Abstract
Patient-reported outcome measures (PROMs) are commonly used to estimate disability of patients with spinal degenerative disease. Emerging technological advances present an opportunity to provide objective measurements of activity. In a prospective, observational study we utilized a low-cost consumer grade wearable accelerometer (LCA) to determine patient activity (steps per day) preoperatively (baseline) and up to one year (Y1) after cervical and lumbar spine surgery. We studied 30 patients (46.7% male; mean age 57 years; 70% Caucasian) with a baseline activity level of 5624 steps per day. The activity level decreased by 71% in the 1st postoperative week (p < 0.001) and remained 37% lower in the 2nd (p < 0.001) and 23% lower in the 4th week (p = 0.015). At no time point until Y1 did patients increase their activity level, compared to baseline. Activity was greater in patients with cervical, as compared to patients with lumbar spine disease. Age, sex, ethnic group, anesthesia risk score and fusion were variables associated with activity. There was no correlation between activity and PROMs, but a strong correlation with depression. Determining activity using LCAs provides real-time and longitudinal information about patient mobility and return of function. Recovery took place over the first eight postoperative weeks, with subtle improvement afterwards.
Subject terms: Physical examination, Rehabilitation, Outcomes research, Neuropathic pain, Skeletal muscle
Introduction
Patients with degenerative spine disease typically suffer from pain and functional disability, which negatively impact their activity levels and health-related quality of life (HRQoL). For a comprehensive patient evaluation, a variety of patient-reported outcome measures (PROMs) can be applied. These multi-dimensional questionnaires are considered the gold-standard of self-assessment1,2. However, these self-reported outcome measures are, at best, an approximation of pain and disability before and after surgery3.
More recently, a variety of functional tests have increasingly been applied to quantify objective functional impairment (OFI)4–7. In a systematic review of the literature, accelerometer and physical activity analyses using wearable devices ranked among the top five most frequently applied methods for objective outcome measurement in spine care4. To date, the activity levels of about 340 patients with lumbar degenerative diseases of the spine have been reported in nine research articles, which included patients with low back pain (LBP), lumbar spinal stenosis (LSS) and lumbar disc herniation (LDH)8–16.
Despite its great potential to contribute to the comprehensive patient evaluation, the current literature on activity tracking in spine patients remains limited. A recent systematic review confirmed that additional studies are needed to determine the strengths and weaknesses of using wearable devices in spine surgery17. To our knowledge, no prior studies have evaluated the use of wearable activity monitors to track postoperative recovery in patients with diseases of the cervical spine. Furthermore, prior studies involving patients with lumbar disorders have had small sample sizes and were restricted to short follow-up periods4,17. This study aimed at determining the feasibility of a low-cost consumer grade accelerometer (LCA) for objective assessment of pre- and postoperative step activity.
Methods
In a prospective feasibility study, we utilized LCAs to provide longitudinal activity-based outcome measurements for patients undergoing elective spine surgery. The study was approved by the institutional review board at Stanford University.
LCA technology
LCAs utilize three-dimensional accelerometers to sense user movement and can continuously measure home and community activity levels. For our study, the Mi Band (Xiaomi, Mountain View, CA (USA)) was utilized. While no device-specific data is available, a body of literature supports the notion that wearable LCAs are generally reliable and are valid indicators of overall physical activity in adults4.
At the time of study inclusion, patients were educated on LCA use by a study physician, including how to properly wear, maintain the device, and sync data to their mobile phones with Bluetooth technology. Patients were encouraged to wear the device as much as possible, though voluntary removal of the device for periods of time did not lead to exclusion from the study. The device was interrogated by study staff for preoperative data extraction and removed on the day of surgery. The device was reapplied immediately post-operatively. Data was extracted again on the day of discharge as well as at each follow-up visit for up to one year post-operatively.
Study Population
Over a 9-month period, we screened adult patients (≥18 years) presenting at our department’s outpatient spine clinic for the first evaluation of a degenerative condition of the subaxial cervical, thoracic or lumbar spine. We offered participation to those owning an iOS or Android smartphone (for compatibility with the Mi Fit app), scheduled for an elective surgical procedure under general anesthesia in more than one week.
Data collection
Activity tracking was conducted longitudinally. PROMs were assessed in the 30-day period before surgery (baseline), as well as at clinic visits at 3 months (M3) and 1 year (Y1) postoperatively. For this, we used our department’s integrated electronic medical record database that collects PROM data18, including a screening for depression using the Patient Health Questionnaire (PHQ-)2 for all patients, the Oswestry Disability Index (ODI) for patients with lumbar spine conditions and the Neck Disability Index (NDI) for patients with cervical spine disease.
The ODI and NDI are the most commonly used PROMs for patients with degenerative diseases of the spine, as they include many relevant aspects of life (pain, personal care, common activities) and their reliability and validity has repeatedly been demonstrated19–21. The PHQ-2 is a brief multipurpose instrument for screening, diagnosing, monitoring and measuring depression in patients and ranges from 0–6 points, with depression likely for scores ≥ 322.
Statistical analysis
Our dependent variable of interest was the mean number of steps per day, determined at baseline, as well as 1, 2, 4, 8, 12 (M3), 26 and 52 weeks (Y1) postoperatively. Descriptive analyses were used to report activity data (daily steps; mean and standard deviation (SD)), as well as feasibility and safety.
Independent variables were patient age (in years), sex, body mass index (BMI; obesity defined according to the WHO as BMI ≥ 30 kg/m2), ethnic background, smoking status (stratified into active/former smokers vs. never smokers), diagnosis, type of procedure (decompression vs. decompression/fusion), number of operated levels (single, two-level or multiple levels), anesthesia risk (American Society of Anesthesiologists (ASA) risk scale; stratified into low (ASA 1 or 2) and high (ASA 3)), as well as disability measures (ODI & NDI) at baseline and follow-up (see Table 1). To evaluate for depressive comorbidity, PHQ-2 scores ≥ 3 were used as recommended cut-offs22. The independent variables’ association with the activity levels at baseline and/or follow-up was tested using linear regression, student’s t-tests and analysis of covariance (ANOVA) models. As we found a marked difference between the activity levels in patients with cervical and lumbar spine disease, we adjusted for this in subsequent analyses using multivariable linear regression or multivariable analysis of covariance (MANOVA) models. Pearson correlation coefficients were calculated between PROMs and activity levels. Analyses were conducted using Stata v14.2 (College Station, Texas (USA)).
Table 1.
Basic demographic information.
| Variable | Result |
|---|---|
| Age in years | 57.1 (14.9) |
| Sex | |
| Female | 15 (50.0%) |
| Male | 15 (50.0%) |
| Ethnicity | |
| Caucasian | 21 (70.0%) |
| Hispanic | 4 (13.3%) |
| Black | 2 (6.7%) |
| Asian | 2 (6.7%) |
| Indian | 1 (3.3%) |
| BMI in kg/m2 | 28.8 (4.9) |
| Smoking status | |
| Active smoker | 1 (3.3%) |
| Former smoker | 10 (33.3%) |
| Never smoker | 19 (63.3%) |
| Depressive comorbidity * | |
| No | 18 (60.0%) |
| Yes | 11 (36.7%) |
| Unknown | 1 (3.3%) |
| Diagnosis | |
| Lumbar spondylolisthesis | 7 (23.3%) |
| LSS | 8 (26.7%) |
| LDH | 3 (10.0%) |
| CDH with radicular pain (soft) | 4 (13.3%) |
| Cervical foraminal stenosis with radicular pain (hard) | 3 (10.0%) |
| Cervical myelopathy for any reason | 5 (16.7%) |
| Surgical procedure | |
| Lumbar discectomy/decompression w/o instrumentation/fusion | 9 (30.0%) |
| Lumbar decompression w/ instrumentation/fusion | 9 (30.0%) |
| Anterior cervical discectomy (and fusion) ** | 9 (30.0%) |
| Posterior cervical decompression (and fusion) | 3 (10.0%) |
| ASA risk scale | |
| 1 | 2 (6.7%) |
| 2 | 18 (60.0%) |
| 3 | 10 (33.3%) |
| Levels treated | |
| 1 | 12 (40.0%) |
| 2 | 13 (43.3%) |
| ≥3 | 5 (16.7%) |
| Preoperative steps per day | 5624 (2776) |
| Total | n = 30 (100%) |
Results are presented as mean (SD) or count (%). ASA = American Society of Anesthesiologists; BMI = body mass index; CDH = cervical disc herniation; LDH = lumbar disc herniation; LSS = lumbar spinal stenosis.
*As determined by PHQ-2 ≥ 3 points.
**The group included two patients with motion preserving total disc arthroplasty.
According to the predefined protocol, we aimed to enroll at least 20 patients in this study. Findings with p ≤ 0.05 were considered statistically significant.
Ethical approval and informed consent
The study was approved by the institutional review board (IRB) at Stanford University and conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed written informed consent prior to inclusion.
Results
Feasibility
In the 9-month period, a total of 3742 outpatient spine clinic visits were conducted, of which 1614 were new patient visits. 607 (37.6%) eligible patients were scheduled for elective spine surgery. Of those, 48 patients were asked to participate (7.9%) and 42 were included. Seven were excluded for non-compliance with the study protocol and in 5 the planned surgery was cancelled. Therefore, 87.5% of patients (42/48) asked to participate in this study consented, and 81.1% of patients (30/37) who consented and underwent surgery provided objective outcome data.
Patient characteristics
Patient demographics are described in Table 1 (n = 30 patients; 46.7% male; mean age: 57.1 ± 14.9 years; 70% Caucasian). The mean baseline activity level was 5624 steps per day (SD 2776; median 5063). Objective outcome data was available for the entire cohort at baseline and at postoperative weeks 2 and 4. 29/30 patients provided outcome data at postoperative week 1, 26/30 at postoperative weeks 8, 21/30 at postoperative weeks 12 (M3) and 26, and 11/30 patients until the Y1 time point. There were no adverse events related to the device.
Activity tracking
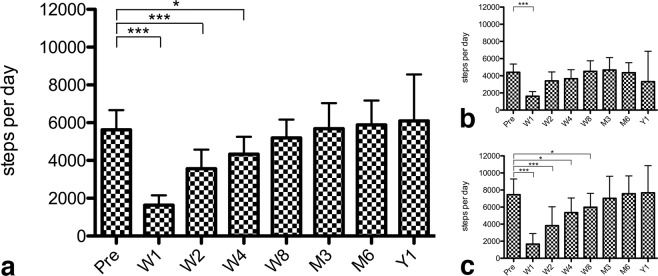
Figure 1 illustrates the change in the number of daily steps for the whole cohort. Compared to baseline, the activity level decreased by 71% to an average of 1635 steps per day (SD 1376; median 1219) in the 1st postoperative week (p < 0.001). It remained 37% lower in the 2nd (mean 3569, SD 2694; p < 0.001; median 3226) and 23% lower in the 4th postoperative week (mean 4335, SD 2469; p = 0.015; median 4487), but the difference lost statistical significance from the postoperative week 8 onwards until Y1 (Fig. 2a; all p > 0.05).
Figure 1.
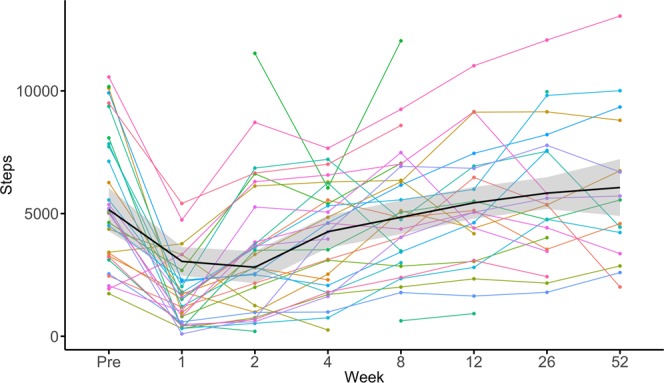
The line graph illustrates the absolute change in the daily steps for the total cohort of n = 30 patients with spine diseases. The x-axis indicates the time from preoperative (Pre) to 52 weeks postoperative. The y-axis indicates the number of steps per day. Individual patient data is illustrated as colored line. The mean of the total cohort is marked as black line with grey 95% confidence interval.
Figure 2.
Bar graphs with whiskers (95% confidence interval), illustrating the absolute change in the daily steps of the total cohort (a), n = 18 patients with lumbar spine diseases (b) or n = 12 patients with cervical spine disease (c). The x-axis indicates the time from preoperative (Pre) to 52 weeks postoperative. The y-axis indicates the mean number of steps. The levels of significance are indicated as *(p ≤ 0.05), **(p ≤ 0.005) or ***(p ≤ 0.001).
Cervical vs. lumbar disease
The mean daily number of steps of 18 patients with lumbar spine disease was 4403 (SD 1936; median 4055) at baseline. This activity decreased by 63% to 1615 (SD 1078; p < 0.001; median 1362) in the first postoperative week. From postoperative week 2 onwards throughout the remaining 1-year follow-up, the number of daily steps was within the range of the baseline assessment (all p > 0.05; Fig. 2b).
The mean daily number of steps of 12 patients with cervical spine disease was 7455 (SD 2903; median 7866) at baseline. This number decreased by 78% to 1667 (SD 1824; p < 0.001; median 953) in the first postoperative week and remained 49% lower during postoperative week 2 (mean 3820; SD 3491; p < 0.001; median 3226), 28% lower during postoperative week 4 (mean 5359; SD 2692; p = 0.015; median 5372) and 20% lower during postoperative week 8 with borderline significance (mean 5983; SD 2559; p = 0.050; median 6207). From M3 onwards throughout the remaining 1-year follow-up, the number of daily steps was within the range of the baseline assessment (all p > 0.05; Fig. 2c).
The mean daily number of steps was significantly greater for the cohort of patients with cervical, as compared to those with lumbar spine disorders at baseline (p = 0.002), as well as on postoperative weeks 26 (p = 0.006) and 52 (Y1; p = 0.05). Activity was comparable at the remaining time points (all p > 0.05).
Factors associated with baseline activity level
Patients with low ASA grade were significantly more active than patients with high ASA grade (6263 ± 2713 (SD) vs. 4345 ± 2562 (SD); p = 0.033). None of the other factors, such as age (p = 0.107), sex (p = 0.104), ethnic group (p = 0.107), obesity (p = 0.140) or smoking status (p = 0.729) were associated with baseline activity levels.
Factors associated with early postoperative mobility
In the first postoperative week, there was a tendency for 32 steps less per day for every year increase in patient age (Coef. −32, 95% CI −66 to 2; p = 0.067). The mean activity differed between ethnic groups (p = 0.039), with highest to lowest activity levels observed in patients of Indian, Black or African-American, Hispanic, Caucasian and Asian ethnicity. There was no significant difference in the number of steps per sex, obesity, smoking status, anesthesia risk or the number of operated levels (all p > 0.05). However, patients who underwent a spinal fusion procedure had a 48% lower activity level (1213 ± 1219 (SD) vs. 2325 ± 1390 (SD); p = 0.032) at the first postoperative week.
In the second postoperative week, every year increase in patient age was associated with lower patient activity by 75 steps (Coef. −75, 95% CI −138 to −11, p = 0.022). No other variable was found to be significantly associated with the number of steps (all p > 0.05).
At the postoperative week 4, 8 and at M3 time points, none of the variables were significantly associated with activity level.
Factors associated with long-term postoperative activity
At postoperative week 26, none of the variables of interest were significantly associated with patient activity. At the Y1 time point, there was a tendency for 158 steps less per day for every year increase in patient age (Coef. −158, 95% CI −328 to 12; p = 0.065), and female patients were more active than their male counterparts (8618 ± 2823 (SD) vs. 3058 ± 1575 (SD); p = 0.016). No other variables of interest were significantly associated with patient activity at Y1.
Convergent validity with PROMs
As shown in Table 2 and Figs. 1 and 2a–c, as ODI/NDI values decreased at M3 and Y1, the activity levels of patients increased. There was no significant association between the activity level and the ODI at baseline or Y1 in patients with lumbar spine disease (all p > 0.05). There was a tendency for moderate negative correlation between the patients’ activity level and the ODI at M3 (r = −0.569, p = 0.054). There was no significant association between the activity level and the NDI at baseline, M3 or Y1 in patients with cervical spine disease (all p > 0.05).
Table 2.
Subjective PROM and objective activity results at baseline (preoperative), as well as at postoperative week 12 (M3) and 52 (Y1).
| Variable | Preoperative | M3 | Y1 | p-value* |
|---|---|---|---|---|
| Cervical | ||||
| Steps per day, mean (SD) | 7455 (2903) | 7020 (3376) | 7678 (3432) |
#p = 0.755 $p = 0.119 |
| NDI, mean (SD) | 37.8 (14.9) | 27.9 (11.7) | 23.5 (16.3) |
#p = 0.071 $p = 0.036 |
| PHQ-2, mean (SD) | 2.3 (2.1) | 1.5 (1.9) | 0.8 (1.6) |
#p = 0.555 $p = 0.129 |
| Steps-ODI, Pearson correlation |
r = 0.063 p = 0.803 |
r = −0.046 p = 0.907 |
r = −0.083 p = 0.876 |
|
| Steps-PHQ-2, Pearson correlation |
r = −0.014 p = 0.957 |
r = −0.739 p = 0.036 |
r = −0.331 p = 0.785 |
|
| Lumbar | ||||
| Steps per day, mean (SD) | 4403 (1936) | 4679 (2265) | 3312 (2228) |
#p = 0.535 $p = 0.179 |
| ODI, mean (SD) | 45.1 (16.6) | 27.1 (17.6) | 25.5 (17.3) |
#p = 0.004 $p = 0.005 |
| PHQ-2, mean (SD) | 1.9 (2.3) | 0.3 (0.8) | 0.7 (0.8) |
#p = 0.396 $p = 0.083 |
| Steps-ODI, Pearson correlation |
r = −0.164 p = 0.612 |
r = −0.569 p = 0.054 |
r = −0.075 p = 0.926 |
|
| Steps-PHQ-2, Pearson correlation |
r = −0.525 p = 0.080 |
n/a** |
r = −0.762 p = 0.238 |
|
| Total cohort | ||||
| Steps-PHQ-2, Pearson |
r = −0.274 p = 0.143 |
r = −0.739 p = 0.003 |
r = −0.474 p = 0.283 |
|
The Pearson correlation coefficients (r) are illustrated to describe the relationship between steps per day and the ODI for patients with lumbar spine disease, and steps per day and NDI for patients with cervical spine disease at all three time points.
*#for comparison of preoperative to M3 and $ for comparison of preoperative to Y1.
**PHQ-2 was 0 points for all patients with available step data, rendering analysis impossible.
With regards to PHQ-2, there was a strong negative correlation between the patients’ activity level and the PHQ-2 score at M3 (r = −0.739, p = 0.003) for the total sample. There was no significant correlation between the activity levels at baseline or Y1 and the PHQ2 (all p > 0.05; see Table 2 for details).
Case vignettes
Illustrative case vignettes highlight the value of LCA-based activity tracking in patients with cervical spine disease (Fig. 3a–d), lumbar spine disease (Fig. 4a–e) and in a patient with a postoperative complication (Fig. 5a–e).
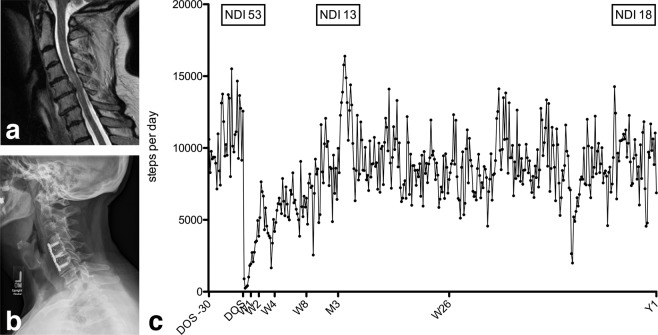
Figure 3.
Case vignette of a 69-year old female (BMI 36.2 kg/m2; ASA 3), who underwent uneventful C4-6 ACDF for cervical spondylitic myelopathy with pain, numbness/tingling and progressive dexterity issues (NDI 53). (a) The preoperative sagittal T2-weighted MRI gives evidence of increased signal intensity at the C4/5 level, consistent with myelopathy, and cervical stenosis at the C5/6 level. (b) Lateral x-rays at Y1 demonstrate a regular postoperative situation after C4-6 ACDF. (c) Individual activity data is illustrated over time, from 30 days before the day of surgery (DOS), over postoperative weeks (W) 1, 2, 4, 8, 12 (M3), 26 and 52 (Y1). At her M3 follow-up visits, she states a nearly 100% improvement of her preoperative symptoms (NDI 13). At her Y1 follow-up visit, she was still significantly better compared to baseline, but she reported a fall from a ladder about 6 months postoperative with minor neck and shoulder pain ever since (NDI 18). The objective activity tracking illustrates remarkable recovery over the first 12 postoperative weeks, with stagnation of further process afterwards.
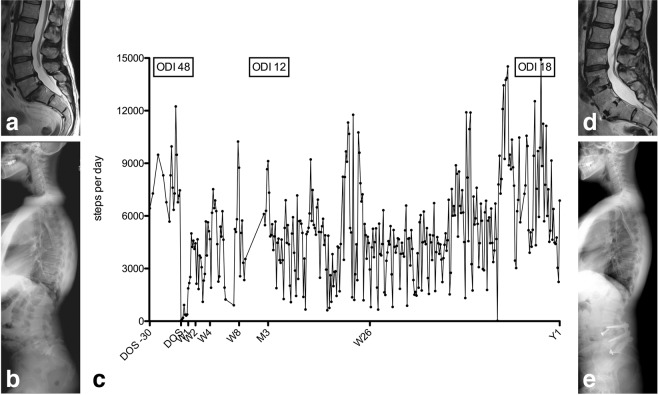
Figure 4.
Case vignette of a 48-year old female former gymnast (BMI 27.6 kg/m2; ASA 2), who underwent uneventful L4-S1 single-stage anterior lumbar interbody fusion and posterior spinal fusion for intractable low back pain and bilateral lower extremity pain (left > right) due to degenerative disc disease at L4-S1 with grade-1 isthmic spondylolisthesis at L5-S1 (ODI 48). (a) Preoperative sagittal T2-weighted MRI. (b) Preoperative long-cassette x-ray show a high pelvic incidence of 70° and a lumbar lordosis (LL) of 62° with preserved sagittal vertical alignment (SVA) of +3.5 cm. (c) Individual activity data is illustrated over time, from 30 days before the day of surgery (DOS), over postoperative weeks (W) 1, 2, 4, 8, 12 (M3), 26 and 52 (Y1). At her M3 follow-up visit the patient reports being 95% better compared to preoperative (ODI 12) and her standing x-rays are unremarkable. At her Y1 follow-up visit, she reports new onset of mild low back pain and some left-sided leg pain, translating into lesser activity and mild increase in the ODI (18). (d) The MRI at Y1 shows a solid fusion at the L4-S1 levels with adjacent segment disease, mild disc protrusion and facet disease at L3-4. The patient improved after additional epidural steroid injection at the L3-4 level and so far no additional surgical treatment was required. (e) Post-operative long-cassette x-ray show a LL of 72°, SVA of +1 cm.
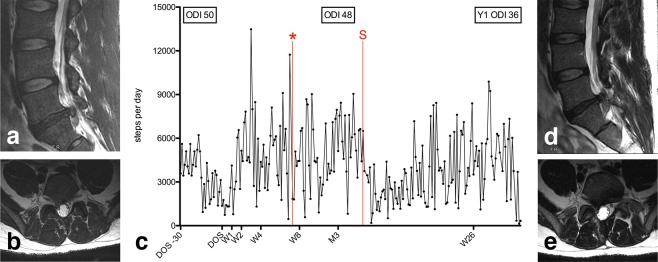
Figure 5.
A 33-year old male patient (BMI 26.2 kg/m2; ASA 2) underwent an uneventful right L5/S1 microdiscectomy for right S1-lumboradicular pain resulting from LDH and non-responsive to conservative management (ODI 50). (a,b) Preoperative sagittal (a) and axial (b) T2-weighted MRI. (c) Individual activity data is illustrated over time, from 30 days before the day of surgery (DOS), over postoperative weeks (W) 1, 2, 4, 8, 12 (M3) and 26. Initially, he experienced complete resolution of his symptoms. After a decrease in the activity level at the 1st postoperative week, he was able to regain (2nd week) and almost double his baseline activity at the 4th week. He then carried a 5-gallon (40-50lbs) jug of paint about seven weeks postoperative and noted new onset of low back and right-sided leg pain with a new plantarflexion weakness (*). A repeat MRI was consistent with a re-herniated right paramedian L5/S1 LDH (d) sagittal view; (e) axial view). The patient underwent repeat surgery (S) about 14 weeks after the initial surgery. The M3 ODI was 48 and the Y1 ODI 36. The patient decided against further objective step count measurements for this study beyond 217 days postoperative.
Discussion
Increased physical activity can lead to better physical and mental outcomes23–26, and level of physical activity can serve as a useful measure of overall health27. Postoperative mobility has long been touted as a reliable indicator of surgical recovery and good post-operative prognosis2,3,6,7,14,28–32. Most mobility data, especially in the perioperative setting, take the form of subjective self-reported questionnaires that suffer from recall bias, conformation to socially desirable responses, and are heavily influenced by mood, depression, anxiety, cognition, and disability3,33,34. Clinical assessments are static, discrete evaluations that can provide some measure of functional ability only at a single time-point. There is a profound lack of data reporting mobility in the perioperative period4,17,32.
In a prospective observational setting, we evaluated the activity levels of a sample of 30 patients before and up to 1 year after elective spine surgery. Objective activity monitoring was safe and well-tolerated by patients. Patients with lumbar spine disease showed generally lower activity levels, compared to patients with cervical spine disease. Activity levels significantly decreased after surgery, and recovery to baseline activity levels took place over the course of several weeks in both patients with cervical and lumbar spine disease. Patients regained their peak activity around 12 weeks postoperatively (M3); additional meaningful improvement after that time was unlikely and patients generally did not improve beyond their preoperative baseline levels. Even though the step count generally increased with progressive recovery documented by the PROMs, the direct statistical correlations between subjective and objective outcome measures were mostly weak and insignificant.
Feasibility and safety
Most patients (87.5%) who were approached about the study agreed to participate and the dropout rate of enrolled patients was fairly low (18.9%). Once included, no additional effort was needed to motivate patients continue wear the LCA, but we reminded patients to upload and send data at predefined intervals. The feasibility of using a LCA is supported by the high acceptance rate and affordability, making its distribution and the determination of solid objective activity results possible in a broader population. In the U.S., the Centers for Medicare and Medicaid Services (CMS) began reimbursing for remote patient monitoring in 2018 via the CPT code 99091, which is likely going to support the increasing use of objective outcome measures further.
There were no complications or (severe) adverse events recorded that could potentially be associated with the LCA use. The one patient who required repeat surgery for a re-herniated disc was involved in non-recommended high physical activity, as described in the case vignette (Fig. 5).
Accuracy, reliability & responsiveness
It was not the aim of this study to investigate or report on reliability measures of the LCA device. Reports from users, who checked the device against other step counters generally testify its accuracy, and research with similar devices found accuracy rates of around 98%10. However, more research is required to determine this and other devices’ accuracy and (test-retest) reliability measures17.
We found the daily step count to be a very responsive marker of overall physical activity, reflecting the reduced activity levels in the first 8–12 weeks after surgery well. Changes in activity were subtle after this time period, which likely mirrors the more gradual further improvement and rehabilitation often described for PROMs in a similar manner29,35. Our findings are in line with a prior study that found improved PROMs but no higher physical activity than baseline, six months after successful surgery for LSS36. The LCAs appeared suitable to detect worsening or low-level activity in patients with obvious unfavorable clinical courses (Fig. 5c). Understanding of responsiveness remains limited as most studies on wearable activity tracking devices only assessed patients at one time point pre- or postoperatively4,17, or included other longitudinal measures but did not analyze daily steps29. This report adds new long-term postoperative outcome data to the current understanding in this emerging field of research. Our current and some previous findings on objective activity tracking indicate that spine surgery increases patients’ capacity and capability (as measured by PROMs), but in order to change lifestyle and increase patient activity beyond the preoperative level additional rehabilitative interventions are needed36.
Association between LCA activity and PROMs
Cross-validating activity data with PROMs in lumbar spine patients (ODI) and cervical spine patients (NDI) revealed weak and non-significant correlation coefficients (Table 2). The current findings are in line with the results of prior studies suggesting weak to moderate correlations between subjective and objective outcome measures, if at all4–6,10,29,31,37,38. In their accelerometer study on n = 28 patients undergoing lumbar spine surgery, Mobbs et al. did not find a significant correlation between improvements in subjective clinical outcome and changes in physical activity measurements at follow-up10. Still, the increase in activity after surgery paralleling the recovery (Figs. 3c, 4c), or lack thereof (Fig. 5c) suggest that steps per day is a valid surrogate marker of outcome. Also, cervical spine patients started from and regained a higher activity level, and patients who underwent a fusion procedure were only half as active as those undergoing minimally-invasive decompression – both findings reflecting our daily clinical experience and suggesting that the objective outcome measurement is valid. They once more underline the fact that objective outcome measurement may not replace the subjective patient evaluation by PROMs, but rather contribute a new dimension to the comprehensive patient evaluation5,6,38.
It is likely that the small sample size, large inter-individual variability in activity, the heterogeneous patient sample, and the patient-specific time course of postoperative recovery made it difficult to formally ascertain the cross-validity of activity data with PROMs. Further studies employing LCAs in larger and more homogenous patient cohorts are needed to study this relationship in more detail. The fact that both patients after lumbar and cervical spine surgery showed a several week-long decrease in activity may indicate that the disability & mobility restriction results less from incisional pain but possibly from general postoperative fatigue or factors not assessed in this study.
It should also be noted that we commonly recommend patients to “take it easy” during the initial 6–8 postoperative weeks, and the reduced activity could be a sign of patient compliance rather than spine-related mobility restriction. After the first 6–8 weeks, all patients received physiotherapy (PT) prescriptions for isometric core muscle strengthening, followed by increasing range of motion and activity. The exact time of starting with PT was not recorded for the scope of this research and therefore its effect on activity could not be measured.
Assessment in patients with depression
The mental health condition of patients with degenerative spine disease is known to bias the subjective, PROM-based outcome assessment39–41. One of the hopes of objective outcome measures is to provide an accurate determination of functional outcome, irrespective of the mental health condition42. In our patient population, depression was associated with reduced physical activity levels at M3 (r = −0.739, p = 0.003). Our findings are consistent with the literature. Ludwig et al. used the PHQ-9 to evaluate 1742 adult patients with cardiovascular disease and showed the daily step count of depressed patients differed significantly (p < 0.001) with moderate to severe depressive symptom patients walking 13.3% (95% CI 18.8%–7.9%) and 15.6% (95% CI 23.7%–6.5%) less23.
Future implications
Recent advances have produced smaller, cheaper, lighter, and smarter devices that have been marketed directly to consumers and have gained widespread popularity4,43. Accelerometers are increasingly integrated into smartphones, smartwatches, and other wearable digital electronics. The objective determination of functional outcome is preferred over questionnaire-based evaluations by most patients with lumbar spine disease44, and tools for the unobtrusive longitudinal assessment of functional outcome have the potential to lower the missing data burden inherent to PROM-based research: an analysis of 13 large prospective spine registries found loss of follow-up rates to range between 21–78%45.
Incentive-based interventions made possible by immediate feedback, Internet or social media have been shown to effectively modify health behavior15,46,47. This is of particular interest for post-operative patients, as improvement in activity levels beyond the preoperative baseline was infrequent (Figs. 1 and 2a–c). Even though disability and pain was improved after surgery, our patients did not maximize the benefits of the intervention by walking more steps. This finding points towards the potential of rehabilitation strategies. Further potential lies in the early detection of complications and/or unfavorable treatment outcomes in patients with decline in activity or lack of postoperative improvement48.
It must be emphasized that the use of LCAs in spine health is likely still in its relative infancy. While PROMs remain the gold standard of outcome assessment, other objective outcome measures such as the Timed-Up and Go (TUG) test5,37,38,42,49,50, the 6-minute walking test (6WT)7,29, the 5-Repetitions Sit-To-Stand (5R-STS) test6 or the Self-Paced Walking Test (SPWT)31,51 are better studied and validated4,17,32. A wide range of activity tracking devices are available on the market enabling objective activity tracking as an experimental, additional outcome marker for now. Our present analysis suggests that daily steps are a sensitive, but nonspecific surrogate marker of spine-related mobility restriction.
Future randomized trails might benefit from implementing both subjective PROM-based and objective outcome measures in order to estimate patient outcome as accurately as possible and herewith support therapeutic decision-making.
Strengths & limitations
This study adds new data to the existing literature on objective activity and outcome analysis. Despite our small sample size, we exceeded most previous similar studies with regards to included patients and length of follow-up4,10,15,17,29,32,51,52. In addition to the objective activity tracking, several well-validated PROMs were applied to assess outcome on subjective scales. Finally, this is the first study to include cervical spine patients and to compare activity levels of patients with degenerative lumbar versus cervical spine diseases.
Certainly, a weakness of this study is the small percentage of potentially eligible patients that were finally asked to participate in this study. The single most important reason behind non-inclusion was lack of dedicated study personnel; incompatibility issues between the LCA and smartphone device were infrequent. A further implication of the sample size and heterogeneity is that we refrain from going into more detail regarding the observed relationship of patient characteristics (e.g., ASA score, obesity, etc.) and objective mobility data. As with many other studies35,45, also this study was impacted by loss of follow-up and inherent missing data in the range of 0–30% at short and 3–63% at long-term follow-up. The inclusion of a heterogeneous patient sample allowed us to gain insights into recovery for a wide range of disease types. Further research should concentrate on characterizing short- and long-term recovery for more well-defined populations. Lastly, we utilized simplified summary data for the main analyses; a more detailed review of day-to-day activity data as illustrated in Figs. 3c, 4c and 5c might identify additional important factors impacting spine-related mobility.
Conclusion
This prospective observational study provides novel insight into the objective activity levels of patients before and after cervical and lumbar spine surgery. Activity tracking provided evidence for progressive recovery over the first 8–12 postoperative weeks, but improvement beyond the preoperative activity level was uncommon up to the Y1 follow-up. The correlation between daily steps and subjective outcome measures was weak, indicating that activity tracking should not replace PROMs but rather add another dimension to the comprehensive outcome assessment of patients undergoing spine surgery.
Author contributions
M.N.S. – data collection, statistical analysis & figures, result interpretation, drafting the first version of the article. P.G.R. – data collection, statistical analysis & figures, result interpretation, revising the article. A.L.H. – organizing funding, data collection, statistical analysis & figures, result interpretation, revising the article. A.V. – contributing patients, data collection, result interpretation, revising the article. C.C.Z. – result interpretation, revising the article. C.T.L. – result interpretation, revising the article. J.P. – contributing patients, data collection, result interpretation, revising the article. J.K.R. – contributing patients, data collection, result interpretation, revising the article. A.M.D. – conceptualizing the project, organizing funding, contributing patients, data collection, result interpretation, revising the article.
Data availability
All data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Martin N. Stienen, Paymon G. Rezaii and Allen L. Ho.
References
- 1.Dansie EJ, Turk DC. Assessment of patients with chronic pain. British Journal of Anaesthesia. 2013;111:19–25. doi: 10.1093/bja/aet124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deyo RA, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine. 1998;23:2003–2013. doi: 10.1097/00007632-199809150-00018. [DOI] [PubMed] [Google Scholar]
- 3.Gautschi OP, Corniola MV, Schaller K, Smoll NR, Stienen MN. The need for an objective outcome measurement in spine surgery–the timed-up-and-go test. The Spine Journal: Official Journal of the North American Spine Society. 2014;14:2521–2522. doi: 10.1016/j.spinee.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Stienen MN, et al. Objective measures of functional impairment for degenerative diseases of the lumbar spine: a systematic review of the literature. The Spine Journal: Official Journal of the North American Spine Society. 2019;19:1276–1293. doi: 10.1016/j.spinee.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Gautschi OP, et al. Validity and Reliability of a Measurement of Objective Functional Impairment in Lumbar Degenerative Disc Disease: The Timed Up and Go (TUG) Test. Neurosurgery. 2016;79:270–278. doi: 10.1227/NEU.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 6.Staartjes, V. E. & Schroder, M. L. The five-repetition sit-to-stand test: evaluation of a simple and objective tool for the assessment of degenerative pathologies of the lumbar spine. Journal of neurosurgery. Spine, 1–8, 10.3171/2018.2.SPINE171416 (2018). [DOI] [PubMed]
- 7.Stienen, M. N. et al. Reliability of the 6-minute Walking Test Smartphone Application. Journal of Neurosurgery (2019). [DOI] [PubMed]
- 8.Conway J, Tomkins CC, Haig AJ. Walking assessment in people with lumbar spinal stenosis: capacity, performance, and self-report measures. The Spine Journal: Official Journal of the North American Spine Society. 2011;11:816–823. doi: 10.1016/j.spinee.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisser ME, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. The Clinical Journal of Pain. 2007;23:780–785. doi: 10.1097/AJP.0b013e31815349bf. [DOI] [PubMed] [Google Scholar]
- 10.Mobbs RJ, Phan K, Maharaj M, Rao PJ. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine Journal. 2016;6:459–464. doi: 10.1055/s-0035-1565259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norden J, Smuck M, Sinha A, Hu R, Tomkins-Lane C. Objective measurement of free-living physical activity (performance) in lumbar spinal stenosis: are physical activity guidelines being met? The Spine Journal: Official Journal of the North American Spine Society. 2017;17:26–33. doi: 10.1016/j.spinee.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pryce R, et al. Relationship between ambulatory performance and self-rated disability in patients with lumbar spinal stenosis. Spine. 2012;37:1316–1323. doi: 10.1097/BRS.0b013e31824a8314. [DOI] [PubMed] [Google Scholar]
- 13.Schulte TL, et al. Step activity monitoring in lumbar stenosis patients undergoing decompressive surgery. European spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2010;19:1855–1864. doi: 10.1007/s00586-010-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomkins-Lane CC, Conway J, Hepler C, Haig AJ. Changes in objectively measured physical activity (performance) after epidural steroid injection for lumbar spinal stenosis. Archives of Physical Medicine and Rehabilitation. 2012;93:2008–2014. doi: 10.1016/j.apmr.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Tomkins-Lane CC, et al. The spinal stenosis pedometer and nutrition lifestyle intervention (SSPANLI): development and pilot. The Spine Journal: Official Journal of the North American Spine Society. 2015;15:577–586. doi: 10.1016/j.spinee.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Zheng CF, et al. Correlations of Japanese Orthopaedic Association Scoring Systems with Gait Parameters in Patients with Degenerative Spinal Diseases. Orthopaedic Surgery. 2016;8:447–453. doi: 10.1111/os.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravorty A, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskeletal Disorders. 2019;20:288. doi: 10.1186/s12891-019-2663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azad TD, et al. Building an electronic health record integrated quality of life outcomes registry for spine surgery. Journal of Neurosurgery. Spine. 2016;24:176–185. doi: 10.3171/2015.3.SPINE141127. [DOI] [PubMed] [Google Scholar]
- 19.Fairbank, J. C. & Pynsent, P. B. The Oswestry Disability Index. Spine25, 2940–2952; discussion 2952 (2000). [DOI] [PubMed]
- 20.Joswig H, Neff A, Ruppert C, Hildebrandt G, Stienen MN. Repeat epidural steroid injections for radicular pain due to lumbar or cervical disc herniation: what happens after ‘salvage treatment’? The Bone & Joint Journal. 2018;100-B:1364–1371. doi: 10.1302/0301-620X.100B10.BJJ-2018-0461.R1. [DOI] [PubMed] [Google Scholar]
- 21.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. Journal of Manipulative and Physiological Therapeutics. 1991;14:409–415. [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Medical Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig VM, et al. Association between depressive symptoms and objectively measured daily step count in individuals at high risk of cardiovascular disease in South London, UK: a cross-sectional study. BMJ Open. 2018;8:e020942. doi: 10.1136/bmjopen-2017-020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuenyongchaiwat K. Effects of 10,000 steps a day on physical and mental health in overweight participants in a community setting: a preliminary study. Brazilian Journal of Physical Therapy. 2016;20:367–373. doi: 10.1590/bjpt-rbf.2014.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Physical Activity Guidelines Advisory Committee report, 2008 To the Secretary of Health and Human Services. Part A: executive summary. Nutrition Reviews. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 26.Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. The International Journal of Behavioral Nutrition and Physical Activity. 2010;7:39. doi: 10.1186/1479-5868-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maddocks M, et al. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1539–1544. doi: 10.1007/s00520-009-0776-2. [DOI] [PubMed] [Google Scholar]
- 28.Chapman JR, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine. 2011;36:S54–68. doi: 10.1097/BRS.0b013e31822ef74d. [DOI] [PubMed] [Google Scholar]
- 29.Loske, S. et al. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. The Spine Journal: Official Journal of the North American Spine Society, 10.1016/j.spinee.2018.04.016 (2018). [DOI] [PubMed]
- 30.Smeets RJ, Hijdra HJ, Kester AD, Hitters MW, Knottnerus JA. The usability of six physical performance tasks in a rehabilitation population with chronic low back pain. Clinical Rehabilitation. 2006;20:989–997. doi: 10.1177/0269215506070698. [DOI] [PubMed] [Google Scholar]
- 31.Tomkins CC, Battie MC, Rogers T, Jiang H, Petersen S. A criterion measure of walking capacity in lumbar spinal stenosis and its comparison with a treadmill protocol. Spine. 2009;34:2444–2449. doi: 10.1097/BRS.0b013e3181b03fc8. [DOI] [PubMed] [Google Scholar]
- 32.Anderson, D. B. et al. Measurement properties of walking outcome measures for neurogenic claudication: a systematic review and meta analysis. The Spine Journal: Official Journal of the North American Spine Society, 10.1016/j.spinee.2019.04.004 (2019). [DOI] [PubMed]
- 33.Rikli RE. Reliability, validity, and methodological issues in assessing physical activity in older adults. Research Quarterly for Exercise and Sport. 2000;71(Suppl 2):89–96. doi: 10.1080/02701367.2000.11082791. [DOI] [PubMed] [Google Scholar]
- 34.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research Quarterly for Exercise and Sport. 2000;71(Suppl 2):1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 35.Stienen MN, et al. Influence of Body Mass Index on Subjective and Objective Measures of Pain, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disc Disease. World Neurosurgery. 2016;96:570–577 e571. doi: 10.1016/j.wneu.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 36.Smuck M, et al. Objective measurement of function following lumbar spinal stenosis decompression reveals improved functional capacity with stagnant real-life physical activity. The Spine Journal: Official Journal of the North American Spine Society. 2018;18:15–21. doi: 10.1016/j.spinee.2017.08.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautschi OP, et al. Pre- and postoperative correlation of patient-reported outcome measures with standardized Timed Up and Go (TUG) test results in lumbar degenerative disc disease. Acta Neurochirurgica. 2016;158:1875–1881. doi: 10.1007/s00701-016-2899-9. [DOI] [PubMed] [Google Scholar]
- 38.Stienen MN, et al. Validation of the baseline severity stratification of objective functional impairment in lumbar degenerative disc disease. Journal of Neurosurgery. Spine. 2017;26:598–604. doi: 10.3171/2016.11.SPINE16683. [DOI] [PubMed] [Google Scholar]
- 39.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 40.Falavigna A, et al. Responsiveness of depression and its influence on surgical outcomes of lumbar degenerative diseases. European Journal of Orthopaedic Surgery & Traumatology: Orthopedie Traumatologie. 2015;25(Suppl 1):S35–41. doi: 10.1007/s00590-015-1651-0. [DOI] [PubMed] [Google Scholar]
- 41.Chaichana KL, Mukherjee D, Adogwa O, Cheng JS, McGirt MJ. Correlation of preoperative depression and somatic perception scales with postoperative disability and quality of life after lumbar discectomy. Journal of Neurosurgery. Spine. 2011;14:261–267. doi: 10.3171/2010.10.SPINE10190. [DOI] [PubMed] [Google Scholar]
- 42.Stienen MN, et al. Influence of the mental health status on a new measure of objective functional impairment in lumbar degenerative disc disease. The Spine Journal: Official Journal of the North American Spine Society. 2017;17:807–813. doi: 10.1016/j.spinee.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Medical Informatics and Decision Making. 2012;12:67. doi: 10.1186/1472-6947-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joswig H, et al. Patients’ Preference of the Timed Up and Go Test or Patient-Reported Outcome Measures Before and After Surgery for Lumbar Degenerative Disk Disease. World Neurosurgery. 2017;99:26–30. doi: 10.1016/j.wneu.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 45.McGirt MJ, et al. Role of prospective registries in defining the value and effectiveness of spine care. Spine. 2014;39:S117–128. doi: 10.1097/BRS.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 46.Kurti AN, Dallery J. Internet-based contingency management increases walking in sedentary adults. Journal of Applied Behavior Analysis. 2013;46:568–581. doi: 10.1002/jaba.58. [DOI] [PubMed] [Google Scholar]
- 47.Washington WD, Banna KM, Gibson AL. Preliminary efficacy of prize-based contingency management to increase activity levels in healthy adults. Journal of Applied Behavior Analysis. 2014;47:231–245. doi: 10.1002/jaba.119. [DOI] [PubMed] [Google Scholar]
- 48.Akeret K, et al. Time to be “smart”-Opportunities Arising From Smartphone-Based Behavioral Analysis in Daily Patient Care. Frontiers in Behavioral Neuroscience. 2018;12:303. doi: 10.3389/fnbeh.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautschi OP, et al. The timed up and go test for lumbar degenerative disc disease. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 2015;22:1943–1948. doi: 10.1016/j.jocn.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 50.Gautschi OP, et al. Assessment of the Minimum Clinically Important Difference in the Timed Up and Go Test After Surgery for Lumbar Degenerative Disc Disease. Neurosurgery. 2017;80:380–385. doi: 10.1227/NEU.0000000000001320. [DOI] [PubMed] [Google Scholar]
- 51.Tomkins-Lane CC, Battie MC, Macedo LG. Longitudinal construct validity and responsiveness of measures of walking capacity in individuals with lumbar spinal stenosis. The Spine Journal: Official Journal of the North American Spine Society. 2014;14:1936–1943. doi: 10.1016/j.spinee.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 52.Lee SI, et al. Objectively quantifying walking ability in degenerative spinal disorder patients using sensor equipped smart shoes. Medical Engineering & Physics. 2016;38:442–449. doi: 10.1016/j.medengphy.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author upon reasonable request.