Abstract
Caveolae have been implicated in a wide range of critical physiological functions. In the past decade, the dominant role of cavin-1 in caveolae formation has been established, and it has been recognized as another master regulator for caveolae biology. Human patients with cavin-1 mutations develop lipodystrophy and muscular dystrophy and have some major pathological dysfunctions in fat tissue, skeleton muscle, heart, lung and other organs. Cavin-1 deficiency animal models consistently show similar phenotypes. However, the underlying molecular mechanisms remain to be elucidated. Recent studies have suggested many possible pathways, including mechanosensing, stress response, signal transduction, exosome secretion, and potential functions in the nucleus. Many excellent and comprehensive review articles already exist on the topics of caveolae structure formation, caveolins, and their pathophysiological functions. We will focus on recent studies using cavin-1 deficiency models, to summarize the pathophysiological changes in adipose, muscle, and other organs, followed by a summary of mechanistic studies about the roles of cavin-1, which includes caveolae formation, ribosomal RNA transcription, mechanical sensing, stress response, and exosome secretion. Further studies may help to elucidate the exact underlying molecular mechanism to explain the pathological changes observed in cavin-1 deficient human patients and animal models, so potential new therapeutic strategies can be developed.
Introduction
Caveolae or little caves were first observed by electronic microscopy in the 1950s [1,2]. The first caveolae protein was discovered in the 1990s, named caveolin-1 [3,4]. Subsequently, two other caveolin isoforms (caveolin-2 and 3) were reported [5–7]. In the past decade another family of caveolae proteins, named cavins (cavin-1, 2, 3, and 4) have also been documented (reviewed in [8]). These cavin proteins were originally studied outside of caveolae and named in the context of the experiments being performed. Briefly, cavin-1 was called polymerase I and transcript release factor (PTRF) [9], cavin-2 was called serum deprivation response gene (SDPR) [10] and cavin-3 was called SRBC for SDPR-related gene that binds PKC [11], and cavin-4 was called muscle-related coiled-coil protein [12]. Caveolins and cavins are expressed in some degree of tissue-specific manner [13–19]. The components of caveolae in non-muscle cells include caveolin-1 and caveolin-2 and cavin-1–3. Caveolin-3 and cavin-4 are expressed only in cardiac and skeletal muscle. Studies from knockout mouse models also demonstrate only caveolin-1 [20,21], caveolin-3 [22], and cavin-1[23] are absolutely necessary for caveolae structure formation.
In 1998, Cavin-1 was first reported as a cofactor of RNA polymerase I (Pol I) in the nucleus [9]. It was subsequently described as a caveolae protein by the Vinten and Tranum–Jensen group from Denmark in 2001 [24]. They isolated a ‘lipid raft’ fraction from adipocytes, followed by applying a hybridoma antibody screening approach. They identified a clone called ‘2F11,’ that specifically labeled adipocyte caveolae using immunogold staining electron microscopy technique. This antibody recognized a target ~ 60-kDa, so the antigenic protein was initially called ‘cav-p60.’ The same group confirmed its caveolae-specific expressions in other tissues [25,26], and it has been called ‘cavin’ since then [27]. Another group used a proteomics approach to locate cavin-1 and its other family members in human adipocyte caveolae and document many key biochemical features [28]. In the past 10 years, studies using cavin-1 deficiency models have helped to established the role of cavin-1 in caveolae biology. In this review, we will summarize the pathophysiological relevance that was learned from these working systems, followed by the insights about the potential cavin-1 dependent molecular and cellular mechanisms that underlie these changes.
Pathophysiological relevance of cavin-1
Cavin-1 in adipocytes: lipodystrophy
One major phenotype found in adult cavin-1 null mice is an abnormal loss of fat (termed lipodystrophy) [23]. The knockout mice developed dramatically reduced adipose tissue throughout the body [23,29] and were resistant to diet-induced obesity; epididymal white fat cells were small and relative insensitive to insulin and β-adrenergic agonists, resulting in dramatically reduced adipocyte lipid storage, lipid tolerance, and lipolysis [29]. This metabolic disorder has also been found in human patients with loss-of-function cavin-1 mutations, which is termed as congenital generalized lipodystrophy type 4 (CGL4) [30–32]. The reduced lipid accumulation in adipocytes was thought to be caused by decreased fatty acid uptake and incorporation and the virtual absence of insulin-stimulated glucose transport [29]. At the molecular level, studies have shown cavin-1 was involved in the regulations of two key enzymes of lipolysis, perilipin and hormone-sensitive lipase (HSL) [29,33]. The phosphorylation of both proteins has been known as key initial steps of lipolysis. Perilipin phosphorylation was impaired in cavin-1 null adipocytes [29]. In the plasma membrane cavin-1 bound to HSL for the regulation of its cellular localization, and loss of cavin-1 caused HSL dislocation [34]. Further functional studies using knockdown and overexpression of cavin-1 suggested the role of cavin-1 in adipocyte lipolysis is dependent on its own phosphorylation as well [33], although the details remain to be determined. Besides cavin-1 phosphorylation, it is possible other mechanisms might be involved in the regulation of lipolysis. One study indicated that acetylated cavin-1 preferentially interacted with HSL and recruited it to the caveolae, thereby promoting lipolysis [35]. Nevertheless, it remains unclear how reduced lipolysis contributes to the lipodystrophy phenotype in cavin-1 deficiency models as one might expect the opposite response to reduced fat cell mass.
Studies also support that there is a close correlative relationship between cavin-1 expression level and adipocyte expansion and lipid storage, although the conclusions from two following reports seem inconsistent. One of the studies shows the increasing density of caveolae accommodates larger lipid droplets and storage with increased adipocyte functionality, and conversely lipid mobilization that induces lipid droplet shrinkage leads to the loss of cavin-1 expression, which is coincident with caveolae disassembly/disappearance [36]. However, another report demonstrated the opposite result: cavin-1 overexpression compromises adipocyte differentiation with decreased lipid storage capacity, and its mRNA levels are positively correlated with cellular senescence and therefore limited expansion in human adipose tissue [37]. This apparent inconsistency is not necessary completely contradictive, since the direct casual or consequence relationship between cavin-1 expression level and adipocyte function has not been firmly established. One also needs to consider the factors involved in the different working systems used at different stages of healthy or diseased states. The role of cavin-1 in physiological healthy adipocyte expansion might be quite different from its function in adipocytes with pathological changes in obesity and metabolic disease conditions.
The role of caveolae and cavin-1 in lipid metabolism has been reviewed elsewhere [18]. In one of our recent studies, we showed that cavin-1 is less significant in adipocyte differentiation or lipolysis signal transduction [38]. However, we believe these were secondary changes due to the loss of growth ability upon nutrient loading. This idea is also supported by the fact that the lipodystrophy phenotype only becomes obvious in adult mice (>6–8 weeks) rather than newborns or infants (unpublished data). In our cavin-1 knockout mice studies [23,29], we saw that the newborn knockout mice looked quite normal and almost identical with wild-type littermates. The loss of fat tissue became increasingly evident as the knockout mice grew older. A similar situation was found in human patients with cavin-1 mutations; almost all reports were about toddlers or children, not newborns [39–42]. These suggest that a direct effect on lipid metabolism (such as lipolysis) may not be the primary mechanism for cavin-1 action. Instead, the functions of cavin-1 in other cellular localization, such as nucleus or cytosol might be the major causes of the lipodystrophy phenotype.
Cavin-1 in muscle: muscular dystrophy
Among cavin family proteins, cavin-4 is the one showing muscle specificity [14,43]. But in this review, we will focus on the findings directly linking cavin-1 to muscle physiology. Human mutations in the cavin-1 gene cause CGL4 associated with myopathy [30]. Long-QT syndrome and fatal cardiac arrhythmia are observed in some patients with CGL4 [44]. Cavin-1 null mice recapitulated these pathological changes, showing impaired exercise ability and muscle hypertrophy with increased muscle fiber size and muscle mass, a compensatory response to muscle weakness and wasting [45]. Skeletal muscles without cavin-1 expression were fibrotic and exhibited impaired membrane integrity accompanied by an apparent compensatory activation of the dystrophin–glycoprotein complex and elevated expression of proteins involved in muscle repair function [45]. One study showed with electron tomography that cavin-1 null muscle fibers had a striking loss of sarcolemmal organization, aberrant T-tubule structures, and increased sensitivity to membrane tension [46].
Studies also demonstrate an essential role of cavin-1 in the cardiovascular system. A progressive cardiomyopathic phenotype with wall thickening of the left ventricle and reduced fractional shortening were observed in 16-week-old cavin-1 null mice [47]. Further histological analysis revealed cardiomyocyte hypertrophy accompanied by progressive interstitial/perivascular fibrosis [47]. Consistently, another study shows cardiac ejection properties were modestly reduced in cavin-1 knockout mice, along with the exaggeration of intrinsic beating rate, diastolic stiffness and stretch-dependent diastolic and systolic forces as well [48]. Similar to skeleton muscle, the hypertrophy phenotype was also observed in cavin-1 null heart, as the increased right ventricle mass of the heart and elevated right ventricular pressure were reported by another group [49].
Cavin-1 in lung and other tissues
The function of cavin-1 in other tissues has also been examined. Cavin-1-deficient mice exhibited an increased lung tissue density (vessel thickness) and hypertrophic remodeling of pulmonary arteries [49]. Consistent with this, a study showed that cavin-1 deficient mice possessed dramatically altered distal lung morphology and increased lung elastance, which were associated with hypercellularity and accumulation of lung macrophages [50]. The bladder weight in male cavin-1 null mice was increased, with a reduction in depolarization-induced contraction but no change of micturition patterns and diuresis [51].
Overall, the whole-body cavin-1 null mice are not embryonic lethal but do develop some major phenotypes in adipose tissue and muscular organs, including the skeleton, cardiovascular system, and smooth muscle. These data raise questions about the underlying molecular mechanisms that are responsible for these pathological changes.
Molecular and cellular functions of cavin-1
An indispensable role in caveolae formation
The major breakthrough about the function of cavin-1 in caveolae biology came from two groups’ studies ~2008, using RNAi knockdown and gene knockout models: knockdown cavin-1 by shRNA in cultured 3T3-L1 adipocyte diminishes caveolin-1 expression [52]; cavin-1 knockdown reduces caveolae density in NIH 3T3 fibroblasts [53]; cavin-1 knockdown via morpholino technology at the stage of zebrafish embryo development shows a dramatic reduction in caveolae [53]; and a whole-body cavin-1 knockout mouse model shows the complete loss of caveolae in fat, skeleton, and smooth muscle tissue, accompanied by diminishing protein expressions of all caveolin isoforms [23]. These results were confirmed by other groups with the same or similar working models [14,54]. Together, these studies well established the essential role of cavin-1 in caveolae formation. So far the dominant roles of cavin-1 and caveolin1/3, two ‘master’ proteins, are indispensable and well accepted in the field. The phenotypes in cavin-1 null mice almost recapitulate the ones in both caveolin-1 and caveolin-3 null mice, suggesting that a caveolae-dependent mechanism might be important to the dysfunctions observed in cavin-1 null mice. However, caveolin-1 and caveolin-3 null mice both show diminished cavin-1 expression, it is also possible that the phenotypes observed in both knockout mouse models were driven by the function of cavin-1 outside of caveolae.
Cavin-1 positively regulates ribosomal RNA transcription in the nucleus
Cavin-1 was first characterized by its Pol I-related regulatory function ~20 years ago by the Grummt group [9]. Cavin-1 was studied for its role in ribosomal RNA transcription and was called ‘Polymerase I and Transcript Release Factor’ (PTRF) [9]. Transcription by all three classes of nuclear RNA polymerases includes initiation, elongation, and termination. Termination of Pol I-mediated pre-ribosomal RNA (pre-rRNA) transcription is a two-step process that involves pausing elongating transcription complexes and releasing both pre-rRNA and Pol I from the template. By using a cell-free in vitro reconstituted transcription assay system, the authors demonstrated that PTRF not only augments the efficiency of transcript release but also increases the overall rate of transcription in a concentration-dependent manner [9]. More detailed mechanistic studies from the same group revealed that PTRF-mediated transcript disassociation could lead to increasing the efficiency of reinitiation, which was potentially regulated through PTRF-phosphorylation [55,56]. However, the physiological relevance of this activity had never been established in cells or in vivo.
Recently, we used a primary mouse and cultured adipocyte experimental systems to show that cavin-1 localized to the nucleus and associated with the Pol I transcription complex, directly affecting metabolically regulated ribosomal DNA transcription [38]. These studies not only show a specific role of cavin-1 on metabolism-regulated ribosomal DNA transcription in the adipocytes but also add another layer of regulation to rDNA transcriptional complexity. This mechanism could explain the lipodystrophy phenotype that was observed in mouse models and human patients lacking functional cavin-1. Considering ribosome biogenesis is a general cellular ‘building’ process, we believe this cavin-1 dependent ribosome biogenesis mechanism may be applied to explain muscle weakness, growth failure and wasting phenotypes in muscular dystrophy, simply because these could be the result of impairment of protein synthesis.
Cavin-1 in mechanical stress response and membrane remodeling
It is known that caveolae are essential in response to mechanical stress and are involved in the regulation of subsequent membrane remodeling [57]. The mirrored expression levels between caveolin-1 and cavin-1 and their indispensable roles in caveolae formation suggest that cavin-1 must be involved in such regulation. The role of cavin-1 in mechanical stress response was established both in vitro and in vivo by the following studies. In cultured cells, upon mechanical stress, the interaction between cavelin-1 and cavin-1 was decreased and cavin-1 was disassociated from caveolae [58]. In muscle, caveolae occupied ~s 50% of the sarcolemmal area and were predominantly assembled into rosette shapes, which can also be preferentially disassembled in response to increased membrane tension [46]. Loss of cavin-1 was associated with abnormal sarcolemma organization and T-tubule structures and increased sensitivity to membrane tension, and these resulted in compromised muscle integrity [46]. Mechanistically this can be explained through various downstream targets or pathways. Cavin-1 could act as a docking protein for MG53, which has been believed to be an essential component of the membrane repair machinery [59]. Cells that do not express endogenous cavin-1 show defective trafficking of MG53 to membrane injury sites [60]. Our recent studies show that upon insulin stimulation, cavin-1 can be acutely translocated to focal complex compartments, where it regulates focal complex formation through an interaction with paxillin [61]. We also found that loss of cavin-1 impairs focal complex remodeling and focal adhesion formation and causes a mechanical stress response, concomitant with activation of proinflammatory and senescence/apoptosis pathways [61]. The mechanical stress response is closely related to cell volume change. Considering the dramatic change of adipocyte size under different metabolic conditions, this cavin-1-dependent mechanical sensing mechanism likely plays a significant role in the regulation of adipocyte lipid storage. Additionally, cell migration is another biological process that requires significant membrane remodeling. Many studies have suggested cavin-1 is involved in this process [62–66]. One of the studies demonstrates cavin-1 can suppress tumor cell migration through the inhibition of focal adhesion dynamics [64].
Cavin-1 in response to oxidative stress and p53 pathway activation
There are many reports that suggest there might be a connection between cavin-1 and stress responses that were caused by cellular genotoxic and oxidative insults. Cavin-1 expression was up-regulated in stressed cells, such as senescent human fibroblasts [67], cells with oxidative stress induced by hydrogen peroxide [68], and cells with diabetes-induced oxidative stress [69]. Hypoxia can reduce its expression in 3T3-L1 adipocytes [70]. A recent study showed evidence that cavin-1 and other family members can physically interact with p53 in the cytosolic compartment [71]. These studies indicate that cavin-1 may directly link stress to the gene transcriptional changes through p53-dependent pathways. Besides the direct interactions between cavins and p53, our studies show that cavin-1-dependent ribosomal RNA transcription contributes to the activation of the p53 pathway through an indirect ‘ribosomal or nuclear’ stress mechanism [38]. Nevertheless, all the data consistently support that cavin-1 might be involved in a p53 pathway-related stress response. As a well-known master regulator, p53 plays significant roles in cell senescence, growth arrest, and apoptosis (reviewed in [72,73]), which are also the common characteristics that can be observed in lipodystrophy and muscular dystrophy. This cavins-p53 stress sensing and response pathway might serve as a common mechanism mediating the pathological changes in adipose tissue and other tissues, such as muscle.
Cavin-1 in secretory pathway and exosome secretion.
There are some recent reports suggesting that cavin-1 may be involved in the secretory pathway. One study using prostate cancer cells (PC-3) showed that cavin-1 can reduce the secretion of a subset of proteins, including proteases, cytokines, and growth regulatory proteins [74]. Another study suggested when the newly synthesized Fam198a precursor (a member of secreted protein kinase family [75]) in the endoplasmic reticulum (ER) was transported to the Golgi apparatus, in which it cleaved into the secreted mature form, cavin-1 was required for Fam198a secretion after its maturation in the Golgi apparatus [75]. Although the exact underlying mechanism remains unclear, it is possible cavin-1 dependent caveolae biogenesis could indirectly impact on protein secretion pathway and the disruption of caveolae system will affect the secreted proteins’ functions.
Other studies also show cavin-1 overexpression increased exosome secretion and stimulated cell growth both in vitro and in vivo [76]. Moreover, cavin-1 itself can be found in human plasma, and is, at least in part, carried by exosomes [77]. However, more studies are needed to clarify how cavin-1 is involved in exosome secretion.
Summary/future directions
As illustrated in Figure 1, multiple functions of cavin-1 at a various subcellular location (plasma membrane, cytosol, and nucleus) could mediate the pathological changes of lipo- and muscular dystrophies that were observed in cavin-1 null mice. However, the precise molecular mechanism(s) remains to be determined in future studies. Cavin-1 undergoes many post-translational modifications, such as phosphorylation, ubiquitination, and acetylation. It will be interesting to see how cavin-1 acting as a signaling molecular mediates the upstream signal(s) to downstream effects. Such research would expand our understandings of caveolae biology.
Figure 1. Potential molecular mechanisms for the pathological changes observed in cavin-1 deficiency.
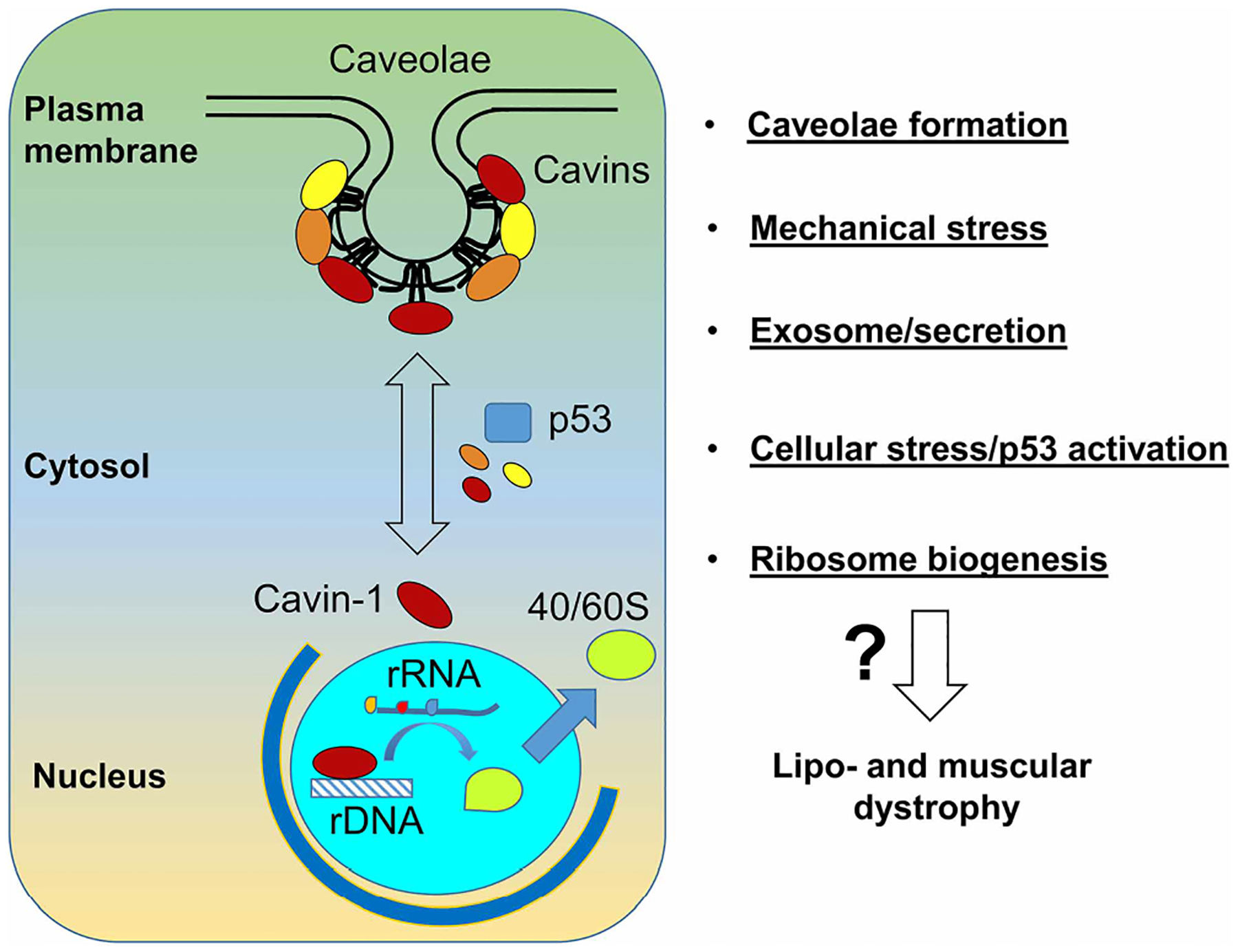
Cavin-1 plays a critical role in caveolae formation. It also has functions in other cellular locations, such as nucleus and cytosol (left panel). Current studies have suggested it is involved in many important cellular processes (right panel). Further research is needed to elucidate which one(s) are responsible for the lipodystrophy and muscular dystrophy phenotypes.
Perspectives.
Importance of the field: Human patients with loss of function in cavin-1 suffer from lipodystrophy and muscular dystrophy. However, the precise molecular actions of cavin-1 remain unclear.
Current thinking: Cavin-1 is indispensable in caveolae formation and involved in ribosomal RNA transcription, mechanical stress response, oxidative and other cellular stress response, exosome and secretion, and many others that are related to caveolae.
Future directions: Results from current studies suggest that cavin-1 might serve as a signaling molecule that integrates nutrients, mechanical stress, oxidation, and other signals with systemic transcriptional regulation. Further studies are needed to address the molecular details (such as multiple post-translational modifications of cavin-1) that are responsible for these functions.
Acknowledgements
The author would like to extend sincere thanks to Dr. Paul Pilch for critically reading the manuscript.
Funding
This work is supported by NIH Grant (DK112945) to L.L.
Abbreviations
- CGL4
congenital generalized lipodystrophy type 4
- ER
endoplasmic reticulum
- HSL
hormone-sensitive lipase
- Pol I
polymerase I
- pre-rRNA
pre-ribosomal RNA
- PTRF
polymerase I and transcript release factor
- SDPR
serum deprivation response gene
Footnotes
Competing Interests
The author declares that there are no competing interests associated with this manuscript.
References
- 1.Palade GE (1953) Fine structure of blood capillaries. J. Appl. Phys 24, 1424 [Google Scholar]
- 2.Yamada E (1955) The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol 1, 445–458 10.1083/jcb.1.5.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR and Anderson RG (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682 10.1016/0092-8674(92)90143-Z [DOI] [PubMed] [Google Scholar]
- 4.Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M et al. (1992) VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J. Cell Biol 118, 1003–1014 10.1083/jcb.118.5.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF and Lisanti MP (1996) Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl Acad. Sci. U.S.A 93, 131–135 10.1073/pnas.93.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS et al. (1996) Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem 271, 2255–2261 10.1074/jbc.271.4.2255 [DOI] [PubMed] [Google Scholar]
- 7.Way M and Parton RG (1996) M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 378, 108–112 10.1016/0014-5793(96)82884-5 [DOI] [PubMed] [Google Scholar]
- 8.Parton RG and del Pozo MA (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol 14, 98–112 10.1038/nrm3512 [DOI] [PubMed] [Google Scholar]
- 9.Jansa P, Mason SW, Hoffmann-Rohrer U and Grummt I (1998) Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 17, 2855–2864 10.1093/emboj/17.10.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustincich S and Schneider C (1993) Serum deprivation response gene is induced by serum starvation but not by contact inhibition. Cell Growth Differ. 4, 753–760 [PubMed] [Google Scholar]
- 11.Mineo C, Ying YS, Chapline C, Jaken S and Anderson RG (1998) Targeting of protein kinase Calpha to caveolae. J. Cell Biol 141, 601–610 10.1083/jcb.141.3.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, Kawashima T et al. (2008) MURC, a muscle-restricted coiled-coil protein that modulates the Rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance. Mol. Cell. Biol 28, 3424–3436 10.1128/MCB.02186-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TM and Lisanti MP (2004) The caveolin proteins. Genome Biol. 5, 214 10.1186/gb-2004-5-3-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP et al. (2009) MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol 185, 1259–1273 10.1083/jcb.200903053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastiani M and Parton RG (2010) Caveolae at a glance. J. Cell Sci 123(Pt 22), 3831–3836 10.1242/jcs.070102 [DOI] [PubMed] [Google Scholar]
- 16.Hansen CG and Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 20, 177–186 10.1016/j.tcb.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Briand N, Dugail I and Le Lay S (2011) Cavin proteins: new players in the caveolae field. Biochimie 93, 71–77 10.1016/j.biochi.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 18.Pilch PF and Liu L (2011) Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metab 22, 318–324 10.1016/j.tem.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilch PF, Meshulam T, Ding S and Liu L (2011) Caveolae and lipid trafficking in adipocytes. Clin. Lipidol 6, 49–58 10.2217/clp.10.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B et al. (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 10.1126/science.1062688 [DOI] [PubMed] [Google Scholar]
- 21.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB et al. (2001) Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem 276, 38121–38138 10.1074/jbc.M008340200 [DOI] [PubMed] [Google Scholar]
- 22.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M et al. (2001) Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem 276, 21425–21433 10.1074/jbc.M100828200 [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH et al. (2008) Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8, 310–317 10.1016/j.cmet.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinten J, Voldstedlund M, Clausen H, Christiansen K, Carlsen J and Tranum-Jensen J (2001) A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 305, 99–106 10.1007/s004410100389 [DOI] [PubMed] [Google Scholar]
- 25.Voldstedlund M and Vinten J (2001) Tranum-Jensen J. cav-p60 expression in rat muscle tissues. Distribution of caveolar proteins. Cell Tissue Res. 306, 265–276 10.1007/s004410100439 [DOI] [PubMed] [Google Scholar]
- 26.Voldstedlund M, Thuneberg L, Tranum-Jensen J, Vinten J and Christensen EI (2003) Caveolae, caveolin and cav-p60 in smooth muscle and renin-producing cells in the rat kidney. Acta Physiol. Scand 179, 179–188 10.1046/j.1365-201X.2003.01183.x [DOI] [PubMed] [Google Scholar]
- 27.Vinten J, Johnsen AH, Roepstorff P, Harpoth J and Tranum-Jensen J (2005) Identification of a major protein on the cytosolic face of caveolae. Biochim. Biophys. Acta 1717, 34–40 10.1016/j.bbamem.2005.09.013 [DOI] [PubMed] [Google Scholar]
- 28.Aboulaich N, Vainonen JP, Stralfors P and Vener AV (2004) Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem. J 383(Pt 2), 237–248 10.1042/BJ20040647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding SY, Lee MJ, Summer R, Liu L, Fried SK and Pilch PF (2014) Pleiotropic effects of cavin-1 deficiency on lipid metabolism. J. Biol. Chem 289, 8473–8483 10.1074/jbc.M113.546242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S et al. (2009) Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest 119, 2623–2633 10.1172/JCI38660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg A (2011) Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab 96, 3313–3325 10.1210/jc.2011-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK and Garg A (2010) Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am. J. Med. Genet. A 152A, 2245–2253 10.1002/ajmg.a.33578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aboulaich N, Chui PC, Asara JM, Flier JS and Maratos-Flier E (2011) Polymerase I and transcript release factor regulates lipolysis via a phosphorylation-dependent mechanism. Diabetes 60, 757–765 10.2337/db10-0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aboulaich N, Ortegren U, Vener AV and Stralfors P (2006) Association and insulin regulated translocation of hormone-sensitive lipase with PTRF. Biochem. Biophys. Res. Commun 350, 657–661 10.1016/j.bbrc.2006.09.094 [DOI] [PubMed] [Google Scholar]
- 35.Zhou SR, Guo L, Wang X, Liu Y, Peng WQ, Liu Y et al. (2017) Acetylation of cavin-1 promotes lipolysis in white adipose tissue. Mol. Cell Biol 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briand N, Prado C, Mabilleau G, Lasnier F, Le Liepvre X, Covington JD et al. (2014) Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes 63, 4032–4044 10.2337/db13-1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Diaz S, Johnson LA, DeKroon RM, Moreno-Navarrete JM, Alzate O, Fernandez-Real JM et al. (2014) Polymerase I and transcript release factor (PTRF) regulates adipocyte differentiation and determines adipose tissue expandability. FASEB J. 28, 3769–3779 10.1096/fj.14-251165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L and Pilch PF (2016) PTRF/Cavin-1 promotes efficient ribosomal RNA transcription in response to metabolic challenges. eLife 5, e17508 10.7554/eLife.17508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami N, Hayashi YK, Oto Y, Shiraishi M, Itabashi H, Kudo K et al. (2013) Congenital generalized lipodystrophy type 4 with muscular dystrophy: clinical and pathological manifestations in early childhood. Neuromuscular Disord. 23, 441–444 10.1016/j.nmd.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 40.Patni N and Garg A (2015) Congenital generalized lipodystrophies — new insights into metabolic dysfunction. Nat. Rev. Endocrinol 11, 522–534 10.1038/nrendo.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Pol RJ, Benninga MA, Magre J, Van Maldergem L, Rotteveel J, van der Knaap MS et al. (2015) Berardinelli-Seip syndrome and achalasia: a shared pathomechanism? Eur. J. Pediatr 174, 975–980 10.1007/s00431-015-2556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salle-Teyssieres L, Auclair M, Terro F, Nemani M, Elsayed SM, Elsobky E et al. (2016) Maladaptative autophagy impairs adipose function in congenital generalized lipodystrophy due to cavin-1 deficiency. J. Clin. Endocrinol. Metab 101, 2892–2904 10.1210/jc.2016-1086 [DOI] [PubMed] [Google Scholar]
- 43.Ogata T, Naito D, Nakanishi N, Hayashi YK, Taniguchi T, Miyagawa K et al. (2014) MURC/Cavin-4 facilitates recruitment of ERK to caveolae and concentric cardiac hypertrophy induced by alpha1-adrenergic receptors. Proc. Natl Acad. Sci. U.S.A 111, 3811–3816 10.1073/pnas.1315359111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R et al. (2010) Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 6, e1000874 10.1371/journal.pgen.1000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding SY, Liu L and Pilch PF (2017) Muscular dystrophy in PTFR/cavin-1 null mice. JCI Insight 2, e91023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo HP, Nixon SJ, Hall TE, Cowling BS, Ferguson C, Morgan GP et al. (2015) The caveolin–cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J. Cell Biol 210, 833–849 10.1083/jcb.201501046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi T, Maruyama N, Ogata T, Kasahara T, Nakanishi N, Miyagawa K et al. (2016) PTRF/cavin-1 deficiency causes cardiac dysfunction accompanied by cardiomyocyte hypertrophy and cardiac fibrosis. PLoS ONE 11, e0162513 10.1371/journal.pone.0162513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaakinen M, Reichelt ME, Ma Z, Ferguson C, Martel N, Porrello ER et al. (2017) Cavin-1 deficiency modifies myocardial and coronary function, stretch responses and ischaemic tolerance: roles of NOS over-activity. Basic Res. Cardiol 112, 24 10.1007/s00395-017-0613-6 [DOI] [PubMed] [Google Scholar]
- 49.Sward K, Sadegh MK, Mori M, Erjefalt JS and Rippe C (2013) Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF. Physiol. Rep 1, e00008 10.1002/PHY2.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govender P, Romero F, Shah D, Paez J, Ding SY, Liu L et al. (2013) Cavin1; a regulator of lung function and macrophage phenotype. PLoS ONE 8, e62045 10.1371/journal.pone.0062045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karbalaei MS, Rippe C, Albinsson S, Ekman M, Mansten A, Uvelius B et al. (2012) Impaired contractility and detrusor hypertrophy in cavin-1-deficient mice. Eur. J. Pharmacol 689, 179–185 10.1016/j.ejphar.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 52.Liu L and Pilch PF (2008) A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem 283, 4314–4322 10.1074/jbc.M707890200 [DOI] [PubMed] [Google Scholar]
- 53.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ et al. (2008) PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 10.1016/j.cell.2007.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen CG, Shvets E, Howard G, Riento K and Nichols BJ (2013) Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae. Nat. Commun 4, 1831 10.1038/ncomms2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansa P and Grummt I (1999) Mechanism of transcription termination: PTRF interacts with the largest subunit of RNA polymerase I and dissociates paused transcription complexes from yeast and mouse. Mol. Gen. Genet 262, 508–514 10.1007/s004380051112 [DOI] [PubMed] [Google Scholar]
- 56.Jansa P, Burek C, Sander EE and Grummt I (2001) The transcript release factor PTRF augments ribosomal gene transcription by facilitating reinitiation of RNA polymerase I. Nucleic Acids Res. 29, 423–429 10.1093/nar/29.2.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parton RG (2018) Caveolae: structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol 34, 111–136 10.1146/annurev-cellbio-100617-062737 [DOI] [PubMed] [Google Scholar]
- 58.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D et al. (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 10.1016/j.cell.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M et al. (2009) MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol 11, 56–64 10.1038/ncb1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N et al. (2011) Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J. Biol. Chem 286, 12820–4 10.1074/jbc.C111.221440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Pilch PF and Liu L (2019) Cavin-1/PTRF mediates insulin-dependent focal adhesion remodeling and ameliorates high-fat diet-induced inflammatory responses in mice. J. Biol. Chem 294, 10544–10552 10.1074/jbc.RA119.008824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai L, Deng X, Li Q, Wang M, An W, Deli A et al. (2012) Down-regulation of the cavin family proteins in breast cancer. J. Cell. Biochem 113, 322–328 10.1002/jcb.23358 [DOI] [PubMed] [Google Scholar]
- 63.Faggi F, Chiarelli N, Colombi M, Mitola S, Ronca R, Madaro L et al. (2015) Cavin-1 and Caveolin-1 are both required to support cell proliferation, migration and anchorage-independent cell growth in rhabdomyosarcoma. Lab. Invest 95, 585–602 10.1038/labinvest.2015.45 [DOI] [PubMed] [Google Scholar]
- 64.Meng F, Joshi B and Nabi IR (2015) Galectin-3 overrides PTRF/cavin-1 reduction of PC3 prostate cancer cell migration. PLoS ONE 10, e0126056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill MM, Daud NH, Aung CS, Loo D, Martin S, Murphy S et al. (2012) Co-regulation of cell polarization and migration by caveolar proteins PTRF/Cavin-1 and caveolin-1. PLoS ONE 7, e43041 10.1371/journal.pone.0043041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davalos A, Fernandez-Hernando C, Sowa G, Derakhshan B, Lin MI, Lee JY et al. (2010) Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase i and transcript release factor/CAVIN-1 IN endothelial cells. Mol. Cell. Proteomics 9, 2109–2124 10.1074/mcp.M110.001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai L, Deng X, Li J, Wang M, Li Q, An W et al. (2011) Regulation of cellular senescence by the essential caveolar component PTRF/Cavin-1. Cell Res. 21, 1088–1101 10.1038/cr.2011.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volonte D and Galbiati F (2011) Polymerase I and transcript release factor (PTRF)/cavin-1 is a novel regulator of stress-induced premature senescence. J. Biol. Chem 286, 28657–28661 10.1074/jbc.C111.235119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bitar MS, Abdel-Halim SM and Al-Mulla F (2013) Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am. J. Physiol. Endocrinol. Metab 305, E951–E963 10.1152/ajpendo.00189.2013 [DOI] [PubMed] [Google Scholar]
- 70.Regazzetti C, Dumas K, Lacas-Gervais S, Pastor F, Peraldi P, Bonnafous S et al. (2015) Hypoxia inhibits Cavin-1 and Cavin-2 expression and down-regulates caveolae in adipocytes. Endocrinology 156, 789–801 10.1210/en.2014-1656 [DOI] [PubMed] [Google Scholar]
- 71.McMahon KA, Wu Y, Gambin Y, Sierecki E, Tillu VA, Hall T et al. (2019) Identification of intracellular cavin target proteins reveals cavin-PP1alpha interactions regulate apoptosis. Nat. Commun 10, 3279 10.1038/s41467-019-11111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vousden KH and Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137, 413–431 10.1016/j.cell.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 73.Kruiswijk F, Labuschagne CF and Vousden KH (2015) P53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol 16, 393–405 10.1038/nrm4007 [DOI] [PubMed] [Google Scholar]
- 74.Inder KL, Zheng YZ, Davis MJ, Moon H, Loo D, Nguyen H et al. (2012) Expression of PTRF in PC-3 cells modulates cholesterol dynamics and the actin cytoskeleton impacting secretion pathways. Mol. Cell. Proteomics 11, M111.012245 10.1074/mcp.M111.012245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei Z, Liu T, Lei J, Wu Y, Wang S and Liao K (2018) Fam198a, a member of secreted kinase, secrets through caveolae biogenesis pathway. Acta Biochim. Biophys. Sin. (Shanghai) 50, 968–975 10.1093/abbs/gmy105 [DOI] [PubMed] [Google Scholar]
- 76.Huang K, Fang C, Yi K, Liu X, Qi H, Tan Y et al. (2018) The role of PTRF/Cavin1 as a biomarker in both glioma and serum exosomes. Theranostics 8, 1540–1557 10.7150/thno.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Diaz S, Garcia-Sobreviela MP, Gonzalez-Irazabal Y, Garcia-Rodriguez B, Espina S, Arenaz I et al. (2018) PTRF acts as an adipokine contributing to adipocyte dysfunctionality and ectopic lipid deposition. J. Physiol. Biochem 74, 613–622 10.1007/s13105-018-0638-9 [DOI] [PubMed] [Google Scholar]


