Abstract
Gout, which results from elevated serum uric acid (SUA), is a common form of arthritis that is induced by urate crystals. A single nucleotide polymorphism, rs2544390, of LDL receptor related protein 2 (LRP2/Megalin), has previously been reported to be associated with SUA by a genome-wide association study in a Japanese population. However, it was controversial as to whether rs2544390 is associated with gout in a Japanese population, since previous studies with Japanese populations have reported an association between gout and rs2544390 both with and without significance. This prompted us to investigate the association between gout and rs2544390 of LRP2. Using 1208 clinically diagnosed gout patients and 1223 controls in a Japanese male population, our results showed that while rs2544390 did not show a significant association with gout susceptibility in the present study (p = 0.0793, odds ratio [OR] with 95% confidential interval [CI] 1.11 [0.99–1.24]). However, a meta-analysis using previous studies on Japanese populations revealed a significant association with gout (pmeta = 0.0314, OR with 95% CI 1.09 [1.01–1.18]). We have therefore for the first time confirmed a positive association between rs2544390 and gout with only a Japanese male population. Our study provides clues to a better understanding of the pathogenesis of gout and has the potential to lead to novel therapeutic strategies against gout using LRP2 as a molecular target.
Keywords: Uric acid, Gout, Hyperuricemia, LRP2, Single nucleotide polymorphism (SNP)
Introduction
Gout is a common disease that results from an increase in serum uric acid (SUA), which can lead to renal failure, hypertension, and cardiovascular disease [1, 2]. rs2544390, a single nucleotide polymorphism (SNP) in LDL receptor related protein 2 (LRP2, also known as Megalin), was found to have an association with SUA in a genome-wide association study (GWAS) with 8868 Japanese [3]. However, the association between rs2544390 and gout remained to be clarified, because some studies, including ours, have reported no association [4, 5], while others have revealed a significant association [5–7]. In this study, we investigated a further association between gout and rs2544390 with clinically diagnosed gout patients and controls, and performed a meta-analysis based on past Japanese population studies [4, 6].
Methods
Patients and controls
1208 male Japanese patients were recruited from outpatients at Ryougoku East Gate Clinic (Tokyo, Japan). All these subjects had been diagnosed with primary gout according to the criteria established by the American College of Rheumatology [8]. As the control group, 1223 Japanese males without a history of gout or hyperuricemia (SUA levels > 7.0 mg/dL) were selected from participants in the Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) [9]. The mean age and standard deviation of cases and controls were 45.4 ± 10.4 and 53.1 ± 8.8 years, respectively, and their mean body mass index was 25.4 ± 3.7 and 23.3 ± 2.7 kg/m2, respectively.
Genetic and statistical analyses
Genomic DNA was extracted from whole peripheral blood [10]. Genotyping of LRP2 polymorphism (rs2544390) was performed using a TaqMan assay (Custom TaqMan MGB, Applied Biosystems) with a Lightcycler 480 (Roche Diagnostics) as previously described [4]. To confirm their genotypes, more than 25 samples were subjected to direct sequencing with the following primers: for rs2544390, forward 5′-CTGTCTGAGACCATGACACAG-3′, and reverse 5′-CCTCACCTGTCATTGTCTTG-3′. DNA sequencing analysis was performed with a 3130xl Genetic Analyzer (Applied Biosystems) [11]. For the calculations in the statistical analyses, we used SPSS v.22.0J (IBM Japan Inc., Tokyo, Japan) and R (version 3.1.1) [12] including a meta-package [13]. The Chi-square test was used for the association and Hardy–Weinberg equilibrium analyses. A p value of < 0.05 was regarded as statistically significant.
Results
Table 1 shows the genotyping results of rs2544390 for 1208 gout cases and 1223 controls. The genotyping call rate for this SNP was more than 98%. In the control group, this SNP was in Hardy–Weinberg equilibrium (p > 0.05), which suggested no mistyping.
Table 1.
Association between gout and LRP2 rs2544390 polymorphism
| Genotype | MAFa | Allele frequency mode | ||||
|---|---|---|---|---|---|---|
| C/C | C/T | T/T | p valueb | OR (95% CI) | ||
| Case | 291 | 587 | 330 | 0.516 | 0.0793 | 1.11 (0.99–1.24) |
| Control | 312 | 608 | 290 | 0.491 | ||
MAF minor allele frequency, OR odds ratio, CI confidence interval
aT: minor allele
bChi-square test of rs2544390 polymorphism
As in our previous study [4], rs2544390 did not show a significant association with gout susceptibility (p = 0.0793, odds ratio [OR] with 95% confidential interval [CI] 1.11 [0.99–1.24]: Table 1). The frequency of the minor risk allele, which in this study was the T allele of rs2544390, in the gout cases (51.6%) was higher than in the controls (49.1%). However, a meta-analysis including previous studies [4, 6] with a Japanese population showed a significant association with gout (pmeta = 0.0314, OR with 95% CI 1.09 [1.01–1.18]; Fig. 1).
Fig. 1.
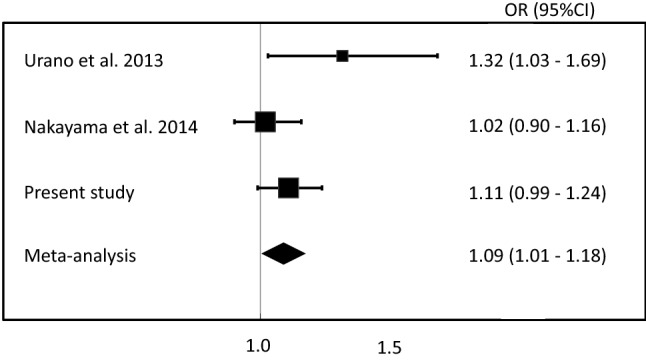
A meta-analysis of rs2544390 of LRP2 for gout in the Japanese male population. The meta-analysis was conducted using the present study and two previous studies of Japanese male populations (Urano et al. [3] and Nakayama et al. [5]). The OR in the meta-analysis was 1.09 (95% CI 1.01–1.18) and was statistically significant (pmeta = 0.0314), indicating a significant association between gout and the LRP2 gene. OR odds ratio, CI confidence interval
Discussion
We confirmed that rs2544390, an SNP of LRP2, has an association with gout in the Japanese population. LRP2 is a member of the low-density lipoprotein receptor [LDLR (MIM606945)] gene family and has been suggested to mediate the endocytosis of multiple ligands [14].
The association between rs2544390 and SUA was first identified by a GWAS of SUA in a Japanese population by Kamatani et al [3]; this was also confirmed by the recent genome-wide meta-analysis of SUA [15]. Since the association between LRP2 and SUA was reported, several association studies for the Japanese population have been conducted, because gout is a consequence of hyperuricemia. From Japanese male populations, Urano et al. [6] reported a positive association between gout and rs2544390 (p = 0.0250, OR with 95% CI 1.32 [1.03–1.69]) in 153 gout cases and 532 controls, whereas Nakayama et al. [4] reported no association with gout (p = 0.758, OR with 95% CI 1.02 [0.90–1.16]) in 741 gout cases and 1302 controls. It therefore remained an open question as to whether rs2544390 has an association with gout in the Japanese population.
In other studies with non-Japanese populations, Dong et al. [7] showed a positive association with a Chinese male population of 483 gout cases and 389 controls (p = 0.020, OR with 95% CI 1.26 [1.03–1.53]). Furthermore, their meta-analysis with the results of Nakayama et al. [4] and Urano et al. [6] revealed a positive association with gout (p = 0.019, OR with 95% CI 1.13 [1.02–1.24]) [7], which is consistent with the conclusions of our present study. Another study from New Zealand by Rasheed et al. [5] with 1431 gout cases and 1205 controls showed a negative association between rs2544390 and gout of all the participants including European Caucasian (p = 0.360, OR with 95% CI 1.06 [0.94–1.18]), in contrast to a sub-analysis of only populations of Māori and Pacific ancestry (p = 0.0090, OR with 95% CI 1.20 [1.05–1.38]). These results suggest there to be ethnic differences for gout risk due to the LRP2 variant and that Asia-Pacific populations should show a positive association between them.
Nakayama et al. [4], who previously reported no association between rs2544390 and gout, pointed out the need for further analysis with a greater number of samples to demonstrate any significant association between the LRP2 variant in question and gout. While the present association study also showed no association, our meta-analysis with a greater number of samples showed a significant positive association between rs2544390 and gout in the Japanese population.
In the present study, we have confirmed for the first time a significant positive association between rs2544390 and gout, which has been shown in other populations by Dong et al. [7] and Rasheed et al. [5], in a Japanese population.
SUA levels are regulated by urate transporters that are expressed in the kidney and intestines. Recent GWASs of clinically defined gout have revealed associations between gout and urate transporter genes, such as ABCG2/BCRP, URAT1/SLC22A12, GLUT9/SLC2A9, NPT1/SLC17A1 [16–19], all of which are expressed in the proximal tubular cells of the kidney [17]. Common variants of the urate excretion transporter ABCG2 are known to significantly elevate gout/hyperuricemia susceptibility and are a major cause of early onset gout [20–22]. ABCG2 is expressed in the human intestine and is associated with intestinal urate excretion in addition to renal urate excretion [23, 24]. Furthermore, some association analyses have also shown associations between gout and urate transporter genes URAT1 [25–27], NPT1 [27–29], OAT4/SLC22A11 [30] and OAT10/SLC22A13 [31], and scaffold protein genes PDZK1 [32] and LRRC16A [33], which bind to various transporters and form transportosomes with them. These genes, including urate transporter genes which are associated with SUA and gout/hyperuricemia, are mostly seen in the kidney, which is one of the urate-excreting organs. The LRP2 gene is also strongly expressed in the kidney [14, 34, 35].
In conclusion, the association between a mutation of LRP2 and gout is confirmed in addition to association with SUA. LRP2 is thought to have a possible association with urate regulation and gout susceptibility by playing an important role in urate mobilization via endocytosis. The clarification of the association between LRP2 and gout progression will lead to a better understanding of the molecular pathogenesis of gout and to novel therapeutic strategies against gout, using LRP2 as a molecular target. Our new insights into LRP2 have the potential to assist with the development of future personalized medicine.
Acknowledgements
The authors would like to thank all the participants for their generous contributions to this study. We are also grateful to members of the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) for their support. We are indebted to K. Gotanda, Y. Morimoto, M. Miyazawa and M. Takahashi for genetic analyses, T. Nakamura and M. Sakiyama for statistical analyses, and K. Ooyama, N. Hamajima, and K. Wakai for sample collection. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, including JSPS Kakenhi Grants (Nos. 16H06279, 16H01808, 17H04128, 18KK0247, 19K22786, and 25293145) and Grants-in-Aid for Scientific Research in Innovative Areas (No. 221S0002), the Ministry of Defense, the Kawano Masanori Memorial Foundation for Promotion of Pediatrics, and the Gout Research Foundation of Japan. The J-MICC Study was supported by Grants-in-Aid for Scientific Research from MEXT, including those for Priority Areas (No. 17015018) and Innovative Areas (No. 221S0001), as well as by JSPS Kakenhi Grant No. 16H06277.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the institutional ethical committees of National Defense Medical College (No. 2914) and Nagoya University (No. 2011-1248-7). All procedures were performed in accordance with the Declaration of Helsinki and its later amendments.
Informed consent
A written informed consent was obtained from each subject participating in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Airi Akashi and Akiyoshi Nakayama contributed equally to this work.
References
- 1.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout Nat Rev Dis Primers. 2019;5(1):69. doi: 10.1038/s41572-019-0115-y. [DOI] [PubMed] [Google Scholar]
- 3.Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42(3):210–215. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama A, Matsuo H, Shimizu T, Takada Y, Nakamura T, Shimizu S, et al. Common variants of a urate-associated gene LRP2 are not associated with gout susceptibility. Rheumatol Int. 2014;34(4):473–476. doi: 10.1007/s00296-013-2924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasheed H, Phipps-Green A, Topless R, Hollis-Moffatt JE, Hindmarsh JH, Franklin C, et al. Association of the lipoprotein receptor-related protein 2 gene with gout and non-additive interaction with alcohol consumption. Arthritis Res Ther. 2013;15(6):R177. doi: 10.1186/ar4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urano W, Taniguchi A, Inoue E, Sekita C, Ichikawa N, Koseki Y, et al. Effect of genetic polymorphisms on development of gout. J Rheumatol. 2013;40(8):1374–1378. doi: 10.3899/jrheum.121244. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Zhao D, Yang C, Zhou J, Qian Q, Ma Y, et al. Common variants in LRP2 and COMT genes affect the susceptibility of gout in a Chinese population. PLoS ONE. 2015;10(7):e0131302. doi: 10.1371/journal.pone.0131302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 9.Hamajima N, J-MICC Study Group The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev. 2007;8(2):317–323. [PubMed] [Google Scholar]
- 10.Nakayama A, Matsuo H, Shimizu T, Ogata H, Takada Y, Nakashima H, et al. Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum Cell. 2013;26(4):133–136. doi: 10.1007/s13577-013-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba T, Matsuo H, Sakiyama M, Nakayama A, Shimizu S, Wakai K, et al. Common variant of ALPK1 is not associated with gout: a replication study. Hum Cell. 2015;28(1):1–4. doi: 10.1007/s13577-014-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Development Core Team R. R: A language and environment for statistical computing. Vienna: R. Foundation for Statistical Computing; 2006. [Google Scholar]
- 13.Schwarzer G. Meta: meta-analysis with R. Berlin: Springer; 2014. [Google Scholar]
- 14.Marzolo MP, Farfan P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. 2011;44(1):89–105. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- 15.Nakatochi M, Kanai M, Nakayama A, Hishida A, Kawamura Y, Ichihara S, et al. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol. 2019;2(1):115. doi: 10.1038/s42003-019-0339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo H, Yamamoto K, Nakaoka H, Nakayama A, Sakiyama M, Chiba T, et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis. 2016;75(4):652–659. doi: 10.1136/annrheumdis-2014-206191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama A, Nakaoka H, Yamamoto K, Sakiyama M, Shaukat A, Toyoda Y, et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis. 2017;76(5):869–877. doi: 10.1136/annrheumdis-2016-209632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SJ, Tsai MH, Ko YC, Tsai PC, Chen CJ, Lai HM. The cyclic GMP-dependent protein kinase II gene associates with gout disease: identified by genome-wide analysis and case-control study. Ann Rheum Dis. 2009;68(7):1213–1219. doi: 10.1136/ard.2008.093252. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Li Z, Liu S, Wang C, Han L, Cui L, et al. Genome-wide association analysis identifies three new risk loci for gout arthritis in Han Chinese. Nat Commun. 2015;6:7041. doi: 10.1038/ncomms8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 21.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep. 2013;3:2014. doi: 10.1038/srep02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo H, Nakayama A, Sakiyama M, Chiba T, Shimizu S, Kawamura Y, et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci Rep. 2014;4:3755. doi: 10.1038/srep03755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi A, Urano W, Yamanaka M, Yamanaka H, Hosoyamada M, Endou H, et al. A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum. 2005;52(8):2576–2577. doi: 10.1002/art.21242. [DOI] [PubMed] [Google Scholar]
- 26.Sakiyama M, Matsuo H, Shimizu S, Nakashima H, Nakamura T, Nakayama A, et al. The effects of URAT1/SLC22A12 nonfunctional variants, R90H and W258X, on serum uric acid levels and gout/hyperuricemia progression. Sci Rep. 2016;6:20148. doi: 10.1038/srep20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phipps-Green AJ, Merriman ME, Topless R, Altaf S, Montgomery GW, Franklin C, et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Ann Rheum Dis. 2016;75(1):124–130. doi: 10.1136/annrheumdis-2014-205877. [DOI] [PubMed] [Google Scholar]
- 28.Urano W, Taniguchi A, Anzai N, Inoue E, Kanai Y, Yamanaka M, et al. Sodium-dependent phosphate cotransporter type 1 sequence polymorphisms in male patients with gout. Ann Rheum Dis. 2010;69(6):1232–1234. doi: 10.1136/ard.2008.106856. [DOI] [PubMed] [Google Scholar]
- 29.Chiba T, Matsuo H, Kawamura Y, Nagamori S, Nishiyama T, Wei L, et al. NPT1/SLC17A1 is a renal urate exporter in humans and its common gain-of-function variant decreases the risk of renal underexcretion gout. Arthritis Rheumatol. 2015;67(1):281–287. doi: 10.1002/art.38884. [DOI] [PubMed] [Google Scholar]
- 30.Sakiyama M, Matsuo H, Shimizu S, Nakashima H, Nakayama A, Chiba T, et al. A common variant of organic anion transporter 4 (OAT4/SLC22A11) gene is associated with renal underexcretion type gout. Drug Metab Pharmacokinet. 2014;29(2):208–210. doi: 10.2133/dmpk.dmpk-13-nt-070. [DOI] [PubMed] [Google Scholar]
- 31.Higashino T, Morimoto K, Nakaoka H, Toyoda Y, Kawamura Y, Shimizu S, et al. Dysfuctional missense variant of OAT10/SLC22A13 decreases gout risk and serum uric acid levels. Ann Rheum Dis. 2020;79:164–166. doi: 10.1136/annrheumdis-2019-216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higashino T, Matsuo H, Sakiyama M, Nakayama A, Nakamura T, Takada T, et al. Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: a replication study and meta-analysis in Japanese population. Drug Metab Pharmacokinet. 2016;31(6):464–466. doi: 10.1016/j.dmpk.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Sakiyama M, Matsuo H, Shimizu S, Chiba T, Nakayama A, Takada Y, et al. Common variant of leucine-rich repeat-containing 16A (LRRC16A) gene is associated with gout susceptibility. Hum Cell. 2014;27(1):1–4. doi: 10.1007/s13577-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 35.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]


