Abstract
Aims
To evaluate the long‐term trends of new psychoactive substance (NPS) detection in pooled urine samples collected across a city centre.
Methods
Pooled urine samples from portable stand‐alone urinals were collected on a monthly basis over 5.5 years (July 2013–December 2018) across a city centre. These were analysed using a high‐performance liquid chromatography system, interfaced to a high‐resolution accurate mass spectrometer. Data were processed against a database containing over 2000 drugs/metabolites including over 800 NPS.
Results
In total, 44 NPS were detected with variation over time including cathinones (15, 34.1%), synthetic cannabinoids (8, 18.2%) and 21 (47.7%) other NPS. Since the introduction of the UK Psychoactive Substances Act (May 2016) cathinone detection has decreased with minimal detection over the last 4 months of the study. Synthetic cannabinoids were not detected on a regular basis until July 2016 with a subsequent variable detection frequency. There was a consistent, low level detection frequency of all other NPS throughout the study, but which appears to have increased alongside the decrease in cathinone detection.
Conclusion
Pooled urine analysis of samples taken from portable urinals in a city centre can be used as an effective monitoring tool to determine long‐term trends in the use of NPS. The results of this study demonstrate the impact of the Psychoactive Substances Act and reflect the findings of population surveys and clinical studies. Triangulation of these data with other data sources will enable greater insight into the NPS phenomenon.
Keywords: new psychoactive substances, pooled urine analysis, cathinones, synthetic cannabinoids, psychoactive substance act
What is already known about this subject
Many diverse new psychoactive substances (NPS) exist within a rapidly changing and dynamic recreational drug market.
Accurate data on NPS is acquired via several epidemiological indicators including general and subpopulation level surveys, web mapping projects, drug seizures, drug related deaths and clinical studies assessing demand for treatment.
This study aimed to evaluate long term trends of NPS detected in pooled urine samples collected from portable street urinals and confirm these trends in detection against other established monitoring tools.
What this study adds
The majority of NPS detected during the study were cathinones and synthetic cannabinoids but also a consistent, lower level detection of several other classes of NPS were found.
Since the introduction of the UK Psychoactive Substance Act (May 2016) cathinone detection has decreased. Synthetic cannabinoids were only detected with a great frequency during the latter half of the study from July 2016 but with considerable variation in detection over time.
Pooled urine analysis of samples taken from portable urinals in a city centre can be used as an effective monitoring tool to determine which substances have been in circulation, and long‐term trends in the use of NPS.
These data triangulate closely with those of other monitoring tools, allowing for greater insight into the NPS phenomenon.
1. BACKGROUND
Recreational drug use has significant consequences for public health and societies across the globe. It was estimated in 2017 that 5.5% of the global population aged 15–64 years had used drugs in the previous year, with the number of people who use drugs being 30% higher than in 2009.1 In the UK, the 2018/2019 Crime Survey for England and Wales (CSEW) estimated that 9.4% of adults aged 16–59 years and 20.3% aged 16–24 years had taken a recreational during the last year. This has been an upward trend since the 2015/2016 survey at 8.3% and 19.8% respectively.2
For over a decade there have been significant changes in the drugs that are available to users both in terms of geographic and temporal variation.1, 3 This is particularly evident for new psychoactive substances (NPS) where a large number of diverse substances exist within a rapidly changing and dynamic market.3 By the end of 2018, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) was monitoring more than 730 NPS, 55 of which were detected for the first time in Europe in 2018.3 The number of new substances identified peaked in 2014 and 2015 with 101 and 98 substances respectively and have since stabilised. The causes for this are unclear but may reflect sustained efforts to control new substances through legislative processes across the world.3
In May 2016 the Psychoactive Substances Act (PSA) came into effect in UK, making the production, distribution, sale and supply of psychoactive substances illegal.4 The main aim of the PSA was to target production and supply rather than possession for individual use. Although restrictions have limited the open sale of their products, they remain available through online shops, the darknet, or may be sold on the illicit drug market.5, 6, 7 The most prevalent classes of NPS are cathinone derivatives, which produce stimulant‐like effects similar to cocaine, amphetamine and 3,4‐methylenedioxymethamphetamine (MDMA); and the synthetic cannabinoids, which produce effects similar to cannabis but are associated with greater toxicity due to their greater potency at endogenous cannabinoid receptors.1, 3, 8 Since the introduction of the UK PSA, clinical studies have assessed its impact on hospital presentations with acute NPS toxicity. In a study in an inner‐city hospital (London, UK), there was no difference in the overall proportion of presentations involving NPS in the 12 months before and after the PSA introduction. However, there were significant changes in the types of NPS reported with a decrease in cathinones and increase in synthetic cannabinoids.9 In a UK multicentre study (Identification Of Novel psychoActive substances [IONA] study) characterising the NPS involved in episodes of severe NPS toxicity, the NPS involved in acute toxicity presentations were again shown to change in the 12 months before and after the PSA. In this study, NPS were analytically confirmed and there were decreases in the detection of synthetic cannabinoids, cathinones and methiopropamine (a synthetic methamphetamine analogue).10 Similar findings are also evident by the number of healthcare telephone enquiries to the UK poisons service (National Poisons Information Service, NPIS) related to emergency hospital admissions with NPS acute toxicity. In the year following the introduction of the PSA there was a reduction in NPS‐related enquiries from 464 during the 12 months prior, to 142 telephone enquiries (69.4% reduction).6 A 50% reduction was also seen in the number of accesses to the NPIS internet database for NPS‐related information. However, no evidence of displacement to other substances was evident as telephone enquiries and database accesses related to conventional drugs of misuse remained stable.6
It is important to have accurate and timely information on changes in the patterns of drug use in order that prevention, educational and harm‐reduction interventions can be appropriately targeted. In Europe, these data are acquired via a number of epidemiological indicators such as general and subpopulation level surveys, web mapping projects, drug seizures and drug‐related deaths.3 Other important indicators include clinical studies assessing demand for treatment such as trends in hospital admissions with acute NPS toxicity.9, 10, 11, 12 The difficulty with some of these indicators, and particularly drug surveys, is that they are based on user self‐report of the substance(s) that they have taken, which may not be accurate. For example, there a number of studies that have shown significant variability in the content of both classical recreational drugs and NPS.13, 14, 15, 16 Therefore, data from these sources may not be truly reflective of drug prevalence and trends, especially considering the dynamic nature of the NPS market with new drug trends becoming established over short time frames before they can be widely identified.3
Wastewater analysis is an alternative method to demonstrate spatial and temporal patterns of illicit drug use. This involves sampling a source of wastewater, such as sewage influent to a wastewater treatment plant, allowing for the analytical detection of illicit drugs and their metabolites and an estimate of the quantity of drugs consumed by a community. Over recent years, wastewater‐based epidemiology has been demonstrated to be an effective approach for monitoring patterns and trends of illicit drug use within communities17 and several countries worldwide.18 Large scale studies have been undertaken across France involving 25 water treatment plants and demonstrated significant geographical differences in drug consumption across the country.19 Since 2010 a Europe‐wide network (Sewage analysis CORe group—Europe [SCORE]) has been established with the aim of standardising the approach used for wastewater analysis and coordinating international studies. In 2018 this group published studies involving 72 cities and 20 countries in Europe directly comparing illicit drug loads over a 1‐week period for 8 consecutive years.20 The project revealed distinct geographical and temporal patterns of drug use across European cities for cocaine, amphetamine, methamphetamine and MDMA.
Wastewater analysis has been proposed as a tool for providing information of the temporal and regional trends in the use of NPS. However, the dynamic NPS market presents several challenges for this methodology. This includes the large number of individual NPS available, their low prevalence of use in comparison to other illicit drugs, the dynamic nature of the market whereby 1 drug may be replaced by another, and the lack of clinical pharmacokinetic studies regarding their metabolism, excretion and stability.21 A solution to these issues is to target sampling of wastewater from populations that are expected to have higher use of NPS than the general population. A technique has been established to identify NPS that involves the collection and analysis of pooled urine from portable public stand‐alone urinals.21 This has been demonstrated to be an effective tool in nightclubs,22 music festivals23 and city centres24, 25, 26 providing confirmatory and timely data on NPS. Whilst this technique does have limitations discussed in these studies, it provides a unique insight into subpopulation use of NPS and can be used to track trends in NPS use over time.19, 20, 21
The objective of this study was to evaluate the trends of NPS detection in pooled urine samples taken from stand‐alone portable urinals in central London, UK over a prolonged detection period (>5 years) and confirm trends in detection against other established monitoring tools.
2. METHOD
2.1. Sample collection
The study was conducted in the City of Westminster, a borough in central London, UK that has a wide variety of venues including bars, late‐night restaurants and nightclubs. Urine samples were taken from 12 4‐bay stand‐alone portable urinals. For 12 months of the study period, only 6 urinals were available for sampling. Urinals were placed in locations as previously described20 and used in areas where street urination may be problematic.
The urinals were made available for use by the general public over a 12‐hour period (1800–0600) on Saturday night. Sampling during this study was undertaken over 5.5 years with a collection taken on the first weekend of every month from July 2013 to December 2018. Sample collection did not occur in August 2015 and January 2016 and data from these months are not included in the analysis. The use of the urinals was voluntary and anonymous and therefore no data on the number of people using the urinals was collected. The urinals were designed for male use but did not preclude use by other genders.
Urine is pooled in a central holding tank with a total capacity of 400 L. The urinals have no flushing mechanism, so samples were not diluted. After each collection period, 100 mL of pooled urine from each urinal was taken using a single‐use manual vacuum system. Urine samples were centrifuged at 3500 g for 5 minutes on the day of collection to remove cellular debris and then stored at −20°C until analysis. This study had approval from Westminster Council and the London Metropolitan Police; it was discussed with the local ethics committee (institutional review board) and deemed not to require formal approval.
2.2. Sample analysis
2.2.1. General drug screen
Urine (2 mL) was mixed with 1 mL phosphate buffer (1 mol/L, pH 6.3) containing EDDP‐D3 (100 μg/L) and morphine‐3‐glucuronide‐D3 (115 μg/L). Beta‐glucuronidase solution (2 + 3, v/v in reagent grade water; 100 μL; 4000 units) was added with thorough mixing and the sample incubated overnight (45°C). All samples were centrifuged (1500× g, 5 min; bench centrifuge) prior to solid phase extraction, which was performed using a Cerex 48 place positive pressure system (Tecan UK, Theale, UK). Solid phase extraction cartridges were conditioned sequentially with 1 mL methanol and 1 mL deionised water before use. The supernatants from the centrifuged samples were loaded onto the cartridges, which were washed with 0.5 mL hexane and dried under a stream of nitrogen (30 s). Analyte elution was performed using 2 × 1 mL methanol: ethyl acetate (1 + 9, v/v). The eluent was mixed with 1.5 mL deionised water, and after centrifugation (3000 rpm, 5 min; bench centrifuge) the organic phase was transferred to a glass tube and evaporated to dryness at ambient temperature (Genevac centrifugal vacuum evaporation system) before reconstitution in 5 μL 2‐propanol +100 μL 0.1% (v/v) aqueous acetic acid. The reconstituted samples were transferred to liquid chromatography vials prior to analysis.
Prepared samples (10 μL) were analysed on a Thermo XRS ultrahigh‐performance liquid chromatography system, interfaced to a Thermo Q Exactive Focus high‐resolution accurate mass spectrometer, operating in heated positive ion electrospray mode. Chromatographic separation was achieved in 5.0 minutes on a Waters Atlantis T3 HPLC column maintained at 40°C using a gradient consisting of a mixture of 0.1% acetic acid and acetonitrile containing 0.1% acetic acid. Data were acquired in full scan mode operating at a mass resolution of 70,000 across a mass range of 50–750 amu. Data dependent scanning was enabled in confirmation mode utilising an inclusion list of over 800 compounds derived from an in‐house accurate mass database. A second scan event using all ion fragmentation (AIF) was performed with a stepped higher collisional dissociation setting of 15, 35 and 50 at a mass resolution of 35,000 across a scan range of 80–500 amu. Acquired data were processed using Toxfinder software (Thermo) against an in‐house database containing over 2000 drugs and metabolites including over 800 NPS. The presence of reported drugs is confirmed in the data through full scan accurate mass determination of molecular ions, identification of accurate mass qualifier ions in AIF, and the automatic generation of accurate mass tandem mass spectrometry data for comparison with mass spectral libraries.
The samples were subsequently analysed again using the same instrumental conditions as above but in heated negative ion electrospray mode to detect compounds only seen in this mode.
2.2.2. Synthetic cannabinoid drug screen
A 2‐mL portion of the sample was prepared for analysis by the addition of 1 mL acetate buffer (1 mol/L, pH 4.7) containing hydroxypentyl JWH‐018‐D5 (5μg/L) and β‐glucuronidase solution (2 + 3, v/v in reagent grade water; 100 μL; 4000 units) with thorough mixing and the sample incubated overnight (45°C). The sample was then acidified through the addition of 750 μL hydrochloric acid (2 mol/L) and liquid/liquid extraction performed, using 1% ethyl acetate in hexane (2 mL). Following rotary mixing for a minimum of 20 minutes, the sample was centrifuged (3000 rpm, 5 min; bench centrifuge) and the supernatant was removed to a glass tube. A second liquid/liquid extraction using an additional 2 mL of the same solvent mix was performed on the urine residue after alkalisation of the urine with 1500 μL sodium hydroxide (2 M). After centrifugation as before, the supernatant was removed and added to the previously recovered supernatant. After evaporating to dryness at ambient temperature (Genevac centrifugal vacuum evaporation system) the combined extracts were reconstituted in 10 μL methanol followed by 90 μL 0.1% acetic acid and transferred to glass liquid chromatography vials for analysis.
Prepared samples (10 μL) were analysed on a Thermo XRS ultrahigh‐performance liquid chromatography system, interfaced to a Thermo Q Exactive Focus high‐resolution accurate mass spectrometer, operating in heated positive ion electrospray mode. Chromatographic separation was achieved in 6.5 minutes on a Phenomenex Luna C182 column maintained at 40°C using a gradient consisting of a mixture of 0.1% acetic acid (A) and methanol containing 0.1% acetic acid (B). Using a flow rate of 400 μL/min, initial solvent conditions were 90% A and 10% B. Solvent B was ramped to 60% over the next minute and then to 98% by 3.5 min. These conditions were held until 6.5 minutes when initial conditions were reinstated. These were held for a further 2 minutes for equilibration prior to the next injection. Data were acquired in full scan mode operating at a mass resolution of 70,000 across a mass range of 240–550 amu. Data dependent scanning was enabled in confirmation mode utilising an inclusion list of over 800 compounds derived from an in‐house accurate mass database. In addition, a second scan event using AIF was performed with a stepped higher collisional dissociation setting of 15, 35, 50 at a mass resolution of 35 000 across a scan range of 95–500 amu. Acquired data were processed using Toxfinder software (Thermo) against a regularly updated in‐house database containing synthetic cannabinoids reported to the EMCDDA early warning system and other forensic drug networks and observed or postulated metabolites.
2.2.3. Data analysis
In this study all NPS and relevant established recreational drugs (cocaine, amphetamine and cannabis) detected are presented. Data are expressed as frequency and percentage or as median (interquartile range, IQR) for nonparametric data. Graphical data represent the amalgamated frequency of urinals positive per month for a drug class identified rather than a single NPS e.g. all cathinones or synthetic cannabinoids detected per urinal.
3. RESULTS
3.1. Background detection
Nicotine/cotinine (a major metabolite of nicotine), caffeine (and metabolites) and hordenine (present in germinated barley, so it was probably from beer) were detected in all pooled urine samples analysed. These substances are commonly used in the general population and their presence helps to affirm that the urine collection and analysis is valid.
3.2. NPS
Table 1 lists the NPS detected over the study period. In total, 44 NPS were detected, with cathinones (15, 34.1%) and synthetic cannabinoids (8, 18.2%) being the most prevalent. All synthetic cannabinoids detected were third generation only. Other NPS groups included arylalkylamines (4, 9%), arylcyclohexylamines (1, 2.3%), phenethylamines (3, 6.8%), piperazine derivatives (5, 11.4%) and others (8, 18.2%). A least 1 or more NPS was detected per month. Figure 1 illustrates the trend in the frequency of detection of NPS over time for cathinones (Figure 1A), synthetic cannabinoids (Figure 1B) and all other NPS (Figure 1C, excluding cathinones and synthetic cannabinoids).
Table 1.
New psychoactive substances detected during the study period July 2013 – December 2018 (n = 44)
| Arylalkylamines (4, 9%) | |
|---|---|
| ▪ 5 or 6 APDB (5‐(2‐aminopropyl)benzofuran) | ▪ 5 or 6 EAPB (1‐(benzofuran‐6‐yl)‐N‐ethylpropan‐2‐amine) |
| ▪ 5‐(2‐Aminopropyl)indole (5‐IT) | ▪ Methiopropamine (MPA; N,α‐dimethyl‐2‐thiopheneethanamine, monohydrochloride) |
| Arylcyclohexylamines (1, 2.3%) | |
| |
| Cathinones (15, 34.1%) | |
| ▪ 4 methylmethcathinone (mephedrone) | ▪ Clephedrone (4‐chloromethcathinone) |
| ▪ 4‐chloro‐N‐ethylcathinone | ▪ Dibutylone (−(1,3‐benzodioxol‐5‐yl)‐2‐(dimethylamino)butan‐1‐one) |
| ▪ 4F‐pentedrone | ▪ Ephylone (N‐ethyl pentylone) |
| ▪ 4‐methylethcathinone | ▪ Ethylone (3,4‐methylenedioxy‐N‐ethylcathinone) |
| ▪ 4‐methyl‐N,N‐dimethylcathinone | ▪ MDPT (tBuONE; 3′,4′‐methylenedioxy‐N‐tert‐butylcathinone) |
| ▪ α‐Pyrrolidinovalerophenone (α‐PVP) | ▪ Methylone (3,4‐methylenedioxy‐N‐methylcathinone) |
| ▪ bk‐MDDMA (dimethylone; 2‐dimethylamino‐3′,4′‐methylenedioxy) | ▪ Mexedrone (3‐methoxy‐2‐(methylamino)‐1‐(4‐methylphenyl) propan‐1‐one) |
| ▪ cathinone (2‐amino‐1‐phenylpropan‐1‐one) | |
| Phenethylamines (3, 6.8%) | |
| ▪ 2C‐B (4‐Bromo‐2,5‐dimethoxyphenethylamine) | ▪ Ethylamphetamine |
| ▪ 4‐methylamphetamine | |
| Piperazine derivative (5, 11.4%) | |
| ▪ 1‐(3‐chlorophenyl) piperazine | ▪ BZP (benzylpiperazine) |
| ▪ TFMPP (1‐(3‐trifluoromethylphenyl)‐piperazine) | ▪ MEOP/MEXP (methoxypiperamide) |
| ▪ DCPP (2,3‐dichlorophenylpiperazine) | |
| Synthetic cannabinoid receptor agonists (8, 18.2%) | |
| ▪ 5F ADB/5F MDMB‐PINACA (methyl 2‐{[1‐(5‐fluoropentyl)‐1H‐indazole‐3‐carbonyl]amino}‐3,3‐ dimethylbutanoate) | ▪ AMB‐ or MMB ‐CHMICA (methyl 2‐[[1‐(cyclohexylmethyl)indole‐3‐carbonyl]amino]‐3‐methyl‐butanoate |
| ▪ 5F AKB‐48 (N‐(1‐adamantyl)‐1‐(5‐fluoropentyl)indazole‐3‐carboxamide) | ▪ Cumyl 5F PINACA (1‐(5‐fluoropentyl)‐N‐(2‐phenylpropan‐2‐yl)‐1H‐ indazole‐3‐carboxamide) |
| ▪ AKB‐48/APINACA (N‐(adamantan‐1‐yl)‐1‐pentyl‐1H‐indazole‐3‐carboxamide) | ▪ MDMB‐CHMICA (methyl 2‐[[1‐(cyclohexylmethyl)‐1H‐indole‐3‐ carbonyl]amino]‐3,3‐dimethylbutanoate) |
| ▪ AB‐FUBINACA N‐[(2S)‐1‐amino‐3‐methyl‐1‐oxobutan‐2‐yl]‐1‐[(4‐fluorophenyl)methyl]‐1H‐indazole‐3‐carboxamide | ▪ STS‐135/5F APICA ((N‐(adamantan‐1‐yl)‐1‐(5‐fluoropentyl)‐1H‐indole‐3‐carboxamide) |
| Other (8, 18.2%) | |
| ▪ N‐ethylphentermine | ▪ methylhexaneamine (1,4‐DMAA) |
| ▪ 3 fluorophenmetrazine (3‐FMP) | ▪ N,α‐diethyl phenethylamine |
| ▪ 4F norephedrine | ▪ Ostarine |
| ▪ Methoxyphenidine (MXP) | ▪ phenylisobutylamine (phenibut) |
Figure 1.
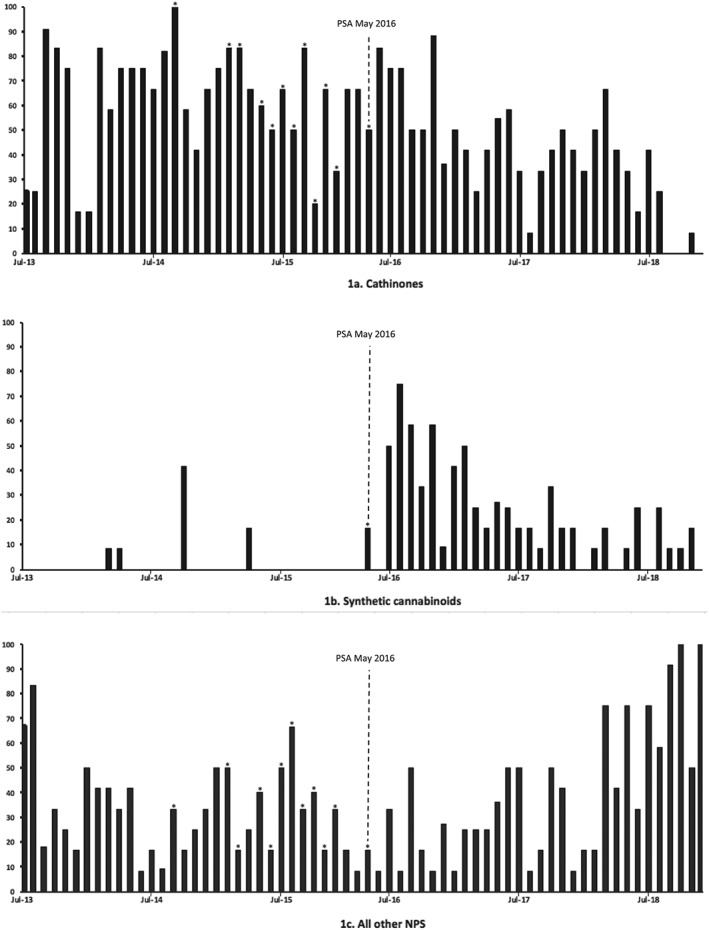
New psychoactive substances (NPS) detection frequency over time for cathinones (A), synthetic cannabinoids (B), all other NPS (C) from July 2013 to December 2018. Figures demonstrate trends of NPS detection frequency shown as percentage of urinals positive per month (x‐axis) against time in months (y‐axis). Sampling was not conducted for august 2015 and January 2016 and these data are not included. The introduction of the Psychoactive Substances Act (PSA) in May 2016 is indicated by a dashed line. An asterisk (*) indicates a month where only 6 urinals were available for sampling
3.2.1. Cathinones
Figure 1A demonstrates a continued but variable trend of detection of cathinones over time.
Prior to the Psychoactive Substances Act that came into effect in the UK in May 2016 the frequency of detection of all cathinones from July 2013 to May 2016 ranged from 16.7 to 100%, with a median of 66.7% (interquartile range, IQR, 50–75). From June 2016 to December 2018 the range of detection was 0 to 84.3%, with a median of 41.7% (IQR 29.2–50). Importantly, as shown in Figure 1A, cathinone detection decreased over the last 18 months of the study period—cathinones had been detected on a monthly basis until September 2018; however, they were not detected in any of the urinals in September, October and December 2018, and only detected in 1 urinal (8.3%) in November 2018.
3.2.2. Synthetic cannabinoids
There was considerable variation in the frequency of detection of synthetic cannabinoids over time (Figure 1B). Between July 2013 and May 2016 synthetic cannabinoid detection was infrequent and they were detected in urinals in only 5 months of sampling including March 2014 (8.3%), April 2014 (8.3%), October 2014 (41.7%), April 2015 (16.7%) and May 2016 (16.7%). Following the introduction of the PSA, after June 2016 through to the study end in December 2018, synthetic cannabinoids were detected in every month apart from January, April, July and December 2018, with a frequency of detection ranging from 9.1 to 75%, median 16.7% (IQR 8.3–31.8).
3.2.3. All other NPS
Twenty‐one of the 44 NPS detected (47.7%) included arylalkylamines (4, 9%), arylcyclohexlamines (1, 2.3%), phenethylamines (3, 6.8%), piperazine derivatives (5, 11.4%), and others (8, 18.2%; Table 1). Figure 1C illustrates the frequency of detection of these other NPS during the study period. From July 2013 to May 2016 (prior to the PSA) the frequency of detection ranged from 16.7 to 83.3% with a median of 33.3% (IQR, 16.7–41.7). Following this from June 2016 to December 2018 the range of detection was 8.3–100% with a similar distribution and a median of 33.3% (IQR, 16.7–50).
3.3. Established recreational drugs
Figure 2 illustrates the detection frequency trends for commonly used, and commonly detected established recreational drugs including cocaine (Figure 2A), amphetamines (Figure 2B) and cannabis (Figure 2C). Cocaine and its major metabolites (benzoylecgonine, ethylbenzoylecgonine) were consistently detected in every urinal on a month‐to‐month basis. Amphetamines (amphetamine, dimethamphetamine, MDMA and its metabolites 4‐hydroxy‐3‐methoxymethamphetamine [HMMA] and 3,4‐methylenedioxyamphetamine, methamphetamine and 3,4‐methylenedioxy‐N‐ethylamphetamine [MDEA]), were consistently present in the majority of urinals with little variation in detection over the study period. From July 2013 to November 2015 carboxy tetrahydrocannabinol (THC, cannabis metabolite), indicating cannabis use, had a variable frequency of detection. However, following this, detection was consistently high and found in the majority of urinals until the study end. The reason for this is believed to have been the change to a more sensitive methodology used to detect specifically carboxy THC.
Figure 2.
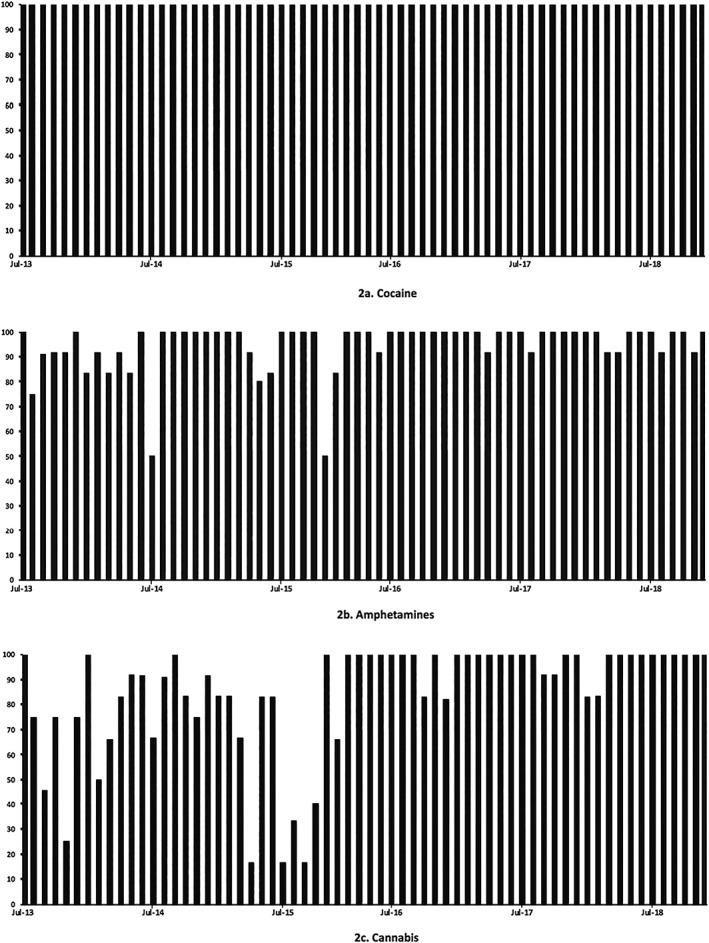
Established recreational drug detection frequency over time for cocaine (A), amphetamine (B) and cannabis (C) from July 2013 to December 2018. Figures demonstrate trends of established recreational drug detection frequency shown as percentage of urinals positive per month (x‐axis) against time in months (y‐axis). Sampling was not conducted for august 2015 and January 2016 and these data are not included
4. DISCUSSION
In this study, we have demonstrated that pooled urine analysis from portable stand‐alone urinals is an effective measure for long term NPS surveillance trends within a city centre. Our results also demonstrate the impact of the PSA as reported in earlier population surveys and clinical studies.2, 5, 6, 9, 10 Although not the main aim of this work, we have also shown that the prevalence of established recreational drugs remains high, including cocaine, amphetamine and cannabis, which is supported by other data sources.2
For NPS, in total we detected 44 substances from the urine samples during 5.5 years of the study. As single classes of NPS, the cathinones and synthetic cannabinoids were the most prevalent at 34.1 and 18.2% respectively. However, the prevalence of their detection has been variable, particularly over the latter stages of the study, with a decrease in detection of the cathinones. Synthetic cannabinoids were not detected on a regular basis until June 2016 and have similarly reduced in prevalence but have remained detectable at a lower level of frequency. A number of other NPS classes were detected during the study including 21 (47.8%) other noncathinone, nonsynthetic cannabinoid NPS in total (Table 1). In general, the detection of these substances has been consistent throughout the study period with a background and regular low‐level frequency. Visual extrapolation of the detection trends from Figure 1 over the last 18 months of the study suggests that there has been an increase in noncathinone, nonsynthetic cannabinoid NPS detection alongside the decrease in cathinone detection.
These data clearly illustrate the dynamic nature of the NPS drug market where the prevalence of different substances is highly variable but overall the number of substances in circulation remains constant. There are several broad reasons including legislative initiatives and drug seizures that may help to explain why synthetic cannabinoid and cathinone NPS may be reducing with other NPS increasing. NPS are predominantly produced in China and shipped for processing in Europe; however, recent restrictions in China and Europe have limited their open sale.3 The detection of new substances identified in Europe peaked in 2014–2015.3 Furthermore, recent drug seizure reports in Europe show that seizures of NPS are typically dominated by cathinones and synthetic cannabinoids3 allowing for other groups of drugs to become more prominent.
There are likely to be several other contributors that have influenced trends in NPS use over time. Sources of data including national surveys, local and national clinical studies and international initiatives each provide a variety of information, which, when triangulated as monitoring tools, can provide a greater insight into the overall pattern and impact of NPS use. Several studies have looked at the potential impact of the UK PSA, which was introduced in May 2016.
In the UK, the PSA was introduced in an attempt to reduce the availably and supply of NPS. One of the leading effects of this has been the reduction in the public availability of NPS through head shops. 6 However, their availability remains more covert particularly through street level drug dealers, online shops or the darknet.3, 5, 27 In these cases, they may be sold under their own name or as adulterants of more established drugs such as cocaine and MDMA. The CSEW provides some evidence of the shift toward online sales before and after the introduction of the PSA. In the 2015/16 survey, 25% of individuals (age 16–59 years) reported the use of NPS, which they said had been obtained from a shop on the last occasion. This has significantly fallen to 13.9% from the last survey of 2018/19.2 Additionally, in 2018/19 respondents were much more likely to have originally sourced NPS from the internet (24.6%) than other drugs (8.6%).2 Further data supporting the impact of the PSA in the UK comes for the Welsh Emerging Drugs and Identification of Novel Substances project (WEDINOS) involving the analytical confirmation of NPS samples voluntarily submitted by individuals from organisations predominantly across Wales but also the UK. The 2016/17 WEDINOS annual report compared 12 months before and after the introduction of the PSA and identified a significant reduction (128 to 72, 43%) in the range of NPS since the PSA, which was thought to be explained predominantly by a drop in synthetic cannabinoids (46 to 13, 74% decrease).28 Post PSA, 5F‐ADB and AMB‐FUBINACA were the most prevalent synthetic cannabinoids detected. 5F‐PB‐22 was the most commonly identified NPS over the 2 years before the PSA introduction but was only detected 4 times during the following 12 months post‐PSA and was not in the top 10 most commonly detected NPS. In December 2016, third generation synthetic cannabinoids were controlled in the UK as Class B drugs under Misuse of Drugs Act, 1971.29 This, in addition to the general dynamic nature of the NPS market, may help to explain the reduction in synthetic cannabinoids seen in the WEDINOS project and in our study.
NPS prevalence data can also be extracted from the CSEW. In 2018/192 approximately 0.5% of adults age 16–59 years had used NPS in the last year which is similar to the 2017/18 and 2016/2017 surveys (both 0.4%). This was, however, a decrease from the 2014/15 survey of 0.9%, which was completed before the introduction of the PSA. As in previous years, around half of all NPS users were aged 16–24 years.2 Further data are available from the IONA study. This is a UK multicentre study of 22 hospitals characterising the NPS involved in episodes of severe toxicity. NPS were confirmed by analytical detection in biological samples and analysis of data from the IONA study has demonstrated changing patterns of NPS over time >1 year before and after the UK PSA.10 The overall prevalence of NPS related hospital admissions appeared to reduce over time with 93 pre‐PSA admissions vs 72 post‐PSA admissions (22.6% reduction). The prevalence of cathinones in this study also appeared to reduce in a time‐wise progression; however, total prevalence over time as per the study parameters demonstrated a stable number of cathinones pre and post PSA. During the IONA study period, 5 third‐generation synthetic cannabinoids were also detected with a variable frequency. For total prevalence over time it showed an increase in 2 synthetic cannabinoids and a decrease in 3. After the PSA introduction, all synthetic cannabinoids remained detectable but generally with a lower level frequency, which is also reflected in the results presented in our study.
Further validation of our study comes from local sources of data—in particular a study looking at the drugs involved in acute drug and NPS toxicity presentations to an inner‐city London, UK emergency department 12 months before and after the PSA introduction.9 In this study, no difference in the overall proportion or average monthly NPS‐related presentations were seen. Average monthly cathinone‐related presentations significantly decreased by approximately 57%, while average monthly synthetic cannabinoid‐related presentations significantly increased by approximately 65%. There was no significant change in average monthly other NPS‐related admissions. Although this study did not analytically confirm the NPS involved with each presentation, on comparing the detection trend data of the current study from Figure 1, the results have comparable similarities in the trends of NPS prevalence.
Wider reaching data sources used for monitoring can also be gained from national poison services and large international studies. Telephone data from the UK NPIS show a year‐on‐year reduction in enquiries related to the cathinone mephedrone since 2014/15.30 The largest reduction of approximately 75% came during the 12 months following the introduction of PSA with the most recent enquiries in 2018/19 being approximately 12% of those in 2014/2015. Similarly, enquires for synthetic cannabinoids have reduced year‐on‐year with the largest reduction being approximately 52% during the 12 months following the introduction of PSA and with current 2018/2019 enquires being approximately 80% of those in 2014/2015.30
There are several limitations associated with this study that have been reported previously.20, 21, 22, 23, 24, 25, 26 Although the urinals used were made available for use by the general public, their design facilitates urine collection from males rather than females. A bias in sample collection therefore exits with over representation from males rather than the general population. It is not known whether this may have led to a difference in the trend of NPS identified; however, qualitatively the NPS detected are likely to be representative of those in use within the geographical area of the sample collection and therefore likely to provide external validation. Pooled urine collection and analysis has the potential to identify parent drugs and or their metabolites as they may be expected to have higher concentrations since there is less risk of dilution in the reservoir tanks of the urinals compared to municipal wastewater systems. However, the urinals are still subject to dilution from rainwater or liquids that have been disposed of during the collection period, which may lead to drug concentrations in samples falling below analytical detection limits. This is particularly true for drugs that may already be detected in users at low concentrations e.g. the synthetic cannabinoids. Dilution or drug degradation prior to analysis may limit detection; however, their absence may not necessarily exclude their use and instead reflect a low concentration in urinal samples collected. Pooled urine analysis from urinals also provides data that are more qualitative than quantitative in nature. Whilst this can still provide valuable data on the drugs being used within a selected area, comparisons over time are likely to be based on frequency of drug detection rather than a magnitude of consumption as in wastewater analysis. Finally, following November 2015 the detection of carboxy‐THC increased during the remainder of the study period. Whilst the analytical methodology over the period of this study did not change, there was a change in where we looked for carboxy‐THC. Originally, this was in the general drug screen that was run, but was found to work better in the synthetic cannabinoid screen hence the improvement in detection. Over the duration of study, the number of analytes increased but the nature of the data is such that it is easily re‐interrogated if there is a feeling that a drug may have been in circulation before it was in the analytical database of this study. In this way we were able to react quickly to updates and findings from new drugs reports from the EMCDDA Early Warning System and other forensic drug networks and observed or postulated metabolites. Furthermore, analysis of samples during this study were completed in batches between 6–12 months. This means that during the lapsed time period between sampling and testing, any new drugs would have already been added to the database.
5. CONCLUSION
The NPS phenomenon is dynamic with rapid emergence of new substances and limited reliable data on prevalence of use. This study demonstrates that analysis of samples collected from portable urinals in a city centre can be used as an effective monitoring tool to determine trends in the use of NPS. Whilst there is stability in the use of established classical drugs such as cocaine and the amphetamines, there have been significant changes in the rate of detection of NPS. In particular, cathinones and synthetic cannabinoids have been decreasing. Triangulation of these data with data from surveys of drug use and studies investigating harm associated with NPS will enable greater insight into the NPS phenomenon and the impact of legislative change.
COMPETING INTERESTS
P.D. is a member of the Scientific Committee of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and until February 2019 was a Member of the UK Advisory Council on the Misuse of Drugs (ACMD). D.W. is an expert advisor to the EMCDDA and ACMD.
CONTRIBUTORS
J.A., D.W. and P.D. made substantial contributions to the conception and design of this study. All authors were involved in the analysis and interpretation of data and in drafting the manuscript. S.H. led sample analysis.
ACKNOWLEDGEMENTS
We would like to thank Rachel Abouchedid, Elizabeth Biswell, James Ho, Hwee Lee, Caitlyn Murch, Nicholas Webb, Joanna White, Caitlin Wolfe and Takahiro Yamamoto for their participation in sample collection.
Archer JRH, Mendes F, Hudson S, Layne K, Dargan PI, Wood DM. Evaluation of long‐term detection trends of new psychoactive substances in pooled urine from city street portable urinals (London, UK). Br J Clin Pharmacol. 2020;86:517–527. 10.1111/bcp.14239
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. World Drug Report 2019 (United Nations publication, Sales No. E.19.XI.8). Available from: https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_1_EXECUTIVE_ SUMMARY.pdf [last accessed OCT 2019]
- 2. Home Office . Drugs Misuse: Findings from the 2018/19 Crime Survey for England and Wales. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832533/drug‐misuse‐2019‐hosb2119.pdf [last accessed OCT 2019]
- 3. European Monitoring Centre for Drugs and Drug Addiction (2019), European Drug Report 2019: Trends and Developments, Publications Office of the European Union, Luxembourg. Available from: http://www.emcdda.europa.eu/system/files/ publications/11364/20191724_TDAT19001ENN_PDF.pdf [last accessed OCT 2019]
- 4. Home Office of the United Kingdom . Psychoactive Substances Act 2016. Available from: https://www. gov.uk/government/collections/psychoactive‐substances‐bill‐2015. [last accessed OCT 2019]
- 5. Haden M, Wood DM, Dargan PI. The impact of the psychoactive substances act 2016 on the online availability of MDMB‐CHMICA. QJM. 2017;110(10):619‐622. [DOI] [PubMed] [Google Scholar]
- 6. Home Office . Review of the Psychoactive Substance Act 2016. November 2018. Available from: https://assets.publishing.service.gov.uk/government/uploads/syste m/uploads/attachment_data/file/756896/Review_of_the_Psychoactive_Substances_Act__2016___web_.pdf [last accessed OCT 2019]
- 7. Scourfield A, Flick C, Ross J, et al. Synthetic cannabinoid availability on darknet drug markets—changes during 2016–2017. Tox Commun. 2019;3(1):7‐15. [Google Scholar]
- 8. Bretteville‐Jensen AL, Tuv SS, Bilgrei OR, Fjeld B, Bachs L. Synthetic cannabinoids and cathinones: prevalence and markets. Forensic Sci Rev. 2013;25(1‐2):7‐26. [PubMed] [Google Scholar]
- 9. Webb NE, Wood DM, Greene SL, et al. Change in the new psychoactive substances associated with emergency department acute toxicity presentations associated with the introduction of the UK 2016 psychoactive substances act. Clin Tox. 2019;57(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 10. Thomas SHL, Dunn MD, Hill SL, et al. Changes with time in analytically confirmed exposure to novel psychoactive substances (NPS) in patients with severe clinical toxicity in the UK. Clin Tox. 2018;56:499‐500. [Google Scholar]
- 11. Dine AM, Wood DM, Yates C, et al. Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European drug emergencies network (euro‐DEN). Clin Toxicol. 2015;53(9):893‐900. [DOI] [PubMed] [Google Scholar]
- 12. Helander A, Bäckberg M. Epidemiology of NPS based confirmed overdose cases: the STRIDA project. Handb Exp Pharmacol. 2018;252:461‐473. [DOI] [PubMed] [Google Scholar]
- 13. Vogels N, Brunt TM, Rigter S, Van Dijk P, Vervaeke H, Niesink RJM. Content of ecstasy in the Netherlands: 1993‐2008. Addiction. 2009;104(12):2057‐2066. [DOI] [PubMed] [Google Scholar]
- 14. Davies S, Wood DM, Smith G, et al. Pruchasing ‘legal highs’ on the internet – is there consistency in what you get? QJM. 2010;103(7):489‐493. [DOI] [PubMed] [Google Scholar]
- 15. Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG 'legal highs' in the UK: identification and formation of novel cathinones. Drug Test Anal. 2011;3(9):569‐567. [DOI] [PubMed] [Google Scholar]
- 16. Araújo AM, Valente MJ, Carvalho M, et al. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of 'legal high' packages containing synthetic cathinones. Arch Toxicol. 2015;89(5):757‐771. [DOI] [PubMed] [Google Scholar]
- 17. Van Nuijs AL, Castiglioni S, Tarcomnicu I, et al. Illicit drug consumption estimations derived from wastewater analysis: a critical review. Sci Total Environ. 2011;409(19):3564‐3577. [DOI] [PubMed] [Google Scholar]
- 18. Castiglioni S, Thomas KV, Kasprzyk‐Hordern B, Vandam L, Griffiths P. Testing wastewater to detect illicit drugs: state of the art, potential and research needs. Sci Total Environ. 2014;487:613‐620. [DOI] [PubMed] [Google Scholar]
- 19. Nefau T, Karolak S, Castillo L, Boireau V, Levi Y. Presence of illicit drugs and metabolites in influents and efluents of 25 sewage water treatment plants and map of drug consumption in France. Sci Total Environ. 2013;461–462:712‐722. [DOI] [PubMed] [Google Scholar]
- 20. The Sewage analysis CORe group — Europe (SCORE) . Perspectives on Drugs (POD): Wastewater analysis and drugs — a European multi‐city study. Avaialble from: http://www.emcdda.europa.eu/topics/pods/ waste‐water‐analysis (last accessed Oct 2019)
- 21. Archer JRH, Hudson S, Wood DM, Dargan PI. Analysis of urine from pooled urinals ‐ a novel method for the detection of novel psychoactive substances. Curr Drug Abuse Rev. 2013;6(2):86‐90. [DOI] [PubMed] [Google Scholar]
- 22. Archer JRH, Dargan PI, Hudson S, et al. Taking the pissoir – a novel and reliable way of knowing what drugs are being used in nightclubs. J Subst Abuse. 2013;19:103‐107. [Google Scholar]
- 23. Kinyua J, Negreira N, Miserez B, et al. Qualitative screening of new psychoactive substances in pooled urine samples from Belgium and United Kingdom. Sci Total Environ. 2016;573:1527‐1535. [DOI] [PubMed] [Google Scholar]
- 24. Archer JRH, Dargan PI, Hudson S, Wood DM. Analysis of anonymous pooled urinals in Central London confirms the signicant use of novel psychoactive substances. QJM. 2013;106(2):147‐152. [DOI] [PubMed] [Google Scholar]
- 25. Archer JRH, Hudson S, Jackson O, et al. Analysis of anonymized pooled urine in nine UK cities: variation in classical recreational drug, novel psychoactive substance and anabolic steroid use. QJM. 2015;108(12):929‐933. [DOI] [PubMed] [Google Scholar]
- 26. Archer JRH, Dagan PI, Lee HMD, Hudson S, Wood DM. Trend analysis of anonymised pooled urine from portable street urinals in Central London identifies variations in the use of novel psychoactive substances. Clin Toxicol. 2014;52(3):160‐165. [DOI] [PubMed] [Google Scholar]
- 27. European Monitoring Centre for Drugs and Drug Addiction and Europol . Drugs and the darknet: perspectives for enforcement, research and policy. Luxembourg: EMCDDA–Europol Joint publi‐ cations, Publications Office of the European Union; 2017. Available from: https://www.europol.europa.eu/publications-documents/drugs-and-darknet-perspectives-for-enforcement-research-and-policy [last accessed Oct 2019]
- 28. WEDNOS . Annual Report 2016/17. Available from: https://www.wedinos.org/ resources/downloads/Philtre_Annual_Report_2016–17.pdf [last accessed Oct 2019]
- 29. Home Office . A change to the Misuse of Drugs Act 1971. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/574495/Home_Office_Circular_-_SC_and_Dienedione.pdf []
- 30. National Poisons Information Service Report 2018/2019. Available from: http://www.npis.org/NPISAnnualReport2018-19.pdf []
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


