24 | 5.65 | 6.09 | 6.52 | 28.32 |
| Initial hospitalizationb | 2.02 | 2.20 | 2.37 | 2.55 | 2.73 | 11.87 | ||
| Professional feesc | 0.75 | 0.81 | 0.88 | 0.95 | 1.01 | 4.39 | ||
| Complications | 1.10 | 1.56 | 1.79 | 1.97 | 2.13 | 8.54 | ||
| Total | 8.66 | 9.81 | 10.69 | 11.56 | 12.40 | 53.13 | ||
| SAVR | Valve | 0.40 | 0.33 | 0.25 | 0.17 | 0.09 | 1.23 | |
| Initial hospitalizationb | 1.15 | 0.94 | 0.72 | 0.49 | 0.25 | 3.55 | ||
| Professional feesc | 0.28 | 0.23 | 0.18 | 0.12 | 0.06 | 0.87 | ||
| Complications | 0.39 | 0.42 | 0.37 | 0.29 | 0.18 | 1.65 | ||
| Total | 2.22 | 1.93 | 1.51 | 1.06 | 0.58 | 7.30 | ||
| Net budget impact | Valve | 3.61 | 3.94 | 4.25 | 4.58 | 4.90 | 21.27 | |
| Initial hospitalizationb | −1.45 | −1.58 | −1.70 | −1.84 | −1.97 | −8.54 | ||
| Professional feesc | −0.10 | −0.11 | −0.12 | −0.13 | −0.14 | −0.61 | ||
| Complications | −0.06 | −0.03 | −0.05 | −0.08 | −0.10 | −0.32 | ||
| Total | 2.00 | 2.22 | 2.37 | 2.53 | 2.69 | 11.80 | ||
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
Includes the cost of intensive care unit and ward stay after the initial procedure.
Includes surgeon, surgical assistant, and anesthesiologist fees.
Table A20:
Budget Impact Analysis, Full Results, Scenario Analyses
| Scenario Analysis | Scenario | Budget Impact, Millionsa | ||||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |||
| Reference case | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | 8.66 | 9.81 | 10.69 | 11.56 | 12.40 | 53.13 | |
| SAVR | 2.22 | 1.93 | 1.51 | 1.06 | 0.58 | 7.30 | ||
| Total | 10.89 | 11.74 | 12.20 | 12.62 | 12.98 | 60.43 | ||
| Net budget impact | 2.00 | 2.22 | 2.37 | 2.53 | 2.69 | 11.80 | ||
| Scenario 1: 20% of SAVR + CABG patients not eligible for TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.22 | 8.82 | 9.12 | 9.34 | 9.56 | 45.07 | ||
| Total | 8.22 | 8.82 | 9.12 | 9.34 | 9.56 | 45.07 | ||
| New scenario | TAVI | 8.01 | 9.09 | 9.91 | 10.70 | 11.53 | 49.25 | |
| SAVR | 2.06 | 1.78 | 1.40 | 0.98 | 0.54 | 6.76 | ||
| Total | 10.07 | 10.88 | 11.32 | 11.68 | 12.07 | 56.01 | ||
| Net budget impact | 1.85 | 2.05 | 2.20 | 2.34 | 2.50 | 10.94 | ||
| Scenario 2: 25% of current SAVR patients at intermediate surgical risk | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 15.94 | 17.08 | 17.67 | 18.11 | 18.48 | 87.29 | ||
| Total | 15.94 | 17.08 | 17.67 | 18.11 | 18.48 | 87.29 | ||
| New scenario | TAVI | 15.53 | 17.61 | 19.21 | 20.74 | 22.28 | 95.37 | |
| SAVR | 3.99 | 3.46 | 2.72 | 1.90 | 1.04 | 13.10 | ||
| Total | 19.52 | 21.06 | 21.92 | 22.64 | 23.32 | 108.47 | ||
| Net budget impact | 3.58 | 3.98 | 4.26 | 4.54 | 4.84 | 21.18 | ||
| Scenario 3: 83% of patients can have transfemoral access TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 7.95 | 8.54 | 8.83 | 9.05 | 9.24 | 43.62 | ||
| Total | 7.95 | 8.54 | 8.83 | 9.05 | 9.24 | 43.62 | ||
| New scenario | TAVI | 7.75 | 8.80 | 9.60 | 10.37 | 11.14 | 47.67 | |
| SAVR | 1.99 | 1.73 | 1.36 | 0.95 | 0.52 | 6.55 | ||
| Total | 9.74 | 10.53 | 10.96 | 11.32 | 11.66 | 54.21 | ||
| Net budget impact | 1.79 | 1.99 | 2.13 | 2.27 | 2.42 | 10.59 | ||
| Scenario 4: all access routes funded | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 9.59 | 10.29 | 10.65 | 10.92 | 11.12 | 52.58 | ||
| Total | 9.59 | 10.29 | 10.65 | 10.92 | 11.12 | 52.58 | ||
| New scenario | TAVI | 9.35 | 10.61 | 11.58 | 12.51 | 13.41 | 57.44 | |
| SAVR | 2.40 | 2.08 | 1.64 | 1.15 | 0.62 | 7.89 | ||
| Total | 11.74 | 12.69 | 13.22 | 13.66 | 14.03 | 65.34 | ||
| Net budget impact | 2.15 | 2.40 | 2.57 | 2.74 | 2.91 | 12.76 | ||
| Scenario 5: higher initial uptake | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | 10.40 | 11.66 | 11.98 | 12.25 | 12.46 | 58.74 | |
| SAVR | 0.89 | 0.50 | 0.50 | 0.51 | 0.52 | 2.91 | ||
| Total | 11.28 | 12.16 | 12.48 | 12.76 | 12.97 | 61.66 | ||
| Net budget impact | 2.39 | 2.63 | 2.65 | 2.67 | 2.68 | 13.03 | ||
| Scenario 6: lower initial uptake | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | 5.78 | 7.48 | 9.06 | 10.70 | 12.34 | 45.35 | |
| SAVR | 4.45 | 3.74 | 2.79 | 1.76 | 0.66 | 13.39 | ||
| Total | 10.22 | 11.22 | 11.85 | 12.45 | 12.99 | 58.74 | ||
| Net budget impact | 1.33 | 1.69 | 2.02 | 2.36 | 2.70 | 10.11 | ||
| Scenario 7: TAVI 3-day hospital stay | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | 7.88 | 8.96 | 9.76 | 10.56 | 11.34 | 48.50 | |
| SAVR | 2.22 | 1.93 | 1.51 | 1.06 | 0.58 | 7.30 | ||
| Total | 10.10 | 10.88 | 11.28 | 11.63 | 11.91 | 55.80 | ||
| Net budget impact | 1.21 | 1.36 | 1.44 | 1.53 | 1.62 | 7.17 | ||
| Scenario 8: TAVI 1-day hospital stay | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| Total | 8.89 | 9.52 | 9.83 | 10.09 | 10.29 | 48.63 | ||
| New scenario | TAVI | 7.06 | 8.06 | 8.80 | 9.53 | 10.23 | 43.67 | |
| SAVR | 2.22 | 1.93 | 1.51 | 1.06 | 0.58 | 7.30 | ||
| Total | 9.28 | 9.99 | 10.31 | 10.59 | 10.80 | 50.97 | ||
| Net budget impact | 0.39 | 0.47 | 0.48 | 0.49 | 0.51 | 2.34 | ||
| Scenario 9: SAVR costs $3,000 | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 8.09 | 8.71 | 9.00 | 9.25 | 9.44 | 44.49 | ||
| Total | 8.09 | 8.71 | 9.00 | 9.25 | 9.44 | 44.49 | ||
| New scenario | TAVI | 8.66 | 9.81 | 10.69 | 11.56 | 12.40 | 53.13 | |
| SAVR | 2.02 | 1.76 | 1.39 | 0.98 | 0.53 | 6.69 | ||
| Total | 10.69 | 11.58 | 12.08 | 12.54 | 12.94 | 59.82 | ||
| Net budget impact | 2.59 | 2.87 | 3.07 | 3.29 | 3.50 | 15.33 | ||
| Scenario 10: costs inflated to 2018 CAD | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 9.25 | 9.91 | 10.23 | 10.50 | 10.70 | 50.58 | ||
| Total | 9.25 | 9.91 | 10.23 | 10.50 | 10.70 | 50.58 | ||
| New scenario | TAVI | 9.01 | 10.21 | 11.12 | 12.03 | 12.90 | 55.27 | |
| SAVR | 2.31 | 2.00 | 1.57 | 1.10 | 0.60 | 7.59 | ||
| Total | 11.32 | 12.21 | 12.69 | 13.13 | 13.50 | 62.86 | ||
| Net budget impact | 2.08 | 2.31 | 2.46 | 2.63 | 2.80 | 12.28 | ||
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers may appear inexact due to rounding.
Appendix 10: Call for Participation
CALL FOR PARTICIPATION
REVIEW OF AORTIC VALVE REPLACEMENT PROCEDURE
Have you or someone you are caring for had experience of narrowing of the aortic heart valve?
If yes, we'd like to speak to you about your experiences and treatment options.
Interviews will take 20 to 30 minutes, either on the phone or in-person, scheduled between now and April 30, 2018.
WHY GET INVOLVED?
Your participation will help Health Quality Ontario with the review of usefulness of Transcatheter Aortic Valve Replacement. This review will result in a recommendation for public funding to Ontario's Ministry of Health and Long-Term Care.
ABOUT US
Health Quality Ontario is a provincial agency with one purpose: better health for all Ontarians. Part of our work involves conducting reviews of various health care technologies and services to gauge their usefulness.
Please feel free to forward this invitation to anyone you know who may be interested in participating.
Appendix 11: Interview Guide
Background
Provide information on the mandate of Health Quality Ontarioa
Explain the health technology assessment program and part of Patient, Caregiver and Public Engagement. Explained the purpose of the interview
Confirm consent for audio-recording
Restate options for withdrawal, freedom of sharing and not sharing of information
Lived Experience
What are the biggest challenges of living/caring for someone with aortic valve stenosis?
How does it impact your day-to-day routine? How would you describe your quality of life?
Currently Available Therapies
What are the current therapies/treatments that you aware of?
What therapies/treatments are accessible to you? Did you face any barriers?
Which therapies/treatments have you explored? Why did you explore these? How did the therapies/treatments meet your needs?
How did the therapies impact your quality of life?
What were the side effects and benefits?
Were there any equity issues related to cost, access, knowledge of the health care system?
TAVI Procedure
Please explain the process of therapy
How did this therapy meet/not meet your needs? How was it adequate/inadequate? Quality of life, empowerment? Ownership? Adherence? Lifestyle?
What were the side effects and benefits? Anxiety, pain, intrusiveness? Length of stay? Recovery?
Were there equity issues related to cost, access, knowledge of health care system, etc.? Travel, repeat visits?
What challenges did this procedure address?
Health Quality Ontario is now the Quality business unit at Ontario Health.
Author contributions
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Vania Costa; the primary health economist was Lindsey Falk; the secondary health economists were Hong Anh Tu and Man Wah Yeung; the patient and public partnership analyst was Arshia Ali; and the medical librarian was Melissa Walter.
KEY MESSAGES
What Is This Health Technology Assessment About?
The aortic valve is located between the left ventricle (the heart's lower left chamber) and the aorta (the main artery that distributes blood from the heart to the body). Aortic valve stenosis is a narrowing of the aortic valve, which prevents it from opening completely and reduces blood flow from the heart. This causes the heart to work harder to pump blood to the body and may lead to symptoms such as chest pain, shortness of breath, and fatigue. Surgical aortic valve replacement (SAVR) is the usual treatment for people who have severe, symptomatic aortic valve stenosis and who are at low or intermediate risk for surgery. With SAVR, surgeons replace the damaged valve with an artificial valve through a cut in the chest.
Transcatheter aortic valve implantation (TAVI) involves placing an artificial valve inside the existing valve using a catheter (a long, flexible tube), most commonly through an artery in the leg. There is no need to open the chest. At present in Ontario, TAVI is not funded in people at intermediate surgical risk.
This health technology assessment looked at how safe and effective TAVI is for people with severe, symptomatic aortic valve stenosis who are at intermediate surgical risk. It looked at the cost-effectiveness and budget impact of publicly funding TAVI in people at intermediate surgical risk. It also looked at the experiences, preferences, and values of people with aortic valve stenosis and their families and caregivers.
What Did This Health Technology Assessment Find?
We found that TAVI and SAVR had similar risks of mortality and disabling stroke, but they had different patterns of complications. The authors of the studies we looked at said that longer-term follow-up is needed to determine how durable the TAVI valve is. The device costs are much higher for TAVI than for SAVR, but they might be offset by lower costs for hospitalization and complications, especially if less invasive surgical approaches can be used. Publicly funding TAVI would lead to additional costs for the health care system, but TAVI may still offer good value for money.
Contributor Information
Ontario Health (Quality):
Vania Costa, Lindsey Falk, Hong Anh Tu, Man Wah Yeung, Arshia Ali, and Melissa Walter
About Us
This health technology assessment was produced by the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1).Health Quality Ontario. Transcatheter aortic valve implantation for treatment of aortic valve stenosis: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2016. Nov [cited 2018 Sep 5]; 16(19):[94 p.]. Available from: http://www.hqontario.ca/evidence-to-improve-care/journal-ontario-health-technology-assessment-series. [PMC free article] [PubMed]
- (2).Liu Z, Duarte R, Kidney E, Bem D, Bramley G, Rooney S, et al. Clinical effectiveness and safety of transcatheter aortic valve implantation (TAVI) for aortic stenosis: a systematic review and meta-analysis [Internet]. Birmingham (UK): University of Birmingham; 2016. [cited 2018 Sep 5]. Available from: https://www.nice.org.uk/guidance/ipg586/documents/supporting-documentation [Google Scholar]
- (3).Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56(6):565–71. [DOI] [PubMed] [Google Scholar]
- (4).Indolfi C, Bartorelli AL, Berti S, Golino P, Esposito G, Musumeci G, et al. Updated clinical indications for transcatheter aortic valve implantation in patients with severe aortic stenosis: expert opinion of the Italian Society of Cardiology and GISE. J Cardiovasc Med. 2018;19(5):197–210. [DOI] [PubMed] [Google Scholar]
- (5).Zakkar M, Bryan AJ, Angelini GD. Aortic stenosis: diagnosis and management. BMJ. 2016;355:i5425. [DOI] [PubMed] [Google Scholar]
- (6).Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–12. [DOI] [PubMed] [Google Scholar]
- (7).Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99(1):55–61. [DOI] [PubMed] [Google Scholar]
- (8).Otto CM, Prendergast B. Aortic-valve stenosis – from patients at risk to severe valve obstruction. N Engl J Med. 2014;371(8):744–56. [DOI] [PubMed] [Google Scholar]
- (9).Webb J, Rodes-Cabau J, Fremes S, Pibarot P, Ruel M, Ibrahim R, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol. 2012;28(5):520–8. [DOI] [PubMed] [Google Scholar]
- (10).Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–20. [DOI] [PubMed] [Google Scholar]
- (11).Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403–18. [DOI] [PubMed] [Google Scholar]
- (12).Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;60(6):483–92. [DOI] [PubMed] [Google Scholar]
- (13).Vahanian A, Himbert D, Brochet E. Transcatheter valve implantation for patients with aortic stenosis. Heart. 2010;96(22):1849–56. [DOI] [PubMed] [Google Scholar]
- (14).Mahtta D, Elgendy IY, Bavry AA. From CoreValve to Evolut PRO: reviewing the journey of self-expanding transcatheter aortic valves. Cardiol Ther. 2017;6(2):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bourantas CV, Serruys PW. Evolution of transcatheter aortic valve replacement. Circ Res. 2014;114(6):1037–51. [DOI] [PubMed] [Google Scholar]
- (16).Tam DY, Hughes A, Fremes SE, Youn S, Hancock-Howard RL, Coyte PC, et al. A cost-utility analysis of transcatheter versus surgical aortic valve replacement for the treatment of aortic stenosis in the population with intermediate surgical risk. J Thorac Cardiovasc Surg. 2018;155(5):1978–88.e1. [DOI] [PubMed] [Google Scholar]
- (17).Tam DY, Hughes A, Wijeysundera HC, Fremes SE. The cost-effectiveness of self-expandable transcatheter aortic valves in intermediate risk patients. Ann Thorac Surg. 2018;106(3):676–83. [DOI] [PubMed] [Google Scholar]
- (18).Health Quality Ontario. Transcatheter aortic valve implantation for treatment of aortic valve stenosis: OHTAC recommendation [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. November [cited 2018 Sep 5]. Available from: http://www.hqontario.ca/evidence-to-improvecare/recommendations-and-reports/OHTAC/aortic-valve-stenosis [Google Scholar]
- (19).Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–31. [DOI] [PubMed] [Google Scholar]
- (20).Costa V, Falk L, Tu H, Ali A, Walter M. Transcatheter aortic valve implantation for the treatment of patients with severe, symptomatic aortic valve stenosis and intermediate surgical risk. PROSPERO 2018 CRD42018093719 [cited 2018 Sep 24] Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018093719.
- (21).Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (23).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (24).Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- (25).Higgins JP, Altman DG, Sterne JA. Assessing risk of bias in included studies. In: Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [Internet]. London (UK): The Cochrane Collaboration, 2011. [updated 2011 Mar; cited 2018 Sep 5]. Available from www.handbook.cochrane.org. [Google Scholar]
- (26).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (27).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Tam DY, Vo TX, Wijeysundera HC, Ko DT, Rocha RV, Friedrich J, et al. Transcatheter vs surgical aortic valve replacement for aortic stenosis in low-intermediate risk patients: a meta-analysis. Can J Cardiol. 2017;33(9):1171–9. [DOI] [PubMed] [Google Scholar]
- (29).Siemieniuk RA, Agoritsas T, Manja V, Devji T, Chang Y, Bala MM, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis. BMJ (Online). 2016;354:i5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Garg A, Rao SV, Visveswaran G, Agrawal S, Sharma A, Garg L, et al. Transcatheter aortic valve replacement versus surgical valve replacement in low-intermediate surgical risk patients: a systematic review and meta-analysis. J Invasive Cardiol. 2017;29(6):209–16. [PubMed] [Google Scholar]
- (31).Singh K, Carson K, Rashid MK, Jayasinghe R, AlQahtani A, Dick A, et al. Transcatheter aortic valve implantation in intermediate surgical risk patients with severe aortic stenosis: a systematic review and meta-analysis. Heart Lung Circ. 2018;27(2):227–34. [DOI] [PubMed] [Google Scholar]
- (32).Sardar P, Kundu A, Chatterjee S, Feldman DN, Owan T, Kakouros N, et al. Transcatheter versus surgical aortic valve replacement in intermediate-risk patients: evidence from a meta-analysis. Catheter Cardiovasc Interv. 2017;90(3):504–15. [DOI] [PubMed] [Google Scholar]
- (33).Khan AR, Khan S, Riaz H, Luni FK, Simo H, Bin Abdulhak A, et al. Efficacy and safety of transcatheter aortic valve replacement in intermediate surgical risk patients: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;88(6):934–44. [DOI] [PubMed] [Google Scholar]
- (34).Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiology. 2017;2(8):837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Thourani VH, Forcillo J, Szeto WY, Kodali SK, Blackstone EH, Lowry AM, et al. Outcomes in 937 intermediate-risk patients undergoing surgical aortic valve replacement in PARTNER-2A. Ann Thorac Surg. 2018;105(5):1322–9. [DOI] [PubMed] [Google Scholar]
- (36).American Heart Association. Classes of heart failure [Internet]. Dallas (TX): American Heart Association; 2017. [cited 2018 Sep 5]. Available from: http://www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classesof-Heart-Failure_UCM_306328_Article.jsp#.WiAvz0qnGUk [Google Scholar]
- (37).Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–7. [DOI] [PubMed] [Google Scholar]
- (38).Reyes M, Reardon MJ. Transcatheter valve replacement: risk levels and contemporary outcomes. Methodist Debakey Cardiovasc J. 2017;13(3):126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kodali S, Thourani VH, White J, Malaisrie SC, Lim S, Greason KL, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37(28):2252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance. Appendix I: Quality appraisal checklist — economic evaluations [Internet]. London (UK): The Institute; 2012. [cited 2018 Sep 5]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [PubMed] [Google Scholar]
- (41).Kodera S, Kiyosue A, Ando J, Komuro I. Cost effectiveness of transcatheter aortic valve implantation in patients with aortic stenosis in Japan. J Cardiol. 2018;71(3):223–9. [DOI] [PubMed] [Google Scholar]
- (42).Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Koyama Y, et al. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. 2016;217:58–63. [DOI] [PubMed] [Google Scholar]
- (43).Watanabe Y, Kozuma K, Hioki H, Kawashima H, Nara Y, Kataoka A, et al. Comparison of results of transcatheter aortic valve implantation in patients with versus without active cancer. Am J Cardiol. 2016;118(4):572–7. [DOI] [PubMed] [Google Scholar]
- (44).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa (ON): The Agency; 2017. [Google Scholar]
- (45).Arnold SV, Reynolds MR, Wang K, Magnuson EA, Baron SJ, Chinnakondepalli KM, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US pivotal trial. JACC Cardiovasc Interv. 2015;8(9):1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol. 2012;60(6):548–58. [DOI] [PubMed] [Google Scholar]
- (47).Cohen DJ. Cost-effectiveness of trancatheter vs. surgical aortic valve replacement in intermediate risk patients: results from the PARTNER 2A and Sapien 3 intermediate risk trials. Paper presented at: TCT 2017 annual conference; 2017. Oct 31; Denver, CO.
- (48).Goodall G, Candolfi P, Zemanova B, Sohlberg A. The cost-effectiveness of TAVI in intermediate risk patients in France. Value Health. 2017;20(9):A588. [Google Scholar]
- (49).Ribera A, Slof J, Andrea R, Falces C, Gutierrez E, Del Valle-Fernandez R, et al. Transfemoral transcatheter aortic valve replacement compared with surgical replacement in patients with severe aortic stenosis and comparable risk: cost-utility and its determinants. Int J Cardiol. 2015;182:321–8. [DOI] [PubMed] [Google Scholar]
- (50).Attizzani GF, Alkhalil A, Padaliya B, Tam CC, Lopes JP, Fares A, et al. Comparison of outcomes of transfemoral transcatheter aortic valve implantation using a minimally invasive versus conventional strategy. Am J Cardiol. 2015;116(11):1731–6. [DOI] [PubMed] [Google Scholar]
- (51).Wijeysundera HC, Li L, Braga V, Pazhaniappan N, Pardhan AM, Lian D, et al. Drivers of healthcare costs associated with the episode of care for surgical aortic valve replacement versus transcatheter aortic valve implantation. Open Heart. 2016;3(2):e000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–84. [DOI] [PubMed] [Google Scholar]
- (53).Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2017;52(3):408–17. [DOI] [PubMed] [Google Scholar]
- (54).Arsalan M, Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol. 2016;13(6):360–7. [DOI] [PubMed] [Google Scholar]
- (55).Sulzenko J, Tousek P, Kocka V, Widimsky P. Transcatheter aortic valve implantation: long-term clinical outcome and valve durability. Expert Rev Med Devices. 2015;12(5):529–35. [DOI] [PubMed] [Google Scholar]
- (56).Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91. [DOI] [PubMed] [Google Scholar]
- (57).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act. Toronto (ON): Queen's Printer for Ontario; 2015. [Google Scholar]
- (58).Ailawadi G, LaPar DJ, Speir AM, Ghanta RK, Yarboro LT, Crosby IK, et al. Contemporary costs associated with transcatheter aortic valve replacement: a propensity-matched cost analysis. Ann Thorac Surg. 2016;101(1):154–60. [DOI] [PubMed] [Google Scholar]
- (59).Osnabrugge RL, Head SJ, Genders TS, Van Mieghem NM, De Jaegere PP, van der Boon RM, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg. 2012;94(6):1954–60. [DOI] [PubMed] [Google Scholar]
- (60).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (61).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. The patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (62).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario — final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2018 Sep]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (63).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients' experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser [Internet]. 2013. [cited 2018 Sep]; 13(15):[33 pp.]. Available from: http://hqontario.ca/Portals/0/Documents/evidence/reports/full-report-qualitative-cd-patients-experience-accessing-health-care-130906-en.pdf. [PMC free article] [PubMed]
- (64).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (65).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Miller WL, Crabtree BF. Thousand Oaks (CA): Sage Publications; 1999. p. 33–45. [Google Scholar]
- (66).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods Thousand Oaks (CA): Sage Publications; 1994. p. 23–41. [Google Scholar]
- (67).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- (68).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage Publications; 1998. [Google Scholar]
- (69).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2016 Jan]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (70).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (71).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Denzin NK, Lincoln YS. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]
- (72).Statistics Canada. Consumer Price Index: Table 18-10-0004-13 [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 July 11]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260020 [Google Scholar]