Abstract
Impairments in social communication (SC) predominate among the core diagnostic features of autism spectrum disorders (ASDs). Neuroimaging has revealed numerous findings of atypical activity and connectivity of ‘social brain’ networks, yet no consensus view on crucial developmental causes of SC deficits has emerged. Aside from methodological challenges, the deeper problem concerns the clinical label of ASD. While genetic studies have not comprehensively explained the causes of nonsyndromic ASDs, they highlight that the clinical label encompasses many etiologically different disorders. The question of how potential causes and etiologies converge onto a comparatively narrow set of SC deficits remains. Only neuroimaging designs searching for subtypes within ASD cohorts (rather than conventional group level designs) can provide translationally informative answers.
An Ever-Increasing Public Health Challenge
ASDs represent a group of neurodevelopmental disorders with increasing prevalence, which has doubled within less than a decade (in large part, as a result of improved detection and growing awareness [1]) and is currently estimated in the USA at 1 in 59 children [2], with similar rates (1.5–2%) reported in other countries and continents [3–5]. Aside from personal suffering in affected individuals and their families, ASDs present a major public health challenge, with an estimated annual cost of ~US$300 billion in the USA, projected in one report to approach 3–4% of the gross domestic product within the next decade [6].
Core diagnostic features of ASDs (see Glossary) are sociocommunicative impairments and restricted and repetitive behaviors and interests, with symptom onset during the first years of life [7]. ASDs are heterogeneous in presenting symptoms, developmental outcomes, and adaptive function, with symptom severity levels ranging from mild (requiring minimal support) to severe (necessitating substantial support throughout the lifespan). According to the most recent diagnostic consensus summarized in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 [7]), core autism symptoms can occur in individuals with or without intellectual or language delays, effectively broadening the diagnosis to include people with the full range of cognitive and language functioning. While delayed language acquisition is no longer among the diagnostic criteria, language skills and IQ remain the strongest predictors of outcomes in both naturalistic studies of ASDs [8,9] and in treatment response [10,11]. Males are affected about four times more frequently than females [2], although females with ASDs may be underidentified [12,13], likely due to gender differences in symptom presentation and comorbid features [14–16] (Box 1). All in all, because of the onset in early childhood, the life-long impact of ASDs on the health, economic well-being, social integration, and quality of life of affected individuals and their families is immense. On the societal level, the direct and indirect costs to the educational, healthcare, and economic sectors emphasize the need to continue the search for neurobiological causes, enhanced detection, effective and targeted interventions, and, ultimately, prevention.
Box 1. Gender-Related Differences in ASDs.
Given the high male:female ratio in ASD prevalence, relatively little is known about female variants. While growing evidence indicates that profiles of SC impairments in ASDs likely differ between genders, it is unclear whether this might be due to different biological mechanisms (e.g., existence of specific factors protecting females from developing ASDs [139]), or an ascertainment bias (i.e., diagnostic criteria biased by the more prevalent male presentation [13,140]). Behaviorally, females with ASDs exhibit fewer restricted and repetitive behaviors [16], more social motivation [141], and less impaired nonverbal communication, including normative eye gaze following behavior [142], compared with males with ASDs. However, some evidence suggests that these gender differences in SC are only observed in higher-functioning samples and are absent altogether in lower-functioning samples [143]. Biologically, females may be more protected from heritable and de novo ASD risk variants, with sex chromosomal genes and sex hormones modulating the effects of genetic variants on the presentation of ASD symptoms [144]. Furthermore, limited evidence suggests differences in brain organization between males and females with ASDs, with the neuroanatomical features of ASDs involving different structures or growth trajectories in females than in males, including distinct patterns of early brain overgrowth (i.e., enlargement of brain volume early in life observed more prominently, or even exclusively in boys [145]), local differences in gray and white matter, and attenuation of neurotypical male > female volumetric differences [146].
Baron-Cohen’s ‘extreme male brain’ theory of autism proposes that autism represents an amplification of specific aspects of typical sexual dimorphism in cognition, with the extreme female brain characterized as ‘empathizing,’ and the extreme male brain as ‘systematizing’ [147]. However, this ‘masculinization’ theory linking the ASD etiology to the masculinizing effects of fetal testosterone [148] remains controversial, with an alternative theory proposing that ASDs are associated with ‘gender incoherence’ or androgyny, rather than with extreme masculinization, at the neurophysiological level [149]. Notably, the two theories make the same neuroanatomical predictions for females with ASDs (i.e., a more masculinized neural signature), but not for males with autism (i.e., more feminized features according to the latter). One study that directly compared brain connectivity patterns in males and females with ASDs reported reduced gender differentiation relative to the differences observed between typically developing (TD) boys and girls, supporting neural androgyny rather than masculinization [150]. Larger studies are required to replicate these findings and to understand differences in brain connectivity that could contribute to the sex differences in SC behaviors.
Social Cognition and the ‘Social Brain’
Despite heterogeneity in behavioral manifestations across sensory, motor, attention, language, and other domains, impaired social functioning is a core feature of ASDs [17]. Deficits in what the DMS-5 calls ‘social communication’, including diminished social responsiveness, difficulty recognizing others’ emotions and intentions, deficits in developing, maintaining, and understanding relationships, or relating to peers in a reciprocal manner, are considered the most universal and specific characteristics of the autism spectrum [18]. Social skills and competence are integral to many aspects of human behavior, laying the foundation for successful functioning in a dynamic, complex social world. The fundamental nature of social engagement and its prominence early in life is evidenced by prenatal sensitivity to human voices [19] and face-like stimuli [20], orientation to social cues, such as human faces and gestures within the first days of life [21,22], and heritability of socially relevant attention to facial eye and mouth regions observed in infants [23]. While it is unknown whether this primal preferential orientation to social cues is absent or reduced in fetuses and neonates who are later diagnosed with ASDs, diminished attention to social cues has been well documented in the first years of life in children with ASDs, including diminished attention to human voices [24], biological motion [25], eye gaze [23,26], and human faces [27], and impaired joint attention skills [28]. This is critical because the typically early-emerging preference for social cues is considered a precursor to the various forms of social understanding and adaptive social communication, arguably the most important attainments of early childhood development [29,30] (Box 2).
Box 2. Competing Accounts and Developmental Trajectories of Social Dysfunction in ASDs.
The neurocognitive accounts of impaired social cognition in ASDs can be largely summed up as belonging to two camps: while domain-specific explanations focus on (neural or cognitive) mechanisms specialized for social inferences and mentalizing (e.g., [151,152]), social orienting or social attention accounts propose that children with ASDs fail to preferentially orient to people, or social contingencies in general due to impairments in bottom-up, low-level attentional processes (e.g., [153]), leading to reduced opportunities for experiential learning. The latter specifically focus on social brain networks and mechanisms supporting processing of social cues, including human faces, voices, and biological motion. These bottom-up accounts emphasize that impoverished social information input (resulting from poor attention to social information) is responsible for diminished social experience and social engagement, which in turn compromise the development of higher order social cognition, such as theory of mind. Mechanistic explanations of diminished sensitivity to stimuli with social contingencies are still lacking, but the apparent indifference to social cues at early developmental stages impedes the development of social interactions, and of supporting social brain networks [17,52]. Potentially orthogonal to these models is the executive dysfunction account, which suggests that impaired executive functions, such as goal-directed initiation, inhibition, working memory, and cognitive flexibility, underlie or serve as moderators of SC impairments in ASDs [154].
Children who develop ASDs do not appear to exhibit clear impairments in SC until the end of the first year of life, with some children continuing on a more typical trajectory until later in the second or third year of life, followed by a regression in SC skills. Among the most common early behavioral signs of ASDs (observable and apparent during the second year of life) are poor eye contact, poor joint or shared attention, and diminished social or shared smiling [155], all key features of social orienting. The evidence for these aberrant social-developmental milestones comes from both retrospective studies of parental reports or home videos, and prospective studies of high-risk infants (i.e., those who have older siblings with ASDs) who are later diagnosed with ASDs. Unfortunately, domain-specific accounts of ‘mindblindness’ in ASDs [156] encounter developmental constraints, because it cannot be detected before 4–5 years of age, when mentalizing, or the ability to infer the contents of other people’s beliefs, intentions, or emotions, first emerges in typical development. Thus, while evidence suggests that mentalizing, or theory of mind, is delayed [157] and remains impaired across the lifespan in ASDs [158], this construct is not measurable during the early stages of human development, preventing any direct comparisons between the two accounts of SC dysfunction in ASDs. Likewise, the executive dysfunction accounts of SC impairment in ASDs are inherently limited by age, given the protracted development of executive functions across the human lifespan.
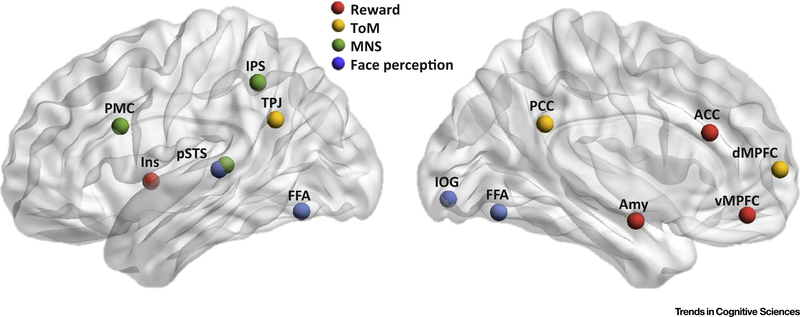
The SC functions associated with social orientation, processing of social cues, and understanding of others are supported by a distributed network of brain regions collectively referred to as the ‘social brain’ [31]. This collection of brain networks involved in processing social signals includes the dorsal and ventral medial prefrontal cortices (dMPFC and vMPFC), anterior and posterior cingulate cortex (ACC and PCC), amygdala, posterior superior temporal sulcus (pSTS), temporoparietal junction (TPJ), inferior occipital gyrus (IOG), fusiform face area (FFA), and the insula [32–34]. Extensive neuropsychological and functional neuroimaging evidence indicates that these social brain regions are related to specific domains of social cognition, such as analysis of faces and gaze by the IOG, FFA, and pSTS [35,36], emotional processing in the amygdala [37], processing of others’ intentions, feelings, and points of view (i.e., theory of mind [ToM] or mentalizing) in the dMPFC, PCC, and TPJ [38], and imitation and understanding of others’ actions, which is crucial for social learning, by the regions associated with the mirror neuron system (MNS), including the premotor cortex (PMC), intraparietal sulcus (IPS), and pSTS [39]. A growing number of connectivity studies show that these social brain regions are organized in large-scale functional networks supporting specific social functions (reward-related system [40], ToM network [41], the MNS [42], and face perception network [43]; Figure 1).
Figure 1.
Social Brain Networks. Core networks known to be involved in social cognition. Only main network regions, simplified as spheres, are shown with approximate location (excluding subcortical structures). Abbreviations: ACC, anterior cingulate cortex; Amy, amygdala; dMPFC, dorsomedial prefrontal cortex; FFA, fusiform face area; Ins, insula; IOG, inferior occipital gyrus; IPS, intraparietal sulcus; MNS, mirror neuron system; PCC, posterior cingulate cortex; PMC, premotor cortex; pSTS, posterior superior temporal sulcus; ToM, theory of mind; TPJ, temporoparietal junction; vMPFC, ventromedial prefrontal cortex.
Abnormalities of Social Brain Networks in ASDs
Many of the abilities supported by the social brain networks overlap with core impairments associated with ASDs, including difficulties inferring other people’s emotions or intentions and recognizing facial expressions, as well as deficits in imitation, social learning, and joint attention [44–46]. While activation likelihood estimation (ALE) meta-analyses have reported consistent dysfunction (primarily hypoactivation during social tasks) of most of the individual ‘social brain’ regions mentioned above in ASDs [47–51], there is a growing consensus that brain abnormalities in ASDs are not localized to one or a few regions but rather implicate alterations in the connectivity of distributed brain networks. Since the early 2000s, a method of choice in the study of network connectivity has been functional connectivity MRI (fcMRI). Aberrant patterns of connectivity have been identified within and between widely distributed networks supporting core social functions [52–54]. For instance, reduced network integration, or weaker inter-regional connectivity, has been observed for the face perception [49], imitation [55], and amygdalar emotional-processing networks [56,57]. Furthermore, there is convergent evidence of reduced segregation (or differentiation) of social networks in ASDs, reflected in excessive connections with extraneous regions that are not part of neurotypical social networks. These findings appear to converge on a pattern of dual impairment, affecting both network integration and segregation, and resulting in reduced network specialization and processing efficiency of brain systems crucial for SC functions. However, not all findings to date have been consistent with this interpretation, with some reports of global overconnectivity across all social and nonsocial networks [58] or weaker connectivity between social brain networks [53,59]. One complicating aspect is that atypical maturational trajectories in ASDs may be associated with underconnectivity of a given network at one developmental stage, but overconnectivity at another [60]. We return to the question of how divergent findings may be explained in the following section.
Particularly relevant to the broad ASD phenotype, most of the regions comprising the social brain networks also support functions outside of the social domain. For instance, TPJ, considered a nexus of theory of mind and self-other distinction [38], is also associated with domain-general attentional reorienting to salient cues [61]. Similarly, while dMPFC, as part of the theory of mind network, is engaged in inferring others’ mental states [62,63], it has also been implicated in abstract tasks without mentalizing content, such as categorization [64,65]. Even the FFA, considered a canonical social brain region for its crucial role in face perception, is involved in domain-general functions beyond the social context, including perceptual expertise for nonface objects [66,67].
Challenges and Perspectives
Many Methods, Uncountable Findings, and Few Answers …
The imaging literature on brain connectivity investigating core SC symptomatology in ASDs has rapidly grown over the past decade. While a gross pattern of reduced network integration and segregation appears to emerge, as described above, the numerous findings have not added up to a fully coherent picture of brain anomalies that are crucial for impaired SC in ASDs (cf. [60,68]). One set of problems is methodological. First, different techniques are sensitive to different neural parameters that are directly or indirectly related to connectivity and brain network organization. Connectivity strictly refers to the presence of anatomical connections and, thus, to axonal and synaptic organization, which in humans can be directly examined only in postmortem studies. Diffusion-weighted imaging allows indirect inferences on anatomical connectivity based on differential water diffusion [69]. By contrast, functional connectivity is a derivative concept based on the often tacit assumption that distal brain regions show statistically related activity patterns only if they communicate (directly or indirectly) via anatomical connections. Within the wide field of functional connectivity, different techniques, from positron emission tomography in early studies [70] to fcMRI, EEG, and magnetoencephalography, are again sensitive to different aspects of neural activity fluctuations. These differ with respect to signal source (e.g., hemodynamic versus electrophysiological), temporal frequency bands, and spatial resolution, making the integration of findings from different techniques challenging [71]. Second, even within the boundaries of a given technique, such as fcMRI, different methodological approaches can result in divergent findings in the same data set [72]. The need for clearly defined best practices in data preprocessing and analysis appears plausible, but is complicated by the fact that potential incremental improvements in processing steps are proposed continuously (e.g., [73]) and any previously accepted set of practices will no longer be ‘best’ after a short time.
… but Can there be Clear Answers to an Ill-Posed Question?
Genetic investigations into ASDs, considered to promise explanatory models of causation decades ago, have failed to provide clear and simple answers as to how and why affected children fail to develop crucial SC skills. The reasons are manifold, having to do with the complexity of gene-behavior relationships, which are somewhat more tractable in syndromic forms that affect only a single locus, such as fragile X or Rett syndrome [74], but largely intractable with the current state of knowledge in polygenic disorders, such as ‘idiopathic’ ASDs. The results of 30 years of genetic ASD research show some analogy to the challenges in neuroimaging described earlier. Large-sample studies have revealed more and more risk genes, now in the hundreds [75]. Although most of these account for only a small causative factor, penetrance (the rate at which individuals with a given genetic variant develop a specific phenotype, such as ASD) varies widely [75]. Thus, causation in most individual cases of ‘idiopathic’ ASDs may be due to some permutation of ‘multiple hits’ among numerous risk genes [76], with added roles of epigenetic [77] and environmental factors [78]. In other words, both in neuroimaging and genetics, there are numerous findings that appear to apply to some cases of ASDs, but not others, and that probably need to occur in some unknown combination in order to result in ASD symptomatology.
Have neuroimaging and genetics research failed the community of families with ASDs and the funding organizations that have subsidized these fields? This question misses the point. If there has been failure that has prevented the science of ASDs to generate mechanistic models of causation and precision medicine, it is a failure to appreciate the inadequacy of a clinical label such as ‘autism spectrum disorder’ (in the singular of the DSM-5 [7]) in the pursuit of neurobiological causes. From a developmental neurobiology perspective, the expectation that a consensus-based catalog of behavioral observations (as listed in the DSM-5) could tractably correspond to a small set of genetic causes or brain features is misguided. This expectation may be prompted by the existence of some single-gene disorders associated with autism symptomatology [74]. The reverse inference – that a disorder defined by a distinct set of behavioral symptoms, such as ASD, could therefore be traced back to a single cause or a small set of causes - is however unfounded.
Specificity of Sociocommunicative Symptomatology: Where is the Convergence?
The breadth of findings, both with respect to neuroimaging features found to be atypical in ASDs and with respect to the numerous genetic (and other) risk factors implicated, is remarkable given the comparatively circumscribed domain of SC core symptoms on which clinical diagnosis is based [7]. Therefore, some convergence may be expected to occur in development, from a very wide range of possible causes onto a narrower range of core symptoms. One proposal has been made in the genetics literature as to the convergence of many ASD risk genes into gene networks or modules [79,80], some of which may specifically affect synapto-genesis, synaptic function, and circuit formation [81,82]. This view may be supported by the convergence of a large number of knockout mouse models (of genes associated with ASDs) onto few distinct neuroanatomical phenotypes [83]. However, the argument could be compelling only if based on quantitative tests showing that the fraction of ASD risk genes involved in connectivity is significantly greater than would be expected from the fraction of genes somehow affecting brain connectivity within the entire genome. A corresponding argument, according to which autism is a disorder at the brain network level, has been made in the imaging literature. Although broadly accepted (cf. [60,68]), this argument is open to an analogous critique: Cognitive neuroscience has generally moved towards understanding all functional systems in terms of distributed networks [84], highlighting the importance of network connectivity, and this view has been generally applied to the study of psychopathology [85]. The view of autism as a connectivity disorder therefore conveys little specific insight.
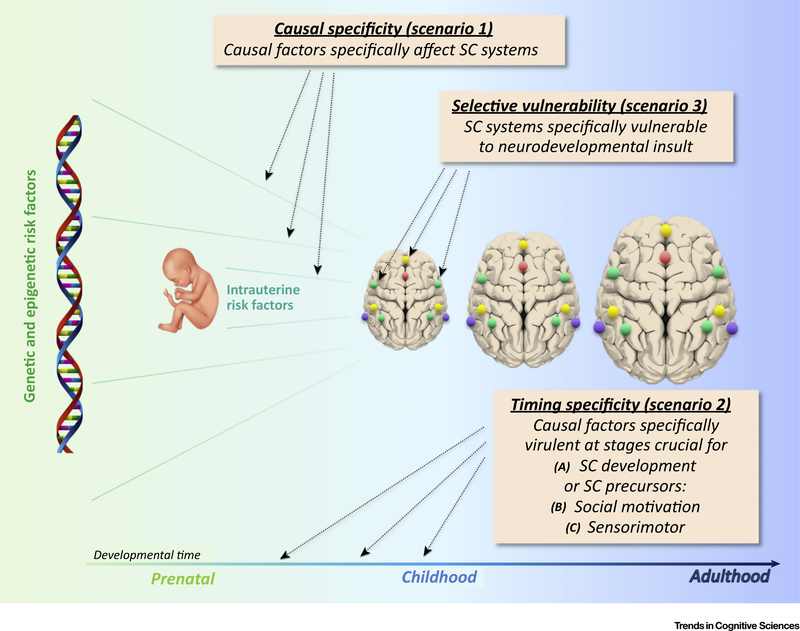
However, even if one accepts a connectivity approach as specifically informative about the causation of ASDs, the convergence question remains. Since all functional systems (sensorimotor, limbic, and higher cognitive supramodal) are organized in distributed interconnected networks, why would a ‘connectivity disorder’ specifically affect SC? The question may have to be slightly softened, as it is known that ASDs are often accompanied by impaired or atypical functioning in many domains other than SC, such as sensory [86], motor [87], attention [88], and executive [89]. Although such evidence is now acknowledged by the inclusion of sensory symptomatology and greater emphasis on restricted interests and behaviors in the DSM-5, deficits in social interaction and communication skills remain the defining core feature of ASDs. What can account for this domain specificity? Several scenarios may be considered (Figure 2).
Figure 2.
Convergence Scenarios. Cartoon of three convergence scenarios described in main text. Colored dots represent social network nodes diagrammatically. For anatomical locations see Figure 1. Abbreviation: SC, social communication.
Scenario 1 implies causal specificity, according to which ‘multiple hits’ ([epi]genetic and/or environmental) causing the emergence of an ASD predominantly affect neural circuits that are crucial for social cognition and communication, for example through region-specific gene expression [90]. This appears to be supported by the predominance of imaging findings implicating sociocommunicative brain networks (as described earlier). However, there is an understandable bias in study designs to preferentially target domains of core symptomatology. This alone may explain why findings for these domains are more abundant [91]. Notably, anomalous connectivity has also been reported for domains beyond core ASD symptomatology, such as motor control [92,93], salience network [94,95], and circuits connecting basal ganglia [96], thalamus [97,98], and cerebellum [99,100] with cerebral cortex. All in all, the neuroimaging literature indicates that atypical connectivity patterns in ASDs are not exclusive to sociocommunicative circuits.
A second set of scenarios relates to timing specificity. In scenario 2a, genetic and other causes have greatest impact at developmental stages crucial to the emergence of SC. Neurodevelopmental disturbances, such as gray [101] and white matter growth anomalies [102] or enlarged cerebrospinal fluid compartments [103], arise during the first 1–2 years of life, or possibly prenatally [104]. This may affect preferential social responses that have been observed prenatally and early postnatally (as described earlier), although some more complex SC abilities known to be affected in ASDs, such as ‘theory of mind’ (e.g., [105]), fully develop later in life [106]. In related scenarios, early disturbances specifically affect precursors crucial for subsequent SC development. In scenario 2b, these may be limbic defects affecting social motivation and reward [46]. Alternatively, in scenario 2c, these are early sensorimotor disturbances. This scenario seems supported by growing evidence of atypical or impaired development of auditory [107], visual [108], somatosensory [86], and motor functions [109] in ASDs. Scenario 2c might imply that core ASD symptomatology is sensorimotor and that SC impairments are secondary and derivative. However, there is no conclusive evidence that sensorimotor deficits are a necessary condition for the emergence of SC symptomatology in ASDs. Even if one acknowledges the plausibility of such developmental links, the specificity of resulting SC impairment would require further explanation, as other developmental disorders have also been related to early sensorimotor impairments (e.g., rapid auditory processing deficits in specific language impairment [110]).
A third scenario implies specific vulnerability and pertains to the domain of impairment itself. If SC abilities are selectively vulnerable to neurodevelopmental disturbances, causal factors that have little domain specificity may result in a phenotype with greater behavioral specificity. However, this scenario on its own is not plausible, because early neurodevelopmental disturbances can also result in other deficit profiles, such as broad impairment of overall level of functioning in intellectual disability [111], or predominant deficits in circumscribed domains, such as spoken [112] or written language [113], or attention and cognitive control [114]. It may be more plausible in combination with scenario 2, in the sense that SC abilities or their crucial building blocks could be selectively vulnerable to specific neurodevelopmental disturbances at specific maturational stages only, possibly because of the highly distributed organization of SC networks in the brain (as described earlier). This would imply that developmental disorders achieve specificity based on the predominant timing of insult or disturbance. However, even this combination of scenarios 2 and 3 has little direct evidence in support. Indeed, based on available evidence, one would be hard pressed to pinpoint the exact timing of crucial neurodevelopmental disturbances in ASDs: Is it several years after birth when brain overgrowth peaks [101] or when, in some cases, developmental regression is observed [115]; or prenatally, when intrauterine risk factors, such as maternal inflammation, occur [116]; or at earliest stages of neuronal proliferation, as suggested by studies using induced pluripotent stem cells [117]? Since the timing of crucial insult in ASDs is undetermined (probably because it cannot actually be pinpointed to a single developmental stage), any argument that timing of insult at a stage of specific vulnerability of SC functions (or their building blocks) could account for domain specificity in outcome symptomatology appears less than compelling.
Finally, returning to questions raised earlier, we may consider whether the construct of ASD itself could be a convenient clinical fiction, serving to categorize children with developmental disorders for the purpose of treatment decisions. This would imply that convergence is an artifact of diagnostic procedures, unrelated to underlying biology. The argument has been made (e.g., [118]), and may be considered with reference to the human mind’s penchant to perceive categorical boundaries even where there are none in the physical world [119], as, for example, in phonemic categorization [120]. This view is substantiated by the contemporary trend towards transdiagnostic approaches to research and clinical practice, focusing on features that cross diagnostic and categorical boundaries (e.g., [121,122]), which highlights, for instance, that SC deficits are also present in schizophrenia spectrum disorders, social anxiety, and other developmental or conduct disorders. However, while clinicians will acknowledge comorbidities and fuzzy lines of demarcation between diagnostic categories, they will likely reject the view of ASD as a convenient fiction because it is incompatible with their everyday clinical experience.
Many ASDs: Are Large Samples the Answer?
For reasons solely to do with logical transparency, we have presented the different convergence scenarios as distinct alternatives. In reality, each of them probably captures an aspect of the true complexity in the relation between causal factors and SC symptomatology, and each scenario may apply to different degrees to the varying etiologies underlying ASDs. It is likely that genetic and epigenetic risk factors affect SC abilities with some specificity at maturational stages of increased vulnerability and in regions with predominant effects on SC functions or its precursors. In addition, as discussed earlier, the DSM-5 presentation of ASD as a singular disorder may indeed be misleading, because it solely reflects a relative convergence at the behavioral outcome level (onto a set of diagnostic criteria and a range of severities), of what at the biological level most likely comprises an array of diverse etiologies. The attempt to understand behavioral convergence made in the previous section is therefore the exact flipside of the need to understand diversity of causation and trajectories of neurodevelopmental disturbances.
From a purely statistical perspective, a plausible remedy for heterogeneity and expected cohort variability is the pursuit of large sample sizes. Indeed, with the increasing availability of large samples, such as the Autism Brain Imaging Data Exchange [123], some recent studies have presented findings that appear definitive solely based on ample statistical power. For one example, a recent ‘mega-analysis’ reported reduced amygdala volume in a cohort of 1508 children and adults with ASDs, compared with 1601 typically developing participants [124]. The finding is significant (P <0.05, even after correction for numerous comparisons), albeit with a miniscule effect size (Cohen’s d = 0.08). Although this finding derives from a large sample that is probably more representative of the ASD population than smaller samples in many other studies, the effect size indicates that the finding may have little significance beyond statistics. It could be meaningful if ASD were associated with a subtle, but functionally critical decrease in amygdalar volume in every or most individuals with the disorder, but this is improbable. More likely, a minority of etiologies grouped under the current ASD diagnostic label are associated with robustly reduced amygdalar volumes, whereas no such effect is seen in most cases. Therefore, any conclusion that ASD is associated with reduced amygdala volume, while statistically accurate, would be misleading.
There is great awareness of the problem of underpowered investigations in neuroimaging [125], but a complementary problem of overpowered group-level comparisons that may lend unsuitable prominence to findings with very small effect size is not generally considered. In this age of emerging large-sample collaborative endeavors (e.g., [123,126]), a careful distinction between statistical and conceptual significance is needed (cf. [127]). However, while large sample studies may generate findings that provide only minimal information about the general ASD population, they are, at the same time, indispensable for the detection of variants of the disorder.
Towards Translational Goals
As discussed earlier, ASDs may encompass a large set of etiologically different disorders [128], each associated with potentially divergent trajectories of neurodevelopmental disturbances. The identification of ASD subvariants is therefore crucial, because targeted treatments may only be achievable for specific variants, rather than for the population as a whole. The approach is often characterized as ‘stratification’, with the misleading implication that subvariants are expected to form ‘layers’ differing in one dimension only. In fact, constructs such as ‘low-functioning’ versus ‘high-functioning’ autism are unlikely to capture the biological diversity of ASD subtypes.
Although it can be assumed that SC symptomatology in ASDs is associated with atypical functional and structural brain organization, etiological diversity implies that the specific features of anomaly may diverge across subvariants of the disorder. Therefore, two individuals with ASDs may show similar profiles of behavioral symptoms, but these may be linked to different brain features. Attempts to subtype ASDs based on behavioral variables have been made (e.g., [129–131]); however, variants defined by shared brain features may relate more closely to etiological variants [132]. This can be presumed because both structural and functional brain organization reflect developmental history, not simply present outcome state. For example, fundamental organizational principles of regional specialization of cerebral cortices result from early developmental processes, such as neuronal migration along specific radial glial cells and early establishment of thalamocortical afferents [133]. Neuroimaging in children (and even in adults) is therefore ‘archeological’ because it can reveal neurodevelopmental disturbances and can unearth ‘intermediate phenotypes’ that are more directly informative of causation than behavior alone [134]. Thus, imaging has the potential to identify brain markers resulting from the convergence of differential etiologies, which may aid the development of targeted behavioral or biological interventions [135].
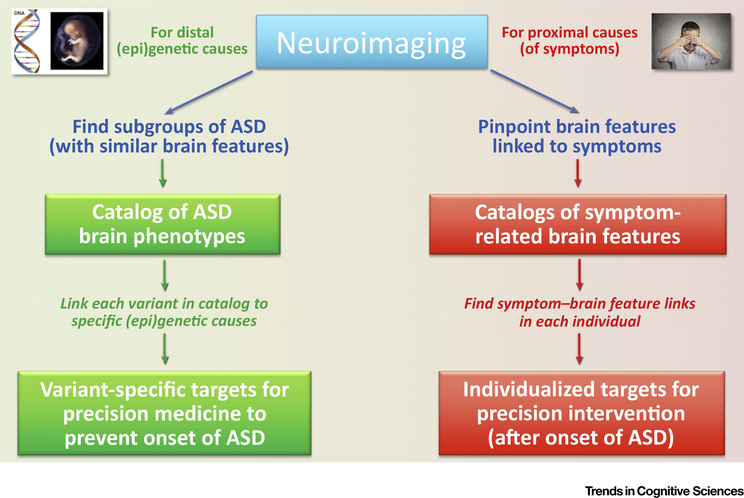
However, imaging measures are compounded by experiential plasticity related to environmental effects in the broadest sense (see Outstanding Questions). Therefore, imaging indices both distally reflect traces of prenatal and early postnatal neurodevelopmental disturbances and, at the same time, proximally show the neural bases of behavioral impairment in the current outcome phenotype of a single child (or adult) with ASDs. This opens two avenues for neuroimaging in ASDs that have traditionally not been clearly distinguished (Figure 3). Per its archeological ability to reveal intermediate phenotypes, neuroimaging can contribute to the identification of etiologically defined variants of ASDs, each of which may be expected to relate more tractably to genetic, epigenetic, and other causative factors. Defining such variants will be a first step towards mechanistic models and the implementation of precision medicine. On the other hand, per its ability to reveal proximal neural causation of outcome SC impairments, neuroimaging can identify targets for brain intervention techniques (e.g., transcranial magnetic or direct cortical stimulation [136,137], neurofeedback training [138]). Neither side is ready for this. The neuroimaging field needs to develop better tools to identify variants within ASD cohorts and pinpoint brain features crucially linked to SC (and other) deficits at the level of the individual; and brain stimulation techniques need to be calibrated for targeted use, in order to affect the function or connectivity of specific brain regions in a desired direction.
Outstanding Questions.
Does atypical neurofunctional organization of social networks in ASDs reflect causation or compensation? The answer for most findings is ‘probably both’. Disentangling the two is crucial for ultimate translational goals, because it will allow the distinction between atypical brain features that one may wish to ameliorate with brain intervention (or other) techniques versus features that may be promoted.
How do atypical maturational trajectories in ASDs affect comparisons with chronological age-matched TD groups? It is likely that connectivity in ASDs is atypical in ways that differ depending on maturational stage (e. g., infancy versus preteens versus adolescence); but there is little consensus on what these differences are. There may be no general pattern, but trajectories may differ across networks. In addition, heterogeneity and suspected existence of etiological subtypes are also likely to affect maturational trajectories in different ways, and there may be no single answer to this question that applies to ASDs across the board.
What does ASD heterogeneity imply for neuroimaging? The conventional focus on similarity within ASD groups, in comparisons with TD groups, should be redirected onto differences. Such focus can reveal subgroups in large samples that may reflect neurodevelopmental etiology more transparently than conventional group-level approaches.
Can machine learning improve our understanding of ASDs? Data-driven techniques are useful and can help in at least two ways: (i) They can identify brain markers that best distinguish ASD from TD participants (diagnostic prediction through supervised learning); and (ii) they can find patterns (clusterings) within large multivariate imaging data sets that may indicate brain-based variants of ASDs (through unsupervised learning).
Figure 3.
Two Distinct Pathways towards Translational Application of Neuroimaging in Autism Spectrum Disorders (ASDs). Left: Since brain features contain information about developmental history, imaging can contribute to the search for distal (epi)genetic causes by providing a catalog of brain phenotypes, each of which may be linked to a specific etiology with specific targets for precision medicine. Right: Neuroimaging can reveal a catalog of proximal causes of ASD symptoms that require intervention. Specific links between brain features and symptoms at the level of the individual can inform targets for precision intervention. While causal neuroimaging may contribute to precision medicine that may prevent the onset of the disorder, it is also possible that such intervention would reverse critical neurobiological conditions after onset and diagnosis. Conversely, intervention targets developed from symptomatic neuroimaging may be developed for earliest signs before the onset of full symptomatology. Therefore, the timing implications of the two imaging pathways are not absolute.
Concluding Remarks
Social impairments predominate among the core diagnostic criteria of ASDs. However, it is likely that these impairments result from the convergence of many different biological etiologies onto a set of phenotypes fulfilling these diagnostic criteria. Neuroimaging studies of atypical brain connectivity linked to social symptomatology must therefore move away from a focus on group-level effects towards the detection of variants within the disorder. Identifying biological subtypes and individualized targets for intervention will be the crucial challenge for neuroimaging in its quest to alleviate social impairments in children and adults with ASDs.
Highlights.
With increasing prevalence, ASDs present a major public health challenge. Many neuroimaging findings indicate that the ‘social brain’ is organized atypically in ASDs.
Growing evidence shows that neural bases of ASDs cannot be pinpointed to specific regions of the brain, but that symptomatology is instead linked to atypical connectivity within and between functionally specialized brain networks (including ‘social brain’ networks). However, few neuroimaging findings have been widely replicated and no clear picture of the brain bases of sociocommunicative impairments in ASDs has emerged.
A main factor that has prevented consensus findings is etiological diversity. While diagnostic criteria focus on social deficits in ASDs, these probably result from the convergence of many different neurodevelopmental trajectories and many different causative factors.
Acknowledgments
This work was supported by the National Institute of Health NIMH R01 MH10173 and R01 MH103494 (R.A.M.), and K01 MH097972 and R01 MH107802 (I.F.). The authors thank Sarah Reynolds for assistance with Figure 1.
Glossary
- Brain network integration and segregation
the human brain is organized into large-scale networks reflecting coordinated neural activity among spatially segregated cortical areas, which takes up a large fraction of metabolism in the brain. Intrinsic networks have been observed during, or in the absence of, overt cognitive tasks or external stimuli, and can be detected during various states of consciousness, including sleep and sedation. Specialized and functionally optimized brain networks are the outcome of prolonged neurodevelopmental processes, including constructive (e.g., synaptogenesis and axonal myelination) and regressive events (e.g., synaptic pruning and axonal loss). Overabundant connectivity during early postnatal years is pruned away based on experience-driven activity patterns, resulting in distinct and functional specialized networks. These developmental principles appear to be impaired in ASDs
- Core diagnostic features of ASDs
a diagnosis of an ASD is based on the presence of two core symptom domains: (i) SC impairments, including deficits in social-emotional reciprocity, in nonverbal communicative behaviors, and in developing, maintaining, and understanding relationships; and (ii) restricted and repetitive patterns of behavior and interests, such as stereotyped or repetitive speech, movements, or use of objects; excessive adherence to routines and insistence on sameness; highly restricted, fixated interests; and atypical reactivity to, or unusual interest in, sensory input. ASD symptoms must be present early during development. While often detected during toddlerhood or preschool, they may not raise concern until social demands exceed a child’s capabilities (e.g., in elementary school with progressively rising academic and social demands)
- Functional connectivity MRI (fcMRI
similar to conventional functional MRI, fcMRI uses times series of brain images acquired every 2 s or faster. The contrast of interest relies on changes in the blood oxygen level-dependent (BOLD) signal associated with local neuronal activity and synaptic transmission. FcMRI detects correlations (or statistical dependence) of BOLD signals between different brain regions, interpreted as functional connectivity. In the most common type of fcMRI, called intrinsic fcMRI, images are acquired during rest (without specific task) and spontaneous synchronization of BOLD fluctuations at low frequencies (typically <0.1 Hz) is detected. However, fcMRI can also be performed to detect BOLD correlations associated with task performance or sensory stimulation
- ‘Idiopathic’ ASD
indicates cases of ASD with no known cause, as opposed to syndromic ASD with established genetic (or other) causes. Although this term (or its equivalent ‘nonsyndromic’ ASD) is still commonly used, it is destined to become less suitable with improving knowledge of causation in variants of the disorder
Footnotes
Appendix A Supplementary data
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tics.2018.09.008.
References
- 1.Hansen SN et al. (2015) Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 169, 56–62 [DOI] [PubMed] [Google Scholar]
- 2.Baio J et al. (2018) Prevalence of autism spectrum disorder among children aged 8 years – Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 67, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS et al. (2011) Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 168, 904–912 [DOI] [PubMed] [Google Scholar]
- 4.Levaot Y et al. (2018) Autism prevalence and severity in Bedouin-Arab and Jewish communities in southern Israel. Community Ment Health J [DOI] [PubMed] [Google Scholar]
- 5.May T et al. (2017) Autism spectrum disorder: updated prevalence and comparison of two birth cohorts in a nationally representative Australian sample. BMJ Open 7, e015549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leigh JP and Du J (2015) Brief report: forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 45, 4135–4139 [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders – 5. (4th edn), American Psychiatric Association [Google Scholar]
- 8.Anderson DK et al. (2014) Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 55, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis Weismer S and Kover ST (2015) Preschool language variation, growth, and predictors in children on the autism spectrum. J. Child Psychol. Psychiatry 56, 1327–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Itzchak E and Zachor DA (2007) The effects of intellectual functioning and autism severity on outcome of early behavioral intervention for children with autism. Res. Dev. Disabil. 28, 287–303 [DOI] [PubMed] [Google Scholar]
- 11.Perry A et al. (2011) Predictors of outcome for children receiving intensive behavioral intervention in a large, community-based program. Res. Autism Spectr. Disord. 5, 592–603 [Google Scholar]
- 12.Frazier TW et al. (2014) Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 53, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomes R et al. (2017) What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474 [DOI] [PubMed] [Google Scholar]
- 14.Dworzynski K et al. (2012) How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 51, 788–797 [DOI] [PubMed] [Google Scholar]
- 15.Solomon M et al. (2012) Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J. Autism Dev. Disord. 42, 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szatmari P et al. (2012) Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159b, 5–12 [DOI] [PubMed] [Google Scholar]
- 17.Pelphrey KA et al. (2011) Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry 52, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tager-Flusberg H (2010) The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Netw. 23, 1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voegtline KM et al. (2013) Near-term fetal response to maternal spoken voice. Infant Behav. Dev. 36, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid VM et al. (2018) The human fetus preferentially engages with face-like visual stimuli. Curr. Biol. 28, 824. [DOI] [PubMed] [Google Scholar]
- 21.Meltzoff AN and Moore MK (1977) Imitation of facial and manual gestures by human neonates. Science 198, 75–78 [DOI] [PubMed] [Google Scholar]
- 22.Simion F et al. (2008) A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. U. S. A. 105, 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantino JN et al. (2017) Infant viewing of social scenes is under genetic control and atypical in autism. Nature 547, 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperdin HF and Schaer M (2016) Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front. Neurosc. 10, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klin A et al. (2009) Two-year-olds with autism orient to nonsocial contingencies rather than biological motion. Nature 459, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsabbagh M et al. (2012) Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr. Biol. 22, 338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grelotti DJ et al. (2002) Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev. Psychobiol. 40, 213–225 [DOI] [PubMed] [Google Scholar]
- 28.Korhonen V et al. (2014) Autism spectrum disorder and impaired joint attention: A review of joint attention research from the past decade. Nordic. Psychology 66, 94–107 [Google Scholar]
- 29.Grossmann T (2015) The development of social brain functions in infancy. Psychol. Bull. 141, 1266–1287 [DOI] [PubMed] [Google Scholar]
- 30.Grossmann T (2017) How to build a helpful baby: a look at the roots of prosociality in infancy. Curr. Opin. Psychol. 20, 21–24 [DOI] [PubMed] [Google Scholar]
- 31.Brothers L (1990) The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1, 27–51 [Google Scholar]
- 32.Adolphs R (2009) The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakemore SJ (2008) The social brain in adolescence. Nat. Rev. Neurosci. 9, 267–277 [DOI] [PubMed] [Google Scholar]
- 34.Frith U and Frith C (2010) The social brain: allowing humans to boldly go where no other species has been. Philos. Trans R. Soc. Lond. B Biol. Sci. 365, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haxby JV et al. (2000) The distributed human neural system for face perception. Trends Cogn. Sci. 4, 223–233 [DOI] [PubMed] [Google Scholar]
- 36.Carlin JD and Calder AJ (2013) The neural basis of eye gaze processing. Curr. Opin. Neurobiol. 23, 450–455 [DOI] [PubMed] [Google Scholar]
- 37.Phelps EA and LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187 [DOI] [PubMed] [Google Scholar]
- 38.Saxe R (2006) Uniquely human social cognition. Curr. Opin. Neurobiol. 16, 235–239 [DOI] [PubMed] [Google Scholar]
- 39.Rizzolatti G and Craighero L (2004) The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 [DOI] [PubMed] [Google Scholar]
- 40.Bickart KC et al. (2012) Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J. Neurosci. 32, 14729–14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett S and Blakemore SJ (2009) Functional connectivity during a social emotion task in adolescents and in adults. Eur. J. Neurosci. 29, 1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babiloni C et al. (2017) Frontal functional connectivity of electrocorticographic delta and theta rhythms during action execution versus action observation in humans. Front. Behav. Neurosc. 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen Kadosh K et al. (2011) Developmental changes in effective connectivity in the emerging core face network. Cereb. Cortex 21, 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillon Q et al. (2014) Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neurosci. Biobehav. Rev. 42, 279–297 [DOI] [PubMed] [Google Scholar]
- 45.Chita-Tegmark M (2016) Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res. Dev. Disabil. 48, 79–93 [DOI] [PubMed] [Google Scholar]
- 46.Chevallier C et al. (2012) The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Martino A et al. (2009) Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry 65, 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickstein DP et al. (2013) Developmental meta-analysis of the functional neural correlates of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 52, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nickl-Jockschat T et al. (2015) Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct. Funct. 220, 2355–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J and Hofmann J (2016) Action observation and imitation in autism spectrum disorders: an ALE meta-analysis of fMRI studies. Brain Imaging Behav. 10, 960–969 [DOI] [PubMed] [Google Scholar]
- 51.Patriquin MA et al. (2016) Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 37, 3957–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotts SJ et al. (2012) Fractionation of social brain circuits in autism spectrum disorders. Brain 135, 2711–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von dem Hagen EAH et al. (2013) Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc. Cogn. Affect. Neurosci. 8, 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fishman I et al. (2014) Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 71, 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fishman I et al. (2015) Reduced integration and differentiation of the imitation network in autism: a combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging study. Ann. Neurol. 78, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudie JD et al. (2012) Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb. Cortex 22, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen MD et al. (2016) Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 55, 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Supekar K et al. (2013) Brain hyper-connectivity in children with autism and its links to social deficits. Cell Rep. 5, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kana RK et al. (2014) Functional brain networks and white matter underlying theory-of-mind in autism. Soc. Cogn. Affect. Neurosci. 9, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picci G et al. (2016) A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Dev. Sci. 19, 524–549 [DOI] [PubMed] [Google Scholar]
- 61.Carter RM and Huettel SA (2013) A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 17, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bzdok D et al. (2013) Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosc. 7, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Overwalle F (2011)A dissociation between social mentalizing and general reasoning. Neuroimage 54, 1589–1599 [DOI] [PubMed] [Google Scholar]
- 64.Baetens K et al. (2014) Involvement of the mentalizing network in social and non-social high construal. Soc. Cogn. Affect. Neurosci. 9, 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baetens KL et al. (2017) The dorsal medial prefrontal cortex is recruited by high construal of non-social stimuli. Front. Behav. Neurosc. 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilalic M (2016) Revisiting the role of the fusiform face area in expertise. J. Cogn. Neurosci. 28, 1345–1357 [DOI] [PubMed] [Google Scholar]
- 67.McGugin RW et al. (2014) Robust expertise effects in right FFA. Neuropsychologia 63, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hull JV et al. (2017) Resting–state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry 7, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lebel C et al. (2017) A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. Published online September 8, 2017 10.1002/nbm.3778 [DOI] [PubMed] [Google Scholar]
- 70.Horwitz B et al. (1988) The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch. Neurol. 45, 749–755 [DOI] [PubMed] [Google Scholar]
- 71.Mash LE et al. (2018) Multimodal approaches to functional connectivity in autism spectrum disorders: an integrative perspective. Dev. Neurobiol. 78, 456–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nair A et al. (2014) Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum. Brain Mapp. 35, 4035–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Power JD et al. (2018) Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. U. S. A. 115, E2105–E2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sztainberg Y and Zoghbi HY (2016) Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 19, 1408–1417 [DOI] [PubMed] [Google Scholar]
- 75.Vorstman JAS et al. (2017) Autism genetics: opportunities and challenges for clinical translation. Nat. Rev. Genet. 18, 362–376 [DOI] [PubMed] [Google Scholar]
- 76.Brandler WM and Sebat J (2015) From de novo mutations to personalized therapeutic interventions in autism. Annu. Rev. Med. 66, 487–507 [DOI] [PubMed] [Google Scholar]
- 77.Ciernia AV and LaSalle J (2016) The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat. Rev. Neurosci. 17, 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandy W and Lai MC (2016) Annual research review: the role of the environment in the developmental psychopathology of autism spectrum condition. J. Child Psychol. Psychiatry 57, 271–292 [DOI] [PubMed] [Google Scholar]
- 79.Parikshak NN et al. (2015) Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat. Rev. Genet. 16, 441–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mosca E et al. (2017) Network diffusion-based prioritization of autism risk genes identifies significantly connected gene modules. Front. Gene. 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ebrahimi-Fakhari D and Sahin M (2015) Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr. Opin. Neurol. 28, 91–102 [DOI] [PubMed] [Google Scholar]
- 82.Sahin M and Sur M (2015) Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science 350, aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ellegood J et al. (2014) Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol. Psychiatry 20, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 28, 597–613 [DOI] [PubMed] [Google Scholar]
- 85.Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 [DOI] [PubMed] [Google Scholar]
- 86.Marco EJ et al. (2011) Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 69, 48R–54R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Setoh P et al. (2017)Autism spectrum disorder and early motor abnormalities: connected or coincidental companions? Res. Dev. Disabil. 60, 13–15 [DOI] [PubMed] [Google Scholar]
- 88.Keehn B et al. (2013) Atypical attentional networks and the emergence of autism. Neurosci. Biobehav. Rev. 37, 164–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craig F et al. (2016) A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 12, 1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ansel A et al. (2017) Variation in gene expression in autism spectrum disorders: an extensive review of transcriptomic studies. Front. Neurosc. 10, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philip RC et al. (2012) A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci. Biobehav. Rev. 36, 901–942 [DOI] [PubMed] [Google Scholar]
- 92.Carper RA et al. (2015) Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. J. Am. Acad. Child Adolesc. Psychiatry 54, 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nebel MB et al. (2014) Disruption of functional organization within the primary motor cortex in children with autism. Hum. Brain Mapp. 35, 567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbott AE et al. (2016) Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb. Cortex 26, 4034–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Green SA et al. (2016) Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J. Am. Acad. Child Adolesc. Psychiatry 55, 618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Martino A et al. (2011) Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry 69, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nair A et al. (2015) Regional specificity of aberrant thalamocortical connectivity in autism. Hum. Brain Mapp. 36, 4497–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woodward ND et al. (2017) Thalamocortical dysconnectivity in autism spectrum disorder: An analysis of the Autism Brain Imaging Data Exchange. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan AJ et al. (2015) Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol. Psychiatry 78, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stoodley CJ et al. (2017) Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci. 20, 1744–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Courchesne E et al. (2011) Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380, 138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolff JJ et al. (2012) Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen MD et al. (2017) Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol. Psychiatry 82, 186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Padilla N et al. (2017) Poor brain growth in extremely preterm neonates long before the onset of autism spectrum disorder symptoms. Cereb. Cortex 27, 1245–1252 [DOI] [PubMed] [Google Scholar]
- 105.Kana RK et al. (2015) Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol Autis. 6, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzales CR et al. (2017) Introspection plays an early role in children’s explicit theory of mind development. Child Dev. 89, 1545–1552 [DOI] [PubMed] [Google Scholar]
- 107.O’Connor K (2012) Auditory processing in autism spectrum disorder: a review. Neurosci. Biobehav. Rev. 36, 836–854 [DOI] [PubMed] [Google Scholar]
- 108.Simmons DR et al. (2009)Vision in autism spectrum disorders. Vision Res. 49, 2705–2739 [DOI] [PubMed] [Google Scholar]
- 109.Chukoskie L et al. (2013) Motor skill in autism spectrum disorders: a subcortical view. Int Rev. Neurobiol. 113, 207–249 [DOI] [PubMed] [Google Scholar]
- 110.Benasich AA et al. (2002) The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev. Psychobiol. 40, 278–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vissers LE et al. (2016) Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 17, 9–18 [DOI] [PubMed] [Google Scholar]
- 112.Rice ML (2013) Language growth and genetics of specific language impairment. Int. J. Speech. Lang. Pathol. 15, 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramus F et al. (2018) Neuroanatomy of developmental dyslexia: pitfalls and promise. Neurosci. Biobehav. Rev. 84, 434–452 [DOI] [PubMed] [Google Scholar]
- 114.Vilor-Tejedor N et al. (2017) Imaging genetics in attention-deficit/hyperactivity disorder and related neurodevelopmental domains: state of the art. Brain Imaging Behav. 11, 1922–1931 [DOI] [PubMed] [Google Scholar]
- 115.Williams K et al. (2015) Regression in autism spectrum disorders. J. Paediatr. Child Health 51, 61–64 [DOI] [PubMed] [Google Scholar]
- 116.Edmiston E et al. (2017) Autoimmunity, autoantibodies, and autism spectrum disorder. Biol. Psychiatry 81, 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marchetto MC et al. (2016) Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry 22, 820–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waterhouse L and Gillberg C (2014) Why autism must be taken apart. J. Autism Dev. Disord. 44, 1788–1792 [DOI] [PubMed] [Google Scholar]
- 119.Goldstone RL (1994) Influences of categorization on perceptual discrimination. J. Exp. Psychol. Gen. 123, 178–200 [DOI] [PubMed] [Google Scholar]
- 120.Dehaene-Lambertz G (1997) Electrophysiological correlates of categorical phoneme perception in adults. Neuroreport 8, 919–924 [DOI] [PubMed] [Google Scholar]
- 121.Sturm A et al. (2018) Are the components of social reciprocity transdiagnostic across pediatric neurodevelopmental disorders? Evidence for common and disorder-specific social impairments. Psychiatry Res. 264, 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whitton AE et al. (2015) Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 28, 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Martino A et al. (2017) Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Dat 4, 170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Rooij D et al. (2017) Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD Working Group. Am. J. Psychiatry 175, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cremers HR et al. (2017) The relation between statistical power and inference in fMRI. PLoS One 12, e0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Loth E et al. (2017) The EU-AIMS Longitudinal European Autism Project (LEAP): design and methodologies to identify and validate stratification biomarkers for autism spectrum disorders. Mol. Autism 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wasserstein RL (2016) ASA statement on statistical significance and P-values. Am. Stat. 70, 129–133 [Google Scholar]
- 128.Geschwind DH and State MW (2015) Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 14, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beglinger LJ and Smith TH (2001) A review of subtyping in autism and proposed dimensional classification model. J. Autism Dev. Disord. 31, 411–422 [DOI] [PubMed] [Google Scholar]
- 130.Campbell DJ et al. (2014) Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: a subtyping analysis. J. Autism Dev. Disord. 44, 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeBoth KK and Reynolds S (2017) A systematic review of sensory-based autism subtypes. Res. Autism Spectr. Disord. 36, 44–56 [Google Scholar]
- 132.Hong SJ et al. (2017) Multidimensional neuroanatomical subtyping of autism spectrum disorder. Cereb. Cortex 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jabaudon D (2017) Fate and freedom in developing neocortical circuits. Nat Commu. 8, 16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rasetti R and Weinberger DR (2011) Intermediate phenotypes in psychiatric disorders. Curr. Opin. Genet. Dev. 21, 340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beversdorf DQ and Missouri Autism Summit Consortium (2016) Phenotyping, etiological factors, and biomarkers. J. Dev. Behav. Pediatr. 37, 659–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Valero-Cabre A et al. (2017) Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 83, 381–404 [DOI] [PubMed] [Google Scholar]
- 137.Philip NS et al. (2017) Low-intensity transcranial current stimulation in psychiatry. Am. J. Psychiatry 174, 628–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sitaram R et al. (2017) Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 18, 86–100 [DOI] [PubMed] [Google Scholar]
- 139.Werling DM and Geschwind DH (2013) Understanding sex bias in autism spectrum disorder. Proc. Natl. Acad. Sci. U. S. A. 110, 4868–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Constantino JN and Charman T (2012) Gender bias, female resilience, and the sex ratio in autism. J. Am. Acad. Child Adolesc. Psychiatry 51, 756–758 [DOI] [PubMed] [Google Scholar]
- 141.Sedgewick F et al. (2016) Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. J. Autism Dev. Disord. 46, 1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Whyte EM and Scherf KS (2018) Gaze following is related to the broader autism phenotype in a sex-specific way: building the case for distinct male and female autism phenotypes. Clin. Psychol. Sci. 6, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Banach R et al. (2009) Brief report: relationship between non-verbal IQ and gender in autism. J. Autism Dev. Disord. 39, 188–193 [DOI] [PubMed] [Google Scholar]
- 144.Werling DM and Geschwind DH (2013) Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nordahl CW et al. (2011) Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A. 108, 20195–20200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lai MC et al. (2017) Imaging sex/gender and autism in the brain: etiological implications. J. Neurosci. Res. 95, 380–397 [DOI] [PubMed] [Google Scholar]
- 147.Baron-Cohen S (2002) The extreme male brain theory of autism. Trends Cogn. Sci. 6, 248–254 [DOI] [PubMed] [Google Scholar]
- 148.Baron-Cohen S et al. (2015) Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 20, 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bejerot S et al. (2012) The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br. J. Psychiatry 201, 116–123 [DOI] [PubMed] [Google Scholar]
- 150.Alaerts K et al. (2016) Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 11, 1002–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baron-Cohen S et al. (1985) Does the autistic child have a ‘theory of mind’? Cognition 21, 37–46 [DOI] [PubMed] [Google Scholar]
- 152.Leslie AM et al. (2004) Core mechanisms in ‘theory of mind’. Trends Cogn. Sci. 8, 528–533 [DOI] [PubMed] [Google Scholar]
- 153.Dawson G et al. (2004) Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 40, 271–283 [DOI] [PubMed] [Google Scholar]
- 154.Demetriou EA et al. (2018) Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry 23, 1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ozonoff S et al. (2010) A prospective study of the emergence of early behavioral signs of autism. J. Am. Acad. Child Adolesc. Psychiatry 49, 256–266 [PMC free article] [PubMed] [Google Scholar]
- 156.Frith U (2001) Mind blindness and the brain in autism. Neuron 32, 969–979 [DOI] [PubMed] [Google Scholar]
- 157.Kaland N et al. (2002) A new ‘advanced’ test of theory of mind: evidence from children and adolescents with Asperger syndrome. J. Child Psychol. Psychiatry 43, 517–528 [DOI] [PubMed] [Google Scholar]
- 158.Schneider D et al. (2013) A temporally sustained implicit theory of mind deficit in autism spectrum disorders. Cognition 129, 410–417 [DOI] [PubMed] [Google Scholar]