Abstract
Abstract
Background
Primary care providers prescribe most long-term opioid therapy and are increasingly asked to taper the opioid doses of these patients to safer levels. A recent systematic review suggests that multiple interventions may facilitate opioid taper, but many of these are not feasible within the usual primary care practice.
Objective
To determine if opioid taper plans documented by primary care providers in the electronic health record are associated with significant and sustained opioid dose reductions among patients on long-term opioid therapy.
Design
A nested case-control design was used to compare cases (patients with a sustained opioid taper defined as average daily opioid dose of ≤ 30 mg morphine equivalent (MME) or a 50% reduction in MME) to controls (patients matched to cases on year and quarter of cohort entry, sex, and age group, who had not achieved a sustained taper). Each case was matched with four controls.
Participants
Two thousand four hundred nine patients receiving a ≥ 60-day supply of opioids with an average daily dose of ≥ 50 MME during 2011–2015.
Main Measures
Opioid taper plans documented in prescription instructions or clinical notes within the electronic health record identified through natural language processing; opioid dosing, patient characteristics, and taper plan components also abstracted from the electronic health record.
Key Results
Primary care taper plans were associated with an increased likelihood of sustained opioid taper after adjusting for all patient covariates and near peak dose (OR = 3.63 [95% CI 2.96–4.46], p < 0.0001). Both taper plans in prescription instructions (OR = 4.03 [95% CI 3.19–5.09], p < 0.0001) and in clinical notes (OR = 2.82 [95% CI 2.00–3.99], p < 0.0001) were associated with sustained taper.
Conclusions
These results suggest that planning for opioid taper during primary care visits may facilitate significant and sustained opioid dose reduction.
Electronic supplementary material
The online version of this article (10.1007/s11606-019-05445-1) contains supplementary material, which is available to authorized users.
KEY WORDS: long-term opioid therapy, case-control study, dose reduction, natural language processing, opioid discontinuation
INTRODUCTION
Primary care providers prescribe most long-term opioid therapy and are increasingly asked to taper patients’ opioid doses to safer levels.1, 2 Decreasing opioid dose among long-term opioid therapy (LTOT) patients may reduce the risks of abuse, addiction, and overdose.3 But, opioid tapering after years of therapy may be difficult for patients and is feared by many of them.4, 5 Primary care providers can become so frustrated with the challenges of opioid therapy and opioid tapering that they discharge or abandon these patients.6, 7 Most patients who continue on LTOT believe they benefit from it.8 Even patients taking LTOT who report high levels of pain and low levels of function may report opioids are helpful.9 Therefore, patients often are reluctant to taper and may resist suggestions by providers to taper their dose. In a recent study, only 15% of veterans who discontinued LTOT had taper plans.10
A recent systematic review of opioid tapering interventions concluded that there is very low-quality evidence that several interventions may be effective at reducing LTOT while improving pain, function, and quality of life.11 But, most of the interventions reviewed are not feasible in usual primary care practice. As prescribers of a controlled substance, providers have the power to unilaterally reduce or discontinue opioid prescribing. But, forced or abrupt opioid tapers have been strongly criticized as abusive and counterproductive12, 13 and the Centers for Disease Control and Prevention (CDC) recently wrote that forced tapering is not the intended result of the CDC opioid guideline.14 Hence, it is not known if opioid taper can be feasibly planned and accomplished within the usual primary care setting. Nor is it known which patient characteristics that may trigger taper planning (e.g., substance use disorders) or what components of taper plans [e.g., prescriptions for serotonin-norepinephrine reuptake inhibitor (SNRI) or tricyclic antidepressant (TCA) antidepressants] may be associated with success.
We therefore conducted a nested case-control study of the role of opioid taper plans as documented by primary care providers in the electronic health record. We assessed if documentation of an opioid taper plan in either the clinical notes or the prescription instructions was associated with subsequent significant sustained opioid taper after accounting for patient characteristics and drug regimen. We also assessed what components of opioid taper plans were associated with successful taper.
METHODS
Study Design
We identified a cohort of 2409 long-term, higher-dose opioid recipients aged 18 years or older enrolled in the Kaiser Permanente Washington (KPWA) integrated group practice (IGP). Patients entered the study cohort after two consecutive quarters of opioid prescriptions during January 2011–December 2015 with ≥ 60-day supply and a daily dose of ≥ 50 mg morphine equivalent (MME) in each quarter. This threshold was chosen as consistent with the CDC opioid guideline and previous research concerning when opioid prescribing becomes long term.15, 16 The daily MME in a quarter was calculated as the total MME dispensed divided by the total days of supply dispensed in the quarter excluding fills greater than 1800 MME or day of supply > 180. Our primary measure of opioid dose in each calendar quarter was the moving average of the current and immediately preceding quarter’s average daily MME. Patients remained in the cohort until the earliest of death, disenrollment from the health plan (≥ 60-day enrollment gap), discontinuation of pharmacy benefit, or the study end date, December 31, 2017. Patients with a cancer diagnosis or less than two calendar quarters of follow-up after cohort entry were excluded. Cancer diagnoses were assessed for the year prior to cohort entry. Codes used were ICD-9 codes 140.* through 172.*, 174.* through 194.*, 200.* through 208.*, and 195.8.*, inclusive.
We used a nested case-control design to compare patients who had a sustained opioid taper to those without a sustained taper. Cases were patients with a sustained taper defined as two consecutive quarters after cohort entry that each met at least one of the following two criteria: (1) average daily opioid dose of ≤ 30 MME (with discontinuation as a special case) or (2) at least a 50% reduction in MME from the patient’s nearest peak opioid average dose. A patient’s nearest peak dose was defined as their highest (two-quarter moving) average dose preceding the current quarter (allowing for peaks to be reset when clinically meaningful as described in Table 5 in Supplementary Material). Sustained taper was defined as meeting taper criteria at the end of the second consecutive quarter.
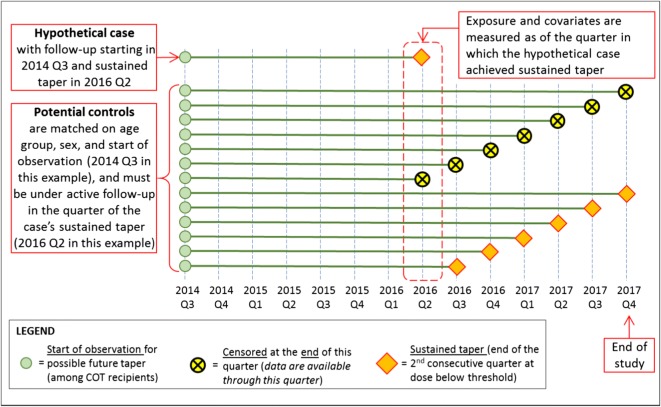
Figure 1 controls were matched to cases on time of cohort entry, sex, and age group. Controls had not achieved a sustained taper as of the same follow-up quarter as the matched case. Matching on calendar time of cohort entry accounted for changes in the healthcare delivery practices and attitudes toward opioid use over time. Matching was also done on sex and age group (18–40, 41–60, and 61+). Since we sought to examine the association of opioid dose and sustained taper, we did not match on dose. We identified 4 controls per case using incidence-density sampling which makes random selections from the pool of eligible controls.17 This procedure allows (1) a patient to serve as a control for more than one case, (2) a control to serve as a future case, and (3) 1:n matching where n is the number of controls per case. Figure 1 illustrates characteristics of controls eligible for matching a hypothetical case.
Fig. 1.
Illustration of a hypothetical case and characteristics of potential matched controls.
A total of 954 patients (40%) in the study cohort achieved a sustained taper. Of these 954 patients, 894 (94%) were matched to four controls and included as cases. Sixty patients with sustained tapers were excluded from analyses because they could not be matched to at least 4 controls. These were more likely male, entered the cohort later, and were younger at cohort entry compared to included cases. The control group included 1524 unique patients comprising a total of 3576 matches, counting each unique combination of one case and one control as a match (i.e., 4 × 894 = 3576). Each control served as a match for from 1 to 10 cases (median = 2).
Taper Plan
Natural language processing (NLP) was used to identify evidence of taper plans from two sources of unstructured clinical text: (1) medication instructions included with prescriptions for opioids (the “SIG” portion of the prescription) or (2) primary care clinical encounter notes as recorded in the electronic health record (EHR). We utilized NLP methods that we have validated and used in multiple previous studies in this population of patients, using this same EHR.18–22 We manually annotated a random sample of 1000 EHR notes and 1000 SIGs to develop and validate an algorithm to identify taper plans. From this review, we determined that the most reliable indications of provider taper plans were (a) SIG simple tapers, which were explicit mentions to “taper,” “reduce,” or “wean” appearing in the medication instructions (e.g., “1 tablet in morning, 1 tablet afternoon, 1/2 tablet evening. 2 weeks only. every 2 weeks wean by 1/2 tab per day”); (b) SIG discontinuations (instructions to stop or discontinue medication use in the medication instructions (e.g., “take one tablet twice daily for two weeks, then once daily for two weeks, then discontinue”); and (c) EHR explicit plans, which contained explicit mentions of a taper plan, taper agreement, or taper schedule in an EHR encounter note (e.g., “Opioid taper plan as below: Week 1: Oxycontin … Week 2: …”).
All occurrences of a taper plan between January 2010 and December 2017 were extracted and summarized at the quarter level. Only taper plans occurring in any of the three quarters preceding the second quarter of sustained taper were considered to have a possible influence on near-term tapering. This time period was chosen because a taper can take up to a year or more and plans are often in place before the taper occurs.
Covariates
Time-invariant covariates included race and Hispanic origin. All time-varying covariates were calculated over a 1-year look-back period and updated quarterly. Covariates included taper plan components and patient clinical characteristics, including (a) any dispensing of SNRI or TCA; (b) any dispensing of gabapentin or pregabalin; (c) any behavioral health specialty visit; (d) any ICD-9 or ICD-10 diagnosis of opioid use disorder (OUD); (e) any ICD-9 or ICD-10 diagnosis of other substance use disorders (SUDs), including alcohol, drug, and cannabis; (f) any ICD-9 or ICD-10 depression diagnosis; (g) any ICD-9 or ICD-10 post-traumatic stress disorder (PTSD) diagnosis; and (h) any dispensing of benzodiazepines. We also assembled a quarterly count of 11 common pain diagnoses (abdominal, arthritis, back, chest, chronic, fibromyalgia, head, limb, neck, neuropathies, and pelvic), ranging from 0 to 11. In previous research, we have found that the type of pain diagnosis has little effect on opioid prescribing and outcomes, but that the number of pain diagnoses has a large effect.23, 24
Statistical Analysis
Each case and four matched controls made up an individual risk set for a total of 894 risk sets. A conditional logistic regression model was used to examine the association between the outcome of a sustained taper and exposure to a taper plan, conditional on the risk sets to account for the case-control matching. Both unadjusted and adjusted models were performed where the adjusted model included near peak dose (see Supplementary Material) and all covariates except Hispanic origin.
Our primary analysis examined the association of a sustained taper from the near peak dose with a qualifying taper plan (defined above). We hypothesized that sustained tapers would be positively associated with the presence of taper plans and, additionally, that SIG taper plans would be more effective because they direct patients to decrease opioid dose via the medication instructions. Therefore, we also conducted separate analyses for SIG taper plans (combining SIG simple and SIG discontinuation) and EHR explicit taper plans, comparing each to no taper plan.
In a secondary analysis, we increased the minimum dose threshold for cohort eligibility to ≥ 90 MME, the maximum opioid daily dose recommended by the CDC guidelines for non-cancer pain.15 We identified corresponding new sets of cases (n = 392) and controls (n = 1568), allowing time of cohort entry to differ by one quarter (to facilitate matching), and otherwise conducted analyses in a manner similar to the primary analysis.
Two sensitivity analyses for the primary analysis were conducted where (1) cases and controls were additionally matched on near peak opioid dose, using three categories (< 90 MME, 90–< 120 MME, ≥ 120 MME), including two controls per case, and (2) the sample was restricted to those patients with an EHR diagnosis of OUD at cohort entry, matching two controls per case when possible, otherwise one control per case.
All analyses were done using SAS. The SAS PHREG procedure was used for modeling.
RESULTS
Study Cohort
As shown in Table 1, the majority of the 2409 patients entered the study in 2011 and were middle-aged (41–60 years). Patients entering the study cohort in 2011 were established or prevalent long-term opioid therapy users; patients entering the study cohort in the subsequent years of the study were new or incident long-term opioid therapy users. The sample was 1446 (60%) female and 1935 (88%) white race. Most of the sample was taking between 50 and 90 MME at study entry. In the year preceding cohort entry, approximately half (1182, 49%) were taking SNRI or TCA medications and 1048 (44%) were taking benzodiazepines. Slightly over a quarter of the sample (629, 26%) was receiving gabapentin or pregabalin, and 656 (27%) received at least one behavioral health visit. OUD was diagnosed in 249 (10%) of the sample, and 277 (12%) were diagnosed with other (non-opioid) SUDs. Depression was diagnosed over the past year in 340 (14%), and PTSD was diagnosed over the past year in 108 (5%) of patients. Patients had a median follow-up of 10 calendar quarters (IQR 5–18) after cohort entry.
Table 1.
Description of Patient Population
| Patient characteristics | Full cohort | Primary model | |
| N = 2409 patients | Cases (N = 894) | Controls (N = 3576) | |
| unique patients with a sustained taper | non-unique patients with no sustained taper at time of case | ||
| N (%) | N (%) | N (%) | |
| Year at cohort entry (matching variable) | |||
| 2011 | 1266 (52.6) | 531 (59.4) | 2124 (59.4) |
| 2012 | 394 (16.4) | 138 (15.4) | 552 (15.4) |
| 2013 | 264 (11.0) | 81 (9.1) | 324 (9.1) |
| 2014 | 253 (10.5) | 79 (8.8) | 316 (8.8) |
| 2015 | 232 (9.6) | 65 (7.3) | 260 (7.3) |
| Age group at cohort entry, years (matching variable) | |||
| 18–40 | 339 (14.1) | 87 (9.7) | 348 (9.7) |
| 41–60 | 1298 (53.9) | 507 (56.7) | 2028 (56.7) |
| 61+ | 772 (32.0) | 300 (33.6) | 1200 (33.6) |
| Median [IQR] | 56 [47–63] | 57 [49–63] | 57 [49–63] |
| Female (matching variable) | 1446 (60.0) | 555 (62.1) | 2220 (62.1) |
| White race1 | 1935 (88.0) | 725 (87.1) | 2930 (88.5) |
| Hispanic origin2 | 81 (3.7) | 33 (4.0) | 154 (4.7) |
| Opioid dose at cohort entry, MME | |||
| 50–< 90 | 1709 (70.9) | 572 (64.0) | 2587 (72.3) |
| 90–< 120 | 317 (13.2) | 134 (15.0) | 443 (12.4) |
| 120–< 150 | 150 (6.2) | 66 (7.4) | 194 (5.4) |
| 150–< 200 | 117 (4.9) | 63 (7.0) | 188 (5.3) |
| 200+ | 116 (4.8) | 59 (6.6) | 164 (4.6) |
| In year prior to cohort entry3 | In year prior to sustained taper for cases and matched follow-up time for controls | ||
| SNRI or TCA medication exposure | 1182 (49.1) | 508 (56.8) | 1804 (50.4) |
| Gabapentin or pregabalin medication exposure | 629 (26.1) | 289 (32.3) | 942 (26.3) |
| Benzodiazepine medication exposure | 1048 (43.5) | 385 (43.1) | 1466 (41.0) |
| Behavioral health treatment | 656 (27.2) | 308 (34.5) | 880 (24.6) |
| Opioid use disorder diagnosis | 249 (10.3) | 146 (16.3) | 361 (10.1) |
| Substance use disorder diagnosis | 277 (11.5) | 204 (22.8) | 412 (11.5) |
| Major depression diagnosis | 340 (14.1) | 216 (24.2) | 635 (17.8) |
| Post-traumatic stress disorder diagnosis | 108 (4.5) | 50 (5.6) | 161 (4.5) |
| Count of anatomical regions with pain diagnoses, median [IQR]4 | 3 [2–4] | 3 [2–4] | 3 [2–4] |
1Race missing for n = 209 patients in full cohort (N = 62 cases, N = 267 controls); % is among non-missing
2Hispanic origin missing for n = 218 patients in full cohort (N = 63 cases, N = 285 controls); % is among non-missing
3Full cohort: 306 patients (13% of total) not enrolled or no pharmacy benefits for a full year prior to study entry (case-control: All patients had 1-year complete history prior to follow-up quarter used for matching)
4Maximum count of 11 common pain diagnoses: abdominal, arthritis, back, chest, chronic, fibromyalgia, head, limb, neck, neuropathies, and pelvic pain
Sustained Taper
Cases with sustained taper (N = 894) are grouped according to the criterion met in their second quarter of sustained taper (see Table 2) with the most common type being low dose (≤ 30 MME; n = 410, 46%) followed by ≥ 50% dose reduction (n = 284, 32%) and discontinuation (n = 200, 22%). The most common near peak dose prior to sustained taper was in the 50–90 MME range. In the year prior to sustained taper, 146 (16%) of tapering patients were diagnosed with OUD, and this rate did not differ significantly by the type of taper achieved (chi-square test, p = 0.25).
Table 2.
Characteristics of Cases
| No. of cases | Sustained taper absolute dose reduction from peak dose, MME | Sustained taper percent dose reduction from peak dose, % | Sustained taper dose, MME | |
|---|---|---|---|---|
| Median [IQR] | Median [IQR] | Median [IQR] | ||
| Total | 894 | 60 [44–90] | 69 [58–91] | 24 [7–38] |
| Type of taper achieved | ||||
| Discontinuation | 200 | 75 [60–100] | 100 [100–100] | 0 [0–0] |
| Low ≤ 30 MME | 410 | 47 [35–62] | 69 [58–81] | 23 [15–28] |
| Reduced ≥ 50% | 284 | 75 [53–122] | 58 [53–66] | 50 [38.5–67.5] |
| Peak dose prior to taper | ||||
| 31–< 50 MME | 21 | 25 [18–40] | 51 [40–100] | 21 [0–27] |
| 50–< 90 MME | 481 | 47 [37–59] | 70 [57–93] | 20 [5–28] |
| 90–< 120 MME | 165 | 72 [60–90] | 71 [61–100] | 27 [0–39] |
| 120–< 150 MME | 84 | 87 [75–114] | 65 [58–92] | 44 [10.5–56] |
| 150–< 200 MME | 66 | 122 [103–145] | 68 [59–83] | 54 [27–67] |
| 200+ MME | 77 | 192 [148–269] | 68 [58–77] | 90 [57–120] |
| Year of cohort entry | ||||
| 2011 | 531 | 66 [49–100] | 68 [57–89] | 27 [8–45] |
| 2012 | 138 | 51 [39–80] | 66 [57–85] | 24.5 [11–31] |
| 2013 | 81 | 53 [42–77] | 73 [60–91] | 20 [7–30] |
| 2014 | 79 | 58 [43–75] | 77 [61–100] | 19 [0–28] |
| 2015 | 65 | 55 [40–70] | 74 [59–100] | 19 [0–29] |
| Age group at cohort entry, years | ||||
| 18–40 | 87 | 61 [44–92] | 77 [62–88] | 20 [7–30] |
| 41–60 | 507 | 62 [45–91] | 68 [57–93] | 26 [6–41] |
| 61+ | 300 | 58 [43–89] | 68 [57–89] | 24 [9–34] |
| Sex | ||||
| Female | 555 | 60 [44–90] | 68 [58–89] | 25 [8–37] |
| Male | 339 | 61 [45–90] | 71 [57–100] | 24 [0–40] |
| Race | ||||
| White | 725 | 60 [44–90] | 69 [58–91] | 24 [7–38] |
| African-American | 39 | 53 [43–90] | 77 [56–100] | 21 [0–30] |
| American Indian | 19 | 61 [39–93] | 69 [54–87] | 23 [18–30] |
| Alaskan Native | ||||
| Asian | 4 | 62.5 [45–87.5] | 63 [61–82] | 26.5 [11.5–41.5] |
| Hawaiian or other | 3 | 50 [34–74] | 56 [52–62] | 40 [32–46] |
| Pacific Islander | ||||
| Other | 16 | 55 [40–88.5] | 61 [53–70] | 30 [24.5–43] |
| Mixed (more than 1) | 26 | 61 [45–68] | 67 [55–84] | 26 [9–41] |
| Hispanic origin | ||||
| No | 798 | 60 [44–90] | 69 [57–90] | 25 [8–37] |
| Yes | 33 | 72 [45–89] | 67 [59–100] | 25 [0–40] |
Among cases achieving sustained taper, 238 (26.6%) had a taper plan in any of the immediately preceding 3 quarters, compared to 306 (8.6%) of the control group (Table 3). The percent of cases with a taper plan from SIG only, EHR note only, or both was 118 (13.2%), 61 (6.8%), and 59 (6.6%), respectively, compared to 161 (4.5%), 95 (2.7%), and 50 (1.4%) for the control group.
Table 3.
Percent of Cases and Controls with a Taper Plan in the Prior Three Quarters
| Patient characteristics | % of case and controls with a taper plan in the 3 quarters prior to matched follow-up time | |
|---|---|---|
| Cases (N = 894) | Controls (N = 3576) | |
| Overall | 26.6 | 8.6 |
| Taper plan source | ||
| SIG discontinuation or simple plan only | 13.2 | 4.5 |
| Clinical explicit note only | 6.8 | 2.7 |
| Both | 6.6 | 1.4 |
| Year at matched follow-up time | ||
| 2011 | 18.2 | 13.6 |
| 2012 | 30.5 | 8 |
| 2013 | 32.4 | 9.9 |
| 2014 | 22.6 | 7.9 |
| 2015 | 26.1 | 8.8 |
| 2016 | 15.7 | 6.1 |
| 2017 | 28.8 | 9.7 |
| Age group at matched follow-up time, years | ||
| 18–40 | 43.1 | 14.5 |
| 41–60 | 29.1 | 8.8 |
| 61+ | 20.9 | 7.3 |
| Sex | ||
| Female | 27.6 | 9.1 |
| Male | 25.1 | 7.7 |
| White race | ||
| No | 32.7 | 11.9 |
| Yes | 26.6 | 8.7 |
| Hispanic origin | ||
| No | 27.2 | 9.1 |
| Yes | 33.3 | 8.4 |
| Peak dose, MME | ||
| 31–< 50 | 14.3 | 11.7 |
| 50–< 90 | 25.2 | 8.2 |
| 90–< 120 | 30.3 | 9.4 |
| 120–< 150 | 27.4 | 6.5 |
| 150–< 200 | 31.8 | 7.9 |
| 200+ | 26 | 11.3 |
| SNRI or TCA medication exposure | 27.6 | 9.1 |
| Gabapentin or pregabalin medication exposure | 27.7 | 8.2 |
| Benzodiazepine medication exposure | 31.4 | 9.7 |
| Behavioral health treatment | 31.8 | 12.5 |
| Opioid use disorder diagnosis | 43.8 | 16.1 |
| Substance use disorder diagnosis | 38.2 | 15 |
| Major depression diagnosis | 32.4 | 13.5 |
| Post-traumatic stress disorder diagnosis | 28 | 16.1 |
| Count of anatomical regions with pain diagnoses1 | ||
| 0 | 20 | 1.6 |
| 1 | 18.8 | 3.1 |
| 2 | 26.6 | 6.3 |
| 3 | 25.3 | 10.1 |
| 4 | 30.9 | 10.4 |
| 5+ | 28.6 | 12.4 |
1Pain is coded as a continuous count of 11 pain diagnoses from 0 to 4 where 4+ = at least 4 (categories include abdominal, arthritis, back, chest, chronic, fibromyalgia, head, limb, neck, neuropathies, and pelvic pain)
Primary Analysis
Table 4 provides results of conditional logistic regression models to examine the association of a sustained taper with patient characteristics, near peak dose, and having a taper plan. In unadjusted models, all patient characteristics other than race, PTSD, and receipt of benzodiazepines were positively associated with a sustained taper as were higher near peak doses and presence of a taper plan. After adjusting for all patient covariates and near peak dose, a taper plan still had a statistically significant association with a sustained taper (OR = 3.63 [95% CI 2.96–4.46]). Both taper plans in prescription instructions alone (OR = 4.03 [95% CI 3.19–5.09]) and taper plans in clinical notes alone (OR = 2.82 [95% CI 2.00–3.99]) were positively associated with sustained taper. Covariates that maintained statistically significant association with sustained taper in the fully adjusted analyses included diagnosis of SUD (OR = 1.74 [95% CI 1.41–2.16]), receipt of gabapentin/pregabalin (OR = 1.26 [95% CI 1.06–1.49]), any behavioral health visits (OR = 1.22 [95% CI 1.00–1.48]), receipt of SNRIs/TCAs (OR = 1.19 [95% CI 1.01–1.40]), near peak dose of 120–< 150 MME (OR = 1.38 [95% CI 1.04–1.83]), and near peak dose of > 200 MME (OR = 1.51 [95% CI 1.12–2.04]).
Table 4.
Conditional Logistic Regression Model Results
| Patient characteristics | Primary model | Primary model | Secondary model | |||
|---|---|---|---|---|---|---|
| Unadjusted results, N = 894 cases, 3576 controls | Adjusted results, N = 894 cases, 3576 controls | Adjusted results, N = 392 cases, 1568 controls | ||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Race | ||||||
| White (reference group) | 1 (–) | 1 (–) | 1 (–) | |||
| Non-white | 1.14 (0.91–1.43) | 0.26 | 1.09 (0.85–1.39) | 0.49 | 0.89 (0.61–1.31) | 0.57 |
| Unknown | 0.94 (0.70–1.25) | 0.67 | 1.10 (0.81–1.48) | 0.56 | 1.38 (0.90–2.14) | 0.14 |
| Overall p value | 0.46 | 0.69 | 0.26 | |||
| Peak dose, MME | ||||||
| 31–< 50 | 1.24 (0.75–2.04) | 0.40 | 1.23 (0.73–2.08) | 0.44 | ||
| 50–< 90 | 1 (–) | 1 (–) | 0.33 (0.12–0.95) | 0.04 | ||
| 90–< 120 | 1.14 (0.94–1.40) | 0.18 | 1.11 (0.90–1.37) | 0.34 | 1 (–) | |
| 120–< 150 | 1.44 (1.10–1.88) | 0.01 | 1.38 (1.04–1.83) | 0.02 | 1.25 (0.90–1.74) | 0.19 |
| 150–< 200 | 1.32 (0.98–1.78) | 0.07 | 1.23 (0.90–1.68) | 0.20 | 1.22 (0.86–1.72) | 0.26 |
| 200+ | 1.51 (1.14–1.99) | 0.004 | 1.51 (1.12–2.04) | 0.01 | 1.45 (1.04–2.03) | 0.03 |
| Overall p value | 0.01 | 0.04 | 0.02 | |||
| SNRI or TCA medication exposure | 1.31 (1.12–1.52) | 0.0005 | 1.19 (1.01–1.40) | 0.04 | 1.24 (0.97–1.58) | 0.09 |
| Gabapentin or pregabalin medication exposure | 1.33 (1.14–1.56) | 0.0004 | 1.26 (1.06–1.49) | 0.008 | 1.17 (0.89–1.54) | 0.26 |
| Benzodiazepine medication exposure | 1.09 (0.94–1.27) | 0.26 | 0.94 (0.80–1.10) | 0.44 | 0.86 (0.66–1.11) | 0.24 |
| Behavioral health treatment | 1.61 (1.37–1.89) | < 0.0001 | 1.22 (1.00–1.48) | 0.05 | 1.04 (0.77–1.40) | 0.79 |
| Opioid use disorder diagnosis | 1.81 (1.46–2.25) | < 0.0001 | 1.13 (0.88–1.45) | 0.33 | 1.56 (1.10–2.20) | 0.01 |
| Substance use disorder diagnosis | 2.26 (1.88–2.73) | < 0.0001 | 1.74 (1.41–2.16) | < 0.0001 | 2.19 (1.60–3.00) | < 0.0001 |
| Major depression diagnosis | 1.48 (1.24–1.77) | < 0.0001 | 1.08 (0.87–1.34) | 0.46 | 1.01 (0.72–1.42) | 0.94 |
| Post-traumatic stress disorder diagnosis | 1.26 (0.91–1.76) | 0.16 | 0.91 (0.63–1.31) | 0.61 | 1.97 (1.11–3.47) | 0.02 |
| Count of anatomical regions with pain diagnoses1 | 1.09 (1.03–1.15) | 0.003 | 0.98 (0.92–1.04) | 0.54 | 1.06 (0.96–1.16) | 0.27 |
| Taper plan in any of the prior 3 quarters | ||||||
| None (reference group) | 1 (–) | 1 (–) | 1 (–) | |||
| Any | 4.00 (3.29–4.87) | < 0.0001 | 3.63 (2.96–4.46) | < 0.0001 | 3.57 (2.65–4.81) | < 0.0001 |
| Taper plan by source | ||||||
| None (reference group) | 1 (–) | 1 (–) | 1 (–) | |||
| SIG discontinuation or simple plan | 4.35 (3.47–5.44) | < 0.0001 | 4.03 (3.19–5.09) | < 0.0001 | 3.60 (2.57–5.04) | < 0.0001 |
| Clinical explicit note only | 3.27 (2.34–4.57) | < 0.0001 | 2.82 (2.00–3.99) | < 0.0001 | 3.50 (2.06–5.92) | < 0.0001 |
1Pain is coded as a continuous count of 11 pain diagnoses from 0 to 5 where 5 = at least 5 (categories include abdominal, arthritis, back, chest, chronic, fibromyalgia, head, limb, neck, neuropathies, and pelvic pain)
Secondary Analysis
In a secondary analysis of patients qualifying for the study at ≥ 90 MME dose, model results were like results for the primary analysis. In adjusted analyses, patients with a taper plan had higher odds of sustained taper (OR = 3.57 [95% CI 2.65–4.81]). Compared to no taper plan, taper plans in prescription instructions (OR = 3.60 [95% CI 2.57–5.04]) were like taper plans in clinical notes alone (OR = 3.50 [95% CI 2.06–5.92]). Among other covariates, SUD was most strongly associated with sustained taper (OR = 2.19 [95% CI 1.60–3.00]), followed by PTSD (1.97 [95% CI 1.11–3.47]) and OUD (OR = 1.56 [95% CI 1.10–2.20]). Compared to near peak doses of 90–< 120 MME, near peak doses of ≥ 200 MME had a higher odds of sustained taper (OR = 1.45 [95% CI 1.04–2.03]) while a lower near peak dose of 50–< 90 MME had a lower odds of sustained taper (OR = 0.33 [95% CI 0.12–0.95]).
Sensitivity Analyses for Primary Model
In a sensitivity analysis where near peak opioid dose was added as a matching variable and cases were matched to two controls, the association between taper plan and successful taper remained statistically significant (OR = 3.72 [95% CI 2.88–4.80]). Both taper plans in the prescription instructions (OR = 4.10 [95% CI 3.06–5.49]) and taper plans in the clinical notes (OR = 2.91 [95% CI 1.89–4.48]) remained significant. In another sensitivity analysis where cases and controls were restricted to those who had an EHR diagnosis of OUD at the time of cohort entry, taper plan continued to have a statistically significant association with successful taper (OR = 6.51 [95% CI 2.22–19.1]). Both taper plans in the prescription instructions (OR = 6.49 [95% CI 2.07–20.4]) and taper plans in the clinical notes (OR = 6.54 [95% CI 1.01–42.6]) remained significant.
DISCUSSION
Among higher-dose LTOT patients, those with an EHR-documented taper plans were more likely to sustain clinically significant opioid tapers. Taper plans noted in prescription instructions (SIGs) and taper plans noted in clinical encounter notes were both associated with an approximately fourfold increased odds of sustained opioid taper. Although taper plans in prescription instructions showed a somewhat stronger relationship with opioid taper than taper plans in clinical notes (OR = 4.03 vs. OR = 2.82), this difference was not statistically significant. These results suggest that there is an association between primary care provider taper plans and the likelihood LTOT patients will taper their opioid doses. This may occur by discussing opioid taper with patients and documenting a taper plan in prescription instructions or clinical notes. The association of sustained tapering with taper plans was stronger than association with patient characteristics or opioid dose. In sensitivity analyses, taper plan remained significantly associated with successful taper when cases and controls were additionally matched on near peak dose and when restricted to those with an EHR diagnosis of OUD at the time of cohort entry.
The concomitant medication and behavioral health therapies we measured as covariates were included for various reasons. Prescriptions for SNRIs/TCAs or gabapentin/pregabalin were hypothesized to make taper easier by providing alternate means of chronic pain relief and reduced affective distress. By contrast, prescription for benzodiazepine was hypothesized to make taper more difficult or to be a marker for distress that might make taper more difficult. Visits to specialty behavioral health were hypothesized to facilitate opioid taper by addressing distress and providing pain self-management skills. Opioid and other substance use disorders were hypothesized to be markers for increased risks of opioid therapy that might prompt primary care providers to initiate discussion and planning for opioid taper. The diagnoses of major depression and post-traumatic stress disorder also increase the risks of opioid therapy and can make opioid taper more difficult. They were hypothesized to decrease the likelihood of successful opioid taper.
While most of these variables significantly increased the likelihood of opioid taper in unadjusted analyses, only a few remained significant in adjusted analyses. These included a marker of risk of problem opioid use (substance use disorder diagnosis) and three therapies that might facilitate opioid taper by providing relief from chronic pain, depression, or anxiety (SNRI/TCA prescription, gabapentin/pregabalin prescription, behavioral health visit). Surprisingly, opioid use disorder did not remain significant in adjusted analyses.
Our secondary analysis, restricted to study-qualifying daily doses ≥ 90 MME, showed similar results. However, in adjusted analyses with this higher-dose sample, the effect of taper mention in prescription instructions was somewhat stronger (OR = 4.30) and the effect of taper mention in clinical notes was somewhat weaker (OR = 2.71) compared to the primary analysis using the 50 MME threshold. Several covariates remained significantly associated with taper in these adjusted analyses including substance use disorder (OR = 2.47), opioid use disorder (OR = 1.61), and behavioral health visit (OR = 1.49). This suggests that substance abuse may be more likely to prompt opioid taper among higher-dose patients. Our sensitivity analyses suggest that taper plans may be effective through the usual opioid dose ranges encountered in clinical care and among patients diagnosed with opioid use disorder.
Our study is the first to examine whether primary care opioid taper plans predict sustained opioid taper among LTOT patients. A recent systematic review by Frank et al.11 examined the effectiveness of a wide variety of interventions to support opioid taper, including interdisciplinary pain programs, buprenorphine-assisted dose reduction, behavioral interventions, other outpatient programs, other interventional programs, detoxification, ketamine-assisted dose reduction, and acupuncture. With the possible exception of the two studies of system-wide interventions in primary care included in the “other outpatient programs” category,2, 25 these are all interventions outside the scope of usual primary care practice. Our study examines the role of documenting a planned taper (by SIG or EHR note), an action available to any primary care provider.
This study has several limitations. First, it was conducted on a sample of patients continuously enrolled in primary care clinics at Kaiser Permanente Washington. These patients were stably enrolled health maintenance organization (HMO) patients who may have more stable social situations than LTOT patients in other care settings. Second, our ascertainment of the presence of a primary care opioid taper plan in prescription instructions or clinical notes depended upon extraction of these plans using NLP, which may have introduced errors. We examined clinical notes and prescription instructions for taper plans, but not after visit summaries. Kaiser Permanente Washington instituted Open Notes, which gave patients access to clinician notes in November 2014. Because our study examined clinician notes from January 2011 to December 2015, we do not believe patient viewing clinician notes played a significant role in our study. Third, our assessment of opioid dose was derived from automated pharmacy records maintained by Kaiser clinical systems. These records have been validated as a complete and reliable record of medication dispensing,26 but we do not have independent records concerning which medications were actually used by patients or if they obtained medications from sources outside Kaiser. We have no information on the use of illicit opioids in our sample, though we assume this to be low based on urine drug testing results in the general LTOT population in this setting.27 Finally, we have no assessment of pain or other patient-reported outcomes for our sample, so we cannot determine whether these worsened or improved with opioid taper.
There are multiple clinical and policy implications of this study. Discussions about and implementation of taper plans for patients on long-term high-dose opioid therapy are often difficult and may be avoided by primary care providers.28 However, our study shows that developing and documenting a taper plan, as reflected in prescription instructions or clinical notes, is associated with a fourfold increase in the chances of a sustained opioid taper. Substance use disorders and common mental health conditions were weaker predictors of successful taper in multivariate analyses than the presence of a taper plan. Other significant components included SNRI/TCA or gabapentin/pregabalin prescription and at least one behavioral health visit. These findings suggest that providing non-opioid medications (Table 7 in Supplementary Material) or psychological support to treat chronic pain, anxiety, or depression may facilitate opioid taper. Primary care providers should be encouraged and supported to have conversations about opioid taper with their patients. As has been shown with smoking cessation, repeated requests to consider and attempt opioid taper may bear fruit.29
Electronic Supplementary Material
(DOCX 237 kb)
Funding Information
This work was supported by an unrestricted research grant from Purdue Pharma (no. 2018103940) who had no role in the study design, analytic strategy, or conclusions drawn.
Compliance with Ethical Standards
Conflict of Interest
Dr. Sullivan reports grants from Purdue in support of the submitted work and grants from Pfizer, personal fees from Aetna, personal fees from Chrono Therapeutics, personal fees from State of Maryland Attorney General, and personal fees from Revon Systems, outside the submitted work. No other authors report conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Centers for Disease C, Prevention. Controlled Substance Prescribing Patterns—Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill Summ. 2015;64(9):1–14. doi: 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- 2.Weimer MB, Hartung DM, Ahmed S, Nicolaidis C. A chronic opioid therapy dose reduction policy in primary care. Subst Abus. 2016;37(1):141–147. doi: 10.1080/08897077.2015.1129526. [DOI] [PubMed] [Google Scholar]
- 3.Nelson LS, Juurlink DN, Perrone J. Addressing the Opioid Epidemic. JAMA. 2015;314(14):1453–1454. doi: 10.1001/jama.2015.12397. [DOI] [PubMed] [Google Scholar]
- 4.Henry SG, Paterniti DA, Feng B, et al. Patients’ Experience With Opioid Tapering: A Conceptual Model With Recommendations for Clinicians. J Pain. 2019;20(2):181–191. doi: 10.1016/j.jpain.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthias MS, Johnson NL, Shields CG, et al. “I’m Not Gonna Pull the Rug out From Under You”: Patient-Provider Communication About Opioid Tapering. J Pain. 2017. [DOI] [PMC free article] [PubMed]
- 6.Wyse JJ, Ganzini L, Dobscha SK, Krebs EE, Morasco BJ. Setting Expectations, Following Orders, Safety, and Standardization: Clinicians’ Strategies to Guide Difficult Conversations About Opioid Prescribing. J Gen Intern Med 2019. [DOI] [PMC free article] [PubMed]
- 7.Ziegler SJ. Patient abandonment in the name of opioid safety. Pain Med. 2013;14(3):323–324. doi: 10.1111/pme.12036. [DOI] [PubMed] [Google Scholar]
- 8.DiJulio B, Wu B, Brodie M. The Washington Post/Kaiser Family Foundation survey of long-term prescription painkiller users and their household members. 2016; https://www.kff.org/report-section/the-washington-post-kaiser-family-foundation-survey-of-long-term-prescription-painkiller-users-and-their-household-members-introduction/. Accessed 15 May 2018.
- 9.Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297–306. doi: 10.1093/pm/pnw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovejoy TI, Morasco BJ, Demidenko MI, Meath THA, Dobscha SK. Clinician Referrals for Non-opioid Pain Care Following Discontinuation of Long-term Opioid Therapy Differ Based on Reasons for Discontinuation. J Gen Intern Med. 2018;33(Suppl 1):24–30. doi: 10.1007/s11606-018-4329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank JW, Lovejoy TI, Becker WC, et al. Patient Outcomes in Dose Reduction or Discontinuation of Long-Term Opioid Therapy: A Systematic Review. Ann Intern Med. 2017;167(3):181–191. doi: 10.7326/M17-0598. [DOI] [PubMed] [Google Scholar]
- 12.Darnall BD, Juurlink D, Kerns RD, et al. International Stakeholder Community of Pain Experts and Leaders Call for an Urgent Action on Forced Opioid Tapering. Pain Med. 2019;20(3):429–433. doi: 10.1093/pm/pny228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kertesz SG, Satel SL, DeMicco J, Dart RC, Alford DP. Opioid discontinuation as an institutional mandate: Questions and answers on why we wrote to the Centers for Disease Control and Prevention. Subst Abus 2019:1–3. [DOI] [PubMed]
- 14.Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med 2019. [DOI] [PMC free article] [PubMed]
- 15.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 16.Von Korff M, Turner JA, Shortreed SM, et al. Timeliness of Care Planning upon Initiation of Chronic Opioid Therapy for Chronic Pain. Pain Med. 2016;17(3):511–520. doi: 10.1093/pm/pnv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12):e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters ET, Ramaprasan A, Mardekian J, et al. Natural Language Processing-Identified Problem Opioid Use and Its Associated Health Care Costs. J Pain Palliat Care Pharmacother 2019:1–10. [DOI] [PubMed]
- 19.Palmer R, Carrell DS, Cronkite D, et al. The Prevalence of Problem Opioid Use in Patients Receiving Chronic Opioid Therapy: Computer Assisted Review of Electronic Health Record Clinical Notes. Pain. 2015. [DOI] [PubMed]
- 20.Carrell DS, Cronkite D, Palmer RE, et al. Using natural language processing to identify problem usage of prescription opioids. Int J Med Inform. 2015;84(12):1057–1064. doi: 10.1016/j.ijmedinf.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Hylan TR, Von Korff M, Saunders K, et al. Automated prediction of risk for problem opioid use in a primary care setting. J Pain. 2015;16(4):380–387. doi: 10.1016/j.jpain.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Carrell DS, Halgrim S, Tran DT, et al. Using natural language processing to improve efficiency of manual chart abstraction in research: the case of breast cancer recurrence. Am J Epidemiol. 2014;179(6):749–758. doi: 10.1093/aje/kwt441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braden JB, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Trends in use of opioids by noncancer pain type 2000-2005 among Arkansas Medicaid and HealthCore enrollees: results from the TROUP study. J Pain. 2008;9(11):1026–1035. doi: 10.1016/j.jpain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112(1–2):90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harden P, Ahmed S, Ang K, Wiedemer N. Clinical Implications of Tapering Chronic Opioids in a Veteran Population. Pain Med. 2015;16(10):1975–1981. doi: 10.1111/pme.12812. [DOI] [PubMed] [Google Scholar]
- 26.Drieling RL, LaCroix AZ, Beresford SA, Boudreau DM, Kooperberg C, Heckbert SR. Validity of Self-Reported Medication Use Compared With Pharmacy Records in a Cohort of Older Women: Findings From the Women’s Health Initiative. Am J Epidemiol. 2016;184(3):233–238. doi: 10.1093/aje/kwv446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JA, Saunders K, Shortreed SM, et al. Chronic opioid therapy urine drug testing in primary care: prevalence and predictors of aberrant results. J Gen Intern Med. 2014;29(12):1663–1671. doi: 10.1007/s11606-014-3010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins EJ, Malte CA, Hagedorn HJ, et al. Survey of Primary Care and Mental Health Prescribers’ Perspectives on Reducing Opioid and Benzodiazepine Co-Prescribing Among Veterans. Pain Med. 2017;18(3):454–467. doi: 10.1093/pm/pnw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013(5):CD000165. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 237 kb)