Abstract
The prognosis of patients with pancreatic cancer continues to remain dismal, even though numerous trials have been conducted to establish more effective therapies in Japan and throughout the world. Recent advances in treatment have been characterized by the use of novel combinations of conventional cytotoxic chemotherapies. Especially in Japan, S-1 has become one of the most widely used cytotoxic agents for the treatment of pancreatic cancer, after clinical evidence was established of the survival benefit offered by this drug for patients with resectable or unresectable pancreatic cancer. Unfortunately, with the exception of erlotinib, no targeted treatment strategies have been approved for pancreatic cancer. However, following an increase in interest in drug development in recent years, proactive attempts have been made to develop new therapeutic strategies, including neoadjuvant chemotherapy for patients with resectable or borderline resectable pancreatic cancer, multi-agent combination chemotherapy for patients with advanced pancreatic cancer, and therapies with new targeted agents or immuno-oncologic agents for patients with pancreatic cancer bearing specific gene mutations.
Keywords: Pancreatic ductal adenocarcinoma, Adjuvant therapy, Immunotherapy, Targeted therapy, Actionable mutation
Introduction
In Japan, along with the rapidly aging population, the number of patients with pancreatic cancer is also rapidly increasing, similar to the case for lung and colorectal cancer [1]. The number of deaths from pancreatic cancer in Japan has recently exceeded that from liver cancer, and pancreatic cancer now ranks as the fourth leading cause of death from cancer. In the majority of patients with pancreatic cancer, the cancer is already at an advanced unresectable stage at the time of diagnosis. Even in patients with resectable tumor at diagnosis who are treated by surgery, recurrence often occurs in the early phase after the operation. Thus, the prognosis of pancreatic cancer remains extremely poor [2].
The two most important approaches to improve the prognosis of pancreatic cancer are (1) to establish a better diagnostic method that would enable detection of this cancer at a resectable stage, and (2) to establish effective non-surgical treatments for patients with recurrent or unresectable pancreatic cancer. Until recently, there were a few effective non-surgical treatments. However, numerous clinical studies have been conducted in recent years, which have led to the establishment of new standard treatments that offer survival advantage. Several phase III studies have also been conducted in Japan, which have led to the establishment of standard treatments unique to pancreatic cancer patients in Japan.
In this article, we review the recent developments in chemotherapy for pancreatic cancer, including those based on evidence from clinical trials conducted in Japan, the current standard therapies recommended in guidelines in Japan (Figs. 1 and 2) and overseas, and global attempts to establish new strategies to overcome this disease with a grim prognosis.
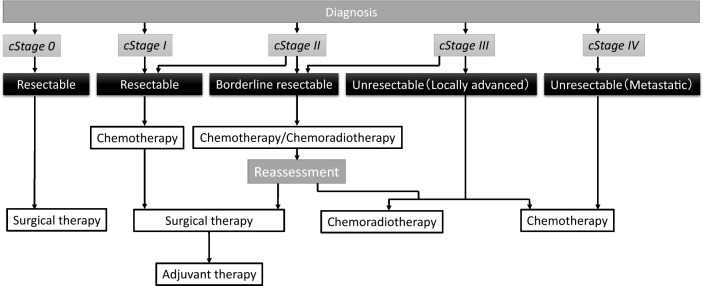
Fig. 1.
Algorithm for the treatment of pancreatic cancer according to the Clinical Practice Guidelines for Pancreatic Cancer 2019 from the Japan Pancreas Society. The clinical cancer stage (cStage) classification and resectability classification are based on the General Rules for the Study of Pancreatic Cancer, Seventh Edition, The Japan Pancreas Society
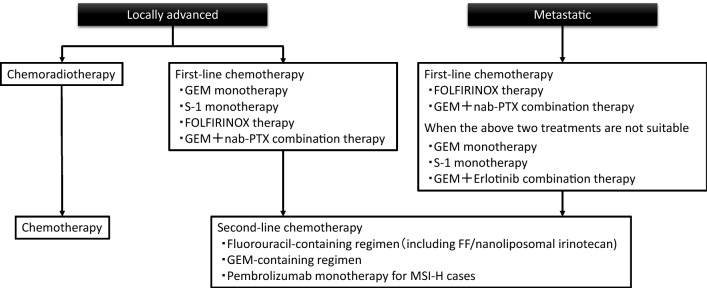
Fig. 2.
Algorithm for chemotherapy of pancreatic cancer according to the Clinical Practice Guidelines for Pancreatic Cancer 2019 published by the Japan Pancreas Society. GEM gemcitabine, nab-PTX nab-paclitaxel, FF fluorouracil + calcium folinate
Neoadjuvant chemotherapy
Patients with pancreatic cancer are known to show high recurrence rates even after curative resection, and the prognosis of patients with recurrent disease is extremely poor. To prevent or delay the development of recurrence after resection and to improve the prognosis in patients with resectable tumor, numerous clinical trials of adjuvant therapy, including chemotherapy and chemoradiotherapy, administered before and/or after resection, have been actively undertaken both in Japan and overseas. Among the several types of adjuvant therapy, postoperative adjuvant chemotherapy has come to be recognized as a standard treatment strategy globally, based on demonstration in recent phase III studies of its ability to improve the long-term prognosis of pancreatic cancer patients. On the other hand, until recently, no solid evidence from large-scale randomized-controlled studies had been established the survival benefit of neoadjuvant (preoperative) therapy. In 2018 to 2019, one phase III study each of neoadjuvant therapy was conducted in Japan and overseas (Table 1).
Table 1.
Major randomized phase III trials of neoadjuvant treatments with reported results for pancreatic cancer
| Study | Treatments | No. of patients | Median disease-free survival (months) | p value | Median survival (months) | p value |
|---|---|---|---|---|---|---|
| Prep-02/JSAP-05 2019 | Gemcitabine/S-1 | 182 | 14.28 | 0.028 | 36.72 | 0.015 |
| Up-front surgery | 180 | 11.28 | 26.65 | |||
| PREOPANC-1 2018 | Gemcitabine/radiation | 119 | 9.9 | 0.023 | 17.1 | 0.074 |
| Up-front surgery | 127 | 7.9 | 13.7 |
Prep Study group of preoperative therapy for pancreatic cancer, JSAP Japanese Study Group of Adjuvant Therapy for Pancreatic cancer, PREOPANC Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer
The results of the phase III study (Prep-02/JSAP-05 Study) of neoadjuvant chemotherapy with gemcitabine plus S-1 for pancreatic cancer patients scheduled for resection conducted in Japan were reported at the American Society of Clinical Oncology-—Gastrointestinal Cancers Symposium (ASCO-GI) 2019; the study showed that the overall survival (OS) was significantly better in the neoadjuvant therapy group as compared to that in the upfront surgery group [hazard ratio (HR) 0.72, p = 0.015] [3–5]. Approximately 80% of patients enrolled in this study had resectable pancreatic cancer at diagnosis, although the study also included patients with borderline resectable pancreatic cancer with portal vein invasion. A subgroup analysis showed better treatment outcomes in patients with resectable tumor and a trend towards better survival in patients with borderline resectable cancer assigned to the neoadjuvant therapy arm [5].
The overseas phase III study (PREOPANC-1 study) to confirm the survival benefit of neoadjuvant chemoradiotherapy was conducted in The Netherlands in patients with resectable and borderline resectable pancreatic cancer [6]. The results, reported at ASCO 2018, showed a trend towards a better OS in the neoadjuvant therapy arm as compared to the immediate surgery arm, although the difference was not statistically significant (HR 0.74, p = 0.074).
Prior to these two studies mentioned above, no phase III study had demonstrated the benefits of neoadjuvant therapy for patients with resectable pancreatic cancer [7, 8]; therefore, Japanese guidelines had not recommended neoadjuvant therapy for patients with pancreatic cancer until recently [9, 10]; the same is true of guidelines in other countries overseas, which still do not recommend neoadjuvant therapy as standard treatment for patients with resectable pancreatic cancer with exceptions for those with high-risk factors [11–14]. Based on the results of the Prep-02/JSAP-05 study, however, the latest Japanese guidelines (Clinical Practice Guidelines for Pancreatic Cancer 2019) recommend gemcitabine plus S-1 combination therapy (GS therapy) as a standard neoadjuvant therapy for patients with resectable pancreatic cancer [15, 16]. Since the Prep-02/JSAP-05 study was conducted only in Japan and use of S-1 is not as feasible in Western populations, as mentioned later, until now, GS therapy is recognized as a standard therapy only in Japan and China [13, 17, 18].
For patients with borderline resectable pancreatic cancer, Japanese guidelines recommend neoadjuvant therapy, in general, but have refrained from recommending any specific regimens [15, 16]. Although guidelines in many other countries also recommend neoadjuvant therapy for borderline resectable pancreatic cancer, no consensus on any standard regimens has been established in any country until date [11–14]. Among the several ongoing randomized-controlled trials of treatments for borderline resectable pancreatic cancer (Table 2) [19–27], a phase II/III study of neoadjuvant therapy with gemcitabine plus nab-paclitaxel therapy versus chemoradiotherapy with S-1 is under way in Japan; this study is expected to provide specific new evidence for the establishment of a standard regimen for borderline resectable pancreatic cancer [21].
Table 2.
Major ongoing randomized trials of neoadjuvant treatments for borderline resectable pancreatic cancer
| Study | Treatments | Eligibility | Phase | No. of patients | Primary endpoint | Study Start | Estimated Study Completion | Country |
|---|---|---|---|---|---|---|---|---|
| UVA-PC-PD101 NCT02305186 | Radiation/capecitabine/pembrolizumab | R BR | Phase 1/2 | 56 | Number of tumor-infiltrating lymphocytes (TILs) | Mar 2015 | Dec 2020 | US |
| Radiation/capecitabine | ||||||||
| NCT02717091 | FOLFIRINOX | BR | Phase 2 | 50 | R0 resection rate | Jul 2015 | Jun 2020 | Japan |
| gemcitabine/nab-paclitaxel | ||||||||
| GABARNANCE Trial UMIN000026858 | S1 + radiation | BR | Phase 2/3 | 110 | Overall survival | Apr 2017 | Sep 2022 | Japan |
| Gemcitabine/nab-paclitaxel | ||||||||
| PANDAS-PRODIGE 44 NCT02676349 | mFOLFIRINOX + radiation/capecitabine | BR | Phase 2 | 90 | R0 resection rate | Oct 2016 | Jan 2026 | France |
| mFOLFIRINOX | ||||||||
| Alliance Trial A021501 NCT02839343 | mFOLFIRINOX + radiation | BR | Phase 2 | 126 | 18 months overall survival rate | Dec 2016 | Mar 2020 | Canada, US |
| mFOLFIRINOX | ||||||||
| BRPCNCC-1 NCT03777462 | Gemcitabine/nab-paclitaxel | BR | Phase 2 | 150 | Overall survival | Apr 2019 | Dec 2021 | China |
| Gemcitabine/nab-paclitaxel + radiation | ||||||||
| S1/Nab-paclitaxel + radiation | ||||||||
| NCT01458717 | radiation/gemcitabine | BR | Phase 2/3 | 58 | 2-year survival rate | Nov 2011 | Jan 2018 | Korea |
| Upfront surgery | ||||||||
| NEOLAP NCT02125136 | Gemcitabine/nab-paclitaxel | BR LA | Phase 2 | 168 | Conversion rate | Nov 2014 | Oct 2020 | US |
| Gemcitabine/nab-paclitaxel + mFOLFIRINOX | ||||||||
| NCT03983057 | mFOLFIRINOX/Anti-PD-1 antibody | BR LA | Phase 3 | 830 | Progression-free survival | Apr 2019 | Apr 2021 | China |
| mFOLFIRINOX |
R resectable, BR borderline resectable, LA locally advanced, mFOLFIRINOX modified-FOLFIRINOX
Adjuvant chemotherapy
Randomized-controlled trials comparing postoperative adjuvant chemotherapy and resection alone have been conducted since the 1990s, mainly in Europe and Japan (Table 3). In the CONKO-001 trial conducted in Germany and Austria, 354 patients who had undergone resection for pancreatic cancer were randomly assigned to receive postoperative adjuvant chemotherapy with gemcitabine alone or resection alone [28, 29]. The results showed a significantly prolonged recurrence-free survival in the adjuvant chemotherapy arm. While no significant prolongation of the OS was noted initially (p = 0.06) [28], a subsequent analysis performed after long-term follow-up revealed significant prolongation of not only the recurrence-free survival, but also of the OS [29]. In the JSAP-02 study conducted in Japan, 118 patients who had undergone resection for pancreatic cancer were randomly assigned to receive postoperative adjuvant chemotherapy with gemcitabine alone or resection alone [30]. Consistent with the initial results of the CONKO-001 trial, significant prolongation of the recurrence-free survival was observed in the gemcitabine-alone arm. The European Study Group of Pancreatic Cancer (ESPAC) conducted the ESPAC-3 Study in Europe, Australia, Japan, and Canada, in which 1088 patients who had undergone resection for pancreatic cancer were randomly assigned to receive postoperative adjuvant chemotherapy with either fluorouracil plus folinate calcium or gemcitabine alone [31]. Although there was no significant difference in the OS between the two groups, the incidence of serious adverse events was significantly lower in the gemcitabine-alone arm than in the fluorouracil plus folinate calcium arm. These results indicate that patients receiving postoperative adjuvant chemotherapy with gemcitabine show significantly better survival outcomes than those undergoing resection alone; in addition, since serious adverse events were also less frequent in the gemcitabine arm than in the fluorouracil plus folinate calcium arm, gemcitabine could be regarded as the global standard treatment agent for postoperative adjuvant chemotherapy.
Table 3.
Pivotal phase III trials of adjuvant treatments for pancreatic cancer
| Study | Regimens | No. of patients | Median disease-free survival (months) | p value | Median survival (months) | p value |
|---|---|---|---|---|---|---|
| ESPAC-1 2004 | Chemoradiotherapy | 73 | NR | NR | 13.9 |
p = 0.009* p = 0.05+ |
| 5-FU/folinic acid | 75 | NR | 21.6 | |||
| Chemoradiotherapy + 5-FU/folinic acid | 72 | NR | 19.9 | |||
| Observation | 69 | NR | 16.9 | |||
| CONKO-001 2007 | Gemcitabine | 179 | 13.4 | 0.001 | 22.1 | 0.06 |
| Observation | 175 | 6.9 | 20.2 | |||
| ESPAC-3 2010 | Gemcitabine | 537 | 14.3 | 0.53 | 23.6 | 0.39 |
| 5-FU/folinic acid | 551 | 14.1 | 23.0 | |||
| JASPAC-01 2016 | S-1 | 192 | 22.9 | 0.0001 | 46.5 | 0.0001 |
| Gemcitabine | 193 | 11.3 | 25.5 | |||
| ESPAC-4 2017 | Gemcitabine plus capecitabine | 364 | 13.9 | 0.082 | 28.0 | 0.032 |
| Gemcitabine | 366 | 13.1 | 25.5 | |||
| CONKO-005 2017 | Gemcitabine plus erlotinib | 219 | 11.4 | 0.26 | 24.5 | 0.61 |
| Gemcitabine | 215 | 11.4 | 26.5 | |||
| Unicancer GI PRODIGE 24/CCTG PA.6 2018 | Modified FOLFIRINOX | 247 | 21.6 | 0.001 | 54.4 | 0.003 |
| Gemcitabine | 246 | 12.8 | 35.0 | |||
| APACT 2019 | Gemcitabine plus nab-paclitaxel | 432 | 19.4 | 0.182 | 40.5 | 0.045 |
| Gemcitabine | 434 | 18.8 | 36.2 |
ESPAC European Study Group for Pancreatic Cancer 1, CONKO Charité Onkologie, JASPAC Japan Adjuvant Study Group of Pancreatic Cancer, GI gastrointestinal, PRODIGE partenariat de recherche en oncologie digestive, CTG PA Clinical Trials Group Pancreatic Adenocarcinoma, APACT adjuvant therapy for patients with resected pancreatic cancer
*Chemotherapy vs. no chemotherapy
+Chemoradiotherapy vs. no chemoradiotherapy
In Japan, the Japan Adjuvant Study Group of Pancreatic Center (JASPAC) conducted a phase III comparative study (JASPAC 01) of postoperative adjuvant chemotherapy with gemcitabine alone versus S-1 alone in patients who had undergone resection for pancreatic cancer [32]. A total of 385 patients were enrolled, and the 5-year survival rate and median survival time were 44.1% and 46.5 months, respectively, in the S-1 group, and 24.4% and 25.5 months, respectively, in the gemcitabine group. The results demonstrated that postoperative adjuvant therapy with S-1 as compared to that with gemcitabine was associated with a significantly improved OS after resection of pancreatic cancer (HR 0.57, p < 0.0001). The ESPAC conducted the ESPAC-4 study, in which 730 patients who had undergone resection for pancreatic cancer were randomly assigned to receive either gemcitabine alone or combined gemcitabine plus capecitabine therapy in England, Scotland, Wales, Germany, France, and Sweden [33]. The median survival time was 25.5 months in the gemcitabine monotherapy arm and 28.0 months in the gemcitabine plus capecitabine arm. The results demonstrated that the combined gemcitabine plus capecitabine regimen yielded a significantly prolonged OS after pancreatic cancer resection as compared to gemcitabine monotherapy (HR: 0.82, p = 0.032). The results of the PRODIGE 24-ACCORD 24/CCTG PA 6 study, conducted in France and Canada, have also been reported; in this study, the modified FOLFIRINOX regimen was compared with gemcitabine alone as adjuvant therapy [34]. A total of 493 patients were enrolled, and the median disease-free survival, which was the primary endpoint, was 21.6 months in the modified FOLFIRINOX arm and 12.8 months in the gemcitabine monotherapy arm, demonstrating superior outcomes in the modified FOLFIRINOX arm (HR 0.58, p < 0.0001). In terms of the OS also, better results were obtained in the modified FOLFIRINOX arm (the median survival time was 54.4 months in the modified FOLFIRINOX arm and 35.0 months in the gemcitabine monotherapy arm; HR 0.64, p = 0.003). Since no clinical study has been conducted to compare S-1 alone with the combined gemcitabine plus capecitabine regimen and modified FOLFIRINOX regimen, and it is still not clear as to which of the three above regimes might be the optimal one for adjuvant therapy. In Japan and China, S-1 is frequently used as the standard treatment agent [13, 15, 16] because of its higher efficacy as compared to gemcitabine monotherapy (as suggested by the superior HR of 0.57) [32], its milder adverse effects in Asians, and its availability as an oral formulation, which can be expected to reduce the burden on the patients. On the other hand, S-1 has not been tested as adjuvant therapy in Western populations [12, 17]. Therefore, in countries including Europe and the US, the gemcitabine plus capecitabine regimen or modified FOLFIRINOX regimen is preferred and regarded as the standard [11, 12, 14]. Thus, this is another difference in the treatment practice for pancreatic cancer between Asian and Western countries.
The global phase III APACT trial evaluated adjuvant treatment with nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with resected pancreatic cancer [35]. Results of the study were reported at ASCO 2019. The primary endpoint, disease-free survival by independent review, was not met. The median disease-free survival was 19.4 months with nab-paclitaxel plus gemcitabine versus 18.8 months with gemcitabine monotherapy (HR = 0.88; p = 0.1824). However, the prespecified sensitivity analysis of investigator-assessed disease-free survival and interim OS were improved with nab-paclitaxel plus gemcitabine versus gemcitabine alone (HR 0.82 for both). Additional OS follow-up may better support nab-paclitaxel plus gemcitabine as an option in the adjuvant setting.
Chemotherapy for locally advanced pancreatic cancer
Locally advanced pancreatic cancer, defined as locally invasive pancreatic cancer without obvious distant metastases, is difficult to resect because of invasion of the major arteries. Both in Japan and other countries, guidelines recommend chemoradiotherapy or chemotherapy alone for locally advanced pancreatic cancer, although there is no consensus yet on which of the two might be preferable [11–16].
In regard to chemoradiotherapy for locally advanced pancreatic cancer, in western countries, induction chemotherapy is undertaken prior to chemoradiotherapy, and is recommended by guidelines as the standard treatment option [11, 12]. The aims of induction chemotherapy are to select patients who are more likely to benefit from chemoradiotherapy and to prevent distant metastases. However, there have been no randomized-controlled trials examining the clinical benefits of induction chemotherapy, except for the JCOG1106 trial, which was a randomized phase II trial conducted in Japan [36]. In this study, the median survival time and 2-year OS in the induction chemotherapy arm receiving gemcitabine monotherapy for 12 weeks before the start of radiotherapy combined with S-1 were 17.2 months and 18.9%, respectively; no statistically significant differences were observed as compared to the corresponding values (19.0 months and 36.9%) in the group that did not receive induction chemotherapy, although a trend towards poorer outcomes was observed in the induction chemotherapy arm. There was no significant difference in the incidence of adverse reactions observed either between the two groups. This study, which is the only trial of induction chemotherapy conducted to date, failed to demonstrate any clinical benefits of induction chemotherapy. Induction chemotherapy is not a common practice in Japan. Therefore, Japanese guidelines do not recommend induction chemotherapy for patients with locally advanced pancreatic cancer [15, 16].
Historically, in clinical trials of systemic chemotherapy for advanced pancreatic cancer, both patients with locally advanced disease and patients with distant metastases have been enrolled under the umbrella term, “unresectable pancreatic cancer,” and treatments that were found to be of survival benefit in these studies have been regarded as the standard treatments for both categories of patients. However, in recent years, these two categories of patients have been classified into separate group in trials, and many phase III studies for systemic chemotherapy are now being conducted in only patients with distant metastases (Table 4). On the other hand, no definitive conclusions have been reached yet as to the standard therapy for this population; chemotherapies demonstrated to show survival benefit in patients with distant metastases are also considered highly likely to be effective in patients with locally advanced pancreatic cancer. FOLFIRINOX and the combined gemcitabine plus nab-paclitaxel regimen have been shown to prolong the survival, as compared to gemcitabine monotherapy, in patients with distant metastases [37, 38], and are, therefore, also the most highly recommended regimens in both Japanese [15, 16] and overseas guidelines [11–13] for locally advanced pancreatic cancer patients with a good performance status (PS) except British guidelines [14]. In Japan, a randomized phase II study (JCOG1407) is under way to compare the efficacy and safety of the modified FOLFIRINOX regimen and combined gemcitabine plus nab-paclitaxel regimen for patients with locally advanced pancreatic cancer, to determine the most promising chemotherapy regimen for this stage of disease [39]. This study was the world’s first randomized-controlled study comparing the two regimens, and a subsequent phase III study is being planned to compare the chemotherapy regimen that is suggested to be promising by this phase II study with chemoradiotherapy, which is also a standard treatment strategy for locally advanced pancreatic cancer. Therefore, this study is expected to contribute greatly to the establishment of evidence-based standard treatment for pancreatic cancer patients with locally advanced disease.
Table 4.
Pivotal phase III trials evaluating first-line treatment for advanced pancreatic cancer
| Study | Regimens | Eligibility | No. of patients | Response rate (%) | Median progression-free survival (months) | p value | Median survival (months) | Hazard ratio | p value |
|---|---|---|---|---|---|---|---|---|---|
| Gemcitabine vs. 5-FU 1997 | Gemcitabine | LA M | 63 | 5.4 | 9 weeks | 0.0002 | 5.65 | NR | 0.0025 |
| 5-FU | 63 | 0 | 4 weeks | 4.41 | |||||
| NCIC CTG PA.3 2007 | Gemcitabine plus erlotinib | LA M | 285 | 8.6 | 3.75 | 0.004 | 6.24 | 0.82 | 0.038 |
| Gemcitabine | 284 | 8.0 | 3.55 | 5.9 | |||||
| GEST 2013 | Gemcitabine plus S-1 | LA M | 275 | 29.3 | 5.7 | < 0.001* | 10.1 | 0.88 | 0.15* |
| S-1 | 280 | 21.0 | 3.8 | 0.02+ | 9.7 | 1.0 | 0.001+ | ||
| Gemcitabine | 277 | 13.3 | 4.1 | 8.8 | |||||
| PRODIGE 4/ ACCORD 11 2011 | FOLFIRINOX | M | 171 | 31.8 | 6.4 | < 0.001 | 11.1 | 0.57 | < 0.001 |
| Gemcitabine | 171 | 11.3 | 3.3 | 6.8 | |||||
| MPACT 2019 | Gemcitabine plus nab-paclitaxel | M | 431 | 23 | 5.5 | < 0.001 | 8.5 | 0.7 | < 0.001 |
| Gemcitabine | 430 | 7 | 3.7 | 6.7 |
L locally advanced, M metastatic, NCIC CTG PA National Cancer Institute of Canada—Clinical Trials Group Pancreatic Adenocarcinoma, GEST gemcitabine and TS-1 Trial, PRODIGE: partenariat de recherche en oncologie digestive, ACCORD actions concertées dans les cancers colorectaux et digestif, MPACT Metastatic Pancreatic Adenocarcinoma Clinical Trial
*Superiority to gemcitabine
+Non-inferiority to gemcitabine
Chemotherapy for metastatic pancreatic cancer
The Japanese Clinical Practice Guidelines for Pancreatic Cancer 2019 recommends FOLFIRINOX therapy or combined gemcitabine plus nab-paclitaxel therapy as the first-line treatment for pancreatic cancer patients with distant metastases [15, 16]. For patients in whom these treatments are unsuitable owing to their systemic condition or age, gemcitabine monotherapy, S-1 monotherapy or gemcitabine plus erlotinib combination therapy is recommended. A phase III study conducted overseas demonstrated the survival benefits of the FOLFIRINOX regimen [37], combined gemcitabine plus nab-paclitaxel regimen [38], gemcitabine monotherapy [40], and the gemcitabine plus erlotinib regimen [41]. Thereafter, clinical trials were also conducted in Japan, and the efficacy and safety of these regimens were also confirmed in Japanese patients [42–45]. On the other hand, S-1 monotherapy has come to be recommended as a standard treatment on the basis of the results of a phase III study (GEST study) conducted in Japan and Taiwan [46, 47]. The GEST study examined the non-inferiority of S-1 monotherapy to gemcitabine monotherapy and the superiority of combined gemcitabine plus S-1 therapy over gemcitabine monotherapy, in terms of the survival outcomes. The non-inferiority of S-1 monotherapy was statistically confirmed (the median survival time was 8.8 months in the gemcitabine arm and 9.7 months in the S-1 arm; HR 0.96, p < 0.001). However, the superiority of the combined gemcitabine plus S-1 regimen could not be confirmed (the median survival time was 8.8 months in the gemcitabine arm and 10.1 months in the gemcitabine plus S-1 arm; HR 0.88, p = 0.15). Therefore, S-1 monotherapy has come to be regarded as a standard treatment in Asian countries [13, 15, 16]. However, S-1 therapy has been reported to have only marginal anti-tumor activity in Western populations [48], and is, therefore, not regarded as a valid treatment option in Western countries.
The second-line treatment regimens recommended in Japan are: (1) a fluorouracil-containing regimen after a first-line gemcitabine-containing regimen, (2) a gemcitabine-containing regimen after a first-line fluorouracil-containing regimen, and (3) pembrolizumab for microsatellite instability-high (MSI-H) [15, 16]. The fluorouracil-containing regimen consists of fluorouracil combined with folinate calcium and MM-398 (nanoliposomal irinotecan).
Tropomyosin receptor kinase (TRK) inhibitors have been reported to be useful for patients with solid tumors harboring neurotrophic receptor tyrosine kinase (NTRK) fusion genes. At the 2018 Annual Meeting of the European Society of Clinical Oncology (ESMO), the results from an integrated analysis of three studies (STARTRK-2 study, STARTRK-1 study and ALKA-372-001 study) conducted in patients with tumors harboring NTRK fusion genes were presented [49]. In these studies, entrectinib, a TRK inhibitor, was administered to 54 patients with soft-tissue sarcomas, non-small cell lung cancer, and others, and the response rate was 57.4%. Entrectinib was also approved in Japan in June 2019. Pancreatic cancer harboring NTRK fusion genes is reported to be extremely rare, accounting for less than 1% of all cases [50]. The Japanese guidelines are being revised to include entrectinib as a treatment option.
Future of pancreatic cancer chemotherapy
Immunotherapy
Programmed death-ligand 1 (PD-L1) (found on the surfaces of cancer cells and stromal cells) and programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (found on the surface of T cells) have been shown to play particularly important roles in the suppression of T-cell activation by cancer cells. Strong efficacy of such monoclonal antibodies against specific types of cancer that are known to show particularly high immunogenicity (e.g., malignant melanoma) has been reported. In 2011, ipilimumab (an anti-CTLA-4 antibody) was approved for the treatment of malignant melanoma in the US, followed by the approval and clinical introduction of two anti-PD-1 antibodies (nivolumab and pembrolizumab) and three anti-PD-L1 antibodies (atezolizumab, avelumab, and durvalumab) for the treatment of several types of cancer. Active efforts have also been made to develop similar therapies for pancreatic cancer. A phase II study of ipilimumab alone for unresectable pancreatic cancer was conducted, and the response rate was 0% (0 of 27 patients) [51]. Similarly, in a phase I study of BMS-936559 (an anti-PD-L1 antibody), the response rate was 0% (0 of 14 patients) [52]. In addition, a randomized phase II study was conducted to compare durvalumab (an anti-PD-L1 antibody) alone with combined durvalumab plus tremelimumab (an anti-CTLA-4 antibody) therapy [53]. The response rate and the median survival time were 0% (0 of 32 patients) and 3.6 months, respectively, in the monotherapy group, and 3% (1 of 32 patients) and 3.1 months, respectively, in the combined therapy group. Thus, the immune checkpoint inhibitors, even the two immune checkpoint inhibitors used in combination, failed to elicit any desirable treatment outcomes. Therefore, the development of immune checkpoint inhibitors for pancreatic cancer is currently focused on combination therapy with chemotherapeutic agents, based on the expectation of possible add-on effects to current standard treatments, such as combined gemcitabine plus nab-paclitaxel therapy and FOLFIRINOX therapy [54–56]. However, in regard to MSI-H pancreatic cancer, some studies of pembrolizumab monotherapy have shown encouraging results [57, 58]. MSI-H is considered as a target against which immune checkpoint inhibitors are effective.
One of the possible reasons why immune checkpoint inhibitors are less effective in patients with pancreatic cancer is that pancreatic cancer contains proliferating interstitial components with a few tumor-infiltrating T cells. Therefore, various studies have been conducted to develop treatments using immune checkpoint inhibitors in combination with drugs targeting the tumor microenvironment characterized by such proliferation of interstitial components and immune responses. Human tumor-infiltrating Treg cells, which suppress anti-tumor immunity, express high levels of the chemokine receptor CCR4. In a phase I study of nivolumab plus mogamulizumab, a monoclonal antibody targeting CCR4, for patients with solid tumors, the partial response, and stable disease rates in 15 patients with pancreatic cancer were 7% and 33%, respectively [59]. It is reported that pancreatic cancer is characterized by a high degree of infiltration by tumor-associated macrophages (TAMs) that inhibit anti-tumor T-cell activity, and that blocking colony-stimulating factor 1 receptor (CSF-1R) signaling—which supports the recruitment, differentiation, and maintenance of immunosuppressive macrophages in tumors—may lead to depletion of the TAMs and upregulation of T-cell checkpoints. In a phase 1a/b study, cabiralizumab, a monoclonal antibody targeting CSF-1R signaling, plus nivolumab four partial responses (13%) were observed in 31 patients with pancreatic cancer [60, 61]. At present, a randomized phase II study to compare the efficacy of nivolumab plus cabiralizumab with or without chemotherapy in patients with pancreatic cancer is in progress [62]. TGFβ is another main contributor to immune evasion and tumor progression. M7824 is a bifunctional fusion protein composed of two extracellular domains of TGF-βRII (a TGF-β “trap”) fused with a human IgG1 monoclonal antibody against PD-L1. In a phase I study of M7824 conducted in patients with advanced solid tumors, one and three of the five pancreatic cancer patients enrolled in the study showed partial response and stable disease, respectively [63]. Furthermore, several trials of immunotherapy-based treatment combinations with targeted agents are ongoing for patients with pancreatic cancer [64–66].
In Japan, clinical studies of immunotherapy using peptide vaccines are actively being conducted [67–69]. Among them, a randomized phase II study for the Wilms’ tumor gene 1 (WT1) vaccine showed promising results [69]; WT1, which is ranked as the top antigen among 75 tumor-associated antigens (TAAs) [70], is one of the most promising TAAs. In this study, gemcitabine plus WT1 vaccine tended to prolong the progression-free survival (HR 0.66; p = 0.084) and improve the OS (HR 0.82; p = 0.363) in comparison with gemcitabine monotherapy. Currently, a phase III study of a dendritic cell vaccine loaded with WT1 peptides (TLP0-001) is being conducted in Japan in patients with advanced pancreatic cancer refractory to standard chemotherapy [71–73]. Many clinical studies of immunotherapy using vaccines have also been conducted overseas. In particular, randomized-controlled trials of prime/boost vaccination with GVAX and CRS-207 have yielded encouraging results [74]. GVAX, which is composed of two irradiated, granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic pancreatic cancer cell lines, administered 24 h after treatment with low-dose cyclophosphamide (Cy) to inhibit regulatory T cells, induced T-cell immunity against cancer antigens, including mesothelin. CRS-207, a live-attenuated Listeria monocytogenes—expressed mesothelin, induces innate and adaptive immunity. On the basis of preclinical synergy, a phase II randomized study was conducted to compare Cy/GVAX followed by CRS-207 (arm A) with Cy/GVAX alone (arm B) in patients with metastatic pancreatic cancer. A total of 90 patients were treated (arm A, n = 61; arm B, n = 29); the OS was 6.1 months in arm A versus 3.9 months in arm B (HR 0.59; p = 0.02). On the basis of the observed survival and favorable safety profile, Cy/GVAX and CRS-207 are being explored further as suitable treatments for pancreatic cancer.
CAR-T cells are engineered T cells from patients, which can recognize tumor antigens, by transfection of genes encoding B-cell epitopes and T-cell activation signals. CAR-T cells infused into patients elicit an immune response that specifically attacks only those cells, including cancer cells, which express the target proteins. Multinational phase II studies (ELIANA study, JULIET study) have demonstrated the efficacy of CAR-T-cell therapy in patients with diffuse large B-cell lymphoma (DLBCL) [75, 76]. Clinical studies of CAR-T-cell therapy for pancreatic cancer are also in progress, and the results are expected. A phase I study was conducted to evaluate the safety and efficacy of adoptive cell therapy with autologous mesothelin-specific CAR-T cells (CARTmeso cells) in six patients with chemotherapy-refractory metastatic pancreatic cancer [77]. The disease stabilized in two patients and the progression-free survival times were 3.8 and 5.4 months. In 18F-2-fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography/computed tomography imaging performed to monitor the metabolic active volume (MAV) of individual tumor lesions, the total MAV remained stable in three patients and decreased by 69.2% in one patient with biopsy-proven mesothelin expression; in this patient, all liver lesions showed complete abrogation of FDG uptake at 1 month as compared to the baseline.
Molecular-targeted therapy
KRAS, CDKN2A, TP53, and SMAD4 have been recognized as major driver genes in pancreatic carcinogenesis. Mutations in these genes are the most commonly encountered mutations in the majority of patients with pancreatic cancer, although potential therapeutic-target genes are limited to KRAS G12C and CDKN2A, which are found only in a small subgroup of patients. Thus, at present, there are no promising therapeutic agents for non-KRAS G12C, TP53, and SMAD4-mutated pancreatic cancer, which account for the majority of the cases. Therefore, selection of the treatment regimen based on gene mutations has yet to become standard strategies for patients with pancreatic cancer. Of the gene mutations, mutations of the DNA repair genes, such as BRCA1/2, PALB2, ATM, ATR, and ATRX, are the most common “highly actionable” alterations. It has been reported that in patients with gene mutations leading to homologous recombination deficiency (HRD), poly (ADP-ribose) polymerase (PARP) inhibitors exert anti-tumor effects by inducing cell death via synthetic lethality. In recent years, promising results have been reported from clinical studies of PARP inhibitors for patients with germline BRCA-mutated pancreatic cancer. According to the findings of the recently completed, international, phase-III POLO (Pancreas cancer OLaparib Ongoing) trial, treatment with the PARP inhibitor olaparib significantly reduced the risk of disease progression in patients with a germline BRCA1 or BRCA2 mutation and metastatic pancreatic cancer and disease that had not progressed during the first-line platinum-based chemotherapy [78]. In this study, 3315 patients with pancreatic cancer were screened for germline BRCA1/2 mutations, and 247 (7.5%) were found to have BRCA1/2 mutations. Of these 247 patients, 154 were randomized to receive olaparib or placebo. The primary endpoint, namely, progression-free survival, was significantly prolonged in the olaparib group as compared to the placebo group (median progression-free survival: 7.4 months vs. 3.8 months, HR 0.53, p = 0.004). Based on this result, the National Comprehensive Cancer Network (NCCN) guidelines in the United States includes the recommendation of olaparib as maintenance treatment for patients who have germline BRCA1/2 mutations, good PS, metastatic disease, and no disease progression after at least 4–6 month first-line chemotherapy [11]. Other PARP inhibitors such as veliparib and rucaparib are also being examined in clinical trials for pancreatic cancer [79–81].
Although KRAS mutations are predominant in pancreatic cancer, no effective therapeutic agent targeting KRAS mutations has been discovered until date. Recently, however, several candidate inhibitors of the KRAS G12C mutant protein have been reported. Among these, encouraging results were reported from a phase I study of AMG510, especially in a non-small cell lung cancer cohort [82]. On the other hand, pancreatic cancers with wild-type KRAS, although rare, account for only about 5% of all cases; BRAF and EGFR gene mutations and some fusion genes (FGFR, ALK, NTRK, NRG1, etc.) have also been reported to be detected at a relatively high frequency [83].
The “Know Your Tumor Project” initiative undertaken by the Pancreatic Cancer Patient Association (PanCAN) in the United States reported that 26% of patients with pancreatic cancer had actionable alterations, suggesting the possibility that therapy targeting these alterations could prolong the survival [84]. Recently, the NCCN guidelines have been updated to include the following: “Germline testing is recommended for any patient with confirmed pancreatic cancer using comprehensive gene panels for hereditary cancer syndromes” and “Tumor/somatic gene profiling is recommended for patients with locally advanced/metastatic disease who are candidates for anti-cancer therapy to identify uncommon mutations.” Thus, the guidelines recommend a search for actionable mutations, even if they are rare [11]. In the near future, guidelines in other countries may also follow these recommendations.
Others
Metastatic pancreatic ductal adenocarcinoma is characterized by excessive hyaluronan (HA) accumulation in the tumor microenvironment, with elevation of the interstitial pressure and impaired perfusion. Preclinical studies have demonstrated that pegvorhyaluronidase alfa (PEGPH20) degrades HA, thereby increasing drug delivery. In a randomized phase II study, patients with previously untreated metastatic pancreatic ductal adenocarcinoma were randomly assigned to treatment with PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) or nab-paclitaxel/gemcitabine (AG) [85]. The progression-free survival was significantly prolonged with PAG treatment in the overall subject population (HR 0.73; p = 0.049) and in patients with HA-high tumors (HR 0.51; p = 0.048). On the other hand, PEGPH20 combination with modified FOLFIRINOX caused increased toxicity and resulted in decreased treatment duration compared with modified FOLFIRINOX alone in another randomized study [86]. A global phase III study of PAG versus AG in patients with HA-high PDA is now ongoing [87].
Circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), as liquid biopsies, are an emerging minimally invasive tool with a unique potential for (a) determining the prognosis, (b) monitoring therapeutic responses and tumor recurrence in real time, (c) exploring therapeutic targets, and (d) potentially developing new drugs by studying metastatic cancer biology and drug resistance mechanisms [88–90]. In addition to CTCs and ctDNA, circulating tumor extracellular vesicles (e.g., exosomes), tumor-educated platelets (TEPs), and blood-based protein and metabolite markers also show early promise as biomarkers that could be used from cancer screening through to targeted treatments for pancreatic cancer. In particular, ctDNA is expected to be clinically useful in noninvasive molecular profiling for the novel actionable mutations [91]. Sequential real-time liquid biopsies could potentially allow early identification of resistance to cancer therapy in individual patients as an important hallmark of personalized cancer medicine. Detection and characterization of minimal residual disease after resection are another important aim and challenge for future studies [92].
Conclusions
Steady progress has been made in the development of non-surgical therapies for advanced pancreatic cancer, and the prognosis of the patients is steadily improving, although the results are still far from satisfactory. Until now, the standard systemic treatment for pancreatic cancer has been limited to existing cytotoxic anti-cancer drugs. Recently, large-scale randomized-controlled studies of pre- and postoperative adjuvant therapies have been actively undertaken in an attempt to improve the prognosis of patients with resectable pancreatic cancer by introducing chemotherapies which have been commonly used for advanced pancreatic cancer, with or without radiotherapy, and this trend is expected to continue in the future. For some pancreatic cancers with actionable mutations, such as germline BRCA1/2 mutations, NTRK fusion mutation, and MSI-H, molecular-targeted therapy and immunotherapy are being introduced. It is expected that the mechanisms of initiation and development of pancreatic cancer will be better elucidated and that targeted treatments that would yield marked tumor shrinkage and survival prolongation will be developed. In addition to developing highly effective and safe anti-cancer treatments, it is also important to ensure that supportive and palliative care is available for efficient implementation of such anti-cancer treatments, and multidisciplinary cooperation and collaboration with the community should be promoted, because both improvement of the patient prognosis and improvement of the quality of life are major goals of treatment of this disease.
Compliance with ethical standards
Conflict of interest
Takuji Okusaka has received research funding from Novartis Pharma, Eli Lilly Japan, AstraZeneca, Chugai Pharmaceutical, Eisai, and Bristol-Myers. Junji Furuse has received honorarium from Eisai, Bayer Yakuhin, Taiho Pharmaceutical, Fujifilm, Astellas Pharma, Yakult Honsha, Ono Pharmaceutical, Shire, Chugai Pharma, Novartis, Teijin pharma, and research funding from Ono Pharmaceutical, MSD, J-Pharma, Taiho Pharmaceutical, Sumitomo Dainippon, Yakult Honsha, and AstraZeneca.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ministry of Health Labour and Welfare. Vital Statistics. https://www.mhlw.go.jp/english/database/db-hw/vs01.html; Accessed 1 Dec 2019.
- 2.National Cancer Center Japan. Cancer Registry and Statistics. Cancer Information Service. https://ganjoho.jp/en/index.html; Accessed 1 Dec 2019.
- 3.Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 4.Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 2019;37 (abstract 189). [DOI] [PubMed]
- 5.Satoi S, Unno M, Motoi F, et al. The effect of neoadjuvant chemotherapy with gemcitabine and S-1 for resectable pancreatic cancer (randomized phase II/III trial; Prep-02/JSAP-05). J Clin Oncol 2019;39 (abstract 4126). [DOI] [PubMed]
- 6.Tienhoven GV, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36 (abstract LBA4002).
- 7.Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. doi: 10.1007/s00066-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadei R, Di Marco M, Ricci C, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg. 2015;19:1802–1812. doi: 10.1007/s11605-015-2890-4. [DOI] [PubMed] [Google Scholar]
- 9.Japan Pancreas Society . Clinical practice guidelines for pancreatic cancer 2016. Tokyo: Kanahara & Co, Ltd; 2016. [Google Scholar]
- 10.Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical practice guidelines for pancreatic cancer 2016 from the Japan pancreas society: a synopsis. Pancreas. 2017;46:595–604. doi: 10.1097/MPA.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Guidelines Version 1.2020, Pancreatic Adenocarcinoma. 2020 https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf; Accessed 1 Dec 2019.
- 12.Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC) Dig Liver Dis. 2018;50:1257–1271. doi: 10.1016/j.dld.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 13.National Health Commission of the People's Republic of C Chinese guidelines for diagnosis and treatment of pancreatic cancer 2018 (English version) Chin J Cancer Res. 2019;31:278–294. doi: 10.21147/j.issn.1000-9604.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. NICE guidance, Pancreatic cancer in adults: diagnosis and management, NICE guideline [NG85], Published date: February 2018. 2018 https://www.nice.org.uk/guidance/ng85; Accessed 1 Dec 2019. [PubMed]
- 15.Japan Pancreas Society . Clinical practice guidelines for pancreatic cancer 2019. Tokyo: Kanahara & Co, Ltd; 2019. [Google Scholar]
- 16.Okusaka T, Nakamura M, Yoshida M, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a Synopsis. Pancreas. 2020 (49:in press). [DOI] [PMC free article] [PubMed]
- 17.Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–6965. doi: 10.1200/JCO.2005.01.917. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 19.NCT02305186, Safety and immunological effect of pembrolizumab in resectable or borderline resectable pancreatic cancer (UVA-PC-PD101). https://clinicaltrials.gov/ct2/show/NCT02305186; Accessed 21 Dec 2019.
- 20.NCT02717091, Neoadjuvant FOLFIRINOX or Nab-paclitaxel With gemcitabine for borderline resectable pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT02717091?term=NCT02717091&draw=2&rank=1; Accessed 21 Dec 2019.
- 21.UMIN000026858, Randomized phase II/III study of gemcitabine and nab-paclitaxel therapy versus S-1 and concurrent radiotherapy as neoadjuvant treatment for Borderline resectable pancreatic cancer. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000030821; Accessed 21 Dec 2019.
- 22.NCT02676349, Neoadjuvant mFolfirinox With or Without Preoperative Concomitant Chemoradiotherapy in Patients With Borderline Resectable Pancreatic Carcinoma (PANDAS-PRODIGE 44). https://clinicaltrials.gov/ct2/show/NCT02676349?term=PANDAS-PRODIGE+44&draw=2&rank=1; Accessed 21 Dec 2019.
- 23.Katz MHG, Ou FS, Herman JM, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017;17:505. [DOI] [PMC free article] [PubMed]
- 24.Gao S, Zhu X, Shi X, et al. Comparisons of different neoadjuvant chemotherapy regimens with or without stereotactic body radiation therapy for borderline resectable pancreatic cancer: study protocol of a prospective, randomized phase II trial (BRPCNCC-1) Radiat Oncol. 2019;14:52. doi: 10.1186/s13014-019-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCT01458717, neoadjuvant chemoradiation in patients with borderline resectable pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT01458717?term=NCT01458717&draw=2&rank=1; Accessed 21 Dec 2019.
- 26.NCT02125136, Trial to investigate intensified neoadjuvant chemotherapy in locally advanced pancreatic cancer (NEOLAP). https://clinicaltrials.gov/ct2/show/NCT02125136?term=NCT02125136&draw=2&rank=1; Accessed 21 Dec 2019.
- 27.NCT03983057, Combination of Anti-PD-1 Antibody and Chemotherapy in Pancreatic Cancer. https://clinicaltrials.gov/ct2/show/NCT03983057?term=NCT03983057&draw=2&rank=1; Accessed 21 Dec 2019.
- 28.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 29.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 30.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–915. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 32.Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 33.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 34.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 35.Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37 (abstract 4000).
- 36.Ioka T, Fukutomi A, Mizusawa J, et al. Randomized phase II study of S-1 and concurrent radiotherapy with versus without induction chemotherapy of gemcitabine for locally advanced pancreatic cancer (LAPC): final analysis of JCOG1106. Ann Oncol. 2016;27 (abstract 621PD).
- 37.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 38.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizusawa J, Fukutomi A, Katayama H, et al. Protocol digest of randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer: Japan clinical oncology group study (JCOG1407) Pancreatology. 2018;18:841–845. doi: 10.1016/j.pan.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 41.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 42.Okada S, Ueno H, Okusaka T, et al. Phase I trial of gemcitabine in patients with advanced pancreatic cancer. Jpn J Clin Oncol. 2001;31:7–12. doi: 10.1093/jjco/hye003. [DOI] [PubMed] [Google Scholar]
- 43.Okusaka T, Furuse J, Funakoshi A, et al. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102:425–431. doi: 10.1111/j.1349-7006.2010.01810.x. [DOI] [PubMed] [Google Scholar]
- 44.Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105:1321–1326. doi: 10.1111/cas.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno H, Ikeda M, Ueno M, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603. doi: 10.1007/s00280-016-2972-3. [DOI] [PubMed] [Google Scholar]
- 46.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 47.Okusaka T, Miyakawa H, Fujii H, et al. Updated results from GEST study: a randomized, three-arm phase III study for advanced pancreatic cancer. J Cancer Res Clin Oncol. 2017;143:1053–1059. doi: 10.1007/s00432-017-2349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultheis B, Strumberg D, Bergmann L, et al. Results of a phase II trial of S-1 as first-line treatment of metastatic pancreatic cancer (CESAR-study group) Invest New Drugs. 2012;30:1184–1192. doi: 10.1007/s10637-011-9665-x. [DOI] [PubMed] [Google Scholar]
- 49.Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive tumours: Pooled analysis of STARTRK-2, STARTRK-1, and ALKA-372-001. Ann Oncol. 2018;29 (abstract LBA4).
- 50.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical Trial. JAMA Oncol. 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 54.Wainberg ZA, Hochster HS, Edward Jae-Hoon Kim, et al. Phase I study of nivolumab (Nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). J Clin Oncol. 2019;37 (abstract 298).
- 55.Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36:96–102. doi: 10.1007/s10637-017-0525-1. [DOI] [PubMed] [Google Scholar]
- 56.JapicCTI-184230, ONO-4538 Phase II Study (ONO-4538–83/TASUKI-83). https://rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-184230; Accessed 21 Dec 2019.
- 57.Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 58.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doi T, Muro K, Ishii H, et al. A phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25:6614–22. [DOI] [PubMed]
- 60.Carleton M, Powers J, Phillips P, et al. Pharmacodynamics (PD) and genomic profiling of pts treated with cabiralizumab (cabira) + nivolumab (NIVO) provide evidence of on-target tumor immune modulations and support future clinical applications. J Clin Oncol 2018;36 (abstract 3020).
- 61.Wainberg ZA, Piha-Paul SA, Luke J, et al. First-in-human phase 1 dose escalation and expansion of a novel combination, anti–CSF-1 receptor (cabiralizumab) plus anti-PD-1 (nivolumab), in patients with advanced solid tumors. J Immunother Cancer. 2017;5 (abstract O42)
- 62.Wang-Gillam A, O'Reilly EM, Bendell JC, et al. A randomized phase II study of cabiralizumab (cabira) + nivolumab (nivo) ± chemotherapy (chemo) in advanced pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 2019;37 (abstract TPS465).
- 63.Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFbeta, in advanced solid tumors. Clin Cancer Res. 2018;24:1287–1295. doi: 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiss KA, Mick R, O'Hara MH, et al. A randomized phase II trial of niraparib plus either nivolumab or ipilimumab in patients with advanced pancreatic cancer whose cancer has not progressed on platinum-based therapy. J Clin Oncol 2019;37 (abstract TPS4161).
- 65.Desai J, Kortmansky JS, Segal NH, et al. MORPHEUS: A phase Ib/II study platform evaluating the safety and clinical efficacy of cancer immunotherapy (CIT)—based combinations in gastrointestinal (GI) cancers. J Clin Oncol 2019;37 (abstract TPS467).
- 66.Wang-Gillam A, Lockhart AC, Tan BR, et al. Phase I study of defactinib combined with pembrolizumab and gemcitabine in patients with advanced cancer. J Clin Oncol 2018;36 (abstract 2561).
- 67.Shima H, Tsurita G, Wada S, et al. Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA-A24-positive pancreatic adenocarcinoma. Cancer Sci. 2019;110:2378–2385. doi: 10.1111/cas.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaue H, Tsunoda T, Tani M, et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci. 2015;106:883–890. doi: 10.1111/cas.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishida S, Ishikawa T, Egawa S, et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: a phase II randomized study. Cancer Immunol Res. 2018;6:320–331. doi: 10.1158/2326-6066.CIR-17-0386. [DOI] [PubMed] [Google Scholar]
- 70.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsuda M, Miyazawa M, Kawai M, et al. A phase III, double-blind, randomized clinical trial comparing S-1 in combination with DC vaccine loaded with WT1 peptides (TLP0-001) or placebo for the patients with advanced pancreatic cancer refractory to standard chemotherapy. J Clin Oncol. 2017;35 (abstract TPS4153).
- 72.Katsuda M, Miyazawa M, Ojima T, et al. A double-blind randomized comparative clinical trial to evaluate the safety and efficacy of dendritic cell vaccine loaded with WT1 peptides (TLP0-001) in combination with S-1 in patients with advanced pancreatic cancer refractory to standard chemotherapy. Trials. 2019;20:242. doi: 10.1186/s13063-019-3332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura Y, Tsukada J, Tomoda T, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 74.Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 77.Beatty GL, O'Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowery MA, Kelsen DP, Capanu M, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Reilly EM, Lee JW, Lowery MA, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124:1374–1382. doi: 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shroff RT, Hendifar A, McWilliams RR, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol. 2018;2018. [DOI] [PMC free article] [PubMed]
- 82.Fakih M, O'Neil B, Price TJ, et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37 (abstract 3003).
- 83.Aung KL, Fischer SE, Denroche RE, et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res. 2018;24:1344–1354. doi: 10.1158/1078-0432.CCR-17-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of patients with pancreatic cancer: initial results from the know your tumor initiative. Clin Cancer Res. 2018;24:5018–5027. doi: 10.1158/1078-0432.CCR-18-2645. [DOI] [PubMed] [Google Scholar]
- 85.Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 86.Ramanathan RK, McDonough SL, Philip PA, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062–1069. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doherty GJ, Tempero M, Corrie PG. HALO-109-301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14:13–22. doi: 10.2217/fon-2017-0338. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374–394. doi: 10.1016/j.molonc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786–4800. doi: 10.1158/1078-0432.CCR-14-1190. [DOI] [PubMed] [Google Scholar]
- 90.Lee JS, Park SS, Lee YK, et al. Liquid biopsy in pancreatic ductal adenocarcinoma: current status of circulating tumor cells and circulating tumor DNA. Mol Oncol. 2019;13:1623–1650. doi: 10.1002/1878-0261.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment and precision medicine in pancreatic cancer. Adv Exp Med Biol. 2016;924:13–17. doi: 10.1007/978-3-319-42044-8_3. [DOI] [PubMed] [Google Scholar]
- 92.Mader S, Pantel K. Liquid biopsy: current status and future perspectives. Oncol Res Treat. 2017;40:404–408. doi: 10.1159/000478018. [DOI] [PubMed] [Google Scholar]