Abstract
Background:
Defensin peptide isolated from plants are often heterogeneous in length, sequence and structure, but they are mostly small, cationic and amphipathic. Plant defensins exhibit broad-spectrum antibacterial and antifungal activities against Gram-positive and Gram-negative bacteria, fungi and etc. Plant defensins also play an important role in innate immunity, such as heavy metal and some abiotic stresses tolerance.
Objectives:
In this paper, in vitro broad-spectrum activities, antimicrobial and heavy metal absorption, of a recombinant plant defensin were studied.
Material and Methods:
SDmod gene, a modified plant defensin gene, was cloned in pBISN1-IN (EU886197) plasmid, recombinant protein was produced by transient expression via Agroinfiltration method in common bean. The recombinant protein was tested for antibacterial activity against Gram-negative, Gram-positive bacteria and Fusarium sp. the effects of different treatments on heavy metal zinc absorption by this peptide were tested.
Results:
We confirmed the antibacterial activities of this peptide against Gram-negative (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus and Bacillus cereus) bacteria, and antifungal activities of this peptide against Fusarium spp. (Fusarium oxysporum and Fusarium solani). High metal absorption coefficient for this peptide was also observed.
Results:
Out of six actinobacterial isolates, VITVAMB 1 possessed the most efficient RO-16 decolorization property. It decolorized 85.6% of RO-16 (250 mg L-1) within 24hrs. Isolate VITVAMB 1 was identified to be Nocardiopsis sp. Maximum dye decolorization occurred at pH 8, temperature 35oC, 3% salt concentration and a dye concentration of 50 mg L-1.
Conclusions:
Results suggesting that modified defensin peptide facilitates a broader range of defense activities. dedefensins are an important part of the innate immune system in eukaryotes. These molecules have multidimensional properties that making them promising agents for therapeutic drugs.
Keywords: Antibacterial Effect, Heavy Metal Adsorption, Plant Defensin, Therapeutic Potential
1. Background
Plant defensins are small cationic peptides consisting of low number (45–54) of amino acid residues with conserved cysteines that can form four disulfide bridges ( 1 ). Plant defensins comprised of tertiary structure including a single α-helix and triple-stranded anti- parallel β- sheet. The complex results in formation of a compact shape by its four disulfide bridges ( 2 , 3 ). Plant defensins were first studied in wheat and barley ( 4 ). Since 1990s, researchers have been studying antibacterial properties of cationic plant cysteine-rich antimicrobial peptides (AMP), the role of defensins in plant immune system is well reported ( 3 , 5 ). Variations in the amino acids sequences result in small conformational changes in the tertiary structure of a protein. Ultimately these possible variations contribute to biological activities in functional properties of these proteins. One single amino acid substitution can lead to change in the functional activities including antifungal, antibacterial, enzyme inhibitory behaviors. These possible changes have been reported to play a role in heavy metal absorption and abiotic stress-tolerance ( 6 ).
2. Objectives
The antimicrobial activities of plant defensins were mainly observed as they apply to fungi ( 7 , 8 ) and less so to bacteria ( 9 ). Plant defensins can be highly expressed or upregulated when plants are exposed to various abiotic stresses such as drought, salt, cold and heavy metals ( 6 ). In this paper the in vitro broad-spectrum activities of recombinant defensin (SDmod) protein have been investigated. The SDmod gene is a modified plant defensin that facilitates a broader range of defensin activities, based on the sd2 gene sequence from Sunflower ( 3 ). In this study the recombinant SDmod protein was produced by transient expression in common bean.
3. Materials and Methods
3.1. Genes, Plasmid and Microorganisms
The SDmod gene, a modified defensin gene ( 3 ), was cloned with the marker gene LicBM2 in pBISN1-IN (EU886197) plasmid (Fig. 1). In this experiment, LicBM2, isolated from the bacterium Clostridium thermocellum, encodes the thermostable lichenase enzyme and was used as a safe reporter gene. Bacterial β-1,3–1,4-glucanases (EC 3.2.1.73; lichenase) specifically cleave the β-1, 4-glycosidic linkage adjacent to 3-O-substituted glucopyranose residues ( 10 ). DNS method was used to measure enzymatic activity and reducing sugars ( 11 ) to define the recombinant protein in transgenic plants. Two constructs of pBISN1-IN plasmid (one with the SDmod gene and one without) was used to eliminate the effects of the marker gene.
Figure 1.
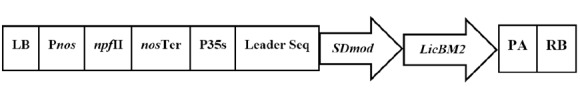
Gene construct of T-DNA region of pBISN1-IN plasmid.
The bacteria Escherichia coli DH5α and Agrobacterium tumefaciens LBA4404 were used for gene cloning and plant transformation respectively.
3.2. Transient Expression by Agroinfiltration Method
The vectors were transferred to A. tumefaciens LBA4404 using the electroporation method ( 12 ). 1 mL of overnight culture of transformed Agrobacterium containing 50 mg.L-1 kanamycin, 100 mg.L-1 rifampicin, was added to 100 mL Infiltration medium containing 100 μM acetosyringone, 50 mg.L-1 kanamycin and 100 mg.L-1 rifampicin, and was incubated in 28 °C with continuous shaking (190 rpm) for 6 hours. The culture centrifuged (5min, 40000 rpm) and the pellet re- suspended in infiltration medium of 200 μM acetosyringone (adjusted to OD600=0.4). Each leaf was injected using syringe infiltration with two types of infiltration medium (transformed bacteria and non- transformed bacteria) (Fig. 2).
Figure 2.

Infiltrating agrobacterium into been epidermal cells for transient expression.
3.3. Protein Extraction
Three days after infiltration, the injected leaves were harvested and grinded in a pestle and mortar with liquid nitrogen. Extraction buffer (250 mL of 150mMTrisHCl pH=8) was added to each sample, homogenized and centrifuged at 12000 rpm for 20 min at 4oC. The supernatant liquid containing the total proteins fraction (protein extract) was transferred to clean micro tubes.
3.4. Selection of Recombinant Proteins
The existence of recombinant protein (defensine- lichenase) in the extracted plant proteins were definded by lichenase enzyme activity and reducing sugars measured by the DNS method ( 11 ). Modified Ellman’s test was used to estimate the recombinant defensing quantities in plant protein solutions ( 13 ). This method can measure the number of cysteine and thiol groups in proteins ( 14 , 15 ). In this study 1M of recombinant protein proved to contain 9M of cysteine.
3.5. Antimicrobial Activity Assay
Following the method of Bhalodia and et al (2011), potato dextrose agar (PDA) and Mueller Hinton Agar (MHA) were used to measure the antifungal and antibacterial susceptibility using the disk diffusion and agar-well diffusion methods ( 16 ). Two recombinant protein(with and without recombinant defensing protein) were produced by transforming two different contracts (LicBM2 and SDmod-LicBM2) using transient expression, to eliminate antimicrobial effect of lichenase enzyme and test the antimicrobial effects of recombinant defensin protein produced in this study. The extracted proteins from the injected leaf by non- transgenic bacteria were used as control to eliminate antimicrobial effect of plant and bacteria proteins. Protein sample aliquot of 150 μL was added to wells with Three recombinant protein defensin-lichenase concentrations (5x10-10, 10x10-10 and 20x10-10 μM) alongside with recombinant protein Lichenase, Kanamycin antibiotic and Nystatin as positive control and protein extraction from the injected leaf by non- transformed bacteria as negative control.
The recombinant proteins were tested for antibacterial activity against known pathogens, Gram-negative Escherichia coli and Pseudomonas aeruginosa bacteria and Gram-positive Staphylococcus aureus and Bacillus cereus bacteria. In addition, recombinant proteins were also tested for antifungal activity of Fusarium spp (Fusarium oxysporum and Fusarium solani).
The halo/ clear zone diameters in bacteria media was measured after 8, 16 and 24 h and in fungal media after 12, 24, 48 and 72 h.
3.6. Preparing Proteins to Measure Metal Absorption Amount
In order to investigate the effect of different treatments on heavy metal zinc absorption, we tested different times (5, 30, 60, 90, 120, 150, and 180 min) ( 17 ), different amounts of active recombinant protein (10×10-9, 30×10-9, 50×10-9, 70×10-9, 90×10-9, 130×10-9, 150×10-9 µM) ( 14 ), different pH treatments of buffer phosphate solution (4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, and 8) and six temperature levels (20, 25, 30, 30, 35, 40, and 50 °C). Here the protein of non-transgenic plant was used as control.
For preparing recombinant protein samples, 3 mL of buffer phosphate solution (137 mM NaCl, 2.7 mM KCL, 4.3 mM Na2Hpo4, and 1.47 mM KH2po4) was incubated with active recombinant protein, 10 percent mercaptoethanol and 2 µl of 0.1 molar zinc sulfate (Zn(So4)27H2O) for 2:30 hours at 35°C. Densities of the zinc (Zn+2) in samples were recorded by the atomic absorption system.
3.7. Statistical Analysis
Triplicated data were analyzed using SAS software. Mean values were compared at 0.05 p value.
4. Results
4.1. Antibacterial Effects
The aim of this study was to investigate the antibacterial properties of the modified defensin protein on the selected gram-positive and -negative pathogenic bacteria.
Defensin-lichenase recombinant protein showed a greater inhibition and larger clear zone on bacterial culture media than recombinant lichenase protein compared with the controls. The highest concentration (20x10-10 μM) of protein formed a significantly larger clear zone compared with the alternate concentrations (Fig. 3).
Figure 3.
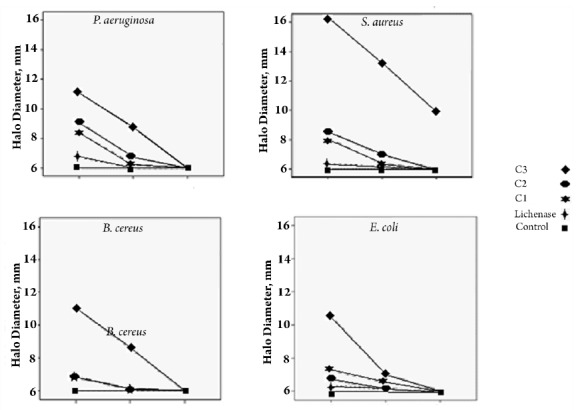
In Vitro antibacterial activity (halo/clear zone diameter) of three concentrations of recombinant defensin protein: C1: 20×10-10 μM. C2: 10×10-10 μM. C3: 5×10-10 μM compared to Lichenase enzyme and control(plant protein) in three times traetment, in gram positive and negative pathogenic bacteria; S. aureus, B. cereus, E. coli and P. aeruginosa.
Analysis of diameter of clear zones at different time points indicated that the halo diagonals were distinctly expressed at higher rates at the first stage of growth (8h) across all bacteria. Over time the antimicrobial activity of the recombinant protein was declined. After 24h, no antibacterial activity of recombinant proteins was recorded, except for S. aureus where the effect was still obvious. In vitro longevity of recombinant protein activities in P. aeruginosa and B. cereus was up to 16h In contrast, due to bacteriostatic effects of the protein, E. coli antibacterial activity was up to 8h. In S. aureus this effect was bactericidal and killed the organism (Fig. 3).
The inhibition zone's mean size for four bacteria showed that E. coli was the least susceptible of the organisms tested, while S. aureus was the most sensitive (Fig. 4). Across all tested bacteria, the inhibitory effects of protein proved to be the greatest at initial times of growth (up to 8h), but these effects declined over time and the bacteria continued to grow after 24h. The suggested concentrations of recombinant defensine protein in this study were bacteriostatic and not bactericidal.
Figure 4.
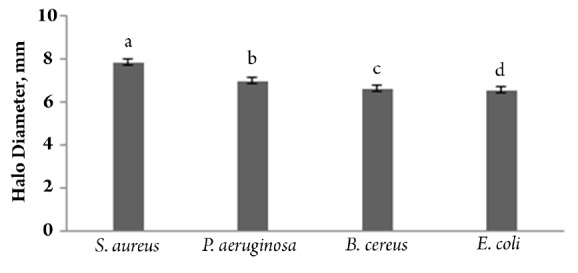
Comparison of antibacterial activity (inhibition halo diameter) of recombinant protein defensin produced by transient expression of SDmod gene in baen plant, against gram positive and negative pathogenic bacteria; S. aureus, B. cereus, E.coli and P. aeruginosa. (Treatments with at laest one letter are not significantly different in 5% LSD test.)
4.2. Antifungal Effects
As for the investigation of the antifungal activity of recombinant protein we used the highest concentration of protein (20×10-10 μM).
Analysis of halo diagonals of clear zone at different growth time points (12, 24, 43 and 72 h) showed that the antimicrobial activity of recombinant protein was reduced over time (Fig. 5).
Figure 5.
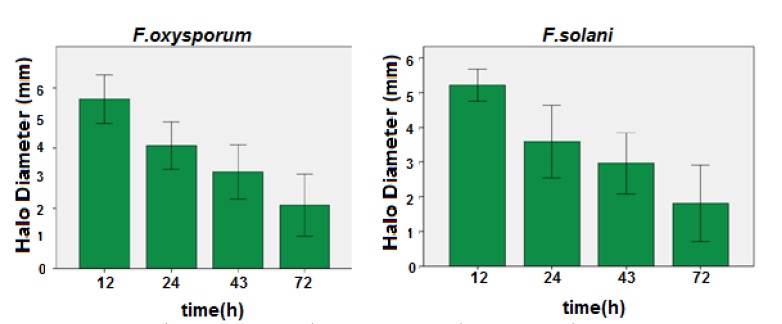
In vitro antifungall activity (halo/clear zone diameter) of recombinant protein (defensing-lichinase) produced by transient expression in four different times (12, 24, 42 and 72 h) against F. oxysporum and F. solani.
In this study, recombinant defensing-lichinase protein antifungal activity similar to Nystatin, while recombinant lichinase protein had an insignificant effect on fungal growth (Fig. 6). In vitro antifungal effect of recombinant protein against f. oxysporum and f. solani are showed.
Figure 6.
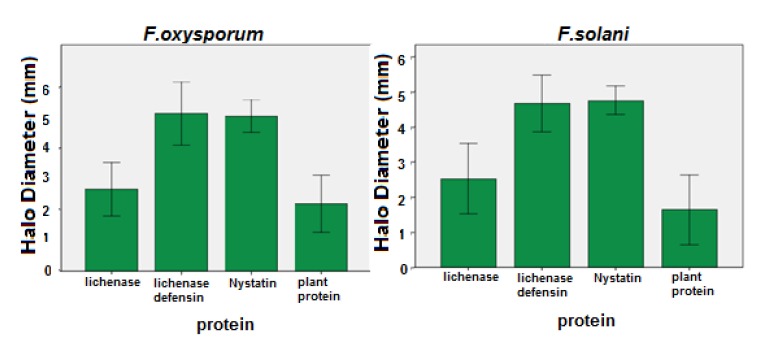
Antifungal activity of recombinant protein defensin-lichenase produced by transient expression compared to Lichenase, + controle (Nystatin) and –control (nontransgenic plant protein) against F. oxysporum and F. solani.
4.3. Heavy Metal Absorption Effect
The highest zinc absorption rate (3.73 ppm) by defensin protein was observed at pH=6 (Fig. 7a). In this study, we found that pH is a key factor in biological absorption of zinc by recombinant defensin protein. Accordingly, pH=6 was selected as suitable pH for the metal absorption experiment and subsequent analysis. Optimal temperature for zinc absorption by recombinant defensin protein was determined to be 35 °C, at which the zinc-absorption reading of 0.774 ppm was observed (Fig. 7b).
Figure 7.
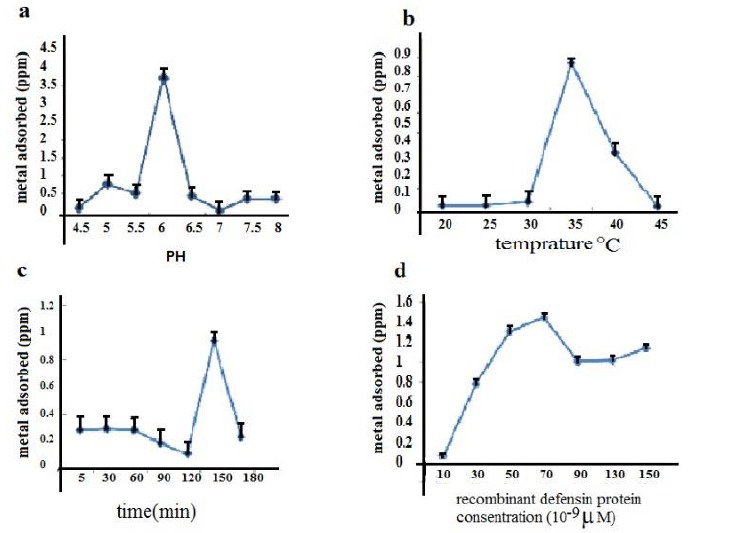
In vitro heavy metal (zinc) adsorption by recombinant defensin protein in different treatments of a: PH, b:tempreture, c:time, d: protein concentrations
We assessed seven time regimes (5, 30, 60, 90, 120, 150, and 180 min) to select optimal absorption. The absorption of zinc was decreased to 0.938 ppm after 150 minutes (Fig. 7c). Density of recombinant defensin protein on zinc absorption was studied. Proteins with different densities were added to solutions containing 2 µM zinc 0.1 molar. The highest absorption rate at the density of 70×10-9 µM of recombinant protein as 1.53 ppm was observed (Fig. 7d).
5. Discussion
The studied recombinant defensin protein showed a broad spectrum of activities against fungi, gram positive and negative bacteria. One of the key function of defensins is its antimicrobial activity that plays a role in plant innate immunity ( 16 , 18 , 19 ).With the exception of defensins with antibacterial ( 10 , 20 , 21 ) and insecticide activities, most plant defensins have a powerful effect conferring systemic resistance against fungi ( 8 ).The cationic peptides, such as defensin, are attracted electrostatically to negatively charged molecules such as anionic phospholipids, lipopolysaccharides (LPS) and teichoic acid, which are located asymmetrically in the membrane structure and cause cell death ( 16 , 22 ). Due to their high biocompatibility potential, moderate biodegradability, and low resistance developed on target microorganisms, defensins might prove to be a forthcoming novel class of antibiotics with application in several fields. Defensins can also be used to control fungal and bacterial infections in humans ( 9 , 23 ). Defensis can be applied in controlling plant diseases as a new tool in crop protection programs ( 5 , 24 , 25 ). Moreover, other applications of defensins have also been reported including: a replacement of or complement to antibiotics in animal nutrition; bio preservatives in food, cosmetics and biomaterials; and a substrate for antifouling ( 2 , 6 , 8 ).
Natural defensins are generally known to have a narrow activity range aimed towards one or a few pathogens. To overcome this limitation, modified novel defensin peptides based on structure-activity studies have been developed ( 3 , 26 ). For example, the SDmod gene has been modified to target a broader range of antimicrobial activities based on sd2 gene sequence isolated from Sunflower ( 3 ). Since heavy metals are absorbed from pollutants without provoking any secondary pollution, biological techniques (ie application of defensins) appear to be one of the best solutions for absorbing heavy metals ( 27 ). Previous studies reported heavy metal cadmium absorption potential of this recombinant defensin protein (SDmod) experimentally and theoretically. Experimental results proved that the best absorption of cadmium by this recombinant defensin protein happens at the concentration of 150×10-9 µM of protein ( 14 ).
Environmental factors such as temperature, salt density, pH, and biomass density influence the absorption level of heavy metal. Runping et al. (2006) introduced the effect of pH to be an important factor in absorption of lead and copper ( 28 ). Results of their study showed that capacity of absorption of heavy metal by yeast mass is increased when pH is increased from 2 to 6. In pH lower than 2, the least absorption was observed. Hence pH=5 was selected as the optimal pH. They also examined the effect of temperature as it influenced absorption of these metal ions by yeast. Temperatures varied between 20 to 50 °C. As temperature increased, more copper- and less lead ions were absorbed by yeast. Therefore, this process appears to be endothermic for copper and exothermic for lead. Cadmium ( 14 ) and zinc absorption potential with recombinant defensin protein proves to be an endothermic process under temperatures up to 35 °C. The amount of absorbed heavy metal ions by biomass is increased as time increases. The examination of the metal absorption in the yeast indicated that as the yeast's biomass increased, the absorption level of metal ions decreased ( 17 ).
6. Conclusions
As results indicate, as the mass of SDmod protein increases up to 70×10-9 µM, the absorption level of zinc is increased. Due to this finding Mirouze et al. (2006) increased the tolerance towards zinc in the transgenic plant through transmission of the defensin gene AhPDF1.1 in A. thaliana ( 29 ). A study by Valls et al. (2000) proved that peptides bound to metals and metallothioneins cause more tolerance and aggregation of cadmium in E. coli cells ( 30 ). Another study by Nemeth et al. (2004) on hemostasis showed that defensins prevent heavy metal cations trafficking and, thus, increase zinc’s tolerance. Environmental pollution by heavy metals and biological methods of absorption may justify the production and utilization of transgenic plants. Phytoremediation is defined as absorption of, or, possibly, recovery of heavy metals and other organic and inorganic materials from soil and water by virtue of engineered green plants. Identification of cysteine-rich proteins and production of metal-absorbent transgenic plants are apparent subjects to be followed up by environmental scientists. SDmod defensin is a cysteine- rich protein that has been used as a model for the development of heavy metal removal systems in order to eliminate environmental pollutions.
Acknowledgement
This work was supported financially by Shahrekord University of Iran.
References
- 1.Carvalho Ade O, Gomes VM. Plant defensins-- prospects for the biological functions and biotechnologicalproperties. Peptides. 2009;30(5):1007–1020. doi: 10.1016/j.peptides.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Gadd GM, de Rome L. Biosorption of copper by fungal melanin. Appl Microbiol Biotechnol. 1988;29(6):610– 617. doi: 10.1007/bf00260993. [DOI] [Google Scholar]
- 3.Sotchenkov DV, Goldenkova IV, Mirakholi N, Volkova LV. [Modification of the sunflower defensin SD2 gene sequence and its expression in bacterial and yeast cells] Genetika. 2005;41(11):1453–1461. doi: 10.1007/s11177-005-0219-1. [DOI] [PubMed] [Google Scholar]
- 4.Colilla FJ, Rocher A, Mendez E. gamma-Purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett. 1990;270(1-2):191–194. doi: 10.1016/0014-5793(90)81265-p. [DOI] [PubMed] [Google Scholar]
- 5.Montesinos E. Antimicrobial peptides and plant disease control. FEMS Microbiol Lett. 2007;270(1):1–11. doi: 10.1111/j.1574-6968.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71(1):1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 7.Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GW, Osborn RW, et al. Antimicrobial Peptides from Plants. Crit Rev Plant Sci. 2010;16(3):297–323. doi: 10.1080/07352689709701952. [DOI] [Google Scholar]
- 8.Kraszewska J, Beckett MC, James TC, Bond U. Comparative Analysis of the Antimicrobial Activities of Plant Defensin-Like and Ultrashort Peptides against Food-Spoiling Bacteria. Appl Environ Microbiol. 2016;82(14):4288–4298. doi: 10.1128/AEM.00558-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1(3):156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 10.Broekaert WF, Terras FR, Cammue BP, Osborn RW. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995;108(4):1353–1358. doi: 10.1104/pp.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller GL. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 12.Lee MW, Yang Y. Transient expression assay by agroinfiltration of leaves. Methods Mol Biol. 2006;323:225–229. doi: 10.1385/1-59745-003-0:225. [DOI] [PubMed] [Google Scholar]
- 13.Ullah H, Khan MF, Jan SU, Hashmat F. Depletion of GSH in human blood plasma and cytosolic fraction during cadmium toxicity is temperature and pH dependent. Pak J Pharm Sci. 2016;29(1):89–95. [PubMed] [Google Scholar]
- 14.Mahnam K, Foruzandeh S, Mirakhorli N, Saffar B. Experimental and theoretical studies of cadmium ions absorption by a new reduced recombinant defensin. J Biomol Struct Dyn. 2018;36(8):2004–2014. doi: 10.1080/07391102.2017.1340851. [DOI] [PubMed] [Google Scholar]
- 15.Riener CK, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman's reagent and with 4,4'- dithiodipyridine. Anal Bioanal Chem. 2002;373(4- 5):266–276. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa Pelegrini P, Del Sarto RP, Silva ON, Franco OL, Grossi-de-Sa MF. Antibacterial peptides from plants: what they are and how they probably work. Biochem Res Int. 2011;2011:250349. doi: 10.1155/2011/250349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han R, Li H, Li Y, Zhang J, Xiao H, Shi J. Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater. 2006;137(3):1569–1576. doi: 10.1016/j.jhazmat.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216(2):193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 20.Osborn RW, De Samblanx GW, Thevissen K, Goderis I, Torrekens S, Van Leuven F, et al. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995;368(2):257–262. doi: 10.1016/0014-5793(95)00666-w. [DOI] [PubMed] [Google Scholar]
- 21.Segura A, Moreno M, Molina A, Garcia-Olmedo F. Novel defensin subfamily from spinach (Spinacia oleracea) FEBS Lett. 1998;435(2-3):159–162. doi: 10.1016/s0014-5793(98)01060-6. [DOI] [PubMed] [Google Scholar]
- 22.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8(1):11–26. [PubMed] [Google Scholar]
- 23.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 24.Coca M, Penas G, Gomez J, Campo S, Bortolotti C, Messeguer J, et al. Enhanced resistance to the rice blast fungus Magnaporthe grisea conferred by expression of a cecropin A gene in transgenic rice. Planta. 2006;223(3):392–406. doi: 10.1007/s00425-005-0069-z. [DOI] [PubMed] [Google Scholar]
- 25.Marcos JF, Munoz A, Perez-Paya E, Misra S, Lopez-Garcia B. Identification and rational design of novel antimicrobial peptides for plant protection. Annu Rev Phytopathol. 2008;46:273–301. doi: 10.1146/annurev.phyto.121307.094843. [DOI] [PubMed] [Google Scholar]
- 26.Badosa E, Moiset G, Montesinos L, Talleda M, Bardaji E, Feliu L, et al. Derivatives of the antimicrobial peptide BP100 for expression in plant systems. PLoS One. 2013;8(12):e85515. doi: 10.1371/journal.pone.0085515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Olmedo F, Molina A, Alamillo JM, Rodríguez-Palenzuéla P. Plant defense peptides. Biopolymers. 1998;47(6):479–491. doi: 10.1002/(sici)1097-0282(1998)47:6<479::aid-bip6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Runping J, Honglin Z, Yan S, Xingguo C, Zhide H. Human serum albumen enhanced resonance light scattering of dyes. Talanta. 2004;64(2):355–360. doi: 10.1016/j.talanta.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Mirouze M, Sels J, Richard O, Czernic P, Loubet S, Jacquier A, et al. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 2006;47(3):329–342. doi: 10.1111/j.1365-313X.2006.02788.x. [DOI] [PubMed] [Google Scholar]
- 30.Valls M, de Lorenzo V, Gonzalez-Duarte R, Atrian S. Engineering outer-membrane proteins in Pseudomonas putida for enhanced heavy-metal bioadsorption. J Inorg Biochem. 2000;79(1-4):219–223. doi: 10.1016/s0162-0134(99)00170-1. [DOI] [PubMed] [Google Scholar]


