Abstract
Spatial signals are prevalent within the hippocampus and its neighboring regions. It is generally accepted that these signals are defined with respect to the external world (i.e., a world-centered, or allocentric, frame of reference). Recently, evidence of egocentric processing (i.e., self-centered, defined relative to the subject) in the extended hippocampal system has accumulated. These results support the idea that egocentric sensory information, derived from primary sensory cortical areas, may be transformed to allocentric representations that interact with the allocentric hippocampal system. We propose a framework to explain the implications of the egocentric-allocentric transformations to the functions of the medial temporal lobe memory system.
Introduction
A majority of the principal cells within the hippocampal formation fire when the subject is located within a localized region in an environment. This spatial selectivity is so robust and striking that it has taken center stage in studies of medial temporal lobe function since the pioneering discovery of place cells by O’Keefe and Dostrovsky [1] and the subsequent discoveries and characterizations of head direction cells by Ranck and Taube [2] and grid cells by Hafting, Fyhn, Moser, and Moser [3] in regions that provide input to the hippocampus. Decades of investigation of these cells have shown that they primarily encode position and direction in an allocentric (i.e., world-centered) frame of reference, independent of the orientation of the body or head (although direction selectivity of place cells can develop with experience, as “local views” of sensory input become associated with allocentric spatial locations [4,5]). Place cells, grid cells, and head direction cells are thought to represent an allocentric location and compass system that functions as a “cognitive map” used for flexible navigation, hypothesis-based problem solving, and episodic memory [1]. It has been proposed that the egocentric representations of the primary sensory cortical areas must be transformed into an allocentric representation in the hippocampus, and then transformed back to an egocentric motor representation for behavioral output [6]. How this transformation is accomplished in the rodent cognitive mapping system has largely been the realm of theory. However, a number of recent papers have investigated how egocentric sensory and motor representations can be transformed into allocentric spatial maps. These studies have revealed a surprising degree of egocentric coding within the hippocampal formation itself, which may underlie the ability of the hippocampus to direct explicit trajectories to goals and to represent the first-person experiences of the world that underlie the formation and retrieval of explicit, episodic memories.
Definitions
A range of phenomena are placed under the umbrella term egocentric coding, and it is important to define this term precisely [7]. Egocentric coding can be used to describe the reference frames of both incoming sensory input as well as motor output. For example, a sound can be perceived as coming from my left, and I can turn my head to my left to orient to the sound. Just as there are different reference frames for allocentric coding that must be specified, such as local vs. global frames [8], there are also different egocentric frames of reference. Egocentric representations may be defined relative to the body, head, or eyes, for example, all of which have been demonstrated in sensorimotor representations in primate parietal cortex [9]. For most studies of spatial navigation and cognition in rodents, both allocentric and egocentric coordinates are defined by tracking the head only in the horizontal plane [7]. Thus, our discussion of this topic involves only this specific form and greater complexity may be uncovered when data from other frames become available in the future.
Theoretical studies about the egocentric-allocentric transformation
Parietal cortex, which is essential for representing peripersonal space (i.e., space next to the body) [10] and visual spatial attention [9] has been a source of inspiration for studying in the hippocampal formation the much larger spatial scale of navigation. First, early models of place cells relied on the egocentric representation of cues (local views [4]) in the environment [11,12], which was hypothesized to be streamed to the hippocampal system from the parietal cortex [13]. Second, great progress has been made in understanding the neural mechanisms underlying transformations between coordinate frames in the parietal cortex. For example, “gain fields” were discovered in the parietal cortex, in which an allocentric representation of location in the visual world is accomplished by modulating the firing rates of retinotopic (i.e., egocentric) receptive fields by the position of the eyes in the orbits [9].
Similar egocentric-to-allocentric transformations were hypothesized to occur in the brain’s navigation system. Burgess and colleagues [6,14] presented a model for spatial memory and imagery, in which the retrosplenial cortex constitutes a site for coordinate transformation, integrating egocentric sensory information from the parietal cortex and an allocentric head direction signal from limbic regions [2] to form an allocentric signal for space. They postulated the existence of cells with tuning for both allocentric head direction and egocentric bearing. Specific wiring circuits among these cells could construct the boundary vector cells or border cells, a type of cell in the subiculum and the medial entorhinal cortex (MEC) that fires when the animals are at a distance and allocentric direction to a boundary [15–17]. This allocentric spatial signal has been suggested to be the basis for the construction of place cells in the hippocampus [18]. Similarly, McNaughton et al. [15] hypothesized that place cells were representations of allocentric bearing and distance to specific external landmarks used to facilitate vector-based flexible navigation. These “landmark vector cells” were hypothesized to be constructed from egocentric bearing and allocentric head direction cells. Despite these theoretical explorations of the coordinate transformation in the hippocampal navigation system, experimental support for these ideas was, until recently, scarce.
Recent experimental results support the models of egocentric-allocentric transformation
In recent years, egocentric representations have been discovered in many brain areas in the navigational system, with characteristics predicted by mechanisms of coordinate transformation in the aforementioned theoretical studies. In parietal cortex, neurons encode egocentric bearing of an external cue, with a proportion of the cells carrying conjunctive allocentric head direction information [16]. Together, these cells could form a transformation circuit for landmark-vector/object-vector cells hypothesized by McNaughton et al. [15] and subsequently discovered in the downstream hippocampus [17] and medial entorhinal cortex [19]. Another parietal downstream target, the retrosplenial cortex, has been considered a hub for coordinating different reference frames [6,20,21]. Indeed, in open arenas, cells in retrosplenial cortex were found to be selective for egocentric bearing of and distance to the environmental boundaries [22,23]. These findings were similar to earlier work in retrosplenial cortex [24], where “direction-dependent place cells” were suggested to be used to guide flexible spatial behavior.
Other studies have proposed sequential processing models by which allocentric and egocentric bearing signals produce allocentric spatial selectivity. Cells in the postsubiculum appear to combine an allocentric head direction signal from the anterior dorsal thalamic nucleus with an egocentric signal about the orientation of the rat relative to a boundary to produce a spatial signal in the form of a directionally modulated boundary (border) cell [25]. In a study of a number of parahippocampal areas, including postrhinal cortex, parasubiculum, and postsubiculum (called dorsal presubiculum in that paper), the postrhinal cortex was the only region that showed a high proportion of pure egocentric boundary cells, whereas the other regions (postsubiculum, parasubiculum, and MEC, as well as postrhinal cortex itself) showed conjunctive coding of both egocentric boundary information and head direction [26]. These authors suggested that this pathway (visual/parietal cortex → postrhinal cortex → MEC/para/postsubiculum) transforms egocentric signals into allocentric border cells. A similar study also reported egocentric bearing representations in postrhinal cortex, commingled with distance information and an allocentric head direction signal [27]. In line with O’Keefe’s centroid model of allocentric spatial map generation [11], these authors suggested that the postrhinal cortex contained all of the cell types necessary to create an allocentric map based on sensory landmarks.
Egocentric responses have been found unexpectedly in the hippocampus and lateral entorhinal cortex
The above-mentioned brain regions are well recognized as playing various roles in spatial information processing, and thus it is not surprising to have found egocentric spatial representations in these regions upstream of the allocentric representations known to reside in the hippocampus and MEC. However, several recent studies have uncovered unexpected egocentric spatial representations in the hippocampus itself, as well as the lateral entorhinal cortex, which was previously thought to convey primarily nonspatial information to the hippocampus.
In Egyptian fruit bats, 19% of hippocampal CA1 cells encoded the egocentric bearing of a goal target located inside the behavioral arena [28]. Some other cells encoded the distance to the goal. Thus, in addition to its allocentric place cell representation, the bat hippocampus contains an egocentric vector representation of the animal’s location relative to a goal. Consistent with this finding, a number of other reports have shown that a fraction of pyramidal cells in the rodent hippocampus show egocentric coding for boundaries or salient local landmarks [23,29,30]. It is possible that prior reports of allocentric coding of head direction in these cells [31,32] may instead reflect egocentric coding to landmarks at a distance from the experimental apparatus, which would be indistinguishable from egocentric coding [22,23,25–27,33,34]. Further investigation of this topic with systematic manipulation of the distant and behaviorally relevant landmarks is needed to disambiguate these two possible interpretations. For example, recordings in a very large environment or in virtual reality may reveal that apparent allocentric head direction coding at the center of the environment is actually egocentric coding relative to a distal landmark when the animal is allowed to move around the distal landmark and sample it from multiple egocentric bearings.
What are the possible sources of egocentric information for the hippocampal neurons? In a study of the two major hippocampal input regions, the lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC), LEC neurons were found to represent egocentric directional information referenced to the center or boundaries and to local objects or goals; in contrast, MEC cells were dominated by allocentric head direction [33]. These results are consistent with theories that hypothesized this egocentric vs. allocentric dichotomy [35] and behavioral studies suggesting egocentric processing in LEC [36]. The egocentric representation of objects in LEC could underlie the generation of allocentric landmark- (or object-) vector cells in CA1 [17] and MEC [19] through egocentric-to-allocentric transformation mechanisms proposed in earlier studies [6,14,15].
Why would a center for allocentric spatial signals, like the hippocampus, be involved in egocentric processing? The ability of the hippocampus to represent salient external items (the sensory contents of an experience) in an egocentric frame of reference fits well with its purported role in episodic memory. Life is experienced from a first-person (egocentric) perspective, and our most salient episodic memories are also retrieved from this same perspective. We argue that these egocentric representations of external items—whether egocentric vectors to navigational goals or the first-person contents of a memory—are conveyed to the hippocampus by the LEC, whereas the allocentric spatial framework used to bind these experiences into a unitary representation is conveyed by the MEC (Fig. 4). The medial temporal lobe has traditionally been divided into “what” (lateral entorhinal cortex) and “where” (medial entorhinal cortex) pathways. Lateral entorhinal cortex was suggested to be dominated by input from the perirhinal cortex. However, both parietal cortex and postrhinal cortex, considered to be part of the “where” pathway, also directly innervate LEC [37–39]. It is possible that the LEC inherits its egocentric tuning from these areas, or conversely, relays this information back to either of these regions.
Based on these new physiological findings and a re-evaluation of classic anatomical findings [37], we propose an updated model of the functional organization of the hippocampus and its entorhinal inputs (see Fig. 4). We propose that the MEC processes self-motion generated (“idiothetic”) signals and environmental landmark (“allothetic”) orienting signals to create an allocentric representation of self-location via path integration computations [40], whereas the LEC processes allothetic sensory information to create an egocentric representation of locations (and perhaps identities) of specific other items in the external world. The hippocampus integrates the allocentric “self” information with the egocentric “other” information to create consistent maps of allocentric space and the landmarks and other items that occupy these spaces during an episode. The egocentric sensory inputs streamed from LEC are best suited for providing the content of memory from an egocentric perspective [35], whereas MEC constitutes the center of allocentric processing with a more rigid set of internal dynamics allowing the expression of a consistent framework across multiple environments/contexts [41]. This rigidity may underlie MEC’s function in navigational computation (e.g., vector navigation) [42,43] and more abstract navigation through other forms of mental space [44]. The output of the memory system could then be used to guide the egocentric action in downstream areas such as striatum and motor cortex, where egocentric representations of boundaries [22,34] and allocentric representations of head direction [45] have been discovered. Although still loosely defined, this framework provides a tentative guide for future investigation into the extended hippocampal system.
Remaining questions
a. Defining the proper egocentric reference frame and correlates.
To study egocentric representations, effort must be made to disambiguate the exact frame of egocentric coding under investigation, e.g., whether neural representations are defined with respect to the body, head, or eyes. This effort may entail monitoring eye and body positions in freely moving rodents [46,47], which is still not common because of technical difficulties. In addition, there are different variables that must be distinguished even when restricting analysis to head-related variables. Head direction, the direction the head is pointing, and movement direction, the direction the head is moving, are strongly correlated in rodents but not identical in either egocentric or allocentric frames [48]. More accurate fits of the data by one vs. the other variable may inform the underlying neural mechanisms or functions of the neural activity. For example, dorsomedial striatum neurons are more strongly correlated with movement direction than head direction, consistent with the role of the dorsomedial striatum in action planning/execution [34]. In contrast, the activity of lateral entorhinal cortex [33] neurons were more strongly correlated with egocentric head direction, reflecting the putative involvement of LEC in sensory information processing.
b. Determining the item/object being represented by egocentric-coding cells.
Allocentric representations of self-location require at most 6 parameters (x, y, z, yaw, pitch, and roll) to capture the 3-D pose of the subject in a given, although perhaps dynamically changing [49], frame of reference (e.g., local vs. global). In stark contrast, egocentric representations suffer a combinatorial explosion of possible reference points (e.g., does a cell represent bearing to the center, the boundaries, a goal, a salient landmark, or any other potential location within an environment?). Defining which external item a neuron represents is a necessary first step in characterizing its behavior. However, except when there are explicit task requirements, the choice of represented item is not an easy task if there is no a priori rationale available. When there are two or more objects in the arena, how does one choose which one to use for an individual cell? Relatedly, do egocentric representations require considerable cognitive load (e.g., attention to specific items or locations that may change on the time scale of seconds) or are they more or less the result of an automatic process? For simplicity, many studies used only a single item for egocentric analysis. However, our daily environments are cluttered with different kinds of objects. It is extremely important, and challenging at the same time, to investigate at the neural level, how the egocentric representations of multiple objects are established and coordinated.
c. Representation of nonstationary items.
Hippocampal neurons can encode the allocentric position not only of the test subject but also of other moving animals or objects being observed by the test subject [50,51]. However, the majority of studies of egocentric phenomena only employed stationary objects. It is important to extend these studies with moving objects/conspecifics. In experiments involving pairs of bats, these “social” place cells were more prominent in the LEC-recipient zone of CA1 (distal CA1) compared to the MEC-recipient zone (proximal CA1). It would be of interest to determine whether these “other”-encoding cells were dependent on the LEC input, as proposed by models of LEC function [35,52].
Conclusions
Egocentric representations of both sensory signals and motor actions are prevalent in many brain regions. Until recently, however, they were largely overlooked in investigations of hippocampus and related areas, where investigators explored and reasoned with an allocentric mindset. The neural mechanisms of allocentric place field generation in the hippocampus is of paramount importance, as it is a key to understanding the functional organization of the medial temporal lobe. Nonetheless, it is also essential to examine the transformation from egocentric to allocentric processing in upstream areas, and several recent studies are leading the way. The manifestation of egocentric coding within hippocampus itself also promises to lead to new insights into the function of the hippocampus in episodic memory, which is inherently egocentric in nature.
Figure 1. Definition of frames of reference for allocentric and egocentric coding.
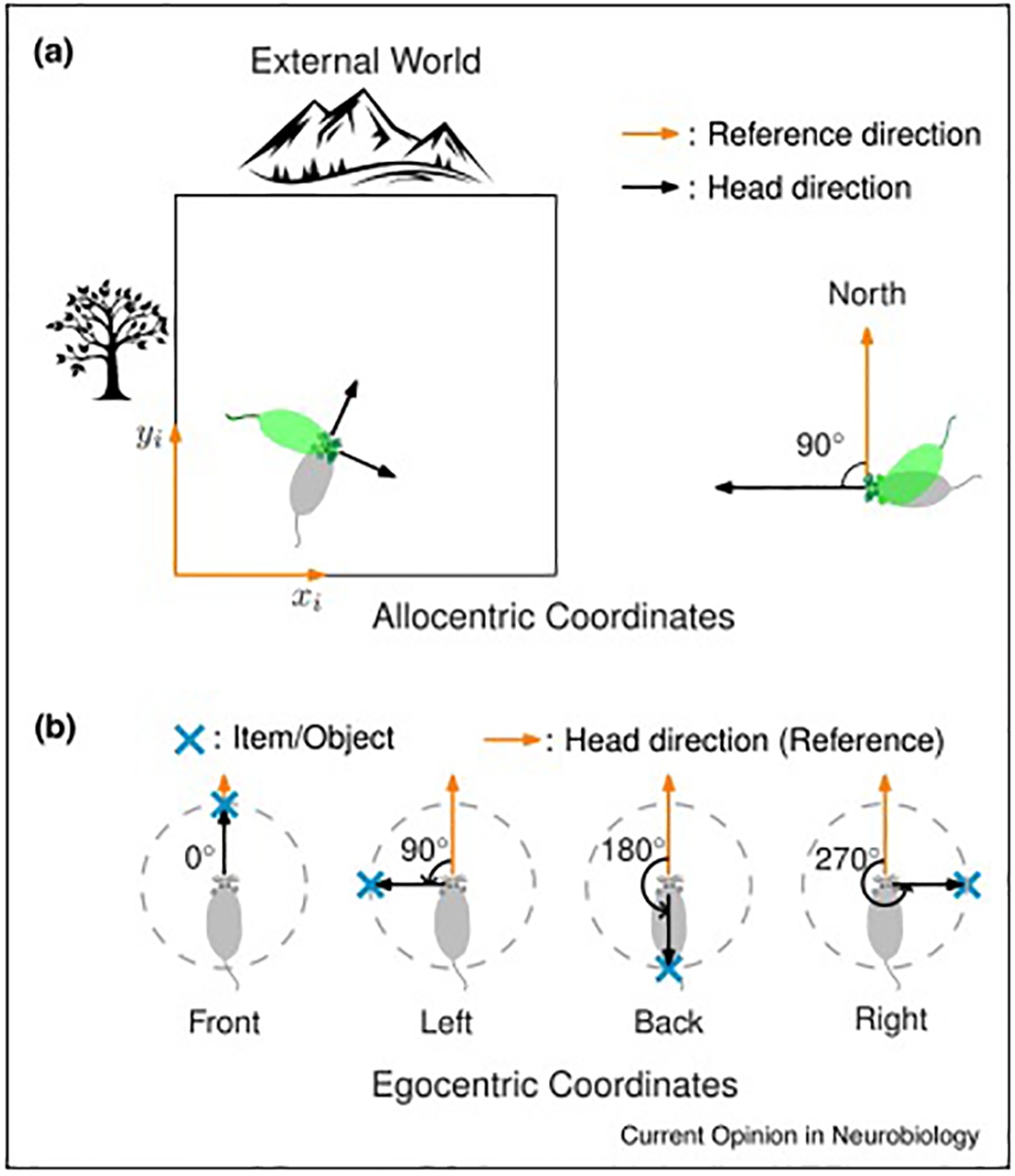
(a) Representation of location and bearing in an allocentric reference frame. Left, in allocentric coordinates, the position of the subject is not dependent on the animal’s body or head orientation. Right, the allocentric head direction representation of the animal is defined with respect to an external reference direction, e.g. North. The allocentric head direction is independent of the body orientation.
(b) Representation of the distance and bearing of external items in an egocentric reference frame. The egocentric representation of an external item/object is defined with respect to the animal itself. For a given item/object, a vector from the head of the subject to the item is constructed. The egocentric bearing of the item is defined as the angle of the vector, referenced to the subject’s allocentric head direction. Reproduced from Wang, Chen et al. [33].
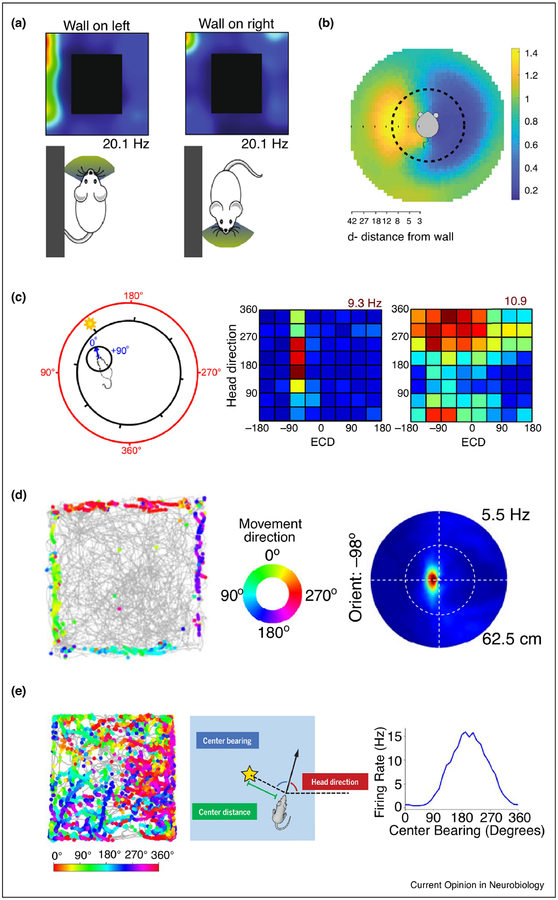
Figure 2. Egocentric and allocentric representations outside the hippocampus.
(a) Activity of some head direction cells in the postsubiculum were modulated by the egocentric bearing of the environmental boundary. In this example cell, the firing rate is much higher when the wall is on the animal’s left than when the wall is on its right. Modified, with permission, from Peyrache et al. [25].
(b) An example cell in postrhinal cortex showed strong activity in response to the presence of a border at a given egocentric direction and distance to the rat. The rat is in the center of this egocentric boundary rate map, in which distance and egocentric direction to the boundaries is indicated by radial distance and angle from the center, respectively, of this map. The yellow area indicates that the cell was most active when there was a border 10 cm to the left of the rat. Reproduced, with permission, from Gofman et al. [26].
(c) Parietal cortex is tuned to both allocentric and egocentric reference frames. Left, Schematic of egocentric cue direction (ECD, inner circle, numbers in blue) and head direction (outer circle, numbers in red). In this frame, the ECD is approximately 10° and head direction is 160°. Middle, a cell showed selectivity for ECD. Right, a cell showed conjunctive selectivity for ECD and head direction. Reproduced, with permission, from Wilber et al. [16].
(d) A substantial portion of cells in the dorsomedial striatum respond to environmental boundaries in an egocentric reference frame. Left, Trajectories of the rat (gray) superimposed with locations of the rat when the cell fires spikes (colored dots). Middle, Color wheel indicates the movement direction of the rat for each colored dot in the left panel. Right, Egocentric boundary rate map similar to that shown in (b). For this cell, the preferred orientation of the boundary is −98° (the boundary is to the left of the rat) and the preferred distance is approximately 5.5 cm from the boundary. Reproduced, with permission, from Hinman et al. [34].
(e) Some cells in POR encode the egocentric bearing of the center of the arena. Left, same as in (d), except that the color bar indicates the head direction instead of movement direction. Middle, Schematic of egocentric bearing of the center of the arena (“center-bearing”). Right, this example cell has the highest firing activity when the center bearing is 180° (i.e., behind the rat). Reproduced, with permission, from LaChance et al. [27].
Figure 3. Egocentric representation in the hippocampus and lateral entorhinal cortex.
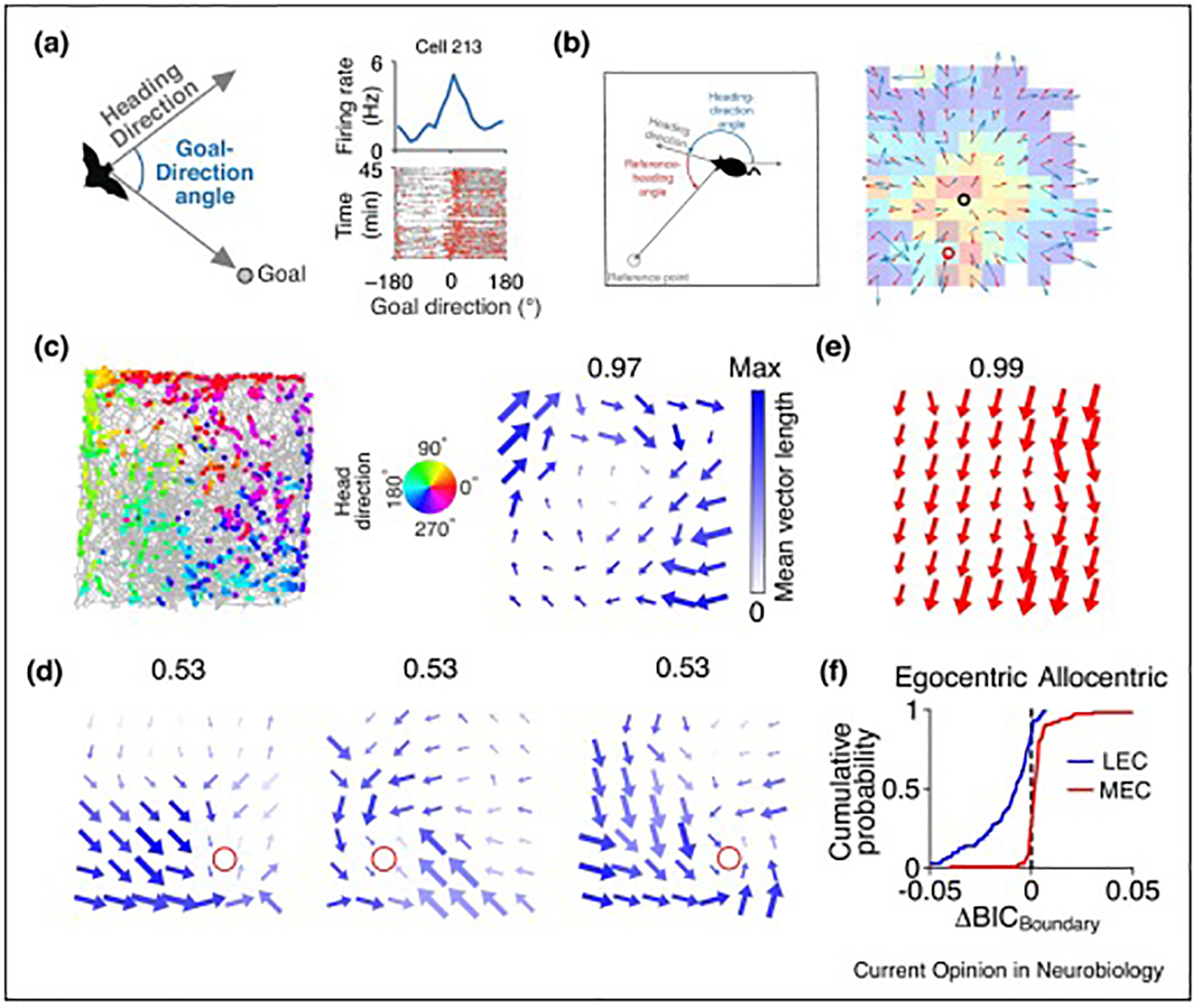
(a) Some hippocampal CA1 cells in bats showed egocentric goal direction selectivity. Left, Schematic for egocentric goal direction. Top right, an example goal direction cell that has the highest firing rate when the goal direction is 0° (the bat flies toward the goal location). Bottom right, trajectories of goal-direction angles along the behavioral session (gray), with spikes overlaid (red). Reproduced, with permission, from Sarel, Finkelstein et al. [28].
(b) Activity of some place cells in mouse hippocampal CA1 can be modulated by egocentric heading direction to a reference point in the environment. Left, Schematic of the egocentric heading direction relative to a specific reference point. Right, Heat map: spatial firing rate map; red circle: center of mass for the rate map; blue arrows: heading direction tuning within each spatial bin; black circle: the reference point obtained by a model based on the real heading direction tuning; red arrows: heading direction tuning fitted by the model in each spatial bin. Reproduced, with permission, from Jercog et al. [29].
(c) An example cell in LEC showed selectivity for egocentric bearing of the arena boundary/center. Left, trajectory (gray lines) and position and head direction of the rat when the cell fired (colored dots). Middle, Color wheel denotes the head direction. Right, Local head direction tuning in each spatial bin. Arrow direction: preferred head direction; arrow size: firing rate; color saturation: the mean vector length of the tuning curve (MVL); number on top: maximum MVL.
(d) An example LEC cell tuned for egocentric bearing of goal location in a goal-oriented task. In this task, a single food well (red circle) was shifted from the standard goal location in session 1 (left) to a different location in session 2 (middle), and then back to the original standard location in session 3 (right). Local head direction tuning is showed as in (c).
(e) An example cell in MEC showed selectivity for allocentric head direction, as demonstrated by all arrows pointing in the same, allocentric direction.
(f) LEC represents spatial information in an egocentric frame of reference, whereas MEC utilizes an allocentric frame of reference. Bayesian information criterion (BICBoundary) indicates the goodness of fit of a cell’s activity to an egocentric bearing model or an allocentric bearing model. ΔBICBoundary describes the difference of goodness of fit between the two models, in which a negative value means the cell prefers the egocentric frame of reference, whereas a positive value means the cell prefers the allocentric frame of reference. (c-f) Reproduced, with permission, from Wang, Chen et al. [33].’
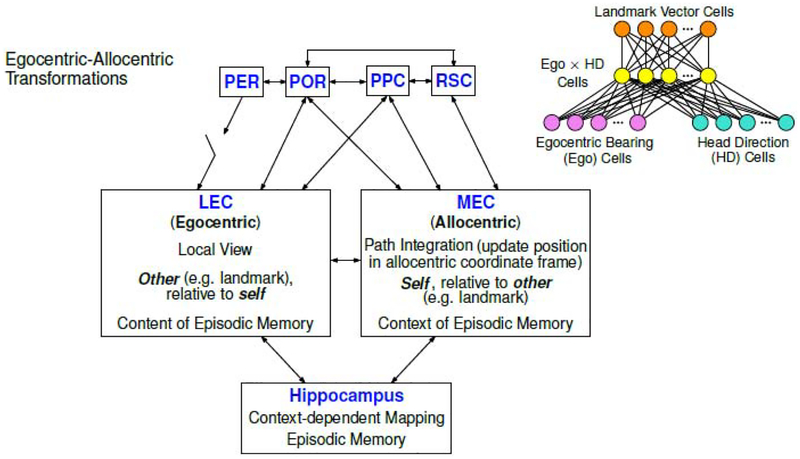
Figure 4. Working model for the egocentric-allocentric transformation in the medial temporal lobe and related brain regions.
In this highly simplified model of functional anatomy, perirhinal cortex (PER) and postrhinal cortex (POR) are two important parahippocampal regions that directly project to the entorhinal cortex (EC). (Note that this diagram dos not attempt to depict the relative strength of any of the connections.) The PER input is “gated” by the necessity for coactive inputs from other regions that presumably convey some type of salience tag (e.g., from amygdala or prefrontal cortex) to the PER sensory input [37]. Two high-order associative brain areas, posterior parietal cortex (PPC) and retrosplenial cortex (RSC), also have connections with the EC. Cells representing egocentric and allocentric reference frames were found recently in these brain regions upstream of the EC-hippocampus system. Upper right, a diagram (modified, with permission, from McNaughton et al. [15]) showing how specific circuits wired by cells encoding egocentric bearing and allocentric head direction information in these areas could construct an allocentric spatial signal (in this case, a landmark vector cell). Lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC), the two major cortical input regions to the hippocampus, show a functional dichotomy in egocentric vs. allocentric spatial frames of reference [33]. This difference is hypothesized to underlie separate roles of these regions in providing information to the hippocampal episodic memory system.
Highlights:
Several recent studies have discovered egocentric spatial representations in the rodent brain coinciding with classic allocentric representations.
These discoveries support previous theories of egocentric-to-allocentric transformation.
The hippocampus and lateral entorhinal cortex show unexpected egocentric coding that may support the hippocampal role in episodic memory.
Acknowledgements
This work was supported by NIH grant R01 MH094146, NIH grant R01 NS039456.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.O’Keefe J, Nadel L: The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 2.Taube JS, Muller RU, Ranck JB: Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 1990, 10:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI: Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436:801–806. [DOI] [PubMed] [Google Scholar]
- 4.McNaughton BL, Leonard B, Chen L: Cortical-hippocampal interactions and cognitive mapping: A hypothesis based on reintegration of the parietal and inferotemporal pathways for visual processing. Psychobiology 1989, 17:230–235. [Google Scholar]
- 5.Navratilova Z, Hoang LT, Schwindel CD, Tatsuno M, McNaughton BL: Experience-dependent firing rate remapping generates directional selectivity in hippocampal place cells. Front Neural Circuits 2012, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne P, Becker S, Burgess N: Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev 2007, 114:340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatzky RL: Allocentric and Egocentric Spatial Representations: Definitions, Distinctions, and Interconnections In Spatial Cognition. Springer, Berlin, Heidelberg; 1998:1–17. [Google Scholar]
- 8.Knierim JJ, Hamilton DA: Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol Rev 2011, 91:1245–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen RA, Buneo CA: Intentional Maps in Posterior Parietal Cortex. Annual Review of Neuroscience 2002, 25:189–220. [DOI] [PubMed] [Google Scholar]
- 10.Makin TR, Holmes NP, Zohary E: Is That Near My Hand? Multisensory Representation of Peripersonal Space in Human Intraparietal Sulcus. J Neurosci 2007, 27:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe J: An allocentric spatial model for the hippocampal cognitive map. Hippocampus 1991, 1:230–235. [DOI] [PubMed] [Google Scholar]
- 12.Sharp PE: Computer simulation of hippocampal place cells. Psychobiology 1991, 19:103–115. [Google Scholar]
- 13.Redish AD: Beyond the Cognitive Map: From Place Cells to Episodic Memory. A Bradford Book; 1999. [Google Scholar]
- 14.Bicanski A, Burgess N: A neural-level model of spatial memory and imagery. eLife 2018, 7:e33752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNaughton BL, Knierim JJ, Wilson MA: Vector encoding and the vestibular foundations of spatial cognition: Neurophysiological and computational mechanisms In The cognitive neurosciences. . The MIT Press; 1995:585–595. [Google Scholar]
- ●16.Wilber AA, Clark BJ, Forster TC, Tatsuno M, McNaughton BL: Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. J Neurosci 2014, 34:5431–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that, in a random spatial sequence task, rat posterior parietal cortex (PPC) contains classes of cells that show tuning for allocentric head direction (HD), egocentric cue bearing (ECD), as well as conjunctive HD and ECD. These results provide strong support for the idea that PPC is a critical brain region engaged in the transformation from egocentric coordinates to allocentric coordinates for landmark-based navigation.
- 17.Deshmukh SS, Knierim JJ: Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus 2013, 23:253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartley T, Burgess N, Lever C, Cacucci F, O’Keefe J: Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 2000, 10:369–379. [DOI] [PubMed] [Google Scholar]
- ●●19.Høydal ØA, Skytøen ER, Andersson SO, Moser M-B, Moser EI: Object-vector coding in the medial entorhinal cortex. Nature 2019, 568:400–404. [DOI] [PubMed] [Google Scholar]; This study reports the existence of object- (or landmark-) vector cells in the medial entorhinal cortex (MEC), similar to previous results from hippocampus [17]. This functional cell type shows a significant response when the animal is at a given distance and allocentric direction from an object in the space, regardless of the animal’s own head direction. These results support the allocentric coding in MEC and suggest a neural correlate of landmark-dependent, vector-based navigation.
- 20.Alexander AS, Nitz DA: Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci 2015, 18:1143–1151. [DOI] [PubMed] [Google Scholar]
- 21.Vann SD, Aggleton JP, Maguire EA: What does the retrosplenial cortex do? Nat Rev Neurosci 2009, 10:792–802. [DOI] [PubMed] [Google Scholar]
- 22.Alexander AS, Carstensen LC, Hinman JR, Raudies F, Chapman GW, Hasselmo ME: Egocentric boundary vector tuning of the retrosplenial cortex. bioRxiv 2019, doi: 10.1101/702712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurens J, Abrego A, Cham H, Popeney B, Yu Y, Rotem N, Aarse J, Dickman D, Angelaki D: Multiplexed code of navigation variables in the anterior limbic system. bioRxiv 2019, doi: 10.1101/684464. [DOI] [Google Scholar]
- 24.Cho J, Sharp PE: Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci 2001, 115:3–25. [DOI] [PubMed] [Google Scholar]
- ●25.Peyrache A, Schieferstein N, Buzsáki G: Transformation of the head-direction signal into a spatial code. Nat Commun 2017, 8:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the firing of head direction cells in the postsubiculum, an important center for allocentric spatial and directional processing, can be modulated by the animal’s egocentric position to the borders. The authors propose that integration of sensory information with head direction input could generate an allocentric spatial code.
- ●26.Gofman X, Tocker G, Weiss S, Boccara CN, Lu L, Moser M-B, Moser EI, Morris G, Derdikman D: Dissociation between Postrhinal Cortex and Downstream Parahippocampal Regions in the Representation of Egocentric Boundaries. Current Biology 2019, 29:2751–2757.e4. [DOI] [PubMed] [Google Scholar]; This paper compares several parahippocampal areas and reveals that boundaries of the environment are represented in postrhinal cortex in an egocentric frame of reference. The authors propose a model that constructed allocentric boundary/border cells from egocentric boundary representations and allocentric head direction signals.
- ●●27.LaChance PA, Todd TP, Taube JS: A sense of space in postrhinal cortex. Science 2019, 365:eaax4192. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that in postrhinal cortex (POR), some cells encode egocentric bearing of the environment center, some cells encode allocentric head direction, and some POR cells show selectivity for the distance to the center of the environment. These cells can show conjunctive selectivity for more than one parameter. However, the egocentric cells type were largely absent in medial entorhinal cortex and parasubiculum. These data provide evidence of the coordinate transformation from the egocentric sensory information to an allocentric spatial map.
- ●28.Sarel A, Finkelstein A, Las L, Ulanovsky N: Vectorial representation of spatial goals in the hippocampus of bats. Science 2017, 355:176–180. [DOI] [PubMed] [Google Scholar]; This study reports that a subpopulation of bat hippocampal CA1 neurons are tuned to egocentric angles to the goal, and some of these egocentric cells are tuned to the distance to the goal. These conjunctive cells make up a vectorial representation of goal location.
- 29.Jercog PE, Ahmadian Y, Woodruff C, Deb-Sen R, Abbott LF, Kandel ER: Heading direction with respect to a reference point modulates place-cell activity. Nature Communications 2019, 10:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhingra S, Sandler R, Rios R, Vuong C, Shahi M, Acharya L, Hachisuka A, Mehta MR: Visual cues evoke object-centric directional tuning across the entire hippocampal place cell ensemble. Washington, DC: Society for Neuroscience, 2017. Online.; 2017:Program No. 523.10 / UU42. [Google Scholar]
- 31.Rubin A, Yartsev MM, Ulanovsky N: Encoding of Head Direction by Hippocampal Place Cells in Bats. J Neurosci 2014, 34:1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acharya L, Aghajan ZM, Vuong C, Moore JJ, Mehta MR: Causal Influence of Visual Cues on Hippocampal Directional Selectivity. Cell 2016, 164:197–207. [DOI] [PubMed] [Google Scholar]
- ●●33.Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, Knierim JJ: Egocentric coding of external items in the lateral entorhinal cortex. Science 2018, 362:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that cells in lateral entorhinal cortex (LEC) encode the egocentric bearing of environmental centers or boundaries, local objects, or goals relative to the animal. In contrast, cells in medial entorhinal cortex (MEC) were dominated by allocentric head direction. These results demonstrated for the first time that, unlike allocentric representation of “self” location in the MEC, the LEC neurons encode “non-self” information in a first-person perspective. This discovery suggested that egocentric information could reach the hippocampus directly to serve episodic memory.
- ●34.Hinman JR, Chapman GW, Hasselmo ME: Neuronal representation of environmental boundaries in egocentric coordinates. Nature Communications 2019, 10:2772. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that in dorsomedial striatum, a substantial portion of cells encode environmental boundaries in an egocentric frame of reference based on the animal’s direction of movement. This egocentric representation of local environmental boundaries is stable across environments. These data support the idea that the allocentric cognitive map in the hippocampal formation is conversed to egocentric frame of reference to guide navigational behavior.
- 35.Lisman JE: Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets. Prog Brain Res 2007, 163:615–625. [DOI] [PubMed] [Google Scholar]
- 36.Wilson DIG, Watanabe S, Milner H, Ainge JA: Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus 2013, 23:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●37.Nilssen ES, Doan TP, Nigro MJ, Ohara S, Witter MP: Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus 2019, 0. [DOI] [PubMed] [Google Scholar]; This paper provides a much-needed new perspective on the anatomical connectivity patterns in the medial temporal lobe. The authors argue against the common model of two segregated, parallel processing streams (one through the postrhinal and medial entorhinal cortices and the other through the perirhinal and lateral entorhinal cortices) and propose instead a model that emphasizes interactions among these brain regions.
- 38.Olsen GM, Ohara S, Iijima T, Witter MP: Parahippocampal and retrosplenial connections of rat posterior parietal cortex. Hippocampus 2017, 27:335–358. [DOI] [PubMed] [Google Scholar]
- 39.Burwell RD, Amaral DG: Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol 1998, 391:293–321. [DOI] [PubMed] [Google Scholar]
- ●40.Savelli F, Knierim JJ: Origin and role of path integration in the cognitive representations of the hippocampus: computational insights into open questions. Journal of Experimental Biology 2019, 222:jeb188912. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reviews computational arguments from robotics and physiological data from animals to argue that path integration involves a necessary interaction between the integration of self-motion cues and localization relative to stable, allothetic landmarks. Solutions derived by roboticists to solve the “simultaneous localization and mapping” (SLAM) problem appear remarkably similar to strategies found by evolution in animal navigation.
- 41.Fyhn M, Hafting T, Treves A, Moser M-B, Moser EI: Hippocampal remapping and grid realignment in entorhinal cortex. Nature 2007, 446:190–194. [DOI] [PubMed] [Google Scholar]
- 42.Bush D, Barry C, Manson D, Burgess N: Using Grid Cells for Navigation. Neuron 2015, 87:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banino A, Barry C, Uria B, Blundell C, Lillicrap T, Mirowski P, Pritzel A, Chadwick MJ, Degris T, Modayil J, et al. : Vector-based navigation using grid-like representations in artificial agents. Nature 2018, 557:429–433. [DOI] [PubMed] [Google Scholar]
- 44.Constantinescu AO, O’Reilly JX, Behrens TEJ: Organizing conceptual knowledge in humans with a gridlike code. Science 2016, 352:1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiener SI: Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci 1993, 13:3802–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer AF, Poort J, O’Keefe J, Sahani M, Linden JF: A Head-Mounted Camera System Integrates Detailed Behavioral Monitoring with Multichannel Electrophysiology in Freely Moving Mice. Neuron 2018, 100:46–60.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mimica B, Dunn BA, Tombaz T, Bojja VPTNCS, Whitlock JR: Efficient cortical coding of 3D posture in freely behaving rats. Science 2018, 362:584–589. [DOI] [PubMed] [Google Scholar]
- 48.Raudies F, Brandon MP, Chapman GW, Hasselmo ME: Head direction is coded more strongly than movement direction in a population of entorhinal neurons. Brain Research 2015, 1621:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelemen E, Fenton AA: Coordinating different representations in the hippocampus. Neurobiology of Learning and Memory 2016, 129:50–59. [DOI] [PubMed] [Google Scholar]
- 50.Danjo T, Toyoizumi T, Fujisawa S: Spatial representations of self and other in the hippocampus. Science 2018, 359:213–218. [DOI] [PubMed] [Google Scholar]
- 51.Omer DB, Maimon SR, Las L, Ulanovsky N: Social place-cells in the bat hippocampus. Science 2018, 359:218–224. [DOI] [PubMed] [Google Scholar]
- 52.Knierim JJ, Neunuebel JP, Deshmukh SS: Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond, B, Biol Sci 2014, 369:20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]