Abstract
The opportunity to eliminate hepatitis C virus (HCV) is at hand, but challenges remain that negatively influence progress through the care continuum, particularly for persons co-infected with HIV who are not well engaged in care. We conducted a randomized controlled trial to test the effect of nurse case management (NCM) on the HCV continuum among adults co-infected with HIV compared to usual care (UC). Primary outcomes included linkage to HCV care (attendance at an HCV practice appointment within 60 days) and time to direct acting antiviral (DAA) initiation (censored at 6 months). Sixty-eight participants were enrolled (NCM n=35; UC n=33). Participants were 81% Black/African American, 85% received Medicaid, 46% reported illicit drug use, 41% alcohol use, and 43% had an undetectable HIV viral load. At day 60, 47% of NCM participants linked to HCV care compared to 25% of UC participants (p=0.031; 95% confidence bound for difference, 3.2-40.9%). Few participants initiated DAAs (12% NCM; 25% UC). There was no significant difference in mean time to treatment initiation (NCM=86 days; UC=110 days; p=0.192). Engagement in HCV care across the continuum was associated with drinking alcohol, knowing someone who cured HCV, and having a higher CD4 cell count (p<0.05). Our results support provision of NCM as a successful strategy to link persons co-infected with HIV to HCV care, but interventions should persist beyond linkage to care. Capitalizing on social networks, treatment pathways for patients who drink alcohol, and integrated substance use services may help improve the HCV care continuum.
Keywords: access to health care, case management, hepatitis C virus, HIV, patient compliance
Hepatitis C virus (HCV) is a major global public health concern, surpassing HIV-related deaths in the United States and Europe.1–4 Risk for HCV-related liver disease is increased in the setting of HIV co-infection.5 Due to similar transmission routes, approximately 2.3 million people living with HIV (PLWH) are co-infected with HCV globally, and greater than 82% of PLWH who inject drugs also have HCV.6 But with the advent of effective and tolerable all-oral direct acting antivirals (DAAs), we have the opportunity to cure HCV in most patients and achieve the World Health Organization’s target of HCV elimination by 2030.7
While necessary, DAAs alone are not sufficient to ensure HCV elimination without first linking patients to HCV care.8 This is particularly true among PLWH in the setting of injection drug use in the United States, Canada, and Europe, who have historically low HCV treatment uptake.9–12 Engagement in HIV care is a major consideration in success across the HCV continuum. Uncontrolled HIV is associated with not being prescribed DAAs,13 despite evidence of high cure rates and HCV treatment guidelines that recommend prioritizing treatment for PLWH.14–18 Other barriers to engaging in HCV care include comorbidities and competing priorities such as substance use, poor access to specialty care, navigating the healthcare system, low knowledge and perceived threat of HCV, lack of provider expertise, and non-referral to specialty care by primary care providers.19–22 High prevalence of drug interactions between antiretroviral therapy (ART) and DAAs which may necessitate modification of ART regimens have introduced an additional barrier to initiating HCV treatment among PLWH.23,24
Strategies to link PLWH to HCV care and minimize barriers to initiating treatment are needed to eliminate HCV. While prior studies have demonstrated improvement in linkage to HIV care with case management and reminder systems,25–27 few interventions to date promote linkage to HCV care in this relatively new era of all-oral DAAs.28–30 Furthermore, interventions that include persons with uncontrolled HIV, mental illness, or substance use are needed to maximize the impact of DAAs among the remaining untreated patients. In the present study, we aimed to investigate whether nurse case management (NCM) improves the HCV care continuum compared to usual care (UC) among PLWH, regardless of comorbidities or HIV viral suppression. We also sought to describe the characteristics associated with engaging in HCV care among a group of high-priority PLWH with prevalent uncontrolled HIV, drug and alcohol use, and psychiatric illness.
MATERIALS AND METHODS
Study Design and Sample
We conducted a randomized controlled trial comparing the effect of HCV NCM to UC on the proportion of participants who link to HCV care and time to HCV treatment initiation. This study was approved by the Johns Hopkins Medicine Institutional Review Board and registered on ClinicalTrials.gov ( NCT02707991). The trial protocol has previously been reported.31
We included individuals age 18 years or older with HIV and chronic HCV (chronic HCV ICD-10 code and most recent HCV plasma RNA >15 IU/mL). Eligible participants were engaged in HIV care with at least one visit to the HIV clinic in the past 12 months, but not engaged in HCV care (no visit to the viral hepatitis practice in the past 12 months). We excluded pregnant women and persons unable to provide independent informed consent.
Procedures.
We enrolled participants from a large, urban infectious disease outpatient practice providing HIV primary care and HCV specialty care in Baltimore, Maryland, USA. At the start of the study, the HIV and viral hepatitis clinics were separate units within the hospital. The two clinics merged into a new co-located space 10 months after recruitment began, but referral to an HCV-treating specialist remained a barrier due to health insurance requirements. One HIV provider was actively treating HCV in his co-infected patients and the rest chose to refer their patients to a viral hepatitis specialist within the practice. Participants self-referred to the study from flyers and clinic advertisements or were referred by their clinical care team during HIV primary care visits.
Eligible individuals provided written informed consent and responded to a sociodemographic survey that included validated self-report measures of alcohol use,32 depressive symptoms,33 and HCV knowledge34 at the enrollment visit. Individual items such as whether the participant knows someone who was cured of HCV and perceived financial strain35,36 were also included in the survey. We collected objective data from the electronic medical record, including CD4 cell count, HIV viral load, prescribed ART, HCV viral load and genotype, liver fibrosis level, receipt of prior HCV treatment, date of last visit with an HCV-treating provider, comorbidities, and health insurance provider at the enrollment visit. We followed participants for 6 months via the electronic medical record to collect follow-up data and study outcomes at 60 and 180 days. The 180-day study period is consistent with simulated linkage to care and treatment initiation HCV case management that predicted substantial improvements in cure rates and cost savings in 6 months.8
Randomization and masking.
Participants were randomized 1:1 using block randomization during the enrollment visit immediately following completion of the baseline survey. The randomization scheme was developed by the study team statistician, who did not enroll participants into the study, but contributed to data analysis and interpretation. A study team member masked to randomization collected all outcome data. In addition, viral hepatitis providers were not informed of allocation as to not influence treatment decisions for the patient.
Usual care.
UC consisted of normal outpatient clinical processes with the addition of receipt of the United States Centers for Disease Control and Prevention (CDC) HCV Fact Sheet.37 After randomization, participants in the UC group were given the HCV fact sheet and referred back to usual clinic care (e.g., provider visit, lab work, check out of appointment and schedule follow-up as needed). The infectious disease practice has HIV nurse case managers and Ryan White-funded social workers, which continued to be available to both groups in the study at the patients’ request. In addition, all patients in the health system receive automatic telephone appointment reminders two days before any scheduled appointment.
Intervention.
The NCM intervention consisted of two phases to address the following study outcomes – 1) Linkage to Care and 2) Time to Treatment Initiation. Phase 1 included a nurse-initiated HCV referral, strengths-based HCV education, patient navigation and clinical coordination, and appointment reminders. The study nurse case manager initiated the referral to HCV care and assisted participants to schedule an appointment in the HCV practice. Strategies to minimize barriers to attending the appointment were discussed. Personalized appointment reminders by phone, email, or text message were offered to participants both 1-week and 1-day before their scheduled HCV practice appointments. Strengths-based HCV education25,38 was directed by a study-developed HCV Basics patient guide, delivered one-on-one with the nurse case manager, and focused on goal-setting and coaching participants to identify their strengths (e.g., social support, adherence to HIV treatment, resilience) IHIV tHand apply them to improving their liver health.
The goal of Phase 2 was to minimize potential ART/DAA drug interactions to reduce time to HCV treatment initiation. After a participant attended an appointment at the HCV practice, the nurse case manager examined the medical record for a potential drug interaction between ART and DAAs. If a contraindicated drug interaction was present, the study protocol directed the nurse case manager to coordinate an ART modification with the participant and his/her primary HIV provider.
Outcomes.
The primary outcome was linkage to care, defined as attendance at an appointment in the viral hepatitis practice within 60 days of study enrollment (yes/no). All attended appointments are registered in the electronic medical record, so absence of a documented appointment or documentation of a no-show in the electronic medical record was considered non-attendance. The secondary outcome was time to HCV treatment initiation, defined as the number of days between study enrollment and DAA start date, according to the electronic medical record. The care team uses standardized documentation to record DAA start dates for every patient, so these data were readily available in the medical record. Exploratory outcomes included whether a participant was referred to HCV care, scheduled for an HCV clinic appointment, prescribed DAAs, started taking DAAs, and achieved an undetectable HCV RNA within the 6-month follow-up period. We also examined patient-level characteristics as predictors of success across these care continuum outcomes.
Statistical Analysis
We summarized continuous variables with mean and standard deviation or median and interquartile range (IQR) and categorical variables with frequencies and percentages. We used a one-tailed intention-to-treat z-test for difference in proportions to estimate the effect of the intervention on linkage to care. A one-tailed test was justified given that NCM was additive to UC and there was no reasonable reason for participants in the NCM group to do worse than those in the UC group. Based on an estimated effect size range of 18-30% absolute difference in linkage to care25,26, needed sample size estimate would range from 66 to 190 to detect a chosen difference (within the range of 18-30%) in the primary outcome between the NCM and UC arms with 80% power. In consideration of the study being pilot in nature with limited resources, we determined that 66 would be a sufficient sample size (G*Power 3.1). We calculated a phi coefficient to determine the effect size of the intervention on our primary outcome.39
We conducted Kaplan Meier estimates to compare time to HCV treatment initiation between participants in the NCM group and the UC group. We examined differences by randomization arm using a log-rank test. Participants who did not start HCV treatment were censored at 180 days.
We evaluated the effect of participant characteristics chosen from the literature on HCV care continuum outcomes for the full participant cohort using binary logistic regression. We performed an analysis for each HCV care continuum outcome separately (i.e., scheduled an appointment, attended an appointment, prescribed DAAs, and initiated DAAs). First, we conducted binary logistic regression with each characteristic of interest. Participant characteristics associated with the outcome with a p-value of less than 0.2 were used to build the final models.40 We employed a stepwise forward method and used a correlation matrix to assess multicollinearity of independent variables. A chi-squared test was used to examine collinearity of binary variables. Randomization arm (NCM vs. UC) was included as a covariate in each model.
P-values <0.05 were considered significant. Ninety-five percent confidence bounds (CB) were calculated for one-sided tests and 95% confidence intervals (CI) for two-sided tests. We conducted all analyses in Stata IC version 15.0 (College Station, TX, USA).41
RESULTS
Between July 2016 and February 2018, 68 participants enrolled and were randomized to receive NCM (n=35) or UC (n=33) (Figure 1). One participant in each arm was excluded after randomization because an undetectable HCV RNA result became available in the medical record shortly after enrollment, indicating they had cleared HCV and no longer needed treatment.
Figure 1:
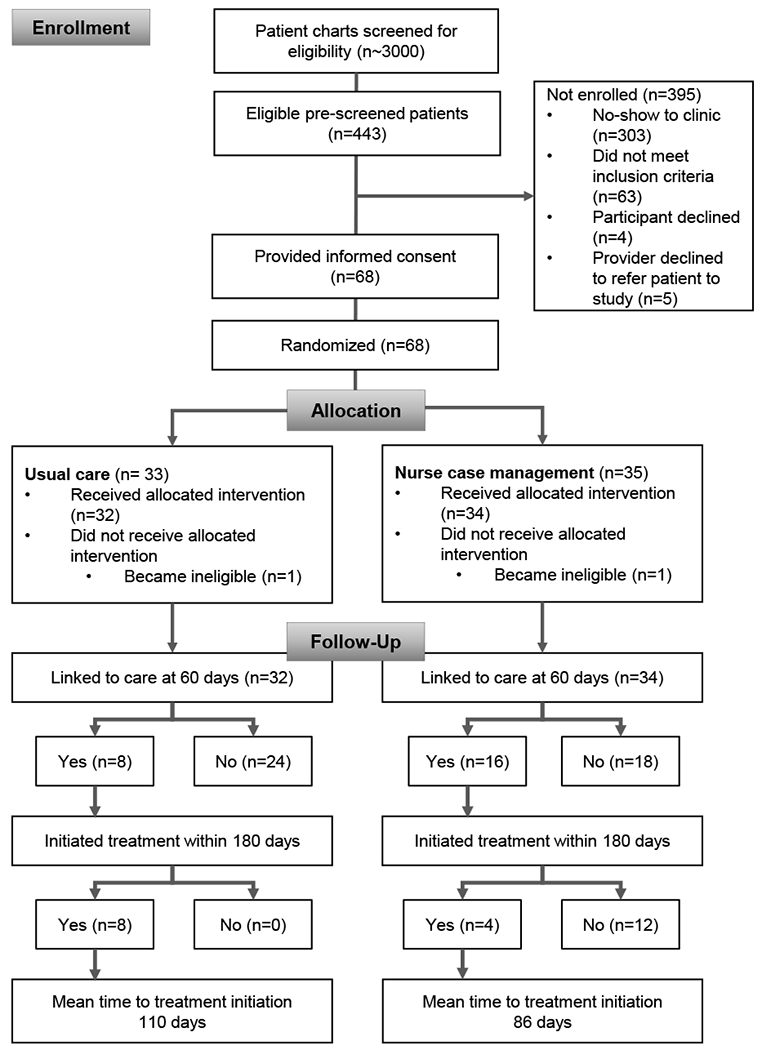
CONSORT Study Flow Diagram. This figure diagrams the enrolment, allocation and follow-up outcomes for study participants in the medical record shortly after enrolment, indicating they had cleared HCV and no longer needed treatment.
Table 1 summarizes the sample characteristics. Prior research with this population reported the most common mode of transmission was injection drug use,42 although participants were not excluded by mode of transmission. There were no significant baseline differences between participants in the NCM and UC groups. No participants were eligible for Phase 2 of the study as no ART/DAA drug-drug interactions were identified among participants in the intervention arm.
Table 1.
Baseline characteristics of study participants.
| Total Study Sample (N=68) |
Usual Care (N=33) |
Nurse Case Management (N=35) |
|||||
|---|---|---|---|---|---|---|---|
| M (SD) | n (%) | M (SD) | n (%) | M (SD) | n (%) | p | |
| Demographic Characteristics | |||||||
| Age, years | 55 (7.65) | 55 (7.73) | 55 (7.68) | 0.762† | |||
| Female | 26 (38) | 13 (39.39) | 13 (37) | 0.617‡ | |||
| Race/Ethnicity | 0.366§ | ||||||
| Black/African American | 55 (81) | 26 (79) | 29 (83) | ||||
| White/Caucasian | 10 (15) | 4 (12) | 6 (17) | ||||
| Native American/American | 1 (2) | 1 (3) | 0 (0) | ||||
| Indian | 2 (3) | 2 (6) | 0 (0) | ||||
| Hispanic/Latino | |||||||
| Education and Employment | |||||||
| Education | 0.393‡ | ||||||
| No high school diploma/No GED | 28 (41) | 11 (33) | 17 (49) | ||||
| High school graduate/GED | 25 (37) | 13 (39) | 12 (34) | ||||
| Some college or higher | 15 (22) | 9 (27) | 6 (17) | ||||
| Income source | 0.130§ | ||||||
| Government benefits | 56 (82) | 25 (76) | 31 (89) | ||||
| Work full- or part-time | 3 (4) | 1 (3) | 2 (6) | ||||
| No income | 9 (13) | 7 (21) | 2 (6) | ||||
| Annual income <$25,000 | 65 (96) | 32 (97) | 33 (94) | >0.999§ | |||
| Financial strain‖ | 0.752§ | ||||||
| Never | 14 (28) | 8 (33) | 6 (23) | ||||
| Once in a while | 9 (18) | 5 (21) | 4 (15) | ||||
| Fairly often | 14 (28) | 6 (25) | 8 (31) | ||||
| Very often | 13 (26) | 5 (21) | 8 (31) | ||||
| Health insurance# | |||||||
| Medicaid | 58 (85) | 26 (79) | 32 (91) | 0.140‡ | |||
| Medicare | 17 (25) | 8 (24) | 9 (26) | 0.889‡ | |||
| Private | 1 (2) | 0 (0) | 1 (3) | 1.000§ | |||
| Health Status | |||||||
| CD4+ T-cell count, median (IQR) | 366 (198-653) | 379 (266-676) | 361 (125-540) | 0.291¶ | |||
| HIV viral load <20 | 29 (43) | 15 (46) | 14 (40) | 0.649‡ | |||
| Currently prescribed ART | 66 (97) | 33 (100) | 33 (94) | 0.493§ | |||
| Fibrosis level | 0.726‡ | ||||||
| Metavir < F2 | 14 (21) | 7 (21) | 7 (20) | ||||
| Metavir ≥ F2 | 24 (35) | 13 (39) | 11 (31) | ||||
| Unknown | 30 (44) | 13 (39) | 17 (49) | ||||
| HCV genotype | 0.249§ | ||||||
| 1a | 44 (67) | 21 (66) | 23 (68) | ||||
| 1b | 14 (21) | 9 (28) | 5 (15) | ||||
| 2b | 1 (2) | 1 (3) | 0 (0) | ||||
| 3a | 1 (2) | 0 (0) | 1 (3) | ||||
| 4 | 1 (2) | 0 (0) | 1 (3) | ||||
| Unknown | 5 (8) | 1 (3) | 4 (12) | ||||
| Previously treated for HCV | 15 (22) | 8 (24) | 7 (20) | 0.810‡ | |||
| Knows someone who cured HCV | 28 (41) | 17 (52) | 11 (31) | 0.093‡ | |||
| PHQ-9 total score, median (IQR) | 6 (3-10) | 7 (3-11) | 6 (2-8) | 0.471¶ | |||
| HCV knowledge total score | 15.1 (2.07) | 15.4 (2.01) | 14.8 (2.12) | 0.264† | |||
| Substance Use | |||||||
| Injection drug use, past 12 months | 16 (24) | 9 (27) | 7 (20) | 0.480‡ | |||
| OAT, currently taking | 35 (52) | 17 (52) | 18 (51) | 0.994‡ | |||
| Alcohol use, past 6 months | 28 (41) | 10 (30) | 18 (51) | 0.077‡ | |||
| AUDIT totalΔ, median (IQR) | 3 (2-9) | 6 (2-12) | 3 (2-7) | 0.650¶ | |||
M, mean; SD, standard deviation; IQR, interquartile range; ART, antiretroviral therapy; HCV, hepatitis C virus; PHQ, patient health questionnaire; OAT, opiate agonist therapy; AUDIT, alcohol use disorders identification test.
t-test.
Chi-square test.
Fisher’s exact test.
Mann-Whitney U test.
Financial strain (how often is your income not enough for food, housing, or medications?) was an added measure for the last 50 participants enrolled.
Nine participants (13%) had both Medicaid and Medicare.
AUDIT total score is reported for the 28 participants who reported any alcohol use in the past 6 months.
A higher proportion of participants who received the NCM intervention (47%) linked to HCV care within 60 days of enrollment compared to those who received UC (25%) (p=0.031; 95% CB for difference 3.2-40.9%; Cramer’s phi medium effect size 0.2339) (Figure 2).
Figure 2:
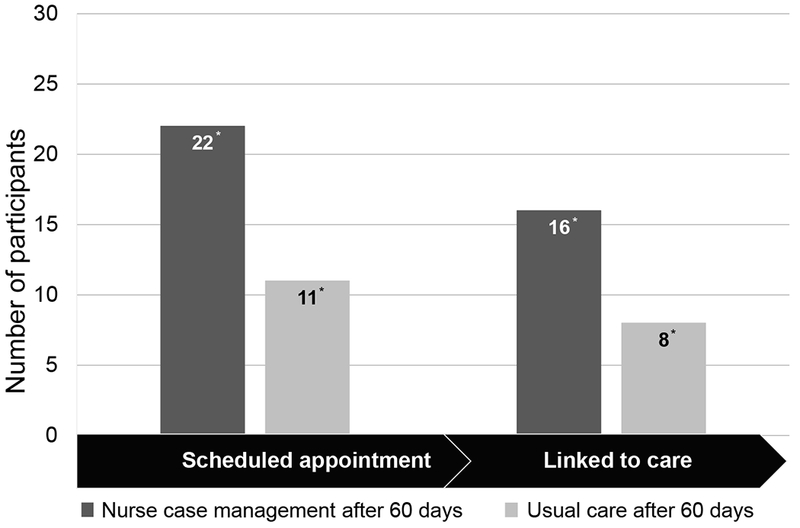
Sixty-day HCV Care Continuum Outcomes Related to the Intervention. This graph shows the number of participants who scheduled an HCV appointment and linked to HCV care within 60 days of randomization. Note: *P < .05 by z test for difference in proportions; linked to care is the primary outcome.
Twelve participants initiated DAA treatment within the study period – eight in UC (25%) and four receiving NCM (12%). Barriers to initiating HCV treatment within 180 days included insurance denial for detectable HIV viral load (11%), insurance denial for fibrosis level <F2 (33%), unresolved prior authorization for DAAs (33%), lost to follow up (11%), and waiting to receive an HCV-positive kidney transplant (11%). At the time of the study, Maryland Medicaid did not restrict DAA prescription authorization for active substance use.43
Among participants who initiated HCV treatment, the median time to initiation was 85.5 days for the NCM group (IQR, 58.5-100) and 110 days for UC (IQR, 72.5-130). The difference in time to treatment initiation between the two groups was not significant (p=0.192).
Overall, a high proportion of participants were referred to HCV specialty care by their primary care provider in the NCM and UC groups during the 6-month follow-up period (76% and 72%, respectively) with no significant difference between the groups (p=0.335). According to the HIV primary care providers, patients were not referred to HCV care because of too many missed visits (31%), competing comorbidities such as cancer (25%), and the need to stabilize HIV first (25%) or the need to decrease substance use (13%). A higher proportion of participants in the NCM group had an appointment scheduled with the viral hepatitis practice within 60 days compared to the UC group (65% vs. 34%, respectively; p=0.007; 95% CI for difference 7%-53%). We found no significant difference between the proportion of participants who were prescribed DAAs (p=0.670), initiated DAAs (p=0.164), or achieved an undetectable HCV RNA (p=0.651) within 180 days between the study arms (Figure 3).
Figure 3:
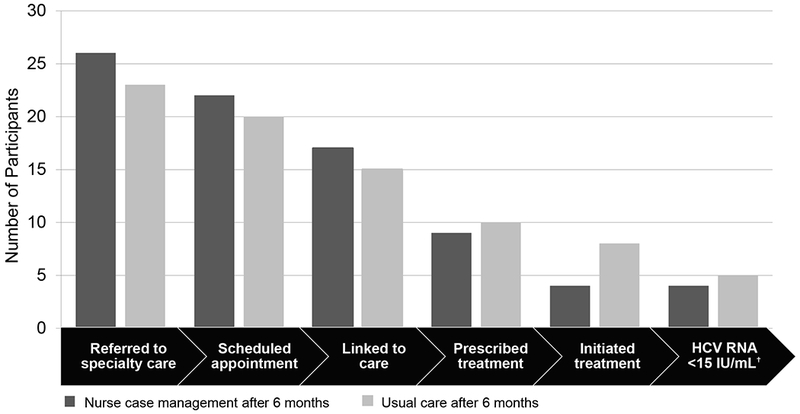
Six-month HCV Care Continuum. This graph shows the hepatitis C virus (HCV) care continuum outcomes for each randomization arm at 6 months. Note: †3 participants in the usual care arm were still taking directacting antivirals at study completion secondary outcome of time to treatment initiation.
Table 2 displays results of adjusted logistic regression models for predictors of success across the HCV care continuum. Drinking alcohol and higher CD4 cell count were associated with greater odds of scheduling an HCV appointment. Odds of both attending an HCV appointment and being prescribed DAAs were 3.8 times higher among participants who drank any alcohol compared to those who did not report drinking alcohol in the past 12 months. Knowing someone who had cured HCV was associated greater odds of being prescribed DAAs and initiating treatment. Being prescribed DAAs was 75% less likely among participants taking opiate agonist therapy (OAT) compared to participants not taking OAT. Psychiatric illness or depressive symptoms were not associated with any outcomes.
Table 2.
Identification of factors predicting HCV care continuum outcomes by logistic regression (n=68).
| Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|
| Odds Ratio | p value | 95% Confidence Interval | Odds Ratio | p value | 95% Confidence Interval | |
| Scheduled an appointment † | ||||||
| CD4+ cell count, 100-unit increase | 1.00 | 0.054 | 1.00 – 1.00 | 1.23 | 0.032 | 1.02 – 1.48 |
| Alcohol use | 3.00 | 0.051 | 0.99 – 9.05 | 3.79 | 0.030 | 1.14 – 12.61 |
| Attended an appointment ‡ | ||||||
| Alcohol use | 3.57 | 0.016 | 1.27 – 10.04 | 3.79 | 0.016 | 1.29 – 11.13 |
| Prescribed direct acting antivirals § | ||||||
| Alcohol use | 3.66 | 0.023 | 1.20 – 11.16 | 4.18 | 0.035 | 1.10 – 15.80 |
| Opiate agonist therapy | 0.34 | 0.062 | 0.11 – 1.06 | 0.25 | 0.036 | 0.07 – 0.91 |
| Know someone who cured HCV | 4.62 | 0.009 | 1.47 – 15.53 | 5.24 | 0.014 | 1.40 – 19.55 |
| Initiated direct acting antivirals ¶ | ||||||
| Financial strain | 0.64 | 0.123 | 0.36 – 1.13 | 0.37 | 0.052 | 0.14 – 1.01 |
| Knows someone who cured HCV | 4.62 | 0.009 | 1.47 – 14.53 | 8.05 | 0.036 | 1.15 – 56.49 |
adjusted model included CD4 cell count, alcohol use, and randomization arm.
adjusted model included alcohol use and randomization arm.
adjusted model included alcohol use, opiate agonist therapy, knowing someone who cured HCV, and randomization arm.
adjusted model included knowing someone who cured HCV, financial strain, and randomization arm.
DISCUSSION
Our results showed a higher proportion of participants who received HCV NCM linked to HCV care compared to UC in a real-world HIV practice setting. This highlights the benefits of additional education, navigation, and care coordination for this population early in the continuum. Although similar in effect to comparable interventions linking individuals to HIV care,25,26 it is noteworthy that less than half of NCM participants and only one-quarter of UC participants attended an HCV appointment, despite >70% having a referral to HCV care. Patient self-scheduling specialty appointments is a known barrier to engaging in care.20,22,44 The NCM intervention bypassed the scheduling barrier, but persistently low scheduling and attendance rates underscore a need to expand HCV treatment to non-specialist or community-based providers who may already have trusting relationships with patients and are equally effective in achieving sustained virologic response (SVR) compared to HCV specialists.45 The lack of integration of HIV and HCV-treating providers in our practice, including few HIV providers choosing to treat HCV, limited follow-up for HCV care regardless of NCM efforts. Additionally, referral and linkage to care alone were not sufficient to initiate HCV treatment in our study, indicating need for interventions that extend through the entire care continuum. The higher rate of treatment initiation among UC participants, although not statistically significant, may indicate a difference in patient motivation between the groups, but this was not measured.
Control of HIV is a critical issue in this population. Although connected to HIV primary care (97% prescribed ART), 57% of our sample was not virally suppressed. Despite high DAA cure rates regardless of HIV viral load,46,47 insurance payers in the United States often require an undetectable HIV viral load to approve DAAs,48,49 creating a barrier to initiating treatment in 44% of participants in our study. HIV providers noted a desire to stabilize HIV or decrease substance use to justify not referring patients to HCV care, consistent with prior research findings.46,49 These misconceptions in payer and provider prerequisites regarding HIV control must be corrected; given the high burden of co-infection, HCV and HIV care cannot happen in silos.
Opportunities exist within HIV care settings to direct resources toward overlapping services for HCV care management. Case management targeting patients with socioeconomic disparities, substance use disorder, and uncontrolled HIV can also reach patients who are the highest priority to cure HCV. Case management services are relatively accessible to PLWH, particularly in the United States in the setting of Ryan White funding50, and effective for improving engagement in HIV care.25–27,51
Alcohol use was associated with likelihood of scheduling and attending an appointment and being prescribed DAAs. These findings conflict with prior studies reporting that a history of alcohol use is associated with lower odds of follow up to HCV appointments and receipt of DAAs.52,53 Our findings may be explained by patient motivation to cure HCV and eliminate one stressor on the liver, potentially decreasing the combined effect from HCV and alcohol use. The positive association between knowing someone who cured HCV and being prescribed and initiating DAAs is not surprising given that nearly half of persons living with HCV access their HCV information from friends and peers.54 Opportunities to capitalize on social networks to eliminate HCV exist, such as HCV support groups55 or peer education and peer navigator interventions.55–58 Recent studies of peer support interventions to improve engagement in HCV care have found similar results to this study of NCM, including improved appointment attendance but little effect on treatment initiation or SVR.57,58 This emphasizes the need for interventions that focus on treatment initiation and achieving cure, as well as future research on the comparative-effectiveness of peer versus nurse-led supportive interventions.
Participants taking OAT may be less likely to be prescribed DAAs due to competing priorities, including managing addiction and clinic visits to receive OAT as frequently as daily59,60, yet care for HCV and substance use are rarely integrated. This population is both at risk for HCV transmission in the setting of ongoing drug use and also under direct observation of a healthcare provider, underscoring a missed opportunity to engage OAT recipients in curing HCV through integrated substance use and HCV treatment programs.61–64 Both integrating HCV treatment into substance use treatment programs and integrating addiction services into HCV care have improved linkage to care and treatment success among people living with HCV and substance use disorder in developed countries.62,65–69
This study has limitations. The small sample and six-month follow-up limited our ability to conduct robust multivariable analyses on the full continuum and we were under-powered to examine the secondary outcome of time to treatment initiation. We did not look at SVR, which should be included in future studies. Our co-located HIV and HCV practice may limit generalizability, but considering the gaps that exist in this high-level clinical setting, our findings remain relevant to clinical settings that do not have this capacity. Although our sample included untreated patients with high rates of uncontrolled HIV, we were only successful in enrolling a limited number of eligible patients because those targeted by this study had low show rates to the clinic. This may present a sampling bias resulting in an overestimate of success across the care continuum in both groups since our participants did attend an HIV clinic visit to be enrolled. The NCM intervention did not require a registered nurse to perform its most effective components (appointment scheduling and navigation); a non-clinician can implement the linkage to care components, saving nursing intervention costs for ART optimization and complex care coordination. We are unable to extract whether the strengths-based element of NCM had any effect. Despite these limitations, this study fills an important gap in efforts to strengthen engagement across the HCV care continuum in a high-priority population co-infected with HIV.
In conclusion, case management can improve linkage to HCV care among vulnerable persons co-infected with HIV by coordinating specialty referrals, navigating appointment scheduling, providing strengths-based education, and tailoring appointment reminders. But without a care delivery system that promotes HCV treatment in this high-priority patient population, such as evidence-based treatment decisions and HIV providers trained and willing to treat HCV, the benefits of case management may be limited. The patient characteristics associated with engaging in the HCV care continuum emphasize the importance of integrated programs for the treatment of HIV, substance use, and HCV, as well as treatment pathways for persons who drink alcohol who are at high risk for liver disease but willing to cure their HCV.
Acknowledgements
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Numbers F31NR016200 and T32NR014205. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study also received funding from the Council for the Advancement of Nursing Science/Southern Nursing Research Society and Sigma Theta Tau International Honor Society for Nursing/Association of Nurses in AIDS Care.
Abbreviations
- ART
antiretroviral therapy
- AUDIT
alcohol use disorders identification test
- CB
confidence bound
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DAA
direct acting antiviral
- HCV
hepatitis C virus
- IQR
interquartile range
- M
mean
- NCM
nurse case management
- OAT
opiate agonist therapy
- OR
odds ratio
- PHQ-9
patient health questionnaire
- PLWH
persons living with HIV
- SD
standard deviation
- SVR
sustained virologic response
- UC
usual care
- USA
United States of America
Footnotes
Declaration of interests
MS has served as an advisor/consultant for Gilead Sciences, Merck, Arbutus, and AbbVie and has received research funding from Gilead Sciences, Merck, AbbVie, and Assembly Biosciences.
REFERENCES
- 1.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated with Hepatitis C Virus in the United States, 2003-2013. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62(10):1287–1288. doi: 10.1093/cid/ciw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke GS, Lemoine M, Thursz M, et al. Viral hepatitis and the Global Burden of Disease: a need to regroup. J Viral Hepat. 2013;20(9):600–601. doi: 10.1111/jvh.12123 [DOI] [PubMed] [Google Scholar]
- 3.Cowie BC, Allard N, MacLachlan JH. European responses in focus: Comparing viral hepatitis and HIV related deaths in Europe 1990–2010 in the Global Burden of Disease Study 2010. J Hepatol. 2014;60(1, Supplement):S35–S36. doi: 10.1016/S0168-8278(14)60088-X [DOI] [Google Scholar]
- 4.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. The Lancet. 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo Re V, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):369–379. doi: 10.7326/M13-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. Geneva; 2016. [Google Scholar]
- 8.Linas BP, Barter DM, Leff JA, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PloS One. 2014;9(5):e97317. doi: 10.1371/journal.pone.0097317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed C, Stuver SO, Tumilty S, et al. Predictors of treatment for hepatitis C virus (HCV) infection in drug users. Subst Abuse. 2008;29(1):5–15. doi: 10.1300/J465v29n01_02 [DOI] [PubMed] [Google Scholar]
- 10.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1-2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS Lond Engl. 2006;20(18):2361–2369. doi: 10.1097/QAD.0b013e32801086da [DOI] [PubMed] [Google Scholar]
- 12.Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402–411. doi: 10.1016/j.jhep.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaweera D, Althoff K, Eron J, et al. Untreated HCV in HIV/HCV co-infection: Data from the TRIO network. J Hepatol. 2018;68:S261. doi: 10.1016/S0168-8278(18)30735-9 [DOI] [Google Scholar]
- 14.American Association for the Study of Liver Diseases & Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org/ Published 2019. Accessed March 14, 2018.
- 15.Naggie S, Cooper C, Saag M, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):705–713. doi: 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyles D, Bräu N, Kottilil S, et al. Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;65(1):6–12. doi: 10.1093/cid/cix260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12):1223–1231. doi: 10.1001/jama.2015.1328 [DOI] [PubMed] [Google Scholar]
- 18.Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153 [DOI] [PubMed] [Google Scholar]
- 19.Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J. 2013;10:7. doi: 10.1186/1477-7517-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zickmund SL, Campbell SA, Tirado CF, Zook CL, Weinrieb RM. Perceived barriers to hepatitis C therapy for patients receiving opioid agonist treatment. J Addict Med. 2012;6(3):233–239. doi: 10.1097/ADM.0b013e31825f491b [DOI] [PubMed] [Google Scholar]
- 21.Clark BT, Garcia-Tsao G, Fraenkel L. Patterns and predictors of treatment initiation and completion in patients with chronic hepatitis C virus infection. Patient Prefer Adherence. 2012;6:285–295. doi: 10.2147/PPA.S30111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGowan CE, Monis A, Bacon BR, et al. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatol Baltim Md. 2013;57(4):1325–1332. doi: 10.1002/hep.26246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel N, Nasiri M, Koroglu A, et al. Prevalence of drug-drug interactions upon addition of simeprevir- or sofosbuvir-containing treatment to medication profiles of patients with HIV and hepatitis C coinfection. AIDS Res Hum Retroviruses. 2015;31(2):189–197. doi: 10.1089/AID.2014.0215 [DOI] [PubMed] [Google Scholar]
- 24.Cope R, Pickering A, Glowa T, Faulds S, Veldkamp P, Prasad R. Majority of HIV/HCV Patients Need to Switch Antiretroviral Therapy to Accommodate Direct Acting Antivirals. AIDS Patient Care STDs. 2015;29(7):379–383. doi: 10.1089/apc.2015.0004 [DOI] [PubMed] [Google Scholar]
- 25.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr 1999. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51 [DOI] [PubMed] [Google Scholar]
- 26.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS Lond Engl. 2005;19(4):423–431. [DOI] [PubMed] [Google Scholar]
- 27.Farmer T, Brook G, McSorley J, Murphy S, Mohamed A. Using short message service text reminders to reduce “did not attend” rates in sexual health and HIV appointment clinics. Int J STD AIDS. 2014;25(4):289–293. doi: 10.1177/0956462413502325 [DOI] [PubMed] [Google Scholar]
- 28.Ford MM, Johnson N, Desai P, Rude E, Laraque F. From Care to Cure: Demonstrating a Model of Clinical Patient Navigation for Hepatitis C Care and Treatment in High-Need Patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;64(5):685–691. doi: 10.1093/cid/ciw806 [DOI] [PubMed] [Google Scholar]
- 29.Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int J Drug Policy. 2015;26(10):922–935. doi: 10.1016/j.drugpo.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou K, Fitzpatrick T, Walsh N, et al. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect Dis. 2016;16(12):1409–1422. doi: 10.1016/S1473-3099(16)30208-0 [DOI] [PubMed] [Google Scholar]
- 31.Starbird LE, Han H-R, Sulkowski MS, Budhathoki C, Reynolds NR, Farley JE. Care2Cure: A randomized controlled trial protocol for evaluating nurse case management to improve the hepatitis C care continuum within HIV primary care. Res Nurs Health. August 2018. doi: 10.1002/nur.21903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, Dependence WHOD of MH and S. AUDIT : the Alcohol Use Disorders Identification Test : guidelines for use in primary health care. Screening and brief intervention for alcohol problems in primary care. 2001. http://www.who.int/iris/handle/10665/67205 Accessed March 16, 2018.
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balfour L, Kowal J, Corace KM, et al. Increasing public awareness about hepatitis C: development and validation of the brief hepatitis C knowledge scale. Scand J Caring Sci. 2009;23(4):801–808. doi: 10.1111/j.1471-6712.2008.00668.x [DOI] [PubMed] [Google Scholar]
- 35.Szanton SL, Allen JK, Thorpe RJ, Seeman T, Bandeen-Roche K, Fried LP. Effect of Financial Strain on Mortality in Community-Dwelling Older Women. J Gerontol B Psychol Sci Soc Sci. 2008;63(6):S369–S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel LJ, Szanton SL, Weiss CO, Thorpe RJ, Semba RD, Fried LP. Financial Strain Is Associated with Malnutrition Risk in Community-Dwelling Older Women. Epidemiol Res Int. 2012;2012:696518. doi: 10.1155/2012/696518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Hepatitis C General Information. 2015. https://www.cdc.gov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf.
- 38.Gottlieb LN. Strengths-based nursing. Am J Nurs. 2014;114(8):24–32; quiz 33,46. doi: 10.1097/01.NAJ.0000453039.70629.e2 [DOI] [PubMed] [Google Scholar]
- 39.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd edition Hillsdale, N.J: Routledge; 1988. [Google Scholar]
- 40.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th edition Boston: Pearson; 2012. [Google Scholar]
- 41.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 42.Falade-Nwulia O, Sutcliffe C, Moon J, et al. High hepatitis C cure rates among black and nonblack human immunodeficiency virus-infected adults in an urban center. Hepatol Baltim Md. 2017;66(5):1402–1412. doi: 10.1002/hep.29308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maryland Department of Health and Mental Hygiene. Clinical Criteria for Hepatitis C (HCV) Therapy. January 2015. https://mmcp.health.maryland.gov/pap/docs/Hep%20C%20clinical%20criteria%20.pdf.
- 44.Coupland H, Day C, Levy MT, Maher L. Promoting equitable access to hepatitis C treatment for Indo-Chinese injecting drug users. Health Promot J Aust Off J Aust Assoc Health Promot Prof. 2009;20(3):234–240. [DOI] [PubMed] [Google Scholar]
- 45.Kattakuzhy S, Gross C, Emmanuel B, et al. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med. 2017;167(5):311. doi: 10.7326/M17-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiberger T, Obermeier M, Payer BA, et al. Considerable under-treatment of chronic HCV infection in HIV patients despite acceptable sustained virological response rates in a real-life setting. Antivir Ther. 2011;16(6):815–824. doi: 10.3851/IMP1831 [DOI] [PubMed] [Google Scholar]
- 47.Jayaweera D, Althoff K, Eron J, et al. Untreated HCV in HIV/HCV co-infection: Data from the TRIO network In: Vol 68 Paris, France: Journal of Hepatology; 2018:S261. [Google Scholar]
- 48.Maryland Department of Health. Clinical Criteria for Hepatitis C (HCV) Therapy. February 2018. https://mmcp.health.maryland.gov/pap/docs/HCV%20%20Clinical%20Criteria%20Feb%2021%202018%20final.pdf.
- 49.Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat. 2010;17(12):839–844. doi: 10.1111/j.1365-2893.2009.01250.x [DOI] [PubMed] [Google Scholar]
- 50.Weiser J, Beer L, Frazier EL, et al. Service Delivery and Patient Outcomes in Ryan White HIV/AIDS Program-Funded and -Nonfunded Health Care Facilities in the United States. JAMA Intern Med. 2015;175(10):1650–1659. doi: 10.1001/jamainternmed.2015.4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PloS One. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992–1000. doi: 10.1111/apt.14328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sims OT, Guo Y, Shoreibah MG, et al. Short article: Alcohol and substance use, race, and insurance status predict nontreatment for hepatitis C virus in the era of direct acting antivirals: a retrospective study in a large urban tertiary center. Eur J Gastroenterol Hepatol. 2017;29(11):1219–1222. doi: 10.1097/MEG.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 54.Watson B, Conigrave KM, Wallace C, Whitfield JB, Wurst F, Haber PS. Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug Alcohol Rev. 2007;26(3):231–239. doi: 10.1080/09595230701247681 [DOI] [PubMed] [Google Scholar]
- 55.Roose RJ, Cockerham-Colas L, Soloway I, Batchelder A, Litwin AH. Reducing Barriers to Hepatitis C Treatment among Drug Users: An Integrated Hepatitis C Peer Education and Support Program. J Health Care Poor Underserved. 2014;25(2):652–662. doi: 10.1353/hpu.2014.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arain A, De Sousa J, Corten K, et al. Pilot Study: Combining Formal and Peer Education with FibroScan to Increase HCV Screening and Treatment in Persons who use Drugs. J Subst Abuse Treat. 2016;67:44–49. doi: 10.1016/j.jsat.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 57.Ward KM, Falade-Nwulia O, Moon J, et al. A Randomized Controlled Trial of Cash Incentives or Peer Support to Increase HCV Treatment for Persons with HIV Who Use Drugs: The CHAMPS Study. Open Forum Infect Dis. 2019;6(4):ofz166. doi: 10.1093/ofid/ofz166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stagg HR, Surey J, Francis M, et al. Improving engagement with healthcare in hepatitis C: a randomised controlled trial of a peer support intervention. BMC Med. 2019;17(1):1–9. doi: 10.1186/s12916-019-1300-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ball SA, Carroll KM, Canning-Ball M, Rounsaville BJ. Reasons for dropout from drug abuse treatment: symptoms, personality, and motivation. Addict Behav. 2006;31(2):320–330. doi: 10.1016/j.addbeh.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 60.Shamsalinia A, Norouzi K, Fallahi-Khoshknab M, Farhoudian A, Ghaffari F. Experiences of substance abusers from methadone maintenance therapy. Med J Islam Repub Iran. 2017;31:45. doi: 10.14196/mjiri.31.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grebely J, Alavi M, Micallef M, et al. Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: the ETHOS Study. Addict Abingdon Engl. 2016;111(2):311–319. doi: 10.1111/add.13197 [DOI] [PubMed] [Google Scholar]
- 62.Alavi M, Grebely J, Micallef M, et al. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57 Suppl 2:S62–69. doi: 10.1093/cid/cit305 [DOI] [PubMed] [Google Scholar]
- 63.Schìtz A, Moser S, Marchart K, Haltmayer H, Gschwantler M. Direct Observed Therapy of Chronic Hepatitis C With Interferon-Free All-Oral Regimens at a Low-Threshold Drug Treatment Facility-a New Concept for Treatment of Patients With Borderline Compliance Receiving Opioid Substitution Therapy. Am J Gastroenterol. 2016;111(6):903–905. doi: 10.1038/ajg.2016.119 [DOI] [PubMed] [Google Scholar]
- 64.Akiyama MJ, Agyemang L, Arnsten JH, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis. 2018;18(1):74. doi: 10.1186/s12879-018-2964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litwin AH, Soloway IJ, Cockerham-Colas L, et al. Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program. Int J Drug Policy. 2015;26(10):1014–1019. doi: 10.1016/j.drugpo.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talal AH, Andrews P, Mcleod A, et al. Integrated, Co-located, Telemedicine-based Treatment Approaches for Hepatitis C Virus Management in Opioid Use Disorder Patients on Methadone. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;69(2):323–331. doi: 10.1093/cid/ciy899 [DOI] [PubMed] [Google Scholar]
- 67.Socías ME, Karamouzian M, Parent S, Barletta J, Bird K, Ti L. Integrated models of care for people who inject drugs and live with hepatitis C virus: A systematic review. Int J Drug Policy. 2019;72:146–159. doi: 10.1016/j.drugpo.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 68.Trabut J-B, Barrault C, Charlot H, et al. Integrated Care for the Use of Direct-acting Antivirals in Patients With Chronic Hepatitis C and Substance Use Disorder. J Addict Med. 2018;12(5):346–352. doi: 10.1097/ADM.0000000000000415 [DOI] [PubMed] [Google Scholar]
- 69.Burton MJ, Voluse AC, Anthony V. Integrating comprehensive hepatitis C virus care within a residential substance use disorder treatment program. J Subst Abuse Treat. 2019;98:9–14. doi: 10.1016/j.jsat.2018.11.008 [DOI] [PubMed] [Google Scholar]


