Abstract
Background
Single-sided deafness refers to profound sensorineural hearing loss or non-functional hearing in one ear, with normal or near-normal hearing in the other ear. Its hallmark is the inability to localize sound and hear in noisy environments. Conductive hearing loss occurs when there is a mechanical problem with the conduction of sound vibrations. Mixed hearing loss is a combination of sensorineural and conductive hearing loss. Conductive and mixed hearing loss, which frequently affect both ears, create additional challenges in learning, employment, and quality of life. Cochlear implants and bone-conduction implants may offer objective and subjective benefits of hearing for people with these conditions who are deemed inappropriate candidates for standard hearing aids and do not meet the current indication (i.e., bilateral deafness) for publicly funded cochlear implants in Canada.
Methods
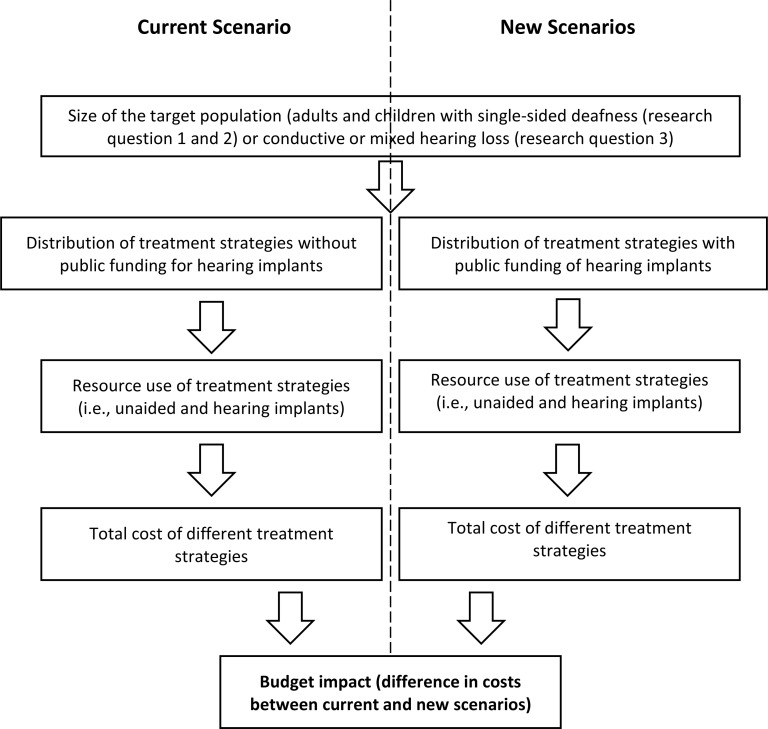
We conducted a health technology assessment, which included an evaluation of clinical benefits and harms, cost-effectiveness, budget impact, and patient preferences and values related to implantable devices for single-sided deafness and conductive or mixed hearing loss. We performed a systematic literature search for systematic reviews and cost-effectiveness studies of cochlear implants and bone-conduction implants, compared to no interventions, for these conditions in adults and children. We conducted cost-utility analyses and budget impact analyses from the perspective of the Ontario Ministry of Health to examine the impact of publicly funding both types of hearing implants for the defined populations. We also interviewed 22 patients and parents of children about their experience with hearing loss and hearing implants.
Results
We included 20 publications in the clinical evidence review. For adults and children with single-sided deafness, cochlear implantation when compared with no treatment improves speech perception in noise (% correct responses: 43% vs. 15%, P < .01; GRADE: Moderate), sound localization (localization error: 14° vs. 41°, P < .01; GRADE: Moderate), tinnitus (Visual Analog Scale, loudness: 3.5 vs. 8.5, P < .01; GRADE: Moderate), and hearing-specific quality of life (Speech Spatial and Qualities of Hearing Scale, speech: 5.8 vs. 2.6, P = .01; spatial: 5.7 vs. 2.3, P < .01; GRADE: Moderate); for children, speech and language development also improve (GRADE: Moderate). For those with single-sided deafness in whom cochlear implantation is contraindicated, bone-conduction implants when compared with no intervention provide clinically important functional gains in hearing thresholds (36–41 dB improvement in pure tone audiometry and 38–56 dB improvement in speech reception threshold, P < .05; GRADE: Moderate) and improve speech perception in noise (signal-to-noise ratio −2.0 vs. 0.6, P < .05 for active percutaneous devices; signal-to-noise ratio improved by 1.3–2.5 dB, P < .05 for active transcutaneous devices; GRADE: Moderate) and hearing-specific quality of life (Abbreviated Profile for Hearing Aid Benefit, ease of communication: 12%–53% vs. 24%–59%; background noise: 18%–48% vs. 33%–79%; listening in reverberant condition: 26%–55% vs. 41%–65%, P < .05 [active percutaneous devices]; ease of communication: 7% vs. 20%; background noise: 46% vs. 69%; listening in reverberant condition: 27% vs. 43%; P < .05 [active transcutaneous devices]; Children's Home Inventory for Listening Difficulties score 7.3 vs. 3.4; P < .05 [passive transcutaneous devices]; GRADE: Moderate). For those with conductive or mixed hearing loss, bone-conduction implants when compared with no intervention improve hearing thresholds (improved 19–45 dB [active percutaneous devices], improved 24–37 dB [active transcutaneous devices], improved 31 dB [passive transcutaneous devices], and improved 21–49 dB [active transcutaneous middle-ear implants]; GRADE: Moderate), speech perception (% correct: 77%–93% vs. < 25%; P < .05 [active transcutaneous devices], % speech recognition: 55%–98% vs. 0–72%; P < .05 [active transcutaneous middle-ear implants]; GRADE: Moderate), and hearing-specific quality of life and subjective benefits of hearing (GRADE: Moderate).
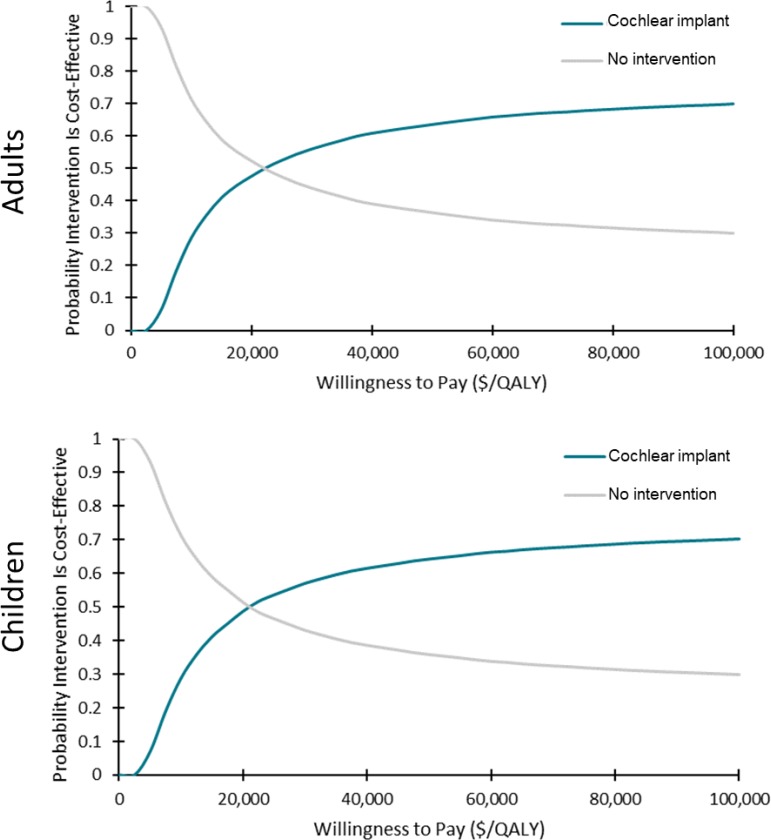
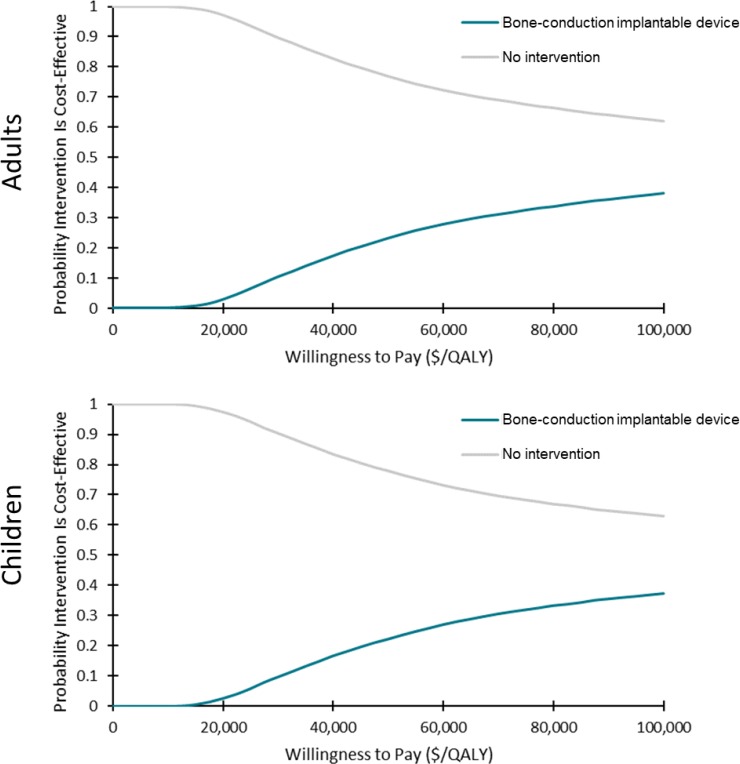
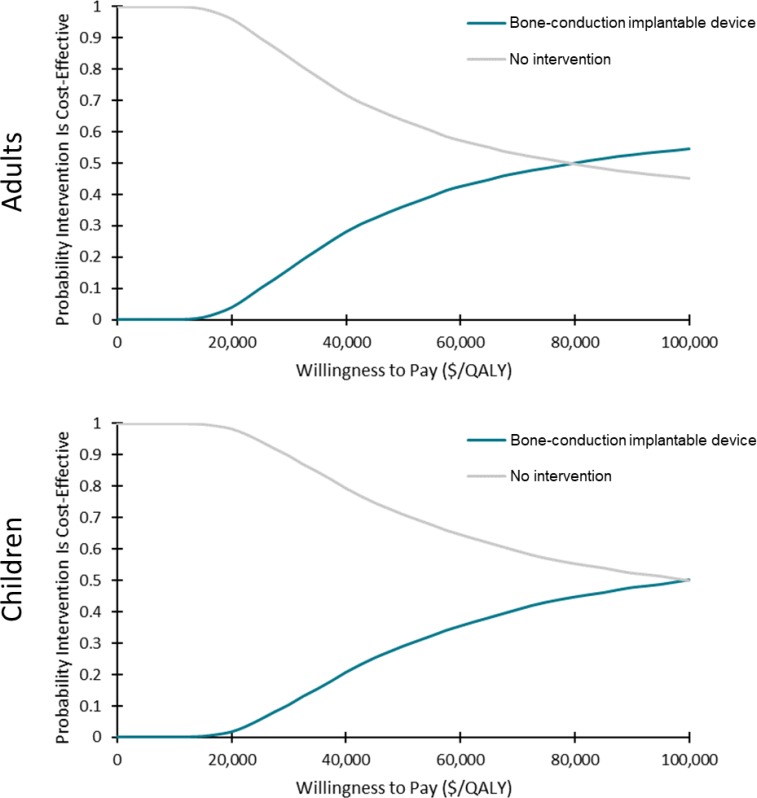
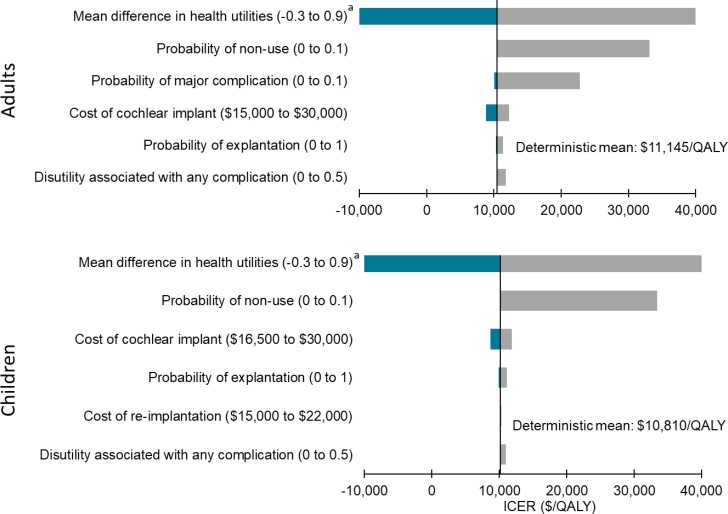
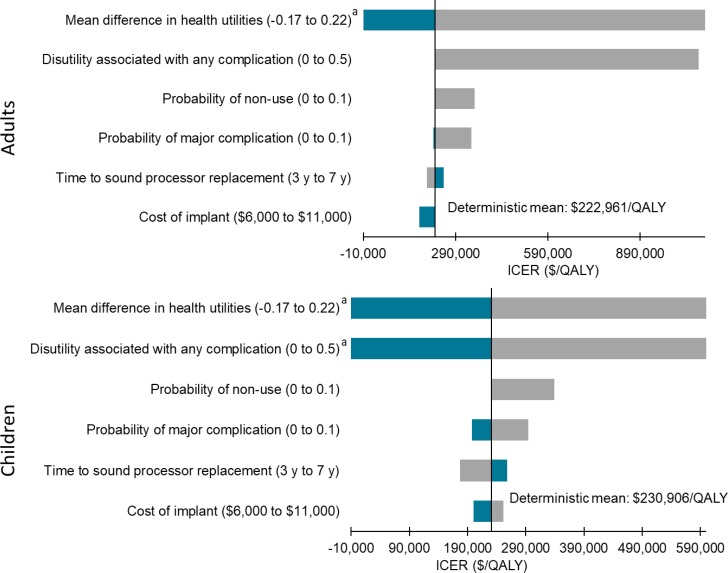
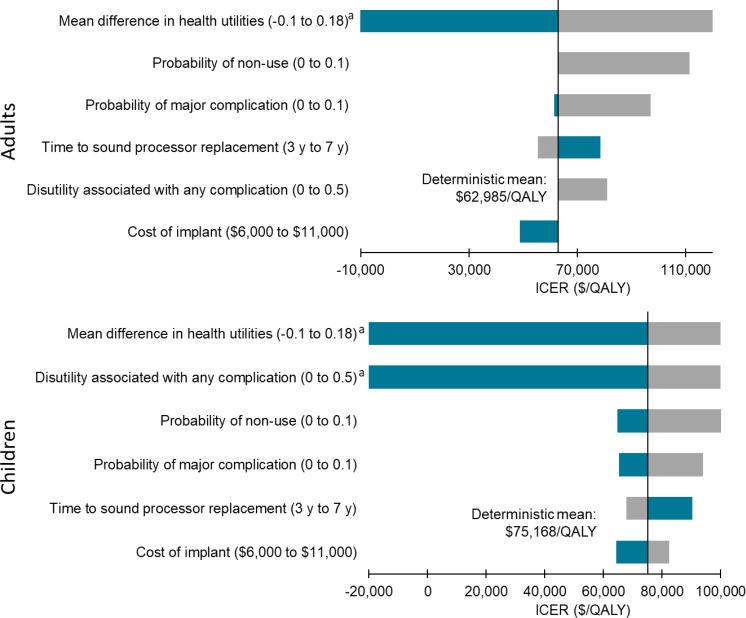
In the cost–utility analyses, cochlear implants for adults and children with single-sided deafness provided greater health gains for an incremental cost, compared with no intervention. On average, the incremental cost-effectiveness ratio (ICER) was between $17,783 and $18,148 per quality-adjusted life-year (QALY). At a willingness-to-pay of $100,000 per QALY, 70% of the simulations were considered cost-effective. For the same population, bone-conduction implants were not likely to be cost-effective compared with no intervention (ICER: $402,899–$408,350/QALY). Only 38% of simulations were considered cost-effective at a willingness-to-pay of $100,000 per QALY. For adults and children with conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no intervention (ICER: $74,155–$87,580/QALY). However, there was considerable uncertainty in the results. At a willingness-to-pay of $100,000 per QALY, only 50% to 55% of simulations were cost-effective. In sensitivity analyses, results were most sensitive to changes in health-related utilities (measured using generic quality-of-life tools), highlighting the limitations of currently published data (i.e., small sample sizes and short follow-up).
For people with single-sided deafness, publicly funding cochlear implants in Ontario would result in an estimated additional cost of $2.8 million to $3.6 million in total over the next 5 years, and an additional $0.8 million would be required for bone-conduction implants for this population. For people with conductive or mixed hearing loss, publicly funding bone-conduction implants would cost an estimated additional $3.1 million to $3.3 million in total over the next 5 years.
In interviews, people with single-sided deafness and conductive or mixed hearing loss reported that standard hearing aids did not meet their expectations; therefore, they chose to undergo surgery for an implantable device. Most participants with experience of a cochlear implant or bone-conduction implant spoke positively about being able to hear better and enjoy a better quality of life. People with a cochlear implant reported additional benefits: binaural hearing, better sound localization, and better hearing in noisy areas. Cost and access were barriers to receiving an implantable device.
Conclusions
Based on evidence of moderate quality, cochlear implantation and bone-conduction implants improve functional and patient-important outcomes in adults and children with single-sided deafness and conductive or mixed hearing loss. Qualitative results of interviews with patients are consistent with the findings of the systematic reviews we examined.
Among people with single-sided deafness, cochlear implants may be cost-effective compared with no intervention, but bone-conduction implants are unlikely to be. Among people with conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no intervention. Results and uncertainty are mainly driven by changes in health utilities associated with having a hearing implant. Hence, further research on utility values in this population is warranted with larger sample sizes and longer follow-up.
The 5-year cost of publicly funding both types of hearing implant for single-sided deafness and conductive or mixed hearing loss in Ontario is estimated to be $6.7 million to $7.8 million.
OBJECTIVE
This health technology assessment looked at the effectiveness, safety, and cost-effectiveness of cochlear implants and bone-conduction implants for adults and children with single-sided deafness and the use of bone-conduction implants for adults and children with conductive or mixed hearing loss. It also looked at the budget impact of publicly funding these implantable devices, as well as the preferences, values, and experiences of people with single-sided deafness, conductive hearing loss, or mixed hearing loss.
BACKGROUND
Health Condition
Single-Sided Deafness
Single-sided deafness is the most severe level of unilateral (single-sided) sensorineural hearing loss, which occurs when there is damage to the hair cells in the cochlea (the sensory organ in the inner ear) or to the neural pathways of hearing (the nerve pathways between the inner ear and the brain). Single-sided deafness is defined as having non-functional hearing or a hearing level of 90 decibels (dB HL) or greater in one ear, with normal or near-normal hearing in the other ear.1 In a hearing test, a person with single-sided deafness cannot hear in their deafened ear until the volume is at least 90 dB, about the noise level of a power lawn mower.
With one hearing ear and one deafened ear, people with single-sided deafness cannot separate sound and noise signals from spatially separated sources (squelch effect), and they do not have the doubling of auditory input (summation effect) that binaural (two-ear) hearing provides.2 In addition, the head creates a baffle or auditory shadow that blocks sounds from reaching the hearing ear (head shadow effect).3 These effects lead to the major deficits associated with single-sided deafness: people have difficulty localizing sound (identifying which direction it comes from) and perceiving speech, particularly in noisy environments or where multiple people are talking at once.
About 1 in 1,000 children is born with some degree of unilateral hearing loss.4,5 The prevalence of the condition increases with age to an estimate of more than 5% in school-aged children as a result of delayed-onset congenital hearing loss and acquired hearing loss.6 About 10% of children born with any degree of unilateral hearing loss eventually progress to bilateral hearing loss (hearing loss in both ears).7 In adults, acquired single-sided deafness is estimated to affect 12 to 27 per 100,000 people in the general population.8
The causes of congenital single-sided deafness in children include temporal bone abnormalities, cochlear dysplasia, cochlear nerve aplasia and hypoplasia, and congenital cytomegalovirus infection. Acquired single-sided deafness in children is caused by meningitis, head trauma, ear surgery, or ototoxic medications (drugs that cause damage to the inner ear).9,10 For adults, most acquired single-sided deafness arises suddenly and has an unknown cause, possibly due to viral or vascular injuries. Other causes include head trauma, ototoxic medications, viral infections, Meniere disease, and complications of surgery (e.g., removal of acoustic neuroma).11
Conductive Hearing Loss and Mixed Hearing Loss
Conductive hearing loss occurs when there is a mechanical problem with the conduction of sound vibrations in the external and middle ear. On testing, this results in an air-bone gap, a diagnostic term describing that the patient's hearing is weak when sound is transmitted through air but normal when transmitted via bone conduction, using a device that vibrates the bones in the head (bypassing the dysfunctional middle ear structures). Conductive hearing loss is defined as having a bone-conduction threshold of less than 20 dB HL with an air-conduction threshold of more than 20 dB HL, creating an air-bone gap of more than 10 dB. The degree of conductive hearing loss is determined by the difference between the air- and bone-conduction thresholds. The maximum air-bone gap possible is about 65 dB.
Mixed hearing loss is a combination of conductive hearing loss and sensorineural hearing loss. Mixed hearing loss occurs when the bone-conduction threshold is more than 20 dB HL and the air-bone gap is more than 10 dB.
Conductive hearing loss results from conditions that affect the ability of the outer or middle ear structures to transmit sound vibrations to the inner ear. Causes include middle ear fluids, trauma, infections, eardrum perforation, aural atresia (the congenital malformation of the ear canal and middle ear), cholesteatoma (a noncancerous skin growth that can destroy the middle ear structure), otosclerosis (abnormal bone growth) that results in stapes fixation (a condition in which the innermost bone in the middle ear cannot vibrate), and other malformations or discontinuities in the ossicles (the three small bones in the middle ear).
Conductive hearing loss accounts for 90% to 95% of all cases of childhood hearing loss, with middle ear effusion (a buildup of fluid behind the eardrum) among the most common causes. Conductive hearing loss is generally self-limiting and resolves over time with or without surgical interventions such as tympanostomy tubes.
Permanent, congenital, or acquired conductive hearing loss caused by obstruction, dysfunction, malformation, or destruction of the outer ear and/or middle ear structures (i.e., conditions such as aural atresia) is relatively rare but may cause lasting deficits in speech and language development and educational outcomes if not managed early.12
Cholesteatomas have both congenital and acquired causes, with the acquired form being associated with chronic otitis media (middle ear infection).13 In children with a history of chronic otitis media, approximately 0.1% to 2% will develop a cholesteatoma within 8 years.14
Otosclerosis initially leads to conductive hearing loss in the lower sound frequencies, but as the disease advances, it comes to affect all frequencies. It usually occurs between the ages of 15 and 40 years.15
Clinical Need and Target Population
Single-Sided Deafness
Single-sided deafness in children has a substantial negative impact on the developing auditory system and on spoken language development.16 Children with single-sided deafness are at higher risk of delayed speech-language development (trouble producing sounds and/or understanding speech), poor academic performance, behavioural problems, and decreased quality of life than their normal-hearing peers.17–20 These learning and psychosocial deficits are likely largely a result of impaired binaural hearing; children with single-sided deafness hear only about one-third of speech around them.21
In early childhood, single-sided deafness can lead to aural preference syndrome. This occurs when the developing auditory pathway reorganizes to prefer the hearing ear, leaving the deafened ear weakly represented in the auditory system.16 The resulting asymmetry makes it difficult for children to process cues about the timing and level of sounds, cues that would help them localize sound and perceive speech in noisy environments.16 Early restoration of hearing symmetry by ensuring both ears receive effective stimulation during a sensitive period of auditory development could secure the function of the deafened ear and restore binaural hearing.22
As noted, adults adapting to the loss of hearing in one ear experience difficulties localizing sound or conversing in an environment with background noise.11 These functional difficulties have been shown to affect social and psychological well-being. Social consequences include reduced social interaction and quality of life.23 Psychological impacts include worry about possible loss of hearing in the opposite ear, embarrassment related to the social stigma of hearing loss, and reduced confidence in one's ability to participate in social activities.24 In addition, listening fatigue plays a substantial role in the negative effects associated with single-sided deafness.25
Conductive Hearing Loss and Mixed Hearing Loss
People with conductive hearing loss perceive sounds as soft, due to dysfunction of the outer or middle ear structures that physically block part of the space within the ear. People with conductive hearing loss due to chronic drainage from middle ear effusion are not able to wear conventional hearing aids to amplify sounds. As with other types of disabling hearing loss, conductive and mixed hearing loss have significant impact on children's language development, educational outcomes, and social development.26,27 For adults, conductive and mixed hearing loss also compromise communication, psychosocial well-being, quality of life, and economic independence.28
Current Treatment Options
Single-Sided Deafness
Treatment for single-sided deafness can focus on redirecting sound to the hearing ear or on trying to revive the deafened ear, through the use of various devices. The choice of treatment depends on the cause and duration of deafness, and the person's needs. Two treatment options overcome the head shadow effect but do not restore binaural hearing: hearing aids that use a wireless microphone technology to divert sound from the deafened ear to the hearing ear (these are known as contralateral routing of signal, or CROS, hearing aids), and a bone-conduction device worn as a hearing aid or implanted into the skull to activate the hearing ear via bone vibration. A third option is to restore binaural hearing by stimulating the deafened ear directly through a cochlear implant (described below, Health Technology Under Review).
Conductive Hearing Loss and Mixed Hearing Loss
Depending on the underlying cause, conductive and mixed hearing loss can be treated medically or surgically. For a subgroup of people who do not benefit from conventional hearing aids, such as those with chronic drainage, congenital aural atresia, or an allergy to hearing aid materials, a bone-conduction implant is indicated to restore hearing.
Health Technology Under Review
This health technology assessment reviewed hearing loss treatment devices that are surgically inserted: cochlear implants and bone-conduction implants. We did not review nonsurgical options for the use of hearing devices, including CROS hearing aids, conventional hearing aids, and bone-conduction hearing aids.
Cochlear implants can be used to treat single-sided deafness. Bone-conduction implants can also be used to treat single-sided deafness and conductive or mixed hearing loss.
Cochlear Implants
The cochlea is a part of the inner ear composed of sensory cells (hair cells) that convert vibrations into neural messages, which are then passed to the auditory nerve and brain and perceived as sound. A cochlear implant bypasses the inner ear to stimulate the auditory nerve with electrical pulses. This is intended to stimulate the afferent auditory pathways (the nerves that carry sensory information from the inner ear up to the brain) and generate sound perception. The device is designed for people with severe to profound sensorineural hearing loss as a result of damage to the cochlea and/or its communication with the primary auditory nerve. People with this type of hearing loss typically still have enough primary auditory neurons to be stimulated by the electrical pulses. More central parts of the afferent auditory pathway, including the auditory cortex, can process the electrical input, translating it into detectible sound. In this way, cochlear implant users can learn to recognize speech, environmental sounds, noise, and music.
A cochlear implant system has two parts. The first part is an external wearable device that contains a microphone, a speech processor, a battery, and a transmitter. It detects sound and assesses its frequency and amplitude components over time. The second part, which is surgically implanted in the cochlea, has a series of electrical contacts placed along an array. The external equipment sends information about external sound to the internal components via radio frequency waves. Instructions are sent regarding which electrodes should provide electrical pulses and at what level over time. High frequencies are allocated to electrodes at the basal end (bottom) of the array with progressively lower frequencies allocated more apically (at the top end of the array).
Bone-Conduction Implants
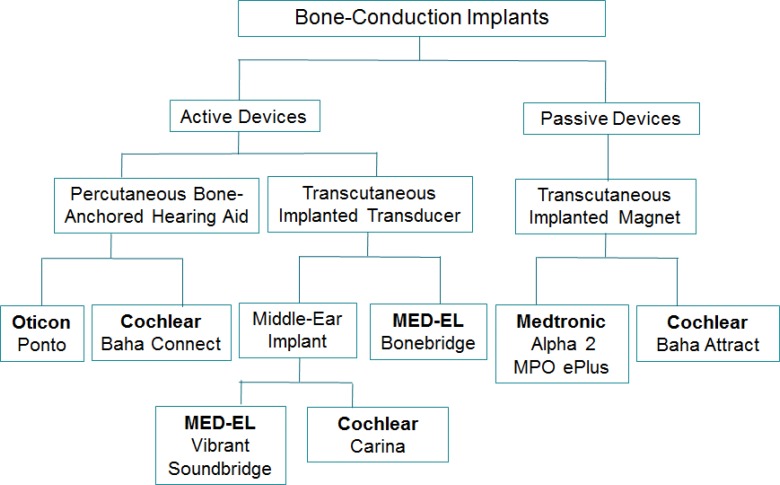
A number of implantable devices are currently available to achieve vibro-conduction bone stimulation for people with single-sided deafness or conductive or mixed hearing loss. These devices rely on the efficient transmission of sound into the cochlea through vibration of the skull or structures of the middle ear (i.e., the ossicular chain and the round window membrane). They are generally categorized as passive or active implants. Figure 1 shows the bone-conduction implant devices currently licensed by Health Canada.
Figure 1: Classification of Bone-Conduction Implants.
Passive Bone-Conduction Implants
A passive device has an implantable magnet unit that is osseointegrated (meaning it anchors to the bone) and an external device that drives sound vibration into the skull through the skin. Leaving the skin intact is clearly advantageous for the person; however, this type of device has limited gain (acoustic power), particularly in the high frequency range, compared with active devices. Currently available passive devices include the Cochlear Baha Attract and the Medtronic Alpha 2 MPO ePlus (formerly known as Medtronic Sophono Alpha 2 MPO). Based on their characteristics, passive devices are generally considered to be adequate and effective for conductive and mixed hearing loss, but of limited gain and function for single-sided deafness.
Active Bone-Conduction Implants
An active device enables sound transmission by directly coupling a transducer's vibro-acoustic properties with the inner ear, either via the skull bones or the structures of the middle ear. A transducer is a device that converts physical changes such as vibration into electrical signals, or vice versa.
The Cochlear Baha Connect and the Oticon Ponto are active percutaneous devices, meaning they include a component (called an abutment) that is placed through the skin. Outside the skull is a transducer that transmits sound by coupling to an osseointegrated screw system. These devices are commonly called bone-anchored hearing aids. The implant base of the Cochlear Baha Connect is a migrational platform that can change the device from being active to passive, or vice versa.
The MED-EL Bonebridge is an active transcutaneous device, meaning the implant remains under intact skin. It has an external wearable audio processor coupled to the skull with a magnet. The device records sound and converts it into signals that are then transferred through the skin to an internal implanted transducer. The implant is embedded in the temporal bone (surrounding the inner and middle ear) which converts the sound signals into mechanical vibrations that are transmitted to the surrounding bone. The bone conducts these vibrations to the inner ear where they are converted to nerve signals and transmitted as impulses to the auditory nerve. MED-EL Bonebridge is the only active transcutaneous bone-conduction implant available in Canada at present. This device received Health Canada approval in 2013. Since it is a relatively new device, there is not much published data available yet.
Middle ear implants are active transcutaneous devices that use vibro-conductive stimulation directed at the structures of the middle ear to achieve inner ear stimulation. This type of device is designed for those with mixed hearing loss, as the gain achieved (acoustic power) is higher. The MED-EL Vibrant Soundbridge has a similar design as the MED-EL Bonebridge; both are partially implantable with an external audio processor and an implant system which is surgically placed under the skin. The Vibrant Soundbridge device requires a more precise placement into structures of the middle ear. The Cochlear Carina is a fully implantable device with an internal microphone and a middle ear transducer attached to the stapes bone.
Regulatory Information
Cochlear implantation systems available in Canada come from at least four manufacturers: Advanced Bionics (Switzerland), Cochlear Limited (Australia), MED-EL AG (Austria), and Oticon Medical (Denmark). They are licensed by Health Canada as Class III devices.
Cochlear implants by MED-EL, Oticon, and Advanced Bionics are approved by Health Canada for treatment of single-sided deafness. As of March 2020, Cochlear Limited is preparing an application for Health Canada approval to use its cochlear implants to treat single-sided deafness. The Cochlear Carina middle ear implant is not being used or promoted for clinical practice in Canada; it has been used only in research. Its Health Canada licence was discontinued in September 2018.
Table 1 lists the bone-conduction implants licensed by Health Canada as Class III devices.
Table 1:
Manufacturer Information on Bone-Conduction Implants Licensed for Use in Canada
| Device Name | Manufacturer | Health Canada Licence Number |
|---|---|---|
| Passive transcutaneous bone-conduction implants | ||
| Baha Attract | Cochlear Limited | 11960 |
| Alpha 2 MPO ePlus | Medtronic | 87657 |
| Active percutaneous bone-conduction implants | ||
| Baha Connect | Cochlear Limited | 11960 |
| Ponto | Oticon Medical | 83679 |
| Active transcutaneous bone-conduction implants | ||
| Bonebridge | MED-EL AG | 90672 |
| Active transcutaneous middle ear implants | ||
| Carina | Cochlear Limited | 87848 |
| Vibrant Soundbridge | MED-EL AG | 74428 |
Ontario Context
In Canada, most provincial ministries have created mechanisms to fund bone-conduction implants for conductive hearing loss in both ears. Implantable devices for single-sided deafness, including cochlear implants and bone-conduction implants, are not publicly funded anywhere in Canada.
In Ontario, cochlear implants are publicly funded for adults and children with severe to profound bilateral sensorineural hearing loss. Historically, one cochlear implant has been publicly funded; more recently, Health Quality Ontario has recommended that a second implant also be publicly funded.29 Cochlear implants are not publicly funded for people with single-sided deafness. Bone-conduction implants are not publicly funded in Ontario for any type of hearing loss. A small number of people have received these devices funded by philanthropy, research grants, or from hospital budgets. In addition to the four implant centres in the Ontario Cochlear Implant Program, one community hospital has been implanting bone-conduction devices for adults with conductive hearing loss.
According to the Ontario Cochlear Implant Program's candidacy guidelines, there are three clinical indications for cochlear implantation in adults with single-sided deafness: (1) single-sided deafness due to acute or chronic causes (e.g., auto-immune disease, idiopathic viral neuropathy, acoustic neuroma or other intracranial tumors) where the other ear is at risk of future deterioration, (2) single-sided deafness from subacute or chronic inner ear disease, where other forms of sound amplification have been unsuccessful (i.e., CROS aids, bone-conduction hearing aids), and (3) a duration of deafness less than 10 years. To have good hearing outcomes, patients must be willing to participate in a program of auditory rehabilitation (speech and sound exercises). In children with single-sided deafness, the duration of deafness (i.e., less than 4 years) and etiology of hearing loss (e.g., meningitis) are major factors to consider for cochlear implantation. The Ontario Cochlear Implant Program estimates the clinical need for cochlear implants for adults and children with single-sided deafness to be 24 devices per year.
Some people with single-sided deafness are not candidates for a cochlear implant, such as those with cochlear nerve aplasia and those whose inner ear is contraindicated for implantation (e.g., prior surgical removal of an acoustic neuroma). These people may be considered for bone-conduction implants to restore hearing. However, a meta-analysis found that approximately 50% of people who tried a bone-conduction implant were not using it after a trial period.30 Therefore, only people with a reasonably successful CROS trial and realistic expectation of improved hearing, and for whom a cochlear implant is not an option, would be considered eligible for a bone-conduction implant. The Ontario Cochlear Implant Program estimates the clinical need for bone-conduction implants for adults and children with single-sided deafness who are contraindicated for cochlear implantation to be 11 devices per year.
For people with conductive or mixed hearing loss, candidates for bone-conduction implants are those who would benefit from sound amplification but cannot use conventional air-conduction hearing aids. Candidacy is based on the person's hearing profile, age, needs, perceived risks, and preference. According to Health Canada's indications, the minimum age for children to receive a bone-conduction implant is 5 years old. The Ontario Cochlear Implant Program estimated the clinical need for bone-conduction implants for adults and children with conductive or mixed hearing loss to be 57 devices per year in both implant centres and community hospitals.
In the United Kingdom, bone-conduction implants have been routinely used and funded for more than 30 years for anyone with any type of hearing loss. The National Health Service (NHS) England clinical commissioning policy on bone-conduction implants states that, despite a lack of high-quality evidence, they are the only treatment option to restore hearing in a small number of patients and it is not appropriate to conduct randomized controlled trials for the clinical conditions that warrant the use of these implants.31
CLINICAL EVIDENCE
Research Questions
-
1.
What are the clinical benefits and harms of cochlear implants in adults and children with single-sided deafness?
-
2.
What are the clinical benefits and harms of bone-conduction implants in adults and children with single-sided deafness?
-
3.
What are the clinical benefits and harms of bone-conduction implants in adults and children with conductive or mixed hearing loss?
Methods
We conducted an overview of systematic reviews. We developed the research questions in consultation with patients, health care providers, clinical experts, and other health system stakeholders.
Clinical Literature Search
We performed a clinical literature search on January 4, 2018, to retrieve studies published from database inception until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database (NHS EED).
Medical librarians developed the search strategy using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. A search filter was applied to limit results to systematic reviews, meta-analyses, and health technology assessments. The final search strategy was peer-reviewed using the PRESS Checklist.32 We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment review.
We performed targeted grey literature searching of health technology assessment agency websites and the PROSPERO register of systematic reviews. See Appendix 1 for the literature search strategies, including all search terms.
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts and obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. We also examined reference lists for any additional relevant studies not identified through the literature search.
Inclusion Criteria
Studies
English-language full-text publications
-
Systematic reviews of any study designs if they met all of the following criteria:
-
–
Specified clearly defined review questions and inclusion and exclusion criteria
-
–
Used a reproducible literature search strategy on two or more electronic databases
-
–
Assessed and reported the methodological quality of the included studies
-
–
Participants
Adults and children with single-sided deafness
Adults and children with conductive or mixed hearing loss
Comparators
No treatment
No conventional or bone-conduction hearing aids
Interventions
Cochlear implants for single-sided deafness
Bone-conduction implants for single-sided deafness and conductive or mixed hearing loss
-
–
Passive transcutaneous bone-conduction implants
-
–
Active percutaneous bone-conduction implants
-
–
Active transcutaneous bone-conduction implants
-
–
Active transcutaneous middle ear implants
Outcomes of Interest
Speech perception
Sound localization
Tinnitus (adults)
Subjective benefits of hearing (patient-reported outcomes)
Quality of life
Adverse events
Speech and language development (children)
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information about the following, when available and applicable:
Source (i.e., citation information)
Methods (i.e., study design, literature search date and databases used, population, interventions, comparators, and method of quality assessment)
Outcomes (i.e., outcomes measured, number of studies for each outcome, quality assessment, outcome definition and source of information, unit of measurement, numeric data on results if reported, description of direction of results if numeric data not reported)
We extracted data relevant to the research questions and comparators and only on devices currently available in Canada. We considered cochlear implants and bone-conduction implants as a class of technology instead of reviewing the devices of individual manufacturers, implant models, or sound processors.
Statistical Analysis
Since this is an overview of systematic reviews, we did not pool the results of the included systematic reviews. Instead, we undertook a qualitative analysis, summarized the results in tables, and described them in the text.
Evidence Synthesis
Data on clinical benefits and harms were tabulated from the published systematic reviews, without reviewing primary studies. If the systematic reviews did not report numeric data from primary studies, we report the outcomes using descriptions such as “no change,” “deterioration,” or “improvement.”
In assessing functional gains in hearing (the measurement of hearing improvement with the use of hearing aids or devices), an improvement of 10 to 15 dB in pure tone average or speech recognition thresholds or an improvement of 10% to 15% of speech discrimination score is generally considered clinically important.33 However, the size of the air–bone gap in conductive or mixed hearing loss directly affects the functional gains.34 For studies that evaluated signal-to-noise ratio in the context of an adaptive hearing test, an improvement of 2 to 3 dB is generally considered clinically important.33
Critical Appraisal of Evidence
We assessed risk of bias of the included systematic reviews using the Risk of Bias in Systematic Reviews (ROBIS) tool35 (Appendix 2). The ROBIS tool includes four key domains: study eligibility criteria; identification and selection of studies; data collection and study appraisal; and synthesis and findings.
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Handbook.36 We used the review authors' quality measures as a guide to assess the overall risk of bias. In particular, we assessed adequate adjustment of confounding and loss to follow-up in observational studies. We determined precision from the presence of a treatment effect and statistical significance reported in the included reviews. We assessed directness based on the studies' target populations and interventions. We assessed consistency by looking at the overall direction of results and the similarity of point estimates across the included reviews. We determined the presence of publication bias by looking at the proportion of small studies and industry-sponsored studies.
The quality score reflects our assessment of the reliability of the evidence. We considered an upgrade of the evidence based on magnitude of effect, dose response, and direction of bias.
Expert Consultation
We consulted clinical experts in otology and audiology from November 2017 to September 2018. Our consulted experts provided advice on research questions, review methods and review results, and helped to place the evidence in clinical context.
Results
Literature Search
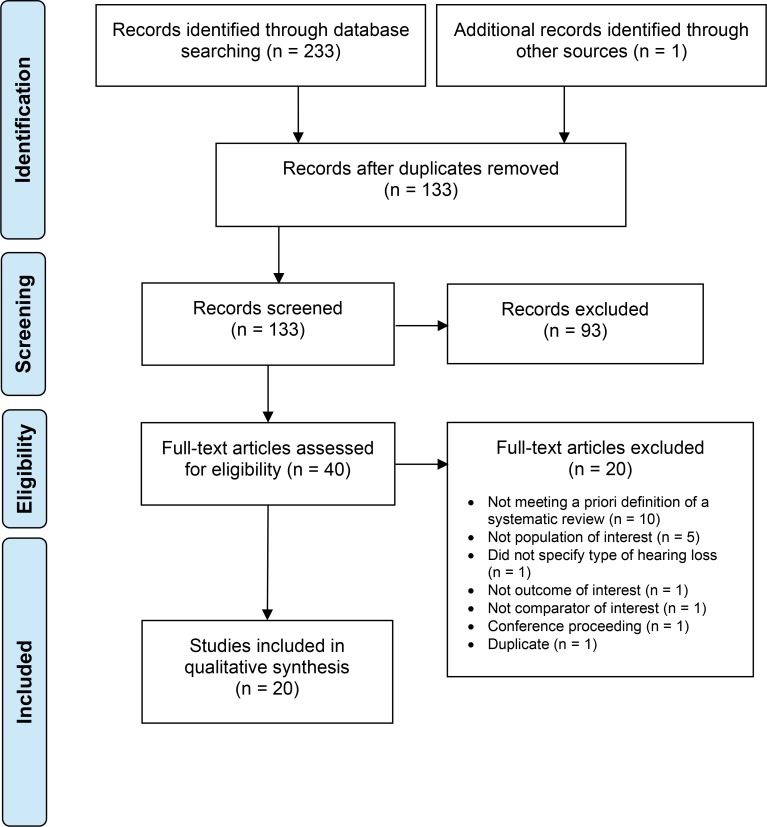
The literature search yielded 133 citations, after removing duplicates. Twenty systematic reviews on clinical benefits met the inclusion criteria. We reviewed the reference lists of the included systematic reviews but did not identify any additional relevant systematic reviews. Appendix 3 provides a list of excluded systematic reviews with reasons for exclusion. Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.37
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
The systematic literature search did not identify any relevant studies that addressed the complications of cochlear implantation. The clinical epidemiologist searched PubMed, Embase and Trip databases from inception to January 2018 using keywords related to complications of cochlear implantation (i.e., complications, adverse events, device failure) and used cross-referencing and input from experts to identify studies on the complications of cochlear implantation. The results of this targeted supplementary literature search retrieved studies to inform the section on the safety of cochlear implantation in the clinical evidence review.
Characteristics of Included Systematic Reviews
We identified 20 systematic reviews that evaluated the clinical benefits of implantable devices for single-sided deafness and/or conductive or mixed hearing loss. Table 2 describes the number of included reviews with respect to their interventions and populations.
Table 2:
Interventions and Populations of Included Systematic Reviews
| Intervention and Population(s) | Number of Reviews | Reference(s) |
|---|---|---|
| Cochlear implants for single-sided deafness | 3 | Peters et al, 201638 van Zon et al, 201539 Vlastarakos et al, 201440 |
| Cochlear implants and bone-conduction implants for single-sided deafness | 1 | Kitterick et al, 201641 |
| Bone-conduction implants for single-sided deafness and conductive or mixed hearing loss | 2 | Mandavia et al, 201742 Sprinzl and Wolf-Magele, 201643 |
| Bone-conduction implants for single-sided deafness | 3 | Appachi et al, 201744 Kim et al, 201745 Peters et al, 201546 |
| Bone-conduction implants for conductive or mixed hearing loss | 10 | University of Alberta, 201147 Australia Medical Services Advisory Committee, 201034 Bezdjian et al, 201748 Colquitt et al, 201149 Danhauer et al, 201050 Ernst et al, 201651 Johnson et al, 200652 Klein et al, 201253 Medical Advisory Secretariat, 200254 Verhaert et al, 201355 |
| Complications of osseointegrated hearing aids | 1 | Kiringoda and Lustig, 201356 |
The included systematic reviews report varied outcome measures. For consistency, we grouped outcomes into audiometry (i.e., hearing thresholds, functional gains), speech audiometry (i.e., speech discrimination, speech recognition, speech perception in quiet and noise), sound localization, tinnitus, hearing-specific quality of life (i.e., patient satisfaction, subjective benefits of hearing), speech and language development in children, and adverse events.
Methodological Quality of Included Systematic Reviews
Appendix 2, Table A1, presents results of our risk of bias assessment for the included systematic reviews. Seven34,40,43,50,54–56 of the 20 systematic reviews were rated as having high risk of bias. The main source of bias was single reviewer in study selection and/or data extraction. Other sources of bias included unclear dates of literature search or the number of databases searched.
Cochlear Implants: Effectiveness for Single-Sided Deafness
Four systematic reviews were on cochlear implantation for single-sided deafness,38–41 and Table 3 summarizes their results. Two of these reviews included studies in adults only,39,41 one included studies in children only,38 and one included studies in both adults and children.40 The characteristics of the included systematic reviews are summarized in Appendix 4.
Table 3:
Summary of Results of Systematic Reviews on Cochlear Implants vs. No Treatment for Single-Sided Deafness in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Speech audiometrya | |||
| Kitterick et al, 201641 | 4 | Significant improvement in speech perception in noise when the implanted ear had a more favorable SNR (numeric data not shown) | Low–moderate quality |
| Peters et al, 201638 | 4 | Improvement in speech perception in noise in most patients (numeric data could not be summarized)b | Directness of evidence: low–moderate Risk of bias: moderate–high |
| van Zon et al, 201539 | 6 | Significant improvement in speech perception in noise when noise is from the better ear side and speech from the cochlear implant side (correctly repeated HSM 42.5% vs. 14.6%, P < .01) | Directness of evidence: moderate–high Risk of bias: moderate–high |
| Improvement or no change in other testing configurations | |||
| Vlastarakos et al, 201440 | 7 | Improvement in speech perception in noise when noise is from the front or the deafened eara (numeric data not shown) | Strength of recommendation: B (directly based on category II evidence or extrapolated recommendations from category I evidence) |
| Sound localizationa | |||
| Kitterick et al, 201641 | 3 | Improvement in sound localizationb (numeric data not shown) | Low–moderate quality |
| Peters et al, 201638 | 4 | Significant improvement in sound localization in most patients (RMS 14° vs. 41°, P < .05) | Directness of evidence: low– moderate Risk of bias: moderate–high |
| van Zon et al, 201539 | 2 | Significant improvement in sound localization (RMS 15° vs. 34°, P < .01) | Directness of evidence: moderate– high Risk of bias: moderate–high |
| Vlastarakos et al, 201440 | 6 | Improvement in sound localizationb (numeric data not shown) | Strength of recommendation: B (directly based on category II evidence or extrapolated recommendations from category I evidence) |
| Tinnitusa | |||
| van Zon et al, 201539 | 6 | Significant suppression of tinnitus loudness and distress (VAS loudness 3.5 vs. 8.5, P < .01) | Directness of evidence: moderate–high Risk of bias: moderate–high |
| Significant decrease in TRQ score (range 77%–100%) | |||
| Vlastarakos et al, 201440 | 8 | Tinnitus improved in 95% of patients | Strength of recommendation: B (directly based on category II evidence or extrapolated recommendations from category I evidence) |
| Hearing-specific quality of lifea | |||
| Kitterick et al, 201641 | 5 | Significant benefits on subjective benefits of hearing measured by SSQ (speech: 1.0 [range 0.6–1.5], spatial: 1.3 (0.7–1.9), qualities: 0.6 [0.1–1.0])c | Low–moderate quality |
| Peters et al, 201638 | 1 | Significant improvement in subjective benefits of hearing measured by child and parent version of SSQ (numeric data not shown) | Directness of evidence: high Risk of bias: moderate—high |
| van Zon et al, 201539 | 3 | Significant improvement in subjective benefits of hearing measured by SSQ (speech: 5.8 vs. 2.6; P = .01; spatial: 5.7 vs. 2.3; P < .01) | Directness of evidence: moderate–high Risk of bias: moderate–high |
| Vlastarakos et al, 201440 | 5 | Improvement in speech and spatial components of the SSQ (numeric data not shown)b | Strength of recommendation: B (directly based on category II evidence or extrapolated recommendations from category I evidence) |
| Speech and language developmenta | |||
| Peters et al, 201638 | 1 | Improvement in CAP-II scores and SIR scores in all childrenb | Directness of evidence: moderate Risk of bias: high |
Abbreviations: CAP-II, Categories of Auditory Performance II; HSM, Hochmair-Schulz-Moser sentence test; RMS, root mean square; SIR, Speech Intelligibility Rating; SNR, signal-to-noise ratio; SSQ, Speech, Spatial and Qualities of Hearing Scale; TRQ, Tinnitus Reaction Questionnaire; VAS, Visual Analog Scale.
Outcomes of speech audiometry, sound localization, and quality of life were for adults and children; outcome of tinnitus was for adults only; outcome of speech and language development was for children only.
Some results were statistically significant while others were not significant or not reported.
Effect sizes are reported as standardized mean differences (SMDs) that express pre–post differences as a multiple of their standard deviations. Positive SMDs indicate more favorable outcomes with the intervention.
Speech Audiometry
Four systematic reviews reported on speech perception in noise in adults and children.38–41 Speech perception in noise was measured using different spatial locations of speech and noise stimuli. All included studies measured the S0N0 configuration, meaning both speech and noise are presented from the front. Outcomes were reported as either the signal-to-noise ratio (in dB) at which participants correctly understood 50% of the speech presented, or the total percentage of correctly repeated words. Despite varied test configurations and results, across reviews there was an overall improvement of speech perception in noise after cochlear implantation.
The quality of the evidence for speech audiometry was moderate (Appendix 2, Table A2).
Sound Localization
Four systematic reviews reported on sound localization in adults and children.38–41 Although all studies used different test set-ups, they all used localization error as the outcome measure. Localization error is the mean difference in degrees between the location of the sound source and the source indicated by the patient. All included studies consistently showed an improvement in sound localization after cochlear implantation.
The quality of the evidence for sound localization was moderate (Appendix 2, Table A2).
Tinnitus
Two systematic reviews reported on tinnitus (perceived noise or ringing in the ear) in adults.39,40 Several subjective scales, including the Visual Analog Scale and Tinnitus Reaction Questionnaire, were used to assess tinnitus distress or loudness. All studies showed a reduction of tinnitus after cochlear implantation.
The quality of the evidence for tinnitus was moderate (Appendix 2, Table A2).
Hearing-Specific Quality of Life
Four systematic reviews reported subjective benefits of hearing as a measure of hearing-specific quality of life in adults and children.38–41 All studies measured subjective benefits of hearing using the Speech, Spatial and Qualities of Hearing Scale (SSQ). For studies in children, the child and parent versions of the SSQ were used.38 Subjective benefits of hearing consistently improved after cochlear implantation.
The quality of the evidence for hearing-specific quality of life was moderate (Appendix 2, Table A2).
Speech and Language Development
One systematic review measured speech and language development in children using the Categories of Auditory Performance II (CAP-II) and Speech Intelligibility Rating (SIR) scores.38 There was an improvement in CAP-II and SIR scores after cochlear implantation.
The quality of the evidence for speech and language development was moderate (Appendix 2, Table A2).
Cochlear Implants: Safety
None of the included systematic reviews on cochlear implantation in single-sided deafness reported adverse events. The supplementary search conducted by the clinical epidemiologist identified four observational studies on complications of cochlear implantation, described below.
A retrospective analysis of 500 consecutive cochlear implantations (178 in adults, 322 in children) from 1989 to 2006 reported an overall rate of complications of 16%. Revision surgery was performed in 10.2% of cases, with the remaining 5.8% managed medically. Among these complications, 7.2% involved re-implantation, 3.2% were major complications, and 5.6% were minor complications. Reasons for revision surgery included device failure, infection, and trauma. Major complications were meningitis and surgery without re-implantation. Minor complications were transient facial palsy, wound hematoma, tinnitus, and infections that resolved with medical treatment.57
In a retrospective analysis of 403 cochlear implantation (168 in adults, 235 in children) between 1993 and 2013, the overall complication rate was 19.9%. Among these, 5% were major complications requiring surgical revision or hospitalization (e.g., device failure) and 14.9% were minor complications requiring conservative management (e.g., infection and vertigo).58
A retrospective review of 2,827 cochlear implantations performed in 2,311 patients between 1982 and 2011 found 235 cases of revision surgery, and device failure accounted for 57.8% of these surgeries. Overall rates of revision surgery and device failure were 8.3% and 4.8%, respectively.59 One study reported a very low rate of re-implantation (2.9%) among 971 devices implanted in 738 children from 1990 to 2010.60
Based on this existing evidence, cochlear implantation surgery is reasonably safe.
Bone-Conduction Implants: Effectiveness for Single-Sided Deafness
Six systematic reviews examined three types of bone-conduction implants for single-sided deafness, including active percutaneous implants (also known as bone-anchored hearing aids), active transcutaneous implants, and passive transcutaneous implants.41–46 The results for each type of device are described separately below. The characteristics of the included systematic reviews are summarized in Appendix 4.
Mandavia et al42 presented the body of evidence available to inform the current UK national policy on bone-conducting hearing devices. This systematic review included 39 studies that evaluated all types of bone-conduction implants for adults and children with single-sided deafness or conductive or mixed hearing loss. It tabulated the overall results of the included studies, instead of by individual outcomes. Therefore, we could not include this systematic review in the tables below, which summarize results by devices and outcomes. The review showed consistent benefits of bone-conducting devices in improving objective and subjective hearing outcomes across studies, given the appropriate indications. Using the GRADE system, the evidence was classified as very low quality. The authors downgraded the quality of evidence from low (observational studies) to very low because of significant limitations: the quality of methodology, consistency of results across studies, limited generalizability, and limited effect size. This systematic review included studies of all designs (e.g., case series, systematic reviews), took an overview approach to summarizing the results and did not grade the quality of evidence by outcomes, which made it difficult to delineate the certainty of the body of evidence and the magnitude of effects.
Kitterick et al41 reviewed hearing instruments for single-sided deafness in adults. The review did not specify the type of implants and reported outcomes of bone-conduction implants as a whole; consequently, these results also could not be included in the tables below. Four studies reported that bone-conduction implants significantly improved speech perception in noise, when noise was presented from the hearing ear. Nine studies showed significant benefits of hearing from bone-conduction implants measured by the Abbreviated Profile for Hearing Aid Benefit. Five studies showed no significant difference in sound localization when comparing bone-conduction implants with no treatment. Two studies reported complication rates of 38% and 13%, all related to skin reactions around abutment sites of bone-anchored hearing aids, and all resolved with medical treatment.
Active Percutaneous Bone-Conduction Implants: Effectiveness for Single-Sided Deafness
Two models of bone-anchored hearing aids (Cochlear Baha Connect and Oticon Ponto) are currently available in Canada. Two systematic reviews reported on these implants for single-sided deafness.45,46 One review was on adults and children45 while the other was on adults only.46 Table 4 summarizes the results.
Table 4:
Summary of Results on Active Percutaneous Bone-Conduction Implants vs. No Treatment for Single-Sided Deafness in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Speech audiometry | |||
| Kim et al, 201745 | 12 | Significant improvement for speech perception in noise in S0N0 configuration (SNR −2.0 vs. 0.6)a | NR |
| Peters et al, 201546 | 3 | Improvement (SpeN0: dB SNR −0.3 vs. 2.2)a or no differences (S0Npe: dB SNR −5.5 vs. −7.1)a in speech perception in noise depending on testing configurationsb | Directness of evidence: moderate–high Risk of bias: low–moderate |
| Sound localization | |||
| Kim et al, 201745 | 6 | No significant difference in sound localization (% correct identification: 15–69 vs. 13–66) | NR |
| Peters et al, 201546 | 4 | No significant difference in sound localization (% correct identification: 17–59 vs. 18–61) | Directness of evidence: moderate–high Risk of bias: low–moderate |
| Hearing-specific quality of life | |||
| Kim et al, 201745 | 12 | Significant improvement in subjective benefits and satisfaction measured by APHAB (EC: 12%–53% vs. 24%–59%; BN: 18%–48% vs. 33%–79%; RV: 26%–55% vs. 41%–65%) | NR |
| Peters et al, 201546 | 4 | Improved subjective benefits of hearing measured by APHABb (numeric data for unaided condition not shown) | Directness of evidence: moderate–high Risk of bias: low–moderate |
Abbreviations: APHAB, Abbreviated Profile of Hearing Aid Benefit; BN, background noise; EC, ease of conversation, NR not reported; RV, listening in reverberant condition; SNR, signal-to-noise ratio.
The lower the SNR, the better the hearing.
Some results were statistically significant while others were not significant or not reported.
Speech Audiometry
Two systematic reviews reported speech perception in noise.45,46 Studies measured speech perception in noise in various configurations of spatially separated speakers, with sound and noise coming from the side of the better ear, the deafened ear, or from the front. Outcomes were reported as either the signal-to-noise ratio (in dB) at which 50% of speech was understood correctly, or the total percentage of correctly repeated words. One review of 12 studies in adults and children45 showed significant improvement in speech perception in noise, while another review of three studies in adults46 showed improvement or no differences, depending on where the noise originated.
The quality of the evidence for speech audiometry was moderate (Appendix 2, Table A3).
Sound Localization
Two systematic reviews reported on sound localization in adults and children.45,46 Although the included studies used different test set-ups, they all used localization error (in degree) as the outcome measure. Both reviews concluded no significant improvement in sound localization after implantation of bone-anchored hearing aids. These results were expected because bone-conduction implants do not restore binaural hearing which is necessary to locate the direction of sound.
The quality of the evidence for sound localization was moderate (Appendix 2, Table A3).
Hearing-Specific Quality of Life
Two systematic reviews reported subjective benefits of hearing and patient satisfaction as measures of hearing-specific quality of life in adults and children.45,46 Various questionnaires were used to measure these outcomes, most frequently the Speech, Spatial and Qualities of Hearing Scale and the Abbreviated Profile of Hearing Aid Benefit. All included studies showed improvement in subjective benefits of hearing and patient satisfaction with bone-anchored hearing aids.
The quality of the evidence for hearing-specific quality of life was moderate (Appendix 2, Table A3).
Active Transcutaneous Bone-Conduction Implants: Effectiveness for Single-Sided Deafness
One systematic review reported clinical outcomes of the Bonebridge device for single-sided deafness in adults and children43 (Table 5). Bonebridge improved speech perception in noise and demonstrated subjective benefits of hearing and patient satisfaction when compared with no treatment.
Table 5:
Summary of Results on Active Transcutaneous Bone-Conduction Implants vs. No Treatment for Single-Sided Deafness in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Speech audiometry | |||
| Sprinzl and Wolf-Magele, 201643 | 2 | Significant improvement in SNR by 1.3–2.5 dBa depending on where the noise originated | Low quality |
| Hearing-specific quality of life | |||
| Sprinzl and Wolf-Magele, 201643 | 1 | Significantly improved subjective benefits of hearing measured by APHAB (EC: 7% vs. 20%; BN: 46% vs. 69%; RV: 27% vs. 43%) | Low quality |
| 2 | Improved patient satisfaction measured by HDSS and GBIb (numeric data not shown) | ||
Abbreviations: APHAB, Abbreviated Profile of Hearing Aid Benefit; BN, background noise; EC, ease of conversation; GBI, Glasgow Benefit Inventory; HDSS, Hearing Device Satisfaction Scale; SNR, single-to-noise ratio; RV, listening in reverberant condition.
An improvement of 2–3 dB in SNR in adaptive hearing test is considered clinically important.
Statistical significance not reported.
The quality of the evidence was moderate for speech audiometry and hearing-specific quality of life (Appendix 2, Table A3).
Passive Transcutaneous Bone-Conduction Implants: Effectiveness for Single-Sided Deafness
Two passive transcutaneous bone-conduction implants (Cochlear Baha Attract and Medtronic Alpha 2 MPO ePlus (formerly known as Medtronic Sophono Alpha) are currently available in Canada. One systematic review reported clinical outcomes of these devices for children with single-sided deafness44 (Table 6). Passive transcutaneous implants improved objective audiological measures including speech recognition threshold, pure tone average, and word recognition scores. In addition, functional auditory outcomes measured using the Children's Home Inventory for Listening Difficulties were improved.
Table 6:
Summary of Results on Passive Transcutaneous Bone-Conduction Implants vs. No Treatment for Single-Sided Deafness in Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Audiometry | |||
| Appachi et al, 201744 | 3 | Significant improvement in pure tone average (average 36–41 dB) and speech reception threshold (average 38–56 dB)a | Moderate risk of bias |
| Speech audiometry | |||
| Appachi et al, 201744 | 3 | Improvement in word recognition scores (HINT-C mean scores 81% vs. 38% at SNR 0 dB and 100% vs. 71% at SNR 10 dB; correctly repeated phonemes 74% vs. 51%)b | Moderate risk of bias |
| Hearing-specific quality of life | |||
| Appachi et al, 201744 | 3 | Significant benefits in functional auditory measures (APHAB: EC 27%, RV 47%, BN 53%; CHILD-child scores 7.3 vs. 3.4; CHILD-parent scores 7.0 vs. 3.4) | Moderate risk of bias |
Abbreviation: APHAB, Abbreviated Profile of Hearing Aid Benefit; BN, background noise; CHILD, Children's Home Inventory for Listening Difficulties; EC, ease of conversation; HINT-C, Hearing in Noise Test for Children; RV, listening in reverberant condition; SNR, signal-to-noise ratio.
An improvement of 10–15 dB in hearing thresholds is considered clinically important.
Some results were statistically significant while others were not significant or not reported.
The quality of the evidence was moderate for audiometry and hearing-specific quality of life, and low for speech audiometry (Appendix 2, Table A3).
Bone-Conduction Implants: Effectiveness for Conductive or Mixed Hearing Loss
Eleven systematic reviews reported on four types of bone-conduction implants for conductive or mixed hearing loss, including active percutaneous implants (bone-anchored hearing aids), active transcutaneous implants, passive transcutaneous implants, and active transcutaneous middle ear implants.34,43,47–55 The results for each type of device are described separately below. The characteristics of the included systematic reviews are summarized in Appendix 4.
The results of the systematic review by Mandavia et al,42 which included all types of bone-conduction implants in adults and children with single-sided deafness and/or conductive or mixed hearing loss, have been summarized in the earlier section on effectiveness for single-sided deafness.
Active Percutaneous Bone-Conduction Implants: Effectiveness for Conductive or Mixed Hearing Loss
Four systematic reviews reported on active percutaneous implantable devices for conductive or mixed hearing loss.49,50,52,54 Table 7 summarizes the results.
Table 7:
Summary of Results on Active Percutaneous Bone-Conduction Implants vs. No Treatment for Conductive or Mixed Hearing Loss in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Audiometry | |||
| Colquitt et al, 201149 | 4 | Significant improvement in hearing thresholds (average sound-field threshold gains: 19–45 dBa) | Weak methodological quality |
| Danhauer et al, 201050 | 3 | Significant improvement in hearing thresholds (aided thresholds in normal range) | Low–moderate quality evidence |
| Medical Advisory Secretariat, 200254 | 3 | Significant improvement in hearing thresholds (sound-field threshold gains: 22–42 dBa) | Low quality evidence |
| Speech audiometry | |||
| Colquitt et al, 201149 | 3 | Improvement in speech perception in noise (SRT 1–3 dB HL vs. 9 dB HL when noise was from the back, SRT 3–4 dB HL vs. 12 dB HL when noise was from the front)b,c | Weak methodological quality |
| Danhauer et al, 201050 | 3 | Improvement or no difference in speech perception in noise (numeric data not shown) | Low–moderate quality evidence |
| Hearing-specific quality of life | |||
| Danhauer et al, 201050 | 3 | Significant improvement in quality of life (numeric data not shown) | Low–moderate quality evidence |
| Johnson et al, 200652 | 7 | Significant improvement measured by hearing-specific QOL questionnaire (GBI, HHDI), but not generic QOL questionnaire (MOS SF-36, EQ-5D) (numeric data not shown) | Limited methodological quality |
| Medical Advisory Secretariat, 200254 | 1 | Significant improvement in quality of life measured by the GBI (31-point increase in total benefit, 37-point increase in general benefit, 24-point increase in social benefit, 14-point increase in physical benefit) | Low quality evidence |
Abbreviations: dB HL, decibel of hearing level; EQ-5D, EuroQoL-5D; GBI, Glasgow Benefits Inventory; HHDI, Hearing Handicap and Disability Inventory; MOS SF-36, Medical Outcomes Study General Survey Instrument, Short Form 36; QOL, quality of life; SRT, speech recognition threshold.
An improvement of 10–15 dB in hearing thresholds is considered clinically important.
Statistical significance not reported.
The lower the SRT, the better the hearing.
Audiometry
Three systematic reviews reported hearing thresholds in adults and children as the outcome measure of audiometry.49,50,54 All included studies measured aided and unaided sound-field warble tone or pure tone thresholds at different frequencies. All studies consistently showed that bone-anchored hearing aids improved hearing thresholds when compared with no treatment.
The quality of the evidence for audiometry was moderate (Appendix 2, Table A4).
Speech Audiometry
Two systematic reviews reported on speech audiometry in adults and children.49,50 Some studies showed improvement while others showed no differences in speech audiometric outcomes when comparing bone-anchored hearing aids to no treatment. Varied test measures and set-ups, as well as different etiology and comparators, may account for the discrepancy in results.
The quality of the evidence for speech audiometry was low (Appendix 2, Table A4).
Hearing-Specific Quality of Life
Three systematic reviews reported on hearing-specific quality of life in adults and children.50,52,54 Patients reported better hearing-specific quality of life when comparing bone-anchored hearing aids with no treatment. Specifically, patients reported a significant reduction in disability after fitting with bone-anchored hearing aids.52,54 Generic quality of life questionnaires showed no improvement.
The quality of the evidence for hearing-specific quality of life was moderate (Appendix 2, Table A4).
Active Transcutaneous Bone-Conduction Implants: Effectiveness for Conductive or Mixed Hearing Loss
Bonebridge is the only active transcutaneous bone-conduction implant currently available in Canada. One systematic review reported the clinical outcomes of the Bonebridge device in conductive or mixed hearing loss in adults and children43 (Table 8).
Table 8:
Summary of Results on Active Transcutaneous Bone-Conduction Implants vs. No Treatment for Conductive or Mixed Hearing Loss in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Audiometry | |||
| Sprinzl and Wolf-Magele, 201643 | 7 | Functional gains: 24–37 dBa | Low quality |
| Speech audiometry | |||
| Sprinzl and Wolf-Magele, 201643 | 5 | Significant improvement in speech perception in quiet (Freiburger disyllabic words 77%–93% vs. < 25%) | Low quality |
| Quality of life | |||
| Sprinzl and Wolf-Magele, 201643 | 1 | Improvement in subjective benefits of hearing (numeric data on unaided condition not shown)b,c | Low quality |
| 1 | Higher patient satisfaction (numeric data not shown)b,d | ||
An improvement of 10–15 dB in hearing thresholds is considered clinically important.
Statistical significance not reported.
Meaured by Glasgow Benefit Inventory.
Meaured by Hearing Device Satisfaction Scale.
Audiometry
One systematic review reported functional gains as a measure of audiometry, comparing Bonebridge to no treatment in adults and children.43 The included studies showed functional gains ranging from 24 to 37 dB. The magnitude of the functional gains was considered clinically important.
The quality of the evidence for audiometry was moderate (Appendix 2, Table A4).
Speech Audiometry
One systematic review reported speech perception in quiet as a measure of speech audiometry in adults and children.43 Patients with the Bonebridge device showed significant improvement in speech perception in quiet when compared with those with no treatment.
The quality of the evidence for speech audiometry was moderate (Appendix 2, Table A4).
Hearing-Specific Quality of Life
One systematic review reported subjective benefits of hearing and patient satisfaction as measures of hearing-specific quality of life in adults and children.43 Within the systematic review, one study measured subjective benefits of hearing using the Glasgow Benefit Inventory and reported an improvement in general health and physical health after Bonebridge implantation. Another study reported that patients were satisfied with the device as measured by the Hearing Device Satisfaction Scale.
The quality of the evidence for hearing-specific quality of life was low (Appendix 2, Table A4).
Passive Transcutaneous Bone-Conduction Implants: Effectiveness for Conductive or Mixed Hearing Loss
One systematic review reported on the Sophono device, a passive transcutaneous bone-conduction implant, for conductive or mixed hearing loss in adults and children48 (Table 9).
Table 9:
Summary of Results on Passive Transcutaneous Bone-Conduction Implants vs. No Treatment for Conductive or Mixed Hearing Loss in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Audiometry | |||
| Bezdjian et al, 201748 | 8 | Average functional gains: 31 dBa | Risk of bias: low–moderate Directness of evidence: high |
An improvement of 10–15 dB in hearing thresholds is considered clinically important.
Audiometry
One systematic review reported functional gains in hearing thresholds as a measure of audiometry in adults and children.48 The included studies showed clinically important average functional gains when comparing Sophono devices with no treatment.
The quality of the evidence for audiometry was moderate (Appendix 2, Table A4).
Active Transcutaneous Middle Ear Implants: Effectiveness for Conductive or Mixed Hearing Loss
Five systematic reviews reported on middle ear implants, including Vibrant Soundbridge and Carina, for conductive or mixed hearing loss in adults and children34,47,51,53,55 (Table 10).
Table 10:
Summary of Results of Systematic Reviews on Active Transcutaneous Middle Ear Implants vs. No Treatment for Conductive or Mixed Hearing Loss in Adults and Children
| Author, Year | No. of Studies | Results | Quality Assessment |
|---|---|---|---|
| Audiometry | |||
| University of Alberta, 201147 | 32 |
Vibrant Soundbridge vs. no treatment Average functional gains: 27 dBa |
Low quality |
| 10 |
Carina vs. no treatment Average functional gains: 21 dBa |
||
| Australia Medical Services Advisory Committee, 201034 | 4 |
Middle ear implant vs. no treatment in mild–moderate mixed hearing loss Functional gains ranged 26–32 dBa |
Low quality |
| 2 |
Middle ear implant vs. no treatment in severe mixed hearing loss Functional gains ranged 35–49 dBa |
||
| 2 |
Middle ear implant vs. no treatment in conductive hearing loss Functional gains ranged 36–46 dBa |
||
| Ernst et al, 201651 | 6 |
Vibrant Soundbridge vs. no treatment Average functional gains: 30 dBa |
Low quality (nonrandomized intervention studies and observational studies) to high quality (systematic reviews) |
| Klein et al, 201253 | 10 |
Carina vs. no treatment Average functional gains: 21 dBa |
Limited methodological quality |
| Verhaert et al, 201355 | 14 |
Vibrant Soundbridge vs. no treatment Average functional gains: 11–58 dBa |
Low–moderate quality |
| Speech audiometry | |||
| University of Alberta, 201147 | 12 |
Vibrant Soundbridge vs. no treatment Range of speech reception thresholds in quiet: 40–61 dB vs. 58–94 dB (P < .05) |
Low quality |
| 16 | Range of speech recognition: 55%–95% vs. 0%–72% (P < .05) | ||
| 4 |
Carina vs. no treatment Average speech reception threshold gain: 20 dB |
||
| 4 | Range of speech recognition: 69%–94% vs. 33%–40% (P < .05) | ||
| Australia Medical Services Advisory Committee, 201034 | 2 |
Middle ear implant vs. no treatment in mild–moderate mixed hearing loss Improvement in speech perception at conversational levela (numeric data not shown) |
Low quality |
| 1 | Improvement in speech reception thresholdc (numeric data not shown) | ||
| Australia Medical Services Advisory Committee, 201034 (continued) | 1 |
Middle ear implant vs. no treatment in severe mixed hearing loss Improvement in speech perception at conversational level by 48%a |
Low quality |
| 2 | Significant improvement in speech reception threshold in quiet (numeric data not shown) | ||
| 2 |
Middle ear implant vs. no treatment in conductive hearing loss Speech perception in quiet improved by 70%–76% |
||
| 1 | Speech reception threshold improved by 32 dB | ||
| Ernst et al, 201651 | 2 |
Vibrant Soundbridge vs. no treatment Significant improvement in speech perception in noise (SNR 3 dB SPL vs. 12 dB SPLb) |
Low quality (nonrandomized intervention studies and observational studies) to high quality (systematic reviews) |
| Klein et al, 201253 | 10 |
Carina vs. no treatment Speech reception threshold gain: 20 dB |
Limited methodological quality |
| 10 | Word recognition: 69%–94% vs. 33%–40% | ||
| Verhaert et al, 201355 | 13 |
Vibrant Soundbridge vs. no treatment Significant improvement in speech perception in quiet (numeric data not shown) |
Low–moderate quality |
| 4 | Significant improvement in speech perception in noise (numeric data not shown) | ||
| Hearing-specific quality of life | |||
| University of Alberta, 201147 | 5 |
Vibrant Soundbridge vs. no treatment Significant benefits reported in GBI and APHAB (numeric data not shown) |
Low quality |
| 3 |
Carina vs. no treatment Hearing benefits reported in APHAB (numeric data not shown)c |
||
| Australia Medical Services Advisory Committee, 201034 | 1 |
Middle ear implants vs. no treatment in mild or moderate mixed hearing loss Significant benefits reported in APHAB (numeric data not shown) |
Low quality |
| Ernst et al, 201651 | 4 |
Soundbridge vs. no treatment Significant benefit of hearing reported in APHAB device satisfaction reported in HDSS and improvement in general health status reported in GBI (numeric data not shown) |
Low quality (nonrandomized intervention studies and observational studies) to high quality (systematic reviews) |
| Klein et al, 201253 | 3 |
Carina vs. no treatment Significant hearing benefits reported in APHAB (numeric data not shown) |
Limited methodological quality |
| Verhaert et al, 201355 | 4 |
Vibrant Soundbridge vs. no treatment Significant subjective benefits of hearing reported in APHAB (numeric data not shown) |
Low–moderate quality |
| 4 | Improvement in quality of life reported in GBI (numeric data not shown)c | ||
Abbreviations: APHAB, Abbreviated Profile of Hearing Aid Benefit; GBI, Glasgow Benefit Inventory; HDSS, Hearing Device Satisfaction Scale; SNR, signal-to-noise ratio; SPL, sound pressure level.
An improvement of 10–15 dB in hearing thresholds is considered clinically important.
The lower the SPL, the better the hearing.
Statistical significance not reported.
Audiometry
Five systematic reviews reported functional gains in hearing thresholds as a measure of audiometry in adults and children.34,47,51,53,55 Middle ear implants improved functional gains in hearing thresholds when compared with no treatment. The magnitude of functional gains was considered clinically important. The Australian health technology assessment also reported clinically important functional gains with middle ear implants across different degrees of mixed or conductive hearing loss.34
The quality of the evidence for audiometry was moderate (Appendix 2, Table A4).
Speech Audiometry
Five systematic reviews reported speech audiometry using various testing set-up and outcome measures in adults and children.34,47,51,53,55 Overall, middle ear implants showed clinically important improvement in speech reception thresholds and word recognition when compared with no treatment. An improvement in speech reception threshold of 10 to 15 dB or 10% to 15% is considered clinically important. The Australian health technology assessment also showed that middle ear implants improved speech perception in patients with different degrees of mixed or conductive hearing loss.34
The quality of the evidence for speech audiometry was moderate (Appendix 2, Table A4).
Hearing-Specific Quality of Life
Five systematic reviews reported patient satisfaction, subjective benefits of hearing, and hearing-specific quality of life in adults and children.34,47,51,53,55 Patient satisfaction was measured by the Hearing Device Satisfaction Scale. Subjective benefits of hearing were measured by the Abbreviated Profile of Hearing Aids Benefits. Hearing-specific quality of life was measured by the Glasgow Benefit Inventory. The included reviews consistently reported that middle ear implants improved subjective benefits of hearing and hearing-specific quality of life.
The quality of the evidence for hearing-specific quality of life was moderate (Appendix 2, Table A4).
Bone-Conduction Implants: Safety
Active Percutaneous Bone-Conduction Implants
Two systematic reviews reported adverse events associated with active percutaneous bone-conduction implants.45,54 Kim et al45 reported a complication rate of 5% to 17% from two studies. All adverse events were minor complications related to skin reactions around the abutment sites, and all resolved with medical treatment.
An evidence-based analysis conducted by the Medical Advisory Secretariat of the Ontario Ministry of Health and Long-Term Care showed an overall success rate of 88% to 99% in maintaining a functional bone-anchored hearing aid, from six studies. The majority of adverse events that led to the removal of implants were related to failed osseointegration, trauma, or infections. The rate of skin reactions around the abutment sites was 8% to 32%.54
Our literature search also identified a publication that reviewed the complications associated with osseointegrated hearing aids. This review included 20 studies involving 2,134 patients who underwent a total of 2,310 osseoimplants. Skin reactions of grades 2 to 4 (moderate to profound signs of infection) in the Holgers classification of skin complication ranged from 2.4% to 38.1%. Failure of osseointegration ranged from 0% to 18% in adult and mixed populations, and 0% to 14.3% in pediatric populations. The rate of revision surgery was 1.7% to 34.5% in adult and mixed populations, and 0% to 44.4% in pediatric populations.56
Active Transcutaneous Bone-Conduction Implants
One systematic review reported adverse events from 12 studies of patients using a Bonebridge device.43 Nine of the 12 studies reported no adverse events after Bonebridge implantation. The remaining three studies reported a rate of minor adverse events of 5.1% and a rate of revision surgery of 0.85%. Minor adverse events included wound pain, dizziness, tinnitus, and headache, all of which resolved on their own or were treated without surgical intervention.
Passive Transcutaneous Bone-Conduction Implants
One systematic review reported adverse events associated with passive transcutaneous bone-conduction implants.48 The eight included studies did not report any intra-operative adverse events. However, 29% of patients experienced postoperative adverse events, with 3.5% of these events considered as serious. Adverse events were deemed serious if surgical intervention was required or if healing took longer than one month. Postoperative adverse events included moderate to severe pain, pressure necrosis or discomfort, skin erythema, and wound infection that resolved with antibiotics.
Active Transcutaneous Middle Ear Implants
Four systematic reviews reported adverse events associated with middle ear implants.34,47,51,53 In the health technology assessment conducted for Alberta Health and Wellness, the overall device failure rate was 4.8% for Vibrant Soundbridge (n = 22) and 17.6% for Carina (n = 8).47 Ernest et al51 reported an overall device failure rate of 2% for Vibrant Soundbridge (n = 13), whereas Klein et al53 reported an overall device failure rate of 18% for Carina (n = 11). As noted (Background, Regulatory Information), Carina is only being used in research protocols and is not available for clinical use in Canada.
The health technology assessment conducted by the Australian Medical Services Advisory Committee reported adverse events of middle ear implants from 50 studies. The rate was less than 4% for each of the following clinical adverse events: infection, pain, hematoma, tinnitus, vertigo, and aural fullness. The rate was less than 2% for each of the following technical adverse events: facial nerve damage, device extrusion, device migration, device failure, electromagnetic interference, and insufficient gain.34
Based on the best available data, which show a lack of major complications associated with the bone-conduction implants currently in clinical use in Canada, surgery for bone-conduction implants is reasonably safe.
Discussion
In this overview of systematic reviews on implantable devices for adults and children with single-sided deafness and conductive or mixed hearing loss, we based our evidence synthesis on data reported in the published reviews. While there were differences in patient characteristics, testing conditions, and outcome measurements, most studies within the systematic reviews showed similar results.
Cochlear implants, but not bone-conduction implants, improve sound localization by restoring binaural hearing in patients with single-sided deafness. Bone-conduction implants do provide clinically important functional gains in hearing thresholds for patients with conductive or mixed hearing loss. The consistency, large magnitude of effects (hearing thresholds), and lack of bias (sound localization) of these biologically plausible results in the literature, despite heterogeneity in study design and conduct, increased our certainty about the body of evidence and allowed us to believe that, overall, the study results represent some true effects.
For children, these benefits in hearing are crucial in optimizing the development of their auditory systems and for speech and language acquisition. In addition, for both children and adults, improved hearing supports better communication and learning, with potential downstream impacts on their educational and employment opportunities. The surgery to implant cochlear and bone-conduction devices is reasonably safe. The selection of the most appropriate device for each patient would be a clinical decision based on the etiology of their hearing loss, perceived gains and risks, and patient preferences and expectations.
In summary, we have a moderate level of certainty that cochlear implants and bone-conduction implants, if clinically indicated, improve functional hearing in adults and children with single-sided deafness, conductive hearing loss, or mixed hearing loss. The benefits of being able to hear better with these implants are substantial for patient-important outcomes, such as decreasing disability and improving quality of life.
Limitations
In the before-and-after studies included in the systematic reviews, heterogeneity existed in patient characteristics (e.g., etiology, duration of deafness), test conditions, follow-up durations, and outcome measurements
Most of the included systematic reviews combined adult and pediatric populations
Statistical significance was not consistently examined or reported
No comparative data were available to allow comparisons between types of bone-conduction implants
Adverse events included different generations of implants; improvements in surgical techniques and device designs could decrease complication rates, which may not be fully captured in our review
Ongoing Reviews
We identified four ongoing systematic reviews that have potential relevance to implantable devices for single-sided deafness and conductive or mixed hearing loss, through a search of the PROSPERO register of systematic reviews (Appendix 5).
Conclusions
Cochlear Implants for Single-Sided Deafness
Based on the best evidence available, when compared with no treatment in adults and children with single-sided deafness, cochlear implants:
Likely improve speech perception in noise (GRADE: Moderate)
Likely result in a large improvement in sound localization (GRADE: Moderate)
Likely improve hearing-specific quality of life (GRADE: Moderate)
Likely improve tinnitus (GRADE: Moderate)
Likely improve speech and language development in children (GRADE: Moderate)
Based on the best evidence available, cochlear implantation is reasonably safe.
Bone-Conduction Implants for Single-Sided Deafness
Based on the best evidence available, when compared with no treatment in adults and children with single-sided deafness who are contraindicated for cochlear implantation, bone-conduction implants:
Likely result in a large improvement in hearing thresholds (GRADE: Moderate)
Likely improve speech perception in noise (GRADE: Moderate)
Likely improve hearing-specific quality of life (GRADE: Moderate)
Likely do not improve sound localization (GRADE: Moderate)
Bone-Conduction Implants for Conductive or Mixed Hearing Loss
Based on the best evidence available, when compared with no treatment in adults and children with conductive or mixed hearing loss, bone-conduction implants:
Likely result in a large improvement in hearing thresholds (GRADE: Moderate)
Likely improve speech perception in noise (GRADE: Moderate)
Likely improve hearing-specific quality of life (GRADE: Moderate)
Based on the best evidence available, surgery to implant bone-conduction devices is reasonably safe.
ECONOMIC EVIDENCE
For the economic evidence review, we considered the following three types of hearing loss, described in the Background section of this report:
Single-sided deafness (severe or profound unilateral sensorineural hearing loss)
Conductive hearing loss
Mixed hearing loss
Research Questions
Based on the published literature:
-
1.
What is the cost-effectiveness of cochlear implants compared with no intervention in adults and children with single-sided deafness?
-
2.
What is the cost-effectiveness of bone-conduction implants compared with no intervention in adults and children with single-sided deafness?
-
3.
What is the cost-effectiveness of bone-conduction implants compared with no intervention in adults and children with conductive or mixed hearing loss (single-sided or bilateral)?
Methods
Economic Literature Search
We performed an economic literature search on January 8, 2018, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment review. We performed targeted grey literature searching of health technology assessment agency websites, the PROSPERO register of systematic reviews, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used and Appendix 1 for the literature search strategies, including all search terms.
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and performed further assessment for eligibility.
Inclusion Criteria
English-language full-text publications
Studies published between database inception and January 8, 2018
Studies in patients with single-sided deafness, conductive hearing loss (single-sided or bilateral), or mixed hearing loss (single-sided or bilateral)
Studies comparing cochlear implants or bone-conduction devices to no intervention
Cost–utility, cost-effectiveness, or cost–benefit analyses
Exclusion Criteria
Cost analyses, cost–consequence analyses, cost-minimization analyses
Abstracts, letters, and editorials
Unpublished studies
Bilateral sensorineural hearing loss
Outcomes of Interest
Incremental costs
Incremental effectiveness outcomes
Incremental quality-adjusted life-years (QALYs)
Incremental cost-effectiveness ratios (ICERs)
Incremental net benefit
Data Extraction
We extracted relevant data on the following from the payer perspective:
Source (i.e., name, location, year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, ICERs)
We stratified results by population and age where possible. Adults were defined as 18 years of age or older. Children were defined as under 18 years old for cochlear implants, and between 5 and 18 years of age for bone-conduction implants, as per Health Canada indications. We contacted authors of the studies to provide clarification as needed. We present original cost figures, without converting to the same currency or inflating to the same year.
Study Applicability and Methodological Quality
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform development of NICE's clinical guidelines.61 We modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). A summary is presented in Appendix 6. In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly or partially applicable.
Results
Literature Search
The literature search yielded 118 citations, after removing duplicates. We excluded a total of 103 articles based on information in the title and abstract. We then obtained the full texts of 15 potentially relevant articles for further assessment. Figure 3 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). One study met the inclusion criteria.
Figure 3: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al, 2009.37
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Review of Included Economic Studies
Question 1: Cochlear Implants for Single-Sided Deafness
No economic evaluations were identified.
Question 2: Bone-Conduction Implants for Single-Sided Deafness
No economic evaluations of bone-conduction implants exclusively in a population with single-sided deafness were identified. One included study (discussed under question 3) examined a heterogeneous population of patients with single-sided, conductive, and mixed hearing loss.62
Question 3: Bone-Conduction Implants for Conductive or Mixed Hearing Loss
We identified one study for a heterogeneous population of patients with conductive or mixed hearing loss (55%), single-sided deafness (44%) and bilateral sensorineural hearing loss, a population not relevant to our health technology assessment (1%).62 This study evaluated bone-anchored hearing aids, also known as active percutaneous devices; these implants penetrate the skin permanently and directly stimulate the bone to transmit sound waves to the cochlea in the inner ear. The comparator in this study was no hearing implant and included some people who use no intervention and some people who use conventional hearing aids. Despite a mixed comparator group, we included the study in our review. Economic evaluations were not identified for other types of bone-conduction implants.
Table 11 summarizes the results of the included study.49,62 It reported incremental cost-effectiveness ratio (ICER) results in terms of cost per quality-adjust life-year (QALY). The study was a piggyback evaluation, based on an observational study (an uncontrolled before-and-after study) in the United Kingdom.62
Table 11:
Results of Economic Literature Review—Summary
| Results | ||||||
|---|---|---|---|---|---|---|
| Name, Year, Location | Analytic Technique, Study Design, Perspective, Time Horizon | Population(s) | Interventions, Comparators | Health Outcomes | Costs | Cost-Effectiveness |
| Monksfield et al, 2011,62 Birmingham, United Kingdom | CUA Piggyback evaluation from uncontrolled before-and-after study UK health care payer perspective Lifetime time horizon | Patients offered a primary bone-anchored hearing aid; likely unilateral implant, although not reported explicitly Tertiary referral, university hospital Mean age = 55 years; Range = NR Male = 39% Indications (type of hearing loss): conductive = 52%; single-sided sensorineural = 44%; mixed = 3%; bilateral sensorineural = 1% | Implant (n = 70): Patients implanted with a primary bone-anchored hearing aid Costs and outcomes were derived from patients after receiving an implant vs. No implant (n = 70): For the counterfactual, patients were assumed to have not received an implant Hearing aid use was assumed to have remained at the same level for the rest of their life expectancy (56% used 1 or 2 standard hearing aids, remaining were unaided) Costs and outcomes were derived from patients prior to implant | Implant: Utility = 0.66 (95% CI 0.60–0.72) No implant: Utility = 0.57 (95% CI 0.51–0.62) for all patients who completed questionnaires before implant Utility = 0.59 (95% CI 0.53–0.65) for patients who completed questionnaires before and after implant Mean difference: 1.89 QALYs (95% CI 0.71–3.23) HUI3 and HUI2 questionnaires completed at baseline (when implant was offered) and 6 months after device fitting QALY = age- and sex-specific life expectancy 1 year after implantation × difference in utility scores before and after Bootstrapped mean QALY gains Discounting 3.5% | Implant: £21,430 (95% CI 20,263–22,535) No implant: £827 (95% CI 644–1,022) Mean difference: £20,604 (95% CI 19,462–21,769) Included costs: assessment, device, surgery, postoperative care, replacement, annual maintenance Bootstrapped mean costs GBP (2008) Discounting 3.5% | Authors concluded bone-anchored hearing aids can be cost-effective Base case analysis: ICER = £17,610 per QALY using HUI3 ICER = £21,688 per QALY using HUI2 Probabilistic sensitivity analysis (WTP = £20,000 per QALY): Probability of being cost-effective = 56% Sensitivity analyses were conducted by varying discount rates (no discounting, discounting costs only). Results did not change qualitatively |
Abbreviations: CI, confidence interval; CUA, cost–utility analysis; HUI2, Health Utilities Index Mark II questionnaire; HUI3, Health Utilities Index Mark III questionnaire; ICER, incremental cost-effectiveness ratio; NR, not reported; QALY, quality-adjusted life-year; WTP, willingness-to-pay.
The study reported that bone-anchored hearing aids were likely cost-effective compared to no hearing implants (i.e., patients with one, two, or no conventional hearing aids).62 The authors reported that results were robust, although they conducted very few sensitivity analyses.
Applicability and Methodological Quality of the Included Studies
Appendix 6, Table A6, shows our assessment of applicability for the included study.62 The study took a UK health care payer perspective and evaluated bone-anchored hearing aids. One aim of the present health technology assessment is to evaluate bone-conduction implants as a device class, which includes active and passive transcutaneous implants, in addition to bone-anchored hearing aids (i.e., active percutaneous implants). Our economic evidence review also aims to stratify the population by type of hearing loss and by age. The study, however, included a mix of hearing loss types, mainly addressing research question 3 (conductive and mixed hearing loss, 55%) and research question 2 (single-sided deafness, 44%).62 The mean age was 55 years, although it is unclear if children were recruited in the observational study. In addition, about 50% of the people wore hearing aids before they received an implant (i.e., the comparator group). Our research question intended to compare implants versus no intervention at all. For these reasons, the study was deemed partially applicable to the present health technology assessment.
Appendix 6, Table A7, shows our assessment of methodological quality. The study design, an uncontrolled before-and-after study where patients volunteered to be enrolled, had limitations. This design assesses the same set of patients before an intervention and then after. It may be prone to self-selection bias (due to the recruitment strategy) and confounding (as it is unable to control for changes that might occur over time). In addition, improvements in health-related quality of life (HRQOL) were only measured 6 months after device fitting, but in the calculations of the economic evaluation, the benefits of the intervention were assumed to last a lifetime.62 To create a comparison group, the economic evaluation relied on counterfactual (“what if”) data: the authors used the “before” group as the comparator, supposing they did not later receive an implant when, in fact, they did. Instead, the study assumed that, for the rest of their lives, these patients had the same costs (e.g., for use of hearing aids) and HRQOL as in the “before” portion of the study.62 In addition, the piggyback study did not adequately examine parameter or structural uncertainty.62
For these reasons, we deemed the study to have potentially serious limitations.
Discussion
The published literature on economic evaluations of hearing implants in people with single-sided deafness and conductive or mixed hearing loss was limited. We identified no studies on cochlear implants for single-sided deafness (research question 1). We identified one study62 on bone-conduction implants (research questions 2 and 3); however, of the entire class of these devices, only bone-anchored hearing aids were evaluated. We identified, but excluded, one additional study that compared bone-anchored hearing aids to non-implanted bone-conduction hearing aids in people with conductive or mixed hearing loss.49 Despite similar objectives of these studies (to assess the cost-effectiveness of bone-anchored hearing aids), the results from the excluded study, concluding that bone-anchored hearing aids were not cost-effective,49 were not consistent with the included study. This may be due to differences in comparator groups, study design, methodological assumptions, setting, patient populations (conductive and mixed hearing loss versus conductive, mixed, and unilateral and bilateral sensorineural hearing loss), cost parameters, and time horizons (10 years versus lifetime).
Strengths and Limitations
The included economic analysis had strengths and limitations. The piggyback study measured costs and HRQOL data from patients in tertiary care who received a bone-conduction implant.62 However, the uncontrolled before-and-after study design did not allow for comparisons of separate groups of patients with and without implantation. Rather, the same group of patients were their own controls and were compared before and after the intervention. Patients also had to volunteer to be in the study. This design is susceptible to self-selection bias, was conducted over a short follow-up period (the “after” assessment was 6 months after device fitting), and cannot control for temporal changes that would otherwise have occurred without the intervention (this could lead to confounding).
Overall, the included study62 had methodological limitations and was not generalizable to the Ontario context. Hence, an analysis for the Ontario population is warranted.
Conclusions
We identified no economic evaluations of cochlear implants for people with single-sided deafness. We identified one economic evaluation of bone-conduction implants (specifically, bone-anchored hearing aids) for people with conductive hearing loss, mixed hearing loss, or single-sided deafness. The study was from the United Kingdom, was not directly applicable to Ontario, and had potentially serious methodological limitations.
PRIMARY ECONOMIC EVALUATION
The included study in the economic evidence review had methodological limitations and was not generalizable to the Ontario context. Therefore, we conducted a primary economic evaluation.
Research Questions
Within the context of the Ontario Ministry of Health, we asked the following questions:
-
1.
What is the cost-effectiveness of cochlear implants compared with no intervention in adults and children with single-sided deafness?
-
2.
What is the cost-effectiveness of bone-conduction implants compared with no intervention in adults and children with single-sided deafness?
-
3.
What is the cost-effectiveness of bone-conduction implants compared with no intervention in adults and children with conductive or mixed hearing loss?
Note that questions 1 and 2 are for the same population. We do not compare cochlear implants with bone-conduction implants because, for this population, neither receives targeted funding from the Ministry of Health; therefore, neither is standard of care in Ontario. Further, as previously described (Background, Ontario Context), people with single-sided deafness would be offered bone-conduction implants only if cochlear implants are contraindicated.
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.63
Type of Analysis
We conducted a cost–utility analysis using a state transition Markov cohort model for each research question. The reference case and sensitivity analyses were conducted probabilistically.
Target Population
We analyzed adults and children as separate subgroups. We included both sexes. Table 12 summarizes the patient characteristic inputs in the three economic models, and descriptions follow.
Table 12:
Patient Characteristics of Target Populations
| Adults | Children | References | |
|---|---|---|---|
| Model 1: Cochlear implantation for single-sided deafness | |||
| Sex | 65% male | 54% male | Clinical experts and estimated from Fitzpatrick et al, 201764 |
| Canadian Health Measures Survey, 2012/201365 | |||
| Statistics Canada, 2012–201566 | |||
| Intervention arm: age at implantation | Mean = 40 years Range = 18 to 80 years | Mean = 18 months Range = 6 months to 17 years | Clinical experts and estimated from Canadian Agency for Drugs and Technologies in Health, 201167 |
| Fitzpatrick et al, 201068 | |||
| Model 2: Bone-conduction implants for single-sided deafness | |||
| Sex | 35% male | 54% male | Clinical experts and estimated from Fitzpatrick et al, 201764 |
| Canadian Health Measures Survey, 2012/201365 | |||
| Statistics Canada, 2012–201566 | |||
| Intervention arm: age at implantation | Mean = 40 years Range = 18 to 80 years | Mean = 10 years Range = 5 to 17 years | Clinical review |
| Model 3: Bone-conduction implants for conductive/mixed hearing loss | |||
| Sex | 50% male | 50% male | Clinical experts and estimated from clinical review, Davids et al, 200769 |
| Intervention arm: age at implantation | Mean = 50 years Range = 18 to 75 years | Mean = 8 years Range = 5 to 17 years | Clinical experts and estimated from clinical review, Davids et al, 200769 Colquitt et al, 201149 |
Our target populations are based on the candidacy criteria specified by the Ontario Cochlear Implant Program, as described previously in this report (Background, Ontario Context).
Model 1: Cochlear Implants for Single-Sided Deafness
In this model, patients have single-sided deafness (one deafened ear) and all other forms of amplification have been unsuccessful. Further, patients are candidates for a cochlear implant (i.e., based on the etiology of hearing loss) and have limited duration of deafness (i.e., less than 4 years in children or 10 years in adults).
Model 2: Bone-Conduction Implants for Single-Sided Deafness
In this model, patients have single-sided deafness (one deafened ear), and all other forms of amplification have been unsuccessful. These patients are contraindicated for cochlear imlantation (e.g., cochlear nerve aplasia). Further, patients are eligble for bone-conduction implants (i.e., based on Health Canada indications, they are at least 5 years of age).
Model 3: Bone-Conduction Implants for Conductive or Mixed Hearing Loss
In this model, patients have conductive or mixed hearing loss in one or both ears, and all other forms of amplification have been unsuccessful. This population combines two types of hearing loss because the majority of relevant clinical studies recruited and reported on patients with conductive hearing loss and mixed hearing loss together.43,48,62,70–73 Patients in this population are also eligble for bone-conduction implants (i.e., based on Health Canada indications, they are at least 5 years of age).
Perspective
For the reference case, we conducted our analysis from the perspective of the Ontario Ministry of Health. This perspective includes direct costs (i.e., device, outpatient care, inpatient care, physician billing). In sensitivity analyses, we used a public payer perspective. This broader perspective incorporated costs borne by other ministries (i.e., Community and Social Services; Child and Youth Services; and Education).
Interventions
Table 13 summarizes the interventions evaluated in the three economic models. We considered two main classes of devices: (1) cochlear implants, which take over the function of the damaged cochlea in the inner ear by converting sound into electrical signals to the hearing nerve; and (2) bone-conduction implants, which transmit sound by bone to the inner ear, effectively bypassing the outer and middle ear. The three types of bone-conduction implants are as follows:
Table 13:
Interventions and Comparators Evaluated in the Primary Economic Models
| Intervention | Comparators | Population | Outcomes |
|---|---|---|---|
| Cochlear implant (in one ear) | Adults and children who are unaided (i.e., do not use standard air-conduction hearing aids) | Single-sided deafness | Cost, QALYs, ICER |
Bone-conduction implant, as a class, which includes:
|
Adults and children who are unaided (i.e., do not use any standard air-conduction or non-implantable bone-conduction hearing aids) | Single-sided deafness | Cost, QALYs, ICER |
| Bone-conduction implant, as a class, which includes: | Adults and children who are unaided (i.e., do not use any standard air-conduction or non-implantable bone-conduction hearing aids) | Conductive or mixed hearing loss | Cost, QALYs, ICER |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Active transcutaneous, including MED-EL Bonebridge and middle ear implants (MED-EL Vibrant Soundbridge and Cochlear Carina)
Active percutaneous, including Oticon Ponto and Cochlear Baha Connect
Passive transcutaneous, including Medtronic Alpha 2 MPO e Plus and Cochlear Baha Attract
All types of bone-conduction implant are suitable for conductive or mixed hearing loss. However, middle ear implants and passive transcutaneous implants are generally considered inadequate or inappropriate for single-sided deafness. For more information, see Figure 1 and Background, Health Technology Under Review.
While our report examines bone-conduction implants as a class of devices, in our reference case analyses we base our model parameters on the most commonly used devices. In adults, we assume the most common device is an active transcutaneous bone-conduction implant (e.g., Bonebridge). In children, we assume the most common type of device is active percutaneous. In sensitivity analyses, we varied parameters based on other types of bone-conduction implants. All hearing implants were compared with no intervention.
Discounting, Cycle Length, and Time Horizon
We applied an annual discount rate of 1.5% to both costs and quality-adjusted life-years (QALYs) and applied a half-cycle correction (a technique to balance the distribution of people who transition between health states at the beginning or end of each cycle). We varied discount rates in sensitivity analyses. For model 1 (cochlear implants), we used a 25-year time horizon in the reference case. For models 2 and 3 (bone-conduction implants), we used a 10-year time horizon in the reference case. These time horizons are in line with other modelling studies conducted for cochlear implants74 and bone-conduction implants.75,49 These time horizons provide sufficient time for differences between interventions to be realized and avoid extrapolating too far beyond available data. In scenario analyses, we used a lifetime time horizon. For all three models, we used a 6-month cycle length, meaning patients may transition to a different health state only once during any 6-month period.
Model Structure
We developed a Markov model for each research question, following patients from the time of implantation until the end of the time horizon. We used the same model structure for all three research questions (Figure 4). However, we varied the natural history parameters, utility parameters, and cost parameters to reflect the specific populations and interventions. The structure was adapted from a previous Health Quality Ontario economic evaluation on bilateral cochlear implantation.29 The health states are described below.
Figure 4: Model Structure—Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss.
Adapted from Health Quality Ontario, 2018.29
Model Health States
Alive with implant—Everyone in the intervention group (cochlear implant or bone-conduction implant) begins in this health state. They may have complications (minor or major), may have their device explanted (removed), may require a re-implantation, may elect not to use their device, may have their sound processor replaced, or may die from background mortality. Patients in this health state also have scheduled health care visits for audiologic management, surgical/wound management, and rehabilitation. The frequency of health care visits differs between adults and children, and between cochlear and bone-conduction implants
-
Alive without implant—This health state refers to:
-
–
Patients in the intervention group who elect to discontinue using their implant by turning off the sound processor (i.e., due to low self-reported benefit from the implant, learning disabilities, etc.)76,77
-
–
Patients in the intervention group who have had their implants removed due to a rare complication
-
–
Patients who never received an implant; everyone in the comparator group (i.e., unaided) begins in this health state
-
–
Patients may remain in this state or transition into the “dead” state
-
–
Dead—At any point during the model timeline, individuals have a probability of death due to age- and sex-specific background mortality (the general population's risk of death)
Model 1: Cochlear Implantation for Single-Sided Deafness
Figure 5 shows the patient pathway in Ontario for adults and children undergoing cochlear implantation. These health care visits are accounted for as events in the model where there may be associated costs and changes to quality of life.
Figure 5: Patient Pathway for Adults and Children Receiving a Cochlear Implant.
Source: Expert opinion; Sunnybrook Cochlear Implant Program, 201878; Chen et al, 2014.74
Model 2 and Model 3: Bone-Conduction Implants for Single-Sided Deafness and Conductive or Mixed Hearing Loss
Figure 6 shows the patient pathway in Ontario for adults and children undergoing bone-conduction implantation. These health care visits are accounted for as events in the model in which there may be associated costs and changes to quality of life. Unlike the cochlear implantation pathway, we did not include rehabilitation with an auditory verbal therapist. Rehabilitation is not required because bone-conduction implants produce a more natural sound, whereas cochlear implants produce a more distorted (sometimes described as “robotic”) sound. Consequently, patients undergoing cochlear implantation need to learn how to hear with their device. We based the patient pathway on active transcutaneous implant procedures for the reference case because these implants are commonly used in Ontario.
Figure 6: Patient Pathway for Children and Adults Receiving a Bone-Conduction Implant.
Sources: Expert opinion; Colquitt et al, 201149; Cochlear Limited Surgery Guides, 2014 and 2015.79,80
Main Assumptions
The major assumptions for this model are:
There are no direct costs associated with hearing implants for patients in the No Intervention arm; this is a conservative assumption as patients or caregivers may have already invested in hearing aids
The same type of hearing implants have the same effectiveness, regardless of the manufacturer; that is, all cochlear implants from all manufacturers have the same effectiveness, all active percutaneous devices from all manufacturers have the same effectiveness, and so on
All patients receive an implant in only one ear; patients with unilateral and bilateral conductive or mixed hearing loss have identical costs and utilities
Disease-specific mortality (i.e., mortality associated with hearing loss) is negligible; we test this assumption in sensitivity analysis (In the reference case, we account only for age- and sex-specific background mortality)
Complication rates are device-specific rather than disease-specific. In other words, complication rates for bone-conduction implants are the same for patients with single-sided deafness and with conductive or mixed hearing loss
We do not account for partial non-use of devices. We assumed people either use their devices over the model's entire time horizon or elect not to use their devices at some point, in which case they remain a non-user for the remainder of the time horizon
We assume non-users are no longer at risk for complications associated with their implant
Patients are vaccinated and do not get meningitis after receiving their cochlear implant
Patients attend all their scheduled health care visits for audiologic management, surgical/wound management, and rehabilitation
The improvement in health-related quality of life due to receiving an implant does not deteriorate over time, as long as individuals continue to use their device
Clinical Outcome and Utility Parameters
We used a number of input parameters to populate the model. These included:
Variables to model the natural history of the disease
Variables to modify the natural history model to account for treatment effects of cochlear implants and bone-conduction implants
Variables to capture people's health-related quality-of-life
Natural History
Model 1: Cochlear Implants for Single-Sided Deafness
Successful surgical procedure. Based on experience of the Ontario Cochlear Implant Program, we assumed all initial surgical procedures would be successful (written communication, Joseph Chen, MD, June 10, 2018).
Complications. None of the systematic reviews on cochlear implantation in single-sided deafness examined in our clinical review reported adverse events. Instead, we based complication parameters on two large, retrospective analyses described in the clinical review. The analyses were conducted in cochlear implant recipients with bilateral sensorineural hearing loss.57,58 Table 14 shows the probability of experiencing complications for patients in the “alive with implant” health state (Appendix 7, Complications, Table A8, shows the corresponding conditional 6-month probabilities). Complications were defined as:
Table 14:
6-Month Probability of Complications After Cochlear Implantation
| Complications | Probability | SD | Distribution | Reference |
|---|---|---|---|---|
| Minor complications (occurrence during first year) | ||||
| Minor complications, adults | 0.0720 | 0.0186 | Beta | Health Quality Ontario, 2018,29 which pooled probability from Venail et al, 2008,57 and Farinetti et al, 201458 |
| Minor complications, children | 0.0338 | 0.0106 | Beta | Health Quality Ontario, 2018,29 which pooled probability from Venail et al, 2008,57 and Farinetti et al, 201458 |
| Major complications (ongoing occurrence) | ||||
| Major complication, adults | 0.0083 | 0.0008a | Beta | Venail et al, 200857 |
| Conditional probability explantationb, adults | 0.77 | 0.077a | Beta | Venail et al, 200857 |
| Conditional probability re-implantationc, adults | 0.92 | 0.092a | Beta | Venail et al, 200857 |
| Major complications, children | 0.0093 | 0.0009a | Beta | Venail et al, 200857 |
| Conditional probability explantationb, children | 0.74 | 0.074a | Beta | Venail et al, 200857 |
| Conditional probability re-implantationb, children | 0.96 | 0.096a | Beta | Venail et al, 200857 |
Abbreviation: SD, standard deviation.
Note: 6-month probabilities are reported unless otherwise stated.
SD assumed to be 10% of mean.
Conditional probability of explantation given a major complication. Calculated as no. of individuals with explantation (either explantation only or re-implantation) ÷ no. of individuals with major complications.
Conditional probability of re-implantation given an explantation. Calculated as no. of individuals with re-implantation ÷ no. of individuals with explantation (either explantation only or re-implantation).
Minor complications—These require conservative management. They include infections resolved by medical treatment (i.e., skin infections, otitis media), neurological complications (i.e., temporary facial palsy, dysgeusia), pain (i.e., facial stimulation, facial or neck pain), tinnitus, vestibular complications (i.e., vertigo, dizziness), and others (i.e., cerebrospinal fluid leak, hematoma). In the model, we assumed minor complications occur only within the first year after surgery. In sensitivity analyses, we assumed a constant probability of minor complications throughout the duration of the model
Major complications—These require hospitalization and/or surgical revisions. They include but are not limited to device failures, infections (i.e., mastoiditis), cholesteatoma, and perforated eardrum after acute otitis media. Meningitis infections were excluded because we assumed everyone was vaccinated prior to implantation. We assumed the probability of major complications remained constant over time. Individuals may have more than one major complication over the course of the model (unless their device is permanently removed)
Explantation (including explantation only or re-implantation)—A subset of major complications included those that require the implant to be removed (e.g., due to device malfunction or persistent infection). We assumed the probability of explantation (conditional on a major complication) remained constant over the course of the model
Re-implantation only—A subset of explantations includes re-implantations. We assumed the probability (conditional on an explantation) was constant over time. Individuals may experience more than one re-implantation over the course of the model
Elective device non-use. People who have received a cochlear or bone-conduction implant may elect to no longer use their sound processor (the external component), for various reasons. It may be due to low self-reported benefit from the implant, learning disabilities, lack of support from family, or lack of support from educational placements.76,77,81 Table 15 shows the probability of elective non-use in children and adults used in the reference case and sensitivity analyses. In our reference case, based on Ontario data, we assumed over 5 years 9% of adults would not use their device. The rate of non-use in children with single-sided deafness has been shown to be very low.82 To be conservative, in our reference case we assumed 5% of children over ten years would not use their devices. We assumed the rate of non-use is constant over the included time frame (Table 15) and negligible after.
Table 15:
Probability of Not Using Cochlear Implant
| Adults | Children | |||
|---|---|---|---|---|
| Probability | Time Frame | Probability | Time Frame | Reference |
| Reference case | ||||
| 9% | 5 y | 5% | 10 y | Data from OCIPa (adults); assumption (children) |
| Sensitivity analysis | ||||
| Time-dependent; see Appendix 7, Table A9 | Bhatt et al, 200983; Ozdemir et al, 201376; Archbold et al, 200984; Ray et al, 200685; Raine et al, 200881 | |||
| 5% | 10 y | 0% | 4 m | Expert opinion (adults); Polonenko et al, 201782 (children) |
Abbreviations: m, month; OCIP, Ontario Cochlear Implant Program; y, year.
Written communication, Joseph Chen, MD, July 12, 2018.
We conducted several sensitivity analyses: we assumed the risk of non-use is constant over the entire time horizon, and we used time-dependent probabilities obtained from the literature, as well as constant probabilities over time obtained from expert opinion. Appendix 7, Elective Non-use, Table A9, shows the corresponding 6-month probabilities.
Models 2 and 3: Bone-Conduction Implants for Single-Sided Deafness, and Bone-Conduction Implants for Conductive or Mixed Hearing Loss
Successful surgical procedure. Based on experience of the Ontario Cochlear Implant Program, we assumed all initial surgical procedures would be successful.
Complications. We assumed that complications are device-specific rather than disease-specific (i.e., same probability of complications for people with single-sided deafness versus conductive or mixed hearing loss). Wherever possible, we based complications on systematic reviews identified in our clinical review and stratified by adults and children.43,48,56 As in model 1, we defined minor complications as requiring conservative management and occurring only in the first year after implantation, given the short follow-up time of clinical studies. In sensitivity analysis, we assumed a constant probability of minor complications throughout the duration of the model because skin-related complications may be expected to occur at any time, not only right after implantation. We defined major complications as requiring hospitalization and/or surgical revisions. A subset of major complications included explantation and re-implantation of the device. We assumed a constant probability of major complications throughout the duration of the model.
-
Minor complications—For single-sided deafness and conductive or mixed hearing loss, the reference case for adults in models 2 and 3 was based on active transcutaneous devices (Table 16). One systematic review pooled the number of patients experiencing adverse events (5.1% over 11 months).43The majority of studies were in adult-only populations. Minor events included skin infections, wound pain, dizziness, tinnitus, and headache.
For children, the reference case in models 2 and 3 was based on two studies conducted in children with active percutaneous devices (Table 16).86,87 One of the studies also included children with passive transcutaneous devices. The rate of minor complications was high (more than 70% over 2 to 3 years).
Sensitivity analyses for the rate of minor complications were based on different device types, as summarized in Appendix 7, Table A8.
-
Major complications—For adults with single-sided deafness or conductive or mixed hearing loss, the reference case in models 2 and 3 was based on studies of active transcutaneous devices (Table 16). One systematic review pooled the total number of people requiring revision surgery after implantation (0.85% over 12 months).43 The vast majority of studies were in adult-only populations.
For children with single-sided deafness or conductive or mixed hearing loss, the reference case in models 2 and 3 was based on the complication rate in three studies of children with active percutaneous devices.86–88 One of the studies also included children with passive transcutaneous devices.
Sensitivity analyses for the rate of minor complications were based on different device types, as summarized in Appendix 7, Table A8.
Table 16:
6-Month Probability of Complications After Receiving a Bone-Conduction Implant
| Complications | Estimate | SD | Distribution | Reference |
|---|---|---|---|---|
| Minor complications (occurrence during first year) | ||||
| Active transcutaneous devices, adults | 0.0283 | 0.0028a | Beta | Sprinzl and Wolf-Magele, 201543 |
| Active percutaneous devices, children | 0.5632 | 0.0563a | Beta | Chan et al, 201786; Kraai et al, 201187 |
| Major complications (ongoing occurrence) | ||||
| Active transcutaneous devices, adults | 0.0043 | 0.0004a | Beta | Sprinzl and Wolf-Magele, 201543 |
| Conditional probability explantation,b adults | 0.54 | ±10% | Uniform | Badran et al, 200970 (assumed same as active percutaneous) |
| Conditional probability re-implantation,c adults | 0.77 | ±10% | Uniform | Badran et al, 200970 (assumed same as active percutaneous) |
| Active percutaneous devices, children | 0.0345 | 0.0035a | Beta | Chan et al, 201786; Kraai et al, 201187; Yellon et al, 200788 |
| Conditional probability explantation,b children | 0.41 | ±10% | Uniform | Chan et al, 201786; Kraai et al, 201187; Yellon et al, 200788 |
| Conditional probability re-implantation,c children | 0.47 | ±10% | Uniform | Chan et al, 201786; Yellon et al, 200788 |
Abbreviation: SD, standard deviation.
Note: 6-month probabilities are reported unless otherwise stated.
SD assumed to be 10% of mean.
Conditional probability of explantation given a major complication. Calculated as no. of individuals with explantation (either explantation only or re-implantation) ÷ no. of individuals with major complications.
Conditional probability of re-implantation given an explantation. Calculated as no. of individuals with re-implantation ÷ no. of individuals with explantation (either explantation only or re-implantation).
Explantation (including explantation only or with re-implantation). A subset of major complications included those that require the implant to be removed and, in some cases, re-implanted (i.e., due to device malfunction or patient request).
Re-implantation only. A subset of explantations includes re-implantations. Patients may have more than one re-implantation over the course of the model.
Elective device non-use. Patients who have received a bone-conduction implant may choose to stop using their sound processor. We stratified by adults and children where possible (Table 17). In the reference case for most subgroups, we used probabilities from Ontario data or literature and assumed non-use was constant over the duration of the follow-up. While expert opinion suggests non-use in adults with conductive or mixed hearing loss is negligible, to be conservative we assumed 5% of patients over 10 years would cease to use their device.
Table 17:
Probability of Not Using Bone-Conduction Implant
| Adults | Children | ||||
|---|---|---|---|---|---|
| Probability | Time Frame | Probability | Time Frame | Reference | |
| Reference case | |||||
| Model 2 (single-sided deafness) | 5% | 4 y | 5% | 4 y | Data from OCIPa (assumed children same as adults) |
| Model 3 (conductive/mixed hearing loss) | 5% | 10 y | 11% | 7.7 m | Assumption (adults); Polonenko et al, 201689 (children) |
| Sensitivity analysis | |||||
| Model 2 (single-sided deafness) | 30% | 7.5 y | 72.7% | 8.6 y | Expert opinion; Kesser et al, 201390 |
| Model 3 (conductive/mixed hearing loss) | 10% | 7.5 y | 10% | 7.5 y | Expert opinion (assumed children same as adults) |
| Model 3 (conductive/mixed hearing loss) | 0% | 4 y | Data from OCIPa | ||
| Model 3 (conductive/mixed hearing loss) | Up to 19% | 3.2 y | Up to 11% | 2.8 y | Gluth et al, 201091; Hobson et al, 201072; Kraai et al, 201187; Kiringoda and Lustig, 201356 |
Abbreviations: OCIP, Ontario Cochlear Implant Program; y, year.
Personal communication, Joseph Chen, MD, July 12, 2018.
In a series of sensitivity analyses, we assumed the risk of non-use is constant over the entire time horizon and we used non-use estimates based on expert opinion and the literature. Appendix 7, Elective Non-use, Table A10, shows the corresponding 6-month probabilities.
Common Parameters Across All Models
Mortality
We assumed hearing loss or hearing implants did not have an impact on mortality. People in all health states had the same mortality (Statistics Canada lifetables, 2014–2016).92 In sensitivity analyses, we assumed an increased risk of mortality associated with hearing loss, which was reported for people aged 70 years or older.93 We applied a statistically nonsignificant hazard ratio to people over age 70 without an implant (1.39; 95% confidence interval = 0.97 to 2.01).93 This hazard ratio is applicable in two scenario analyses: when the time horizon is extended, and when the age of implantation in adults is at the upper range. In the reference case, the models terminate before people reach age 70.
Sound Processor
Given the time horizon of 25 years (for model 1) and 10 years (for models 2 and 3), the external component of a hearing implant (the sound processor) may need replacement or upgrading due to personal choice or malfunction. Table 18 summarizes the time to replacement, manufacturer warranty, and funding provided for sound processors by the Assistive Devices Program of the Ministry of Health. In the reference case, the program pays for replacement up to a certain limit ($5,444 or $3,000, depending on device). In the scenario analysis using a public payer perspective, we assumed 40% of patients would be considered low-income and, therefore, the Ministry of Community and Social Services would pay remaining costs not covered by the Assistive Devices Program. In another scenario analysis, we assumed the program would pay the full cost of replacement. We assumed that the cost of a sound processer is approximately half of the total device cost (i.e., internal implant and sound processor).
Table 18:
Model Inputs for Replacement of Sound Processors for Hearing Implants
| Model 1 | Models 2 and 3 | |
|---|---|---|
| Sound processor replacement time (uniform distribution) | 5 to 10 y | 3 to 7 y |
| Manufacturer warranty | 5 y for initial; 3 y for subsequent | 2 to 3 y |
| Current ADP funding outside warranty period | 75% of cost, up to $5,44494 | 75% of cost, up to $3,00094 for active percutaneous devices; none for other bone-conduction implants |
| Reference case: ADP funds up to a limit | $5,444 | $3,000 for all bone-conduction implants |
| Scenario: Public payer (40% eligible for MCSS funding) | $5,444 for 60%; $11,000a for 40% | $3,000 for 60%; $4,500–$5,500 for 40% |
| Scenario: ADP funds full list price | $11,000 | $4,500-$5,500 |
Abbreviations: ADP, Ministry of Health Assistive Devices Program; MCSS, Ministry of Community and Social Services.
Utilities
Utilities are measures of patients' preferences about quality of life in different health states. While the clinical review identified several studies on hearing-specific quality of life, these cannot be translated into utility values without validated mapping algorithms. In the absence of mapping studies, we performed a targeted literature search for utility values on February 26, 2018, for studies published from inception to the search date in MEDLINE. The search was based on the clinical search strategy with a methodological filter applied to limit retrieval to health state utility values.95 See Appendix 1 for the literature search strategies, including all search terms. In addition, the health economist searched the Cost-Effectiveness Analysis Registry published by Tufts Medical Center for utilities and disutilities (decreases, or decrements, in quality of life) associated with the model health states. We screened for preference-based quality of life measures, which differ from other quality of life measures (i.e., acoustic measures) reported in the clinical review.
We restricted our reference case to utilities derived from the Health Utilities Index Mark III (HUI3) questionnaire (a generic health-related quality-of-life tool), for two reasons. First, preferences in this questionnaire are derived from the Canadian general public.96 Second, it includes a question on hearing among other health attributes such as pain and mobility. The hearing attribute allows the HUI3 to be more responsive than other generic questionnaires (i.e., SF-36 or EQ-5D) in estimating utility related to hearing loss.97 Utilities range from 0 (equivalent to being dead) to 1 (equivalent to perfect health). A mean difference in health utilities of more than 0.03 in the HUI score is considered clinically relevant.98 We constrained the total health utility per year to a value between 0 and 1.
We were unable to obtain disutilities associated with complications in our populations of interest from these searches. In the reference case, we did not apply disutilities. In sensitivity analyses, we applied disutilities obtained from the Health Quality Ontario health technology assessment on bilateral cochlear implants.29
Model 1: Cochlear Implants for Single-Sided Deafness
In the reference case, we derived health utilities from the literature (Table 19). According to a systematic review in 2016,41 only one study reported preference measures for people with single-sided deafness receiving cochlear implantation.99 These utilities were derived from a single centre in Germany, where adults responded to the HUI3 before receiving a cochlear implant and 6 months after the implant was activated. We did not identify relevant studies for children with single-sided deafness, so we assumed utilities were the same as for adults (Table 19).
Table 19:
Health Utilities Used in Economic Model for Adults and Children With Single-Sided Deafness Receiving a Cochlear Implant
| Estimate | SD | Distribution | Reference | |
|---|---|---|---|---|
| Reference case | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.56 | 0.3101 | Beta | Arndt et al, 201199 (based on HUI3) |
| Impact of intervention (mean differencea) | ||||
| Cochlear implant vs. unaided | 0.24 | 0.3106 | Normal | Derived from Arndt et al, 201199 (based on HUI3) |
| Sensitivity analyses | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.80 | 0.0153 | Beta | Lucas et al, 2015100 (based on TTO in general public) |
| Impact of intervention (mean differencea,b) | ||||
| Cochlear implant vs. unaided | 0.050 | 0.0073 | Normal | Derived from Lucas et al, 2015100 (based on TTO in general public) |
| Disutilitiesc | ||||
| Minor complication | 0.020 | 0.0015 | Beta | Health Quality Ontario, 201829 |
| Major complication | 0.020 | 0.0015 | Beta | Health Quality Ontario, 201829 |
Abbreviations: HUI3, Health Utilities Index Mark III questionnaire; SD, standard deviation; TTO, time trade-off methodology.
Utility values derived from adults but assumed to be the same in children.
Mean difference = utilities of intervention group minus comparator group. Health utilities range from 0 (equivalent to dead) to 1 (perfect health).
Disutilities are decrements to quality of life.
From the grey literature, we identified a conference poster reporting utilities, measured using the time trade-off methodology, among the general public in the United Kingdom.100 We used these estimates in sensitivity analyses.
Model 2: Bone-Conduction Implants for Single-Sided Deafness
In the reference case, we used time-dependent HUI3 data provided by an Ontario hospital (written communication, Joseph Chen, MD, June 13, 2018). The study sample was adults who had received an active transcutaneous implant (n = 17) and were surveyed up to 12 months after implantation. We assumed the improvement realized at 12 months (not statistically significant) remained constant thereafter, if the individuals continued to use their device. In the literature, we were unable to identify relevant data for children, and so we assumed they experienced the same baseline quality of life and implant benefit (Table 20).
Table 20:
Health Utilities Used in Economic Model for Adults and Children With Single-Sided Deafness Receiving a Bone-Conduction Implant
| Estimate | SD | Distribution | Reference | |
|---|---|---|---|---|
| Reference case | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.78 | 0.1693 | Beta | Data from Ontario hospital (based on HUI3)b |
| Impact of intervention (mean differencea,c) | ||||
| Active transcutaneous vs. unaided, months after implantation: | ||||
| 6 m | 0.06 | 0.0526 | Normal | Data from Ontario hospital |
| 12 m and onwards | 0.01 | 0.0701 | (based on HUI3)b | |
| Sensitivity analyses | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.56 | 0.3101 | Beta | Arndt et al, 201199 (based on HUI3) |
| Impact of intervention (mean differencea,c) | ||||
| Bone-conduction hearing aid (i.e., softband/tension clamp) vs. unaided | 0.11 | 0.3539 | Normal | Derived from Arndt et al, 201199 (based on HUI3); assumed bone-conduction implant produced the same improvement as hearing aid |
| Disutilitiesd | ||||
| Minor complication (1-month duration) | 0.020 | 0.0015 | Beta | Assumed same as model 1 |
| Major complication | 0.020 | 0.0015 | Beta | Assumed same as model 1 |
Abbreviations: EQ-5D VAS, EuroQol 5 Dimensions questionnaire visual analogue scale; HUI3, Health Utilities Index Mark III questionnaire; SD, standard deviation.
Utility values derived from adults but assumed to be the same in adults.
Written communication, Joseph Chen, MD, June 13, 2018.
Mean difference = utilities of intervention group minus comparator group. Health utilities range from 0 (equivalent to dead) to 1 (perfect health).
Disutilities are decrements to quality of life.
For sensitivity analyses, we used utilities from the literature (see Appendix 7, Utilities, for description).
Model 3: Bone-Conduction Implants for Conductive or Mixed Hearing Loss
In the reference case, we used time-dependent HUI3 data provided by an Ontario hospital (written communication, Joseph Chen, MD, June 13, 2018) to reflect benefits for adults implanted with an active transcutaneous device (n = 33). Again, we assumed the improvement realized at 12 months remained constant for the remainder of time, as long as the individuals continued to use their device. Given the lack of data for children, we assumed they had the same health utilities as adults (Table 21).
Table 21:
Health Utilities Used in Economic Model for Adults and Children With Conductive or Mixed Hearing Loss Receiving a Bone-Conduction Implant
| Estimate | SD | Distribution | Reference | |
|---|---|---|---|---|
| Reference case | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.69 | 0.1972 | Beta | Data from Ontario hospital (based on HUI3)b |
| Impact of intervention (mean differencea,c) | ||||
| Active transcutaneous vs. unaided, months after implantation: | ||||
| 6m | 0.07 | 0.0559 | Normal | Data from Ontario hospital (based on |
| 12 m and onwards | 0.04 | 0.0538 | HUI3)b | |
| Sensitivity analysis | ||||
| Health states for adults and childrena | ||||
| Alive without implant | 0.57 | 0.028 | Beta | Monksfield et al, 201162 (based on HUI3, mix of conductive/mixed and single sided sensorineural hearing loss) |
| Impact of intervention (mean differencea,c) | ||||
| Active percutaneous vs. unaided | 0.09 | 0.042 | Normal | Monksfield et al, 201162 (based on HUI3, mix of conductive/mixed and single sided sensorineural hearing loss) |
| Disutilitiesd | ||||
| Minor complication (1-month duration) | 0.020 | 0.0015 | Beta | Assumed same as model 1 |
| Major complication | 0.020 | 0.0015 | Beta | Assumed same as model 1 |
Abbreviation: HUI3, Health Utilities Index Mark III questionnaire.
Utility values derived from adults but assumed to be the same in adults.
Written communication, Joseph Chen, MD, June 13, 2018.
Mean difference = utilities of intervention group minus comparator group. Health utilities range from 0 (equivalent to dead) to 1 (perfect health).
Disutilities are decrements to quality of life.
For sensitivity analyses, we used utilities from the literature (see Appendix 7, Utilities, for description).
Cost and Resource Use Parameters
We report currency in Canadian dollars in the costing index year of 2018 (Consumer Price Index for Canada health and personal care).101 We included direct medical costs associated with hearing implants. Our target population were those who did not benefit from other amplification methods (i.e., hearing aids); therefore we assumed the comparator arm (i.e., no hearing implant) did not have any associated costs. For the intervention arm (i.e., hearing implant), we included the following costs:
Preprocedural costs—candidacy assessment, imaging, and consultations
Procedural costs—cost of device, surgeon fees, and hospital costs (e.g., operating room costs, post-anesthetic care unit costs)
Postprocedural costs—surgical/wound management (i.e., follow-up with the surgeon), audiologic management, as well as rehabilitation for cochlear implant recipients.
Complication costs—minor, major, explantation, and re-implantation
In current practice, Ontario provides no targeted public funding for hearing implants in these populations, but they are still made available to a very limited extent through hospital funding provided by the Ministry of Health or research grants. Some costs may be covered by the individuals (or families) receiving the devices (i.e., batteries, partial device costs, and processor upgrades). Assumptions about funding mechanisms considered in our model are as follows:
Internal device bundled with initial sound processor—Some manufacturers provided a list price for the internal device and external sound processor combined, while others provided them separately. We assumed a bundled cost in the economic models, fully paid for by the Ministry.
Sound processor upgrades/replacement—Replacing or upgrading the sound processor is currently covered, in part, by the Assistive Devices Program for some devices (cochlear implants and active percutaneous bone-conduction implants only). In our reference case analysis, we assumed the program would pay up to a maximum amount as outlined in Table 18. In scenario analyses, we assumed the program would pay the full amount.
Battery replacement—We assumed manufacturers or patients would cover the cost of battery replacements. Hence, we did not include these in our economic model.
Preprocedural, Procedural, and Postprocedural Costs
Model 1: Cochlear Implants for Single-Sided Deafness
Based on expert consultation, the patient pathway for model 1 is the same as that for unilateral cochlear implantation in bilateral sensorineural hearing loss. Hence, we used many of the same assumptions on frequency of health care visits and cost and resource use parameters from the previous Health Quality Ontario health technology assessment.29 The previous assessment was based on Chen et al, 2014,74 and expert opinion.
Table 22:
Preprocedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness Receiving a Cochlear Implant
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Preprocedural assessment tests, adults | ||||
| Audiologic assessments | 63.78 | 1 | 63.78 | Health Quality Ontario, 201829 (based on Chen et al, 201474 and clinical experts) |
| Vestibular assessment | 116.93 | 1 | 116.93 | |
| Preprocedural assessment tests, children | ||||
| Audiologic assessment | 48.54 | 3 (3 hours) | 145.62 | Health Quality Ontario, 201829 (based on OPSEU collective agreement102 and clinical experts); expert opinion |
| Vestibular assessment | 116.93 | 1 | 116.93 | |
| Language assessment | 48.54 | 1 (1 hour) | 48.54 | |
| Social worker | 48.54 | 1 (1 hour) | 48.54 | |
| Other preprocedural costs | ||||
| MRIa | 223.45 | 1 | 223.45 | Schedule of Benefits (X421, Z430)103; Health Quality Ontario, 201829 |
| CT scanb | 43.15 | 1 | 43.15 | Schedule of Benefits (X001)103; Health Quality Ontario, 201829 |
| Surgical consult | 160.00 | 1 | 160.00 | Schedule of Benefits (A935)103; Health Quality Ontario, 201829 |
| Preoperative general assessment | 65.05 | 1 | 65.05 | Schedule of Benefits (A903)103; Health Quality Ontario, 201829 |
| Total preprocedural costs, adults, $ | 448.91 | |||
| Total preprocedural costs, children, $ | 808.13 | |||
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; OPSEU, Ontario Public Service Employees Union.
MRI with anesthesia used for children.
CT scan used for adults.
Table 23:
Procedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness Receiving a Cochlear Implant
| Variable | Cost, $ | SD | Distribution | Reference |
|---|---|---|---|---|
| Device cost (internal device + sound processor) | 25,000.00 | 2,500 | Gamma | Health Quality Ontario, 201829 |
| Hospital costsa | 4,722.74 | 940 | Gamma | Merdad et al, 2014104 |
| Physician fees for cochlear implant | 1,524.16 | N/A | N/A | Schedule of Benefits (E341, E320)103 |
| Total procedural costs, adults and children | 31,246.90 |
Abbreviations: N/A, not applicable; SD, standard deviation.
Includes operating room time, nursing, anesthesiology, supplies, etc. Does not include indirect costs such as overhead.
In line with the previous Health Quality Ontario health technology assessment,29 we incorporated the following postprocedural costs. As part of surgical/wound management, patients have a follow-up visit with the clinician. As part of audiologic management, there is follow-up with an audiologist to program and optimize the cochlear implant. As part of rehabilitation to retrain hearing, patients often receive auditory-verbal therapy. In the reference case, we assumed no adults received rehabilitation (neither hospital-based nor community-based). We assumed 5% of children received hospital-based rehabilitation and the remaining 95% received community-based rehabilitation (funded by the Ministry of Child and Youth Services Infant Hearing Program and the Ministry of Education). The length of rehabilitation is expected to vary, but conservatively we assumed for children it would consist of 1 appointment per week over 18 months. In sensitivity analyses, we varied the proportion of adults and children receiving auditory-verbal therapy. We assumed rehabilitation for adults would consist of 2 to 3 one-hour sessions funded by hospitals.
Table 24:
Postprocedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness Receiving a Cochlear Implant
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Follow-up visit with ENT specialist (for adults and children) | 31.00 | 1 | 31.00 | Schedule of Benefits (C242)103 |
| Follow-up costs, audiologist appointments, adults | ||||
| Year 1 | 48.54 | 5 (5 hours) | 242.70 | Health Quality Ontario, 201829 (based on OPSEU collective agreement102 and clinical experts) |
| Year 2 | 48.54 | 1 (1 hour) | 48.54 | |
| After year 2 | 48.54 | Every other year (1 hour) | 48.54/2 years | |
| Follow-up costs, audiologist appointments, children | ||||
| Year 1 | 48.54 | 4 (4 hours) | 194.16 | Health Quality Ontario, 201829 (based on OPSEU collective agreement102 and clinical experts); expert opinion |
| Year 2 | 48.54 | 2 (2 hours) | 97.08 | |
| After year 2 | 48.54 | Every year (1 hour) | 48.54/1 year | |
| Rehabilitationa (audio-verbal therapist) | ||||
| Children | 48.54 | Every week (1 hour) for 18 monthsa | 3,786.12 | Health Quality Ontario, 201829 (based on OPSEU collective agreement102 and clinical experts); expert opinion |
| Total postprocedural costs, adults (first 2 years, not including rehabilitation) | 322.24 | |||
| Total postprocedural costs, children (first 2 years, not including rehabilitation) | 322.24 | |||
Abbreviations: OPSEU, Ontario Public Service Employees Union.
Occurs in hospital for 5% of children in the reference case analysis.
Models 2 and 3: Bone-Conduction Implants for Single-Sided Deafness and Conductive or Mixed Hearing Loss
The costs associated with a bone-conduction implant should be the same regardless of whether the patient has single-sided deafness versus conductive or mixed hearing loss (Tables 25, 26, 27). For procedural costs (Table 26), mean estimates for device costs and physician fees were based on active transcutaneous devices for adults and active percutaneous devices for children. Ranges were based on other bone-conduction implant costs. In some cases, in children, bone-conduction devices may be implanted in a two-stage surgery. The range of physician fees account for the additional physician fees that would be associated with a two-stage surgery. For pre- and postprocedural costs (Tables 25 and 27), the unit costs were informed by collective agreements or the Schedule of Benefits. The quantities (number of hours or visits) were informed by a health technology assessment conducted by the University of Alberta47 as well as clinical experts. We assumed there would be no rehabilitation required for patients receiving bone-conduction implants.
Table 25:
Preprocedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness or Conductive/Mixed Hearing Loss Receiving a Bone-Conduction Implant
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Preprocedural assessment tests, adults | ||||
| Audiologic assessment (including counselling) | 48.54 | 6 hours | 291.24 | OPSEU collective agreement102 and clinical experts |
| Preprocedural assessment tests, children | ||||
| Audiologic assessment | 48.54 | 2 hours | 97.08 | University of Alberta, 201147; OPSEU collective agreement102 and clinical experts |
| Other preprocedural costs | ||||
| ENT specialist visit | 77.90 | 1 | 77.90 | Schedule of Benefits (C245)103 and clinical experts |
| Total preprocedural costs, adults | 369.14 | |||
| Total preprocedural costs, children | 174.98 | |||
Abbreviations: ENT, ear, nose, and throat; OPSEU, Ontario Public Service Employees Union.
Table 26:
Procedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness or Conductive/Mixed Hearing Loss Receiving a Bone-Conduction Implant
| Variable | Cost, $ | SD or Range | Distribution | Reference |
|---|---|---|---|---|
| Adults, active transcutaneous device | ||||
| Internal device + sound processor | 11,000.00 | Range: 6,000 to 11,000 | N/A | Manufacturers |
| Physician fee | 733.55 | Range: 345.15 to 1,556.74 | N/A | Reference case: Ministry of Health |
| Range: Schedule of Benefits (E346, E322+R005)103 | ||||
| Hospital costsa | 3,701.93 | SD: 2,129.16 | Gamma | Ontario Case Costing day surgery (2016/2017)105b |
| Children, active percutaneous device | ||||
| Internal device + sound processor | 9,000.00 | Range: 6,000 to 11,000 | N/A | Manufacturers |
| Hospital costsa | 3,701.93 | SD: 2,129.16 | Gamma | Ontario Case Costing day surgery (2016/2017)105b |
| Physician fee | 733.55 | Range: 345.15 to 1,556.74 | N/A | Reference case: Ministry of Health |
| Range: Schedule of Benefits (E346, E322+R005)103 | ||||
| Total procedural costs, adults | 15,435.48 | |||
| Total procedural costs, adults | 13,435.48 | |||
Abbreviations: N/A, not applicable; SA, sensitivity analysis; SD, standard deviation
Includes operating room time, nursing, anesthesiology, supplies, etc. Does not include indirect costs such as overhead.
Including Canadian Classification of Health Initiatives codes 1DL53LAEF,1DL53LAEFA,1DL53LAEG,1DL53LAEGB,1DL53LAEJ.
Table 27:
Postprocedural Costs Included in the Economic Model for Adults and Children With Single-Sided Deafness or Conductive/Mixed Hearing Loss Receiving a Bone-Conduction Implant
| Variable | Unit Cost, $ | Quantity (Total Duration) | Total Cost, $ | Reference |
|---|---|---|---|---|
| Follow-up visitsa with ENT specialist (for adults and children) | 31.00 | 3 | 93.00 | Schedule of Benefits (C242, C247, C249)103 and clinical experts |
| Follow-up costs, audiologist appointments, adults | ||||
| Year 1 | 48.54 | 3 (3 hours) | 145.62 | OPSEU collective agreement102 and clinical experts |
| After year 1 | 48.54 | 1 (1 hour) each year | 48.54 | |
| Follow-up costs, audiologist appointments, children | ||||
| Year 1 | 48.54 | 4 (4 hours) | 194.16 | OPSEU collective agreement102 and clinical experts |
| After year 1 | 48.54 | 1 (1 hour) each year | 48.54 | |
| Total postprocedural costs (in first 2 years), adults | 287.16 | |||
| Total postprocedural costs (in first 2 years), children | 335.70 | |||
Abbreviations: ENT, otolaryngologist; OPSEU, Ontario Public Service Employees Union.
ENT visits at 3 weeks, 3 to 6 months, and 1 year post-implantation.
Complication Costs
We had four broad categories of costs related to complications: (i) minor complications (requiring conservative management); (ii) major complications (requiring hospitalization or revision surgery without explantation or re-implantation); (iii) explantation without re-implantation; and (iv) explantation followed by re-implantation. We weighted common types of minor events to generate an average cost for minor complications. We weighted common types of surgical revisions to generate an average cost for major complications (for calculations, see Appendix 7, Costs Associated With Complications, and Table A11). Table 28 shows the average costs of complications used in the reference case for the three economic models.
Table 28:
Complication Costs for Adults and Children Receiving a Hearing Implant
| Average Cost Adults, $ | Average Cost Children, $ | References | |
|---|---|---|---|
| Minor complication, all models | 73.66 (Range: 72.78–93.88) | 87.84 (Range: 83.93–93.88) | Schedule of Benefits103; Ontario Drug Benefit Formulary106 |
| Major complication, all models | 2,928.78a | 2,761.26a | Ontario Case Costing (2016/2017)105; Merdad et al, 2014104 |
| Explantation only, all models | 4,427.05a | 4,427.05a | Gaboury et al, 2010107 |
| Re-implantation only, model 1 | 18,427.05a,b | 18,427.05a,b | Derived based on Health Quality Ontario 201829 |
| Re-implantation only, models 2 and 3 | 10,516.28a,b | 9,516.28a,b | Reference case: University of Alberta, 2001,47 for hospital costs plus physician fees and internal device costs from Table 26 |
Abbreviations: SA, sensitivity analysis.
Assumed a standard deviation of 10% for gamma distribution.
Assumed only cost of internal device required, no external processor cost included.
Based on active transcutaneous in adults and active percutaneous in children.
Analysis
We conducted all analyses in TreeAge Pro 2018. Outcomes are reported as incremental cost-effectiveness ratios (ICER), which represent the incremental cost for each additional quality-adjusted life-year (QALY) gained.
In the reference case, we analyzed each model probabilistically using Monte Carlo simulations. To capture parameter uncertainty, we randomly sampled parameter distributions 10,000 times. Results of the probabilistic analyses are presented as cost-effectiveness acceptability curves.
Scenario analyses were also run probabilistically. We ran one-way deterministic sensitivity analyses to examine the impact of varying one parameter on the results. Deterministic results are presented as a tornado diagram. Table 29 summarizes the analyses described in the Methods section above.
Table 29:
Sensitivity Analyses and Scenario Analyses, Primary Economic Evaluation
| Scenario | Parameter(s) Used in Reference Case | Parameter(s) Used in Scenario Analysis |
|---|---|---|
| Age of implantation (all models) | Mean age (Table 12) | Lower and upper range (Table 12) |
| Time horizon (all models) | 25 years for cochlear implants; 10 years for bone-conduction devices | Lifetime |
| Perspective (all models) | Ministry of Health | Public payer (includes Ministry Health, Ministry of Community and Social Services, Ministry of Child and Youth Services, Ministry of Education); additional costs include sound processor replacement for people with low income, and rehabilitation for children with cochlear implants |
| Complication rates (models 2 and 3) | Rates associated with:
|
Rates associated with other types of bone-conduction implants (Appendix 7, Table A8) |
| Risk of minor complications (all models) Device non-use (all models) | Minor complications occur in first year of implantation As described in Table 15, Table 17, Appendix Table A9, and Appendix Table A10 | All models: minor complications occur over entire duration of model As described in Table 15, Table 17, Appendix Table A9, and Appendix Table A10; includes time-dependent data from the literature |
| Risk of non-use (all models) | Non-use occurs over duration of follow-up data from Ontario Cochlear Implant Program (4 m to 5 y) | Non-use occurs over entire duration of model |
| Mortality (all models) | Background mortality only | Increased risk of mortality associated with hearing loss in elderly ≥ 70 years (HR = 1.39; 95% CI = 0.97 to 2.01) |
| Health utilities (models 2 and 3) | HUI3 data from Ontario hospital based on active transcutaneous bone-conduction implants | Utilities from systematic search; based on other bone-conduction implants |
| Disutilities (all models) | No disutilities applied to complications | Applied disutilities to complications |
| Rehabilitation costs (model 1) | Community-based rehabilitation (every week for 18 months) for children; none for adults | Same as reference case for children; hospital-based rehabilitation (2 to 3 1-hour sessions) for adults |
| Sound processor (all models) | Assistive Devices Program pays up to a maximum amount for replacement sound processors | Assistive Devices Program pays the full amount for replacement sound processors |
| Discount rate (all models) | 1.5% | 0%, 3%, 5% |
Abbreviations: CI, confidence interval; EQ-5D VAS, EuroQol 5 Dimensions questionnaire visual analogue scale; HR, hazard ratio; HUI3, Health Utilities Index Mark III questionnaire; m, month; TTO, time trade-off; y, year.
Generalizability
The findings of this economic analysis cannot be generalized to all patients with single-sided deafness or conductive or mixed hearing loss. They may, however, be used to guide decision-making about the specific patient populations addressed in the trials investigated by Health Quality Ontario.
Results
Reference Case Analysis
Table 30 presents the reference case results. Among adults and children with single-sided deafness, cochlear implants provided greater health gains for an incremental cost compared with no intervention. Expressed as an incremental cost-effectiveness ratio (ICER), these estimates can be considered cost-effective under commonly used willingness-to-pay values of $50,000 and $100,000 per quality-adjusted life-year (QALY). Figure 7 shows the cost-effectiveness acceptability curves for adults and children. Over a range of willingness-to-pay values on the x-axis, the curves show the proportion of the 10,000 simulated ICERs that are considered to be cost-effective. At a willingness-to-pay of $100,000 per QALY, 70% of the simulations were considered cost-effective.
Table 30:
Reference Case Analysis Results
| Strategy | Average Total Costs (±SD), $ | Incremental Cost,a $ | Average Total Effects (±SD), QALYs | Incremental Effect,b,c QALYs | ICER,c,d $/QALY | Probability of Being Cost-Effective (WTP $100,00/QALY) |
|---|---|---|---|---|---|---|
| Model 1: Cochlear implants for single-sided deafness | ||||||
| Adults | ||||||
| No intervention | 0 | 11.30 (± 6.20) | ||||
| Cochlear implant | 50,089 (± 4,894) | 50,089 | 14.06 (± 6.00) | 2.76 | 18,148 | 70% |
| Children | ||||||
| No intervention | 0 | 11.55 (± 6.34) | ||||
| Cochlear implant | 53,497 (± 5,285) | 53,497 | 14.56 (± 6.32) | 3.01 | 17,783 | 70% |
| Model 2: Bone-conduction implants for single-sided deafness | ||||||
| Adults | ||||||
| No intervention | 0 | 6.89 (± 1.46) | ||||
| Bone-conduction implant | 22,436 (± 3,391) | 22,436 | 6.95 (± 1.58) | 0.06 | 408,350 | 38% |
| Children | ||||||
| No intervention | 0 | 6.90 (± 1.51) | ||||
| Bone-conduction implant | 22,798 (± 3,420) | 22,798 | 6.95 (± 1.57) | 0.05 | 402,899 | 37% |
| Model 3: Bone-conduction implants for conductive/mixed hearing loss | ||||||
| Adults | ||||||
| No intervention | 0 | 6.08 (± 1.74) | ||||
| Bone-conduction implant | 22,478 (± 3,322) | 22,478 | 6.38 (± 1.72) | 0.30 | 74,155 | 55% |
| Children | ||||||
| No intervention | 0 | 6.14 (± 1.79) | ||||
| Bone-conduction implant | 21,114 (± 3,438) | 21,114 | 6.38 (± 1.77) | 0.24 | 87,580 | 50% |
Abbreviations: CE, cost-effective; ICER, incremental cost-effectiveness ratio; Q1 and Q2, quadrants 1 and 2 of cost-effectiveness plane; QALY, quality-adjusted life-years; WTP, willingness-to-pay.
Incremental cost = average cost (hearing implant) – average cost (no intervention).
Incremental effect = average effect (hearing implant) – average effect (no intervention).
Numbers may appear off due to rounding.
ICER = incremental cost ÷ incremental effect.
Figure 7: Cost-Effectiveness Acceptability Curve for People With Single-Sided Deafness Receiving a Cochlear Implant.
Abbreviation: QALY, quality-adjusted life-year.
For people with single-sided deafness, bone-conduction implants provided minimal health gains (based on utilities derived from generic health-related quality-of-life tools) at an incremental cost compared with no intervention. Estimates of ICERs for both adults and children were not considered cost-effective under the commonly used willingness-to-pay value of $100,000 per QALY. At that willingness-to-pay, about 38% of the simulations were considered cost-effective. In about 45% of the simulations, bone-conduction implants were more costly and less effective than no intervention (Figure 8).
Figure 8: Cost-Effectiveness Acceptability Curve for People with Single-Sided Deafness Receiving a Bone-Conduction Implant.
Abbreviation: QALY, quality-adjusted life-year.
For people with conductive or mixed hearing loss, bone-conduction implants provided health gains at an incremental cost compared with no hearing implants. At a willingness-to-pay value of $100,000, about 50% to 55% of the simulations were considered cost-effective, and in 27% of the simulations were more costly and less effective than no intervention (Figure 9).
Figure 9: Cost-Effectiveness Acceptability Curve for People with Conductive/Mixed Hearing Loss Receiving a Bone-Conduction Implant.
Abbreviation: QALY, quality-adjusted life-year.
Sensitivity Analysis
Table 31 shows the results from scenario analyses where we tested alternate estimates of health-related quality of life. In the reference case analysis, we used health utilities derived from the HUI3 instrument, and we based estimates for models 2 and 3 on Ontario data. In scenario analyses, we used alternate estimates derived from a different way of capturing health-related quality of life (i.e., trade-off technique) for model 1, and different published sources for models 2 and 3. These scenarios are presented in detail because they consistently produced the greatest fluctuations in ICER estimates compared with the reference case. Despite the wide fluctuations, the scenario results did not qualitatively change the conclusions of cost-effectiveness demonstrated in models 1 and 3. ICER estimates in model 2 did change qualitatively if we assumed a greater improvement in health-related quality of life than we assumed in the reference case.
Table 31:
Scenario Analyses Using Alternate Mean Differences in Health Utilities, Results
| Scenario Analysis | Reference Case | |||||
|---|---|---|---|---|---|---|
| Average Total Costs (± SD), $ | Incremental Cost,a $ | Average Total Effects (± SD), QALYs | Incremental Effect,b,c QALYs | ICER,c,d $/QALY | ICER,d $/QALY | |
| Model 1: Cochlear implants for single-sided deafness | ||||||
| Adults: Based on time trade-off method, MDe: 0.050 | MDe: 0.24 | |||||
| No intervention | 0 | 16.00 (± 0.31) | ||||
| Cochlear implant | 50,090 (± 4,894) | 50,090 | 16.90 (± 0.34) | 0.90 | 55,655 | 18,148 |
| Children: Based on time trade-off method in adults, MDe: 0.050 | MDe: 0.24 | |||||
| No implant | 0 | 16.40 (± 0.31) | ||||
| Cochlear implant | 53,497 (± 5,285) | 53,497 | 17.39 (± 0.35) | 0.99 | 54,038 | 17,783 |
| Model 2: Bone-conduction implants for single-sided deafness | ||||||
| Adults: Based on bone-conduction hearing aid (i.e., softband/tension clamp), MDe: 0.11 | MDe: 0.01 | |||||
| No intervention | 0 | 4.92 (± 2.75) | ||||
| Bone-conduction implant | 22,436 (± 3,391) | 22,436 | 5.45 (± 3.01) | 0.53 | 42,332 | 408,350 |
| Children: Based on bone-conduction hearing aid (i.e., softband/tension clamp), MDe: 0.11 | MDe: 0.01 | |||||
| No intervention | 0 | 4.94 (± 2.76) | ||||
| Bone-conduction implant | 22,798 (± 3,420) | 22,798 | 5.46 (± 3.00) | 0.52 | 43,842 | 402,899 |
| Model 3: Bone-conduction implants for conductive/mixed hearing loss | ||||||
| Adults: Based on active percutaneous device, MDe: 0.09 | MDe: 0.04 | |||||
| No intervention | 0 | 4.98 (± 0.25) | ||||
| Bone-conduction implant | 22,478 (± 3,322) | 22,478 | 5.76 (± 0.44) | 0.78 | 28,818 | 74,155 |
| Children: Based on active percutaneous device, MDe: 0.09 | MDe: 0.04 | |||||
| No intervention | 0 | 5.05 (± 0.25) | ||||
| Bone-conduction implant | 21,114 (± 3,438) | 21,114 | 5.67 (± 0.41) | 0.62 | 30,054 | 87,580 |
Abbreviations: ICER, incremental cost-effectiveness ratio; MD, mean difference; QALY, quality-adjusted life-years.
Incremental cost = average cost (hearing implant) – average cost (no intervention).
Incremental effect = average effect (hearing implant) – average effect (no intervention).
Numbers may appear off due to rounding.
ICER = incremental cost ÷ incremental effect.
Represents the mean difference in utility values before and after implantation.
The other scenario analyses produced generally robust results and, in most cases, did not qualitatively change the conclusions about cost-effectiveness. Hence, we present only the range of ICERs from scenario analyses in Appendix 7, Table A12. Several of the lower ICER estimates were generated by the scenario with a lifetime time horizon plus an increased risk of death associated with having no intervention. This is expected because most costs are upfront (i.e., preprocedural, procedural, rehabilitation), so a longer time horizon allows for greater health benefits to accrue without many more costs over the long term. Applying an increased risk of death to those without hearing implants consistently improved the ICER, as expected, because more people without hearing implants transition to the “dead” health state and, therefore, do not accrue as many quality-adjusted life-years. Several of the upper ICER estimates were generated by applying disutilities for complications and assuming that the Assistive Devices Program would cover the full cost of sound processor replacements.
Appendix 7, Table A12, also presents results of the public payer scenario analysis, which includes costs we expected other ministries to cover, in addition to the Ministry of Health.
Figures A1 to A3 in Appendix 7 present the one-way deterministic sensitivity analyses as tornado diagrams. The cost-effectiveness results were most sensitive to variations in the mean difference for health utilities and to variations in the cost of devices. Health utilities ranged from positive (in favour of hearing implants) to negative (in favour of no intervention) and, subsequently, the hearing implants ranged from very cost-effective (in association with positive utilities) to inferior (not cost-effective, in association with negative utilities). The results were also sensitive, although less so, to the probability of major complications and device non-use.
Discussion
Results from the reference case and scenario analyses suggested that cochlear implants may be cost-effective compared with no intervention for people with single-sided deafness, but bone-conduction implants are unlikely to be cost-effective in this population. Among those with conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no intervention. For all three economic models, the simulated ICERs were all determined to be within quadrants 1 and 2 of the cost-effectiveness plane. Falling in quadrant 1 means that the hearing implant is costlier but more effective compared with no intervention. Being in quadrant 2 means the ICER is inferior; that is, the hearing implant is costlier and less effective than no intervention. Cochlear implants for single-sided deafness had about 70% of the simulations falling in quadrant 1 below a willingness-to-pay of $100,000 per QALY. There was greater uncertainty associated with bone-conduction implants for single-sided deafness (about 38% fell in quadrant 1 below the willingness-to-pay), and conductive or mixed hearing loss (50% to 55% fell in quadrant 1 below the willingness-to-pay).
As noted in the economic evidence review, we did not identify studies on cochlear implants in single-sided deafness to compare our results with. Two studies on bone-anchored hearing aids (active percutaneous devices) examined cost-effectiveness in populations with single-sided deafness and with conductive or mixed hearing loss, with conflicting conclusions.49,62 We were also unable to compare our results to other health technology assessments. The National Health Service assessment in 2016 did not identify any cost-effectiveness studies on active or passive transcutaneous bone-conduction implants or middle ear implants.31 The University of Alberta was unable to conduct a primary economic evaluation on middle ear implants.47
Impact of Model Inputs for Health-Related Quality of Life
Changes in generic health-related quality of life associated with a hearing implant were the main driver of the cost-effectiveness results in all three economic models. Results were not as sensitive to rates of complications and of device non-use. Even in scenario analyses using higher rates of complication derived from various types of bone-conduction implant (i.e., active percutaneous devices), cost-effectiveness results did not qualitatively change. Given the importance of changes to health utilities in our results, we elaborate below on the data sources. Note that a mean difference in health utilities of more than 0.03 in the HUI score is considered clinically relevant.98
Model 1—The mean difference in health utilities of 0.24 used in the reference case is considered very high. This model input was based on data from 11 individuals at a single centre in Germany, measured at baseline and 6 months after cochlear implant fitting.99 Despite it's small sample size, this was the only study identified in our systematic search for quality of life data associated with cochlear implants in single-sided deafness. No Ontario-specific data were available, unlike for the other two models. The reference case ICER was very favourable to cochlear implants, at $17,783 to 18,148 per QALY. In a scenario analysis, we used utilities obtained using the time trade-off technique, from a poster presentation. Under the reported mean difference of 0.050, the ICER may still be favourable, although more expensive, at $54,083 to 55,655 per QALY.
Model 2—The Ontario Cochlear Implant Program had HUI3 data available for bone-conduction implants in single-sided deafness (mean difference of 0.01 at 12 months, which we assumed remained constant onward). The reference case ICER was not favourable to bone-conduction implants for single-sided deafness, at $402,899 to 408,350 per QALY. In scenario analysis for adults and children, we used a utility gain of 0.11 derived from the same German study described above.99 Arndt et al99 measured health utilities in adults before any intervention and after an unspecified duration of testing with a bone-conduction hearing aid (i.e., Softband/tension clamp often used in a trial period prior to proceeding with an implantation). The utility gain appears very high, especially for an intervention that is not an implant but a bone-conduction hearing aid used to imitate an implant in testing. The scenario analysis ICERs became favourable at $42,322 to 43,842 per QALY, qualitatively changing the results of the analysis. This shows a great need for further research into health-related quality of life for this intervention, particularly in children, to reduce the uncertainty around its true health impact for specific populations.
Model 3—The Ontario Cochlear Implant Program had HUI3 data available for bone-conduction implants in conductive and mixed hearing loss (mean difference of 0.04 at 12 months, which we assumed remained constant onward). The reference case ICERs, on average, were favourable to bone-conduction implants, at $74,155 to $87,580 per QALY. However, there was significant uncertainty in the results, driven by uncertainty in the quality of life data. This can, in part, be attributed to the small sample size of the Ontario data. The scenario analysis used a mean difference of 0.09 derived from active percutaneous devices.62 Monksfield et al62 measured baseline utilities around 2 to 4 months after surgery and after 3 to 6 months of using the device at a single centre in the United Kingdom (n = 70). The scenario analysis ICERs became more favourable to bone-conduction devices, at $28,818 to $34,054 per QALY. The uncertainty was also reduced when using the utilities from this study.
Despite wide fluctuation in our results, our clinical review found that implants improved quality of life when measured by disease-specific quality-of-life tools. However, converting these measures to utilities is controversial, and mapping algorithms are not currently available. Thus, we used generic health-related quality-of-life measures, which may not be as sensitive as changes in hearing. Given the challenges involved in capturing quality-of-life benefits in this population, our results should be considered along with all clinical outcomes (including those we were unable to incorporate into the model).
Additional Limitations and Strengths
There are several additional limitations to our study. The scope of the health technology assessment was broad, examining two classes of intervention (cochlear implants and bone-conduction implants) for three populations (single-sided deafness, conductive hearing loss, and mixed hearing loss). We considered all types of bone-conduction implants (i.e., active transcutaneous, active percutaneous, passive transcutaneous devices, middle ear implants) as a single device class. An alternate approach would be to build a separate economic model for each type of device. However, we were limited by the availability of research in the peer-reviewed literature and would unlikely have sufficient data to populate each economic model with device-specific parameters (i.e., health utilities, device non-use, complications). For instance, our systematic search found no studies of heath utilities associated with active and passive transcutaneous devices. For these reasons, our reference analysis for research questions 2 and 3 used data from Ontario adults. In sensitivity analyses, we varied parameters based on other types of bone-conduction implants.
In our analysis, we assumed people either used their hearing implant devices over the entire model time horizon or stopped at some point and remained a non-user. In actuality, people may use their device for parts of the day, or some days of the week, or stop using their device for years and then use it again. We were unable to estimate the utility gains associated with regular use versus partial use because the primary studies reported health utilities for samples with a hearing implant in general, not broken down by usage.
The quality of our analysis is dependent on the availability and quality of the literature from which we drew many of our model parameters. For instance, we did not identify any applicable quality-of-life studies in children; hence, we had to assume their health state utility and benefit after receiving an implant were the same as for adults, butt is unclear whether these populations would gain similar benefit from hearing implants. In addition, as noted above, the primary clinical studies on generic health-related quality of life had fairly small sample sizes, which would be prone to being influenced by data outliers. This contributed to the large amount of uncertainty in models 2 and 3. Note that the baseline health utilities (without an implant) varied widely from one source to another. In some cases, the upper range of baseline health utility approached 1 (perfect health). This produces a ceiling effect, whereby potential benefits due to a hearing implant may not be realized, as the health utility could not exceed 1 in our model. Device non-use provides another example of outlier effects in small sample sizes. In particular, the probability of non-use in children was 11% over 7.7 months.89 This very high rate is an artefact of having 1 non-user among 9 children over a short follow-up time. To partially address this problem, we did not extrapolate non-use beyond the follow-up period (which was as short at 7.7 months) in the reference case, and we extrapolated the risk of non-use over the entire time horizon in scenario analysis.
In addition, the primary clinical studies on bone-conduction implants that we identified did not have very long follow-up. The adult reference cases in models 2 and 3 were predominantly based on active transcutaneous devices because these are commonly used in Ontario today. Due to the short follow-up in the relevant studies, however, we had to extrapolate data over the long-term in our analyses. For instance, major complications were extrapolated to 10 years from follow-up of 12 months or less.43 This assumes that the risk of complications does not increase or decrease after the 12 months. Our results could be underestimated if complications increased or overestimated if complications decreased after the follow-up period. To partially address this issue for minor complications, the reference case for models 2 and 3 assumed the risk of minor complications dropped to zero after 1 year.
Our analysis used two perspectives of relevance: that of the Ministry of Health and a broader public payer perspective that added costs borne by the Ministries of Community and Social Services, Child and Youth Services, and Education. These perspectives do not incorporate indirect costs associated with productivity loss for adults and educational outcomes for children. Nor do they incorporate out-of-pocket costs for individuals such as travel to clinic. Information on indirect costs from Canadian sources is limited.
In the model, we assumed there were no direct costs associated with the comparator arm (i.e., no hearing implants). This is a conservative assumption, in line with the Ontario Cochlear Implant Program candidacy criteria, which is specific to people who do not benefit from conventional hearing aids or who have a condition (i.e., chronically draining ears, narrow/no ear canal) that precludes wearing them. If the comparator arm had associated costs, such as from using hearing aids, then the difference in costs between the intervention arm and comparator arm would be smaller. Consequently, the ICER estimate would also be smaller, showing more favourable cost-effectiveness results for hearing implants.
There are several strengths to this analysis. Firstly, model parameters were specific to Ontario wherever possible (namely, costs, health utilities, and patient demographics) to support funding recommendations in the province. Note that generalizability outside of Ontario may be limited. Secondly, we considered numerous parameter and methodological uncertainties. Our results remained generally robust across most scenarios. We noted that changes in health-related quality of life associated with a hearing implant were very important to the cost-effectiveness results. Thirdly, study methodology was based on consultations with stakeholders including clinical experts, manufacturers, and the Ontario Ministry of Health.
Conclusions
Among people with single-sided deafness, cochlear implants may be cost-effective compared with no intervention, but bone-conduction implants are unlikely to be. Among people with conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no intervention. Results are mainly driven by changes in health utilities associated with having a hearing implant. Hence, further research on health utilities in this population is warranted with larger sample sizes and longer follow-up.
BUDGET IMPACT ANALYSIS
Research Questions
From the perspective of the Ontario Ministry of Health, we asked the following questions:
-
1.
What is the potential budget impact in Ontario of publicly funding cochlear implants in adults and children with single-sided deafness?
-
2.
What is the potential budget impact in Ontario of publicly funding bone-conduction implants in adults and children with single-sided deafness?
-
3.
What is the potential budget impact in Ontario of publicly funding bone-conduction implants in adults and children with conductive or mixed hearing loss?
Methods
Analytic Framework
The budget impact of hearing implants (cochlear implants and bone-conduction implants) was estimated as the cost difference between two scenarios: current clinical practice without public funding for hearing implants (the current scenario), and the anticipated clinical practice with public funding for hearing implants (the new scenarios). Figure 10 shows the model schematic for this budget impact analysis.
Figure 10: Budget Impact Analysis Framework.
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. In sensitivity analyses we explore how the results are affected by varying input parameters and model assumptions.
Key Assumptions
There are no direct medical costs for people who do not have a hearing implant
Currently, hospitals pay 100% of the cochlear implant costs for children, through global budgets. Research funding pays 100% of cochlear implants in adults (which we assumed was neither a cost to the hospital nor the Ministry of Health). In the new scenarios, the Ministry of Health will pay the full implant cost through targeted public funding
Currently, hospitals pay for 60% of the bone-conduction implant costs through hospital global budgets while individuals pay out-of-pocket for 40% of the device cost in the adult population. For children, hospitals pay for 100% of the bone-conduction implant costs through global budgets. In the new scenarios, the Ministry of Health will pay for the full implant cost in both adults and children through targeted public funding
We assume patients attend all scheduled health care visits before and after their implantation procedure
We do not distinguish between manufacturers. Cochlear implants are considered as a device class. Bone-conduction implants are also considered as a device class
Hearing loss and hearing implants do not affect disease-specific mortality
All individuals receive one hearing implant, whether they have unilateral or bilateral hearing loss
Target Population
The target populations were adults and children with single-sided deafness (research questions 1 and 2) or conductive or mixed hearing loss (research question 3). Further, they were people who do not benefit from conventional hearing aids or who have a condition (i.e., chronically draining ears, narrow or no ear canal) that precludes wearing them. Hence, an implant is warranted.
For the population with single-sided deafness, people were eligible for a cochlear implant (research question 1) or, if their condition was not suitable for a cochlear implant, they would be offered an appropriate bone-conduction implant—active transcutaneous or active percutaneous devices (research question 2). For the population with conductive or mixed hearing loss, people were eligible for any type of bone-conduction implant (i.e., active transcutaneous, active percutaneous, passive transcutaneous, and middle ear implants) (research question 3).
Current Intervention Mix
Currently, through the Ontario Cochlear Implant Program, four implant centres in Ontario receive fixed volume-based funding from the Ontario Ministry of Health to provide cochlear implants to people with bilateral sensorineural hearing loss. Through this funding, adults (n = 270, annually) currently receive one cochlear implant and children (n = 98, annually) receive two implants.
Currently, Ontario does not provide targeted public funding for hearing implants for people with single-sided deafness and conductive or mixed hearing loss. However, in these populations, some hospitals have made hearing implants available to a limited extent through research funding or through their global budgets, which are provided by the Ministry of Health.
More specifically, a limited number of adults with single-sided deafness have received cochlear implants through research funding (written communication, Joseph Chen, MD, May 25, 2018). Thus, the Ministry is currently not funding cochlear implants, directly or indirectly, for adults with single-sided deafness. For children with single-sided deafness, cochlear implants have been funded through hospital global budgets (n = 8 per year for Ontario residents) (in-person communication, Blake Papsin, MD; Sharon Cushing, MD; and Vicky Papaioannou, M.Cl.Sc, April 11, 2018; written communication, Karen Gordon, PhD, June 22, 2018).
Implant centres have capped bone-conduction implants for adults at 10 implants per year. Based on clinical consultations, we assumed 20% of these implants were for single-sided deafness (n = 2) and 80% were for conductive or mixed hearing loss (n = 8) (written communication, Joseph Chen, MD, May 25, 2018). As noted, hospitals pay for 60% of the device cost (internal and external components) for adults, and individuals pay out of pocket for the remaining 40% (e.g., for a $25,000 device, patients would pay about $10,000) (written communication, Joseph Chen, MD, May 25, 2018). Implant centres have paid for a total of 10 bone-conduction implantations each year for children with single-sided deafness (1 implant) and conductive or mixed hearing loss (9 implants) (in-person communication, Blake Papsin, MD; Sharon Cushing, MD; and Vicky Papaioannou, M.Cl.Sc, April 11, 2018). No copayments are required for children; hospitals pay the full cost.
Cochlear implantation can only be performed at one of four implant centres in Ontario, whereas bone-conduction implantation can also be performed at community hospitals. To our knowledge, only one community hospital is currently implanting active percutaneous devices, using hospital funding (N = 10 adults per year for conductive or mixed hearing loss) (written communication, Joseph Chen, MD, May 25, 2018).
For the current scenario in the budget impact analysis, we assumed that, without targeted public funding, the situation described above would continue over the next 5 years. In summary, each year 8 people would receive a cochlear implant for single-sided deafness, 3 people would receive a bone-conduction implant for single-sided deafness, and 27 people would receive a bone-conduction implant for conductive or mixed hearing loss (Table 32).
Table 32:
Current Scenario (Without Public Funding): Number of Hearing Implants Each Year, by Indication
| Years 1 to 5 | Single-Sided Deafness | Conductive/Mixed Hearing Loss | ||
|---|---|---|---|---|
| Cochlear Implant | Bone-Conduction Implant at Implant Centre | Bone-Conduction Implant at Implant Centre | Bone-Conduction Implant at Community Hospital | |
| Adults, N | 0a | 2b | 8b | 10 |
| Children, N | 8 | 1 | 9 | 0 |
| Total, N | 8 | 3 | 27 | |
Assumes that all cochlear implants in adults with single-sided deafness are paid for through research funding and, therefore, the costs are not borne by the Ministry of Health.
Assumes that 20% of bone-conduction implants were for single-sided deafness (n = 2) and 80% were for conductive or mixed hearing loss (n = 8).
Future Intervention Mix—New Scenario 1 (Same Increase for Adults and Children)
Given public funding, the Ontario Cochlear Implant Program projected their new implant volumes for single-sided deafness and conductive or mixed hearing loss as a percentage of the current total volumes funded for bilateral sensorineural hearing loss for the first 3 years. Those projections used the same percentage for adults and children, and we used that increase as the basis for our first new scenario.
Table 33 shows the number of hearing implants projected in new scenario 1. The Ontario Cochlear Implant Program plans to fund 24 cochlear implants per year for people with single-sided deafness. This represents approximately 6% of the total currently funded cochlear implant volumes (N = 368 patients).
Table 33:
New Scenario 1 (With Public Funding): Number of People to Receive a Hearing Implant Each Year, by Indication
| Years 1 to 3 | Single-Sided Deafness | Conductive/Mixed Hearing Loss | ||
|---|---|---|---|---|
| Cochlear Implants (6% of Current Totala) | Bone-Conduction Implants at Implant Centres (15% of Current Totalb) | Bone-Conduction Implants at Implant Centres (15% of Current Totalc) | Bone-Conduction Implants at Community Hospital (15% Increased) | |
| Adults, n | 16 | 8 | 33 | 11 |
| Children, ne | 8 | 3 | 13 | 0 |
| Total, N | 24 | 11 | 46 | 11 |
| Years 4 to 5 | Cochlear Implants (10% of New Volumef) | Bone-Conduction Implants (10% of New Volumef) | Bone-Conduction Implants (10% of New Volumef) | Bone-Conduction Implants (10% of New Volumef) |
| Adults, n | 18 | 9 | 36 | 12 |
| Children, n | 9 | 4 | 14 | 0 |
| Total, N | 27 | 13 | 50 | 12 |
New volumes are derived from taking approximately 6% of current total volumes at implant centres for bilateral sensorineural hearing loss.
New volumes are derived from taking approximately 15% of current total volumes at implant centres for bilateral sensorineural hearing loss, then assuming 20% of the bone-conduction implants are for single-sided deafness.
New volumes at implant centres are derived from taking approximately 15% of current total volumes for bilateral sensorineural hearing loss, then assuming 80% of the bone-conduction implants are for conductive or mixed hearing loss.
New volumes at community hospital are derived by assuming approximately 15% increase to current volume of bone-conduction implants (N = 10 adults).
Volumes for children may appear incorrect due to rounding and a cushioning added to handle a surge after public funding.
Volumes for years 4 to 5 are derived by assuming a 10% increase to the volumes for years 1 to 3.
As shown in Table 33, the additional projected volume of bone-conduction implants (n = 57) represents approximately 15% of the total cochlear implant volumes in bilateral sensorineural hearing loss (N = 368 patients), assuming 20% would continue to be for single-sided deafness (n = 11 people) and 80% would be for conductive or mixed hearing loss (n = 46 people). For the community hospital performing bone-conduction implants, we assumed a 15% increase to their current volumes for conductive or mixed hearing loss (n = 1 additional to the current 10 bone-conduction implants).
For years 4 and 5, the Ontario Cochlear Implant Program projected a one-time 10% increase to the volumes for years 1 to 3. These projections are consistent with how the Ministry has historically funded the implant centres: constant volumes for three years before reassessing for additional volumes for the next several years.
Future Intervention Mix—New Scenario 2 (Differential Increase for Adults and Children)
In new scenario 2, we calculated the new adult volumes by applying the same percentages as in new scenario 1. For new volumes in children, we did not apply the same formula but instead consulted with experts to project new volumes: 13 children requiring cochlear implants and 20 children requiring bone-conduction implants (where 20% were for single-sided deafness and 80% were for conductive or mixed hearing loss) (in-person communication, Blake Papsin, MD; Sharon Cushing, MD; and Vicky Papaioannou, M.Cl.Sc, April 11, 2018). After 3 years, we assumed a 10% increase to these new volumes, similar to new scenario 1. Table 34 shows projected volumes in new scenario 2.
Table 34:
New Scenario 2 (With Public Funding): Number of Hearing Implants Each Year, by Indication
| Years 1 to 3 | Single-Sided Deafness | Conductive/Mixed Hearing Loss | ||
|---|---|---|---|---|
| Cochlear Implants | Bone-Conduction Implants at Implant Centre | Bone-Conduction Implants at Implant Centre | Bone-Conduction Implants at Community Hospital | |
| Adults, n | 16 | 8 | 33 | 11 |
| Children, n | 13a | 4a | 16a | 0 |
| Total, N | 29 | 12 | 49 | 11 |
| Years 4 to 5 | Cochlear Implants (10% of New Volumeb) | Bone-Conduction Implants (10% of New Volumeb) | Bone-Conduction Implants (10% of New Volumeb) | Bone-Conduction Implants (10% of New Volumeb) |
| Adults, n | 18 | 9 | 36 | 12 |
| Children, n | 14a | 5a | 18a | 0 |
| Total, N | 32 | 14 | 54 | 12 |
Projections in children different from new scenario 1
Volumes for years 4 to 5 are derived by assuming a 10% increase to the volumes for years 1 to 3.
Resources and Costs
This analysis included direct health care costs to the Ministry of Health, either billed directly to the Ministry or indirectly through hospital global budgets. Annual undiscounted costs for adults and children were extracted from our primary economic evaluations. As noted, without targeted public funding, the device cost is currently paid for in part or in full by hospital budgets. Also as noted, we assumed 40% of the bone-conduction device costs in adults are paid for out-of-pocket by individuals. We assumed no out-of-pocket costs for bone-conduction implants in children, or for cochlear implants in adults and children. Given targeted public funding, we assumed the Ministry of Health would pay the full device cost (i.e., internal component and initial sound processor). We assume most non-device costs (i.e., physician fees, assessments, operating and complication costs) would be covered by the Ministry directly or through hospital global budgets. We excluded costs related to rehabilitation and the sound processor that would be paid for through ministries other than the Ministry of Health (i.e., for patients with a low income).
All costs are reported in 2018 Canadian dollars. When 2018 costs were not available, we used the health care component of the Statistics Canada Consumer Price Index was used to adjust costs.101 Appendix 8, Table A13, shows annual per-patient costs.
Analysis
In the reference case analysis, we calculated the required budget to publicly fund hearing implants in adults and children with single-sided deafness, and conductive/mixed hearing loss in Ontario. To do so, we extracted costs of hearing implants from the primary economic evaluations (for adults and children). Costs were multiplied by the projected volumes. We calculated the net budget impact as the cost difference between our new scenarios (public funding for hearing implants) and the current scenario (no public funding for hearing implants).
Results
Table 35 to Table 37 show the total and net budget impacts for the various scenarios over a 5-year projection. Publicly funding cochlear implants for people with single-sided deafness would result in an estimated additional budget of $2.8 million to $3.6 million. Publicly funding bone-conduction implants would result in an estimated additional budget of $0.8 million in single-sided deafness, and an additional $3.1 million to $3.3 million in conductive or mixed hearing loss. In total, funding both hearing types of implants for the two types of hearing loss over 5 years would result in an estimated additional budget of $6.7 million to $7.8 million.
Table 35:
Results of Budget Impact Analysis for Adults and Children With Single-Sided Deafness Receiving a Cochlear Implant
| Scenario | Total Cost to Ministry, $, Millionsa | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Total budget impact | ||||||
| Current scenario | 0.260 | 0.264 | 0.267 | 0.269 | 0.271 | 1.331 |
| New scenario 1b | 0.776 | 0.784 | 0.791 | 0.893 | 0.901 | 4.144 |
| New scenario 2c | 0.939 | 0.949 | 0.957 | 1.061 | 1.070 | 4.977 |
| Net budget impact | ||||||
| New scenario 1 – Current scenario | 0.515 | 0.520 | 0.524 | 0.624 | 0.630 | 2.813 |
| New scenario 2 – Current scenario | 0.678 | 0.685 | 0.691 | 0.793 | 0.799 | 3.645 |
Notes: All costs are reported in 2018 Canadian dollars.
Numbers may appear off due to rounding.
New scenario 1 assumes implants for adults and children will increase by the same percentage, given public funding (see Table 33 for details).
New scenario 2 assumes implants for adults and children will increase differently, given public funding (see Table 34 for details).
Table 37:
Results of Budget Impact Analysis for Adults and Children With Conductive/Mixed Hearing Loss Receiving a Bone-Conduction Implant
| Scenario | Total Cost to Ministry, $, Millionsa | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Total budget impact | ||||||
| Current scenario | 0.339 | 0.344 | 0.349 | 0.353 | 0.431 | 1.817 |
| New scenario 1b | 0.894 | 0.904 | 0.912 | 0.999 | 1.166 | 4.875 |
| New scenario 2c | 0.937 | 0.948 | 0.957 | 1.059 | 1.234 | 5.135 |
| Net budget impact | ||||||
| New scenario 1 – Current scenario | 0.555 | 0.559 | 0.563 | 0.645 | 0.735 | 3.058 |
| New scenario 2 – Current scenario | 0.598 | 0.603 | 0.608 | 0.706 | 0.803 | 3.318 |
Note: All costs are reported in 2018 Canadian dollars.
Numbers may appear off due to rounding.
New scenario 1 assumes implants for adults and children will increase by the same percentage, given public funding (see Table 33 for details).
New scenario 2 assumes implants for adults and children will increase differently, given public funding (see Table 34 for details).
Table 36:
Results of Budget Impact Analysis for Adults and Children With Single-Sided Deafness Receiving a Bone-Conduction Implant
| Scenario | Total Cost to Ministry, $, Millionsa | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Total budget impact | ||||||
| Current scenario | 0.038 | 0.038 | 0.039 | 0.040 | 0.049 | 0.204 |
| New scenario 1b | 0.172 | 0.174 | 0.176 | 0.208 | 0.243 | 0.974 |
| New scenario 2c | 0.186 | 0.189 | 0.191 | 0.224 | 0.262 | 1.052 |
| Net budget impact | ||||||
| New scenario 1 – Current scenario | 0.134 | 0.136 | 0.137 | 0.169 | 0.194 | 0.770 |
| New scenario 2 – Current scenario | 0.148 | 0.150 | 0.152 | 0.184 | 0.213 | 0.849 |
Note: All costs are reported in 2018 Canadian dollars.
Numbers may appear off due to rounding.
New scenario 1 assumes implants for adults and children will increase by the same percentage, given public funding (see Table 33 for details).
New scenario 2 assumes implants for adults and children will increase differently, given public funding (see Table 34 for details).
Discussion
The net budget impact for Ontario's Ministry of Health to provide targeted public funding of hearing implants for people with single-sided deafness and conductive or mixed hearing loss, all told, is projected to range between $6.7 million and $7.8 million over 5 years. Specifically, funding cochlear implants in single-sided deafness may cost $2.8 million to $3.6 million more, compared to current public costs; funding bone-conduction implants in the same population may cost an additional $0.8 million; and funding bone-conduction implants in conductive or mixed hearing loss may cost $3.1 million to $3.3 million more, over current funding.
Given the small target populations, the total 5-year budget impact (total costs, not just the additional costs) would be relatively small, ranging from $10.0 million to $11.2 million, across the three research questions and two scenarios.
We projected volumes using data from the Ontario Cochlear Implant Program and applied the same formula the program plans to use if targeted public funding is provided (i.e., basing new volumes on a percentage of the current total volumes for bilateral sensorineural hearing loss). We did not derive new volumes using a burden of disease approach (i.e., starting with the total number of people with single-sided deafness and conductive or mixed hearing loss in the province, eligible for a hearing implant). Prevalence data on hearing loss in Canada are lacking. Statistics Canada has prevalence data on sensorineural hearing loss only, but not specific to single-sided deafness or conductive or mixed hearing loss.66 We were unable to identify public reports on prevalence from the Canadian Institute for Health Information or other sources. While there may be more people with hearing loss eligible for implantation in Ontario, the increases projected by the Ontario Cochlear Implant Program are based on patients who have exhausted other options for implantation. If hearing implants were to be offered to a broader range of patients, we could expect a much higher budget impact.
There are several limitations to this analysis. Unlike the primary economic evaluations which modelled costs probabilistically (i.e., more than 10,000 simulations), costs for the budget impact analyses were modelled deterministically, and therefore did not capture parameter uncertainty.
The same limitations discussed in the primary economic evaluation apply to the budget impact analysis, such as the short follow-up time and small sample sizes of primary studies used to inform the risk of complications and the risk of device non-use.
There are also several strengths to this analysis. We explored different scenarios for the projected volumes over the next 5 years: first, based on current volumes of cochlear implants in bilateral sensorineural hearing loss; second, based on expert opinion. In addition, the per-patient costs were derived from our primary economic evaluations, which capture background mortality, clinical events, and disability. The per-patient costs were predominantly from Ontario-specific data sources. While this may limit generalizability outside of Ontario, our work supports funding recommendations in the province.
Conclusions
For people with single-sided deafness, publicly funding cochlear implants would result in an estimated new cost of $2.8 million to $3.6 million over the next 5 years, and an additional $0.8 million would be required for bone-conduction implants for this population. For people with conductive or mixed hearing loss, publicly funding bone-conduction implants would cost an estimated additional $3.1 million to $3.3 million over the next 5 years.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, preferences, and priorities of those who have lived experience with single-sided deafness and conductive or mixed hearing loss. The treatment focus was cochlear implants and bone-conduction implants.
Background
Patient, caregiver, and public engagement provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. This information includes the impact of the condition and its treatment on the patient, the patient's family and other caregivers, and the patient's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).108–110 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in published literature, we contact and speak directly with people who live with a given health condition, including those who may have experience with the intervention we are exploring.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with single-sided deafness and conductive or mixed hearing loss and those of their caregivers.111 We focused particularly on their perceptions and experiences of using devices to improve their hearing. We engaged people via face-to-face and telephone interviews.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with single-sided deafness and conductive or mixed hearing loss, as well as those of their families and caregivers. Our main task in interviewing is to understand what people tell us and to gain an understanding of the meaning of their experiences.112 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
Participant Outreach
We used an approach called purposive sampling,113–116 which involves actively reaching out to patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We contacted more than 30 clinicians, organizations, and groups affiliated with single-sided deafness and conductive or mixed hearing loss to spread the word about this engagement opportunity.
Inclusion Criteria
We sought to speak with people and caregivers who have been actively managing single-sided deafness and conductive or mixed hearing loss by using implantable devices.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
For this project, we spoke with 20 people living in Ontario with single-sided deafness, conductive hearing loss, or mixed hearing loss, as well as two parents of children over 5 years old with one of these types of hearing loss.
Of the 22 participants, 18 had received or were caring for someone who had received a cochlear implant or bone-conduction implant to treat their hearing loss. Both types of devices have a component that is surgically implanted. People who, for various reasons, cannot benefit from externally worn hearing aids may be offered an implantable device: people with single-sided deafness may benefit from a cochlear implant or a bone-conduction device, and bone-conduction implants may also be appropriate for people with conductive or mixed hearing loss.
The remaining participants had experience of considering an implantable device to treat their hearing loss. Participants varied in their socioeconomic background, place of residence, and gender and language preferences.
Approach
At the beginning of the interview, we explained the role of our organization, the purpose of the health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 9). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 20 to 40 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.117 Questions focused on the impact of hearing loss on patients' and families' quality of life, and their perceptions of the benefits or limitations of implantable devices. See Appendix 10 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.118,119 We used the qualitative data analysis software program NVivo (QSR International, Doncaster, Victoria, Australia) to identify and interpret patterns in the interview data. The patterns we identified allowed us to highlight the impact of single-sided deafness and conductive or mixed hearing loss on the patients, family members, and caregivers we interviewed.
Results
Summary
During the interviews, people with single-sided deafness or conductive or mixed hearing loss and their family members emphasized the struggle of living with hearing loss. People with experience of a cochlear implant or a bone-conduction implant were able to compare the impact of these devices with other currently available treatments.
People who had received an implantable device expressed the positive impact it has made in their lives, particularly in communicating with others. However, they also described certain limitations of these devices, and some barriers make it difficult for people to receive one.
Day-to-Day Impact of Hearing Loss
Participants noted the immense impact that hearing loss has on their day-to-day lives. Most interviewees discussed struggles with their ability to effectively communicate and to work, enjoy life, and stay safe in their surroundings. Some people noted that they had coexisting health conditions, including chronic pain and multiple disabilities, which also affected their quality of life and the type of treatments they sought for hearing loss.
Participants recalled the time in their lives when they started experiencing hearing loss. For some, the loss was gradual; others noted a sudden loss. For both groups, the impacts were physical, psychological, social, and financial.
Physical Impact
People who had experienced gradual hearing loss reported that they often found themselves asking others to repeat things. With sudden hearing loss, participants described it as “someone had turned down the volume of the radio” or “my ear was plugged.” Hearing loss led to a decreased awareness of their surroundings. People spoke of feeling that there was a “dead zone” on the affected side.
It's funny how your awareness is gone when you can't hear movement, like there's—it's like a dead zone; there's just nothing there.
People with single-sided deafness reported the difficulty of positioning themselves physically to maximize their ability to hear. They struggled in noisy places such as restaurants where they could not differentiate between the sound of interest and other sounds.
Like, I would not position myself on the far end of the table so that my ear was facing the opposite direction. If I was sitting in a room, I would corner myself so that no one could go on my right side or anything like that.
A few participants with single-sided deafness associated the onset of their hearing loss with viral infections or the onset of Meniere disease, as they experienced symptoms of vertigo, tinnitus, vomiting, dizziness, and balance issues. Others were unable to pinpoint the reason for their hearing loss. One participant noted, “In my situation literally [I] just kind of woke up one morning and literally couldn't hear out of my one ear.”
Symptoms of Meniere disease had a substantial effect on their daily functioning and ability to complete simple tasks such as getting out of bed or enjoy activities such as playing sports.
For me, and I know it doesn't necessarily happen to everyone, the tinnitus … was a big issue for myself. I know for some people thinking sometimes when you go deaf that there's just silence. For me there was some significant, significant ringing, so much to the point that at times, when the ringing would get really bad, it would almost overpower any hearing out of my good ear as well. Like that was the only thing I could hear in my head was that ringing.
Now the dizziness, fortunately, the vertigo did subside with the pills. I know for some people that does continue on. For me after the first maybe three or four weeks that dizziness stuff—it was usually more times if I would get up really quick … But getting up fairly quickly or leaning backwards or anything like that sometimes would trigger some dizziness with the system. But I never used to experience kind of before.
People with conductive hearing loss experienced needing medical treatment for ear infections, ear drum perforations, and fluid in the ear canal. These physical difficulties affected their hearing function and quality of life.
Psychological Impact
A few participants reported mental health issues such as anxiety and depression, which they associated with their hearing loss. People with sudden hearing loss noted it was devastating and life changing. They also shared feelings of anger, shock, and fear. People with gradual hearing loss said they felt frustrated and depressed without proper hearing.
Having perfect hearing in both ears and then having to cope with one gone, it's—for lack of better term—it's absolutely devastating and life changing.
The longer I am going without hearing … the more frustrating and depressing it is.
I know as far as going through different emotions—anger, depression—absolutely … But I think there was probably for me, I think anger issues was probably a little bit bigger … It's … a combination of a few things in regards to stress, anger, slight depression …
Most participants noted a high level of stress related to the effort of coping with the condition, the increased effort of listening, and the resulting fatigue, particularly while they were adapting to the hearing loss.
And the other thing with hearing loss is: hearing loss is enervating. It takes energy to try to understand what the other person is saying because you're always wondering, “Did I miss something? Did I misinterpret?” It's very easy to misinterpret.
I mean I think overall energy levels, tiredness, and all that sort of stuff all comes and plays into a factor. I mean absolutely it's tough …
Social Impact
Most participants reported having difficulty holding intimate conversations and enjoying movies, music, or surrounding sounds. The inability to localize sound affected activities they had previously enjoyed such as bird watching. There were safety implications to not being able to locate sound when it came to crossing a road or walking in a parking lot or on a sidewalk.
You can't identify the location of the sound … you can't tell when you hear birds singing, you have no idea where it is. I used to be able to pinpoint when [I] hear a robin singing and … follow the song and … find the bird. Now I have to do a 360-degree turn looking for them. And there's some dangers too. Cars would, like, sneak up on [me], [and I] wouldn't hear them coming …
You … hear absolutely nothing on that side, and it does become a danger … I remember walking in a parking lot … and a car came up behind me, and I didn't even hear it … Or sometimes people … whisper in my ear … you hear nothing. Or if somebody is on that right side, you know, you hear nothing, you hear noises, and you can't really distinguish where they're coming from.
… if you're walking on the sidewalk and somebody's coming up behind you with a bike or something, you can't hear that.
Participants noted a social stigma against people with hearing loss. Most participants had experienced being perceived as rude or bossy by strangers or coworkers who did not understand their condition.
I get into depression, I was so isolated in all of that time because you can't go anywhere. Even to grocery shop was scary because if someone's talking to you, you can't [pick them out] and then they get so mad at you because you're standing there and your cart is probably in their way or something.
Participants reported that their inability to follow the conversation in social circles made them feel withdrawn. They noted withdrawing themselves from events and within events. They reported that they progressively stopped socializing and going out with friends and family. This impacted their ability to maintain and make new friendships.
So we'd always go in a restaurant, ask them for the quietest spot in the corner table … I finally said, I just want you to know when we're out for dinner, we're not out for dinner. You guys are; I'm alone. I can't participate in the conversation because I can't hear you guys.
I didn't want to be there, I didn't want to go out to restaurant, as far as still being social absolutely, but I wouldn't want to go to a bar for a couple of drinks, I wouldn't want to go to a restaurant with a group of friends because I couldn't hear.
The biggest impact, I'd have to say, was when we moved from a place where we had a group of friends to Ottawa where we essentially knew nobody, and that's actually when I felt that my quality of life went down considerably because I was unable to really get involved in conversation and make friends …
Parents of children with hearing loss expressed concern about the impact it has on their child and the way they communicate with others in their day-to-day lives.
The bigger challenges for her are around communication … she has limited communication skills. She's very reliant on a combination of sign language and verbal skills … People don't understand also other aspects of hearing loss, such as … needing to be clear when you're speaking to the person, that you need to be facing them …
Financial Impact
Participants with single-sided deafness or conductive or mixed hearing loss noted the impact on their work and communications with their clients, coworkers and colleagues. They noted straining to listen to conversations to properly conduct their work. Participants said that hearing loss led them to change how they worked; for some, hearing loss was one of the reasons they changed careers.
The only thing I would say is that, because of the patients that I deal with and because we're talking about some very incredibly serious matters, I'm worried that I will miss part of their conversation. So what I end up by doing is, I ask them to send me their information in writing. Now, that's a two-fold reason. One, it tells me whether they're serious or not and, two, is that I can't misinterpret something that they put in writing.
There were several factors that made me think that perhaps I should get out of private practice, veterinary practice; so I made a career change and one of those factors was my hearing loss. I felt that with it worsening over time, that I may start missing things during physical exams or even listening to clients, which would then impact my ability to practice veterinary medicine and potentially even put the lives of my patients at risk.
Current Treatment Options
People with single-sided deafness or conductive or mixed hearing loss described being given the opportunity to try out different hearing aids to determine whether they would meet their needs. Most people interviewed discussed how the various non-implantable options did not meet their needs with respect to hearing from both ears, sound localization, hearing in loud environments, safety, and self-esteem, and this led them to consider implantable devices.
Single-Sided Deafness: Perspectives on Current Treatment Options
Participants identified bone-conduction hearing aids worn as headbands and contralateral routing of signals (CROS) hearing aids as currently available treatments for people with single-sided deafness. Some patients noted that their doctors offered them these devices to try for a designated time. Some patients were offered these as the only available options, but a few patients reported that, if these treatments did not meet their needs, their doctors offered them other treatments that were currently not funded.
They [hearing aids] just make the sound louder that was still not intelligible to me, and so it … seemed like CROS was my only option, and had I been a patient that wasn't aware of different possibilities through my research and whatnot, then I might have just stopped there; and I worry that there are a lot of patients who are in that boat, that they don't pursue other options and so they end up with devices that potentially aren't as beneficial for them.
Patients who had experience with CROS hearing aids or bone-conduction headbands noted that these devices helped them hear better by leveraging the hearing they had left. The bone-conduction headband was perceived to be better than the CROS hearing aid but still did not address the need to localize sound.
Apparently the hearing aids that I use now work primarily with sound, and they use whatever hearing system, whatever I have left over in my ears. All it does is augment what I have left over …
I felt, I think, it [bone-conduction headband] was a little bit better than the CROS hearing aid, but I still think that … they were similar ideas as to going back to why I was frustrated in the first place with some of the other stuff [directionality] before.
Conductive or Mixed Hearing Loss: Perspectives on Current Treatment Options
People with conductive or mixed hearing loss noted that hearing aids and bone-conduction headbands leveraged the hearing they currently had but did nothing to recover the remaining hearing ability.
Participants with conductive and mixed hearing loss noted that conventional hearing aids made them prone to ear infections.
… I got a hearing aid in my ear and … I started hearing a little bit. That was nice and good but the big problem that I had, after a couple of months using it, it created moisture inside and then I started getting a lot of infections …
Participants with mixed hearing loss noted that wearing hearing aids was a nuisance as putting on heavy clothes such as coats, sweaters, and hats made the device make sounds. One participant who works with children noted they often pull on her bone-conduction headband, and she was concerned it might break.
I cannot wear coats or heavy sweaters with [a hearing aid]. It makes a lot of sound. Bonebridge [a type of bone-conduction implant] lays flat on head.
Right now, working with children, they grab on the headband. If I can have something that is not noticeable, it will be easier to work with children, so they don't break the device.
Current Treatment Options: Overall Perspectives of Unmet Need
A few participants noted that CROS hearing aids and bone-conduction headbands were not discreet. People noted they were self-conscious when they were wearing these devices.
I think [the implantable device] will help with self-esteem and appearance. [Going from] something that is pretty noticeable to something that can be hid underneath my hair. [Current hearing aid] is not meeting my needs. People should feel that they could look their best. It helps with their self-esteem.
People with conductive or mixed hearing loss noted that CROS hearing aids and bone-conduction headbands did not address the issue of deciphering sounds in noisy areas.
I personally was pretty disappointed … I didn't see much benefit … I used it [CROS] I believe for about, I'm trying to remember if it was four weeks or six weeks or exactly how long … I personally didn't see much benefit for it. To me, I kept going back to some of the challenges … being in louder situations, being at restaurants … it's like anytime you were in a larger space or a louder area, it's the loudest noise was just so overpowering and it's just—it didn't help. It really didn't help.
People with single-sided deafness noted they were looking for ways to hear from both ears. They reported that binaural (two-sided) hearing would enhance their ability to localize sound, perform at work, socialize with friends and family, and stay out of danger. Participants noted that simply augmenting the hearing they have left does not address their needs.
And of course for me I wanted to … I specifically was interested in a device that would provide me with binaural hearing again so that I could hear sound, recognize that sounds came from different locations …
Health Technology Under Review
Participants were asked for their perspectives on implantable hearing devices. People with single-sided deafness were asked to reflect on their experience with cochlear implants and bone-conduction implants, and people with conductive or mixed hearing loss shared their thoughts on bone-conduction implants.
Participants who had received a bone-conduction implant did not distinguish between active and passive devices, but many referred to their implant as BAHA, short for bone-anchored hearing aids, or as Bonebridge, a specific device. As outlined in the Background section of this health technology assessment, bone-anchored hearing aids have a component that is implanted through the skin (percutaneous) and completely under the skin (transcutaneous); with the Bonebridge device, the implant remains completely under the skin (transcutaneous). This difference is important in understanding the challenges and barriers that participants described regarding their bone-conduction implants.
Treatment Decision-Making
Cochlear Implants
Some patients noted distress in deciding whether to receive a cochlear implant, perceiving it as an “irreversible” process. People who had gone through the challenges of hearing loss were concerned by the possible risk of living with an embedded device, worrying that it could damage other parts of their ear.
For me, I think the idea of certain things being irreversible, well potentially irreversible, when you're putting the cochlear in with the electrodes, the idea of potentially of damaging a lot of stuff in there if it wasn't already currently damaged. To me that was slightly kind of concerning on that side.
Parents of children with hearing loss expressed distress as they discussed their decision-making about the choice of device, type of surgery, the surgeon, and place of surgery. Parents noted they had to research their options to understand how to minimize their child's risks during the procedure.
Yeah, [there is] significant difference in the surgical technique and surgical approaches, and that had a big impact for our child—to not be under anaesthesia for longer than a few hours, rather than being under anaesthesia for eight hours. But that was at our own, with our own research and our own sort of gaining knowledge and our initiative of contacting that surgeon …. So I think we were supported in the conversation whether or not we should do it; but I think in terms of finding the right expertise, we weren't as supported as we could be.
Bone-Conduction Implants
People with single-sided deafness preferred a cochlear implant over bone-conduction implants based on the risks and benefits. Those unable to receive a cochlear implant either due to cost or contraindications chose to receive a bone-conduction implant.
… cochlear implant was off the table because it wasn't funded; and so … I went with the Bonebridge implant.
… I specifically was interested in a device that would provide me with binaural hearing again so that I could hear and recognize that sounds came from different locations, and the only device that potentially would offer that was the cochlear implant. But of course it wasn't funded … but the next best thing was either the BAHA or the Bonebridge …
Treatment Process
Cochlear Implants
People who had received a cochlear implant described the invasive surgery it involved. The size of the incision was regarded as “massive,” and the recovery was “month long” as patients eagerly waited to hear again. The month of healing was considered “uncomfortable,” but participants reflected that they recovered well and noted that the long-term gain outweighed the short-term losses such as discomfort.
It probably hurt a little bit more than I was expecting. But … they're drilling into your skull. I had about 30 staples in the side of my head there … it's a fairly invasive process … I was out for about four or five days after that.
I remember … after the surgery … I didn't realize how big of an incision they were going to have to do … When they finally took the bandage off … I went holy something … I actually did well with the surgery … it was successful. And then … you have to wait a month for them to … turn it on and place the exterior piece on. So that had to be the longest month in my life, I swear, because I just couldn't wait to get that.
Patients and caregivers noted that, once the month of healing was over, the receiver was placed on the implant.
The receiver is magnetized; it goes onto the implant and then there's a … an ear piece that kind of hangs onto your ear as well with the battery and the receiver. And that's removable, so the batteries are rechargeable, they usually lasts for a full day. And then as soon as you take that off, you essentially are deaf again.
Bone-Conduction Implants
Participants mentioned that they went through multiple steps to be diagnosed and receive an implantable device: injections, referrals, and wait lists.
… [my hearing] was just gone, and so I went to an audiologist and she said that I needed to see my family doctor right away and get a referral to a hearing specialist, and so I did … she gave me—over the course of three weeks she gave me three … I'm trying to think what was it, it was injections, steroids, three steroid injections. She thought that that might help but it didn't.
So, he [doctor] put me on a waiting list … to get a BAHA. It took a couple of years to have that happen.
People with experience of a bone-conduction implant also noted it required invasive surgery. They were more content with the newer versions of the bone-conduction devices compared to older versions in which the implant protruded from the skin, making the site susceptible to infection.
… they drill a hole in your head and then they implant the receive[r] part and they screw it in, and mine was/is totally under the skin…The older ones had a little tab that it came out through your skin and you hooked your transmitter onto that, but that's because it was protruding through, your skin was always susceptible to infection. Mine is 100% under my skin so it's totally covered so there's no infections which is a really, really big deal.
Benefits of Implantable Devices
Participants felt that implantable devices enhanced their day-to-day lives. Most said they were able to hear much better and to locate sounds. They were able to focus better. The implants made it easier for most participants to communicate while driving, hear in noisy environments, and enjoy activities such as bird watching, movies, and music.
Being able to hear people more clearly, being more comfortable, and not having to concentrate and focus as much as I've done before has been a significant help in kind of medium to loud situations. Even driving let's say on a three-hour drive with my wife, if I didn't have my … implant it can be a little more challenging trying to … communicate and talk with her when I'm driving, so my bad ear is facing her. Just with the noises in the car and all that sort of stuff. If she's not speaking very loud and very clear, it used to be very challenging, where it's become a lot easier on that stuff.
Parents of children with hearing loss reflected on the importance of reducing ear infections and having full hearing during the child's developmental years. They noted the hearing sense was just as important as the sight sense for developing children.
Most people don't realize … a basic sense—hearing and vision—how important that can be [for a child's development].
Cochlear Implants: Perceptions of Benefits
Most people cherished their cochlear implant for its ability to help them hear from both ears and localize sounds, provide tinnitus relief, feel safer in their environment, and socialize. Participants mentioned they wore their cochlear implant as soon as they woke up and took precautions to keep it safe.
I mean I pretty much wear it from as soon as I wake up to as soon as I go to bed, other than when I do sports I do take it off.
… I will fight someone to the death if you think you're going to take it away from me.
Participants mentioned that the cochlear implant improved hearing from both ears. It made them more aware of their surroundings, and they were able to hold more fluid conversations. It gave them the “gift of hearing.”
Because if I walk around at home, I don't have it on, then all of a sudden, I put it on, it's like, the TV's louder, the radio is louder, I can hear somebody upstairs … it gave me hearing on my deaf side, which was … amazing … It gave me the gift of hearing, so that was wonderful … I have the surround sound now.
Hearing-wise, I now hear from both sides of my head, versus only the one side.
So, with the cochlear implant, … the biggest thing for me, was—it almost has created like surround sound, meaning that now when I'm in the louder situations when someone's talking across for me it's much, much easier to be able to hear them …
People also were able to identify sound direction better with cochlear implants. One interviewee mentioned that he participated in a research trial studying the improvement in sound localization with cochlear implant, and he reported a notable difference.
When I got the CI, so first thing was… I can actually hear more now, point one. Point two, … I've got some of my localization back.
I … went over [to] the Department of Defence building over at [location in Toronto] … with some professor that works for Defence on hearing for the troops … they've got a circle of speakers and a chair in the middle. she had me … with and without the CI, [to study] could I figure out where the sound was coming from? And … it was clear that the CI made a difference. …I think she said about three-quarters of the time with the CI I was accurate on where the noise came from. Without, I was all over the map.
Some people noted that their cochlear implant also relieved their tinnitus. One patient said the ringing sound they had been experiencing significantly reduced when the implant was turned on. However, some people noted that their tinnitus was not completely relieved.
Before the CI, I swore I had a jet engine in my head. My wife will never forget, I think I scared the hell out of her, we were in the kitchen and I'm standing there and it was roaring, okay? And it just got the point where I said, “Shut up” [laughs] and my wife thought I was yelling at her. And I'm no, I'm yelling at the tinnitus. It's driving me crazy. But as soon as I put the CI, on the tinnitus goes away. Again, that's part of my love for this thing.
So, it [the ringing] didn't go back to normal, unfortunately, but it has helped, absolutely. I would definitely agree that it has helped for myself …
People whose ability to work was affected by the onset of single-sided deafness were able to rejoin their line of work with the help of the cochlear implant.
You have these life-changing things happen to you, and you have to really hope and, luckily for me, I wasn't pushed into poverty. I was able to go back to work, but I have to say it's because of the cochlear implant. If I didn't have that, I wasn't able to do that job. I'd have to go do something else. I would not have been able to make the kind of money that I was making.
Parents of children noted that the implantable device became part of the child's identity. One mother indicated that her daughter equated the implant to her ears and believed it was part of her body.
A parent of a child with hearing loss and coexisting conditions mentioned that a cochlear implant helped the child use her hearing fully, to listen and visualize more effectively. These abilities were noted as an essential requirement for the development of the child
She was at a better advantage for her learning or be able to engage with her environment. … You know, the simple fact is that someone who's interacting as significant as our child with the health care system and needing to interact with her world, had she not had cochlear implants, that would have been significantly compromised.
Bone-Conduction Implants: Perception of Benefits
People with single-sided deafness and conductive or mixed hearing loss expressed more confidence in social situation with background noise with bone-conduction implants. They no longer felt the need to specially position themselves in social situations, as they had done prior to receiving the implant.
It had quite a significant impact on my leisure time and activities, too, I'll say … obviously … it's not giving me back my hearing … So, what I find beneficial is … this year, when we had the same party, I could engage in conversation with most of the people at the table. It wasn't perfect, but at least I could hear my husband.
It's helped my confidence. It's helped … reduce the anxiety, made me more social. I still try to avoid some of those situations if I can, if it's optional, because it can be … uncomfortable, the loud environments, but I'm able to cope.
Oh, it's made a huge difference in my work life and … even in my personal life, things that made a difference. Like going to a movie, … even sitting in a restaurant … as simple as walking down the sidewalk …
Participants felt they were fully dependent on the implantable device to hear, function at work, and conduct usual activities of daily living.
And then if I don't wear them, if I don't wear the damn things, I can't hear properly.
… the most important thing in my life is my work. And I am really afraid [at] some point that if something happened to this device, then I will not be able to work.
Bone-Conduction Implants for Single-Sided Deafness
People with single-sided deafness noted the bone-conduction implant made a subtle but “big difference” to their hearing.
Okay, it's a very subtle … I think it's a very good quality but it is very subtle and you can almost think that it's not doing its job, but when you then block your right ear from hearing, you then know how much of your hearing is coming from the Bonebridge which is quite substantial; but it is very subtle and you can think, “Oh well, I'm not sure this is all that great,” but you take it off and yeah it's like, “Oh, that makes a big difference.”
Bone-Conduction Implants for Conductive or Mixed Hearing Loss
People with conductive or mixed hearing loss noted that their bone-conduction implants reduced the risk of infection, which they perceived they were facing with external hearing aids.
The reason I ended up with the BAHA is that I had traditional hearing aids, where they were—the ones that were fit in my ear. I was getting a lot of ear infections. And I'm prone to ear infections and have been since childhood, but I think that those types of hearing aids were impacting, causing more ear infections. So it [the bone-conduction implant] definitely helped my hearing. I still do get some ear infections, but not … the way that I used to. They've certainly reduced at that point.
Barriers to Implantable Devices
Participants reported that cost and access to implantable devices for hearing loss were barriers to receiving these treatments.
Cost
Some participants with lived experience of a cochlear implant or a bone-conduction implant noted that the high cost of the device was a barrier to receiving the implant, for themselves or potentially for others.
[Doctor] told me that I fell out of the inclusion criteria in the trial; he said to me, he said, if you want to do it we can still do it but you have to buy the implant. And I go okay, how much is that? He says $30,000 [for a cochlear implant]. And I went well, next …
If one of my kids had deafness in one ear … and what if I couldn't afford the $6,000 [for a bone-conduction implant] and not be able to give my child this advantage—it would be heartbreaking.
Coverage for the device was variable among the participants. Some people had partial coverage through private insurance or the provincial Assistive Devices Program; some had full coverage through a research grant; and others were expected to pay the full price.
I did not pay from my pocket … the doctor … said to me that I will get that free. And there was a confusion about that —I got the device without any cost; but it … is very expensive.
[The BAHA] was over $5,000. Three thousand of that was actually covered by the program, through the government. And then I had just over $2,000 … So I'm still out of pocket, but not a lot.
I know if the Adaptive [Assistive Devices] Program, if it hadn't have been available, even for partial payment [of Bonebridge], it would have been a financial hardship.
People who were exploring a cochlear implant noted the larger price tag compared to bone-conduction devices.
But [cochlear implant] wasn't funded, it wasn't covered by OHIP [the Ontario Health Insurance Plan] but the next best thing was either the BAHA or the Bonebridge… But, at that time, I also was still a graduate student and didn't have the funds to be able to purchase the processor that was required for either the BAHA or the Bonebridge.
As far as out-of-pocket costs, I'd say it's really the price of the processor … so really, compared to the cochlear implant, I think out-of-pocket it's the $6,500 that we had to pay for the processor, and for me my private health care plan, health insurance, covered $500 of that.
Patients who received full or partial coverage for the device noted they also incurred out-of-pocket costs to travel to the implant centre, which were not covered.
It was day surgery [to receive a Bonebridge] … we did spend six nights in the hotel in Toronto, a couple of nights before the operation and four nights after the operation, to make sure everything was fine. We live … a thousand miles from Toronto. … the Northern Ontario Travel Grant covered one night in the hotel only and that was only $100 so, you know a hotel room in Toronto costs a lot more than $100. So it cost us six nights hotel at about $250 a night, and the plane fare to and from Toronto was covered by the Northern Ontario Travel Grant.
There were costs that we covered and there were costs that were out of pocket. So surgery and that type of thing were covered. Our travel to was not covered, and our time there as family, which was about a week, was not covered.
The out-of-pocket costs had an impact on participants' personal lives as they had to adjust their savings and earnings, and sometimes had to borrow money which would be paid off later, adding to the financial burden on the patient.
It required some juggling of finances to be able to ensure that we had the funds for all that we wanted to do at that time in our lives. Yeah, but thankfully it all worked out with lines of credit and whatnot. But it definitely was a substantial and significant impact on our financial abilities for the year.
But I can't afford half of that because they said, you know, the beginning, like $6,300, something like that, right? … there's no way we can afford, me and my family, to do this … Well, they mentioned they're going to go through my insurance, too, because they
can help me, too, and they said I'm going to pay the minimum, of course, if the government helps me with this problem. Of course I'm going to pay a little bit but if now I have to borrow money to do this …
It [Bonebrige] cost me, what, about—the receiver itself was about $6,000. Which is about the costs of a hearing aid. I mean, I have to say I don't—like it's—I have extended the health coverage through my job … which of course doesn't cover any of this … [The] amount that it gives you for hearing aids is $500. … It's an essential thing that you need and, if there are assistive devices out there, it should be accessible to a certain degree … So, I didn't, to be very honest, I couldn't just fork out $6,000 all at once …
Patients also had out-of-pocket costs to maintain and upgrade the implantable device they had received.
It is a lifelong—the Bonebridge is in my head but the receiver is only good for so many years. It's going to, it will probably require maintenance or I'm going to require a new one, and then there's the cost of that as well.
The device itself [cochlear implant], the internal and the external device, had coverage. But if there was any loss or damage or extra material required … anything in terms of adaptors, or batteries or chargers, anything like that, that was all out of pocket for us … [… we did suffer damage to one device and loss of another, and we had to cover that.
… I think the warranty is something like five years. And so, beyond that, if you need to purchase another one …
Parents of children with implantable hearing devices noted additional costs related to making the device child-friendly.
… so, ear hooks, or what they call “snuggies,” which on a young child holds the devices, the microphone part of device, on more securely.
Access
Many participants commented that implantable devices were not available or easily accessible across Ontario. One participant stated that they learned about the Bonebridge device by chance when receiving treatment for a different health problem.
A few participants felt they had no option but to travel to Toronto for a consultation with the specialist, the implant surgery, and follow-up visits.
I am currently living in [a northern town]. At that time that I was doing my graduate studies, I was in [a smaller town] which anyway is north of Toronto by about an hour and a half … so I would drive down to Toronto to [implant centre].
If I needed anything done with it [the device] or all of a sudden something didn't work on it, I'd have to probably go to Toronto to get it fixed, right? … Make it more accessible in more centres would be much better. So be able to do that here in [northern city] instead of having to go to Toronto for that, to make it more accessible to other people, that would be amazing.
This is going to be a lifelong appliance … so, I'll have this and then in five years I'll probably need to get another receiver because they will wear out, and then I'll have to do that … to be able to not necessarily have to go to Toronto every time I need a new one.
Limitations of Implantable Devices
Although people enjoyed hearing better via implantable devices, they also reported limitations in using them.
Cochlear Implants: Perceptions of Limitations
Participants stated that a cochlear implant was not like wearing prescription eye glasses. It did not fully restore hearing in the deafened ear. People still struggled with understanding unfamiliar accents and tones.
When you wear a prescription glass, your eyesight is restored 100%, right? A cochlear implant is different, you don't get 100% replacement of your lost hearing … the sound is different. But it did address my challenge because … I'm able to hear people from my left but although the sound is not—
So it … definitely helps me to hear better. It's not perfect and it will never ever be perfect. Anyone with a cochlear implant doesn't get back 100% of what they had. But it was about 70% that I did my last test. If you put me in a room alone with my good ear not able to hear, it's still not perfect. Words will be mumbled and certain accents you can't understand, like the British accent … Some different tones I still can't understand, even with the cochlear implant.
A few people noted that localizing sound, especially loud sounds, continued to be a challenge.
The sound I think has gotten a little bit better but it's still one of those things, like, if I hear a fire engine or a police car or something like that it, I still have to move my head 360 degrees to figure out where it's actually coming from because essentially any real loud noise always sounds like it's coming from the left side.
A few people reported disappointment because the quality of the sound generated from their cochlear implant was not like the sound generated naturally by their good ear.
But that sound, especially if you have good hearing or have heard before, is not very good. It's a single tone, a single sound, it doesn't matter if it's someone talking, it doesn't matter if it's music playing, it doesn't matter if there's water running or if you drop something onto the floor, it all sounds the exact same. It's like a, almost like a robotic static crackly tone. It's not very pleasant compared to good hearing.
I'm not trying to build up my expectations too much on that side, but I think at the beginning I was a little disappointed on that side.
Participants with experience of cochlear implants noted that the device required a long training period. They reported having to spend time training their brain how to decipher sounds and learning how to hear from one good ear and the cochlear implant (which some patients referred to as CI). The sounds were different.
I put on a receiver for the first time and I'm just thinking, oh, thank you, I can, I'm going to be able to hear again, this was all worth it, like I was going to be done. And then it's just like, oh yeah, I'm probably going to have to spend another year of training.
I mean it just doesn't sound good … one of the challenges is that your brain's trying to interpret. You've got your good hearing and now you've got this simulated sound [from the cochlear implant] that's coming at the same time. It's like if you've ever spoken … into a microphone and you hear your voice, you're—almost right at the beginning you're doing a lot of that. Where your kind of hearing that feedback out of the receiver out of the new cochlear implant and it's kind of—it doesn't sound the greatest. I don't think people realize that there is a lot of training. You really, really have to put time into wearing it.
But just being able to kind of regain hearing and being able to kind of understand, I think it's very, very tough to be able to simulate what it's like or to explain what it's like to get a cochlear implant and what it's like to hear.
Participants also reflected that a cochlear implant may not be right for everyone as it requires a lot of effort and commitment to retrain the brain to comprehend the different types of sounds generated from the good ear and the cochlear implant.
Don't get me wrong; everyone likes the idea of being able to hear again out of their ear, but it's not like that at all. I mean between going through the surgery, between going through the training and wearing it and that learning curve and retraining your brain and all that extra stuff, I don't think people would realize how tough all that is …
Some participants expected the cochlear implant surgery would address their vertigo and tinnitus but were disappointed it did not.
The biggest thing for me in recovery is I didn't lose the vertigo or the tinnitus.
So it [the ringing] didn't go back to normal, unfortunately, but it [the cochlear implant] has helped, absolutely.
A few participants experienced some side effects such as perceived impairment of their sense of taste after the cochlear implant surgery.
My taste was … that was actually very different … after the surgery as well as continuing for the next, I would say, month … It's like whether or not I just became used to the new tastes or whether or not my taste buds did go back to normal, I'm never 100% sure on that. But I know for sure after surgery that was probably one of the side effects that were a little more surprising to myself that I wasn't necessarily suspecting.
Parents of children with a cochlear implant mentioned that receiving the implant was not the end of their journey to hear. Children still required adjustments or accommodations at school and at home to function well with the implant.
I think people forget that or make the assumption that, “You've got a cochlear implant. You hear just like the typical person,” but you don't. There's accommodations that they also require.
Bone-Conduction Implants: Perceptions of Limitations
Participants who had received a bone-conduction implant discussed the limitations of their device. These limitations are presented below as general, specific to single-sided deafness, and specific to conductive or mixed hearing loss.
General Limitations of Bone-Conduction Implants: People with lived experience of a bone-conduction implant noted some side effects after the surgery, such as long-term pain in their head and neck.
So it's not been quite a year yet since my surgery but I do feel—well, I've been put on pregabalin [a pain medication] for possible neuralgia of my head and neck area, and I may not have needed to go on that if I had not had the Bonebridge implanted.
After the Bonebridge surgery, I didn't feel that I was able to conduct my job at as high a level as I thought I would be able to, and some of that was the result of the pain and having to take pain killers …
The follow-up visits to adjust the volume on the device had a significant impact on people who lived far from centres that provide this service.
There were follow-ups in the beginning and it's a bit of a distance to go to Toronto but, you know, that was fine, and I went back for adjustments, volume adjustments and that sort of thing. You have to practice with it to see what it is—which setting is best for you—so they switched that and I haven't been back, I don't need any adjustments …
Some participants recognized that the bone-conduction implant could not completely restore normal hearing in the damaged ear. Most participants noted their continued struggle with understanding unfamiliar accents and tones with the bone-conduction implant.
My part-time job is behind a glass, so … sometimes I have challenges when clients talk to me. And then that's five-layers [of] glass, I'm having a hard time hearing them because not everybody has the same pitch and modulate … Some women, some men, they speak louder; and some, they speak very low.
Most people also described the challenges of maintaining and caring for the bone-conduction device. This was especially seen as a limitation among people with experience of BAHA devices. They also noted they maintained it by removing the daily accumulation of skin around the implant.
I have some challenges to remove the skin … The [problem] is my skin is growing. My skin is growing around the [abutment site] and installation implant.
In the interviews, there was a consistent theme of worry about damaging the device while travelling (such as on the subway), playing sports, or putting on hats and jackets. Some participants talked about how they had to be careful to avoid damaging it by not sleeping on the side with the implant and to keep it dry while showering or socializing at the beach.
… because it's external … sometimes it [gets] knocked off if I don't think about where I'm putting my head and ducking under something, I might knock it off or … someone [else can] knock it off me, as well.
Sometimes I just want to sleep on my right side, which is where the implant is…I try so the pillow is not directly under that part of my head…when I'm sleeping… After I shower, I put a little bit of … hydrogen peroxide…to clean around the site of the [abutment]. And I put a little bit of cream around it, like a prescription, just to make sure there's no infection.
Some people noted they also needed always carried extra batteries in case they needed replacing.
I keep my battery with me all the time, 24/7 when I leave my home. So, in case my battery is finished, then I need to replace it right away. So I do have [some needs]. So I just go to washroom, and then just take off and change, replace the battery; and then I [go] back to normal routine, so then back to work.
Limitations in Single-Sided Deafness. Most patients with single-sided deafness noted that a bone-conduction implant could not restore sound localization.
You can't identify the location of the sound and that is still the way it is whether I have the implant on or not… Like you can't tell when you hear birds singing, you have no idea where it is…
My left ear doesn't hear anything but the Bonebridge picks it up and sends it to my right ear, so everything that I'm hearing is coming in through my right ear, so I still can't tell … where the sound is coming from.
Participants with single-sided deafness noted that the bone-conduction implant depends on the good ear for hearing. This can “overwhelm” the good ear and make it difficult to hear in noisy situations.
There are situations where it [the implant] doesn't help, so, where there's a lot of background noise it overwhelms me. The device, it transmits the sound to my good ear, of course, and so all of the noise in the room is then—it's all being driven into that one ear … Definitely in lower noise environments even, I do find that the Bonebridge can cause my good ear to become overwhelmed.
Some participants with single-sided deafness were also disappointed the implant did not help restore their balance.
So, he [doctor] did the surgery to remove the balance nerve but at the same time then he put the BAHA in. So I thought okay let's try this, let's see what happens. I didn't—the BAHA was not very effective for me, it really didn't help a lot, okay? Because again the reality is you're still only hearing out of one ear, right?
Limitations in Conductive or Mixed Hearing Loss. Depending on the degree of their hearing loss, some people with conductive or mixed hearing loss who had received a bone-conduction implant noted that localizing sound continued to be a challenge, particularly for loud sounds.
I still sort of try to avoid those [group situations] when I can … It [the implant] hasn't really helped with my ability to locate sounds …
Although the bone-conduction implants helped with hearing, depending on the degree and type of hearing loss, participants noted they did not completely restore their confidence at work. They found themselves staying away from certain roles that involved communicating with or leading large groups of people.
And I would still say that, even with the Bonebridge, I'm not sure that I would have the confidence to take on a role where I was leading lots of people, had to appear in front of lots of people and big meetings with lots of noise. I find that I'm very sensitive to noise, you know, since the hearing loss … I'd say the Bonebridge has helped … improve my social ability … my confidence.
Discussion
People with experience of single-sided deafness and conductive or mixed hearing loss shared personal experiences, either their own or as a caregiver, about of the physical, psychological, social, financial, and positive impact of hearing loss on their daily lives, well-being, work, and relationships. Parents of children with hearing loss noted the developmental and behavioural impact on the children.
Participants described the perceived benefits and limitations of currently available treatments— CROS hearing aids and bone-conduction headbands. They noted that these devices work by enhancing their remaining hearing but noted several limitations that led them to explore implantable devices.
People with experience of a cochlear implant or bone-conduction implant could compare the device with other treatments they had used. Many participants with single-sided deafness reported that the advantages of a cochlear implant were binaural hearing, the potential reduction of tinnitus, and improved sound localization. Participants with single-sided deafness reported bone-conduction devices improved their hearing ability but did not help them in noisy environments or with sound localization. Participants with mixed or conductive hearing loss reported that bone-conduction implants enhanced their hearing and reduced the problem of skin infections associated with hearing aids.
People who had received an implantable device expressed the positive impact it had made in their lives, particularly in communicating with others, either at home or at work, and in improving their self-esteem.
Despite these benefits, some participants noted limitations of the devices. They did not meet their expectations in terms of improving the ability to localize sound, identify different tones and accents, or hear in noisy areas, and they did not address tinnitus or balance problems. The effort and out-of-pocket costs required to maintain the device were additional challenges. Some barriers made it difficult for people to receive an implantable device, primarily the cost of the devices and access to centres that provide the surgery and follow-up services.
Conclusions
People with single-sided deafness and conductive or mixed hearing loss reported that the currently available treatments did not meet their expectations and therefore they chose to undergo surgery for an implantable device. Most participants with experience of either a cochlear implant or bone-conduction implant spoke positively about being able to hear better and enjoy a better quality of life. People with a cochlear implant reported additional benefits: binaural hearing, better sound localization, and hearing in noisy areas.
Cost and access were barriers to the implantable devices. Some people noted limitations of the devices.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
Based on evidence of moderate quality from systematic reviews of clinical studies, cochlear implants and bone-conduction implants improve functional and patient-important outcomes in adults and children with single-sided deafness and conductive or mixed hearing loss.
We did not identify any cost-effectiveness studies in the literature that were directly applicable to our research questions.
Among people with single-sided deafness, cochlear implants may be cost-effective compared with no intervention, but bone-conduction implants are unlikely to be. Among people with conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no intervention. Results and uncertainty are mainly driven by changes in health utilities associated with having a hearing implant. Further research on health-related quality of life is warranted, with larger sample sizes and longer follow-up.
We estimate that publicly funding cochlear and bone-conduction implants as indicated for people with single-sided deafness and conductive or mixed hearing loss would cost Ontario approximately $6.7 million to $7.8 million in total over the next 5 years. Hearing implants for single-sided deafness account about half of this budget impact ($2.8 million to $3.6 million for cochlear implants and an additional $0.8 million for bone-conduction implants). Bone-conduction implants for conductive or mixed hearing loss account for the remaining 5-year budget impact ($3.1 million to $3.3 million).
In interviews, people with single-sided deafness or conductive or mixed hearing loss reported that the currently available treatments did not meet their expectations and therefore they chose to undergo surgery for an implantable device. Despite describing some limitations to the devices, most participants with experience of either a cochlear implant or bone-conduction implant spoke positively about being able to hear better and enjoy a better quality of life. People with a cochlear implant reported additional benefits: binaural hearing, better sound localization, and better hearing in noisy areas. Cost and access were important barriers to receiving a hearing implant.
Acknowledgments
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Christine Lee, the health economists were Man Wah Yeung and Lindsey Falk, the patient and public partnering analyst was Arshia Ali, and the medical librarian was Melissa Walter.
The medical editor was Amy Zierler. Others involved in the development and production of this report were Harrison Heft, Claude Soulodre, Kara Cowan, Ana Laing, Saleemeh Abdolzahraei, Kathryn Schwarz, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to the following individuals for their expert advice in the preparation of this report: Shelly Armstrong (The Ottawa Hospital), Rex Banks (Canadian Hearing Society), Paul Bradley (MedTech Consulting), Joseph Chen (Sunnybrook Health Sciences Centre), Sharon Cushing (Hospital for Sick Children), Karen Gordon (Hospital for Sick Children), Gordon Guyatt (McMaster University), Vincent Lin (Sunnybrook Health Sciences Centre), Muhammad Mamdani (St. Michael's Hospital), Vicky Papaioannou (Hospital for Sick Children), Blake Papsin (Hospital for Sick Children), and David Schramm (The Ottawa Hospital).
We thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- BAHA
Bone-anchored hearing aid
- CROS
Contralateral routing of systems (a type of hearing aid)
- dB
Decibel
- dB HL
Decibel of hearing level
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HRQOL
Health-related quality of life
- HUI3
Health Utilities Index Mark III questionnaire
- ICER
Incremental cost-effectiveness ratio
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- QALY
Quality-adjusted life-year
- ROBIS
Risk of Bias in Systematic Reviews
GLOSSARY
- Adaptive hearing test
A hearing test used to estimate a person's speech reception threshold (the intensity or decibel level at which a person can understand 50% of spoken words). The test follows an adaptive procedure in which the next stimulus presented to the test-taker is adjusted based on the test-taker's response to the previous stimulus. For example, the first sentence in a list is presented at a level below the expected speech reception threshold of the test-taker. The level of this sentence is then gradually increased until the test-taker can repeat it correctly. For the remaining sentences, the level is dependent on the accuracy of the previous response: it is increased following an incorrect repetition and decreased after a correct response. The test-taker's speech reception threshold is estimated as the average presentation level of sentences in the last part of the list.
- Audiometry
A method of assessing a person's hearing. Audiometry tests both the intensity and the tone of sounds, as well as balance and other issues related to the function of the inner ear.
- Binaural hearing
The ability to hear with both ears.
- Congenital
Describes a condition or trait that develops during fetal development and is present at birth. A congenital condition or trait may result from an infection, genetic factors, and/or environmental factors.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- Health-related quality of life (HRQOL)
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Listening fatigue
A condition caused by an increased effort to listen and understand owing to untreated hearing loss. Symptoms may include tiredness, discomfort, and pain.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Pure tone average (or threshold)
The average of hearing threshold levels at a set of specified frequencies, typically 500, 1,000, 2,000, and 4,000 Hz. This value helps assess a person's hearing level in each ear. As the pure tone average increases, hearing ability decreases.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Signal-to-noise ratio
A measure that compares the level of a desired signal (e.g., speech) to the level of background noise. It is commonly used in adaptive hearing testing to assess how well a person can understand speech when it is presented along with background noise.
- Speech audiometry
Speech audiometry typically refers to two speech tests done in a standard audiometric evaluation. The speech reception threshold is a measure that determines the level at which a person can repeat 50% of familiar spondaic words (two syllables with equal emphasis on both syllables). It is used to check the validity of the pure tone air-conduction thresholds obtained. If the two measures do not coincide, this suggests that the pure tone thresholds are not accurate. The second speech measure is word recognition ability, which is a measure to provide information about how clearly a person can hear. Hearing loss often affects clarity as well as volume, especially when the hearing loss falls within the severe to profound range. Speech audiometry does not give information about the type of hearing loss.
- Speech discrimination score
A measure of how well a person understands what they hear when speech is loud enough for the person to hear comfortably. Speech discrimination is measured as a percentage; a score of 100% means a person understands everything they hear.
- Speech recognition threshold
The faintest level at which a person can understand simple two-syllable words 50% of the time.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Warble tone
A tone whose frequency varies several times per second over a small range. Warble tones are typically used during sound field testing for calibration purposes. They prevent standing waves from forming in a sound field, which ensures a more consistent stimulus during sound field testing. Sound field testing using warble tones refers to providing a stimulus out of a speaker as opposed to through headphones.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: January 4, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to December 28, 2017>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews -NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 01>, All Ovid MEDLINE(R) <1946 to Present>
Search strategy:
-
1
Hearing Loss, Unilateral/ (1078)
-
2
((single side∗ or one side∗ or single ear or one ear or unilateral∗ or uni lateral∗ or monolateral∗ or mono lateral∗ or monoaural∗ or mono aural∗ or asymmetric∗) adj7 (deaf∗ or hearing)).ti,ab,kf. (8358)
-
3
1 or 2 (8654)
-
4
Cochlear Implantation/ (7919)
-
5
Cochlear Implants/ (20817)
-
6
(Cochlea∗ adj (implant∗ or device∗ or prosthes#s or prosthetic∗ or stimulator∗)).ti,ab,kf. (27193)
-
7
or/4–6 (30873)
-
8
3 and 7 (1308)
-
9
Bone Conduction/ (7790)
-
10
Osseointegration/ (28659)
-
11
(bone∗ adj3 (conduct∗ or anchor∗ or integrat∗)).ti,ab,kf. (13725)
-
12
(osseointegrat∗ or osseo integrat∗).ti,ab,kf. (17194)
-
13
or/9–12 (53118)
-
14
Hearing Aids/ (18308)
-
15
Correction of hearing impairment/ (4562)
-
16
(hearing adj3 (aid∗1 or device∗ or system∗ or implant∗ or technolog∗)).ti,ab,kf. (22689)
-
17
or/14–16 (30846)
-
18
13 and 17 (2628)
-
19
(Bonebridge∗ or Soundbridge∗).ti,ab,kf. (568)
-
20
((BAHA or BAHAs or BAHS or BAHSs or BAHI or BAHIs or BAHD or BAHDs or BCHI or BCHIs) adj5 (cochlea∗ or implant∗ or device∗ or system∗1)).ti,ab,kf. (629)
-
21
((Ponto or Carina or Sophono) adj5 (cochlea∗ or implant∗ or device∗)).ti,ab,kf. (129)
-
22
(middle ear adj2 (implant∗ or prosthetic∗ or prosthes#s or device∗ or transducer∗)).ti,ab,kf. (1362)
-
23
(implantable hearing or implanted hearing).ti,ab,kf. (682)
-
24
or/19–23 (2766)
-
25
18 or 24 (4463)
-
26
8 or 25 (5668)
-
27
exp Animals/ not Humans/ (15062120)
-
28
26 not 27 (4357)
-
29
limit 28 to english language [Limit not valid in CDSR; records were retained] (3734)
-
30
29 use coch,clhta (13)
-
31
Meta Analysis.pt. (98161)
-
32
Meta-Analysis/ or Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/(302244)
-
33
(((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)) or pooled analysis or published studies or published literature or hand search∗ or handsearch∗ or medline or pubmed or embase or cochrane or cinahl or data synthes∗ or data extraction∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab. (633695)
-
34
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗).mp. (432077)
-
35
or/31–34 (860163)
-
36
26 and 35 (243)
-
37
exp Animals/ not Humans/ (15062120)
-
38
36 not 37 (180)
-
39
limit 38 to english language [Limit not valid in CDSR; records were retained] (157)
-
40
39 use ppez,cleed (98)
-
41
unilateral hearing loss/ (1621)
-
42
single sided deafness/ (51)
-
43
((single side∗ or one side∗ or single ear or one ear or unilateral∗ or uni lateral∗ or monolateral∗ or mono lateral∗ or monoaural∗ or mono aural∗ or asymmetric∗) adj7 (deaf∗ or hearing)).tw,kw. (8417)
-
44
or/41–43 (8899)
-
45
cochlear implantation/ (7919)
-
46
cochlea prosthesis/ (12862)
-
47
(Cochlea∗ adj (implant∗ or device∗ or prosthes#s or prosthetic∗ or stimulator∗)).tw,kw,dv. (27506)
-
48
or/45–47 (30077)
-
49
44 and 48 (1328)
-
50
Bone Conduction/ (7790)
-
51
(bone∗ adj3 (conduct∗ or anchor∗ or integrat∗)).tw,kw,dv. (13861)
-
52
(osseointegrat∗ or osseo integrat∗).tw,kw,dv. (17778)
-
53
or/50–52 (34280)
-
54
Hearing Aid/ (19158)
-
55
auditory rehabilitation/ (2569)
-
56
(hearing adj3 (aid∗1 or device∗ or system∗ or implant∗ or technolog∗)).tw,kw,dv. (22851)
-
57
or/54–56 (30335)
-
58
53 and 57 (2669)
-
59
exp bone conduction hearing aid/ (553)
-
60
middle ear implant/ (1804)
-
61
(Bonebridge∗ or Soundbridge∗).tw,kw,dv. (611)
-
62
((BAHA or BAHAs or BAHS or BAHSs or BAHI or BAHIs or BCHI or BCHIs) adj5 (cochlea∗ or implant∗ or device∗ or system∗1)).tw,kw,dv. (641)
-
63
((Ponto or Carina or Sophono) adj5 (cochlea∗ or implant∗ or device∗)).tw,kw,dv. (134)
-
64
(middle ear adj2 (implant∗ or prosthetic∗ or prosthes#s or device∗ or transducer∗)).tw,kw,dv. (1395)
-
65
(implantable hearing or implanted hearing).tw,kw,dv. (705)
-
66
or/59–65 (4295)
-
67
58 or 66 (5785)
-
68
49 or 67 (6995)
-
69
Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (295424)
-
70
(((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)) or pooled analysis or published studies or published literature or hand search∗ or handsearch∗ or medline or pubmed or embase or cochrane or cinahl or data synthes∗ or data extraction∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab. (633695)
-
71
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗).mp. (432077)
-
72
or/69–71 (859035)
-
73
68 and 72 (281)
-
74
(exp animal/ or nonhuman/) not exp human/ (10770964)
-
75
73 not 74 (279)
-
76
limit 75 to english language [Limit not valid in CDSR; records were retained] (254)
-
77
76 use emez (122)
-
78
30 or 40 or 77 (233)
-
79
78 use ppez (96)
-
80
78 use coch (0)
-
81
78 use clhta (13)
-
82
78 use cleed (2)
-
83
78 use emez (122)
-
84
remove duplicates from 78 (135)
Economic Evidence Search
Search date: January 8, 2018
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <December 2017>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to January 4, 2018>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 02>, All Ovid MEDLINE(R) <1946 to Present>
Search strategy:
-
1
Hearing Loss, Unilateral/ (1091)
-
2
((single side∗ or one side∗ or single ear or one ear or unilateral∗ or uni lateral∗ or monolateral∗ or mono lateral∗ or monoaural∗ or mono aural∗ or asymmetric∗) adj7 (deaf∗ or hearing)).ti,ab,kf. (8433)
-
3
1 or 2 (8729)
-
4
Cochlear Implantation/ (7868)
-
5
Cochlear Implants/ (20804)
-
6
(Cochlea∗ adj (implant∗ or device∗ or prosthes#s or prosthetic∗ or stimulator∗)).ti,ab,kf. (27307)
-
7
or/4–6 (30962)
-
8
3 and 7 (1318)
-
9
Bone Conduction/ (7775)
-
10
Osseointegration/ (10242)
-
11
(bone∗ adj3 (conduct∗ or anchor∗ or integrat∗)).ti,ab,kf. (13937)
-
12
(osseointegrat∗ or osseo integrat∗).ti,ab,kf. (17341)
-
13
or/9–12 (39326)
-
14
Hearing Aids/ (18472)
-
15
Correction of hearing impairment/ (4607)
-
16
(hearing adj3 (aid∗1 or device∗ or system∗ or implant∗ or technolog∗)).ti,ab,kf. (23176)
-
17
or/14–16 (31334)
-
18
13 and 17 (2650)
-
19
(Bonebridge∗ or Soundbridge∗).ti,ab,kf. (569)
-
20
((BAHA or BAHAs or BAHS or BAHSs or BAHI or BAHIs or BAHD or BAHDs or BCHI or BCHIs) adj5 (cochlea∗ or implant∗ or device∗ or system∗1)).ti,ab,kf. (640)
-
21
((Ponto or Carina or Sophono) adj5 (cochlea∗ or implant∗ or device∗)).ti,ab,kf. (135)
-
22
(middle ear adj2 (implant∗ or prosthetic∗ or prosthes#s or device∗ or transducer∗)).ti,ab,kf. (1368)
-
23
(implantable hearing or implanted hearing).ti,ab,kf. (686)
-
24
or/19–23 (2795)
-
25
18 or 24 (4503)
-
26
8 or 25 (5720)
-
27
limit 26 to english language [Limit not valid in CDSR; records were retained] (4965)
-
28
27 use cleed (4)
-
29
economics/ (255815)
-
30
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (792383)
-
31
economics.fs. (427504)
-
32
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (806273)
-
33
exp “costs and cost analysis”/ (563405)
-
34
(cost or costs or costing or costly).ti. (246354)
-
35
cost effective∗.ti,ab,kf. (289859)
-
36
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab. (188735)
-
37
models, economic/ (12235)
-
38
markov chains/ or monte carlo method/ (75540)
-
39
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (37630)
-
40
(markov or markow or monte carlo).ti,ab,kf. (118357)
-
41
quality-adjusted life years/ (35822)
-
42
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (62579)
-
43
((adjusted adj (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (100273)
-
44
or/29–43 (2387050)
-
45
27 and 44 (185)
-
46
exp Animals/ not Humans/ (14601203)
-
47
45 not 46 (141)
-
48
Case Reports/ (2031765)
-
49
47 not 48 (141)
-
50
49 use ppez,coch,cctr,clhta (80)
-
51
28 or 50 (84)
-
52
unilateral hearing loss/ (1607)
-
53
single sided deafness/ (46)
-
54
((single side∗ or one side∗ or single ear or one ear or unilateral∗ or uni lateral∗ or monolateral∗ or mono lateral∗ or monoaural∗ or mono aural∗ or asymmetric∗) adj7 (deaf∗ or hearing)).tw,kw. (8492)
-
55
or/52–54 (8962)
-
56
cochlear implantation/ (7868)
-
57
cochlea prosthesis/ (12660)
-
58
(Cochlea∗ adj (implant∗ or device∗ or prosthes#s or prosthetic∗ or stimulator∗)).tw,kw,dv. (27612)
-
59
or/56–58 (30150)
-
60
55 and 59 (1336)
-
61
Bone Conduction/ (7775)
-
62
(bone∗ adj3 (conduct∗ or anchor∗ or integrat∗)).tw,kw,dv. (14085)
-
63
(osseointegrat∗ or osseo integrat∗).tw,kw,dv. (17928)
-
64
or/61–63 (34626)
-
65
Hearing Aid/ (19300)
-
66
auditory rehabilitation/ (2549)
-
67
(hearing adj3 (aid∗1 or device∗ or system∗ or implant∗ or technolog∗)).tw,kw,dv. (23385)
-
68
or/65–67 (30843)
-
69
64 and 68 (2700)
-
70
exp bone conduction hearing aid/ (529)
-
71
middle ear implant/ (1813)
-
72
(Bonebridge∗ or Soundbridge∗).tw,kw,dv. (610)
-
73
((BAHA or BAHAs or BAHS or BAHSs or BAHI or BAHIs or BCHI or BCHIs) adj5 (cochlea∗ or implant∗ or device∗ or system∗1)).tw,kw,dv. (650)
-
74
((Ponto or Carina or Sophono) adj5 (cochlea∗ or implant∗ or device∗)).tw,kw,dv. (140)
-
75
(middle ear adj2 (implant∗ or prosthetic∗ or prosthes#s or device∗ or transducer∗)).tw,kw,dv. (1405)
-
76
(implantable hearing or implanted hearing).tw,kw,dv. (709)
-
77
or/70–76 (4323)
-
78
69 or 77 (5841)
-
79
60 or 78 (7061)
-
80
Economics/ (255815)
-
81
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (131199)
-
82
Economic Aspect/ or exp Economic Evaluation/ (429744)
-
83
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (830503)
-
84
exp “Cost”/ (563405)
-
85
(cost or costs or costing or costly).ti. (246354)
-
86
cost effective∗.tw,kw. (300758)
-
87
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab. (189882)
-
88
Monte Carlo Method/ (60499)
-
89
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (41330)
-
90
(markov or markow or monte carlo).tw,kw. (123244)
-
91
Quality-Adjusted Life Years/ (35822)
-
92
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (66335)
-
93
((adjusted adj (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (119592)
-
94
or/80–93 (2023089)
-
95
79 and 94 (230)
-
96
(exp animal/ or nonhuman/) not exp human/ (10651858)
-
97
95 not 96 (228)
-
98
Case Report/ (4221497)
-
99
97 not 98 (223)
-
100
limit 99 to english language [Limit not valid in CDSR; records were retained] (204)
-
101
100 use emez (106)
-
102
51 or 101 (190)
-
103
102 use ppez (72)
-
104
102 use emez (106)
-
105
102 use coch (0)
-
106
102 use cctr (5)
-
107
102 use clhta (3)
-
108
102 use cleed (4)
-
109
remove duplicates from 102 (134)
Grey Literature Search
Performed: January 3–8, 2018
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, PROSPERO International prospective register of systematic reviews, Tufts Cost-Effectiveness Analysis Registry, Sick Kids Paediatric Economic Database Evaluation (PEDE)
Keywords used:
Cochlea∗, single sided, Bonebridge, Soundbridge, Carina, Ponto, Sophono, bone anchored, bone conducted, bone conduction, bone integrated, middle ear, ear implant, ear implants
Results: 1 (6 PROSPERO systematic review protocols not counted in PRISMA)
Search for Intervention-Related Health State Utilities
Search date: February 26, 2018
Database: All Ovid MEDLINE(R) <1946 to Present>
Search strategy:
-
1
Hearing Loss, Unilateral/ (664)
-
2
((single side∗ or one side∗ or single ear or one ear or unilateral∗ or uni lateral∗ or monolateral∗ or mono lateral∗ or monoaural∗ or mono aural∗ or asymmetric∗) adj7 (deaf∗ or hearing)).ti,ab,kf. (3814)
-
3
1 or 2 (3992)
-
4
Cochlear Implantation/ (5436)
-
5
Cochlear Implants/ (8626)
-
6
(Cochlea∗ adj (implant∗ or device∗ or prosthes#s or prosthetic∗ or stimulator∗)).ti,ab,kf. (12544)
-
7
or/4–6 (14031)
-
8
3 and 7 (621)
-
9
Bone Conduction/ (3039)
-
10
Osseointegration/ (8876)
-
11
(bone∗ adj3 (conduct∗ or anchor∗ or integrat∗)).ti,ab,kf. (6177)
-
12
(osseointegrat∗ or osseo integrat∗).ti,ab,kf. (8404)
-
13
or/9–12 (20146)
-
14
Hearing Aids/ (7748)
-
15
Correction of hearing impairment/ (1905)
-
16
(hearing adj3 (aid∗1 or device∗ or system∗ or implant∗ or technolog∗)).ti,ab,kf. (10794)
-
17
or/14–16 (13750)
-
18
13 and 17 (1204)
-
19
(Bonebridge∗ or Soundbridge∗).ti,ab,kf. (254)
-
20
((BAHA or BAHAs or BAHS or BAHSs or BAHI or BAHIs or BAHD or BAHDs or BCHI or BCHIs) adj5 (cochlea∗ or implant∗ or device∗ or system∗1)).ti,ab,kf. (277)
-
21
((Ponto or Carina or Sophono) adj5 (cochlea∗ or implant∗ or device∗)).ti,ab,kf. (56)
-
22
(middle ear adj2 (implant∗ or prosthetic∗ or prosthes#s or device∗ or transducer∗)).ti,ab,kf. (617)
-
23
(implantable hearing or implanted hearing).ti,ab,kf. (320)
-
24
or/19–23 (1240)
-
25
18 or 24 (2017)
-
26
8 or 25 (2591)
-
27
Quality-Adjusted Life Years/ (9855)
-
28
(quality adjusted or adjusted life year∗).tw. (12791)
-
29
(qaly∗ or qald∗ or qale∗ or qtime∗).tw. (8241)
-
30
(illness state$1 or health state$1).tw. (5330)
-
31
(hui or hui1 or hui2 or hui3).tw. (1230)
-
32
(multiattribute∗ or multi attribute∗).tw. (728)
-
33
(utility adj3 (score$1 or valu∗ or health∗ or cost∗ or measure∗ or disease∗ or mean or gain or gains or index∗)).tw. (11501)
-
34
utilities.tw. (5783)
-
35
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eurqol5d or euro?qul or eur?qul5d or euro∗ quality of life or European qol).tw. (8180)
-
36
(euro∗ adj3 (5 d or 5d or 5 dimension∗ or 5dimension∗ or 5 domain∗ or 5domain∗)).tw. (2805)
-
37
(sf36∗ or sf 36∗ or sf thirtysix or sf thirty six).tw. (18823)
-
38
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw. (1606)
-
39
((qol or hrqol or quality of life).ti. or ∗quality of life/) and ((qol or hrqol∗ or quality of life) adj2 (increas∗ or decreas∗ or improve∗ or declin∗ or reduc∗ or high∗ or low∗ or effect or effects of worse or score or scores or change$1 or impact$1 or impacted or deteriorate$)).ab. (25169)
-
40
Cost-Benefit Analysis/ and (cost effectiveness ratio∗ and (perspective∗ or life expectanc∗)).tw. (2681)
-
41
∗quality of life/ and (quality of life or qol).ti. (45054)
-
42
quality of life/ and ((quality of life or qol) adj3 (improve∗ or chang∗)).tw. (19662)
-
43
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw. (9707)
-
44
quality of life/ and health-related quality of life.tw. (24881)
-
45
quality of life/ and ec.fs. (8745)
-
46
quality of life/ and (health adj3 status).tw. (7466)
-
47
(quality of life or qol).tw. and cost-benefit analysis/ (10088)
-
48
models, economic/ (8552)
-
49
or/27–48 (130585)
-
50
26 and 49 (96)
Appendix 2: Critical Appraisal of the Clinical Evidence
Table A1:
Risk of Biasa Among Included Systematic Reviews (ROBIS Tool)
| Author, Year | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|
| Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review | |
| University of Alberta, 201147 | Low | Low | Highb | Low | Low |
| Appachi et al, 201744 | Low | Low | Low | Low | Low |
| Australia Medical Services Advisory Committee, 201034 | Low | Highc | Highd | Low | High |
| Bezdjian et al, 201748 | Low | Low | Low | Low | Low |
| Colquitt et al, 201149 | Low | Low | Low | Low | Low |
| Danhauer et al, 201050 | Low | Highe | Highf | Low | High |
| Ernst et al, 201651 | Low | Highf | Low | Low | Low |
| Kim et al, 201745 | Low | Low | Highh | Low | Low |
| Kiringoda and Lustig, 201356 | Low | Highe | Highf | Low | High |
| Kitterick et al, 201641 | Low | Low | Low | Low | Low |
| Klein et al, 201253 | Low | Low | Highb | Low | Low |
| Johnson et al, 200652 | Low | Highi | Low | Low | Low |
| Mandavia et al, 201742 | Low | Low | Low | Low | Low |
| Medical Advisory Secretariat, 200254 | Low | Highj | Highk,l | Low | High |
| Peters et al, 201546 | Low | Low | Low | Low | Low |
| Peters et al, 201638 | Low | Low | Low | Low | Low |
| Sprinzl and Wolf-Magele, 201643 | Low | Highj | Highk | Low | High |
| van Zon et al, 201539 | Low | Low | Low | Low | Low |
| Verhaert et al, 201355 | Low | Highe | Highf | Low | High |
| Vlastarakos et al, 201440 | Low | Highe,m | Highf,l | Low | High |
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Single reviewer for data extraction and study quality assessment, with data from 10% of the studies being extracted by two reviewers.
Single reviewer for study selection with another assessing those studies over which there was doubt.
Single reviewer for data extraction and checked by a second reviewer.
Unclear on the number of reviewers for study selection.
Unclear on the number of reviewers for data extraction.
Did not specify inclusion and exclusion criteria.
Appraisal of methodological quality was planned a priori but was not performed.
No description on search dates.
Single reviewer for study selection.
Single reviewer for data extraction and quality assessment.
No assessment of the methodological quality of included studies. Only assessed the evidence based on study designs.
No description of databases searched.
Table A2:
GRADE Evidence Profile for Comparison of Cochlear Implantation and No Intervention in Adults and Children With Single-Sided Deafness
| Number of Studies (Designs) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Speech audiometry | |||||||
| 4 (systematic reviews of observational studies)38–41 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Sound localization | |||||||
| 4 (systematic reviews of observational studies)38–41 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Tinnitus | |||||||
| 2 (systematic reviews of observational studies)39,40 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Hearing-specific quality of life | |||||||
| 4 (systematic reviews of observational studies)38–41 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Speech and language development | |||||||
| 1 (systematic review of observational studies)38 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f,h | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
Observational studies started at a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding). No further downgrade of GRADE was made unless there were more substantial limitations in how the study was conducted. Most included studies used a before-and-after design; thus no risk of bias on adjustment of confounding. Loss to follow-up was not identified by authors of systematic reviews as a limitation.
Some inconsistencies in results of speech perception in noise likely related to clinical heterogeneity from different diagnosis, duration of deafness, testing conditions, or outcome measures, instead of different treatment effects.
Specific target populations and interventions related to the research questions in all included studies. Authors of the included systematic reviews rated directness of evidence as moderate–high.
Most studies showed positive effects and significant statistical differences in these outcomes.
Not dominated by small studies and very few studies funded by industry.
Upgraded because of large magnitude of effect (from deafness to hearing as soon as the sound processor is turned on) and because the ability to hear leads to improvement in both objective and patient-important outcomes.
Most results of these outcomes were consistent across studies.
Early restoration of hearing symmetry by cochlear implantation during a sensitive period of auditory development could secure the function of the deafened ear and restore binaural hearing to optimize speech and language development in children.
Table A3:
GRADE Evidence Profile for Comparison of Bone-Conduction Implants and No Intervention in Adults and Children With Single-Sided Deafness
| Number of Studies (Designs) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Active percutaneous implantable devices | |||||||
| Speech audiometry | |||||||
| 2 (systematic reviews of observational studies)45,46 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Sound localization | |||||||
| 2 (systematic reviews of observational studies)45,46 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsh | Undetectede | Other considerations (+1)i | ⊕⊕⊕ Moderate |
| Hearing-specific quality of life | |||||||
| 2 (systematic reviews of observational studies)45,46 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Active transcutaneous implantable devices | |||||||
| Speech audiometry | |||||||
| 1 (systematic review of observational studies)43 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Quality of life | |||||||
| 1 (systematic review of observational studies)43 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Passive transcutaneous implantable devices | |||||||
| Audiometry | |||||||
| 1 (systematic review of observational studies)44 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Speech audiometry | |||||||
| 1 (systematic review of observational studies)44 | No serious limitationsa | No serious limitationsf | No serious limitationsc | Serious limitations (−1)j | Undetectede | Other considerations (+1)f | ⊕⊕ Low |
| Hearing-specific quality of life | |||||||
| 1 (systematic review of observational studies)44 | No serious limitationsa | No serious limitationsf | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development and Evaluation. (Notes on next page.)
Notes for Table A3:
Observational studies started at a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding). No further downgrade of GRADE was made unless there were more substantial limitations in how the study was conducted. Most included studies used a before-and-after design; thus no risk of bias on adjustment of confounding. Loss to follow-up was not identified by authors of systematic reviews as a limitation.
Some inconsistencies in results of speech perception in noise likely related to clinical heterogeneity from different diagnosis, duration of deafness, testing conditions, or outcome measures, instead of different treatment effects.
Specific target populations and interventions related to the research questions in all included studies. Authors of the included systematic reviews rated directness of evidence as moderate–high.
Most studies showed positive effects and significant statistical differences in these outcomes.
Not dominated by small studies and very few studies funded by industry.
Upgraded because of large magnitude of effect (from deafness to hearing as soon as the sound processor is turned on) and because the ability to hear leads to improvement in both objective and patient-important outcomes.
Most results of these outcomes were consistent across studies.
All included studies showed no statistically significant differences in sound localization; this was expected biologically because bone-conduction implants only reroute sounds from the deafened ear to the better ear and do not restore binaural hearing.
Upgraded because of no bias in results. If there were bias, we would observe an effect in sound localization; however, a bone-conduction implant would not improve sound localization biologically.
Most studies did not report statistical significance; thus we are unable to determine the precision of estimates.
Table A4:
GRADE Evidence Profile for Comparison of Bone-Conduction Implants and No Intervention in Adults and Children With Conductive or Mixed Hearing Loss
| Number of Studies (Designs) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Active percutaneous implantable devices | |||||||
| Audiometry | |||||||
| 3 (systematic reviews of observational studies)49,50,54 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Speech audiometry | |||||||
| 2 (systematic reviews of observational studies)49,50 | No serious limitationsa | No serious limitationsf | No serious limitationsc | Serious limitations (−1)h | Undetectede | Other considerations (+1)f | ⊕⊕ Low |
| Hearing-specific quality of life | |||||||
| 3 (systematic reviews of observational studies)50,52,54 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Active transcutaneous implantable devices | |||||||
| Audiometry | |||||||
| 1 (systematic review of observational studies)43 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Speech audiometry | |||||||
| 1 (systematic review of observational studies)43 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Hearing-specific quality of life | |||||||
| 1 (systematic review of observational studies)43 | No serious limitationsa | No serious limitationsb | No serious limitationsc | Serious limitations (−1)h | Undetectede | Other considerations (+1)f | ⊕⊕ Low |
| Passive transcutaneous implantable devices | |||||||
| Audiometry | |||||||
| 1 (systematic review of observational studies)48 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Active transcutaneous middle ear implants | |||||||
| Audiometry | |||||||
| 5 (systematic reviews of observational studies)34,47,51,53,55 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Speech audiometry | |||||||
| 5 (systematic reviews of observational studies)34,47,51,53,55 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
| Hearing-specific quality of life | |||||||
| 5 (systematic reviews of observational studies)34,47,51,53,55 | No serious limitationsa | No serious limitationsb | No serious limitationsc | No serious limitationsd | Undetectede | Other considerations (+1)f | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
Observational studies started at a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding). No further downgrade of GRADE was made unless there were more substantial limitations in how the study was conducted. Most included studies used a before-and-after design; thus, no risk of bias on adjustment of confounding. Loss to follow-up was not identified by authors of systematic reviews as a limitation.
Most results of these outcomes were consistent across studies.
Specific target populations and interventions related to the research questions in all included studies. Authors of the included systematic reviews rated directness of evidence as moderate–high.
Most studies showed positive effects and significant statistical differences in these outcomes.
Not dominant by small studies and very few studies funded by industry.
Upgraded because of large magnitude of effect (from deafness to hearing as soon as the sound processor is turned on) and because the ability to hear leads to improvement in both objective and patient-important outcomes.
Some inconsistencies in results of speech perception in noise likely related to clinical heterogeneity from different diagnosis, duration of deafness, testing conditions, or outcome measures, instead of different treatment effects.
Most studies did not report statistical significance; thus we are unable to determine the precision of estimates.
Appendix 3: Excluded Systematic Reviews—Clinical Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Baguley DM, et al. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol. 2006;31:6–14. | No quality assessment |
| Blasco MA, et al. Cochlear implantation in unilateral sudden deafness improves tinnitus and speech comprehension: meta-analysis and systematic review. Otol Neurotol. 2014;351:1426–32. | No quality assessment |
| Bruchhage KL et al. Systematic review to evaluate the safety, efficacy and economical outcomes of the Vibrant SoundBridge for the treatment of sensorineural hearing loss. Eur Arch Otorhinolaryngol 2017;274:1797–806. | Not population of interest |
| Butler CL, et al. Efficacy of the active middle-ear implant in patients with sensorineural hearing loss. J Laryngol Otol. 2013; 127 Suppl 2: S8–16. | Not population of interest |
| Cabral Junior F, et al. Cochlear implantation and single-sided deafness: a systematic review of the literature. Int Arch Otohinolaryngol. 2016;20:69–75. | No quality assessment |
| Canadian Agency for Drugs and Technologies in Health. Completely-in-the-canal and bone anchored hearing aids: a review of the clinical effectiveness and cost-effectiveness. 2010. | Limited literature search |
| Colquitt JL, et al. Bone-anchored hearing aids for people with bilateral hearing impairment: a systematic review. Clin Otolaryngol. 2011;36:419–11. | Duplicates |
| Cooper T et al. Passive transcutaneous bone-conduction hearing implants: a systematic review. Otol Neurotol. 2017;38:1225–32. | No quality assessment |
| Dimitriadis PA, et al. Three year experience with the cochlear BAHA attract implant: a systematic review of the literature. BMC Ear, Nose & Throat Disorders, 2016;16:12. | No quality assessment |
| Janssen RM, et al. Bilateral bone-anchored hearing aids for bilateral permanent conductive hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2012;147(3):412–22. | Not comparator of interest |
| Kahue CN, et al. Middle ear implants for rehabilitation of sensorineural hearing loss: a systematic review of FDA approved devices. Otol Neurotol. 2014;35:1228–37. | Not population of interest |
| Kitterick, PT, et al. Improving health-related quality of life in single-sided deafness: a systematic review and meta-analysis. Audiol Neurotol 2015;20(suppl 1):79–86. | Conference proceeding |
| Liu CC, et al. The role of bone-conduction hearing aids in congenital unilateral hearing loss: a systematic review. Int J Pediatr Otorhinolaryngol. 2017;94:45–51. | No quality assessment |
| Luers JC, et al. Vibroplasty for mixed and conductive hearing loss. Otol Neurotol. 2013;34:1005–12. | No quality assessment |
| Nadaraja GS, et al. Hearing outcomes of atresia surgery versus osseointegrated bone-conduction devise in patients with congenital aural atresia: a systematic review | No quality assessment |
| Pulcherio JO, et al. Carina and Esteem: a systematic review of fully implantable hearing devices. PLoS One. 2014;9:e110636. | No quality assessment |
| Roland L, et al. Quality of life in children with hearing loss: systematic review and meta-analysis. Otolaryngology. 2016;155:208–19. | Unspecified type of hearing loss |
| Tysome JR. Systematic review of middle ear implants; do they improve hearing as much as conventional hearing aids? Otol Neurotol. 2013;31(9):1369–75. | Not population of interest |
| Wendrich AW, et al. Systematic review on the trial period for bone-conduction devices in single-sided deafness: rates and reasons for rejection. Otol Neurotol. 2017;38:632–41. | Not outcome of interest |
| Zernotti ME, et al. Active bone-conduction prosthesis: Bonebridge. Int Arch Otorhainolaryngol. 2014;19:343–8. | Narrative review |
Appendix 4: Characteristics of Included Systematic Reviews
Table A5:
Characteristics of Systematic Reviews Included in the Clinical Evidence Review
| Author, Year | Objective(s) | Search Date and Databases Used | Inclusion Criteria | Method of Quality Assessment | ||||
|---|---|---|---|---|---|---|---|---|
| Study Design | Population | Intervention(s) | Comparator(s) | Outcome(s) | ||||
| University of Alberta, 201147 | To systematically review the evidence on middle ear implants for the treatment of hearing loss | Search date Inception to September 2011 Databases searched MEDLINE Embase | All except editorials, comments and case reports | Adults and children with sensorineural, conductive or mixed hearing loss | Middle ear implants (Vibrant Soundbridge, Esteem, Carina) | No treatment Conventional hearing aids Bone-anchored hearing aids Cochlear implants | Functional gains Speech reception Speech recognition Quality of life Adverse events | Levels of evidence from Oxford Centre for Evidence-Based Medicine120 |
| Cochrane library Web of Science CINAHL PsycINFO CRD | ||||||||
| Unpublished and non-peer-reviewed literature was located through internet searches and included manufacturer and association websites. | ||||||||
| Electronic search was supplemented with a manual search of the reference lists from included articles, recent health technology assessments and systematic reviews. | ||||||||
| Appachi et al, 201744 | To systematically review the current literature to characterize auditory outcomes of hearing rehabilitation options in children with unilateral hearing loss | Search date Inception to January 2016 Databases searched PubMed Cochrane library CINAHL MEDLINE Embase | All except case reports | Children with unilateral hearing loss | Baha Attract Sophono | No treatment | Functional auditory measures Objective auditory measures Word recognition scores | Newcastle-Ottawa scale121 |
| References of identified studies were reviewed to identify additional studies. | ||||||||
| Australia Medical Services Advisory Committee, 201034 | To systematically review the evidence on the clinical effectiveness of middle ear implants for patients with mild to severe sensorineural, conductive, or mixed hearing loss | Search date Inception to August 2009 Databases searched PubMed Cochrane library Embase Current Contents | All designs except non-systematic reviews, case reports, letters, editorials, and animal, in vitro, and laboratory studies | Adults with mild to severe sensorineural, conductive, or mixed hearing loss | Middle ear implants (Vibrant Soundbridge, Otologics middle ear transducer, Envoy Esteem, Rion device, Soundtec Direct Drive Hearing System) | Bone-anchored hearing aids Cochlear implants | Functional gains Speech perception Quality of life Adverse events | Dimensions of evidence from the National Health and Medical Research Councila |
| Bezdjian et al, 201748 | To systematically review published papers presenting Sophono implanted patients to delineate the device's functional improvement and perioperative outcomes | Search date 1975 to August 2016 Databases searched PubMed Embase Cross-reference checking was conducted to retrieve studies not identified in the initial search strategy. | All designs except case reports, letters, commentaries, literature reviews, abstracts | Adults and children with single-sided deafness or conductive or mixed hearing loss | Sophono | No treatment | Hearing thresholds Adverse events | Critical appraisal checklistb |
| Colquitt et al, 201149 | To assess the clinical effectiveness of bone-anchored hearing aids for people who are bilaterally deaf | Search date Inception to November 2009 Databases searched Web of science CENTRAL Cochrane library DARE Embase CRD Health Management Information Consortium MEDLINE Web of knowledge Reference lists of retrieved articles were examined for additional studies. Expert advisory group and manufacturers were contacted for additional references. | RCTs Controlled clinical trials Prospective cohort studies and case series Cross-sectional studies | Adults and children with bilateral hearing loss | Bone-anchored hearing aids | No treatment Conventional hearing aids Bone-conduction hearing aids | Audiometry Speech audiometry Self-reported measures | Quality assessment criteria by Thomas and colleagues122 |
| Danhauer et al, 201050 | To determine if the evidence supports the recommendation of bone-anchored hearing aids over unaided conditions in persons with conductive hearing loss | Search date Inception to 2010 Databases searched PubMed ComDisDome CINAHL CDSR | Systematic reviews RCTs Nonrandomized interventional studies | Children with congenital unilateral aural atresia | Bone-anchored hearing aids | No treatment | Audibility Speech perception Sound localization Quality of life | Quality assessment criteria by Chisolm and colleagues123 |
| Reference lists of retrieved articles were hand-searched for additional relevant studies. | ||||||||
| Ernst et al, 201651 | To systematically review the safety and effectiveness of the Vibrant Soundbridge in treating conductive and mixed hearing loss | Search date January 2006 to April 2014 Databases searched PubMed MEDLINE Embase CRD Cochrane library | Did not specify | Adults and children with conductive or mixed hearing loss | Middle ear implants (Vibrant Soundbridge) | No treatment Bone-conduction hearing implantable devices Middle ear surgery with conventional hearing aids | Hearing thresholds Functional gains Speech recognition Subjective outcomes Adverse events | Levels of evidence from Oxford Centre for Evidence-Based Medicine120 Checklist from the Evidence Analysis Library, Academy of Nutrition and Dieteticsc |
| The list of study titles was supplemented with potentially relevant publications already known by the research team. | ||||||||
| The bibliographic references of reviews were searched to locate additional relevant materials. | ||||||||
| Johnson et al, 200652 | To systematically review the nonacoustic benefits for adult patients receiving bone-anchored hearing aids relative to other forms of amplification | Search date Did not specify Databases searched PubMed ComDisDome | Randomized controlled trials Nonrandomized interventional studies Cohort studies | Adults and children with conductive or mixed hearing loss | Bone-anchored hearing aids | No treatment | Quality of life | Quality assessment criteria by Taylor et al124 |
| Kim et al, 201745 | To analyze the present capabilities of bone-anchored hearing aids in the context of single-sided deafness and to evaluate their efficacy in improving speech recognition in noisy condition, sound localization, and subjective outcomes | Search date Inception to August 2015 Databases searched Cochrane library MEDLINE Embase Relevant conference proceedings were hand-searched. | RCT Non-RCT Cohort studies Before-and-after studies Case–control studies | Adults and children with single-sided deafness or unilateral hearing loss | Bone-anchored hearing aids | No treatment | Speech discrimination in noise Sound localization Subjective benefits Adverse events | Critical appraisal checklists of the Scottish Intercollegiate Guidelines Network125 |
| Kiringoda and Lustig, 201356 | To summarize available peer-reviewed literature to describe the range and rate of complications related to osseointegrated hearing aids in adult and pediatric patients | Search date Between 2000 and 2011 Databases searched PubMed Embase | All except case reports, general reviews, commentaries, and studies that did not include patient outcomes, that reported outcomes associated with nonstandard implantation, or were of poor study or reporting quality | Adults and children who were implanted with bone-anchored hearing aids | Bone-anchored hearing aids | No treatment | Complications | Sackett levels of evidence126 |
| Kitterick et al, 201641 | To assess the nature and quality of the evidence for the use of hearing instruments in adults with unilateral severe to profound sensorineural hearing loss | Search date Inception to February 2015 Databases searched PubMed Cochrane library CINAHL DARE MEDLINE Embase Other databases were searched using their public-facing websites. | All except published abstracts, articles published in non-peer-reviewed publications and unpublished studies | Adults with unilateral severe to profound sensorineural hearing loss | Cochlear implants Bone-conduction devices | No treatment Contralateral routing of signals | Speech perception Sound localization Quality of life Adverse events | Downs and Black risk of bias checklist127 |
| Reference lists of articles that met the inclusion criteria were searched for potentially eligible articles. | ||||||||
| Klein et al, 201253 | To examine the safety and effectiveness of fully implantable middle ear devices in the treatment of hearing loss | Search date Inception to September 2011 Databases searched MEDLINE Embase Cochrane library Web of science CINAHL PsycINFO CRD | All except editorials and comments | Adults and children with sensorineural, conductive or mixed hearing loss | Middle ear implants (Carina, Esteem) | No treatment Conventional hearing aids | Functional gains Speech reception Speech recognition Quality of life Averse events | Levels of evidence from Oxford Centre for Evidence-Based Medicine120 |
| Unpublished and non-peer-reviewed literature was located through Internet searches using Google and scans of websites of manufacturers and professional associations. | ||||||||
| The electronic search was supplemented by a manual search of the references lists from included studies. | ||||||||
| Mandavia et al, 201742 | To provide stakeholders with a transparent and pragmatic assessment of the quality of the body of evidence available to inform current UK national policy on bone-conducting hearing devices | Search date Inception to September 2016 Databases searched MEDLINE Embase | All except conference proceedings and letters | Adults and children with single-sided deafness or conductive or mixed hearing loss | Bone-conduction devices | Did not specify | Did not specify | GRADE36 |
| Medical Advisory Secretariat, 200254 | To assess the effectiveness and cost-effectiveness of bone-anchored hearing aid in improving the hearing of people with conductive or mixed hearing loss | Search date January 1990 to May 2002 Databases searched Cochrane library MEDLINE Embase CCOHTA reports INAHTA AHRQ | Systematic reviews RCTs Nonrandomized controlled studies Case series | Adults and children with conductive or mixed hearing loss | Bone-anchored hearing aids | No treatment Conventional hearing aids Bone-conduction hearing aids | Hearing thresholds Speech recognition in quiet and noise Patient satisfaction Adverse events | Goodman's hierarchy of levels of evidence128 |
| Websites of Health Canada, Food and Drug Administration, and manufacturers were searched. | ||||||||
| Peters et al, 201546 | To systematically review the literature on the clinical outcomes of bone-conduction devices and contralateral routing of sound systems for patients with single-sided deafness | Search date Inception to April 2014 Databases searched PubMed Embase Cochrane library CINAHL | All except narrative reviews, case reports, and symposium programs | Adults with single-sided deafness | Bone-conduction devices Contralateral routing of signals | No treatment | Speech perception in noise Sound localization Subjective benefits | Critical appraisal checklistd |
| Cross-reference checking and related article search were performed. | ||||||||
| Peters et al, 201638 | To systematically review the literature on cochlear implantation for children with unilateral hearing loss | Search date Inception to June 2015 Databases searched PubMed Cochrane library CINAHL Embase | All except abstracts, non-peer-reviewed articles | Children with unilateral or asymmetrical hearing loss | Cochlear implants | No treatment | Speech perception Sound localization Quality of life Speech and language development | Critical appraisal checklistd |
| Sprinzl and Wolf-Magele, 201643 | To assess the safety and effectiveness of the Bonebridge for individuals with conductive or mixed hearing loss and single-sided deafness | Search date Inception to June 2014 Databases searched PubMed Cochrane library MEDLINE Embase | All | Adults and children with conductive or mixed hearing loss and single-sided deafness | Bonebridge | No treatment Conventional hearing aids | Functional gains Speech perception Subjective benefits Patient satisfaction Adverse events | National Health and Medical Research Council levels and grades of evidencee |
| Literature was supplemented by presentations from relevant conferences. | ||||||||
| van Zon et al, 201539 | To systematically review the literature to evaluate clinical outcomes of cochlear implantation for patients with single-sided deafness or asymmetrical hearing loss | Search date Inception to December 2013 Databases searched PubMed Cochrane library CINAHL Embase | All except narrative reviews, case reports | Adults with single-sided deafness or asymmetrical hearing loss | Cochlear implants | No treatment | Speech perception Sound localization Quality of life Tinnitus | Critical appraisal checklistd |
| Cross-reference and related article search was performed. | ||||||||
| Verhaert et al, 201355 | To systematically review the literature on clinical outcomes and safety of acoustic hearing implants in adults with mixed hearing loss | Search date Inception to March 2013 Databases searched MEDLINE Cochrane library Embase | All except case reports, narrative reviews, editorials | Adults with mixed hearing loss | Cochlear implants Bone-anchored hearing aids Middle ear implants | No treatment Conventional hearing aids | Functional gains Speech perception Self-reported outcomes Adverse events | Levels of evidence from Oxford Centre for Evidence-based Medicine120 Quality assessment criteriaf |
| Vlastarako et al, 201440 | To critically review the current evidence on the efficacy of cochlear implantation as a treatment modality for single-sided deafness and/or unilateral tinnitus | Search date Inception to May 2013 Databases searched MEDLINE Other available database sources | Did not specify | Adults and children with postlingual single-sided deafness and/or unilateral tinnitus | Cochlear implants | No treatment | Speech perception Sound localization Tinnitus Quality of life | Evidence-based guidelines for the categorization of medical studies129 |
| Reference lists from the retrieved articles were manually search. | ||||||||
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CCOHTA, Canadian Coordinating Office for Health Technology Assessment; CDR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CRD, Centre for Reviews and Dissemination; DARE, Database of Abstracts and Reviews of Effects; GRADE, Grading of Recommendations Assessment, Development and Evaluation; INAHTA, International Network of Agencies for Health Technology Assessment; RCT, randomized controlled trial.
Methodological quality was assessed in 3 domains: strength of the evidence, size of the effect, and relevance of the evidence.
Directness of evidence was assessed using 6 criteria: indication for surgery (clearly reported diagnosis), demographic data (including age at surgery, gender, implant laterality), description of surgical technique, complications, audiological improvement (in dB), and follow-up time (in months). Risk of bias was assessed using 5 criteria: loss to follow-up, standardization of treatment, standardization of complication (according to the Holgers classification), missing data and standardization of audiological tests (audiological performance assessed according to a protocol and by an individual other than the surgeon).
Quality assessment criteria included conflict of interest, power analysis, confounding factors considered, appropriate statistical analysis, sufficient follow-up, outcomes clearly defined, extra/unplanned treatment described, interventions specified, withdrawals/excluded or lost to follow-up, sample characteristics described, inclusion/exclusion criteria defined, research question clearly specified, prospective study.
Risk of bias was assessed by blinding, randomization, allocation concealment, standardization of interventions, standardization of outcome measures, and completeness of outcome data for primary outcome. Directness of evidence assessed outcome measurements with respect to patient population, treatment intervention, and outcome measurements.
Quality of evidence was assessed by evidence base (number of studies, level of evidence and risk of bias), consistency, clinical impact, generalizability, and applicability.
Quality of studies was assessed based on ethical approval, prospective study, eligibility criteria specified, power calculation made, appropriate controls and outcome measures used, confounding factors reported and controlled for, appropriate analysis made, and any missing data accounted for.
Appendix 5: Ongoing Reviews of Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss
| ID (Registry) | Title | Review Question(s) |
|---|---|---|
| CRD42017078285 (PROSPERO) | The efficacy of bone-anchored hearing implants in children | What is the efficacy of bone-anchored hearing implants in children with an indication for a percutaneously applied bone-conduction device? |
| CRD42017080811 (PROSPERO) | Quality of life and hearing with the use of middle ear implant: a systematic review and meta-analysis | In patients with conductive hearing loss, mixed hearing loss or unilateral hearing loss, how do middle ear implants compare to pre-implantation hearing aids in quality of life and hearing? |
| CRD42017075696 (PROSPERO) | Bone-anchored hearing aids skin complications in the pediatric population: systematic review with meta-analysis | How does the rate of skin complications of percutaneous bone-conduction implants compare to transcutaneous bone-conduction implants? How does the rate of implant loss of percutaneous bone-conduction implants compare to transcutaneous bone-conduction implants? How does the rate of re-operation related to implantation of percutaneous bone-conduction implants compare to transcutaneous bone-conduction implants? |
| CRD42017079675 (PROSPERO) | Quality of life and hearing after the implant of bone anchored hearing aid: a systematic review and meta-analysis | In patients with conductive hearing loss, mixed hearing loss or unilateral hearing loss, how do bone-anchored hearing aids compare to pre-implantation hearing aids in quality of life and hearing? |
Appendix 6: Critical Appraisal of the Economic Evidence Review
Table A6:
Results of Applicability Checklist for Studies Included in the Economic Evidence Review
| Objective: To assess the cost-effectiveness of hearing implants | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Were the perspectives clearly stated and what were they? | Are estimates of relative treatment effect from the best available source? |
| Monksfield et al, 201162 | Partly | Partly | Partly | Yes. UK health care payer | Partly |
| Author, Year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgement (directly applicable/partially applicable/not applicable) |
|---|---|---|---|---|
| Monksfield et al, 201162 | Yes. 3.5% discounting | Yes | No | Partially applicable |
Table A7:
Methodological Quality of Studies Included in the Economic Evidence Review
| Objective: To assess the cost-effectiveness of hearing implants | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the estimates of relative treatment effects obtained from best available sources? | Do the estimates of relative treatment effect match the estimates contained in the clinical report? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from best available sources? |
| Monksfield et al, 201162 | N/A. Piggybacked evaluation | Yes. Lifetime | Partly | Partly. Small sample size; both HUI2 and HUI3 used | Unclear. Unclear if authors used individual utility data or the mean utility to derive QALYs | Partly | Yes |
| Author, Year | Are the unit costs of resources obtained from best available resources? | Is an appropriate incremental analysis presented or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall assessment including applicability to the project (Minor limitations/potentially serious limitations/very serious limitations) |
|---|---|---|---|---|---|
| Monksfield et al, 201162 | Yes | Yes | No. Only deterministic sensitivity analyses for discount rates | No | Potentially serious limitations. Based on uncontrolled before-and-after study |
Abbreviations: HRQOL, health-related quality of life; HUI2, Health Utilities Index Mark II questionnaire; HUI3, Health Utilities Index Mark III questionnaire; N/A, not applicable; QALY, quality-adjusted life-year.
Appendix 7: Inputs and Results for the Primary Economic Evaluation Complications
Complications
Table A8 presents the inputs for major and minor complications used in the three economic models. Note that the probabilities of explantation and re-implantation are conditional probabilities (explantation is conditional on having had a major complication, and re-implantation is conditional on having had a device removed). Also note that some of the distributions used for probabilities for those two variables were uniform rather beta. Given the small sample sizes in data sources used to estimate the probability of explantation or re-implantation conditional on having had a major complication, we used uniform distributions to place equal weighting across a range of probabilities.
Table A8:
6-Month Probability of Complications After a Hearing Implant, Sensitivity Analyses
| Complications | Probability | SD | Distribut ion | Reference |
|---|---|---|---|---|
| Sensitivity analyses | ||||
| Models 2 and 3: Bone-conduction implants for single-sided deafness and conductive/mixed hearing loss | ||||
| Minor complications, active percutaneous devices, adults | 0.072 | 0.0072a | Beta | Gluth et al, 201091 |
| Minor complications, active transcutaneous devices, children | 0.0283 | 0.0028a | Beta | Sprinzl and Wolf-Magele, 201543 |
| Major complications, active percutaneous devices, adults | 0.0150 | 0.0015a | Beta | Badran et al, 200970 |
| Conditional probability explantationb | 0.54 | ±10% | Uniform | Badran et al, 200970 |
| Conditional probability re-implantationc | 0.77 | ±10% | Uniform | Badran et al, 200970 |
| Major complications, active transcutaneous devices, children | 0.0043 | 0.0004a | Beta | Sprinzl and Wolf-Magele, 201543 |
| Conditional probability explantationb | 0.41 | ±10% | Uniform | Kraai et al, 201187; Chan et al, 201786; Yellon et al, 200788 (assume same as active percutaneous) |
| Conditional probability re-implantationc | 0.47 | ±10% | Uniform | Chan et al, 201786; Yellon et al, 200788 (assume same as active percutaneous) |
| Model 3 only: Bone-conduction implants for conductive/mixed hearing loss | ||||
| Minor complications, passive transcutaneous devices, adults and children | 0.1320 | 0.0132a | Beta | Bezdjian et al, 201748 |
| Major complications, passive transcutaneous devices, adults and children | 0.0169 | 0.0017a | Beta | Bezdjian et al, 201748 |
| Conditional probability explantationb | 0.5 | ±10% | Uniform | MED-EL, 2017130 |
| Conditional probability re-implantationc | 0.5 | ±10% | Uniform | MED-EL, 2017130 |
Note: 6-month probabilities are reported unless otherwise stated.
SD assumed to be 10% of mean.
Conditional probability of explantation given a major complication. Calculated as no. of individuals with explantation (either explantation only or re-implantation) ÷ no. of individuals with major complications.
Conditional probability of re-implantation given an explantation. Calculated as no. of individuals with re-implantation ÷ no. of individuals with explantation (either explantation only or re-implantation).
Elective Non-use
Model 1: Cochlear Implants for Single-Sided Deafness
Table A9:
6-Month Probabilities of Elective Non-use of Cochlear Implant
| Years After Implantation | Probability of Elective Non-use of Device | ||
|---|---|---|---|
| Adults | Children | References | |
| Reference case | |||
| All | 0.0095 (assume 10% SD) | 0.0026 (SD: 0.001) | Data from OCIPa (adults) and assumption (children) |
| Sensitivity analysis | |||
| 0.5 | 0.0023 | 0.0012 | Bhatt et al, 200983; Ozdemir et al, 201376 |
| 1 | 0.0023 | 0.0012 | |
| 1.5 | 0.0023 | 0.0012 | |
| 2 | 0.0023 | 0.0012 | |
| 2.5 | 0.0023 | 0.0012 | |
| 3 | 0.0023 | 0.0029 | Bhatt et al, 200983; Archbold et al, 200984 |
| 3.5 | 0.0023 | 0.0029 | |
| 4 | 0.0014 | 0.0029 | Ray et al, 200685; Archbold et al, 200984 |
| 4.5 | 0.0014 | 0.0029 | |
| 5 | 0.0014 | 0.0029 | |
| 5.5 | 0.0014 | 0.0029 | |
| 6 | 0.0014 | 0.0029 | |
| 6.5 | 0.0014 | 0.0029 | |
| 7 | 0.0014 | 0.0029 | |
| 7.5 | 0.0014 | 0.0029 | |
| 8 | 0.0014 | 0.0631 | Ray et al, 200685; Raine et al, 200881 |
| 8.5 | 0.0014 | 0.0631 | |
| 9 | 0.0014 | 0.0881 | |
| 9.5 | 0.0014 | 0.0881 | |
| ≥ 10 | 0.0014 | 0.1611 | |
| Sensitivity analysis | |||
| All | 0.0026 (SD: 0.001) | 0 | Expert opinion (adults); Polonenko et al, 2017 (children)82 |
Abbreviations: OCIP, Ontario Cochlear Implant Program; SD, standard deviation.
Written communication, Joseph Chen, MD, July 12, 2018.
Models 2 and 3: Bone-Conduction Implants for Single-Sided Deafness and Conductive or Mixed Hearing Loss
Table A10:
6-Month Probabilities of Elective Non-use of Bone-Conduction Implant
| Probability of Elective Non-use of Device | ||||
|---|---|---|---|---|
| Mean | SD | Distribution | Reference | |
| Reference case | ||||
| Model 2: Bone-conduction implants for single-sided deafness | ||||
| Adults | 0.0064 | 0.0487 | Beta | Data from OCIPa |
| Children | 0.0064 | 0.0487 | Beta | No OCIP data; assume same as adults |
| Model 3: Bone-conduction implants for conductive/mixed hearing loss | ||||
| Adults | 0.0026 | 0.0010 | Beta | Assumption |
| Children | 0.0877 | 0.0943 | Beta | Polonenko et al, 201689 |
| Sensitivity analysis | ||||
| Models 2 and 3: Bone-conduction implants for single-sided deafness and conductive/mixed hearing loss | ||||
| Adults, SSD | 0.0235 | 0.0043 | Beta | Expert opinion |
| Children, SSD | 0.0728 | 0.0783 | Beta | Kesser et al, 201390 |
| Adults and children, CHL/MHL | 0.0070 | 0.0013 | Beta | Expert opinion |
| Adults, CHL/MHL | 0 | N/A | N/A | Data from OCIPa |
| Adults, CHL/MHL | 0.0162 | 0.0081 | Beta | Gluth et al, 201091; Hobson et al, 201072 |
| Children, CHL/MHL | 0.0104 | 0.0052 | Beta | Kraai et al, 2011,87 Kiringoda and Lustig, 201356 |
Abbreviations: CHL/MHL, conductive/mixed hearing loss; OCIP, Ontario Cochlear Implant Program; N/A, not applicable; SSD, single-sided deafness.
Personal communication, Joseph Chen, MD, July 12, 2018.
Utilities
Model 1: Cochlear Implants for Single-Sided Deafness
In the reference case, we used utilities derived by a small study (n = 11) using the HUI3 in patients with unilateral hearing.99 While we recognize the limitations (i.e., imprecision) of the small sample size, this was the only study we identified that was published in full text and/or conducted using the HUI3. We have previously described the benefits of using the HUI3 to derive utilities for hearing loss and in an Ontario population (Primary Economic Evaluation, Methods, Utilities). In sensitivity analyses, we used utilities estimated using the time trade-off technique, obtained from a poster presentation.100
Model 2: Bone-Conduction Implants for Single-Sided Deafness
In the reference case, we used health utilities from a small Ontario study of adults (n = 16) who had received active transcutaneous implants (i.e., Bonebridge) (written communication, Joseph Chen, MD, June 13, 2018). In sensitivity analyses, we used health utilities identified from the literature.99 While we acknowledge these data may not be fully generalizable to children or other device classes, we explore alternative sources through sensitivity analyses.
In adults, we identified one study in single-sided deafness that reported utilities after bone-conduction implantation.131 The study did not report utilities before implantation and for this reason we could not calculate a mean difference associated with bone-conduction implantation. Instead, we used a study by Arndt et al99 who measured utilities before any hearing aid intervention, and after a testing period with a non-implantable, bone-conduction hearing aid (i.e., softband/tension clamp). We assumed the benefits seen after the testing period (mean difference = 0.11) are the same as benefits seen after bone-conduction implantation. The sample size in this study was also small (n = 11). We did not identify any relevant studies specific to children, so we used these values in sensitivity analyses as well.
Model 3: Bone-Conduction Implants for Conductive/Mixed Hearing Loss
In the reference case, we used health utilities of adults in Ontario (n = 34) using active transcutaneous implants. In sensitivity analyses, we used health utilities identified from the literature.
We identified two partially relevant studies from the literature that used the HUI3. Both were conducted in adults with mixed types of hearing loss (i.e., including conductive, mixed, unilateral and/or bilateral sensorineural hearing loss). One assessed quality of life after patients received a middle ear implant71 and the other after bone-anchored hearing devices62. Both studies reported a mean HUI3 difference of 0.09 after implantation. Edfeldt et al71 included some patients with bilateral sensorineural hearing loss and some patients who had used conventional hearing aids. Therefore, in our sensitivity analyses, we used the values derived from Monksfield et al,62 which were based on active percutaneous devices. We did not identify any relevant studies specific to children, so we used these values in sensitivity analyses as well.
Costs Associated with Complications
We calculated the “average” cost of a minor complication and a major complication for each economic model. This was done by finding the frequency of different complications among adults and children (minor complications included tinnitus and pain; major complications included revision surgery for infections and cholesteatoma). The relative frequencies were used as weights to multiply by the cost of each type of complication. The relative frequencies used were based on the previous Health Quality Ontario health technology assessment.29 The sum of these products provided the final weighted average cost. We used only one set of average costs as a simplifying assumption across all three economic models, and tested the other average costs in sensitivity analyses. Table A11 provides details for these calculations.
Table A11:
Costs of Complications for Adults and Children Receiving a Hearing Implant, Weighted Average Calculations
| Variable | Unit Cost, $ | Weight (Adults) | Weight (Children) | Cost Components | References for Costs | References for Weights |
|---|---|---|---|---|---|---|
| Minor complications | ||||||
| Infection (skin infections, otitis media) | 93.88 | 0.1458 | 0.6486 | Emergency department assessment and antibioticsa,b | Schedule of Benefits (H065)103 | Farinetti et al, 201458; Venail et al, 200857 |
| Neurological complications (facial palsy, dysgeusia) | 49.23 | 0.1250 | 0.0541 | Specific assessment and corticosteroidsb,c | Schedule of Benefits (A013)103; Ontario Drug Benefit Formulary106 | Farinetti et al, 201458; Venail et al, 200857 |
| Pain (facial stimulation, other) | 74.25 | 0.1458 | 0.0541 | Emergency department assessment | Schedule of Benefits (H065)103 | Farinetti et al, 201458; Venail et al, 200857 |
| Tinnitus (worsening or new occurrence) | 47.50 | 0.2292 | 0.0000 | Specific assessment | Schedule of Benefits (A013)103 | Farinetti et al, 201458; Venail et al, 200857 |
| Vestibular complications (vertigo, dizziness) | 90.65 | 0.3542 | 0.1351 | Specific assessment and CT scan | Schedule of Benefits (A013, X001)103 | Farinetti et al, 201458; Venail et al, 200857 |
| Other complications (cerebrospinal fluid leak hematoma, atlantoaxial subluxation) | 74.25 | 0.0000 | 0.1081 | Emergency department assessment | Schedule of Benefits (H065)103 | Farinetti et al, 201458; Venail et al, 200857 |
| Average cost, minor complications: | ||||||
| Adults | 73.66 | |||||
| Children | 87.84 | |||||
| Major complications | ||||||
| Revision (infection)d | 670.08 | 0.25 | 0.57 | Surgical drainage | OCC day surgery 2016/2017105 | Farinetti et al, 201458 |
| Revision (cholesteatoma)e | 2,190.93 | 0.25 | 0.14 | Surgery | OCC day surgery 2016/2017105 | Farinetti et al, 201458 |
| Revision (other) | 4,427.05 | 0.50 | 0.29 | Surgery | Merdad et al, 2014104 | |
| Average cost, major complications: | ||||||
| Adults | 2,928.78 | |||||
| Children | 2,761.26 | |||||
Abbreviations: CT, computed tomography; OCC, Ontario Case Costing.
Outpatient drug costs not included for individuals > 24 years or < 65 years.
Drug cost of treating otitis media in Ontario using amoxicillin in all patients.107
Assumes prednisolone 50 mg/day × 10 days.132
Including Canadian Classification of Health Initiatives codes 1DA52, 1DE52, 1DK52, 1DL52, 1DN52, 1DR52.
Including Canadian Classification of Health Initiatives codes 1DK87, 1DL87.
Results
Table A12:
Selected Results From Scenario Analyses: Public Payer Scenario and Lower and Upper Range of Incremental Cost-Effectiveness Ratios
| Economic Model | Scenario | ICER, $/QALY |
|---|---|---|
| Model 1, adults | Reference case | 18,148 |
| Lower ICER: lifetime time horizon and increased risk of mortality associated with no intervention | 13,165 | |
| Upper ICER: mean difference in utilities based on time trade-off method | 55,655 | |
| Public payer,a plus 100% receiving rehabilitation (2 one-hour sessions) | 20,237 | |
| Model 1, children | Reference case | 17,783 |
| Lower ICER: lifetime time horizon and increased risk of mortality associated with no intervention | 11,293 | |
| Upper ICER: mean difference in utilities based on time trade-off method | 54,038 | |
| Public payer,a 100% receiving rehabilitation (weekly for 18 months) | 20,645 | |
| Model 2, adults | Reference case | 408,350 |
| Lower ICER: age of implantation at 80 years and increased risk of mortality associated with no intervention | 29,071 | |
| Upper ICER: ADP pays for full price of sound processor replacements | 450,927 | |
| Public payera | 404,732 | |
| Model 2: children | Reference case | 402,899 |
| Lower ICER: utilities based on literature | 43,842 | |
| Upper ICER: disutilities applied to all complications | 2,277,930 | |
| Public payera | 477,640 | |
| Model 3, adults | Reference case | 74,155 |
| Lower ICER: lifetime time horizon and increased risk of mortality associated with no intervention | 24,730 | |
| Upper ICER: ADP pays for full price of sound processor replacements | 90,372 | |
| Public payera | 81,104 | |
| Model 3: children | Reference case | 87,580 |
| Lower ICER: utilities based on literature | 34,054 | |
| Upper ICER: disutilities applied to all complications | 105,569 | |
| Public payera | 91,591 |
Abbreviations: ADP, assistive devices program; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
In the public payer scenario, the Ministry of Community and Social Services pays the remainder of the sound processor replacement cost not covered by the Ministry of Health's Assistive Devices Program, in the 40% of individuals who are considered low-income.
Note: Model 1 examined cochlear implants for single-sided deafness; Model 2, bone-conduction implants for single-sided deafness; Model 3, bone-conduction implants for conductive/mixed hearing loss.
Sensitivity Analysis Results
The tornado diagrams in Figures A1 to A3 summarize the one-way deterministic sensitivity analyses. Each horizontal bar represents the variation of incremental cost-effectiveness ratios (ICERs) around the deterministic reference case ICER (vertical line) as one model input was varied over a range of values. Inputs that generate the widest bars have the greatest influence on cost-effectiveness results. These inputs are considered sensitive. The figures present only the most sensitive inputs.
Figure A1: Tornado Diagram for Cost-Effectiveness of Cochlear Implants for People With Single-Sided Deafness.
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
aParameter led to range from negative infinity to positive infinity.
Figure A2: Tornado Diagram for Cost-Effectiveness of Bone-Conduction Implants for People With Single-Sided Deafness.
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
aParameter led to range from negative infinity to positive infinity.
Figure A3: Tornado Diagram for Cost-Effectiveness of Bone-Conduction Implants for People With Conductive/Mixed Hearing Loss.
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
aParameter led to range from negative infinity to positive infinity.
Appendix 8: Budget Impact Analysis
Annual Per-Patient Costs
Table A13:
Per-Patient Costs to the Ministry of Health With and Without Targeted Public Funding of Hearing Implants, Years 1 to 5
| Scenario | Per-Patient Costs to Ministry, $ | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Model 1: Cochlear implants for single-sided deafness | |||||
| With targeted public funding | |||||
| Adults | 32,209 | 273 | 267 | 216 | 256 |
| Children | 32,553 | 463 | 303 | 301 | 299 |
| No targeted public funding | |||||
| Adults | 7,209 | 273 | 267 | 216 | 256 |
| Children | 32,553 | 463 | 303 | 301 | 299 |
| Model 2: Bone-conduction implants for single-sided deafness | |||||
| With targeted public funding | |||||
| Adults | 16,101 | 104 | 101 | 101 | 3,076 |
| Children | 14,344 | 443 | 391 | 389 | 3,308 |
| No targeted public funding | |||||
| Adults | 11,701 | 104 | 101 | 101 | 3,076 |
| Children | 14,344 | 443 | 391 | 389 | 3,308 |
| Model 3: Bone-conduction implants for conductive/mixed hearing loss | |||||
| With targeted public funding | |||||
| Adults | 16,100 | 103 | 100 | 100 | 3,032 |
| Children | 14,313 | 356 | 297 | 295 | 2,512 |
| No targeted public funding | |||||
| Adults | 11,700 | 103 | 100 | 100 | 3,032 |
| Children | 14,313 | 356 | 297 | 295 | 2,512 |
Appendix 9: Call for Participationa
HEALTH QUALITY ONTARIO REQUESTS YOUR PARTICIPATION
REVIEW OF DEVICES IMPLANTED FOR HEARING LOSS
Do you have a surgically implanted hearing device? Are you considering one?
If you or someone you are caring for has this experience, we'd like to speak to you.
Interviews will take 20 to 30 minutes, either on the phone or in-person, scheduled between now and April 30, 2018.
WHY GET INVOLVED?
Your participation will help Health Quality Ontario with the review of usefulness of Implantable Devices for Single-sided deafness and Conductive/Mixed Hearing Loss. This review will result in a recommendation for public funding to the Ministry of Health and Long-Term Care.
ABOUT US
Health Quality Ontario is a provincial agency with one purpose: better health for all Ontarians. Part of our work involves conducting reviews of various health care technologies and services to gauge their usefulness.
If you're interested in participating, or have questions, please contact:
Health Quality Ontario is now the Quality business unit at Ontario Health.
Appendix 10: Interview Guide
Overview: What is Health Quality Ontario'sb mandate? What is health technology assessment?
Health Quality Ontariob is a provincial agency dedicated to ensuring our health care system delivers a better experience of care and better outcomes for Ontarians at better value for money. Part of this role includes evaluating the effectiveness of health care technologies and services through a process called health technology assessment.
Health technology assessment projects involve rigorous clinical and economic evidence review on the effective, safety, and cost of technologies while considering the perspectives of patients and caregivers who have experience with the condition or technology in question. We are currently reviewing hearing implants in adults. I am calling you to hear about your experience with hearing loss and the treatment options available.
Question 1:
What kind of health conditions do you (your loved one) have?
What are the biggest challenges of living/caring for someone with hearing loss?
How does it impact your day-to-day routine? How would you describe your quality of life?
Question 2:
What kind of treatments are you aware of and which are the ones you have explored?
Question 3: If patients/caregivers are waiting for treatment:
What are the potential benefits and risks of the different treatments in their opinion?
Was it difficult to weigh potential risks/benefits with the type of treatment?
Question 4: If patients/caregivers have not had hearing implants:
How did that procedure meet/not meet their needs? How was it adequate/inadequate? QOL, Empowerment? Ownership? Adherence? Lifestyle?
What were the side effects and benefits? Anxiety, painful, intrusiveness?
Were there issues related to cost, access, knowledge of health care system, etc.? Travel, repeat visits
What challenges did this treatment address?
Question 5: If patients/caregivers have had hearing implants:
How did it meet/not meet their needs? QOL, Empowerment? Ownership? Adherence? Lifestyle?
What were the side effects and benefits? Invasiveness, follow-ups? Anxiety, painful?
Were there issues related to cost, access, knowledge of health care system, etc.? Travel, repeat visits
What challenges would hearing implant address? How will it be beneficial or not beneficial?
Thank you for sharing your story and your insights on this condition and the available technologies. We will use these insights to draft a report and recommendation for funding. The draft report will be posted on our public website for comments, and we would welcome you to review and share your thoughts on it. If you wish, we could email you to alert you about this posting.
If we do not have their email, request it and add to the stakeholder list.
Health Quality Ontario is now the Quality business unit at Ontario Health.
Author contributions
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The clinical epidemiologist was Christine Lee, the health economists were Man Wah Yeung and Lindsey Falk, the patient and public partnering analyst was Arshia Ali, and the medical librarian was Melissa Walter.
KEY MESSAGES
What Is This Health Technology Assessment About?
Single-sided deafness is profound sensorineural hearing loss (damage to the hearing organ or hearing nerve in the inner ear) or non-functional hearing in one ear, with normal or near-normal hearing in the other ear. Hearing in only one ear makes it hard to tell where sound is coming from and to hear in noisy environments. Conductive hearing loss is a mechanical problem with the ear's ability to conduct sound vibrations. Mixed hearing loss is a combination of sensorineural and conductive hearing loss. Conductive and mixed hearing loss frequently affect both ears, which creates additional challenges for people in school, work, and social life. Cochlear and bone-conduction implants may help some people who cannot use standard hearing aids. Currently in Ontario, a limited number of people with hearing loss receive implantable hearing devices at no cost, whereas some others pay part or all of the cost of the device.
This health technology assessment looked at how safe, effective, and cost-effective surgically implanted hearing devices are for three types of hearing loss and what the budget impact of publicly funding these devices would be. It also looked at the experiences, preferences, and values of people with single-sided deafness or conductive or mixed hearing loss.
What Did This Health Technology Assessment Find?
The best available evidence shows that cochlear and bone-conduction implants helped people with single-sided deafness or conductive or mixed hearing loss hear better and improved their hearing-specific quality of life. For people with single-sided deafness, cochlear implants may be cost-effective compared with no hearing aids or no implant. Bone-conduction implants are not as attractive from a cost-effectiveness perspective but are acceptable to patients who cannot use cochlear implants. For conductive or mixed hearing loss, bone-conduction implants may be cost-effective compared with no hearing aids or no implant. However, these findings depend largely on limited data about how people's overall quality of life (measured by generic quality-of-life measures, often less sensitive than hearing-specific measures) changes after an implant. We estimate that publicly funding cochlear implants for people with single-sided deafness would cost $2.8 million to $3.6 million over the next 5 years, with an additional $0.8 million needed for bone-conduction implants. We estimate that publicly funding bone-conduction implants for people with conductive or mixed hearing loss would cost an additional $3.1 million to $3.3 million over the next 5 years.
People with hearing loss with whom we spoke reported believing that implantable devices are better than standard hearing aids even if the implants have some limitations. Some people experienced high out-of-pocket costs to get or maintain their device.
Contributor Information
Ontario Health (Quality):
Christine Lee, Man Wah Yeung, Lindsey Falk, Arshia Ali, and Melissa Walter
About Us
This health technology assessment was produced by the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1).Van de Heyning P, Tavora-Vieira D, Mertens G, Van Rompaey V, Rajan GP, Muller J, et al. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiol Neurootol. 2016;21(6):391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Akeroyd MA. The psychoacoustics of binaural hearing. Int J Audiol. 2006;45 Suppl 1:S25–33. [DOI] [PubMed] [Google Scholar]
- (3).Van Wanrooij MM, Van Opstal AJ. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24(17):4163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–54. [DOI] [PubMed] [Google Scholar]
- (5).Johnson JL, White KR, Widen JE, Gravel JS, James M, Kennalley T, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116(3):663–72. [DOI] [PubMed] [Google Scholar]
- (6).Bess FH, Tharpe AM. Unilateral hearing impairment in children. Pediatrics. 1984;74(2):206–16. [PubMed] [Google Scholar]
- (7).Uwiera TC, DeAlarcon A, Meinzen-Derr J, Cohen AP, Rasmussen B, Shott G, et al. Hearing loss progression and contralateral involvement in children with unilateral sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2009;118(11):781–5. [PubMed] [Google Scholar]
- (8).Baguley DM, Bird J, Humphriss RL, Prevost AT. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol. 2006;31(1):6–14. [DOI] [PubMed] [Google Scholar]
- (9).Bamiou DE, Savy L, O'Mahoney C, Phelps P, Sirimanna T. Unilateral sensorineural hearing loss and its aetiology in childhood: the contribution of computerised tomography in aetiological diagnosis and management. Int J Pediatr Otorhinolaryngol. 1999;51(2):91–9. [DOI] [PubMed] [Google Scholar]
- (10).Laury AM, Casey S, McKay S, Germiller JA. Etiology of unilateral neural hearing loss in children. Int J Pediatr Otorhinolaryngol. 2009;73(3):417–27. [DOI] [PubMed] [Google Scholar]
- (11).Voelker CC, Chole RA. Unilateral sensorineural hearing loss in adults: etology and managment. Semin Hear. 2010;31(4):313–25. [Google Scholar]
- (12).Dougherty W, Kesser BW. Management of conductive hearing loss in children. Otolaryngol Clin North Am. 2015;48(6):955–74. [DOI] [PubMed] [Google Scholar]
- (13).Bhutta MF, Williamson IG, Sudhoff HH. Cholesteatoma. BMJ. 2011;342:d1088. [DOI] [PubMed] [Google Scholar]
- (14).Schilder AG, Zielhuis GA, Haggard MP, van den Broek P. Long-term effects of otitis media with effusion: otomicroscopic findings. Am J Otol. 1995;16(3):365–72. [PubMed] [Google Scholar]
- (15).Zahnert T. The differential diagnosis of hearing loss. Dtsch Arztebl Int. 2011;108(25):433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136(1):141–53. [DOI] [PubMed] [Google Scholar]
- (17).Lieu JE. Management of children with unilateral hearing loss. Otolaryngol Clin North Am. 2015;48(6):1011–26. [DOI] [PubMed] [Google Scholar]
- (18).Dornhoffer JR, Dornhoffer JL. Pediatric unilateral sensorineural hearing loss: implications and management. Curr Opin Otolaryngol Head Neck Surg. 2016;24(6):522–8. [DOI] [PubMed] [Google Scholar]
- (19).Kuppler K, Lewis M, Evans AK. A review of unilateral hearing loss and academic performance: is it time to reassess traditional dogmata? Int J Pediatr Otorhinolaryngol. 2013;77(5):617–22. [DOI] [PubMed] [Google Scholar]
- (20).Purcell PL, Shinn JR, Davis GE, Sie KC. Children with unilateral hearing loss may have lower intelligence quotient scores: a meta-analysis. Laryngoscope. 2016;126(3):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dincer D'Alessandro H, Sennaroglu G, Yucel E, Belgin E, Mancini P. Binaural squelch and head shadow effects in children with unilateral cochlear implants and contralateral hearing aids. Acta Otorhinolaryngol Ital. 2015;35(5):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Polonenko MJ, Gordon KA, Cushing SL, Papsin BC. Cortical organization restored by cochlear implantation in young children with single sided deafness. Sci Rep. 2017;7(1):16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol. 2010;119(11):772–81. [PubMed] [Google Scholar]
- (24).Lucas L, Katiri R, Kitterick PT. The psychological and social consequences of single-sided deafness in adulthood. Int J Audiol. 2018;57(1):21–30. [DOI] [PubMed] [Google Scholar]
- (25).Alhanbali S, Dawes P, Lloyd S, Munro KJ. Self-reported listening-related effort and fatigue in hearing-impaired adults. Ear Hear. 2017;38(1):e39–e48. [DOI] [PubMed] [Google Scholar]
- (26).Rapin I. Conductive hearing loss effects on children's language and scholastic skills. A review of the literature. Ann Otol Rhinol Laryngol Suppl. 1979;88(5 Pt 2 Suppl 60):3–12. [DOI] [PubMed] [Google Scholar]
- (27).Bidadi S, Nejadkazem M, Naderpour M. The relationship between chronic otitis media-induced hearing loss and the acquisition of social skills. Otolaryngol Head Neck Surg. 2008;139(5):665–70. [DOI] [PubMed] [Google Scholar]
- (28).Olusanya BO, Neumann KJ, Saunders JE. The global burden of disabling hearing impairment: a call to action. Bull World Health Organ. 2014;92(5):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Health Quality Ontario. Bilateral cochlear implantation: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2018. Oct;18(6):1–139. Available from: http://www.hqontario.ca/evidence-to-improve-care/journal-ontario-health-technology-assessment-series. [PMC free article] [PubMed] [Google Scholar]
- (30).Wendrich AW, Kroese TE, Peters JPM, Cattani G, Grolman W. Systematic review on the trial period for bone conduction devices in single-sided deafness: rates and reasons for rejection. Otol Neurotol. 2017;38(5):632–41. [DOI] [PubMed] [Google Scholar]
- (31).National Health Service England. Clinical commissioning policy: bone conducting hearing implants (BCHIs) for hearing loss (all ages) [Internet]. London (UK): NHS England; 2016. [cited 2018 Aug 21]. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2013/05/16041_FINAL.pdf. [Google Scholar]
- (32).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (33).Carlsson PU, Hakansson BE. The bone-anchored hearing aid: reference quantities and functional gain. Ear Hear. 1997;18(1):34–41. [DOI] [PubMed] [Google Scholar]
- (34).Australia Medical Services Advisory Committee. Middle ear implant for sensorineural, conductive and mixed hearing losses. MSAC application 1137 assessment report [Internet]. Canberra (AU): Commonwealth of Australia; 2010. [cited 2018 March 6]. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/FD11A73F9DB18F7ECA25801000123B78/Φle/1137-Assessment%20_Report.pdf [Google Scholar]
- (35).Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): Grade Working Group; 2013. [cited 2018 Apr 17]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- (37).Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- (38).Peters JP, Ramakers GG, Smit AL, Grolman W. Cochlear implantation in children with unilateral hearing loss: a systematic review. Laryngoscope. 2016;126(3):713–21. [DOI] [PubMed] [Google Scholar]
- (39).van Zon A, Peters JP, Stegeman I, Smit AL, Grolman W. Cochlear implantation for patients with single-sided deafness or asymmetrical hearing loss: a systematic review of the evidence. Otol Neurotol. 2015;36(2):209–19. [DOI] [PubMed] [Google Scholar]
- (40).Vlastarakos PV, Nazos K, Tavoulari EF, Nikolopoulos TP. Cochlear implantation for single-sided deafness: the outcomes. An evidence-based approach. Eur Arch Otorhinolaryngol. 2014;271(8):2119–26. [DOI] [PubMed] [Google Scholar]
- (41).Kitterick PT, Smith SN, Lucas L. Hearing instruments for unilateral severe-to-profound sensorineural hearing loss in adults: a systematic review and meta-analysis. Ear Hear. 2016;37(5):495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Mandavia R, Carter AW, Haram N, Mossialos E, Schilder AGM. An evaluation of the quality of evidence available to inform current bone conducting hearing device national policy. Clin Otolaryngol. 2017;42(5):1000–24. [DOI] [PubMed] [Google Scholar]
- (43).Sprinzl GM, Wolf-Magele A. The Bonebridge bone conduction hearing implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol. 2016;41(2):131–43. [DOI] [PubMed] [Google Scholar]
- (44).Appachi S, Specht JL, Raol N, Lieu JEC, Cohen MS, Dedhia K, et al. Auditory outcomes with hearing rehabilitation in children with unilateral hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2017;157(4):565–71. [DOI] [PubMed] [Google Scholar]
- (45).Kim G, Ju HM, Lee SH, Kim HS, Kwon JA, Seo YJ. Efficacy of bone-anchored hearing aids in single-sided deafness: a systematic review. Otol Neurotol. 2017;38(4):473–83. [DOI] [PubMed] [Google Scholar]
- (46).Peters JP, Smit AL, Stegeman I, Grolman W. Bone conduction devices and contralateral routing of sound systems in single-sided deafness. Laryngoscope. 2015;125(1):218–26. [DOI] [PubMed] [Google Scholar]
- (47).University of Alberta. Middle ear implants for the treatment of hearing loss [Internet]. Edmonton (AB): Alberta Health and Wellness; 2011. [cited 2018 Mar 6]. Available from: https://open.alberta.ca/publications/middle-ear-implants-for-the-treatment-of-hearing-loss-final-ste-report [Google Scholar]
- (48).Bezdjian A, Bruijnzeel H, Daniel SJ, Grolman W, Thomeer H. Preliminary audiologic and peri-operative outcomes of the Sophono transcutaneous bone conduction device: a systematic review. Int J Pediatr Otorhinolaryngol. 2017;101:196–203. [DOI] [PubMed] [Google Scholar]
- (49).Colquitt JL, Jones J, Harris P, Loveman E, Bird A, Clegg AJ, et al. Bone-anchored hearing aids (BAHAs) for people who are bilaterally deaf: a systematic review and economic evaluation. Health Technol Assess. 2011;15(26):1–200, iii-iv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Danhauer JL, Johnson CE, Mixon M. Does the evidence support use of the Baha implant system (Baha) in patients with congenital unilateral aural atresia? J Am Acad Audiol. 2010;21(4):274–86. [DOI] [PubMed] [Google Scholar]
- (51).Ernst A, Todt I, Wagner J. Safety and effectiveness of the Vibrant Soundbridge in treating conductive and mixed hearing loss: a systematic review. Laryngoscope. 2016;126(6):1451–7. [DOI] [PubMed] [Google Scholar]
- (52).Johnson CE, Danhauer JL, Reith AC, Latiolais LN. A systematic review of the nonacoustic benefits of bone-anchored hearing aids. Ear Hear. 2006;27(6):703–13. [DOI] [PubMed] [Google Scholar]
- (53).Klein K, Nardelli A, Stafinski T. A systematic review of the safety and effectiveness of fully implantable middle ear hearing devices: the Carina and Esteem systems. Otol Neurotol. 2012;33(6):916–21. [DOI] [PubMed] [Google Scholar]
- (54).Medical Advisory Secretariat. Bone anchored hearing aid: an evidence-based analysis. Ont Health Technol Assess Ser. 2002;2(3):1–47. [PMC free article] [PubMed] [Google Scholar]
- (55).Verhaert N, Desloovere C, Wouters J. Acoustic hearing implants for mixed hearing loss: a systematic review. Otol Neurotol. 2013;34(7):1201–9. [DOI] [PubMed] [Google Scholar]
- (56).Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. 2013;34(5):790–4. [DOI] [PubMed] [Google Scholar]
- (57).Venail F, Sicard M, Piron JP, Levi A, Artieres F, Uziel A, et al. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg. 2008;134(12):1276–81. [DOI] [PubMed] [Google Scholar]
- (58).Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(3):177–82. [DOI] [PubMed] [Google Scholar]
- (59).Wang JT, Wang AY, Psarros C, Da Cruz M. Rates of revision and device failure in cochlear implant surgery: a 30-year experience. Laryngoscope. 2014;124(10):2393–9. [DOI] [PubMed] [Google Scholar]
- (60).Eskander A, Gordon KA, Kadhim L, Papaioannou V, Cushing SL, James AL, et al. Low pediatric cochlear implant failure rate: contributing factors in large-volume practice. Arch Otolaryngol Head Neck Surg. 2011;137(12):1190–6. [DOI] [PubMed] [Google Scholar]
- (61).National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance. 3rd ed. London (UK): The Institute; 2012. Appendix I, quality appraisal checklist—economic evaluations. [PubMed] [Google Scholar]
- (62).Monksfield P, Jowett S, Reid A, Proops D. Cost-effectiveness analysis of the bone-anchored hearing device. Otol Neurotol. 2011;32(8):1192–7. [DOI] [PubMed] [Google Scholar]
- (63).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (64).Fitzpatrick EM, Al-Essa RS, Whittingham J, Fitzpatrick J. Characteristics of children with unilateral hearing loss. Int J Audiol. 2017;56(11):819–28. [DOI] [PubMed] [Google Scholar]
- (65).Feder KP, Michaud D, McNamee J, Fitzpatrick E, Ramage-Morin P, Beauregard Y. Prevalence of hearing loss among a representative sample of Canadian children and adolescents, 3 to 19 years of age. Ear Hear. 2017;38(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Statistics Canada. Health fact sheets: hearing loss of Canadians, 2012 to 2015 [Internet]. Ottawa (ON): Statistics Canada; 2016. [cited 2018 Jul]. Available from: http://www.statcan.gc.ca/pub/82-625-x/2016001/article/14658-eng.htm [Google Scholar]
- (67).Hanrahan C. Cochlear implants in the pediatric population: a scan of Canadian provinces. Environmental scan issue 25 [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2011. [cited 2018 Jul]. Available from: https://www.cadth.ca/media/pdf/Cochlear_Implants_es_25_e.pdf [Google Scholar]
- (68).Fitzpatrick EM, Brewster L. Adult cochlear implant survey. Can J Speech Lang Path Audiol [Internet]. 2010. [cited 2018 Aug]; 34(4). Available from: https://www.cjslpa.ca/files/2010_CJSLPA_Vol_34/No_04_226-303/Fitzpatrick_Brewster_CJSLPA_2010.pdf [Google Scholar]
- (69).Davids T, Gordon KA, Clutton D, Papsin BC. Bone-anchored hearing aids in infants and children younger than 5 years. Arch Otolaryngol Head Neck Surg. 2007;133(1):51–5. [DOI] [PubMed] [Google Scholar]
- (70).Badran K, Arya AK, Bunstone D, Mackinnon N. Long-term complications of bone-anchored hearing aids: a 14-year experience. J Laryngol Otol. 2009;123(2):170–6. [DOI] [PubMed] [Google Scholar]
- (71).Edfeldt L, Stromback K, Grendin J, Bunne M, Harder H, Peebo M, et al. Evaluation of cost-utility in middle ear implantation in the ‘Nordic School’: a multicenter study in Sweden and Norway. Cochlear Implants Int. 2014;15:S65–S7. [DOI] [PubMed] [Google Scholar]
- (72).Hobson JC, Roper AJ, Andrew R, Rothera MP, Hill P, Green KM. Complications of bone-anchored hearing aid implantation. J Laryngol Otol. 2010;124(2):132–6. [DOI] [PubMed] [Google Scholar]
- (73).Lenarz T, Verhaert N, Desloovere C, Desmet J, D'Hondt C, Gonzalez JC, et al. A comparative study on speech in noise understanding with a direct acoustic cochlear implant in subjects with severe to profound mixed hearing loss. Audiol Neurootol. 2014;19(3):164–74. [DOI] [PubMed] [Google Scholar]
- (74).Chen JM, Amoodi H, Mittmann N. Cost-utility analysis of bilateral cochlear implantation in adults: a health economic assessment from the perspective of a publicly funded program. Laryngoscope. 2014;124(6):1452–8. [DOI] [PubMed] [Google Scholar]
- (75).Kosaner Kliess M, Kluibenschaedl M, Zoehrer R, Schlick B, Scandurra F, Urban M. Cost-utility of partially implantable active middle ear implants for sensorineural hearing loss: a decision analysis. Value Health. 2017;20(8):1092–9. [DOI] [PubMed] [Google Scholar]
- (76).Ozdemir S, Tuncer U, Tarkan O, Kiroglu M, Cetik F, Akar F. Factors contributing to limited or non-use in the cochlear implant systems in children: 11 years experience. Int J Pediatr Otorhinolaryngol. 2013;77(3):407–9. [DOI] [PubMed] [Google Scholar]
- (77).Summerfield AQ, Marshall DH. Non-use of cochlear implants by post-lingually deafened adults. Cochlear Implants Int. 2000;1(1):18–38. [DOI] [PubMed] [Google Scholar]
- (78).Sunnybrook Health Sciences Centre, Otolaryngology – Head and Neck Surgery. Cochlear implant process [Internet]. Toronto (ON): Sunnybrook Health Sciences Centre; 2018. [cited 2018 Jul]. Available from: https://sunnybrook.ca/content/?page=cochlear-implant-program-process [Google Scholar]
- (79).Cochlear Limited. Surgery guide: Cochlear Baha 4 Attract System surgical procedure [Internet]. Centennial (CO): Cochlear Limited; 2014. [cited 2018 Jul]. Available from: https://www.cochlear.com/8eb51452-641c-47a5-b6b9-9223f25686c5/BUN226%E2%80%89+%E2%80%89ISS2%E2%80%89+%E2%80%89JAN14%E2%80%89+%E2%80%89Attract+Surgery+Guide+US+TP.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=8eb51452-641c-47a5-b6b9-9223f25686c5 [Google Scholar]
- (80).Cochlear Limited. Surgery guide: Cochlear Baha DermaLock surgical procedure [Internet]. Centennial (CO): Cochlear Limited; 2015. [cited 2018 Jul]. Available from: https://www.cochlear.com/66b43e66-3e0b-453b-9751-bc904f3961fd/BUN128+ISS4+NOV30+-+Baha+Connect+Surgery+Guide+FINAL.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE-66b43e66-3e0b-453b-9751-bc904f3961fd-llOXgkw [Google Scholar]
- (81).Raine CH, Summerfield Q, Strachan DR, Martin JM, Totten C. The cost and analysis of nonuse of cochlear implants. Otol Neurotol. 2008;29(2):221–4. [DOI] [PubMed] [Google Scholar]
- (82).Polonenko MJ, Papsin BC, Gordon KA. Children with single-sided deafness use their cochlear implant. Ear Hear. 2017;38(6):681–9. [DOI] [PubMed] [Google Scholar]
- (83).Bhatt YM, Green KM, Mawman DJ, Aplin Y, O'Driscoll M P, Saeed SR, et al. Device nonuse among adult cochlear implant recipients. Otol Neurotol. 2005;26(2):183–7. [DOI] [PubMed] [Google Scholar]
- (84).Archbold SM, Nikolopoulos TP, Lloyd-Richmond H. Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use. Cochlear Implants Int. 2009;10(1):25–40. [DOI] [PubMed] [Google Scholar]
- (85).Ray J, Wright T, Fielden C, Cooper H, Donaldson I, Proops DW. Non-users and limited users of cochlear implants. Cochlear Implants Int. 2006;7(1):49–58. [DOI] [PubMed] [Google Scholar]
- (86).Chan KH, Gao D, Jensen EL, Allen GC, Cass SP. Complications and parent satisfaction in pediatric osseointegrated bone-conduction hearing implants. Laryngoscope. 2017;127(9):2165–70. [DOI] [PubMed] [Google Scholar]
- (87).Kraai T, Brown C, Neeff M, Fisher K. Complications of bone-anchored hearing aids in pediatric patients. Int J Pediatr Otorhinolaryngol. 2011;75(6):749–53. [DOI] [PubMed] [Google Scholar]
- (88).Yellon RF. Bone anchored hearing aid in children–prevention of complications. Int J Pediatr Otorhinolaryngol. 2007;71(5):823–6. [DOI] [PubMed] [Google Scholar]
- (89).Polonenko MJ, Carinci L, Gordon KA, Papsin BC, Cushing SL. Hearing benefit and rated satisfaction in children with unilateral conductive hearing loss using a transcutaneous magnetic-coupled bone-conduction hearing aid. J Am Acad Audiol. 2016;27(10):790–804. [DOI] [PubMed] [Google Scholar]
- (90).Kesser BW, Krook K, Gray LC. Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope. 2013;123(9):2270–5. [DOI] [PubMed] [Google Scholar]
- (91).Gluth MB, Eager KM, Eikelboom RH, Atlas MD. Long-term benefit perception, complications, and device malfunction rate of bone-anchored hearing aid implantation for profound unilateral sensorineural hearing loss. Otol Neurotol. 2010;31(9):1427–34. [DOI] [PubMed] [Google Scholar]
- (92).Statistics Canada. Life expectancy and other elements of the life table, Canada, all provinces except Prince Edward Island (2014–2016), Table 13-10-0114-01 [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 Jul]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401 [Google Scholar]
- (93).Contrera KJ, Betz J, Genther DJ, Lin FR. Association of hearing impairment and mortality in the National Health and Nutrition Examination Survey. JAMA Otolaryngol Head Neck Surg. 2015;141(10):944–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Assistive Devices Program, Ministry of Health and Long-Term Care. Hearing devices policy and administration manual [Internet]. Toronto (ON): The Ministry; 2016. [cited 2018 Aug]. Available from: http://www.health.gov.on.ca/en/pro/programs/adp/policies_procedures_manuals/docs/hearing_devices_manual.pdf [Google Scholar]
- (95).Glanville J, Arber M, Veale T, Garcia S. Sensitivity of a search filter designed to identify studies reporting health state utility values. Paper presented at: Mosaic, 116th Annual Meeting, Medical Library Association; 2016. May 15–20; Toronto, ON.
- (96).Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Grutters J, Joore M, Horst F, Verschuure H, Dreschler W, Anteunis L. Choosing between measures: comparison of EQ-5D, HUI2 and HUI3 in persons with hearing complaints. Qual Life Res. 2007;16:1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Drummond M. Introducing economic and quality of life measurements into clinical studies. Ann Med. 2001;33(5):344–9. [DOI] [PubMed] [Google Scholar]
- (99).Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. [DOI] [PubMed] [Google Scholar]
- (100).Lucas L, McKay H, Kitterick P. Valuing the health of individuals with a single-sided deafness. 2015. Audiol Neurotol, 20 Suppl 1:79–86. [DOI] [PubMed] [Google Scholar]
- (101).Statistics Canada. Consumer Price Index, Table 326-0020 [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 Apr 30]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260020 [Google Scholar]
- (102).Ontario Public Service Employees Union. 2016–2019 OPSEU Hospital Profesionals Division central agreement [Internet]. North York (ON): The Union; 2016. Available from: https://opseu.org/sites/default/files/hpd_collective_agreement_20190331.pdf. [Google Scholar]
- (103).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act. Toronto (ON): Queen's Printer for Ontario; 2015. p. 746. [Google Scholar]
- (104).Merdad M, Wolter NE, Cushing SL, Gordon KA, Papsin BC. Surgical efficiency in bilateral cochlear implantation: a cost analysis. Cochlear Implants Int. 2014;15(1):43–7. [DOI] [PubMed] [Google Scholar]
- (105).Ministry of Health and Long-Term Care. Health data branch web portal: Ontario case costing [Internet]. Toronto (ON): Queen's Printer for Ontario; 2017. [cited 2018 Apr 30]. Available from: https://hsim.health.gov.on.ca/HDBPortal [Google Scholar]
- (106).Ministry of Health and Long-Term Care. Ontario Drug Benefit Formulary/Comparative Drug Index [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. [cited 2018 Apr 30]. Available from: https://www.formulary.health.gov.on.ca/formulary [Google Scholar]
- (107).Gaboury I, Coyle K, Coyle D, Le Saux N. Treatment cost effectiveness in acute otitis media: a watch-and-wait approach versus amoxicillin. Paediatr Child Health. 2010;15(7):e14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (109).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (110).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (111).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients' experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser [Internet]. 2013. Sep 1 [cited 2018 Apr 30]; 13(15):1–33. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3817950/ [PMC free article] [PubMed] [Google Scholar]
- (112).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (113).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Miller WL, Crabtree BF. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (114).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (115).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (116).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (117).Health Technology Assessment International. FAQ for HTA agencies and policy makers [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/interest-groups/patient-and-citizen-involvement/resources/for-hta-agencies-and-policy-makers/faq-for-hta-agencies-and-policy-makers.html#c3229 [Google Scholar]
- (118).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (119).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Denzin NK, Lincoln YS. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (120).Centre for Evidence-Based Medicine. Levels of evidence [Internet]. Oxford (UK): The Centre; 2009. [cited 2018 April 17]. Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ [Google Scholar]
- (121).Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): The Ottawa Hospital Research Institute; 2014. [cited 2018 April 16]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- (122).Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–84. [DOI] [PubMed] [Google Scholar]
- (123).Chisolm TH, Johnson CE, Danhauer JL, Portz LJ, Abrams HB, Lesner S, et al. A systematic review of health-related quality of life and hearing aids: final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol. 2007;18(2):151–83. [DOI] [PubMed] [Google Scholar]
- (124).Taylor RS, Paisley S, Davis A. Systematic review of the clinical and cost effectiveness of digital hearing aids. Br J Audiol. 2001;35(5):271–88. [DOI] [PubMed] [Google Scholar]
- (125).Scottish Intercollegiate Guidelines Network. Critical apprasial notes and checklists [Internet]. Edinburgh (UK): Healthcare Improvement Scotland; 2017. [cited 2018 April 16]. Available from: http://www.sign.ac.uk/checklists-and-notes.html [Google Scholar]
- (126).Sackett DL, Straus SE, Richardson WS, Roseberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Edinburgh (UK): Churchill Livingston; 2000. [Google Scholar]
- (127).Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm (SE): Swedish Council on Technology Assessment in Health Care; 1993. [DOI] [PubMed] [Google Scholar]
- (129).Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(7183):593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Zoehrer R. Safety outcomes for implantable bone conduction and middle ear devices: a systematic review [Internet]. Innsbruck (Austria): MED-EL Medical Electronics; 2017. [cited 2018 Jul]. Available from: kategorizacia.mzsr.sk/Pomocky/Download/RequestAttachment/77590 [Google Scholar]
- (131).Schwartz SR, Kobylk D. Outcomes of bone anchored hearing aids (BAHA) for single sided deafness in nontraditional candidates. Otol Neurotol. 2016;37(10):1608–13. [DOI] [PubMed] [Google Scholar]
- (132).Murthy JMK, Saxena AB. Bell's palsy: treatment guidelines. Ann Ind Acad Neurol. 2011;14 Suppl 1:S70–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]