Abstract
Purpose:
To estimate the rate and magnitude of neurologic symptom change during radiation therapy (RT) and impact of symptom change on survival outcomes in patients with diffuse intrinsic pontine glioma (DIPG).
Methods:
From 2006 to 2014, 108 patients with newly diagnosed DIPG were treated with conventionally fractionated radiation therapy (RT) to 54 Gy (median) at our institution. The presence and severity of neurologic symptoms related to cranial neuropathy (CN) and cerebellar (CB) and long-tract (LT) signs was reviewed before and weekly during RT for each patient. The rate and magnitude of change for each symptom category was evaluated according to accumulated RT dose. The impact of clinical factors and radiation dose-volume parameters was determined using Cox proportional hazards models.
Results:
Median dose to first sign of symptomatic improvement was 16.2 Gy (CN), 19.8 Gy (LT) and 21.6 Gy (CB). Most patients showed an improvement by 20 Gy. Larger uninvolved brainstem volume, alone or normalized to total brain (TB) or posterior fossa volume (PF), was associated with shorter time to LT sign improvement (P = 0.044, P = 0.033, and P = 0.05, respectively). Patients with any improvement in CN experienced significantly, yet modestly, prolonged progression-free survival (PFS) and overall survival (OS) (P = 0.002 and P = 0.008, respectively). Tumor volume, with or without normalization to TB or PF, was not significantly associated with PFS or OS.
Conclusions:
Low cumulative RT doses resulted in neurologic improvement in most patients with DIPG. The volume of brainstem spared by tumor influenced time to symptomatic improvement. Neurologic improvement during RT was associated with superior survival.
Keywords: Diffuse intrinsic pontine glioma, diffuse midline glioma, neurologic symptoms, radiation therapy, dose response
INTRODUCTION
Diffuse intrinsic pontine glioma (DIPG) is the deadliest brain tumor in children, with a median survival of <1 year [1]. Its clinical presentation is characterized by the acute onset (generally <3 months) of stereotypic neurologic symptoms, including cranial nerve deficits, long-tract signs, and cerebellar signs—the “classic triad” of clinical symptoms [2]. Symptoms are thought to result from compressive effects of the growing tumor and/or dysfunction of anatomic structures due to infiltrative tumor in and around the pons, although the pathophysiology of neurologic deficits in DIPG and therapy-related changes are poorly understood [3]. Radiation therapy (RT) results in neurologic improvement in 70%-80% of patients with DIPG, but the clinical gains are short lived [4].
Historic studies using RT suggest that conventionally fractionated doses of >45 Gy result in improved survival [5]; however, the association between RT dose and neurologic symptom change in DIPG has not been characterized. Prior studies suggested that improvements in neurologic symptoms after RT influence survival [6,7], yet the impact on survival of early changes during RT is less well established. Furthermore, the relation between the baseline tumor extent and anatomic brain subregions and neurologic symptom change during RT is poorly understood. The primary objective of this study was to estimate the incidence of neurologic symptom change over the course of conformal irradiation and explore the association between cumulative RT dose and this change in a large population of patients with DIPG. Secondarily, we evaluated the impact of patient- and treatment-related factors and the volume of the brain subregions and tumor on the kinetics of neurologic symptoms and patient survival.
METHODS
Study cohort
A total of 110 pediatric patients with clinically or pathologically diagnosed treatment-naive, non-metastatic DIPG treated at our institution from August 2006 to November 2014 were retrospectively reviewed. Of those patients, two required intubation throughout irradiation and were excluded from this analysis (CONSORT diagram, Fig. 1). This study was approved by our Institutional Review Board (XPD15-114).
Fig 1.
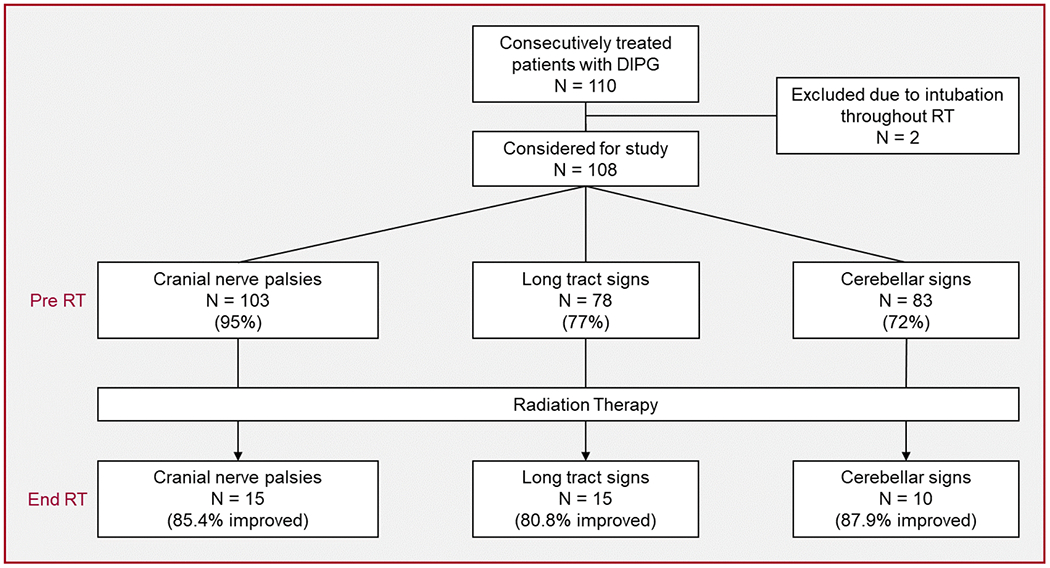
Study CONSORT diagram and neurologic symptom course
Primary therapy and follow-up
Patients were enrolled on institutional prospective phase I clinical trials [8–10] (83%) or individualized treatment plans (17%). Conventional fractionation (1.8 Gy/day) was delivered to all but three patients (97%), with a cumulative prescription dose of 54 Gy to all but five patients (95%). Exceptions included two patients treated to a cumulative dose of 42 Gy delivered in 14 daily fractions of 3 Gy, and one patient who was treated to 53.8 Gy, with an initial phase of the treatment using 2.5 Gy daily fractions. Two patients received 55.8 Gy to account for a treatment interruption of 3 days. Focal conformal methods were used in 106 patients (98%), and two patients received whole-brain irradiation for extensive contiguous tumor spread at diagnosis. Target volumes were delineated based on coregistration of CT and MRI treatment-planning datasets obtained with the patient in the treatment position. An anatomically constrained clinical target volume (CTV) margin was added to the gross tumor volume (GTV), consisting of a 1- to 2-cm transverse and caudal expansion and a 1- to 3-cm cranial expansion. A patient-specific planning target volume (PTV) margin of 0.3-0.5 cm was added to the CTV. Three-dimensional (3D) conformal radiotherapy was delivered to 95% of the patients and intensity-modulated radiotherapy to 5%.
A complete history and physical exam, including a detailed neurologic exam, and a diagnostic brain MRI scan were obtained before the start of (chemo)RT, approximately 4-8 weeks after the completion of (chemo)RT, and every 8-10 weeks thereafter for the duration of therapy or until disease progression. Additional clinical assessments with physical exams were obtained weekly during RT, every other week during adjuvant systemic therapy, and monthly thereafter. Radiographic disease progression was defined as a >25% increase in the product of the maximum perpendicular diameters of the tumor lesion or the presence of any new metastatic disease.
Neurologic symptom and brain subregion assessment
The presence and severity (improved, stable, or declined) of neurologic symptoms related to cranial nerve deficits, long-tract signs, and cerebellar signs were subjectively scored based on medical record review before the start of RT and at each weekly therapy visit during RT. The administered radiation dose was scored at the time of each weekly on-therapy visit. Long-tract signs included muscle paresis, hyperreflexia, clonus, upgoing plantar reflex, and spasticity. Cerebellar signs included ataxia (gait and posture), dysmetria, dysdiadochokinesia, dysarthria, nystagmus, tremor, and hyporeflexia. Baseline and weekly corticosteroid use, use of concurrent systemic therapy, CSF shunt placement before or during RT, and volumetric data for 14 brain subregions and the tumor were extracted from available data sets. Brain subregions were delineated on diagnostic MR images before irradiation, using MIM Software (Cleveland, OH), and included the total brain, brainstem, cerebellum, posterior fossa (the union of the brainstem and cerebellum), and GTV (the union of the abnormal T2 hyperintensity and enhancement, when present, observed on T2-weighted and T1-weighted post-contrast images, respectively) (Supplemental Fig. 1). The subregion volumes (mL) were extracted, and the proportions of the following subregions relative to the total brain or posterior fossa were derived: the brainstem, the cerebellum, the GTV, and the portion of the brainstem uninvolved by tumor (brainstem volume minus GTV) (Supplemental Fig. 1) .
Statistical analysis
The distribution of patients with a baseline neurologic symptom type who experienced an improvement and that of patients who experienced a decline in a neurologic symptom type were depicted by histograms according to cumulative RT dose, censoring patients with missing symptom data. Median daily dexamethasone dose over the course of RT was determined and smoothed using quantile regression. Time to first neurologic improvement by each neurologic symptom category or to any first neurologic improvement was defined as the time from pre-irradiation evaluation to first improvement or to the last visit for patients with no improvement during RT. Progression-free survival (PFS) was defined as the time from diagnosis to progression, i.e., local failure, distant failure, or death, whichever occurred first. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Patients who experienced no event were censored at the last follow-up date for survival analysis. Probability estimates of time to first neurologic improvement, PFS, and OS were calculated by the Kaplan-Meier (KM) method and compared using the log-rank test. A Cox proportional hazards model was used to assess the association of patient age and brain subregion and tumor volumes with first neurologic improvement and survival. Risk estimates, estimated by hazard ratios (HRs), and P-values and 95% CIs were reported. A two-sided significance level of P< 0.05 was considered statistically significant.
RESULTS
Patient and treatment characteristics
One hundred and eight patients with newly diagnosed DIPG treated with conformal RT were evaluated for neurologic symptom change before and weekly during irradiation. Median age at the start of RT was 6.3 years (range, 2.1-17.4 years). Most patients (71%) were white and 56% were female. Cerebrospinal fluid (CSF) diversion before or during RT, primarily through ventriculoperitoneal shunting, was performed in 17% of the patients. Eighty-one percent of the patients received dexamethasone before RT (median dose, 8 mg/day; range, 1-16 mg/day). Eighty-nine percent received concurrent systemic therapy during RT, of whom 94% received investigational therapies targeting receptor tyrosine kinases. Eight patients underwent diagnostic biopsy prior to RT, confirming grade II-IV diffuse glioma in all but one patient whose biopsy was non-diagnostic. Histone H3 status was evaluable in three of these samples, confirming H3.3 K27M mutation in one case, H3.1 K27M mutation in one case, and H3 K27M mutation, not otherwise specified, in one case. Patient and treatment characteristics are summarized in Table 1.
Table 1.
Patient, treatment, and neurologic symptom characteristics
| Characteristics | N (%) | Characteristics | N (%) |
|---|---|---|---|
| Sex | Concurrent chemotherapy | ||
| Male | 47 (44%) | Yes | 96 (89%) |
| Female | 61 (56%) | No | 12 (11%) |
| Race | TKI | 90 (94%) | |
| White | 77 (71%) | HDAC inhibitor | 3 (3%) |
| Black | 19 (18%) | Temozolomide | 2 (2%) |
| Other | 12 (11%) | Motexafin-Gad | 1 (1%) |
| Age at RT | RT Dose | ||
| Median | 6.3 years | Median | 54.0 Gy |
| Range | 2.1–17.4 years | Range | 42.0–55.8 Gy |
| Cerebrospinal fluid diversion | Clinical target volume | ||
| Yes | 18 (17%) | 1 cm | 59 (54%) |
| No | 90 (83%) | >1 cm | 49 (45%) |
| Pre-RT biopsy | Histone H3 status (biopsy) | ||
| Yes | 8 (7%) | H3.3/H3.1/H3 NOS | 1 (1%)/1 (1%)/1(1%) |
| No | 100 (93%) | Unknown | 5 |
| Characteristics | Median (range) | ||
| Improvement | Cranial nerve deficits | Long tract signs | Cerebellar signs |
| RT dose to improvement | 16.2 Gy (1.8–54.0 Gy) | 19.8 Gy (3.6–54 Gy) | 21.6 Gy (1.8–54 Gy) |
| Decline | Cranial nerve deficits | Long tract signs | Cerebellar signs |
| # Patients (%) | 15 (13.9%) | 19 (17.6%) | 16 (14.8%) |
| RT dose to decline | 41.4 Gy (23.4–50.4 Gy) | 31.5 Gy (23.4 Gy–50.4 Gy) | 35.1 Gy (14.4–54.0 Gy) |
| Corticosteroids | Dose before RT | Dose at end of RT | RT dose to complete cessation |
| Dexamethasone | 8.0 mg/day (1–16 mg/day) | 0.5 mg/day (0–16 mg/day) | 45.0 Gy (1.8–54 Gy) |
Abbreviations: TKI, tyrosine kinase inhibitor; HDAC, histone deacetylase; Motexafin-Gad, motexafin-gadolinium
Neurologic symptom changes during conformal irradiation
Before irradiation, cranial nerve deficits, long-tract signs, or cerebellar signs were observed in most patients, with cranial neuropathies the most frequently observed (95%) (Fig. 1). Eighty-five percent of the patients experienced two or more neurologic deficits, and the classic triad of deficits was observed in 57%. Assessment at completion of conformal radiotherapy revealed improvement in each symptom category in >80% of the patients with a baseline deficit (Fig. 1).
The distribution of the first neurologic symptom improvement by cumulative RT dose for each symptom category is shown in Figure 2. Median dose to first sign of symptomatic improvement was 16.2 Gy (for cranial nerve deficits), 19.8 Gy (for long-tract signs), and 21.6 Gy (for cerebellar signs) (Table 1). Most patients showed an improvement in neurologic symptoms by 20 Gy, despite decreasing daily dexamethasone dose (Figure 2). Conversely, worsening of any neurologic symptoms was observed in only 14%-18% of patients, occurring at higher RT doses (31.5-41.4 Gy) and lower corticosteroid doses (Supplemental Fig. 2). The median daily dexamethasone dose dropped over the course of RT from 8.0 mg/day at the start of RT to 0.5 mg/day at completion, and the median RT dose to complete corticosteroid cessation was 45.0 Gy (Table 1). Of the patients receiving corticosteroids before RT, 41% were able to cease steroid use by the completion of RT. Of the 20 patients who were not administered dexamethasone prior to RT and had a neurologic deficit, the median RT dose to first neurologic symptom improvement was similar to the overall cohort of patients (range, 12.6 – 25.2 Gy; Supplemental Table 1), yet the proportion of patients who experienced improvements in long-tract and cerebellar signs was less (50% and 45%, respectively; Supplemental Table 1).
Fig 2.
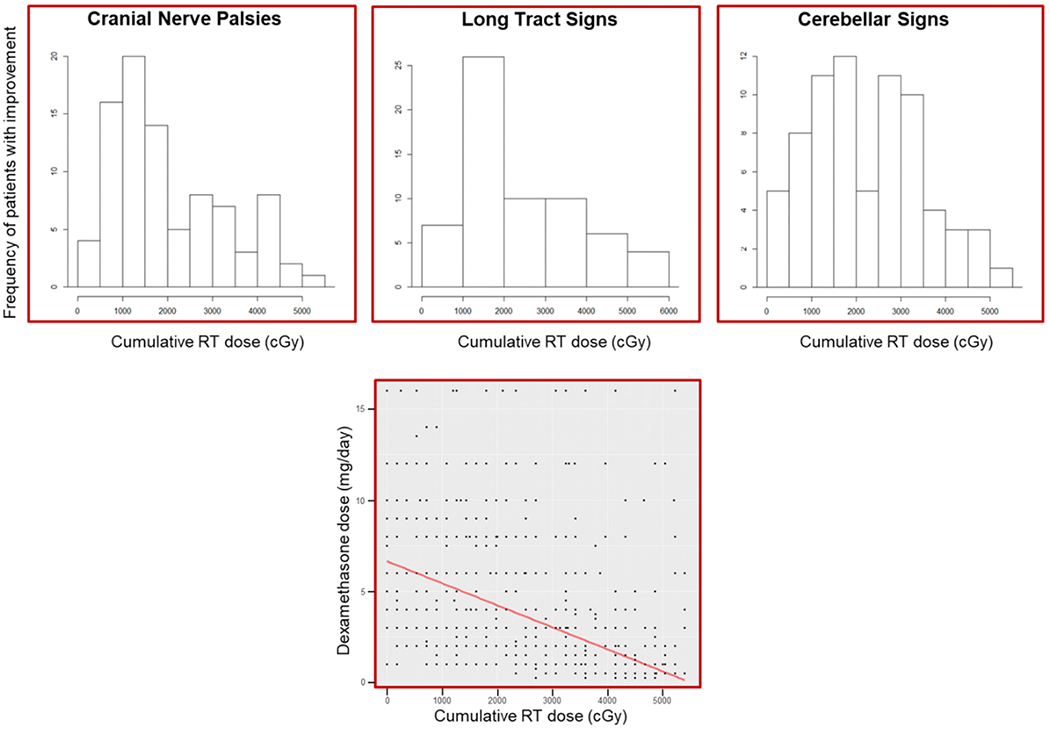
Distribution of first neurologic symptom category improvement by RT dose and dexamethasone dose during RT with smoothed median dose (red line) and dose distribution (black dots) (far right).
Factors associated with neurologic symptom change and associations with survival
The proportion of patients with deficits by cumulative RT dose for each neurologic symptom category is shown in Figure 3. The impact of clinical variables (including patient age, sex, and race; CSF diversion before or during RT; receipt of concurrent chemotherapy; and clinical target volume) on the RT dose associated with first neurologic symptom improvement was evaluated via Cox regression or the log-rank test. Except for race, for which black patients demonstrated improved long-tract signs at lower cumulative doses when compared to non-black patients (P = 0.027), no clinical variables significantly influenced the association between RT dose and any first or individual neurologic symptom-type improvement (Supplemental Figs. 3–5).
Fig 3.

Kaplan–Meier estimates of improvement in neurologic deficit with cumulative RT dose
Volumetric assessment of brain subregions and tumor based on pre-irradiation MRI revealed a significant association between increasing volume of the brainstem spared by tumor at baseline and shorter time to improvement of long-tract signs (P = 0.04) (Table 2). When normalized to the posterior fossa or total brain volumes to account for interpatient variability, larger volume of spared brainstem was significantly associated with shorter time to improvement in long-tract signs (P = 0.05 and P = 0.03, respectively). Other brain regions, including the cerebellum and brainstem, as well as the tumor volume, alone or as proportions of the posterior fossa or total brain, were not significantly associated with time to any first or individual first neurologic symptom-type improvement.
Table 2.
Significant associations with time to symptom improvement and survival outcomes
| Characteristics | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Time to long tract sign improvement* | |||
| Uninvolved brainstem (mL) | 1.05 | 1.001–1.10 | 0.04 |
| Uninvolved brainstem relative to posterior fossa (mL) | 1.09 | 1.000–1.18 | 0.05 |
| Uninvolved brainstem relative to whole brain (mL) | 2.16 | 1.065–4.36 | 0.03 |
| Progression-free survival# | |||
| Improvement in cranial nerve deficits | 0.40 | 0.23–0.72 | <0.01 |
| Overall survival# | |||
| Improvement in cranial nerve deficits | 0.46 | 0.25–0.82 | <0.01 |
Hazard ratios > 1 indicate shorter time to neurologic symptom improvement with larger volumes of uninvolved brainstem;
Hazard ratios <1 indicate longer survival duration with improvement in neurologic symptom.
The influence of neurologic symptom change during RT on survival outcomes was assessed by measuring the improvement in any or an individual neurologic symptom type or the RT dose at first improvement. Both PFS and OS were significantly, yet modestly, longer in patients who experienced an improvement in cranial nerve deficits, as compared to patients who experienced no such improvement (HR = 0.40, P < 0.01, 7.6 months vs. 6.3 months; and HR = 0.46, P < 0.01, 11.3 months vs. 9.8 months, respectively). Significantly longer PFS was associated with lower RT doses at first improvement of cranial nerve deficits (P = 0.04). Apparent trends toward improved OS and lower RT doses for cranial nerve deficits (P = 0.082) and improved PFS and lower RT doses for improvement in cerebellar signs (P = 0.067) were not significant. Finally, no significant associations were observed between the 14 delineated brain subregion volumes, including the baseline tumor volume, and survival outcomes.
DISCUSSION
The primary goal of this study was to characterize neurologic symptom change over the course of conformal RT for patients with newly diagnosed DIPG and to assess the RT dose response governing these changes. After a pre-irradiation consultation, we assessed first improvement in or worsening of the classic triad of neurologic symptoms weekly over the course of RT. For each neurologic symptom category, initial improvements were noted early during RT and at doses of <20 Gy for most patients, with the lowest dose threshold being observed for improvements in cranial nerve deficits. Consistent with prior reports [4], most patients (>80%) experienced an improvement in neurologic symptoms and 41% were able to come off of corticosteroids completely over the course of RT. However, in 14%-18% of the patients, first declines in neurologic status were observed, most frequently towards the completion of RT, when steroid usage was lowest and RT doses were relatively high.
Most studies of the clinical response to RT in patients with DIPG have focused on time-points after the completion of irradiation and have often included composite outcomes with corticosteroid administration rates and/or imaging responses [4]. Establishing the RT dose response for neurologic symptoms during irradiation may be useful for evaluating the clinical impact of alterations to RT delivery (e.g., using hypofractionated RT or altering the target volumes), as well as of the combination of radiosensitizing or immune-modulating systemic agents, with which significant shifts in survival outcomes may not be realized but meaningful changes in neurologic function may be observed. For patients with DIPG, and patients with brain tumors generally, neurologic integrity significantly affects their quality of life and is a key priority in the expectations regarding therapy among patients with brain tumors [11]. In an early study of the use of hypofractionated RT in DIPG, mostly delivered in 13 fractions of 3 Gy, all patients showed improvement within the first 2 weeks of radiotherapy [12]. This timeframe is similar to that observed in our study using conventional fractionation, although the magnitude was greater. In a randomized trial of hypofractionated versus conventionally fractionated RT for patients with newly diagnosed DIPG, there was a trend toward more rapid improvement in cranial nerve deficits during hypofractionated RT (P = 0.06) [13], suggesting additional utility to this approach beyond patient/family convenience factors. This information may also assist providers in counselling patients and families regarding the expected kinetics of neurologic symptom palliation, and it may influence post-RT treatment planning. As a small number of patients experienced neurologic decline during irradiation, most commonly towards the end of RT, some patients may benefit from closer observation following RT and/or a more protracted course of steroid administration.
A secondary aim of this study was to evaluate the impact of clinical and treatment characteristics, as well as brain subregion and tumor volume, on the cumulative dose or treatment time to neurologic symptom improvement and patient survival. Except for patient race and improvement in long-tract signs, baseline clinical variables had little influence on the dose to neurologic improvement. However, volumetric assessment revealed a significant association between the time to improvement of long-tract signs and the normal brainstem volume. This was also observed when interpatient volumetric variability was accounted for by normalizing normal brainstem volume to the cranial and posterior fossa volumes. This suggests a correlation between the initial tumor extent within the brainstem and the kinetics of neurologic symptom improvement. With advances in MRI, including diffusion tensor imaging, it may be interesting to characterize more fully brainstem cranial nerve nuclei and neural pathways within the brainstem in relation to tumor infiltration and/or associated edema in patients with DIPG [14,15]. Finally, although reports vary, studies of very large populations of patients with consistently defined DIPG have also demonstrated no correlation between initial tumor size and survival [16,17], something we also observed even when accounting for initial cranial or posterior fossa extent.
Importantly, any improvement in cranial nerve deficits during RT was associated with prolonged survival, and prolonged PFS was associated with lower cumulative RT doses at first improvement of cranial nerve deficits. Using an endpoint of CT-based radiographic response and/or resolution of at least one of the cardinal neurologic signs after completion of irradiation, Hibi et al. observed a positive correlation with the response rate to initial RT up to doses of 6499 cGy, with declines being observed with higher doses for patients with brainstem glioma [7]. Similar findings were reported more recently for pediatric patients with specific clinicoradiologic criteria of DIPG, with imaging based primarily on MRI, after treatment with concurrent RT and temozolomide [6]. Early identification of patients who may experience prolonged survival after RT may not only assist in guiding post-irradiation clinical trial participation but also have an impact on experimental trial design, in which adjuvant systemic therapy after standard RT is increasingly common. Thus, it is important to determine prospectively the prognostic value of early clinical responses during radiotherapy and the neurologic symptom(s) that are most reproducibly associated with survival gains.
The observation that a relatively low RT dose led to neurologic symptom improvement in most patients, coupled with the finding that patients who did experience neurologic improvement had prolonged survival, raises the possibility of developing a more patient-tailored RT regimen for DIPG within a clinical trial context. Although limited retrospective studies conducted largely in the 1960s and 1970s suggested an association of RT dose response with survival for patients with brainstem glioma [5], rigorous prospective data is lacking regarding the lowest dose threshold necessary to provide a survival benefit for patients with DIPG. A clinical trial that may test this hypothesis, at least indirectly, is the recently launched Italian INT 94/15 clinical trial [18]. In the experimental arm of this trial, the conventionally fractionated RT regimen is split into an initial course of 36 Gy followed by protracted systemic therapy and then by a second and third course of 19.8 Gy if patients do not exhibit tumor progression after the first and second courses of RT, respectively. The dose response we have observed in our cohort would suggest that most patients will experience neurologic symptom palliation with the initial RT course, implying clinical utility at this dose. It will be interesting to assess the completion rate of the subsequent scheduled courses of RT and its impact on neurologic symptoms and survival.
An obvious limitation of the present study is the retrospective and subjective nature of the neurologic assessments. Despite its specification within the Response Assessment in Neuro-Oncology (RANO) criteria [19] and the preceding Macdonald criteria [20] for assessing treatment efficacy for adult patients with brain tumors, the assessment of neurologic function remains subjective and is categorized simply as the same, better, or worse. Although several tools to characterize the neurologic status of adult and pediatric patients with brain tumors are currently used, including assessments of performance status, symptoms and burden, quality of life, and neurocognitive aspects, formal neurologic function is not objectively evaluated with these assessment tools. To address this shortcoming and the subjectivity of assessments and to provide an objective and quantifiable measure of neurologic function, the Neurologic Assessment in Neuro-Oncology (NANO) scale has been developed for adult patients with brain tumors through an international, multidisciplinary working group [21]. This scale was recently tested in a multicenter study that demonstrated a >90% inter-observer agreement rate with a kappa statistic ranging from 0.35 to 0.83 (fair to almost perfect agreement) and a median assessment time of 4 minutes [21]. Although the NANO scale was specifically designed for adult patients and tested in patients aged 18 years or older, its extension to the pediatric population or the formalization of a pediatric brain tumor-specific neurologic assessment tool may help reduce the subjectivity of these assessments and enhance both overall treatment response criteria and functional clinical trial endpoints for our pediatric patients.
CONCLUSION
This descriptive analysis suggests that low cumulative doses of RT provide neurologic improvement in most patients with DIPG, as measured by clinical assessment of common deficits. A few patients experienced progressive neurologic symptoms, and this was correlated with higher cumulative RT doses and lower corticosteroid intake. Black race was associated with improvement in long-tract signs at significantly lower cumulative RT doses, whereas other clinical factors did not significantly alter the impact of cumulative RT dose on neurologic symptom improvement. Brain subregion volumes influenced time to symptomatic improvement, and neurologic improvement was associated with superior survival outcomes.
Supplementary Material
ACKNOWLEDGMENTS:
The authors would like to thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript.
Funding: This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), the National Cancer Institute grant P30 CA021765 (St. Jude Cancer Center Support Grant), and the Dunagan MD Medical Education Fund (to K.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
REFERENCES
- 1.Cooney T, Lane A, Bartels U, et al. Contemporary survival endpoints: an International Diffuse Intrinsic Pontine Glioma Registry study. Neuro Oncol. 2017;19(9):1279–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J. Clin Oncol 2006;24(8):1266–1272. [DOI] [PubMed] [Google Scholar]
- 3.Veldhuijzen van Zanten SE, Cruz O, Kaspers GJ, Hargrave DR, van Vuurden DG, SIOPE DIPG Network. State of affairs in use of steroids in diffuse intrinsic pontine glioma: an international survey and a review of the literature. J Neurooncol 2016;128(3):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 2006;7(3):241–248. [DOI] [PubMed] [Google Scholar]
- 5.Whyte TR, Colby MY Jr, Layton DD Jr. Radiation therapy of brain-stem tumors. Radiology. 1969;93(2):413–416 passim. [DOI] [PubMed] [Google Scholar]
- 6.Jalali R, Raut N, Arora B, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys 2010;77(1):113–118. [DOI] [PubMed] [Google Scholar]
- 7.Hibi T, Shitara N, Genka S, et al. Radiotherapy for pediatric brain stem glioma: radiation dose, response, and survival. Neurosurgery 1992;31(4):643–650; discussion 650–641. [DOI] [PubMed] [Google Scholar]
- 8.Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol 2010;28(31):4762–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broniscer A, Baker SD, Wetmore C, et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 2013;19(11):3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetmore C, Broniscer A, Turner D, et al. First-in-pediatrics phase I study of crenolanib besylate (CP-868,596-26) administered during and after radiation therapy (RT) in newly diagnosed diffuse intrinsic pontine glioma (DIPG) and recurrent high-grade glioma (HGG). J Clin Oncol 2014; 32(15 suppl):10064. [Google Scholar]
- 11.Armstrong TS, Bishof AM, Brown PD, Klein M, Taphoorn MJ, Theodore-Oklota C. Determining priority signs and symptoms for use as clinical outcomes assessments in trials including patients with malignant gliomas: Panel 1 Report. Neuro Oncol 2016;18 Suppl 2:ii1–ii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssens GO, Gidding CE, Van Lindert EJ, et al. The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int J Radiat Oncol Biol Phys 2009;73(3):722–726. [DOI] [PubMed] [Google Scholar]
- 13.Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 2014;111(1):35–40. [DOI] [PubMed] [Google Scholar]
- 14.Helton KJ, Phillips NS, Khan RB, et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am J Neuroradiol 2006;27(4):786–793. [PMC free article] [PubMed] [Google Scholar]
- 15.Helton KJ, Weeks JK, Phillips NS, et al. Diffusion tensor imaging of brainstem tumors: axonal degeneration of motor and sensory tracts. J Neurosurg Pediatr 2008;1(4):270–276. [DOI] [PubMed] [Google Scholar]
- 16.Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, et al. Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol . 2015; 17(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 2018;36(19):1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massimino M Study of Re-Irradiation at Relapse versus RT and Multiple Elective RT Courses (DIPG). Clinical Trials.gov Identifier: NCT03620032, 2015. [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990; 8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 21.Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol 2017;19(5):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


