Abstract
Purpose:
Although intracranial meningiomas are the most common primary brain tumor in adults, treatment options are few and have traditionally been limited to surgical resection and radiotherapy. Additional targeted therapies and biomarkers are needed, especially as complete surgical resection is frequently not feasible in many patients.
Methods:
Non-pathologic brain tissue from 3 patients undergoing routine autopsies and tumor specimens from 16 patients requiring surgical resection for meningioma were collected. EMP2 protein expression was evaluated by immunohistochemistry and western blot analysis. EMP2 mRNA expression was also investigated using surgical specimens and validated by analysis of several independent NCBI GEO databases.
Results:
EMP2 mRNA expression levels were found to be higher in meningioma relative to non-pathologic meninges (P=0.0013) and brain (P=0.0011). Concordantly, strong EMP2 protein expression was demonstrated in 100% of meningioma specimens from all 16 patients, with no observable protein expression in normal brain tissue samples from 3 subjects (P<0.001). EMP2 expression was confirmed by western blot analysis in five samples, with EMP2 protein intensity positively correlating with histologic staining score (R2=0.780; P=0.047). No association was found between EMP2 mRNA or protein levels and WHO grade or markers of proliferation. However, EMP2 expression was positively associated with an angiomatous pattern on histologic evaluation (P=0.0597), VEGF-A mRNA expression (P<0.001), and clinical markers of tumor vascularity such as operative blood loss (P=0.037).
Conclusions:
EMP2 is not found in normal brain tissue, yet has shown consistently high mRNA and protein expression in meningiomas, and may serve as a useful molecular marker for these tumors.
Keywords: angiogenesis, EMP-2, immunohistochemistry, meningioma
Introduction
Meningiomas are the most common primary brain tumor in adults, accounting for 34% of all primary brain tumors, with a prevalence of 97.5 per 100,000 people in the United States as determined by the Central Brain Tumor Registry of the United States.[1] The already high frequency of this tumor may yet be underrepresenting true population burden, as up to an additional 2-3% of the general population have undetected subclinical meningiomas.[2, 3] The vast majority of meningiomas (90%) are benign WHO grade I, slow growing tumors with long latency[1, 4]. Despite the frequently benign course, the high prevalence of this disease contributes to an overall large medical burden. Current standard of care treatment of meningiomas is relatively uniform, with management primarily involving surgical resection with or without radiotherapy.[4]
Etiologic risk factors for meningiomas are relatively understudied. Meningiomas are known to be twice as common in females, and with sex hormone dependent growth rates [1],[5, 6]. Recent studies have identified additional candidate pathways involved in tumor maintenance and growth, including the NF2[7, 8], p53/pRb[9, 10], hedgehog[11], notch[12], PI3K/Akt[13-15], VEGF[16, 17], progesterone receptor[18-20], and cyclooxygenase[21, 22] pathways. Further work has identified epigenetic modifications in tumor suppressor genes, developmental factors, growth factors, and angiogenetic factors as playing roles in meningioma growth.[23] Despite these new genetic associations in meningioma development, preliminary phase II studies using chemotherapeutic agents, hormonal agents, and growth receptor inhibitors have shown no significant improvement in survival of patients with recurrent meningiomas.[24] There remains a pressing need for identification of robust therapeutic targets and systemic therapy for meningioma to expand options beyond surgery and radiation and allow for individualization of treatment.
Epithelial membrane protein 2 (EMP2) is a protein first found to be upregulated in ovarian, breast, and endometrial tumors[25-28]. Normal brain tissues are not known to express EMP2, however, EMP2 is expressed in gliomas[29, 30], where it is thought to promote cell migration and invasion,[29] as well as increase angiogenesis via upregulation of VEGF-A[29, 31]. Treatment of mice with anti-EMP2 antibodies has been shown to reduce tumor burden and decrease tumor vasculature in mouse models of glioblastoma [29, 31]. The expression of EMP2 has not been evaluated in other central nervous system tumors. This study characterizes EMP2 expression in meningiomas and relates expression levels with alterations in tumor angiogenesis. EMP2 may be useful as a novel biomarker and possible therapeutic molecular target for meningiomas.
Methods and Materials
Patient Data
UCLA Institutional review board approval was obtained for this study. Patients who had undergone surgical meningioma resection between 2015-2019 were identified. Inclusion criteria included: 1) adult patients, age >18; 2) pathologic diagnosis of meningioma; and 3) tumor tissue available for immunohistochemistry (IHC). Implementation of these criteria yielded 16 patients available for analysis. The electronic medical record of these patients was queried for pre-operative, operative, and post-operative characteristics. Data regarding gross and microscopic findings were abstracted from pathology reports. Five patients also had frozen specimens available which were analyzed by western blot analysis as detailed below. Patient demographics and clinical course were also collected.
Multiple brain sections from 3 patients undergoing routine autopsy were obtained for comparison. Tissues were obtained from meninges, cortical gray matter, cortical white matter, cerebellum, and choroid plexus from all control patients. Immunohistochemical and blotting processes are described in Supplemental 1.
Gene Expression Omnibus Repository
The NCBI Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/gds) was queried for publicly available mRNA expression data from fresh-frozen meningiomas and normal tissues. We identified a total of 5 datasets with mRNA Affymetrix microarray expression data from total RNA extracted from clinical specimens. (GSE43290; GSE88720; GSE54934; GSE77259; GSE12530).[32-36] In each case, microarray expression data underwent RMA normalization to yield log2 RMA signal values for each gene. Normalized mRNA expression data and pathologic diagnosis were downloaded from each of these 5 datasets. Two of these datasets also had normal controls that were obtained and processed in the same way as tumor specimens and these studies were used to evaluate differences in mRNA expression between meningioma and normal tissue. Four of the datasets had documentation of pathologic grade for each specimen.
Gene Ontology
Differential gene expression analysis associated with EMP2 expression was performed using NCBI GEO2R (https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html). GEO2R uses the Linear Models of Microarray Analysis packing in R to identify differentially expressed genes and applies multiple-testing corrections on p-values to correct for false positives. In each of the five datasets with mRNA expression data, we used the median EMP2 expression level to stratify specimens. We identified genes that were differentially expressed in high and low EMP2 meningiomas and positively correlated with EMP2 expression. All genes that had a p-value of < 0.05 after correction for multiple comparisons were selected. We used the PANTHER Gene Ontology Classification System via the Gene Ontology Resource (https://www.geneontology.org) to identify biological processes associated with the previously mentioned genes. All biological processes with p-value of < 0.05 after correction for multiple comparisons were selected and the top 5 processes reported.
Statistical Analysis
Unpaired t-tests with and without Welch’s correction for unequal variance were used to test differences in mRNA and protein expression levels as well as with pathologic and clinical factors. Three factor ANOVA tests were used to test differences in mRNA and protein expression levels by WHO grade. Linear regression analysis was used to test the relationship between VEFG-A and EMP2 mRNA expression levels. Correlation Stratified by EMP2 staining levels, progression free survival was evaluated using log-rank analyses on Kaplan Meier data. No corrections for multiple comparisons were performed. Statistical analyses were performed with GraphPad Prism v6.0h (GraphPad Software, Inc., La Jolla, CA). All errors are presented in standard error of the mean (S.E.M.).
Results
Patient characteristics
A total of sixteen patients who underwent meningioma resection were included in this study. Patient characteristics are described in Table 1. Twelve females and four males were identified who ranged from 34-70 years (average age, 53 years) at time of surgery. Three patients had surgery for recurrent tumor. No patients met clinical criteria for diagnosis of NF2, and genomic sequencing was not performed. Presenting clinical symptoms included headache (5/16, 31%), seizure (3/16, 18.8%), speech issues (2/16, 12.5%), weakness (2/16, 12.5%), proptosis/physical lesion (2/16, 12.5%) or incidental discovery of tumor (4/16, 25%). Tumor was located most commonly in the frontal lobe (8/16, 50%). Other tumor locations represented included the temporal lobe (2/16), olfactory groove (2/16), parietal lobe (1/16), occipital lobe (1/16), cerebellum (1/16) and cerebellopontine angle (1/16). Average preoperative tumor size was 4.3 cm (range 2.4 – 7.7 cm). The three patients presenting with recurrent tumors all had prior resection surgery, with two having also received previous fractionated stereotactic radiotherapy. All patients had surgical craniotomy performed for resection of tumor, with eight patients (50%) also receiving pre-operative embolization. Ten patients (63%) underwent gross total resection of the tumor, while the remaining six patients had subtotal resections (38%). Average estimated blood loss from surgery was 263 cc (range 50-500 cc). Nine patients had subsequent postoperative radiotherapy (56%). No tumor recurrence was reported in this cohort, with an average follow up of 10 months.
Table 1.
Patient characteristics
| All Patients (% of Total) |
EMP2 Low (% of Total) |
EMP2 High (% of Total) |
P-Value | ||
|---|---|---|---|---|---|
| Total | 16 | 8 (50%) | 8 (50%) | ||
| Gender | |||||
| Women | 12 (75%) | 6 (75%) | 6 (75%) | 1.00 | |
| Men | 4 (25%) | 2 (25%) | 2 (25%) | 1.00 | |
| Age at Diagnosis [year] | 52.5 | 50.4 | 54.7 | 0.403 | |
| Ethnicity | |||||
| Caucasian | 11 (69%) | 4 (50%) | 7 (88%) | 0.120 | |
| Smoking History | 3 (19%) | 1 (13%) | 2 (25%) | 0.554 | |
| Diabetes Mellitus | 1 (6.3%) | 1 (13%) | 0 (0%) | 0.334 | |
| Recurrent Tumor | 3 (19%) | 1 (13%) | 2 (25%) | 0.206 | |
| Grade | |||||
| WHO Grade I | 5 (31%) | 3 (38%) | 2 (25%) | 0.619 | |
| WHO Grade II | 10 (63%) | 4 (50%) | 6 (75%) | 0.334 | |
| WHO Grade III | 1 (6.3%) | 1 (13%) | 0 (0%) | 0.334 | |
| Tumor Diameter [mm] | 43.1 | 42.3 | 44.0 | 0.800 |
Pathologic analysis
All tumor samples were evaluated by neuropathologists. Tumors were graded in accordance with the WHO meningioma grading scale with the following results: 5 of 16 samples grade I, 10 of 16 grade II, and 1 of 16 grade III. Microscopic analysis of clinical specimens documented angiomatous features in 3/16 (18.8%), brain invasion (confirmed with GFAP stain showing tumor invasion into glial tissue) in 4/16 (25%), necrosis in 4/16 (25%), and collagen deposition in 1/16 (6.3%). Mitoses ranged from 4-8 per 10 high power fields (HPFs) in all tumor samples. Immunostaining for Ki67 was carried out in 13 samples. The overall Ki67 proliferation index was 1% in 3/13 samples, 5% in 4/13, 10% in 4/13, 15% in 1/13, and 30% in 1/13 samples. Focal Ki67 positivity was 1% in 3/13 samples, 5% in 1/13, 10% in 3/13, 15% in 5/13, and 30% in 1/13 samples.
EMP2 is highly expressed in new and recurrent meningioma
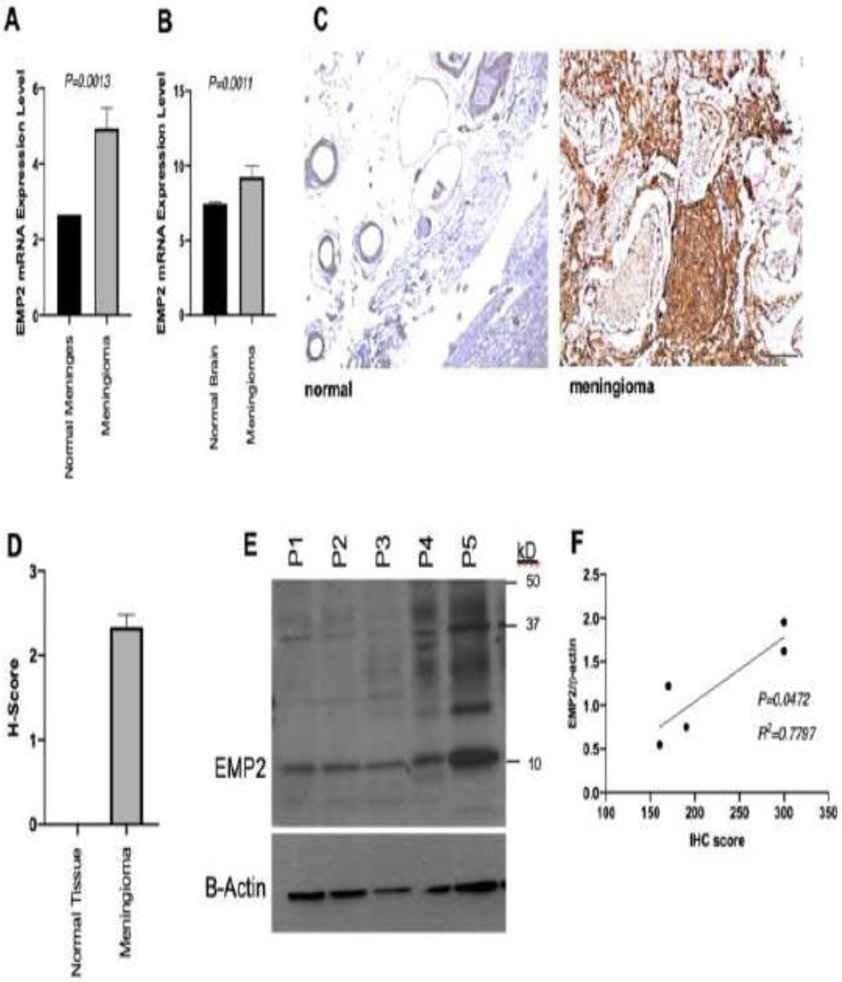
Two publicly available datasets on the NCBI GEO database with RMA normalized mRNA expression data from similarly processed meningioma and non-pathologic brain or non-pathologic meninges mRNA expression data were selected for analysis. EMP2 mRNA expression levels were found to be significantly higher in tumor relative to meninges (log2 RMA value 4.93 versus 2.66; P=0.0013; Figure 1A) and brain (log2 RMA value 9.3 versus 7.5; P=0.0011; Figure 1B). To validate this observation in our patient samples, multiple sections from non-pathologic autopsy tissue (n=3) and resected meningioma tumor samples (n=16) were stained for EMP2. EMP2 staining was not detectable in non-pathologic brain tissue and meninges (Figure 1C top panel, Supplemental 2). In contrast, all 16 tumor specimens (100%) showed positive EMP2 staining (Figure 1C, bottom panel). The intensity of staining was scored using an intensity scale (see Methods) ranging from 0-3. No meningioma patients had a staining intensity score of 0 or 1. Nine patients (56.3%) received an intensity score of 2 while 7 patients (43.8%) were scored at the highest intensity. H score for EMP2 with first time surgery was 2.36 and H score for EMP2 with previous surgery was 2.23 (P=0.75). H score for EMP2 with prior irradiation was 1.85 and without prior irradiation was 2.40 (P=0.22).
Figure 1:
EMP2 is highly expressed in meningiomas. RMA normalized EMP2 mRNA expression [log2 RMA signal] is increased in tumor specimens (N=16) relative to non-pathologic meninges (A) and brain tissue (B) from three patients. (C) Representative slides showing IHC staining with anti-EMP2 antibodies in meningeal (left) and meningioma (right) specimens. H-score was calculated from these IHC slides showing higher H-score in meningioma relative to non-pathologic tissue (D). Parallel samples from a portion of the same tumor specimens were used for western blot analysis (E, n=5). H-score calculated from IHC slides was positively correlated with quantitative protein expression via western blot (F).
The overall percentage of total cells in the tumor specimen with positive EMP2 staining was also quantified, with the meningioma samples demonstrating 95% of cells with positive staining on average (range 80-100%). The mean H-score of meningioma specimens was 234 (Range 160-300), significantly higher than normal brain specimens (H-score 234 vs 0; p< 0.001; Figure 1D). To validate EMP2 expression, western blot analysis was also performed on five surgical specimens to quantify and validate protein levels of EMP2, with 100% of samples showing positive EMP2 protein levels. Two tumors with significantly higher EMP2 protein expression were identified (P4 and P5, Figure 1E), which appropriately also had the highest immunohistochemical staining for EMP2 (mean H score 300 versus 173.3; P=0.002). Immunohistochemical H-score was positively correlated with quantitative protein expression on western blot (R2=0.7797; P=0.0472; Figure 1F).
We further investigated whether EMP2 staining varied by pre-operative patient characteristics. Gender, age at surgery, primary tumor vs recurrence, previous surgery or radiation, presenting symptoms, tumor location, and tumor size did not significantly correlate with EMP2 staining intensity (Table 1).
EMP2 expression is not associated with increased proliferation
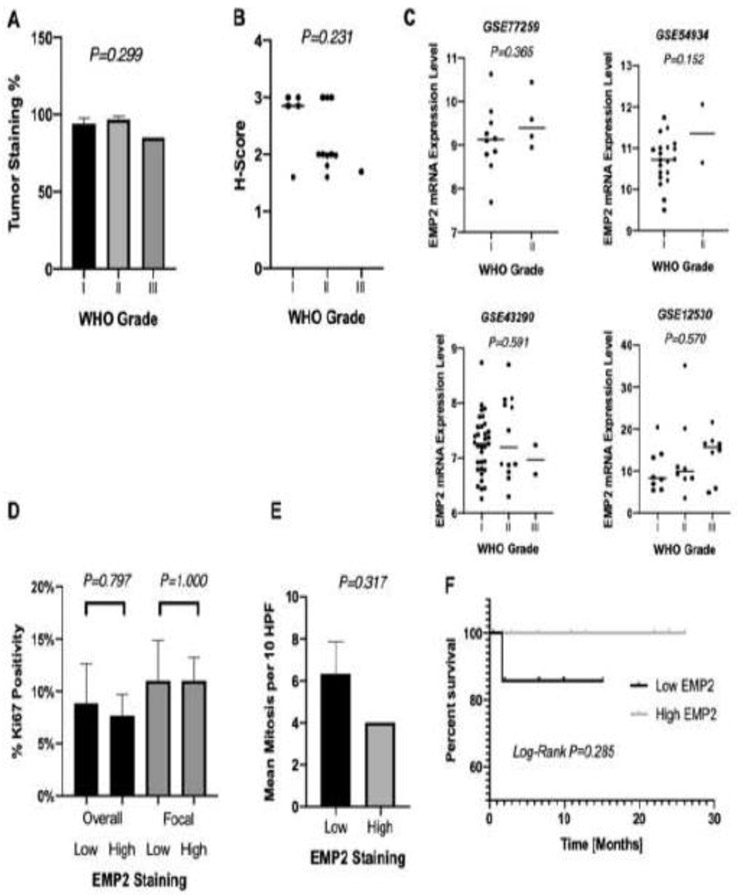
WHO Grade I, II, and III tumors all demonstrated EMP2 staining in the 80-100% range, with no significant difference in percentage staining or H-score based on WHO classification (ANOVA P=0.299; Figure 2A & ANOVA P=0.231; Figure 2B). To further understand the role of EMP2 in meningiomas, four publicly available independent datasets with mRNA expression data in meningiomas and corresponding WHO classification were additionally queried with no significant difference in EMP2 RMA normalized mRNA expression levels by WHO grade in all datasets (log 2 RMA value; P=0.365; P=0.152; P=0.591; P=0.570; Figure 2C). The proliferative index was further evaluated for all specimens using immunohistochemical staining for Ki67. Neither focal nor overall Ki67 positivity percentages were correlated to EMP2 staining levels. In patients with higher (grade 3) EMP2 staining, average focal and overall Ki67 positivity were 11% and 8% respectively, while in patients with lower (grade 2) EMP2 staining, focal and overall Ki67 positivity were 11% and 9% respectively (Overall P=0.797; Focal P=1.00; Figure 2D). Consistent with this lack of association of EMP2 expression with the proliferative index by Ki67 staining, no positive association was found with number of mitoses on microscopic examination. There were on average 4 mitoses per 10 HFPs in specimens with higher (grade 3) EMP2 staining and 6 mitoses per 10 HFPs in specimens with lower (grade 2) EMP2 staining (P=0.317; Figure 2E). Survival analysis was also carried out, with progression free survival not significantly altered when stratified by EMP2 levels (median PFS = 10.8 versus 6.5 months; Log-Rank P=0.285; Figure 2F).
Figure 2:
Increased EMP2 is not associated with increased cell proliferation. Differences in percent EMP2 staining (A) and EMP2 H-score (B) stratified by WHO histological grade. Four NCBI GEO datasets (GEO accession number listed) with RMA normalized mRNA expression data and histologic grade were identified. In each dataset, there was no difference between EMP2 expression [log2 RMA signal] and WHO Grade (C). Percent Ki67 immunopositivity (D) both throughout the specimen (overall) and at focal points (focal) as well as mean mitoses per 10 HPFs (E) as seen on pathologic evaluation were not significantly different between specimens with high and low EMP2 staining. Kaplan Meier curve evaluating progression free survival in patients with high and low EMP2 staining (F).
EMP2 expression is correlated with clinical and pathologic factors associated with increased vascularity
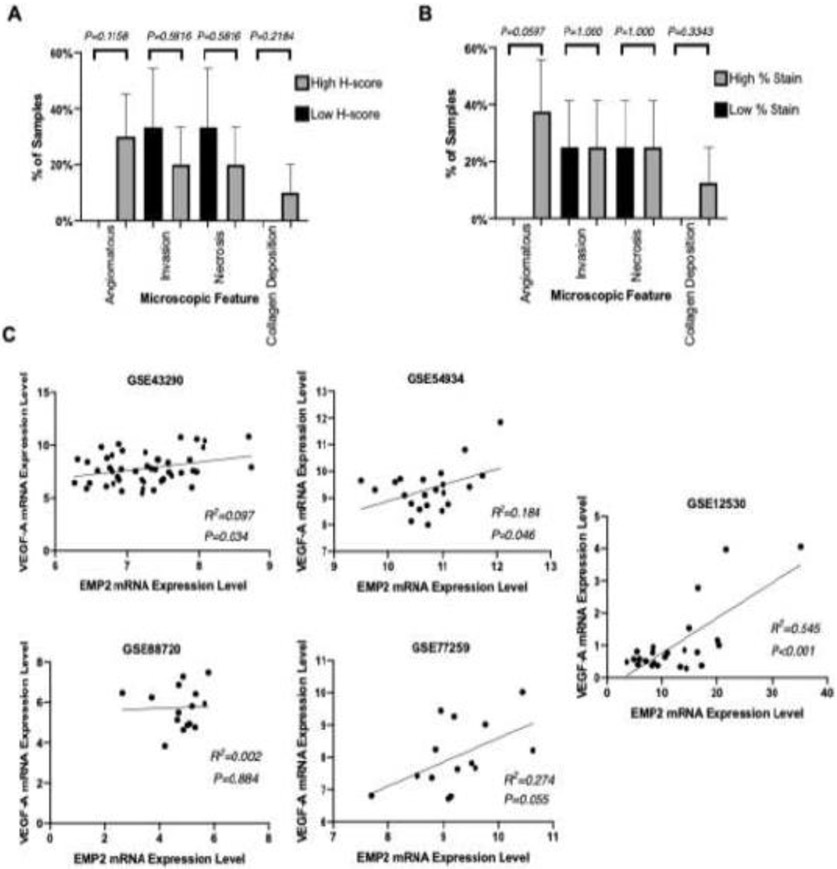
All expression readings and analyses were performed by a senior neuropatholgist. When tumors were stratified based on higher EMP2 levels (H score>200) or lower (H score<200), tumors with high EMP2 levels correlated with changes in angiogenesis. An angiomatous pattern was seen in 30% of cases with high EMP2 H-scores (n=8) compared to 0% of cases with lower EMP2 H-scores (n=8) (Chi-Square P=0.1158; Figure 3A). These findings are consistent when reanalyzed based on percent of cellular EMP2 staining instead of H-score. Angiomatous features were described in 40% of tumors that had higher EMP2 percent staining based on a median cut off(n=8), while the angiomatous pattern was reported in 0% of cases with lower EMP2 staining (n=8) (Chi-Square P=0.0597; Figure 3B). In contrast, both invasion and tumor necrosis did not associate with EMP2 expression levels. Invasion was seen in 25% of both higher and lower EMP2 staining cases. In contrast, while collagen deposition was observed in 13% of cases with higher EMP2 levels and 0% of lower EMP2 staining cases, this effect was not significant (Chi Square P=0.3343; Figure 3B) but this feature is not critical to diagnosis and may be infrequently reported.
Figure 3:
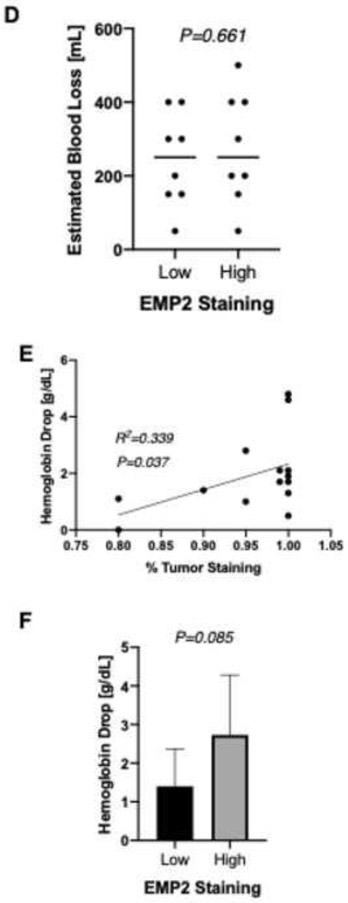
EMP2 expression is correlated with clinical and pathologic factors associated with increased vascularity. Differences between high and low H-score (A) and percent EMP2 staining (B) of specimens by microscopic histopathologic analysis of various microscopic features. A positive correlation was observed between mRNA expression levels of EMP2 and VEGF-A [log2 RMA signal] using 5 available NCBI GEO datasets (GEO accession number listed) (C). Differences in operative blood loss between high and low EMP2 staining specimens (D). Correlation between tumor staining percentage and difference in hemoglobin level pre and post surgery (E, F).
We have previously shown that EMP2 upregulates VEGFA in glioblastoma [31]. We therefore examined five independent NCBI GEO meningioma datasets to further evaluate the correlation between EMP2 and VEGFA mRNA expression levels. All five datasets demonstrated a positive correlation between EMP2 and VEGFA (Figure 3C). Three of the five datasets reached statistical significance (P=0.034; P<0.001; P=0.046), while two datasets with small sample sizes (n=14 each) did not (P=0.33; P=0.055). To extend on these studies, we carried out additional differential gene expression analyses to identify genes associated with increased EMP2 expression. Using a gene ontology platform (Gene Ontology Resource; PANTHER; Biological Processes), the top five biological processes identified included: positive regulation of axon regeneration, intermediate filament cytoskeleton organization, vasculogenesis, ephrin receptor pathway, and regulation of blood pressure (Table 2).
Table 2.
Top 5 biological processes identified with association of increased EMP2 expression
| Biologic Process | Association | Corrected p-value |
|---|---|---|
| Positive Regulation of Axon Regeneration | + | 0.0365 |
| Intermediate Filament | + | 0.0103 |
| Cytoskeleton Organization Vasculogenesis | + | 0.0231 |
| Ephrin receptor pathway | + | 0.0103 |
| Regulation of blood pressure | + | 0.0176 |
Our results thus far suggested that EMP2 may help regulate tumor associated neoangiogenesis. To further translate how EMP2 levels may affect clinical outcomes, we next evaluated variables that may serve as clinical proxy for increased angiogenesis and vascularity. Of note, patients who had successful preoperative tumor embolization were eliminated in this analysis. Estimated operative blood loss was higher in cases with increased EMP2 staining (n=8) (333 cc versus 236 cc; P=0.661; Figure 3D). Given the low accuracy of reporting of operative blood loss, we concurrently evaluated changes in hemoglobin levels after surgery. A linear correlation was observed between percentage of EMP2 staining and difference between preoperative and postoperative hemoglobin, or hemoglobin drop (R2=0.339; P=0.037; Figure 3E). Similarly, loss of hemoglobin was generally greater in cases with higher EMP2 (score 3) staining even with no significant difference in surgery type or tumor volume (2.9 g/dL versus 1.4 g/dL; P=0.085; Figure 3F).
Discussion
Current therapeutic options for meningiomas are few and limited to surgery and radiation. While high Simpson grade resection often leads to excellent outcomes, surgical resection is frequently not feasible in cases with poor location, for example with tumors in close proximity to the dural venous sinuses. In addition, WHO grade II and III meningiomas have higher rates of recurrence and can progress despite adjuvant radiation. There exists a pressing need for additional molecular targeted therapy in the treatment of meningiomas. Despite a large amount of literature characterizing the genetic and epigenetic landscape of meningioma tumors, there have been no successful molecular targets to date[24].
In this study, we introduce EMP2 as a possible biomarker for meningiomas. We report consistent and high EMP2 protein expression in all meningiomas tested with no evidence of significant expression in non-neoplastic cortex and normal meningeal tissues. We also report increased levels of mRNA expression in tumors relative to non-pathologic tissues in our clinical samples as well as multiple publicly available datasets, consistent with our observed protein expression. We have found that EMP2 protein is consistently expressed in 100% of all tested meningiomas, highlighting EMP2 as a viable potential target. In contrast, other proposed targets, including NF2, are only modified in a subset of meningiomas[37-39].
We found no correlation between EMP2 mRNA expression and histologic grade, Ki67 immunostaining, or number of mitoses. This suggests that EMP2 may not play a major role in tumor cell proliferation. However, our clinical tumor samples exhibited high levels of staining (grade 2 or 3) in all specimens, and the differences in EMP2 expression between these high expressing tumors may not be sufficient to produce alterations in proliferation.
EMP2 has been shown to promote angiogenesis in glioblastoma multiforme through upregulation of VEGF-A. [29] We propose that EMP2 plays a similar role in meningiomas. Consistent with this hypothesis, we have shown that EMP2 expression correlates with the presence of angiomatous areas as well as clinical factors associated with increased tumor vascularity such as operative blood loss. While reported estimated blood loss is an inconsistent measure, blood loss as captured by pre and post-operative laboratory analysis is better validated and was significantly associated with increased EMP2 expression. Additionally, EMP2 was positively correlated with VEGF-A in all 5 publicly available mRNA expression datasets. We hypothesize that similar to the case in glioblastomas, EMP2 upregulates VEGF-A in meningiomas, although further experiments need to be completed to confirm the mechanistic pathways for EMP2 in these tumors. Differential gene expression analysis and gene ontology queries identified axon regeneration, cytoskeleton organization, vasculogenesis, regulation of blood pressure, and ephrin receptor pathways as the top biological processes upregulated with EMP2. The implications and importance of these associations remain to be clarified. However, these identified pathways are congruous with previous associations of EMP2 with cell migration and invasion45The ephrin receptor pathway has also been strongly associated with VEGF induced angiogenesis45. Our data collectively support the hypothesis that EMP2 is involved in angiogenesis in meningiomas through interactions with the VEGF pathway, and is associated with both pathologic and clinical correlates of increased tumor vascularity.
Bevacizumab, an anti-VEGF monoclonal antibody, has been evaluated as a treatment option for meningiomas in preliminary trials [41]. While initial reports show improvements in tumor size and FLAIR hyperintensity, there is as yet insufficient data to determine the true effect of anti-VEGF therapy on progression free survival. Our data suggest that therapy targeted against EMP2, alone or in combination with anti-VEGF therapy like bevacizumab, may have potential additional benefit for the treatment of meningioma. Further work with larger sample sizes is needed to clarify the role of EMP2 in angiogenesis, its exact mechanisms, associations with VEGF-A, and the effects of EMP2 inhibition on new, residual, and recurrent meningiomas. The investigation of EMP2 as a novel biomarker for meningiomas has the potential to lead to enhanced understanding of tumor angiogenesis with extensive clinical applications.
Conclusions
EMP2 shows increased mRNA expression and protein expression in meningiomas relative to normal controls and may serve as a molecular marker for all meningiomas. EMP2 is associated with upregulation of genes associated with angiogenesis such as VEGF-A, and both pathologic and clinical correlates of increased tumor vascularity. The exact mechanism and effect of inhibition of EMP2 in meningioma needs to be elucidated.
Supplementary Material
Acknowledgments
This work was generously supported by Eli & Edythe Broad Stem Cell Institute at UCLA Fellowship (K.P.) NIH/NCI P50-CA211015 (M.W. and W.Y.) and NCI R01 CA163971 (M. W.).
Footnotes
Conflict of interest
M.W and L.K.G. are inventors on the University of California patents related to anti-EMP2 monoclonal antibodies.
References
- 1.Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99:307–314. 10.1007/s11060-010-0386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernooij MW, Ikram MA, Tanghe HL, et al. (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357:1821–1828. 10.1056/NEJMoa070972 [DOI] [PubMed] [Google Scholar]
- 3.Krampla W, Newrkla S, Pfisterer W, et al. (2004) Frequency and risk factors for meningioma in clinically healthy 75-year-old patients: results of the Transdanube Ageing Study (VITA). Cancer 100:1208–1212. 10.1002/cncr.20088 [DOI] [PubMed] [Google Scholar]
- 4.Rockhill J, Mrugala M, Chamberlain MC (2007) Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus 23:E1 10.3171/FOC-07/10/E1 [DOI] [PubMed] [Google Scholar]
- 5.Bickerstaff ER, Small JM, Guest IA (1958) The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry 21:89–91. 10.1136/jnnp.21.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud DS, Gallo V, Schlehofer B, et al. (2010) Reproductive factors and exogenous hormone use in relation to risk of glioma and meningioma in a large European cohort study. Cancer Epidemiol Biomarkers Prev 19:2562–2569. 10.1158/1055-9965.EPI-10-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y, Nunes F, Stemmer-Rachamimov A, et al. (2009) Genomic profiling distinguishes familial multiple and sporadic multiple meningiomas. BMC Med Genomics 2:42 10.1186/1755-8794-2-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Liu J, Patel S, et al. (2010) Genomic landscape of meningiomas. Brain Pathol 20:751–762. 10.1111/j.1750-3639.2009.00356.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boström J, Meyer-Puttlitz B, Wolter M, et al. (2001) Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol 159:661–669. 10.1016/S0002-9440(10)61737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates S, Phillips AC, Clark PA, et al. (1998) p14ARF links the tumour suppressors RB and p53. Nature 395:124–125. 10.1038/25867 [DOI] [PubMed] [Google Scholar]
- 11.Laurendeau I, Ferrer M, Garrido D, et al. (2010) Gene expression profiling of the hedgehog signaling pathway in human meningiomas. Mol Med 16:262–270. 10.2119/molmed.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baia GS, Stifani S, Kimura ET, et al. (2008) Notch activation is associated with tetraploidy and enhanced chromosomal instability in meningiomas. Neoplasia 10:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawrin C, Sasse T, Kirches E, et al. (2005) Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 11:4074–4082. 10.1158/1078-0432.CCR-04-2550 [DOI] [PubMed] [Google Scholar]
- 14.Johnson MD, Woodard A, Kim P, Frexes-Steed M (2001) Evidence for mitogen-associated protein kinase activation and transduction of mitogenic signals by platelet-derived growth factor in human meningioma cells. J Neurosurg 94:293–300. https://doi.Org/10.3171/jns.2001.94.2.0293 [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, Woodard A, Okediji EJ, et al. (2002) Lovastatin is a potent inhibitor of meningioma cell proliferation: evidence for inhibition of a mitogen associated protein kinase. J Neurooncol 56:133–142 [DOI] [PubMed] [Google Scholar]
- 16.Lamszus K, Lengler U, Schmidt NO, et al. (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46:938–947; discussion 947-948. 10.1097/00006123-200004000-00033 [DOI] [PubMed] [Google Scholar]
- 17.Samoto K, Ikezaki K, Ono M, et al. (1995) Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res 55:1189–1193 [PubMed] [Google Scholar]
- 18.Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86:113–120. 10.3171/jns.1997.86.1.0113 [DOI] [PubMed] [Google Scholar]
- 19.Verhagen A, Go KG, Visser GM, et al. (1995) The presence of progesterone receptors in arachnoid granulations and in the lining of arachnoid cysts: its relevance to expression of progesterone receptors in meningiomas. Br J Neurosurg 9:47–50 [PubMed] [Google Scholar]
- 20.Konstantinidou AE, Korkolopoulou P, Mahera H, et al. (2003) Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology 43:280–290 [DOI] [PubMed] [Google Scholar]
- 21.Ragel BT, Jensen RL, Gillespie DL, et al. (2005) Ubiquitous expression of cyclooxygenase-2 in meningiomas and decrease in cell growth following in vitro treatment with the inhibitor celecoxib: potential therapeutic application. J Neurosurg 103:508–517. 10.3171/jns.2005.103.3.0508 [DOI] [PubMed] [Google Scholar]
- 22.Ragel BT, Jensen RL, Couldwell WT (2007) Inflammatory response and meningioma tumorigenesis and the effect of cyclooxygenase-2 inhibitors. Neurosurg Focus 23:E7 10.3171/FOC-07/10/E7 [DOI] [PubMed] [Google Scholar]
- 23.He S, Pham MH, Pease M, et al. (2013) A review of epigenetic and gene expression alterations associated with intracranial meningiomas. Neurosurg Focus 35:E5 10.3171/2013.10.FOCUS13360 [DOI] [PubMed] [Google Scholar]
- 24.Le Rhun E, Taillibert S, Chamberlain MC (2016) Systemic therapy for recurrent meningioma. Expert Rev Neurother 16:889–901. 10.1080/14737175.2016.1184087 [DOI] [PubMed] [Google Scholar]
- 25.Fu M, Maresh EL, Helguera GF, et al. (2014) Rationale and preclinical efficacy of a novel anti-EMP2 antibody for the treatment of invasive breast cancer. Mol Cancer Ther 13:902–915. 10.1158/1535-7163.MCT-13-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu M, Maresh EL, Soslow RA, et al. (2010) Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin Cancer Res 16:3954–3963. 10.1158/1078-0432.CCR-10-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habeeb O, Goodglick L, Soslow RA, et al. (2010) Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer 116:4718–4726. 10.1002/cncr.25259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadehra M, Natarajan S, Seligson DB, et al. (2006) Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer 107:90–98. 10.1002/cncr.21957 [DOI] [PubMed] [Google Scholar]
- 29.Qin Y, Fu M, Takahashi M, et al. (2014) Epithelial membrane protein-2 (EMP2) activates Src protein and is a novel therapeutic target for glioblastoma. J Biol Chem 289:13974–13985. 10.1074/jbc.M113.543728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freije WA, Castro-Vargas FE, Fang Z, et al. (2004) Gene expression profiling of gliomas strongly predicts survival. Cancer Res 64:6503–6510. 10.1158/0008-5472.CAN-04-0452 [DOI] [PubMed] [Google Scholar]
- 31.Qin Y, Takahashi M, Sheets K, et al. (2017) Epithelial membrane protein-2 (EMP2) promotes angiogenesis in glioblastoma multiforme. J Neurooncol 134:29–40. 10.1007/s11060-017-2507-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabernero MD, Maillo A, Gil-Bellosta CJ, et al. (2009) Gene expression profiles of meningiomas are associated with tumor cytogenetics and patient outcome. Brain Pathol 19:409–420. https://doi.Org/10.1111/j.1750-3639.2008.00191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres-Martin M, Lassaletta L, Isla A, et al. (2014) Global expression profile in low grade meningiomas and schwannomas shows upregulation of PDGFD, CDH1 and SLIT2 compared to their healthy tissue. Oncol Rep 32:2327–2334. 10.3892/or.2014.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalan AB, Gulluoglu S, Tuysuz EC, et al. (2017) Simultaneous analysis of miRNA-mRNA in human meningiomas by integrating transcriptome: A relationship between PTX3 and miR-29c. BMC Cancer 17:207 10.1186/s12885-017-3198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan I, Baeesa S, Bangash M, et al. (2017) Pleomorphism and drug resistant cancer stem cells are characteristic of aggressive primary meningioma cell lines. Cancer Cell Int 17:72 10.1186/s12935-017-0441-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller A, Ludwig N, Backes C, et al. (2009) Genome wide expression profiling identifies specific deregulated pathways in meningioma. Int J Cancer 124:346–351. 10.1002/ijc.23942 [DOI] [PubMed] [Google Scholar]
- 37.Ragel BT, Jensen RL (2005) Molecular genetics of meningiomas. Neurosurg Focus 19:E9. [DOI] [PubMed] [Google Scholar]
- 38.Choy W, Kim W, Nagasawa D, et al. (2011) The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus 30:E6 https://doi.Org/10.3171/2011.2.FOCUS1116 [DOI] [PubMed] [Google Scholar]
- 39.Ragel BT, Jensen RL (2010) Aberrant signaling pathways in meningiomas. J Neurooncol 99:315–324. 10.1007/s11060-010-0381-8 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Nakayama M, Pitulescu ME, et al. (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465:483–486. 10.1038/nature09002 [DOI] [PubMed] [Google Scholar]
- 41.Franke AJ, Skelton WP, Woody LE, et al. (2018) Role of bevacizumab for treatment-refractory meningiomas: A systematic analysis and literature review. Surg Neurol Int 9:133 10.4103/sni.sni_264_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.