Abstract
Opioid-sparing adjuncts are treatments that aim to reduce the overall dose of opioids needed to achieve analgesia, hence decreasing the burden of side effects through alternative mechanisms of action. Lorcaserin is a serotonin 5-HT2C receptor (5-HT2CR) agonist that has recently been reported to reduce abuse-related effects of the opioid analgesic oxycodone. The goal of our studies was to evaluate the effects of adjunctive lorcaserin on opioid-induced analgesic-like behavior using the tail-flick reflex (TFR) test as a mouse model of acute thermal nociception. We show that whereas subcutaneous (s.c.) administration of lorcaserin alone was inactive on the TFR test, adjunctive lorcaserin (s.c.) significantly increased the potency of oxycodone as an antinociceptive drug. This effect was prevented by the 5-HT2CR antagonist SB242084. A similar lorcaserin (s.c.)-induced adjunctive phenotype was observed upon administration of the opioid analgesics morphine and fentanyl. Remarkably, we also show that, opposite to the effects observed via s.c. administration, intrathecal (i.t.) administration of lorcaserin alone induced antinociceptive TFR behavior, an effect that was not prevented by the opioid receptor antagonist naloxone. This route of administration (i.t.) also led to a significant augmentation of oxycodone-induced antinociception. Lorcaserin (s.c.) did not alter the brain or blood concentrations of oxycodone, which suggests that its adjunctive effects on opioid-induced antinociception do not depend upon changes in opioid metabolism. Together, these data indicate that lorcaserin-mediated activation of the 5-HT2CR may represent a new pharmacological approach to augment opioid-induced antinociception.
Keywords: lorcaserin, serotonin 5-HT2C receptor, opioid receptor, G protein-coupled receptor (GPCR), oxycodone, morphine, fentanyl, analgesia, pain, antinociception
1. Introduction
Prescription opioid misuse, a serious public health issue in the United States (U.S.), skyrocketed the past decade (Jalal et al., 2018). In 2017, more than 70,000 people died from an opioid overdose; almost two-thirds of these deaths involved a prescription or illicit opioid (Scholl et al., 2018). In response to this “opioid crisis”, medical research agencies, including the National Institutes of Health (NIH), implemented measures to boost research on preventing or treating addiction following pain treatment (Volkow and Collins, 2017). Unfortunately, opioids are still the cornerstone of moderate and severe pain management (Twycross and Lickiss, 1996). Acute pain in patients with opioid tolerance makes pain management a challenge. Identification of new pharmacological targets to augment analgesia in opioid tolerant patients with an acute pain condition without increasing the incidence of overdose or side effects is therefore a major need in antinociceptive drug development (Coluzzi et al., 2017).
The use of non-opioid adjuvants, defined as drugs that enhance the analgesic efficacy when used in conjunction with opioids, is a promising strategy to reduce opioid doses (Kumar et al., 2017). The rationale behind the combination of opioid and non-opioid adjuvants lies in the capacity to modulate pain pathways thereby increasing analgesia and reducing opioid doses as well as their side effects. Commonly used non-opioid adjuvants include glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDS), acetaminophen, tricyclic antidepressants (TCAs), anticonvulsants, serotonin and norepinephrine reuptake inhibitors, and cannabinoids (Ghosh and Berger, 2014).
Early studies have shown a functionally relevant interaction between the opioid and serotonergic systems. For instance, it has been reported that the release of spinal serotonin underlies the antinociceptive effects of morphine (Crisp et al., 1991; Yaksh and Wilson, 1979; Yaksh and Tyce, 1979). Serotonin (or 5-hydroxytryptamine, 5-HT) has both excitatory and inhibitory effects and exerts its analgesic action by modulating the pain circuitry through a descending modulatory pathway at the dorsal horn and supraspinal nuclei (Eide and Hole, 1991; Fasmer et al., 1986; Sengupta et al., 2014; Zhao et al., 2007). However, the diversity of subtypes of the 5-HT receptors together with the cytoarchitectural complexity of the central nervous system complicates the understanding of the role of each serotonin receptor subtype in pain behavior. The effects of 5-HT are mediated by a large number of receptor subtypes, most of which belonging to the G protein-coupled receptor (GPCR) family. Activation of receptors within the 5-HT2 group, which includes 5-HT2AR, 5-HT2BR and 5-HT2CR, results in activation of phospholipase C and release of Ca2+ from the endoplasmic reticulum (Hoyer et al., 2002). In the past few years, 5-HT2CR has emerged as a novel target for treating drug addiction and pain (Baptista-de-Souza et al., 2014; Kohut and Bergman, 2018). Although originally described in the choroid plexus (Pazos et al., 1984), we are now aware that expression of the 5-HT2CR is widely distributed in brain regions that include the frontal cortex, amygdala, thalamic nuclei, periaqueductal gray (PAG), and the dorsal spinal cord (Backstrom et al., 1995; Clemett et al., 2000). Density of the 5-HT2CR is upregulated at both the PAG and spinal cord following neuropathic pain (Baptista-de-Souza et al., 2014). Lorcaserin, a 5-HT2CR agonist with 18-fold greater sensitivity over 5-HT2AR (Thomsen et al., 2008), was originally developed as anti-obesity drug (Smith et al., 2010; Fidler et al., 2011) and recently evaluated as a possible treatment for addiction disorders. In preclinical studies, lorcaserin was reported to reduce abuse-related properties of several drugs including alcohol, cocaine, nicotine and opioids (Collins et al., 2016; Higgins et al., 2012; Neelakantan et al., 2017; Wu et al., 2015; Zhang et al., 2016; Gerak et al. 2019). Available data also suggest that lorcaserin may reduce antinociception through an alternative non-opioid pathway (Nakai et al., 2010; Ogino et al., 2013), although its mechanisms of action as a potential antinociceptive drug remain largely unexplored.
The warm water tail immersion test, one of the most accepted assays to study nociception, depends on the spinal reflex (i.e., tail-flick reflex), which is a monosynaptic circuit initiated by the activation of the cutaneous nociceptor (Danneman et al., 1994). The tail-flick reflex (TFR) is modulated by descending pathways originating in the brainstem (raphe nucleus and periaqueductal gray) and local inhibitory neurons (Hall et al., 1982; Morton et al., 1983; Takazawa and MacDermott, 2010). Using the TFR, here we tested the analgesic efficacy of lorcaserin and its potential use as an adjunct therapy for pain management in mice.
2. Materials and Methods
2.1. Drugs
Oxycodone hydrochloride (National Institutes on Drug Abuse, Bethesda, MD) was prepared in pyrogen-free isotonic saline (Hospira, Lake Forest, IL) and administered via oral gavage (o.g.). Morphine sulfate and fentanyl (National Institute on Drug Abuse, Bethesda, MD) were dissolved in pyrogen-free isotonic saline and administered subcutaneously (s.c.). Lorcaserin hydrochloride and 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride; SB242084) were purchased from Cayman Chemicals (Ann Arbor, MI). Lorcaserin was prepared in isotonic saline and injected s.c. For intrathecal (i.t.) administration, lorcaserin was prepared in deionized water. SB242084 was prepared in an 0.9% saline solution containing 8% hydroxypropyl-β-cyclodextrin.
2.2. Animals
Male, Swiss Webster mice (8 to 10-week-old, Harlan Laboratories, Indianapolis, IN) weighing 25 – 35 g, were housed in groups of five in 500 cm2 Plexiglas cages (Allentown, NJ) in the animal care facilities (22 ± 2°C, 12-hour light-dark cycle) with a d libitum access to food (Teklad LM-485 mouse diet, Envigo) and water. The cage contained bedding (Teklad 1/4” Corncob bedding, Envigo) and one Nestlests™ (Ancare, Bellmore, NY, USA). On the day prior to experimentation, the mice were moved to the laboratory and allowed to acclimate overnight. Different animals were used for each experiment (e.g., accumulative dose response or time course). Animal care and experimental procedures were performed according to an Institutional Animal Care and Use Committee (IACUC) approved protocol at Virginia Commonwealth University.
2.3. Intrathecal Injections
Intrathecal (i.t.) injections were performed according to the protocol of Hylden and Wilcox (Hylden and Wilcox, 1983). Unanesthetized mice were injected with a volume of 5 μL between the L5 and L6 area of the spinal cord using a 30-gauge, ½-inch needle. Based on the time course experiments of lorcaserin’s i.t. activity, all antinociceptive testing was conducted 10 minutes after intrathecal injection.
2.4. Warm Water Tail-Withdrawal Test
The warm water tail-withdrawal test (hereinafter TFR test) used to assess antinociception in mice was developed by D’Amour and Smith (D’ Amour and Smith, 1941), but modified by Dewey et al. (Dewey et al., 1970). In all experiments (unless otherwise stated), mice were tested using a 52° C water bath. Before drug administration, the baseline (control) latency for each mouse was determined and only mice with a control reaction time from 2 – 4 seconds were used. The test latency after drug treatment was assessed 20 minutes after drug administration, with a maximum cut-off value of 10 seconds to prevent tissue damage to the tail. Antinociception was quantified according to the method of Harris & Pierson (Harris and Pierson, 1964) as the percentage of maximum possible effect (%MPE) which was calculated as: %MPE = [(test – baseline)/(10 – baseline)] ×100.
2.5. Cumulative dose-response assays
Drugs were administered using a cumulative dosing technique in an ascending order during one session to the same animal. In the drug-combination studies, saline or lorcaserin were administered at doses of 0.25, 0.5, 1, 2, and 4 mg/kg (s.c.), 30 minutes prior to the first opioid treatment. After lorcaserin pretreatment, the first dose of opioid was administered via oral gavage or subcutaneous injection, and animals were tested 20 minutes later. After each round of testing, animals received an additional cumulative dose of opioid and tested again 20 minutes later. Testing and dosing continued until the animal reached the maximum cut-off time of 10 seconds.
2.6. Time Course assays
Mice were first administered (s.c.) saline or lorcaserin (0.5, 1 or 2 mg/kg), 30 minutes prior to opioid treatment. After the lorcaserin pretreatment, mice were administered saline or oxycodone (10 mg/kg, o.g.) and then tested at the following time points: 15, 30, 60, 120 minutes for the TFR.
2.7. Locomotor activity assay
Locomotor activity was monitored in enclosed, sound attenuating, photo beam activity monitors (Med Associates., St. Albans, VT) that record “ambulatory counts” via photo beam breaks. Numbers of beam breaks were recorded in 5-minute time blocks. Mice were administered saline or lorcaserin (0.5, 1, or 2 mg/kg, s.c.), and immediately placed in the chamber for 40 minutes of recording. Activity chambers were thoroughly cleaned between subjects with cleaning solution and then dried.
2.8. Lorcaserin and oxycodone distribution
Seven-point calibration curves for both lorcaserin and oxycodone at 10 –1000 ng/mL for blood and 10 – 1000 ng/kg for brain tissue homogenate along with negative controls with or without internal standard (ISTD) were prepared in drug-free mouse blood and brain tissue with each analytical run. Lorcaserin was extracted using the addition of acetonitrile and oxycodone was extracted using an ISOLUTE® PLD+ Protein and Phospholipid Removal 96 well plate (Biotage, Uppsala, Sweden). Lorcaserin analysis took place on a Shimadzu UPLC system (Kyoto, Japan) attached to a Sciex 6500+ QTRAP system with an IonDrive Turbo V source for TurbolonSpray® (Sciex, Ontario, Canada) controlled by Analyst software (Sciex, Ontario, Canada). Chromatographic separation of lorcaserin and its ISTD, cocaine-d3, was performed using a Thermo Hypersil Gold column, 50 × 2.1 mm, 3 micron (Thermofisher Scientific, Waltham, MA). The mobile phase consisted of water/methanol (40:60, v/v) with 0.1 mM ammonium formate and was delivered at a flow rate of 1 mL/min. The source temperature was set at 600°C, and curtain gas had a flow rate of 30 mL/ min. The ionspray voltage was 5500 V, with the ion source gases 1 and 2 having flow rates of 60 and 45 mL/min, respectively. The declustering potential and collection energy were for lorcaserin and the ISTD were 100 and 38 V, respectively. The acquisition mode used was multiple reaction monitoring (MRM). The following transition ions (m/z) with their corresponding collision energy (eV) in parentheses were monitored in negative mode for lorcaserin: 196>144 (34) & 196>129 (40) and in positive mode for cocaine-d3: 307>185 (28) & 307>105 (50). The total run time for the analytical method was 4.0 minutes. The oxycodone analysis took place on an AcQuity XEVO-TQ-S Micro UPLC-MS/MS system (Wates, Milford, Massachusetts). The Chromatographic separation of Oxycodone and its ISTD, oxycodone d6, was performed using an Ultra Biphenyl 3 mm, 100 × 2.1 mm column (Restek, Bellefonte, PA). Mobile phase A was 20 mM ammonium formate in water and mobile phase B was 20 mM ammonium formate in methanol. The flow rate of 0.6 mL/min with the following gradient was used: 95% A changed to 60% A at 1.5 min. Then ramped to 0% A and held for 0.5 min and returning to 95% A at 3.6 min. The source temperature was set at 150°C with a desolvat ion temperature of 500°C. The cone flow rate was 100 L/h and the desolvation gas had a flow rate of 40°C L/h. The acquisition mode used was multiple reaction monitoring (MRM). The following transition ions were monitored in positive mode: 316>241 & 316>212 for oxycodone and 322>247 & 322>218 for oxycodone-d6. The total run time for the analytical method was 4.0 minutes. Linear regression of the peak area of ratios of the quantification transition ions of lorcaserin or oxycodone and there ISTDs were used to construct the calibration curves.
2.9. Data Analysis
Statistical analyses were performed with GraphPad Prism software version 8. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in our previous publications. Animals were randomly allocated into the different experimental groups. Statistical significance of experiments involving three or more groups and two or more treatments was assessed by two-way ANOVA followed by Dunnett’s multiple comparison test. Statistical significance of experiments involving time courses or cumulative dose-response assays was assessed by two-way repeated measures ANOVA followed by Dunnett’s multiple comparison test. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Dunnett’s multiple comparison test. The level of significance was chosen at p = 0.05. All values included in the figures represent mean ± S.E.M.
3. Results
3.1. Intrathecal administration of lorcaserin leads to antinociceptive effects
We first tested the potential antinociceptive effect of lorcaserin when administered intrathecally (i.t.). Our data show that lorcaserin (i.t.) by itself was able to induce a dose-dependent antinociceptive effect (Fig. 1A) (F[4,21] = 24.98, p < 0.001), an interesting behavioral phenotype that was corroborated by time-course assays with peak effects at 10 min from drug injection (Fig. 1B) (lorcaserin [64 μg/5 μl] vs vehicle, F[4,40] = 5.42, p < 0.01). Importantly, this effect of lorcaserin (i.t.) alone on the TFR test was not prevented by the opioid receptor antagonist naloxone (Fig. 1C) (lorcaserin [32 μg/5 μl] vs lorcaserin [64 μg/5 μl], F[1,19] = 4.80, p < 0.05; naloxone vs vehicle, F[1,19] = 8.50 , p > 0.05). Additionally, lorcaserin (i.t.) showed an adjunctive effect on the antinociceptive action induced by oxycodone (10 mg/kg, o.g.) (Fig. 1D) (F[2,32] = 7.84, p < 0.01).
Fig. 1. Effect of lorcaserin (i.t.) on response latencies over time on the TFR test.
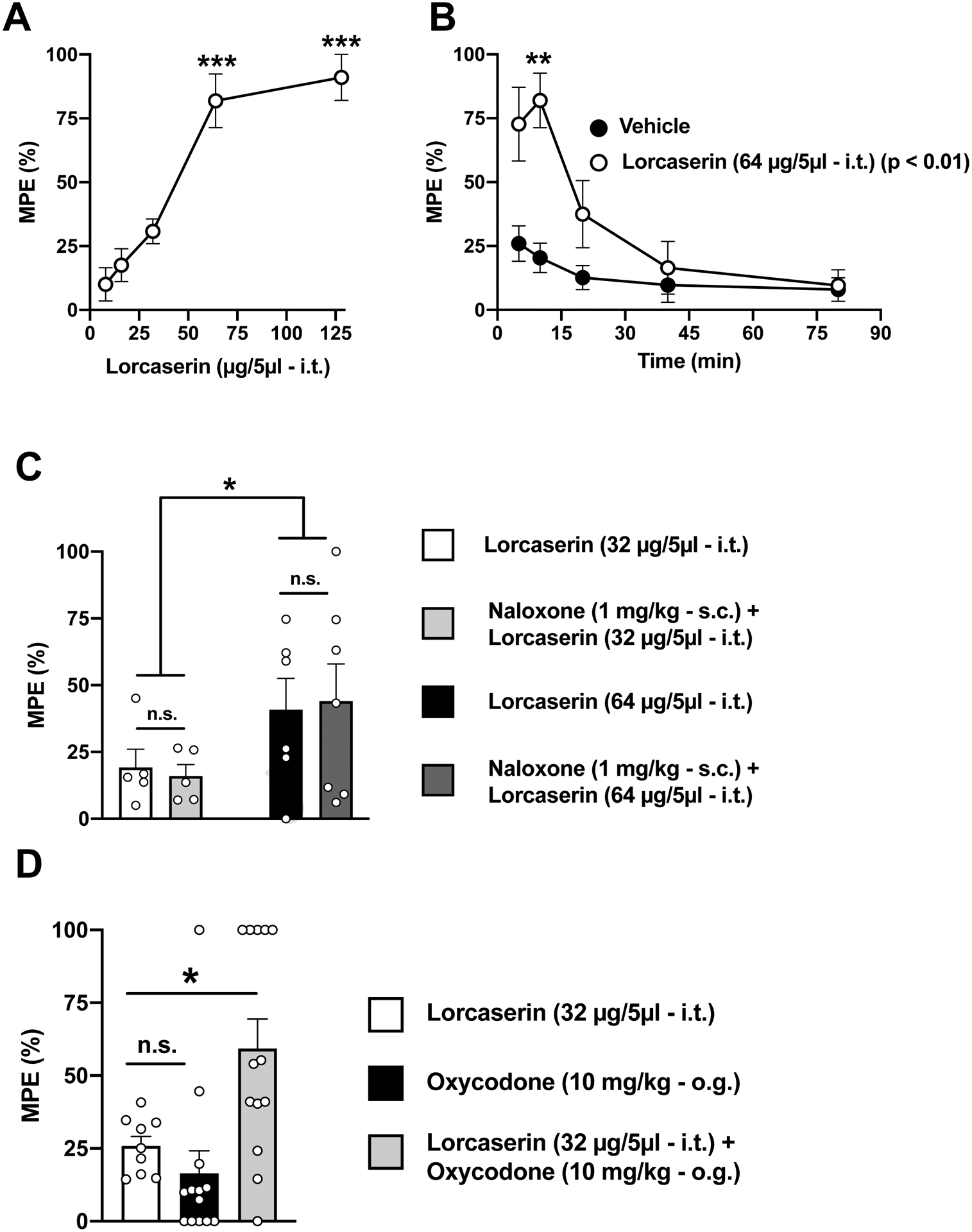
(A) Dose-dependent antinociceptive effect of lorcaserin (i.t.) in the TFR test (n = 5–8). Data were evaluated with a one-way ANOVA (F[4,21] = 24.98, p < 0.001) followed by Dunnet’s post hoc test. Asterisks in the graph show post hoc p values comparing different doses against the lowest dose. (B) Time-course of the effect of lorcaserin (i.t.) on the TFR test with peak effects at ~10 min (n = 6–7). Data were evaluated with a two-way repeated measures ANOVA (vehicle/lorcaserin × time) followed by Dunnet’s post hoc test. Lorcaserin, F[1,10] = 12.23, p < 0.01; time, F[1.4,14.02] = 13, p < 0.01; interaction of lorcaserin and time, F[4,40] = 5.42, p < 0.01. Asterisks in the graph show post hoc p values comparing vehicle versus lorcaserin at different time points. (C) Lack of effect of naloxone (s.c.) on lorcaserin (i.t.)-induced antinociception in the TFR test (n = 5–7). Data were evaluated with a two-way ANOVA (dose of lorcaserin, F[1,19] = 4.86, p < 0.05; naloxone, F[1,19] = 8.52 × 10−11, p > 0.05; interaction of lorcaserin and naloxone, F[1,19] = 0.08, p > 0.05). (D) A subthreshold dose (32 μg) of lorcaserin (i.t.) augments oxycodone (o.g)-induced antinociception (n = 9–13). Data were evaluated with a one-way ANOVA (F[2,32] = 7.84, p < 0.01) followed by Dunnet’s post hoc test. *p<0.05, **p<0.01 ***p<0.001. n.s., not significant. Data are mean ± S.E.M response latencies expressed as percentage of MPE for the different experimental conditions.
3.2. Subcutaneous administration of lorcaserin lacks antinociceptive effects, but augments opioid-induced antinociception via 5-HT2C receptors
We tested the effect of the subcutaneous (s.c.) administration of lorcaserin on the TFR test. Our time-course data show that lorcaserin (s.c.) alone did not affect TFR test at any of the doses, as compared to vehicle (Fig. 2A) (F[15,65] = 1.366, p > 0.05). However, as expected based on previous findings by other groups (Rezvani et al., 2014), these doses affected locomotion in a dose-dependent manner (Fig. S1). We next evaluated the effect of several doses (0.25 – 4 mg/kg) of lorcaserin (s.c.) in combination with oxycodone (10 mg/kg, o.g.). Interestingly, there was a dose-dependent significant effect of lorcaserin (s.c.) that shifted to the left (higher potency) the dose-response curve of oxycodone (Fig. 2B and Fig. S2A) (lorcaserin, F[5,50] = 3.77, p < 0.01). Additionally, lorcaserin (0.5 and 2.0 mg/kg, s.c.) also increased the time-course antinociceptive effect of oxycodone (10 mg/kg, o.g.) (Fig. 2C) (F[2,25] = 8.36, p < 0.01).
Fig. 2. Effect of lorcaserin (s.c.) on the TFR test.
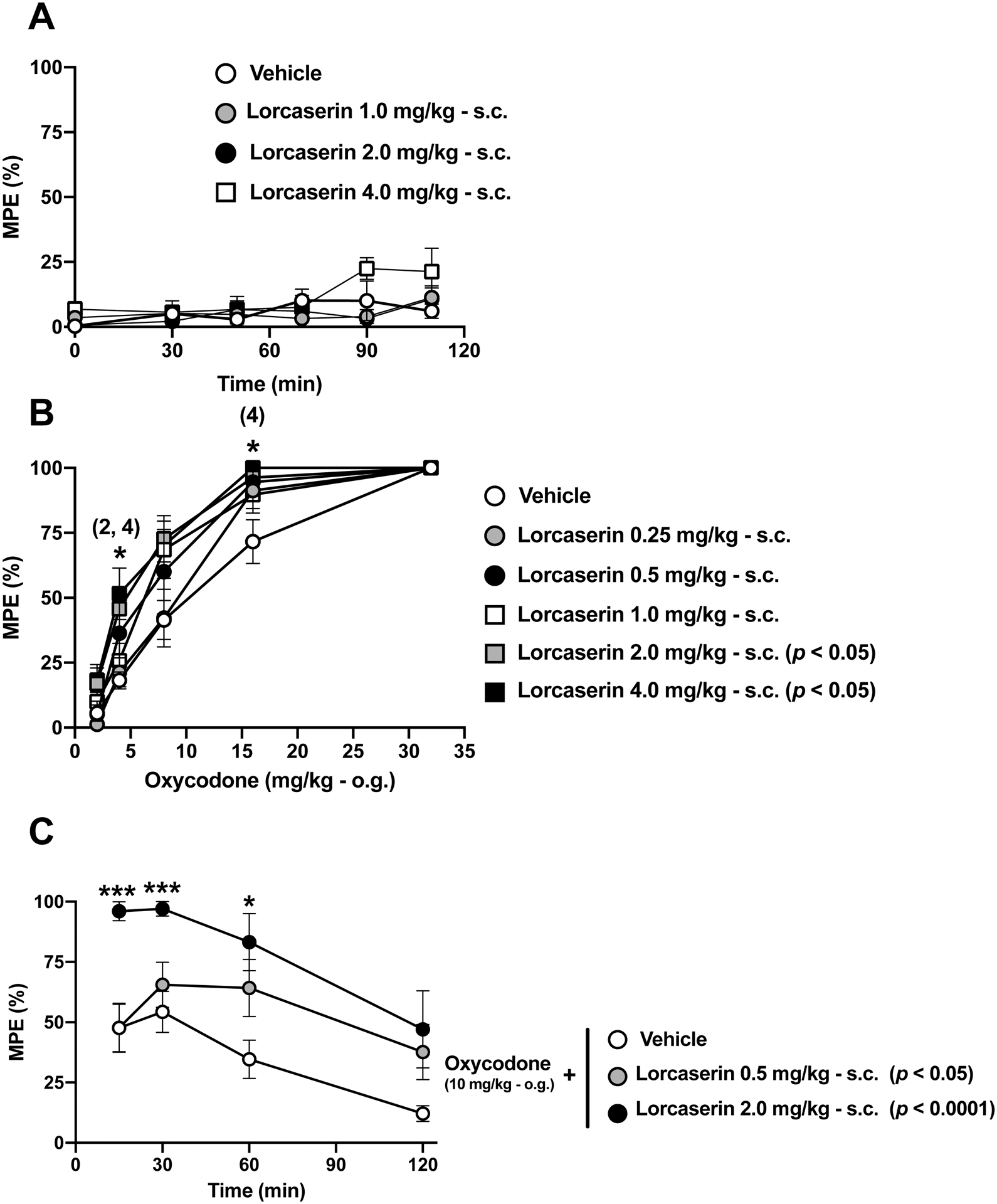
(A) Absence of effect of lorcaserin (s.c.) on the TFR test (n = 4–5). Lorcaserin, F[3,13] = 1.44, p > 0.05; time, F[2.99,38.96] = 4.27, p < 0.05; interaction of lorcaserin and time, F[15,65] = 1.36, p > 0.05. (B) Lorcaserin (s.c.) augments oxycodone (o.g.)-induced antinociception (n = 4–15). Lorcaserin, F[5,50] = 3.77, p < 0.01; time, F[3.05,152.4] = 207, p < 0.001; interaction of lorcaserin and time, F[20,200] = 1.576, p > 0.05. (C) Time-course of the effect of lorcaserin (s.c.) on oxycodone (10 mg/kg, o.g.)-induced antinociception (n = 5–13). Lorcaserin, F[2,25] = 8.36, p < 0.01; time, F[2.64,66.15] = 11.72, p < 0.001; interaction of lorcaserin and time, F[6,75] = 1.18, p > 0.05. All the data were evaluated with a repeated measures two-way ANOVA (vehicle/lorcaserin × time or vehicle/lorcaserin ´ oxycodone) followed by Dunnett’s multiple comparison test. Asterisks in B show post hoc p values comparing different doses of lorcaserin against its vehicle at different doses of oxycodone. Numbers between brackets in B show the dose of lorcaserin in which a statistically significant post hoc test comparing different doses of lorcaserin against its vehicle at different doses of oxycodone was observed. Asterisks in C show post hoc p values comparing different doses of lorcaserin against vehicle at different time points. Next to the doses of lorcaserin, post hoc p values comparing different doses against its vehicle are depicted. *p<0.05, ***p<0.001. Data are mean ± S.E.M response latencies expressed as percentage of MPE for the different experimental conditions.
This effect of adjunctive lorcaserin (s.c.) on oxycodone-induced time-course antinociceptive effect was prevented by the 5-HT2CR antagonist SB242084 (Fig. 3) (F[3,34] = 11.3, p < 0.001). The 5-HT2CR antagonist alone, however, did not affect the antinociceptive effect induced by oxycodone (Fig. 3).
Fig. 3. Adjunct effect of lorcaserin on oxycodone-induced antinociception in the TFR test is blocked by the 5-HT2CR antagonist SB242084.
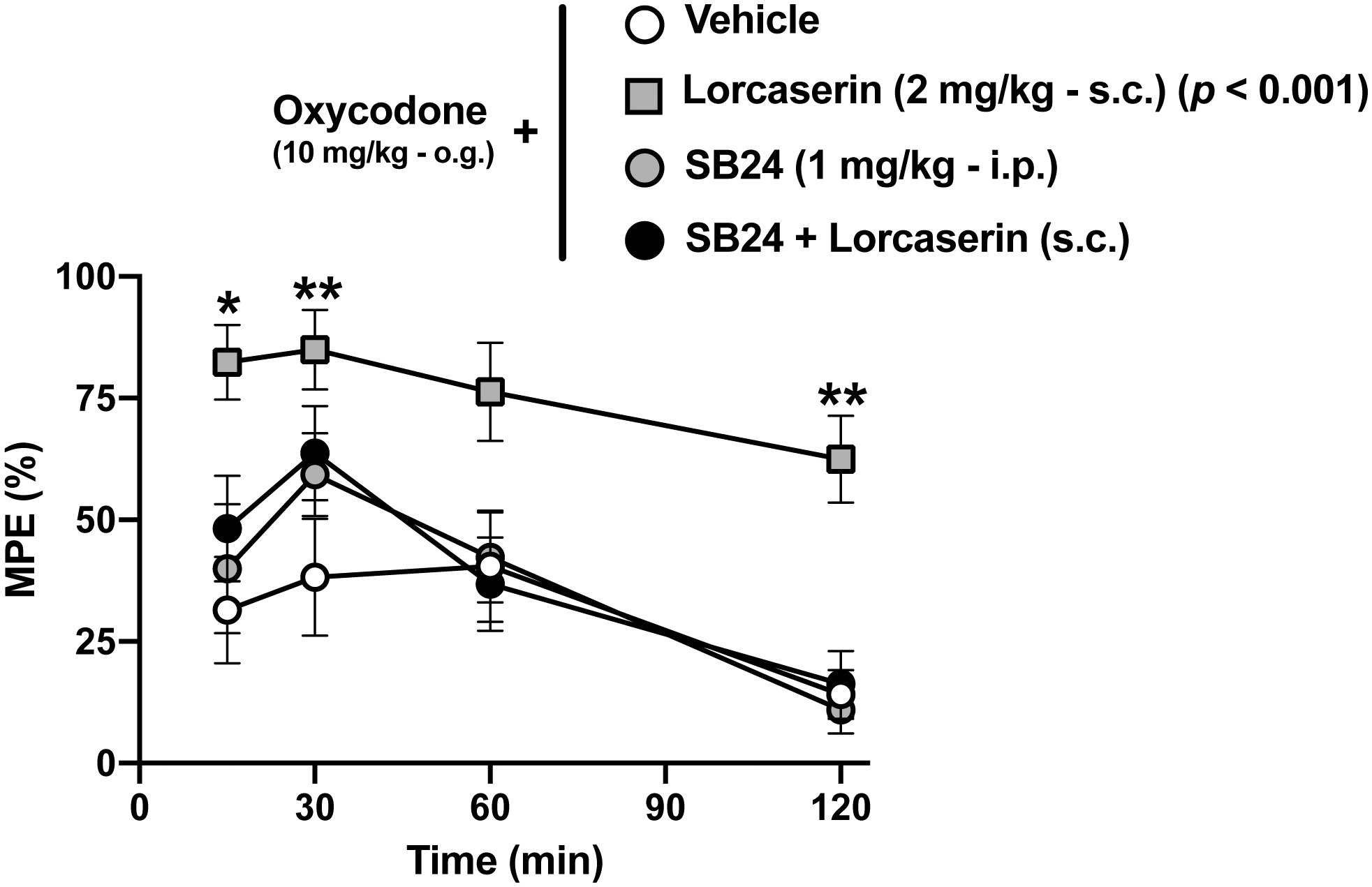
Time-course of the effect of lorcaserin (1 mg/kg, s.c.) on oxycodone (10 mg/kg, o.g.)-induced antinociception in the presence or in the absence of SB242084 (1 mg/kg, i.p.) (n = 9–10). Drugs, F[3,34] = 11.3, p < 0.001; time, F[2.85, 96.97] = 12.07, p < 0.001; interaction of drug and time, F[9,102] = 0.68, p > 0.05. Data were evaluated with repeated measures two-way ANOVA (vehicle/lorcaserin/SB24 × time) followed by Dunnett’s multiple comparison test. Asterisks in the graph show post hoc p values comparing drug treatments against vehicle at different time points. Next to lorcaserin and/or SB24, post hoc p values comparing different drug treatments against vehicle are depicted. *p<0.05, **p<0.01. Data are mean ± S.E.M response latencies expressed as percentage of MPE for the different experimental conditions.
We next compared the adjunctive effects of lorcaserin on oxycodone-induced antinociception with those induce by the opioid analgesics morphine and fentanyl. Our data show that the analgesic-like effects of both cumulative morphine (s.c.) (Fig. 4A and Fig. S2B) and cumulative fentanyl (s.c.) (Fig. 4B and Fig. S2C) on the TFR test were increased by the 5-HT2CR agonist lorcaserin (effect of lorcaserin on morphine-induced antinociception: F[2,25] = 7.449, p < 0.01; effect of lorcaserin on fentanyl-induced antinociception: F[2,24] = 4.35, p < 0.05).
Fig. 4. Adjunct effect of lorcaserin antinociceptive effect on morphine- and fentanyl-induced antinociception in TFR test.
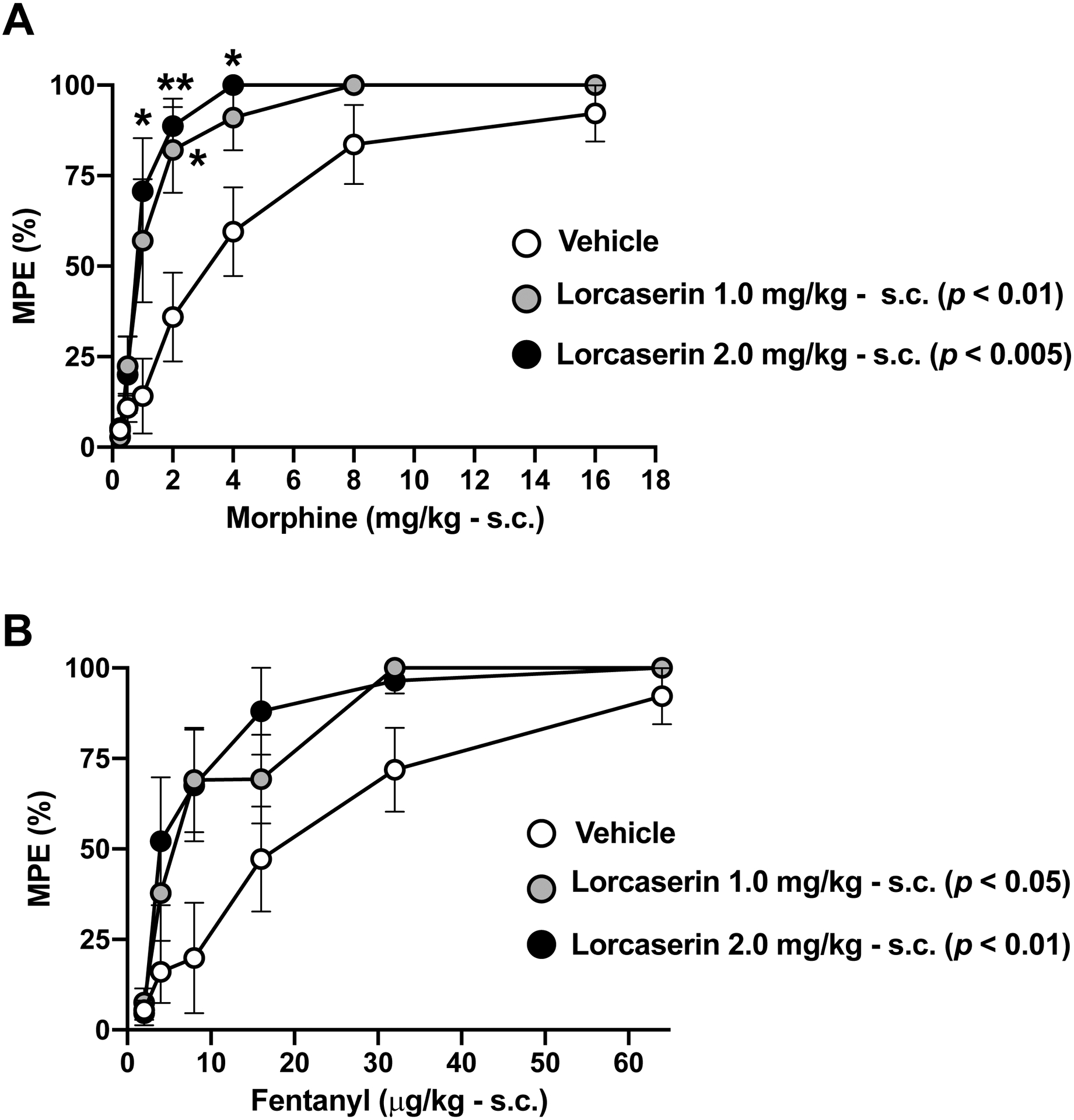
(A) Lorcaserin (s.c.) augments morphine (s.c.)-induced antinociception (n = 9–10). Lorcaserin, F[2,25] = 7.44, p < 0.01; time, F[3.27,81.71] = 72.68, p < 0.001; interaction of lorcaserin and time, F[12,150] = 2.74, p < 0.01. (B) Lorcaserin (s.c.) augments fentanyl (s.c.)-induced antinociception (n = 8–10). Lorcaserin, F[2,24] = 4.35, p < 0.05; time, F[2.92, 70.19] = 44.93, p < 0.001; interaction of lorcaserin and time, F[10,120] = 1.61, p > 0.05. Data were evaluated with repeated measures two-way ANOVA (vehicle/lorcaserin × morphine or vehicle/lorcaserin × fentanyl) followed by Dunnett’s multiple comparison test. Asterisks in the graph show post hoc p values comparing drug treatments against vehicle at different doses of morphine (A) or fentanyl (B). Next to the doses of lorcaserin, post hoc p values comparing different doses against its vehicle are depicted. *p<0.05, **p<0.01. Data are mean ± S.E.M response latencies expressed as percentage of MPE for the different experimental conditions.
3.3. Lorcaserin (s.c.) preferentially accumulates in the CNS and does not affect plasma or brain concentrations of oxycodone
Next, we studied the distribution of lorcaserin and its effects on the concentrations of oxycodone in the CNS and plasma. Using UPLC-MS/MS assays 30 and 120 min after drug(s) administration, we found that lorcaserin is preferentially accumulated in brain and spinal cord, as compared to plasma (Fig. 5A) (p < 0.001). Additionally, the concentrations of oxycodone in either brain (Fig. 5B) or plasma (Fig. 5C) were unaffected by lorcaserin (s.c.) injection. As expected, concentrations of both lorcaserin and oxycodone in either CNS or plasma were lower 120-min after injection (s.c.) (Figs. 5A–C) (lorcaserin: F[1,44] = 107.0, p < 0.001; oxycodone-brain: F[1,28] = 5.9, p < 0.05; oxycodone-plasma: F[1,32] = 14.30, p < 0.001).
Fig. 5. Lorcaserin preferentially accumulates in the CNS relative to plasma upon subcutaneous administration.
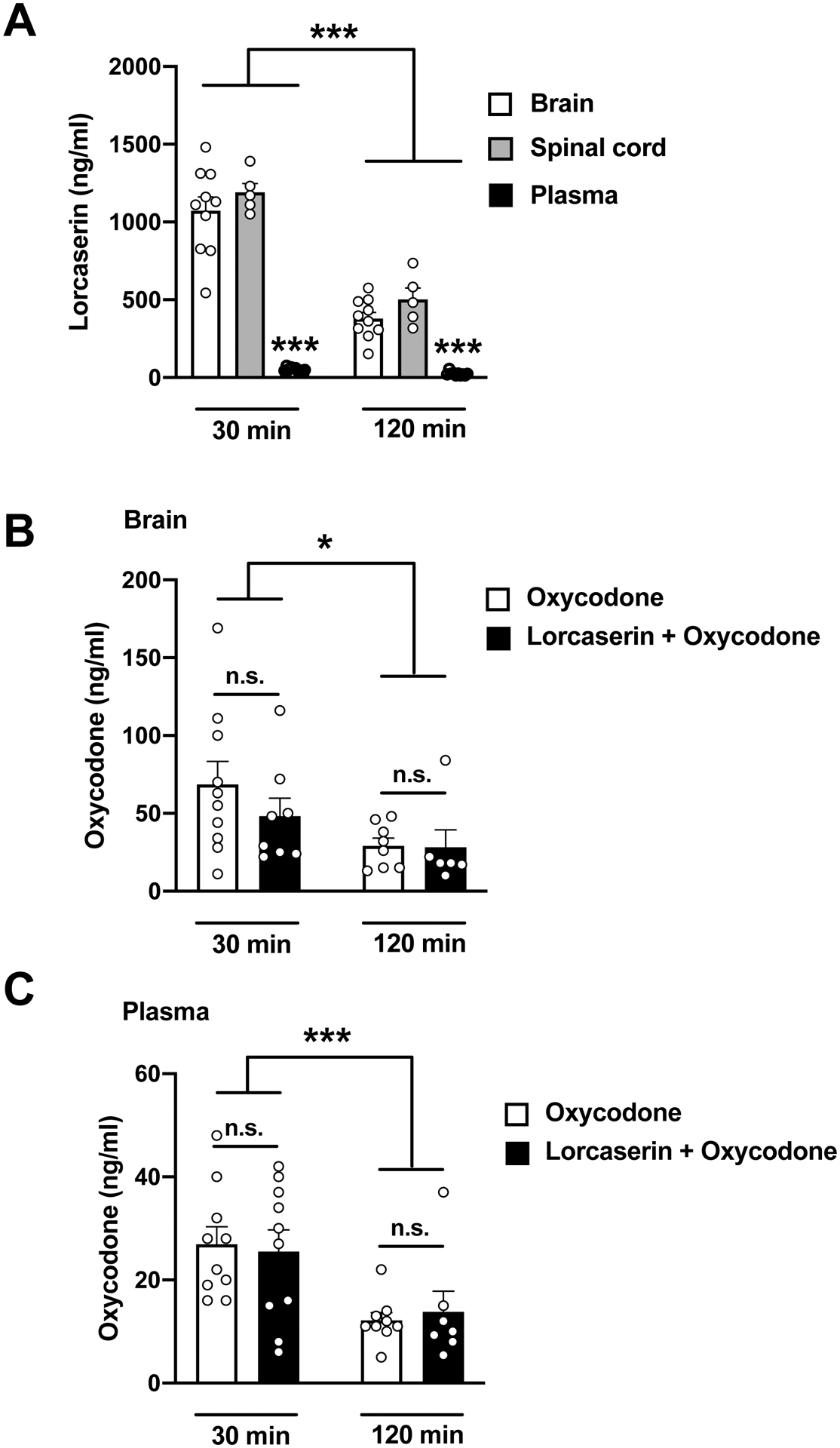
(A) Brain, spinal cord, and plasma concentrations of lorcaserin 30 and 120 min after a single administration (s.c., 2 mg/kg) (n = 5–10). Tissue, F[2,44] = 140, p < 0.001; time, F[1,44] = 107, p < 0.001; interaction of tissue and time, F[2,44] = 28.71, p < 0.001. (B) Brain concentrations of oxycodone after a single dose of oxycodone (o.g., 10 mg/kg) alone, or together with lorcaserin (s.c., 2 mg/kg) (n = 6–10). Drug F[1,28] = 0.75, p >0.05 ; time, F[1,28] = 5.91, p < 0.05; interaction of drug and time, F[1,28] = 0.62, p >0.05. (C) Plasma concentrations of oxycodone after a single dose of oxycodone (o.g., 10 mg/kg) alone, or together with lorcaserin (s.c., 2 mg/kg) (n = 7–10). Drug F[1,32] = 0.002, p >0.05 ; time, F[1,232] = 14.30, p < 0.001; interaction of drug and time, F[1,32] = 0.19, p > 0.05. Data were evaluated with a two-way ANOVA followed by Dunnett’s multiple comparison test. *p<0.05, ***p<0.001. n.s., not significant. Data are mean ± S.E.M
4. Discussion
The main goal of our study was to determine the antinociceptive effects of lorcaserin and its potential use as an adjunctive non-opioid analgesic agent in rodents. We show that lorcaserin, when injected locally (i.t.), produced antinociceptive effects in a dose-dependent manner with a peak action after 10 min of injection; and that this effect was opioid receptor-independent. On the contrary, when lorcaserin is injected systemically (s.c.) up to 4 mg/kg, it did not show any significant antinociceptive effect on the TFR test. Interestingly, however, lorcaserin (s.c.) potentiated oxycodone-induced antinociception in a dose-dependent manner; a similar adjunctive effect was observed when morphine and fentanyl were administered. Furthermore, administration of lorcaserin (s.c.) did not significantly affect the distribution of oxycodone within the brain or spinal cord.
Opioid antinociception is mediated through stimulation of central (PAG, nucleus reticularis paragigantocellularis and dorsal horn) and peripheral (afferent nociceptive fibers) mechanisms (Pathan and Williams, 2012) . It is well established that the serotonin and opioid systems share similar anatomical locations within the PAG (Basbaum and Fields, 1984) and the dorsal horn of the spinal cord (Song et al., 2007). Within the dorsal horn, opioid receptors are located both pre- and postsynaptically (Fields et al., 1980; Besse et al., 1990), preventing transmission of noxious information from Ad and C fibers. Similarly, serotonin receptors are present in primary afferent terminals and interneurons facilitating or inhibiting nociceptive transmission depending on the receptor subtype. For instance, noxious stimuli activate opioidergic neurons in the PAG, which in turn modulate serotonergic projections to supraspinal nuclei including the nucleus accumbens and amygdala (Ma and Han, 1992; Grahn et al., 1999). In addition, morphine administration increases serotonin in the spinal cord (Kimura et al., 2014). By contrast, the activation of 5-HT1A receptor in the spinal cord and hypothalamus inhibits the release of endogenous opioids (Song et al., 2007) . Moreover, targeting the 5-HT3 receptor can attenuate or enhance morphine-induced analgesia depending on the animal model tested (Kimura et al., 2014). In the present study, we showed that lorcaserin, a highly selective 5-HT2C receptor agonist, induced a dose-dependent antinociceptive effect when administered locally at the spinal canal, whereas systemic lorcaserin administration did not elicit a significant antinociceptive response. Additionally, we found that activation of the 5-HT2C receptor upon lorcaserin administration augmented opioid-mediated antinociceptive effects. This adjunctive effect of lorcaserin was observed via both parenteral injection (s.c.) and local administration at the spinal canal (i.t.). Early reports proved the effectiveness of 5-HT2C receptor agonism in nicotine, cocaine and alcohol addiction (reviewed in Palacios et al., 2017), and a recent study showed that lorcaserin administration inhibits oxycodone intake in rats (Neelakantan et al., 2017). Our findings presented here have important implications for the development of adjunctive therapies to improve opioid-induced analgesia.
Intrathecal delivery bypasses the blood brain barrier (BBB) and provides direct access to the spinal canal. This route of administration allows for pharmacological investigations at local neural circuits in the lumbar spinal cord (Lu and Schmidtko, 2013). Our data here show the interesting finding that intrathecal lorcaserin administration alone produced an opioid-independent dose-dependent antinociceptive effect in mice. Similar antinociceptive phenotypes have been observed by administration of 5-HT2C receptor agonists directly at the brainstem (Baptista-de-Souza et al., 2014) and amygdala (Tavares et al., 2018). Previous observations suggest that the 5-HT2C receptor is expressed postsynaptically in the dorsal horn of the spinal cord, probably within GABAergic interneurons (Fonseca et al., 2001; Nakae et al., 2008; Tysseling et al., 2017), thereby modulating synaptic transmission (Takazawa and MacDermott, 2010). These antinociceptive effects of 5-HT2C receptor agonists were also previously proven in murine models of trigeminal and spinal nerve ligation (Nakai et al., 2010; Obata et al., 2004) . Interestingly, while the 5-HT2C receptor is not present in naïve DRGs, previous findings demonstrate that de novo 5-HT2C receptor expression following treatment with complete Freund’s adjuvant (CFA) as a rodent model of inflammatory pain (Wu et al., 2001) might be responsible for neuron hyperexcitability through the sensitization of TRPV1 channels (Salzer et al., 2016) . Together, these data suggest that activation of 5-HT2C receptor under inflammatory conditions may increase rather than alleviate nociception. However, to our knowledge, intrathecal injection of selective 5-HT2C agonists has not been tested in this animal model of inflammatory pain. Additionally, in our study we found that sub-analgesic doses of intrathecal lorcaserin increased the antinociceptive effects of oxycodone. Whether this adjunctive effect via i.t. administration is due to an additive or synergistic mechanism remains to be investigated. Still, these findings advocate for a μ-opioid-5-HT2C receptor crosstalk at the spinal cord.
A more valuable clinical approach may be the systemic administration of a drug that potentiates the effects of opioids thereby reducing their dosage (i.e., opioid sparing effect). Interestingly, we show that concomitant administration of lorcaserin dose- dependently potentiated the antinociceptive effect of commonly used opioids including oxycodone, morphine and fentanyl, an adjunctive effect that lasted at least two hours. Since this effect was attenuated with the 5-HT2C antagonist SB242084, we can conclude that these opioid-sparing effects are most probably mediated via 5-HT2C receptor activation. Additionally, and opposite to that observed via intrathecal administration, we also found here that subcutaneous administration of lorcaserin alone (up to 4 mg/kg) did not affect the tail-flick response. Considering our findings showing the effect of lorcaserin (s.c.) on locomotor activity and the distribution of the drug in the CNS, we speculate that the lack of antinociceptive response by lorcaserin (s.c.) cannot be attributed to a restriction in BBB diffusion. It is possible that 5-HT2C receptor activation in the periphery counteracts the antinociceptive effects of the centrally administered drug (Tian et al., 2016), but further investigation will be needed to address this question.
Drugs targeting both serotonin and noradrenaline transporters (SNRIs) such as duloxetine have proven efficacy and are recommended in neuropathic pain (Attal, 2019a, b). On the contrary, selective serotonin transporter inhibitors have shown a modest clinical analgesic effect and thus are not indicated in pain management (Finnerup, et al. 2015). However, it is well established that serotonin receptors are involved in modulating nociceptive processing along the neuroaxis including spinal afferences and descending modulatory pathways from more complex neural circuits within the CNS (Ji et al., 2017; Kiefel et al., 1992; Lin et al., 2011). A reason that can explain this apparent paradox lies in the fact that different serotonin receptors, such as 5-HT2A and 5-HT2C, can exert opposite actions despite their similar cellular distributions and signaling pathways downstream. Thus, their concomitant activation by the endogenous ligand serotonin may blur the contribution of each receptor to serotonin-dependent transmission. For instance, while intrathecal serotonin administration shows a net antinociceptive effect (Bardin et al., 1997) , ex vivo treatment of dorsal root ganglion (DRG) explants with serotonin increases the excitability of DRG neurons via 5-HT2 receptors (Salzer et al., 2016) . The 5-HT2A/2C agonist DOI exerts pronociceptive responses (Rahman et al., 2011) . On the contrary, the 5-HT2A/2C agonist α-methyl-5-HT exerts antinociceptive effects in a rodent model of inflammatory and neuropathic pain (Sasaki et al., 2003) . This apparent contradiction may be due to pain-induced changes in expression of serotonin receptors that cause a disruption of the physiological nociceptive mechanisms under pathological conditions (Wu et al., 2001). Our findings here, together with previous findings by other groups, suggest that activation of either 5-HT2C or 5-HT2A receptors produces antinociceptive (Nakai et al., 2010) or pronociceptive (Rahman et al., 2011) effects, respectively. Additional work will be needed to better characterize the signaling pathway and neural/glial circuits responsible for the unique effects of 5-HT2C vs 5-HT2A receptor agonism on preclinical models of nociception.
However, some limitations should be noted. First, since lorcaserin induced a decrease in locomotor activity, we cannot exclude the possibility that the tail-flick responses observed in our study might be biased by the effect of lorcaserin in spinal cord motor efferent neurons. Second, our study is limited to male mice, while a growing body of work suggests that differences in responsivity to analgesic treatment is sex dependent (Bartley et al. 2013). For instance, one study showed that male rats require a lower dose of morphine in the hot plate test compared to female rats while no differences were found in the tail-flick assay (South et al. 2009). In regards to the serotonin system, Lei et al. found opposite roles of serotonin on descending inhibitory pathways in males and females (Lei et al. 2011). Future studies will explore whether lorcaserin induces an increase in opioid-induced antinociception in a sex-dependent manner.
In conclusion, we provide evidence for the antinociceptive role of spinal 5-HT2C receptors on the warm-water tail withdrawal assay in mice. More importantly, systemic co-administration of lorcaserin with an opioid drug produced a dose-dependent augmentation of opioid-mediated antinociception. Together, these data pave the way for the clinical evaluation of lorcaserin as a potential opiate adjunct. Numerous studies reveal a possible association of family A GPCRs into dimers/oligomers (Gonzalez-Maeso, 2011; Gomes et al., 2016). Whether this antinociceptive potentiation is due to independent actions at different CNS levels or consequence of a direct physical interaction via GPCR heteromerization still remains to be investigated.
Supplementary Material
Supplementary Fig S1. Effect of lorcaserin on locomotor activity. Mice were injected (s.c.) with lorcaserin (0.5, 1.0, or 2.0 mg/kg), or vehicle (n = 4–5). Immediately after injection, mice were placed in the locomotor chamber where locomotor activity was measured for 40 min. (A) Cumulative ambulatory counts of lorcaserin-induced locomotion in 5 min blocks. Data were evaluated with repeated measures two-way ANOVA (vehicle/lorcaserin × time) followed by Dunnett’s multiple comparison test. Lorcaserin, F[3,15] = 5.43, p < 0.01; time, F[1.35, 20.35] = 147.2, p < 0.001; interaction of lorcaserin and time, F[21,105] = 15.76, p < 0.001. (B) Data summary of the total lorcaserin-induced locomotion. Data were evaluated with a one ANOVA (vehicle × lorcaserin) followed by Dunnett’s multiple comparison test. Lorcaserin, F[3,15] = 9.32, p < 0.01. Asterisks in the graphs show post hoc p values comparing drug treatments against vehicle at different time points (A) or at different doses (B). Numbers between brackets in A show the dose of lorcaserin in which a statistically significant post hoc test comparing different doses of lorcaserin against vehicle at different time points was observed. Next to the doses of lorcaserin, post hoc p values comparing different doses against its vehicle are depicted. *p < 0.05, **p < 0.01, ***p<0.001. Data are mean ± S.E.M.
Supplementary Fig S2. Percentage of animals exhibiting at any point the cut-off latency for vehicle and different doses (s.c.) of lorcaserin (1.0, or 2.0 mg/kg) and cumulative doses of oxycodone (A), morphine (B) and fentanyl (C).
HIGHLIGHTS.
Intrathecal administration of lorcaserin induces antinociception whereas subcutaneous injection lacks antinociceptive effect
Adjunctive subcutaneous administration of lorcaserin augments opioid-induced antinociception
These included oxycodone, morphine and fentanyl.
Adjunctive antinociceptive effect of lorcaserin is prevented by a 5-HT2C receptor antagonist
Lorcaserin may serve as a new approach to augment opioid-induced analgesia
Funding
NIH P30 DA033934 (W.L.D), NIH R01 DA036975 (W.L.D.), NIH T32 DA007027 (W.L.D), NIH R01 MH084894 (J.G.-M.), and NIH R01 MH111940 (J.G.-M) participated in the funding of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The Authors report no conflict of interest
References
- Attal N, Didier B, 2019a. Translational neuropathic pain research. Pain 160, S23–S28. doi: 10.1097/j.pain.0000000000001522 [DOI] [PubMed] [Google Scholar]
- Attal N, 2019b. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev Neurol (Paris) 175, 46–50. Doi: 10.1016/j.neurol.2018.08.005. Epub 2018 Oct 11 [DOI] [PubMed] [Google Scholar]
- Backstrom JR, Westphal RS, Canton H, Sanders-Bush E, 1995. Identification of rat serotonin 5-HT2C receptors as glycoproteins containing N-linked oligosaccharides. Brain Res. Mol. Brain Res 33, 311–318. doi: 10.1016/0169-328x(95)00156-m [DOI] [PubMed] [Google Scholar]
- Baptista-de-Souza D, Di Cesare Mannelli L, Zanardelli M, Micheli L, Nunes-de-Souza RL, Canto-de-Souza A, Ghelardini C, 2014. Serotonergic modulation in neuropathy induced by oxaliplatin: effect on the 5HT2C receptor. Eur. J. Pharmacol 735, 141–149. doi: 10.1016/j.ejphar.2014.04.028 [DOI] [PubMed] [Google Scholar]
- Bardin L, Bardin M, Lavarenne J, Eschalier A, 1997. Effect of intrathecal serotonin on nociception in rats: influence of the pain test used. Exp. Brain Res 113(1): 81–7 [DOI] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB, 2013. Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth 111 (1), 52–8. doi: 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL, 1984. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci 7: 309–38 [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM, 1990. Pre- and postsynaptic location of mu, delta and kappa opioid receptors in the superficial layers of the dorsal horn of the rat spinal cord. Prog. Clin. Biol. Res 328: 183–6 [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC, 2000. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39, 123–132. doi: 10.1016/s0028-3908(99)00086-6 [DOI] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP, 2016. Lorcaserin Reduces the Discriminative Stimulus and Reinforcing Effects of Cocaine in Rhesus Monkeys. J. Pharmacol. Exp. Ther 356, 85–95. doi: 10.1124/jpet.115.228833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluzzi F, Bifulco F, Cuomo A, Dauri M, Leonardi C, Melotti RM, Natoli S, Romualdi P, Savoia G, Corcione A, 2017. The challenge of perioperative pain management in opioid-tolerant patients. Ther. Clin. Risk. Manag 13, 1163–1173. doi: 10.2147/TCRM.S141332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Uram M, Perni VC, Weaver MF, Spanos LJ, 1991. Serotonin contributes to the spinal antinociceptive effects of morphine. Pharmacol. Biochem. Behav 39, 591–595. [DOI] [PubMed] [Google Scholar]
- Danneman PJ, Kiritsy-Roy JA, Morrow TJ, Casey KL, 1994. Central delay of the laser-activated rat tail-flick reflex. Pain 58, 39–44. doi: 10.1016/0304-3959(94)90183-x [DOI] [PubMed] [Google Scholar]
- D’ Amour FE, Smith DL, 1941. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther 72, 74. [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Nuite JA, 1970. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J. Pharmacol. Exp. Ther 175, 435–442. [PubMed] [Google Scholar]
- Eide PK, Hole K, 1991. Different role of 5-HT1A and 5-HT2 receptors in spinal cord in the control of nociceptive responsiveness. Neuropharmacology 30, 727–731. [DOI] [PubMed] [Google Scholar]
- Fasmer OB, Berge OG, Post C, Hole K, 1986. Effects of the putative 5-HT1A receptor agonist 8-OH-2-(di-n-propylamino)tetralin on nociceptive sensitivity in mice. Pharmacol. Biochem. Behav 25, 883–888. doi: 10.1016/0091-3057(86)90402-8 [DOI] [PubMed] [Google Scholar]
- Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM, BLOSSOM Clinical Trial Group, 2011. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab 96, 3067–3077. doi: 10.1210/jc.2011-1256 [DOI] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL, 1980. Multiple opiate receptor sites on primary afferent fibres. Nature. 27; 284 (5754): 351–3. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M, 2015. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14 (2), 162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Ni YG, Dunning DD, Miledi R, 2001. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Brain Res. Mol. Brain Res 18; 89 (1–2): 11–9 [DOI] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, Maguire DR, France CP, 2019. Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp. Clin. Psychopharmacol 27 (1), 78–86. doi: 10.1037/pha0000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Berger A, 2014. Opioids, adjuvants, and interventional options for pain management of symptomatic metastases. Ann. Palliat. Med 3, 172–191. doi: 10.3978/j.issn.2224-5820.2014.07.07 [DOI] [PubMed] [Google Scholar]
- Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KDG, Devi LA, 2016. G Protein-Coupled Receptor Heteromers. Annu. Rev. Pharmacol. Toxicol 56, 403–425. doi: 10.1146/annurev-pharmtox-011613-135952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, 2011. GPCR oligomers in pharmacology and signaling. Mol. Brain 4, 20–7. doi: 10.1186/1756-6606-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn RE, Maswood S, McQueen MB, Watkins LR, Maier SF, 1999. Opioid-dependent effects of inescapable shock on escape behavior and conditioned fear responding are mediated by the dorsal raphe nucleus. Behav. Brain Res 99(2):153–67 [DOI] [PubMed] [Google Scholar]
- Hall JG, Duggan AW, Morton CR, Johnson SM, 1982. The location of brainstem neurones tonically inhibiting dorsal horn neurones of the cat. Brain Res. 29; 244(2): 215–22 [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK, 1964. Some narcotic antagonists in the benzomorphan series. J. Pharmacol. Exp. Ther 143, 141–148. [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ, 2012. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37, 1177–1191. doi: 10.1038/npp.2011.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR, 2002. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav 71, 533–554. doi: 10.1016/s0091-3057(01)00746-8 [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL, 1983. Intrathecal serotonin in mice: analgesia and inhibition of a spinal action of substance P. Life Sci. 33, 789–795. [DOI] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS, 2018. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 361, eaau1184. doi: 10.1126/science.aau1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Zhang W, Mahimainathan L, Narasimhan M, Kiritoshi T, Fan X, Wang J, Green TA, Neugebauer V, 2017. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci 8;37(6):1378–1393. doi: 10.1523/JNEUROSCI.2468-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefel JM, Cooper ML, Bodnar RJ, 1992. Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res. 4;597(2):331–8 [DOI] [PubMed] [Google Scholar]
- Kimura M, Obata H, Saito S, 2014. Peripheral Nerve Injury Reduces Analgesic Effectsof Systemic Morphine via Spinal 5-Hydroxytryptamine 3 Receptors. Anesthesiology 121 (2): 362–71 [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, 2018. Lorcaserin decreases the reinforcing effects of heroin, but not food, in rhesus monkeys. Eur. J. Pharmacol 840, 28–32. doi: 10.1016/j.ejphar.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Kirksey MA, Duong S, Wu CL, 2017. A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth. Analg 125, 1749–1760. doi: 10.1213/ANE.0000000000002497 [DOI] [PubMed] [Google Scholar]
- Lei J, Jin L, Zhao Y, Sui MY, Huang L, Tan YX, Chen YK, You HJ, 2011. Sex-related differences in descending norepinephrine and serotonin controls of spinal withdrawal reflex during intramuscular saline induced muscle nociception in rats. Exp. Neurol 228 (2), 206–14. doi 10.1016/j.expneurol.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH, 2011. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hiperalgesia. J. Neurosci 26;31(4):1410–8. doi: 10.1523/JNEUROSCI.4682-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Schmidtko A, 2013. Direct intrathecal drug delivery in mice for detecting in vivo effects of cGMP on pain processing. Methods Mol. Biol 1020: 215–21. doi: 10.1007/978-1-62703-459-3_14, [DOI] [PubMed] [Google Scholar]
- Ma QP, Han JS, 1992. Neurochemical and morphological evidence of an antinociceptive neural pathway from nucleus raphe dorsalis to nucleus accumbens in the rabbit. Brain Res. Bull 28 (6): 931–6 [DOI] [PubMed] [Google Scholar]
- Morton CR, Johnson SM, Duggan AW, 1983. Lateral reticular regions and the descending control of dorsal horn neurones of the cat: selective inhibition by electrical stimulation. Brain Res. 19;275(1):13–21 [DOI] [PubMed] [Google Scholar]
- Nakae A, Nakai K, Tanaka T, Takashina M, Hagihira S, Shibata M, Ueda K, Mashimo T, 2008. Serotonin2C receptor mRNA editing in neuropathic pain model. Neurosci. Res 60 (2): 228–31 [DOI] [PubMed] [Google Scholar]
- Nakai K, Nakae A, Oba S, Mashimo T, Ueda K, 2010. 5-HT2C receptor agonists attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Eur. J. Pain 14, 999–1006. doi: 10.1016/j.ejpain.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA, 2017. Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem. Neurosci 8, 1065–1073. doi: 10.1021/acschemneuro.6b00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Saito S, Sakurazawa S, Sasaki M, Usui T, Goto F, 2004. Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury. Pain 108, 163–169. doi: 10.1016/j.pain.2003.12.019 [DOI] [PubMed] [Google Scholar]
- Ogino S, Nagakura Y, Tsukamoto M, Watabiki T, Ozawa T, Oe T, Shimizu Y, Ito H, 2013. Systemic administration of 5-HT(2C) receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol. Biochem. Behav 108, 8–15. doi: 10.1016/j.pbb.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Palacios JM, Pazos A, Hoyer D, 2017. A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl). 234(9–10):1395–1418. doi: 10.1007/s00213-017-4545-5 [DOI] [PubMed] [Google Scholar]
- Pathan H, Williams J, 2012. Basic opioid pharmacology: an update. Br. J. Pain 6 (1): 11–6. doi: 10.1177/2049463712438493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Hoyer D, Palacios JM, 1984. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur. J. Pharmacol 106, 539–546. doi: 10.1016/0014-2999(84)90057-8 [DOI] [PubMed] [Google Scholar]
- Rahman W, Bannister K, Bee LA, Dickenson AH, 2011. A pronociceptive role for the 5-HT2 receptor on spinal nociceptive transmission: an in vivo electrophysiological study in the rat. Brain Res. 25;1382:29–36. doi: 10.1016/j.brainres.2011.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED, 2014. Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol. Biochem. Behav 125, 8–14. doi: 10.1016/j.pbb.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Salzer I, Gantumur E, Yousuf A, Boehm S, 2016. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca(2+)-activated chloride channels. Neuropharmacology 110(Pt A):277–286. doi: 10.1016/j.neuropharm.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Obata H, Saito S, Goto F, 2003. Antinociception with intrathecal alpha-methyl-5-hydroxytryptamine, a 5-hydroxytryptamine 2A/2C receptor agonist, in two rat models of sustained pain. Anesth. Analg 96(4):1072–8 [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2018. Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep 67, 1419–1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Mickle A, Kannampalli P, Spruell R, McRorie J, Shaker R, Miranda A, 2014. Visceral analgesic effect of 5-HT(4) receptor agonist in rats involves the rostroventral medulla (RVM). Neuropharmacology 79, 345–358. doi: 10.1016/j.neuropharm.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group, 2010. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med 363, 245–256. doi: 10.1056/NEJMoa0909809 [DOI] [PubMed] [Google Scholar]
- Song B, Chen W, Marvizón JC, 2007. Inhibition of opioid release in the rat spinal cord by serotonin 5-HT (1A) receptors. Brain Res. 16; 1158: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SM, Edwards SR, Smith MT,2009. Antinociception Versus Serum Concentration Relationships Following Acute Administration of Intravenous Morphine in Male and Female Sprague-Dawley Rats: Differences Between the Tail Flick and Hot Plate Nociceptive Tests. Clin. Exp. Pharmacol. Physiol 36 (1), 20–8. doi: 10.1111/j.1440-1681.2008.05019.x [DOI] [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB, 2010. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci 1198: 153–8. doi: 10.1111/j.1749-6632.2010.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares LRR, Baptista-de-Souza D, Canto-de-Souza A, 2018. Activation of 5-HT2C (but not 5-HT1A) receptors in the amygdala enhances fear-induced antinociception: Blockade with local 5-HT2C antagonist or systemic fluoxetine. Neuropharmacology 135: 376–385. doi: 10.1016/j.neuropharm.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D, 2008. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther 325, 577–587. doi: 10.1124/jpet.107.133348 [DOI] [PubMed] [Google Scholar]
- Tian B, Wang XL, Huang Y, Chen LH, Cheng RX, Zhou FM, Guo R, Li JC, Liu T, 2016. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci. Rep 8;6:36286. doi: 10.1038/srep36286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twycross R, Lickiss N, 1996. Pain control and the World Health Organization analgesic ladder. JAMA 275, 835–author reply 836. doi: 10.1001/jama.275.11.835b [DOI] [PubMed] [Google Scholar]
- Tysseling VM, Klein DA, Imhoff-Manuel R, Manuel M, Heckman CJ, Tresch MC, 2017. Constitutive activity of 5-HT2C receptors is present after incomplete spinal cord injury but is not modified after chronic SSRI or baclofen treatment. J. Neurophysiol 118, 2944–2952. doi: 10.1152/jn.00190.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Collins FS, 2017. The Role of Science in the Opioid Crisis. N. Engl. J. Med 377, 1798–1798. doi: 10.1056/NEJMc1711494 [DOI] [PubMed] [Google Scholar]
- Wu S, Zhu M, Wang W, Wang Y, Li Y, Yew DT, 2001. Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund’s adjuvant-induced inflammation. Neurosci. Lett 20: 307(3): 183–6 [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang Y-M, Li G, Xu S, Dong L, Stackman RW, Zhang G, 2015. Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci. Lett 607, 23–28. doi: 10.1016/j.neulet.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Tyce GM, 1979. Microinjection of morphine into the periaqueductal gray evokes the release of serotonin from spinal cord. Brain. Res 171, 176–181. doi: 10.1016/0006-8993(79)90747-9 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Wilson PR, 1979. Spinal serotonin terminal system mediates antinociception. J. Pharmacol. Exp. Ther 208, 446–453. [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang Y-M, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW, 2016. Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology 101, 246–254. doi: 10.1016/j.neuropharm.2015.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RW 4th, Chen ZF, 2007. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc. Natl. Acad. Sci. U S A 104 (36), 14519–14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig S1. Effect of lorcaserin on locomotor activity. Mice were injected (s.c.) with lorcaserin (0.5, 1.0, or 2.0 mg/kg), or vehicle (n = 4–5). Immediately after injection, mice were placed in the locomotor chamber where locomotor activity was measured for 40 min. (A) Cumulative ambulatory counts of lorcaserin-induced locomotion in 5 min blocks. Data were evaluated with repeated measures two-way ANOVA (vehicle/lorcaserin × time) followed by Dunnett’s multiple comparison test. Lorcaserin, F[3,15] = 5.43, p < 0.01; time, F[1.35, 20.35] = 147.2, p < 0.001; interaction of lorcaserin and time, F[21,105] = 15.76, p < 0.001. (B) Data summary of the total lorcaserin-induced locomotion. Data were evaluated with a one ANOVA (vehicle × lorcaserin) followed by Dunnett’s multiple comparison test. Lorcaserin, F[3,15] = 9.32, p < 0.01. Asterisks in the graphs show post hoc p values comparing drug treatments against vehicle at different time points (A) or at different doses (B). Numbers between brackets in A show the dose of lorcaserin in which a statistically significant post hoc test comparing different doses of lorcaserin against vehicle at different time points was observed. Next to the doses of lorcaserin, post hoc p values comparing different doses against its vehicle are depicted. *p < 0.05, **p < 0.01, ***p<0.001. Data are mean ± S.E.M.
Supplementary Fig S2. Percentage of animals exhibiting at any point the cut-off latency for vehicle and different doses (s.c.) of lorcaserin (1.0, or 2.0 mg/kg) and cumulative doses of oxycodone (A), morphine (B) and fentanyl (C).


