Abstract
Binge drinking is the most common pattern of excessive alcohol consumption and is a significant contributor to the development of Alcohol Use Disorder and dependence. Previous studies demonstrated involvement of kappa opioid receptors (KOR) in binge-like drinking in mice using the Drinking-in-the-Dark model. The current studies examined the role of KOR specifically in the bed nucleus of the stria terminals (BNST) in binge-like alcohol consumption in male and female mice. Direct administration of the long lasting KOR antagonist, nor-BNI, into the BNST decreased binge-like alcohol consumption and blood alcohol concentrations in male and female C57BL/6J mice. Similarly, direct nor-BNI administration into the BNST modestly reduced sucrose consumption and the suppression of fluid intake was not related to reduced locomotor activity. To further determine the role of KOR within the BNST on binge-like alcohol consumption, the KOR agonist U50,488 was administered systemically which resulted in a robust increase in alcohol intake. Microinjection of nor-BNI into the BNST blocked the high level of alcohol intake after systemic U50,488 challenge reducing intake and resultant blood alcohol concentrations. Together, these data suggest that KOR activity in the BNST contributes to binge-like alcohol consumption in both male and female mice.
Keywords: Binge Alcohol Drinking; Kappa Opioid Receptor; Dynorphin; Nor-BNI; U50,488; BNST
1. Introduction
Binge drinking significantly contributes to the enormous economic, societal, and individual burden of alcohol abuse (Sacks et al., 2015). Defined as the consumption of 4 drinks for women or 5 drinks for men in about two hours, binge drinking results in a rapid elevation in blood alcohol concentration (BAC) above the 80 mg/dL legal limit of intoxication (Alcoholism, 2004; Centers for Disease and Prevention, 2012). Binge drinking is not only the most common pattern of excessive drinking, but the high level of intoxication and resultant BACs achieved positively correlates with the development of Alcohol Use Disorder (AUD) (Addolorato et al., 2018; Jennison, 2004; McCarty et al., 2004). In preclinical models of AUD, repeated bouts of alcohol exposure that yield BACs above 80 mg/dL have been shown to produce negative affective-like behaviors and perpetuation of excessive alcohol consumption in mice, rats, and non-human primates (Baker et al., 2014; Lee et al., 2015; Lopez and Becker, 2005). Given that alcohol consumption in a binge-like manner represents a risk factor for developing AUD, it is important to understand the neural mechanisms underlying this pattern of drinking in order to develop effective strategies for tempering such risky behavior. Recent attention has focused on the dynorphin/kappa opioid receptor (DYN/KOR) system in mediating excessive drinking associated with alcohol dependence as well as binge patterns of consumption (Anderson and Becker, 2017; Koob and Le Moal, 2008; Walker and Koob, 2008).
Dynorphin, an opioid peptide derived from the precursor prodynorphin, acts as the endogenous ligand for KOR (Chavkin et al., 1982; Mansour et al., 1995). KORs are Gi-coupled receptors expressed in abundance throughout the cortex, striatum, and extended amygdala and generally inhibit neuronal activity (Al-Hasani et al., 2015; Chavkin et al., 1982). Signaling through the DYN/KOR system has been shown to play a role in motivational behaviors involving reward, aversion, and stress, and the DYN/KOR system has been implicated in the regulation of alcohol consumption (Al-Hasani et al., 2015; Anderson and Becker, 2017; Bruchas et al., 2010). Activation of the DYN/KOR system following chronic alcohol exposure has been associated with behaviors reflective of a negative affective state experienced during alcohol withdrawal, and this has been suggested to increase relapse vulnerability as well as promote excessive levels of drinking (Karkhanis et al., 2017; Sirohi et al., 2012). In fact, KOR antagonists have garnered much interest as a potential therapeutic intervention for the treatment of AUD (Karkhanis et al., 2017). In support of this idea, studies have shown that systemic administration of the KOR antagonist, nor-BNI, attenuates dependence-related escalation of alcohol consumption and alleviates withdrawal symptomology in rats (Walker et al., 2011). These effects appear to be mediated by blockade of KORs in the extended amygdala as direct injection of nor-BNI into the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), or nucleus accumbens shell (NAcShell) reduced elevated drinking and anxiety-like behavior in alcohol dependent animals (Erikson et al., 2018; Kissler et al., 2014; Rose et al., 2016).
While these findings strongly implicate a role for the DYN/KOR system within the extended amygdala in alcohol dependence, recent findings suggest a more general role for KORs in the regulation of alcohol consumption in non-dependent animals. For example, mice lacking prodynorphin or KORs show decreased alcohol drinking and preference (Blednov et al., 2006; Kovacs et al., 2005; Van’t Veer et al., 2016). However, few studies have probed the role of KORs within the context of binge drinking. The Drinking-in-the-Dark (DID) paradigm models binge drinking in rodents by producing high levels of alcohol consumption within a relatively short period of time such that subjects reliably achieve BACs above the 80 mg/dL threshold of intoxication (Rhodes et al., 2005; Thiele et al., 2014; Thiele and Navarro, 2014). We previously demonstrated that systemic administration of a KOR agonist increased, while a KOR antagonist decreased, binge-like alcohol consumption in male C57BL/6J mice (Anderson et al., 2018a).
Since the BNST is rich in KORs and sensitive to alcohol (Burnham and Thiele, 2017; Poulin et al., 2009), the present study examined whether manipulation of KORs in the BNST influence binge-like alcohol consumption using the DID model. Here we show that blockade of KORs in the BNST via direct injection of a KOR antagonist significantly reduced binge-like alcohol consumption and blocked the ability of a systemically-administered KOR agonist to elevate binge-drinking in male and female mice.
2. Methods and Materials
2.1. Animals
Male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) ranging from 10–14 weeks old were singly housed and tested in a temperature and humidity controlled AAALAC approved facility on a reverse 12-hr light/dark cycle with food and water continuously available. For all experiments, mice were treated in accordance with both the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and the Institutional Animal Care and Use Committee at MUSC.
2.2. Surgical Procedures
Mice were anaesthetized with isoflurane and bilateral guide cannula (Plastics One, Inc.) were positioned above the BNST (AP: +0.8, ML: +/− 0.75, DV: −3.6) and secured to the skull with a light-cured resin system (Haun et al., 2018). Once inserted, microinjector tips extended 1 mm beyond the guide to target the BNST for microinjection. After surgery, all mice were given 2 weeks to recover prior to the start of experiments.
2.3. Alcohol Binge Drinking Procedure
After recovery from surgery, mice were habituated to handling and injection procedures by administering daily intraperitoneal (ip.) injections of vehicle for several days before alcohol access and then at 30 minutes prior to drinking sessions. Mice were trained to drink alcohol in their home cage in the limited access “Drinking-in-the-Dark” (DID) procedure, as previously described (Anderson et al., 2018a). A single bottle of alcohol (20% v/v) was presented 3 hours into the dark cycle in place of the water bottle. Access to alcohol was for 2 hours on 3 consecutive days, and then extended for 4 hours on the 4th day. Alcohol intake was determined for each 2-hour session and then for the 0–2 and 2–4 hour time periods during the final (4th day) 4-hour drinking session. An identical procedure was used for assessing sucrose (0.5% w/v) consumption. All mice were given one 4-day cycle of binge drinking, 3 days rest in the home cage, followed by a second 4-day binge cycle. For all experiments, drug challenges occurred during the second binge cycle, prior to the 4-hour access (test) session. Average drinking across the first 3 days of the second binge cycle were used to separate subjects evenly into drug treatment groups. Immediately after the 4-hour test drinking session, blood samples were collected, plasma extracted, and blood alcohol concentrations determined using an AM1 Alcohol Analyzer (Analox Instruments, Stourbridge, UK). Separate, experimentally-naïve groups of mice were used in each experiment to ensure that a history of prior alcohol or sucrose consumption did not influence drinking or locomotor activity.
2.4. Locomotor Activity Test
Activity chambers (ENV-510; Med Associates) were used to assess locomotor activity as previously described (May et al., 2015). Briefly, the open field arenas measured 27.5 cm wide × 27.5 cm long × 20.5 cm deep. Mice were placed into the locomotor activity apparatus 16-hours after microinjection and distance traveled (cm) was measured in 1 min bins for 10 minutes. Additionally, cumulative time spent in the center (10 cm) of the open field arenas was collected to assess possible pharmacological effects on anxiety-like behavior.
2.5. Drugs
The KOR antagonist nor-Binaltorphimine dihydrochloride (nor-BNI; 2.5 μg/side, Tocris) was dissolved in 1xPBS for microinjection. The KOR agonist U50,488 (5 mg/kg; Tocris) was dissolved in 0.9% saline. Doses of nor-BNI and U50,488 were based on previous studies (Anderson et al., 2018a).
2.6. Microinjection Procedures
Microinjections were administered 16 hours before Day 4 of drinking during the second binge cycle as described above. Vehicle or nor-BNI (2.5 μg/side) was delivered bilaterally into the BNST at 0.25 μL/min for 2-min, followed by a 2-min diffusion period before microinjector removal (Anderson et al., 2018a; Griffin et al., 2014; Haun et al., 2018). Obdurators were replaced and mice returned to their home cage where they remained undisturbed until the following day. For Experiment 5, saline or U50,488 (5 mg/kg) was administered via intraperitoneal (ip) injection (10 ml/kg) 30-min prior to the 4-hour test binge session.
2.7. Histology
At the conclusion of all experiments, mice were euthanized with urethane and transcardially perfused with 10 mL saline followed by 10 mL of paraformaldehyde (PFA; 4%). Brains were extracted, post fixed in 4% PFA for 24 hours and cryoprotected in sucrose (30% wt/vol) until sectioning. Tissue was sliced in serial 40 μM sections and mounted on Permafrost slides. The tissue was then dehydrated in alcohol and stained with Cresyl Violet for histological verification of microinjector placement within the BNST, as previously described (Haun et al., 2018). Only mice with verified bilateral placements in the BNST in reference to a mouse stereotaxic atlas were included in the final analyses (Franklin and Paxinos, 2008).
2.8. Statistical Analysis
The primary dependent variables were alcohol intake (g/kg), BEC (mg/dL), sucrose intake (mL/kg), and distance traveled (cm). All data were analyzed by ANOVA, with Time as a repeated factor as necessary. Significant factor interactions were further evaluated using the Student-Newman–Keuls (SNK) for post-hoc comparisons. Alpha was set to p< 0.05 for all analyses.
3. Results
3.1. Experiment 1: Effect of KOR antagonist microinjection into the BNST on binge-like alcohol consumption.
Male (N= 17) and female (N= 17) mice were split evenly into drug treatment groups based on alcohol intake averaged across the three preceding 2-hour limited-access drinking sessions. During these three sessions, males consumed an average of 2.01 ± 0.11 g/kg and females 3.01 ± 0.10 g/kg alcohol, consistent with other reports indicating female mice consume more alcohol than males (Finn et al., 2005; Rhodes et al., 2005; Sneddon et al., 2019).
Alcohol intake during the test drinking session for the vehicle and KOR antagonist (nor-BNI) treatment groups are summarized in Figure 1. As shown in Figure 1A, females consumed more alcohol than males during the 0–2 and 2–4 hour time periods following vehicle treatment. Further, nor-BNI strongly reduced alcohol consumption in both male and female mice. Although ANOVA did not indicate a significant 3-way interaction (Sex × Drug × Time; F= 0.43), there was a significant Sex × Drug interaction [F(1,30)= 6.53, p< 0.025], as well as main effects of Sex [F(1,30)= 19.06, p< 0.001] and Drug [F(1,30)= 67.50, p< 0.001]. Subsequent post-hoc analyses showed that nor-BNI significantly reduced alcohol intake in both male in female mice during both time periods (* p< 0.001). Also, vehicle-treated females consumed significantly more alcohol than vehicle-treated males (^ p< 0.001). Lastly, both sexes generally consumed more alcohol during the first 2-hour time period compared to the second 2-hour time period, which was supported by a main effect of Time [F(1,30)= 10.34, p< 0.01].
Fig. 1:
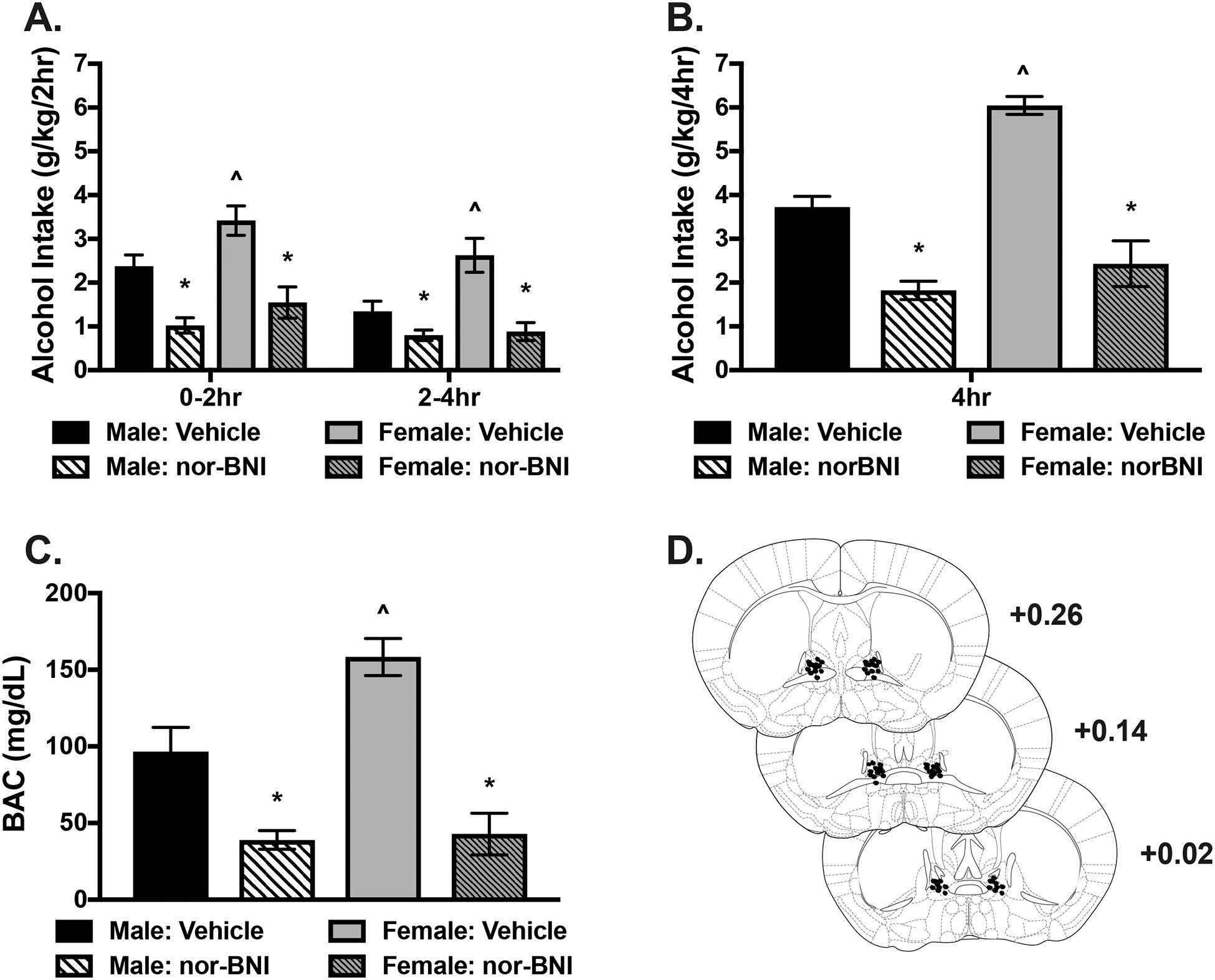
KOR antagonist infusion into the BNST reduces binge-like alcohol consumption. Male and female mice received a bilateral microinjection of vehicle or the KOR antagonist, nor-BNI (2.5 μg/side), 16 hours before a binge drinking session. A) Alcohol intake (g/kg) during the consecutive 2-hour time periods (0–2 and 2–4 hour) in the 4-hour access session. Females consumed more alcohol than males receiving vehicle across both timepoints (^ p< 0.001). Nor-BNI significantly reduced alcohol intake in males and females (*ps<0.001) relative to vehicle but drinking did not differ between sexes treated with nor-BNI at either time point. B) Cumulative alcohol intake across the 4-hour session. When treated with vehicle, females consumed more alcohol than males (^ p< 0.001). Nor-BNI reduced alcohol intake in both males and females compared to their respective vehicle (* ps< 0.001). However, no difference was observed between males and females receiving nor-BNI. C) Blood alcohol concentration (BAC; mg/dL) was assessed at the end of the 4-hour session. Vehicle treated females had greater BACs than males receiving vehicle (^ p< 0.001). BACs were significantly reduced in both male and females compared to their respective vehicle groups (* ps< 0.001) but no difference was observed between males and females treated with nor-BNI. D) Representative microinjector tip placements within the BNST.
Analysis of alcohol intake during the entire 4-hour test session showed that intra-BNST injection of nor-BNI significantly reduced alcohol consumption (Figure 1B). This was supported by a significant Sex × Drug interaction [F(1,30)= 6.53, p< 0.025], as well as main effects of Drug [F(1,30)= 67.50, p< 0.001] and Sex [F(1,30)= 19.06, p< 0.001]. Post-hoc analyses showed that nor-BNI decreased alcohol intake in both sexes compared to vehicle (* p< 0.001), and female mice consumed significantly more alcohol than males following vehicle treatment (^ p< 0.001).
At the conclusion of the 4-hour test drinking session, blood samples were collected and blood alcohol concentrations (BACs) were assessed. Consistent with the alcohol intake data, BACs were significantly lower in both sexes after nor-BNI treatment and, under vehicle conditions, the higher alcohol intake in females resulted in significantly higher BACs compared to males (Figure 1C). This was supported by 2-way ANOVA, which revealed a Sex × Drug interaction [F(1,30)= 5.54, p< 0.025] as well as main effects of Drug [F(1,30)= 49.64, p< 0.001] and Sex [F(1,30)= 7.15, p< 0.02]. Post hoc analyses revealed significantly reduced BACs in male and female mice as a result of nor-BNI treatment compared to vehicle (* p< 0.001) and higher average BACs in females compared to males receiving vehicle (^ p< 0.001). Finally, a schematic representation of microinjector guide placements within the BNST is represented in Figure 1D.
3.2. Experiment 2: Effect of KOR antagonist microinjection into the BNST on binge-like sucrose consumption.
Male (N= 16) and female (N= 16) mice were split into treatment groups based on average intake across the preceding three days of 2-hour sucrose drinking. Males consumed an average of 36.94 ± 1.97 mL/kg and females consumed 47.85 ± 2.22 mL/kg during these three days prior to drug challenge. During the test session, female mice generally consumed more sucrose than males and nor-BNI treatment produced a modest reduction in intake in both sexes (Figure 2A). ANOVA indicated main effects of Drug [F(1,28)= 9.29, * p< 0.005] and Sex [F(1,28)= 10.47, ^ p< 0.01], but the Dug × Sex and Drug × Sex × Time interaction terms did not achieve statistical significance (Fs< 2.2). Likewise, analysis of sucrose intake during the entire 4-hour test session indicated that nor-BNI reduced sucrose consumption in both sexes and females generally consumed more sucrose than males (Figure 2B). This was supported by significant main effects of Drug [F(1,28)= 9.29, * p< 0.01] and Sex [F(1,28)= 10.47, ^ p< 0.01]. BNST microinjector placements for mice in this study are represented in Figure 2C.
Fig. 2:
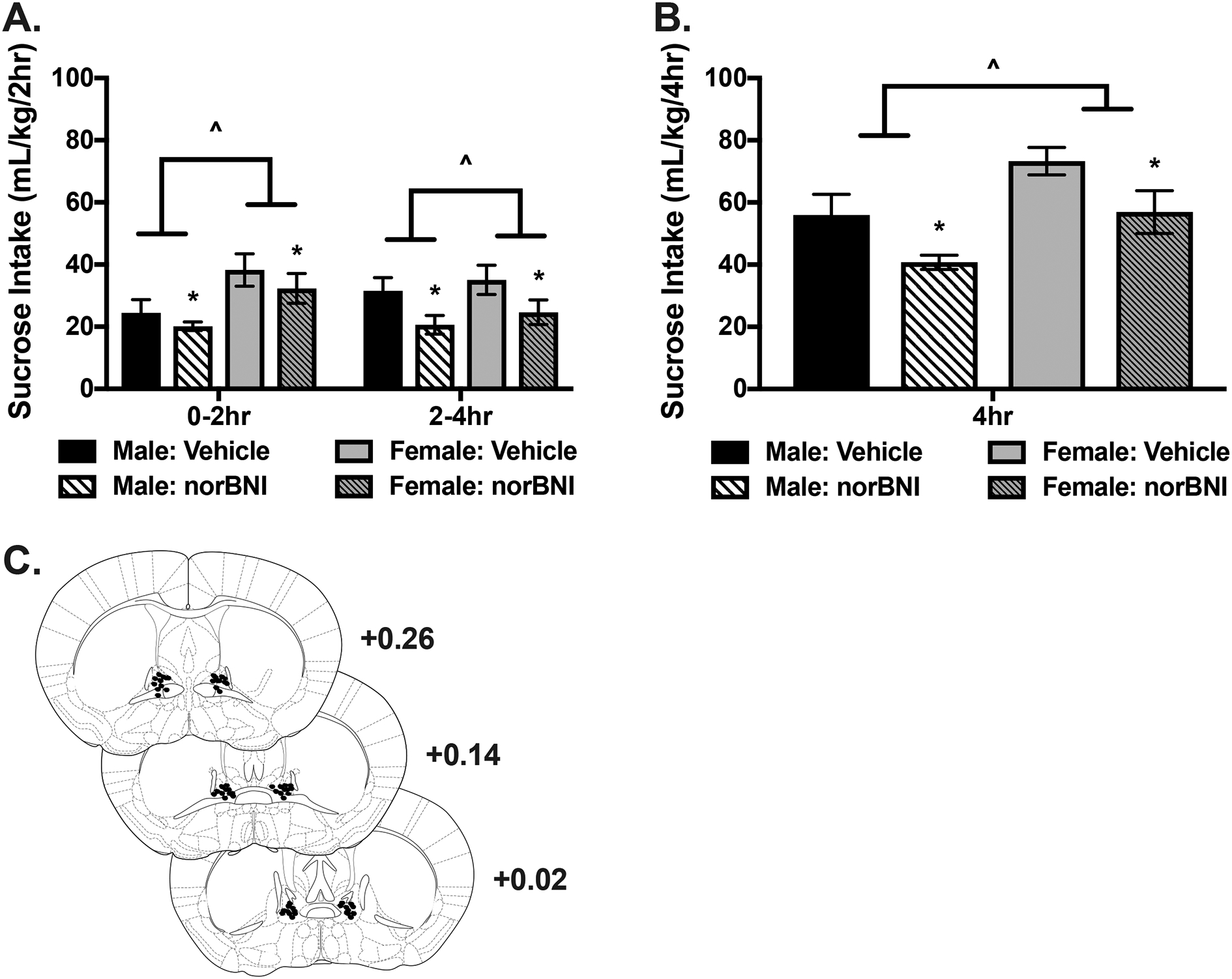
KOR antagonist infusion into the BNST reduces binge-like sucrose consumption. A) Sucrose intake (mL/kg) during the consecutive 2-hour time periods (0–2 and 2–4 hour) in the 4-hour access session. Females consumed more sucrose than males (^ p< 0.01) and infusion of nor-BNI into the BNST resulted in a general suppression of sucrose intake (* p= 0.005). B) Cumulative sucrose intake across the 4-hour session. Sucrose intake was greater in females (^ p< 0.01) while intake was lower in mice treated with nor-BNI compared to vehicle across sexes (* p= 0.005). C) Representative microinjector tip placements within the BNST.
3.3. Experiment 3: Comparison of nor-BNI’s effects in the BNST on alcohol and sucrose consumption.
Because direct administration of nor-BNI into the BNST decreased consumption of both alcohol (Figure 1) and sucrose (Figure 2), the effect size was compared by expressing data from these 2 experiments as the percent change from vehicle (mL/kg). As can be seen in Figure 3, intra-BNST nor-BNI injection reduced alcohol consumption to a greater extent than sucrose. This was supported by ANOVA, which indicated a significant Solution × Drug interaction [F(1,58)= 8.99, p< 0.005] as well as a main effect of Drug [F(1,58)= 61.45, p< 0.001] and Solution [F(1,58)= 8.50, p< 0.005]. There was no main effect of Sex or interactions with this variable. Post-hoc analyses showed that the percent decrease in intake after nor-BNI was significantly greater for alcohol compared to sucrose (# p< 0.001). This suggests that the overall suppressive effect of local KOR antagonist treatment in the BNST is more pronounced for alcohol compared to sucrose.
Fig 3:
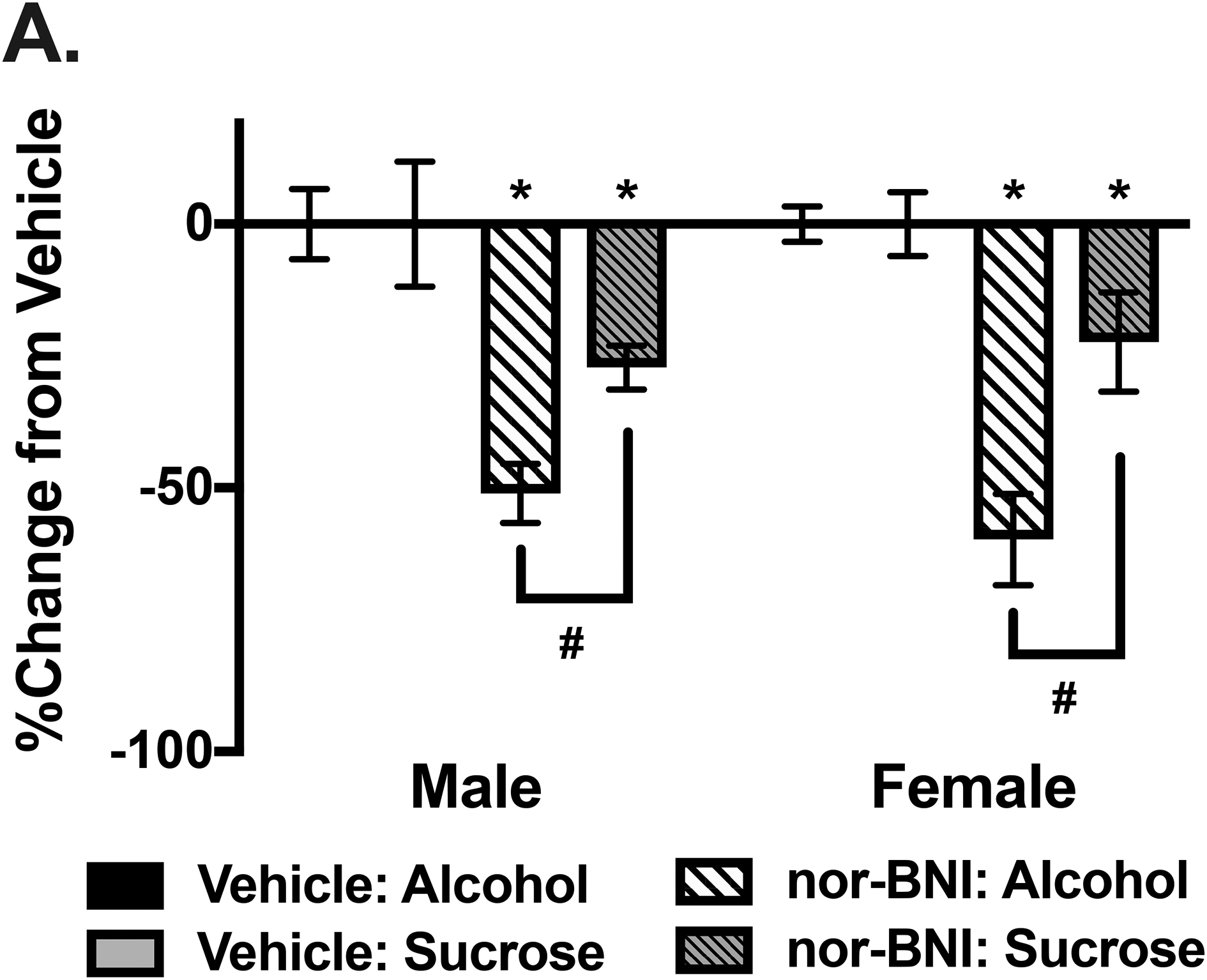
Suppressed intake after KOR antagonist infusion into the BNST is greater for alcohol compared to sucrose. A) Using data collected in Experiments 1 and 2, a percent change from vehicle calculation was made based on 4-hour intake of alcohol or sucrose for each sex. Nor-BNI microinjection into the BNST resulted in a significant decrease compared to vehicle for intake of alcohol and sucrose (* ps< 0.001). However, the percent change from vehicle after nor-BNI treatment was significantly greater in mice consuming alcohol compared to those drinking sucrose (# ps< 0.001).
3.4. Experiment 4: Examination of non-specific locomotor effects of KOR antagonist microinjection in the BNST.
A separate cohort of male (N= 17) and female (N= 16) mice were used to determine if the decrease in alcohol and sucrose consumption following intra-BNST nor-BNI microinjection resulted from non-specific sedative effects by examining locomotor activity in an open-field arena. While females were generally more active than males [F(1,29)= 31.40, p< 0.001], nor-BNI infused into the BNST 16 hours prior to testing did not affect distance traveled in either sex (Figure 4A). This was supported by the fact that ANOVA did not reveal a main effect of Drug (F= 0.03) or an interaction of Drug with Sex or Time variables. This suggests that the suppressive effect of nor-BNI on drinking behavior is not likely related to a general sedative-like effect. Further, analysis of time spent in the center portion of the open field indicated no significant main effects of Sex (F= 0.53) or Drug (F= 0.02). This suggests that KOR blockade within the BNST did not affect anxiety-like behavior under these behavioral testing conditions (Figure 4B). Microinjector placements within the BNST for this study are represented in Figure 4C.
Fig. 4:
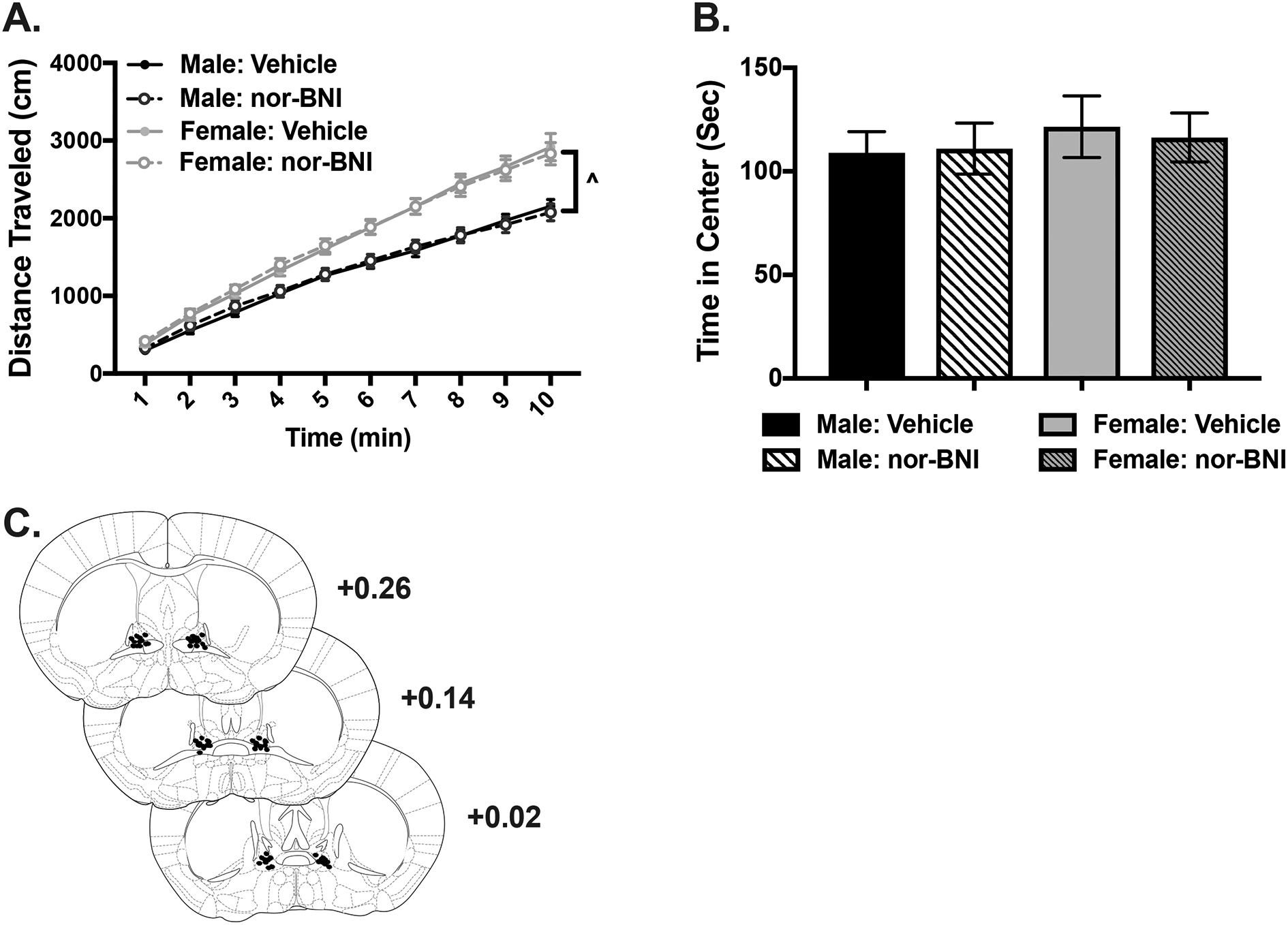
KOR antagonist infusion into the BNST does not affect locomotor activity. A) Cumulative distance traveled (cm) after vehicle or nor-BNI microinjection into the BNST 16 hours before open field testing. Females generally traveled a greater distance than males (^ p< 0.001) but nor-BNI did not affect locomotor activity. B) Cumulative time spent (sec) in center portion of open field arena after vehicle or nor-BNI microinjection into the BNST. C) Representative microinjector tip placements in the BNST.
3.5. Experiment 5: Effect of systemic KOR agonist challenge on binge-like alcohol consumption after microinjection of a KOR antagonist into the BNST.
We previously showed that systemic administration of the KOR agonist, U50,488, increased binge-like alcohol consumption in male mice (Anderson et al., 2019). Experiment 5 was conducted to determine whether KOR within the BNST contribute to U50,488’s ability to increase alcohol intake in male and female mice.
Male (N= 35) and female (N= 34) mice were separated into drug treatment groups based on average intake across the three 2-hour drinking sessions preceding testing on Day 4. During these sessions, males consumed an average of 2.42 ± 0.08 g/kg and females consumed 2.93 ± 0.12g/kg alcohol. Initial analysis indicated that there was no main effect of Sex [F(1,60)= 3.20, p= 0.08] and since Sex did not interact with the other factors, data for the 4-hour test binge session were averaged across males and females. Consistent with our previous report, systemic administration of U50,488 robustly increased binge-like alcohol consumption (Anderson et al., 2018a). Further, microinjection of nor-BNI into the BNST decreased alcohol intake and blocked the ability of U50,488 to increase alcohol consumption in this binge-drinking model (Figure 5A). ANOVA indicated a Systemic Treatment × Microinjection × Time interaction [F(1,60)= 24.52, p< 0.001] and subsequent post hoc analysis showed that U50,488 robustly increased alcohol intake during the first 2 hours of the test binge session relative to vehicle (* p< 0.001). In contrast, alcohol consumption was lower in mice treated with U50,488 compared to vehicle (* p< 0.001) during the latter half (2–4 hour) of the binge session, an effect likely due to a ceiling effect reached in the first 2 hours of drinking. As observed in Experiment 1, nor-BNI microinjection into the BNST significantly decreased alcohol intake compared to vehicle during both time periods of the binge session (* p< 0.001). Further, blocking KOR in the BNST via direct injection of nor-BNI completely blocked increased drinking following systemic administration of the KOR agonist U50,488 during the first 2 hours of the session (+ p< 0.001).
Fig. 5:
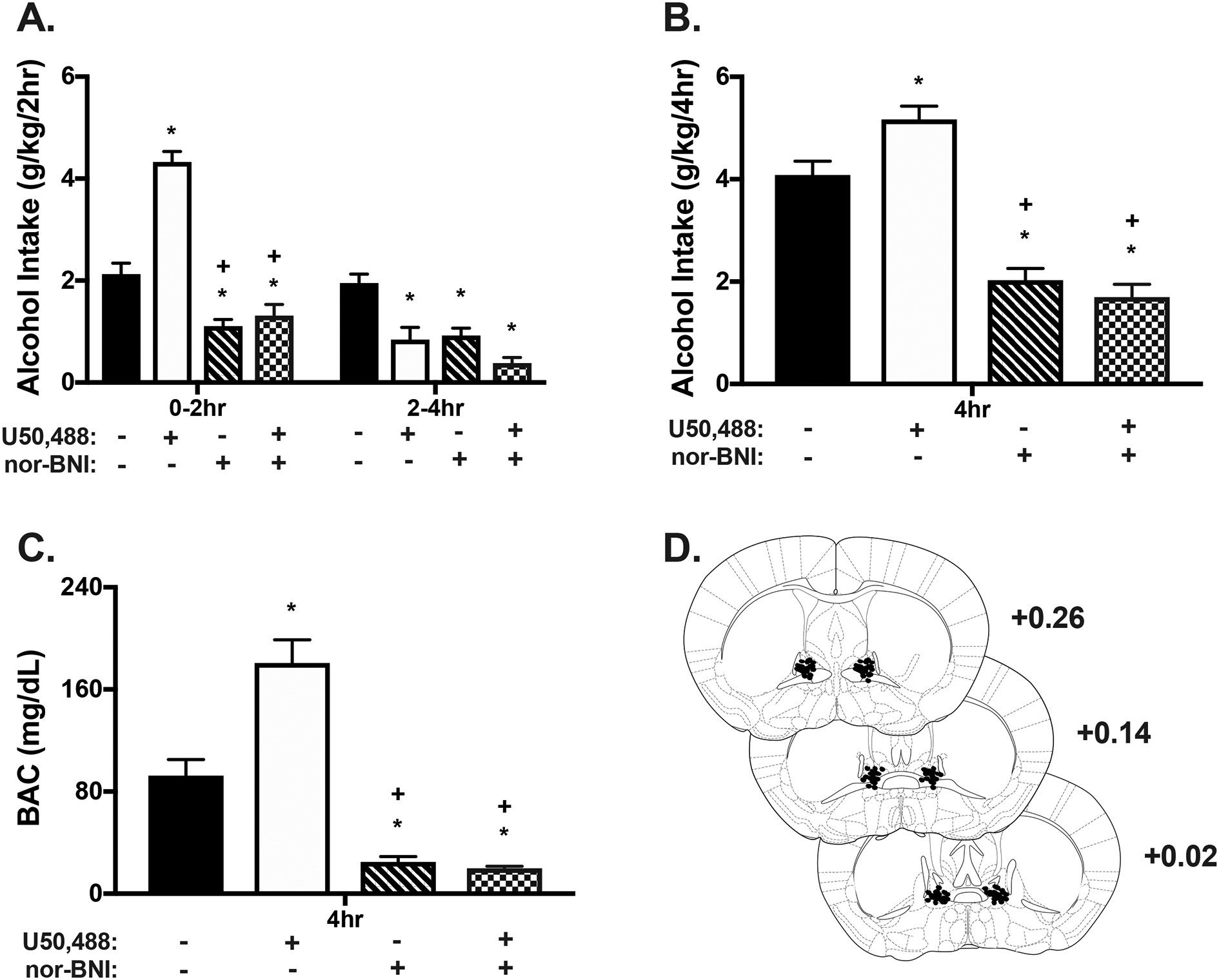
KOR antagonist infusion into the BNST blocks elevated alcohol consumption after systemic administration of a KOR agonist. Nor-BNI was microinjected into the BNST 16 hour prior to the 4-hour drinking session and the KOR agonist, U50,488 (5 mg/kg; ip.) was administered 30 min prior to drinking. Alcohol drinking data are collapsed across sex given a nonsignificant main effect (p= 0.079). A) Systemic U50,488 treatment resulted in a significant increase in alcohol intake during the first 2 hours of drinking compared to vehicle (*p<0.001). However, a ceiling effect was likely reached given that intake was lower in the 2–4 hour period (* p< 0.001). Microinjection of nor-BNI into the BNST resulted in significantly lower alcohol consumption compared to vehicle (* p< 0.001) and U50,488 (+ p< 0.001) during the first 2 hours. In mice receiving nor-BNI infusion into the BNST combined with systemic U50,488, alcohol intake was reduced compared to vehicle (* p< 0.001) and U50,488 alone (+ p< 0.001) during the first 2 hours. Drinking was also lower in the drug combination group than vehicle during the final 2 hours of testing (*p<0.001). B) A similar pattern of drinking was observed for cumulative intake across the 4-hour session. U50,488 (ip.) resulted in a significant increase in alcohol intake (* p< 0.01) while nor-BNI microinjection in the BNST decreased intake compared to vehicle (*<0.001). Intake was significantly less than both vehicle and U50,488 in mice receiving the combined U50,488 and nor-BNI (* p< 0.001; + p< 0.001). C) BAC was assessed at the end of the 4-hour drinking session. Systemic U50,488 resulted in significantly greater BACs compared to vehicle while mice treated with nor-BNI microinjection into the BNST or combined nor-BNI and U50,488 (ip.) had lower BACs relative to vehicle (* ps< 0.001). Furthermore, mice receiving nor-BNI in the BNST or nor-BNI and U50,488 had lower BACs compared to U50,488 alone (+ps < 0.001). D) Representative microinjector tip placements within the BNST.
Cumulative alcohol intake during the entire 4-hour test session is presented in Figure 5B, also collapsed across Sex. Analysis revealed a significant Systemic Treatment × Microinjection interaction [F(1,60)= 7.33, p= 0.009]. Post hoc tests indicated that systemic administration of U50,488 increased alcohol intake compared to vehicle (* p< 0.01), intra-BNST nor-BNI treatment alone reduced alcohol intake (* p< 0.001), and blocking KOR in the BNST blocked the ability of systemic U50,488 to elevate drinking (+ p< 0.001). Analysis of BACs determined immediately following the test binge-drinking session indicated a significant Systemic Treatment × Microinjection interaction [F(1,60)= 16.46, p< 0.001]. Resultant BACs mirrored cumulative drinking data in that BACs were elevated in mice treated with U50,488, reduced in mice that received nor-BNI in the BNST alone, and lower than U50,488 or vehicle levels in mice that received both systemic U50,488 and intra-BNST injection of nor-BNI (ps< 0.001) (Figure 5C). Representative microinjector placements are depicted in Figure 5D.
4. Discussion
Results from these studies show that KORs in the BNST play a role in regulating binge-like alcohol drinking in male and female mice. Direct administration of the KOR antagonist nor-BNI into the BNST significantly reduced alcohol consumption in the DID model. Further, intra-BNST nor-BNI injection completely blocked the ability of a systemically administered KOR agonist (U50,488) to increase binge-like alcohol consumption in the model. While nor-BNI injection into the BNST also reduced sucrose consumption, the magnitude of this effect was smaller than the suppression of alcohol intake. Collectively, these data are consistent with our earlier report showing that systemic administration of KOR drugs bi-directionally alter alcohol consumption in the DID model (Anderson et al., 2018a) and extend those findings to show that KOR signaling in the BNST is significantly involved in regulating binge-like alcohol consumption.
These findings agree with several reports suggesting that KOR antagonists attenuate excessive alcohol consumption in several rodent models, and the effects are mediated by action within the extended amygdala (Anderson et al., 2018b; Crowley and Kash, 2015; Koob, 2013). For example, nor-BNI was shown to decrease binge-like alcohol intake when administered systemically, as well as via microinjection into the CeA, or as reported here, within the BNST (Anderson et al., 2018a). The CeA is of particular relevance because it expresses dynorphinergic (DYN+) neurons that send dense projections to the BNST (Ahrens et al., 2018; Al-Hasani et al., 2015; Mansour et al., 1994; Marchant et al., 2007; Normandeau et al., 2018). Chemogenetic inhibition of DYN+ neurons in the CeA decreased binge alcohol consumption and, thus, it is possible that reduced activity of KORs within the BNST may mediate this effect (Anderson et al., 2018a). Since there is a high degree of co-expression of DYN and other neuropeptides in the CeA (Kim et al., 2017; McCullough et al., 2018; Pomrenze et al., 2019a), it is possible that targeted chemogenetic inhibition of the CeA-BNST pathway may produce its effects by also altering release of other peptides. For example, DYN is co-expressed with CRF within the CeA and inactivation of the CeA-BNST-CRF+ pathway decreased dependence-related drinking and anxiety-like behavior (de Guglielmo et al., 2019; Marchant et al., 2007; Pomrenze et al., 2019b). Further, increased anxiety-like behavior induced by CeA-BNST excitation is dependent upon KOR signaling within the BNST, suggesting involvement of the CeA-BNST-DYN+ circuit (Ahrens et al., 2018). It is likely that CeA-BNST-DYN+ projections are similarly recruited during binge drinking and influence KOR actions in the BNST. Future studies will need to selectively target the CeA-BNST-DYN+ pathway using the DID model to more directly address this issue. Nevertheless, while it is difficult to rule out the involvement of other neuropeptide systems, results from the present study support the notion that KOR signaling in the BNST contributes to regulating alcohol drinking in the DID binge model.
While KOR blockade within the BNST decreased alcohol consumption in a robust manner (55% reduction), nor-BNI injection into the BNST also produced a modest decrease (25% reduction) in sucrose consumption under the same binge-drinking conditions. Interestingly, systemic administration of nor-BNI did not affect sucrose intake in the DID model, suggesting that KORs in the BNST may play a more prominent role in general consummatory behavior (Anderson et al., 2018a). The DYN/KOR system is known to influence feeding behavior, including consumption of natural (palatable) rewards (Karkhanis et al., 2017; Nogueiras et al., 2012). However, the mechanisms and site of action for these effects are not entirely clear. For example, neither systemic nor intracerebroventricular (ICV) administration of nor-BNI altered sucrose or saccharine intake (Lopez et al., 2011). In another study, ICV infusion of nor-BNI reduced feeding behavior, but targeted blockade of KORs in the BNST did not alter responding for palatable food pellets (Le et al., 2018; Lopez et al., 2011). Thus, it is unclear to what extent pharmacological antagonism of KORs produce general reductions in food intake. Results from the present study suggest that alcohol intake is more sensitive to KOR antagonism, but since only a single dose of nor-BNI was evaluated, future studies are needed to determine whether an alcohol-selective effect reflects a shift to the left in the dose-response function. In addition, since KOR antagonists do not appear to influence water intake (Lindholm et al., 2001; Zhou and Kreek, 2019), reduced binge-drinking in this study is not likely due to a general effect on fluid consumption. Finally, it is possible that intra-BNST nor-BNI reduced alcohol and sucrose consumption in the present study by altering their rewarding effects (Anderson and Becker, 2017; Crowley and Kash, 2015; Karkhanis et al., 2017). However, few studies have examined the role of KOR signaling in the BNST in relation to reward processes.
Heightened anxiety is thought to promote excessive drinking and KOR antagonists have been shown to exert anxiolytic effects (Koob, 2013; Van’t Veer and Carlezon, 2013). More specifically, systemic administration of nor-BNI decreased anxiety-like behavior in open field (OF) and elevated plus-maze (EPM) tests (Knoll et al., 2007; Wittmann et al., 2009). Additionally, KOR antagonists have been shown to produce anti-anxiety effects in novelty suppressed feeding (Carr and Lucki, 2010), fear-potentiated startle (Knoll et al., 2007), and forced swim stress procedures (Mague et al., 2003). Also, nor-BNI administration into the BNST attenuated ultrasonic vocalizations, physiological withdrawal scores, and alcohol intake in rats with a history of alcohol dependence (Erikson et al., 2018). Given the expression of KOR within the BNST, a structure known to be involved in anxiety (Kash et al., 2015), it is reasonable to suspect that nor-BNI administration into the BNST would result in an anxiolytic phenotype in the present studies. However, we observed no difference in the amount of time spent in the center portion of the open field arena between vehicle and nor-BNI treated mice. This is consistent with another report that showed a similar dose of nor-BNI (2 μg/side) microinjected into the BNST of male mice had no effect on center time in the OF nor in time spent in the open arm of an EPM (Ahrens et al., 2018). Thus, it is likely that KOR activity in the BNST mediates anxiety-like behavior, but they may be selectively recruited under specific testing conditions and/or the state of the subjects.
The present studies found, in agreement with others, that female mice generally consume more alcohol than males, resulting in significantly greater BACs (Finn et al., 2005; Rhodes et al., 2005; Sneddon et al., 2019). Females also exhibited greater sucrose intake and higher levels of locomotor activity upon first introduction to an open-field arena, which others have reported (Archer, 1975; Crabbe et al., 1999; Kaur et al., 2012; Tucker et al., 2016). Interestingly, no studies have directly explored sex differences in the effect of KOR antagonists on binge-drinking. We observed a proportionately larger suppression of alcohol intake resulting from nor-BNI microinjection in the BNST for female mice compared to males. This finding points to potential sex differences in the ability of KOR antagonists to modulate alcohol intake and merits further exploration including more thorough testing of nor-BNI dosing. While sex differences were observed in alcohol consumption in Experiment 1, no significant differences were observed in Experiment 5. In the latter case, the observation of females consuming more alcohol than males neared significance (p=0.08), with females consuming roughly 0.5 g/kg more than males. Sex differences in alcohol intake are not consistently observed in different models of excessive alcohol consumption and, in this case, may be due to differences in experimental design between Experiment 1 and 5 (Hilderbrand and Lasek, 2018). Mice in Experiment 1 received a single microinjection into the BNST prior to drinking while mice in Experiment 5 received a microinjection combined with an ip. injection 30-min prior to the test drinking session. The more robust handling procedure may have resulted in slightly lower intake, obscuring potential sex differences in alcohol intake.
In contrast to KOR antagonists, sex differences in response to KOR agonists have been reported but vary by brain region. For example, male guinea pigs exhibit greater U50,488-induced GTPγS activity in the cortex, claustrum, periaqueductal gray, and substantia nigra while females show greater GTPγS activity in the dentate gyrus and hypothalamus (Wang et al., 2011). Females are also less sensitive than males to U50,488-induced responding for intracranial self-stimulation suggesting sex differences in the ability of KOR to mediate motivated behaviors, such as binge-like alcohol consumption (Russell et al., 2014). The BNST is a sexually dimorphic region of particular interest in relation to KOR activation given that after U50,488 administration, expression of the immediate early gene c-Fos is greater in females than males, and sex differences in KOR mRNA expression have been reported (Conway et al., 2019; Russell et al., 2014). In the context of binge drinking, however, our studies indicate no sex differences in the ability of U50,488 to enhance alcohol drinking to a high level, nor in the ability of nor-BNI to counter U50,488’s effects within the BNST. The effect of KOR agonists to increase intake appears to be selective to alcohol under binge conditions given that the same dose of U50,488 (5 mg/kg; ip.) does not affect sucrose intake (Anderson et al., 2018a). Others have similarly reported no change in sucrose consumption after challenge with the KOR agonist, Mesyl Salvinorin B (Zhou et al., 2017). These findings support a role for KOR in the BNST in the motivation to consume alcohol in both males and females.
Taken together, these findings point to a role for KOR within the BNST in regulating excessive alcohol drinking. However, it remains unclear as to how DYN/KOR activity influences drinking in the context of non-dependent binge-like alcohol consumption when the majority of studies suggest efficacy of KOR antagonists only under conditions of alcohol dependence. Interestingly, many neuropeptide systems associated with dependence-related drinking also affect binge-like drinking in non-dependent subjects. For example, antagonists targeting the CRF1 receptor (Funk et al., 2007), orexin1 receptor (Lopez et al., 2016), or KOR (Walker and Koob, 2008) all attenuate dependence-related drinking without affecting more moderate levels of consumption in non-dependent animals. And yet, these systems are also engaged in and promote binge-like drinking prior to the development of dependence. Selective antagonists targeting the CRF1 receptor (Lowery-Gionta et al., 2012), orexin1 receptor (Olney et al., 2015), or KOR (Anderson et al., 2018a) decrease binge-drinking, effectively normalizing intake to a moderate level. Since all of these neuropeptide systems are responsive to stress, they may become engaged under drinking-in-the-dark conditions that engenders high levels of alcohol intake, which presents as a potent stressful event (Anderson et al., 2018b; Koob, 2013; Rivier, 1996; Stephens and Wand, 2012). In the context of binge drinking, the high level of alcohol intake achieved results in a rapid elevation in BAC which may recruit the DYN/KOR system. For example, systemic administration of alcohol at doses generating BACs only in excess of 80 mg/dL results in increased extracellular DYN release within the nucleus accumbens (Marinelli et al., 2006). This increase in DYN occurs during the acute effects of alcohol intoxication suggesting a dynamic response of DYN to alcohol beyond the contribution of DYN/KOR signaling to dysphoria experienced during withdrawal (Chavkin and Koob, 2016). Therefore, KOR antagonists may modulate the stress response to alcohol or anxiety associated with binge drinking in non-dependent mice resulting in a normalization of intake to more moderate levels. This is supported by the fact that nor-BNI has no effect on moderate alcohol consumption in non-dependent subjects when delivered by systemic injection (Walker et al., 2011) or into extended amygdala structures (Erikson et al., 2018; Kissler et al., 2014).
Thus, the DYN/KOR system, along with other stress-related peptides contribute to excessive alcohol consumption, and are further recruited as bouts of binge drinking and intoxication increase in frequency, leading to escalated and uncontrolled drinking observed in dependence. This provides a potential prophylactic window during binge drinking to selectively target these systems early in the trajectory of the addiction cycle to normalize intake such that regular bouts of binging become less frequent, thereby reducing the likelihood of escalation of intake over time.
In conclusion, the present series of studies demonstrate that targeted KOR antagonist treatment within the BNST attenuates binge-like alcohol consumption and normalizes KOR agonistpotentiated drinking in both male and female mice. Future studies aimed at targeting the precise dynorphinergic circuity within the extended amygdala that contributes to activity at KOR within the BNST will shed further light on the role the DYN/KOR system plays in mediating excessive binge-like alcohol drinking.
Highlights.
KOR antagonist in the BNST decreased binge-like alcohol consumption.
KOR antagonist in the BNST modestly reduced binge-like sucrose consumption.
KOR antagonist in the BNST does not affect locomotor activity.
Systemic administration of a KOR agonist increased binge-like alcohol consumption.
Effect of systemic KOR agonist was blocked by KOR antagonist in the BNST.
Acknowledgements
This work was supported by NIH grants P50 AA10716 (HCB), U01 AA014095 (HCB, MFL, WCG, HLH), R01 AA02653 (HCB), U01 AA020929 (HCB), F31 AA027420 (HLH), T32 AA007474 (HLH) and VA grant BX000813 (HCB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Addolorato G, Vassallo GA, Antonelli G, Antonelli M, Tarli C, Mirijello A, Agyei-Nkansah A, Mentella MC, Ferrarese D, Mora V, Barbara M, Maida M, Camma C, Gasbarrini A, Alcohol Related Disease C, 2018. Binge Drinking among adolescents is related to the development of Alcohol Use Disorders: results from a Cross-Sectional Study. Sci Rep 8, 12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens S, Wu MV, Furlan A, Hwang GR, Paik R, Li H, Penzo MA, Tollkuhn J, Li B, 2018. A Central Extended Amygdala Circuit That Modulates Anxiety. J Neurosci 38, 5567–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, 2015. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoholism, N. I. o. A. A. a., 2004. NIAAA council approves definition of binge drinking. NIAAA Newsletter 3.
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP, 2013. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience 249, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, 2017. Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol. Alcohol Clin Exp Res 41, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Griffin WC, Haun HL, Bloodgood DW, Pati D, Boyt KM, Kash TL, Becker HC, 2018a. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Moorman DE, Becker HC, 2018b. Contribution of Dynorphin and Orexin Neuropeptide Systems to the Motivational Effects of Alcohol. Handb Exp Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J, 1975. Rodent sex differences in emotional and related behavior. Behav Biol 14, 451–479. [DOI] [PubMed] [Google Scholar]
- Baker EJ, Farro J, Gonzales S, Helms C, Grant KA, 2014. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res 38, 2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA, 2006. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol 40, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C, 2010. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham NW, Thiele TE, 2017. Voluntary Binge-like Ethanol Consumption Site-specifically Increases c-Fos Immunoexpression in Male C57BL6/J Mice. Neuroscience 367, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I, 2010. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology (Berl) 210, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention, 2012. Vital signs: binge drinking prevalence, frequency, and intensity among adults - United States, 2010. MMWR Morb Mortal Wkly Rep 61, 14–19. [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A, 1982. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215, 413–415. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Koob GF, 2016. Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacology 41, 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SM, Puttick D, Russell S, Potter D, Roitman MF, Chartoff EH, 2019. Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology 146, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC, 1999. Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Kash TL, 2015. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 62, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, Koob GF, Messing RO, George O, 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun 10, 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson CM, Wei G, Walker BM, 2018. Maladaptive behavioral regulation in alcohol dependence: Role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology 140, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC, 2005. A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl) 178, 471–480. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, Third Edition San Diego: Academic Press Third Edition. [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF, 2007. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry 61, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun HL, Griffin WC, Lopez MF, Solomon MG, Mulholland PJ, Woodward JJ, McGinty JF, Ron D, Becker HC, 2018. Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology 140, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW, 2018. Studying Sex Differences in Animal Models of Addiction: An Emphasis on Alcohol-Related Behaviors. ACS Chem Neurosci 9, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison KM, 2004. The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abuse 30, 659–684. [DOI] [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, Jones SR, 2017. Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction. Int Rev Neurobiol 136, 53–88. [DOI] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA, 2015. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells 38, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Li J, Stenzel-Poore MP, Ryabinin AE, 2012. Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin Exp Res 36, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, 2017. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479 e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM, 2014. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA Jr., 2007. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323, 838–845. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2013. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 4, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008. Addiction and the brain antireward system. Annu Rev Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, Simonin F, Kieffer BL, Vadasz C, 2005. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res 29, 730–738. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Tamadon S, Shaham Y, 2018. Role of kappa-Opioid Receptors in the Bed Nucleus of Stria Terminalis in Reinstatement of Alcohol Seeking. Neuropsychopharmacology 43, 838–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK, 2015. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J, 2001. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res 120, 137–146. [DOI] [PubMed] [Google Scholar]
- Lopez CA, Guesdon B, Baraboi ED, Roffarello BM, Hetu M, Richard D, 2011. Involvement of the opioid system in the orexigenic and hedonic effects of melanin-concentrating hormone. Am J Physiol Regul Integr Comp Physiol 301, R1105–1111. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, 2005. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181, 688–696. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Moorman DE, Aston-Jones G, Becker HC, 2016. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res 1636, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE, 2012. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci 32, 3405–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr., Jones RM, Portoghese PS, Carlezon WA Jr., 2003. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305, 323–330. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ, 1994. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci 5, 124–144. [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H, 1995. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res 700, 89–98. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB, 2007. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol 504, 702–715. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C, 2006. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 30, 982–990. [DOI] [PubMed] [Google Scholar]
- McCarty CA, Ebel BE, Garrison MM, DiGiuseppe DL, Christakis DA, Rivara FP, 2004. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics 114, 714–719. [DOI] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Hartmann J, Carlezon WA Jr., Ressler KJ, 2018. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Romero-Pico A, Vazquez MJ, Novelle MG, Lopez M, Dieguez C, 2012. The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts 5, 196–207. [DOI] [PubMed] [Google Scholar]
- Normandeau CP, Torruella Suarez ML, Sarret P, McElligott ZA, Dumont EC, 2018. Neurotensin and dynorphin Bi-Directionally modulate CeA inhibition of oval BNST neurons in male mice. Neuropharmacology 143, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE, 2015. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res 39, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO, 2019a. Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning. Cell Rep 29, 13–21 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, Morikawa H, Hopf FW, Messing RO, 2019b. A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J Neurosci 39, 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G, 2009. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry 33, 1356–1365. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Rivier C, 1996. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res 20, 240–254. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR, 2016. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH, 2014. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM, 2012. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcohol Clin Exp Res 43, 243–249. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G, 2012. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34, 468–483. [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL 2nd, 2014. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68, 9 49 41–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, 2014. “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT, 2016. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J Neurotrauma 33, 880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA Jr., 2013. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 229, 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Smith KL, Cohen BM, Carlezon WA Jr., Bechtholt AJ, 2016. Kappa-opioid receptors differentially regulate low and high levels of ethanol intake in female mice. Brain Behav 6, e00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF, 2008. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF, 2011. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY, 2011. Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther 339, 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C, 2009. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology 34, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Crowley RS, Ben K, Prisinzano TE, Kreek MJ, 2017. Synergistic blockade of alcohol escalation drinking in mice by a combination of novel kappa opioid receptor agonist Mesyl Salvinorin B and naltrexone. Brain Res 1662, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ, 2019. Combination of Clinically Utilized Kappa-Opioid Receptor Agonist Nalfurafine With Low-Dose Naltrexone Reduces Excessive Alcohol Drinking in Male and Female Mice. Alcohol Clin Exp Res 43, 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]


