Abstract
Non-invasive detection of cirrhosis via vibration-controlled transient elastography (VCTE) has revolutionized the management of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. However, VCTE has not been studied in chronic hepatitis D virus (HDV) infection and accuracy remains in question due to the significant hepatic inflammation associated with this infection. Consecutive HBV, HCV, and HDV patients who underwent VCTE (2006–2019) were evaluated. Diagnosis of cirrhosis was made via liver biopsy or clinical findings. VCTE was compared to other non-invasive serum fibrosis tests using AUROC curves. The performance of VCTE in HBV/HCV/HDV was also compared. We evaluated 319 patients (HBV-112; HCV-132; HDV-75), 278(87%) patients had histology for evaluation. HDV patients had evidence of higher hepatic inflammation as evidence by aspartate aminotransferase, alanine aminotransferase, and histology activity index. Cirrhotic HDV patients had higher mean liver stiffness measurements compared to non-cirrhotic patients (29.0 vs 8.3 kPa, P<0.0001). VCTE demonstrated excellent diagnostic accuracy for the detection of cirrhosis with an AUROC of 0.90 compared to APRI (0.83), FIB-4 (0.88), AAR (0.73), and RPR (0.85). Performance of VCTE in HDV was comparable to HBV (0.93) and HCV (0.94). At the optimized cut-off value of ≥14.0 kPa for determining cirrhosis in HDV, VCTE had a sensitivity of 0.78, specificity of 0.86, NPV of 0.93, and PPV of 0.64. Hence VCTE is a useful non-invasive test in HDV for determining cirrhosis despite the presence of significant hepatic inflammation.
Keywords: hepatitis D, hepatitis B, cirrhosis, transient elastography, non-invasive marker
INTRODUCTION
The Hepatitis D virus (HDV) is a defective RNA virus that requires the presence an established Hepatitis B virus (HBV) infection to be pathogenic in humans.1 HDV infection has recently been estimated to carry a global disease burden of 62–72 million2 and results in the most aggressive form of chronic viral hepatitis with progression to cirrhosis in 70–80% of patients within 5 to 10 years.3 In addition, HDV has also been associated with an increased risk of hepatic decompensation4, hepatocellular carcinoma (HCC)5,6, liver transplantation7, and mortality6,8 compared to HBV mono-infection.
Due to the seriousness of HDV-related liver disease, accurate assessment of disease stage (fibrosis) is critical because of prognostic implications related to progressive liver disease leading to cirrhosis, portal hypertensive sequelae, mortality, and HCC.9,10 Additionally, staging of liver in HDV plays an important part in determining the need for nucleos(t)ide analog therapy to treat the concomitant HBV infection, eligibility for pegylated-interferon or an investigational therapy through a clinical trial, and the need to consider referral to a transplant center.11,12
Although liver biopsy has traditionally been the gold-standard diagnostic method for the staging of liver disease, it is subject to complications due to its invasive nature and requires allocation of additional resources.13 Moreover, the advent of accurate, non-invasive tests for fibrosis assessment in Hepatitis C (HCV) has resulted in changes in medical practice such that liver biopsies can be avoided in most circumstances with this disease.14 These tests have also increased the proportion of asymptomatic patients diagnosed with cirrhosis who are at risk for decompensation and thus should undergo routine endoscopic variceal and HCC screening.11,15 The most commonly utilized non-invasive fibrosis tests include serum tests that are derived from commonly ordered markers such as the aspartate aminotransferase (AST) to platelet ratio index (APRI)16 and the fibrosis-4 score (FIB-4)17 as well as liver stiffness measurements (LSM) via vibration-controlled transient elastography (VCTE)18,19 (Fibroscan®, Echosens, Paris, France).
However, these non-invasive tests are not without shortcomings. Both types of testing (serologic and VCTE) can be limited by hepatic inflammation.20–24 For example, serologic formulas such as the APRI or FIB-4 incorporate markers associated with hepatic inflammation such as AST and alanine aminotransferase (ALT). Rise in these markers in the setting of hepatitis can cause artificial elevations in these scores. These serum markers are also non-specific to the liver and systemic processes can also result in artificial elevations.25 In like manner, LSM can also be falsely increased in several settings such as ongoing hepatic inflammation, cholestasis, and congestion.26 Nonetheless, VCTE has been well validated in HBV and HCV and can be considered as the preferred non-invasive fibrosis testing modality with these two liver diseases due to its superior performance compared to serologic tests.27 However, studies have demonstrated that the performance of VCTE and the LSM cut-offs to diagnose different stages of fibrosis have differed between HBV and HCV indicating that the validation of VCTE in different liver diseases is crucial.28–30
In HDV, VCTE has not yet been validated and two recent studies evaluating non-invasive serum tests have demonstrated inadequate performance.31,32 Concerns exist on whether these non-invasive tests can be clinically useful in chronic HDV since HDV is associated with severe necroinflammation which may skew testing results.33,34 It remains unclear if non-invasive testing, especially VCTE, has any clinical value in chronic HDV and if the pre-existing VCTE cut-offs for HBV or HCV are applicable to HDV. Thus, the aim of this study was to compare the performance of VCTE in HDV to HBV and HCV as well as to commonly used non-invasive serum tests in HDV.
METHODS
Patients
This study included enrolled consecutive patients with chronic HBV, HCV, and HDV infection who underwent LSM via VCTE at the National Institutes of Health Clinical Center between the years of 2006 to 2019. All patients were enrolled in a natural history of liver diseases protocol [NCT00001971] that has been approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board and gave written informed consent for participation.
All patients underwent a comprehensive hepatologic evaluation including serological testing, imaging, and liver biopsy as clinically indicated. HCV infection was defined as the presence of HCV-RNA in serum prior to the liver biopsy. HBV infection was defined as the presence of hepatitis B surface antigen (HBsAg) in serum. HDV infection was defined either by HDV-RNA PCR or positive staining for hepatitis delta antigen in hepatocytes on histology. Chronicity of at least 6 months was established for each of the liver diseases based on clinical and laboratory findings. Data regarding demographic, laboratory, imaging (Ultrasound, CT, MRI), VCTE, liver biopsy, and viral treatment (interferon or nucleos(t)ide analog therapy at the time of VCTE) was collected. LSM values were accepted only if there were at least 10 validated readings, had a success rate ≥ 60%, and an interquartile range (IQR) of all validated measurements under 30% of the median value. Results were reported in kilopascals (kPa). Laboratory, imaging, and liver biopsy data was only used if it was within 3, 6, and 12 months of the VCTE date.
Patients were determined to have cirrhosis either by histology, when available, (Ishak fibrosis score ≥ 5)35 or by imaging consistent with cirrhosis (i.e. nodular liver) with one or more signs of portal hypertension (i.e. thrombocytopenia (platelet count < 150 × 109/L), collaterals, splenomegaly, varices, or mild ascites).19,36 Splenomegaly was defined based on cut-offs established based on age and height.37 Exclusion criteria included: inadequate LSMs, lack of imaging in HDV patients without liver biopsy, moderate to severe ascites, any evidence of other acute or chronic liver diseases which includes, but is not limited to: alcoholic liver disease, non-alcoholic steatohepatitis (NASH), acute viral hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis. Human immunodeficiency virus (HIV) infection was not an exclusion.
Liver histopathology
Liver biopsy was performed either via the percutaneous or transjugular approach as clinically indicated. All liver biopsy specimens were read and scored by an expert hepatopathologist (DEK). Hepatic fibrosis was assessed using the Ishak fibrosis score (0–6) with cirrhosis defined as an Ishak score of 5 or 6.35 Hepatic inflammation was assessed using the histology activity index (HAI) (0–18).38 Active HDV infection in patients who underwent liver biopsy was confirmed via Hepatitis D antigen staining.
Comparisons of VCTE to other non-invasive tests
The performance of VCTE was compared to commonly used serum non-invasive fibrosis tests for the detection of cirrhosis. The rationale for this comparison was to explore whether VCTE would outperform these readily available serum tests. The tests that were evaluated include the APRI16 and FIB-417 index, AST-to-ALT ratio (AAR)39, and the red cell distribution width (RDW) to platelet ratio (RPR)40. Formulas for these scores are shown below:
Statistical analysis
Baseline patient characteristics were described using frequencies for categorical variables and means versus medians (depending on distribution) for continuous variables. All statistical analysis was performed using SAS 9.4 (Cary, NC) Wilcoxon rank-sum tests, chi-squared tests and fisher’s exact tests were used to compare baseline variables across competing groups. Post-hoc analysis was conducted using Tukey’s multiple comparisons tests. The following variables were included in the univariate analysis: demographics (age, sex, race), laboratory results (platelet count, prothrombin time, total bilirubin, albumin, ALT, AST, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT)), further clinical characteristics (HBeAg status, nucleos(t)ide analog therapy, histology (fibrosis stage, and HAI), and LSM). A 2-sided P value of less than .05 was considered statistically significant.
Area under the receiver operating characteristic (AUROC) curves was used to assess the performance of VCTE and the other non-invasive serum tests in detecting cirrhosis among the entire HDV cohort. Since patients without liver biopsy or clinical cirrhosis could still have sub-clinical cirrhosis, additional sub-analysis using AUROC curves was performed on the cohort after excluding patients without liver biopsy who does not have clinical cirrhosis.
All variables used were logarithmically transformed to adjust for normality. Chi-sq test was used to compare the performance of VCTE across HBV, HCV, and HDV. The optimal cut-off for VCTE to identify cirrhosis in HDV was determined using the distance criterion. This criterion chooses the point closest to the point on the ROC graph where 1-Specificity = 0 and Sensitivity = 1 (http://support.sas.com/kb/25/018.html). Sensitivity (Se), specificity (Sp), positive and negative predictive value (PPV and NPV), positive and negative likelihood ratio (LR+ and LR-) were calculated using previously defined cut-offs with VCTE30: <12.5 or ≥12.5 kPa; APRI16: <1 or >2; FIB-441: <1.6 or >3.6; AAR39 <1 or ≥1; and RPR40: <0.16 or ≥0.16. Indeterminate results were considered “not classified”.
RESULTS
Patient characteristics
Three hundred and nineteen patients with chronic viral hepatitis: HBV (n = 112); HCV (n = 132); HDV (n = 75) were included in this study. 241 HBV, 44 HDV patients were not included due to the lack of a valid VCTE result. The characteristics of the whole cohort are shown in Table 1. Patients with HBV and HDV tended to be younger, mean age 46.3 (standard deviation (SD):14.0) and 43.4 (SD:11.5) years, respectively, compared to HCV patients, 53.5 (SD:10.7) years, P<0.0001. HBV and HDV patients also were more likely to be Asian compared to HCV patients. Breakdown of race by country of birth and HBV genotype is shown in Supplemental Table 1.
Table 1.
Clinical characteristics and laboratory values of all patients evaluated
| HDV (n = 75) |
HBV (n = 112) |
HCV (n = 132) |
P | |
|---|---|---|---|---|
| Age at VCTE (years) | 43.4 (11.5) | 46.3 (14.0) | 53.5 (10.7) | <0.0001 |
| Gender Male Female |
46 (61.3%) 29 (38.7%) |
76 (67.9%) 36 (32.1%) |
74 (56.1%) 58 (44.0%) |
0.16 |
| Race White Black Asian Other |
26 (34.7%) 8 (8.0%) 41 (54.7%) 0 (0%) |
27 (24.1%) 18 (16.1%) 66 (58.9%) 1 (0.8%) |
67 (50.8%) 33 (25.0%) 22 (16.7%) 10 (7.5%) |
<0.0001 |
| Laboratory | ||||
| ALP (IU/L) | 84.7 (30.1) | 69.4 (23.3) | 76.0 (25.3) | 0.002 |
| AST (IU/L) | 73.1 (64.3) | 43.7 (43.2) | 47.5 (37.3) | <0.0001 |
| ALT (IU/L) | 107.5 (124.0) | 71.3 (89.3) | 65.9 (60.0) | <0.0001 |
| Total bilirubin (mg/dL) | 0.77 (1.1) | 0.71 (0.4) | 0.67 (0.5) | 0.42 |
| GGT(IU/L) | 59.3 (48.8) | 41.3 (63.0) | 77.3 (83.1) | <0.0001 |
| Albumin (g/dL) | 4.0 (0.44) | 4.1 (0.4) | 3.9 (0.4) | 0.01 |
| PT (seconds) | 14.3 (1.4) | 13.7 (1.0) | 13.8 (0.9) | 0.01 |
| Platelet count (K/μL) | 161.6 (76.0) | 185.3 (57.3) | 183.3 (70.7) | 0.04 |
| HBeAg positivity | 11 (14.7%) | 31 (27.7%) | N/A | 0.03 |
| HBeAb positivity | 53 (70.7%) | 63 (56.3%) | N/A | 0.02 |
| Nucleos(t)ide analog therapy | 45 (60.0%) | 49 (43.8%) | N/A | 0.06 |
| Histology | 34 (45.3%) | 112 (100%) | 132 (100%) | |
| HAI (0–18) | 9.4 (2.4) | 4.3 (3.3) | 6.8 (2.9) | <0.0001 |
| Ishak Fibrosis Stage (0–6) | <0.0001 | |||
| 0 | 1 (2.9%) | 51 (45.5%) | 31 (23.5%) | |
| 1 | 2 (5.9%) | 24 (21.4%) | 24 (18.2%) | |
| 2 | 8 (23.5%) | 10 (8.9%) | 23 (17.4%) | |
| 3 | 9 (26.5%) | 10 (8.9%) | 21 (15.9%) | |
| 4 | 4 (11.8%) | 5 (4.5%) | 9 (6.8%) | |
| 5 | 6 (17.7%) | 3 (2.7%) | 2 (1.5%) | |
| 6 | 4 (11.8%) | 9 (8.0%) | 22 (16.7%) | |
| LSM (kPa) | 13.3 (14.5) | 7.2 (4.7) | 11.1 (9.7) | <0.0001 |
| Cirrhosis (Clinical + Histology) | 18 (24.0%) | 12 (10.7%) | 24 (18.2%) | 0.05 |
Values expressed as mean (standard deviation) or N (%) unless otherwise stated. HBV and HCV were compared to HDV for statistical analysis.
Abbreviations: ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; PT, prothrombin time; HBeAg, hepatitis B e antigen; HBeAb, hepatitis B e antibody; HAI, histology activity index; LSM, liver stiffness measurement; N/A, not applicable
Patients with HDV had significantly higher hepatic inflammation on laboratory parameters compared to HBV and HCV patients. These include higher ALT levels in HDV - 107.5 (SD:124.0) IU/L compared to HBV – 71.3 (SD:89.3) IU/L and HCV – 65.9 (SD:60.0) IU/L, P<0.0001. Similarly, HDV patients also had higher AST levels – 73.1 (SD:64.3) IU/L compared to HBV – 43.7 (SD:43.2) IU/L and HCV 47.5 (SD:37.3) IU/L, P<0.0001.
Compared to HBV, HDV patients were less likely to be hepatitis B e-Antigen (HBeAg) positive: 11 of 74 (14.5%) vs 31 of 112 (27.7%), P=0.03, but more likely to be hepatitis B e-Antibody (HBeAb) positive: 53 of 74 (70.7%) vs 63 of 112 (56.3%), P=0.02.
45 of 76 (60.0%) HDV patients were on nucleos(t)ide analog therapy at the time of VCTE compared to 49 of 112 (43.8%) HBV patients, P=0.06. There was no statistical difference in LSM (P=0.27), ALT (P=0.54), or HAI (0.25) between HDV patients on nucleos(t)ide analog therapy compared to those who were not. 3 of 76 (5.3%) HDV patients were on long term interferon therapy at the time of VCTE compared to 1 of 112 (0.9%) HBV patients.
Histology and cirrhosis classification
Histologic evaluation was available for all HBV (n = 112) and HCV (n = 132) patients and 34 of 75 (45.3%) HDV patients (Table 1). HDV patients had significantly more necroinflammation on biopsy with a HAI of 9.4 (SD:2.4) compared to HBV - 4.3 (SD:3.3) and HCV - 6.8 (SD:2.9), P<0.0001.
Cirrhosis was identified in 18 of 75 (24.0%) HDV patients, 10 by liver biopsy (Ishak ≥ 5) and 8 by clinical findings (See Supplemental Table 1). Notably, all patients that were clinically classified with cirrhosis had at least two signs of portal hypertension (splenomegaly and thrombocytopenia) with 5 of the 8 also having varices and/or ascites. Comparatively, 12 of 112 HBV (10.7%) patients and 24 of 132 (18.2%) HCV patients were cirrhotic.
Comparative performance of non-invasive tests
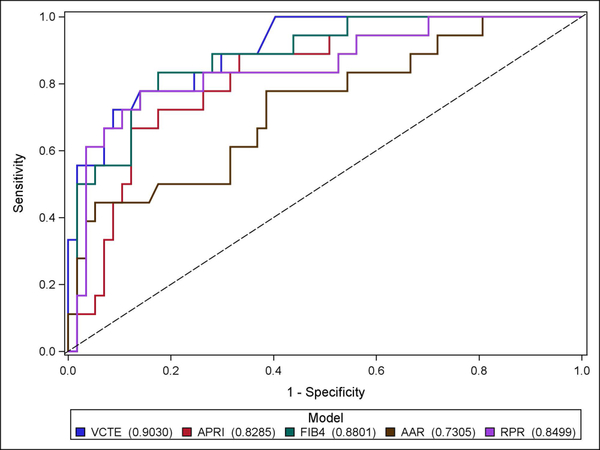
Results of the diagnostic performance of VCTE compared to serum tests in HDV are depicted in Figure 1 and Table 2. The serum tests that were evaluated displayed varying performance in the detection of cirrhosis. The AUROC for the detection of cirrhosis in HDV were as follows: FIB-4 = 0.88, RPR = 0.85, APRI = 0.83 and AAR = 0.73. Among these tests, RPR correctly classified the most patients with HDV (86.7%) followed by the AAR (74.7%). The APRI and FIB-4 correctly classified the least number of HDV patients at 61.3% and 56.0%, respectively. Indeterminate results with the FIB-4 and APRI were common at 34.7% and 26.7%. The FIB-4 and APRI had the highest NPV among the serologic tests at 97.0% and 94.6%, respectively, while the AAR had the lowest NPV at 83.9%. LR+ of the serum tests ranged from 2.86–15.89 while the LR- ranged from 0.11–0.61.
Fig. 1.
ROC curves of VCTE compared to serologic fibrosis markers in differentiating “cirrhosis” from “no cirrhosis” in HDV (n = 75). VCTE had an AUROC of 0.90 compared to APRI (0.83), FIB-4 (0.88), AAR (0.73), and RPR (0.85).
Abbreviations: ROC, receiver operating characteristic, VCTE, vibration-controlled transient elastography; AUROC, area under receiver operating characteristic, APRI, aspartate aminotransferase (AST) to platelet ratio index; FIB-4, fibrosis-4 index; AAR, aspartate aminotransferase to alanine aminotransferase ratio; RPR, red cell distribution width to platelet ratio
Table 2.
Comparative performance of non-invasive tests for the diagnosis of cirrhosis in Hepatitis D
| Non- invasive tests |
Cut-offs for cirrhosis |
Patients with cirrhosis (n = 18) |
Patients without cirrhosis (n = 57) |
Se (%) |
Sp (%) |
PPV (%) |
NPV (%) |
LR+ | LR- | Correctly classified |
|---|---|---|---|---|---|---|---|---|---|---|
| VCTE – Ideal Cut-off (kPa) | ≥14.0 | 14 | 8 | 77.8% | 86.0% | 63.6% | 92.5% | 5.55 | 0.26 | 63 (84.0%) |
| <14.0 | 4 | 49 | ||||||||
| VCTE (kPa)30 |
≥12.5 | 14 | 10 | 77.8% | 82.5% | 58.3% | 92.2% | 4.45 | 0.27 | 61 (81.3%) |
| <12.5 | 4 | 47 | ||||||||
| FIB-441 |
>3.6 | 10 | 6 | 90.9% | 84.2% | 62.5% | 97.0% | 5.75 | 0.11 | 42 (56.0%) |
| NC | 7 | 19 | ||||||||
| <1.6 | 1 | 32 | ||||||||
| APRI16 | >2.0 | 11 | 7 | 84.6% | 83.3% | 61.1% | 94.6% | 5.1 | 0.19 | 46 (61.3%) |
| NC | 5 | 15 | ||||||||
| <1.0 | 2 | 35 | ||||||||
| AAR39 | ≥1 | 9 | 10 | 50.0% | 82.5% | 47.4% | 83.9% | 2.86 | 0.61 | 56 (74.7%) |
| <1 | 9 | 47 | ||||||||
| RPR40 | ≥0.16 | 10 | 2 | 55.6% | 96.5% | 83.3% | 87.3% | 15.89 | 0.46 | 65 (86.7%) |
| <0.16 | 8 | 55 |
Values expressed as n or n (%);
Abbreviations: Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; NC, not categorized
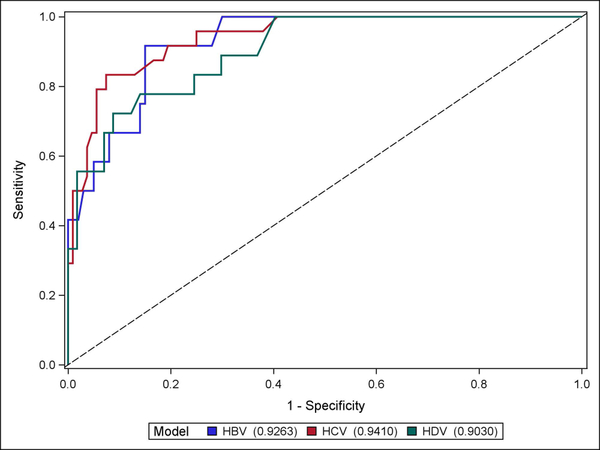
In HDV, compared to the performance of the serum noninvasive fibrosis markers, VCTE demonstrated the highest AUROC for the detection of cirrhosis at 0.90. This result was comparable to AUROC of VCTE in HBV (0.93) and HCV (0.94). (See Figure 2) Of note, HDV patients had a significantly higher mean LSMs compared to HBV patients (13.3 (SD:14.5) vs 7.2 (SD:4.7) kPa, P<0.0001) but not HCV patients. In addition, the performances of all of the non-invasive serum tests are shown in Supplemental Figure 1 and 2.
Fig. 2.
ROC curves of VCTE in HBV (n = 112, AUROC 0.93), HCV (n = 132, AUROC 0.94), and HDV (n = 75, AUROC 0.90) in differentiating “cirrhosis” from “no cirrhosis”.
Abbreviations: ROC, receiver operating characteristic; VCTE, vibration-controlled transient elastography; AUROC, area under receiver operating characteristic; HBV, hepatitis B, HCV, hepatitis C, HDV, hepatitis D
At a cut-off of 12.5 kPa30, 81.3% of HDV patients were correctly classified with “cirrhosis” compared to “no cirrhosis”. At this cut-off, VCTE predicted cirrhosis with 77.8% Se, 82.5% Sp, 58.3% PPV, and 92.2% NPV. At the calculated ideal cut-off of 14.0 kPa, 84.2% of patients were correctly classified with “cirrhosis” compared to “no cirrhosis”. VCTE at this cut-off had the same Se but higher Sp (86.0%), PPV (63.6%), and NPV (92.5%) for predicting cirrhosis. In VCTE, LR+ ranged from 4.48–5.55 and LR- ranged from 0.26–0.27.
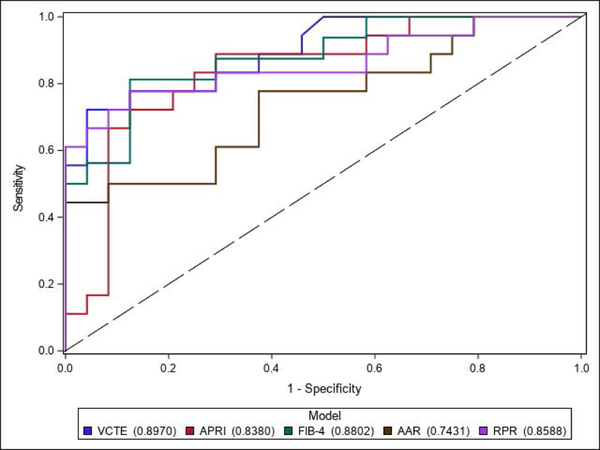
In the sub-analysis excluding patients without liver biopsy who did not have clinical cirrhosis, all of the non-invasive tests performed similarly to their performance in the entire cohort. AUROC curves: VCTE = 0.90, FIB-4 = 0.88, RPR = 0.86, APRI = 0.84, and AAR 0.74. (See Figure 3)
Fig. 3.
ROC curves of VCTE compared to serologic fibrosis markers in differentiating “cirrhosis” from “no cirrhosis” excluding patients without liver biopsy who does not have clinical cirrhosis (n = 42). VCTE had an AUROC of 0.90 compared to APRI (0.84), FIB-4 (0.88), AAR (0.74), and RPR (0.86).
Abbreviations: ROC, receiver operating characteristic, VCTE, vibration-controlled transient elastography; AUROC, area under receiver operating characteristic, APRI, aspartate aminotransferase (AST) to platelet ratio index; FIB-4, fibrosis-4 index; AAR, aspartate aminotransferase to alanine aminotransferase ratio; RPR, red cell distribution width to platelet ratio
DISCUSSION
In the first study evaluating the utility of VCTE in chronic HDV patients, we showed that VCTE has excellent diagnostic accuracy (AUROC - 0.90) in detecting cirrhosis, which is comparable to the performance of VCTE in HBV and HCV. However, to account for the substantial necroinflammation present in HDV compared to HBV/HCV patients (as evidenced by the significantly higher laboratory (ALT/AST) and histologic (HAI) parameters), we have also identified a new, higher cut-off of 14.0 kPa in HDV for the detection of cirrhosis compared to HBV and HCV which seemingly improves the diagnostic performance of VCTE in HDV. Impressively, VCTE at an ideal cut-off was able to correctly classify 84.0% of HDV patients. Necroinflammatory activity has been reported as one of the factors that causes discordance between liver biopsy and LSM and in the setting of elevated ALT levels, higher cut-offs for LSM has been previously proposed in HBV.22,42,43
Furthermore, in HDV, we found that VCTE seems to outperform commonly used serum noninvasive fibrosis markers including the APRI, FIB-4, AAR, and RPR. These results in HDV is congruent with findings of previous studies in HBV and HCV that have demonstrated the superiority of VCTE over serologic tests.19,30 Interestingly, we arrived at this finding despite the better than expected performance of serologic tests in our study. The performance of the APRI and FIB-4 in our study was notably better than what has been described in two prior studies in HDV including our previous study.31,32 This is likely because the cohort used in the present study is significantly different compared to our previous cohort. The present cohort comes entirely from a post-VCTE era compared to our previous study which included a significant number of pre-VCTE era patients.31 Moreover, performance of the APRI and FIB-4 in HDV in the current study are similar to prior studies evaluating their performance in chronic HBV.44 Thus, our findings may represent a “regression to the mean”. Meanwhile, the poor performance of AAR in our study is consistent with those prior studies and the RPR score has never been investigated in HDV.
VCTE offers several advantages to these serologic tests in the assessment of fibrosis. The serologic tests that were evaluated are all considered indirect fibrosis markers which incorporates laboratory parameters such as AST, ALT, and platelet count which are not liver-specific.45 These tests can often be considerably affected by systemic conditions (i.e. rhabdomyolysis, renal failure) or medications (that causes thrombocytopenia) while VCTE, which measures liver stiffness directly via shear wave imaging, is usually not affected.46,47 In addition, serologic algorithms that incorporate AST and ALT can also in influenced by acute hepatitis flares as previously mentioned.20 Lastly, indeterminate or “unable to classify” results are common with these serologic tests that incorporates two sets of cut-offs (to rule in “cirrhosis” and rule out “no cirrhosis”) when test scores fall outside the cut-offs. In our study, the FIB-4 and APRI was unable to classify 34.7% and 26.7% of patients, respectively. Thus, even though the FIB-4 and APRI had the highest AUROC among non-invasive fibrosis markers at 0.88 and 0.83, due to the high-rates of indeterminate results, these two tests performed the worst in correctly classifying cirrhosis versus non-cirrhosis. However, VCTE is not without limitations itself. These advantages need to be weighed against several limitations. For example, VCTE is often unreliable or not attainable due to certain anatomic issues (obesity, ascites), the presence of iron overload, inexperience of the operator.48–50
Our study has several limitations. First, approximately half of the HDV patients included in this study did not undergo histologic evaluation to confirm cirrhosis. However, we feel confident that our pre-defined, strict, clinical definition of cirrhosis (imaging of the liver compatible with cirrhosis plus one or more sign of portal hypertension) is adequate in correctly classifying patients with cirrhosis (likely underestimating the number of patients with cirrhosis). Another limitation of this study is the use of platelet count as part of the cirrhosis definition may explain the improved performance of the serum tests compared to prior studies in HDV and possibly account for the lack of more differences in diagnostic performance between VCTE and serum tests. Nevertheless, this does not blemish the excellent ability of VCTE to detect cirrhosis in HDV. In addition, we did not evaluate the performance of non-invasive testing in diagnosing intermediate stages of fibrosis such as significant fibrosis due to lack of a liver biopsy in every HDV patient. However, the use of testing in this area remains controversial even in HBV and HCV due to poor diagnostic performance.30 Finally, we did not evaluate the impact of nucleos(t)ide analog therapy on the performance of VCTE. However, there we found no difference in necroinflammation or LSM between those who were on nucleos(t)ide analog therapy compared to those who were not suggesting there is no effect. This is consistent with prior data that have shown that nucleos(t)ide analog therapy do not improve ALT in chronic HDV.51
In summary, this study shows that VCTE is a useful non-invasive test in HDV for determining cirrhosis despite the presence of significant necroinflammation (histologically and by laboratory parameters) in this patient population. Additionally, VCTE compared favorably to commonly used non-invasive serologic tests such as the APRI, FIB-4, AAR, and RPR. Further validation of our findings in other HDV populations are needed. The development of novel fibrosis algorithms incorporating VCTE should be explored.
Supplementary Material
Acknowledgments
Financial support statement: Supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, National Cancer Institute, and National Institutes of Health Clinical Center.
Footnotes
Writing Assistance: none
Conflict of interest statement: The authors have no relevant conflicts of interest.
REFERENCES
- 1.Taylor JM. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol. 2006;307:1–23. [DOI] [PubMed] [Google Scholar]
- 2.Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Yurdaydin C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat. 2010;17(11):749–756. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Giustina G, Christensen E, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut. 2000;46(3):420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toukan AU, Abu-el-Rub OA, Abu-Laban SA, et al. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology. 1987;7(6):1340–1345. [DOI] [PubMed] [Google Scholar]
- 6.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136(5):1629–1638. [DOI] [PubMed] [Google Scholar]
- 7.Coghill S, McNamara J, Woods M, Hajkowicz K. Epidemiology and clinical outcomes of hepatitis delta (D) virus infection in Queensland, Australia. Int J Infect Dis. 2018;74:123–127. [DOI] [PubMed] [Google Scholar]
- 8.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buti M, Homs M, Rodriguez-Frias F, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat. 2011;18(6):434–442. [DOI] [PubMed] [Google Scholar]
- 10.Niro GA, Smedile A, Ippolito AM, et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53(5):834–840. [DOI] [PubMed] [Google Scholar]
- 11.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh C, Heller T, Glenn JS. Pathogenesis of and New Therapies for Hepatitis D. Gastroenterology. 2019;156(2):461–476 e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(10):877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanai FM, Keeffe EB. Liver biopsy for histological assessment: The case against. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association. 2010;16(2):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. [DOI] [PubMed] [Google Scholar]
- 16.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 18.Berzigotti A Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67(2):399–411. [DOI] [PubMed] [Google Scholar]
- 19.Castera L Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16(5):300–314. [DOI] [PubMed] [Google Scholar]
- 20.European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. [DOI] [PubMed] [Google Scholar]
- 21.Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64(4):773–780. [DOI] [PubMed] [Google Scholar]
- 22.Chan HL, Wong GL, Choi PC, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16(1):36–44. [DOI] [PubMed] [Google Scholar]
- 23.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47(2):380–384. [DOI] [PubMed] [Google Scholar]
- 24.Vispo E, Barreiro P, Del Valle J, et al. Overestimation of liver fibrosis staging using transient elastography in patients with chronic hepatitis C and significant liver inflammation. Antivir Ther. 2009;14(2):187–193. [DOI] [PubMed] [Google Scholar]
- 25.Chin JL, Pavlides M, Moolla A, Ryan JD. Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front Pharmacol. 2016;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7(5):1303–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim JK, Flamm SL, Singh S, Falck-Ytter YT, Clinical Guidelines Committee of the American Gastroenterological A. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152(6):1536–1543. [DOI] [PubMed] [Google Scholar]
- 28.Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16(2):372. [DOI] [PubMed] [Google Scholar]
- 29.Castera L Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–1302 e1294. [DOI] [PubMed] [Google Scholar]
- 30.Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53(6):1013–1021. [DOI] [PubMed] [Google Scholar]
- 31.Takyar V, Surana P, Kleiner DE, et al. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther. 2017;45(1):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutterkort GL, Wranke A, Yurdaydin C, et al. Non-invasive fibrosis score for hepatitis delta. Liver Int. 2017;37(2):196–204. [DOI] [PubMed] [Google Scholar]
- 33.Pascarella S, Negro F. Hepatitis D virus: an update. Liver Int. 2011;31(1):7–21. [DOI] [PubMed] [Google Scholar]
- 34.Negro F Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med. 2014;4(11):a021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. [DOI] [PubMed] [Google Scholar]
- 36.Lu SN, Wang JH, Liu SL, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107(9):2212–2222. [DOI] [PubMed] [Google Scholar]
- 37.Chow KU, Luxembourg B, Seifried E, Bonig H. Spleen Size Is Significantly Influenced by Body Height and Sex: Establishment of Normal Values for Spleen Size at US with a Cohort of 1200 Healthy Individuals. Radiology. 2016;279(1):306–313. [DOI] [PubMed] [Google Scholar]
- 38.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. [DOI] [PubMed] [Google Scholar]
- 39.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95(3):734–739. [DOI] [PubMed] [Google Scholar]
- 40.Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8(7):e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546–553. [DOI] [PubMed] [Google Scholar]
- 42.Vigano M, Paggi S, Lampertico P, et al. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther. 2011;34(3):353–362. [DOI] [PubMed] [Google Scholar]
- 43.Kim SU, Kim JK, Park YN, Han KH. Discordance between liver biopsy and Fibroscan(R) in assessing liver fibrosis in chronic hepatitis b: risk factors and influence of necroinflammation. PLoS One. 2012;7(2):e32233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parikh P, Ryan JD, Tsochatzis EA. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med. 2017;5(3):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 46.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847. [DOI] [PubMed] [Google Scholar]
- 47.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367–1384. [DOI] [PubMed] [Google Scholar]
- 48.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–835. [DOI] [PubMed] [Google Scholar]
- 49.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Discordance in fibrosis staging between liver biopsy and transient elastography using the FibroScan XL probe. J Hepatol. 2012;56(3):564–570. [DOI] [PubMed] [Google Scholar]
- 50.Adhoute X, Foucher J, Laharie D, et al. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol. 2008;32(2):180–187. [DOI] [PubMed] [Google Scholar]
- 51.Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364(4):322–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.