Abstract
Aims
Use and misuse of benzodiazepine might be very prevalent in patients with acute psychiatric symptoms, whereas they might be associated with specific adverse events in this population. The study investigated their prevalence in these patients. Secondary objectives were to identify risk factors for misuse of benzodiazepines, and its impact.
Methods
A cohort study was based on the hospital's electronic patient records and conducted in patients aged 18 years and over and admitted to a psychiatric hospital. They were followed up for 84 days or until the end of hospitalisation. Four variables of misuse were built: excessive duration of treatment, type of product, excessive dosage and concomitant benzodiazepines. Backward stepwise multivariate logistic regression analysis was used to assess risk factors for each misuse criterion, on the 1 hand, and impact of benzodiazepine misuse, on the other.
Results
In total, 511 psychiatric inpatients were included with 89.0% of them exposed to benzodiazepine. Discharge prescription included no benzodiazepine or a dosage lower than the maximum dosage prescribed during hospitalisation for 78.2% of patients exposed to benzodiazepine during their stay. Of benzodiazepine treatments, 31.4% were associated with at least 1 misuse criterion. Excessive dosage was associated with age ≥65 years (OR 11.57; 95% confidence interval 4.92–27.17), substance/alcohol use disorders (3.35; 95% confidence interval 1.70–6.62) and parenthood (0.49; 0.25–0.97). Some criteria of benzodiazepine misuse were associated with a higher frequency of adverse events occurring after treatment initiation.
Conclusions
Misuse of benzodiazepines is very common in inpatients with psychiatric disorders. These findings should alert clinicians to comply with clinical recommendations.
Keywords: benzodiazepines, pharmacoepidemiology, psychiatry, quality use of medicines
What is already known about this subject
The use and the misuse of benzodiazepine drugs are widespread and associated with an increase in potentially serious adverse events in the general population.
Patients with psychiatric disorders are highly exposed to benzodiazepines, while they are likely to present with specific adverse events.
There are no specific clinical recommendations for patients with psychiatric disorders despite their particular vulnerability.
What this study adds
One third of inpatients who used benzodiazepine drugs were in a misuse situation during their stay in psychiatric hospital, whereas some criteria of misuse were associated with a higher frequency of some adverse events.
Clinicians should be alerted to the need to prescribe benzodiazepines only as directed by clinical recommendations, especially in the most serious patients.
Withdrawal from benzodiazepines or at least dose reduction were performed in the majority of inpatients with acute symptoms; thus current clinical guidelines may be applicable for patients with psychiatric disorders.
1. INTRODUCTION
Benzodiazepines, which are mainly used to treat anxiety symptoms and insomnia, have been associated with adverse events such as falls1, 2, 3 and road accidents.4 Misuse of benzodiazepines, defined as a use noncompliant with guidelines, has been associated with an increased risk of fractures when they were used over a longer period or at a higher dosage than recommended,5 and with an increased risk of cognitive decline and dementia in older patients when treatment duration was > 3 months and long half‐life products were prescribed.6, 7, 8, 9 Based on the National Institute for Health and Care Excellence guidelines, many countries recommend that benzodiazepine treatment should not exceed 4 consecutive weeks.10, 11, 12
Patients with psychiatric disorders are particularly exposed to benzodiazepines13, 14 and represent a very different population study than those usually considered when assessing the patterns of benzodiazepine use even when considering only older patients.15 Misuse of benzodiazepines might be more frequent when they are prescribed by a psychiatrist.16 Thus, patients with psychiatric disorders might be more likely to misuse these drugs, especially since they present with intense and prolonged anxiety symptoms as well as sleep disorders. Moreover, they may be vulnerable to some adverse effects of benzodiazepines. In patients with schizophrenia, benzodiazepines were associated with a 70% increased risk of death, including suicide, when prescribed at a dosage >0.5× defined daily dose17 and with higher scores of aggression when associated with second‐generation antipsychotics.18 Benzodiazepines are suspected of inducing sedation, ataxia, cognitive impairment and behavioural disinhibition, and of exacerbating psychotic symptoms in schizophrenia patients.19 In borderline personality disorder, disinhibition, loss of control and aggression were observed in persons exposed to benzodiazepines in randomised controlled clinical trials.19 In adolescents with refractory depression, the addition of benzodiazepines to treatment was associated with a higher rate of self‐injurious behaviours, suicidal or not.20 In patients with major depressive disorders, other potential risks reported are falls, cognitive impairment and paradoxical activation.19
Thus, the use and misuse of benzodiazepine might be very prevalent in patients with psychiatric disorders, particularly during periods associated with acute psychiatric symptoms, justifying their prescription such as during psychiatric hospitalisations, whereas they might be associated with specific adverse events in this population. The paucity of studies addressing this issue raise questions about the definitions of the proper use and misuse of benzodiazepines in the psychiatric population, as well as the relevance of applying the same recommendations to this population as those used in the general population.
The objective of the study was to investigate the prevalence of use and misuse of benzodiazepines in patients admitted to a psychiatric hospital. The secondary objectives were: (i) to identify the sociodemographic and clinical characteristics associated with misuse of benzodiazepines; and (ii) to assess the impact of benzodiazepine misuse with respect to the occurrence of some adverse events that have been previously reported to be associated with benzodiazepines.
2. METHODS
2.1. Study design, data source and study population
A cohort study was conducted including all patients aged 18 years and over and admitted to all the acute wards of the Charles Perrens psychiatric hospital (Bordeaux, France), corresponding to around 420 hospital beds, in February or June 2016. These 2 months were chosen to include patients admitted over 2 different periods and seasons to be representative. Patients were included only once. This public hospital includes university and nonuniversity departments. It serves an urban and semirural geographical area of 815 000 inhabitants and has a total active file of 25 000 patients. Included patients were hospitalised for at least 24 h regardless of the indication. Patients who were hospitalised in the emergency unit only for 1 night were excluded. They were followed up for 84 days after the first prescription of a benzodiazepine drug if they were still hospitalised or if they were outpatients in the same hospital allowing data registration, or until the end of hospitalisation. Data collection was based on the hospital's electronic patient records. The latter included all medical, paramedical and nursing observations, as well as the results of paraclinical examinations and drug prescriptions. The study complied with French ethical rules.
2.2. Exposure to benzodiazepines
Benzodiazepines were categorised as hypnotics (lormetazepam, loprazolam, nitrazepam, estazolam, flunitrazepam) or anxiolytics (alprazolam, bromazepam, clobazam, clorazepate, clotiazepam, diazepam, ethyl loflazepate, lorazepam, nordazepam, oxazepam) according to the WHO ATC classification (N05 CD and CF for hypnotics, and N05 BA for anxiolytics).21 Z‐drugs (zolpidem, zopiclone) are hereafter termed hypnotic benzodiazepines. A patient with at least 1 prescription over their hospitalisation was considered as exposed. Because of their nonpsychiatric‐labelled indications in France, injectable forms of benzodiazepines (i.e. diazepam and prazepam) and clonazepam were not considered. We collected information on the prescribed drug, the duration of prescription, the maximum dosage over the hospitalisation and the presence and the dosage on the discharge prescription. The duration of exposure to benzodiazepines included the duration of treatment during the hospitalisation and the duration of treatment that was prescribed on the discharge prescription for patients who had no outpatient follow‐up in the hospital. For patients with an outpatient follow‐up, we added the duration of ambulatory prescriptions over the follow‐up period.
2.3. Benzodiazepine misuse
Misuse of benzodiazepines was defined according to French clinical guidelines22, 23 and summary of product characteristics. Four variables were built and defined as present/absent. The first variable concerned an excessive duration of treatment. The duration of treatment with hypnotic and anxiolytic benzodiazepines should not exceed 28 and 84 consecutive days, respectively. The second variable concerned the type of product: benzodiazepines with a long half‐life (20 h or more: bromazepam, clobazam, clorazepate, diazepam, loflazepate, nordazepam, prazepam, nitrazepam and flunitrazepam) should not be used in patients older than 75 years or in patients older than 65 years and presenting with polypathology. Since the psychiatric population is generally more ill than the general population, the use of long half‐life benzodiazepine in patients older than 65 years was considered as misuse. The last 2 variables concerned dosage and number of concomitant drugs. The daily dosage should not be higher than the maximum dosage recommended in the summary of product characteristics. When a dosage was specified for patients with psychiatric conditions and those older than 65 years this was taken as the reference. Only 1 benzodiazepine drug was to be prescribed at the same time.
2.4. Other collected information
For each patient, demographic variables were collected: age, sex, living alone or not, marital status (couple or single), professional status (working or not) and parenting status (having children or not). Main psychiatric diagnosis (i.e. psychotic disorders, bipolar disorder, alcohol or other substances use disorders, anxiety, depressive, personality, adaptation or eating disorders) and comorbid psychiatric diagnoses (i.e. alcohol or other substances use disorders, anxiety, depressive, personality, adaptation or eating disorders) were defined according to the ICD‐10 classification.24 Clinical variables were also collected: mode of hospitalisation (free or compulsory), length of stay (in days), presence of a chronic somatic disease, number of previous psychiatric hospitalisations (categorised as none, only 1 and ≥2), number of previous suicide attempts (categorised as none, only 1 and ≥2) and coprescription of psychotropic drugs over the present hospitalisation (i.e. antidepressants, antipsychotics, mood stabilisers, drugs used to treat addictions and other sedatives). Information was collected on occurrence of some adverse events that were associated with benzodiazepine use in previous studies: agitation, seclusion and restraints, falls, cognitive problems and confusion, insomnia, suicidal thoughts, delusions, and hallucinations.17, 18, 19, 20, 25, 26 An event was considered as potentially induced by benzodiazepine exposure only if it occurred during the present hospitalisation and after the initiation of a benzodiazepine drug and was collected only in this case.
2.5. Data analysis
Stratification was based on the use of at least 1 benzodiazepine, at least 1 benzodiazepine with hypnotic indication and at least 1 benzodiazepine with anxiolytic indication. Criteria for misuse were reported separately for patients exposed to anxiolytic and hypnotic benzodiazepines. Regarding criteria for duration, only patients with a follow‐up longer than the maximum treatment duration recommended were considered (28 days for hypnotic exposure and 84 days for anxiolytic exposure), to avoid right‐censoring. Data on misuse of a benzodiazepine with a long elimination half‐life in the elderly was gathered at the same time for anxiolytic and hypnotic benzodiazepines owing to the small number of elderly patients. Finally, the presence of at least 1 misuse criterion during benzodiazepine treatments was also reported. Confidence intervals for misuse criteria were computed and shown in Table S2.
Misusers of benzodiazepines were then compared to users compliant with guidelines to determine the characteristics associated with each misuse criterion by using backward stepwise multivariate logistic regression analysis. A model was built for each criterion: duration, dosage, concomitant use of >1 drug and use of long half‐life drugs in older patients. Some independent variables were forced in the models: age, sex and psychiatric diagnoses (psychotic disorders, bipolar disorder, alcohol or other substances use disorders, other psychiatric disorder i.e. anxiety, depressive, personality, adaptation or eating disorders). Other covariates tested were sex, age, living alone, marital status, occupational status, parenting status, history of compulsory hospitalisation, history of attempted suicide, and presence of at least a chronic somatic disease.
The impact of benzodiazepine misuse was assessed by investigating the associations between each misuse criterion and the occurrence of each adverse event when exposed to benzodiazepine, among patients exposed to benzodiazepine, using backward stepwise multivariate logistic regression analysis. All models were adjusted for age, sex, and other psychotropic drugs. Owing to the low number of medical events that occurred, they were grouped into agitation (i.e. seclusion, restraint, agitation), neurological symptoms (i.e. falls, cognitive disturbances), insomnia, psychotic symptoms (i.e. delusions, hallucinations) and suicidal thoughts. The risk of falls and cognitive disturbances was considered altogether because they were described in previous studies as high in the first 2 weeks of benzodiazepine treatment, decreasing thereafter without ever disappearing (28,29). As hypnotic and anxiolytic drugs have similar pharmacodynamics properties, they were pooled in the logistic regression models. Level of significance for tests was .05. Confidence intervals were computed using normal approximation. Statistical analyses were performed using R software (version 3.5.1).
3. RESULTS
3.1. Description of inpatients using benzodiazepines
Based on our inclusion criteria, a study population of 511 psychiatric inpatients was selected over the 2 months of inclusion. Among them, 89.0% were exposed to benzodiazepine. Of these patients, almost all were exposed to benzodiazepine with anxiolytic indications (98.2%), and 14.1% to benzodiazepine with hypnotic indications. The characteristics of patients exposed to benzodiazepine were similar to those of the study population (Table 1): 29.2% had a main diagnosis of psychotic disorder, nearly a half had a mood disorder, bipolar disorder (22.0%) or unipolar depressive disorder (22.0%), and 73.0% had only 1 psychiatric diagnosis. An antipsychotic treatment was prescribed in 60.7% of patients exposed to benzodiazepine, an antidepressant treatment in 27.3% and a mood stabiliser treatment in 21.8%. Of the medical events that occurred after benzodiazepine therapy was initiated, the most common was insomnia, which affected 10.8% of patients (Table 2).
Table 1.
Characteristics of study population according to their benzodiazepine use
| Cohort (n = 511) | Benzodiazepine users (n = 455) | Benzodiazepine hypnotic users (n = 64) | Benzodiazepine anxiolytic users (n = 447) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sociodemographic characteristics | ||||||||
| Female | 253 | 49.5 | 228 | 50.1 | 28 | 43.8 | 223 | 49.9 |
| Age (y), mean (SD) | 43 | (16.5) | 43 | (16.5) | 51 | (16.6) | 44 | (16.4) |
| 18–64 y | 458 | 89.6 | 404 | 88.8 | 50 | 78.1 | 398 | 89.0 |
| 65+ y | 53 | 10.4 | 51 | 11.2 | 14 | 21.9 | 49 | 11.0 |
| Working | 121 | 23.7 | 107 | 23.6 | 12 | 18.8 | 106 | 23.7 |
| Single | 321 | 62.8 | 281 | 61.8 | 41 | 64.1 | 278 | 62.2 |
| Living alone | 233 | 45.6 | 212 | 46.6 | 39 | 60.9 | 209 | 46.8 |
| Medical characteristics | ||||||||
| Compulsory hospitalisation | 140 | 27.4 | 123 | 27.0 | 21 | 32.8 | 119 | 26.6 |
| Hospital stay duration (days), median (IQR) | 16 | (6–36) | 18 | (7–37) | 23 | (6–53) | 18 | (7–37) |
| Previous hospitalisation | ||||||||
| 0 | 206 | 40.3 | 182 | 40.0 | 24 | 37.5 | 178 | 39.8 |
| 1 | 107 | 20.9 | 97 | 21.3 | 16 | 25 | 96 | 21.5 |
| 2+ | 186 | 36.4 | 167 | 36.7 | 23 | 35.9 | 164 | 36.7 |
| Unknown | 12 | 2.3 | 9 | 2.0 | 1 | 1.6 | 9 | 2.0 |
| Suicide attempt | ||||||||
| 0 | 310 | 60.7 | 275 | 60.4 | 36 | 56.3 | 271 | 60.6 |
| 1 | 124 | 24.3 | 109 | 24.0 | 17 | 26.6 | 107 | 23.9 |
| 2+ | 43 | 8.4 | 39 | 8.6 | 4 | 6.3 | 39 | 8.7 |
| Unknown | 34 | 6.7 | 32 | 7.0 | 7 | 10.9 | 30 | 6.7 |
| Main psychiatric diagnosis | ||||||||
| Psychotic disorder | 153 | 29.9 | 133 | 29.2 | 12 | 18.8 | 130 | 29.1 |
| Bipolar disorder | 113 | 22.1 | 100 | 22.0 | 14 | 21.9 | 96 | 21.5 |
| Anxiety disorder | 28 | 5.5 | 26 | 5.7 | 4 | 6.3 | 26 | 5.8 |
| Personality disorder | 26 | 5.1 | 24 | 5.3 | 4 | 6.3 | 23 | 5.1 |
| Adaptation disorder | 34 | 6.7 | 26 | 5.7 | 1 | 1.6 | 26 | 5.8 |
| Unipolar depressive disorder | 107 | 20.9 | 100 | 22.0 | 23 | 35.9 | 100 | 22.4 |
| Alcohol use disorder | 38 | 7.4 | 26 | 5.7 | 6 | 9.4 | 36 | 8.1 |
| Substance use disorder | 11 | 2.2 | 10 | 2.2 | 0 | 0.0 | 10 | 2.2 |
| Eating disorder | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Comorbid psychiatric diagnosis | ||||||||
| Anxiety disorder | 13 | 2.5 | 13 | 2.9 | 1 | 1.6 | 13 | 2.9 |
| Personality disorder | 39 | 7.6 | 37 | 8.1 | 6 | 9.4 | 36 | 8.1 |
| Adaptation disorder | 28 | 5.5 | 26 | 5.7 | 4 | 6.3 | 26 | 5.8 |
| Unipolar depressive disorder | 24 | 4.7 | 22 | 4.8 | 5 | 7.8 | 22 | 4.9 |
| Alcohol use disorder | 41 | 8.0 | 40 | 8.8 | 5 | 7.8 | 40 | 8.9 |
| Substance use disorder | 30 | 5.9 | 28 | 6.2 | 1 | 1.6 | 28 | 6.3 |
| Eating disorder | 2 | 0.4 | 2 | 0.4 | 0 | 0.0 | 2 | 0.4 |
| Number of psychiatric comorbid diagnoses | ||||||||
| 0 | 382 | 74.8 | 332 | 73.0 | 48 | 75.0 | 325 | 72.7 |
| 1 | 86 | 16.8 | 83 | 18.2 | 11 | 17.2 | 82 | 18.3 |
| ≥ 2 | 43 | 8.4 | 40 | 8.8 | 5 | 7.8 | 40 | 8.9 |
| Chronic somatic disease | 141 | 27.6 | 132 | 29.0 | 21 | 32.8 | 129 | 28.9 |
| Other psychotropic drugs prescribed during hospitalisation | ||||||||
| Antidepressant | 129 | 25.2 | 124 | 27.3 | 30 | 46.9 | 123 | 27.5 |
| Antipsychotic | 311 | 60.9 | 276 | 60.7 | 37 | 57.8 | 270 | 60.4 |
| Mood stabilisers | 108 | 21.1 | 99 | 21.8 | 11 | 17.2 | 96 | 21.5 |
| Drug to treat addiction | 72 | 14.1 | 69 | 15.2 | 10 | 15.6 | 67 | 15.0 |
| Other sedative drugs | 36 | 7.0 | 27 | 5.9 | 7 | 10.9 | 25 | 5.6 |
SD: standard deviation; IQR: interquartile range
Table 2.
Medical events that occurred after benzodiazepine treatment was initiated
| Cohort (n = 511) | Benzodiazepine users (n = 455) | Benzodiazepine hypnotic users (n = 64) | Benzodiazepine anxiolytic users (n = 447) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| At least 1 medical event | 130 | 25.4 | 123 | 27.0 | 29 | 45.3 | 120 | 26.8 |
| Agitation criteria | 44 | 8.6 | 42 | 9.2 | 6 | 9.4 | 40 | 8.9 |
| Seclusion | 28 | 5.5 | 28 | 6.2 | 5 | 7.8 | 26 | 5.8 |
| Restraint | 32 | 6.3 | 30 | 6.6 | 5 | 7.8 | 28 | 6.3 |
| Agitation | 18 | 3.5 | 18 | 4.0 | 3 | 4.7 | 17 | 3.8 |
| Neurological symptoms | 20 | 3.9 | 19 | 4.2 | 3 | 4.7 | 18 | 4.0 |
| Fall | 7 | 1.4 | 6 | 1.3 | 0 | 0.0 | 6 | 1.3 |
| Cognitive disturbance | 15 | 2.9 | 15 | 3.3 | 3 | 4.7 | 14 | 3.1 |
| Insomnia | 50 | 9.8 | 49 | 10.8 | 10 | 15.6 | 49 | 11.0 |
| Psychotic symptoms | 52 | 10.2 | 49 | 10.8 | 9 | 14.1 | 49 | 11.0 |
| Delusion | 45 | 8.8 | 42 | 9.2 | 6 | 9.4 | 42 | 9.4 |
| Hallucination | 15 | 2.9 | 15 | 3.3 | 2 | 3.1 | 15 | 3.4 |
| Suicidal thoughts | 17 | 3.3 | 17 | 3.7 | 3 | 4.7 | 17 | 3.8 |
| Misuse criteria | ||||||||
| At least 1 misuse criterion | NA | 143 | 31.4 | 55 | 85.9 | 121 | 27.1 | |
| Too long treatmenta | NA | 59 | 13.0 | 22 | 34.4 | 45 | 91.8 | |
| Concomitanceb | NA | 63 | 13.8 | 49 | 76.6 | 63 | 14.1 | |
| Excessive dosageb | NA | 68 | 14.9 | 28 | 43.8 | 64 | 14.3 | |
| Long half‐life in the elderlyc | NA | 11 | 21.6 | NA | NA | |||
n = 64 hypnotic users who were followed‐up for at least 1 month and n = 49 anxiolytic users who were followed‐up for at least 3 months;
n = 64 hypnotic users and n = 447 anxiolytic users included in the study;
n = 51 patients aged >65 years, hypnotic users and anxiolytic users combined.
3.2. Course of benzodiazepine treatment
The median duration of hospital stay was 18 days (interquartile range [6–36]; Table 1). The maximum dosage over the hospitalisation and dosage of benzodiazepine treatment at the end of hospitalisation are shown in Figure 1. Most patients exposed to benzodiazepine during their hospital stay had a discharge prescription with no benzodiazepine or a dosage lower than the maximum dosage prescribed during hospitalisation (78.2%) and 57.6% no longer had a benzodiazepine on their discharge prescription.
Figure 1.
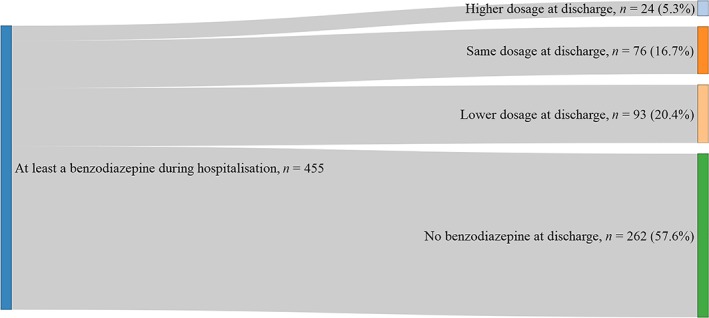
Dosage of benzodiazepine treatment at discharge from hospital in comparison with the maximum dosage during the hospitalisation in patients who were prescribed benzodiazepine at least once during the hospitalisation
Of the medical events that occurred after benzodiazepine therapy was initiated, the most common were insomnia and psychotic symptoms (Table 2). The occurrence of agitation was almost as high. Neurological symptoms (cognitive problems or falls) and suicidal thoughts were the 2 events that occurred least frequently. Among patients exposed to benzodiazepine, 27% presented with at least 1 adverse event occurring after treatment initiation.
3.3. Benzodiazepine misuse, associated factors and adverse events
Among patients exposed to benzodiazepines, 31.4% had at least 1 misuse criterion irrespective of the indication (Table 2). Treatment duration was longer than recommended in 34.4% of hypnotic treatments and 91.8% of anxiolytic treatments (Table 2). The prescription of at least 2 concomitant benzodiazepines concerned 76.6% of patients exposed to hypnotics versus 14.1% of patients with anxiolytics. At least a prescription of benzodiazepine with a dosage exceeding recommendations was present in 43.8% of hypnotic treatment and 14.3% of anxiolytic treatment. The use of long half‐life benzodiazepines (diazepam, prazepam or bromazepam) was found in 21.6% of the 51 patients aged ≥65 years.
Concerning factors associated with misuse, we did not find any factor associated with an excessive duration of benzodiazepine treatment (Table 3). The use of at least 2 benzodiazepines simultaneously was more frequent in patients living alone. Benzodiazepine use at a higher dose than recommended was more frequent in patients older than 65 years and in patients with substance or alcohol use disorders. However, having children was associated with a decreased risk of having this misuse criterion.
Table 3.
Factors associated with each criterion of benzodiazepine misuse among benzodiazepine users (final multivariate regression models)
| Too long treatment (n = 105) | Concomitant treatment (n = 428) | Excessive dosage (n = 428) | ||||
|---|---|---|---|---|---|---|
| aOR | 95%CI | aOR | 95%CI | aOR | 95%CI | |
| Male | 0.86 | 0.37–2.02 | 1.34 | 0.75–2.39 | 1.57 | 0.83–2.94 |
| ≥65 y | 1.37 | 0.49–3.83 | 1.67 | 0.75–3.71 | 11.57 | 4.92–27.17 |
| Psychotic disorders | 2.52 | 0.49–13.10 | 0.54 | 0.19–1.48 | 0.75 | 0.29–1.92 |
| Bipolar disorders | 2.29 | 0.52–9.96 | 0.69 | 0.28–1.73 | 1.02 | 0.43–2.45 |
| Substance or alcohol use disorders | 0.53 | 0.15–1.87 | 0.66 | 0.31–1.43 | 3.35 | 1.70–6.62 |
| Other psychiatric disorders | 2.07 | 0.51–8.49 | 1.35 | 0.58–3.17 | 1.06 | 0.49–2.30 |
| Living alone | 2.21 | 0.92–5.34 | 2.43 | 1.36–4.33 | ||
| Working | 0.58 | 0.28–1.22 | ||||
| Single | 1.92 | 0.96–3.87 | ||||
| Having child | 0.49 | 0.25–0.97 | ||||
| Chronic somatic disease | 0.56 | 0.28–1.12 | ||||
aOR: odds ratio adjusted for age, sex, psychiatric diagnoses; 95%CI: 95% confidence interval.
Some criteria of benzodiazepine misuse were associated with a higher frequency of adverse events occurring after treatment initiation (Table 4 and Table S1 for complete models). Among patients exposed to benzodiazepines, receiving a treatment longer than recommended was associated with an increased risk of agitation and suicidal thoughts, while receiving several benzodiazepines simultaneously was associated with an increased risk of psychotic symptoms. Finally, the use of benzodiazepines at doses above the recommendations was associated with an increase in insomnia.
Table 4.
Association between each criterion of benzodiazepine misuse and adverse events among benzodiazepine users (final multivariate regression models) a
| Too long treatment (n = 105) | Concomitant treatment (n = 428) | Excessive dosage (n = 428) | ||||
|---|---|---|---|---|---|---|
| aOR | 95%CI | aOR | 95%CI | aOR | 95%CI | |
| Agitation | 7.88 | 1.63–38.12 | 2.05 | 0.84–4.98 | 1.58 | 0.63–3.96 |
| Neurological symptoms | 0.63 | 0.12–3.20 | 0.90 | 0.19–4.21 | 0.70 | 0.18–2.66 |
| Insomnia | 1.23 | 0.43–3.52 | 1.13 | 0.49–2.63 | 2.18 | 1.00–4.73 |
| Psychotic symptoms | 1.39 | 0.48–4.06 | 2.58 | 1.16–5.75 | 1.07 | 0.40–2.87 |
| Suicidal thoughts | 11.24 | 1.01–125.08 | 2.25 | 0.73–6.99 | 2.95 | 0.89–9.75 |
Full results of analysis are available in supplementary file.
aOR: odds ratio adjusted for age, sex and psychotropic coprescriptions (i.e. antidepressants, antipsychotics, mood stabilisers, drugs used to treat addiction and other sedatives).
4. DISCUSSION
Benzodiazepine use was highly prevalent in inpatients with psychiatric disorders and almost 1/3 of the patients included in the study were misusing them. The proportion of misusers was high for all criteria of misuse, involving between 1/3 and 3/4 of patients prescribed a hypnotic benzodiazepine and between 1/7 and 3/4 of patients prescribed an anxiolytic benzodiazepine. The most common misuse criterion in patients treated with a hypnotic benzodiazepine was the concomitant use of another benzodiazepine, while the most frequent misuse criterion in patients treated with an anxiolytic benzodiazepine was a duration of treatment longer than recommended. The frequency of misuse contrasted with the course of benzodiazepine treatment during hospitalisation. Almost 80% of patients exposed to benzodiazepine had a discharge prescription with no benzodiazepine or a lower dosage than the maximum dosage prescribed during hospitalisation, which is a first step towards withdrawal. This finding demonstrates that extending benzodiazepine treatment might be avoidable in patients with acute psychiatric symptoms. The paucity of previous studies and methodological differences make it difficult to compare past and present findings concerning the frequency of misuse. Most studies to date included outpatients and did not consider the indication (hypnotic or anxiolytic) of the benzodiazepine treatment, but simply limited the treatment duration to a maximum of 4 weeks of treatment.27, 28, 29 This underlines the lack of an international definition of benzodiazepine misuse, even in the general population.
Thus, the proportion of outpatients with psychiatric disorders who are considered long‐term benzodiazepine users is very variable, ranging from 8% of chronic users in Germany30 to 63% in Taiwan.31 Chronic benzodiazepine use (defined as >28 defined daily doses of benzodiazepine treatment on a discharge prescription) was far higher in patients hospitalised for a first schizophrenic episode in Finland, concerning 90% of them.32 Compared to previous findings, the present figures are not as high. In our population, a duration of treatment longer than recommended was associated with agitation and suicidal thoughts. These adverse events occurred after benzodiazepine initiation but might have led to an extension of the prescription duration, so we cannot rule out that the duration was a consequence of these symptoms. However, such events were attributed to this type of treatment in previous studies and the direction of these associations remained unclear. Both directions might co‐exist, i.e. benzodiazepines might induce disinhibition symptoms that might lead to longer treatment duration with the persistence of adverse effects. A long duration of treatment was not associated with falls or cognitive disturbances, which might be due to pharmacological tolerance. These symptoms were reported to be frequent in the first 2 weeks of benzodiazepine treatment and to decrease thereafter, but never disappeared.27, 28
An excessive dosage was not frequent in patients exposed to anxiolytic and the dosage was often reduced before discharge. In a previous study conducted in southern Europe, almost 3/4 of benzodiazepine treatments had a dosage higher than recommended.33 That study found that an excessive dosage was more frequent in patients living alone or presenting with alcohol or substance use disorders. In the present study, it was associated with alcohol or substance use disorders and with older age. As guidelines recommend that older patients suffering or not from psychiatric disorders receive half of the maximum dosage, the association might mean that this recommendation is not applied in hospital settings. The association with alcohol or substance use disorders might be linked to pharmacological tolerance as well as to the occurrence of insomnia under benzodiazepine treatment. However, a paradoxical effect cannot be ruled out as insomnia occurring when exposed to benzodiazepines might be falsely attributed to an insufficient dosage and lead to an increase in the dosage. Patients with children received an excessive dosage less often than the others. They might have been patients newly exposed to benzodiazepine as this prescription might not have been given in ambulatory care in order to preserve the smooth functioning of the family and/or they may present with less severe psychiatric conditions as parenthood may be a proxy of relatively good social adjustment. Living alone was not associated with excessive dosage but was associated with other criteria of misuse, i.e. concomitant use of at least 2 benzodiazepines and a tendency for an excessive duration of treatment. This might reflect the severity of the psychiatric condition that impaired social functioning and/or the difficulty of loneliness.
The use of at least 2 concomitant benzodiazepines was frequent during hypnotic treatments but rare during anxiolytic 1s. This is consistent with a New Zealand study that found that 16% of psychiatric inpatients with benzodiazepine treatment also received zopiclone treatment.34 It is also consistent with a study conducted in southern Europe that found the simultaneous use of 2 benzodiazepines in 22% of patients prescribed benzodiazepines.33 The concomitant use of several benzodiazepines was associated with the occurrence of psychotic symptoms. This might be due to a greater severity of the psychiatric condition that might lead to a prescription channelling bias. Although such a bias cannot be ruled out, the coprescription of psychotropic prescriptions was taken into account in order to minimise it. An alternative explanation might be the induction by benzodiazepines of psychotic symptoms, particularly hallucinations, as previously described,35 particularly with Z‐drugs that are the most often involved in these associations with several benzodiazepines.
The present study has some methodological limitations. First, the associations of benzodiazepine misuse with adverse medical events should be considered with caution regarding a hypothesis of causality. Only events reported as having occurred after initiation of hospital treatment were considered. However, the recording date might not have been the date of occurrence of the event and protopathic biases might be suspected. Moreover, even though some confounding variables were taken into account, residual confounding cannot be ruled out, particularly the 1 linked to psychotropic polypharmacy. Second, the size of our population limited the statistical power of some analyses, particularly concerning the duration of treatment. Hence, only 10% of patients prescribed anxiolytic benzodiazepines could be considered in the models owing to the number of patients lost to follow‐up after hospitalisation, which weakened the identification of factors and events associated with long‐term benzodiazepine use. Moreover, although this public hospital includes university and nonuniversity departments and serves a wide urban and semi‐rural geographical area, only a single hospital was involved in the study, limiting the generalisability of the findings. A selection bias due to a potential season effect on admission to psychiatric hospital might not be excluded despite the choice of 2 different seasons of inclusion. Finally, we considered that benzodiazepines were prescribed for anxiolytic or hypnotic purposes and not for other indications such as to control epilepsy. However, clonazepam and the injectable forms of benzodiazepines, which are mainly used in epilepsy, were not included and benzodiazepines are rarely used in epilepsy as long‐term treatment. Therefore, this bias might concern only very few inpatients in this study.
Despite these limitations, this study is the first to investigate the prevalence, associated factors and adverse events of the use of benzodiazepines noncompliant with guidelines regarding duration, number of products and dosage in a population with psychiatric disorders, whatever the diagnosis. Another strength was the availability of sociodemographic, clinical and diagnostic information.
The use of benzodiazepines noncompliant with guidelines was very common in this population of inpatients with psychiatric disorders. However, benzodiazepine withdrawal or, at least, dose reduction (which is a first step towards withdrawal) was feasible after short periods of time even in most inpatients with acute psychiatric symptoms. As previous studies did not conclude in the effectiveness of the use of long‐term treatment, of excessive doses or of several benzodiazepines used simultaneously in the treatment of psychiatric disorders and that withdrawal seemed possible after short periods of treatment in patients with acute symptoms, current clinical guidelines may be applicable for patients with psychiatric disorders.19, 25, 26 Moreover, some criteria of benzodiazepine misuse were associated with a higher frequency of adverse events. These findings should alert clinicians to the need to prescribe benzodiazepines only as directed by clinical recommendations, especially in the most serious patients.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
A.F.R. and D.B. wrote the study protocol, took part in the discussion of the findings and revised the manuscript.
H.V. and A. Pariente revised the study protocol, took part in the discussion of the findings and revised the manuscript.
A. Panes wrote the study protocol, collected the data, performed statistical analyses and wrote the manuscript.
M.T. wrote the study protocol, took part in the discussion of the findings and supervised the writing of the manuscript.
Supporting information
TABLE S1 Multivariate analysis of association between benzodiazepine misuse and medical events among benzodiazepine users
TABLE S2 Percentages and confidence intervals for the misuse criteria
ACKNOWLEDGEMENTS
The authors thank Ray Cooke for copyediting the manuscript.
Arnaud Panes is a recipient of an award from the University of Bordeaux to fund his PhD.
Panes A, Verdoux H, Fourrier‐Réglat A, Berdaï D, Pariente A, Tournier M. Misuse of benzodiazepines: Prevalence and impact in an inpatient population with psychiatric disorders. Br J Clin Pharmacol. 2020;86:601–610. 10.1111/bcp.14165
The authors confirm that the Principal Investigator for this paper is Marie Tournier and that she had direct clinical responsibility for patients
DATA AVAILABILITY STATEMENT
The computerised patient record data used in this study are not freely accessible to external staff of the Charles Perrens psychiatric hospital.
REFERENCES
- 1. Rossat A, Fantino B, Bongue B, et al. Association between benzodiazepines and recurrent falls: a cross‐sectional elderly population‐based study. J Nutr Health Aging. 2011;15(1):72‐77. [DOI] [PubMed] [Google Scholar]
- 2. Pariente A, Dartigues J‐F, Benichou J, Letenneur L, Moore N, Fourrier‐Réglat A. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 3. Uhart M, Odouard E, Carlier C, Maire P, Ducher M, Bourguignon L. Relationship between benzodiazepines use and falls in the elderly: a multicenter study in three geriatric centers of a university hospital. Ann Pharm Fr. 2012;70(1):46‐52. 10.1016/j.pharma.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 4. Gustavsen I, Bramness JG, Skurtveit S, Engeland A, Neutel I, Mørland J. Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Med. 2008;9(8):818‐822. 10.1016/j.sleep.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 5. van der Hooft CS, Schoofs MWCJ, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276‐282. 10.1111/j.1365-2125.2008.03185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer's disease: case‐control study. BMJ. 2014;349:g5205 10.1136/bmj.g5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231 10.1136/bmj.e6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Billioti de Gage S, Pariente A, Bégaud B. Is there really a link between benzodiazepine use and the risk of dementia? Expert Opin Drug Saf. 2015;14(5):733‐747. 10.1517/14740338.2015.1014796 [DOI] [PubMed] [Google Scholar]
- 9. Pariente A, de Gage SB, Moore N, Bégaud B. The benzodiazepine‐dementia disorders link: current state of knowledge. CNS Drugs. 2016;30(1):1‐7. 10.1007/s40263-015-0305-4 [DOI] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence . Guidance on the Use of Zaleplon, Zolpidem and Zopiclone for the Short‐Term Management of Insomnia.; 2004. https://www.nice.org.uk/guidance/ta77.
- 11. National Institute for Health and Care Excellence . Generalised Anxiety Disorder and Panic Disorder in Adults: Management. Nice Guideline (CG113).; 2011. https://www.nice.org.uk/guidance/CG113. [PubMed]
- 12. Kurko TA, Saastamoinen LK, Tähkäpää S, et al. Long‐term use of benzodiazepines: definitions, prevalence and usage patterns ‐ a systematic review of register‐based studies. Eur Psychiatry. 2015;30(8):1037‐1047. 10.1016/j.eurpsy.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Peters SM, Knauf KQ, Derbidge CM, Kimmel R, Vannoy S. Demographic and clinical factors associated with benzodiazepine prescription at discharge from psychiatric inpatient treatment. Gen Hosp Psychiatry. 2015;37(6):595‐600. 10.1016/j.genhosppsych.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etchepare F, Pambrun E, Bégaud B, Verdoux H, Tournier M. Compliance of psychotropic drug prescription with clinical practice guidelines in older inpatients. Fundam Clin Pharmacol. 2016;30(1):82‐92. 10.1111/fcp.12167 [DOI] [PubMed] [Google Scholar]
- 15. Franchi C, Rossio R, Ardoino I, Mannucci PM, Nobili A, REPOSI collaborators . Inappropriate prescription of benzodiazepines in acutely hospitalized older patients. Eur Neuropsychopharmacol. 2019;29(7):871‐879. 10.1016/j.euroneuro.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 16. Panes A, Pariente A, Bénard‐Laribière A, et al. Use of benzodiazepines and z‐drugs not compliant with guidelines and associated factors: a population‐based study. Eur Arch Psychiatry Clin Neurosci. 2018;1:1‐8. 10.1007/s00406-018-0966-3 [DOI] [PubMed] [Google Scholar]
- 17. Tiihonen J, Mittendorfer‐Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow‐up study. Am J Psychiatry. 2016;173(6):600‐606. 10.1176/appi.ajp.2015.15050618 [DOI] [PubMed] [Google Scholar]
- 18. Fond G, Boyer L, Favez M, et al. Medication and aggressiveness in real‐world schizophrenia. Results from the FACE‐SZ dataset. Psychopharmacologia. 2016;233(4):571‐578. 10.1007/s00213-015-4167-8 [DOI] [PubMed] [Google Scholar]
- 19. Dell'Osso B, Albert U, Atti AR, et al. Bridging the gap between education and appropriate use of benzodiazepines in psychiatric clinical practice. Neuropsychiatr Dis Treat. 2015;11:1885‐1909. 10.2147/NDT.S83130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brent DA, Greenhill LL, Compton S, et al. The treatment of adolescent suicide attempters study (TASA): predictors of suicidal events in an open treatment trial. J Amn Acad Child Adolesc Psychiatry. 2009;48(10):987‐996. 10.1097/CHI.0b013e3181b5dbe4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2017. https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf. Accessed July 24, 2019.
- 22. Haute Autorité de Santé . Quelle place pour les benzodiazépines dans l'insomnie? 2015. https://www.has-sante.fr/portail/upload/docs/application/pdf/2015-03/bzd_insomnie_v2.pdf.
- 23. Haute Autorité de Santé . Quelle place pour les benzodiazépines dans l'anxiété ? 2018. https://webzine.has-sante.fr/portail/upload/docs/application/pdf/2018-07/fiche_bum_benzodiazepines_anxiete_cd_27062018.pdf.
- 24. World Health Organisation . ICD‐10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organisation; 1992. [Google Scholar]
- 25. Dold M, Li C, Tardy M, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2012;11:CD006391 10.1002/14651858.CD006391.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youssef NA, Rich CL. Does acute treatment with sedatives/hypnotics for anxiety in depressed patients affect suicide risk? A literature review. Ann Clin Psychiatry. 2008;20(3):157‐169. 10.1080/10401230802177698 [DOI] [PubMed] [Google Scholar]
- 27. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295‐301. 10.1111/j.1365-2125.2012.04418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Ann Epidemiol. 1995;5(3):239‐244. [DOI] [PubMed] [Google Scholar]
- 29. Cooperstock R, Hill J. The Effects of Tranquillization: Benzodiazepine Use in Canada. Canada: Minister of National Health and Welfare; 1982. [Google Scholar]
- 30. Smith A. Benzodiazepines ‐ Use & Abuse, A Guide for Prescribers, New Zealand Dept of Health.; 1989. https://www.benzo.org.uk/bznz.htm.
- 31. Wu C‐S, Lin Y‐J, Liu S‐K. Benzodiazepine use among patients with schizophrenia in Taiwan: a nationwide population‐based survey. Psychiatr Serv. 2011;62(8):908‐914. 10.1176/ps.62.8.pss6208_0908 [DOI] [PubMed] [Google Scholar]
- 32. Wiegand HF, Sievers C, Schillinger M, Godemann F. Major depression treatment in Germany‐descriptive analysis of health insurance fund routine data and assessment of guideline‐adherence. J Affect Disord. 2016;189:246‐253. 10.1016/j.jad.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 33. Maric NP, Latas M, Andric Petrovic S, et al. Prescribing practices in southeastern Europe ‐ focus on benzodiazepine prescription at discharge from nine university psychiatric hospitals. Psychiatry Res. 2017;258:59‐65. 10.1016/j.psychres.2017.09.059 [DOI] [PubMed] [Google Scholar]
- 34. Wheeler A, Kairuz T, Sheridan J, McPhee E. Sedative‐hypnotic treatment in an acute psychiatric setting: comparison with best practice guidance. Pharm World Sci. 2007;29(6):603‐610. 10.1007/s11096-007-9096-0 [DOI] [PubMed] [Google Scholar]
- 35. Birnie KI, Stewart R, Kolliakou A. Recorded atypical hallucinations in psychotic and affective disorders and associations with non‐benzodiazepine hypnotic use: the South London and Maudsley case register. BMJ Open. 2018;8(9):e025216 10.1136/bmjopen-2018-025216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Multivariate analysis of association between benzodiazepine misuse and medical events among benzodiazepine users
TABLE S2 Percentages and confidence intervals for the misuse criteria
Data Availability Statement
The computerised patient record data used in this study are not freely accessible to external staff of the Charles Perrens psychiatric hospital.


