Abstract
Scope
The diphenol curcumin from turmeric is rapidly metabolized into phase II conjugates following oral administration, resulting in negligible plasma concentration of the free compound which is considered the bioactive form. Total plasma concentration of curcumin is often quantified after treatment with β-glucuronidase to hydrolyze curcumin-glucuronide, the most abundant conjugate in vivo. The efficiency of enzymatic hydrolysis has not been tested.
Methods and Results
Using LC-MS analyses we compared the efficiency of β-glucuronidase and sulfatase from Helix pomatia to hydrolyze curcumin conjugates in human and mouse plasma after oral administration of turmeric. Both β-glucuronidase and sulfatase completely hydrolyzed curcumin-glucuronide. Unexpectedly, β-glucuronidase hydrolysis was incomplete, affording a large amount of curcumin-sulfate whereas sulfatase hydrolyzed both glucuronide and sulfate conjugates. With sulfatase, the concentration of free curcumin was doubled in human and increased in mouse plasma compared to β-glucuronidase treatment. Incomplete hydrolysis by β-glucuronidase suggested the presence of mixed glucuronide-sulfate conjugates. LC-MS-based searches detected diglucuronide, disulfate and mixed sulfate-glucuronide and sulfate-diglucuronide conjugates in plasma that likely contribute to the increase of free curcumin upon sulfatase treatment.
Conclusion
β-Glucuronidase incompletely hydrolyzes complex sulfate-containing conjugates that appear to be major metabolites, resulting in an underestimation of the total plasma concentration of curcumin.
Keywords: Turmeric, sulfatase, sulfate conjugate, glucuronide, bioavailability
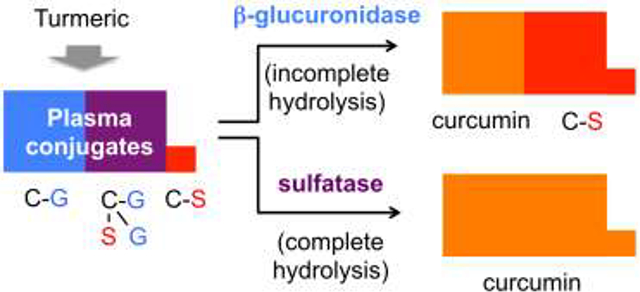
Graphical Abstract
Plasma conjugates of curcumin containing sulfate (C-S, red) are incompletely hydrolyzed using β-glucuronidase. Sulfatase, in contrast, hydrolyzes both curcumin-glucuronide (C-G, blue) and curcumin-sulfate conjugates, resulting in higher plasma levels of free curcumin (C, orange) than when samples are hydrolyzed with β-glucuronidase. To explain this finding we searched for novel metabolites and detected four higher order and mixed glucuronide and sulfate conjugates (purple) of curcumin in human and mouse plasma.
1. Introduction
Polyphenols are a large family of plant secondary metabolites with diverse biologic activities [1–5]. Polyphenols are consumed as part of the regular human diet or as dietary supplements [6]. Curcumin has emerged as one of the dietary polyphenols most widely consumed in the United States in recent years [7]. Deliberate consumption of curcumin or other polyphenols is often coupled to the expectation of therapeutic or otherwise health-promoting effects. Scientific assessment of purported benefits requires to know the plasma concentration of polyphenols and their metabolites in order to quantify overall exposure and bioavailability, and to determine whether plasma concentrations are consistent with those required for activity in bioassays performed in vitro and in animal models [8–10].
Upon oral administration, polyphenols are rapidly converted to glucuronidated, sulfated, and methylated metabolites often resulting in negligible abundance of the free aglycones in plasma [11, 12]. The diversity of plasma metabolites of curcumin and other polyphenols presents a challenge for the analytical procedures used for quantification. In many instances, therefore, plasma samples are treated with β-glucuronidase to hydrolyze conjugates in order to uncover the otherwise nearly undetectable aglycone, especially with curcumin [13, 14], and to decrease the complexity of metabolites that need to be dealt with [15]. While simplifying the analyte profile, this practice also introduces novel sources of variability: Whether hydrolysis of plasma conjugates is complete is difficult to assess in the absence of direct analysis of the remaining parent conjugates. Also, polyphenols may undergo ex vivo hydrolysis or secondary transformation during the hydrolysis process, further contributing to analytical variability [16, 17].
Thus, when the goal is to determine overall exposure to a polyphenol, it is preferred to conduct a direct analysis of all possible plasma metabolites, i.e., without hydrolysis. Such analyses, however, are often not practical and difficult to perform. For one, not all conjugates formed in vivo may have been identified. In addition, direct analysis of phase-II conjugates by LC-MS faces technical challenges including incomplete recovery from the biological matrix (the conjugates are more polar than the polyphenols), “bad” chromatographic peak shapes (low signal-to-noise), inadvertent fragmentation during the ionization process in the mass spectrometer [18], and often a lack of suitable isotopic standards for quantification [19]. Most of these problems are avoided when plasma metabolites are analyzed following hydrolysis of their phase-II conjugates [15].
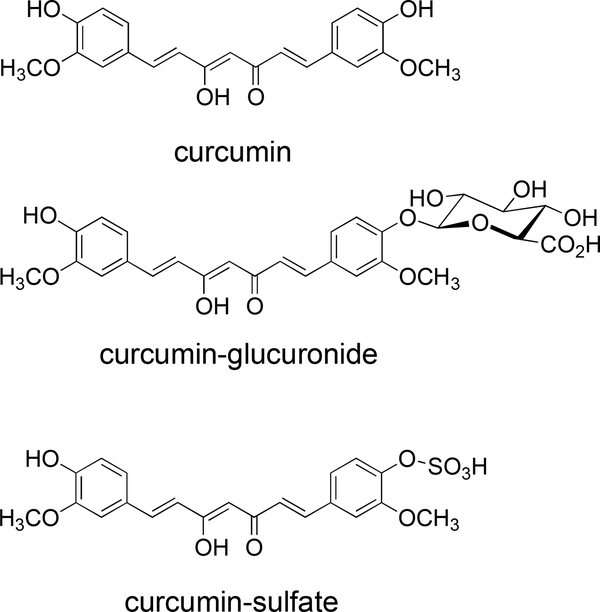
For curcumin and other polyphenols hydrolysis is commonly performed using β-glucuronidase, in accord with information that the major plasma conjugates are glucuronides [20] although sulfate conjugates have been described also [21]. The specification of the most widely used β-glucuronidase, a crude protein fraction prepared from the snail Helix pomatia, also lists sulfatase activity, suggesting sulfate conjugates would be hydrolyzed as well. While we were developing methods for quantitative analysis of curcumin and its glucuronide and sulfate conjugates (Fig. 1) we noticed a discrepancy in the amount of curcumin released from plasma samples treated with Helix pomatia-derived β-glucuronidase versus sulfatase enzyme preparations. Results from our investigation into this discrepancy are reported here.
Fig. 1.
Structures of curcumin and its glucuronic acid and sulfate conjugates.
2. Experimental Section
2.1. Materials
The turmeric-derived curcuminoid product (> 83% curcumin) for human administration was formulated to enhance curcumin bioavailability by creating a water dispersible nanoparticle complex containing glycerin and gum ghatti (Theracurmin HP; Theravalues Corp.) and labeled to contain 90 mg curcumin per 300 mg capsule [22]. Mice were administered a turmeric-derived curcuminoid product (218580100, Fisher) with curcuminoid content verified using standard LC-MS methods (80.6% curcumin, 13.5% demethoxycurcumin, and 2.4% bisdemethoxycurcumin by weight) [23]. Curcumin, d6-curcumin, and d6-curcumin-glucuronide were synthesized as described [18, 24]. D6-curcumin-sulfate was obtained from Toronto Research Chemicals. β-Glucuronidase (2742 U/mg; containing 7.3 U/mg sulfatase; cat.-no. 152284) and sulfatase (23.3 U/mg; containing >300 kU/g β-glucuronidase; cat.-no. S9626–5KU), both from Helix pomatia, were purchased from MP Biomedicals or Sigma, respectively.
2.2. Dosage information for human samples
The human study was approved by the Vanderbilt University IRB (IRB #121493), and volunteers signed a consent form before enrolling in the study. Six healthy volunteers (2 female, 4 males, ages 29–57 years) received 1 serving (2 capsules, 180 mg curcumin) of Theracurmin. Blood was collected into citrate tubes 2 h after administration of the supplement, the reported Tmax for this formulation [25]. Plasma was obtained by centrifugation. Plasma samples were stored at −80°C until extraction and analysis.
2.3. Dosage information for mouse samples
The animal studies were conducted under separate IACUC-approved protocols by the University of Arizona and Vanderbilt University.
For the administration of d6-curcumin one C57BL/6J female mouse was treated with a 1:1-mixture of d0/d6-curcumin (100 mg/kg) in DMSO by intraperitoneal (i.p.) injection. Blood was collected by cardiac puncture 30 min after treatment and added to BD Vacutainer serum separator tubes and inverted 5 times. The tubes were left at room temperature for 30 min to allow clotting. Tubes were centrifuged for 10 min at 1200 × g, and serum was stored at −80°C.
Male C57BL/6J mice (15 weeks old) were administered curcuminoids in DMSO by i.p. injection (100 mg/kg, 0.5 g human equivalent dose [HED]) or by oral gavage (500 mg/kg, 2.5 g HED). Blood was harvested by cardiac puncture after 20 min for i.p. injection or after 30 min for gavage, and serum was stored at −80°C until analyzed.
2.4. Sample extraction
Mouse serum was divided in 3 aliquots of 10 or 20 μl. Each aliquot was diluted 10 times with 20 mM ammonium acetate buffer pH 5 and further acidified to pH 5 with HCl (1 N). Human plasma was divided in 3 aliquots of 200 μl each and acidified to pH 5 with HCl (1 N). Mouse serum and human plasma samples were treated or not with 46.6 U of sulfatase or 745.8 U of β-glucuronidase for 1 h at 37°C. After hydrolysis samples were acidified to pH 3 with HCl (1 N) and 192 pmol each of d6-curcumin, d6-curcumin-glucuronide and d6-curcumin-sulfate were added. Samples were loaded into a paper filter strip (4 cm x 1 cm) placed in a plastic microcentrifuge tube and dried overnight under vacuum. For elution of metabolites, paper strips were incubated with a solvent of acetonitrile/H2O/formic acid (80:20:0.1) for 30 min at 4°C. Samples were centrifuged and the supernatant was analyzed by LC-MS. Samples from the mouse treated with d0/d6 curcumin were processed as described except that paper strips were eluted with a solvent containing 25%, 50% or 80% acetonitrile in H2O/formic acid (0.1%).
2.5. LC-MS analysis
LC-MS analyses were performed using a Thermo Scientific TSQ Vantage triple stage quadrupole mass spectrometer equipped with an electrospray interface operated in the positive or negative ion modes. Ionization parameters were optimized by direct infusion of a solution of curcumin. For chromatography a Waters Symmetry Shield C18 column (2.1 × 50 mm, 1.8 μm) was eluted at room temperature with a gradient of acetonitrile in water/0.1% formic acid changed from 15% acetonitrile to 85% in 3 min and further increased to 95% in 1 min at a flow rate of 0.4 ml/min. Metabolites were analyzed in the full scan and selected reaction-monitoring (SRM) modes. Ion polarities, fragmentation energies, and ion transitions used in the SRM modes are listed in Table 1 and in SI Tables 1 and 2.
Table 1.
Ionization mode and selected reaction-monitoring ion pairs for the detection of curcumin, d6-curcumin, and their conjugates in plasma samples.
| Aglycon/conjugate | Ionization mode | Molecular ion(m/z) | Fragment ion(m/z) | Molecular ion(m/z) | Fragment ion(m/z) |
|---|---|---|---|---|---|
| curcumin | d6-curcumin | ||||
| Curcumina | pos. | 369.0 | 177.0 | 375.25 | 180.24 |
| C-Gb | neg. | 543.48 | 216.99 | 549.48 | 220.42 |
| C-Sa | pos. | 449.38 | 285.38 | 455.38 | 291.0 |
| C-diGb | pos. | 721.60 | 369.20 | 727.60 | 375.18 |
| C-diSa | pos. | 529.51 | 449.38 | 535.50 | 375.18 |
| C-SGa | neg. | 623.60 | 216.99 | 629.60 | 220.42 |
| C-SdiGb | pos. | 801.70 | 369.20 | 807.70 | 375.18 |
C, curcumin; G, glucuronide; S, sulfate; pos., positive; neg., negative.
Paper strips were eluted with a H2O/acetonitrile (50:50) or b H2O/acetonitrile (20:80).
3. Results
3.1. Curcumin and conjugates in human plasma
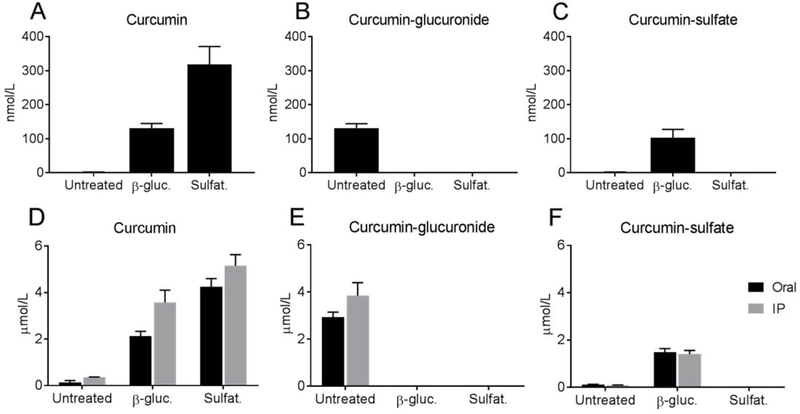
Free curcumin and its glucuronide and sulfate conjugates were analyzed in plasma samples of volunteers obtained 2 h after ingestion of a single oral dose of curcumin. The dose provided 180 mg of curcumin in a nano-dispersed colloidal suspension with reported 27-fold increase in bioavailability [22]. This resulted in low but quantifiable plasma concentration of free curcumin (2.1±0.21 nmol/L) (Fig. 2A). Direct quantification of mono-glucuronide and mono-sulfate conjugates showed that curcumin-glucuronide was the major conjugate (131.6±12.6 nmol/L) while curcumin-sulfate (1.8±0.2 nmol/L) was about two orders of magnitude lower (Fig. 2).
Fig. 2.
Concentration of curcumin and its glucuronide and sulfate conjugates in plasma before and after hydrolysis with β-glucuronidase or sulfatase. Turmeric was administered to human volunteers (A-C), and plasma was obtained after 2 h, Mice (D-F) received turmeric extract by oral gavage or i.p. injection and serum was obtained after 30 or 20 min, respectively. Plasma or serum samples were left untreated or hydrolyzed with β-glucuronidase (β-gluc.) or sulfatase (Sulfat.) prior to extraction, and concentration of curcumin (A,D), curcumin-glucuronide (B,E), and curcumin-sulfate (C, F) was quantified using LC-SRM-MS. All values in A-C are different from each other at p<0.05 (n = 6). In D-F all values are different from each other within the oral and i.p. series, respectively, at p<0.05 (n = 5). Statistical analysis was performed using one-way ANOVA followed by Tukey multiple comparison tests of every pair. Error bars indicate SEM.
3.2. Curcumin concentration in human plasma after hydrolysis
Aliquots of human plasma samples were hydrolyzed with β-glucuronidase or sulfatase. According to the specifications, β-glucuronidase contained sulfatase activity, and the sulfatase preparation also exhibited β-glucuronidase activity. Hydrolysis with β-glucuronidase gave 130.7±14.2 nmol/L free curcumin while hydrolysis with sulfatase gave more than twice the amount (318.4±52.9 nmol/L) (Fig. 2A). This indicated a pool of curcumin conjugates in plasma that was not or not completely hydrolyzed by β-glucuronidase, representing about half of the total hydrolysable conjugates.
3.3. Hydrolysis of conjugates in human plasma
β-Glucuronidase completely hydrolyzed curcumin-glucuronide but, unexpectedly, generated a large amount of curcumin-sulfate (Fig. 2B,C). This indicated that β-glucuronidase is only efficient when deconjugating glucuronidated substrates but not with sulfate conjugates (or much less so), suggesting the existence of a mixed conjugate containing both glucuronic acid and sulfate as an abundant plasma metabolite. Such a mixed conjugate could best explain the low concentration of curcumin-sulfate from non-hydrolase treated plasma together with prominent emergence upon treatment with β-glucuronidase. Based on the amount of curcumin-sulfate formed by β-glucuronidase hydrolysis, one or several mixed conjugates were present at up to 100 nmol/L in human plasma 2 h following administration of turmeric. In contrast, sulfatase completely hydrolyzed curcumin-glucuronide and curcumin-sulfate conjugates (Fig. 2B,C). Resulting curcumin concentrations exceeded those obtained from ß-glucuronidase hydrolysis by about twofold, consistent with a postulated mixed conjugate that is completely hydrolyzed by sulfatase.
3.4. Analysis of curcumin and conjugates in mouse serum
Mice were treated with unformulated turmeric extract by i.p. injection or oral gavage, and blood was collected after 20 or 30 min, respectively. The concentration of free curcumin in mouse serum was low prior to hydrolysis (<0.4 μmol/L), and increased ≈15 to 30-fold upon treatment with β-glucuronidase or sulfatase, respectively (Fig. 2D). Similar to the human samples, higher concentration of free curcumin was achieved by treatment of serum with sulfatase than with β-glucuronidase (Fig. 2D). Curcumin-glucuronide, a major conjugate in untreated serum, was fully hydrolyzed by treatment with both β-glucuronidase or sulfatase, respectively (Fig. 2E). The amount of curcumin-glucuronide in untreated serum matched the amount of curcumin released by β-glucuronidase. Curcumin-sulfate, present at low concentration in untreated plasma (<0.1 μmol/L), was completely hydrolyzed by sulfatase but increased more than 10-fold by β-glucuronidase (Fig. 2F). There was no difference in relative metabolite abundance between oral and i.p. administration of turmeric. The detection of conjugated metabolites upon i.p. administration of curcumin was consistent with the very fast one-pass circulation time of blood in the mouse that was previously determined to be approximately 15 s [26]. Thus, even upon i.p. administration there was sufficient time for curcumin to pass multiple times through the liver and be conjugated prior to sample collection. These results indicated the presence also in mouse serum of mixed conjugates of curcumin that were of higher order, i.e., that were at least doubly conjugated. The predicted mixed conjugates were completely hydrolyzed by sulfatase but converted to simple sulfate conjugates by β-glucuronidase.
3.5. Higher order glucuronide/sulfate conjugates in mouse serum
We developed an LC-MS based strategy to search for putative higher order conjugates of curcumin with glucuronic acid and sulfate in mouse serum. SRM transitions for detection of such conjugates were predicted based on the observed fragmentation of curcumin glucuronide and sulfate conjugates, respectively, in MS2 analyses (Table 1 and SI Tables 1 and 2).
In order to minimize false positive detection of putative conjugates a 1:1 mixture of curcumin and d6-curcumin was administered to mice, and the M/M+6 isotopic pattern was used for positive identification. Serum samples were extracted using paper strip extraction [27] which achieved higher recovery of polar curcumin conjugates than solid-phase or liquid-liquid extraction.
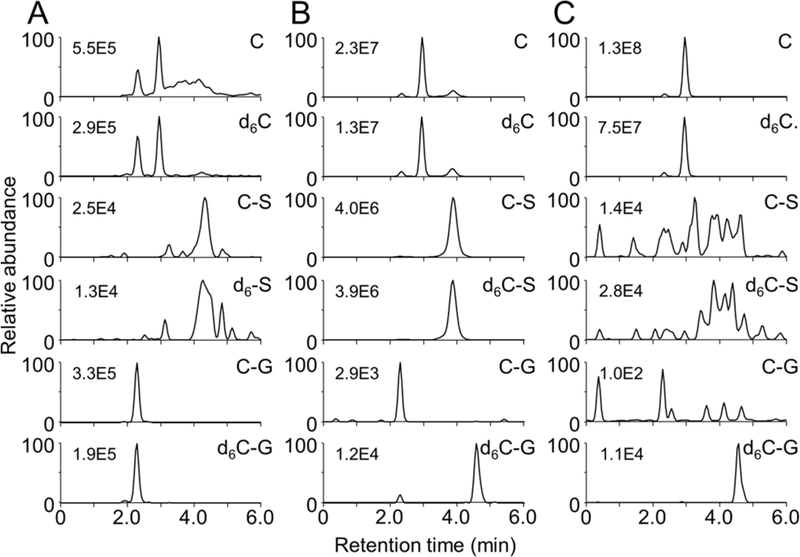
LC-SRM-MS analysis showed parallel M/M+6 ion chromatograms for curcumin, curcumin-sulfate, and curcumin-glucuronide in non-hydrolyzed mouse serum (Fig. 3A). As expected, hydrolysis with β-glucuronidase decreased curcumin-glucuronide and increased curcumin and curcumin-sulfate (Fig. 3B). Sulfatase increased curcumin while decreasing both sulfate and glucuronide conjugates (Fig. 3C). The results obtained with isotope-labeled curcumin corroborated the quantitative analyses (Fig. 2).
Fig. 3.
LC-MS analysis of curcumin and its conjugate metabolites in serum of mice administered a 1:1 mixture of curcumin and d6-curcumin by i.p. injection. Serum samples were (A) untreated or hydrolyzed with (B) β-glucuronidase or (C) sulfatase prior to extraction. Ion trace chromatograms were acquired using LC-SRM-MS analysis of curcumin (C), curcumin-sulfate (C-S), curcumin-glucuronide (C-G), and their respective d6-isotopologues. Analytical conditions are listed in Table 1. The numbers indicate the highest ion intensity in each trace. The first peak eluting in the C and d6-C chromatograms in (A) is derived from in-source fragmentation of the glucuronide conjugates C-G and d6C-G, respectively, cf. ref. [18].
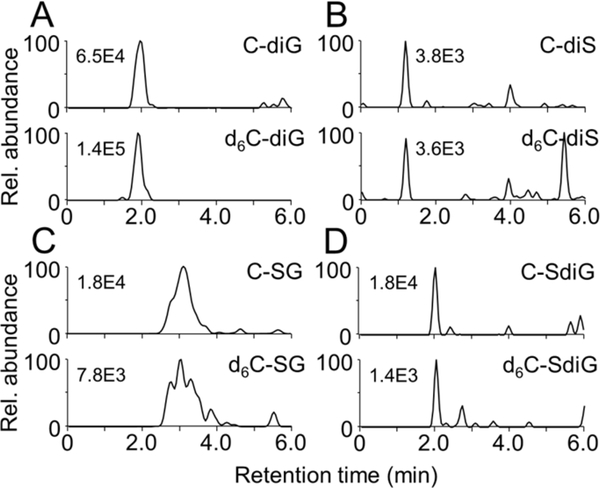
Using predicted ion transitions a curcumin-diglucuronide conjugate was detected in the corresponding M and M+6 SRM chromatograms, respectively, in non-hydrolyzed mouse serum (Fig. 4A). The peak disappeared upon treatment with β-glucuronidase or sulfatase (SI Fig. 1), consistent with a conjugate that only contained glucuronic acid. The curcumin-diglucuronide conjugate was detected in LC-MS analyses conducted in both positive and negative ion modes, providing further support for positive identification. The diglucuronide represents a novel conjugate of curcumin, but the fact that it was hydrolyzed completely by β-glucuronidase suggested this conjugate did not account for the discrepancy in free curcumin concentration achieved by the two hydrolases.
Fig. 4.
Detection of complex conjugates in serum of mice treated with a 1:1 mixture of curcumin and d6-curcumin by i.p. injection. Ion trace chromatograms were acquired using selected reaction-monitoring analysis for (A) curcumin-diglucuronide (C-diG), (B) curcumin-disulfate (C-diS), (C) curcumin-sulfate-glucuronide (C-SG), (D) curcumin-sulfate-diglucuronide (C-SdiG) and their respective d6-isotopologues. Analytical conditions are listed in Table 1. The numbers indicate the highest ion intensity in each chromatogram.
Using the isotope-based approach facilitated detection of additional novel curcumin metabolites, namely curcumin-disulfate, curcumin-sulfate-glucuronide, and curcumin-sulfate-diglucuronide conjugates (Fig. 4B–D). All conjugates showed the M/M+6 isotopic pattern, and peak abundances were markedly reduced upon hydrolysis (SI Fig. 1). Hydrolysis of the higher-order sulfate mixed conjugates likely accounts for additional curcumin released by treatment of plasma with sulfatase compared to β-glucuronidase. Direct quantification of the higher order mixed conjugates in serum was not achieved due to the lack of suitable standards.
4. Discussion
We provide evidence for formation of novel conjugates of curcumin in human and mouse plasma following administration of turmeric. The metabolites are higher order and mixed conjugates containing glucuronic acid and sulfate moieties. The novel metabolites identified were diglucuronide, disulfate, sulfate-glucuronide, and sulfate-diglucuronide conjugates of curcumin. We also show that higher order mixed conjugates as well as the simple sulfate conjugate of curcumin are incompletely hydrolyzed by the standard treatment of plasma with β-glucuronidase. Thus, use of β-glucuronidase may result in an underestimation of the bioavailability of curcumin upon oral administration. If curcumin plasma concentration is to be quantified following conjugate hydrolysis, substrate specificity and efficiency of the hydrolytic enzyme need to be considered. Our analyses suggest that sulfatase treatment will yield higher concentration of free curcumin than β-glucuronidase.
Hydrolysis of plasma samples was performed using standard treatment, i.e., the pH was adjusted to the optimum for β-glucuronidase (pH 5). The same pH was used for sulfatase to enable direct comparison although the enzyme optimum is at pH 6.2. Since both enzymes are mainly used as crude preparations from H. pomatia, activities are only enriched, and specificity is somewhat increased when the pH value optimal for the respective enzyme is used. Since both activities are present in either preparation (cf. section 2.1 Materials) it is conceivable that in reactions using the optimum pH for β-glucuronidase there was less sulfatase activity, and the sulfate conjugates were not hydrolyzed. In contrast, sulfatase treatment (also performed at pH 5) was optimal for β-glucuronidase activity, and this resulted in hydrolysis of both types of conjugates. Nevertheless, standard hydrolysis of plasma samples is performed at only one pH value, and this pH is usually optimal for β-glucuronidase (pH 5), thus precluding hydrolysis of sulfate conjugates.
Our data only allowed to determine the composition of the novel conjugates but not the position at which the moieties were connected to parent curcumin or to each other. Determination of the connectivity would require NMR analyses of the isolated conjugates, provided that sufficient amounts can be obtained, or MS fragmentation data in comparison to authentic standards that are challenging to synthesize. From comparison to authentic (synthesized) standards, it is clear that the initial conjugation occurs at one of the phenolic hydroxyls of curcumin. Given the preference for binding to the phenolic hydroxyl, a glucuronide-sulfate mixed conjugate likely carries the two moieties at the opposite ends of curcumin. Whether the third moiety in the sulfate-diglucuronide is attached to the already present glucuronide or sulfate or connected at the central hydroxyl is unknown. Work by Pfeifer and co-workers suggested that glucuronic acid may be attached to the central β-diketone (keto-enol) by microsomes, based on detection of a glucuronide of 4’,4”-O-dimethylcurcumin, a substrate in which both phenolic hydroxyls are blocked by methylation and thus unavailable for conjugation [28].
Addition of multiple glucuronide or sulfate moieties to a xenobiotic compound to enhance metabolic elimination seems a waste of cellular resources, given the energetic requirements for sulfation and glucuronidation [29, 30]. Nevertheless, dressing of endogenous or exogenous compounds with more than one glucuronide or sulfate moiety is not uncommon [31]. One of the first diglucuronide conjugates to be detected and structurally identified was bilirubin-diglucuronide [32, 33]. A number of diglucuronides of prototypical polyphenols have been described [31], and, for example, mixed glucuronide-sulfate conjugates of quercetin have been detected in urine after oral administration [34, 35]. Formation of a mixed conjugate was also suggested for curcumin [21] but had not yet been proven by direct detection. Resveratrol, in addition to monoglucuronides and monosulfates, also formed disulfates and diglucuronides after oral administration to humans [36]. Interestingly, in both diglucuronides, one of the glucuronic acid moieties was attached to C-2 of the 3,5-diphenolic ring of resveratrol, i.e., via a C-C linkage rather than the usual C-O coupling to a hydroxyl. It remains to be tested whether the C-C linked glucuronide can be hydrolyzed by β-glucuronidase.
Incomplete hydrolysis of sulfate conjugates together with their unexpectedly large abundance has important implications for the determination of curcumin plasma concentrations. Quantification of curcumin plasma concentration with help of a β-glucuronidase hydrolysis step is common practice. Our findings suggest that hydrolysis of curcumin-sulfate and the mixed glucuronide-sulfates may have been incomplete in such studies. It is likely that the true total plasma concentration of curcumin (i.e., including the conjugates) may be higher than what is reported in the literature to date – if the analysis was based on β-glucuronidase hydrolysis. Our findings may also be relevant in the context of studies focusing on site-specific deconjugation of curcumin and other polyphenols by hydrolases in inflammatory tissues [37–39]. While β-glucuronidase is the hydrolase of main interest in this context, additional insight may be gained by analyzing the contribution of sulfatase to site-specific hydrolysis of polyphenol conjugates
Supplementary Material
Acknowledgments
This work was supported by awards from the National Center for Complementary and Integrative Health (NCCIH), the Office of Dietary Supplements (ODS), and the National Cancer Institute of the National Institutes of Health (NIH) (R01AT006896 to CS, R01CA174926 and R34AT007837 to JLF, and F31AT009938 to AGK). Mass spectrometric analyses were performed in part through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404 Core Scholarship.
Abbreviations
- HED
human equivalent dose
- LC-MS
liquid chromatography-mass spectrometry
- SRM
selected reaction monitoring
Footnotes
Conflict of interest statement
The authors declare that no competing financial or other conflicts of interest exist.
5 References
- [1].Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE, Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A, Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kerimi A, Williamson G, At the interface of antioxidant signalling and cellular function: Key polyphenol effects. Mol. Nutr. Food Res. 2016, 60, 1770–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rothwell JA, Knaze V, Zamora-Ros R, Polyphenols: dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [DOI] [PubMed] [Google Scholar]
- [5].Guasch-Ferre M, Merino J, Sun Q, Fito M, Salas-Salvado J, Dietary polyphenols, mediterranean diet, prediabetes, and type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell. Longev 2017, 2017, 6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Manach C, Scalbert A, Morand C, Remesy C, Jimenez L, Polyphenols: food sources and bioavailability. The American journal of clinical nutrition 2004, 79, 727–747. [DOI] [PubMed] [Google Scholar]
- [7].Skiba MB, Luis PB, Alfafara C, Billheimer D, Schneider C, Funk JL, Curcuminoid content and safety-related markers of quality of turmeric dietary supplements sold in an urban retail marketplace in the United States. Mol. Nutr. Food Res 2018, e1800143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Williamson G, The use of flavonoid aglycones in in vitro systems to test biological activities: based on bioavailability data, is this a valid approach? Phytochem. Rev 2002, 1, 215–222. [Google Scholar]
- [9].Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G, How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr 2004, 80, 15–21. [DOI] [PubMed] [Google Scholar]
- [10].Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A, Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol 2014, 88, 1803–1853. [DOI] [PubMed] [Google Scholar]
- [11].Hollman PC, Katan MB, Bioavailability and health effects of dietary flavonols in man. Arch. Toxicol. Suppl. 1998, 20, 237–248. [DOI] [PubMed] [Google Scholar]
- [12].Crozier A, Del Rio D, Clifford MN, Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med 2010, 31, 446–467. [DOI] [PubMed] [Google Scholar]
- [13].Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE, Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev 2008, 17, 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J, The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res 2014, 58, 516–527. [DOI] [PubMed] [Google Scholar]
- [15].Ding Y, Peng M, Zhang T, Tao JS, Cai ZZ, Zhang Y, Quantification of conjugated metabolites of drugs in biological matrices after the hydrolysis with beta-glucuronidase and sufatase: a review of bio-analytical methods. Biomed. Chromatogr 2013, 27, 1280–1295. [DOI] [PubMed] [Google Scholar]
- [16].Quifer-Rada P, Martinez-Huelamo M, Lamuela-Raventos RM, Is enzymatic hydrolysis a reliable analytical strategy to quantify glucuronidated and sulfated polyphenol metabolites in human fluids? Food Funct. 2017, 8, 2419–2424. [DOI] [PubMed] [Google Scholar]
- [17].Lu QY, Zhang L, Eibl G, Go VL, Overestimation of flavonoid aglycones as a result of the ex vivo deconjugation of glucuronides by the tissue beta-glucuronidase. J. Pharm. Biomed. Anal 2014, 88, 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luis PB, Gordon ON, Nakashima F, Joseph AI, Shibata T, Uchida K, Schneider C, Oxidative metabolism of curcumin-glucuronide by peroxidases and isolated human leukocytes. Biochem. Pharmacol 2017, 132, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Achaintre D, Bulete A, Cren-Olive C, Li L, Rinaldi S, Scalbert A, Differential isotope labeling of 38 dietary polyphenols and their quantification in urine by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical chemistry 2016, 88, 2637–2644. [DOI] [PubMed] [Google Scholar]
- [20].Pan MH, Huang TM, Lin JK, Biotransformation of curcumin through reduction and glucuronidation in mice. Drug. Metab. Dispos 1999, 27, 486–494. [PubMed] [Google Scholar]
- [21].Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A, Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [PubMed] [Google Scholar]
- [22].Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T, Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull 2011, 34, 660–665. [DOI] [PubMed] [Google Scholar]
- [23].Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Sólyom AM, Timmermann BN, Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod 2006, 69, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gordon ON, Graham LA, Schneider C, Facile synthesis of deuterated and [(14) C]labeled analogs of vanillin and curcumin for use as mechanistic and analytical tools. J. Labelled Comp. Radiopharm 2013, 56, 696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kanai M, Imaizumi A, Otsuka Y, Sasaki H, Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H, Chiba T, Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol 2012, 69, 65–70. [DOI] [PubMed] [Google Scholar]
- [26].Debbage PL, Griebel J, Ried M, Gneiting T, DeVries A, Hutzler P, Lectin intravital perfusion studies in tumor-bearing mice: micrometer-resolution, wide-area mapping of microvascular labeling, distinguishing efficiently and inefficiently perfused microregions in the tumor. J. Histochem. Cytochem 1998, 46, 627–639. [DOI] [PubMed] [Google Scholar]
- [27].Legette LL, Reed RL, Murty L, Maier CS, Stevens JF, Application of paper strip extraction in combination with LC-MS-MS in pharmacokinetics. Spectroscopy 2013, 39, s18–s25. [PMC free article] [PubMed] [Google Scholar]
- [28].Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M, Curcuminoids form reactive glucuronides in vitro. J. Agric. Food Chem 2007, 55, 538–544. [DOI] [PubMed] [Google Scholar]
- [29].Chapman E, Best MD, Hanson SR, Wong CH, Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. Engl 2004, 43, 3526–3548. [DOI] [PubMed] [Google Scholar]
- [30].Wells PG, Mackenzie PI, Chowdhury JR, Guillemette C, Gregory PA, Ishii Y, Hansen AJ, Kessler FK, Kim PM, Chowdhury NR, Ritter JK, Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. 2004, 32, 281–290. [DOI] [PubMed] [Google Scholar]
- [31].Argikar UA, Unusual glucuronides. Drug Metab. Dispos 2012, 40, 1239–1251. [DOI] [PubMed] [Google Scholar]
- [32].Billing BH, Cole PG, Lathe GH, The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem. J 1957, 65, 774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gordon ER, Goresky CA, Chang TH, Perlin AS, The isolation and characterization of bilirubin diglucuronide, the major bilirubin conjugate in dog and human bile. Biochem. J 1976, 155, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Graf BA, Mullen W, Caldwell ST, Hartley RC, Duthie GG, Lean ME, Crozier A, Edwards CA, Disposition and metabolism of [2–14C]quercetin-4’-glucoside in rats. Drug Metab. Dispos 2005, 33, 1036–1043. [DOI] [PubMed] [Google Scholar]
- [35].Hong YJ, Mitchell AE, Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 6794–6801. [DOI] [PubMed] [Google Scholar]
- [36].Burkon A, Somoza V, Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides - two novel resveratrol metabolites in human plasma. Mol. Nutr. Food Res 2008, 52, 549–557. [DOI] [PubMed] [Google Scholar]
- [37].Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J, Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem 2008, 283, 9424–9434. [DOI] [PubMed] [Google Scholar]
- [38].Kunihiro AG, Brickley JA, Frye JB, Luis PB, Schneider C, Funk JL, Curcumin, but not curcumin-glucuronide, inhibits Smad-signaling in TGFβ-dependent bone metastatic breast cancer cells and is locally enriched in bone. J. Nutr. Biochem 2018, 63, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kunihiro AG, Luis PB, Brickey JA, Frye JB, Chow HS, Schneider C, Funk JL, Beta-glucuronidase catalyzes deconjugation and activation of curcumin-glucuronide in bone. J. Nat. Prod 2019, 82, 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.