Abstract
Testosterone has been shown to have dose-dependent effects on spatial memory in males, but the effects of aging upon this relationship remain unclear. Additionally, the mechanism by which testosterone regulates memory is unknown, but may involve changes in brain-derived neurotrophic factor (BDNF) within specific brain regions. We tested the effects of age and testosterone on spatial memory among male rats using two spatial memory tasks: an object-location memory task (OLMT) and the radial-arm maze (RAM). Castration had minimal effect on performance on the RAM, but young rats (2 months) performed significantly fewer working memory errors than aged rats (20 months), and aged rats performed significantly fewer reference memory errors. Both age and castration impaired performance on the OLMT, with only the young rats with intact gonads successfully performing the task. Subsequent experiments involved daily injections of either drug vehicle or one of four doses of testosterone propionate (0.125, 0.250, 0.500, and 1.00 mg/rat) given to castrated aged males. On the RAM, a low physiological dose (0.125 mg) and high doses (0.500–1.000 mg) of testosterone improved working memory, while an intermediate dose (0.250 mg) did not. On the OLMT, only the 0.250 mg T group showed a significant increase in exploration ratios from the exposure trials to the testing trials, indicating that this group remembered the position of the objects. Brain tissue (prefrontal cortex, hippocampus, and striatum) was collected from all subjects to assay BDNF. We found no evidence that testosterone influenced BDNF, indicating that it is unlikely that testosterone regulates spatial memory through changes in BDNF levels.
Keywords: testosterone, spatial memory, aging, radial arm maze, object location memory, BDNF
1. Introduction
Reduced endogenous testosterone may be an important contributor to age-related memory loss in men (Handelsman et al., 2015). Testosterone decline begins as early as age 30, and hypogonadism occurs in 20% of men 60–69 year olds and in 50% of men over 80 (Feldman et al., 2002). Numerous studies have shown a positive correlation between testosterone levels and cognitive ability in older men, including better spatial memory, suggesting that a reduction in testosterone levels may be partially responsible for age-related memory deficits in men (Barrett-Connor et al., 1999; Yaffe et al., 2002; Moffat et al., 2002). Additionally, testosterone reduction through age-related hypogonadism or artificial androgen deprivation has been identified as a risk factor for dementia (Lv et al., 2016; Nead et al., 2017). Testosterone supplementation improved spatial memory among patients with Alzheimer’s disease or mild cognitive impairment (Cherrier et al., 2005; Tan and Pu, 2003), but the benefits of androgen replacement therapies have been inconsistent (Kenny et al., 2004; Lu et al., 2006). Studies with healthy older men have also produced mixed results regarding the effects of androgen supplementation on cognitive ability (Cherrier et al., 2001; Vaughan et al., 2007; Wolf et al., 2000). These discrepancies may be due to differences among studies in the duration of hormone exposure, doses of testosterone used, and the specific aspects of spatial memory tested. Controlled experiments with animal models provide a useful way to disentangle these variables.
Spatial memory is one cognitive process that seems to be particularly influenced by both circulating testosterone and aging (Holland et al., 2011). Involving aspects of procedural, declarative, as well as both short-term and long-term memory, spatial memory is the process of recognizing, encoding, storing, and recalling spatial information about surroundings, positions of objects, or specific routes (Moscovitch et al., 2006). Testosterone seems to differentially influence the working and reference components of spatial memory. Working memory is a form of short-term memory that involves storage of relevant information while completing a specific task, whereas reference memory involves long-term storage of information from one task to be used for the next task (Cowan, 2008). Using a rat model, we recently demonstrated that testosterone enhances spatial working memory but has no effect on spatial reference memory (Wagner et al., 2018), which corroborates previous findings (Bimonte-Nelson et al., 2003; Gibbs and Johnson, 2008; Spritzer et al., 2011, 2008). However, experiments using the object location memory task (OLMT) suggest that testosterone influences some forms of long-term memory. Specifically, testosterone replacement was shown to improve spatial memory among castrated young adult male rats on the OLMT following both a 30 min retention period (McConnell et al., 2012) and a 2 h retention period (Jacome et al., 2016; Wagner et al., 2018). Thus, the length of the retention period may be a critical variable influencing the memory-enhancing effects of testosterone.
Considerable evidence from studies with humans and rodents suggests that the relationship between circulating testosterone levels and spatial memory is non-linear (Muller et al., 2005; Nowak et al., 2014). Testing male rats on the Morris Water Maze (MWM), we previously demonstrated that certain doses of testosterone (0.250 and 1.00 mg/rat) impaired reversal learning (Spritzer et al., 2011). Using other memory tasks (RAM and OLMT), we demonstrated that high and low physiological doses of testosterone (0.125 and 0.500 mg/rat) improved spatial memory, whereas an intermediate dose (0.250 mg/rat) did not (Wagner et al., 2018). Another study with rats suggested a curvilinear relationship between testosterone and spatial reference memory, with a high physiological dose (0.750 mg/rat) of testosterone optimal for performance (Jia et al., 2013). Administering supra-physiological doses of testosterone has either no effect on memory (Gibbs, 2005) or impairs spatial memory (Emamian et al., 2010). Additionally, human studies suggest that the optimal level of testosterone for spatial memory varies with age (Holland et al., 2011), but this hypothesis has yet to be tested experimentally.
The physiological mechanism by which testosterone influences spatial memory remains unknown. The hippocampus, striatum, and prefrontal cortex have all been implicated in spatial memory (Compton et al., 1997; Liljeholm and O’Doherty, 2012), making them potential direct targets for testosterone. The hippocampus is essential for place learning (i.e., the processing of information about the positions of environmental cues relative to a goal and one’s own position), whereas the striatum is essential for response learning (i.e., the use of stimulus-response relationships to locate a goal). Initially, both the hippocampus and striatum are engaged in parallel to solve a spatial task, and then the striatum becomes more important as the task becomes habitual (Chang and Gold, 2003; Jacobson et al., 2012; Packard and McGaugh, 1996). The pre-frontal cortex seems to be more involved in response learning, and is specifically engaged during spatial working memory tasks to plan the next choice (de Bruin et al., 1997; Floresco et al., 1997). Evidence suggests that the pre-frontal cortex is not essential for performance on the OLMT (Chao et al., 2016) but is employed in parallel with other brain regions for solving RAM tasks (Floresco et al., 1997; Kolb et al., 1994). Due to its critical role in neural plasticity in spatial cognition, brain-derived neurotrophic factor (BDNF) could play a key role in the connection between and testosterone cognitive processing in select brain regions (Egan et al., 2003; Tyler et al., 2002). Some evidence indicates that male rats have higher hippocampal levels of BDNF than do females (Franklin and Perrot-Sinal, 2006). Although BDNF levels have been found to decrease with age in male rats, this decline does not seem to correlate with age-related changes in testosterone (Bimonte-Nelson et al., 2008). Consequently, the relationship between testosterone and BDNF remains unclear and, therefore, merits further investigation.
Few past studies have tested the effects of testosterone on spatial memory in aged rodents (Bimonte-Nelson et al., 2003; Goudsmit et al., 1990), and none have used a broad dose range. Building upon our past research with young rats (Wagner et al., 2018), the present study tested the effects of both age and testosterone dose on spatial memory. Initial experiments tested the effects of castration and age by comparing the performance of 20-month-old and 2-month-old male rats on the RAM and OLMT. Subsequent experiments used the same tasks to test the cognitive effects of testosterone replacement on aged males. We also quantified BDNF levels in the hippocampus, striatum, and prefrontal cortex to assess a possible mechanistic link between testosterone and BDNF.
2. Materials and methods
2.1. Subjects
Young (2 months) and aged (20–22 months) Fischer 344 rats were obtained from the Aged Rodent Colony within National Institute on Aging (Bethesda, MD, USA). Rats were either pair housed or triple housed in opaque polypropylene cages (21 × 42 × 21 cm) filled with Tek-Fresh bedding (Harlan Laboratories, Indianapolis, IN, USA). Animals had free access to tap water from glass bottles and were fed a soy-protein-free rodent diet (Harlan Teklad Diet 2020X). Housing rooms and testing rooms were maintained at constant temperature (24±5 °C) and humidity (50% humidity) with a 12:12 hour light-dark cycle (lights on at 0700 h EST). All animal procedures were approved by the Middlebury College Institutional Animal Care and Use Committee (IACUC) and were carried out in accordance with the ethical guidelines set by the National Institutes of Health.
Rats were operated on following aseptic technique under isoflurane anesthesia (3.5% in oxygen during induction; 2.0–3.0% in oxygen during maintenance). Just prior to surgery, animals were injected with analgesic (Ketofen, 5 mg/kg body mass, s.c.). For castration surgeries, both testes were removed through a single incision at the posterior end of the scrotum and then cauterized and ligated with chromic gut suture material. Sham castrations involved incisions into the skin and muscular sheath without removal of the testes. For all surgeries, the muscle and skin layers were closed with chromic gut sutures and nylon sutures, respectively. Directly following surgeries, topical antibiotic was applied to the surgical site and subjects were administered an injection of lactated Ringer’s solution (10 ml/kg, s.c.). An additional Ketofen injection (5 mg/kg) was administered within 24 h or surgery.
2.2. Apparatus
Radial-arm maze (RAM) testing was conducted in a 250 × 280 cm room with dim lighting (50 lux). The RAM consisted of eight polycarbonate arms (57 × 10 cm) radiating out of a central platform (115 cm diameter) 54 cm above the floor. Reward cups (1.5 cm height) at the end of each arm prevented rats from seeing the reward pellets until they reached the end of an arm. Large, high-contrast posters were mounted on two adjacent walls as visual cues. Following placement of the rat in the maze, the researcher remained in the same corner of the room during all testing. To reduce intra-maze cues, the maze was rotated clockwise a random number of arms (increments of 45°) each day before testing. The maze was wiped with 70% ethanol between all trials.
The Object location memory task (OLMT) was conducted in a larger dimly lit room (85 lux) in an open field arena that was an open-topped grey-painted polycarbonate box (100 × 100 × 46 cm) elevated 33 cm above the floor. A curtain separated the testing area (210 × 420 cm) from the rest of the room. Two posters, used as visual cues, were mounted on two adjoining walls. Immediately after placing animals in the arena for each trial, the researcher moved behind the curtain. The arena was wiped with 70% ethanol between all trials. All behavioral data were recorded using a digital USB camera mounted on the ceiling above the arena and interfaced with a video tracking program on a laptop computer (ANY-maze™ Video Tracking System, 4.115; Stoelting Co., Wood Dale, IL, USA).
2.3. Experiment 1: RAM
Castrated (GDX) and Sham-castrated (Sham) rats were used for two sub-experiments (Experiments 1A and 1B) on the RAM. For Experiment 1A, we tested the relative effects of castration and age (2 months old vs. 20 months old) on spatial memory using four groups of rats (n = 10–12/group): Old/Sham, Old/GDX, Young/Sham, and Young/GDX. For Experiment 1B, we divided 20-month-old rats into six groups (n = 12/group). Sham-castrated and castrated control groups received daily s.c. injections of 0.1 ml sesame oil (Oil Sham and Oil GDX). The other treatment groups were all castrated and received daily s.c. injections of one of four doses (mg/rat) of testosterone propionate (Sigma-Aldrich, St. Louis, MO) dissolved in 0.1 ml of sesame oil: 0.125, 0.250, 0.500, and 1.000 mg T. The rats used for Experiment 1B had an average body mass of 435.7 ± 3.43 g just prior to starting injections, and there was no significant difference in mass among the treatment groups (p = 0.10). These doses produce serum testosterone concentrations ranging from physiological to supra-physiological levels (Wagner et al., 2018). All injections were performed in the morning (0800–1000 h), approximately 4 h before the start of each testing session. Injections began 15–16 days after surgery and continued until the day of euthanasia (Table 1). Seven days of injections were given prior to the start of any behavioral testing (Spritzer et al., 2011b).
Table 1.
Timelines for Experiments 1 and 2, with the sequential days shown during which each procedure was conducted.
| Experiment task | GDX | Handle | Habituate and shape* | Testosterone injections | Testing | Euthanasia |
|---|---|---|---|---|---|---|
| 1A RAM | 1–2 | 10–13 | 16–22 | NA | 23–47 | 48 |
| 1B RAM | 1–2 | 10–13 | 16–22 | 16–48 (33 days) | 23–47 | 48 |
| 2A OLMT | 1 | 9–12 | 11–14; 16–19 | NA | 15; 20 | 21 |
| 2B OLMT | 1 | 9–12 | 11–14; 16–19 | 8–21 (14 days) | 15; 20 | 21 |
NA (not applicable) refers to experiments in which no testosterone injections were used.
GDX (gonadectomy) refers to the days when castration surgeries were performed.
For Experiment 2 there was no shaping period and the two habituation periods were discontinuous.
Following a seven-day surgery recovery period, rats were handled for 4–5 min per day for four days. Subjects were placed on food restriction (85% of free-feeding body mass) starting 7–8 days after surgery and continuing through the day of euthanasia. The first day of maze habituation began after one week of food restriction (i.e., 15–16 days after castration surgeries). Dustless reward pellets (45 mg; Bio-Serv, Frenchtown, NJ) were used to motivate rats on the maze. Our protocol consisted of two habituation days, 4–5 shaping days, and 25 consecutive testing days. Each rat was tested in the afternoon (1200–1700 h) by first placing it in the center of the maze. An arm entry was scored when the rat’s hind legs passed from the center of the maze into an arm. During maze habituation, rats were allowed to explore the maze for 5 min on the first day and 10 min on the second day. No reward pellets were present on the maze during habituation. Subjects received 10 reward pellets in their home cages during each day of habituation and shaping. During the first day of shaping, three reward pellets were placed at equidistant intervals along all eight maze arms, and one pellet was placed in each reward cup. On subsequent shaping days, the number of reward pellets along each arm was reduced by one each day. If a rat had not entered every arm at least once within the trial period (10 min maximum) by the end of the fourth shaping day, it was given a fifth shaping day, and a rat was excluded from the experiment if it did not enter every arm on the fifth shaping day. During testing days, rats were assigned a pseudo-random set of four baited arms that remained consistent for all testing days, with no more than two adjacent arms baited. At the beginning of each trial, a single reward pellet was placed in each of the pre-determined goal cups, and the rat was placed in the center of the maze. A trial ended when a rat had entered all four baited arms or 10 min had elapsed.
2.4. Experiment 2: OLMT
The OLMT measures an animal’s ability to remember the location of objects in space based on exploration time. Two sub-experiments were run using the OLMT (Experiments 2A and 2B), involving groups identical to those described for Experiment 1. Experiment 2A tested the effects of age and castration on spatial memory (n = 9–11/group), and Experiment 2B tested the effects of testosterone dose on spatial memory using only aged rats (n = 9–13/group). All injections were performed in the morning (0800–1000 h), and injections began 8 days after surgery and continued until the day of euthanasia (Table 1). The rats used for Experiment 2B had an average body mass of 434.1 ± 4.08 g just prior to starting injections, and there was no significant difference in mass among the treatment groups (p = 0.62).
Rats were given a seven-day surgery recovery period and were handled for 4–5 min per day during the last four days of recovery. Testing consisted of four habituation days, one OLMT day, four more habituation days, and a second OLMT day. Habituation began between 0900–1300 h, while testing began between 1200–1300 h. Rats were placed in the center of the box with the same orientation at the start of each trial. During habituation, rats were allowed to explore the empty open field for 10 min. OLMT days involved an exposure trial followed by a testing trial. For the two testing days, two separate pairs of identical objects were used (weighted Nalgene containers of different shapes and colors). During each exposure trial, one pair of identical objects was placed 20 cm from the back wall and nearest side wall, and each rat was allowed to explore for 5 min. Once time expired, the rat was removed and the box and objects were cleaned with the 70% ethanol. After an inter-trial interval of 2 h, each rat was returned to the arena and allowed to explore the same objects during a 3 min testing trial. For the testing trials, one of the two objects was moved to a quadrant diagonal from the other object, with the location of the moved object (left or right side) counterbalanced across treatments. A rat was considered to be exploring an object when its nose was within 2 cm of the object for a least 1 s. Testing days were excluded for a subject if: 1) the rat failed to explore both objects during the exposure trial, or 2) the rat failed to explore at least one object during the testing trial. If a rat failed to meet these criteria on both testing days, then it was eliminated from the experiment. If data from both testing days were retained for a particular rat, then the data from the two days were averaged.
2.5. Tissue assays
For Experiments 1B and 2B, testosterone injections were administered in the morning on the day after the completion of behavioral testing, and tissue samples were collected in the afternoon (1300–1600 h) for all experiments. Subjects were euthanized with a lethal i.p. injection of Fatal-Plus (approximately 150 mg/kg of sodium pentobarbital; Vortech Pharmaceuticals, Dearborn, MI). Blood was collected via heart puncture and placed in Protein LoBind microcentrifuge tubes (Eppendorf, Hamburg, Germany) on ice. Immediately following blood collection, brains were extracted and dissected into hippocampus, frontal cortex, and striatum. For the cortex dissection, the olfactory bulbs were discarded and a section of cortex from approximately interaural 12.20 to the anterior tip of the brain was collected (Paxinos and Watson, 1986). Samples were weighed, flash frozen in liquid nitrogen, and stored at −80 °C. Blood was refrigerated overnight at 4 °C, centrifuged for 15 min at 2000 g, and serum was extracted and stored at −20 °C. To extract proteins from brain tissue, samples were diluted with lysis buffer to 0.05 g/ml. The lysis buffer consisted of: 100 mM TrizmaHCl, 4 mM EDTA, 0.1% NaN3, 2% Triton X-100, 2% bovine serum albumin (BSA), 0.1 mM phenylmethyl-sulphonyl fluoride (PMSF), and 2.5% by volume of a 10× protease inhibitor cocktail (P-2714, Sigma-Aldrich). Final concentrations of protease inhibitors from the cocktail were: 0.5 mM AEBSF, 0.075 μM aprotinin, 32.5 μM bestatin, 3.5 mM E-64, and 0.25 mM leupeptin. Tissue was fully homogenized in lysis buffer using a hand-held homogenizer, centrifuged for 30 min at 14,000 g at 4 °C, and stored at −80 °C.
Serum testosterone was assayed in duplicate using a testosterone enzyme immunoassay (EIA) kit (MP Biomedicals Diagnostics Division, Solon, OH, USA). Absorbance was measured at 450 nm by a microplate reader (Synergy HT; Biotek, Winooski, VT, USA). The EIA kit detection limit was 0.05 ng/ml, and all samples below this limit were assigned a concentration of 0 ng/ml. The testosterone antibody was noted to have minimal cross-reactivity with other androgens (0.86–1.0%), and trace cross-reactivity with other steroids (<0.05%). The mean intra-assay coefficient of variance for our samples was 12.6%, and the inter-assay coefficient of variance reported by the manufacturer was < 8.4%.
Total brain-derived neurotrophic factor (BDNF) was assayed in duplicate using a chromogenic ChemiKine™ BDNF sandwich enzyme-linked immunosorbent assay (ELISA) kit (Millipore Corporation, Billerica, MA, USA). Extracts from the two hemispheres were mixed 1:1 for each brain region (cortex, hippocampus, and striatum) and then diluted by half using the kit’s diluent buffer. Absorbance was measured at 450 nm by a microplate reader (Synergy HT). The mean intra-assay coefficient of variance for our samples was 5.47%, and the inter-assay coefficient of variance reported by the manufacturer was < 8.5%.
2.6. Statistical analysis
Two types of memory errors were scored during testing trials on the RAM: reference memory errors (RME), defined as first entries into non-baited arms, and working memory errors (WME), defined as repeated entries into arms within a trial. We also scored working-reference errors (WRE), a sub-set of WMEs defined as within-trial reentries into arms that were never baited. The number of arm entries divided by the total amount of time a rat spent on the maze per trial (arm entry rate) was used as an index of motivation and motor ability. In general, the aged rats tended to reach the 10-min trial limit more frequently than what we previously observed using young rats on the same testing protocol (Wagner et al., 2018). Such trials are difficult to analyze because a rat that remained relatively immobile in the maze for 10 min could make few errors, inaccurately suggesting that it had good memory. Therefore, we developed exclusion criteria, which excluded data from relatively immobile rats and retained data from mobile rats that reached the time limit due to poor memory. Specifically, data from a particular trial were excluded from analyses if the entry rate (entries/min) of a rat that reached the 10 min trial maximum was slower than one standard deviation below the average entry rate of all rats on that day. A small number of such exclusions for a particular rat were unlikely to influence the results because the data were analyzed in 5-day blocks. However, if a rat failed to meet entry rate criteria for 5 or more days (20% of testing days), it was eliminated from the experiment entirely.
RAM data were divided into five 5-day testing blocks for analyses. The various types of memory errors (WME, RME, and WRE) and entry rate (entries/min) were analyzed using repeated measures analysis of variance (ANOVA), with trial block as the within-subjects factor and treatment and age as the between-subjects factors. For each dependent variable, all five blocks of trials were also analyzed individually using univariate ANOVAs. Analyzing each block separately allowed us to detect subtle effects that may not have been detected by the repeated-measures ANOVA.
For the OLMT experiments, the total distance traveled by rats during the first four habituation trials was analyzed by repeated measures ANOVA, with habituation day as the within-subjects factor and treatment as the between-subjects factor. Each day of habituation was analyzed individually using a univariate ANOVA with treatment as the fixed factor. Repeated measures ANOVA was used to compare the total exploration times of both objects, with trial (exposure vs. testing) as a within-subjects effect and treatment and age as between-subjects effects. An exploration ratio was calculated for each rat during both the exposure trials (time exploring to-be-moved object/total time exploring both objects) and the testing trials (time exploring moved object/total time exploring both objects). Paired t-tests were used to compare the exploration ratios between the exposure and testing trials within each group. The advantage of this analysis is that it takes into account any pre-existing bias by a rat to investigate a particular object or particular side of the arena (Jablonski et al., 2013; Wagner et al., 2018). We also compared the exploration ratios from the testing trials within each group to a value of 0.5 (indicative of no preference for one object over the other) using one-sample t-tests.
Serum testosterone concentrations were analyzed using the univariate ANOVAs. The Oil GDX groups were not included in these analyses because most of the samples for all experiments had testosterone levels below the detection limit of the assays. BDNF concentrations were analyzed using repeated measures ANOVA, with brain region (cortex, hippocampus, or striatum) as the within-subjects factor and treatment and age as the between-subjects factors. Fisher’s LSD was used for all post-hoc comparisons, and all statistical analyses were performed using SPSS 24.0 software (SPSS, Chicago, IL, USA) with α = 0.05.
3. Results
3.1. Experiment 1A: Age and castration influenced RAM performance
Castration eliminated most of the circulating testosterone in rats used for Experiment 1A (Table 2). Specifically, seven rats from the Old/GDX group had serum testosterone concentrations below the detection limit of the assay (5 ng/ml), and the other five rats from the group had relatively low testosterone concentrations (0.06–0.16 ng/ml). Similarly, for the Young/GDX group, only three of the ten rats had serum testosterone concentrations above the detection limit (0.06–0.11 ng/ml). As expected, serum testosterone concentrations were significantly higher in the Young/Sham group than in the Old/Sham group (t20 = 3.39, p = 0.003).
Table 2.
Testosterone concentrations (mean ± SEM) in serum collected 4–6 h after testosterone or oil injections on the day after the last day of memory testing for each experiment.
| Experiment | Treatment | n | Serum testosterone (ng/ml) |
|---|---|---|---|
| 1A | Old/Sham | 12 | 0.43 ± 0.013 |
| Old/GDX | 12 | 0.06 ± 0.02 | |
| Young/Sham | 10 | 4.15 ± 1.31* | |
| Young/GDX | 10 | 0.04 ± 0.01 | |
| 1B | Oil GDX | 12 | 0.04 ± 0.02 |
| Oil Sham | 12 | 0.24 ± 0.07 | |
| 0.125 mg T | 12 | 2.78 ± 0.80 | |
| 0.250 mg T | 12 | 8.57 ± 2.40⁑ | |
| 0.500 mg T | 12 | 13.79 ± 3.98⁑ | |
| 1.000 mg T | 12 | 23.91 ± 6.90⁑ | |
| 2A | Old/Sham | 10 | 0.73 ± 0.18 |
| Old/GDX | 11 | 0.08 ± 0.03 | |
| Young/Sham | 9 | 2.06 ± 0.62* | |
| Young/GDX | 10 | 0.08 ± 0.03 | |
| 2B | Oil GDX | 13 | 0.06 ± 0.02 |
| Oil Sham | 11 | 0.50 ± 0.18 | |
| 0.125 mg T | 11 | 3.96 ± 0.70⁑ | |
| 0.250 mg T | 9 | 7.36 ± 1.49⁑ | |
| 0.500 mg T | 11 | 11.34 ± 0.89⁑ | |
| 1.000 mg T | 9 | 14.60 ± 1.99⁑ |
Significantly different from the Old/Sham group (p < 0.05).
Significantly different from the Oil Sham group (p < 0.05).
For arm entry rate, there was a significant block × age interaction (Fig. 1A; F4, 160 = 9.73, p < 0.0005) and significant main effects of block (F4, 160 = 48.89, p <0.0005) and age (F1,40 = 37.13, p <0.0005). There were no other significant main or interaction effects for entry rate (all p > 0.20). Analyses within blocks indicated that the two old groups had significantly slower entry rates than the two young groups during every testing block (Fisher’s LSD, all p < 0.02), but castration had no effect on entry rate within each age group (Fisher’s LSD, both p > 0.30).
Fig. 1.
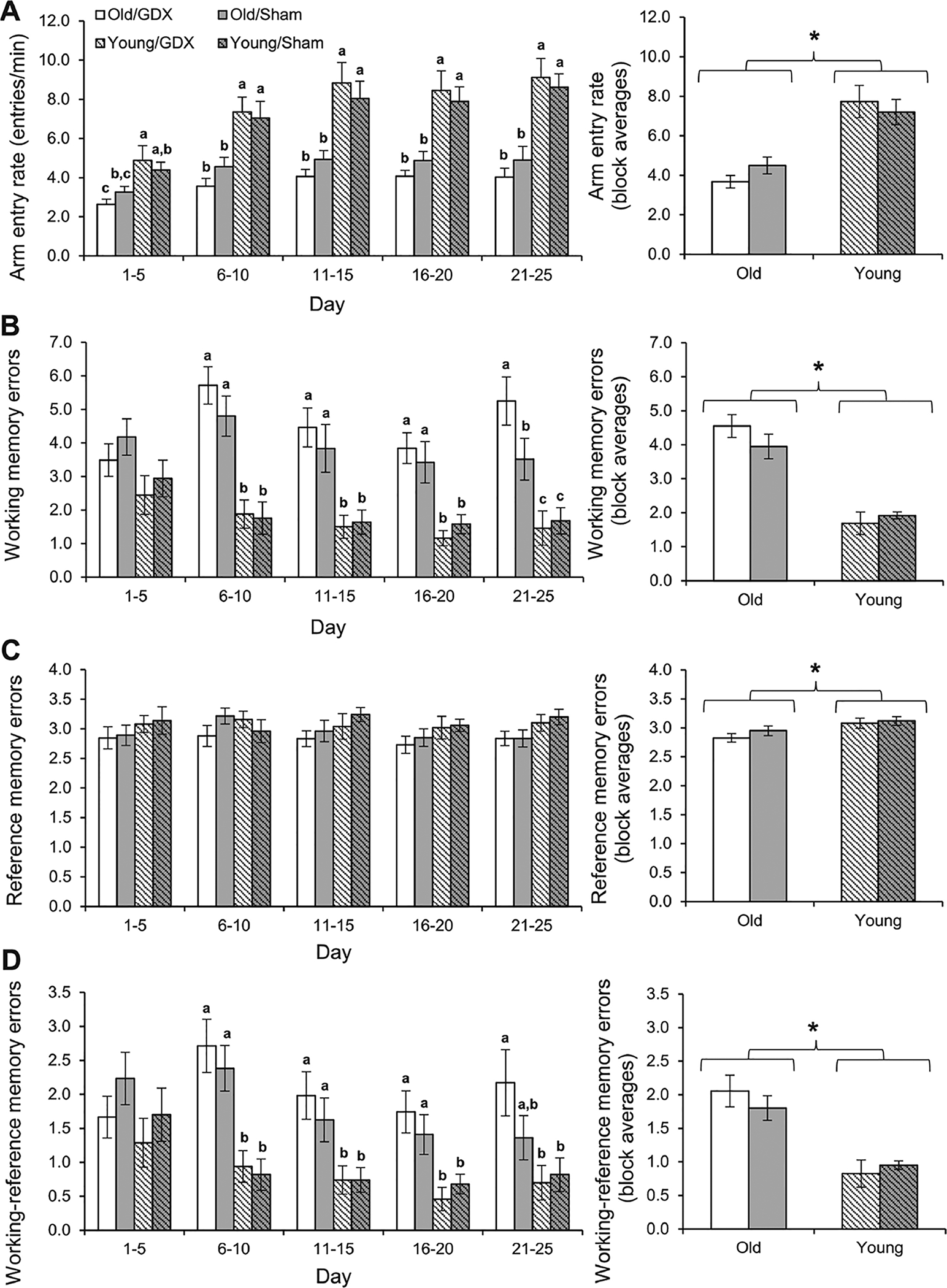
Performance by Young (2 months old) and Old (20 months old) male rats that were either castrated (GDX) or sham castrated (Sham) during 25 days of testing in an 8-arm radial maze (n = 10–12/group). Entry rates and memory errors (mean ± SEM) are divided into 5-day blocks in the left-hand graphs and averaged across blocks in the right-hand graphs. Letters designate groups that differed significantly from each other within blocks (p < 0.05). (A) Arm entry rate increased significantly over the testing blocks (p < 0.0005), and Young rats entered arms at a significantly faster rate than Old rats (*p < 0.0005). There was no effect of castration on arm entry rate. (B) Rats performed significantly fewer WMEs on later testing blocks (p = 0.037), and Young rats performed significantly fewer WMEs than Old rats (*p < 0.0005). There was no main effect of castration, but on the final testing block (days 21–25), the Old/GDX group performed significantly more WMEs than all of the other groups. (C) There was no effect of block or castration on RMEs, but the Old rats performed significantly fewer RMEs than the Young rats (*p < 0.0005). (D) Rats performed significantly fewer WREs on later testing blocks (p = 0.003), and Young rats performed significantly fewer WREs than Old rats (*p < 0.0005). There was no main effect of castration for WREs, but on the final testing block the Old/Sham group performed at a level intermediate between the Old/GDX group and the Young groups.
Overall, older age impaired working memory on the RAM, while the effects of castration were subtle. Specifically, there was a significant block × age interaction for WMEs (Fig. 1B; F4, 160 = 7.84, p = 0.020). There was also a significant main effect of block, with rats performing fewer errors on later days of testing (F4, 160 = 2.62, p = 0.037), and aged rats performed significantly more WMEs than young rats (F1,40 = 60.08, p < 0.005). There was no main effect of castration (p = 0.56), or significant interaction effects involving castration for WMEs (all p > 0.19). Analyses within blocks indicated that the two old groups performed more WMEs than the young groups on every testing block except the first one (all p < 0.01). On the final block of testing (days 21–25), the Old/GDX group performed significantly more errors than all other groups (F3,40 = 8.91, p < 0.0005; Fisher’s LSD, all p < 0.04).
Old rats performed significantly fewer RMEs than young rats (Fig. 1C; F1,40 = 7.04, p = 0.011). There were no other significant main effects or interactions for RMEs (all p > 0.30). Analyses within blocks showed no significant differences among the groups for any of the 5-day blocks (all p > 0.15).
For WREs, there was a significant block effect, with rats generally performing fewer WREs on later testing blocks (Fig. 1D; F4, 160 = 4.16, p = 0.003). Aged rats performed significantly more WREs than young rats (F1,40 = 29.60, p < 0.0005). No other main effects or interactions were significant (all p > 0.07). Analyses within blocks indicated that the two old groups performed more WREs than the young groups on every testing block except the first and last blocks (all p < 0.05). On the first block (days 1–5), there was no difference between the groups (p =0.33). On the final block (days 21–25), the Old/GDX group performed more WREs than the young groups (both p < 0.02), but the Old/Sham group was not significantly different from any of the other groups (all p > 0.1).
3.2. Experiment 1B: Testosterone had dose-dependent effects on RAM performance in aged males
Among the Oil GDX group, only three of the 12 subjects had serum testosterone levels above the detection limit (Table 2; 0.11–0.17 ng/ml). Serum testosterone concentrations differed significantly among the other groups (F4, 55 = 60.92, p < 0.0005). Post-hoc comparisons showed that each group had significantly different serum testosterone concentrations than all other groups (all p < 0.01), with the exception that the 0.125 mg T group did not differ from the Oil Sham group (p = 0.14).
Arm entry rate increased significantly over the five testing blocks (Fig 2A; F4, 264 = 177.37, p < 0.0005). There was no main effect of treatment (p = 0.87) or significant block × treatment interaction for entry rate (p = 0.15). Analyses within blocks indicated no significant effects of treatment for arm entry rate (all p > 0.65).
Fig. 2.
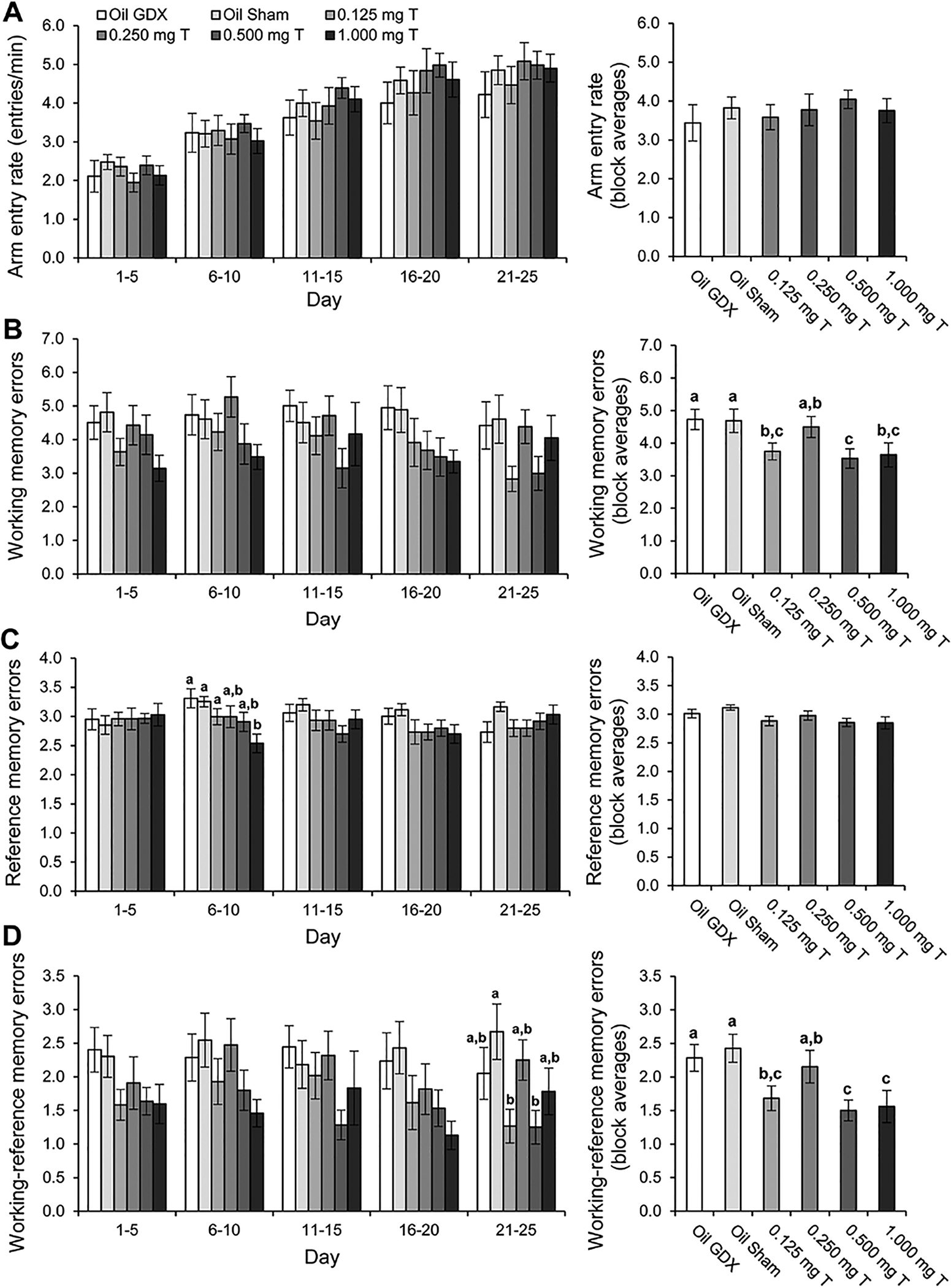
Performance by male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during 25 days of testing in an 8-arm radial maze (n = 12/group). All rats in the GDX and testosterone-injected groups were bilaterally castrated, and the Sham group underwent sham castrations. Entry rates and memory errors (mean ± SEM) are divided into 5-day blocks in the left-hand graphs and averaged across blocks in the right-hand graphs. Letters designate groups that differed significantly from each other within blocks (p < 0.05). (A) Arm entry rate increased significantly over the testing blocks (p < 0.0005), but there were no effects of treatment on arm entry rates. (B) Treatment had a significant effect on WMEs (p = 0.017), with the 0.500 mg T group performing the fewest WMEs and the Oil GDX and Sham GDX groups performing the most WMEs. There was no effect of testing block on WMEs. (C) There was no main effect of block or treatment on RMEs, but during the second block of testing (days 6–10) the 1.000 mg T group performed fewer RMEs than the Oil GDX and Sham GDX groups. (D) Treatment had a significant effect on WREs (p = 0.005), with the 0.500 mg T and 1.000 mg T groups performing the fewest WREs and the Oil GDX and Sham GDX groups performing the most WMEs overall. A significant effect of treatment on WREs was also observed during the final block of testing, with differences between groups comparable to the effect of treatment averaged across blocks.
Testosterone injections caused dose-dependent enhancement of spatial working memory among aged male rats (Fig. 2B). For WMEs, there was no significant block × treatment interaction (p = 0.82) and there was no significant effect of block (p = 0.52). However, treatment had a significant main effect on WMEs (F5, 66 = 2.98, p = 0.017). Post-hoc analysis indicated that the Oil GDX and Oil Sham groups performed significantly more WMEs than did the 0.125 mg T, 0.500 mg T and 1.000 mg T groups (all p < 0.05). Additionally, the 0.250 mg T group performed significantly more WMEs than did the 0.500 mg T group (p = 0.037). No other pairwise comparisons were significant (all p > 0.10). Analyzing each 5-day block separately revealed no significant effects of treatment (all p > 0.14).
For RMEs (Fig. 2C), there was no significant block × treatment interaction (p = 0.088) and there was no significant main effect of block (p = 0.67) or treatment (p = 0.13). Analyses within blocks indicated a significant effect of treatment on days 6–10 (F5, 66 = 3.35, p = 0.009) but not for any of the other four testing blocks (all p > 0.12). Post-hoc analyses within the day 6–10 testing block showed that the 1.00 mg T group performed significantly fewer RMEs than the 0.125 mg T, Oil GDX, and Oil Sham groups (all p < 0.04). None of the other pairwise comparisons for the 6–10 day testing block were significant (all p > 0.06).
The results for WREs paralleled those obtained for WMEs (Fig. 2D). There was no significant block × treatment interaction (p = 0.88) and there was no significant effect of block (p = 0.49) on WREs. Treatment did, however, have a significant effect on WREs (F1,66 = 3.75, p = 0.005). Post-hoc comparisons indicated that the Oil GDX and Oil Sham groups performed significantly more WREs than did the 0.125 mg T, 0.500 mg T, and 1.000 mg T groups (all p < 0.05). The 0.250 mg T group performed significantly more WMEs than the 0.500 mg T and 1.00 mg T groups (both p < 0.05). No other pairwise comparisons were significant (all p > 0.11). Analyses within blocks indicated a significant effect of treatment during the final testing block (F5, 66 = 2.89, p = 0.02), but not for the other four testing blocks (all p > 0.11). Post-hoc analyses within the 20–25 day testing block showed that both the Oil Sham and the 0.250 mg T groups performed significantly more RMEs than the 0.125 mg T and the 0.500 mg T groups (both p < 0.04). None of the other pairwise comparisons for the 20–25 day testing block were significant (all p > 0.06).
3.3. Experiment 2A: Age and castration influenced OLMT performance
Castration eliminated most of the circulating testosterone in rats used for Experiment 2B (Table 2). Four of the 11 rats in the Old/GDX group had serum testosterone concentrations below the detection limit, while the other seven rats had very low testosterone concentrations (0.07–0.26 ng/ml). Similarly, four of the ten rats in the Young/GDX group had serum testosterone concentrations below the detection limit, while the other six rats had low testosterone concentrations (0.08–0.32 ng/ml). Serum testosterone concentrations were significantly higher in the Young/Sham group than in the Old/Sham group (t22 = 2.68, p = 0.014).
Over the first four days of habituation in the open field, rats showed a significant decrease in path length (Fig. 3A; F3, 108 = 10.04, p < 0.0005). Old rats had significantly shorter path lengths during habituation (F1, 36 = 18.7, p < 0.005), and there was a significant day × age interaction effect (F3,108 = 5.55, p = 0.001). Castration did not have a significant main effect on path length (p = 0.42) and there were no significant interaction effects with castration (all p > 0.35). Analyses within days showed significant differences in path length among groups on habituation days 1, 2, and 4 (all p < 0.02), and pairwise comparisons within these days showed that young rats had longer path lengths than old rats (Fig. 3A; all p < 0.0005), but no other pairwise comparisons were significant (both p >0.45).
Fig. 3.
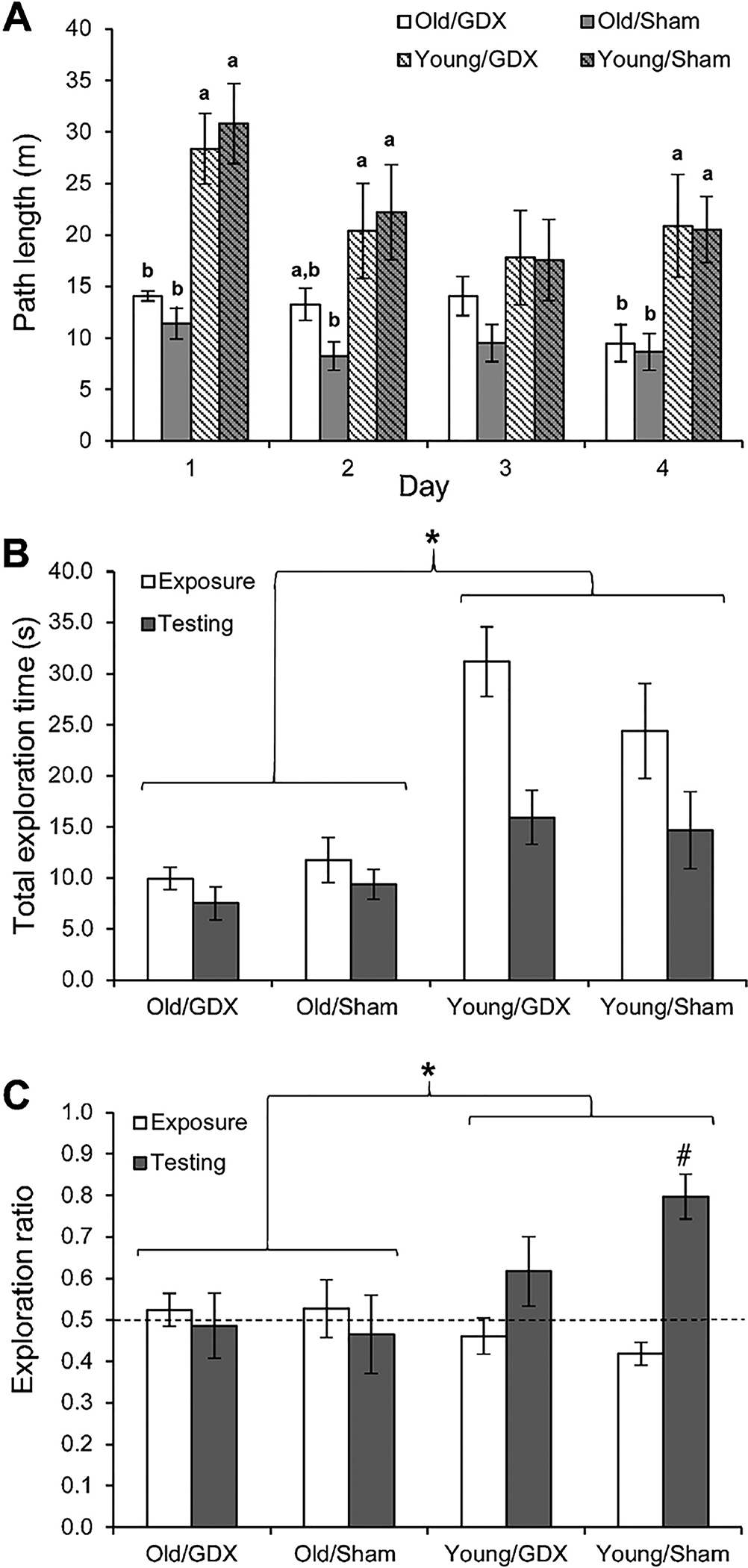
Behavioral data (mean ± SEM) for Young (2 months old) and Old (20 months old) male rats that were either castrated (GDX) or sham castrated (Sham) during habituation and testing on the OLMT (n = 9–11/group). (A) Over the four days of habituation to the open field prior to testing, all groups showed a significant decrease in the distance traveled (p < 0.0005). Old rats had significantly shorter path lengths than young rats overall (p < 0.0005), and the effect of age was significant within each day except day 3 of habituation. Letters designate groups that differed significantly from each other within blocks (p < 0.05). (B) During OLMT testing, the total exploration time for both objects was significantly greater during the exposure trials than during the testing trials (p < 0.0005) and the young rats engaged in significantly more exploration of the two objects than did the old rats (*p < 0.0005). (C) During OLMT testing, Young rats had a significantly higher exploration ratios than old rats during the testing trials (*p = 0.007). Only the Young/Sham group showed a significant increase in exploration ratios from the exposure trials to the testing trials (#p < 0.0005) and was the only group to have a mean exploration ratio during the testing trials that was significantly greater than the chance level of 0.5, indicated by the dashed line (p = 0.001).
Rats explored the two objects significantly more during the exposure trials than during the testing trials (Fig. 3B; F1,36 = 32.14, p < 0.0005), and the young rats explored the objects significantly more than the old rats (F1,36 = 24.86, p < 0.0005). There was also a significant age × trial interaction, with the difference based on age being less during the testing trails (F1,36 = 14.63, p = 0.001). Note that this analysis does not assess memory, but simply indicates that the young rats were more exploratory than the old rats. There was no significant effect of castration or interaction effects involving castration for the total exploration times (all p > 0.23).
Young rats had a significantly higher exploration ratios than old rats during the testing trials (Fig. 3C; F1, 36 = 8.20, p = 0.007), indicating better memory of the location of the two objects. During testing trials, there was no main effect of castration or an age × castration interaction effect for the exploration ratios (both p > 0.35). The Young/Sham group showed significantly higher exploration ratios during the testing trials than during the exposure trials (Fig. 3C; t8 = 6.06, p < 0.0005), and the difference in exploration ratios between the exposure and testing trials was nearly significant for the Young/GDX group (p = 0.053). The two old groups showed no significant difference in their exploration ratios between the exposure and testing trials (both p > 0.60). The exploration ratio for the Young/Sham group was significantly greater than chance level (0.5) during the testing trials (t8 = 5.50, p = 0.001), indicating a strong preference for exploring the moved object. None of the other groups had exploration ratios significantly greater than 0.5 during the testing trials (all p > 0.19).
3.4. Experiment 2B: Testosterone had dose-dependent effects on OLMT performance
Within the Oil GDX group, seven of the 13 subjects had serum testosterone levels above the detection limit (Table 2; 0.05–0.20 ng/ml). Serum testosterone concentrations differed significantly among the other groups (F4, 45 = 26.30, p < 0.0005). Post-hoc comparisons showed that each group had significantly different serum testosterone concentrations than all of the other groups (all p < 0.05), with the Sham GDX group having the lowest serum testosterone concentration.
Rats showed a significant decrease in path length during the first four days of habituation in the open field, (Fig. 4A; F3, 174 = 22.47, p < 0.0005). There was no significant effect of treatment on path length d (p = 0.94) and there was no significant day × treatment interaction (p = 0.89). Analyses within each day revealed no significant effect of treatment on path length (all p > 0.77).
Fig. 4.
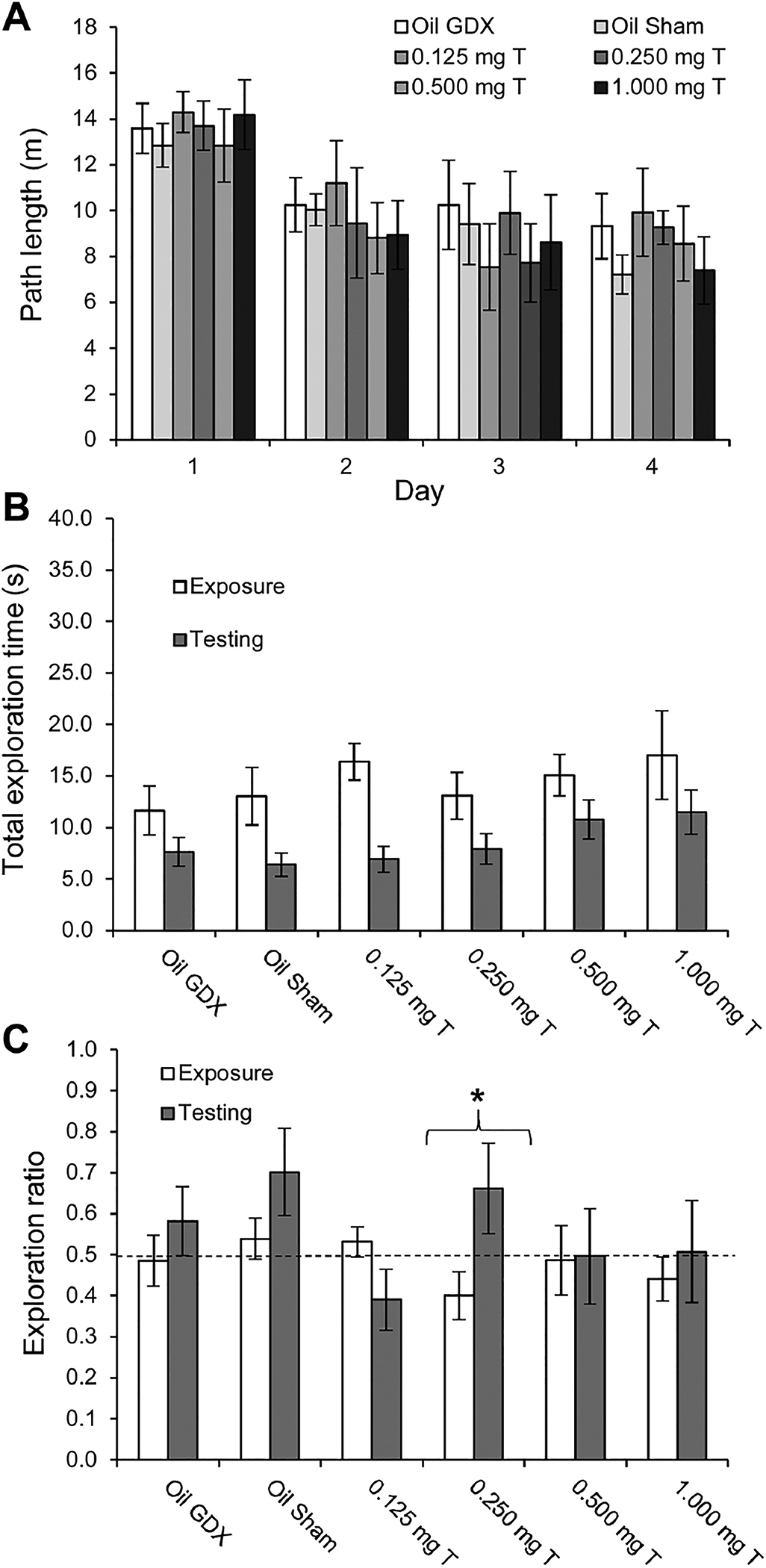
Behavioral data (mean ± SEM) for male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during habituation and testing on the OLMT (n = 9–13/group). All rats in the GDX and testosterone-injected groups were bilaterally castrated, and the Sham group underwent sham castrations. (A) Over the four days of habituation to the open field prior to testing, all groups showed a significant decrease in the distance traveled (p < 0.0005). There was no effect of treatment on path length during habituation. (B) During OLMT testing, the total exploration time for both objects was significantly greater during the exposure trials than during the testing trials (p < 0.0005), but there was no significant differences between the groups for total time exploring the objects. (C) During OLMT testing, only the 0.250 mg T group showed a significant increase in exploration ratios from the exposure trials to the testing trials (*p < 0.05). None of the groups showed exploration ratios during the testing trials that were significantly greater than the chance level of 0.5, indicated by the dashed line.
Rats explored the two objects significantly more during the exposure trials than during the testing trials (Fig. 4B; F1,58 = 26.39, p < 0.0005). There was no significant main effect of treatment (p = 0.31) or a trial × treatment interaction (p = 0.73). Treatment had no significant effect on the exploration ratios during testing trials (Fig. 4C; p = 0.32). The 0.250 mg T group had a significantly higher exploration ratio during testing than during exposure (Fig. 4C; t8 = 2.37, p = 0.046). None of the other groups showed significant differences in their exploration ratios between the two phases of testing (all p > 0.18). Additionally, none of the groups had exploration ratios that were significantly greater than the chance level (0.5) during the testing trials (all p > 0.09).
3.5. Testosterone treatment had no effect on BDNF concentrations
For all four experiments, there were significant differences in BDNF concentrations among the brain regions (Table 3; all p < 0.0005). Post-hoc analyses showed that BDNF concentrations were significantly higher in the hippocampus than in both the striatum and the frontal cortex for all of the experiments (all p < 0.0005). BDNF concentrations did not differ significantly between the striatum and the frontal cortex (all p > 0.060).
Table 3.
Mean ± SEM BDNF concentrations (pg/mg wet mass) in brain tissue (hippocampus, frontal cortex, or striatum) collected 4–6 h after testosterone or oil injections on the day after the last day of memory testing for each experiment.
| Experiment | Treatment | n | Hippocampus | Striatum | Frontal Cortex |
|---|---|---|---|---|---|
| 1A | Old/, sham | 12 | 15.04 ± 1.38 | 5.76 ± 0.34 | 5.62 ± 0.32 |
| Old/GDX | 12 | 16.05 ± 1.42 | 5.54 ± 0.39 | 6.67 ± 0.85 | |
| Young/Sham | 10 | 14.38 ± 2.21 | 6.97 ± 1.62 | 4.97 ± 0.39 | |
| Young/GDX | 10 | 11.37 ± 1.23 | 5.14 ± 0.63 | 3.89 ± 0.58 | |
| 1B | Oil GDX | 12 | 20.86 ± 1.89 | 5.44 ± 0.63 | 5.82 ± 0.32 |
| Oil Sham | 12 | 19.85 ± 2.03 | 6.04 ± 0.65 | 5.63 ± 0.63 | |
| 0.125 mg T | 12 | 21.14 ± 2.18 | 5.78 ± 0.40 | 6.21 ± 0.58 | |
| 0.250 mg T | 12 | 18.27 ± 1.47 | 4.54 ± 1.47 | 4.53 ± 0.50 | |
| 0.500 mg T | 12 | 21.69 ± 2.21 | 5.59 ± 0.74 | 5.41 ± 0.65 | |
| 1.000 mg T | 12 | 16.89 ± 1.81 | 4.59 ± 0.63 | 4.65 ± 0.62 | |
| 2A | Old/Sham* | 10 | 21.47 ± 2.84 | 9.17 ± 0.70 | 9.28 ± 1.19 |
| Old/GDX* | 11 | 14.82 ± 2.95 | 7.02 ± 1.25 | 6.32 ± 1.06 | |
| Young/Sham | 9 | 10.46 ± 2.15 | 4.63 ± 0.82 | 5.07 ± 0.93 | |
| Young/GDX | 10 | 14.75 ± 2.59 | 6.90 ± 1.20 | 7.61 ± 1.27 | |
| 2B | Oil GDX | 13 | 27.66 ± 3.99 | 7.80 ± 1.32 | 7.61 ± 1.22 |
| Oil Sham | 11 | 23.76 ± 4.75 | 5.79 ± 1.16 | 6.04 ± 1.42 | |
| 0.125 mg T | 11 | 26.75 ± 3.18 | 8.09 ± 1.25 | 8.22 ± 1.43 | |
| 0.250 mg T | 9 | 27.21 ± 2.71 | 9.80 ± 1.09 | 10.72 ± 1.38 | |
| 0.500 mg T | 11 | 26.47 ± 3.86 | 6.85 ± 1.55 | 7.55 ± 1.77 | |
| 1.000 mg T | 9 | 27.58 ± 3.08 | 7.52 ± 1.09 | 8.83 ± 1.24 |
Old rats had significantly higher BDNF concentrations in the brain than young rats in Experiment 2A.
For Experiment 2A, old males had significantly higher overall BDNF concentrations in the brain than did young males (Table 3; F2,36 = 7.66, p = 0.009). There was a significant age × castration interaction for Experiment 2A (F1,36 = 9.55, p = 0.004), with castration decreasing BDNF concentration in all brain regions among the old males, while slightly increasing BDNF concentrations in all brain regions among young males. However, castration had no significant main effect on BDNF concentrations for Experiment 2A (p = 0.70) and there were no other significant interaction effects for this experiment (all p > 0.22). Although old rats tended to have higher BDNF concentrations than young rats for Experiment 1A, in this case there was neither a significant main effect of age (p = 0.10) nor a significant age × castration interaction (all p > 0.09). No other main effects of interactions effects were significant for Experiment 1A (all p > 0.08).
With regards to the two experiments involving testosterone propionate injections (Table 3), there was no effect of treatment for either experiment (Experiment 1B, p = 0.34; Experiment 2B, p = 0.84). There was also no significant region × treatment interaction for either of these experiments (both p > .78).
4. Discussion
Clinical studies have produced mixed results regarding the potential benefits of testosterone for the prevention and treatment of age-related memory loss and dementia (Fuller et al., 2007; Holland et al., 2011). Our results showed that testosterone replacement can improve spatial memory in an aged rat model, but the effects are dose-dependent and vary with the assessed form of memory (i.e., working memory or long-term memory). Old rats (20 months old) showed significant impairments in spatial memory relative to young rats (2 months old). On the RAM (Experiment 1A), castration had no effect on spatial working memory among young males and caused a minor impairment among old males (significant difference only on the final 5-day testing block). On the OLMT (Experiment 2A), castration impaired long-term memory in the young rats, while both castrated and sham-castrated old males failed to recognize the moved object. Testosterone injections had dose-dependent effects on spatial working memory in old male rats. Specifically, a low physiological dose (0.125 mg) and high doses (0.500–1.000 mg) of testosterone improved working memory on the RAM, while an intermediate dose (0.250 mg) did not improve working memory relative to that observed in the Oil GDX group. In contrast, the intermediate dose of testosterone (0.250 mg) was the only dose that improved long-term memory on the OLMT.
4.1. Aging impairs spatial memory and testosterone production
In support of past results, Experiment 1A demonstrated that age impairs spatial working memory among rats. A number of past studies have shown that increased age impairs performance by male rats on the MWM (Begega et al., 2001; Bizon et al., 2009; Frick et al., 1995; Goudsmit et al., 1990). Increased age also impaired working memory on the water-escape RAM (Bimonte-Nelson et al., 2003), a water-reward version of the RAM (Barnes, 1980), and on a food-reward versions of the RAM similar to our protocol (Oler and Markus, 1998; Stewart et al., 1989).
Contrasting the effects of age on working memory, we observed that aged males performed significantly fewer RMEs than young males on the RAM. The aged rats also had significantly slower entry rates, so they may have been slower and less impulsive than young rats when choosing which arm to enter, leading to the observed difference in RMEs (Simon et al., 2010). However, WREs have both a working memory and reference memory component, and aged rats performed significantly more WREs than did the young rats in Experiment 1A. Bimonte-Nelson et al. (2003) observed an age-related impairment in both working memory and reference memory on the water-escape RAM, where all rats were likely motivated to make their first choice quickly. Additionally, Experiment 2A demonstrated that aged rats were impaired on the OLMT with a 2 h inter-trial interval. One potential confound is the fact that the aged rats explored the objects significantly less overall than did the young rats. Analysis of the exploration ratios partially accounts for this, because this analysis involved comparing relative interest in the two objects during the exposure and testing phases rather than relying upon comparisons of absolute time exploring the objects. Some past work with rats indicates that age-related deficits in spatial memory correlate with age-related deficits in attention (Guidi et al., 2015), and it is especially difficult to disentangle the effects of age on attention vs. spatial memory with the OLMT. However, Bizon et al. (2009) demonstrated that the impairing effect of age increased as the retention period between exposure and testing increased on a water maze task that relies less on attention. In summary, current evidence suggests that increased age impairs both spatial working memory and long-term memory, but future experiments should assess how increasing age impacts the length of memory retention.
The old male rats had significantly lower serum testosterone levels than did the young rats. The fact that the aged rats showed both decreased testosterone and impaired spatial memory provides correlational support for a mechanistic connection between testosterone and memory. Past studies have demonstrated a similar age-related decline in testosterone in male rats (Chen et al., 1994; Wang et al., 1993). Chen et al. (1994) found that while cultured Leydig cells from aged rats responded to an LH pulse, they produced significantly less testosterone than cultured Leydig cells from young rats. This suggests that the inability of aged rats to produce testosterone is due to testicular dysfunction.
4.2. Testosterone replacement improves spatial memory in aged males
Our results using the RAM demonstrated that daily testosterone injections reduced the number of WMEs among castrated aged males. These results support past findings with young male rats that castration impairs spatial working memory (Bimonte-Nelson et al., 2008; Daniel et al., 2003; Gibbs and Johnson, 2008; Spritzer et al., 2008; Wagner et al., 2018) and that testosterone treatment improves working memory relative to castrated animals (Locklear and Kritzer, 2014; Spritzer et al., 2011; Wagner et al., 2018). We observed minimal effects of castration alone on the RAM, but castration did increase WME’s among the aged rats on the RAM during the final testing block in Experiment 1A. This effect of castration was not replicated in Experiment 1B, possibly due to the higher testosterone levels in the Old/Sham group in Experiment 1A compared to the Oil Sham group in Experiment 1B (Table 2). In general, castration may have had minimal effect on RAM performance among the aged rats because even without castration, circulating testosterone levels were quite low. Contradicting our past results (Spritzer et al., 2008; Wagner et al., 2018), we found that castration had no effect on WMEs among young rats, with both groups learning the task at the same rate. This may be due to subtle differences in experimental design, including differences in rat strain (Fischer 344 vs Sprague-Dawley). A direct comparison to the results of Wagner et al. (2018) shows that during each block of testing on the RAM their group of young sham-castrated rats were performing about half as many WMEs compared to the Young Sham group in our Experiment 1A. Past work indicates that these two strains perform comparably on the MWM (Harker, 2002), but perhaps Fisher 344 rats have cognitive deficits that prevent them from performing as well as the Sprague-Dawley rats on the working memory component of the RAM. Such a floor effect could obscure subtle effects of castration among Fisher 344 rats. Another important consideration is that we had no control over the daily testosterone levels of the Young/Sham group, and testosterone was assayed only at the end of our experiment, so perhaps relatively low testosterone levels in that group during the early trials prevented them from out-performing the Young/GDX group. Past studies using the RAM have shown main effects of castration to occur during the early testing blocks (Gibbs and Johnson, 2008; Spritzer et al., 2008; Wagner et al., 2018).
During the RAM experiments, we found that testosterone injections had little effect on RMEs, with the only significant finding being that the highest dose of testosterone (1.00 mg/rat) reduced RMEs on just one 5-day testing block (days 6–10). This result suggests a possible benefit of supra-physiological doses of testosterone, but the transient nature of the effect (i.e., only one early testing block) suggests that the effect of testosterone on reference memory is minimal. Our past work with young rats using an identical RAM task showed no effect of testosterone injections on RMEs during any block of testing (Spritzer et al., 2011; Wagner et al., 2018). Testosterone injections also reduced WMEs but had no effect on RMEs for aged male rats tested on a water-escape RAM (Bimonte-Nelson et al., 2008). We previously demonstrated that testosterone injections can cause small improvements in reference memory on the MWM (Spritzer et al., 2011), but there are also numerous studies showing that castration has no effect on MWM performance (Hodosy et al., 2010; Sandstrom et al., 2006; Spritzer et al., 2008). Reference memory, as assessed by most RAM and MWM protocols, can be considered a form of long-term memory with a 24 h delay period (Cowan, 2008). Thus, there is considerable evidence from rat studies indicating that testosterone has no effect on long-term memory. However, the duration of memory retention should be considered, and our OLMT protocol allowed us to assess long-term memory with a 2 h delay rather than a 24 h delay. Our results from the OLMT showed that castration impaired long-term memory and testosterone replacement restored memory. This supports past OLMT experiments indicating that testosterone improves long-term memory when there is a 30 min delay (McConnell et al., 2012) or 2 h delay between exposure and testing (Jacome et al., 2016; Wagner et al., 2018). Perhaps the benefits of testosterone for spatial memory decline as the period of memory retention increases—this would reconcile our observation that that the 0.250 mg T group performed well on the OLMT (2 h delay) but had no apparent advantage in terms of reference memory on the RAM (24 h delay).
We observed dose-dependent effects of testosterone on memory for both the RAM and OLMT. On the RAM, we found that low (0.125 mg/rat) and high (0.500–1.00 mg/rat) doses of testosterone reduced WMEs relative to the Oil GDX and Oil Sham groups, while an intermediate dose of testosterone (0.250 mg/rat) did not. This interesting pattern is remarkably similar to the dose-dependent effects that we observed with young rats (Wagner et al., 2018). In spite of this similarity, the young rats in our previous experiment performed about half as many WMEs per trial compared to the aged rats in the current study, indicating that even with testosterone injections, the spatial memory of aged males was impaired. A low dose of testosterone (0.125 mg) biases male rats toward using a striatum-dependent response strategy to solve spatial tasks and a high dose (0.500 mg) of testosterone biases rats toward using a hippocampus-dependent place strategy (Spritzer et al., 2013). The poor performance of the 0.250 mg T group on the RAM may be due to their inability to generate either a response or a place strategy effectively. Comparable to the effects of estradiol on memory in female rats (Zurkovsky et al., 2007), we hypothesize that low physiological doses of testosterone improve response learning through maintained striatal activity, whereas high physiological doses of testosterone improve place learning through enhanced hippocampal activity and suppressed striatal activity. It is also noteworthy that male rats and humans shift to increased reliance on a response strategy over a place strategy with increasing age (Begega et al., 2001; Oler and Markus, 1998; Wiener et al., 2013). In males, this shift could be partially due to declining testosterone levels.
In our previous work, we observed a dose-response relationship on the OLMT very similar to that observed on the RAM, with the 0.250 mg dose having no restorative effects on long-term memory (Wagner et al., 2018). In contrast, the current results with aged males (Experiment 2B) showed that the 0.250 mg T group was the only group that recognized the moved object on the OLMT. This suggests that the effects of age on spatial working memory differ from its effects on long-term memory. Perhaps neurological damage associated with increased age necessitates a higher dose of testosterone to improve long-term memory. Thus, the long-term memory-enhancing effects of the 0.125 mg dose of testosterone observed in 2-month-old rats may correspond with the memory-enhancing effects of the 0.250 mg dose among 20-month-old rats. Why such a shift in the necessary dose was not apparent for working memory on the RAM is unclear, but this difference may be due to differential effects of aging on brain regions that encode separate components of spatial memory (Floresco et al., 1997). Rats received more than twice as many days of injections in Experiment 1B than in Experiment 2B (Table 1), but it seems unlikely that this would be the cause of the difference in the optimal dose between the two experiments given that no such difference in the optimal dose was observed when identical behavioral protocols were used with young rats (Wagner et al., 2018). Another methodological concern is the fact that all of our drug dosing was per rat rather than per unit body mass, and the rats in Experiment 2B weighed approximately 100 g more on average than those used by Wagner et al. (2018). This difference seems fairly unimportant, however, as the serum testosterone concentrations at the end of the experiments were very similar for rats receiving the same doses in the two experiments (Wagner et al., 2018). Thus, increased age is the most plausible explanation for the increased dosing needed for older rats to perform well on the OLMT relative to younger rats. Finally, it should be noted that even the optimal dose of testosterone from the current study (0.250 mg/rat) did not increase the exploration ratio significantly above chance levels, contrasting the strong performance for the Young/Sham group from Experiment 1A. Thus, as for working memory on the RAM, testosterone injections were not sufficient to restore long-term memory levels on the OLMT to that which is observed in young rats with natural testosterone levels.
4.3. Testosterone replacement did not influence BDNF concentrations
As expected, BDNF levels were significantly higher in the hippocampus than in the striatum and frontal cortex in all of our experiments, supporting the accuracy of our assay. However, we observed no effect of testosterone injections on BDNF concentrations in any of the brain regions analyzed. This suggests that BDNF is not the molecular mechanism by which testosterone influences spatial memory in aged males. Similarly, Bimonte-Nelson et al. (2003) observed no effects of testosterone injection on BDNF levels within the hippocampus of young and aged male rats. Past evidence that testosterone influences BDNF in the hippocampus suggests that the effects vary among the sub-regions of the hippocampus. Castration increased BDNF levels within the mossy fibers extending from the dentate gyrus to the CA3 layer of the hippocampus (Skucas et al., 2013) but decreased BDNF in the CA1 layer (Li et al., 2012). Thus, our assays of BDNF from the entire hippocampus may have been too coarse to capture changes occurring at a smaller scale. Additionally, we measured total BDNF levels, with no distinction between pro-BDNF and mature BDNF. Mature BDNF and pro-BDNF have differential effects on cell survival and synaptic plasticity, so it is possible that testosterone influences spatial memory by changing the ratio of mature BDNF to pro-BDNF (Hill, 2012; Teng et al., 2005).
Rather than engaging neurotrophic pathways, testosterone may be acting primarily as a neuroprotectant to improve memory. Cell culture studies have shown that androgens prevent apoptosis (Hammond et al., 2001; Nguyen et al., 2010), and interestingly the effects of androgens in activating the mitogen-activated protein kinase (MAPK) and phophoinositide 3-kinase pathways were non-linear (Gatson et al., 2006), providing a possible mechanism for the neuroprotective effects of testosterone at differing concentrations. Androgens have also been shown to activate cAMP response element binding protein (CREB), likely downstream of the MAPK activation (Nguyen et al., 2009), and CREB is known to activate many aspects of neuroplasticity and neuroprotection. In rodent models, dihydrotestosterone treatment reduced the impact of kainite lesions (Ramsden et al., 2003) and ischemic damage to brain tissue (Uchida et al., 2009). An indirect mechanism by which testosterone may be acting as a neuroprotectant is through its negative feedback effects upon luteinizing hormone (LH), which has neurodegenerative properties (Barron et al., 2006). Injections of an LH agonist impaired spatial memory among female rats (Berry et al., 2008), and an LH antagonist was effective in improving spatial memory among castrated male rats on the OLMT (McConnell et al., 2012). Thus, testosterone may improve memory by blocking the neurodegenerative effects of LH, but is seems unlikely that this mechanism would fully explain the complex dose-response relationship that we observed on the RAM.
Unexpectedly, the old rats had significantly higher BDNF concentrations than did the young rats in Experiment 2A. Bimonte-Nelson et al. (2008) observed a similar increase in BDNF levels in the hippocampus of 20-month-old male rats relative to 7-month-old males. Another study with male rats found that increased age caused BDNF levels to increase in the hippocampus and decrease in the cortex and striatum (Katoh-Semba et al., 1998). Apparent age-related increases in BDNF, at least in the hippocampus, may be due to transient increases in BDNF associated with neuronal damage brought on by aging (Ballarín et al., 1991).
4.4. Conclusion
We demonstrated that 20-month-old rats exhibit decreased testosterone and impaired spatial memory, suggesting a connection between declining testosterone in age-related memory loss. We found that low and high doses of testosterone restored spatial working memory, while an intermediate dose did not, replicating our previous findings with young male rats (Wagner et al., 2018). The physiological cause of this unusual dose-response relationship remains untested, but may involve differential effects of the high and low doses of testosterone on different memory systems (Gold et al., 2013). We found no evidence that testosterone replacement enhanced BDNF levels, making it unlikely that BDNF is the molecular link between testosterone and memory. However, future work should assess whether testosterone induces differences in the relative levels of pro-BDNF and mature BDNF or acts in a more selective manner on specific cells layers within the hippocampus.
Research Highlights.
Aged rats had impaired spatial working memory relative to young rats.
Aging and castration impaired performance on an object location memory task.
High and low, but not intermediate, doses of testosterone improved working memory.
An intermediate dose of testosterone improved long-term spatial memory.
Testosterone manipulations had no effect on BDNF levels in the brains of aged rats.
Acknowledgements
We thank Lucy Botswick, Eliza Ferrari, Sedge Lucas, Laura Nelson, Leslie Panella, Maddie Pronovost, Ethan Roy, and Mark Sinks for their assistance with data collection. Melissa Glen and Clarissa Parker provided guidance on brain tissue processing. Mark Stefani assisted with maze construction and behavioral protocols. Jan Thornton provided guidance on the OLMT protocol. We also thank Vicki Major, Alexis Paquette, and the rest of the Middlebury College animal care staff.
Funding
This project was funded by Middlebury College and the National Institute of Aging (NIH AREA grant number 1R15AG042155). The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
None.
References
- Ballarín M, Ernfors P, Lindefors N, Persson H, 1991. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp. Neurol 114, 35–43. 10.1016/0014-4886(91)90082-N [DOI] [PubMed] [Google Scholar]
- Barnes CA, 1980. Spatial memory deficit in senescent rats. Can. J. Psychol 34, 29; 29–39; 39. [DOI] [PubMed] [Google Scholar]
- Barron AM, Fuller SJ, Verdile G, Martins RN, 2006. Reproductive hromones modulate oxidative stress in Alzheimer’s disease. Antioxid. Redox Signal 8, 2047–2059. [DOI] [PubMed] [Google Scholar]
- Begega A, Cienfuegos S, Rubio S, Santıń JL, Miranda R, Arias JL, 2001. Effects of ageing on allocentric and egocentric spatial strategies in the Wistar rat. Behav. Processes 53, 75–85. 10.1016/S0376-6357(00)00150-9 [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J, 2008. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases amyloid-β levels in female rats. Horm. Behav 54, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Granholm ACE, Nelson ME, Moore AB, 2008. Patterns of neurotrophin protein levels in male and female Fisher 344 rats from adulthood to senescence: how young is “young” and how old is “old”? Exp. Aging Res 34, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE, 2003. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol 181, 301–312. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH, 2009. Spatial reference and working memory across the lifespan of male Fisher 344 rats. Neurobiol Aging 30, 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE, 2003. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J. Neurosci 23, 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, Huston JP, Li J-S, Wang A-L, de Souza Silva MA, 2016. The medial prefrontal cortex-lateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus 26, 633–645. 10.1002/hipo.22547 [DOI] [PubMed] [Google Scholar]
- Chen HL, Hardy MP, Huhtaniemi I, Zirkin BR, 1994. Age-related decreased leydig cell testosterone production in the brown Norway rat. J. Androl 15, 551–557. [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S, 2001. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57, 80–88. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S, 2005. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 64, 2063–2068. [DOI] [PubMed] [Google Scholar]
- Compton DM, Griffith HR, McDaniel WF, Foster RA, Davis BK, 1997. The flexible use of multiple cue relationships in spatial navigation: a comparison of water maze performance following hippocampal, medial septal, prefrontal cortex, or posterior parietal cortex lesions. Neurobiol. Learn. Mem 68, 117–132. 10.1006/nlme.1997.3793 [DOI] [PubMed] [Google Scholar]
- Cowan N, 2008. What are the differences between long-term, short-term, and working memory? Prog. Brain Res 169, 323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM, 2003. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl.) 170, 294–300. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Swinkels WA, de Brabander JM, 1997. Response learning of rats in a Morris water maze: involvement of the medical prefrontal cortex. Behav. Brain Res 85, 47–55. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR, 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269. [DOI] [PubMed] [Google Scholar]
- Emamian S, Naghdi N, Sepehri H, Jahanshahi M, Sadeghi Y, Choopani S, 2010. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav. Brain Res 208, 30–37. 10.1016/j.bbr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB, 2002. Age trends in the level of serum testosterone and other hormone in middle-aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab 87, 589–598. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG, 1997. Selective roles of hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci 17, 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS, 2006. Sex and ovarian steroids modulate brain-derived neruotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology 31, 38–48. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL, 1995. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging 16, 149–160. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Tan RS, Martins RN, 2007. Androgens in the etiology of Alzheimer’s disease in aging men and possible therapeutic interventions. J. Alzheimers Dis 12, 129–142. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M, 2006. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phophoinositide 3-kinase/Akt pathways through the nuclear and novel membrane andorgen receptor in C6 cells. Endocrinology 147, 2028–2034. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, 2005. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm. Behav 48, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA, 2008. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149, 3176–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, Korol DL, 2013. Modulation of multiple memory systems: From neurotransmitters to metabolic substrates. Hippocampus 23, 1053–1065. 10.1002/hipo.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit E, Vandepoll NE, Swaab DF, 1990. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in your and middle-aged animals. Behav. Neural Biol 53, 6–20. [DOI] [PubMed] [Google Scholar]
- Guidi M, Rani A, Karic S, Severance B, Kumar A, Foster TC, 2015. Contribution of N-methyl-D-aspartate receptors to attention and episodic spatial memory during senescence. Neurobiol. Learn. Mem 125, 36–46. 10.1016/j.nlm.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A, 2001. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem 77, 1319–1326. 10.1046/j.1471-4159.2001.00345.x [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP, 2015. Age-specific population centiles for androgen status in men. Eur. J. Endocrinol 173, 809–817. 10.1530/EJE-15-0380 [DOI] [PubMed] [Google Scholar]
- Harker KT, 2002. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fishcer 344) and albinism (Wistar, Sprague-Dawley) but no domestication (wild rat vs. Long-Evans, Fischer-Norway). Behav. Brain Res 234, 467–477. [DOI] [PubMed] [Google Scholar]
- Hill RA, 2012. Interaction of Sex Steroid Hormones and Brain- Derived Neurotrophic Factor-Tyrosine Kinase B Signalling: Relevance to Schizophrenia and Depression. J. Neuroendocrinol 24, 1553–1561. 10.1111/j.1365-2826.2012.02365.x [DOI] [PubMed] [Google Scholar]
- Hodosy J, Pales J, Ostatnikova D, Celec P, 2010. The effects of exogenous testosterone on spatial memory in rats. Cent. Eur. J. Biol 5, 466–471. [Google Scholar]
- Holland J, Bandelow S, Hogervorst E, 2011. Testosterone levels and cognition in elderly men: A review. Maturitas 69, 322–337. 10.1016/j.maturitas.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME, 2013. Determinants of novel object and location recognition during development. Behav. Brain Res 256, 140–150. 10.1016/j.bbr.2013.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson TK, Gruenbaum BF, Markus EJ, 2012. Extensive training and hippocampus or striatum lesions: Effect on place and response strategies. Physiol. Behav 105, 645–652. 10.1016/j.physbeh.2011.09.027 [DOI] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN, 2016. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157, 1357–1362. 10.1210/en.2015-1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Kang L, Li S, Geng D, Fan P, Wang L, Cui H, 2013. Amelioratory effects of testosterone treatment on cognitive performance deficits induced by soluble Aβ1–42 oligomers injected into the hippocampus. Horm. Behav 64, 477–486. 10.1016/j.yhbeh.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K, 1998. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci. Res 31, 227–234. 10.1016/S0168-0102(98)00040-6 [DOI] [PubMed] [Google Scholar]
- Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S, 2004. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J. Gerontol 59A, 75–78. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ, 1994. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb. Cortex N. Y. N 1991 4, 664–680. 10.1093/cercor/4.6.664 [DOI] [PubMed] [Google Scholar]
- Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M, 2012. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res 1484, 76–84. 10.1016/j.brainres.2012.09.028 [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP, 2012. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn. Sci 16, 467–475. 10.1016/j.tics.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF, 2014. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm. Behav 66, 298–308. 10.1016/j.yhbeh.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Mosterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL, 2006. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch. Neurol 63, 177–185. [DOI] [PubMed] [Google Scholar]
- Lv W, Du N, Liu Y, Fan X, Wang Y, Jia X, Hou X, Wang B, 2016. Low testosterone level and risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Mol. Neurobiol 53, 2679–2684. 10.1007/s12035-015-9315-y [DOI] [PubMed] [Google Scholar]
- McConnell SEA, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE, 2012. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm. Behav 61, 479–486. 10.1016/j.yhbeh.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM, 2002. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J. Clin. Endocrinol. Metab 87, 5001–5007. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS, 2006. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol 16, 179–190. 10.1016/j.conb.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Muller M, Aleman A, Grobbee DE, de Haan EHF, van der Schouw YT, 2005. Endogenous sex hormone levels and cognitive function in aging men. Neurology 64, 866–871. [DOI] [PubMed] [Google Scholar]
- Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH, 2017. Association between androgen deprivation therapy and risk of dementia. JAMA Oncol 3, 49–55. 10.1001/jamaoncol.2016.3662 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Jayaraman A, Quaglino A, Pike CJ, 2010. Androgens selectively protect against apoptosis in hippocampal neurones. J. Neuroendocrinol 22, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ, 2009. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res 1298, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak NT, Diamond MP, Land SJ, Moffat SD, 2014. Contributions of sex, testosterone, and androgen receptor CAG repeat number to virtual Morris water maze performance. Psychoneuroendocrinology 41, 13–22. 10.1016/j.psyneuen.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ, 1998. Age-related deficits on the radial maze and in fear conditioning: Hippocampal processing and consolidation. Hippocampus 8, 402–415. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL, 1996. Inactivation of the hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem 65, 65–72. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1986. The Rat Brain in Stereotaxic Coordinates Academic Press Inc., San Diego, CA. [Google Scholar]
- Ramsden M, Shin TM, Pike CJ, 2003. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience 122, 573–578. 10.1016/j.neuroscience.2003.08.048 [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA, 2006. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav 50, 18–26. [DOI] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL, 2010. Good things come to those who wait: Attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol. Aging 31, 853–862. 10.1016/j.neurobiolaging.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE, 2013. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, and sprouting in area CA3 of hippocampus. J. Neurosci 33, 2338–2355. 10.1523/JNEUROSCI.3857-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN, 2011. Effects of testosterone on spatial learning and memory in adult male rats. Horm. Behav 59, 484–496. 10.1016/j.yhbeh.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J, 2013. Testosterone influences spatial strategy preferences among adult male rats. Horm. Behav 63, 800–812. 10.1016/j.yhbeh.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LAM, 2008. Castration differentially affects spatial working and reference memory in male rats. Arch. Sex Behav 37, 19–29. [DOI] [PubMed] [Google Scholar]
- Stewart J, Mitchell J, Kalant N, 1989. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol. Aging 10, 669–675. [DOI] [PubMed] [Google Scholar]
- Tan RS, Pu SJ, 2003. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 6, 13–17. [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen Z-Y, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL, 2005. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. Off. J. Soc. Neurosci 25, 5455–5463. 10.1523/JNEUROSCI.5123-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD, 2002. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem 9, 224–237. 10.1101/lm.51202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD, 2009. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J. Cereb. Blood Flow Metab 29, 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan C, Goldstein FC, Tenover JL, 2007. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in older men with low serum testosterone. J. Androl 28, 875–881. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Braddick VC, Batson CG, Cullen BH, Miller LE, Spritzer MD, 2018. Effects of testosterone dose on spatial memory among castrated adult male rats. Psychoneuroendocrinology 89, 120–130. 10.1016/j.psyneuen.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP, 1993. Reproductive aging in the male brown-norway rat: a model for the human. Endocrinology 133, 2773–2781. [DOI] [PubMed] [Google Scholar]
- Wiener JM, de Condappa O, Harris MA, Wolbers T, 2013. Maladaptive Bias for Extrahippocampal Navigation Strategies in Aging Humans. J. Neurosci 33, 6012–6017. 10.1523/JNEUROSCI.0717-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Preut R, Hellhammer DH, Kudielka BM, Schurmeyer TH, Krischbaum C, 2000. Testosterone and cognition in elderly men: a single testosterone injection blocks the practice effect in verbal fluency, but has no effect on spatial or verbal memory. Biol. Psychiatry 47, 650–654. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL, 2007. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 144, 26–37. 10.1016/j.neuroscience.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


