Abstract
Background
The central vein sign (CVS) has been shown to help in the differential diagnosis of MS, but most prior studies are retrospective.
Objectives:
To prospectively assess the diagnostic predictive value of the CVS in diagnostically difficult cases.
Methods
In this prospective-multicenter-study, 51 patients with suspected MS who had clinical, imaging, or laboratory “red flags” (i.e., features atypical for MS) underwent 3T FLAIR* MRI for CVS assessment. After the diagnostic work-up, expert clinicians blinded to the results of the CVS assessment came to a clinical diagnosis. The value of the CVS to prospectively predict an MS diagnosis was assessed.
Results
Of the 39 patients who received a clinical diagnosis by the end of the study, 27 had MS and 12 received a non-MS diagnosis that included systemic lupus erythematosus, sarcoidosis, migraine, Sjögren disease, SPG4-spastic-paraparesis, neuromyelitis optica, and Susac syndrome. The percentage of perivenular lesions was higher in MS (median=86%) compared to non-MS (median=21%; p<0.0001) patients. A 40% perivenular lesion cut-off was associated with 97% accuracy and 96% positive/100% negative predictive value.
Conclusions
The CVS detected on 3T FLAIR* images can accurately predict a MS diagnosis in patients suspected to have MS, but with atypical clinical, laboratory, and imaging features.
Keywords: Central vein sign, Red flags, MS diagnosis
Introduction
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system (CNS) characterized by a relapsing or progressing clinical course and associated with characteristic hyperintensities on T2-weighted magnetic resonance imaging (MRI) of the brain and spinal cord.1 There is no single diagnostic test for MS, and current diagnostic criteria rest upon the demonstration of disease dissemination in space (DIS) and time (DIT) using clinical, laboratory, and MRI criteria.2 Although highly useful, the specificity of the current diagnostic imaging criteria is limited, and the risk of diagnosing MS in individuals affected by other disorders is still substantial.3,4
Increasing scientific evidence suggests that novel imaging techniques could improve the specificity of the current diagnostic criteria.2,5 The presence of a vein at the center of brain white matter (WM) lesions, the “central vein sign” (CVS), is a specific feature of MS and can now be depicted at clinical MRI field strength using specialized gradient-echo MRI sequences.5–7 Several studies have shown how this promising imaging biomarker can differentiate MS from other disorders showing similar WM lesions on MRI, including migraine,8,9 cerebral small vessel disease,10 neuromyelitis optica,11 Susac syndrome,12 and primary or secondary vasculitis of the central nervous system.6 However, most prior studies are retrospective. Prospective, multicenter studies starting from the time of initial work-up are needed to assess the true diagnostic value of the CVS, especially in diagnostically difficult cases.
Cases presenting with syndromes typical for MS but with concurrent clinical, laboratory or imaging features atypical for MS are particularly challenging for the treating neurologist in clinical practice. A number of important reviews have identified differentiating clinical, laboratory or imaging features (“red flags”) to guide clinicians during the diagnostic work-up of patients with suspected MS but with atypical features for the diagnosis.2,4,13–18 Data regarding the prospective diagnostic value of the CVS in these challenging conditions are lacking. In this multicenter study, we prospectively tested the diagnostic value of the CVS at clinical 3T MRI in patients with possible MS but with atypical clinical, laboratory, or imaging features.
Methods
Patients
Between September 2016 and December 2018, patients with a clinical presentation suggestive of MS but who had clinical, imaging, or laboratory features atypical for MS15,17,18 were prospectively enrolled in 4 academic research hospitals: the Lausanne University Hospital (Lausanne, Switzerland), the Erasme and Brugmann University Hospitals (Brussels, Belgium), and the San Raffaele University Hospital (Milan, Italy). Patients were excluded from the study if (i) they did not experience at least one clinical episode compatible with a focal or multifocal demyelinating event in the CNS; (ii) they did not reach a clinical diagnosis at the end of the study period; (iii) they had a contraindication for MRI or intravenous injection of gadolinium-based contrast material; (iv) MRI images quality was suboptimal because of motion artifact.
The study received approval from ethical standards committees on human experimentation at all centers. Written informed consent was obtained from all participants.
Diagnostic work-up
All enrolled patients received an extensive work-up, including clinical, laboratory, and radiological assessment. Laboratory testing included serological screening for autoimmune and infectious diseases and cerebrospinal fluid (CSF) examination with oligoclonal bands (OCB) testing. Radiological assessment included 3T brain MRI with imaging sequences for central vein assessment (FLAIR* MRI, see below). Other paraclinical tests, including anti-aquaporin-4 IgG (AQP4-antibody), neuro-ophthalmological assessment, salivary gland biopsy, spinal cord MRI, chest and abdominal CT, and whole-body PET-CT, were also performed when necessary.
MRI acquisition protocol
All patients underwent a single brain MRI on a 3T Magnetom Skyra or Prisma scanner (Siemens Healthcare, Erlangen, Germany) in Lausanne and two 3T Philips MRI scanners (Philips, Best, The Netherlands) in Brussels (Ingenia) and Milan (Intera). A single MRI protocol was adopted in all centers, including high resolution 3D T2*-weighted echo-planar-imaging (EPI) and 3D T2-FLAIR images acquired respectively during or after intravenous injection of a single dose (0.1 mmol/kg) of gadolinium-based contrast material, as previously described.6,19 Isotropic resolution of the 3D T2*-EPI was 0.55 mm3 in Brussels/Milan and 0.65 mm3 in Lausanne. 3D T2*-EPI and 3D T2 FLAIR sequence parameters were identical for the 3T Philips MRI scanners in Brussels and Milan and very similar for the 3T Siemens MRI scanners in Lausanne (Table 1).
Table 1.
MRI sequence parameters in Brussels and Milan (Philips scanners), and Lausanne (Siemens scanners), healthcare systems.
| Sequence | 3D T2*-EPI | 3D T2-FLAIR | ||
|---|---|---|---|---|
| Magnet strength | 3T | 3T | 3T | 3T |
| Manufacturer | Siemens | Philips | Siemens | Philips |
| Model | Prisma/Skyra | Ingenia/Intera | Prisma/Skyra | Ingenia/Intera |
| Receive channels | 64 | 8 | 64 | 8 |
| Imaging plane | Sagittal | Sagittal | Sagittal | Sagittal |
| Imaging resolution (mm) | 0.65 | 0.55 | 1 | 1 |
| # slices | 288 | 336 | 176 | 180 |
| Repetition time (TR, ms) | 64 | 53 | 5000 | 4800 |
| Echo time (TE, ms) | 35 | 29 | 391 | 373 |
| Inversion time (TI, ms) | - | - | 1800 | 1600 |
| Flip angle (deg) | 10 | 10 | variable | 90 |
| Averages | 1 | 1 | 1 | 1 |
| Acquisition time (min:sec) | 6:20 | 4:40 | 4:47 | 6:00 |
MRI post-processing and analysis
For the “central vein sign” (CVS) assessment, FLAIR* images were generated by coregistration (up-sampling of the T2-FLAIR to match the T2* resolution) and voxel-wise multiplication of the high-resolution 3D T2* EPI and the 3D T2-FLAIR, as previously described.6,19 For each subject, WM lesions were manually segmented on 3D FLAIR* images using Medical Image Processing, Analysis, and Visualization (MIPAV; NIH; http://mipav.cit.nih.gov), and, for each lesion, the presence/absence of the CVS was assessed according to the NAIMS guidelines.5 Cases were dichotomized as perivenular positive vs. perivenular negative based on 4 previously proposed criteria : (i) the “50% rule”6 and (ii) the “40% rule,”10 whereby a 50% or 40% perivenular lesion cutoff distinguishes MS from its mimics; (iii) the “3-lesion rule”9 and (iv) the “6-lesion rule,”6,20 whereby 3 lesions or 10 lesions are randomly selected and MS is diagnosed if at least 2 or 6 lesions are, respectively, perivenular. For each patient, two investigators (P.M., M.A.) independently assessed the percentage of perivenular lesions for inter-rater reliability. Disagreements were adjudicated by an expert neuroradiologist (D.S.R.). For each patient, fulfillment of MS MRI criteria for dissemination in space (DIS) and time (DIT) according to the most recent criteria2 were also recorded.
Clinical diagnosis and predictive value of the central vein sign.
After the 3D FLAIR* MRI scan for perivenular assessment, patients were prospectively followed to allow for a clinical diagnosis to be achieved. Patients received (i) a MS diagnosis if fulfilling MS diagnostic criteria and no better explanation for the clinical presentation was found, despite red flags; (ii) a non-MS diagnosis if an alternative diagnosis better explained the clinical presentation. After follow-up, expert clinicians in each center, blinded to the results of the CVS assessment, came to an eventual clinical diagnosis. The value of the CVS to prospectively predict MS diagnosis was assessed.
Statistical Analysis.
Difference in perivenular frequency between MS and non-MS patients was tested using Mann-Whitney U test. Inter-rater reliability for the perivenular assessment was computed using Cohen’s κ.
Results
Patients
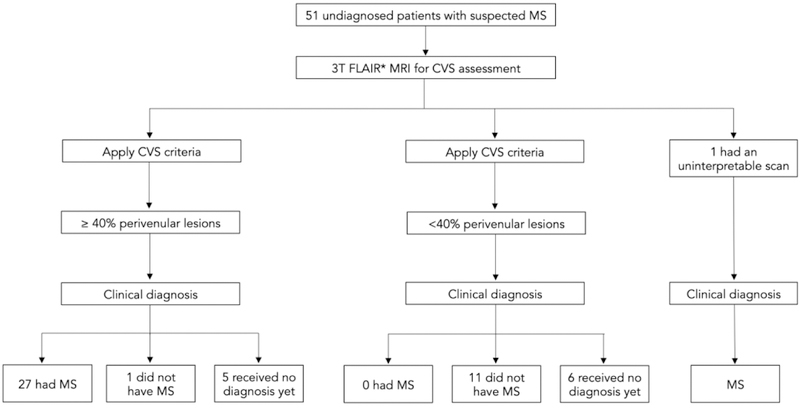
We prospectively included 51 patients. All patients underwent a single standardized 3T imaging research protocol including 3D FLAIR* MRI for CVS assessment. Of the 51 recruited patients, 1 had an uninterpretable scan due to motion artifact, and 11 did not receive a clinical diagnosis by the end of the study (Figure 1). All 39 patients who received a clinical diagnosis by the end of the study (30 females and 9 males), median age 46 years (range, 19–74 years), experienced at least one clinical episode compatible with a focal or multifocal demyelinating event in the CNS2 and had at least one clinical, laboratory, or imaging feature atypical for MS, hereafter termed “red flags” (Table 2).2,15,17,18 Of note, minor clinical red flags (in Table 2 denoted “Minor”), were features not specific of a disease involving the CNS but potentially associated with another systemic inflammatory/autoimmune disorder (SAD) involving the CNS. Patients carrying minor red flags needed at least another red flag to be included in this study.
Figure 1. Patients flow diagram summarizing the study design and main results.
Abbreviation: MS: multiple sclerosis; CVS: central vein sign.
Table 2:
Atypical features for MS diagnosis, i.e. “red flags”,
| Red flags | Patients | |
|---|---|---|
| Red flag type | Red flags (ID) | # patients (%) |
| Clinical | ||
| Age at symptoms onset >50 years old | C(1) | 7 (18%) |
| History of SAD | C(2) | 6 (15%) |
| History of oral/genital aphthosis or VT | C(3) | 5 (13%) |
| Poor recovery or bilateral ON | C(4) | 4 (10%) |
| Uveitis and/or retinal vasculitis | C(5) | 4 (10%) |
| Hearing loss and branch retinal artery occlusion | C(6) | 1 (3%) |
| Cognitive decline at onset | C(7) | 1 (3%) |
| Minor | C(8) | 6 (15%) |
| Laboratory | ||
| Absence of OCB | L(1) | 14 (36%) |
| Abnormal biomarkers of SAD | L(2) | 12 (31%) |
| Proteinorrachia >100 mg/dL | L(3) | 4 (10%) |
| Positive IgM Borrelia burgdoferi serology | L(4) | 1 (3%) |
| Imaging | ||
| Atypical morphology*/distribution WM lesions | I(1) | 12 (31%) |
| Longitudinal extensive transverse myelitis | I(2) | 3 (8%) |
| Diffuse meningeal contrast enhancement | I(3) | 3 (8%) |
| Absence of ≥ 2 spinal cord MRI lesions in OCB negative suspected PPMS | I(4) | 3 (8%) |
Abbreviations: SAD: systemic inflammatory/autoimmune disorder; VT: venous thrombosis; ON: optic neuritis; OCB: CSF specific oligoclonal bands; RRMS: relapsing remitting MS; PPMS: primary progressive MS; Minor (i.e. “minor” red flags): spondyloarthritis, fibromyalgia, Raynaud’s phenomenon and history of joint inflammation with good response to corticosteroids.
including large brainstem lesions.
Atypical MS diagnostic features for each patient are shown in Table 3.
Table 3.
Red flags, fulfillment of MRI DIS and DIT MS diagnostic criteria, clinical diagnosis, and frequency of perivenular lesions
| Patient ID | Age | Red flags ID | Clinical onset | MRI DIS | MRI DIT | OCB | diagnosis | Treatment | % Perivenular |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | C(8), I(1) | Ataxia | fulfilled | fulfilled | present | RRMS | ocrelizumab | 96 |
| 2 | 54 | C(1), L(2) | Limb weakness | fulfilled | fulfilled | present | RRMS | ocrelizumab | 67 |
| 3 | 25 | C(5), L(2) | Visual impairment | fulfilled | not fulfilled | present | Sjögren | tocilizumab | 33 |
| 4 | 52 | C(8), L(1), L(3) | Facial numbness | fulfilled | fulfilled | absent | RRMS | ocrelizumab | 100 |
| 5 | 54 | L(1), I(1) | Facial numbness | fulfilled | fulfilled | absent | RRMS | glatiramer acetate | 71 |
| 6 | 43 | C(2), L(2) | Limb weakness | fulfilled | fulfilled | present | RRMS | azathioprine | 100 |
| 7 | 34 | C(2), C(3), L(2) | Limb weakness | fulfilled | not fulfilled | present | RRMS | NA | 83 |
| 8 | 68 | C(1), L(1), I(4) | Limb weakness | fulfilled | not fulfilled | absent | SPG4 HSP | none ** | 13 |
| 9 | 29 | I(1) | Visual impairment | fulfilled | fulfilled | ND | RRMS | rituximab | 80 |
| 10 | 28 | C(2), C(3), C(5) | Visual impairment | fulfilled | fulfilled | present | RRMS | methotrexate | 93 |
| 11 | 44 | C(5), C(8) | Visual impairment | fulfilled | fulfilled | present | RRMS | cyclophosphamide | 98 |
| 12 | 20 | C(4), L(1) | Visual impairment | fulfilled | fulfilled | absent | RRMS | mitoxantrone | 92 |
| 13 | 42 | C(4), L(1) | Visual impairment | fulfilled | fulfilled | absent | RRMS | interferon beta-1a | 86 |
| 14 | 60 | C(1), L(1) | Limb weakness | fulfilled | fulfilled | absent | RRMS | none | 75 |
| 15 | 64 | C(1), I(1) | Vertigo | fulfilled | not fulfilled | ND | migraine | none | 6 |
| 16 | 54 | I(1) | Visual impairment | fulfilled | fulfilled | present | SPMS | rituximab | 52 |
| 17 | 53 | L(1), L(2), I(2) | Limb numbness | fulfilled | fulfilled | absent | SLE | azathioprine | 0 |
| 18 | 62 | C(1), C(8), L(1) | Limb weakness* | fulfilled | fulfilled | absent | PPMS | ocrelizumab | 67 |
| 19 | 29 | C(3), C(8) | Visual impairment | fulfilled | fulfilled | present | RRMS | fingolimod | 91 |
| 20 | 33 | C(8), L(1) | Visual impairment | fulfilled | fulfilled | absent | RRMS | teriflunomide | 100 |
| 21 | 45 | C(4), L(1), I(2) | Limb weakness | fulfilled | fulfilled | absent | NMO | mycophenolate | 29 |
| 22 | 44 | C(2), L(1), L(2) | Limb weakness* | fulfilled | fulfilled | absent | PPMS | ocrelizumab | 88 |
| 23 | 51 | L(1), I(4) | Limb numbness* | fulfilled | not fulfilled | absent | PPMS | none ** | 59 |
| 24 | 46 | I(1) | Visual impairment | fulfilled | fulfilled | present | RRMS | ocrelizumab | 80 |
| 25 | 48 | C(2), L(2) | Limb numbness | fulfilled | fulfilled | present | SLE | mycophenolate | 25 |
| 26 | 55 | C(6), L(2), I(1), I(3) | Limb weakness* | fulfilled | fulfilled | present | Susac | cyclophosphamide | 20 |
| 27 | 53 | C(4), L(2), L(3), I(3) | Visual impairment | fulfilled | fulfilled | present | sarcoidosis | azathioprine | 57 |
| 28 | 47 | L(3), L(4), I(1) | Limb weakness | fulfilled | fulfilled | present | RRMS | ocrelizumab | 40 |
| 29 | 51 | L(1), I(1) | Vertigo | fulfilled | fulfilled | absent | migraine | none ** | 25 |
| 30 | 74 | C(1), L(2), L(3), I(3) | Limb numbness | fulfilled | fulfilled | present | sarcoidosis | methotrexate | 12 |
| 31 | 61 | C(1), I(1) | Limb numbness | fulfilled | not fulfilled | ND | migraine | none ** | 23 |
| 32 | 39 | C(3) | Limb numbness | fulfilled | fulfilled | present | RRMS | ocrelizumab | 100 |
| 33 | 39 | C(2), L(2) | Limb numbness | fulfilled | fulfilled | present | SLE | azathioprine | 0 |
| 34 | 46 | C(7), I(1) | Vertigo | fulfilled | fulfilled | present | RRMS | alemtuzumab | 71 |
| 35 | 38 | C(3) | Limb numbness | fulfilled | fulfilled | present | RRMS | teriflunomide | 100 |
| 36 | 19 | I(2) | Limb numbness | fulfilled | fulfilled | present | RRMS | fingolimod | 83 |
| 37 | 23 | I(1) | Ataxia | fulfilled | fulfilled | present | RRMS | interferon beta-1a | 100 |
| 38 | 44 | C(5), L(2) | Visual impairment | fulfilled | fulfilled | present | RRMS | none | 100 |
| 39 | 51 | L(1), I(4) | Limb weakness* | fulfilled | not fulfilled | absent | PPMS | NA | 78 |
Abbreviations: Clinical onset: neurological symptoms at initial presentation; DIS: dissemination in space; DIT: dissemination in time; OCB: CSF-specific oligoclonal bands; % Perivenular: frequency of perivenular lesions; Treatment: immunomodulatory/immunosuppressive treatment; NA: not available; ND: not done; RRMS: relapsing remitting MS; PPMS: primary progressive MS; SPG4 HSP: hereditary spastic paraplegia; NMO: neuromyelitis optica; SLE: systemic lupus erythematosus; Susac: Susac syndrome.
progressive course at onset;
not immunomodulatory/immunosuppressive treatment.
Fulfillment of DIS and DIT 2017 McDonald revised criteria for MS
The most common clinical presentations were visual impairment (12 of 39 patients, 31%), followed by limb weakness (11 of 39 patients, 28%) or numbness (9 of 39 patients, 23%; Table 2). All patients fulfilled the MRI criteria for DIS, and 32 of 39 (82%) the MRI criteria for DIT.2 CSF-specific OCB were detected in 22 of the 36 patients tested (Table 3). When taking into account CSF results, 34 of 39 patients (87%) fulfilled the 2017 MS diagnostic criteria for both DIS and DIT in the context of a clinical presentation compatible with inflammatory demyelination (Table 3);2
Clinical diagnosis
The median follow-up period between the FLAIR* MRI scan and the eventual clinical diagnosis was 3 months (range 1–7 months). Clinical diagnosis did not change after a median post-diagnosis follow-up period of 13 months (range 7–31 months). MS was diagnosed in 27 patients, 2 of whom were strongly suspected of having primary progressive MS (PPMS) even if they did not fulfill the most recent criteria for primary progressive MS,2 (patients ID 23 and 39; Table 3). The remaining 12 patients received an alternative diagnosis: systemic lupus erythematosus (SLE)21 (n=3), sarcoidosis22 (n=2), migraine (n=3), Sjögren disease23 (n=1), SPG4-spastic-paraparesis24 (n=1), AQP4-antibody positive neuromyelitis optica 25 (n=1), and Susac syndrome26 (n=1). Nine of the 12 patients (75%) who eventually received an alternative diagnosis, still fulfilled the 2017 McDonald DIS and DIT criteria (Table 3).2
In 4 patients diagnosed with a systemic inflammatory disorder with involvement of the CNS (patients ID 3, 17, 27, 30), the neurological manifestation was the first manifestation of the disease. Four patients who received a diagnosis of MS had a concomitant systemic inflammatory disorder (“history of SAD” in Table 2) potentially affecting the CNS (patients ID 6, 7, 10, 22). Of note, none of these 4 patients harbored MS atypical clinical, laboratory, or imaging features at the level of the CNS.
Central vein sign assessment and predictive value of the diagnosis
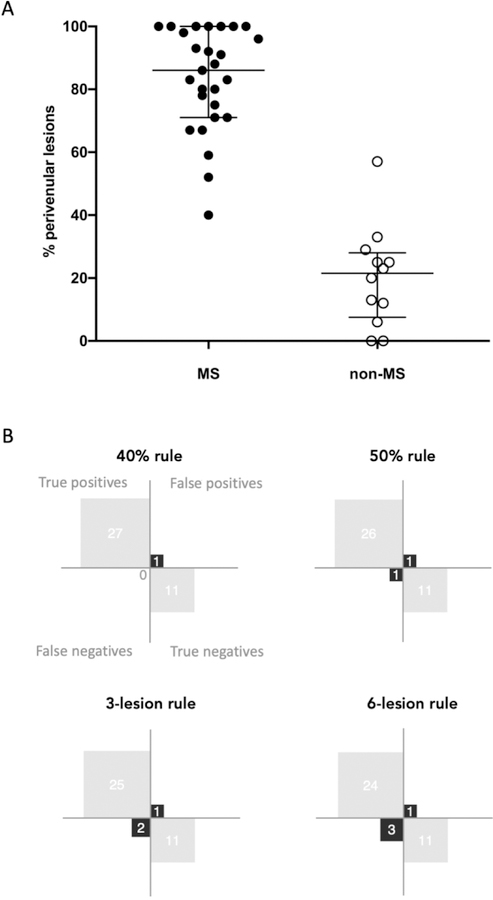
The percentage of perivenular lesions was significantly higher in the 27 patients who received a diagnosis of MS (median=86%, range 40–100%) as compared with the 12 non-MS patients (median=21%, range 0–57%; Mann-Whitney U test, p<0.0001; Figure 2). Representative cases are shown in Figure 3 and 4. The inter-rater agreement for the percentage of perivenular lesions was “substantial/good” with a Cohen’s k of 0.7 and agreement of 85%.
Figure 2. Frequency of perivenular lesions in MS and non-MS patients.
Frequency (median and interquartile range) of perivenular lesions in patients who did (“MS”) and did not (“non-MS”) receive an MS diagnosis (A) and confusion matrices for the differentiation between MS and non-MS based on the different CVS diagnostic tests (B).
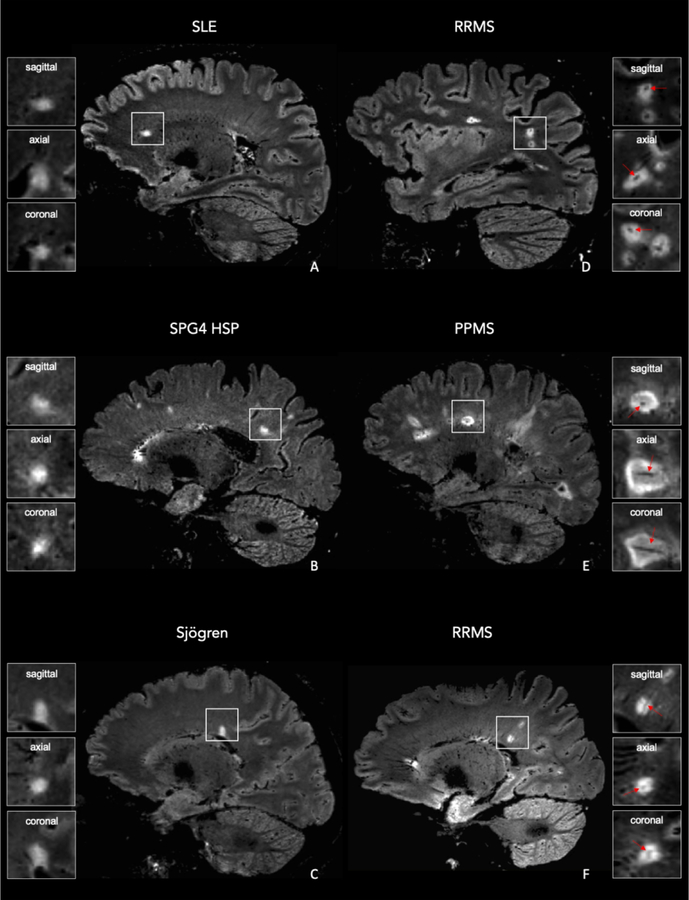
Figure 3. 3D FLAIR* MRI images in individuals who did and did not receive an MS diagnosis.
Representative sagittal FLAIR* images of a woman (A) who received a diagnosis of systemic lupus erythematosus (SLE; subject ID 17), a woman (B) who received a diagnosis of SPG4-spastic-paraparesis (SPG4 HSP; subject ID 8), a woman (C) who received a diagnosis of Sjögren disease (Sjögren; subject ID 3), a man (D) who received a diagnosis of relapsing remitting MS (RRMS; subject ID 19), a woman (E) who received a diagnosis of primary progressive MS (PPMS; subject ID 22), and a man (F) who received a diagnosis of RRMS (subject ID 6). A central vein running through the lesion (arrows) is visible in the majority of MS lesions but is not typical in non-MS lesions.
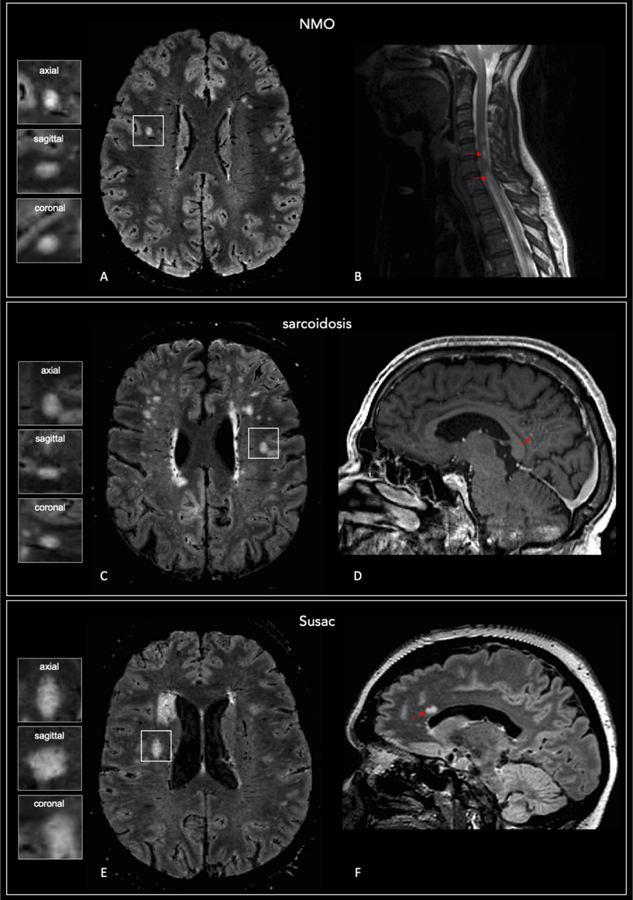
Figure 4. Central vein sign negative lesions in patients who did not receive an MS diagnosis.
Representative axial brain 3D FLAIR* (A) and sagittal spinal cord T2-w (B) images from a subject who received a diagnosis of AQP4-antibody positive neuromyelitis optica (NMO; subject ID 21). Longitudinal extensive transverse myelitis can be seen in the spinal cord image (arrows). Axial 3D FLAIR* (C) and sagittal post-gadolinium MPRAGE (D) images from an individual who received a diagnosis of sarcoidosis (subject ID 30); the arrow shows leptomeningeal enhancement. Axial 3D FLAIR* (E) and sagittal T2-FLAIR images in a subject who received a diagnosis of Susac syndrome (Susac; patient ID 26). Callosal “snowball-shaped” T2 hyperintense lesions (arrow). A central vein running through the lesion is not visible in the majority of white matter lesions in these cases (magnified boxes).
When patients were dichotomized based on the 40% rule (presence of ≥40% perivenular lesions), all MS patients were perivenular positive except for only one non-MS patient (patient ID 27). This patient fulfilled the McDonald 2017 DIS and DIT MRI criteria, had CSF-specific oligoclonal bands, but presented a history of severe optic neuritis with poor visual recovery despite steroids, elevated abnormal proteinorrachia, and leptomeningeal enhancement on brain MRI. The biopsy of a hilar adenopathy confirmed the diagnosis of systemic sarcoidosis with CNS involvement (of note, the neurological manifestation was the first manifestation of the SAD).
When the 50% perivenular rule was applied (presence of ≥50% perivenular lesions), 26 of the 27 MS patients were perivenular positive except for one non-MS patient (patient ID 27, see above). The only MS patient who had less than 50% perivenular lesions (patient ID 28) fulfilled the diagnostic criteria for MS but had a history of IgM-positive Borrelia burgdoferi serology (on two repeated samples) and a Balò-like WM lesion;27 after an extensive work-up, he received a diagnosis of relapsing remitting MS (RRMS). The 40% rule performed slightly better than the 50% rule, with a diagnostic sensitivity of 100%, specificity of 92%, accuracy of 97%, positive and negative predictive value of 96% and 100%, respectively.
Among the simplified CVS lesion algorithms, the 3-lesion rule performed better than the 6-lesion rule, with a diagnostic sensitivity of 93%, specificity of 92%, accuracy of 92%, positive and negative predictive value of 96% and 85%, respectively. Diagnostic test performance is shown in Figure 2. Of note, diagnostic test performance results did not change when patients ID 23 and 39, suspected of PPMS but not fulfilling the McDonald 2017 criteria were excluded from the analysis.
Discussion
The main finding of this prospective multicenter study is that the central vein sign detected on a single 3D FLAIR* MRI scan at clinical 3T field strength can accurately predict a diagnosis of MS in patients with atypical clinical, laboratory, or imaging features for the disease. In particular, we found that using a 40% perivenular lesion threshold, the CVS could predict an MS diagnosis with 97% accuracy and 96% positive/100% negative predictive value. Most existing studies focusing on the clinical value of the CVS for the differential diagnosis of MS included patients with an already known clinical diagnosis.6,8–12 One prior study investigated the diagnostic predictive value of the CVS in patients with possible MS, showing promising results.28 However, this pilot study was done on a research 7T MRI scanner, and not all included patients experienced a neurological syndrome suggestive of MS. Moreover, the presence of specific clinical, laboratory, or imaging features for MS was not an inclusion criterion, and patients who did not receive an MS diagnosis mostly had non-inflammatory diseases of the CNS, such as small vessel disease or migraine. Our prospective study was specifically designed to demonstrate the value of the CVS in the routine work-up of atypical cases and strongly suggests that the CVS could be an imaging biomarker for MS and could be used in routine practice to help neurologists in diagnostically challenging cases.
Our results are particularly relevant considering that the specificity of the current diagnostic imaging criteria for MS is limited,29 and that the prevalence of MS misdiagnosis is high in clinical practice.3 The 2017 McDonald criteria were designed to facilitate an earlier diagnosis in patients presenting with typical clinical, laboratory or imaging features for MS.2 Using these criteria to differentiate MS from other conditions or to diagnose MS in patients harboring red flags may lead to misdiagnosis.15 In our series, 75% of the patients who did not receive an MS diagnosis still fulfilled the most recent DIS and DIT 2017 McDonald criteria, in the context of a clinical presentation compatible with inflammatory demyelination.2 In those cases, additional MRI criteria, such as the frequency of perivenular lesions, are of great value. Indeed, the frequency of perivenular lesions was significantly lower in these patients compared with those who received an MS diagnosis, even though diagnosis was made blinded to the CVS. In a subgroup of our patients who did receive a diagnosis of SAD with secondary CNS involvement, the neurological manifestation was the first manifestation of the disease, making it hard to differentiate such conditions from MS. Even in this challenging clinical scenario, the CVS was able to correctly predict the non-MS diagnosis in most (3 of 4) patients. The only one case where the CVS failed to predict the non-MS diagnosis presented clinical, imaging and laboratory features compatible with CNS inflammatory demyelination and fulfilled the DIS and DIT diagnostic criteria for MS.30 However, the patient harbored significant MS-atypical features at the level of the CNS, and finally received a diagnosis of systemic sarcoidosis with secondary CNS involvement. In the subgroup of patients with a progressive clinical course from onset suggestive of PPMS but who do not fulfill the McDonald 2017 criteria for PPMS2, appropriate diagnosis is also challenging. In this context, after exclusion of all other possible diagnosis, MS experts eventually considered a diagnosis of PPMS in two patients. Interestingly in both cases the CVS assessment was also suggestive of an MS diagnosis.
Regarding the available existing criteria for perivenular assessment, a 40% perivenular lesion cut-off10 and a simplified 3 lesion algorithm9 best differentiated MS from non-MS, and our prospective results are in line with those of a recent large retrospective multicenter study.31
This study presents some limitations. Despite the multicenter setting of our study, our cohort is rather small, because challenging patients presenting with symptoms suggestive of MS and red flags for the diagnosis are rare. A definitive diagnosis is often hard to achieve, and in the absence of one highly specific biomarker for MS, it depends on MS experts’ opinion. Even though we already reported a significant median post-diagnosis follow-up of 13 months, further follow-up of this study cohort will be required to corroborate the eventual clinical diagnosis. Another limitation is the lack of a real objective gold standard for clinical diagnosis between centers, although an extensive work-up was carried out in each center, and all centers are highly experienced in the area of neuroimmunological disorders. Four patients who received a diagnosis of MS also had a concomitant SAD potentially affecting the CNS. Although the CVS correctly predicted the clinical MS diagnosis in all cases, to assess whether the CNS disease results at least in part from the coexisting SAD remains impossible without biopsy. (Of note, none of these patients harbored MS-atypical clinical, laboratory, or imaging features at the level of the CNS.) Similarly, only biopsy could reveal whether an inflammatory demyelinating process (typical of MS) was responsible, at least in part, for the observed CNS disease in the single patient with a relatively high proportion of perivenular lesions who finally received a clinical non-MS diagnosis (sarcoidosis).32 Lastly, because our study was not designed to quantify the delay between initial clinical presentation, first MRI scan and FLAIR* MRI scan for CVS assessment, we cannot demonstrate that the FLAIR* scan is able to shorten the delay of MS diagnosis in atypical presentations.
In conclusion, our prospective multicenter study shows that the CVS can accurately predict an MS diagnosis in diagnostically difficult cases using 3T clinical scanners. Multisite availability of an optimized MRI sequence,33 such as FLAIR* imaging, is required to promote the larger multicenter clinical studies needed to confirm the value of introducing this promising imaging biomarker into everyday clinical practice.
Acknowledgments
The authors thank Dr. Camillo Ribi (Division of Immunology and Allergology, Lausanne University Hospital), Dr. Vasiliki Pantazou (Neuroimmunology Unit, Lausanne University Hospital), Dr. Prochore S. Kamgang, Dr. Monica Ferreira (Division of Internal Medicine, Hôpital Brugmann, Brussels, Belgium) and Dr. Raphael Hourez (Neurology Department, Hôpital Brugmann, Brussels, Belgium), Pr. Vincent van Pesch (Neurology Department, Clinique Universitaires Saint Luc, Brussels, Belgium) for their time and help in patient recruitment and management; Géraldine le Goff (MS research nurse, Neuroimmunology Unit, Lausanne University Hospital) for helping with patients clinical and biological data collection; Tobias Kober, Mario Fartaria de Oliveira, and Jonas Richiardi (MRI research lab, Siemens Helthineers, Lausanne) and Julie Absil and Thierry Methens (Hôpital Erasme, Brussels, Belgium) for helping with MRI sequence implementation and image post-processing; Jean-Baptiste Ledoux (TRM coordinator, service de “radiodiagnostic et radiologie interventionnelle”, Lausanne University Hospital) for helping with MRI acquisition; Dr. Vittorio Martinelli (Neurology department, San Raffaele Hospital, Milan, Italy) for patient recruitment and management; Dr. Roberta Scotti and Antonella Iadanza (Neuroradiology department, San Raffaele Hospital Milan, Italy) for helping with MRI images acquisition.
Funding/Support
Dr. Pietro Maggi is supported by the ECTRIMS Clinical Training Fellowship Program and by the University of Lausanne “relève académique” grant.
Dr. Absinta is supported by the National Multiple Sclerosis Society (NMSS) (grant #FG 2093-A-1) and the Conrad N. Hilton Foundation (grant #17313).
Drs. Absinta, Sati, and Reich are supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
Dr. Bernard Dachy reports no funding or support directly relevant to the manuscript.
Role of the Funder/Sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures
Prof. Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Dr. Théaudin has no conflict of interest involving the work under consideration for publication and no relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing what is written in the submitted work. Outside the submitted work, she received speaker honoraria from Merck, BiogenIdec, Genzyme, Roche; fees for advisory boards from Merck, BiogenIdec and Novartis; and travel grants from Merck, BiogenIdec, Genzyme, Roche and Novartis.
All the other authors declare no competing interests.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 3.Solomon AJ, Corboy JR. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat Rev Neurol 2017; 13: 567–572. [DOI] [PubMed] [Google Scholar]
- 4.Geraldes R, Ciccarelli O, Barkhof F, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol 2018; 14: 199–213. [DOI] [PubMed] [Google Scholar]
- 5.on behalf of the NAIMS Cooperative, Sati P, Oh J, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12: 714–722. [DOI] [PubMed] [Google Scholar]
- 6.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies: Central Vein Sign. Ann Neurol 2018; 83: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Absinta M, Nair G, Monaco MCG, et al. The “central vein sign” in inflammatory demyelination: The role of fibrillar collagen type I. Ann Neurol 2019; 85: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon AJ, Schindler MK, Howard DB, et al. ‘Central vessel sign’ on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine 2016; 3: 82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler 2017; 1352458517726383. [DOI] [PMC free article] [PubMed]
- 10.Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011; 76: 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortese R, Magnollay L, Tur C, et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology 2018; 90: e1183–e1190. [DOI] [PubMed] [Google Scholar]
- 12.Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler J 2012; 18: 1592–1599. [DOI] [PubMed] [Google Scholar]
- 13.Miller D, Weinshenker B, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler J 2008; 14: 1157–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toledano M, Weinshenker BG, Solomon AJ. A Clinical Approach to the Differential Diagnosis of Multiple Sclerosis. Curr Neurol Neurosci Rep; 15 Epub ahead of print August 2015. DOI: 10.1007/s11910-015-0576-7. [DOI] [PubMed] [Google Scholar]
- 15.Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: Impact of the 2017 McDonald criteria on clinical practice. Neurology 2019; 92: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of ‘no better explanation’. Lancet Neurol 2006; 5: 841–52. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 2019; 142: 1858–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stangel M, Fredrikson S, Meinl E, et al. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol 2013; 9: 267–276. [DOI] [PubMed] [Google Scholar]
- 19.Sati P, George IC, Shea CD, et al. FLAIR*: A Combined MR Contrast Technique for Visualizing White Matter Lesions and Parenchymal Veins. Radiology 2012; 265: 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mistry N, Abdel-Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler J 2016; 22: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 21.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern BJ, Royal W, Gelfand JM, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol 2018; 75: 1546. [DOI] [PubMed] [Google Scholar]
- 23.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts: ACR/EULAR CLASSIFICATION CRITERIA FOR PRIMARY SS. Arthritis Rheumatol 2017; 69: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depienne C, Brice A, Durr A. Chapter 14 SPG4, the Most Frequent Hereditary Spastic Paraplegia: Clinical and Genetic Aspects. In: Blue Books of Neurology Elsevier, pp. 296–307. [Google Scholar]
- 25.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleffner I, Dörr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry 2016; 87: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 27.Hardy TA, Miller DH. Baló’s concentric sclerosis. Lancet Neurol 2014; 13: 740–746. [DOI] [PubMed] [Google Scholar]
- 28.Mistry N, Dixon J, Tallantyre E, et al. Central Veins in Brain Lesions Visualized With High-Field Magnetic Resonance Imaging: A Pathologically Specific Diagnostic Biomarker for Inflammatory Demyelination in the Brain. JAMA Neurol 2013; 70: 623. [DOI] [PubMed] [Google Scholar]
- 29.van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, et al. Application of the 2017 Revised McDonald Criteria for Multiple Sclerosis to Patients With a Typical Clinically Isolated Syndrome. JAMA Neurol 2018; 75: 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The central vein sign on cerebral 3T MRI in multiple sclerosis - a multi-center investigation under the auspices of the MAGNIMS study group. In: Free Communications 1 - New diagnostic criteria 138 Presented at the 24th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (2018), pp. 8–120. [Google Scholar]
- 32.Tyshkov C, Pawate S, Bradshaw MJ, et al. Multiple sclerosis and sarcoidosis: A case for coexistence. Neurol Clin Pract 2019; 9: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz DL, Tagge I, Powers K, et al. Multisite reliability and repeatability of an advanced brain MRI protocol: Reliability of an Advanced Brain MRI Protocol. J Magn Reson Imaging Epub ahead of print 16 January 2019. DOI: 10.1002/jmri.26652. [DOI] [PMC free article] [PubMed] [Google Scholar]