Abstract
Purpose:
To evaluate the safety and efficacy of 177Lu-DOTA-EB-TATE, a radiolabeled somatostatin analogue modified by Evans Blue, at escalating doses, to increase tumor retention in patients with progressive metastatic neuroendocrine tumors (NETs).
Methods:
Thirty-three patients with metastatic NETs were prospectively enrolled into four groups: Group A (n=6, 43±12y) administered approximately 3.7 GBq (100 mCi) 177Lu-DOTATATE as controls; Group B (n=7, 55±7y) administered approximately 1.11 GBq (30 mCi) 177Lu-DOTA-EB-TATE; Group C (n=6, 55±10y) administered approximately 1.85 GBq (50 mCi) 177Lu-DOTA-EB-TATE; Group D (n=14, 50±10y) administered approximately 3.7 GBq (100 mCi) 177Lu-DOTA-EB-TATE. Treatment-related adverse events were graded according to the CTCAE v.5.0. 68Ga-DOTATATE PET/CT were performed at baseline and 2–3 months after treatment for response evaluation.
Results:
Administration was well tolerated. No CTC 3/4 hematotoxicity, nephrotoxicity or hepatotoxicity was observed during or after treatment in groups A-C. In group D, CTC-3 hematotoxicity was recorded in 2 patients with multicourse chemotherapy previously. After one-cycle treatment, the SUVmax decreased in group C (Δ%=−17.4±29.3%) and group D (Δ%=−15.1±39.1%), but greatly increased in Group B (Δ%=30.0±68.0%) and mildly increased in group A (Δ%=5.4±45.9%). Referring to EORTC criteria, 16.7% (1/6), 0% (0/7), 50% (3/6) and 50% (7/14) were evaluated as partial response in groups A, B, C and D, respectively. When selecting lesions with comparable baseline SUVmax ranging from 15 to 40, SUVmax showed no significant decrease in group B (Δ%=−7.3±24.5%) (P=0.214), significant decrease in group C (Δ%=−34.9±12.4%) (P=0.001) and in group D (Δ%=−17.9±19.7%) (P=0.012) as compared to group A with increased SUVmax (Δ%=8.4±48.8%). SUVmax significantly decreased in the EBTATE groups (groups B-D combined) (Δ%=−19.0±21.5%) as compared to the TATE group (P=0.045).
Conclusion:
177Lu-DOTA-EB-TATE is well tolerated and is more effective than 177Lu-DOTATATE. Both 1.85GBq (50 mCi) and 3.7 GBq (100 mCi) doses appear to be more effective than 1.11 GBq (30 mCi) dose. Further investigation with more cycles of 177Lu-DOTA-EB-TATE treatment and longer follow-up is warranted.
Keywords: peptide receptor radionuclide therapy (PRRT), 177Lu-DOTA-EB-TATE, 177Lu-DOTATATE, neuroendocrine tumor
Introduction
Neuroendocrine tumors (NETs) are a group of heterogeneous tumors originated from the diffuse neuroendocrine system, and their occurrence has been increasing in recent years [1]. However, due to its insidious onset and heterogeneity, NET is often at late stage when diagnosed. Well-differentiated NETs are not sensitive to chemoradiotherapy with a moderate response rate at the level of 20–35% [2], and only effective in small number of patients with poorly differentiated NETs. More than 70% NETs express somatostatin receptors (SSTRs), which enables the receptor imaging with radiolabeled somatostatin analogs and somatostatin-based peptide receptor radionuclide therapy (PRRT) for NETs [2–4].
SSTR2-targeted PRRT has been used in the clinic for more than two decades as an option for the treatment of NETs. 111In and 90Y were the most important therapeutic radioisotopes used in the past, but the applications have not in general been particularly successful due to low efficacy and/or toxicity [5–7]. 177Lu, a β- and γ-emitting radionuclide with a maximum particle range of 2 mm and a half-life of 160 h, is currently the most successfully radionuclide for both well tolerability and tumor response. 177Lu-DOTATATE is now the most widely clinically used radiopharmaceutical. The results of the milestone phase III Neuroendocrine Tumors Therapy (NETTER-1) trial showed favorable outcomes with respect to the primary endpoint of progression-free survival and a host of secondary objectives, including overall survival, objective response rate, and quality of life [8], which paved the way for approval of clinical application.
However, somatostatin analog octreotide or octreotate can be quickly cleared from the blood through the kidneys, showing suboptimal retention within tumors. 177Lu-DOTA-EB-TATE, compared with 177Lu-DOTATATE, with Evans blue motif, uses endogenous albumin as a reversible carrier to effectively extend the half-life in the blood and substantially increase targeted accumulation and retention within the tumor [9]. Our previous dosimetry study demonstrated that, compared with 177Lu-DOTATATE, 177Lu-DOTA-EB-TATE showed extended circulation in the blood and achieved 7.9-fold increase of tumor dose delivery [10]. Based on these promising preliminary results, we assume that 177Lu-DOTA-EB-TATE has great potential to be a highly effective radiopharmaceutical, requiring lower dose of administration than 177Lu-DOTATATE. In this study, we aim to evaluate the safety and efficacy of 177Lu-DOTA-EB-TATE with escalating doses after one cycle treatment.
Materials and methods
Patients
This study was approved by the Institute Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and registered at the Clinicaltrials.gov ( NCT03478358). All patients gave written informed consent. From August 2017 to February 2019, 33 patients with histological confirmed NETs were enrolled in the study. The inclusion criteria were: (a) high tracer uptake in tumor on 68Ga-DOTATATE PET/CT, evaluated within 1 week before inclusion, (b) histological confirmed or inoperable/metastatic NETs, (c) white blood cell (WBC) ≥ 3 × 109/L, (d) platelet (PLT) ≥ 60 × 109/L, (e) hemoglobin (Hb) ≥ 10 g/dL, (f) serum creatinine clearance (SCr) > 40 mL/min, (g) no pregnancy or lactation, (h) age > 18.
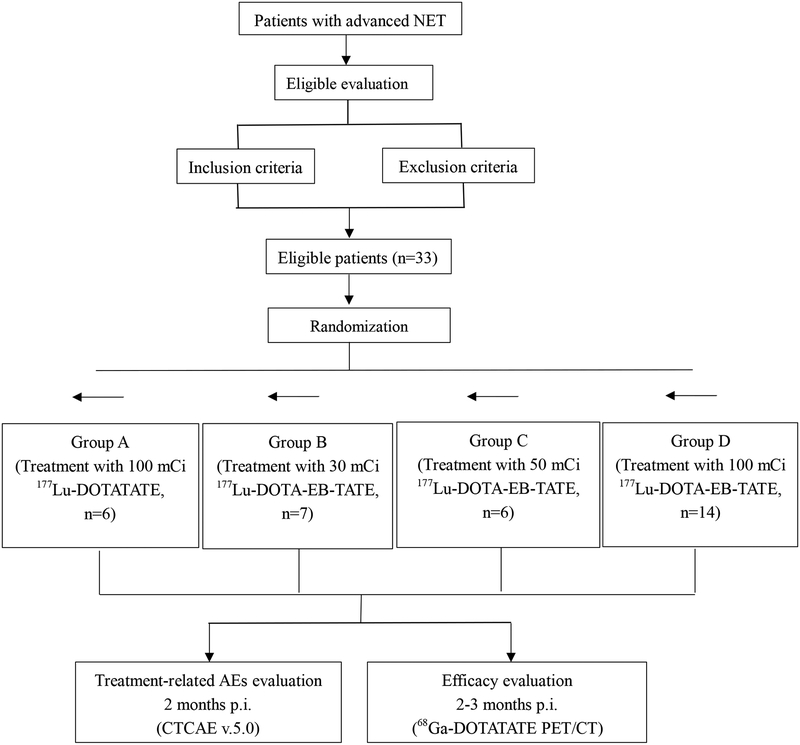
Patients were randomly divided into four groups using sequentially numbered, opaque sealed envelopes. Randomized sequence was generated by computer and was put into opaque, sealed envelopes by the one who wasn’t the investigator or assessor of the study. Qualified patients were divided into four groups with these sequences secretly (allocation concealment) by different people. The setting number of patients was 7 per group in groups A-C and 14 in group D. Finally, group A consisted of 6 patients (4 men/ 2 women, 43 ± 12 years old) were treated with approximately 3.7 GBq) 177Lu-DOTATATE. Group B consisted of 7 patients (5 men/2 women, 55 ± 7 years old) were treated with approximately 1.11 GBq (30 mCi) 177Lu-DOTA-EB-TATE. Group C consisted of 6 patients (4 men/2 women, 55 ± 10 years old) were treated with approximately 1.85 GBq (50 mCi) 177Lu-DOTA-EB-TATE. Group D consisted of 14 patients (7 men/7 women, 50 ± 10 years old) were treated with approximately 3.7 GBq (100 mCi) 177Lu-DOTA-EB-TATE. Participant flow chart of the 4 randomized groups was showed in Fig 1.
Fig.1.
Participant flow chart of the four randomized groups.
Treatment
DOTA-TATE/DOTA-EB-TATE (100 μg dissolved in 20 μL absolute ethyl alcohol) was added to 200 μL 0.5 M NaOAc (pH 5.6) and then 177LuCl3 purchased from LuMark®, IDB, Holland was added. The mixture was heated for 30 min at 100 °C and then purified by C18 cartridge and passed through a 0.22-μm aseptic filtration membrane. The quality control was performed with analytical thin-layer chromatography (Bioscan, USA). CH3OH:NH4OAc (v/v 1:1) was used as the developing solution. The radiolabeling yield was greater than 90% and the radiochemical purity was more than 95%.
Radiopharmaceutical administration was performed in the ward and was preceded by oral medication of an antiemetic (ondansetron 8 mg) to prevent vomiting. For renal protection, arginine was added to 5% glucose solution (25 g/L, 1000 mL). The mixture was administered concomitantly for at least 4 h, starting 30 min before infusion of the radiopharmaceutical. All patients received pre-set dosage of 177Lu-DOTATATE/177Lu-DOTA-EB-TATE via intravenous infusion over 30 min. The mean administered radioactivities in groups A, B, C, D were 110.0 ± 7.5 mCi, 30.0 ± 3.0 mCi, 49.1 ± 5.4 mCi and 102.9 ± 15.9 mCi, respectively.
Safety Evaluation
Treatment-related adverse events (AEs) were recorded over a period of 2 months after the administration of PRRT. Hematological parameters, liver and renal function at baseline, 1 week and 4 weeks post-therapy were tested. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0).
68Ga-DOTATATE PET/CT Response Evaluation
68Ga-DOTATATE PET/CT were performed at baseline and 2–3 months post-therapy for response evaluation. No specific preparation was requested before intravenous injection of 68Ga-DOTATATE at a dosage of approximately 1.85 MBq (0.05 mCi) per kilogram body weight. PET/CT scan was performed at 40 min after tracer administration. After a low-dose CT scan, whole-body PET was performed with 2 min/bed position (5–6 bed positions depending on the height of the patient) from the pelvic bottom to the skull base. The emission data were corrected for randoms, dead time, scattering and attenuation. A Siemens MMWP workstation was used for post-processing.
All images were measured by the same physician who was masked to the clinical data. The regions of interest (ROI) of tumor lesions were drawn manually and the software automatically obtained the radioactivity concentration and standard uptake of value (SUV) in the ROI. The SUVmax of lesions with longest diameter ≥2.0 cm was calculated and no more than 5 lesions (no more than 2 lesions in an organ as well) in one patient were measured. Molecular tumor response was evaluated referring to EORTC criteria.
Statistical Analysis
ΔSUVmax of tumor lesions between baseline and post-therapy were calculated. Percentage of tumor SUVmax decrease (ΔSUVmax%) was obtained by dividing the ΔSUVmax by baseline SUVmax. Quantitative data were expressed as means ± standard deviations. Differences between two independent groups were performed by Student’s t-tests. Differences among four independent groups were compared with one-way ANOVA analysis. All statistical tests were 2-tailed, and P< 0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS 23.0 software (IBM SPSS, Chicago, IL, USA).
Results
Patients
In group A, 3 patients were diagnosed with pancreatic NET, 1 patient with pulmonary NET, 1 patient with ovary NET and 1 patient with carcinoma of unknown primary (CUP). In group B, 3 patients were diagnosed with pancreatic NET, 2 patients with duodenum NET, 1 patient with rectal NET, and 1 patient with pheochromocytoma. In group C, 3 patients were diagnosed with pancreas NET, 1 patient with duodenum NET, 1 patient with pulmonary NET and 1 patient with multiple endocrine neoplasia (MEN 1). In group D, 7 patients were diagnosed with pancreatic NET, 3 patients with duodenum NET, 2 patients with rectal NET, 1 patient with CUP, and 1 patient with paraganglioma.
According to the World Health Organization grades of tumor differentiation, group A included grade 1 (Ki-67 index 2% or less) in 3 patients, grade 2 (Ki-67 index 3% to 20%) in 2 patients and grade 3 (Ki-67 index greater than 20%) in 1 patient. Group B included grade 1, grade 2 and grade 3 in 2, 4 and 1 patients respectively. Group C included grade 1 in 1 patient, grade 2 in 4 patients and grade 3 in 1 patient. Group D included grade 1 in 3 patients, grade 2 in 10 patients and grade 3 in 1 patient.
In group A, 6 patients had liver metastases, 2 patients with bone metastases, 4 patients with lymph node metastases and 1 patient with pulmonary metastases. In group B, patients with liver, bone, lymph node and lung metastases were 7, 3, 3 and 1 patients respectively. In group C, patients with liver, bone, lymph node and lung metastases were 5, 2, 2 and 1 patients respectively and in group D, 14, 6, 5 and 3 patients respectively.
In group A, 1 patient underwent surgical resection of primary lesion or metastases, 5 patients received somatostatin analog and 3 patients received chemotherapy before PRRT. In group B, 5 patients were pretreated with surgery, 2 patients with somatostatin analog and 3 patients with chemotherapy. In group C, 2 patients underwent surgery, 5 patients received somatostatin analog and 3 patients received chemotherapy previously. In group D, 7, 5 and 6 patients underwent surgery, somatostatin analog and chemotherapy respectively.
Detailed baseline characteristics were listed in Table 1 and there was no statistical difference in the listed comparable parameters.
Table 1.
Demographic and baseline clinical characteristics of NET patients.
| Characteristic | 100mCi TATE (n=6) | 30mCi EBTATE (n=7) | 50mCi EBTATE (n=6) | 100mCi EBTATE (n=14) |
|---|---|---|---|---|
| Male/Female | 4/2 | 5/2 | 4/2 | 7/7 |
| Age-y | 43 ± 12 | 55 ± 7 | 55 ± 10 | 50 ± 10 |
| Primary tumor site | ||||
| Pancreas | 3 | 3 | 3 | 7 |
| Duodenum | 0 | 2 | 1 | 3 |
| Rectum | 0 | 1 | 0 | 2 |
| Lung | 1 | 0 | 1 | 0 |
| Ovary | 1 | 0 | 0 | 0 |
| CUP | 1 | 0 | 0 | 1 |
| MEN1 | 0 | 0 | 1 | 0 |
| Paraganglioma | 0 | 0 | 0 | 1 |
| Pheochromocytoma | 0 | 1 | 0 | 0 |
| Tumor grade | ||||
| G1 | 3 | 2 | 1 | 3 |
| G2 | 2 | 4 | 4 | 10 |
| G3 | 1 | 1 | 1 | 1 |
| Number of lesions | ||||
| 1–10 | 1 | 4 | 2 | 6 |
| 11–20 | 2 | 1 | 2 | 1 |
| >20 | 3 | 2 | 2 | 7 |
| Metastases | ||||
| Liver | 6 | 7 | 5 | 14 |
| Bone | 2 | 3 | 2 | 6 |
| Lymph node | 4 | 3 | 2 | 5 |
| Lung | 1 | 1 | 1 | 3 |
| Prior treatment | ||||
| Surgery | 1 | 5 | 2 | 7 |
| Somatostatin analog | 5 | 2 | 5 | 5 |
| Everolimus | 1 | 0 | 3 | 1 |
| TKI* | 1 | 3 | 5 | 9 |
| Chemotherapy | 3 | 3 | 3 | 6 |
| Radiotherapy | 0 | 0 | 1 | 1 |
| TACE | 1 | 1 | 1 | 3 |
| Disease course(month) | 51 ± 28 | 51 ± 36 | 58 ± 26 | 55 ± 25 |
Tyrosine kinase inhibitor
Safety Evaluation
Patients tolerated PRRT procedures well and no irritating pain, allergy or fever occurred during administration. No life-threatening AEs (CTC-4) were reported during the observation period. Only 1 patient had tolerable nausea and vomiting after administration but recovered within 2 weeks.
Hematological parameters, liver and renal functions at baseline, 1 week and 4 weeks after administration were recorded except for 1 patient in group A who died within 1 month after receiving 3.9 GBq 177Lu-DOTATATE. No CTC 3/4 hematotoxicity, nephrotoxicity or hepatotoxicity was observed during or after treatment in groups A-C. As for thrombocytopenia, reversible CTC-3 thrombocytopenia) occurred in 14.3% (2/14) patients in group D, both of whom had poor bone marrow function due to previous multi-courses of chemotherapy. In group C, one patient with CTC-2 thrombocytopenia at baseline, administration of approximately 2.0 GBq 177Lu-DOTA-EB-TATE did not affect platelets. As for anemia, one patient in group D with CTC-3 anemia at baseline, administration of approximately 4.1 GBq did not worsen the condition either. As for leukopenia, all patients were maintained at CTC-0 after treatment. Details were shown in Table 2.
Table 2.
Hematotoxicity, hepatotoxicity and nephrotoxicity after PRRT according to CTCAE v.5.0.
| Group | Status | WBC | PLT | Hb | ALT | AST | ALP | Cr |
|---|---|---|---|---|---|---|---|---|
| 100mCiTATE | Baseline | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 |
| Post-therapy | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | 5 CTC-0 | |
| 30mCi EBTATE | Baseline | 7 CTC-0 | 7 CTC-0 | 7 CTC-0 | 7 CTC-0 | 7 CTC-0 | 7 CTC-0 | 7 CTC-0 |
| Post-therapy | 7 CTC-0 | 6 CTC-0 1 CTC-1 |
7 CTC-0 | 6 CTC-0 1 CTC-2 |
6 CTC-0 1 CTC-2 |
7 CTC-0 | 7 CTC-0 | |
| 50mCi EBTATE | Baseline | 6 CTC-0 | 5 CTC-0 1 CTC-2 |
6 CTC-0 | 6 CTC-0 | 6 CTC-0 | 6 CTC-0 | 6 CTC-0 |
| Post-therapy | 6 CTC-0 | 5 CTC-0 1 CTC-2 |
6 CTC-0 | 6 CTC-0 | 6 CTC-0 | 6 CTC-0 | 6 CTC-0 | |
| 100mCi EBTATE | Baseline | 14 CTC-0 | 14 CTC-0 | 13 CTC-0 1 CTC-3 |
14 CTC-0 | 14 CTC-0 | 14 CTC-0 | 14 CTC-0 |
| Post-therapy | 14 CTC-0 | 12 CTC-0 2 CTC-3 |
12 CTC-0 1 CTC-1 1 CTC-3 |
13 CTC-0 1 CTC-2 |
13 CTC-0 1 CTC-2 |
13 CTC-0 1 CTC-1 |
14 CTC-0 |
The percentage of changes in WBC, PLT, Hb, alanine aminotransferase (ALT), aspartate transferase (AST), alkaline phosphatase (ALP) and Scr between baseline and 4 weeks after administration were listed in Table 3. Significant difference in ΔHb% was observed among four groups with Hb significantly increasing in group C when compared with group B (P=0.019) and group D (P=0.015). No significant change was observable in other parameters.
Table 3.
Changes of hematological parameters and liver and renal function between baseline and 4 weeks after administration.
| Group | 100 mCi TATE | 30 mCi EBTATE | 50 mCi EBTATE | 100 mCi EBTATE |
|---|---|---|---|---|
| ΔWBC% | 9.5 ± 28.6 | −0.4 ± 31.7 | −3.4 ± 42.1 | −12.2 ± 29.8 |
| ΔPLT% | −6.5 ± 10.4 | −27.7 ± 24.9 | −0.1 ± 23.0 | −26.3 ± 27.0 |
| ΔHb%* | −1.1 ± 2.9 | −8.7 ± 9.9 | 2.8 ± 8.5 | −7.7 ± 8.5 |
| ΔALT% | 0.8 ± 18.6 | 37.0 ± 98.2 | −22.4 ± 25.1 | 53.0 ± 102.2 |
| ΔAST% | −4.2 ± 20.0 | 65.0 ± 163.2 | −24.9 ± 27.5 | 35.1 ± 120.2 |
| ΔALP% | 4.1 ± 16.2 | 15.7 ± 33.2 | −0.8 ± 19.5 | −0.5 ± 31.8 |
| ΔScr % | 2.7 ± 12.4 | 7.4 ± 19.3 | −6.6 ± 16.7 | −6.4 ± 19.0 |
P value= 0.044.
Response evaluation
The rates of partial response (PR) were 16.7% (1/6), 0% (0/7), 50% (3/6) and 50% (7/14), respectively; stable disease (SD) were 50% (3/6), 42.9% (3/7), 50% (3/6) and 42.9% (6/14); progressive disease (PD) were 33.3% (2/6), 57.1% (4/7), 0% (0/6) and 1 (7.1%) in groups A, B, C and D. Details were shown in Table 4. Examples of treatment efficacy on 68Ga-DOTATATE were shown in Fig 5 and 6. In group A, 1 patient died within 4 weeks and another patient died shortly before the 2nd PRRT. We believe one death occurred due to high tumor burden with extensive involvement of liver, spleen, lymph nodes and bones. The other death had low tumor burden, but was complicated with extremely dangerous cholangiocarcinoma. The other five patients with PD had new tumor lesions or increased SUVmax.
Table 4.
Response evaluation by molecular imaging (68Ga-DOTATATE PET/CT) referring to EORTC criteria
| Group | CR | PR | SD | PD |
|---|---|---|---|---|
| 100 mCi TATE | 0% (0/6) | 16.7% (1/6) | 50% (3/6) | 33.3% (2/6) |
| 30 mCi EBTATE | 0% (0/7) | 0% (0/7) | 42.9% (3/7) | 57.1% (4/7) |
| 50 mCi EBTATE | 0% (0/6) | 50% (3/6) | 50% (3/6) | 0% (0/6) |
| 100 mCi EBTATE | 0% (0/14) | 50% (7/14) | 42.9% (6/14) | 7.1% (1/14) |
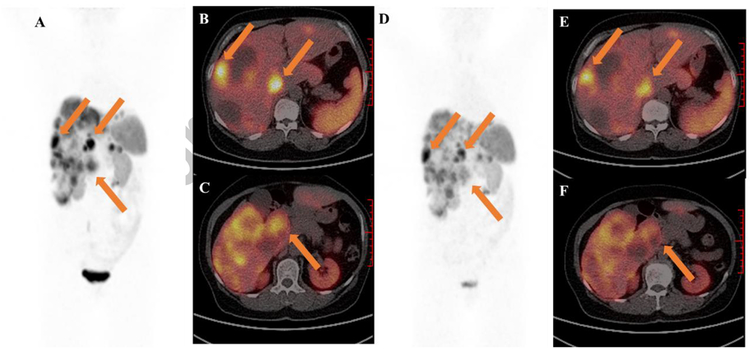
Fig. 5.
A 65-year-old woman with G2 duodenum NET, had extensive involvement of liver. (A-C) Baseline 68Ga-DOTATATE PET/CT before administration with 1.96 GBq 177Lu-DOTA-EB-TATE. (D-F) 2 months post-therapy 68Ga-DOTATATE PET/CT showed diffuse decreased uptake in primary tumor and liver metastases from 21.3 to 10.8 (lower arrow) and 38.3 to 22.2 (upper arrows), respectively.
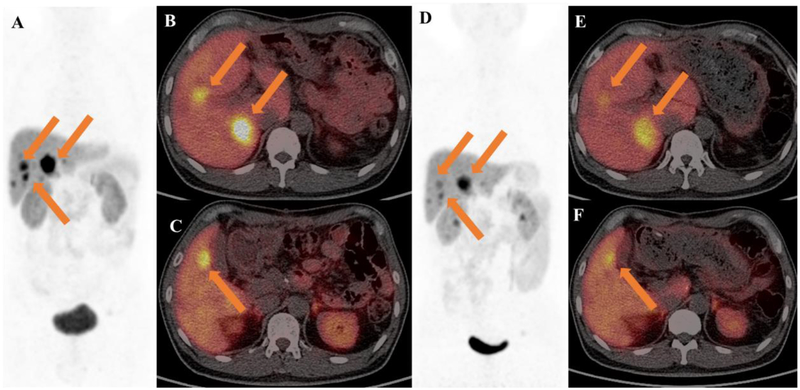
Fig. 6.
A 45-year-old man with pancreatic neuroendocrine tumor (G2, primary tumor removal), was accompanied with diarrhea, flushing and hyperglycemia. (A-C) Baseline 68Ga-DOTATATE PET/CT before administration with 4.14 GBq 177Lu-DOTA-EB-TATE. (D-F) 2 months post-therapy 68Ga-DOTATATE PET/CT showed volume reduction and decreased uptake of metastatic liver lesions(arrows) from 54.4 to 26.5.
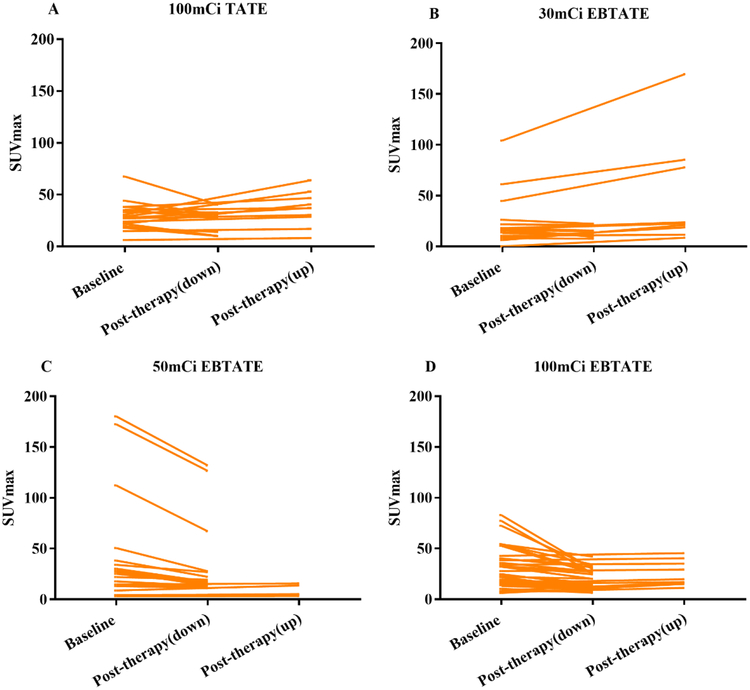
The SUVmax of tumor lesions with longest diameter ≥2.0 cm was calculated and no more than 5 lesions (no more than 2 lesions in an organ as well) were measured in one patient. The number of qualified lesions detected by 68Ga-DOTATATE PET/CT in groups A, B, C and D were 20, 18, 20 and 39, respectively. In a lesion-by-lesion analysis, the changes of SUVmax between baseline and post-therapy were shown in Fig 2. Baseline SUVmax in groups A, B, C and D were 29.3±13.0, 39.0±32.0, 41.4±51.9 and 29.6±19.5, respectively (P>0.05). The percentages of tumor lesions with decreased SUVmax were 50.0% (10/20), 50.0% (9/18), 75.0% (15/20) and 76.9% (30/39) in groups A, B, C and D, respectively.
Fig. 2.
Changes of SUVmax between baseline and post-therapy in each group. (A-D) 50.0% (10/20), 50.0% (9/18), 75.0% (15/20) and 76.9% (30/39) tumor lesions had decreased SUVmax in group A-D, respectively
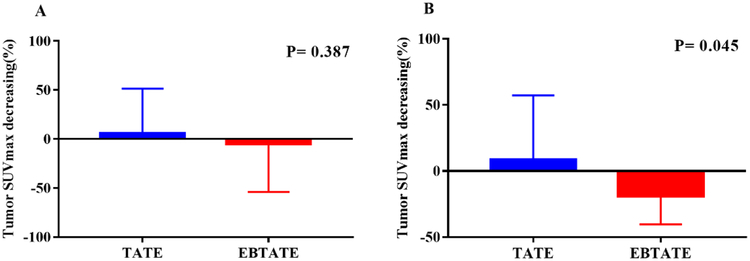
Fig 3 showed the comparison of ΔSUVmax% between 177Lu-DOTATATE group and 177Lu-DOTA-EB-TATE groups (groups B-D combined). In all qualified tumor lesions, ΔSUVmax% in 177Lu-DOTATATE group was 5.4±45.9 and in 77Lu-DOTA-EB-TATE groups was −5.2±49.0 (P =0.387). When selecting comparable baseline SUVmax ranging from 15 to 40, SUVmax significantly decreased in 177Lu-DOTA-EB-TATE groups, but increased in 177Lu-DOTATATE group (ΔSUVmax% is −19.0±21.5 and 8.4±48.8, respectively, P=0.045).
Fig. 3.
Comparison of ΔSUVmax% between 177Lu-DOTATATE group and 177Lu-DOTA-EB-TATE groups (group B-D combined). (A) In all qualified lesions, ΔSUVmax% in 177Lu-DOTATATE group is 5.4 ± 45.9 and in 177Lu-DOTA-EB-TATE groups is −5.2 ± 49.0 (P=0.387); (B) Selecting comparable baseline SUVmax ranging from 15 to 40, mean ΔSUVmax% in 177Lu-DOTATATE group is 8.4 ± 48.8, in177Lu-DOTA-EB-TATE group is −19.0 ± 21.5 (P=0.045).
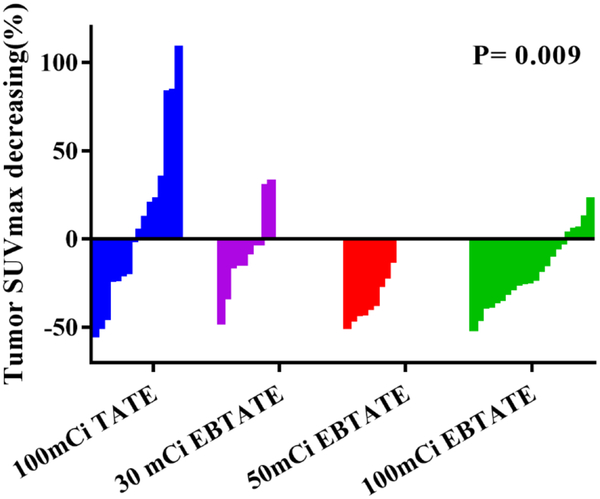
By taking all qualified lesions into account, the SUVmax decreased in group C (Δ%= −17.4±29.3%) and group D (Δ%= −15.1±39.1%), but increased in group B (Δ%= 30.0±68.0%) and group A (Δ%= 5.4±45.9%) with no statistically significant difference. But when selecting lesions with comparable baseline SUVmax ranging from 15 to 40, SUVmax showed no significant decrease in group B (Δ%= −7.3 ± 24.5%, P=0.214), significant decrease in group C (Δ%= −34.9±12.4%, P=0.001) and in group D (Δ%= −17.9±19.7% , P=0.012) as compared to group A with increased SUVmax (Δ%= 8.4±48.8%). Details were shown in Fig 4.
Fig. 4.
When selecting lesions with comparable baseline SUVmax ranging from 15 to 40, SUVmax showed no significant decrease in group B (Δ%= −7.3 ± 24.5%, P=0.214), significant decrease in group C (Δ%= −34.9 ± 12.4%, P=0.001) and group D (Δ%= −17.9 ± 19.7%, P=0.012) as compared to group A with increased SUVmax (Δ%= 8.4 ± 48.8%).
Discussion
177Lu-DOTATATE is effective in treating patients with neuroendocrine tumors, but their doses of radiation being delivered to the tumor is relatively low (23–29 Gy) due to the rapid blood clearance[11–13]. Albumin makes up approximately 55–60% of the serum proteins in the body with a biological half-life of 19 days[14]. Evans blue (EB) as a typical reversible albumin binding molecule with good affinity (Kd = 2.5 μ M), is predicted to have 14 binding sites for albumin[15–18]. Thus, we hypothesized that 177Lu-DOTATATE could be further improved therapeutic efficacy by incorporating EB dye molecule to leverage the circulation half-life of albumin to prolong the blood-circulation time.
One preclinical study performed in mice bearing A427–7 human non-small-cell lung carcinoma xenografts was for 177Lu-DOTA-EB-TATE in comparison with 177Lu-DOTATATE[19]. The tumor uptake of 177Lu-DOTA-EB-TATE was significantly greater than that of 177Lu-DOTATATE. For 177Lu-DOTA-EB-TATE, it reached a maximum of 78.8 ± 4.1% ID/g at 24 h and had a slight decrease with 64.5 ± 7.39% ID/g at 48 h. Whereas for 177Lu-DOTATATE, it reached its peak at 4h with 9.25 ± 0.81% ID/g and then decreased to 3.02 ± 0.20% ID/g at 24 h. As expected, the therapeutic effect of the 177Lu-DOTA-EB-TATE was superior to that of the 177Lu-DOTATATE. Complete remission of tumors was achieved in four out of five mice after administration of 177Lu-DOTA-EB-TATE (1 × 18.5 MBq), and tumor volumes were similar to the starting volumes after administration of 177Lu-DOTA-EB-TATE (1 × 7.4 MBq). But tumors continued to grow even with the higher dose of 177Lu-DOTATATE (1 × 18.5 MBq). In another preclinical study, similar results showed that 86Y-DOTA-EB-TATE had approximately 35-fold higher uptake in HCT116/SSTR2+ tumor xenografts than that of 86Y-DOTATATE[9]. What’s more, significantly greater tumor growth inhibition was observed when using 90Y-DOTA-EB-TATE compared with 90Y-DOTATATE at the same dose of 7.4 MBq. However, in the meantime, the prolonged circulation time also leaded to increase uptake in normal tissues.
Our previous dosimetry study demonstrated that 177Lu-DOTA-EB-TATE had significantly higher retention in circulation compared to 177Lu-DOTATATE with relatively long circulation half-life with t1/2α around 9.47 h and t1/2β around 236 h in the blood [10]. As a result, tumor uptake of 177Lu-DOTATATE reached the peak at 3 h after injection with average SUVs of 8.37±5.63 and decreased over time, whereas that of 177Lu-DOTA-EB-TATE kept increasing from 2 to 120 h with maximum average SUVs of 22.46±12.95 and remained high at 168 h after injection with average SUVs of 21.94±11.63. Consequently, 177Lu-DOTA-EB-TATE delivered 7.9-fold higher dose to tumor than 177Lu-DOTATATE. Prolonged blood retention of 177Lu-DOTA-EB-TATE led to significant increase of dose delivery to the kidneys and red marrow, which is 3.2 and 18.2-fold, respectively more than those of 177Lu-DOTATATE [10], but still far less than the general accepted maximum absorbed doses [12, 20, 21].
As expected, none of our patients receiving 177Lu-DOTA-EB-TATE experienced life-threating AEs (CTC-4). Only 14.3% (2/14) patients in group D had reversible thrombocytopenia of CTC-3, both of whom had poor bone marrow function due to previous multi-courses of chemotherapy. This rate is in accordance with other cohort studies with 3.1%–12% CTC 3/4 haematotoxicity in patients who underwent standard 177Lu-PRRT [22–26]. Heavy pretreatment with alkalizing chemotherapeutics is likely one of the most important risk factors for long-term haematotoxicity of PRRT for NET patients [27]. Notably, in one patient with CTC-2 thrombocytopenia at baseline, administration of approximately 2.0 GBq 177Lu-DOTA-EB-TATE did not affect platelets. In another patient with CTC-3 anemia at baseline, administration of approximately 4.1 GBq did not worsen the condition either. What’s more, there was significant difference in ΔHb% among four groups with Hb significantly increasing in group C compared with group B (P=0.019) and group D (P=0.015). For 177Lu-labeled somatostatin analogues, the rate of nephrotoxicity is typically less than 3 % [28–30]. In a large cohort of 323 patients, 3 (1%) developed CTC-2 nephrotoxicity and no CTC-3/4 nephrotoxicity was observed [31]. However, in another large cohort study, 13.4% patients suffered nephrotoxicity [22]. In this study, no nephrotoxicity of any grade was reported. Nephrotoxicity and hematotoxicity are of the greatest concerns regarding PRRT and as for hepatotoxicity, the occurrence is rare. In a large cohort with 504 patients, only 3 (0.6%) patients had severe hepatotoxicity [11]. In this study, only 2 patients with extensive liver metastases and normal baseline liver function, had transient simultaneous rises in serum ALT, AST and ALP (CTC-1/2 hepatotoxicity) and recovered before the 2nd PRRT. However, we tend to believe this is a transient reaction due to compression of normal liver tissue after edema and necrosis of tumors, rather than radiotoxicity.
Our preliminary study with single low dose (approximately 0.66 GBq) 177Lu-DOTA-EB-TATE demonstrated that compared with 3.7 GBq 177Lu-DOTATATE, no significant difference in efficacy was observed [32]. Based on these inspiring results, we assumed that higher dosage of 177Lu-DOTA-EB-TATE would achieve better efficacy. Patients with NET treated with 177Lu-DOTATATE had a low response rate. GEP-NETs have a better response rate, with as much as 31% objective response (CR plus PR) with high cumulative activity of 29 GBq [11]. This study showed PR in 16.7% cases and SD in 50% cases in group A, which were in line with existing literatures [8, 33]. In 177Lu-DOTA-EB-TATE groups (groups B-D combined), the rates of PR and SD were 37.0% and 44.4%, respectively, which were much higher than those in group A. When selecting comparable baseline lesions with SUVmax ranging from 15 to 40, SUVmax significantly decreased in 177Lu-DOTA-EB-TATE groups (ΔSUVmax%= −19.0 ± 21.5%), but increased in 177Lu-DOTATATE group (ΔSUVmax%=8.4 ± 48.8). The rates of SD among groups B, C and D were similar, but the rates of PR in groups C and D were significantly higher than that in group B. It appears that 1.85GBq and 3.7 GBq doses are more effective than 1.11 GBq dose.
Despite all the encouraging findings, there are several limitations to this study. One is the relatively small scale of patients with just one single dose. Second is the large variations of baseline characteristics of patients, such as the origin of primary tumor and tumor burden.
Conclusion
177Lu-DOTA-EB-TATE was well tolerated in NET patients and more effective than 177Lu-DOTATATE. Both 1.85 GBq (50 mCi) and 3.7 GBq (100 mCi) doses appear to be more effective than 1.11 GBq (30 mCi) dose. Further investigation with more cycles of 177Lu-DOTA-EB-TATE and longer follow-up is warranted.
Acknowledgement
This study was supported by the Key Project on Inter-Governmental International Scientific and Technological Innovation Cooperation in National Key Projects of Research and Development Plan (2016YFE0115400), the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), and was also partly supported by the Chinese Academy of Medical Science Major Collaborative Innovation Project (2016-I2M-1-011), Capital Health Development Scientific Research Project (2018-1-4011), National Nature Science Foundation (81871392, 81741142, 3316 81701742) and Beijing Municipal Natural Science Foundation (7161012).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest None
Ethical approval All procedures involving human participants in studies were in accordance with the ethical standards of the Institute Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all participants included in the study.
Name of the registry:
Treatment Using 177Lu-DOTA-EB-TATE in Patients with Advanced Neuroendocrine Tumors ( NCT03478358)
References
- 1.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 2.Werner RA, Weich A, Kircher M, Solnes LB, Javadi MS, Higuchi T, et al. The theranostic promise for Neuroendocrine Tumors in the late 2010s - Where do we stand, where do we go? Theranostics. 2018;8:6088–100. doi: 10.7150/thno.30357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 4.Luke C, Price T, Townsend A, Karapetis C, Kotasek D, Singhal N, et al. Epidemiology of neuroendocrine cancers in an Australian population. Cancer Causes Control. 2010;21:931–8. doi: 10.1007/s10552-010-9519-4. [DOI] [PubMed] [Google Scholar]
- 5.Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–34. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy KE, Woltering EA, Espenan GD, Cronin M, Maloney TJ, Anthony LB. In situ radiotherapy with 111In-pentetreotide: initial observations and future directions. Cancer J Sci Am. 1998;4:94–102. [PubMed] [Google Scholar]
- 7.Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–22. [DOI] [PubMed] [Google Scholar]
- 8.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G, et al. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics. 2018;8:735–45. doi: 10.7150/thno.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Wang H, Jacobson Weiss O, Cheng Y, Niu G, Li F, et al. Safety, Pharmacokinetics and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analogue 177Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J Nucl Med. 2018;59:1699–705. doi: 10.2967/jnumed.118.209841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 12.Cives M, Strosberg J. Radionuclide Therapy for Neuroendocrine Tumors. Curr Oncol Rep. 2017;19:9. doi: 10.1007/s11912-017-0567-8. [DOI] [PubMed] [Google Scholar]
- 13.Del Prete M, Buteau FA, Beauregard JM. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging. 2017;44:1490–500. doi: 10.1007/s00259-017-3688-2. [DOI] [PubMed] [Google Scholar]
- 14.Ehlerding EB, Lan X, Cai W. Albumin Hitchhiking” with an Evans Blue Analog for Cancer Theranostics. Theranostics. 2018;8:812–4. doi: 10.7150/thno.24183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–56. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans HM, Schulemann W. The Action of Vital Stains Belonging to the Benzidine Group. Science. 1914;39:443–54. doi: 10.1126/science.39.1004.443. [DOI] [PubMed] [Google Scholar]
- 17.Freedman FB, Johnson JA. Equilibrium and kinetic properties of the Evans blue-albumin system. Am J Physiol. 1969;216:675–81. doi: 10.1152/ajplegacy.1969.216.3.675. [DOI] [PubMed] [Google Scholar]
- 18.Lau J, Jacobson O, Niu G, Lin KS, Benard F, Chen X. Bench to Bedside: Albumin Binders for Improved Cancer Radioligand Therapies. Bioconjug Chem. 2019;30:487–502. doi: 10.1021/acs.bioconjchem.8b00919. [DOI] [PubMed] [Google Scholar]
- 19.Bandara N, Jacobson O, Mpoy C, Chen X, Rogers BE. Novel Structural Modification Based on Evans Blue Dye to Improve Pharmacokinetics of a Somastostatin-Receptor-Based Theranostic Agent. Bioconjug Chem. 2018;29:2448–54. doi: 10.1021/acs.bioconjchem.8b00341. [DOI] [PubMed] [Google Scholar]
- 20.Rolleman EJ, Kooij PP, de Herder WW, Valkema R, Krenning EP, de Jong M. Somatostatin receptor subtype 2-mediated uptake of radiolabelled somatostatin analogues in the human kidney. Eur J Nucl Med Mol Imaging. 2007;34:1854–60. doi: 10.1007/s00259-007-0457-7. [DOI] [PubMed] [Google Scholar]
- 21.Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46. doi: 10.1007/s00259-009-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 23.Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014;41:1845–51. doi: 10.1007/s00259-014-2735-5. [DOI] [PubMed] [Google Scholar]
- 24.Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43:453–63. doi: 10.1007/s00259-015-3193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Poppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–61. doi: 10.2967/jnumed.112.119347. [DOI] [PubMed] [Google Scholar]
- 26.NETTER-1 Phase III in Patients With Midgut Neuroendocrine Tumors Treated With 177Lu-DOTATATE: Efficacy and Safety Results. Clin Adv Hematol Oncol. 2016;14:8–9. [PubMed] [Google Scholar]
- 27.Brieau B, Hentic O, Lebtahi R, Palazzo M, Ben Reguiga M, Rebours V, et al. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr Relat Cancer. 2016;23:L17–23. doi: 10.1530/ERC-15-0543. [DOI] [PubMed] [Google Scholar]
- 28.Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46 Suppl 1:83S–91S. [PubMed] [Google Scholar]
- 29.Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62. doi: 10.1200/JCO.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 30.Forrer F, Uusijarvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med. 2005;46:1310–6. [PubMed] [Google Scholar]
- 31.Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, et al. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43:1802–11. doi: 10.1007/s00259-016-3382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Cheng Y, Zhang J, Zang J, Li H, Liu Q, et al. Response to Single Low-dose (177)Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics. 2018;8:3308–16. doi: 10.7150/thno.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pencharz D, Walker M, Yalchin M, Quigley AM, Caplin M, Toumpanakis C, et al. Early efficacy of and toxicity from lutetium-177-DOTATATE treatment in patients with progressive metastatic NET. Nucl Med Commun. 2017;38:593–600. doi: 10.1097/MNM.0000000000000685. [DOI] [PubMed] [Google Scholar]