Abstract
A new study reveals that a high-sugar diet acutely alters human sperm small RNA profiles after 1 week and that these changes are associated with changes in sperm motility. This rapid response by sperm to nutritional fluctuation raises intriguing questions regarding the underlying mechanisms and the potential effects on offspring metabolic health.
Mammalian sperm carries diverse forms of large and small RNAs in addition to a haploid DNA1. Sperm RNAs were initially considered remnants of spermatogenesis that could be used as biomarkers for fertility. In the 2010s, however, it became clear that sperm RNAs are responsive to dynamic environmental conditions and can act as causal factors for the intergenerational transmission of certain paternal phenotypes acquired from environmental stressors, such as diet-induced metabolic disorders and mental stressor-induced phenotypes1. These research advances have promoted the concept of ‘sperm RNA code’, suggesting that a specific environmental input can be encoded in the form of sperm RNA and RNA modification profiles, which represent epigenetic signatures that influence offspring health, in addition to being biomarkers of sperm quality1.
Previous studies from mice have shown that the mature sperm carry a specific set of small non-coding RNAs (sncRNAs) enriched by tRNA-derived small RNAs (tsRNAs) and rRNA-derived small RNAs (rsRNAs), in addition to the well-known microRNAs (miRNAs) and piwi-interacting RNAs (piRNAs)2–4. Mouse studies have also shown that sperm tsRNAs and rsRNAs are responsive to nutritional interventions such as a high-fat diet, and the injection of tsRNA-enriched and rsRNA-enriched sperm RNA fractions from males exposed to a high-fat diet can induce offspring metabolic phenotypes such as altered sugar metabolism2,3. Human sperm are also known to have tsRNAs and rsRNAs, the signature of which can represent sperm quality for fertilization from retrospective samples1. However, detailed time-course analyses of sperm sncRNA profiles in the same individual human under defined diet intervention has been lacking.
In a new study, Nätt and colleagues carefully investigated the human sperm sncRNA profile over a 2-week diet intervention. During the first week, a healthy diet was introduced, which was followed by 1 week of a high-sugar diet (HSD)5. The most notable finding is that a subset of sperm tsRNAs and rsRNAs, mostly of mitochondrial origin, are sensitively upregulated after 1 week of a HSD. Although the majority of genomic tsRNAs and rsRNAs are not changed, the authors identified a specific type of tsRNA, which is derived from the internal T-loop of genomic tRNAs (known as nitRNAs), that are also elevated after HSD. In addition, sperm motility showed an overall increase after the 2-week diet intervention, and the increased sperm motility was associated with an increase in mitochondrial tsRNAs and the sugar-sensitive nitRNAs5 (Fig. 1). These data from humans not only resonate with previous observations in mice that sperm tsRNA and rsRNA are modulated by diet, but also raise new questions that await addressing.
Fig. 1 |. Potential modulation of sperm by high-sugar diet.
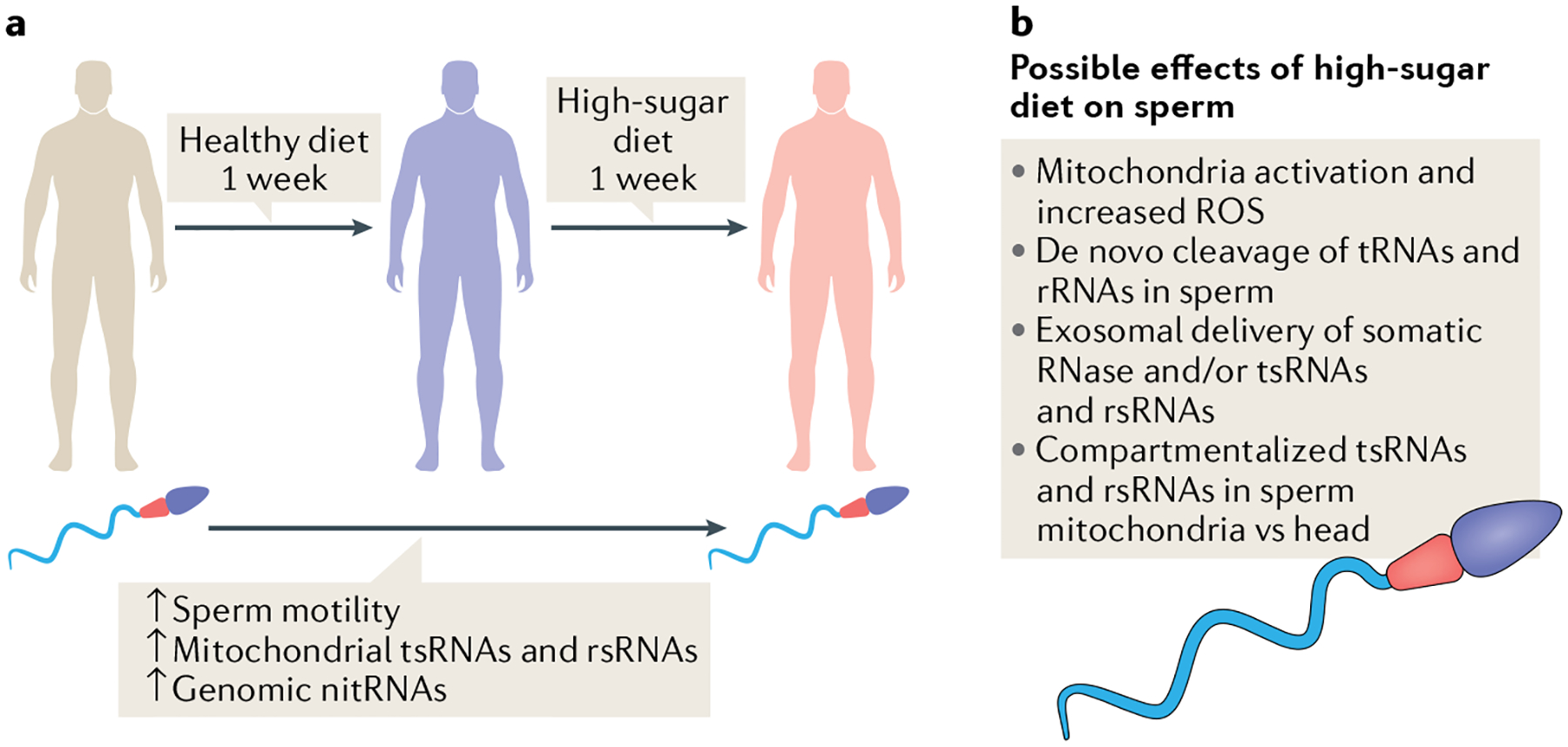
a | The acute human sperm response to high-sugar diet. b | The potential mechanisms underlying the response of sperm to a high-sugar diet. Red regions in sperm represent mitochondria-containing segments. Blue regions represent the sperm tail. Purple regions represent the sperm head. nitRNAs, tsRNAs derived from the internal T-loop of genomic tRNAs.
The first question concerns the cellular and functional compartmentalization of sperm sncRNAs. The observation that mitochondrial tsRNAs and rsRNAs are the most sensitively elevated types of RNA is somewhat surprising because they exist at a much lower level than genomic tsRNAs and/or rsRNAs5. The logical assumptions that follow this observation could be twofold. Firstly, the mitochondrial and genomic tsRNA and rsRNA might be differentially compartmentalized in a sperm cell. For example, the molecules could be present in different sub-cellular local environments upon HSD and thus undergo different regulation (such as cleavage of tRNA or rRNA). However, it remains unclear whether mitochondrial tsRNA and rsRNA exclusively exist in the sperm mitochondria-containing tail and whether the sperm head only contains genomic tsRNA and rsRNA. This question can be tested in the future by separately sequencing the sncRNAs from the sperm head and tail. Secondly, the mitochondrial and genomic tRNA and rRNA might respond differently to the same (currently undefined) HSD-associated factors, which would result in different cleavage rates and/or patterns for generating tsRNAs and rsRNAs. This concept is supported by the fact that mitochondria and genomic tRNAs have different sets of RNA modifications6, which might underline different sensitivities to enzymatic cleavage. The above-mentioned two scenarios are not mutually exclusive and can co-exist.
The second interesting question concerns the origin and mechanism of regulation for sperm tsRNA and rsRNA (both genomic and mitochondrial) after HSD. Mitochondria are actively involved in the generation of reactive oxygen species (ROS) under elevated glucose levels7. Elevated oxidative stress could accumulate in sperm mitochondria after daily transient windows of hyperglycaemia after HSD, although the fasting blood levels of glucose of the participants was unaffected at the end of the study5. Oxidative stress is a well-known factor that induces the cleavage of both tRNAs and rRNAs, which generate extra tsRNAs and rsRNAs8. The regulation of tRNA and rRNA cleavage under oxidative stress is not fully understood, but it involves the mobilization and/or activation of RNases that are sequestered and/or inactivated under normal conditions8 and the recognition of damaged (oxidized) RNAs as targets (Fig. 1).
The source of RNases that cleave the sperm tRNAs and/or rRNAs upon HSD remains unknown. Different kinds of RNase could be either mobilized from sequestered parts inside the sperm (mitochondria), delivered from nearby somatic cells, or both. According to Nätt and colleages, the enzyme responsible for generating the sugar-sensitive genomic nitRNAs (derived from internal T-loops) might be different from the previously well-identified Angiogenin, which cleaves tRNA at anti-codons6. It is also possible that the nitRNAs are generated in nearby somatic cells and delivered to sperm by exosomes, as previously suggested in mice4. On the other hand, the specific upregulation of mitochondrial tsRNAs and rsRNAs after HSD supports a scenario of their de novo generation by tRNA and/or rRNA cleavage within sperm mitochondria.
Interestingly, oxidized nucleotides (for example, caused by ROS) show higher binding capacity to human Y box-binding protein 1 (YB1) than do non-oxidized nucleotides9, and YB1 is known to bind with tsRNAs to regulate various oxidative stress responses at the translational level6. Whether HSD-induced sperm tsRNAs bind to YB1 or other proteins in the sperm or after fertilization remains unknown and warrants further investigation, as this binding relates to their functions in the early embryo which potentially affect offspring health.
The third question raised by the study concerns the potential shared factors that regulate both sperm motility and sncRNA profiles. Altered mitochondrial function induced by HSD might be the mechanism of both observations, that is, possibly a result of increased ROS, in addition to ATP production. It remains unknown whether there is an evolutionary trade-off between elevated sperm motility and altered sncRNAs upon enhanced mitochondrial metabolism, as the former relates to the chance of sperm getting fertilized, while the latter concerns the epigenetic information carried by the sperm. Notably, some studies suggest that sperm motility alone is not a good predictive factor for pregnancy success; other factors, such as population-based sperm behaviour, can also be involved.
Finally, the present study also cross-analysed previous sperm sncRNA datasets from people with obesity or who were lean10 and compared them with their HSD datasets. The researchers found that sperm from males with obesity (compared with lean status) and sperm from males who were placed on a HSD for 1 week (compared with healthy diet) showed opposite responses in tsRNA and rsRNA5. Although it is realized that the widely-used sncRNA-sequencing (sncRNA-seq) protocol used by the authors of the study (also in other previous studies of sperm sncRNA-seq) shows sequencing bias due to heavy RNA modifications in tsRNAs and rsRNAs1, the specific changes observed in the present study enhanced the concept of a sperm RNA code by demonstrating that different nutritional statuses generate different sperm sncRNA signatures. If the influence of diet on sperm is further validated in multiple cohorts and the role of tsRNA and/or rsRNA in offspring health is clarified, the sperm RNA code can be monitored before a planned pregnancy as a clinical translational application for precision medicine.
Acknowledgements
The authors thank Xudong Zhang for discussion. Y.Z. is supported by MOST (2019YFA0802600 and 2018YFC1004500) and Q.C. is supported by NIH (R01HD092431).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Zhang Y, Shi J, Rassoulzadegan M, Tuorto F & Chen Q Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol 15, 489–498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 20, 535–5402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma U et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nätt D et al. Human sperm displays rapid responses to diet. PLoS Biol. 17, e3000559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schimmel P The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 19, 45–58 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Thompson DM & Parker R Stressing out over tRNA cleavage. Cell 138, 215–219 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa H et al. Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry 41, 12739–12744 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Donkin I et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell. Metab. 23, 369–378 (2016). [DOI] [PubMed] [Google Scholar]


