Aims
It remains uncertain whether statin use is associated with the risks of tuberculosis (TB) and herpes zoster in patients with type 2 diabetes. This study aims to assess the effects of statins vs nonstatin lipid‐lowering agents on the risk of these infectious diseases in patients with diabetes.
Methods
Participants in the Taiwan National Health Insurance Research Database diagnosed with type 2 diabetes in 2001–2013 were classified as statin users, nonstatin users and lipid‐lowering drug‐free groups. Participants were observed for incident TB and herpes zoster from diabetes diagnosis until treatment crossover or December 2013. Statin user and nonstatin user were the time‐dependent variables in Cox regression analysis.
Results
Over 240 782 person‐years of observation, statin users (n = 17 696) were associated with a lower TB risk than nonstatin users (n = 5327) and the drug‐free group (n = 22 316) (adjusted hazard ratio [aHR]: 0.66; 95% confidence interval [CI]: 0.44–0.99 and aHR: 0.57; 95% CI: 0.44–0.73). Compared with nonstatin users, statin users showed a dose‐dependent association with TB risk (low‐potency statin users, aHR: 0.692; 95% CI: 0.455–1.053; high‐potency users, aHR: 0.491; 95% CI: 0.241–0.999). Statin users presented with a higher risk of herpes zoster than nonstatin users and the drug‐free group (aHR: 1.23; 95% CI: 1.01–1.50 and aHR: 1.20; 95% CI: 1.09–1.33). The risks of TB and herpes zoster were not statistically different between nonstatin users and the drug‐free group.
Conclusion
Compared with nonstatin drugs, statin use was specifically associated with a decreased risk of TB but a moderately increased risk of herpes zoster in this cohort study.
Keywords: diabetes mellitus, herpes zoster, lipid‐lowering agent, statins, tuberculosis
What is already known about this subject
Statins have demonstrated associations with decreased tuberculosis (TB) risk and increased risk of herpes zoster in observational studies without active comparator design.
It remains uncertain whether the use of statins vs nonstatin lipid lowering agents is associated with the risks of both infection, especially in high‐risk patients with diabetes.
What this study adds
Among patients with diabetes, statin users presented with a decreased risk of TB compared with nonstatin users although they carried an increased risk of herpes zoster.
With respect to host‐direct therapy for TB, further prospective studies are warranted to assess the effect of statins on TB prevention and treatment.
1. INTRODUCTION
Diabetes is a metabolic disease affecting 422 million people globally, and type 2 diabetes mellitus accounts for 90% of cases.1 Patients with diabetes have a higher risk of contracting infection diseases than those without.2 Besides bacterial infection,3 patients with diabetes are prone to viral diseases including herpes zoster, a common illness due to reactivation of latent varicella‐zoster virus infection.4 More importantly, patients with diabetes carry a 3‐fold increased risk for tuberculosis (TB), a contagious disease caused by Mycobacterium tuberculosis (Mtb).5 Although the population attribution fraction of type 2 diabetes for TB is considerably high at 11–15%, preventive strategies for TB in patients with diabetes remain uncertain.5, 6
Statins are lipid‐lowering agents and are commonly prescribed to patients with diabetes who have hyperlipidaemia and are at risk of vascular events.7 Statins have immunomodulatory properties but the effect of statins on prevention of infections remains inconclusive.8 Notably, statins have demonstrated anti‐TB effects in in vitro and animal studies and been considered as a potential host‐direct anti‐TB therapy.9, 10 Subsequent studies have reported that statins were associated with a lower risk of TB in the general population,11, 12 although one cohort study in patients with diabetes found no difference in the risk between statin‐users and nonusers.13 Nevertheless, no active‐drug comparator has been employed to avoid biased estimates in these studies. Interestingly, despite the potential benefits, studies have noted that statin‐users carry an increased risk of herpes zoster compared with nonusers, although the pathogenic role of statins or hyperlipidaemia itself remains uncertain without using an active comparator design.4, 14, 15 To clarify the opposite‐direction effects of statin on TB and herpes zoster in an at‐risk population, particularly patients with diabetes, it is necessary to assess both outcomes simultaneously in a cohort using an active‐drug comparator as a control group.8, 16 Nonstatin lipid‐lowering agents, which include fibrates, cholesterol absorption inhibitors, niacin and bile acid sequestrants, are available alternatives for statin‐intolerant patients. However, although statins and nonstatin drugs have been widely used in patients with diabetes and the general population,17 no studies have compared the effects of statins and nonstatin drugs on risk of TB and herpes zoster in patients with diabetes.
It is important to clarify the TB‐reducing effects of statin vs nonstatin lipid‐lowering agents before statins are prescribed as host‐direct therapies for TB in high risk patients with diabetes. In addition, it is worthwhile to assess the contrary in effects of statins on the risk of TB and herpes zoster in the same cohort. Hence, we designed an active‐comparator cohort study in subjects with diabetes to assess the effects of statins on the risks of TB and herpes zoster.
2. METHODS
2.1. Design and data source
This was a retrospective cohort study in subjects with and without statins or nonstatin lipid‐lowering agent treatment after diagnosis of type 2 diabetes. The data source was the Longitudinal Health Insurance Database (LHID), which contains the comprehensive healthcare data of 1 000 000 representative samples from all the beneficiaries of the National Health Insurance program in Taiwan. The LHID is one subset of the National Health Insurance Research Database (NHIRD), and the accuracy of the major diagnosis codes in the NHIRD has been assessed previously.12, 18 The Institutional Review Board of Taipei Veterans General Hospital approved this study employing de‐identified secondary data (2015–04‐004 AC).
2.2. Population
Participants aged ≥20 years with a type 2 diabetes diagnosis that presented in ≥1 inpatient or ≥ 4 outpatient records (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9] codes: 250.x0 and 250.x2) without type 1 diabetes (codes: 250.x1 and 250.X3) were identified in records from 2000 to 2013.18 All study participants were validated as having oral antidiabetic agent and/or injectable insulin prescriptions.19 The date of first‐time diabetes diagnosis was the index date. Because participants should have a preceding year for comorbidities and comedication review, subjects with diabetes diagnosed during 2001–2013 were included and those in 2000 were excluded. Participant with chronic kidney disease (CKD), a prevalent comorbidity in patients with diabetes, were excluded before analysis to reduce confounders because: (i) CKD is a strong risk factor for infectious diseases and advanced CKD itself is a contraindication for certain nonstatin lipid‐lowering drugs, including fenofibrate20, 21; and (ii) the severity of CKD was difficult to obtain from the NHIRD. Since incident TB, herpes zoster and liver abscess were the outcomes of interest, participants having those conditions before the index date were excluded. Finally, participants receiving any kinds of lipid‐lowering drugs before diabetes diagnosis were excluded to avoid prevalent user bias,22 and those who diagnosed with diabetes in 2001 were confirmed to have no prior use of statins in 2000.
2.3. Exposure classification
All eligible participants were classified according to lipid‐lowering drug exposure status (drug‐free, statin user or nonstatin user) during follow‐up (Figure 1). Statins in this study included the following 7 drugs: simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, and pitavastatin. High‐potency statins were rosuvastatin of ≥10 mg, atorvastatin ≥20 mg or simvastatin ≥40 mg, whereas low‐potency statins were all the others.23 Nonstatin lipid‐lowering agents consisted of several drugs, including fibric acid derivatives (bezafibrate, ciprofibrate, clofibrate, gemfibrozil and fenofibrate), nicotinic acid (niacin), bile acid sequestrants (cholestyramine, colesevelam and colestipol), cholesterol absorption inhibitors (ezetimibe) and others (acipimox, inositol nicotinate and xantinol nicotinate). Drug‐free study subjects were defined as those without using any statins or nonstatin drugs before the primary endpoints. After the exclusion of combined initiators, who started both statins and nonstatin drugs within a 90‐day window, statin initiators were identified as those who initiated statins first, with or without subsequent use of nonstatin drugs, and only those who received ≥28 cumulative defined daily doses (cDDD) of statins were selected as statin users.24 Using a similar rule, nonstatin initiators were defined as those who initiated nonstatin lipid lowering agents first and, among them, nonstatin users were selected if they received ≥28 cDDD of nonstatin drugs. Here, the cDDD of drugs were standardized by using the Anatomical Therapeutic Chemical Classification System with Defined Daily Doses scheme of the World Health Organization. In this study with a new‐user design, statin users and nonstatin users were confirmed to have no period of using statin or nonstatin drugs before therapy initiation.
Figure 1.
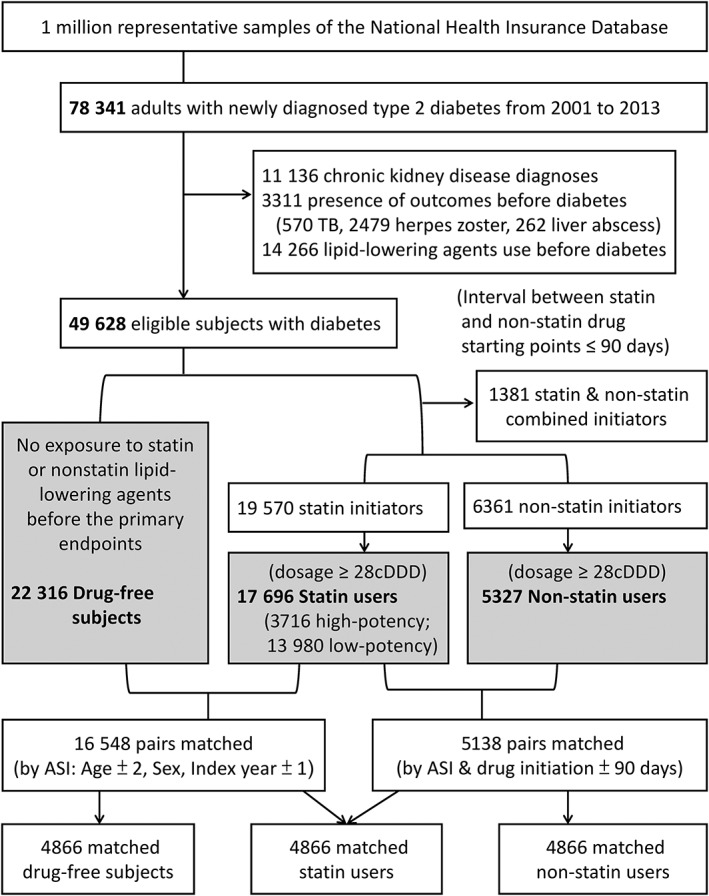
Flow chart of the study. A 1:1:1 matching process is presented in the bottom part of Figure 1. Drug‐free subjects and statin users were matched by age ± 2 years, sex, index calendar year ±1 (3 factors: ASI, n = 16 548), while statin users and nonstatin users were matched by these factors and the length (± 90 days) between the index date and drug‐initiation (n = 5138). Finally, statin users who could be simultaneously matched to subjects in both the drug‐free group and nonstatin user group, and their matched participants in both groups, were selected for further analysis (trios, n = 4866). ASI indicates Age, Sex, and Index calendar year; cDDD, cumulative defined daily dose; TB, tuberculosis
To explore a possible dose–response association between statin use and TB, statin users were further classified into high‐potency and low‐potency statin users. A high‐potency user was defined as a subject using high‐potency statins for >30 cDDD within the 90 days after statin initiation; a statin user not meeting that criterion was classified as a low‐potency user. Nonstatin lipid‐lowering agents were chosen as the active‐drug comparators for statins because they are alternatives to statins and similar methods have been previously introduced.25, 26 Finally, to make the treatment groups appearing comparable regarding certain important covariate,27 the 3 groups of subjects were 1:1:1 matched by age ± 2 years, sex, index calendar year ±1 and the length (± 90 days) between the index date and drug‐initiation (see Figure 1 for detailed matching process).
In this study with a modified intention to treat approach, a statin user or nonstatin user was considered exposed from the date of drug‐initiation forward, even if therapy was discontinued, in a manner as previously described.28 To avoid misclassification, statin users' person‐times were censored on the dates of treatment crossover if they received combined or switched therapy with nonstatin lipid‐lowering agents; they were not considered to have exposure to nonstatin drugs after the time points. This rule was the same for the person‐times of nonstatin users. To test the effects of drug exposure with more accurate observation period, a sensitivity analysis was conducted by using the date of drug discontinuation as another endpoint, which was defined as the date of drug dispensing without further prescriptions for at least 12 months.29
2.4. Outcomes and measurement
The primary outcome was incident TB, which was defined as the presence of ICD‐9 codes 010–018 in ≥1 inpatient or ≥2 outpatient records along with the prescription of ≥2 anti‐TB drugs for ≥28 days within a 180‐day interval, as previously validated.12 The secondary outcomes was incident herpes zoster (codes 053.0–053.9),30 considered as an adverse effect of statins, which should occur before the primary endpoints including TB, along with diagnosis in ≥1 inpatient or ≥ 2 outpatient records. All subjects were observed from the index date to the end points, including the occurrence of outcomes, treatment crossover, death/withdrawal from the national health insurance programme and 31 December 2013.
To test the specificity of the findings by using a negative control outcome, the association between statin use and pyogenic liver abscess (code 572.0) was examined,31 in a manner as previously described.4, 32 Pyogenic liver abscess was selected because: (i) it is common in patients with diabetes; (ii) its diagnostic accuracy in NHIRD may be more reliable than those of other infectious diseases, including pneumonia, because a sonography or computed tomography scan is an essential survey in Taiwan33; and (iii) almost no studies have reported its association with statin use. Thus, a null association with this negative control outcome would support causal inference of our findings.
The covariates of interest included age, sex, severity of diabetes, social economic status as indicated by income level and urbanization, and early exposure to metformin, reported to be associated with a decreased risk of TB.19 The severity of diabetes at baseline was assessed with the adapted diabetes complication severity index (aDCSI) score, as previously used in claim‐data studies.19, 26, 34 Here, the aDCSI score was calculated by diabetes‐related complications that presented in ≥2 outpatient or 1 inpatient records in the preceding and following years of diabetes diagnosis. A metformin early‐initiator was defined if a patient started ≥30 cDDD of metformin treatment in the initial 6 months after diabetes diagnosis. Income level was classified into 3 levels in this study, and urbanization was categorized into urban, suburban and rural areas.19 The comorbidities of interest were chronic obstructive pulmonary disease (ICD‐9 codes 491, 492, and 496) and/or asthma (code 493), malignancy (codes 140–208), liver cirrhosis (codes 571.2 and 571.5) and rheumatoid arthritis (code 714). These covariates and comorbidities were selected and adjusted in the models because they may be associated with the exposure and outcomes. Additionally, to evaluate for residual confounding by lipid control status and by the use of comedication at baseline, primary analysis was repeated in sensitivity analyses to adjust for these cofactors.28 Accordingly, the frequency of lipid profile monitoring at baseline, a potential surrogate for lipid control status, were also identified and assessed in this study (see supplementary material for details).
The nomenclature of drugs listed in this article was confirmed to adhere to the IUPHAR/BPS Guide to PHARMACOLOGY.35
2.5. Statistical analysis
Data are presented as n (%) or as mean (± standard deviation) as appropriate. Because the exposure of statins or nonstatin drugs may start after an immortal‐time from the index date, for all analysis in the original cohort and the matched subcohort, statin users and nonstatin users were coded as drug‐free (unexposed) before drug‐initiation and then recoded as statin users or nonstatin users after the date of drug‐initiation, in a manner as previously described.27, 33 Thus, the person‐years before drug‐initiation in statin users and nonstatin users were classified as drug‐free to calculate the incidence rate of outcomes in the study groups.
Time‐dependent Cox regression models for statin user and nonstatin user were used to compare the risk of outcomes.36, 37 The 95% confidence intervals (95% CIs) were calculated for the adjusted hazard ratios (HRs), and P values of <.05 were considered significant. A marginal Cox model for the matched subcohort was used to account for potential correlation between paired outcomes.27 To account for death as a competing risk for TB disease in this study, a subdistribution hazard model was used in the competing risk analysis (see online supplements for definition of death). The data were analysed in SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and SAS 9.4 software package (SAS Institute, Cary, NC, USA) after data extraction using the SAS.
3. RESULTS
3.1. Characteristics and TB rate in study participants
Among 49 628 eligible study subjects with diabetes, 1381 (2.7%) combined initiators of statins and nonstatin lipid‐lowering agents were excluded, after which 22 316 drug‐free subjects, 17 696 statin users and 5327 nonstatin users were selected (Figure 1). The mean age of participants was 57.7 ± 13.7 years, 54% were male, and the mean follow‐up length was 5.3 ± 5.6 years. The statin users were older than the nonstatin users and had more comorbidities. Statin users and nonstatin users initiated lipid‐lowering agents around 1.9 ± 2.5 and 1.7 ± 2.2 years after diabetes diagnosis (Table 1). Notably, 2993 (16.9%) statin users switched to nonstatin lipid lowering agents and 727 (4.1%) combined statins with nonstatin drugs during follow‐up periods. For the remaining 13 976 statin users without subsequent nonstatin drugs, 43 and 67% of them discontinued statins after their first and third years, respectively (see supplementary material for details).
Table 1.
Characteristics of diabetes patients without lipid lowering agents (drug‐free), with the use of statin (statin users) and nonstatin drugs (nonstatin users); n = 45 339
| Variables | Drug‐free group (n = 22 316)a | Lipid‐lowering drug users | ||
|---|---|---|---|---|
| Statin users (n = 17 696) | Nonstatin (n = 5327) | P valueb | ||
| Age, years | 59.9 ± 15.1 | 56.5 ± 11.7 | 52.7 ± 12.1 | <.001 |
| Male sex | 12 448 (55.8) | 8625 (48.7) | 3330 (62.5) | <.001 |
| aDCSI score | <.001 | |||
| aDCSI = 0 | 14 267 (63.9) | 11 093 (62.7) | 3546 (66.6) | |
| aDCSI = 1 | 3384 (15.2) | 3330 (18.8) | 966 (18.1) | |
| aDCSI ≥2 | 4665 (20.9) | 3273 (18.5) | 815 (15.3) | |
| Level of income | .004 | |||
| High income | 3877 (17.4) | 3373 (19.1) | 929 (17.4) | |
| Intermediate | 8490 (38) | 6730 (38) | 1922 (36.1) | |
| Low income | 9949 (44.6) | 7593 (42.9) | 2476 (46.5) | |
| Urbanization | <.001 | |||
| Urban area | 12.557 (56.3) | 10792 (61) | 3113 (58.4) | |
| Suburban area | 7137 (32) | 5161 (29.2) | 1651 (31) | |
| Rural area | 2622 (11.7) | 1743 (9.8) | 563 (10.6) | |
| Metformin early‐initiator | 6929 (31.0) | 7902 (44.7) | 2443 (45.9) | .124 |
| Comorbidities | ||||
| COPD and/or asthma | 2144 (9.6) | 791 (4.5) | 182 (3.4) | .001 |
| Malignancy | 1389 (6.2) | 390 (2.2) | 72 (1.4) | <.001 |
| Liver cirrhosis | 605 (2.7) | 59 (0.3) | 28 (0.5) | .055 |
| Rheumatoid arthritis | 127 (0.6) | 80 (0.5) | 21 (0.4) | .637 |
| AIDS | 17 (0.1) | 2 | 0 | >.999 |
| Comedication at baselinec | ||||
| Sulfonylurea | 6178 (27.7) | 7604 (43.0) | 2523 (47.4) | <.001 |
| Insulin | 987 (4.4) | 581 (3.3) | 187 (3.5) | .433 |
| DPP‐4 inhibitor | 409 (1.8) | 373 (2.1) | 82 (1.5) | .010 |
| Systemic steroid | 202 (0.9) | 23 (0.1) | 7 (0.1) | >.999 |
| Time to drug initiation, years | ‐ | 1.9 ± 2.5 | 1.7 ± 2.2 | <.001 |
| Total follow‐up years | 5.0 ± 3.6 | 5.9 ± 3.5 | 5.0 ± 3.4 | <.001 |
| Tuberculosis events | 426 (1.9) | 81 (0.5) | 32 (0.6) | .218 |
Continuous data are expressed as mean ± standard deviation and categorical data as number (%).
aDCSI, adapted diabetes complication severity index; AIDS, acquired immunodeficiency syndrome; COPD, chronic obstructive pulmonary disease; DPP‐4 inhibitor, dipeptidyl peptidase‐4 inhibitor.
All comparisons between lipid‐lowering agent‐free patients and statin‐users have P values <.001, except rheumatoid arthritis (P = .107).
Comparisons between statin‐users and nonstatin lipid‐lowering agent‐users.
Defined as initiation of certain drug for ≥30 cumulative defined daily dose in the first 6 months after diabetes diagnosis.
Examination of 240 782 person‐years of follow‐up revealed 539 TB events (81 [0.5%] in statin users, 32 [0.6%] in nonstatin users, and 426 [1.9%] in the drug‐free group), which contributed to an estimated TB incidence of 224 per 100 000 person‐years. After recording of the drug‐unexposed person‐years from statin users and nonstatin users into the drug‐free group, the TB incidences in the statin user, nonstatin user, and drug‐free groups were 115.1, 181.6 and 278.7 per 100 000 person‐years, respectively.
3.2. TB risk in different exposure groups
Using the drug‐free group as the reference group, the risk of TB decreased for the statin users (crude HR, 0.475; 95% CI, 0.373–0.605) but not for the nonstatin users (crude HR, 0.733; 95% CI, 0.511–1.051) in a univariate analysis. Notably, with nonstatin users as the reference group, statin users were associated with a 35% decreased risk of TB (crude HR, 0.648; 95% CI, 0.430–0.976).
In a multivariable analysis, the risk of TB remained low for the statin users compared with the drug‐free group (adjusted HR, 0.567; 95% CI, 0.444–0.725) after adjustment for potential confounders including age, sex, aDCSI, income levels, urbanization, metformin early‐initiator, and comorbidities including chronic obstructive pulmonary disease and/or asthma, malignancy, liver cirrhosis and rheumatoid arthritis. Again, the statin users still had a 34% reduced risk of TB compared with nonstatin users after adjustment (adjusted HR, 0.659; 95% CI, 0.437–0.994; Table 2). When accounting for death as a competing risk for TB, the adjusted subdistribution hazards of TB were 0.658 for statin users (95% CI, 0.437–0.992; P = .045) and 1.159 for the drug‐free group (95% CI, 0.801–1.677; P = .434) compared with nonstatin users (see supplemental material for detailed numbers of deaths in groups).
Table 2.
Incidence of outcomes and multivariate cox regression analysis
| Compared groups | Number of events | Exposure PYsa | Incidence per 100,000 PYs | Drug‐free group as reference | Nonstatin users as reference | ||
|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI)b | P value | Adjusted HR (95% CI)b | P value | ||||
| Primary outcome: tuberculosis | |||||||
| Before matching (original) | |||||||
| Drug‐free (n = 22,316) | 426 | 152 821.8 | 278.7 | 1 | 1.161 (0.806–1.674) | .423 | |
| Statin users (n = 17,696) | 81 | 70 344.7 | 115.1 | 0.567 (0.444–0.725) | <.001 | 0.659 (0.437–0.994) | .047 |
| Nonstatin users (n = 5,327) | 32 | 17 615.4 | 181.6 | 0.861 (0.597–1.241) | .423 | 1 | |
| After 1:1:1 matching | |||||||
| Drug‐free (n = 4,866) | 106 | 42 927.1 | 246.9 | 1c | 1.168 (0.494–2.761)c | .724 | |
| Statin users (n = 4,866) | 19 | 20 837.5 | 91.2 | 0.429 (0.185–0.996) | .049 | 0.501 (0.488–0.515) | <.001 |
| Nonstatin‐users (n = 4,866) | 31 | 15 953.8 | 194.3 | 0.856 (0.362–2.025) | .724 | 1 | |
| Secondary outcome: herpes zoster | |||||||
| Drug‐free (n = 22,639)d | 1209 | 148 986.3 | 811.5 | 1 | 1.025 (0.846–1.241) | .804 | |
| Statin users (n = 17,433) | 647 | 67 620.8 | 956.8 | 1.202 (1.088–1.327) | <.001 | 1.231 (1.011–1.500) | .039 |
| Nonstatin users (n = 5,267) | 118 | 17 107.1 | 689.8 | 0.976 (0.806–1.182) | .804 | 1 | |
| Control outcome: liver abscess | |||||||
| Drug‐free (n = 22,357)d | 160 | 152 360.4 | 105.0 | 1 | 0.902 (0.542–1.502) | .692 | |
| Statin users (n = 17,664) | 60 | 70 124.0 | 85.6 | 0.979 (0.717–1.337) | .894 | 0.883 (0.513–1.519) | .653 |
| Nonstatin users (n = 5,318) | 17 | 17 550.0 | 96.9 | 1.109 (0.666–1.846) | .692 | 1 | |
CI, confidence interval; HR, hazard ratio; PYs, person‐years.
Person‐years of statin users and nonstatin users were coded as drug‐free before initiation of lipid lowering agents and then recoded as statin users or nonstatin users after drug initiation.
The covariates included in the adjusted model were age, sex, aDCSI, income level, urbanization, metformin early‐initiator, and comorbidities including chronic obstructive pulmonary disease and/or asthma, malignancy, liver cirrhosis, and rheumatoid arthritis.
Marginal Cox regression models were used in the analysis for matched subcohort.
The numbers of patients in each group were redefined because patients who developed herpes zoster or pyogenic liver abscess before initiation of lipid lowering agents were reclassified from original tuberculosis‐outcome defined statin users and nonstain users groups into a drug‐free group for secondary outcome analysis. For example, a patient developing both TB after statin‐initiation and herpes zoster before statin‐initiation was defined as a statin user for primary outcome analysis but redefined as drug‐free for herpes‐zoster (secondary) outcome analysis.
3.3. Dose response association, matched analysis and sensitivity analysis
Observation of 13 980 low‐potency statin users and 3716 high‐potency statin users revealed that 71 (0.51%) and 10 (0.27%) subjects developed TB in the two subgroups (P = .056), respectively. Here, for high‐potency users, the mean dosage of high‐potency statins was 85.5 ± 41.4 cDDD within the initial 90 days of therapy. In a multivariable Cox regression analysis using nonstatin users as the reference groups, the risk of TB moderately declined for low‐potency statin users (adjusted HR, 0.692; 95% CI, 0.455–1.053) and markedly decreased for high‐potency statin users (adjusted HR, 0.491; 95% CI, 0.241–0.999).
As shown in Figure 1, in the 3 matched groups, there were 4866 trios, in which mean age (53.1 ± 11.8 years), ratio of male sex (66%) and index year were similar (see supplemental Table S1 for baseline characteristics after matching). In a multivariable analysis using a marginal Cox model, the risk of TB for matched statin users was significantly reduced compared with the matched drug‐free group and nonstatin users, respectively (adjusted HR, 0.429; 95% CI, 0.185–0.996 and adjusted HR, 0.501; 95% CI, 0.488–0.515). The TB risk was not statistically different between the nonstatin user and drug‐free groups (Table 2).
As shown in Table 3, for TB outcome, the results of sensitivity analyses were similar to these of our primary analysis. Notably, the baseline lipid monitoring frequency, a surrogate for lipid control status, was included in an adjusted model but was not associated with TB development (adjusted HR 1.007 [0.965–1.052], P = .734). To assess the synergistic effect between metformin and stains on TB risk, and effect modification by metformin in the relationship between statins and TB risk, subgroups analyses were conducted. However, the results showed no evidence of synergistic effect and effect modification by metformin in our analysis (see supplemental material for details).
Table 3.
Sensitivity analyses of the risk of tuberculosis (TB) and herpes zoster in comparison groups
| Compared groups | Events number | Drug‐free group as reference | Nonstatin users as reference | ||
|---|---|---|---|---|---|
| Adjusted HR (95% CI)a | P value | Adjusted HR (95% CI)a | P value | ||
| Sensitivity analyses for the primary outcome (TB) | |||||
| Drug‐discontinuation date as an additional end point | |||||
| Drug‐free (n = 22 316) | 426 | 1 | 0.830 (0.516–1.337) | .445 | |
| Statin users (n = 17 696) | 38 | 0.602 (0.430–0.842) | .003 | 0.500 (0.285–0.878) | .016 |
| Nonstatin users (n = 5327) | 18 | 1.204 (0.748–1.939) | .445 | 1 | |
| Adjustment for lipid profiles monitoring frequencyb | |||||
| Drug‐free (n = 22 316) | 426 | 1 | 1.179 (0.809–1.718) | .390 | |
| Statin users (n = 17 696) | 81 | 0.558 (0.429–0.725) | <.001 | 0.658 (0.436–0.993) | .046 |
| Nonstatin users (n = 5327) | 32 | 0.848 (0.582–1.235) | .390 | 1 | |
| Adjustment for use of comedications at baselinec | |||||
| Drug‐free (n = 22 316) | 426 | 1 | 1.199 (0.832–1.728) | .331 | |
| Statin users (n = 17 696) | 81 | 0.551 (0.431–0.705) | <.001 | 0.661 (0.438–0.997) | .049 |
| Nonstatin users (n = 5327) | 32 | 0.834 (0.579–1.202) | .331 | 1 | |
| Sensitivity analyses for secondary outcome (herpes zoster) | |||||
| Drug‐discontinuation date as an additional end point | |||||
| Drug‐free (n = 22 639) | 1209 | 1 | 1.061 (0.773–1.456) | .716 | |
| Statin users (n = 17 433) | 284 | 1.288 (1.129–1.469) | <.001 | 1.366 (0.980–1.904) | .066 |
| Nonstatin users (n = 5267) | 40 | 0.943 (0.687–1.294) | .716 | 1 | |
| Accounting for all herpes zoster events (before and after TB) | |||||
| Drug‐free (n = 22 639) | 1224 | 1 | 1.024 (0.847–1.239) | .806 | |
| Statin users (n = 17 433) | 651 | 1.202 (1.089–1.327) | <.001 | 1.231 (1.012–1.499) | .038 |
| Nonstatin users (n = 5267) | 119 | 0.976 (0.807–1.181) | .806 | 1 | |
CI indicates confidence intervals; HR, hazard ratio.
In the Cox model with time‐dependent covariates, person‐years of statin users and nonstatin users were coded as drug‐free before initiation of lipid lowering agents and then recoded as statin users or nonstatin users after drug initiation. The adjusted cofactors in the models were those listed in the footnote b of Table 2.
The lipid profiles monitoring frequency at baseline referred to the sum of numbers of monitoring lipid profiles (including total cholesterol, high‐density lipoprotein, and triglyceride) within the first post‐treatment 6 months in statin users and nonstatin users, and within the initial 6 months after diabetes diagnosis in drug‐free group.
The additional comedication entered into the model were sulfonylurea, insulin, dipeptidyl peptidase‐4 inhibitor and systemic steroid use at baseline.
3.4. Secondary outcomes in different exposure groups
As shown in Table 2, the incidence of herpes zoster was highest in the statin users, followed by the drug‐free group and nonstatin users (956.8, 811.5 and 689.8 per 100 000 person‐years, respectively). The risk of herpes zoster for statin users was significantly increased compared with both the drug‐free and nonstatin user groups, respectively (adjusted HR, 1.202; 95% CI, 1.088–1.327 and adjusted HR, 1.231; 95% CI, 1.011–1.500). The HR of herpes zoster for nonstatin users did not increase compared with the drug‐free group. As presented in Table 3, for herpes zoster outcome, the results of sensitivity analyses showed similar trends to those of the original scenario (see details in supplemental material). The risks of pyogenic liver abscess (the negative control outcome) were not statistically different among the 3 groups (Table 2).
4. DISCUSSION
This large‐scale cohort study found that, among patients with diabetes, statin users presented with a decreased risk of TB compared with nonstatin users. In contrast, statin users carried an increased risk of herpes zoster, but not pyogenic liver abscess. Notably, the risks of TB and herpes zoster were not statistically different between the nonstatin user and drug‐free groups. Thus, our study participants receiving statins were specifically associated with a decreased risk of TB but an increased risk of herpes zoster relative to their comparators.
Patients with diabetes are prone to infectious disease because of complications of diabetes and hyperglycaemia, which are associated with dysfunction of immune cells.2 Particularly, an impaired innate immunity and delayed adaptive immune effector response to Mtb infection could lead to active TB in diabetes.38 For TB prevention by immunomodulation, statins are candidate drugs because they have demonstrated a host‐direct anti‐TB effect and are widely used for all patients with diabetes at sufficient risk of vascular events.7, 10 Ensuing studies have discovered that statin‐users had a decreased risk of incident TB in the general population, although the nondrug comparator design may weaken the robustness of the results.11, 12, 39 Unusually, one cohort study in patients with diabetes reported no TB‐risk difference between statin‐users and nonusers.13 However, this discordant finding may have resulted from time‐lagging bias due to the different time points of the follow‐up starts (index dates) for comparative groups, and a modified design may be needed for clarification.25, 40
In contrast, our study included statin users and nonstatin users, who were the active‐drug comparator reference group, and used a time‐dependent method to assess the treatment effects given universal index dates on diabetes diagnosis.37 Moreover, we adjusted diabetes severity by aDCSI and metformin usage in the analysis because of their associations with TB risk even after exclusion of CKD.19 With these efforts, we demonstrated that statin users were associated with a 34% decreased risk of TB compared with nonstatin users.
Statins, inhibitors of 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase, have both lipid‐lowering and anti‐inflammation effects. In basic research, statins reduce the survival of Mtb in macrophages by promoting autophagy and maturation of the phagosome against Mtb‐induced phagosome immaturation.41 Moreover, combined with a standard anti‐TB regimen, statin accelerates bacillary clearance in the lungs of mice.9 In contrast, nonstatin lipid‐lowering agents are unknown to have anti‐TB effects, except ezetimibe, which has recently presented potential benefits.42 Since statin users and nonstatin users were both likely to have dyslipidaemia and the former were older and had more comorbidities than the latter in this study, statins' effect on TB cannot simply be explained by hyperlipidaemia itself or the healthy user effect.16 Even after controlling for time‐related biases and balancing certain variables in the matched subcohort analysis, the results remained significant.33 Although the underlying mechanism remains unclear, the plausible dose–response relationship between statin and TB risk in our study may also imply the anti‐TB effect of statin. The similar results of a series of sensitivity analyses to our primary analysis may support the robustness of our findings. Thus, this hypothesis‐driven study presents valid findings that may fill the knowledge gap between basic studies and clinical reports without active‐drug comparators.
Despite the potential benefits, statin use may be associated with risks of persistent infections of certain viruses.43 Particularly, observational studies have reported that statin‐users have an increased risk of herpes zoster in both general populations and patients with diabetes, and one of the proposed mechanisms is statin‐induced enhanced immune‐tolerance of regulatory T cells.4, 15, 44 However, without an active comparator, whether hyperlipidaemia itself or immunomodulation of statins plays a pathogenic role in the mechanism of herpes zoster remained unanswered.14 In our study, statin users had a significantly higher risk of herpes zoster than nonstatin users, but this risk was not different between nonstatin users and drug‐free subjects. These findings may imply that the increased risk of herpes zoster is associated with the use of statins but not the use of nonstatin drug. Moreover, the effect of statins should be specific, given that the risks of pyogenic liver abscess were not different between groups in this study. This null association may be supported by findings of a meta‐analysis of data from randomized placebo controlled trials,8 although one case–control study reported an association between statin use and liver abscess in analyses without controlling for severity of diabetes and CKD.30 In brief, our results may imply that statins have an immune‐specific role on incident TB and herpes zoster.
Several limitations of this study should be noted. The first is the possibility of confounding by indication for statins vs nonstatin lipid‐lowering agents. To minimize the deviation from the truth in our primary analysis for TB outcome, we used an active‐drug comparative design as previously reported,25 accounted for death as a competing risk, assessed the dose–response association, and additionally conducted a matched analysis and sensitivity analyses to confirm the results. Second, potential confounders, including herpes zoster vaccine, glycaemic levels, lipid profiles, latent TB infection (LTBI), and smoking status, were not available in the NHIRD. However, vaccines for herpes zoster became available in Taiwan in October 2013, which only covered a 2‐month period of this study, so their impact on our results may be neglected. Regarding diabetes severity, we adjusted the baseline aDCSI score as a surrogate for glycaemic control status in the analysis. Notably, a cross‐sectional study reported that diabetes patients receiving both metformin and statins had a reduced prevalence of LTBI,45 which is a risk factor for active TB.46 Although we observed no synergistic effect between metformin and statins on active TB prevention in our cohort study, this result should be verified by further studies with information on participants' LTBI status. Third, the proportions of statin users who discontinued statins were relatively high (up to 43% and 67% after the first and third years), although the findings may be in line with another cohort study reporting a continuation rate of 53.1%.29 Further study is warranted to assess the reasons for statin discontinuation in patients with diabetes and the impact thereof on the risk of TB development. Finally, our exclusion of CKD patients to reduce confounding may limit the generalizability of our findings. Thus, the positive and negative effects of statins on these infectious diseases in CKD patients and the general population warrant further study.
In conclusion, this cohort study in subjects with diabetes discovered that statin users were specifically associated with a decreased risk of TB but a moderately increased risk of herpes zoster when compared with nonstatin users and a drug‐free group. With respect to statin's role as a candidate host‐direct therapy for TB in patients with diabetes, further prospective studies are warranted to accurately assess the effect of statins on TB prevention and treatment.
COMPETING INTERESTS
The authors do not have any conflicts of interest.
CONTRIBUTORS
Pan S.W., Yen Y.F., Su W.J. and Chan Y.J. conceived the study concept and design. Pan S.W., Chuang P.H. and Yen Y.F. acquired the data. All authors conducted data analysis and interpretation. Pan S.W., Kou Y.R., Yen Y.F. and Chan Y.J. contributed to critical revision of the manuscript. Pan S.W., Yen Y.F., Su W.J. and Chan Y.J. are the guarantors of the paper, taking responsibility for the integrity of the work as a whole.
Supporting information
TABLE S1 Characteristics of diabetes patients of matched subcohort
ACKNOWLEDGEMENTS
The authors thank the National Health Research Institute of Taiwan for providing the National Health Insurance Research Database. The authors also would like to thank Professors I‐Feng Lin and Yaa‐Hui Dong, and colleagues at the Institute of Public Health of National Yang‐Ming University, Taiwan, for statistics consultations.
The authors thank the Taipei Veterans General Hospital for support under grant V107B‐016 and V108B‐039, and the Ministry of Science and Technology, Taiwan, for support under grant MOST 106‐2314‐B‐075‐007, 107‐2314‐B‐075‐057 and 108‐2314‐B‐075‐001.
Pan S‐W, Yen Y‐F, Feng J‐Y, et al. Opposite effects of statins on the risk of tuberculosis and herpes zoster in patients with diabetes: A population‐based cohort study. Br J Clin Pharmacol. 2020;86:569–579. 10.1111/bcp.14142
The authors confirm that the PI for this paper is Sheng‐Wei Pan, and that he is the guarantor of the paper and takes responsibility for the integrity of the work as a whole.
The preliminary results of this study were presented as an abstract in the American Thoracic Society Annual Conference 2019 (ATS‐2019) on 18 May 2019 in Dallas, Texas.
Contributor Information
Wei‐Juin Su, Email: wjsu.mail@gmail.com.
Yu‐Jiun Chan, Email: yjchan@vghtpe.gov.tw.
DATA AVAILABILITY STATEMENT
The dataset used in this study is National Health Insurance Research Database in Taiwan (nhird@nhri.org.tw). This dataset cannot be shared in public but the database is available for investigators who meet the criteria for access to confidential data
REFERENCES
- 1. World Health Organization . Global report on diabetes 2016. http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=6DB95D7B3651050E0BA883AF9C93F79D?sequence=1. Accessed Apr 7, 2016.
- 2. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513‐521. [DOI] [PubMed] [Google Scholar]
- 3. Thomsen RW, Jepsen P, Sørensen HT. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis. 2007;44(9):1194‐1201. [DOI] [PubMed] [Google Scholar]
- 4. Antoniou T, Zheng H, Singh S, Juurlink DN, Mamdani MM, Gomes T. Statins and the risk of herpes zoster: a population‐based cohort study. Clin Infect Dis. 2014;58(3):350‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Global tuberculosis report 2016. http://apps.who.int/medicinedocs/documents/s23098en/s23098en.pdf. Accessed Dec 28, 2016.
- 6. Walker C, Unwin N. Estimates of the impact of diabetes on the incidence of pulmonary tuberculosis in different ethnic groups in England. Thorax. 2010;65(7):578‐581. [DOI] [PubMed] [Google Scholar]
- 7. Cholesterol Treatment Trialists' (CTT) Collaborators , Kearney PM, Blackwell L, et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371(9607):117‐125. [DOI] [PubMed] [Google Scholar]
- 8. van den Hoek HL, Bos WJ, de Boer A, van de Garde EM. Statins and prevention of infections: systematic review and meta‐analysis of data from large randomised placebo controlled trials. BMJ. 2011;343(nov 29):d7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skerry C, Pinn ML, Bruiners N, Pine R, Gennaro ML, Karakousis PC. Simvastatin increases the in vivo activity of the first‐line tuberculosis regimen. J Antimicrob Chemother. 2014;69(9):2453‐2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallis RS, Hafner R. Advancing host‐directed therapy for tuberculosis. Nat Rev Immunol. 2015;15(4):255‐263. [DOI] [PubMed] [Google Scholar]
- 11. Lai CC, Lee MT, Lee SH, et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax. 2016;71(7):646‐651. [DOI] [PubMed] [Google Scholar]
- 12. Su VY, Su WJ, Yen YF, et al. Statin use is associated with a lower risk of TB. Chest. 2017;152(3):598‐606. [DOI] [PubMed] [Google Scholar]
- 13. Kang YA, Choi NK, Seong JM, et al. The effects of statin use on the development of tuberculosis among patients with diabetes mellitus. Int J Tuberc Lung Dis. 2014;18(6):717‐724. [DOI] [PubMed] [Google Scholar]
- 14. Wennerås C, Bloemberg G, Bogdan C. Reply to Raoult. Clin Infect Dis. 2014;59(7):1042‐1043. [DOI] [PubMed] [Google Scholar]
- 15. Matthews A, Turkson M, Forbes H, Langan SM, Smeeth L, Bhaskaran K. Statin use and the risk of herpes zoster: a nested case‐control study using primary care data from the U.K. clinical research practice datalink. Br J Dermatol. 2016;175(6):1183‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salami JA, Warraich HJ, Valero‐Elizondo J, et al. National Trends in nonstatin use and expenditures among the US adult population from 2002 to 2013: insights from medical expenditure panel survey. J Am Heart Assoc. 2018;7 pii: e007132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157‐163. [PubMed] [Google Scholar]
- 19. Pan SW, Yen YF, Kou YR, et al. The risk of TB in patients with type 2 diabetes initiating metformin vs sulfonylurea treatment. Chest. 2018;153(6):1347‐1357. [DOI] [PubMed] [Google Scholar]
- 20. Romanowski K, Clark EG, Levin A, Cook VJ, Johnston JC. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int. 2016;90(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889‐2934. [DOI] [PubMed] [Google Scholar]
- 22. Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta‐analysis of statins. Am J Epidemiol. 2012;175(4):250‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin JY, Eberg M, Ernst P, Filion KB. Statin potency and the risk of hospitalization for community‐acquired pneumonia. Br J Clin Pharmacol. 2017;83(6):1319‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose‐dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64(1):47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strom BL, Schinnar R, Karlawish J, Hennessy S, Teal V, Bilker WB. Statin therapy and risk of acute memory impairment. JAMA Intern Med. 2015;175(8):1399‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu CY, Chen YT, Su YW, Chang CC, Huang PH, Lin SJ. Statin therapy reduces future risk of lower‐limb amputation in patients with diabetes and peripheral artery disease. J Clin Endocrinol Metab. 2017;102(7):2373‐2381. [DOI] [PubMed] [Google Scholar]
- 27. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22(3):557‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mamtani R, Pfanzelter N, Haynes K, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care. 2014;37(7):1910‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang WS, Hu FC, Chen MK, et al. High risk of herpes zoster among patients with advance acute kidney injury‐‐a population‐based study. Sci Rep. 2015;5(1):13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao KF, Cheng KC, Lin CL, Lai SW. Statin use correlates with reduced risk of pyogenic liver abscess: a population‐based case‐control study. Basic Clin Pharmacol Toxicol. 2017;121(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 32. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung SD, Tsai MC, Lin HC. Increased risk of pneumonia following pyogenic liver abscess: a nationwide population‐based study. Int J Infect Dis. 2013;17(8):e634‐e637. [DOI] [PubMed] [Google Scholar]
- 34. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care. 2012;18(11):721‐726. [PubMed] [Google Scholar]
- 35. Alexander SP, Kelly E, Marrion NV, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: overview. Br J Pharmacol. 2017;174(Suppl 1):S1‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaiteerakij R, Petersen GM, Bamlet WR, et al. Metformin use and survival of patients with pancreatic cancer: a cautionary lesson. J Clin Oncol. 2016;34(16):1898‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time‐to‐treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016‐1023. [DOI] [PubMed] [Google Scholar]
- 38. Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berdaï D, Hotton JM, Lechat P, participates of Round Table n° 1 Giens XXV . Comparators (medicinal and non medicinal) for marketing authorization, for public health, for payers and at the European level. Therapie. 2010;65(4):329‐334. [DOI] [PubMed] [Google Scholar]
- 40. Suissa S, Azoulay L. Metformin and the risk of cancer: time‐related biases in observational studies. Diabetes Care. 2012;35(12):2665‐2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parihar SP, Guler R, Khutlang R, et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209(5):754‐763. [DOI] [PubMed] [Google Scholar]
- 42. Tsai IF, Kuo CP, Lin AB, et al. Potential effect of ezetimibe against mycobacterium tuberculosis infection in type II diabetes. Respirology. 2017;22(3):559‐566. [DOI] [PubMed] [Google Scholar]
- 43. Goldstein MR, Mascitelli L, Pezzetta F. The double‐edged sword of statin immunomodulation. Int J Cardiol. 2009;135(1):128‐130. [DOI] [PubMed] [Google Scholar]
- 44. Forero‐Peña DA, Gutierrez FR. Statins as modulators of regulatory T‐cell biology. Mediators Inflamm. 2013;2013:167086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Magee MJ, Salindri AD, Kornfeld H, Singhal A. Reduced prevalence of latent tuberculosis infection in diabetes patients using metformin and statins. Eur Respir J. 2019;53 pii: 1801695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan SW, Kou YR, Hu TM, et al. Assessment of latent tuberculosis infection in psychiatric inpatients: a survey after tuberculosis outbreaks. J Microbiol Immunol Infect. 2016;49(4):575‐583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Characteristics of diabetes patients of matched subcohort
Data Availability Statement
The dataset used in this study is National Health Insurance Research Database in Taiwan (nhird@nhri.org.tw). This dataset cannot be shared in public but the database is available for investigators who meet the criteria for access to confidential data


