Abstract
Hyperacusis is a debilitating hearing condition in which normal everyday sounds are perceived as exceedingly loud, annoying, aversive or even painful. The prevalence of hyperacusis approaches 10%, making it an important, but understudied medical condition. To noninvasively identify the neural correlates of hyperacusis in an animal model, we used sound-evoked functional magnetic resonance imaging (fMRI) to locate regions of abnormal activity in the central nervous system of rats with behavioral evidence of hyperacusis induced with an ototoxic drug (sodium salicylate, 250 mg/kg, i.p.). Reaction time-intensity measures of loudness-growth revealed behavioral evidence of salicylate-induced hyperacusis at high intensities. fMRI revealed significantly enhanced sound-evoked responses in the auditory cortex (AC) to 80 dB SPL tone bursts presented at 8 and 16 kHz. Sound-evoked responses in the inferior colliculus (IC) were also enhanced, but to a lesser extent. To confirm the main results, electrophysiological recordings of spike discharges from multi-unit clusters were obtained from the central auditory pathway. Salicylate significantly enhanced tone-evoked spike-discharges from multi-unit clusters in the AC from 4 – 30 kHz at intensities ≥ 60 dB SPL; less enhancement occurred in the medial geniculate body (MGB), and even less in the IC. Our results demonstrate for the first time that non-invasive sound-evoked fMRI can be used to identify regions of neural hyperactivity throughout the brain in an animal model of hyperacusis.
Keywords: functional magnetic resonance imaging, fMRI, hyperacusis, central auditory gain, rat
Introduction
Hyperacusis is a auditory hypersensitivity disorder in which moderate intensity everyday sounds are perceived as intolerably loud (Fagelson et al., 2018; Tyler et al., 2014). Unlike loudness recruitment, moderate intensity sounds are perceived as much louder than normal. Epidemiological studies indicate that 5.9 – 9.2% of adults have hyperacusis (Andersson et al., 2002; Paulin et al., 2016). Hyperacusis is not only associated with hearing loss, but also a broad range of medical conditions such as acoustic shock, drug reactions, autism spectrum disorder, migraine, William’s syndrome, and head trauma (Khalfa et al., 2004; Miani et al., 2001; Suhnan et al., 2017). In some cases, hyperacusis can be a debilitating condition causing considerable discomfort, anger, tension, distress, anxiety, and even pain (Baguley, 2003; Tyler et al., 2014). Fear and avoidance of loud sounds often leads to social isolation. While there are no universally accepted or effective treatments for hyperacusis, sound therapy and cognitive behavioral therapy sometimes reduce symptom severity (Baguley et al., 2018; Formby et al., 2015; Juris et al., 2014), whereas the use of earplugs generally exacerbates sound sensitivity.
A major obstacle to identifying more effective treatments for hyperacusis is our limited understanding of the basic neural mechanisms underlying this disorder. A leading model for loudness hyperacusis is increased central auditory gain (i.e., sound-evoked hyperactivity) (Brotherton et al., 2015; Diehl et al., 2015; Knipper et al., 2013; Pienkowski et al., 2014; Zeng, 2013). The increase in central gain is likely related to homeostatic plasticity that attempts to compensate for the reduction in the neural output of the cochlea. In the central gain model, the weak sound-evoked responses emanating from the auditory nerve are progressively amplified as the neural signals are relayed along the ascending auditory pathway. By the time the signal reaches the auditory cortex, sound-evoked responses are often much larger than normal (Chen et al., 2013; Jiang et al., 2017; Salvi et al., 2016).
Most studies of central gain enhancement and hyperacusis have involved electrophysiological recordings from specific regions of the auditory pathway of animal models presumed to have hyperacusis. Most of these detailed electrophysiological studies make a priori assumptions about which auditory brain regions are likely involved in hyperacusis while ignoring other potentially important brain regions that could trigger or exacerbate hyperacusis (Chen et al., 2015). Therefore, whole-brain methods for mapping sound-evoked hyperactivity could aid in elucidating the full extent of the hyperacusis network in animal models with clear behavioral evidence of hyperacusis. In this study, we used noninvasive, sound-evoked functional magnetic resonance imaging (fMRI) to test for regions of sound-evoked hyperactivity in a rat animal model with behavioral evidence of hyperacusis (Radziwon et al., 2017). We used a high-dose of sodium salicylate, an ototoxic drug that causes ~20 dB of transient cochlear hearing loss, and confirmed that this dose induced hyperacusis using a behavioral reaction time-intensity model of loudness growth (Chen et al., 2015; Guitton et al., 2003; Hayes et al., 2014; Turner et al., 2008). Blood oxygenation level dependent (BOLD) fMRI was performed on the rats to test for sound-evoked enhanced central gain. The fMRI findings were cross validated by obtaining sound-evoked electrophysiological recordings from affected brain regions (See Fig. 1 for a graphical description of the study). Our methods and results complement human MRI research on aberrant sound sensitivities in patients (Kumar et al., 2017). Our animal model fMRI method can act as a bridge between animal model findings and human patient findings, many of which are acquired with fMRI (Ghazaleh et al., 2017; Gu et al., 2010; Lanting et al., 2008; Melcher et al., 2000; Melcher et al., 2009).
Figure 1:
Experimental design figure. (A) Number of subjects (sample size) for different parts of the study. (B) Schematic of the go/no-go behavioral conditioning paradigm used to measure reaction time to a loud sound. (C) Functional magnetic resonance imaging (fMRI) hardware and setup. (D) Acoustic power spectra of the three tonal stimuli used during fMRI. (E) Representative block-design paradigm used in fMRI. Note that the order of presentation of tones is pseudorandom. (F) Location of MRI slices used during fMRI with bregma coordinates (in mm). (G) Electrode locations (green dots) used in electrophysiological recordings from the inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC). The boundaries of each structure are traced from the rat brain atlas (Paxinos et al., 2005).
Material and Methods
Animal subjects
All aspects of this study were approved by the animal research ethics committees of the City University of Hong Kong, The University of Hong Kong, and the State University of New York at Buffalo. Normal male Sprague-Dawley rats, 300 – 400 g, were the subjects in this study. Subjects underwent behavioral reaction time-intensity measurement (N=4), sound-evoked functional magnetic resonance imaging (fMRI) (N=6), or electrophysiological recordings (N=10) (see Fig. 1A). Subjects were housed under standard conditions of 2–4 per cage, 12 hr light/dark cycle, and background noise < 40 dB sound pressure level (SPL). Each subject was administered saline to obtain the control measurements. Subjects were than administered salicylate to obtain data associated with drug-induced hyperacusis as described below. The time between saline and salicylate administration depended on the experiment (see details of salicylate administration below). All subjects were euthanized after testing was completed.
Sodium salicylate administration
Subjects were given an intraperitoneal injection of sodium salicylate (Sigma-Aldrich). Salicylate was dissolved in saline solution at a dose of 250 mg/kg body weight (1 mL volume). Control measurements were performed with an equal volume saline. Behavioral measurements were obtained 2 hrs after injection. For behavioral reaction time-intensity measurements, saline and salicylate injections were 1-week apart and the order was alternated across subjects. For fMRI measurements, the injections were 3 hrs apart; saline was given first followed by salicylate. For electrophysiological recordings, the injections were 5 hrs apart; saline was given first followed by salicylate. For the behavioral, fMRI and electrophysiological studies, data collection for the salicylate condition began approximately 2 hrs following salicylate treatment when salicylate-induced hyperacusis and hyperactivity are present.
Reaction time-intensity measurements
Auditory reaction time-intensity measurements are strongly correlated with perceived loudness in humans and have been used as a reliable surrogate of loudness perception in animals (Arieh et al., 2003; Lauer et al., 2007; Moody, 1970; Wagner et al., 2004). In this study, we obtained reaction time-intensity functions using broadband noise bursts to confirm that high-dose salicylate induced changes consistent with hyperacusis (i.e., shorter than normal reaction times at high sound levels) (Fig. 1B) (Radziwon et al., 2017). Briefly, subjects were food restricted to >85% of free-feeding weight, but had unrestricted access to water, except during testing. Testing sessions were ~1 hr/day, 6–7 days/week. Measurements occurred in an acoustically transparent cage (28×30×38 cm) inside a sound attenuating chamber (76×71×71 cm) lined with 5 cm sound attenuating foam (Illbruck, Inc.). Behavior during sessions was monitored by a digital camera (Fire-i). The cage contained a speaker (FT28D Dome Tweeter, Fostex), feeder (Med Associates Model ENV-203M), and nose-poke hole with infrared sensors (Vulintus). The equipment was controlled by Tucker-Davis Technologies (TDT) system-3 hardware, consisting of an RX6 processor (100 kHz) for generating sound stimuli and RPvds software, along with custom Matlab (MathWorks) scripts. Stimuli levels were calibrated using a sound level meter (Larson Davis System 824) and microphone (1/2” free field microphone, model 2520, Larson-Davis) placed at the location of the rat’s head when its nose was in the nose-poke hole.
Sound stimuli were broadband noise bursts (1 – 42 kHz, 300 ms duration, 5 ms cosine rise/fall) presented at 30, 40, 50, 60, 70, 80, and 90 dB SPL. Subjects were trained to detect the noise bursts using a go/no-go operant conditioning procedure (Fig. 1B). During training, subjects first learned to poke into the nose-poke hole to obtain a food reward from the feeder. Once the subjects were reliably nose-poking, sound stimuli were introduced. As training progressed, the waiting interval between a nose poke and presentation of the noise burst was systematically increased from 1 to 4 s, and catch trials were added. If the rat removed its nose during the waiting interval, the trial was aborted. If the subject held its nose through the waiting interval, either a single noise burst was presented (“go” condition) or a catch trial was presented (“no-go” condition). For each block of 10 trials, stimuli were presented in pseudo-random order from a list with the seven noise burst SPLs (“go” condition), along with three catch trials without sound (“no-go” condition). If the subject removed its nose within 2 s after the broadband noise stimulus during a “go” trial, a food pellet (45 mg, Bio-Serv) was delivered to the feeder and a “hit” was recorded. If the subject failed to remove its nose during a “go” trial, a “miss” was recorded and no food was given. If the subject continued to hold its nose during a catch trial, a “correct rejection” was recorded. No food was given for a correct rejection, but the next trial began immediately. However, if the subject removed its nose during a catch trial, a “false alarm” was recorded and a timeout (4 s with lights off and a new trial could not be initiated) was administered.
After reliable baseline reaction time-intensity functions were obtained, salicylate testing began. Two of the rats received a saline injection the first week while the other two rats were given salicylate. The order was reversed one week later; rats that were previously administered saline were given salicylate, and rats previously given salicylate received saline. Reaction times (RT) were measured from the onset of the noise burst to the time the rat removed its nose from the nose-poke hole. Only RTs for hits were analyzed. In addition, data were excluded if the false alarm rate was >30% during a testing session.
fMRI – preparation
Our group has extensive experience performing sound-evoked fMRI on rat subjects (Cheung et al., 2012a; Cheung et al., 2012b; Gao et al., 2014; Gao et al., 2015b; Gao et al., 2015c; Lau et al., 2015a; Lau et al., 2015b; Lau et al., 2013; Wong et al., 2017; Yang et al., 2018; Zhang et al., 2013). Subjects (N=6) were initially anesthetized with 3% isoflurane. Isoflurane dosages were controlled by a vaporizer (SurgiVet, Smiths Medical). Light isoflurane anesthesia was maintained at 1.0% throughout the course of setup and scanning to maintain a stable plane of anesthesia. We avoided the use of injectable anesthetics to minimize the fluctuations in anesthetic state that occur with a drug that requires multiple injections during long-term MRI scanning. A custom delivery tube with a needle was inserted into the peritoneum of the subject for delivering saline and salicylate. The tube was attached to the syringe containing the solution. Subjects were placed in the prone position on a body holder with head restraint and tooth bar to further restrict motion (Fig. 1C). For sound stimulation delivery, a 1.7 m long, 8 mm inner diameter (tapered to 2 mm over the last 5 cm) nylon monaural sound delivery tube was connected to a 6.5 cm long, 2 mm inner diameter polyurethane tube that entered the left ear canal (Zhang et al., 2013). The right ear was occluded with cotton wool, and Vaseline was applied over the wool to reduce the scanner noise reaching the ear. Subjects were kept warm with a circulating 37 °C water pad throughout the experiment while rectal temperature was monitored. Intubation and mechanical ventilation were applied by connecting the endotracheal tube to a ventilator (TOPO Dual Mode, Kent Scientific) and respiration rate was monitored with a pressure sensor (SA instruments) attached to the abdominal area. End-tidal CO2 was monitored by capnography (V9400, Surgivet). Heart rate and oxygen saturation were monitored with a pulse oximeter (SA instruments) attached to one of the hind paws. An actively decoupled receive-only surface coil (Bruker Biospin) was placed over the head, centered on the auditory cortex (AC). The entire assembly was placed inside a 7 T MRI scanner with maximum gradient of 360 mT/m (70/16 PharmaScan, Bruker Biospin) using a transmit-only birdcage coil (Bruker Biospin).
fMRI – stimulation
Sound stimuli were produced using a closed-field magnetic loudspeaker (MF1, Tucker-Davis Technologies) placed at the proximal end of the sound delivery tube, outside of the 5 G line. The speaker was driven by a power amplifier (SA1 Stereo Power Amp, Tucker-Davis Technologies). Subjects were stimulated with pulsed, tonal sound (Fig. 1D). The pulse rate was 10 Hz and the duty cycle was 80% (duration of each tone pulse = 80 ms). The block-design stimulation paradigm consisted of an initial 40 s stimulus-off followed by six blocks of 20 s stimulus-on interleaved with 40 s stimulus-off periods (Fig. 1E). Six such paradigm presentations were played to each subject after each injection (saline and salicylate). Subjects were given one minute of rest between presentations. During each 20 s sound period, tonal sounds generated by the speaker were used to measure the BOLD response. Tones at one of three frequencies (8, 16, and 32 kHz) were played during each period. The frequencies were presented in pseudo-random, interleaved order such that each frequency occupied two blocks in each presentation (Fig. 1E). The SPL of each frequency was calibrated to 80 dB SPL (0.5 cm from the distal end of the sound tube that enters the ear canal) by an omnidirectional condenser microphone with uniform sensitivity up to 50 kHz (M50, Earthworks) and a 192 kHz recorder (FR-2, Fostex). The acoustic power spectra are shown in Fig. 1D.
fMRI – acquisition
After the subject was positioned in the scanner, scout images were acquired along the axial, coronal, and sagittal views to position MRI slices accurately. The scan geometry was eight 1.0 mm thick slices along the coronal view, with 0.2 mm inter-slice distance. The first and last slices were discarded during image processing as they contain artifacts from realignment and registration. Slices were positioned according to the rat brain atlas (Paxinos et al., 2005) such that the third slice was centered on the inferior colliculus (IC) at bregma −8.5 mm (Fig. 1F). An anatomical image was then acquired using a 2D rapid acquisition with refocused echoes (RARE) sequence. The sequence parameters were RARE factor = 8, field of view = 32 × 32 mm2, data matrix = 256 × 256, repetition time (TR) = 4200 ms, and echo time (TE) = 36 ms. Blood oxygenation level dependent (BOLD) fMRI images were acquired with a gradient echo-echo planar imaging (GE-EPI) sequence with the following parameters: field of view = 32 × 32 mm2, data matrix = 64 × 64, TR = 1 s, TE = 20 ms, and 400 acquisitions to match the stimulation paradigm. The EPI scan geometry was imported from the anatomical scan geometry. Twelve EPI sessions were performed, six starting 2 hrs after saline administration and six starting 2 hrs after salicylate administration. A six session scan took approximately 1 hr and the time between the injections was approximately 3 hrs. In total, a subject spent approximately 6 hrs in the scanner.
fMRI – processing
EPI images from each animal were realigned to the mean image of the first fMRI session using SPM8 (Wellcome Department of Imaging Neuroscience, University College London). Images obtained from different animals were co-registered to a custom-made brain template using affine transformation, Gaussian smoothing (full-width at half-maximum (FWHM) = 0.5 mm), and non-brain structures masked out, with the criteria of maximizing normalized mutual information. Images from each fMRI session were split into six 60 image sets (starting 5 s before the onset of each 20 s sound period and ending 35 s after cessation of the sound). Each image corresponded to 1 s in time. Sets with the same stimulation frequency (8, 16, or 32 kHz) were averaged. This led to one 60 image long set for each subject, at each frequency, and for the saline and salicylate administration conditions. The image sets were averaged across subjects and a general linear model (GLM) was applied to calculate the response coefficient (β) maps for each frequency and condition. Student’s t test was performed to identify activated voxels using the threshold p < 0.001 with cluster size ≥ 3. This resulted in six activation maps (saline or salicylate × 3 frequencies).
Functionally defined regions of interest (ROIs) were used to quantify the amplitude of fMRI signals from different structures in the auditory system. Activation maps were calculated after averaging image sets across subjects and saline and salicylate. This resulted in three averaged t-value maps, one for each stimulation frequency (8, 16, and 32 kHz), to account for the tonotopic organization of the central auditory system. ROIs of the contralateral (right) lateral lemniscus (LL), inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC) were finalized by comparing with the rat brain atlas, as those were the largest auditory structures. ROIs were defined by the selection of activated voxels (p < 0.001) in each structure using the averaged t-value maps. Voxels which passed the activation threshold and were within one voxel width of an auditory structure according to the brain atlas were included in the ROI of that structure. This ROI definition ensured that ROIs spanned the same voxels in images acquired after saline and salicylate.
fMRI signals were computed for each auditory structure from the original image sets by averaging the 60 s time course from all voxels in the respective ROI. This resulted in six signals per ROI (saline or salicylate × 3 frequencies). The corresponding fMRI signal amplitude was defined as ((Average value of the signal during the response period) / (Average value of signal during first 5 s of the set) – 1) × 100%. This presents the signal amplitudes in units of % baseline MRI signal. For the subcortical structures (e.g., IC), the response period was observed to be approximately 0 – 50 s after the sound was first presented. For the AC, the response period was approximately 0 – 10 s.
Electrophysiological recordings
Sound-evoked multi-unit spike discharge recordings and local field potentials (LFP) were obtained from the IC, MGB, and AC to corroborate fMRI findings. Subjects were anesthetized with a ketamine/xylazine mixture (50/6 mg/kg, i.p.), instead of isoflurane, as ketamine/xylazine works well and was more suitable for electrophysiological recordings (Auerbach et al., 2019; Stolzberg et al., 2011; Sun et al., 2009). The anesthetized rat was placed in a stereotaxic apparatus using a head holder and two blunt ear bars. The electrophysiological recordings were obtained in an electrically shielded sound booth. The methodology is similar to that described in our earlier studies (Chen et al., 2014; Chen et al., 2013; Chen et al., 2015; Jiang et al., 2017). The dorsal surface of the skull was exposed and a head bar firmly attached to the skull using screws and dental cement. The head bar was attached to a rod mounted on a magnetic base. The head bar assembly was used to hold the rat’s head in the stereotaxic frame after removing the left stereotaxic ear bar. This allowed the left ear to be acoustically stimulated free-field using a loudspeaker. An opening was made in the skull at the appropriate location(s) to gain access to the contralateral IC, MGB or AC (see Fig. 1G). The dura was removed from the surface of the brain overlying the IC, MGB or AC. Afterwards, a 16 channel linear silicon microelectrode array (A-1×16–10mm100e177, NeuroNexus Technologies) was used to record spike discharges from multiunit clusters and LFP from the IC (N=4), MGB (N=3) or AC (N=3). The distance between adjacent electrodes on the array was 100 μm and the diameter of each electrode was 15 μm. The electrode array was inserted slowly using a FHC hydraulic microdrive until reaching an appropriate site within the target region as indicated by robust auditory responses on nearly all 16 channels. Broadband noise bursts (50 ms duration, 1 ms rise/fall time, cosine2-gating, 1, 1.5, 2.3, 3.5, 8, 12.1, 18.3, 27.7, 42, and 65 kHz) were generated using a TDT RX6 multifunction processor (100 kHz sampling) and presented with an interstimulus interval of 300 ms. The stimuli were delivered through a loudspeaker (FT28D, Fostex) 10 cm from the left ear. Sound levels at the ear were measured with a 1/4 inch microphone (Larson Davis, model 2520) and preamplifier (Larson Davis, model 2221). Body temperature was maintained at 37 °C using a heating blanket (Harvard Apparatus). Stable anesthesia was maintained with supplemental doses (0.1 ml) of ketamine/xylazine (10:1 ratio) hourly.
Signals were sampled with a resolution of 40.96 ms using a RA16PA preamplifier and RX5–2 Pentusa base station (Tucker-Davis Technologies System-3). Signals were low-pass filtered (2–300 Hz) to obtain LFPs. The data was down-sampled online with resolution of 1.6393 ms. LFPs to tone bursts were obtained at multiple frequency (1 – 65 kHz) and intensity (60, 70, 80, 90 dB SPL) combinations (50 repetitions each combination, pseudo-random presentation). Three hours of recordings were performed beginning 2 hrs after saline administration and 2 hrs after salicylate administration.
Statistical analysis
Reaction time measurements were statistically analyzed using two-way, repeated measures analysis of variance (ANOVA). The factors were SPL and condition (baseline, saline, salicylate). Post-hoc analyses using Tukey’s pairwise comparisons were performed between saline and salicylate at each SPL. fMRI amplitudes were also analyzed by two-way, repeated measures ANOVA with factors of frequency and brain region (LL, IC, MGB, AC). Post-hoc analysis was performed between saline and salicylate at each frequency and region. LFP root-mean-square amplitudes were also analyzed by two-way, repeated measures ANOVA with factors of frequency and SPL. Different brain regions were separately analyzed as different subjects were used. Post-hoc analysis was performed between saline and salicylate at each frequency, SPL, and region.
Results
Faster reaction times for high sound level stimuli
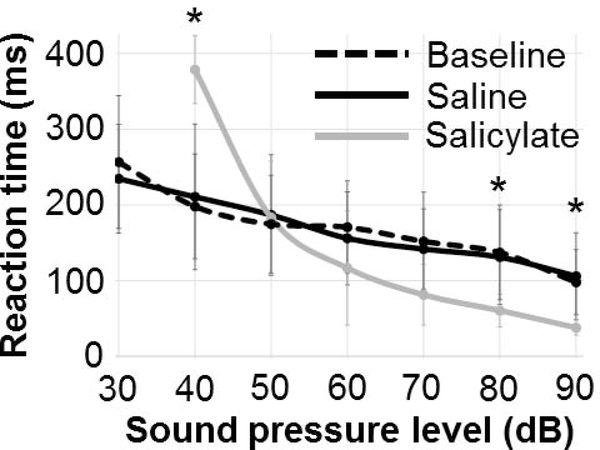
To determine the effects of salicylate on loudness perception, reaction time-intensity functions were obtained in each rat before drug administration (baseline), following saline injection (control), and following salicylate administration. Figure 2 shows reaction times (RTs) to broadband noise bursts measured at baseline (no injection), 2 hrs after saline injection, and 2 hrs after salicylate injection. We found a significant main effect of sound level (F (3,6) = 62.19, p < 0.001), indicating that RTs get faster with increasing stimulus intensity. Importantly, we observed that high-dose salicylate has a significant impact on loudness growth. We found a significant interaction between experimental condition (baseline, saline, and salicylate) and sound level (F (3,12) = 24.18, p < 0.001). Pairwise comparisons between salicylate and saline RTs indicated a significant effect of salicylate on RT at 90 dB (p < 0.05) 80 dB (p < 0.05), and 40 dB (p < 0.01). There was no significant difference between baseline and saline RTs at any sound level. As in our previous studies (Chen et al., 2014; Radziwon et al., 2017), RTs after high-dose salicylate administration were longer for low SPLs (< 50 dB), likely due to temporary outer hair cell dysfunction and associated hearing loss. In contrast, at high SPLs (≥ 80 dB), RTs were shorter than normal, indicative of a hyperacusis-like percept (i.e., shorter RTs indicate louder than normal percept) consistent with our earlier publications in which salicylate shortened RTs at high sound levels for both broadband noise as well as tone bursts (Radziwon et al., 2017).
Figure 2:
Reaction time (RT) measurements. RTs were measured to broadband noise bursts with a “go”-“no-go” behavioral conditioning paradigm (see Methods for full details). Baseline measurements were acquired first without any drug administration. One week later, saline or sodium salicylate (250 mg/kg, i.p.) was administered and RT measured again 2 hrs later. After another week, the other measurement was performed. Salicylate reduces RTs at high sound pressure levels (SPLs), behavioral evidence of hyperacusis. The RT increase during salicylate at low SPLs is likely due to a salicylate-induced temporary threshold shift of approximately 20 dB (Chen et al., 2014; Radziwon et al., 2017). * indicates p < 0.05 (saline vs. salicylate).
fMRI observes enhanced central auditory gain
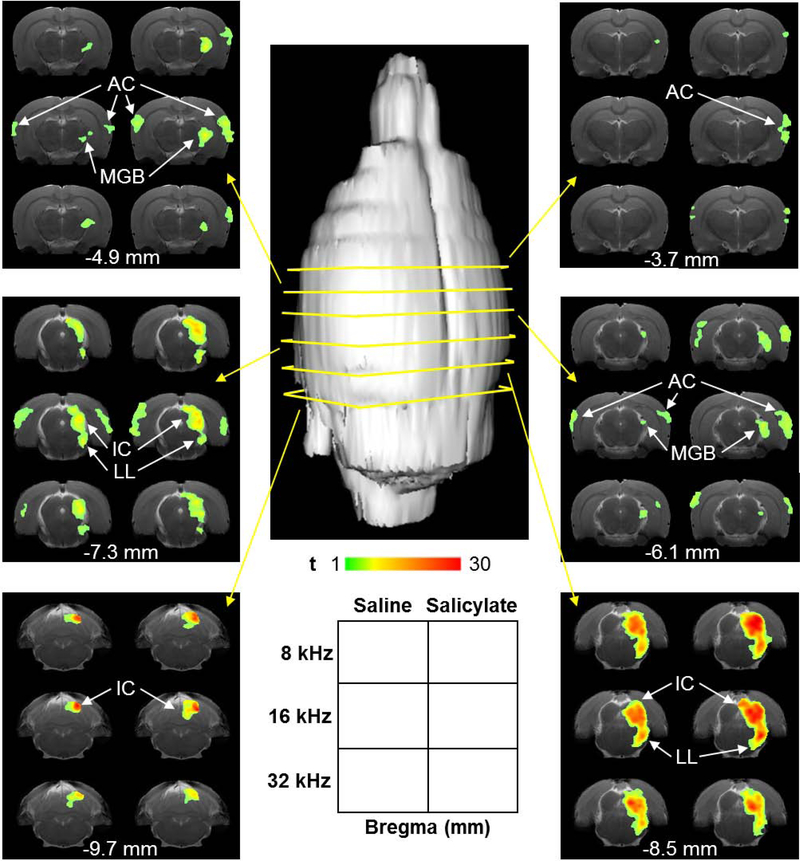
Sound-evoked fMRI was performed to map out change in brain activity following saline and salicylate injections. Figure 3 shows the sound-evoked activation maps recorded at 80 dB SPL for 8, 16 and 32 kHz. Imaging began 2 hrs after injection. Responses were observed in the contralateral lateral lemniscus (LL), inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC), key regions along the ascending auditory pathway. In the midbrain (LL and IC), t-values were slightly higher during salicylate, compared with saline, at 8 and 16 kHz. Higher up the auditory pathway in the MGB, the number of activated voxels at 8 and 16 kHz was greater during salicylate. In the AC, both t-values and number of activated voxels were considerably greater during salicylate. This enhancement occurred across all three frequencies.
Figure 3:
Sound-evoked fMRI activation maps. FMRI was started 2 hrs after saline administration. Afterwards, salicylate was administered and fMRI repeated starting 2 hrs later. fMRI was performed with 80 dB SPL tones at 8, 16, and 32 kHz (see Methods for full details). The t-value maps show responses across the central auditory system, including the contralateral lateral lemniscus (LL), inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC). Responses are noticeably higher during salicylate, versus saline, in the AC, particularly at 8 and 16 kHz. Responses also appear increased in the IC at 8 and 16 kHz. Each of the six panels with six copies of the same brain slice image was acquired from a coronal section of the brain (indicated by yellow arrows). The legend at the bottom center of the figure describes the arrangement of each panel. The Bregma coordinate of the center of each slice is indicated (in mm) at the bottom of each panel. The left column of each panel represents the saline maps. The right column represents the salicylate maps. The top row of each panel represents the 8 kHz maps, the middle row the 16 kHz maps, and the bottom row the 32 kHz maps. T-value is coded by colored heat map.
Previous studies have shown that high dose salicylate significantly reduces (> 50%) the sound-evoked neural output of the cochlea (Chen et al., 2017; Chen et al., 2015). To assess its effects on the auditory brainstem, we measured the BOLD fMRI amplitude in the LL after salicylate treatment to determine if the BOLD response was altered due to a reduced neural output of the cochlea. The mean (+/−SD) tone-evoked BOLD fMRI signal amplitudes, expressed as a percent of the baseline response, are shown in Figure 4A for 8, 16 and 32 kHz stimuli. The BOLD responses for saline and salicylate were similar. Thus, by the time the depressed neural activity from the cochlea was relayed to more rostral regions of the brainstem, there was no evidence of reduced sound-evoked activity in the LL BOLD response.
Figure 4:
(A) Mean (±SD) percent change in the sound-evoked blood oxygenation level dependent (BOLD) fMRI amplitude in LL. Data expressed as percent change relative to baseline. No significant differences between saline or salicylate for 8, 16 and 32 kHz stimuli. (B) Mean percent change in BOLD fMRI time courses in the LL during the 20 s stimulus (pink shaded area) and after the stimulus. Data shown for 8, 16 and 32 kHz stimuli (see legend). Solid lines indicate saline time courses and dashed lines represent salicylate time courses. Response amplitudes increased and decreased sooner for salicylate than saline, but maximum amplitudes were similar across conditions.
Figure 4B shows percent change in the LL BOLD signal during 20-s presentation of the 8, 16 and 32 kHz stimuli. The LL BOLD responses gradually increased above baseline near the beginning of the 20-s stimulus (pink shaded area), reached its peak near the end of the stimulus and then gradually declined back to baseline approximately 20 s after stimulus offset. The increase in the sound-evoked LL BOLD response was greatest for 16 kHz and least for 32 kHz. Salicylate treatment led to a more rapid rise in the LL BOLD response particularly at 8 and 16 kHz, but had little effect on the maximum amplitude of the BOLD response occurring near stimulus offset. Similarly, salicylate led to a more rapid decrease in the response after stimulus cessation.
Figure 5 (A and B) shows tone-evoked BOLD fMRI signal amplitudes from the IC and AC during saline and salicylate. Salicylate enhanced the BOLD responses compared to saline (F (5,25) = 14.39, p < 0.0001). Pairwise comparisons between saline and salicylate indicated that IC responses to 16 kHz tones presented at 80 dB SPL were greater during salicylate (p < 0.001) than saline. Further, AC responses to 8 and 16 kHz sounds were considerably greater during salicylate (p < 0.001 and 0.001, respectively). Given that salicylate reduces the neural output of the cochlea by more than 50%, these results are consistent with enhanced central auditory gain in higher auditory structures during salicylate-induced hyperacusis (Chen et al., 2017; Chen et al., 2015).
Figure 5:
Mean (±SD) percent change in sound-evoked blood oxygenation level dependent (BOLD) fMRI amplitudes in the IC (A) and AC (B). Data expressed as percent change relative to baseline; note difference in ordinate maximum in A versus B. Salicylate responses in IC significantly greater than saline at 16 kHz. Salicylate response in AC greater than saline at 8 and 16 kHz (***, p<0.001). (C and D) Mean percent change in BOLD fMRI time courses during the 20 s stimulus (pink shades area) and after the stimulus. Solid lines indicate saline time courses and dashed lines indicate salicylate time courses for 8, 16 or 32 kHz stimuli (see legend in panels). In the IC, BOLD responses were prolonged and were characterized by a gradually increase and decline. Salicylate caused the response amplitudes to increase and decrease sooner than saline, but maximum amplitudes were largely unchanged. In AC, BOLD responses were characterized by a narrow peak that occurred ~5 s after stimulus onset. Salicylate caused the peak to increase.
Figure 5 (C and D) shows percent change in the BOLD fMRI signal in the IC and AC during and after the 20 s acoustic stimulus. The IC BOLD response began to increase shortly after the start of the 20 s stimulus, reached a peak near stimulus offset and then started to decline at stimulus offset (Fig. 5C). The BOLD response returned to baseline approximately 20 s after stimulus offset. Salicylate treatment led to a more rapid increase in the onset of IC BOLD during the first 10 s following stimulus onset; these changes were more pronounced at 16 and 32 kHz than at 8 kHz. Similarly, salicylate led to a more rapid decrease in the response after stimulus cessation. Salicylate, however, did not substantially alter the maximum amplitude of the BOLD response that occurred near stimulus offset. The time course of the AC BOLD response was much different from the IC even though the measurements were obtained at the same time under identical conditions. The AC BOLD signal increased to a peak approximately 5 s after stimulus onset and then declined to baseline 8–10 s after stimulus onset (Figure 5D). The time course and amplitude of the BOLD responses were similar for 8, 16 and 32 kHz. The rapid decrease of the AC BOLD response could be due to greater cortical habituation or adaptation compared to the IC (Ayala et al., 2012; Mutschler et al., 2010; Westenberg et al., 1976).
We also obtained measurements from the MGB, but the sound-evoked BOLD signal amplitudes were just barely above the baseline response for 8, 16 and 32 kHz during the saline condition. BOLD response amplitudes were slightly greater during salicylate than saline, but the differences were not significantly different (data not shown).
Electrophysiology confirms fMRI
Electrophysiological recordings were carried out to confirm that salicylate enhanced sound-evoked neural responses in higher auditory centers. Figure 6 shows local field potentials (LFPs) measured from the contralateral IC, MGB and AC after saline and salicylate treatments. LFP amplitudes were measured with tone bursts from 1 – 65 kHz. Results are shown for intensities from 60 to 90 dB SPL. In the IC (Fig. 6A), LFP amplitudes measured after saline and salicylate treatments were generally similar across all SPLs and frequencies except for a small, but statistically-significant increase after salicylate treatment at 60 dB (F (39,273) = 2.79, p < 0.0001) and 12.1 kHz (p < 0.01). In the MGB (Fig. 6B), salicylate LFPs were slightly larger than saline LFPs. Statistical significance (F (71,355) = 4.365, p < 0.0001) was observed at 60 dB (4, 8, 16, 24 kHz; p < 0.05, 0.01, 0.001, 0.01), 70 dB (8, 16, 20 kHz; p < 0.001, 0.001, 0.05), 80 dB (8, 12, 30 kHz; p < 0.01, 0.001, 0.05), and 90 dB (3, 4, 16, 30 kHz; p < 0.001, 0.001, 0.001, 0.01). In the AC (Fig. 6C), salicylate LFPs were considerably larger than saline LFPs, particularly at higher intensities where the responses had increased by almost 2-fold. The salicylate-induced increases relative to saline increased with SPL with largest differences occurring between 4–30 kHz. Statistical significance (F (39,117) = 18.68, p < 0.0001) was observed at 60 dB (2.3, 12.1, 18.3, 27.7 kHz; p < 0.05, 0.001, 0.001, 0.01), 70 dB (1.0, 5.3, 8, 12.1, 18.3, 27.7 kHz; p < 0.05, 0.001, 0.001, 0.001, 0.001, 0.001), 80 dB (3.5, 5.3, 8, 12.1, 18.3, 27.7 kHz; p < 0.001, 0.001, 0.001, 0.001, 0.001, 0.001), and 90 dB (1.5, 2.3, 3.5, 5.3, 8, 12.1, 18.3, 27.7, 42 kHz; p < 0.05, 0.001, 0.001, 0.001, 0.001, 0.001, 0.001, 0.001, 0.05). The LFP data presented here was largely consistent with the spike discharge rates recorded from multiunit clusters (data not shown). Altogether, our LFP results show that suprathreshold response amplitudes in the IC were generally normal (i.e., similar saline) even though this dose of salicylate has been shown to reduce the neural output of the cochlea by more than 50%. Normal responses in the IC provide evidence of significant gain enhancement between the cochlea and IC (Chen et al., 2015; Jiang et al., 2017). LFP amplitudes in the IC are largely consistent with the noninvasive fMRI results. LFP amplitudes in the AC were greatly enhanced at suprathreshold intensities, particularly between 8 and 16 kHz, consistent with our noninvasive fMRI data which showed a robust increase in fMRI amplitude at 8 and 16 kHz.
Figure 6:
Sound-evoked local field potentials (LFPs). Recordings were made in the contralateral (A) IC, (B) MGB and (C) AC at intensities from 60 – 90 dB SPL and frequencies from 1 – 65 kHz. LFP amplitudes in the IC were largely similar between saline and salicylate conditions except around 12 kHz at 60 dB, where the salicylate responses were slightly higher than saline. In the MGB, LFP amplitudes during salicylate treatment were generally larger than saline at the mid-frequencies. In the AC, salicylate LFPs were significantly higher than saline, especially at SPLs > 70 dB and frequencies between 4 – 30 kHz. Statistically significant differences noted in figures by *, **, and ***, which indicate p < 0.05, 0.01, and 0.001, respectively (see text for details).
Discussion
Loudness Growth and Hyperacusis
RT-I intensity functions have been used to assess loudness growth in both human and animals (Marshall et al., 1980; Moody, 1973; Pfingst et al., 1975a; Pfingst et al., 1975b; Schlittenlacher et al., 2014). Our RT-I functions were unaffected by saline treatment, supporting the stability and reliability of this technique for assessing loudness growth (Chen et al., 2017; Radziwon et al., 2017). The RT-I function changed in predictable ways after high-dose salicylate treatment. RT to broadband noise became longer than normal at low intensities because of the ~20 dB salicylate-induced hearing loss, which pushed the low-intensity sounds closer to threshold. However, as intensity increased, RT decreased at a rapid rate catching up to the RT-I function at 50 dB SPL. At 80 – 90 dB SPL, post-salicylate RT were significantly shorter than normal, indicating that these suprathreshold intensities were perceived as louder than normal (i.e., hyperacusis), consistent with previous reports in which we showed that high dose sodium salicylate caused a shortening of RTs at high sound levels for both broadband noise and tone bursts (Chen et al., 2017; Radziwon et al., 2017).
Enhanced fMRI central gain
With our fMRI imaging technique, we were able to reliably measure sound-evoked BOLD responses from the rat LL, IC and AC during the saline-control condition, establishing the utility of this approach. However, the sound-evoked BOLD responses from the MGB during saline and salicylate treatments were much smaller and more variable than in other brain regions, minimizing the utility for MGB measurements. Therefore, the discussion will focus on the salicylate-induced changes in the LL, IC and AC where we obtained reliable BOLD responses with and without sound stimulation. Previous electrophysiological studies have found that high dose salicylate significantly reduces (>50%) the amplitude of the cochlear compound action potential, but by the time these signals reach the IC, the amplitude of the neural responses are essentially normal because of their progressive amplification along the central auditory pathway (Chen et al., 2013; Jiang et al., 2017; Stolzberg et al., 2011). Viewed in this context, it is not surprising that our 80 dB SPL BOLD responses from the LL and IC obtained during salicylate treatment were similar to saline control values (Figs. 4 and 5). The IC BOLD data are consistent with our IC LFP results which revealed little difference between salicylate and saline LFP amplitudes (Fig. 6A). In contrast to the LL and IC, AC BOLD responses during salicylate treatment were consistently larger than saline, providing clear evidence of enhanced central gain using fMRI imaging. These results are generally consistent with our AC LFP data (Fig. 6C) and prior reports. One factor that could have influenced the magnitude of the sound-evoked BOLD response in our study was the use of low-dose, 1% isoflurane anesthetic. LFP from the AC of rats anesthetized with 1.5% isoflurane were much smaller than those recorded from conscious rats (Sun et al., 2009). Moreover, 1.5% isoflurane anesthesia also greatly reduced the salicylate-induced enhancement of LFP amplitude. Enhanced electrophysiological responses have been observed in the AC of unanesthetized guinea pigs after intense noise exposure, but the enhancement of AC amplitudes following the exposure were abolished by urethane anesthesia (Popelar et al., 1987). These anesthetic suppressive effects presumably occur because isoflurane and urethane enhance GABA mediated inhibition (Hara et al., 2002; Neumahr et al., 2000; Ranft et al., 2004). Isoflurane induces a stable and well-controlled level of anesthesia that is highly desirable for fMRI imaging studies; however, the BOLD amplitudes obtained under 1% isoflurane anesthesia are likely an underestimate to those that would be seen awake animals.
Time course of sound-evoked BOLD responses
The time courses of the sound-evoked BOLD responses were strikingly different in subcortical and cortical regions. The BOLD temporal profiles in the LL and IC were very similar. The BOLD responses showed a moderate increase near stimulus onset followed by a gradual 2- to 3-fold amplitude increase above the initial value by the end of the 20 s stimulus. Thus in the LL and IC, the duration and maximum amplitude of the BOLD response increased over the duration of the 20 s stimulus. The BOLD response then gradually declined to baseline approximately 20 s after stimulus offset. In contrast to the LL and IC, the AC BOLD response increased shortly after stimulus onset, reached a peak around 4 s, and then returned to baseline roughly 10 s after the onset of the 20 s stimulus (Fig. 5D). The BOLD temporal profiles from the LL and IC of isoflurane anesthetized rats are substantially different from those seen in the AC of conscious humans (Jancke et al., 1999). The human AC BOLD responses to tone burst ranging in duration from 200 to 800 ms were characterized by an onset peak followed by a decline to a plateau of roughly half amplitude. IC and AC BOLD temporal response profiles were also measured in conscious human subjects using noise bursts (Backes et al., 2002). The BOLD responses reached a peak approximately 3 s after stimulus onset and returned to baseline after 8 s. The BOLD temporal profiles and amplitudes in this human study were largely the same in both the IC and AC. The phasic BOLD responses from the rat AC obtained with tone bursts presented at 10 Hz (80 ms duration, 80% duty cycle) resemble the phasic responses seen in the human AC when 25 ms noise bursts were presented at a high rate of 35/second (Harms et al., 2002). However, the human fMRI BOLD response takes on a more sustained profile when the repetition rate is reduced to 2/second. Based on these findings, our results suggest that the rat IC BOLD temporal profile are different from the BOLD response profiles measured in conscious human subjects. A number of factors could account for these differences, the most likely being the anesthetic used in our rat study, the limited range of durations used to evaluate IC responses in humans and differences in the imaging protocols.
Neural hyperactivity mechanisms
The neural mechanisms responsible for enhanced central gain are poorly understood, but could result from an increase in excitation and/or a decrease in inhibition. Increased excitatory drive has been seen in the AC as a result of cochlear hearing loss (Kotak et al., 2005), while changes in both excitatory and inhibitory synaptic function have been seen after cochlear destruction. Others have suggested that enhanced central gain could arise from decreased GABAergic or glycinergic inhibition (Auerbach et al., 2014; Milbrandt et al., 2000; Salvi et al., 2016) at various locations from the cochlear nucleus to the AC (Godfrey et al., 2015; Llano et al., 2012; Middleton et al., 2011; Wang et al., 2009). Advances in magnetic resonance spectroscopy (MRS) combined with sound-evoked BOLD imaging like those employed in this study could be used to determine if excitatory and inhibitory neurotransmitters levels are correlated with changes in central gain at various locations in the auditory pathway. MRS studies have revealed decreased GABA levels in patient with age-related hearing loss (Gao et al., 2015a). In human and animal studies, MRS imaging of neurotransmitter levels could be combined with hyperacusis questionnaires, psychophysical or behavioral measures of loudness growth to determine if changes in neurotransmitter levels are correlated with hyperacusis.
Sound-evoked fMRI of hyperacusis and tinnitus in humans
Studies of “pure” hyperacusis-only human subjects are rare because hyperacusis is generally accompanied by tinnitus. Instead, sound-evoked fMRI studies of tinnitus are often co-morbid with hyperacusis, due to the fact that tinnitus patients are often unaware of their loudness intolerance (Ghazaleh et al., 2017; Gu et al., 2010; Lanting et al., 2008; Melcher et al., 2000; Melcher et al., 2009). In general, such fMRI studies have observed enhanced sound-evoked responses in brain regions from the auditory midbrain up to the cortex. These findings are largely in agreement with the present study as the rat salicylate model of hyperacusis is accompanied by tinnitus. However, a major distinction between hyperacusis and tinnitus relevant to our current sound-evoked fMRI result is that hyperacusis requires sound stimulation to occur whereas tinnitus occurs in the absence of controlled sound stimulation. Interestingly, studies that examined subjects with tinnitus, but not hyperacusis, obtained slightly different results (Gu et al., 2010; Hofmeier et al., 2018). In tinnitus-only subjects without hearing loss, Gu and colleagues found that sound-evoked responses were only enhanced in the AC, but not subcortical responses. In contrast, Hofmeier and associates observed in tinnitus-only subjects with mild hearing loss that subcortical and cortical responses were reduced. These differences may be related to the degree, duration or type of cochlear hearing loss.
Technical considerations
During fMRI and electrophysiological recordings, saline was administered before salicylate. Saline was used to control for the effects of non-salicylate factors (e.g., injection and increase in body fluid). However, this meant that the salicylate results were obtained after a longer time under anesthesia. For multiple reasons, we do not believe that anesthesia duration significantly affected the main findings of this study, which are (1) enhanced central auditory gain in hyperacusis and (2) noninvasive fMRI can observe this gain increase. First, longer anesthesia duration typically does not increase response amplitudes, as observed in this study. In fact, the opposite likely occurs (i.e., reduced sound-evoked responses). Second, these consistent results were obtained with two different methods (fMRI and electrophysiology) and two different anesthetics (isoflurane and ketamine/xylazine).
This study provides new insights on the use of noninvasive sound-evoked fMRI to assess the magnitude, location and time course of sound-evoked neural activity patterns in animal subjects with hyperacusis. Our fMRI techniques can be used to detect changes in sound evoked activity throughout the central nervous system in an animal model in which it is possible to experimentally manipulate the degree and type of hearing loss and to objectively quantify biomarkers of hyperacusis that could be used for clinical diagnosis. One significant impediment of using fMRI to investigate hyperacusis in humans is that hyperacusis patients cannot tolerate the loud sounds generated by MRI scanners. These problems can be overcome to some extent by employing better passive or active sound attenuating ear protective devices and special fMRI scanning techniques, but further improvements are clearly needed for those subjects with severe hyperacusis. One advantage of using mice and rats for these studies is that they have poor low-frequency hearing in regions where there is considerable scanner noise. This misalignment of the rodent audiogram with MRI scanner noise greatly reduces the potential confounding effect of scanner noise.
Supplementary Material
Supplementary Figure 1: BOLD fMRI amplitudes in the LL (top row), IC (middle), and AC (bottom) for 8 (left column), 16 (middle), and 32 (right) kHz stimuli. This data is expanded from that shown in Fig. 4A and 5 (A and B) by plotting individual data points for each of the N = 6 subjects. For each of the nine panels, the N = 6 data points during saline and salicylate administration are shown. The horizontal lines indicate paired measurements from the same subject. The vertical lines adjacent to each set of six dots indicate the mean ± 2 × standard error.
In hyperacusis, sounds are perceived as exceedingly loud, frightening, or painful
Prevalence of hyperacusis approaches 10%
Sound-evoked fMRI developed to locate abnormal brain activity in hyperacusis rats
Enhanced central auditory gain observed and confirmed electrophysiologically
Animal fMRI advances basic research and connects human fMRI data with animal data
Acknowledgements
This work was supported by the Hong Kong Research Grants Council (ECS #21201217 to C.L. and C7048-16G to E.X.W.), Guangdong Provincial Brain Research Key Projects (No. 2018B030332001 and No. 2018B030336001 to E.X.W), City University of Hong Kong (7005107 to C.L.), NIH/NIDCD (F32DC015160 to B.A. and R01DC014452 to R.S.), and Hearing Health Foundation (to K.R.). The authors thank Jay Cheuk for assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson G, Lindvall N, Hursti T, Carlbring P 2002. Hypersensitivity to sound (hyperacusis): a prevalence study conducted via the Internet and post. Int J Audiol 41, 545–54. [DOI] [PubMed] [Google Scholar]
- Arieh Y, Marks LE 2003. Recalibrating the auditory system: a speed-accuracy analysis of intensity perception. J Exp Psychol Hum Percept Perform 29, 523–36. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, Salvi RJ 2014. Central gain control in tinnitus and hyperacusis. Frontiers in neurology 5, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Radziwon K, Salvi R 2019. Testing the Central Gain Model: Loudness Growth Correlates with Central Auditory Gain Enhancement in a Rodent Model of Hyperacusis. Neuroscience 407, 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YA, Malmierca MS 2012. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front Neural Circuits 6, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes WH, van Dijk P 2002. Simultaneous sampling of event-related BOLD responses in auditory cortex and brainstem. Magn Reson Med 47, 90–6. [DOI] [PubMed] [Google Scholar]
- Baguley DM 2003. Hyperacusis. J R Soc Med 96, 582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM, Hoare DJ 2018. Hyperacusis: major research questions. HNO 66, 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton H, Plack CJ, Maslin M, Schaette R, Munro KJ 2015. Pump Up the Volume: Could Excessive Neural Gain Explain Tinnitus and Hyperacusis? Audiol Neuro-Otol 20, 273–282. [DOI] [PubMed] [Google Scholar]
- Chen GD, Radziwon KE, Kashanian N, Manohar S, Salvi R 2014. Salicylate-induced auditory perceptual disorders and plastic changes in nonclassical auditory centers in rats. Neural plasticity 2014, 658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Stolzberg D, Lobarinas E, Sun W, Ding D, Salvi R 2013. Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear Res 295, 100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chen GD, Auerbach BD, Manohar S, Radziwon K, Salvi R 2017. Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress. Hear Res 349, 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ 2015. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife 4, e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Lau C, Zhou IY, Chan KC, Zhang JW, Fan SJ, Wu EX 2012a. High fidelity tonotopic mapping using swept source functional magnetic resonance imaging. Neuroimage 61, 978–86. [DOI] [PubMed] [Google Scholar]
- Cheung MM, Lau C, Zhou IY, Chan KC, Cheng JS, Zhang JW, Ho LC, Wu EX 2012b. BOLD fMRI investigation of the rat auditory pathway and tonotopic organization. Neuroimage 60, 1205–11. [DOI] [PubMed] [Google Scholar]
- Diehl PU, Schaette R 2015. Abnormal Auditory Gain in Hyperacusis: Investigation with a Computational Model. Frontiers in neurology 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagelson M, Baguley D 2018. Hyperacusis and Disorders of Sound Intolerance Plural Publishing, San Diego, USA. [Google Scholar]
- Formby C, Hawley ML, Sherlock LP, Gold S, Payne J, Brooks R, Parton JM, Juneau R, Desporte EJ, Siegle GR 2015. A Sound Therapy-Based Intervention to Expand the Auditory Dynamic Range for Loudness among Persons with Sensorineural Hearing Losses: A Randomized Placebo-Controlled Clinical Trial. Semin Hear 36, 77–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, Edden RA 2015a. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage 106, 311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Zhang JW, Cheng JS, Zhou IY, Wu EX 2014. The inferior colliculus is involved in deviant sound detection as revealed by BOLD fMRI. Neuroimage 91, 220–7. [DOI] [PubMed] [Google Scholar]
- Gao PP, Zhang JW, Chan RW, Leong AT, Wu EX 2015b. BOLD fMRI study of ultrahigh frequency encoding in the inferior colliculus. Neuroimage 114, 427–37. [DOI] [PubMed] [Google Scholar]
- Gao PP, Zhang JW, Fan SJ, Sanes DH, Wu EX 2015c. Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. Neuroimage 123, 22–32. [DOI] [PubMed] [Google Scholar]
- Ghazaleh N, Zwaag WV, Clarke S, Ville DV, Maire R, Saenz M 2017. High-Resolution fMRI of Auditory Cortical Map Changes in Unilateral Hearing Loss and Tinnitus. Brain Topogr 30, 685–697. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Chen K, Godfrey MA, Lee AC, Crass SP, Shipp D, Simo H, Robinson KT 2015. Cochlear ablation effects on amino acid levels in the chinchilla cochlear nucleus. Neuroscience 297, 137–59. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR 2010. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104, 3361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL 2003. Salicylate induces tinnitus through activation of cochlear NMDA receptors. Journal of Neuroscience 23, 3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Harris RA 2002. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94, 313–8, table of contents. [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR 2002. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol 88, 1433–50. [DOI] [PubMed] [Google Scholar]
- Hayes SH, Radziwon KE, Stolzberg DJ, Salvi RJ 2014. Behavioral models of tinnitus and hyperacusis in animals. Frontiers in neurology 5, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeier B, Wolpert S, Aldamer ES, Walter M, Thiericke J, Braun C, Zelle D, Ruttiger L, Klose U, Knipper M 2018. Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. Neuroimage Clin 20, 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Buchanan T, Lutz K, Specht K, Mirzazade S, Shah NJ 1999. The time course of the BOLD response in the human auditory cortex to acoustic stimuli of different duration. Brain research. Cognitive brain research 8, 117–24. [DOI] [PubMed] [Google Scholar]
- Jiang C, Luo B, Manohar S, Chen GD, Salvi R 2017. Plastic changes along auditory pathway during salicylate-induced ototoxicity: Hyperactivity and CF shifts. Hear Res 347, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juris L, Andersson G, Larsen HC, Ekselius L 2014. Cognitive behaviour therapy for hyperacusis: a randomized controlled trial. Behav Res Ther 54, 30–7. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien JL, Barthelemy C, Collet L 2004. Increased perception of loudness in autism. Hear Res 198, 87–92. [DOI] [PubMed] [Google Scholar]
- Knipper L, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U 2013. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Progress in Neurobiology 111, 17–33. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH 2005. Hearing loss raises excitability in the auditory cortex. J Neurosci 25, 3908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tansley-Hancock O, Sedley W, Winston JS, Callaghan MF, Allen M, Cope TE, Gander PE, Bamiou DE, Griffiths TD 2017. The Brain Basis for Misophonia. Current biology : CB 27, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, De Kleine E, Bartels H, Van Dijk P 2008. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol 128, 415–21. [DOI] [PubMed] [Google Scholar]
- Lau C, Zhang JW, McPherson B, Pienkowski M, Wu EX 2015a. Long-term, passive exposure to non-traumatic acoustic noise induces neural adaptation in the adult rat medial geniculate body and auditory cortex. Neuroimage 107, 1–9. [DOI] [PubMed] [Google Scholar]
- Lau C, Pienkowski M, Zhang JW, McPherson B, Wu EX 2015b. Chronic exposure to broadband noise at moderate sound pressure levels spatially shifts tone-evoked responses in the rat auditory midbrain. Neuroimage 122, 44–51. [DOI] [PubMed] [Google Scholar]
- Lau C, Zhang JW, Cheng JS, Zhou IY, Cheung MM, Wu EX 2013. Noninvasive fMRI investigation of interaural level difference processing in the rat auditory subcortex. PLoS One 8, e70706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer A, Dooling R 2007. Evidence of hyperacusis in canaries with permanent hereditary high-frequency hearing loss. Seminars in Hearing 28, 8. [Google Scholar]
- Llano DA, Turner J, Caspary DM 2012. Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J Neurosci 32, 16141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Brandt JF 1980. The relationship between loudness and reaction time in normal hearing listeners. Acta Otolaryngol 90, 244–9. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Levine RA 2000. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83, 1058–72. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B 2009. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miani C, Passon P, Bracale AM, Barotti A, Panzolli N 2001. Treatment of hyperacusis in Williams syndrome with bilateral conductive hearing loss. Eur Arch Otorhinolaryngol 258, 341–4. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T 2011. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A 108, 7601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM 2000. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147, 251–60. [DOI] [PubMed] [Google Scholar]
- Moody D 1970. Reaction time as an index of sensory function, Animal psychophysics: The design and conduct of sensory experiments. Springer, Boston, MA. [Google Scholar]
- Moody DB 1973. Behavioral studies of noise-induced hearing loss in primates: loudness recruitment. Adv Otorhinolaryngol 20, 82–101. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Speck O, Schulze-Bonhage A, Hennig J, Seifritz E, Ball T 2010. Time scales of auditory habituation in the amygdala and cerebral cortex. Cereb Cortex 20, 2531–9. [DOI] [PubMed] [Google Scholar]
- Neumahr S, Hapfelmeier G, Scheller M, Schneck H, Franke C, Kochs E 2000. Dual action of isoflurane on the gamma-aminobutyric acid (GABA)-mediated currents through recombinant alpha(1)beta(2)gamma(2L)-GABA(A)-receptor channels. Anesth Analg 90, 1184–90. [DOI] [PubMed] [Google Scholar]
- Paulin J, Andersson L, Nordin S 2016. Characteristics of hyperacusis in the general population. Noise & health 18, 178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 2005. The Rat Brain in Stereotaxic Coordinates. 5th ed. Elsevier Academic Press. [Google Scholar]
- Pfingst BE, Hienz R, Miller J 1975a. Reaction-time procedure for measurement of hearing. II. Threshold functions. J Acoust Soc Am 57, 431–6. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Hienz R, Kimm J, Miller J 1975b. Reaction-time procedure for measurement of hearing. I. Suprathreshold functions. J Acoust Soc Am 57, 421–30. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Tyler RS, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Coelho CB, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC 2014. A review of hyperacusis and future directions: part II. Measurement, mechanisms, and treatment. Am J Audiol 23, 420–36. [DOI] [PubMed] [Google Scholar]
- Popelar J, Syka J, Berndt H 1987. Effect of noise on auditory evoked responses in awake guinea pigs. Hear Res 26, 239–47. [DOI] [PubMed] [Google Scholar]
- Radziwon K, Holfoth D, Lindner J, Kaier-Green Z, Bowler R, Urban M, Salvi R 2017. Salicylate-induced hyperacusis in rats: Dose- and frequency-dependent effects. Hear Res 350, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgansberger W, Kochs E, Eder M, Hapfelmeier G 2004. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci 20, 1276–80. [DOI] [PubMed] [Google Scholar]
- Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, Radziwon K, Auerbach BD 2016. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Frontiers in neuroscience 10, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlittenlacher J, Ellermeier W, Arseneau J 2014. Binaural loudness gain measured by simple reaction time. Atten Percept Psychophys 76, 1465–72. [DOI] [PubMed] [Google Scholar]
- Stolzberg D, Chen GD, Allman BL, Salvi RJ 2011. Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience 180, 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhnan AP, Finch PM, Drummond PD 2017. Hyperacusis in chronic pain: neural interactions between the auditory and nociceptive systems. Int J Audiol 56, 801–809. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ 2009. Salicylate increases the gain of the central auditory system. Neuroscience 159, 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Parrish J 2008. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol 17, S185–92. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Dauman N, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC 2014. A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol 23, 402–19. [DOI] [PubMed] [Google Scholar]
- Wagner E, Florentine M, Buus S, McCormack J 2004. Spectral loudness summation and simple reaction time. J Acoust Soc Am 116, 1681–6. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM 2009. Plasticity at Glycinergic Synapses in Dorsal Cochlear Nucleus of Rats with Behavioral Evidence of Tinnitus. Neuroscience 164, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg IS, Weinberger NM 1976. Evoked potential decrements in auditory cortex. II. Critical test for habituation. Electroencephalogr Clin Neurophysiol 40, 356–69. [DOI] [PubMed] [Google Scholar]
- Wong E, Yang B, Du L, Ho WH, Lau C, Ke Y, Chan YS, Yung WH, Wu EX 2017. The multi-level impact of chronic intermittent hypoxia on central auditory processing. Neuroimage 156, 232–239. [DOI] [PubMed] [Google Scholar]
- Yang B, Wong E, Ho WH, Lau C, Chan YS, Wu EX 2018. Reduction of sound-evoked midbrain responses observed by functional magnetic resonance imaging following acute acoustic noise exposure. J Acoust Soc Am 143, 2184. [DOI] [PubMed] [Google Scholar]
- Zeng FG 2013. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hearing Res 295, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Lau C, Cheng JS, Xing KK, Zhou IY, Cheung MM, Wu EX 2013. Functional magnetic resonance imaging of sound pressure level encoding in the rat central auditory system. Neuroimage 65, 119–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: BOLD fMRI amplitudes in the LL (top row), IC (middle), and AC (bottom) for 8 (left column), 16 (middle), and 32 (right) kHz stimuli. This data is expanded from that shown in Fig. 4A and 5 (A and B) by plotting individual data points for each of the N = 6 subjects. For each of the nine panels, the N = 6 data points during saline and salicylate administration are shown. The horizontal lines indicate paired measurements from the same subject. The vertical lines adjacent to each set of six dots indicate the mean ± 2 × standard error.