Abstract
Spontaneous wine fermentation is characterized by yeast population evolution, modulated by complex physical and metabolic interactions amongst various species. The contribution of any given species to the final wine character and aroma will depend on its numerical persistence during the fermentation process. Studies have primarily evaluated the effect of physical and chemical factors such as osmotic pressure, pH, temperature and nutrient availability on mono- or mixed-cultures comprising 2–3 species, but information about how interspecies ecological interactions in the wine fermentation ecosystem contribute to population dynamics remains scant. Therefore, in the current study, the effect of temperature and sulphur dioxide (SO2) on the dynamics of a multi-species yeast consortium was evaluated in three different matrices including synthetic grape juice, Chenin blanc and Grechetto bianco. The population dynamics were affected by temperature and SO2, reflecting differences in stress resistance and habitat preferences of the different species and influencing the production of most volatile aroma compounds. Evidently at 15 °C and in the absence of SO2 non-Saccharomyces species were dominant, whereas at 25 °C and when 30 mg/L SO2 was added S. cerevisiae dominated. Population growth followed similar patterns in the three matrices independently of the conditions. The data show that fermentation stresses lead to an individual response of each species, but that this response is strongly influenced by the interactions between species within the ecosystem. Thus, our data suggest that ecological interactions, and not only physico-chemical conditions, are a dominant factor in determining the contribution of individual species to the outcome of the fermentation.
Subject terms: Applied microbiology, Microbial ecology
Introduction
Alcoholic fermentation of grape juice or “grape must” is commonly initiated by complex microbial consortia composed of oxidative, weakly fermentative and strongly fermentative species1–4. During the fermentation process, and dependent on the specific microbiota composition of each must, weakly fermentative yeast species such as Pichia terricola, Metschnikowia pulcherrima and Hanseniaspora uvarum are usually replaced by more strongly fermentative species such as Lachancea thermotolerans and Torulaspora delbrueckii, while Saccharomyces cerevisiae will generally complete the process when alcohol levels are high and oxygen is depleted5–10. Many of these weakly and more strongly fermentative species, however, can contribute significantly to the final composition of wine, and impact the sensory perception of the product11–13. The contribution of each individual species will depend on its numerical presence and length of persistence throughout alcoholic fermentation. These two parameters, i.e absolute numerical presence, and persistence over time, are affected by parameters such as temperature, sulphur dioxide (SO2), pH, osmotic pressure, oxygen, and nutrient availability, and the response of individual species to these parameters has in many cases been investigated, either in single species systems or in co-cultivation with S. cerevisiae11,14–20. The results indicate that abiotic parameters such as oxygen, temperature and SO2 addition strongly affect the fermentation kinetics and yeast population dynamics of the wine fermentation ecosystem16,21–28. For instance, low temperatures (10–15 °C) have been shown to enhance the growth of some non-Saccharomyces species such as Starmerella bacillaris, formerly Candida stellata29, L. thermotolerans12 and Hanseniaspora uvarum30,31 whereas, fermentation at 25–30 °C has been shown to favour the growth of S. cerevisiae28,32. Addition of 30–50 mg/L SO2 as an antiseptic agent to must is a common practice in the wine industry that aims to suppress the growth of undesired yeast and bacterial species such as Brettanomyces bruxellensis, Lactobacillus and Pediococcus species19,24,25,32,33. Previous studies suggest that SO2 addition may positively or negatively affect growth of several yeast species during wine fermentation. For instance, SO2 addition of between 40–80 mg/L has been shown to negatively affect growth of H. uvarum, L. thermotolerans and T. delbrueckii while supporting growth of S. cerevisiae25,34–36.
To this end, few studies have attempted to elucidate the impact of ecological interactions between species and of the broader wine ecosystem on the survival and persistence of individual species and their response to temperature and SO2. Recent data suggest that such biotic impacts may be significant and that their role in defining population dynamics and fermentation outcomes may have been underestimated. A number of recent articles, for instance, suggest that physical contact between species has significant impact on the performance of these species37,38. The data by Rossouw et al.38 suggest that modifying the level of physical contact will lead to complex changes in population dynamics, either favouring or inhibiting certain species. Other ecological interactions have been reported12,20,28. For instance, Alonso del Real et al.28 showed that cryotolerant Saccharomyces yeasts, such as Saccharomyces uvarum, compromise the relative fitness of S. cerevisiae at lower temperatures. Bagheri et al.8,13 reported that the presence of certain species can be detrimental to, or may improve, the survival and numerical persistence of other yeast species within the wine ecosystem.
Taken together, the data suggest that biotic selection pressure exerted by other species is a strong driver of population dynamics that has been mostly ignored in literature thus far. The wine ecosystem constitutes likely a good model system to evaluate such impacts since a relatively limited number of yeast species tends to dominate the system, and interactions between these species have occurred within an evolutionarily significant framework. It is therefore highly likely that ecological interspecies interactions within this system would have shaped the genetic makeup and phenotypes of many of these species. Thus, it is essential to ascertain to what degree the specific species composition of any given wine yeast ecosystem will modulate the growth and survival of individual species. The current study aims to evaluate the response of a constructed multispecies wine yeast ecosystem to temperature and SO2 addition during alcoholic fermentation of three different grape matrices.
Materials and Methods
Yeast consortium and culture conditions
The yeast consortium comprising seven yeast strains obtained from the culture collection of the Institute for Wine Biotechnology (IWBT) and a commercial yeast S. cerevisiae Lalvin EC1118 (Lallemand, Canada) was prepared (Table 1) as described by Bagheri et al.8. The yeast stock cultures were maintained in 20% (v/v) glycerol at −80 °C and were streaked out on Wallerstein Laboratory Nutrient agar (WLN) (Sigma–Aldrich, Spain) when required. The plates were incubated at 30 °C for 3–5 days.
Table 1.
Strains used in the multi-species yeast consortium.
| Species | Strains code | Strains number |
|---|---|---|
| Hanseniaspora vineae | Hv | Y980 |
| Pichia terricola | Pt | Y974 |
| Starmerella bacillaris | Sb | Y975 |
| Candida parapsilosis | Cp | Y842 |
| Lachancea thermotolerans | Lt | Y973 |
| Saccharomyces cerevisiae | Sc | EC1118 |
| Wickerhamomyces anomalus | Wa | Y934 |
| Metschnikowia pulcherrima | Mp | Y981 |
The strain codes are the abbreviation of the name of each strain.
Micro-fermentations
Fermentations were performed in synthetic grape juice (SGJ) medium, Chenin blanc (CHN) and Grechetto bianco (GRC) grape juice. The SGJ medium adapted from Henschke and Jiranek39, and Bely et al.40, contained 200 g/L sugars (100 g/L glucose and 100 g/L fructose) and 300 mg/L assimilable nitrogen (460 mg/L NH4Cl and 180 mg/L amino acids), and was adjusted to pH 3.5.
The effect of temperature (15, 25 and 30 °C) and SO2 (30 mg/L) on the dynamics of the yeast consortium (Table 1) was evaluated in the presence (NS-SC) and absence of S. cerevisiae (NS), as illustrated in Fig. 1. Non-Saccharomyces yeast species were inoculated at 106 cfu/mL while S. cerevisiae was inoculated at 103 cfu/mL in 500 mL of juice in NS-SC fermentations whereas the six Non-Saccharomyces species were each inoculated at 106 cfu/mL in NS fermentation. Static fermentations were conducted in Erlenmeyer flasks fitted with fermentation locks. The samples were withdrawn at 2-day intervals to monitor the fermentation kinetics. Glucose and fructose were measured, using enzymatic kits, Enzytec™Fluid D-glucose (E5140), fructose (E5120) (Boehringer Mannheim, R-biopharm, Darmstadt, Germany). The fermentations were considered dry when the residual sugar (glucose and fructose) was less than 2 g/L.
Figure 1.
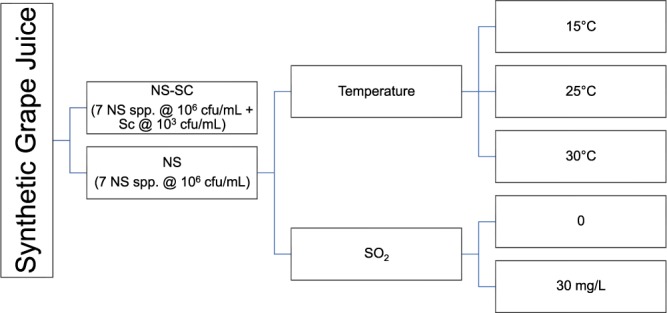
Schematic representation of fermentations conducted in the synthetic juice. Non-Saccharomyces species (NS) were either inoculated alone at 106 cfu/mL (NS) or together with S. cerevisiae added at 103 cfu/mL (NS-SC).
For the Chenin blanc and Grechetto bianco fermentations, fifty liters of clarified juice was obtained from a commercial cellar in South Africa and Italy, respectively. Furthermore, viable counts on Wallerstein Nutrient (WLN) agar were performed to enumerate the natural yeast population in each grape juice. Yeast species were isolated and identified by PCR amplification, Restriction fragment length polymorphisms, and sequencing of the ITS-5.8S rRNA gene as described in Bagheri et al.3. A total of 184 and 212 isolates were obtained from Chenin blanc and Grechetto bianco, respectively.
The Chenin blanc juice was divided into 1.5 L samples and fermentations were conducted in 2 L fermentation bottles in South Africa whereas, in Italy, based on equipment availability, the fermentations were conducted by dividing juice in 500 mL Erlenmeyer flasks. Fermentations were conducted at 15 °C and 25 °C, with 30 mg/L SO2 and without SO2. Three sets of static fermentations were conducted in triplicate: (i) spontaneous (SP), (ii) inoculated with S. cerevisiae EC1118 at 103 cells/mL (EC) and (iii) inoculated with a consortium of 7 non-Saccharomyces yeast strains at 106 cells/mL and S. cerevisiae EC1118 at 103 cells/mL (NS-SC). Fermentation progress was determined by monitoring CO2 release and sugar consumption throughout fermentation. Fermentations were considered complete when residual sugar was less than 2 g/L.
Analysis of yeast population dynamics
Two mL of synthetic and grape must samples were withdrawn to analyze the population species composition at different stages of the fermentation. Four stages of fermentation including the first day of fermentation (T0) for spontaneous fermentation or the inoculation day (IN) for inoculated fermentations, and the beginning (BF), middle (MF) and end (EF) of fermentations, were selected as sampling points, with BF defined as between 10–20%, MF between 40–60% and EF as more than 95% of sugar consumed, respectively. In case of stuck fermentations, the last sampling point was considered as the end of fermentation. Population dynamics in synthetic must fermentations were monitored using ARISA analysis as previously described in Bagheri et al.8. We evaluated the efficiency of four different DNA extraction methods as previously described by Siret et al.41, Garcia-Beneytez et al.42, Savazzini et al.43, and Sambrook and Russell44. However, low quantity (≤15 ng/uL) and quality (260/280 ratios ≤1) DNA were obtained in all scenarios. An efficient DNA extraction from grape and wine has remained a challenge in grape and wine research; such difficulties have been attributed to the presence of large quantities of polyphenols and polysaccharides45,46. In order to eliminate the potential bias introduced by the low quality of DNA, the population dynamics of grape must fermentations were also monitored by direct plating on WL-agar.
Chemical analysis
Sugar concentrations throughout the fermentation were determined using enzymatic kits, Enzytec™Fluid d-glucose (E5140), d-fructose (E5120) (Boehringer Mannheim, R-biopharm, Darmstadt, Germany). The chemical composition of Chenin blanc and Grechetto bianco juice was analyzed by Fourier transform infrared (FT-IR) spectroscopy using the GrapeScan 2000 instrument (Foss Electric, Denmark). Twenty-four hours after the addition of SO2 (30 mg/L) in grape juice, the concentration of free and bound SO2 was measured, using Ripper method (SM Titrino 702, Metrohm, Switzerland). The yeast species were inoculated 24 hours after SO2 addition.
Major volatile compounds were determined using GC-FID as described by Louw et al.47. Briefly, the extraction was carried out by adding 4-methyl-2-pentanol with a final concentration of 5 mg/L, as the internal standard and 1 mL diethyl ether to each sample. This was followed by sonication for 5 min and centrifugation at 4000 × g for 5 min. The ether layer (supernatant) was removed and dried over Na2SO4. Separation of compounds was done, using a DB-FFAP capillary column (Agilent, Little Falls, Wilmington, USA) with dimensions 60 m length × 0.32 mm i.d. × 0.5 μm film thickness. Furthermore, a Hewlett Packard 6890 Plus GC instrument (Little Falls, USA) equipped with a split/splitless injector and a flame ionization detector (FID) were used for gas chromatography (GC). The gas chromatography was performed under the following conditions: an initial oven temperature of 33 °C for 17 min, followed by an increase in temperature up to 240 °C, for 5 min (12 °C/min). Lastly, three microliters of the diethyl-ether extract were injected at 200 °C in split mode, with a split ratio of 15:1 and the split flow rate of 49.5 mL/min. The column flow rate was 3.3 mL/min, using hydrogen as carrier gas. The detector temperature was 250 °C.
Statistical analysis
All fermentations and chemical analysis were reported as means ± SD of three repeats. The effect of temperature and SO2 on yeast population dynamics and wine aroma was evaluated by conducting an analysis of variance (ANOVA) using the statistical software, Statistica version 13.0 (StatSoft Inc., Tulsa, Oklahoma, USA). The treatments were considered significant should the p-values be equal to or less than 0.05. For multivariate data analysis, principal component analysis was performed using the default setting of XLSTAT in Microsoft® Excel (2016).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Grape juice chemical parameters and yeast diversity in grape juice fermentations
Chenin blanc was used for natural grape juice fermentation in South Africa and Grechetto bianco was used in Italy. The Chenin blanc had at a sugar level of 210.7 g/L, pH of 3.37, with a total acidity of 3.23 g/L and 195 mg/L yeast assimilable nitrogen (YAN), while the Grechetto bianco was at a sugar level of 250 g/L, pH 3.17, with a total acidity of 4.41 g/L, and 191 mg/L YAN. The native yeast population in the Chenin blanc juice comprised S. cerevisiae (4.85 × 103 cfu/mL), H. uvarum (4.20 × 103 cfu/mL), W. anomalus (3.34 × 103 cfu/mL), L. thermotolerans (2.60 × 103 cfu/mL), and M. pulcherrima (2.20 × 103 cfu/mL). In contrast, the Grechetto bianco yeast population consisted of H. uvarum (5.20 × 104 cfu/mL), R. mucilaginosa (4.8 × 104 cfu/ mL), P. terricola (4.30 × 104 cfu/mL), M. pulcherrima (3.70 × 104 cfu/mL) and S. cerevisiae (2.10 × 103 cfu/mL). Both grape matrices contained some of the species present in the yeast consortium. However, they were present at levels considerably below the inoculation density of the strains in the yeast consortium.
Impact of temperature and SO2 on fermentation kinetics
The effect of temperature (15, 25 and 30 °C) and SO2 (0 and 30 mg/L) on fermentation kinetics was evaluated in the three matrices. The concentration of free SO2 after 24 hours of SO2 was between 22–32 mg/L in all three matrices.
All fermentations fermented to dryness, with the previously reported exception of the synthetic grape juice inoculated without S. cerevisiae8. In all cases, the fermentation rate increased proportionally with temperature. In synthetic juice, fermentation at 30 °C (NS-SC-T30-S0) was completed in 20 days, followed by fermentation at 25 °C (NS-SC-T25-S0) and 15 °C (NS-SC-T15-S0) which finished in 22 and 28 days, respectively (Fig. S1A). SO2 addition enhanced the fermentation rate in synthetic grape juice, resulting in the NS-SC-T25-S30 completing fermentation within 16 days, compared to the NS-SC-T25-S0 which took 22 days (Fig. S1B). Fermentations performed on Chenin blanc and Grechetto bianco at 25 °C, with 30 mg/L SO2 displayed the fastest kinetics whereas the fermentations conducted at 15 °C without SO2 showed the slowest kinetics (Fig. S2). Furthermore, fermentations inoculated with EC1118 (EC) were consistently faster than fermentations inoculated with the yeast consortium (NS-SC) and the spontaneous fermentations (SP).
Impact of temperature and SO2 on population dynamics
Comparison of population dynamics among the three matrices (synthetic grape juice, Chenin blanc and Grechetto bianco) revealed that the population dynamics observed within the NS-SC consortium in Chenin blanc and Grechetto bianco closely resembled the trends observed in the synthetic grape juice fermentations (Fig. 2A,B). For instance, regardless of the grape matrix, non-Saccharomyces species including L. thermotolerans, S. bacillaris, H. vineae, W. anomalus, and P. terricola accounted for 80–90% of the population by the end of all NS-SC-T15 fermentations whereas they made up less than 30% of the population by the end of all NS-SC-T25 fermentations, independently of the presence or absence of SO2.
Figure 2.
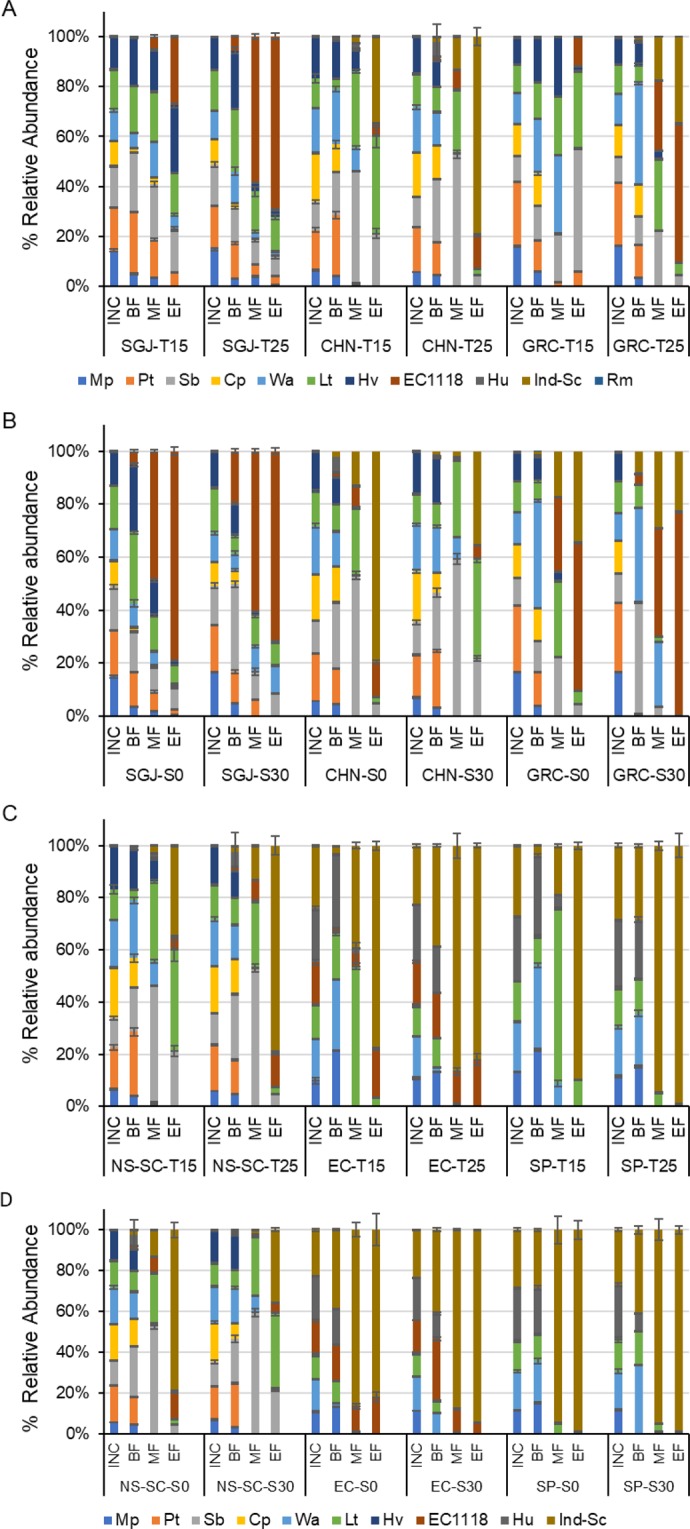
Yeast dynamics in response to temperature (T15 and T25) and SO2 (S0 and S30) addition. (A,B) show the effect of temperature and SO2 on the yeast population dynamics in synthetic grape juice (SGJ), Chenin blanc (CHN) and Grechetto bianco (GRC) at different stages of fermentation. IN shows relative abundance at inoculation, BF, MF and EF is the beginning, middle and end of fermentation with the NS-SC consortium. Fig (C) shows the effect of temperature the population dynamics in spontaneous fermentation (SP), the NS-SC consortium and S. cerevisiae EC1118 inoculated (EC) Chenin blanc while (D) shows the effect of SO2. The yeast species monitored were Metschnikowia pulcherrima (MP), Pichia terricola (Pt), Starmerella bacillaris (Sb), Candida parapsilosis (Cp), Wickerhamomyces anomalus (Wa), Lachancea thermotolerans (Lt), Hanseniaspora vineae (Hv), commercial Saccharomyces cerevisiae (EC1118), indigenous S. cerevisiae (Ind-Sc).
The behaviour of indigenous strains in spontaneous fermentations and fermentations inoculated with EC1118 was similar to that of the same species in NS-SC fermentations. For instance, the behaviour of the indigenous S. cerevisiae, M. pulcherrima, L. thermotolerans and W. anomalus in the Chenin blanc SP fermentation was similar to that of the same species in the NS-SC inoculated in synthetic grape juice and in Chenin blanc (Fig. 2C,D). Similarly, the growth of indigenous strains such as M. pulcherrima and P. terricola in Grechetto bianco fermentations followed similar trends to the inoculated strains in NS-SC fermentations (data not shown). Briefly, M. pulcherrima declined rapidly in all fermentations, irrespective of fermentation temperature or SO2 addition. However, M. pulcherrima strains persisted slightly longer in absence of SO2. Results of a two-way ANOVA confirmed that growth of M. pulcherrima strains was significantly affected by SO2 (Tables S1 and S2). On the other hand, growth of both indigenous and inoculated W. anomalus strains was enhanced at 15 °C and in the presence of SO2. However, the survival of W. anomalus was severely curtailed by high S. cerevisiae levels (e.g. EC-T25 and NS-SC-T25). Inoculated and indigenous L. thermotolerans strains persisted until the end of fermentation in all fermentation scenarios. However, relative growth of this species was significantly increased at 15 °C in NS-SC and SP treatments (Tables S1 and S2). In contrast, the growth of Ind-Sc and EC1118 was enhanced at the higher temperature and in the presence of SO2.
The remaining species in the NS-SC consortium viz. S. bacillaris, P. terricola, C. parapsilosis, and H. vineae maintained similar growth patterns in Chenin blanc and Grechetto fermentations as in synthetic grape juice. Lastly, despite the fact that fermentations were performed under different conditions (T15&T25/S0&S30), the pattern of population dynamics in all NS-SC fermentations remained similar, including the survival of L. thermotolerans and S. bacillaris, along with S. cerevisiae, until middle and end of fermentations and the rapid decline of M. pulcherrima and C. parapsilosis; W. anomalus, P. terricola, and H, vineae persisted in most cases until beginning and middle of fermentations.
The fermentation conducted in the absence of S. cerevisiae (NS) in synthetic grape juice, displayed different patterns of population dynamics, irrespective of fermentation temperature. For instance, W. anomalus was the most dominant non-Saccharomyces species by middle of NS-T25 and NS-T30, accounting for 65 and 73% of the total population whereas this species accounted for less than 2% of the population by middle of NS-SC-T25 and NS-SC-T30 fermentations. Furthermore, S. bacillaris was the most abundant species by the end of NS-T15 whereas H. vineae was the most dominant species in NS-SC-T15 (Fig. S3).
Production of major volatiles
Synthetic grape juice fermentations at 15, 25, and 30 °C produced wines with different aroma profiles. Overall, wine generated from the fermentation at 30 °C (NS-SC-T30) produced the highest level of ethyl esters, mainly due to the high levels of ethyl lactate. However, ethyl caprate and ethyl caprylate were produced at significantly higher concentrations at 15 °C (Table 2). Furthermore, acetate esters were produced at significantly higher levels in the wine generated from the fermentation at 15 °C. In contrast, the fermentation at 25 °C displayed the highest production of higher alcohols (188.9 mg/L), with isoamyl alcohol, 1-propanol, 2-phenyl ethanol, and isobutanol being the main contributors. The production of acetic acid increased proportionally with temperature. However, the NS-SC-T30 fermentation resulted in lower concentrations of total volatile acids, excluding acetic acid. The concentration of some compounds such as ethyl lactate increased proportionally with temperature while others such as 3-ethoxy-1-propanol displayed an inverse relationship (Table 2). Other compounds mainly within the group of volatile acids seemed to not be affected by fermentation temperature
Table 2.
The concentration of volatile compounds at fermentations conducted at 15 °C (NS-SC-T15), 25 °C (NS-SC-T25) and 30 °C (NS-SC-T30) in the synthetic grape juice.
| Compound | NS-SC-T15 | NS-SC-T25 | NS-SC-T30 | p-values | RTS |
|---|---|---|---|---|---|
| Ethyl Esters | |||||
| Ethyl caprylate | 0.5 ± 0.07b | 0.2 ± 0.02a | 0.2 ± 0.01a | 0.00 | DEC |
| Ethyl caprate | 0.6 ± 0.01c | 0 ± 0a | 0.3 ± 0.04b | 0.00 | NP |
| Ethyl lactate | 3.2 ± 0.09a | 5.6 ± 0.06ab | 9.5 ± 0.07b | 0.02 | INC |
| Diethyl succinate | 0.1 ± 0.01a | 0.6 ± 0.13b | 0a | 0.00 | NP |
| ∑ Esters | 4.5 ± 1.98 | 6.5 ± 0.21 | 10.1 ± 0.12 | 0.00 | INC |
| Acetate esters | |||||
| Ethyl acetate | 98.8 ± 2.87b | 82.7 ± 4.57a | 84.3 ± 8.83a | 0.00 | NP |
| Ethyl phenyl acetate | 0.3 ± 0.01a | 1 ± 0.1b | 0.6 ± 0.03ab | 0.00 | NP |
| 2-Phenylethyl acetate | 1 ± 0.1b | 0.8 ± 0.03a | 0.7 ± 0.05a | 0.00 | NP |
| Isoamyl acetate | 0.2 ± 0.06a | 0.6 ± 0.04b | 0.2 ± 0.05a | 0.00 | NP |
| ∑ Acetates | 100.4 ± 3.04 | 85.2 ± 4.74 | 86 ± 8.96 | 0.00 | DEC |
| Alcohols | |||||
| Isoamyl alcohol | 71.9 ± 4.38a | 100.3 ± 4.81b | 78.2 ± 5.12a | 0.01 | NP |
| 2-Phenyl ethanol | 16.2 ± 2.16a | 22.5 ± 3.13b | 18.7 ± 1.24ab | 0.00 | NP |
| Isobutanol | 13.7 ± 6.54a | 15.9 ± 0.35a | 20.9 ± 0.8b | 0.00 | INC |
| Butanol | 0.2 ± 0a | 0.8 ± 0.09b | 0.7 ± 0.04b | 0.00 | NP |
| Propanol | 20.4 ± 2.89a | 46.5 ± 6.03b | 23.3 ± 2.10a | 0.00 | NP |
| 3-ethoxy-1-propanol | 3.9 ± 0.32b | 2.6 ± 0.27a | 2.3 ± 0.28a | 0.00 | DEC |
| ∑ Higher alcohols (no methanol) | 126.6 ± 16.29 | 188.9 ± 14.68 | 144.3 ± 9.58 | 0.03 | NP |
| Volatile acids | |||||
| Acetic acid | 411.5 ± 3.88a | 614.3 ± 8.95ab | 693.2 ± 39.65b | 0.00 | INC |
| Propionic acid | 1.7 ± 0.1b | 0.9 ± 0.04a | 1.2 ± 0.02a | 0.01 | NP |
| Isobutyric acid | 0.5 ± 0.03a | 0.8 ± 0.02b | 0.7 ± 0.04ab | 0.00 | NP |
| Butyric acid | 0.9 ± 0.07b | 2.2 ± 0.06c | 0.7 ± 0.01a | 0.00 | NP |
| Iso-valeric acid | 1 ± 0.03b | 0.8 ± 0.19a | 1.2 ± 0.01b | 0.00 | NP |
| Valeric acid | 0.4 ± 0.55a | 1.1 ± 0.03b | 0.5 ± 0.18a | 0.00 | NP |
| Hexanoic acid | 1.3 ± 0.85b | 1.2 ± 0.17b | 0.7 ± 0.2a | 0.00 | NP |
| Octanoic acid | 1.9 ± 1.29b | 1.7 ± 0.01b | 1.2 ± 0.3a | 0.00 | NP |
| Decanoic acid | 1.6 ± 0.18c | 0.9 ± 0.01a | 1.4 ± 0.02b | 0.00 | NP |
| ∑ Volatile acids without acetic acid | 9.7 ± 3.1 | 9.9 ± 0.53 | 8 ± 0.77 | 0.01 | NP |
| Ketones | |||||
| Acetoin | 7.8 ± 0.57b | 2 ± 1.07a | 13.8 ± 2.09c | 0.00 | NP |
Values are represented in mg/L ± standard deviations. A response pattern of each compound to increasing temperatures (15, 25 and 30 °C) is indicated in a response to temperature (RTS) column. “INC” indicates an increase in concentration of a compound, “DEC” displays a decrease in concentration of a compound in response to increasing temperatures whereas a compound that represent no increasing or decreasing pattern is presented with no pattern (NP). Superscript letters denote statistical differences (p < 0.05). Different letters in the same row indicate significant differences in the compound concentration between the three fermentation temperatures.
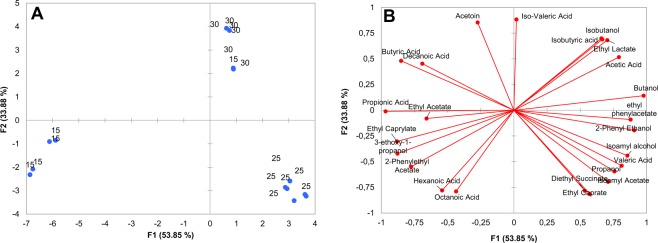
Principal component analysis was applied to all quantifiable major volatiles to determine the compounds that drive the differences between wines as a function of different temperatures. PC1 and PC2 explained 53.85% and 33.88% of the variance (Fig. 3). The PCA plot indicated that the wine produced at 15 °C (NS-SC-T15) was mainly associated with fatty acids (hexanoic acid and octanoic acid), esters (2-phenylethyl acetate, ethyl acetate, and ethyl caprate and ethyl caprylate) as well as 3-ethoxy-1-propanol. In contrast, wine produced at 30 °C (NS-SC-T30) was characterized by higher alcohols (1-butanol and isobutanol), short chain fatty acids (isobutyric acid, isovaleric acid, and acetic acid), acetoin and ethyl lactate. NS-SC-T25, on the other hand, was mainly associated with esters (ethyl phenylacetate, isoamyl acetate, and diethyl succinate) and alcohols (isoamyl alcohol, 2-phenyl ethanol, and 1-propanol) as well as butyric acid. The results of a one-way analysis of variance (ANOVA), indicated that with the exception of pentanol and ethyl butyrate and, the production of all other major volatiles was significantly affected by temperature.
Figure 3.
Principal component analysis showing the observations plot (A) and loadings plot (B) for the major volatile compounds accumulated at the end of fermentation by the NS-SC consortium in synthetic grape juice at 15, 25 and 30 °C.
Chenin blanc grape juice fermented under different temperature and SO2 conditions produced different aroma profiles. A two-way ANOVA confirmed that the production of some major volatiles, mainly within the group volatile acids (propionic acid, butyric acid, hexanoic acid, octanoic acid), higher alcohols (iso-butanol, 2-phenyl ethanol, and isoamyl alcohol), few esters (isoamyl acetate and ethyl lactate), and acetoin was significantly affected by temperature. For instance, ethyl lactate was produced at a significantly higher concentration in NS-SC-T25 (25.9 mg/L) compared to NS-SC-T15 (0) whereas isoamyl acetate was significantly produced at a lower concentration in NS-SC-T25 (66.3 mg/L) compared to NS-C-T15 (76.6 mg/L). The production of some compounds such as ethyl lactate, isoamyl alcohol, 2-phenyl ethanol, and acetic acid was significantly affected with both temperature and SO2 addition (Table S3). For instance, the highest concentration of acetic acid was produced in EC-T25-S0 (433.26 mg/L), while the EC-T15-S30 represented the lowest content of acetic acid.
Evaluating the production of major volatiles in Chenin blanc wines produced from the NS-SC consortium, revealed that the production of some compounds followed a similar pattern as observed in the NS-SC synthetic wines. For instance, higher concentrations of some compounds such as ethyl lactate, 2-phenyl ethanol, acetic acid, and total ethyl esters were produced at 25 °C fermentations compared to 15 °C in synthetic grape juice and Chenin blanc (Table 3). Inconsistent patterns in the production of other compounds such as ethyl caprate, propionic, octanoic and decanoic acids were observed between the two matrices. Furthermore, production of some major volatile compounds, such as ethyl lactate, isoamyl acetate, 2-phenyl ethanol and isobutanol, followed a same trend in Chenin blanc NS-SC, EC and SP treatments whereas production of other compounds such as ethyl caprylate, isobutyric acid, and iso-valeric acid represented a different pattern in the SP or EC treatments compared to the NS-SC treatments.
Table 3.
The concentration of major volatile compounds obtained in wines produced from spontaneous fermentation (SP), S. cerevisiae EC1118 inoculated fermentation (EC) and the model consortium comprising 7 non-Saccharomyces species and S. cerevisiae EC1118 (NS-SC) at 15 and 25 °C (T15, T25), without sulphur (S0) and with 30 mg/L SO2 (S30).
| Compound | Spontaneous fermentation | Inoculated with EC1118 | Inoculated with consortium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP-T25- S0 | SP- T25-S30 | SP- T15-S0 | SP-T15-S30 | EC-T25-S0 | EC-T25- S30 | EC-T15-S0 | EC-T15- S30 | NS-SC-T25-S0 | NS-SC- T25-S30 | NS-SC-T15-S0 | NS-SC- T15- S30 | |
| Ethyl esters | ||||||||||||
| Ethyl caprylate | 0.9 ± 0.09ab | 0.8 ± 0.06a | 1 ± 0.18abc | 1 ± 0.07abc | 1.1 ± 0.02bcd | 0.9 ± 0.04ab | 1.2 ± 0.15de | 1.1 ± 0.01cde | 2 ± 0.03g | 1 ± 0.01ef | 1.5 ± 0.04f | 1.3 ± 0.05de |
| Ethyl caprate | 0.6 ± 0.14ab | 0.6 ± 0.02a | 0.6 ± 0.06a | 0.5 ± 0.12a | 0.9 ± 0.06cde | 0.5 ± 0.13a | 0.7 ± 0.25abc | 0.5 ± 0.07a | 1.3 ± 0.09f | 1 ± 0.08def | 1.1 ± 0.1ef | 0.8 ± 0.06bcd |
| Ethyl lactate | 8.5 ± 0.05b | 8.5 ± 0.05b | 0a | 0a | 28.1 ± 5.06c | 8.4 ± 0.09b | 0a | 0a | 25.9 ± 1.42c | 9.2 ± 0.5b | 0a | 0a |
| Diethyl succinate | 0.4 ± 0.02ab | 0.4 ± 0.02ab | 0.3 ± 0.01ab | 0.4 ± 0.04ab | 0.5 ± 0.05bc | 0.5 ± 0.06abc | 0.5 ± 0.13bc | 0.2 ± 0.02a | 0.4 ± 0.01ab | 0.4 ± 0.01ab | 0.7 ± 0.25c | 0.5 ± 0.13bc |
| Ethyl butyrate | 0.2 ± 0.02a | 0.2 ± 0.02a | 0.3 ± 0.04bc | 0.3 ± 0.01bcd | 0.2 ± 0.01a | 0.3 ± 0.02a | 0.3 ± 0.02cd | 0.4 ± 0.04fg | 0.3 ± 0.01bcd | 0.3 ± 0.011bcd | 0.4 ± 0.03g | 0.4 ± 0.01dfg |
| Ethyl hexanoate | 0.8 ± 0.03a | 0.7 ± 0.03a | 0.9 ± 0.17bc | 1 ± 0.01bc | 0.8 ± 0.03ab | 0.9 ± 0.03ab | 1.1 ± 0.01cd | 1.3 ± 0.04d | 1 ± 0b | 0.9 ± 0.04ab | 1.2 ± 0.06d | 1.1 ± 0.09d |
| ∑ Esters | 11.7 ± 0.33 | 11.5 ± 0.18 | 3.3 ± 0.46 | 3.3 ± 0.25 | 31.9 ± 5.23 | 11.7 ± 0.37 | 4.1 ± 0.56 | 3.7 ± 0.18 | 31.1 ± 1.56 | 13.4 ± 1.44 | 5.1 ± 0.48 | 4.2 ± 0.34 |
| Acetates | ||||||||||||
| Ethyl acetate | 39.8 ± 1.08ab | 37.6 ± 1.84a | 36.3 ± 1.79a | 36.1 ± 0.78a | 34.3 ± 3.93a | 40.3 ± 1.36ab | 35.5 ± 0.52a | 38.2 ± 3.11ab | 66.3 ± 3.75b | 93 ± 15.40c | 76.6 ± 6.08c | 47.1 ± 8.72ab |
| 2-Phenylethyl Acetate | 0.8 ± 0.04a | 0.7 ± 0.01a | 0.6 ± 0.11a | 0.7 ± 0.01a | 0.7 ± 0.07a | 0.9 ± 0.1ab | 0.7 ± 0.01a | 0.7 ± 0.04a | 1 ± 0.06ab | 1.8 ± 0.86c | 1.6 ± 0.67bc | 1.1 ± 0.48abc |
| Isoamyl acetate | 2.2 ± 0.17abc | 2 ± 0.05a | 2.4 ± 0.54abcd | 2.7 ± 0.02bcd | 2.1 ± 0.13ab | 2.7 ± 0.24bcd | 2.8 ± 0.12cd | 3.1 ± 0.32de | 2 ± 0.06a | 2.2 ± 0.08abc | 3.6 ± 0.68e | 3 ± 0.3d |
| ∑ Acetates | 43.1 ± 1.36 | 40.5 ± 1.98 | 39.8 ± 2.46 | 39.8 ± 0.85 | 37.4 ± 4.13 | 44.3 ± 1.87 | 39.5 ± 0.68 | 42.6 ± 3.73 | 69.5 ± 3.88 | 97.3 ± 16.42 | 82.2 ± 7.48 | 51.6 ± 9.54 |
| Alcohols | ||||||||||||
| Isoamyl alcohol | 187.6 ± 4.1d | 170.9 ± 3cd | 146.6 ± 4.72abc | 154.49 ± 3.14bcd | 161.5 ± 5.5bcd | 198.6 ± 5d | 161 ± 2.19bcd | 181.4 ± 6.35cd | 113.4 ± 3.70a | 177.5 ± 13.98cd | 138.7 ± 4.45abc | 129.8 ± 4.11ab |
| 2-Phenyl ethanol | 41.8 ± 2.41gh | 37.5 ± 0.84fgh | 23.8 ± 7.71cd | 26.1 ± 0.67cd | 35.7 ± 6.23efgh | 46 ± 8.09h | 27 ± 2.44cdef | 31.1 ± 4.37efd | 16.1 ± 0.54ab | 33.4 ± 3.08efg | 14.2 ± 1.30a | 19.5 ± 5.62abc |
| Isobutanol | 29.6 ± 1.56bd | 25.7 ± 1.08bcd | 17.9 ± 4.02a | 17.5 ± 2.50a | 22.9 ± 2.29abc | 29 ± 3.44bd | 18.4 ± 2.18ac | 19.1 ± 3.47ac | 28 ± 5.26bd | 32.9 ± 2.31d | 23.6 ± 4.86abcd | 17 ± 2.91a |
| 1-Butanol | 0.7 ± 0.05ab | 0.7 ± 0.04ab | 0.6 ± 0.05a | 0.6 ± 0.0a | 0.7 ± 0.0ab | 0.7 ± 0.07ab | 0.6 ± 0.0a | 0.7 ± 0.09ab | 0.9 ± 0.0c | 0.9 ± 0.1c | 0.8 ± 0.04c | 0.6 ± 0.0a |
| 1-Propanol | 10.6 ± 0.48a | 10 ± 0.3a | 11.3 ± 1.30ab | 10.5 ± 0.64a | 9.8 ± 0.33a | 9.1 ± 0.39a | 9.8 ± 0.27a | 9.4 ± 0.37a | 16 ± 0.91c | 14.5 ± 1.55b | 13.5 ± 0.48b | 10.3 ± 0.54a |
| Hexanol | 1.2 ± 0.03ab | 1.2 ± 0.04a | 1.2 ± 0.06ab | 1.2 ± 0.03ab | 1.2 ± 0ab | 1.2 ± 0.04ab | 1.3 ± 0.01b | 1.3 ± 0.04b | 1.2 ± 0.02ab | 1.3 ± 0.03ab | 1.2 ± 0.04ab | 1.2 ± 0.05ab |
| 3-ethoxy-1-propanol | 2.8 ± 0.1ab | 2.8 ± 0.08ab | 2.8 ± 0.14a | 3 ± 0.07bcd | 2.9 ± 0.06abcd | 2.9 ± 0.02abc | 2.9 ± 0.03abc | 3.1 ± 0.05de | 3.2 ± 0.06e | 2.9 ± 0.04abcd | 3 ± 0.04bcde | 3 ± 0.02cde |
| ∑ Higher alcohols (no methanol) | 274.6 ± 8.73 | 249.1 ± 5.38 | 204.4 ± 18.00 | 213.6 ± 17.91 | 234.9 ± 14.41 | 287.7 ± 17.05 | 221.2 ± 7.12 | 246.4 ± 14.74 | 179 ± 10.49 | 263.7 ± 21.09 | 195.4 ± 11.21 | 181.7 ± 13.25 |
| Volatile acids | ||||||||||||
| Acetic acid | 300.6 ± 4.94a | 296.9 ± 21.47a | 285.8 ± 4.55a | 302.4 ± 16.38a | 433.4 ± 3.09b | 261 ± 15.38a | 265.3 ± 8.73a | 257.1 ± 10.12a | 428.8 ± 2.36b | 272.5 ± 17.11a | 266.4 ± 9.89a | 292.0 ± 12.42a |
| Propionic acid | 1.2 ± 0.02bc | 1.1 ± 0.03abc | 0.6 ± 0.02a | 1.1 ± 0.03abc | 1 ± 0.01abc | 1.1 ± 0.01abc | 1.1 ± 0.03abc | 1.1 ± 0.02abc | 1.1 ± 0.01abc | 1.5 ± 0.41c | 1.1 ± 0.04abc | 0.9 ± 0.02ab |
| Isobutyric acid | 2 ± 0.1b | 1.8 ± 0.04b | 1.1 ± 0.22a | 1.1 ± 0.05a | 1.8 ± 0.28b | 2.2 ± 0.42b | 1.2 ± 0.14a | 1.2 ± 0.16a | 1 ± 0.02a | 2.2 ± 0.12b | 0.8 ± 0.1a | 1.1 ± 0.12a |
| Butyric acid | 1.2 ± 0ab | 1.1 ± 0.02a | 1.3 ± 0.14cde | 1.3 ± 0.06cde | 1.1 ± 0.03ab | 1.2 ± 0.05ab | 1.4 ± 0.04bcd | 1.4 ± 0.09ef | 1.3 ± 0.04bcd | 1.2 ± 0abc | 1.6 ± 0.05f | 1.4 ± 0.01e |
| Iso-valeric acid | 1.6 ± 0.07ef | 1.5 ± 0.01def | 1.2 ± 0.23bc | 1.2 ± 0.02bcd | 1.5 ± 0.19cdef | 1.8 ± 0.27f | 1.3 ± 0.15bcde | 1.4 ± 0.16cde | 0.8 ± 0.01a | 1.4 ± 0.05cde | 0.9 ± 0.05a | 1.1 ± 0.14ab |
| Hexanoic acid | 4.1 ± 0.25a | 4.1 ± 0.21a | 4.7 ± 0.52bc | 4.8 ± 0.09bc | 4.3 ± 0.02abc | 4.4 ± 0.09abc | 5.3 ± 0.08de | 5.4 ± 0.15de | 4.9 ± 0.14cd | 4.2 ± 0.14ab | 5.7 ± 0.06e | 5.7 ± 0.13e |
| Octanoic acid | 6.5 ± 0.31ab | 6.2 ± 0.28a | 7.4 ± 0.73c | 7.7 ± 0.07c | 7.3 ± 0.15bc | 7.4 ± 0.22bc | 8.5 ± 0.18ef | 8.9 ± 0.49e | 7 ± 0.18abc | 6.6 ± 0.34ab | 8.3 ± 0.17de | 8.9 ± 0.57e |
| Decanoic acid | 2.5 ± 0.09bcd | 2.4 ± 0.04ab | 2.5 ± 0.28bcd | 2.6 ± 0.12bcd | 2.7 ± 0.06bcde | 2.9 ± 0.12de | 2.9 ± 0.14cde | 3.1 ± 0.05e | 2.2 ± 0.03a | 2.4 ± 0.2ab | 2.5 ± 0.12bc | 2.8 ± 0.26cde |
| ∑ Volatile acids without acetic acid | 19.7 ± 0.85 | 18.9 ± 0.64 | 19.1 ± 2.19 | 20.4 ± 0.46 | 20.4 ± 0.78 | 21.5 ± 1.2 | 22.1 ± 0.77 | 23 ± 1.16 | 18.6 ± 0.43 | 20.1 ± 1.27 | 21.1 ± 0.59 | 22.3 ± 1.25 |
| Ketones | ||||||||||||
| Acetoin | 0a | 0a | 0a | 0a | 14.6 ± 5.87ab | 2 ± 0.03a | 0a | 0a | 11.7 ± 1.95ab | 24.6 ± 8.16b | 0a | 0a |
Values are represented in mg/L ± standard deviations. Superscript letters denote statistical differences (p < 0.05). Different letters in the same row indicate significant differences in compound concentration across the fermentation modalities.
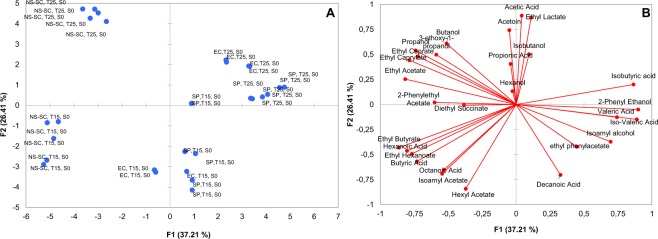
Principal component analysis (PCA) was applied on all the major volatiles obtained from the Chenin blanc wines fermented at 25 °C and 15 °C to determine the effect of temperature on the production of major volatiles (Fig. 4). PC1 explained 37.21% of the variation and separated the fermentations which were conducted at 25 °C and 15 °C in the positive and negative dimension of PC, respectively. In contrast, PC2 separated the NS-SC treatments from EC and SP treatments. EC-T25-S0 and SP-T25-S0 made a small cluster and were mainly associated with the short-chain fatty acids (valeric, isovaleric, isobutyric acid and acetic acid), alcohols (isobutanol and 2-phenyl ethanol) and ethyl lactate whereas EC-T15-S0 and SP-T15-S0 formed a group together and were characterized by the high accumulation of acetate esters (hexyl acetate and ethyl phenylacetate), medium chain fatty acids (octanoic acid and decanoic acid) and isoamyl alcohol. On the other hand, NS-SC-T15-S0 was associated with esters including 2-phenyl ethyl acetate, diethyl succinate, ethyl hexanoate, ethyl butyrate, and isoamyl acetate, as well as hexanoic acid. NS-SC-T25-S0 was characterized by high production of ethyl esters (ethyl acetate, ethyl caprate, and ethyl caprylate), alcohols including aliphatic alcohols (butanol and hexanol), propanol and 3-ethoxy-1-propanol as well as propionic acid and acetoin.
Figure 4.
Principal component analysis showing the observations plot (A) and loadings plot (B) for the major volatile compounds accumulated at the end of fermentation of Chenin blanc. The fermentations were conducted spontaneously (SP), inoculated with EC1118 (EC) or inoculated with the consortium (NS-SC) at 15 °C (T15) and 25°(T25), mg/L (S30) and without SO2 (S0).
Discussion
The current study evaluated the influence of temperature and SO2 on fermentation kinetics and yeast population dynamics in a complex multi-species community. Furthermore, the study aimed to evaluate how changes in population dynamics may affect production of major volatiles in wine. For this purpose, three grape matrices including synthetic juice, Chenin blanc, and Grechetto bianco were selected. Interestingly, some non-Saccharomyces species (e.g. P. terricola and M. pulcherrima) were present in the natural grape juice (indigenous strains) as well as the model consortium (inoculated strains) which allowed us to evaluate whether the effect of temperature and SO2 on the growth of yeast species in NS-SC and control fermentations (SP and EC) remains constant.
As for all approaches evaluating impacts and behaviour of multispecies consortia, choices had to be made regarding the specific settings to be evaluated. Consequently, environmental parameters such as pH could not be included although previous studies have shown that pH affects the growth rate, cell biomass and fermentation behaviour of S. cerevisiae and non-Saccharomyces yeasts in synthetic grape juice48,49. Low or high pH can exert chemical stress on yeast cells and depending on the tolerance levels, it would affect yeast population dynamics. Indeed, at 25 °C pH 3.0 and 3.5, a dominance of S. cerevisiae with concomitant decline of non-Saccharomyces species was reported, suggesting that S. cerevisiae is more resilient at these pH levels50. Unfortunately, due to logistical constraints we could not investigate this parameter in the current study. Similarly, it would obviously have been useful if additional data on interactions between individual pairings of all of the species, and data on drop-out inoculations (where single species would have been omitted), could have been added. However, this would have represented a very large number of fermentations and would logistically not have been feasible, in particular because we evaluate the impact of the treatments in conditions similar to real industrial wine fermentations.
The data generated with our specific settings nevertheless result in several novel and important insights regarding the behaviour of multispecies yeast ecosystem during fermentation. In particular, they show that, while absolute population numbers of each species are condition dependent, the broad trends in population dynamics are independent of the nature of the specific matrix and of conditions, and are therefore a result of the biotic interactions between the contributing species. The data show that temperature and SO2 treatments affected yeast metabolism and growth as reported previously. Fermentations performed at 25 °C and with SO2 enhanced the growth of S. cerevisiae whereas some non-Saccharomyces species persisted longer in the fermentations conducted at 15 °C and without SO2. Most of the previous studies have confirmed that non-Saccharomyces species can persist longer in the fermentations performed at 10–15 °C14,28,30,33. This ability has been linked to modifications in cell wall lipid composition23,33,51–54.
Regarding the effect of SO2 on population dynamics, it is well documented that antimicrobial activity of SO2 is highly pH dependent. At a relatively low pH (<3.5), as was the case in Chenin blanc and Grechetto bianco grape musts, a relatively-higher percentage of molecular SO2 is available in grape must55,56. Our data confirmed that SO2 affects the growth of yeast species in a species- or strain-specific manner. SO2 addition displayed inhibitory (M. pulcherrima), and supportive (S. cerevisiae) effect on the growth of some yeast species while it had a marginal effect on the growth of others (L. thermotolerans). Previous studies have reported that SO2 affects the growth of organisms in a dose-dependent manner, inhibiting the growth of some species (P. terricola and H. uvarum) at high concentrations (60 mg/L–100 mg/L) while showing no effect on the growth of other species (S. bacillaris) at low concentrations (30–50 mg/L)24,35,57,58. Furthermore, the better implantation of S. cerevisiae in the yeast consortium in the presence of SO2 was in agreement with a previous report by Albertin et al.35. S. cerevisiae has been shown to apply different strategies to adapt to SO259–61. However, the supportive effect of SO2 at a low dosage (30 mg/L) on the growth of non-Saccharomyces species was not reported previously. For instance, W. anomalus persisted longer in presence of SO2 in all NS-SC and SP fermentations, suggesting that this species is able to detoxify SO2 efficiently.
In general, despite the differences among three matrices and the differences in yeast community compositions in NS-SC and the control fermentations (SP and EC), a similar trend of population dynamics was observed in all NS-SC fermentation as well as among NS-SC and control fermentations, even though the strains present in the consortium and grape juice were most probably different. Indigenous non-Saccharomyces species Such as M. pulcherrima, H. uvarum, and L. thermotolerans accounted for a higher proportion of yeast population in SP and EC fermentations compared to the NS-SC fermentation. For instance, indigenous L. thermotolerans fermentation, accounted for 53–67% of the total yeast population in the middle of EC-T15 and SP-T15 Chenin blanc fermentations whereas this species accounted for 30% of the population by the middle of NS-SC fermentation. This result can be explained by differences in yeast community composition of SP, EC, and NS-SC which stimulates different yeast-yeast interactions in these communities. Thus, our data underlined the importance of microbial community composition and ecological interaction in wine fermentations.
Another important observation was that despite the differences among the three matrices, synthetic grape juice and the two real grape musts, and the variation in temperature and SO2, the survival of S. bacillaris and L. thermotolerans until middle and end of fermentation was not affected. However, fermentations in absence of S. cerevisiae displayed different population dynamics compared to fermentation in presence of S. cerevisiae, irrespective of fermentation temperature. The data suggest that the population dynamics in a multi-species consortium is modulated strongly by the response of each species to the presence of other species, rather than by temperature and SO2 addition alone. Consequently, the species that evolves better adaptation mechanisms to biotic (competition) stresses present in wine fermentation can outcompete the remaining population and survive longer. Therefore, the dynamics observed in the yeast consortium under different treatments seemed to mainly rely on the ecological interactions, rather than abiotic factors such as temperature and SO2 addition.
Regarding the aromatic profiles of wines, our data confirm that the production of some compounds followed a similar trend in all fermentations independently of the microbial consortia, which suggests that the production of these compounds is primary dependent on physical parameters irrespective of the grape matrix. For instance, the production of acetic acid displayed a linear correlation with the fermentation temperature in all of fermentations which is in agreement with a previous report by Torija et al.21. Since acetic acid is mainly derived from the oxidation of acetaldehyde by aldehyde dehydrogenases (ALDH) as suggested by Remize et al.62, we can speculate that increase in temperature may have enhanced the activity of ALDH. Secondly, the production of other compounds displayed inconsistent patterns in wines obtained from NS-SC fermentations of synthetic grape juice and Chenin blanc juice, which can be explained by the differences between the two matrices and the differences in the microbial ecosystems. For instance, some yeast-derived volatile compounds such as hexanol and hexyl acetate which require the presence of a grape precursor were only detected in Chenin blanc wine. Lastly, the PC analysis displayed a separation of the fermentations based on temperature (PC1) whereas a further separation was observed between NS-SC fermentations and their respective controls (Fig. 4). This observation suggests that the aromatic profile of wine is mainly affected by the presence of non-Saccharomyces species in NS-SC consortium, in spite of the fact that these fermentations were conducted at two different temperatures. Thus, our data show that production of major volatiles is significantly affected by the yeast species composition and the ecological interactions among the species rather than temperature and SO2. This result is in agreement with our previous work by Bagheri et al.13, who confirmed that the aromatic profile of wine is significantly affected by the presence of some non-Saccharomyces (e.g. C. parapsilosis) in grape juice, irrespective of their rapid declines at an early stage of fermentation.
In conclusion, our data suggest that ecological interactions are a dominant factor in determining the outcome of the fermentation. Furthermore, the data demonstrate that the constructed consortium is a robust model that can be used as a tool to evaluate the contribution of ecological interactions in fermentation dynamics and in the metabolic outcome of the fermentation process.
Supplementary information
Acknowledgements
This work was supported by the Wine Industry Network for Expertise and Technology (Winetech), grant SU IWBT 16/02, the National Research Foundation-Technology and Human Resources for Industry Programme (grant number TP14080184824), the Department of Science and Technology/National Research Foundation SARChI Grant UID 83471 as well as the YeSVitE consortium (FP7-IRSES-2013-GA no. 612441). Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the funding agencies.
Author contributions
B.B., F.F.B. and M.E.S. concenptualized the experimental design. B.B. performed all the experiments, data analyses and preparation of Tables and Figures. F.F.B., M.E.S. and G.C. supervised the work. B.B., F.F.B., M.E.S. and G.C. edited and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61690-z.
References
- 1.Jolly NP, Augustyn OPH, Pretorius IS. The occurrence of non-Saccharomyces cerevisiae yeast species over three vintages in four vineyards and grape musts from four production regions of the Western Cape, South Africa. S. Afr. J. Enol. Vitic. 2003;24:63–69. doi: 10.21548/24-2-2640. [DOI] [Google Scholar]
- 2.Ghosh S, Bagheri B, Morgan HH, Divol B, Setati ME. Assessment of wine microbial diversity using ARISA and cultivation-based methods. Ann. Microbiol. 2015;65:1833–1840. doi: 10.1007/s13213-014-1021-x. [DOI] [Google Scholar]
- 3.Bagheri B, Bauer FF, Setati ME. The diversity and dynamics of indigenous yeast communities in grape must from vineyards employing different agronomic practices and their influence on wine fermentation. S. Afr. J. Enol. Vitic. 2015;36:243–251. doi: 10.21548/36-2-957. [DOI] [Google Scholar]
- 4.Wang C, Esteve-Zarzoso B, Cocolin L, Mas A, Rantsiou K. Viable and culturable populations of Saccharomyces cerevisiae, Hanseniaspora uvarum and Starmerella bacillaris (synonym Candida zemplinina) during Barbera must fermentation. Food Res. Int. 2015;78:195–200. doi: 10.1016/j.foodres.2015.10.0. [DOI] [PubMed] [Google Scholar]
- 5.González-Royo E, et al. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015;240:999–1012. doi: 10.1007/s00217-014-2404-8. [DOI] [Google Scholar]
- 6.Lleixà J, et al. Analysis of the NCR mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae during winemaking. Front. Genet. 2019;9:747. doi: 10.3389/fgene.2018.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mane, S. S., Ghormade, V., Tupe, S. G. & Deshpande, M. V. Diversity of natural yeast flora of grapes and its significance in wine making. [Satyanarayana, T. & Kunze, G. (eds)] Yeast diversity in human welfare. 1–27, 10.1007/978-981-10-2621-81 (Springer, Singapore, 2017).
- 8.Bagheri B, Bauer FF, Setati ME. The impact of Saccharomyces cerevisiae on a wine yeast consortium in natural and inoculated fermentations. Front. Microbiol. 2017;8:1988. doi: 10.3389/fmicb.2017.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Çelik ZD, Erten H, Darici M, Cabaroğlu T. Molecular characterization and technological properties of wine yeasts isolated during spontaneous fermentation of Vitis vinifera L.cv. Narince grape juice grown in ancient wine making area Tokat, Anatolia. BIO Web Conf. 2017;9:02017. doi: 10.1051/bioconf/20170902017. [DOI] [Google Scholar]
- 10.Shekhawat K, Patterton H, Bauer FF, Setati ME. RNA-seq based transcriptional analysis of Saccharomyces cerevisiae and Lachancea thermotolerans in mixed-culture fermentations under anaerobic conditions. BMC Genomics. 2019;20:145. doi: 10.1186/s12864-019-5511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciani M, Comitini F, Mannazzu I, Domizio P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010;10:123–133. doi: 10.1111/j.1567-1364.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 12.Gobbi M, et al. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of the wine. Food Microbiol. 2013;33:271–81. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Bagheri B, Zambelli P, Vigentini I, Bauer FF, Setati ME. Investigating the effect of selected non-Saccharomyces species on wine ecosystem function and major volatiles. Front. Bioeng. Biotechnol. 2018;6:169. doi: 10.3389/fbioe.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills DA, Johannsen EA, Cocolin L. Yeast diversity and persistence in botrytis-affected wine fermentations. Appl. Environ. Microbiol. 2002;68:4884–4893. doi: 10.1128/AEM.68.10.4884-4893.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciani M, et al. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016;7:555. doi: 10.3389/fmicb.2016.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollero S, et al. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015;99:2291–2304. doi: 10.1007/s00253-014-6210-9. [DOI] [PubMed] [Google Scholar]
- 17.Padilla B, Gil JV, Manzanares P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016;7:411. doi: 10.3389/fmicb.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Torrado R, et al. Ecological interactions among Saccharomyces cerevisiae strains: insight into the dominance phenomenon. Sci. Rep. 2017;7:43603. doi: 10.1038/srep43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards CG, Oswald TA. Interactive effects between total SO2, ethanol and storage, temperature against Brettanomyces bruxellensis. Lett. Appl. Microbiol. 2017;66:71–76. doi: 10.1111/lam.12816. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017;57:856–873. doi: 10.1080/10408398.2014.983591. [DOI] [PubMed] [Google Scholar]
- 21.Torija MJ, Rozès N, Poblet M, Guillamón JM, Mas A. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003;80:47–53. doi: 10.1016/S0168-1605(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 22.Reddy LVA, Reddy OVS. Effect of fermentation conditions on yeast growth and volatile composition of wine produced from mango (Mangifera indica L.) fruit juice. FBP. 2011;89:487–491. doi: 10.1016/j.fbp.2010.11.007. [DOI] [Google Scholar]
- 23.García-Ríos E, Gutiérrez A, Salvadó ZZ, Arroyo-López FN, Guillamon JM. The fitness advantage of commercial wine yeasts in relation to the nitrogen concentration, temperature, and ethanol content under microvinification conditions. Appl. Environ. Microbiol. 2014;80:704–713. doi: 10.1128/AEM.03405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokulich NA, Swadener M, Sakamoto K, Mills DA, Bisson LF. Sulfur dioxide treatment alters wine microbial diversity and fermentation progression in a dose-dependent fashion. Am. J. Enol. Vitic. 2015;66:73–79. doi: 10.5344/ajev.2014.14096. [DOI] [Google Scholar]
- 25.Sun Y, Zhang T, Lu H, Yu Z, Li X. Effect of added sulfur dioxide levels on the fermentation characteristics of strawberry wine. J. Inst. Brew. 2016;122:446–451. doi: 10.1002/jib.342. [DOI] [Google Scholar]
- 26.Maturano YP, et al. Culture-dependent and independent techniques to monitor yeast species during cold soak carried out at different temperatures in winemaking. Int. J. Food Microbiol. 2016;237:142–149. doi: 10.1016/j.ijfoodmicro.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Shekhawat Kirti, Bauer Florian F., Setati Mathabatha E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Applied Microbiology and Biotechnology. 2016;101(6):2479–2491. doi: 10.1007/s00253-016-8001-y. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-del-Real J, Lairón-Peris M, Barrio E, Querol A. Effect of temperature on the prevalence of Saccharomyces non cerevisiae species against a S. cerevisiae wine strain in wine fermentation: Competition, physiological fitness, and influence in final wine composition. Front. Microbiol. 2017;8:150. doi: 10.3389/fmicb.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao C, Fleet GH. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata, and Kloeckera apiculata. J. Appl. Bacteriol. 1988;65:405–410. doi: 10.1111/j.1365-2672.1988.tb01909.x. [DOI] [Google Scholar]
- 30.Erten H. Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J. Microbiol. Biotechnol. 2002;18:373–378. doi: 10.1023/A:1015221406411. [DOI] [Google Scholar]
- 31.Mendoza M, de Nadra MC, Bru E, Farías ME. Influence of wine-related physicochemical factors on the growth and metabolism of non-Saccharomyces and Saccharomyces yeasts in mixed culture. J. Ind. Microbiol. Biotechnol. 2009;36:229–237. doi: 10.1007/s10295-008-0489-4. [DOI] [PubMed] [Google Scholar]
- 32.Bartowsky EJ. Bacterial spoilage of wine and approaches to minimize it. Lett. Appl. Microbiol. 2009;48:149–56. doi: 10.1111/j.1472-765X.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvadó Z, Arroyo-López FN, Barrio E, Querol A, Guillamón JM. Quantifying the individual effects of ethanol and temperature on the fitness advantage of Saccharomyces cerevisiae. Food Microbiol. 2011;28:1155–1161. doi: 10.1016/j.fm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Cocolin L, Mills DA. Wine yeast inhibition by sulfur dioxide: A comparison of culture-dependent and independent methods. Am. J. Enol. Vitic. 2003;54:125–130. [Google Scholar]
- 35.Albertin W, et al. Oenological pre-fermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int. J. Food Microbiol. 2014;178:87–97. doi: 10.1016/j.ijfoodmicro.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Chandra M, Oro I, Ferreira-Dias S, Malfeito-Ferreira M. Effect of ethanol, sulfur dioxide and glucose on the growth of wine spoilage yeasts using response surface methodology. PLOS One. 2015;10(6):e0128702. doi: 10.1371/journal.pone.0128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossouw D, Bagheri B, Setati ME, Bauer FF. Co-Flocculation of yeast species, a new mechanism to govern population dynamics in microbial ecosystems. PLOS One. 2015;10(8):e0136249. doi: 10.1371/journal.pone.0136249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossouw D, Meiring SP, Bauer FF. Modifying Saccharomyces cerevisiae adhesion properties regulates yeast ecosystem dynamics. mSphere. 2018;3:e00383–18. doi: 10.1128/mSphere.00383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henschke, P. A. & Jiranek, V. Yeasts metabolism of nitrogen compounds. [Fleet, G. H. (ed.)] Wine Microbiology and Biotechnology. 77–164 (Harwood Academic: Chur, Switzerland, 1993).
- 40.Bely M, Sablayrolles JM, Barre P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990;70:246–252. doi: 10.1016/0922-338X(90)90057-4. [DOI] [Google Scholar]
- 41.Siret R, Gigaud O, Rosec PJ, This P. Analysis of grape Vitis vinifera L. DNA in must mixtures and experimental mixed wines using microsatellite markers. Agric. Food. Chem. 2002;50:3822–3827. doi: 10.1021/jf011462e. [DOI] [PubMed] [Google Scholar]
- 42.García-Beneytez E, Moreno-Arribas MV, Borrego J, Polo MC, Ibáñez J. Application of a DNA analysis method for the cultivar identification of grape musts and experimental and commercial wines of Vitis vinifera L. using microsatellite markers. J. Agric. Food Chem. 2002;50:6090–6096. doi: 10.1021/jf0202077. [DOI] [PubMed] [Google Scholar]
- 43.Savazzini F, Martinelli L. DNA analysis in wines: Development of methods for enhanced extraction and real-time polymerase chain reaction quantification. Anal. Chim. Acta. 2006;563:274–282. doi: 10.1016/j.aca.2005.10.078. [DOI] [Google Scholar]
- 44.Sambrook Joseph, Russell David W. Rapid Isolation of Yeast DNA. Cold Spring Harbor Protocols. 2006;2006(1):pdb.prot4039. doi: 10.1101/pdb.prot4039. [DOI] [PubMed] [Google Scholar]
- 45.Briciu D, et al. Development of methods for DNA extraction from leaves and must grapes. Bulletin UASVM. Anim. Sci. Biotechnol. 2010;67:1843–1849. doi: 10.15835/buasvmcn-asb:67:1-2:5369. [DOI] [Google Scholar]
- 46.Işçi B, Yildirim Kalkan H, Altindisli A. Evaluation of methods for DNA extraction from must and wine. J. Inst. Brew. 2014;120:238–243. doi: 10.1002/jib.129. [DOI] [Google Scholar]
- 47.Louw L, et al. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010;31:213–225. doi: 10.21548/31-2-1418. [DOI] [Google Scholar]
- 48.Charoenchai C, Fleet GH, Henschke PA. Effects of temperature, pH and sugar concentration on the growth rates and cell biomass of wine yeasts. Am. J. Enol. Vitic. 1998;49:283–288. doi: 10.1016/j.ijfoodmicro.2009.01.035. [DOI] [Google Scholar]
- 49.Arroyo-Lopez FN, Orlic S, Querol A, Barrio E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009;131:120–127. doi: 10.1016/j.ijfoodmicro.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 50.Heard GM, Fleet GH. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Microbiol. 1988;65:23–28. doi: 10.1111/j.1365-2672.1988.tb04312.x. [DOI] [Google Scholar]
- 51.Alexandre H, Long TN, Feuillat M, Charpentier C. Contribution à l′étude des bourbes: influence sur a fermentiscibilité des moûts. Rev. Fr. Enol. 1994;146:11–19. [Google Scholar]
- 52.Pina C, Couto JA, António J, Hogg T. Inferring ethanol tolerance of Saccharomyces and non-Saccharomyces yeasts by progressive inactivation. Biotechnol. Lett. 2004;26:1521–7. doi: 10.1023/B:BILE.0000044456.72347.9f. [DOI] [PubMed] [Google Scholar]
- 53.Arroyo-Lopez FN, et al. Susceptibility and resistance to ethanol on Saccharomyces strains isolated from wild and fermentative environments. Yeast. 2010;27:1005–1015. doi: 10.1002/yea.1809. [DOI] [PubMed] [Google Scholar]
- 54.Redón M, Guillamón JM, Mas A, cRozès N. Effect of growth temperature on yeast lipid composition and alcoholic fermentation at low temperature. Eur. Food Res. Technol. 2011;232:517–527. doi: 10.1007/s00217-010-1415-3. [DOI] [Google Scholar]
- 55.Jordão António Manuel, Cosme Fernanda., editors. Grapes and Wines - Advances in Production, Processing, Analysis and Valorization. 2018. [Google Scholar]
- 56.Howe P, Worobo R, Sacks GL. Conventional measurements of sulfur dioxide (SO2) in red wine overestimate SO2 antimicrobial activity. Am. J. Enol. Vitic. 2018;69:210–220. doi: 10.5344/ajev.2018.17037. [DOI] [Google Scholar]
- 57.Jolly NP, Augustyn OPH, Pretorius IS. The use of Candida pulcherrima in combination with Saccharomyces cerevisiae for the production of Chenin blanc wine. S. Afr. J. Enol. Vitic. 2003;24:63–69. doi: 10.21548/24-2-2641. [DOI] [Google Scholar]
- 58.Morgan SC, Scholl CM, Benson NL, Stone ML, Durall DM. Sulfur dioxide addition at crush alters Saccharomyces cerevisiae strain composition in spontaneous fermentations at two Canadian wineries. Int. J. Food Microbiol. 2017;244:96–102. doi: 10.1016/j.ijfoodmicro.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Casalone E, et al. Mechanism of resistance to sulfite in Saccharomyces cerevisiae. Curr. Genet. 1992;22:435–440. doi: 10.1007/bf00326407. [DOI] [PubMed] [Google Scholar]
- 60.Park, H. & Bakalinsky, A. T. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast16, 881–888, 10.1002/1097-0061(200007)16:10<881::AID-YEA576>3.0.CO;2-3 (2000). [DOI] [PubMed]
- 61.Divol B, du Toit M, Duckitt E. Surviving in the presence of sulfur dioxide: strategies developed by wine yeasts. Appl. Microbiol. Biotechnol. 2012;95:601–613. doi: 10.1007/s00253-012-4186-x. [DOI] [PubMed] [Google Scholar]
- 62.Remize F, Andriru E, Dequin S. Engineering of the pyruvate dehydrogenase by pass in Saccharomyces cerevisiae: role of the cytosolic Mg(2+) and mitochondrial K(+) acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl. Environ. Microbiol. 2000;66:3151–3159. doi: 10.1128/AEM.66.8.3151-3159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.