Abstract
Radiotherapeutic treatment consists of targeted application of radiation beams to a tumor but exposure of surrounding healthy tissue is inevitable. In the brain, ionizing radiation induces breakdown of the blood–brain barrier by effects on brain microvascular endothelial cells. Damage from directly irradiated cells can be transferred to surrounding non-exposed bystander cells, known as the radiation-induced bystander effect. We investigated involvement of connexin channels and paracrine signaling in radiation-induced bystander DNA damage in brain microvascular endothelial cells exposed to focused X-rays. Irradiation caused DNA damage in the directly exposed area, which propagated over several millimeters in the bystander area. DNA damage was significantly reduced by the connexin channel-targeting peptide Gap26 and the Cx43 hemichannel blocker TAT-Gap19. ATP release, dye uptake, and patch clamp experiments showed that hemichannels opened within 5 min post irradiation in both irradiated and bystander areas. Bystander signaling involved cellular Ca2+ dynamics and IP3, ATP, ROS, and NO signaling, with Ca2+, IP3, and ROS as crucial propagators of DNA damage. We conclude that bystander effects are communicated by a concerted cascade involving connexin channels, and IP3/Ca2+, ATP, ROS, and NO as major contributors of regenerative signal expansion.
Subject terms: Cell signalling, Physiology
Introduction
Ionizing radiation, in particular X-rays, is frequently used for diagnostic and therapeutic purposes. Although the precision of selectively irradiating tumor tissues is steadily increasing, radiation toxicity in healthy tissue remains a major issue affecting the potency of radiation treatment1. Tissue toxicity can be linked to vascular damage since microvascular endothelial cells, especially those forming the blood–brain barrier, are vulnerable to ionizing radiation. Microvascular endothelial cells respond with early production of reactive oxygen species (ROS) and DNA damage, including DNA double strand breaks (DSB), and late responses leading to apoptosis and senescence, resulting in ischemia, necrosis, and tissue fibrosis in normal surrounding tissues2. The damage inflicted to directly irradiated cells can be propagated to unexposed surrounding cells, known as the radiation-induced bystander effect, and mimics the direct effects of ionizing radiation encompassing induction of apoptosis, micronucleus formation, DSBs, and mutations3,4. Among the different effects of ionizing radiation exposure, DSBs are the most deleterious because of the high risk of misrepair5,6 and their potential to induce early and late radiation-induced effects7. A useful marker to detect DSBs is the Ser-139 phosphorylated form of the histone H2AX, γ-H2AX, which occurs rapidly after DSB formation8,9 and is followed by dephosphorylation (γ-H2AX disappearance) upon DSB repair. γ-H2AX foci not only appear in the irradiated area but also spread to bystander cells and form an elegant readout to investigate bystander signaling10,11. Although radiation-induced bystander effects have been investigated extensively, the signaling pathways, mechanisms, and molecules involved remain ill-defined. It also appears that considerable differences are observed depending on the cell type, radiation quality, and experimental approach used. Bystander effects are assumed to propagate via signals transmitted through gap junction channels, or via paracrine soluble factors released by irradiated cells4,6. Gap junctions are channels that directly connect two adjacent cells and are composed of two hemichannels, each formed by six connexin subunits. They allow the passage of substances up to ~1.5 kDa including atomic ions (Na+, K+, Ca2+) and important signaling or metabolic molecules like e.g., adenosine triphosphate (ATP), inositol-1,4,5-trisphosphate (IP3), glucose, and glutamate12. Before being incorporated into gap junctions, hemichannels are present as closed precursor channels that are known to open under ischemic or pro-inflammatory conditions13, oxidative stress14, decreased extracellular Ca2+, or increased intracellular Ca2+ 15. When open, hemichannels shunt the plasma membrane and facilitate bidirectional passage of below 1.5 kDa substances, leading to ionic shifts, depletion of important metabolites, or release of paracrine signaling molecules like ATP and glutamate16. Since connexins have a short half-life of several hours, they respond rapidly to stress and environmental changes. In humans 21 different connexin isotypes are known; vascular endothelial cells express Cx37, Cx40, and Cx43 depending on the vessel type17. The expression of Cx43, the most abundant connexin isotype in the human body18, increases in response to ionizing radiation, which results in enhanced gap junctional communication and contributes to radiation-induced bystander signaling in fibroblasts and epithelial cells19,20. Moreover, Cx43 hemichannels have been shown to be involved in ATP release in γ-ray-irradiated melanoma cells21 but the role of these channels in bystander signaling is currently unknown. Ca2+, ROS, and nitric oxide (NO) have been proposed as candidate bystander messengers, but ROS and NO are short-lived and have a limited effective diffusion distance making them unlikely as major propagating messengers of DNA damage22–24. Although the radiation-induced bystander effect gained significant interest over the past decade, the exact mechanisms underlying bystander propagation still remain incompletely understood especially with regard to the concerted action of the many signaling substances involved. Here, we aimed to elucidate the role of connexin channels and the Ca2+/ROS/NO signaling in brain endothelial cell bystander signaling. We hypothesized that ROS/NO may be responsible for the bystander effects, but the spreading is mediated by Ca2+ fluxes carried by extracellular ATP and intracellular IP3 facilitated by connexin channels.
Materials and methods
Cell cultures
The RBE4 (rat brain endothelial) cell line was kindly provided by Dr F. Roux (Neurotech, Evry, France). The RBE4 cells were grown on collagen (rat-tail collagen; Roche diagnostics, Vilvoorde, Belgium) coated recipients in alfa-MEM + F10 (1/1) medium supplemented with 0.6% geneticin, 1% L-glutamax, 10% fetal bovine serum (FBS, Gibco, Invitrogen, Merelbeke, Belgium), and 1 ng/mL human recombinant basic fibroblast growth factor (hbFGF, Roche diagnostics). Cells plated for the radiation experiments were grown without hbFGF. Next to this cell line, also primary brain microvascular endothelial cells (pBMECs) isolated from C57BL6 and C57BL/6 Cx43:fl/flTie2-Cre mice, were used. Primary BMECs were grown in DMEM (Gibco, Invitrogen, Merelbeke, Belgium) supplemented with 20% newborn calf serum (PAN Biotech, Aidenbach, Germany), 1% glutamax (Gibco, Invitrogen, Merelbeke, Belgium), 0.5% gentamicin (Gibco, Invitrogen, Merelbeke, Belgium), 1% vitamins, 2% amino acids, and 1 ng/mL hbFGF; hbFGF was removed from the cultures 24 h prior to irradiation. Primary BMECs cultures were grown on plates coated with matrigel (3.5 µg/cm²). Patch clamp experiments were performed on HeLa cells stably transfected with Cx4325–27, cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Ghent, Belgium), supplemented with 10% FBS, 2 mM glutamine, 10 µg/ml streptomycin, 10 U/ml penicillin, 0.25 µg/ml fungizone (Invitrogen, Ghent, Belgium), and 1 µg/ml puromycin (Sigma-Aldrich, Bornem, Belgium). HelaWT cells were grown in the medium without puromycin. Mouse Cx43 gene was cloned into the EcoRI/BamHI restricted cloning site of the expression vector pMJgreen. Cytomegalovirus promoter was used. The vector also contains a puromycin N-acetyltransferase gene encoding region.
Agents
2ʹ,6ʹ-diamidino-2-phenylindole (DAPI), carbenoxolone (Cbx), N-acetyl-L-cystein (NALC), pyridoxalphosphate-6-azopehyl-2ʹ,4ʹ-disolfonic acid (PPADS), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt, 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yloxy-3-oxide (C-PTIO), and 4% formaldehyde were purchased from Sigma-Aldrich. 1.2-bis-(2-aminophynoxy)-ethane-N,N,Nʹ,Nʹ, tetraacetic acid acetoxy methyl ester (BAPTA-AM) originates from molecular probes (Invitrogen, Merelbeke, Belgium). Following connexin mimetic peptides were used: gap26 (VCYDKSFPISHVR, amino acids 64–76 from the first extracellular loop of Cx43) and TAT-gap19 (YGRKKRRQRRR-KQIEIKKFK, amino acids 128–136 in the second half of the CL of Cx43) obtained from Genosphere biotechnologies (Genosphere biotechnologies, Paris, France) with a purity >85%.
Isolation of brain microvascular endothelial cells
Cortices from 10 to 12-week-old mice, were isolated by removing cerebellum, striatum, optic nerves, brain white matter, outer vessels, and meninges. After homogenization with a Dounce homogenizer in Washing Buffer B (WBB: Hank’s Balanced Salt Solution (HBSS), 10 mM HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid), 0.35 g/L NaHCO3, 0.1% bovine serum albumin (BSA)), the homogenate was mixed with 30% dextran in WBB and centrifuged at 3000 × g for 25 min at 4 °C. The pellet containing the vascular component was then resuspended in WBB and filtered through a 60 µm NY60 Nylon Net Filter (Millipore, Darmstadt, Germany). Following centrifugation at 1000 × g for 7 min at room temperature, the pellet was digested in collagenase/dispase supplement with DNase I (Roche Diagnostics, Vilvoorde, Belgium) and Tosyl, lysin chloromethyl ketone (Sigma-Aldrich) for 33 min at 37 °C in a shaking water bath. The digested capillary suspension was then seeded after multiple washing steps on wells coated with matrigel or glass coated with Corning Cell-Tak (VWR, Leuven, Belgium).
X-ray irradiation
A defined area of the cell dishes were exposed to X-rays (1 Gy or 20 Gy) by using a small animal radiation research platform (SARRP, Xstrahl, Camberley, UK, 220 kV, 13 mA), making use of a 3 × 3 mm collimator. Whole dish irradiation was done using a 10 × 10 cm broad-beam collimator, while focused irradiation was performed with a 3 × 3 mm collimator. A Gafchromic RTQA2 film, with a sensitivity of 0.02 Gy, was placed underneath the cell dishes in order to delineate the irradiated zone. Control experiments with cells exposed to 0.02 Gy did not produce any detectable effect on the γ-H2AX scores (data not shown), excluding the possibility that scattered irradiation not detected by the irradiated film would influence the results in the bystander area.
γ-H2AX immunostaining and counting procedure
Cells were fixed for 25 min with 4% formaldehyde (VWR) and blocked for 30 min with blocking buffer (5% normal goat serum, 1% BSA), 0.2% Triton X-100 in PBS D+). Overnight incubation with primary antibody (1/500 anti-γ-H2AX in dilution buffer, 1/10 blocking buffer in PBS D+) was followed by a 1 h incubation with 1/400 XX-biotin-goat anti-mouse antibody in dilution buffer, followed by a 1 h incubation with 1/400 streptavidin-alexa 488 in PBS D+. Nuclei were stained with 1 µg/mL DAPI in PBS D+ for 10 min and cells were kept in PBS D+ supplemented with NaN3 at 4 °C. All steps except the overnight incubation that was carried out at 4 °C, were performed at room temperature, and cell dishes were rinsed thoroughly with PBS D+ between all incubation steps. Imaging was done with an automated BD Pathway 435 imaging system (×10 objective, 10 × 10 montage resulting in an overall image size of 8.5 × 6.5 mm). The border between the irradiated zone and the bystander area was defined as the full width at two thirds of maximal radiation.
We tested how counting γ-H2AX-positive nuclei over a large surface area was related to the more classical cell-based approach of quantifying the number of γ-H2AX foci per nucleus. To that purpose, we acquired high magnification (x63 objective) images and quantified the relative area occupied by γ-H2AX foci per nucleus in the irradiated zone. We found a linear relation between the low (×10 objective) and high magnification-based quantifications in the range of 0.1–1 Gy; at higher 10–20 Gy doses, the relation flattened (Supplementary Fig. 1). In the bystander zone, the fraction of γ-H2AX-positive nuclei was in the range of 4–12%, which fell within the linear range.
We quantified the number of γ-H2AX foci-positive nuclei in the directly irradiated and bystander areas and expressed the count as a percentage relative to the number of nuclei and subtracted the percentage of background γ-H2AX signal in non-irradiated paired control cells for each experiment; this analysis was done with ImageJ. For counting γ-H2AX foci, a threshold was applied to remove background pixel noise below the foci level. Where applicable, results were normalized against vehicle (bar charts with a 100% vehicle bar without statistical variability or a horizontal line). Gamma-H2AX foci counts of Fig. 1g, h were calculated in a different way: for Fig. 1g, we calculated the summed raw counts of foci-positive vertical pixel columns for each pixel row position along the image x-axis. For Fig. 1h, counting was done as for Fig. 1g but data were normalized to the counts at the border of the irradiation zone (100%). The γ-H2AX foci images of Figs. 1a–d, f and 2a are raw unprocessed immunofluorescence images; foci images of Fig. 7e were processed by a digital dilation operation (ImageJ) to improve visibility of individual foci-positive dots.
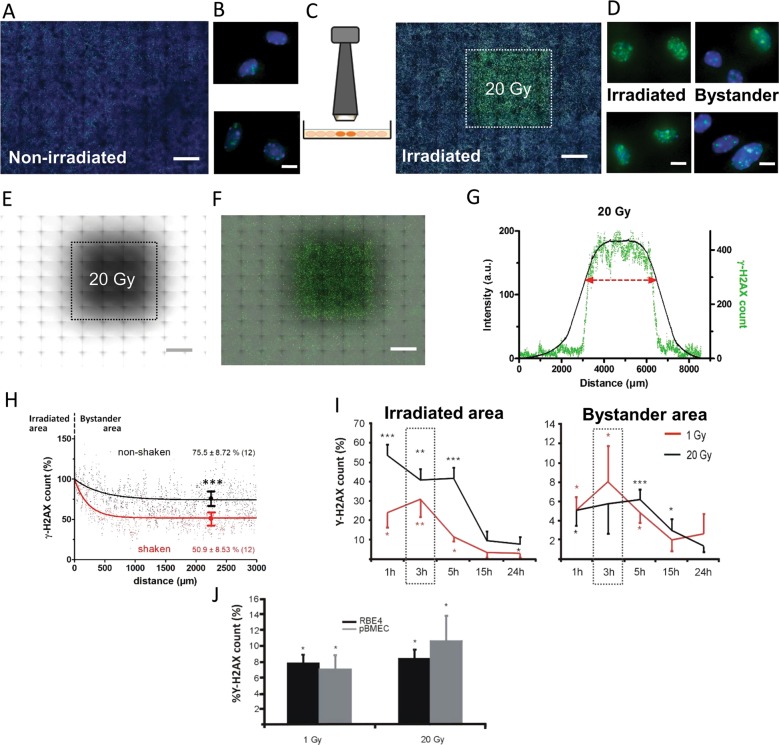
Fig. 1. Investigating radiation-induced bystander effects in response to focused X-ray irradiation.
a Control non-irradiated example image of RBE4 cells with DAPI nuclear staining in blue and background γ-H2AX staining in green (×10 objective; scale bar 1 mm). b Magnifications showing nuclear detail (×40; scale bar 10 µm). c Small aperture 3 × 3 mm collimator used for focused irradiation, with corresponding image taken 3 h post irradiation showing high density γ-H2AX foci (green) in the irradiated zone (dotted line; 20 Gy), and lower density in the surrounding bystander area (DAPI staining in blue; scale bar 1 mm). d Nuclear detail magnifications in irradiated and bystander areas (scale bar 10 µm). e A radiosensitive film placed underneath the cell dish was used to locate the irradiated zone (scale bar 1 mm). f Overlay of the radiosensitive film and the γ-H2AX image (scale bar 1 mm). g Demarcation of the irradiated zone was defined as the full width at two thirds of the maximum radiation intensity (red double arrowed line; ~3.4 mm wide) delineating the zone with the highest γ-H2AX foci count (raw counts, green curve). h Spatial profile of γ-H2AX foci in the bystander area in shaken and non-shaken cells 30 min after irradiation (raw counts normalized to 100% at the border with the irradiated zone). Gamma-H2AX counts averaged over the 1500–3000 µm interval were significantly different from each other. i Time dependence of γ-H2AX signal appearance in the irradiated and bystander areas (γ-H2AX counts relative to the number of nuclei and corrected for background in non-irradiated paired cell dishes) for 1 and 20 Gy irradiation. An asterisk (*) vs. non-irradiated (n = 5–11). j Gamma-H2AX counts in the bystander area recorded 3 h post irradiation were not different between RBE4 cells and pBMECs isolated from C57Bl6 mice. An asterisk (*) vs. non-irradiated.
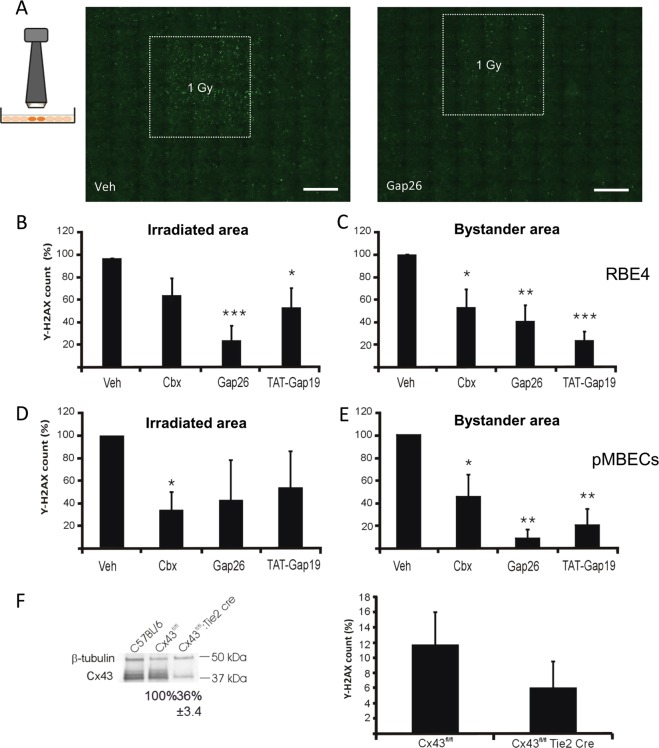
Fig. 2. Connexin channel inhibitors reduce γ-H2AX responses in the irradiated and bystander areas.
a Gamma-H2AX staining in RBE4 cells irradiated with the 3 × 3 mm collimator, treated with vehicle (left) or Gap26 (right; scale bars 1 mm). b Effect of connexin channel inhibition on γ-H2AX scores (normalized to vehicle) in RBE4 cells in the irradiated zone (n = 8–9). c Effect of connexin channel inhibition on bystander γ-H2AX (normalized to vehicle) in RBE4 (n = 8–9). d, e Experiments as in b and c but performed on pBMECs (n = 4–10). f γ-H2AX scores in the bystander area in pBMECs from C57BL/6 Cx43fl/fl:Tie2-Cre mice (n = 12); western blotting analysis (left) demonstrating decreased Cx43 expression in C57BL/6 Cx43fl/fl:Tie2-Cre.
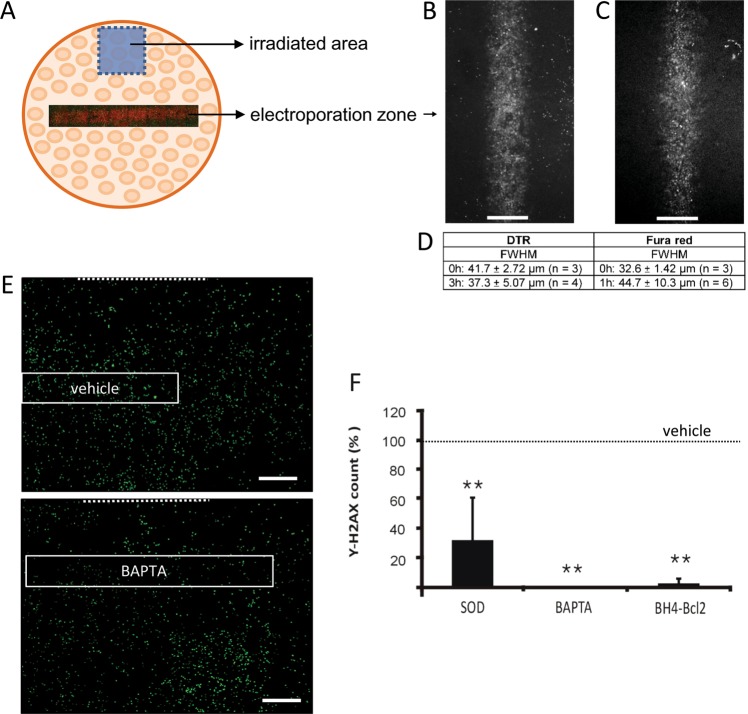
Fig. 7. Localized electroporation loading of cells within the bystander zone with cell-impermeable inhibitors of Ca2+ and ROS signaling pathways strongly reduce γ-H2AX scores.
a Schematic overview of the electroporation experiment, with indication of the irradiated zone and electroporation zone located in the bystander area. b Fluorescence image of the electroporation zone in RBE4 cells as visualized by 10 kDa dextran Texas red (DTR) immediately after electroporation (0 h time point; size bar 50 µm). c Experiment as in b but loaded with fura red as a diffusion reporter (0 h time point). d Full width at half maximum (FWHM) for both dyes at 0 h and later. e Representative γ-H2AX images with indication of the electroporation zone loaded with vehicle or BAPTA (size bar 1 mm). Dotted line indicates the border of the irradiated area. f Summary γ-H2AX data in the electroporation zone, demonstrating that SOD, BAPTA, and BH4-Bcl2 significantly decreased γ-H2AX counts compared with vehicle. An asterisk (*) vs. vehicle (n = 4–5).
Gel electrophoresis and western blotting
RBE4 cells or pBMECs were seeded in 25 cm² falcons or 8 cm² petridishes. Lysates were made with RIPA (Cx43 and Cx37) and laemmli (Cx40) buffer. Protein concentration was determined using the BioRad DC protein assay kit (BioRad, Nazareth, Belgium). The lysate was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), over a mini-protean TGX stain-free gel (BioRad, Nazareth, Belgium) and transferred to a nitrocellulose membrane (Amersham, Buckinghamshire, UK). Membranes were blocked in TBS supplemented with 5% (Cx43 and Cx40) or 2% nonfat milk (Cx37) and 1% (Cx43 and Cx40) or 0.05% (Cx37) Tween20. The following primary antibodies were used: rabbit-anti-Cx43 (sigma), rabbit-anti-Cx37 (anti-rat and anti-mouse, Alpha Diagnositcs, Reinach, Switzerland), goat-anti-Cx40 (Santa Cruz), or rabbit-anti-β-tubulin antibody (Abcam, Cambridge, UK). Membranes were subsequently incubated with an alkaline phosphatase-conjugated goat anti-rabbit (Cx43, Cx37) or donkey anti-goat (Cx40) IgG antibody (Sigma-Aldrich). Detection was done using the nitro-blue-tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate reagent (NBT/BCIP kit, Zymed, Invitrogen). Total protein staining was carried out with SYPRO Ruby protein blot dye (Invitrogen, Molecular Probes, Merelbeke, Belgium).
Immunocytochemistry
For Cx immunoscytochemistry (Cx37, Cx40, and Cx37), fixation was as described for γ-H2AX immunostaining, followed by a 30 min blocking step with blocking buffer B (0.2% Tx100, 0.4% gelatin). Cells were incubated overnight with sheep anti-Cx37 (1/1000, Invitrogen), goat anti-Cx40 (1/50, Santa Cruz), or rabbit-anti-Cx43 (1/500), sigma) combined with rat anti-CD31 (combination of two antibodies, each 1/100, BD and Invitrogen) at 4 °C. In a next step, secondary antibodies were administered for 1 h (donkey anti-sheep alexa 594, chicken anti-goat alexa 594, goat anti-rabbit alexa 594, and goat anti-rat alexa 488, respectively, each 1/400 in blocking buffer B). Confocal images were taken with a Leica SP8 X confocal microscope (×63 water immersion objective) and analyzed using FiJi software.
ATP measurements
For ATP measurements the supernatant present on the cells during irradiation was transferred to a 96-well plate for analysis. ATP was measured using a luciferin–luciferase assay kit following the manufacturer’s instructions (Sigma-Aldrich) in combination with a luminometer plate reader (Victor3 1420 multi label counter; Perkin Elmer, Zaventem, Belgium).
Dye uptake
Cells were grown to near confluency in four-well plates and were incubated with 1 mM propidium iodide (PI) and 200 µM 10 kDa dextran-FITC before focal irradiation and dye uptake was measured 5 min post irradiation by measuring the fluorescence intensity of both dyes following image acquisition with a BD pathway 435 imaging system.
Scrape loading and dye transfer (SLDT)
Confluent cell monolayers were washed three times with nominally calcium-free SLDT buffer (137 mM NaCl, 5.36 mM KCl, 0.81 mM MgCl2.6H2O, 5.55 mM D-glucose, 25 mM HEPES, pH 7.4). Cells were incubated for 1 min in SLDT buffer containing 400 μM 6-carboxyfluorescein; a linear scratch (one per dish) was made across the cell layer using a syringe needle, and the cells were left for another minute in the same solution. Cells were then washed six times with HBSS-HEPES and left for 15 min at room temperature, and images were taken with an inverted epifluorescence microscope equipped with a Nikon DS Ri1 camera.
Electrophysiological recording
Subconfluent HeLa-Cx43 cell cultures were seeded on 13 mm diameter glass coverslips (Knittel Glaser, Novolab, Geraardsbergen, Belgium) and experiments were performed at subconfluency the next day. Recordings were performed in the presence of extracellular Ca2+ and Mg2+ and under conditions of K+-channel blockade with Cs+, Ba2+, and TEA+. Cells were bathed in a recording chamber filled with a modified Krebs–Ringer solution, consisting of (in mM): 150 NaCl, 6 CsCl, 2 MgCl2, 2 CaCl2, 5 glucose, 5 HEPES, 1 BaCl2, and 2 pyruvate, with pH adjusted to 7.4. The whole-cell recording pipette solution was composed of (in mM): 130 CsCl, 10 NaAsp, 0.26 CaCl2, 5 HEPES, 2 EGTA, 5 TEA-Cl, and 1 MgCl2, with an adjusted pH of 7.2. Free intracellular Ca2+ was 50 nM, as calculated with Webmax Standard (http://www.stanford.edu/~cpatton/webmaxcS.htm) software application. An EPC 7 PLUS patch clamp amplifier (HEKA Elektronik, Lambrecht/ Pfalz, Germany) was used to perform single-channel recordings. Data were acquired at 6 kHz using a NI USB-6221 data acquisition device from National Instruments (Austin, TX, USA) and WinWCP acquisition software (designed by Dr J. Dempster; University of Strathclyde, UK). All currents in whole-cell configuration were filtered at 1 kHz (seven-pole Bessel filter). For single-channel analysis, holding currents were subtracted from the recorded current traces, giving traces that only contained unitary current events. Unitary conductances were calculated from the elementary current transitions Δi as: γ = Δi/Vm. From these data, we constructed all-point conductance histograms that displayed one or more Gaussian distributions. These were fit by a probability density function assuming independent channel opening25,27–29. Channel activity was quantified from the charge transfer Qm associated with unitary currents; this was done by integrating the unitary current traces (i.e., a function of time) over the duration of the voltage step as: .
Calcium imaging
Cells were seeded on glass coverslips (18 mm ø) coated with 3.5 µg/cm² Corning cell-tak (VWR, Leuven, Belgium) and ester loaded for 45 min with 10 µM Fluo-3-AM in HBSS-HEPES supplemented with 1 mM of probenecid and 0.01% of pluronic F127 at room temperature followed by de-esterification over 15 min. Imaging was performed using an inverted fluorescence microscope equipped with a ×40 oil immersion objective and an EM-CCD camera (QuantEM™ 512SC CCD camera, Photometrics, Tucson, AZ). For direct Ca2+ measurements the loaded cells were irradiated and transferred to the microscopy stage. For medium transfer, the loaded cells were superfused for 1 min with HBSS-HEPES (1 mM), followed by 4 min with HBSS-HEPES solution retrieved from irradiated cells. Fluorescence intensity changes in the cells were analyzed with custom-developed FluoFrames software (L. Leybaert, Ghent, Belgium). Ca2+ changes were quantified as the area under the curve (AUC) of the Ca2+ traces.
ROS measurements
Cells were seeded in 96-well plates coated with collagen and broad-beam irradiated at different doses. For ROS measurements the cells were loaded with 10 µM of CM-H2DCFDA in HBSS-HEPES. Imaging was performed with a BD Pathway 435 microscope equipped with an automated imaging focus system, avoiding ROS generation associated with long exposures to excitation30. Results were corrected for the ROS produced in the irradiated medium alone.
Cytotox Glo assay
To evaluate cell death, RBE4 cells were seeded in 96-well plates coated with collagen and irradiated using broad-beam at different doses. The CytoTox-GloTM Cytotoxicity assay (Promega, Madison, USA) was used following the manufacturers protocol. The CytoTox-Glo™ Cytotoxicity Assay uses a luminogenic peptide substrate, the AAF-Glo™ substrate, to measure dead-cell protease activity, which is released from cells that have lost membrane integrity. Measurements were performed with a luminometer plate reader.
Electroporation
Cells were grown to near confluency on four-well plates coated with collagen. Cell monolayer dishes were washed three times with HBSS-HEPES 25 mM (pH 7.2–7.4) and subsequently three times with a low conductivity electroporation buffer (4.02 mM KH2PO4, 10.8 mM K2HPO4, 1.0 mM MgCl2, 300 mM sorbitol, 2.0 mM HEPES, pH 7.4). They were placed 400 μm underneath a two-wire Pt–Ir electrode on the microscopic stage and electroporated in the presence of a tiny amount of electroporation solution (10 μl) containing 100 µg/mL superoxide dismutase (SOD), 60 µM BAPTA, or 20 µM BH4-Bcl2 combined with 100 μM 10 kDa dextran Texas red (DTR). Control cell dishes were electroporated with solution containing only 100 μM DTR vehicle solution. Electroporation was carried out with 50 kHz bipolar pulses applied as trains of ten pulses of 2 ms duration each and repeated 15 times. The field strength was 100 V peak-to-peak applied over a 500 μm electrode separation distance. After electroporation, cells were thoroughly washed with HBSS-HEPES 25 mM followed by addition of CO2-independent medium (Invitrogen), which was also present on the cells during and following irradiation. The irradiated area was chosen away from the electroporation area (1555 ± 1468 µm border-to-border distance; n = 15) in order to investigate the effect of the blockers in the bystander area.
Data and statistical analysis
Data are expressed as mean ± SEM, with “n” denoting the number of independent experiments. Multiple groups were compared by one-way ANOVA and a Bonferroni posttest, using GraphPad Instat Software (Graphpad Software). P < 0.05 was considered statistically significant. Statistical significance is indicated in the graphs by one symbol for P < 0.05, two symbols for P < 0.01, and three symbols for P < 0.001.
Results
X-ray-induced DNA damage is propagated from irradiated to non-irradiated bystander cells
To investigate the role of connexin-mediated intercellular communication in radiation-induced bystander responses, we irradiated a defined area of adherent brain microvascular endothelial cells. Both RBE4 cells, a rat brain microvascular endothelial cell line, as well as pBMECs isolated from mouse brains, grown to confluency, were used to that purpose. These cells express Cx37, Cx40, and Cx43 based on western blotting and immunocytochemical studies (Supplementary Fig. 2). Endothelial cell monolayers were locally exposed to X-rays (1 and 20 Gy) using a 3 × 3 mm collimator (Fig. 1), resulting in an irradiated area of ~9 mm2 within a total 190 mm² cell dish surface area. The irradiated zone was identified by a radiosensitive film positioned below the cell dish (Fig. 1e). After irradiation, γ-H2AX immunostainings were performed and imaged in a 55.25 mm2 large area that included the irradiated and surrounding non-irradiated zones. Overlays between the radiosensitive film and γ-H2AX staining allowed to distinguish irradiated and non-irradiated surrounding zones (Fig. 1f). As inferred from Fig. 1f, the irradiated but also non-irradiated zone showed clearly discernable γ-H2AX foci, which were further spatially quantified in terms of radiation intensity and γ-H2AX signal (Fig. 1g).The radiation intensity curve had smoothly varying slopes, while the γ-H2AX profile showed sharp falling edges at the collimator borders, indicating a threshold-like γ-H2AX response. The borders of the irradiated zone were defined based on the full width at two thirds of the maximal radiation intensity (red arrowed line in Fig. 1g), reliably delineating a zone of ~3.4 × 3.4 mm (11.6 mm²). After irradiation, cell dishes were transferred to the lab for subsequent analysis and we thus verified whether leaving the cells immobile would give different γ-H2AX scores in the bystander zone. We found that movement and associated cell dish shaking gave significantly lower γ-H2AX scores in the bystander zone (Fig. 1h) compared with the non-shaken condition (30 min after irradiation). This suggests involvement of paracrine bystander communication, with shaking disturbing an unstirred layer thereby diluting locally released bystander signaling molecules.
In a next experiment, we determined the kinetics of the γ-H2AX signal by counting γ-H2AX-positive nuclei expressing them relative to the total number of nuclei and background correcting them by subtracting relative counts from non-irradiated paired control cell dishes (γ-H2AX count expressed as a percentage). We observed a dose-dependent increase in γ-H2AX scores in the irradiated zone at 1–3 h after irradiation (1 Gy dose), followed by a gradual recovery over the following 24 h (Fig. 1i). We also observed increased γ-H2AX scores in the bystander area, which peaked at 3–5 h post irradiation followed by recovery 24 h later. Interestingly, γ-H2AX responses in the bystander zone were not different between 1 and 20 Gy irradiation (8 ± 3.8% vs. 6.7 ± 2.9%, respectively, 3 h post irradiation; n = 5–10) suggesting that bystander responses saturate between 1 and 20 Gy, as reported by others31–33. The γ-H2AX scores recorded 3 h post irradiation in the bystander area were not different in pBMECs compared with RBE4 cells (Fig. 1j). As 1 and 20 Gy gave equipotent bystander effects, we chose the lower 1 Gy dose for further studies.
Gap junction and hemichannel inhibitors reduce bystander γ-H2AX scores
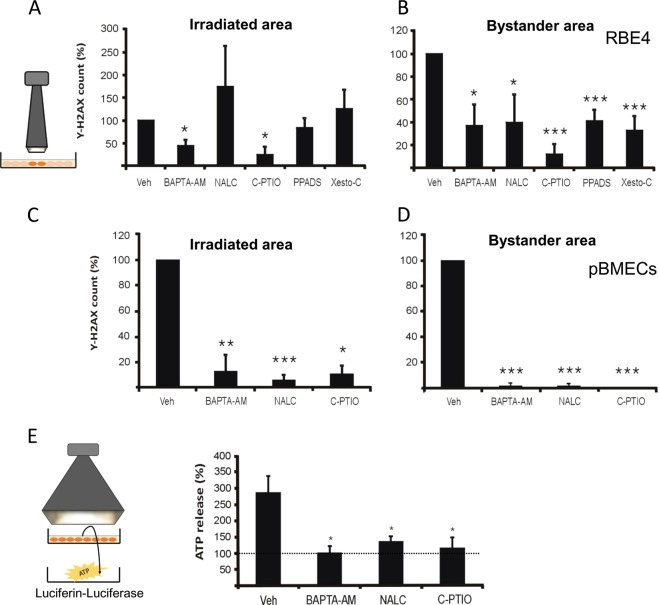
Paracrine factors and direct cell–cell communication via gap junctions are both involved in mediating bystander effects4. As such, not only gap junctions, but also hemichannels could contribute to the DNA damage propagation process. To investigate the contribution of both channel types, we applied several connexin channel inhibitors: the general connexin channel blocker Cbx, Gap26 that targets Cx43, Cx40, and Cx3734 and inhibits hemichannels within minutes and gap junctions with longer exposures27, and TAT-Gap19 that blocks Cx43 hemichannels within 2 min, without inhibiting gap junctions25 (reviewed in Leybaert et al.35,36). Cbx (50 µM), applied 30 min before 1 Gy irradiation and also present in the medium thereafter (30 min + 3 h post irradiation), significantly inhibited γ-H2AX-positive cell counts in the bystander zone while its effect in the irradiated zone were non-significant (RBE4 cells; Fig. 2b, c). By contrast, Gap26 and TAT-Gap19 (161 µM and 200 µM resp.; 30 min + 3 h post irradiation) significantly inhibited γ-H2AX counts in both irradiated and bystander zones. Control experiments with addition of the TAT translocation sequence lacking the active Gap19 moiety did not significantly affect the γ-H2AX counts in both zones (irradiated zone: 110 ± 24%; bystander zone: 104 ± 48%; n = 15). Experiments on primary isolated BMECs demonstrated significantly decreased γ-H2AX counts in both irradiated and bystander zones with Cbx, whereas Gap26 and TAT-Gap19 significantly inhibited only in the bystander zone (Fig. 2d, e). Both Cbx and Gap26 inhibit channels formed by different connexin isotypes, while TAT-Gap19 specifically targets Cx43-based hemichannels. To further substantiate the contribution of Cx43, we isolated pBMECs from C57BL/6 Cx43:fl/flTie2-Cre mice that have targeted Cx43 knockout in endothelial and hematopoetic cells under control of the Tie2 promoter. pBMECs derived from the latter animals displayed strongly decreased Cx43 expression compared with Cx43fl/fl mice, but the bystander response 3 h post irradiation only showed a non-significant trend to decrease (Fig. 2f). Of note, western blotting analysis demonstrated a trend for increased Cx37 expression in pBMECs from C57BL/6 Cx43:fl/flTie2-Cre mice (Supplementary Fig. 3b), suggesting compensatory Cx37 upregulation which may mask the Cx43 knockout effect.
Irradiation induces Cx43 hemichannel opening
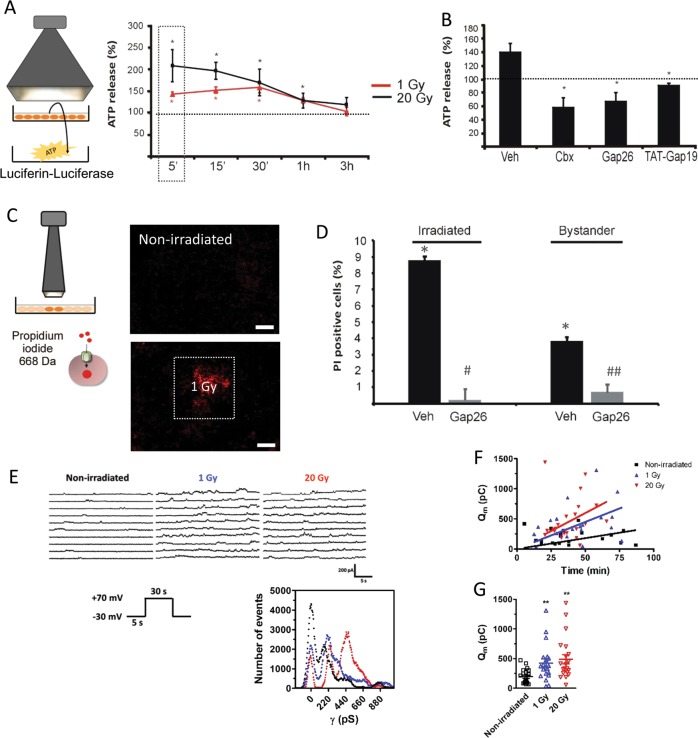
We examined whether X-rays open hemichannels by three different approaches: (i) measuring ATP release in the supernatant, (ii) determining the cellular uptake of the hemichannel-permeable dye PI, and (iii) patch clamp experiments. For ATP release, we irradiated RBE4 cells with a whole-field broad-beam collimator and measured ATP 5 min post irradiation. Both 1 and 20 Gy irradiation significantly increased extracellular ATP release compared with non-irradiated cell dishes and then gradually returned to baseline levels within 3 h (Fig. 3a). ATP release was inhibited by Cbx, Gap26, and TAT-Gap19 (added 30 min before and present until the end; Fig. 3b). In line with this, we found significantly increased cellular uptake of the hemichannel-permeable fluorescent dye PI in irradiated RBE4 cells, in both the irradiated and bystander zones 5 min post irradiation (1 Gy; Fig. 3c). PI uptake was significantly reduced by Gap26 in both areas (Fig. 3d). Irradiation did not affect the uptake of the hemichannel-impermeable dye 10 kDa dextran-FITC (data not shown), indicating membrane integrity was preserved; this was confirmed by the absence of detectable release of a high molecular weight protease (Supplementary Fig. 3e). For patch clamp experiments, both HeLa cells stably transfected with Cx43 (HeLa-Cx43) and HeLaWT were exposed to broad-beam irradiation (1 and 20 Gy) followed by rapid transfer to the electrophysiology setup for single-channel recording. Both 1 and 20 Gy doses resulted in increased unitary current events triggered by voltage steps from −30 to +70 mV applied to activate hemichannel opening (Fig. 3e). All-point histograms demonstrated a unitary conductance of ~220 pS typical for Cx43 hemichannels25,27,37. Potentiation of unitary current activities increased with post-irradiation time and radiation dose (Fig. 3f), and averaging the activity over all time points (range 5–87 min) demonstrated significantly increased membrane charge transfer (Qm) compared with non-irradiated controls (Fig. 3g). Of note, SLDT experiments did not show significant alterations of gap junctional coupling after 1 Gy irradiation (Supplementary Fig. 3c).
Fig. 3. Irradiation triggers hemichannel opening.
a Broad-beam irradiation triggers ATP release in RBE4 cells. The luciferin–luciferase signal is normalized to control non-irradiated (100% dotted line). An asterisk (*) vs. nonirradiated control (n = 6). b Summary data from the 5 min point 1 Gy irradiation (boxed area in a) illustrating the effect of connexin channel inhibition. An asterisk (*) vs. vehicle (n = 4). c Irradiation triggers propidium iodide dye uptake in the irradiated and bystander areas. Dye uptake 5 min post irradiation with the 3 × 3 mm collimator (1 Gy, scale bar 1 mm). d Summary data demonstrating radiation-induced dye uptake (relative to number of nuclei and background corrected for signal in non-irradiated cells) that is inhibited by Gap26 in both irradiated and bystander areas. An asterisk (*) vs. non-irradiated, The symbol “#” vs. vehicle, (n = 4). e Patch clamp experiments on HeLa-Cx43 cells demonstrating traces and matching all-point histograms depicting typical Vm-induced (+70 mV, 30 s) Cx43 hemichannel unitary currents without irradiation and after 1 or 20 Gy irradiation. The histogram illustrates 220 pS and 440 pS peaks that are typical for Cx43 hemichannel opening. f Time course of unitary current activities for the conditions explained in e. Points represent membrane charge transfer (Qm) recorded at different time points after irradiation. Linear regression analysis demonstrates that the slopes increase from 0 to 20 Gy, indicating that hemichannel opening increases with time after radiation exposure. g Qm summary data for repeated Vm steps to +70 mV (n = 5). An asterisk (*) vs. non-irradiated.
Irradiation-induced intracellular Ca2+ elevation and ROS production lead to hemichannel opening
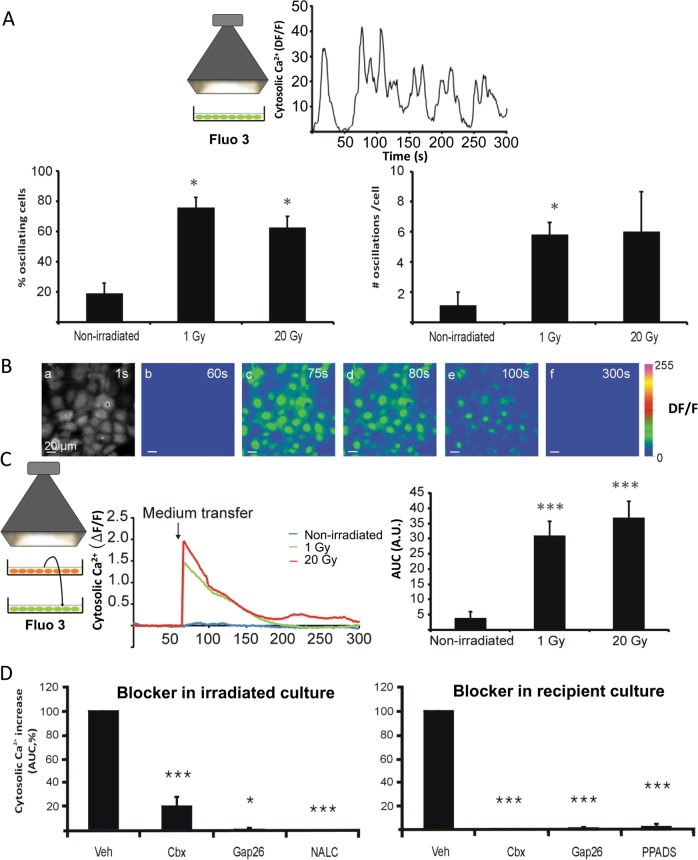
Ionizing radiation has been shown to rapidly generate ROS as well as a rise of cytoplasmic Ca2+ (Ca2+-signal)38, which are two important triggers leading to hemichannel opening14,35. We performed live cell Ca2+-signal imaging experiments starting 5 min after broad-beam irradiation of RBE4 cells, which demonstrated significantly increased Ca2+ oscillations in the cells, both in the percentage of oscillating cells and in the number of oscillations per cell compared with non-irradiated controls; no differences were observed between 1 and 20 Gy (Fig. 4a). In a second approach, transfer of medium collected 5 min after RBE4 irradiation to non-irradiated RBE4 cells induced transient Ca2+-signal elevation in the recipient cells (Fig. 4b, c). Addition of Cbx, Gap26, or the ROS scavenger NALC (1 mM) to the irradiated cells (30 min before irradiation) reduced the AUC of the Ca2+ transients in recipient bystander cells (Fig. 4d). Addition of Cbx, Gap26, or the purinergic receptor blocker PPADS (50 µM) to the recipient cells, also inhibited the Ca2+-signal responses (Fig. 4d).
Fig. 4. Irradiation triggers cytosolic Ca2+ dynamics by direct and paracrine factors.
a Broad-beam irradiation of RBE4 cells triggers cytosolic Ca2+ oscillations recorded 5 min after 1 Gy irradiation. Both the percentage of oscillating cells and the number of oscillations per cell increased upon irradiation. An asterisk (*) vs. non-irradiated (n = 2 cell dishes). b Cytosolic Ca2+ imaging demonstrating Ca2+ dynamics in response to medium transfer from 1 Gy broad-beam irradiated RBE4 cells to reporter RBE4 cells loaded with fluo-3-AM (scale bar measures 20 µm). Image a shows resting fluo-3 fluorescence; b–f are ΔF/F images with b just before medium transfer and subsequent images at time points indicated. c Broad-beam irradiation and medium transfer as used for the experiment in b. Graph shows representative time courses of cytosolic Ca2+ changes upon medium transfer. Bar chart right summarizes average values of area under the curve (AUC) of the Ca2+ changes, demonstrating irradiation significantly increased the AUC compared with medium transferred from non-irradiated cells. An asterisk (*) vs. non-irradiated (n = 3–7). d Connexin channel inhibitors added either to the irradiated (left) or recipient cells (right) strongly reduced the Ca2+ response in the recipient cells (AUC, expressed relative to vehicle). An asterisk (*) vs. vehicle (n = 3–8).
In a third instance, RBE4 cells were preloaded with the oxidative stress marker CM-H2DCFDA-AM, after which the whole cell dish was exposed to X-rays at 1 or 20 Gy. Irradiation induced a significant increase in signal intensity of the CM-H2DCFDA probe measured 5 min after irradiation (Fig. 5). The signal had a trend to increase with dose but this did not attain statistical significance, as observed for the Ca2+-signal responses to medium transfer from irradiated cells (Fig. 4c).
Fig. 5. Irradiation triggers ROS production.
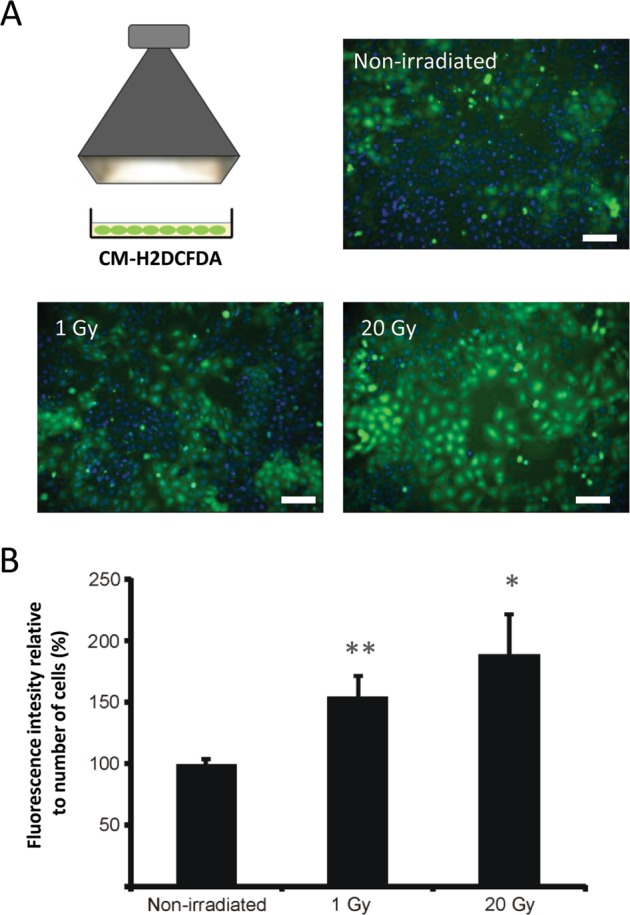
a Broad-beam irradiation increases the fluorescence of CM-H2DFDA-loaded RBE4 cells measured 5 min after irradiation (1 and 20 Gy; scale bar 100 µm). b Quantification of fluorescence intensity relative to the nuclei count and normalized to the non-irradiated condition, showing significantly increased signal for 1 and 20 Gy irradiation. An asterisk (*) vs. non-irradiated (n = 10–11).
Finally, a contribution of Ca2+-signal elevation and ROS production on hemichannel opening was investigated by recording the effect of BAPTA-AM (10 µM; 1 h preloading) and NALC (1 mM; 30 min preloading) on radiation-induced ATP release. Both compounds significantly decreased ATP release 5 min after 1 Gy irradiation (Fig. 6e). NO is involved in the response to ionizing radiation33,39 as well as in hemichannel opening40,41 and we further tested the effect of NO scavenging by preloading the cells with C-PTIO (100 µM; 30 min prior to 1 Gy irradiation); this strongly reduced irradiation-induced ATP release (Fig. 6e) while having no effect on gap junctional coupling (Supplementary Fig. 3f). Taken together, these findings indicate that the opening of hemichannels is an early step in the response of cells to X-rays which involves Ca2+, ROS, and NO signaling pathways.
Fig. 6. Inhibitors of signaling via Ca2+, ROS, NO, ATP, and IP3 inhibit γ-H2AX responses in the irradiated and bystander areas.
a Focused 3 × 3 mm beam irradiation experiments and the effect of cytosolic Ca2+-chelation with BAPTA-AM, ROS scavenging with NALC, NO scavenging with C-PTIO, purinergic P2X antagonism with PPADS, and IP3 receptor antagonism with xestospongin C (Xesto C) on γ-H2AX counts (normalized to vehicle) in RBE4 cells in the irradiated zone. An asterisk (*) vs. vehicle (n = 7–10). b Effects in the bystander area. An asterisk (*) vs. vehicle (n = 7–10). c, d Effect in irradiated and bystander areas in pBMECs. An asterisk (*) vs. vehicle (n = 4–7). e BAPTA-AM, NALC, and C-PTIO also inhibited irradiation-induced ATP release (normalized to non-irradiated control). An asterisk (*) vs. vehicle (n = 3–6).
Interfering with Ca2+, ROS, NO, or ATP signaling reduces irradiation-induced γ-H2AX scores in irradiated and bystander zones
To further document the role of Ca2+, ROS, and NO signaling in the generation of γ-H2AX bystander effects, we applied BAPTA-AM, NALC, and C-PTIO before focused irradiation (3 × 3 collimator) and analyzed their effect in the irradiated and bystander areas. These inhibitors significantly reduced the γ-H2AX scores in both areas, most clearly in primary pBMEC (Fig. 6a–d) with less clear responses being observed in RBE4 cells, especially in the irradiated area (Fig. 6a, c). Interestingly, the IP3 receptor antagonist Xesto C (5 µM) significantly reduced γ-H2AX counts in the bystander zone of RBE4 cells but not in the irradiated area, suggesting IP3 generation in the irradiated zone that is transferred to the bystander zone for subsequent effects. In line with the irradiation-induced ATP release (Fig. 6e), we found that blocking purinergic receptor signaling with PPADS significantly reduced γ-H2AX scores in the bystander area in RBE4 cells (Fig. 6b).
In addition to effects in the bystander area, some of the pharmacological blockers also had significant effects in the directly irradiated area (Fig. 6a, c). Consequently, the reduction of the bystander effects may partly be caused by inhibitory effects in both irradiated and bystander zones. We thus set out to determine the effect of interfering with IP3, Ca2+, and ROS signaling in the bystander zone only, without exposing the irradiated zone to the inhibitors used. To that purpose, we applied in situ electroporation to load a small and defined zone of cells within the bystander area with membrane-impermeable inhibitors of IP3, Ca2+, or ROS signaling42 (Fig. 7). To interfere with IP3 signaling, we loaded the cells with the IP3 receptor blocking peptide BH4-Bcl2 (amino acids 6-30 of Bcl2; MW 3.6 kDa; 20 µM), which interacts with the regulatory/coupling domain of the IP3R43–45. To interfere with downstream Ca2+ signaling, we loaded cell-impermeable BAPTA (MW 476 Da in the absence of Ca2+) into the cells. For ROS, we used the high MW scavenger SOD (MW 32.5 kDa; 3 µM). Of all these substances, BAPTA is the only substance that may potentially pass through gap junctions and thus spread beyond the loaded cell zone. To visualize the spatial distribution of BAPTA and the other high MW substances, we included fura red (809 Da) and 10 kDa DTR in the electroporation solutions. Immediately after electroporation, the full width at half maximum (FWHM) of the electroporation zone was ~42 µm for the 10 kDa DTR and ~33 µm for fura red (Fig. 7d). For DTR, the electroporated zone was not significantly wider 3 h later (~37 µm). For fura red, the fluorescence was too low to estimate the zone width at 3 h; at 1 h we obtained reliable images giving a FWHM of ~45 µm, which is slightly but not significantly larger compared with the width recorded immediately after electroporation. Based on these results, we reasonably concluded that BAPTA diffusion via gap junctions must be limited. We next quantified the γ-H2AX counts in the zone indicated by the 10 kDa DTR tracer, which was included in the electroporation vehicle solution for all experiments. γ-H2AX scoring was done as in all previous experiments, but here we normalized to the score measured in vehicle-electroporated experiments performed in parallel. Fig. 7f summarizes average data of these experiments, demonstrating that BH4-Bcl2, BAPTA, and SOD all strongly reduced the γ-H2AX scores in the electroporation-loaded bystander zone. The strongest effects were observed for the IP3/Ca2+ signaling axis (BH4-Bcl2 and BAPTA). In a last step, we investigated the role of IP3 diffusion via gap junctions, making use of C6 cells stably expressing the V84L mutant Cx26 that is characterized by strongly reduced gap junctional IP3 permeability43. Focused collimator-based irradiation of C6-Cx26 V84L mutant cells demonstrated significantly decreased γ-H2AX scores in the irradiated and bystander zones compared with C6-Cx26 WT cells (Supplementary Fig. 3d), indicating that gap junctional IP3 diffusion is involved in propagating DNA damage.
Discussion
The results demonstrate that bystander communication of DNA damage involves connexin signaling via gap junctions and hemichannels, the canonical IP3/Ca2+ signaling cascade, extracellular ATP, and ROS/NO signaling. Each of these signals has been implicated in radiation-induced bystander signaling38,39,46–50, but a coherent framework is currently lacking. Of note, most of these signals play a role in cell–cell communication of Ca2+ signals51 and all of them have been linked to hemichannels, either as a trigger for their opening14,40,52,53 or as a substance released in a manner facilitated by hemichannels54,55. Specifically, Ca2+ signal communication between cells is mediated by gap junctions that pass IP3 and Ca2+56 and by paracrine signaling that involves extracellular ATP as well as NO released via various mechanisms that include hemichannel-related pathways51,54,57,58. Below we discuss the present findings in the perspective of this Ca2+ signal communication framework.
Irradiation had dose-dependent effects on γ-H2AX scoring in the directly irradiated zone, while a saturation effect was observed in the bystander area (Fig. 1i), as reported by others31–33. Saturation effects in the bystander zone were also observed in the Ca2+-signal responses of recipient cells to medium transfer collected from irradiated cells (Fig. 4a), as observed with γ-rays46,59,60; superoxide production triggered by α-particles likewise displayed saturation effects32. Saturation may result from rate-limiting processes in the signaling cascade and/or regenerative mechanisms that generate maximal signal regardless of the input magnitude. Accordingly, gap junctions may function as a rate-limiting step while regeneration may occur by Ca2+ activation of Phospholipase C (PLC) thereby regenerating IP3, and by ATP-induced ATP release51.
IP3 diffusion through gap junctions contributed to bystander signaling as demonstrated by the reduced γ-H2AX spread in C6 glioma cells expressing IP3-impermeable Cx26 channels. Cx26 is not expressed in microvascular brain endothelial cells but inhibition of IP3 receptors with Xestospongin C (Fig. 6a, b) or BH4-Bcl2 (Fig. 7f) significantly reduced γ-H2AX spreading, pointing to a role for IP3 either diffusing through gap junctions or regenerated as a result of paracrine messengers that activate PLC in distant cells. The gap junctional IP3 diffusion route is certainly involved as junctional coupling was intact and maintained during the time frame of bystander spreading (Supplementary Fig. 3c, d). Also hemichannels contributed and we found that Cx43 hemichannels were open 5 min post irradiation (Fig. 3). Connexin-linked ATP release triggered by γ-rays has been reported in various cell types21,48,49 based on work with non-specific blockers like lindane48 or more specific connexin-targeting peptides like Gap2621. In vivo evidence from Cx43 heterozygous mice (Cx43+/−) exposed to X-rays has furthermore demonstrated a strong linkage between connexin-based ATP release and γ-H2AX bystander responses61. Here, we report that Cbx, Gap26, and TAT-Gap19 suppress γ-H2AX appearance in the bystander zone, with stronger effect size and statistical significance as compared with the irradiated zone (Fig. 2b–e). The smaller effect size in the directly irradiated zone is likely the consequence of the fact that cells are directly hit by radiation in this zone. As Cbx also inhibits Panx1 channels (IC50 in the range of 2–5 µM; presently used concentration 50 µM) and RBE4 cells express Panx162, we cannot exclude Panx1 channel involvement in the results obtained with Cbx. By contrast, TAT-Gap19, which targets Cx43 hemichannels, has no effect on Panx1 channels25 and results obtained with this peptide inhibitor therefore genuinely reflect Cx involvement. Conditional endothelial Cx43 knockout had non-significant effects on bystander communication (Fig. 2f), which may relate to the concomitant increase of Cx37 that can in part compensate for the lost Cx43 (Supplementary Fig. 3b). The strong potency of TAT-Gap19 to inhibit bystander area DNA damage (Fig. 2c, e) indicates a dominant effect of Cx43 hemichannels while general Cx channel block with Cbx proved less effective and of similar magnitude as in endothelial Cx43 knockout (Fig. 2f).
The irradiated cells showed clear oscillatory Ca2+-signal dynamics that play a role in hemichannel opening and ATP release53. Blocking connexin channels furthermore strongly inhibited Ca2+-signal dynamics induced by medium transfer from irradiated cells to recipient naive cells, irrespective whether the inhibitors were applied to irradiated or recipient cell dishes (Fig. 4d). This suggests that connexins, as hemichannels, facilitate the paracrine release of agents that activate Ca2+-signal changes in recipient cells16,63 and facilitate Ca2+ entry at the recipient side53. ROS-induced Ca2+ entry has indeed been demonstrated to be a crucial factor in bystander effects59. In addition to ATP, the irradiated cells also produced ROS (Fig. 5a, b), which may act as a trigger for ATP release48; accordingly, ROS inhibition with NALC suppressed ATP release (Fig. 6e). Moreover, NALC inhibition in irradiated cell dishes and PPADS purinergic receptor inhibition in recipient dishes suppressed Ca2+-signal changes in recipient cells after medium transfer (Fig. 4d). ROS can influence Ca2+ signal by inducing ER Ca2+ release, inhibition of plasma membrane/ER Ca2+ ATPases, stimulation of Ca2+-induced Ca2+ release, or mitochondrial permeability transition pore opening64,65. Conversely, Ca2+ influences ROS signaling in opposite ways: it induces secondary ROS generation in mitochondria via increased oxidative phosphorylation or mitochondrial permeability transition pore opening46,59,66, but can also mitigate ROS signaling by activating antioxidant enzymes such as catalase and SOD64,65. Taken together, these data indicate a tightly connected ATP-ROS-Ca2+-signal signaling triad with strong impact on bystander γ-H2AX responses as judged from the suppressive effect size of PPADS, NALC, BAPTA-AM Ca2+-signal buffering and Xesto C inhibition of IP3 receptors (Fig. 7a, b). NO may be part of this triad, as it is activated by Ca2+-calmodulin signaling, facilitates intercellular Ca2+ signal communication57, contributes to bystander Ca2+-signal signaling in photodynamic therapy67, and may induce DSBs via peroxynitrite (ONOO−) formed by its reaction with ROS39. Accordingly, C-PTIO-based NO scavenging inhibited radiation-induced ATP release (Fig. 6e) as well as bystander γ-H2AX generation (Fig. 6b). Interestingly, the effects of C-PTIO were, like BAPTA-AM and NALC, stronger in pBMECs compared with RBE4, bringing down the γ-H2AX counts to almost zero (Fig. 6a–d); equally strong effects were observed for Gap26 (Fig. 2b–e)68–71.
All things considered, we propose that irradiation activates ATP-ROS-Ca2+-signal signaling with ROS as the primary generated signal, which, given its very short lifetime (10−9 s for the hydroxyl radical23) and diffusion distance (4 nm for the hydroxyl radical24)72, has a limited role in long-range bystander consequences. Ca2+ hereby acts as an intracellular and ATP as an extracellular propagator of bystander effects. Both ATP and Ca2+ diffuse and are actively regenerated by Ca2+-activated IP3 regeneration51 and ATP-induced ATP release73,74. Moreover, Ca2+ may also contribute as an extracellular signal that enters the cells via membrane channels, including open hemichannels and Ca2+ channels. As concerns the role of NO, this messenger has an estimated diffusion distance in the order of 160 µm24 and may be involved in propagation but this needs to be balanced with the fact that low NO concentrations may also mitigate bystander effects75,76. During propagation, extensive crosstalk between these messengers will effectively assemble a robust signaling network linked by hemichannels or gap junctions (Fig. 8). Collectively, the data indicate feed-forward propagation of secondary ROS generation in the bystander zone, driven by the IP3/Ca2+ signaling axis and leading to DNA damage, as apparent from the local interference with ROS/IP3/Ca2+ signaling in the bystander zone (Fig. 7f). Mitochondria likely contribute to secondary ROS generation, as documented for bystander communication of DNA mutations, autophagy, and apoptosis77, which may involve Ca2+-dependent mitochondrial permeability transition78. As targeting IP3/Ca2+ signaling in the bystander zone tended to be more efficient in preventing DNA damage than targeting ROS (Fig. 7f), direct Ca2+/calmodulin-dependent effects on chromatin structure79 may additionally contribute.
Fig. 8. Schematic view of the bystander signal communication network.
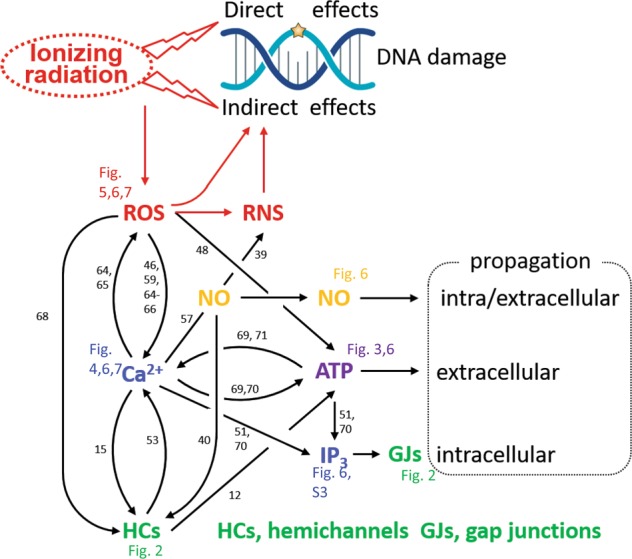
Ionizing radiation directly interacts with DNA molecules and indirectly via radiolysis of water generating ROS and reactive nitrogen species (RNS) produced by ROS interaction with nitric oxide (NO), leading to indirect DNA damage. ROS elevates intracellular Ca2+, and Ca2+ on its turn triggers ROS; Ca2+ also activates synthesis of NO and IP3, and the release of ATP, which drive intracellular and extracellular bystander propagation. IP3 and Ca2+ pass through gap junctions (GJs) while ATP is released via various mechanisms including hemichannels (HCs) that are opened by ROS, Ca2+, and NO. Numbers refer to reference list; Figure numbers refer to results reported here.
Significance for the field. Brain irradiation after glioblastoma tumor removal not only targets remaining tumor cell niches but also brain vascular endothelial cells that, given their close presence to blood oxygen, are highly susceptible to radiation damage. As a result, bystander effects propagating along the network of microvascular endothelial cells and negatively affecting their function or leading to various modes of cell death, will disturb vascular function, resulting in ischemic alterations that compromise normal brain function in the involved areas. Brain endothelial cells not only form the capillary blood–brain barrier but also interact with numerous other cell types, including blood cells, vascular wall cells (pericytes, smooth muscle cells), and parenchymal cells (microglial cells, astrocytes and neuronal cells)80 the interaction with which may be altered at bystander distance, resulting in disturbed neuro-glio-vascular unit functioning.
Supplementary information
Acknowledgements
This work is supported by the Fund for Scientific Research Flanders (FWO-Vlaanderen), Belgium (grants G.0A54.13N, FWO12/PDO/031, FWO15/ASP/080, and FWO15/ASP/124) and BOF, Ghent University, Belgium (01B03142, BOF/13/DOC/227), and the Geneeskundige Stichting Koningin Elisabeth grant A17/OC/0008, DVK is supported by FWO G051918N and Ghent University Special Research Fund IOP 01/O3618.
Conflict of interest
A patent application at the United States Patent and Trademark Office is pending for intellectual property protection of some of the material presented in this study.
Footnotes
Edited by M. Daugaard
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2392-5).
References
- 1.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corre I, Guillonneau M, Paris F. Membrane signaling induced by high doses of ionizing radiation in the endothelial compartment. Relevance in radiation toxicity. Int. J. Mol. Sci. 2013;14:22678–22696. doi: 10.3390/ijms141122678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 4.Verma N, Tiku AB. Significance and nature of bystander responses induced by various agents. Mutat. Res. 2017;773:104–121. doi: 10.1016/j.mrrev.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. 2013;25:578–585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Klammer H, Mladenov E, Li F, Iliakis G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 2015;356:58–71. doi: 10.1016/j.canlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Sokolov MV, et al. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 8.Foster ER, Downs JA. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J. 2005;272:3231–3240. doi: 10.1111/j.1742-4658.2005.04741.x. [DOI] [PubMed] [Google Scholar]
- 9.Hatzi VI, et al. Non-targeted radiation effects in vivo: a critical glance of the future in radiobiology. Cancer Lett. 2015;356:34–42. doi: 10.1016/j.canlet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Firsanov D, Vasilishina A, Kropotov A, Mikhailov V. Dynamics of gammaH2AX formation and elimination in mammalian cells after X-irradiation. Biochimie. 2012;94:2416–2422. doi: 10.1016/j.biochi.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Sedelnikova OA, et al. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- 12.Herve JC, Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013;352:21–31. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- 13.Contreras JE, et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS ONE. 2007;2:e712. doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vuyst, et al. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, et al. Paracrine signaling through plasma membrane hemichannels. Biochim. Biophys. Acta. 2013;1828:35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagasawa K, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 18.Laird DW. Life cycle of connexins in health and disease. Biochem. J. 2006;394(Pt 3):527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzam EI, de Toledo SM, Little JB. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003;63:7128–7135. [PubMed] [Google Scholar]
- 20.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohshima Y, Tsukimoto M, Harada H, Kojima S. Involvement of connexin43 hemichannel in ATP release after gamma-irradiation. J. Radiat. Res. 2012;53:551–557. doi: 10.1093/jrr/rrs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 23.Sarma L, Devasagayam TP, Mohan H, Mittal JP, Kesavan PC. Mechanisms of protection by buthionine sulphoximine against gamma-ray-induced micronuclei in polychromatic erythrocytes of mouse bone marrow. Int. J. Radiat. Biol. 1996;69:633–643. doi: 10.1080/095530096145643. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl Acad. Sci. USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elfgang C, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N, et al. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res. Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vuyst E, et al. In situ bipolar electroporation for localized cell loading with reporter dyes and investigating gap junctional coupling. Biophys. J. 2008;94:469–479. doi: 10.1529/biophysj.107.109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira R, et al. Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol. 2005;6:7. doi: 10.1186/1471-2121-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieprath T, et al. Sustained accumulation of prelamin A and depletion of lamin A/C both cause oxidative stress and mitochondrial dysfunction but induce different cell fates. Nucleus. 2015;6:236–246. doi: 10.1080/19491034.2015.1050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, et al. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- 32.Temme J, Bauer G. Low-dose gamma irradiation enhances superoxide anion production by nonirradiated cells through TGF-beta1-dependent bystander signaling. Radiat. Res. 2013;179:422–432. doi: 10.1667/RR3161.2. [DOI] [PubMed] [Google Scholar]
- 33.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 34.Salameh A. Life cycle of connexins: regulation of connexin synthesis and degradation. Adv. Cardiol. 2006;42:57–70. doi: 10.1159/000092562. [DOI] [PubMed] [Google Scholar]
- 35.Leybaert L, et al. Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharm. Rev. 2017;69:396–478. doi: 10.1124/pr.115.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delvaeye T, Vandenabeele P, Bultynck G, Leybaert L, Krysko DV. Therapeutic targeting of connexin channels: new views and challenges. Trends Mol. Med. 2018;24:1036–1053. doi: 10.1016/j.molmed.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decrock E, et al. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? Biochim. Biophys. Acta. 2017;1864:1099–1120. doi: 10.1016/j.bbamcr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Tomita M, et al. Nitric oxide-mediated bystander signal transduction induced by heavy-ion microbeam irradiation. Life Sci. Space Res (Amst.) 2015;6:36–43. doi: 10.1016/j.lssr.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decrock E, et al. Electroporation loading of membrane-impermeable molecules to investigate intra- and intercellular Ca2+ signaling. Cold Spring Harb. Protoc. 2015;2015:284–288. doi: 10.1101/pdb.prot076562. [DOI] [PubMed] [Google Scholar]
- 43.Decrock E, et al. Transfer of IP(3) through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ. 2012;19:947–957. doi: 10.1038/cdd.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanova H, et al. The BH4 domain of Bcl-2 orthologues from different classes of vertebrates can act as an evolutionary conserved inhibitor of IP3 receptor channels. Cell Calcium. 2017;62:41–46. doi: 10.1016/j.ceca.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Monaco G, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat. Res. 2006;165:400–409. doi: 10.1667/RR3527.1. [DOI] [PubMed] [Google Scholar]
- 47.Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 48.Tsukimoto M, Homma T, Ohshima Y, Kojima S. Involvement of purinergic signaling in cellular response to gamma radiation. Radiat. Res. 2010;173:298–309. doi: 10.1667/RR1732.1. [DOI] [PubMed] [Google Scholar]
- 49.Nishimaki N, Tsukimoto M, Kitami A, Kojima S. Autocrine regulation of gamma-irradiation-induced DNA damage response via extracellular nucleotides-mediated activation of P2Y6 and P2Y12 receptors. DNA Repair. 2012;11:657–665. doi: 10.1016/j.dnarep.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Jella KK, Moriarty R, McClean B, Byrne HJ, Lyng FM. Reactive oxygen species and nitric oxide signaling in bystander cells. PLoS ONE. 2018;13:e0195371. doi: 10.1371/journal.pone.0195371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leybaert L, Sanderson MJ. Intercellular Ca(2+) waves: mechanisms and function. Physiol. Rev. 2012;92:1359–1392. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/S0143-4160(02)00180-X. [DOI] [PubMed] [Google Scholar]
- 53.De Bock M, et al. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J. Biol. Chem. 2012;287:12250–12266. doi: 10.1074/jbc.M111.299610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang J, et al. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bol M, et al. At the cross-point of connexins, calcium, and ATP: blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries. Cardiovascular Res. 2017;113:195–206. doi: 10.1093/cvr/cvw215. [DOI] [PubMed] [Google Scholar]
- 56.Leybaert L. IP3, still on the move but now in the slow lane. Sci. Signal. 2016;9:fs17. doi: 10.1126/scisignal.aal1929. [DOI] [PubMed] [Google Scholar]
- 57.Willmott NJ, Wong K, Strong AJ. A fundamental role for the nitric oxide-G-kinase signaling pathway in mediating intercellular Ca(2+) waves in glia. J. Neurosci. 2000;20:1767–1779. doi: 10.1523/JNEUROSCI.20-05-01767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Saez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology. 2013;75:471–478. doi: 10.1016/j.neuropharm.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 59.Lyng FM, Howe OL, McClean B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int. J. Radiat. Biol. 2011;87:683–695. doi: 10.3109/09553002.2010.549533. [DOI] [PubMed] [Google Scholar]
- 60.Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mancuso M, et al. Role of connexin43 and ATP in long-range bystander radiation damage and oncogenesis in vivo. Oncogene. 2011;30:4601–4608. doi: 10.1038/onc.2011.176. [DOI] [PubMed] [Google Scholar]
- 62.Branca JJV, et al. Oxaliplatin-induced blood brain barrier loosening: a new point of view on chemotherapy-induced neurotoxicity. Oncotarget. 2018;9:23426–23438. doi: 10.18632/oncotarget.25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 64.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharm. Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 65.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao C, Lyng FM, Folkard M, Prise KM. Calcium fluxes modulate the radiation-induced bystander responses in targeted glioma and fibroblast cells. Radiat. Res. 2006;166:479–487. doi: 10.1667/RR3600.1. [DOI] [PubMed] [Google Scholar]
- 67.Cali B, et al. Critical role of gap junction communication, calcium and nitric oxide signaling in bystander responses to focal photodynamic injury. Oncotarget. 2015;6:10161–10174. doi: 10.18632/oncotarget.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Retamal MA. Connexin and pannexin hemichannels are regulated by redox potential. Front. Physiol. 2014;5:80. doi: 10.3389/fphys.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Bock M, et al. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog. Neurobiol. 2013;108:1–20. doi: 10.1016/j.pneurobio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012;1:789–803. doi: 10.1002/wmts.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiong Y, Sun S, Teng S, Jin M, Zhou Z. Ca(2+)-dependent and Ca(2+)-independent ATP release in astrocytes. Front. Mol. Neurosci. 2018;11:224. doi: 10.3389/fnmol.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sawal HA, Asghar K, Bureik M, Jalal N. Bystander signaling via oxidative metabolism. Onco Targets Ther. 2017;10:3925–3940. doi: 10.2147/OTT.S136076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ceriani F, Pozzan T, Mammano F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proc. Natl. Acad. Sci. USA. 2016;113:E7194–E7201. doi: 10.1073/pnas.1616061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J. Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto H, et al. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:IORBAN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 76.Thomas DD, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Yu KN, Hou J, Liu Q, Han W. Radiation-induced bystander effect: early process and rapid assessment. Cancer Lett. 2015;356:137–144. doi: 10.1016/j.canlet.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 78.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 79.Du YC, et al. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell. Proteom. 2006;5:1033–1044. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- 80.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.