Abstract
Aims
Worldwide observational studies are evidencing discordance between guidelines and real‐world practice regarding direct oral anticoagulant drug (DOAC) doses. This systematic review summarizes and evaluate DOACs use in real‐world practice.
Methods
This review was performed following the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines searching PubMed (MEDLINE) and Medscape databases.
Results
Data from 75 studies showed that most of the patients treated with DOACs for stroke prevention in atrial fibrillation received doses in accordance to the guidelines. However, a significant number of patients received off‐label doses (25–50% in most of the studies evaluated). DOAC overdosing was associated with increased all‐cause mortality and worse bleeding events while underdosing was associated with increased cardiovascular hospitalization and, particularly for apixaban, with a nearly 5‐fold increased risk of stroke.
Conclusion
Patients prescribed with off‐label DOAC doses did not receive the full benefit of anticoagulation and presented an increased risk of stroke, bleeding and/or adverse effects.
Keywords: anticoagulants, atrial fibrillation, off‐label use, outcomes assessment, real‐world
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. With a prevalence that has been rising over the last decades, AF is expected to double by 2050.1, 2 AF is associated with heart failure, hospitalization and a 5‐fold increase in the risk of stroke. Therefore, stroke prevention with oral anticoagulants is a critical point in AF management, particularly in patients with additional risk factors.1, 3 Nowadays, the first‐line therapies recommended for stroke prevention in nonvalvular AF (NVAF) are the direct oral anticoagulants (DOACs).4 In Europe, 4 DOACs are currently marketed: dabigatran (a thrombin inhibitor) and rivaroxaban, apixaban and edoxaban (factor Xa inhibitors). DOACs have been preferred over vitamin K antagonists, such as warfarin, due to clinical trials demonstrating that they have a more predictable pharmacokinetic profile, fixed dosing regimen and a lower potential to develop drug–drug interactions. Also, unlike to warfarin, therapeutic drug monitoring was not initially demanded for DOACs.5, 6 However, there is concern about the extrapolation of randomized clinical trials to real‐life patients7 since the reality observed in the idealized clinical trial setting is different from the real clinical practice, where patient characteristics and outcomes differ amongst distinct trials.8 In fact, although fixed doses of DOACs are defined in the guidelines, dose adjustments are currently recommended in the presence of specific clinical conditions, including renal function,9, 10, 11, 12, 13 age and body weight,14, 15 increased bleeding risk (e.g. gastritis, oesophagitis, gastroesophageal reflux)16 and concomitantly administration with other drugs that compromise DOACs systemic exposition and pharmacological effects (e.g. modulators of P‐glycoprotein and/or of cytochrome P450 isoenzymes).10, 17 Therefore, it is important to guarantee that the doses administered to each patient will attain the therapeutic plasma concentration window and are, consequently, therapeutic and safe.6, 15 Overdosing of DOACs is expected to compromise drug safety, by increasing the risk of major bleeding occurrence, while underdosing is expected to increase the risk of systemic embolism or ischaemic stroke.11
Importantly, recent real‐life cases report off‐label DOAC dosing, questioning the impact of underdosing or overdosing on clinical outcomes. Surprisingly, this question remains currently unanswered, prompting the development of the present review. Herein DOACs use will be investigated in real‐world practice in order to assess the correlation between DOAC off‐label doses and treatment effectiveness and safety. To accurately identify underdosed or overdosed patients, the dose adjustment criteria defined by international guidelines must be considered. However, these recommendations are not uniform worldwide, hampering the selection of the preferable posology and the identification of DOAC suboptimal use. Indeed, the approved doses and dose adjustment criteria defined by European Medicine Agency (EMA) and Food and Drugs Administration (FDA) are summarized in Table 1, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 while the recommendations reported by Health Canada regulatory agency, European Society of Cardiology and Japanese guidelines, regarding DOAC dose adjustment in patients with AF, are presented in Table 2.7, 19, 32, 33, 34, 35, 36, 37, 38, 39, 40 Note that Japanese guidelines mention some differences in relation to EMA and FDA, particularly related to the smaller body size, different pharmacokinetic and genetic profiles of Asian populations when compared with European or American patients. All these guidelines were, hence, herein taken into consideration to assess whether DOAC dose adjustment were justified or not and, therefore infer about the impact of DOAC off‐label doses on clinical outcomes of patients with AF.
Table 1.
Dose adjustments for each direct oral anticoagulant drug in accordance with European Medicines Agency and Food and Drugs Administration guidelines16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
| European Medicines Agency (EMA) | Food and Drugs Administration (FDA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | ||
| Recommended dose | 150 mg BID | 20 mg OD | 5 mg BID | 60 mg OD | Recommended dose | 150 mg BID | 20 mg OD | 5 mg BID | 60 mg OD |
| Reduced dose | 110 mg BID | 15 mg OD | 2.5 mg BID | 30 mg OD | Reduced dose | 75 mg BID b | 15 mg OD | 2.5 mg BID | 30 mg OD |
| Dose reduction recommended: | Dose reduction recommended: | ||||||||
| Renal function | – | CrCl 15 − 50 mL/min | CrCl 15 − 29 mL/min | CrCl 15 − 50 mL/min | Renal function | CrCl 15 − 30 mL/min | CrCl 15 − 50 mL/min | Scr ≥ 1.5 mg/dLa or under dialysisb | CrCl 15 − 50 mL/min |
| Scr ≥ 1.5 mg/dLa | |||||||||
| Older age | ≥ 80 y | – | ≥ 80 ya | – | |||||
| Body weight | – | – | ≤ 60 kga | ≤ 60 kg | P‐gp inhibitors |
‐ In patients with CrCl < 30 mL/min: Not recommended ‐ In patients with CrCl 30–50 mL/min: Reduce dose or avoid |
– | Use should be avoided (Also with strong CYP3A4 inhibitors) | – |
| P‐gp inhibitors | Example: Verapamil | – | – | Ciclosporin, dronedaron, eritromicin, cetoconazol | |||||
| Additional conditions when dose reduction need to be considered: | Older age | – | – | ≥ 80 ya | – | ||||
| Older age | 75 − 80 y | – | – | – | |||||
| Renal function | CrCl 30 − 50 mL/min | – | – | – | Body weight | – | – | ≤ 60 kga | – |
| Increased bleeding risk | Patients with gastritis, esophagitis or gastroesophageal reflux | – | – | – | Increased bleeding risk | – | – | – | – |
| Contraindicated | CrCl < 30 mL/min | CrCl < 15 mL/min | No clinical experience in patients with CrCl < 15 mL/min or under dialysis | CrCl < 15 mL/min or under dialysis | Not recommended | CrCl < 15 mL/min | CrCl < 15 mL/min | – | CrCl > 95 mL/minc |
For apixaban 2 of these 3 conditions are required to reduce the dose.
The reduced dose authorization and use in special populations was based on pharmacokinetic data.
This black box warning suggests the use of another anticoagulant for patients with CrCl >95 mL/min and was based on the reported higher rates of ischaemic stroke of these patients.
BID = twice‐a‐day; OD = once‐daily; CrCl = creatinine clearance; y = years; P‐gp = P‐ glycoprotein.
Table 2.
Differences from European Medicines Agency dose adjustments for each direct oral anticoagulant drug according to Health Canada, European Society of Cardiology and Japanese guidelines7, 19, 32, 33, 34, 35, 36, 37, 38, 39, 40
| Health Canada | European Society of Cardiology | Japanese guidelines | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Edoxaban | Dabigatran | Rivaroxaban | Dabigatran | Rivaroxabanb | |||
| Dose reduction recommended: | Dose reduction recommended: |
Recommended dose Reduced dose |
– – |
15 mg OD 10 mg OD |
|||||
| Older age | ≥ 80 y | – | – | Older age | ≥ 80 y | – | |||
| Or older than 75 y plus one risk factor for bleeding: | |||||||||
| Renal impairment | CrCl 30 − 50 mL/min | Renal impairment | CrCl 30 − 49 mL/min | CrCl 30 − 49 mL/min | Dose reduction recommended: | ||||
| Older age | ≥ 70 y | – | |||||||
| Concomitant drugs | P‐gp inhibitors, nonsteroidal anti‐inflammatory drugs and antiplatelet agents | P‐gp inhibitors | Example: Verapamil | – | Renal impairment | CrCl 30 − 50 mL/min | – | ||
| Increased bleeding risk | Predisposition to GI bleeding | Risk of bleeding | HAS‐BLED score ≥ 3a | HAS‐BLED score ≥ 3 | Increased bleeding risk | Previous history of GI bleeding | – | ||
| Not recommended | – | CrCl < 30 mL/min | CrCl < 30 mL/min | – | – | – | P‐gp inhibitors | – | – |
Only described in 2010 ESC guidelines. Other recommendations are according to 2016 ESC guidelines.
Recommendation based on the J‐ROCKET AF trial (Japanese Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), where it was emphasized that the pharmacokinetic profile obtained after administering a 15 mg dose of rivaroxaban to Japanese patients was similar to the 1 reported after an OD oral dose administration of 20 mg to Caucasian patients.
CrCl = creatinine clearance; P‐gp = P‐ glycoprotein; OD = once‐daily, y = years, GI = gastrointestinal, ESC = European Society of Cardiology.
2. METHODS
The present review was conducted following the Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) guidelines. Literature search was systematically performed using PubMed (MEDLINE) and Medscape and there were no date limitations. The searching terms were “atrial fibrillation” AND “dabigatran” OR “rivaroxaban” OR “apixaban” OR “edoxaban” OR “direct oral anticoagulants” OR “DOACs” OR “NOACs” OR “non‐vitamin K antagonist” in combination with “outcomes” OR “stroke” OR “hemorrhage” OR “prognosis” OR “hospitalization” OR “mortality” AND “real‐world” OR “humans”. Exclusion criteria were applied and were excluded all articles in which DOACs were not used to prevent stroke and systemic embolism in patients with NVAF (e.g. use in prevention or treatment of venous thromboembolism and pulmonary embolism and off‐label indications for DOACs). Also excluded were non‐real‐world studies (including information exclusively from randomized controlled trial data), preclinical studies, specific cost‐effectiveness evaluations, perioperative utilization of DOACs, and reversing techniques or antidotes. The search results were limited to human.
Considering the inclusion criteria, all retrospective or prospective real‐world studies, randomized and nonrandomized or observational studies on adult patients with AF and treated with any dose of any of the DOACs, and where DOAC dose adjustments were evaluated were included in this review.
3. RESULTS
3.1. Study identification
After removing duplicates, 750 records were considered for screening based on their title and abstract. Accordingly, 37 articles were excluded (26 because they were not written in English or Spanish and the other 11 because full texts were not available) and, therefore, 713 complete articles were assessed for eligibility. Considering inclusion and exclusion criteria, 75 studies were considered for analysis. A PRISMA diagram of study identification is presented in Figure 1.
Figure 1.
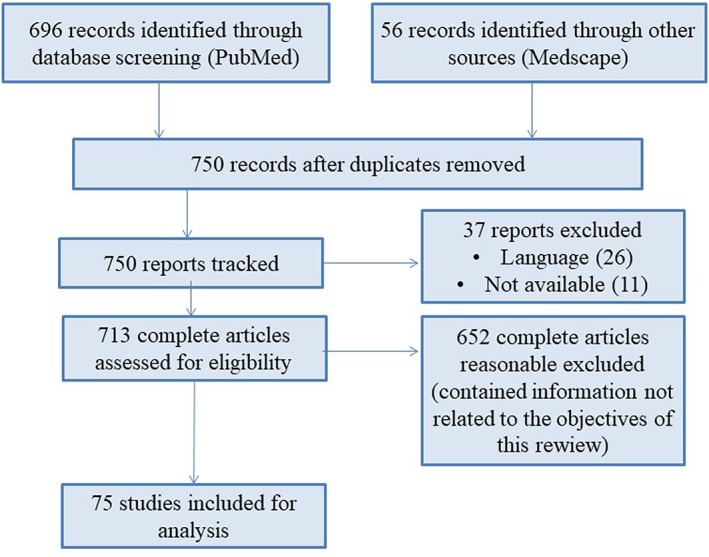
Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) flow diagram detailing study selection
The included studies were all observational, except 2 that can be considered interventional because they assessed the impact of pharmacists in dose appropriateness adjustments. Most of the studies were retrospective; however, some were prospective and some reported results from international registries. Real‐world studies and review articles were analysed to evaluate and compare clinical practice and guideline recommendations. Randomized controlled trials were just used as base of comparison to the presented results.
3.2. Suboptimal DOACs use in clinical practice
Real‐world studies such as REVISIT‐US (real‐world evidence on stroke prevention in patients with atrial fibrillation in the USA)41 highlighted that reduced doses are more frequent in routine clinical practice when compared with clinical trials.42 In accordance with REVISIT‐US, 15.5% patients administered with apixaban received a reduced dose of 2.5 mg, whereas this was only 4.6% in the ARISTOTLE trial. Likewise, a real‐world study conducted by Nguyen et al.43 reported a reduced‐dose prescribing rate of 20.8%, which is also significantly higher than the percentage reported in ARISTOTLE. Regarding rivaroxaban, 17.3% of the patients from REVISIT‐US study41 and 21.7% from Nguyen et al.43 study received a dose of 15 mg, similarly to 20.7% of the patients enrolled in ROCKET AF (reference clinical trial of rivaroxaban).42 SPRINT AF also assessed the prevalence of Canadian patients eligible for the reduced dose of apixaban or rivaroxaban. Accordingly, and in agreement with the mentioned studies, more patients were being prescribed with a reduced dose of the factor Xa inhibitors than those who met the reduction criteria.19 Choosing lower doses reflects a greater concern by the physician to avoid bleeding complications rather than thromboembolic events.44
Real‐world studies demonstrated that low doses of dabigatran and edoxaban are preferentially prescribed and their inappropriate low dosing (ILD) is greater than appropriate low dosing. This trend towards the use of ILD may be due to the reported evidence of the effectiveness of both drugs at low doses.25, 45, 46
3.3. Under and overdosing in clinical practice: Results from real‐world studies
In the present section, data from real‐world studies was collected to evaluate the real use of DOACs in clinical practice (summarized in Table 3).
Table 3.
Real‐world studies summary: quantitative data from patients with atrial fibrillation
| Authors/study (country) | n | DOACS TOTAL | DABIGATRAN | RIVAROXABAN | APIXABAN | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted | Not adjusted | Underdosed | Appropriate | Overdosed | Underdosed | Appropriate | Overdosed | Underdosed | Appropriate | Overdosed | ||
| Barra et al. 5 (USA) | 205 | 94 (45.9%) | 111 patients (54.1%) | NA | 19/59 (32.2% of dabigatran users) | NA | 48/122 (39.3% of rivaroxaban users) | 72/122 (59.0% of rivaroxaban users) | 2/122 (1.7% of rivaroxaban users) | 21/24 (87.5% of apixaban users | 3/24 (12.5% of apixaban users) | NA |
| Andreu Cayuelas et al. 9 (Spain) | 692 | 485 (70.1%) | 207 (29.9%) | NA | 77% of dabigatran users | NA | NA | 69% of rivaroxaban users | NA | NA | 67% of apixaban users | NA |
| Yao et al. 11 (USA) | 14 865 | 12 487 (84%) | 2378 (16%): 12%underdosed; 4% overdosed | 414/4653 (8.9% of patients without RIn) | Total of 3 DOACs combined: 84% | 28/71 (39.4% of those with RIn) | 815/5399 (15.1% of patients without RIn) | Total of 3 DOACs combined: 84% | 425/1029 (41.3% of those with RIn) | 551/3340 (16.5% of patients without RIn) | Total of 3 DOACs combined: 84% | 181/373 (48.5% of those with RIn) |
| Shin et al. 28 (USA) | 3206 | 1811 patients (57%) | 1367 patients (43%): 28% overdosed; 15% underdosed | 107/890 (12% of dabigatran users) | 83% (reduced dose) and 88% (full dose) | 17% (among those who needed dose reduction) | 155/1546 (10% of rivaroxaban users) | 65% (reduced dose) and 90% (full dose) | 35% (among those who needed dose reduction) | 235/1307 (18% of apixaban users) | 85% (reduced dose) and 82% (full dose) | 15% (among those who needed reduced dose) |
| Carlin et al. 44 (Canada) | 47 | 22 (46.8%) | 25 (53.2%) | NA | NA | NA | NA | NA | NA | 19/25 (76.0% of patients that are not adjusted) | 22/47 (46.8%) | 6/25 (24.0%) |
| Bando et al. 47 /SRRT (Japan) | 1339 (453 elderly) | 333/453 (73.5% of elderly group) | 120/453 (26.5% of elderly group) | NA | NA | NA | 93/453 (20.5%)a | 333/453 (73.5%)a | 19/453 (4.0%)a | NA | NA | NA |
| Pattullo et al. 48 (Australia) | 131 | 78 (59.5%) | 53 (40.5%): 13 overdosed (9.9%); 19 underdosed (14.5%); 21 CIn (16.1%) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Camm et al. 49 /XANTUS (Europe, Israel and Canada) | 6784 | 3650 (53.8%) | NA | NA | NA | NA | 592/6784 (8.7%) | 3650/6784 (53.8%) | 230/640 (36.0% of patients with RIn) | NA | NA | NA |
| Brun Guinda et al. 50 (Spain) | 137 | 94 (68.6%) | 43 (31.4%) | NA | NA | NA | 43/112 (38.4% of patients without RIn) | 94/137 (68.6%) | 0 | NA | NA | NA |
| Pharithi et al. 51 (Ireland) | 301 | 154 (51.2%) | 147 (48.8%): 65 underdosed (21.6%); 5 overdosed (1.7%); 36 CIn (11.9%)b | 24/106 (22.7% of dabigatran users)c | 45/106 (42.5% of dabigatran users) | 21/106 (19.8% of dabigatran users): 20 CIn (18.9%); 1 overdosed (< 1.0%) | 24/154 (15.6% of rivaroxaban users)d | 87/154 (56.5% of rivaroxaban users) | 20/155 (13.0% of rivaroxaban users): 16 CIn (10.4%); 4 overdosed (2.6%) | 9/41 (22.0% of apixaban users) | 30/41 (73.2% of apixaban users) | 0/41 (0% of apixaban users)e |
| EDOXABAN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | n | Underdosed | Appropriate | Overdosed | ||||||||
| Okumura et al. 25 /SAKURA AF (Japan) | 1689 | 9/30 (30.0% of edodoxaban users | 19/30 (63.3% of edoxaban users) | 2 patients (6.7% of edoxaban users) | ||||||||
ClCr was unknown in 8 patients (from the 453) so the dose adjustment was not evaluated.
Also in 10 patients (3.3%) was founded inappropriate frequency and in 31 (10.3%) possible drug interactions that can lead to not adjusted doses.
Also 8 patients (7.5%) had inappropriate frequency and 8 (7.5%) had possible drug interactions that can result in a not adjusted dose.
In 2 patients (1.3%), there was an inappropriate frequency and in 21 (13.6%) possible drug interactions that can be considered nonadjustment.
Also 2 patients (4.8%) were taking drugs that can affect DOACs action and consequently the appropriateness of dose adjustment.
AF = atrial fibrillation; CIn = contraindicated; CrCI = creatinine clearance; DOACs = direct oral anticoagulants; NA = nonavailable data; RIn = renal indication for dose reduction.
When analysing the administered DOAC doses, the following must be firstly defined: underdosing—ILD, which corresponds to the administration of low doses of DOACs despite recommendation for standard dose; and overdosing—inappropriate standard dosing, which corresponds to the administration of standard dose of DOACs despite recommendation for dose reduction.
3.3.1. Dabigatran
Results from dose adjustment of the more relevant studies about dabigatran are schemed in Figure 2A. In these studies, frequencies were reported of potentially inappropriate dabigatran prescriptions from 8 to 49% and underdosing was more common than overdosing.52, 53, 62, 63
Figure 2.
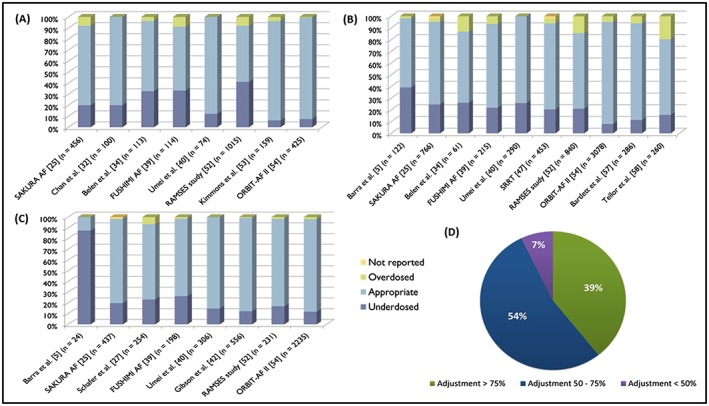
Real‐world studies results concerning dose adjustments of direct oral anticoagulants. (A) Results from dabigatran dose adjustment studies. (B) Results concerning dose adjustments of rivaroxaban. (C) Real‐world studies results concerning dose adjustments of apixaban. (D) Results from direct oral anticoagulants overall adjustments regarding dose appropriateness5, 9, 11, 25, 27, 28, 32, 34, 37, 39, 40, 42, 44, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64
Other real‐world studies with dabigatran, such as that published by Graham et al18 in 2015, concluded that several patients were treated with 75 mg twice a day without having a severe renal impairment that justified dose reduction. In the setting of moderate, mild or no renal impairment, off‐label use of the 75 mg dose might result in underdosed patients and may explain why no differences were observed in risk of ischaemic stroke, major gastrointestinal bleeding or mortality between warfarin and the lower dose of dabigatran. This was also seen in a retrospective study conducted in Japan,37 where 54 patients from 228 (23.7%) presented inappropriate dabigatran prescriptions.
Similarly, data from the GLORIA‐AF registry showed that AF guideline adherence requires improvement, as nearly half of low‐risk patients are overtreated, and that patients at high risk of stroke were undertreated with oral anticoagulants.65 Studies from Larock et al66 McDonald et al67 and Chowdry et al68 also reported high values of potentially inappropriate dabigatran prescriptions (31.2–51.1%), which corroborates the problem of off‐label doses with dabigatran.
However, the study performed by Olesen et al69 used Danish nationwide descriptive data from 2011 to 2013 and concluded that dabigatran is dosed according to guidelines.
3.3.2. Rivaroxaban
Figure 2B schematizes the results from real‐world studies related to rivaroxaban dose adjustments. In the same way, underdosing seems to be more common than overdosing. Studies regarding rivaroxaban reported frequencies of inappropriate prescriptions from 13 to 41%. This underdosing effect was also reported in another Spanish study enrolling 137 patients. Accordingly, all patients with a creatinine clearance (CrCl) <50 mL/min took 15 mg of rivaroxaban, as well as 38.4% of the patients with a CrCl ≥50 mL/min. Moreover, rivaroxaban 15 mg was prescribed incorrectly in 60.1% of the patients older than 75 years. This study showed that the dose of 15 mg was wrongly prescribed, with age being the main cause (87.8%). However, this was not limited to rivaroxaban, but also to the other DOACs50 as will be discussed in subsequent sections. Indeed, in a study with 140 elderly patients taking DOACs, 21 patients (15%) were on an ILD of rivaroxaban (7 patients with 10‐mg dose and 14 patients with 15‐mg dose).12
In the EXPAND study (Evaluation of effectiveness and safety of Xa inhibitor for the prevention of stroke and systemic embolism in a nationwide cohort of Japanese patients diagnosed as NVAF), 1609 of 5326 patients (30.2%) with CrCl ≥50 mL/min used rivaroxaban 10 mg once‐daily (OD), which may have affected the efficacy and safety endpoints.70 Also regarding underdosing, a preplanned pooled analysis of XANTUS (Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation), XANAP (Asia) and XANTUS‐EL (Latin America and EMA Region) evaluated the rivaroxaban dose, according to the label. The results showed that, amongst 5798 patients with a documented CrCl ≥50 mL/min, 1061 (18.3%) received an inappropriate 15‐mg dose of rivaroxaban and 58 (1.0%) received other nonrecommended doses.71
However, overdosing is also an important problem with rivaroxaban use. It can be notice in XANTUS, a relevant Phase IV observational study enrolling patients with AF and prescribed rivaroxaban (results in Table 3).49, 72 Also, Coleman et al73 studied 3319 rivaroxaban treated patients receiving 20 mg of rivaroxaban and stated that >5% of the enrolled patients had a history of stage III or lower chronic kidney disease, and they were not treated in accordance to rivaroxaban labelling, which calls for dose reduction to 15 mg OD.
A real‐world study revealed that, among 1290 NVAF patients treated with rivaroxaban with CrCl ≤50 mL/min (including patients with CrCl <30 mL/min that had been excluded from ROCKET‐AF trial), 35.4% were prescribed with standard dose.74 Similar potential overdosing was found in a Spanish study including 230 patients where, among patients under the dose of 20 mg, 15.3% had a CrCl <50 mL/min and should have been treated with rivaroxaban 15 mg. By contrast, 36.4% of the patients administered with 15 mg had a CrCl ≥50 mL/min when they should have been treated with 20 mg of rivaroxaban,75 showing that underdosing is also significantly present. Similarly, another real‐world study highlighted that 17 patients with a CrCl ≤50 mL/min were prescribed with 20 mg OD and 33 patients with a CrCl >50 mL/min were prescribed with reduced dose of rivaroxaban at baseline.57 Recently, a study performed in Asturias revealed that among patients treated with rivaroxaban 20 and 15 mg, 3.6 and 22.5%, respectively, received inadequate doses.59
3.3.3. Apixaban
Figure 2C summarizes the results of the most relevant studies concerning apixaban dose adjustments in real practice. Like the other 2 DOACs, apixaban underdose was more common than overdose in all these studies. Frequencies of potentially inappropriate apixaban prescriptions were reported to be from 12.9 to 87.5%.5
It is important to note that, in ORBIT‐AF registry II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation phase II), apixaban was the most commonly underdosed DOAC.54 Also, these findings are corroborated by Barra et al.5 study and also by a small Canadian study,44 where a total of 47 patients were included, among whom 25 (53%) were receiving apixaban inconsistent with the ARISTOTLE trial and the product monograph.
In a retrospective study in 3 US hospitals,42 with a total of 556 patients, apixaban was dosed according to FDA labelling by providers in 83.4% (n = 464) of orders. After pharmacist review, 87.1% (n = 484) of orders were at the approved dose, 12.2% (n = 68) were underdosed, and 0.7% (n = 4) were overdosed. Age ≥80 years was the most common reason for underdosing (56%). Of those prescribed a reduced dose in this study, 50% were considered to be underdosed according to the FDA‐approved dose‐reduction criteria, emphasizing the problem of the underdosing with this DOAC.
3.3.4. Edoxaban
SAKURA AF25 is the only study that evaluated edoxaban dose adjustments regarding underdosing and overdosing (Table 3).
3.3.5. Overall
Gathering the information of all these studies, it can be noted that most had adjustment percentages between 50 and 75% (Figure 2D), evidencing the discrepancy between recommended doses and those used in clinical practice.
However, some studies presented lower percentages of off‐label doses, such as a Spanish study conducted in 223 patients from Sant Pau Hospital (137 taking dabigatran and 86 taking rivaroxaban), where only 1.5% of dabigatran users and 14% of the patients taking rivaroxaban initiated the treatment with inappropriate doses.76
From another point of view, in a study at Marshfield Clinic, the most common reasons for nonadherence to protocol for apixaban and rivaroxaban were off‐label indications (11 and 13%, respectively) but also dose too low (11 and 11%, respectively). Age ≥75 years (35%) and off‐label indication (5%) were the most common reasons for dabigatran prescriptions not being per protocol.63
3.4. Impact on clinical outcomes
The previous section evidenced that, in clinical practice, DOAC doses are often prescribed inconsistently with drug labelling specified by FDA, EMA or other organizations. This section aims to understand how that reality affects safety and efficacy of DOACs. Although some real‐world studies do not enrol enough patients to evaluate outcomes, some real‐world data may help to understand the use of off‐label doses and its consequences.
The only 3 studies that report concrete quantitative data linking DOACs dose adjustments (apart from edoxaban) and consequent outcomes, were conducted by Yao et al., 11 Steinberg et al.54 and, only regarding apixaban, Shinoda et al. 77 Their combined results are summarized in Figure 3.
Figure 3.
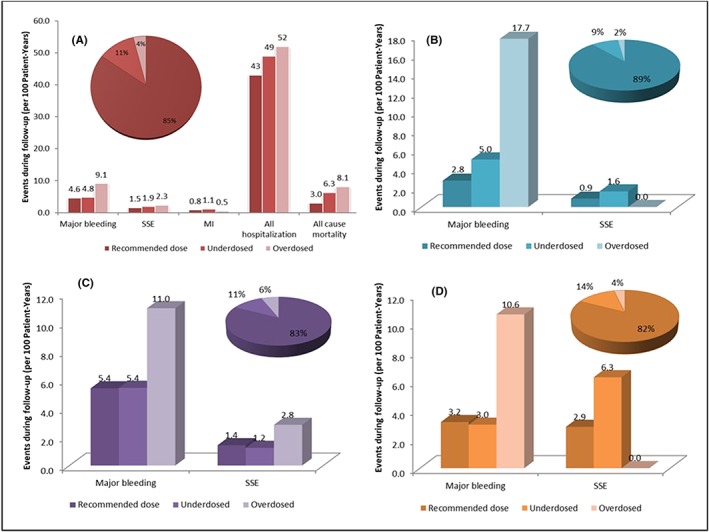
Results from studies that evaluated outcomes according to direct oral anticoagulant dose adjustment. (A) Outcomes results for overall direct oral anticoagulants.11, 54 (B) Outcomes regarding dabigatran dose adjustments.11 (C) Outcomes evaluated according rivaroxaban dose adjustment.11 (D) Results from studies with outcomes regarding apixaban dose adjustments.11, 77 MI = myocardial infarction; SSE = stroke or systemic embolism
3.4.1. Overdosing
DOAC overdosing can be associated with worse outcomes of major bleeding, stroke/systemic embolism, all‐hospitalization and all‐cause mortality (Figure 3A). Among patients taking potentially overdosed DOAC, Yao et al.11 reported higher risk of major bleeding (hazard ratio [HR]: 2.19; 95% confidence interval [CI]: 1.07 to 4.46; P = .03) but with no statistically significant difference benefit in effectiveness (3 DOACs pooled; P = .48). Also, in ORBIT‐AF, DOAC overdosing was, in fact, associated with increased all‐cause mortality compared with recommended doses (adjusted HR: 1.91; 95% CI: 1.02 to 3.60; P = .04). Rates of adverse events were higher in underdosed and overdosed patients than in those receiving the recommended DOAC doses.11, 36, 54
This is particularly important for rivaroxaban, for which overdosing seems to be a concerning problem because this can be related to more cases of major bleeding and stroke/systemic embolism (Figure 3C). In a retrospective study, Bartlett et al57 found that of the 17 patients with a baseline CrCl ≤50 mL/min and no rivaroxaban renal adjustment, 35.3% of the patients presented a bleeding event. Similarly, Burgos et al78 evaluated the characteristics and management of 60 major bleeding events in AF patients taking rivaroxaban, highlighting that higher dose than recommended based on renal function was present in 35% of the cases. Russo‐Alvarez et al79 reported that 29% of rivaroxaban doses were too high at the time of a thromboembolic event, and 33% of the doses were too high at the time of a major bleeding event. Also, the subgroup of patients receiving off‐label doses of rivaroxaban in the XANTUS study71 experienced less favourable outcomes, which was found to be related to both inappropriate dosing and baseline patient characteristics.
However, studies with dabigatran and apixaban also show that overdosing may lead to an increase of bleeding complications when compared with recommended doses (Figures 3B and 3D). Smythe et al80 studied 35 cases of identified major bleedings under dabigatran treatment and concluded that over 1/3 (37.9%) of the patients had an excessive dose considering their renal function. Approximately 50% of dabigatran major bleeding events occurred in those with a CrCl <30 mL/min (contraindication for dabigatran treatment according to EMA and Canadian guidelines). Moreover, in a study that evaluated life‐threatening dabigatran‐related bleeding at a medical centre, Ross et al81 reported a case of gastrointestinal bleeding in a patient receiving an inappropriate standard dose of dabigatran.
3.4.2. Underdosing
By contrast, DOAC underdosing, compared to recommended doses, seems to be related to more myocardial infarction and all‐cause mortality and hospitalization (Figure 3A). It can be seen in ORBIT‐AF,54 where DOAC underdosing was associated with increased cardiovascular hospitalization (Adjusted HR: 1.26; 95% CI: 1.07 to 1.50; P = .007).
Likewise, studies show that underdosing may lead to an ineffective protection against stroke (Figures 3B and 3C). Yao et al. reported that underdosing was associated with a higher risk of stroke particularly in apixaban‐treated patients, where the difference was statistically significant (HR: 4.87; 95% CI: 1.30 to 18.26; P = .02).11 In dabigatran‐ or rivaroxaban‐treated patients without a renal indication there was no statistically significant difference.9 Furthermore, studies suggested that off‐label underdosing of apixaban without following the dose‐reduction criteria is associated with a nearly 5‐fold increased risk of stroke.5, 54 An important result from Ioannou et al82 is that using a reduced dose of apixaban in patients with normal renal function and younger than 75 years was associated with 50% lower plasma concentrations than when treated with standard dose. This can lead to subtherapeutic apixaban plasma levels and predisposes those patients to a higher risk of developing thromboembolic events. Also, Shinoda et al76 reported the difference between the number of patients with stroke episodes receiving appropriate apixaban doses and those receiving intended or unintended ILD doses (1.9% [2 of 105] vs 20.0% [3 of 15], P = .014). These findings corroborate that receiving ILD doses, especially unintended, might be a risk factor for recurrent ischaemic stroke (Figure 3D). Similarly, Kilickiran Avci et al83 also reported an inappropriate reduced dose in 1/3 of the studied patients with NVAF, with a high risk of stroke, what was considered a worrying pattern of practice.
However, Hoyer et al62 studied 99 patients who had stroke receiving DOAC and found no significant difference, at admission, in National Institute of Health Stroke Scale scores—scale of stroke severity—between patients receiving adequately dosed DOAC medication and those with inadequate preadmission DOAC dosage (median National Institute of Health Stroke Scale score 5.5 vs 7, P = .65). When considering SRRT (Shikoku Rivaroxaban Registry Trial) results,47 2 of the 4 elderly patients taking rivaroxaban with cerebral infarction were underdosed. Elderly patients showed high disability at the time of discharge and the risk of death or severe disabilities was high, compared with younger ones, corroborating that dosing criteria should be adhered.
Conversely, bleeding can also be a problem in underdosed patients. Kawabata et al84 collected data from 11 patients with minor bleeding events taking dabigatran and 3 of them were revealed to be underdosed. In a Japanese study, in 5 cases of symptomatic intracranial haemorrhage receiving rivaroxaban 2 of them received an inappropriately reduced dose.38 In SRRT, 4 cases of intracranial haemorrhage were reported in the elderly patients' group, all the cases were of cerebral haemorrhage and 2 of the 4 patients were treated with an inappropriately reduced rivaroxaban dose. Moreover, Schafer et al.27 also studied 33 cases of bleeding where 27% were on a subtherapeutic dose of apixaban. In a retrospective cohort study24 that evaluated 50 major bleeding events in AF patients taking apixaban and more than 1/4 of the patients were on a dose lower than that recommended.
Regarding edoxaban, an Asian study46 reported that 44% of the patients prescribed with 30 mg of edoxaban did not meet the dose‐reduction criteria, which might have affected the outcomes of the study.
4. DISCUSSION
Real‐world studies are currently regarded as an essential resource for evaluating the long‐term safety and effectiveness of DOACs in routine clinical practice, including those patients who may not be represented in randomized controlled trials.85 Few real‐world data are currently available on edoxaban, requiring more studies and clinical reports to be generated for this newest approved DOAC in Europe.
Mild to moderate renal dysfunction, older age a past medical history of bleeding events, and concomitant use of medications that increase bleeding risk, were frequently observed in patients prescribed with a reduced DOAC dose. Renal function dose adjustment has already proven to be a focal point. In a real‐world study with 142 patients, all the 5 observed major bleeding episodes were associated with a decline in renal function compared to baseline.12 Andreu Cayuelas et al.9 also suggest that improvement in monitoring of kidney function in those undergoing therapy with DOAC would be expected to reduce risk for major haemorrhage while also protecting patients from excessive risk for thromboembolism. Globally, it remains crucial to regularly assess the renal function of patients treated with DOACs in order personalize DOAC dose or change to another OAC if renal function declines. These findings indicate a need for continued education on appropriate renal dosing and ongoing monitoring of renal function. Likewise, it is noteworthy that clinical data evidence that administering standard doses of DOACs to patients with severe renal impairment (potential overdosing) double the risk of bleeding with any decrease in stroke risk. This observation suggests that the protection against stroke plateaus with increasing drug exposure. However, DOAC dosing is complex, and the proof is that, despite dose reductions, bleeding events still occur in approximately 20% of the patients. Real‐world bleeding event rates were slightly higher than those reported in randomized clinical trials of DOACs.11 There are several possible reasons for justifying the fact that the real‐world results do not exactly match the results of randomized clinical trials. Frequent use of reduced doses of DOACs may have some influence on unfavourable clinical outcomes. Although reduced doses of DOACs may have a favourable bleeding profile, it may also result in insufficient stroke prevention. Off‐label underdosing of DOAC is also associated with an increased risk for cardiovascular hospitalization.39
In the real‐world studies included in this review, most patients with AF treated with a DOAC in the community were receiving doses according to approved labelling but off‐label doses still very common across all DOACs and dose levels. Likewise, in this review, most of the studies had dose adjustment percentages between 50 and 75% (Figure 2D), which can show the discrepancy between recommended doses by the guidelines and those that are used in clinical practice. The fact that guidelines and drug labelling have some differences in Europe, USA, Japan and Canada undoubtedly complicate the interpretation of the results and the consequent conclusions herein presented. Moreover, another limitation of the studies herein considered is that the majority did not evaluate outcomes for adjusted and non‐adjusted doses separately, hampering understanding of whether the difference were a result of the suboptimal use of DOACs or of the DOAC itself. Some studies also exhibited a very small sample size to successfully detect outcomes, preventing them from being included here. Today, data regarding the potential effects of underdosing or overdosing regimens on DOACs safety and effectiveness are extremely scarce and more investigations must be performed.
Also, by the end of this review, it is evident that a critically important question emerges: are the labelled dose reduction parameters defined in package inserts of DOACs the best practices or should they be overridden? DOAC dose approval and adjustment is different around the world and many DOAC users in real life would not have been eligible to participate in the clinical trials that led to the formulation of dose reduction guidelines.
International and local registries, including ORBIT‐AF I and II, and SAKURA AF and real‐world studies, such as XANTUS, brought new information and highlighted that the management of thromboembolism continues to be suboptimal in certain situations.57 However, these studies are still selective, namely regarding patient recruitment, so the extent of inappropriate dosing, in everyday clinical practice, may be underestimated.11 Another important point is that studies indicated that low‐dose rivaroxaban may be less effective than standard‐dose rivaroxaban without lowering the adverse effect of bleeding.86 However, this can be explained, in part, by clinicians not always prescribing according to the guidelines, introducing this reduced‐dose regimen in patients who should be receiving the standard dose. Therefore, these patients become unintentionally undertreated, which increases their risk of thromboembolic complications.82 Underdosing may have important implications, as rates of systemic embolism, stroke, major bleeding and mortality are generally higher for patients receiving the lower vs higher DOAC dose.39 However, some studies suggest that, in the future, suboptimal low‐dose DOAC therapy may serve as an appropriate choice for some patients with a high risk of stroke and bleeding.40
Pharmacists, doctors and other healthcare professionals are expected to play an important role in ensuring that patients with AF receive the appropriate dosage of DOAC to optimize the risk–benefit ratio of this therapy. They should actively participate on detecting problems such as inadequate doses, nonadherence to the treatment, inadequate patient follow‐up and stopping the DOAC too soon before a surgical procedure—problems that can be the basis of half of haemorrhagic or thrombotic complications.76 In a multicentre retrospective study, pharmacist order review resulted in an overall 3.6% increase in appropriately dosed apixaban orders.42
5. CONCLUSION
The present review supports that most patients treated with DOACs for stroke prevention in AF receive doses according to the guidelines followed in the correspondent country. However, despite approved drug labelling, a significant use of off‐label doses has been identified, revealing suboptimal use of DOAC in clinical practice.
Among the studies included here, only 3 directly correlated DOAC dose adjustment with their specific outcome. In addition, they suggested that under‐ and overdosing of DOACs are associated with increased risk for adverse events. Furthermore, underdosing seems to be associated with increased cardiovascular hospitalization, especially when treated with apixaban. Indeed, its off‐label underdosing was related with an almost 5‐fold increased risk of stroke. DOAC overdosing was associated with increased all‐cause mortality, particularly in patients with impaired renal function, who exhibited worse bleeding outcomes.
More real‐world studies and registries must be performed to understand the impact of under and overdosing of DOACs on clinical outcomes.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
Wrote or contributed to the writing of the manuscript: J.S., M.R., N.A., A.F. The principal investigator for this paper is J.S.
Santos J, António N, Rocha M, Fortuna A. Impact of direct oral anticoagulant off‐label doses on clinical outcomes of atrial fibrillation patients: A systematic review. Br J Clin Pharmacol. 2020;86:533–547. 10.1111/bcp.14127
REFERENCES
- 1. Alamneh EA, Chalmers L, Bereznicki LR. Suboptimal Use of Oral Anticoagulants in Atrial Fibrillation: Has the Introduction of Direct Oral Anticoagulants Improved Prescribing Practices? Am J Cardiovasc Drugs. 2016;16(3):183‐200. 10.1007/s40256-016-0161-8 [DOI] [PubMed] [Google Scholar]
- 2. Lip GY, Mitchell SA, Liu X, et al. Relative efficacy and safety of non‐Vitamin K oral anticoagulants for non‐valvular atrial fibrillation: Network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88‐94. 10.1016/j.ijcard.2015.11.084 [DOI] [PubMed] [Google Scholar]
- 3. Raval AN, Cigarroa JE, Chung MK, et al. Management of Patients on Non‐Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. Circulation. 2017;135(10):e604‐e633. 10.1161/cir.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17(10):1467‐1507. 10.1093/europace/euv309 [DOI] [PubMed] [Google Scholar]
- 5. Barra ME, Fanikos J, Connors JM, Sylvester KW, Piazza G, Goldhaber SZ. Evaluation of Dose‐Reduced Direct Oral Anticoagulant Therapy. Am J Med. 2016;129(11):1198‐1204. 10.1016/j.amjmed.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 6. Vrijens B, Heidbuchel H. Non‐vitamin K antagonist oral anticoagulants: considerations on once‐ vs. twice‐daily regimens and their potential impact on medication adherence. Europace. 2015;17(4):514‐523. 10.1093/europace/euu311 [DOI] [PubMed] [Google Scholar]
- 7. Sanmartin‐Fernandez M, Marzal‐Martin D. Safety of Non‐Vitamin K Antagonist Oral Anticoagulants in Clinical Practice: Focus on Rivaroxaban in Stroke Prevention in Patients With Atrial Fibrillation. Clin Appl Thromb Hemost. 2017;23(7):711‐724. 10.1177/1076029616668404 [DOI] [PubMed] [Google Scholar]
- 8. Noseworthy PA, Yao X, Gersh BJ, Hargraves I, Shah ND, Montori VM. Long‐term stroke and bleeding risk in patients with atrial fibrillation treated with oral anticoagulants in contemporary practice: Providing evidence for shared decision‐making. Int J Cardiol. 2017;245:174‐177. 10.1016/j.ijcard.2017.07.043 [DOI] [PubMed] [Google Scholar]
- 9. Andreu Cayuelas JM, Caro Martinez C, Flores Blanco PJ, et al. Kidney function monitoring and nonvitamin K oral anticoagulant dosage in atrial fibrillation. Eur J Clin Invest. 2018;48(6):e12907 10.1111/eci.12907 [DOI] [PubMed] [Google Scholar]
- 10. Kundu A, Sardar P, Chatterjee S, Aronow WS, Owan T, Ryan JJ. Minimizing the Risk of Bleeding with NOACs in the Elderly. Drugs Aging. 2016;33(7):491‐500. 10.1007/s40266-016-0376-z [DOI] [PubMed] [Google Scholar]
- 11. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non‐Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol. 2017;69(23):2779‐2790. 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 12. Khan F, Huang H, Datta YH. Direct oral anticoagulant use and the incidence of bleeding in the very elderly with atrial fibrillation. J Thromb Thrombolysis. 2016;42(4):573‐578. 10.1007/s11239-016-1410-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz JB. Potential Effect of Substituting Estimated Glomerular Filtration Rate for Estimated Creatinine Clearance for Dosing of Direct Oral Anticoagulants. J Am Geriatr Soc. 2016;64(10):1996‐2002. 10.1111/jgs.14288 [DOI] [PubMed] [Google Scholar]
- 14. Guler E, Babur Guler G, Demir GG, Hatipoglu S. A review of the fixed dose use of new oral anticoagulants in obese patients: Is it really enough? Anatol J Cardiol. 2015;15(12):1020‐1029. 10.5152/AnatolJCardiol.2015.6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Bajorek B. New oral anticoagulants in practice: pharmacological and practical considerations. Am J Cardiovasc Drugs. 2014;14(3):175‐189. 10.1007/s40256-013-0061-0 [DOI] [PubMed] [Google Scholar]
- 16. EMA . Summary of Product Characteristics ‐ Pradaxa. 2018.
- 17. Lippi G, Favaloro EJ, Mattiuzzi C. Combined administration of antibiotics and direct oral anticoagulants: a renewed indication for laboratory monitoring? Semin Thromb Hemost. 2014;40(7):756‐765. 10.1055/s-0034-1381233 [DOI] [PubMed] [Google Scholar]
- 18. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157‐164. 10.1161/circulationaha.114.012061 [DOI] [PubMed] [Google Scholar]
- 19. Turpie AGG, Purdham D, Ciaccia A. Nonvitamin K antagonist oral anticoagulant use in patients with renal impairment. Ther Adv Cardiovasc Dis. 2017;11(9):243‐256. 10.1177/1753944717714921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. FDA . Prescribing Information for Pradaxa. 2018.
- 21. EMA . Summary of Product Characteristics ‐ Xarelto. 2018.
- 22. EMA . Summary of Product Characteristics ‐ Eliquis. 2016.
- 23. EMA . Summary of Product Characteristics ‐ Lixiana. 2015.
- 24. Eisho S, Salem NM, Hoffman JL, Koerber JM, Smythe MA. Major bleeding with apixaban in atrial fibrillation: patient characteristics, management, and outcomes. Hosp Pract (1995). 2018;46(4):165‐169. 10.1080/21548331.2018.1506675 [DOI] [PubMed] [Google Scholar]
- 25. Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythm. 2017;33(4):289‐296. 10.1016/j.joa.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. FDA . Prescribing Information for Xarelto. 2017.
- 27. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and Efficacy of Apixaban Versus Warfarin in Patients With Advanced Chronic Kidney Disease. Ann Pharmacother. 2018;52(11):1078‐1084. 10.1177/1060028018781853 [DOI] [PubMed] [Google Scholar]
- 28. Shin JI, Secora A, Alexander GC, et al. Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation. Clin J Am Soc Nephrol. 2018;13(8):1144‐1152. 10.2215/cjn.13811217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanton BE, Barasch NS, Tellor KB. Comparison of the Safety and Effectiveness of Apixaban versus Warfarin in Patients with Severe Renal Impairment. Pharmacotherapy. 2017;37(4):412‐419. 10.1002/phar.1905 [DOI] [PubMed] [Google Scholar]
- 30. FDA . Prescribing Information for Eliquis. 2018.
- 31. FDA . Prescribing Information for Savaysa. 2017.
- 32. Chan NC, Coppens M, Hirsh J, et al. Real‐world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;13(3):353‐359. 10.1111/jth.12823 [DOI] [PubMed] [Google Scholar]
- 33. Avgil‐Tsadok M, Jackevicius CA, Essebag V, et al. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost. 2016;115(1):152‐160. 10.1160/th15-03-0247 [DOI] [PubMed] [Google Scholar]
- 34. Belen E, Canbolat IP, Bayyigit A, Helvaci A, Pusuroglu H, Kilickesmez K. A new gap in the novel anticoagulants' era: undertreatment. Blood Coagul Fibrinolysis. 2015;26(7):793‐797. 10.1097/mbc.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 35. Farmakis D, Davlouros P, Giamouzis G, et al. Direct Oral Anticoagulants in Nonvalvular Atrial Fibrillation: Practical Considerations on the Choice of Agent and Dosing. Cardiology. 2018;140(2):126‐132. 10.1159/000489922 [DOI] [PubMed] [Google Scholar]
- 36. Chan YH, See LC, Tu HT, et al. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2018;7(8). 10.1161/jaha.117.008150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimizu T, Momose Y, Ogawa R, Takahashi M, Echizen H. Impact of Pharmacists' audit on improving the quality of prescription of dabigatran etexilate methanesulfonate: a retrospective study. J Pharm Health Care Sci. 2017;3(1):4 10.1186/s40780-017-0077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akiyama H, Uchino K, Hasegawa Y. Characteristics of Symptomatic Intracranial Hemorrhage in Patients Receiving Non‐Vitamin K Antagonist Oral Anticoagulant Therapy. PLoS One. 2015;10(7):e0132900 10.1371/journal.pone.0132900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamashita Y, Uozumi R, Hamatani Y, et al. Current Status and Outcomes of Direct Oral Anticoagulant Use in Real‐World Atrial Fibrillation Patients‐ Fushimi AF Registry. Circ J. 2017;81(9):1278‐1285. 10.1253/circj.CJ-16-1337 [DOI] [PubMed] [Google Scholar]
- 40. Umei M, Kishi M, Sato T, et al. Indications for suboptimal low‐dose direct oral anticoagulants for non‐valvular atrial fibrillation patients. J Arrhythm. 2017;33(5):475‐482. 10.1016/j.joa.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coleman CI, Antz M, Bowrin K, et al. Real‐world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT‐US study. Curr Med Res Opin. 2016;32(12):2047‐2053. 10.1080/03007995.2016.1237937 [DOI] [PubMed] [Google Scholar]
- 42. Gibson CM, Smith CB, Davis S, Scalese MJ. Assessment of Apixaban Prescribing Patterns for Nonvalvular Atrial Fibrillation in Hospitalized Patients. Ann Pharmacother. 2018;52(1):54‐59. 10.1177/1060028017726795 [DOI] [PubMed] [Google Scholar]
- 43. Nguyen E, White CM, Patel MR, et al. Doses of apixaban and rivaroxaban prescribed in real‐world United States cardiology practices compared to registration trials. Curr Med Res Opin. 2016;32(7):1277‐1279. 10.1185/03007995.2016.1170672 [DOI] [PubMed] [Google Scholar]
- 44. Carlin S, Pickering J, Schulman S. Appropriateness of Apixaban Dosing to Prevent Stroke in Patients with Atrial Fibrillation: A Pilot Study. Can J Hosp Pharm. 2016;69(6):449‐453. 10.4212/cjhp.v69i6.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post‐hoc analysis from the RE‐LY database. Thromb Haemost. 2014;111(5):933‐942. 10.1160/th13-09-0734 [DOI] [PubMed] [Google Scholar]
- 46. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in Asian Patients With Atrial Fibrillation: Effectiveness and Safety. J Am Coll Cardiol. 2018;72(8):838‐853. 10.1016/j.jacc.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 47. Bando S, Nishikado A, Hiura N, et al. Efficacy and safety of rivaroxaban in extreme elderly patients with atrial fibrillation: Analysis of the Shikoku Rivaroxaban Registry Trial (SRRT). J Cardiol. 2018;71(2):197‐201. 10.1016/j.jjcc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 48. Pattullo CS, Barras M, Tai B, McKean M, Donovan P. New oral anticoagulants: appropriateness of prescribing in real‐world setting. Intern Med J. 2016;46(7):812‐818. 10.1111/imj.13118 [DOI] [PubMed] [Google Scholar]
- 49. Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2015;37(14):1145‐1153. 10.1093/eurheartj/ehv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brun Guinda D, Callen Garcia O, Ondiviela Perez J, et al. Clinical profile, management and outcomes in a cohort of elderly and highly comorbid patients with nonvalvular atrial fibrillation treated with rivaroxaban in routine practice. Future Cardiol. 2018;14(3s):39‐45. 10.2217/fca-2018-0025 [DOI] [PubMed] [Google Scholar]
- 51. Pharithi RB, Ranganathan D, O'Brien J, et al. Is the prescription right? A review of non‐vitamin K antagonist anticoagulant (NOAC) prescriptions in patients with non‐valvular atrial fibrillation. Safe prescribing in atrial fibrillation and evaluation of non‐vitamin K oral anticoagulants in stroke prevention (SAFE‐NOACS) group. Ir J Med Sci. 2019;188(1):101‐108. 10.1007/s11845-018-1837-7 [DOI] [PubMed] [Google Scholar]
- 52. Basaran O, Dogan V, Beton O, et al. Suboptimal use of non‐vitamin K antagonist oral anticoagulants: Results from the RAMSES study. Medicine (Baltimore). 2016;95(35):e4672 10.1097/md.0000000000004672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kimmons LA, Kabra R, Davis M, Segars BV, Oliphant CS. Dabigatran Use in the Real World: A Multihospital System Experience. J Pharm Pract. 2014;27(4):384‐388. 10.1177/0897190013513616 [DOI] [PubMed] [Google Scholar]
- 54. Steinberg BA, Shrader P, Thomas L, et al. Off‐Label Dosing of Non‐Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT‐AF II Registry. J Am Coll Cardiol. 2016;68(24):2597‐2604. 10.1016/j.jacc.2016.09.966 [DOI] [PubMed] [Google Scholar]
- 55. Armbruster AL, Buehler KS, Min SH, Riley M, Daly MW. Evaluation of dabigatran for appropriateness of use and bleeding events in a community hospital setting. Am Health Drug Benefits. 2014;7(7):376‐384. [PMC free article] [PubMed] [Google Scholar]
- 56. Simon J, Hawes E, Deyo Z, Bryant SB. Evaluation of prescribing and patient use of target‐specific oral anticoagulants in the outpatient setting. J Clin Pharm Ther. 2015;40(5):525‐530. 10.1111/jcpt.12296 [DOI] [PubMed] [Google Scholar]
- 57. Bartlett JW, Renner E, Mouland E, Barnes GD, Kuo L, Ha NB. Clinical Safety Outcomes in Patients With Nonvalvular Atrial Fibrillation on Rivaroxaban and Diltiazem. Ann Pharmacother. 2019;53(1):21‐27. 10.1177/1060028018795140 [DOI] [PubMed] [Google Scholar]
- 58. Tellor KB, Patel S, Armbruster AL, Daly MW. Evaluation of the appropriateness of dosing, indication and safety of rivaroxaban in a community hospital. J Clin Pharm Ther. 2015;40(4):447‐451. 10.1111/jcpt.12288 [DOI] [PubMed] [Google Scholar]
- 59. Muniz Lobato S, Tarrazo Tarrazo C, Gonzalez Fernandez E, Moran AM. Clinical profile, adequacy of dosage and thromboembolic and bleeding outcomes in patients with nonvalvular atrial fibrillation treated with rivaroxaban in a regional hospital of Asturias. Spain Future Cardiol. 2018;14(3s):17‐24. 10.2217/fca-2018-0022 [DOI] [PubMed] [Google Scholar]
- 60. Grant SJ, Kothari S, Gimotty PA, Gooneratne NS, Cuker A. Quality of direct oral anticoagulant prescribing in elderly patients with non‐valvular atrial fibrillation: results from a large urban health system. J Thromb Thrombolysis. 2018;46(1):1‐6. 10.1007/s11239-018-1651-0 [DOI] [PubMed] [Google Scholar]
- 61. Kawabata M, Goya M, Sasaki T, et al. Left Atrial Appendage Thrombi Formation in Japanese Non‐Valvular Atrial Fibrillation Patients During Anticoagulation Therapy‐ Warfarin vs. Direct Oral Anticoagulants. Circ J. 2017;81(5):645‐651. 10.1253/circj.CJ-16-1089 [DOI] [PubMed] [Google Scholar]
- 62. Hoyer C, Filipov A, Neumaier‐Probst E, Szabo K, Ebert A, Alonso A. Impact of pre‐admission treatment with non‐vitamin K oral anticoagulants on stroke severity in patients with acute ischemic stroke. J Thromb Thrombolysis. 2018;45(4):529‐535. 10.1007/s11239-018-1634-1 [DOI] [PubMed] [Google Scholar]
- 63. Draper E, Parkhurst B, Carley B, Krueger K, Larson T, Griesbach S. Comparison of Prescribing Practices with Direct Acting Oral Anticoagulant Protocols. Am J Cardiovasc Drugs. 2017;17(6):475‐479. 10.1007/s40256-017-0243-2 [DOI] [PubMed] [Google Scholar]
- 64. Basaran O, Filiz Basaran N, Cekic EG, et al. PRescriptiOn PattERns of Oral Anticoagulants in Nonvalvular Atrial Fibrillation (PROPER study). Clin Appl Thromb Hemost. 2017;23(4):384‐391. 10.1177/1076029615614395 [DOI] [PubMed] [Google Scholar]
- 65. Mazurek M, Huisman MV, Rothman KJ, et al. Regional Differences in Antithrombotic Treatment for Atrial Fibrillation: Insights from the GLORIA‐AF Phase II Registry. Thromb Haemost. 2017;117(12):2376‐2388. 10.1160/th17-08-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Larock AS, Mullier F, Sennesael AL, et al. Appropriateness of prescribing dabigatran etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective study. Ann Pharmacother. 2014;48(10):1258‐1268. 10.1177/1060028014540868 [DOI] [PubMed] [Google Scholar]
- 67. McDonald CJ, Kalisch Ellett LM, Barratt JD, Caughey GE. An international comparison of spontaneous adverse event reports and potentially inappropriate medicine use associated with dabigatran. Pharmacoepidemiol Drug Saf. 2014;24(4):399‐405. 10.1002/pds.3648 [DOI] [PubMed] [Google Scholar]
- 68. Chowdhry U, Jacques A, Karovitch A, Giguere P, Nguyen ML. Appropriateness of Dabigatran and Rivaroxaban Prescribing for Hospital Inpatients. Can J Hosp Pharm. 2016;69(3):194‐201. 10.4212/cjhp.v69i3.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Olesen JB, Sorensen R, Hansen ML, et al. Non‐vitamin K antagonist oral anticoagulation agents in anticoagulant naive atrial fibrillation patients: Danish nationwide descriptive data 2011‐2013. Europace. 2015;17(2):187‐193. 10.1093/europace/euu225 [DOI] [PubMed] [Google Scholar]
- 70. Shimokawa H, Yamashita T, Uchiyama S, et al. The EXPAND study: Efficacy and safety of rivaroxaban in Japanese patients with non‐valvular atrial fibrillation. Int J Cardiol. 2018;258:126‐132. 10.1016/j.ijcard.2018.01.141 [DOI] [PubMed] [Google Scholar]
- 71. Kirchhof P, Radaideh G, Kim YH, et al. Global Prospective Safety Analysis of Rivaroxaban. J Am Coll Cardiol. 2018;72(2):141‐153. 10.1016/j.jacc.2018.04.058 [DOI] [PubMed] [Google Scholar]
- 72. Beyer‐Westendorf J, Camm AJ, Coleman CI, Tamayo S. Rivaroxaban real‐world evidence: Validating safety and effectiveness in clinical practice. Thromb Haemost. 2016;116(Suppl. 2):S13‐s23. 10.1160/th16-06-0485 [DOI] [PubMed] [Google Scholar]
- 73. Coleman CI, Turpie AGG, Bunz TJ, Eriksson D, Sood NA, Baker WL. Effectiveness and safety of rivaroxaban vs. warfarin in non‐valvular atrial fibrillation patients with a non‐sex‐related CHA2DS2‐VASc score of 1. Eur Heart J Cardiovasc Pharmacother. 2019;5(2):64‐69. 10.1093/ehjcvp/pvy025 [DOI] [PubMed] [Google Scholar]
- 74. Weir MR, Berger JS, Ashton V, et al. Impact of renal function on ischemic stroke and major bleeding rates in nonvalvular atrial fibrillation patients treated with warfarin or rivaroxaban: a retrospective cohort study using real‐world evidence. Curr Med Res Opin. 2017;33(10):1891‐1900. 10.1080/03007995.2017.1339674 [DOI] [PubMed] [Google Scholar]
- 75. Marti E, Segado A, Pastor‐Galan I, et al. Use of rivaroxaban for the prevention of stroke in patients with nonvalvular atrial fibrillation in Spain. Future Cardiol. 2018;14(3s):3‐8. 10.2217/fca-2018-0020 [DOI] [PubMed] [Google Scholar]
- 76. Millon Cano JA, Vilalta Seto N, Mateo Arranz J, Souto Andres JC. Importance of appropiate clinical management of direct oral anticoagulants. Med Clin (Barc). 2016;146(1):41‐42. 10.1016/j.medcli.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 77. Shinoda N, Mori M, Tamura S, Korosue K, Kose S, Kohmura E. Risk of Recurrent Ischemic Stroke with Unintended Low‐Dose Oral Anticoagulant Therapy and Optimal Timing of Review. J Stroke Cerebrovasc Dis. 2018;27(6):1546‐1551. 10.1016/j.jstrokecerebrovasdis.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 78. Burgos KD, Sienko SE, Hoffman JL, Koerber JM, Smythe MA. Characteristics, Management, and Outcomes of Patients with Atrial Fibrillation Experiencing a Major Bleeding Event While on Rivaroxaban. Clin Appl Thromb Hemost. 2018;24(2):372‐378. 10.1177/1076029616684030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Russo‐Alvarez G, Martinez KA, Valente M, et al. Thromboembolic and Major Bleeding Events With Rivaroxaban Versus Warfarin Use in a Real‐World Setting. Ann Pharmacother. 2018;52(1):19‐25. 10.1177/1060028017727290 [DOI] [PubMed] [Google Scholar]
- 80. Smythe MA, Forman MJ, Bertran EA, Hoffman JL, Priziola JL, Koerber JM. Dabigatran versus warfarin major bleeding in practice: an observational comparison of patient characteristics, management and outcomes in atrial fibrillation patients. J Thromb Thrombolysis. 2015;40(3):280‐287. 10.1007/s11239-015-1213-7 [DOI] [PubMed] [Google Scholar]
- 81. Ross B, Miller MA, Ditch K, Tran M. Clinical experience of life‐threatening dabigatran‐related bleeding at a large, tertiary care, academic medical center: a case series. J Med Toxicol. 2014;10(2):223‐228. 10.1007/s13181-013-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ioannou A, Tsappa I, Metaxa S, Missouris CG. Non‐valvular atrial fibrillation: impact of apixaban on patient outcomes. Patient Relat Outcome Meas. 2017;8:121‐131. 10.2147/prom.s117549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kilickiran Avci B, Vatan B, Ozden Tok O, et al. The Trends in Utilizing Nonvitamin K Antagonist Oral Anticoagulants in Patients With Nonvalvular Atrial Fibrillation: A Real‐Life Experience. Clin Appl Thromb Hemost. 2016;22(8):785‐791. 10.1177/1076029615581365 [DOI] [PubMed] [Google Scholar]
- 84. Kawabata M, Yokoyama Y, Sasano T, et al. Bleeding events and activated partial thromboplastin time with dabigatran in clinical practice. J Cardiol. 2013;62(2):121‐126. 10.1016/j.jjcc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 85. Camm AJ, Coleman CI, Larsen TB, Nielsen PB, Tamayo CS. Understanding the Value of Real‐World Evidence: Focus on Stroke Prevention in Atrial Fibrillation with Rivaroxaban. Thromb Haemost. 2018;118(S 01):S45‐s60. 10.1055/s-0038-1635084 [DOI] [PubMed] [Google Scholar]
- 86. Lin YC, Chien SC, Hsieh YC, et al. Effectiveness and Safety of Standard‐ and Low‐Dose Rivaroxaban in Asians With Atrial Fibrillation. J Am Coll Cardiol. 2018;72(5):477‐485. 10.1016/j.jacc.2018.04.084 [DOI] [PubMed] [Google Scholar]


