Abstract
Background:
Exercise intensity can influence functional recovery after stroke, but the mechanisms remain poorly understood.
Objective:
In chronic stroke, an intensity-dependent increase in circulating brain-derived neurotrophic factor (BDNF) was previously found during vigorous exercise. Using the same serum samples, this study tested acute effects of exercise intensity on other circulating molecules related to neuroplasticity, including vascular-endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF1) and cortisol, with some updated analyses involving BDNF.
Methods:
Using a repeated-measures design, 16 participants with chronic stroke performed three exercise protocols in random order: treadmill high-intensity interval training (HIT-treadmill), seated-stepper HIT (HIT-stepper) and treadmill moderate-intensity continuous exercise (MCT-treadmill). Serum molecular changes were compared between protocols. Mediation and effect modification analyses were also performed.
Results:
VEGF significantly increased during HIT-treadmill, IGF1 increased during both HIT protocols and cortisol non-significantly decreased during each protocol. VEGF response was significantly greater for HIT-treadmill vs. MCT-treadmill when controlling for baseline. Blood lactate positively mediated the effect of HIT on BDNF and cortisol. Peak treadmill speed positively mediated effects on BDNF and VEGF. Participants with comfortable gait speed ≥ 0.4m/s had significantly lower VEGF and higher IGF1 responses, with a lower cortisol response during MCT-treadmill.
Conclusions:
BDNF and VEGF are promising serum molecules to include in future studies testing intensity-dependent mechanisms of exercise on neurologic recovery. Fast training speed and anaerobic intensity appear to be critical ingredients for eliciting these molecular responses. Serum molecular response differences between gait speed subgroups provide a possible biologic basis for previously observed differences in training responsiveness.
Keywords: vascular endothelial growth factor, insulin-like growth factor, cortisol, brain-derived neurotrophic factor, high-intensity interval training, locomotion
Introduction
Endurance exercise can improve fitness, gait, balance, cognition and cardiovascular risk factors after stroke.1 Accumulating longitudinal evidence suggests that a vigorous training intensity (e.g. ≥ 60% heart rate reserve) is a critical ingredient for improving at least some of these outcomes (e.g. fitness, gait and balance),2–5 and that pairing exercise with task-specific training may be essential for improving outcomes related to central nervous system function (e.g. gait).4,6 However, the potential mechanisms underlying an intensity-dependent effect of exercise on neurologic function remain poorly understood. Identifying these mechanisms could potentially lead to more effective rehabilitation interventions, greater clinical implementation, novel therapeutic targets (e.g. for pharmacotherapy) and better prognostic measures to identify likely responders.6,7
Lately, increasing attention has been focused on brain-derived neurotrophic factor (BDNF) as a possible mediator of the neurologic benefits of exercise.6 BDNF is an abundant growth factor that is involved with activity-induced neuroplasticity,6,8 is upregulated in the animal brain by exercise,9–11 and shows acute increases in human blood during vigorous exercise, which have been correlated with enhanced cognitive and motor learning among healthy adults.12,13 Among persons with chronic stroke, it was recently found that treadmill high-intensity interval training elicited a significantly greater acute BDNF increase than moderate intensity treadmill exercise, which showed no significant effect.14 A secondary analyses found that an exercise intensity around the onset of blood lactate accumulation was the optimal threshold for discriminating large acute BDNF responses.14
While these results provide preliminary evidence that BDNF could potentially mediate some intensity-dependent effects of exercise on neurologic function, there are also several other less-studied molecules that could be involved. For example, vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF1) are two additional neurotrophins that accumulate in human blood during exercise,15,16 and have some evidence of intensity-dependence.15,17,18 VEGF is a protein involved with angiogenesis and neurogenesis, including in the brain after stroke.19 Circulating VEGF increases during exercise are thought to be primarily triggered by hypoxia and vascular shear stress in skeletal muscles.15,17 Similar peripheral increases in VEGF have been shown to promote perilesional angiogenesis and neurologic recovery in animal models of post-acute stroke.20,21 IGF1 is a protein involved with tissue remodeling, including in the brain, where it plays roles in neurogenesis, angiogenesis, neuroprotection and neuroplasticity,22 including after stroke.19 Circulating IGF1 primarily originates in the liver, but is the main IGF1 source for the central nervous system.22 Peripheral IGF1 administration has been shown to have a variety of positive effects on the brain in animal studies.23,24 Further, acute exercise-induced increases in circulating levels of VEGF and IGF1 have each been shown to mediate hippocampal neurogenesis in animals.25,26 Cortisol is another molecule that could mediate intensity-dependent effects of exercise on neurologic function, but one that could have negative effects. Circulating cortisol can acutely increase during psychological stress or high-volume strenuous exercise, due to activation of the hypothalamic-pituitary-adrenal axis,27 and can impair central neuroplasticity and learning if excessively upregulated.28–31
It remains unknown whether acute upregulation of circulating BDNF, VEGF, IGF1 and/or cortisol meaningfully influences stroke rehabilitation outcomes. However, before costly longitudinal trials are conducted, two important steps are to: 1) confirm whether exercise actually causes acute increases in circulating VEGF, IGF1 and/or cortisol among humans with stroke; and 2) evaluate the influence of exercise intensity on these responses, to guide protocol selection. No previous studies have addressed these aims.
In addition, it remains poorly understood how exercise causes different acute circulating molecular responses. Mediation analysis can be used to assess pathways by which an intervention (e.g. exercise protocol) leads to an outcome (e.g. acute circulating BDNF response) through an intermediate or mediating variable (e.g. blood lactate during training).32 Developments in statistical methodology have made it possible to test for causal mediation with lower sample sizes33 and to directly quantify mediated and non-mediated effects in the same model.34 To our knowledge, this newer methodology for mediation analysis has not yet been applied to neurologic rehabilitation research.
The purpose of this study was to determine the effect of exercise intensity on acute circulating VEGF, IGF1 and cortisol responses post-stroke, using the same dataset and serum samples as a previous BDNF analysis.14 We hypothesized that high-intensity interval training (HIT), whether performed on a treadmill (HIT-treadmill) or seated stepper (HIT-stepper), would elicit significantly greater acute increases in VEGF and IGF1 compared with moderate-intensity continuous exercise (MCT-treadmill). We also hypothesized that there would be no significant between-protocol difference in cortisol response, based on the limited evidence of psychological stress found in a previous qualitative assessment of treadmill high-intensity interval training after stroke.3 We also sought to evaluate mediating effects of different types of intensity and modifying effects of different clinical characteristics on these serum molecular responses. BDNF response was included as an additional dependent variable in the mediation analyses, because the novel statistical methods used herein provided more direct quantification of mediated effects than the previous BDNF analysis that used the same samples.14
Methods
The data collection methods for this project have been more extensively reported elsewhere.14 Pertinent information for this analysis is provided below.
Participants
This research was approved by the University of Cincinnati Institutional Review Board. Participants were recruited from the community between April and August of 2015 and provided written informed consent prior to participation. Inclusion criteria were: age 21–80 years; unilateral paresis from stroke experienced >6 months prior to enrollment (to help ensure stable deficits);35 residual gait impairment; able to walk with assistive devices as needed and no physical assistance; stable cardiovascular condition; discharged from formal rehabilitation; able to communicate with investigators and correctly answer consent comprehension questions. Exclusion criteria were: evidence of significant arrhythmia or myocardial ischemia on treadmill exercise test;36 hospitalization for cardiopulmonary disease within 3 months; pacemaker or implanted defibrillator; lower extremity claudication; severe lower limb spasticity i.e. Ashworth >2;37 weight bearing pain >4/10; pregnancy.
Baseline Testing
Each participant had a clinical examination and a symptom-limited graded exercise test38 with electrocardiography in a clinical cardiac stress laboratory to determine eligibility and baseline characteristics. This was followed by a repeated exercise test with gas exchange analysis (on a separate day) to determine peak oxygen consumption rate and heart rate. The exercise test was repeated so that the participant could acclimate to exercise testing before wearing a facemask for gas exchange analysis during the test. During each exercise test, speed was held constant at the participant’s pre-determined, individualized fastest comfortable speed, while incline was increased 2–4% every two minutes until the participant could not keep up, reached a cardiovascular safety limit36,39 or exhibited severe gait instability.
Study Design
We then used a within-participant repeated-measures experimental design. Each participant performed single sessions of three different exercise protocols in random order with approximately one week between sessions. Within participants, each session was scheduled at the same time of day to minimize random error from diurnal fluctuations. Participants were instructed to stay hydrated and to eat a meal approximately 2 hours prior to arrival for each session. All protocols were performed individually in a rehabilitation research lab by the same physical therapist (who was experienced in stroke rehabilitation), with support from an experienced phlebotomy nurse and research assistants for data collection. Personnel training was performed prior to data collection for approximately two months, including data collection practice for each protocol with two healthy participants.
Exercise Protocols
During all sessions, participants wore their habitual orthotic devices and were closely monitored and questioned to identify any adverse events. A fall protection harness (without weight support) and a height-adjusted handrail were used during treadmill sessions. Each protocol included a 3 min warm up at 30–50% heart rate reserve, 20 min of exercise (including recovery periods during HIT) and a 2 min cool down.
Treadmill high-intensity interval training (HIT-treadmill)
HIT-treadmill involved repeated 30 second bursts at maximum tolerated speed (0% incline), alternated with recovery periods where the treadmill was stopped. Recovery duration was decreased from 60 to 30 seconds after the first 5 minutes.3,40 Initial burst speed was determined by a rapid acceleration test towards the end of the warmup, when speed was increased by 0.1 mph every 5 seconds until reaching the limit where the participant drifted backward or exhibited gait instability. Speed was continuously progressed, maintained or regressed between bursts based on performance criteria.3,40 Participants were given verbal encouragement to stay to the front of the treadmill during each burst. Target mean intensity was >60% heart rate reserve. Unlike continuous exercise, the nature of this HIT protocol precludes the use of an instantaneous heart rate target during training. More specifically, the 30 second bursts are too brief for heart rate to reach steady state, so it fluctuates between burst and recovery periods, and has an upward trend over the session.40 However, this HIT protocol was designed to enable persons with stroke to sustain vigorous aerobic intensities (i.e >60% heart rate reserve) and has done so consistently.3,14,40
Seated stepper high-intensity interval training (HIT-stepper)
HIT-stepper used the same burst and recovery durations as HIT-treadmill, but on a seated stepper (NuStep T5XR). Bursts were performed at maximum possible cadence against 50% of maximal resistance (rounded down). Maximal resistance was predetermined with a steep ramp test14 where resistance was increased by one level every 15 seconds until the participant could not maintain a cadence of 80 steps/min (40 cycles/min). During bursts, visual feedback about cadence and verbal encouragement were provided to increase or maintain maximal cadence.
Treadmill moderate-intensity continuous exercise (MCT-treadmill)
MCT-treadmill involved continuous treadmill walking with speed continuously adjusted to achieve a mean intensity of 45 ± 5% heart rate reserve,41 with intermittent verbal encouragement.
Exercise intensity measures
Blood lactate was measured multiple times during each session (see below for details) and values obtained after 5, 10 and 20 minutes of exercise were averaged. Oxygen consumption rate was measured from expired air throughout each session using a Parvomedics TrueOne 2400 metabolic cart with a facemask interface.42 Heart rate responses were captured by electrocardiography. Data were averaged across the 20 minutes of exercise, except for treadmill speed, which used the peak value for analysis to better reflect the sprint-like nature of interval training without omitting the recovery periods (which would make the measurement methods differ between HIT and MCT).
Blood collection, processing and analysis
An antecubital IV line was placed in the non-paretic arm upon participant arrival for each session. Baseline samples were taken 30 minutes after IV insertion, to minimize any potential effect of IV insertion-related stress. For the exercise testing session, 10 mL blood samples were obtained at 3 baseline time points (T−25, T−20, T0) and one minute after the exercise test. For each of the exercise training sessions, 10 mL blood samples were taken before the warm up (T0), after 5, 10 and 20 minutes of exercise (T5, T10 and T20), then 30 and 60 minutes after finishing the exercise (T50 and T80).
One drop from each sample was immediately tested with a point of care analysis system to measure blood lactate.43 The remaining sample was clotted and centrifuged to obtain serum, which was aliquoted and stored in a −80°C freezer. BDNF, VEGF and IGF1 analyses were done in batches using Quantikine sandwich enzyme-linked immunosorbent assay kits (R&D Systems; Minneapolis, MN). Cortisol analysis was done in a batch with electrochemiluminescence immunoassays and a cobas e 411 analyzer (Roche Diagnostics; Basel, Switzerland). Laboratory staff were blinded to the study aims, design and exercise protocol associated with the samples.
Statistical analysis
Primary hypothesis testing
The primary analysis used a separate fixed-effects general linear model for each serum molecule to compare responses between protocols. The model included fixed effects for protocol, time, protocol × time, session number, session number × time, hemolysis and participant ID (to account for within-participant repeated sessions), with an unconstrained covariance matrix to account for within-participant, within-session repeated measures.44 Protocol × time interaction contrasts were obtained to test the hypothesis that HIT-treadmill and HIT-stepper would each elicit significantly different changes compared with MCT-treadmill from T0 to the average of T5, T10 and T20.
Sample size determination
The software GLIMMPSE45 was used to estimate power to detect a significant protocol × time interaction with the above model, based on a two-tailed significance level of 0.05. The expected effect size and covariance parameters were estimated from the lactate data and a preliminary BDNF data subset. (The data and aliquots from the preliminary BDNF assay were not re-used for the full analysis.) This analysis indicated an expected power of 0.90 with 15 participants. Target sample size was 16 to account for potential missing samples.
Intensity variable mediation analysis
Statistical mediation analyses were performed to better understand potential causal mechanisms32 of any between-protocol differences (or lack thereof) in serum molecular responses. This approach uses a mediation model to test how much of an independent variable’s effect on a dependent variable occurs through (or is nullified by) an intermediate variable (mediator). While the validity of any causal inference depends on the appropriateness of the model used (e.g. no residual confounding), statistical mediation analysis can provide relatively strong evidence of causality when used in combination with a prospective randomized experimental design.46 We tested mediating effects of four different exercise intensity variables on serum molecular responses, including: mean blood lactate, peak treadmill speed (for the treadmill protocols only), mean oxygen consumption rate and mean heart rate.
These analyses used natural effects models34 to estimate the mediated (indirect), non-mediated (direct) and total effects of protocol on serum molecular responses. For each protocol-mediator-response observation, an additional observation was imputed for each of the other two protocols, with the mediator level matched to the observed response.47 This imputation modeled molecular response as a function of the protocol, mediator, participant ID, baseline molecular concentration and session number. A natural effects model was then fit to this expanded dataset,34 using molecular response as the dependent variable, with fixed effects for the mediated effect, non-mediated effect, participant ID, baseline molecular concentration and session number. Robust standard errors were calculated using the sandwich estimator.48
Clinical characteristic effect modification analysis
We also tested the modifying effects of different clinical characteristics on the training intensity variables and molecular responses. Tested characteristics were: age, sex, stroke chronicity, body mass index, comfortable gait speed, comfortable gait speed subgroup (≥ 0.4 m/s or < 0.4 m/s),49,50 fast gait speed, peak oxygen consumption rate and peak heart rate (from exercise testing). Each characteristic was tested separately with the intensity variable or molecular response as the dependent variable, fixed effects for the clinical characteristic, protocol and baseline molecular concentration, and an unconstrained covariance matrix. We also tested for characteristic by protocol interactions. Since comfortable gait speed > 0.4 m/s has been shown to predict greater responsiveness to treadmill training,49 we also tested molecular responses to each protocol within each gait speed subgroup.
All analyses were done in SAS v9.4, except for the intraclass correlation coefficients and mediation analyses, which were done in R v3.5.1 with the “psych”51 and “medflex”48 packages, respectively.
Results
Summary of relevant previously reported results14
Sixteen participants were enrolled and all 16 completed the study. Participants were 57.4 ± 9.7 (mean ± SD) years old and 6.5 ± 4.1 years post stroke with a comfortable gait speed of 0.72 ± 0.33 m/s. Out of 352 total planned blood collections, 340 (96.6%) were successfully tested for lactate and 331 (94.0%) were obtained with sufficient volume for the current serum molecular analysis. Signs of hemolysis were observed in 71 (21.6%) of the 331 serum collections.
The HIT-stepper protocol was modified after two of the first nine participants experienced near syncope during recovery from this protocol only. These events were thought to be partially related to higher than expected intensities enabled by the stepper (e.g. lactate values of 17.0 and 12.8 mmol/L). Therefore, for the remaining 7 participants, we did not use resistance and enforced an 85% heart rate reserve limit for the HIT-stepper protocol. No other serious adverse events occurred, and the rate of non-serious events was similar between protocols.
HIT elicited significantly (p<0.05) greater mean responses than MCT for blood lactate (HIT-treadmill, 4.6 mmol/L; HIT-stepper, 6.8; MCT-treadmill, 2.0), mean heart rate (HIT-treadmill, 59.0 % heart rate reserve; HIT-stepper, 67.5; MCT-treadmill, 43.8) and peak treadmill speed (HIT-treadmill, 1.30 m/s; MCT-treadmill, 0.68). The revised HIT-stepper protocol (n=7) had significantly lower responses than the original HIT-stepper protocol (n=9) for lactate (original, 8.5 mmol/L; revised, 3.3) and mean heart rate (original, 79.1 % heart rate reserve; revised, 43.6), so we analyzed these protocols separately in a sensitivity analysis.
Acute effects of exercise on serum molecules
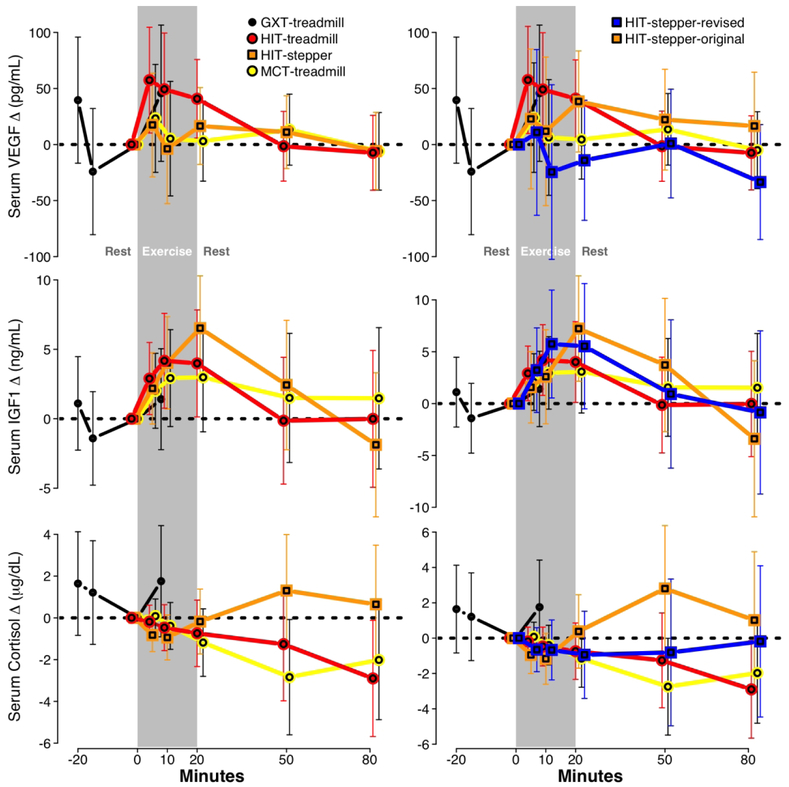
During the graded exercise test, each molecule showed a non-significant increase (Fig 1; Table 1). For the exercise protocols, VEGF significantly increased during HIT-treadmill only, with non-significant increases during HIT-stepper and MCT-treadmill. IGF1 significantly increased during HIT-treadmill and HIT-stepper, with a non-significant increase during MCT-treadmill. Cortisol showed non-significant decreases during all three protocols.
Figure 1. Time course of changes in serum molecules by protocol.
(N=16; sessions=64; samples=331). Results from the primary analysis (left) and a sensitivity analysis separating HIT-stepper into original and revised protocols (right), presented as change from T0, with 95% confidence interval error bars. GXT, graded exercise test; HIT, high-intensity interval training; MCT, moderate-intensity continuous training
Table 1.
Acute exercise effects on serum molecules
| VEGF, pg/mL | IGF1, ng/mL | Cortisol, μg/dL | |
|---|---|---|---|
| Treadmill GXT session (N=16; sessions=16; samples=64) | |||
| GXT | +45.8 [−15.0, 106.5] | +1.4 [−2.2, 5.0] | +1.8 [−0.9, 4.4] |
| Primary analysis (N=16; sessions=48; samples=267) | |||
| HIT-treadmill | +49.2 [8.2, 90.2] | +3.7 [0.8, 6.6] | −0.5 [−1.5, 0.6] |
| HIT-stepper | +10.1 [−30.0, 50.2] | +4.2 [1.4, 7.1] | −0.6 [−1.7, 0.4] |
| MCT-treadmill | +10.5 [−31.3, 52.3] | +2.6 [−0.3, 5.6] | −0.5 [−1.6, 0.6] |
| HIT-treadmill vs MCT-treadmill | +38.7 [−19.8, 97.2] | +1.0 [−3.1, 5.2] | +0.0 [−1.5, 1.6] |
| HIT-stepper vs MCT-treadmill | −0.4 [−59.0, 58.2] | +1.6 [−2.5, 5.7] | −0.2 [−1.7, 1.4] |
| Comparison of HIT-stepper protocols (N=16; sessions=48; samples=267) | |||
| HIT-stepper-original (N=9) | +24.4 [−29.5, 78.2] | +3.8 [−0.0, 7.6] | −0.6 [−2.0, 0.8] |
| HIT-stepper-revised (N=7) | −9.3 [−73.1, 54.5] | +4.8 [0.3, 9.3] | −0.8 [−2.4, 0.9] |
| Revised vs. original | −33.6 [−118.6, 51.3] | +1.0 [−5.0, 7.1] | −0.2 [−2.4, 2.1] |
| Subgroup analysis: (N=16; sessions=48) | |||
| Comfortable gait speed ≥ 0.4 m/s | |||
| HIT-treadmill | +43.8 [5.8, 81.9] | +7.0 [3.7, 10.2] | −0.8 [−1.7, 0.2] |
| HIT-stepper | +28.9 [−9.6, 67.5] | +6.3 [3.0, 9.6] | −0.6 [−1.6, 0.4] |
| MCT-treadmill | −7.7 [−44.1, 28.7] | +4.9 [1.7, 8.1] | −2.0 [−3.0, −1.0] |
| Comfortable gait speed < 0.4 m/s | |||
| HIT-treadmill | +117.1 [34.2, 199.9] | −0.9 [−7.6, 5.7] | 1.0 [−1.1, 3.1] |
| HIT-stepper | +28.1 [−103.2, 159.5] | −0.6 [−8.1, 6.8] | −1.0 [−3.2, 1.2] |
| MCT-treadmill | +5.7 [−54.6, 66.1] | 0.8 [−5.3, 6.9] | 3.0 [1.1, 4.8] |
Values are estimated mean molecular responses [95% CI] with significant (p<0.05) values bolded. For the GXT, models included fixed effects for time and hemolysis with a compound symmetry covariance matrix to account for within-participant repeated measures. Biomarker response was the time contrast from T0 to post-GXT. For the primary analysis and comparison of HIT-stepper protocols, models included fixed effects for protocol, time, protocol × time, session number, session number × time, hemolysis and participant ID, with an unconstrained covariance matrix to account for within-participant, within-session repeated measures. Biomarker response was the time contrast from T0 to the mean of T5, T10 and T20. For the subgroup analysis, molecular response was the mean of T5, T10 and T20 minus T0 and models included fixed effects for subgroup, protocol, subgroup × protocol and baseline molecular concentration, with an unconstrained covariance matrix to account for within-participant repeated sessions.
GXT, graded exercise test; VEGF, vascular-endothelial growth factor; IGF1, insulin-like growth factor 1; HIT, high-intensity interval training; MCT, moderate-intensity continuous training
There were no significant between-protocol differences for any molecular response in the a priori primary analysis (Table 1). However, T0 VEGF was a significant predictor of VEGF exercise response (β=−0.03 [−0.06, −0.01]), and adjusting for T0 VEGF made the HIT-treadmill vs. MCT-treadmill response comparison statistically significant (+32.8 [9.9, 55.7]). T0 level was not a significant predictor of exercise responses for IGF1 (β=0.06 [−0.02, 0.14]) or cortisol (β=−0.13 [−0.28, 0.02]) and adjusting for T0 level did not make any between-protocol comparisons statistically significant for these molecules.
Biomarker responses to original and revised HIT-stepper protocols
In the sensitivity analysis separating the original and revised HIT-stepper protocols, there were no significant between-protocol differences in serum molecular responses (Fig 1 right; Table 1).
Mediating effects of different intensity measures on between-protocol differences in molecular responses
Mediating effects of blood lactate
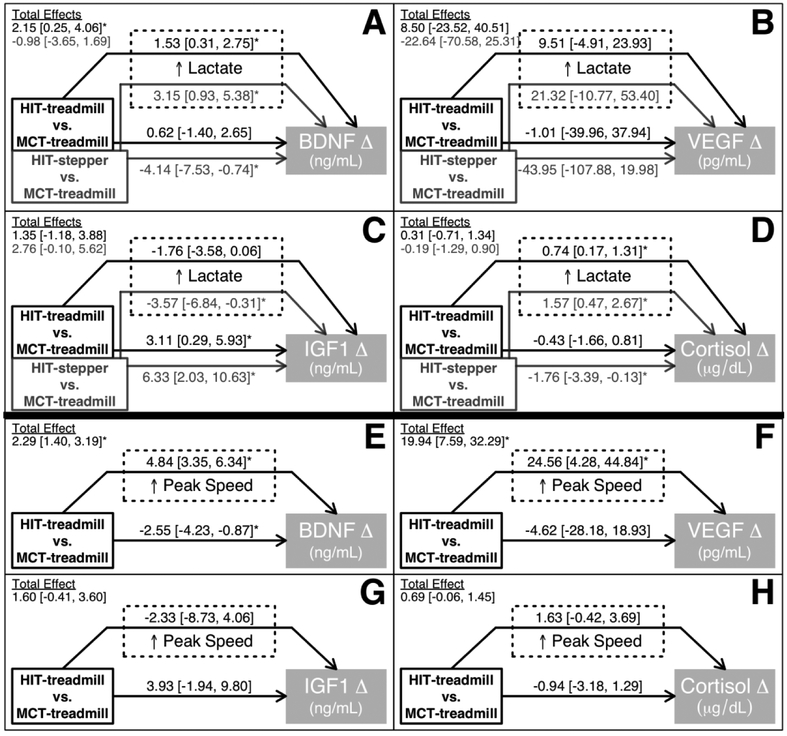
The higher lactate levels during HIT-treadmill and HIT-stepper (vs. MCT-treadmill) significantly increased BDNF responses by an estimated +1.53 and +3.15 ng/mL, respectively (Fig 2A). Outside of this lactate-mediated effect, other between-protocol differences non-significantly increased BDNF response by an estimated +0.62 ng/mL for HIT-treadmill (vs. MCT-treadmill) and significantly decreased BDNF response by an estimated −4.14 ng/mL for HIT-stepper (vs. MCT-treadmill). Consequently, the total effect estimate was a significantly greater BDNF response for HIT-treadmill vs. MCT-treadmill (+2.15 ng/mL) and no significant difference in BDNF response for HIT-stepper vs. MCT treadmill (−0.98 ng/mL).
Figure 2. Intensity mediators of between-protocol differences in serum molecular responses.
Values are model estimates [95% CI] in the units of the molecular response. A mediated effect (arrow going through a dashed box) is the estimated amount of molecular response that is attributable to between-protocol differences in lactate (panels A-D) or peak speed (panels E-H). A non-mediated effect (arrow not going through a dashed box) is the estimated amount of molecular response that is attributable to between-protocol differences other than lactate (panels A-D) or peak speed (panels E-H). A total effect (shown in upper left corner of each panel) is the estimated between-protocol difference in molecular response from the model and is the sum of the mediated and non-mediated effects. For example, panel A shows that the higher lactate levels for HIT-treadmill vs. MCT-treadmill increased the BDNF response by an estimated +1.53 ng/mL, while other between-protocol differences non-significantly increased the BDNF response by an estimated +0.62 ng/mL, making the total estimated between-protocol difference +2.15 ng/mL. Biomarker response was the mean of T5, T10 and T20 minus T0. Blood lactate was the mean of T5, T10 and T20. Covariates included protocol, session number, baseline molecular concentration and participant ID. Controlling for participant ID effectively adjusts for each participant’s mean mediator level and molecular response, so the results are based on within-participant between-protocol differences. *Statistically significant (p<0.05) effect. BDNF, brain-derived neurotrophic factor; VEGF, vascular-endothelial growth factor; IGF1, insulin-like growth factor 1.
The effect of HIT (vs. MCT) on VEGF was not significantly mediated through lactate (Fig 2B). For IGF1, the higher lactate levels during HIT (vs. MCT) decreased responses (significant for HIT-stepper only; Fig 2C). Outside of this negative lactate-mediated effect, other between-protocol differences significantly increased IGF1 responses for HIT, resulting in no significant total difference in IGF1 response for either HIT protocol compared with MCT-treadmill.
For cortisol, the higher lactate levels during HIT significantly increased responses (Fig 2D). Outside of this lactate-mediated effect, other between-protocol differences decreased cortisol responses for HIT (significant for HIT-stepper only), resulting in no significant total difference in cortisol response for either HIT protocol compared with MCT-treadmill.
Mediating effects of peak treadmill speed
The higher peak treadmill speeds for HIT-treadmill vs. MCT-treadmill significantly increased BDNF responses by an estimated +4.84 ng/mL (Fig 2E) and VEGF responses by an estimated +24.56 pg/mL (Fig 2F). Outside of these speed-mediated effects, other between-protocol differences decreased molecular responses for HIT (significant for BDNF only), but the total effects from these models still indicated that HIT-treadmill increased BDNF and VEGF significantly more than MCT treadmill. Peak speed did not significantly mediate the effects of HIT (vs. MCT) on IGF1 or cortisol. (Fig 2G–H)
Mediating effects of oxygen consumption rate and heart rate
Mean oxygen consumption rate and mean heart rate did not significantly mediate the effects of protocol on any serum molecular responses.
Effect modification based on clinical characteristics
Modifying effects of clinical characteristics on training intensity
Greater blood lactate during training was significantly associated with greater stroke chronicity for HIT-stepper only (Table 2). Faster peak treadmill speed during training was significantly associated with faster baseline gait speeds, female sex and higher peak oxygen consumption rate, but the associations with female sex and peak oxygen consumption rate were confounded by differences in baseline comfortable gait speed (Table 2). Mean oxygen consumption rate and mean heart rate during training were not significantly modified by any of the tested baseline characteristics, except that higher mean oxygen consumption rate was significantly associated with greater stroke chronicity for HIT-stepper only (like blood lactate).
Table 2. Modifying effects of clinical characteristics on training intensity and serum molecular responses.
(N=16; sessions=48)
| Clinical characteristics | Modifying effects on intensity | Modifying effects on serum molecular responses | ||||
|---|---|---|---|---|---|---|
| Mean lactate, mmol/L | Peak treadmill speed, m/s | VEGF, pg/mL | IGF1, ng/mL | Cortisol, μg/dL | ||
| Age (years) | 57.4 ± 9.7 | 0.0 [−0.0, 0.1] | −0.0 [−0.0, 0.0] | 0.2 [−1.6, 1.9] | 0.0 [−0.2, 0.2] | 0.1 [−0.0, 0.1] |
| Female sex | 7 (43.8%) | −0.3 [−1.8, 1.2] | 0.4 [0.1, 0.8]* | −14.5 [−47.8, 18.7] | −2.2 [−5.2, 0.8] | −1.3 [−3.0, 0.4] |
| Years post stroke | 6.5 ± 4.1 | 0.2 [0.0, 0.3]a | −0.0 [−0.1, 0.0] | −1.6 [−5.6, 2.4] | −0.1 [−0.5, 0.3] | −0.0 [−0.2, 0.2] |
| Body mass index (kg/m2) | 27.6 ± 3.7 | −0.1 [−0.3, 0.1] | 0.0 [−0.1, 0.1] | −0.3 [−5.6, 5.1] | −0.0 [−0.6, 0.5] | 0.0 [−0.2, 0.3] |
| Comfortable gait speed (m/s) | 0.72 ± 0.33 | −0.6 [−2.9, 1.8] | 0.9 [0.5, 1.2] | −37.6 [−77.9, 2.8] | 5.1 [0.2, 10.1] | −3.5 [−5.5, −1.6]b |
| Comfortable gait speed ≥ 0.4 m/s | 13 (81.3%) | −0.9 [−2.8, 1.0] | 0.6 [0.1, 1.0] | −30.4 [−59.2, −1.5] | 6.4 [3.4, 9.4] | −2.7 [−4.1, −1.4]b |
| Fast gait speed (m/s) | 0.90 ± 0.41 | −0.9 [−2.7, 1.0] | 0.7 [0.4, 1.0] | −22.6 [−57.8, 12.6] | 3.4 [−0.7, 7.5]a | −2.7 [−4.4, −0.9]b |
| VO2peak (mL/kg/min) | 17.2 ± 3.3 | 0.1 [−0.1, 0.3]b | 0.1 [0.0, 0.1]* | −3.0 [−7.8, 1.8] | 0.4 [−0.1, 0.9] | −0.2 [−0.4, 0.1]b |
| HRpeak (bpm) | 139 ± 21 | 0.0 [−0.0, 0.0] | 0.0 [−0.0, 0.0] | 0.5 [−0.2, 1.2] | −0.0 [−0.1, 0.0] | 0.0 [−0.0, 0.1] |
Values are mean ± SD, N (%) or estimated mean [95% CI] with significant (p<0.05) values bolded. Lactate is the mean of T5, T10 and T20. Biomarker response is the mean of T5, T10 and T20 minus T0. Analyses were adjusted for baseline molecular concentration (serum molecular analyses only) and protocol and included an unconstrained covariance matrix to account for within-participant repeated sessions.
VEGF, vascular-endothelial growth factor; IGF1, insulin-like growth factor 1; VO2, oxygen consumption rate; peak, maximum value reached during symptom-limited, graded exercise test; HRR, heart rate reserve
Effects found to be confounded by gait speed. Regression coefficients for female sex and VO2peak decreased by 85% and 50%, respectively, and became non-significant after adding comfortable gait speed to the model, which remained a significant factor in both cases.
Significant (p<0.05) protocol × characteristic interaction. Effect modification significant for aHIT-stepper only or bMCT-treadmill only.
Modifying effects of clinical characteristics on serum molecular responses
Baseline comfortable gait speed category was a significant effect modifier for VEGF, IGF1 and cortisol responses and explained more variability in these responses than any other characteristic, including continuous measures of gait speed (Table 2). Compared with participants who had gait speed < 0.4 m/s, participants with gait speed ≥ 0.4 m/s had significantly lower VEGF responses and higher IGF1 responses, which were not protocol-specific, and significantly lower cortisol responses for MCT-treadmill only.
VEGF significantly increased during HIT-treadmill for both gait speed subgroups (Table 1), but the magnitude was significantly greater for the low gait speed subgroup. IGF1 only increased significantly for the higher gait speed subgroup, and these increases were significant for all three protocols. Cortisol significantly decreased during MCT-treadmill for the higher gait speed subgroup, but significantly increased during MCT-treadmill for the low gait speed subgroup.
Discussion
Treadmill HIT was recently found to elicit significantly greater increases in serum BDNF and corticospinal excitability compared with conventional treadmill MCT.14 Using the same serum samples, the current study tested the intensity-dependence of additional molecular responses related to neuroplasticity, and incorporated novel mediation and effect modification analyses. For the first time among humans with stroke, we now report significant acute increases in serum VEGF and IGF1 during exercise, without a concomitant cortisol stress response, using treadmill HIT. A significant IGF1 increase was also found during seated stepper HIT. Neither VEGF nor IGF1 significantly increased during conventional treadmill MCT (for the study sample as a whole).
Adding to the previous report,14 serum BDNF response also showed additional evidence of intensity-dependence in the current analysis, as it was positively mediated by both blood lactate and peak treadmill speed during training. Aside from BDNF, VEGF showed the strongest evidence of intensity-dependence. Although the between-protocol difference did not exceed the statistical significance threshold in the primary (unadjusted) analysis, VEGF response was over 300% greater for HIT-treadmill vs. MCT-treadmill. Further, this difference was statistically significant when controlling for baseline VEGF level and was significantly mediated by faster treadmill speeds during HIT. Relative to BDNF and VEGF, IGF1 had less evidence of intensity dependence. While the IGF1 response was 42% greater for HIT-treadmill vs. MCT-treadmill, this difference was not statistically significant and was not positively mediated by any intensity variable. In fact, higher lactate values during HIT were found to have decreased the IGF1 response. When statistically controlling for lactate effects, both HIT protocols elicited significantly greater IGF1 responses than MCT through other unknown mechanisms, but these between-protocol differences were cancelled out by the negative lactate effects. Thus, BDNF and VEGF appear to be the most promising serum molecules tested for potentially explaining some of the intensity-dependent effects of exercise on neurologic recovery from stroke. Future longitudinal trials are now warranted to determine the extent to which these molecules mediate the effects of exercise on rehabilitation outcomes.
Human stroke studies have consistently shown significant benefits or at least trends in neurologic function outcomes favoring higher intensity exercise over moderate or low intensity.2–5 Conversely, non-human studies have sometimes found worse outcomes with high-intensity exercise.52–56 In these animals, high intensity exercise also typically elicits significant stress-related elevations in serum corticosterone (the animal analog of cortisol)30,56 and aggression.57 While small increases in cortisol or corticosterone can have positive effects on central neuroplasticity,29 excessive elevations have been shown to have negative effects.28,30,31 The lack of cortisol stress response during human post-stroke HIT in the current study could explain some of these observed between-species differences in optimal intensity. We speculate that humans may have less psychological stress during high-intensity exercise because of voluntary participation and easier communication between researchers and participants.
However, some previous human (non-stroke) studies have observed significant cortisol increases during exercise, generally using continuous and vigorous exercise protocols lasting at least 60 minutes, or shorter protocols with very high intensity.27,58 In the current study, higher lactate during HIT (vs. MAT) did appear to mediate a greater cortisol response, but this between-protocol difference was cancelled out by other factors (potentially the recovery intervals during HIT59). In the subgroup analysis, participants with comfortable gait speed <0.4 m/s had a relatively small but significant cortisol increase during treadmill MCT, but not HIT. It is possible that treadmill exercise provoked anxiety for these participants and that the recovery intervals during HIT helped mitigate that stress, despite the faster speeds.
Persons with stroke who have baseline comfortable gait speed > 0.4 m/s have been previously shown to have greater improvements in walking capacity from continuous treadmill training.49 In the current study, this higher gait speed subgroup had greater serum IGF1 response and a lower serum cortisol response. This raises the hypothesis that greater serum IGF1 responses and/or lower serum cortisol responses could be related to greater training responsiveness. Contrary to our expectations, these differences in serum molecular responses between gait speed subgroups were not due to differences in training speed (see Supplemental Methods and Results), and the mechanisms remain unknown.
Another surprise was that the low gait speed subgroup had a 167% greater serum VEGF response to HIT-treadmill. Based on animal research, an acute exercise-induced increase in circulating VEGF could have beneficial effects on vascular and cognitive outcomes.25 If an acute circulating VEGF response has similar benefits in humans, and if those effects are dose-dependent, it is possible that persons with low gait speed could have preferential vascular or cognitive benefits from HIT.
One issue with the present subgroup analysis is that there were only three participants with gait speed <0.4 m/s. While the repeated measures helped provide sufficient power to detect significant subgroup effects, the response estimates for this subgroup may be particularly prone to fluctuate in future studies. Still, significant effect modification was also present in the study sample as a whole when modeling gait speed as a continuous variable. Further, the dichotomous gait speed subgrouping explained more of the variance in serum molecular responses than the continuous measure. This provides novel biochemical evidence of an important comfortable gait speed threshold around 0.4 m/s.
Limitations and future studies
The primary limitation to this study is that we did not test the effects of the protocols or serum molecular responses on learning or longitudinal outcomes. Previous non-stroke studies have reported exercise-induced learning enhancement with similar or lesser serum molecular responses than those we found with HIT-treadmill.12,13 However, it is still unclear how much different types of learning can be enhanced through upregulation of BDNF, VEGF and IGF1. The current results provide foundational evidence that HIT-treadmill and MCT-treadmill protocols would be useful to include in future longitudinal trials to test mediating effects of BDNF, VEGF and possibly IGF1 on rehabilitation outcomes.
Future stroke studies might also consider testing the mediating effects of circulating catecholamines (e.g. dopamine, epinephrine, norepinephrine) on exercise-induced learning enhancement. Catecholamines were not tested in this study because they must be measured in plasma (not serum), which would have increased the blood collection volume and time. However, catecholamine concentrations have been shown to acutely rise during exercise among healthy adults and their post-exercise concentrations have been correlated with learning enhancement.12,13 Future studies are also needed to provide a stronger base of normative data for comparison to the current results. Most previous studies testing the acute effects of exercise on circulating neurotrophins have recruited young healthy adults, and it is still unclear whether large age differences or stroke might affect these responses.
We acknowledge that serum assays provide imperfect measures of central nervous system processes.13 For example, acute exercise-related brain changes in animals typically outlast corresponding increases in circulating molecular concentrations,10,11 so the lack of sustained serum molecular changes in Figure 1 should be interpreted with caution. Brain levels of these molecules would likely be more related to learning but are not obtainable non-invasively.
For the mediation models to be truly causal, there cannot be any confounding factors affecting both the mediator (e.g. lactate) and outcome (e.g. BDNF change),34 aside from cofactors included in the model (protocol, session number, baseline molecular concentration and participant ID). We cannot completely rule out this possibility but believe that uncontrolled confounding was unlikely to be a major problem because the models were able to discriminate between different mediators for different molecules in ways that are biologically plausible. For example, lactate infusion at rest has been shown to increase circulating BDNF,60 and the models identified blood lactate, but not oxygen consumption rate or heart rate, as a positive mediator of BDNF response.
Conversely, the mediation analysis also found that blood lactate positively mediated cortisol response and there is no established causal pathway through which lactate directly upregulates circulating cortisol. However, correlations between blood lactate and serum cortisol responses have been previously observed among healthy adults,59 anaerobic exercise has a greater effect on cortisol than aerobic exercise,61 and it has been hypothesized that circulating lactate could act as a chemical messenger to stimulate the hypothalamic-pituitary-adrenal axis.62 Thus, it is possible that this finding reflects true causal mediation.
The mediation analysis also does not rule out the possibility of additional upstream, downstream or parallel mediators. For example, we speculate that faster treadmill training speed may have mediated greater VEGF response because faster speeds cause greater blood pressure fluctuations and vascular shearing forces.63
In a previous analysis,14 BDNF response was not significantly associated with any clinical characteristics. However, in the current subgroup effect mediation analysis of the same data (which included additional covariates and only the treadmill protocols), the low comfortable gait speed subgroup showed a significantly greater total BDNF response (see Supplemental Methods and Results). It is possible that this subgroup effect was somehow obscured by confounding in the previous analysis, and this could be explored in future studies.
Conclusions
A treadmill HIT protocol can elicit acute increases in circulating BDNF, VEGF and IGF1 without a significant cortisol stress response, among ambulatory persons with chronic stroke. The faster treadmill speeds and higher blood lactate levels reached during HIT appear to be critical ingredients for eliciting BDNF and VEGF responses. A similar HIT protocol on a seated stepper enabled even higher intensities and similar BDNF and IGF1 responses but needs further testing to balance safety and benefit. BDNF and VEGF appear to be the most promising serum molecules to include in future studies testing intensity-dependent mechanisms of exercise on neurologic recovery from stroke. We also found significantly greater IGF1 responses and lesser cortisol responses for participants with comfortable gait speed > 0.4 (vs ≤ 0.4), leading to the hypothesis that these molecular responses could explain previously reported differences in walking capacity responsiveness between these subgroups.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Heart Association [grant number 17MCPRP33670446]; the National Institutes of Health [grant numbers UL1TR000077, KL2TR001426 and R01HD093694); the Foundation for Physical Therapy [Promotion of Doctoral Studies Scholarship]; and the University of Cincinnati Neuroscience Institute [Pilot Research Award].
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: Review of multiple meaningful benefits. Stroke. 2014;45(12):3742–3747. [DOI] [PubMed] [Google Scholar]
- 2.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. High-intensity interval training in stroke rehabilitation. Top Stroke Rehabil. 2013;20(4):317–330. [DOI] [PubMed] [Google Scholar]
- 3.Boyne P, Dunning K, Carl D, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: A feasibility study. Phys Ther. 2016;96(10):1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyne P, Welge J, Kissela B, Dunning K. Factors influencing the efficacy of aerobic exercise for improving fitness and walking capacity after stroke: A meta-analysis with meta-regression. Arch Phys Med Rehabil. 2017;98(3):581–595. doi: S0003-9993(16)31143-1 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornby TG, Holleran CL, Hennessy PW, et al. Variable intensive early walking poststroke (VIEWS): A randomized controlled trial. Neurorehabil Neural Repair. 2016;30(5):440–50. [DOI] [PubMed] [Google Scholar]
- 6.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93(12):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt J, Borschmann K, Boyd L, et al. Moving rehabilitation research forward: Developing consensus statements for rehabilitation and recovery research. Neurorehabil Neural Repair. 2017;31(8):694–698. doi: 10.1177/1545968317724290 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.VandenBerg PM, Bruneau RM. BDNF is required for maintaining motor map integrity in adult cerebral cortex. Soc Neuroci Abst. 2004;681:5. [Google Scholar]
- 9.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. [DOI] [PubMed] [Google Scholar]
- 10.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. [DOI] [PubMed] [Google Scholar]
- 11.Quirie A, Hervieu M, Garnier P, et al. Comparative effect of treadmill exercise on mature BDNF production in control versus stroke rats. PLoS One. 2012;7(9):e44218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter B, Breitenstein C, Mooren FC, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87(4):597–609. [DOI] [PubMed] [Google Scholar]
- 13.Skriver K, Roig M, Lundbye-Jensen J, et al. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol Learn Mem. 2014;116:46–58. [DOI] [PubMed] [Google Scholar]
- 14.Boyne P, Meyrose C, Westover J, et al. Exercise intensity affects acute neurotrophic and neurophysiologic responses post-stroke. Journal of Applied Physiology. 2019;126(2):431–433. doi: 10.1152/japplphysiol.00594.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahl P, Jansen F, Achtzehn S, et al. Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors. PLoS One. 2014;9(4):e96024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonoli C, Heyman E, Buyse L, et al. Neurotrophins and cognitive functions in T1D compared with healthy controls: Effects of a high-intensity exercise. Appl Physiol Nutr Metab. 2015;40(1):20–27. [DOI] [PubMed] [Google Scholar]
- 17.Wahl P, Zinner C, Achtzehn S, Behringer M, Bloch W, Mester J. Effects of acid-base balance and high or low intensity exercise on VEGF and bFGF. Eur J Appl Physiol. 2011;111(7):1405–1413. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief lowand high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81(10):3492–3497. [DOI] [PubMed] [Google Scholar]
- 19.Ruan L, Wang B, ZhuGe Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg. 2010;23(3):149–155. doi: 10.3109/08941930903469482 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Aleman I Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70(5):384–396. [DOI] [PubMed] [Google Scholar]
- 23.Llorens-Martin M, Torres-Aleman I, Trejo JL. Growth factors as mediators of exercise actions on the brain. Neuromolecular Med. 2008;10(2):99–107. [DOI] [PubMed] [Google Scholar]
- 24.De Geyter D, De Smedt A, Stoop W, De Keyser J, Kooijman R. Central IGF-I receptors in the brain are instrumental to neuroprotection by systemically injected IGF-I in a rat model for ischemic stroke. CNS Neurosci Ther. 2016;22(7):611–616. doi: 10.1111/cns.12550 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabel K, Fabel K, Tam B, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. [DOI] [PubMed] [Google Scholar]
- 26.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duclos M, Tabarin A. Exercise and the hypothalamo-pituitary-adrenal axis. Front Horm Res. 2016;47:12–26. doi: 10.1159/000445149 [doi]. [DOI] [PubMed] [Google Scholar]
- 28.Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28(33):8285–8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray JD, Milner TA, McEwen BS. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goekint M, Bos I, Heyman E, Meeusen R, Michotte Y, Sarre S. Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain-derived neurotrophic factor. J Appl Physiol (1985). 2012;112(4):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813(1):112–120. [DOI] [PubMed] [Google Scholar]
- 32.Corraini P, Olsen M, Pedersen L, Dekkers OM, Vandenbroucke JP. Effect modification, interaction and mediation: An overview of theoretical insights for clinical investigators. Clin Epidemiol. 2017;9:331–338. doi: 10.2147/CLEP.S129728 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerin E, Taylor LM, Leslie E, Owen N. Small-scale randomized controlled trials need more powerful methods of mediational analysis than the baron-kenny method. J Clin Epidemiol. 2006;59(5):457–464. doi: S0895-4356(05)00395-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 34.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–195. doi: 10.1093/aje/kwr525 [doi]. [DOI] [PubMed] [Google Scholar]
- 35.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair. 2017;31(9):793–799. doi: 10.1177/1545968317732668 [doi]. [DOI] [PubMed] [Google Scholar]
- 36.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadephia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 37.Ashworth B Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 38.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: Training protocols and treatment effects. Topics in Stroke Rehabilitation. 2005;12(1):45–57. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: A scientific statement from the american heart association. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 40.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. Within-session responses to high-intensity interval training in chronic stroke. Med Sci Sports Exerc. 2015;47(3):476–84. [DOI] [PubMed] [Google Scholar]
- 41.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: A randomized, controlled trial. Stroke. 2005;36(10):2206–2211. [DOI] [PubMed] [Google Scholar]
- 42.Crouter SE, Antczak A, Hudak JR, DellaValle DM, Haas JD. Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol. 2006;98(2):139–151. [DOI] [PubMed] [Google Scholar]
- 43.Stotler BA, Kratz A. Analytical and clinical performance of the epoc blood analysis system: Experience at a large tertiary academic medical center. Am J Clin Pathol. 2013;140(5):715–720. [DOI] [PubMed] [Google Scholar]
- 44.Jones B, Kenward MG. Design and analysis of cross-over trials. 3rd ed. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 45.Guo Y, Logan HL, Glueck DH, Muller KE. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol. 2013;13:10–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stillman CM, Cohen J, Lehman ME, Erickson KI. Mediators of physical activity on neurocognitive function: A review at multiple levels of analysis. Front Hum Neurosci. 2016;10:626. doi: 10.3389/fnhum.2016.00626 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stijn V, Maarten B, Theis L. Imputation strategies for the estimation of natural direct and indirect effects. Epidemiologic Methods. 2012;1:131. doi: 10.1515/2161-962X.1014. [DOI] [Google Scholar]
- 48.Steen J, Loeys T, Moerkerke B, Vansteelandt S. Medflex: Flexible mediation analysis using natural effect models. R package version 0.6-0. 2015. [Google Scholar]
- 49.Dean CM, Ada L, Lindley RI. Treadmill training provides greater benefit to the subgroup of community-dwelling people after stroke who walk faster than 0.4m/s: A randomised trial. J Physiother. 2014;60(2):97–101. [DOI] [PubMed] [Google Scholar]
- 50.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–2100. [DOI] [PubMed] [Google Scholar]
- 51.Revelle W Psych: Procedures for personality and psychological research. North-Western University, Evanston. R package version 1.8.6. 2018. [Google Scholar]
- 52.Hasan SM, Rancourt SN, Austin MW, Ploughman M. Defining optimal aerobic exercise parameters to affect complex motor and cognitive outcomes after stroke: A systematic review and synthesis. Neural Plast. 2016;2016:2961573. doi: 10.1155/2016/2961573 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennard JA, Woodruff-Pak DS. A comparison of low- and high-impact forced exercise: Effects of training paradigm on learning and memory. Physiol Behav. 2012;106(4):423–427. doi: 10.1016/j.physbeh.2012.02.023 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086 [doi]. [DOI] [PubMed] [Google Scholar]
- 55.Nokia MS, Lensu S, Ahtiainen JP, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594(7):1855–1873. doi: 10.1113/JP271552 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ploughman M, Granter-Button S, Chernenko G, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: S0006-8993(07)00495-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 57.Svensson M, Lexell J, Deierborg T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: What we can learn from animal models in clinical settings. Neurorehabil Neural Repair. 2015;29(6):577–589. doi: 10.1177/1545968314562108 [doi]. [DOI] [PubMed] [Google Scholar]
- 58.Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121(1):59–65. [DOI] [PubMed] [Google Scholar]
- 59.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 3544 [pii]. [DOI] [PubMed] [Google Scholar]
- 60.Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Struder HK. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett. 2011;488(3):234–237. [DOI] [PubMed] [Google Scholar]
- 61.Hackney AC, Premo MC, McMurray RG. Influence of aerobic versus anaerobic exercise on the relationship between reproductive hormones in men. J Sports Sci. 1995;13(4):305–311. doi: 10.1080/02640419508732244 [doi]. [DOI] [PubMed] [Google Scholar]
- 62.Luger A, Deuster PA, Kyle SB, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. physiologic adaptations to physical training. N Engl J Med. 1987;316(21):1309–1315. doi: 10.1056/NEJM198705213162105 [doi]. [DOI] [PubMed] [Google Scholar]
- 63.Lucas SJ, Cotter JD, Brassard P, Bailey DM. High-intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J Cereb Blood Flow Metab. 2015;35(6):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.