Abstract
Background
The purpose of palliative medicine is to optimize the quality of life of patients with incurable, progressive diseases. The care delivered in actual clinical practice is not uniform and often takes insufficient account of the currently available scientific evidence.
Methods
In accordance with the methodological directives on systematic literature reviews and consensus-finding that have been issued by the German Oncology Guideline Program (Leitlinienprogramm Onkologie), a nationwide, representative group of experts updated the previously published seven chapters of the S3 (evidence-based and consensus-based) guideline and formulated new recommendations on a further eight topics in palliative care.
Results
Non-drug options for the treatment of fatigue include aerobic exercise and psycho-educative methods, particularly cognitive behavioral therapy. Sleep disturbances can be treated with improved sleep hygiene and relaxation techniques, as well as with drugs: Z substances for short-term and sedating antidepressants for intermediate-term treatment. For nausea and vomiting, the first line of treatment consists of antidopaminergic drugs, such as haloperidol, or drugs with an antidopaminergic effect combined with a further receptor affinity, such as metoclopramide. For patients suffering from malignant intestinal obstruction (MIO), an important consideration for further treatment is whether the obstruction is complete or incomplete. Psychotherapeutic interventions are indicated for the treatment of anxiety.
Conclusion
Multiple studies have confirmed the benefit of the early integration of palliative care for achieving the goals of better symptom control and maintenance of quality of life. There is currently a low or moderate quality of evidence supporting the management of certain symptoms in patients with incurable cancers.
In the palliative care of a patient with incurable cancer, the goal is to maintain the best possible quality of life. Palliative care starts when cancer has been diagnosed as incurable, and is delivered by primary care providers or by specialized palliative care professionals (general versus specialist palliative care).
The first version of the S3 Guideline for Palliative Care covered seven topics: breathlessness, cancer pain, constipation, depression, communication, the dying phase, and the organization of palliative care (1).
For the second version of the Guideline, eight new topics were chosen for their clinical relevance or their prevalence in the palliative situation (as detailed below). In addition, the seven existing sections were updated, giving a new, expanded, and updated S3 Guideline containing 15 sections, the principles of palliative care, a glossary, and a list of quality indicators (2). The aim of the Guideline is to ensure universal high-quality palliative care for all patients with incurable cancer in Germany. Moreover, many key recommendations can also be applied to other patient groups.
Method
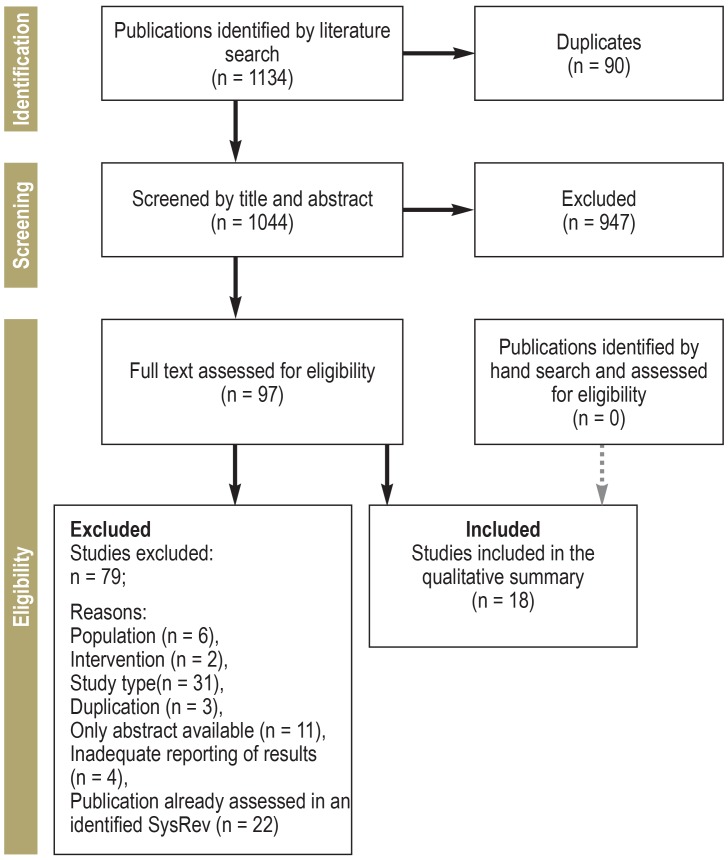
For this planned expansion and update of the S3 Guideline on Palliative Care, a representative group of experts was drawn from 61 medical specialty societies and institutions, including patient representatives. First, we searched for existing guidelines. Next, a literature search for systematic reviews and primary studies was conducted in the databases Medline, Cochrane Library, and—according to the topic—in Embase, PsycInfo and/or CINAHL, until 2016 or 2018, respectively. Publications were selected according to the PRISMA criteria (eFigure 1). The recommendations that were developed were formally agreed at consensus conferences by the elected representatives of the medical specialist societies.
eFigure 1.
PRISMA flow diagram: This example is for the topic “efficacy of drug therapies for symptomatic treatment of malignant bowel obstruction”
PRISMA, preferred reporting items for systematic reviews and meta-analyses; SysRev, systematic review
Results
A total of 212 key recommendations relating to the eight new topics were formulated and are summarized here. Overall, the expanded Guideline now contains 442 recommendations, 162 of which are evidence-based (recommendation grades and evidence levels as defined in eTable 1 are given in parentheses in the text). The remainder of the recommendations are based on expert consensus (EC). Eleven quality indicators were agreed on.
eTABLE 1. Grading system for levels of evidence (LoE; SIGN) and recommendation grades.
| LoE | Description | ||
| 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias | ||
| 1+ | Well-conducted meta-analyses, systematic reviews, or RCTs with a low risk of bias | ||
| 1- | Meta-analyses, systematic reviews, or RCTs with a high risk of bias | ||
| 2++ | High-quality systematic reviews of case control or cohort or studies orHigh quality case control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal | ||
| 2+ | Well-conducted case control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal | ||
| 2- | Case control or cohort studies with a high risk of confounding or bias and a significant risk that the relationship is not causal | ||
| 3 | Non-analytic studies, e.g., case reports, case series | ||
| 4 | Expert opinion | ||
| Recommendation grade | Description | Expression in words | |
| A | Strong recommendation | „Shall“ | |
| B | Recommendation | “Should“ | |
| 0 | Weak recommendation | „Can“ | |
Source: https://www.sign.ac.uk/assets/sign_grading_system_1999_2012.pdf and www.awmf.org/leitlinien/awmf-regelwerk/ll-entwicklung/awmf-regelwerk-03-leitlinienentwicklung/ll-entwicklung-graduierung-der-empfehlungen.html; RCT, randomized controlled study; SIGN, Scottish Intercollegiate Guidelines Network
Identifying treatment goals and decision-making criteria
The process by which decisions are reached about treatment goals and carrying out medical interventions should be participatory—i.e., it should include the patient. It should be started early or even anticipatory (Advance Care Planning).
Goals of care
Goals of care should be set by the doctor and the patient together on the basis of the current state of the patient’s disease and the therapeutic options available (EC). The primary goal of care in palliative care is to maintain or improve of the patient’s quality of life. This includes symptom control and dying with dignity; it does not exclude the goal of prolonging life.
Decisions about starting, continuing, or ending medical interventions
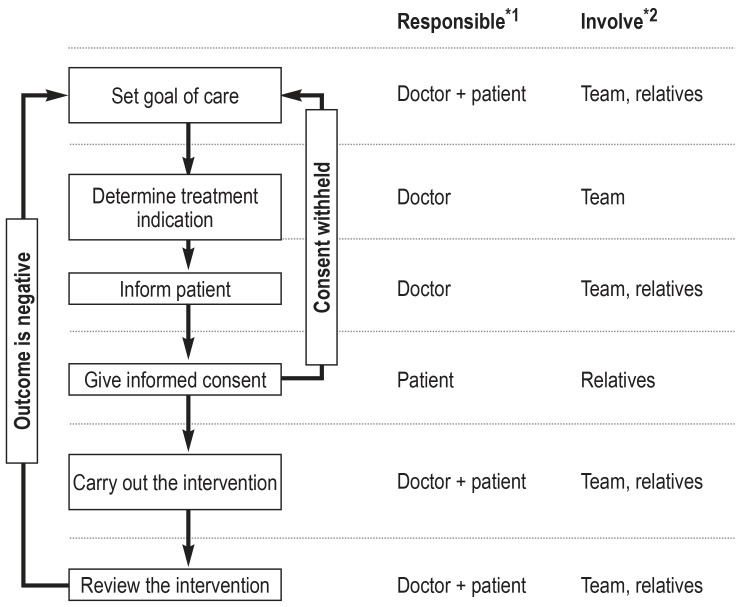
The main criteria in reaching these decisions are medical indication and patient consent (EC statement). The decision to treat should be made on the basis that the goal of care is realistically achievable and that the benefits have been weighed against the harms (EC). For the patient or his or her personal representative to give consent, the medical intervention and its complications must first have been sufficiently explained and information given about other possible treatment options (EC). If the patient does not have capacity to give consent, close relatives should be involved (EC). The goals of care and interventions decided upon should be reviewed regularly (figure 1).
Figure 1.
Decision tree for determining on and carrying out a medical intervention.
*1 If there is reason to suspect that the patient may lack capacity, the patient’s personal representative or healthcare proxy should be involved in the decision. The role of the representative is to support the patient through the decision-making process, and to represent the patient as necessary.
*2 If medically desirable or if desired by the patient
Fatigue
If “fatigue” is not a word the patient can relate to, questions about “weakness” and “tiredness” can be used: for example, “Do you feel unusually tired and/or weak?” or “How tired are you? How weak are you?” (EC).
Treatment
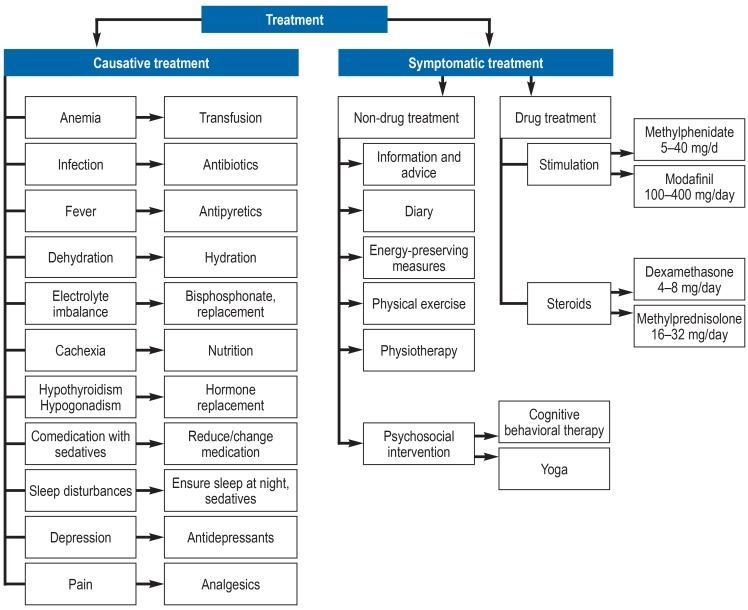
If fatigue has become a burden or is debilitating, the patient should be offered treatment (EC). The first step is to try to identify potentially treatable causes (EC). Secondary fatigue can be caused by, for example, anemia, infection, electrolyte imbalance, cachexia, depression, or the use of sedatives (efigure 2).
eFigure 2.
Treatment options in patients with primary and secondary fatigue
Various evidence-based non-drug interventions are available for symptomatic management of fatigue. Regular aerobic exercise was given only a moderate recommendation (“should”), to allow the functional status of palliative patients to be taken into account (B/1-). Nine out of 13 randomized, controlled studies (RCTs) (n = 744) showed that physical activity significantly reduced fatigue in palliative patients, although no meta-analysis could be conducted ([3]; Mochamat et al., personal communication). The largest RCT (n = 269) demonstrated a decrease of 6.6 points in a 100-point scale (EORTC QLQ-C30; 95% confidence interval [-12.3; -0.9]; p = 0.02; Cohen’s d = 0.33 [0.04; 0.61]) (4). Psychoeducational techniques, especially cognitive behavioral therapy, and energy management strategies are also given a weak recommendation (B/1-) because they are based on weak evidence ([5]; Mochamat et al., personal communication).
Symptomatic drug therapy with psychostimulants and corticosteroids can be tried (0/1-). The efficacy of methylphenidate in patients with advanced cancer was confirmed in a systematic review with a meta-analysis of two RCTs (standardized mean difference = 0.49; [0.15; 0.83]) and in two other RCTs (6). Modafinil has only been investigated in two RCTs with contradictory findings (6). Finally, there is weak evidence for the efficacy of corticosteroids in the treatment of fatigue in patients with advanced cancer (6).
Sleep-related disorders/nocturnal restlessness
Patients with incurable cancer often do not spontaneously report difficulties in sleeping as the focus is on other symptoms (7). For this reason, patients should be actively asked about sleep disorders along with other symptoms (EC) (etable 2). Any causes that can perhaps be addressed—pain, for instance, or drug side effects, fear (fear of dying), depression, or guilt feelings—should also be explored (EC), and, if appropriate, opportunities to talk or possible treatments should be offered (EC).
eTABLE 2. Definition of terms for sleep-related symptoms and related terms.
| Term | Definition | Burden on the patient |
| Sleep disturbance | Disturbed sleep in the narrower sense | Insomnia; consequent daytime tiredness or unusual behavior (sleepwalking) leading to symptoms. |
| Daytime sleepiness | Reduced central nervous activation; patient feels urgent need to sleep and may actually fall asleep; also called narcolepsy; can be measured using vigilance tests | Daytime sleepiness can reduce patients‘ ability to live life actively, and can impair the ability to drive. |
| Daytime tiredness | General term describing stress-related reduction in mental and physical performance | Daytime tiredness is a normal experience for everyone, so long as it does not restrict everyday living. If it does lead to restrictions, or if compensating mechanisms are no longer effective, it can be pathological. |
| Fatigue | Subjective sense of physical and/or mental exhaustion. It occurs in the absence of stress or out of proportion to the amount of stress undergone. | Fatigue reduces patients‘ ability to live life actively, and their ability to perceive active living as enjoyable rather than a burden. |
Source: „S3-Leitlinie Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung“ (2)
Treatment
In addition to general sleep hygiene and stimulus control (EC), cognitive behavioral therapy for insomnia can be initiated (Guideline adaptation, 0/2++). Good indirect evidence for this in a general population has been shown in several meta-analyses (8). Relaxation techniques are also recommended (EC).
For short-term drug treatment of insomnia, Z-drugs such as zopiclone or zolpidem should be used (B/1-) (9), while sedating antidepressants should be used for medium-term treatment (B/1-) (10, 11). The evidence for this is available only for a general patient population with cancer and is very weak—apart from results for paroxetine versus placebo (n = 426; difference in sleep problems [Hamilton Depression Index] in intergroup comparison: χ2(1) = 5.97; p = 0.01; Cohen’s d = 0.23) (11). Benzodiazepines are not indicated primarily, except when there are other indications for their use (B/4). Sedating antipsychotics can also be given where there is another indication for their use or where there is no alternative (0/3) (12). Melatonin can also be used as last-line treatment (0/1+) (13).
Nausea and vomiting (not tumor-therapy-induced)
Every time a patient’s symptoms are assessed, nausea and vomiting should always be included (EC)—preferably using validated recording instruments such as MIDOS or IPOS (B/4).
Treatment
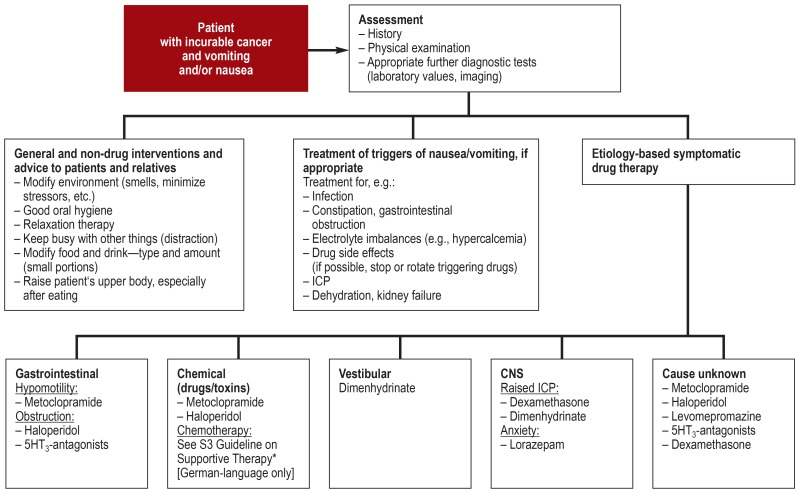
Treatment options are many and varied (figure 2). Fundamentally, aggravating factors such as stress or particular odors should be avoided, and if drugs are a trigger, consideration should be given to whether they can be reduced or stopped (EC). Where nausea and/or vomiting are opioid-induced, it may be possible to switch the opioid (0/1-) (14, 15). Mouth care should be offered after every episode of vomiting (EC). A choice of relaxation techniques should be offered (EC). If vomiting is refractory to treatment, a nasogastric tube may be considered (EC).
Figure 2.
Treatment algorithm for nausea and vomiting
*http://leitlinienprogramm-onkologie.de/Supportive-Therapie.95.0.html; CNS, central nervous system; ICP, intracranial pressure
Regarding drug therapy, the evidence for patients in the palliative setting is limited. Thus, indirect evidence from patients undergoing tumour-specific therapy is mostly used.. Drug treatment should be etiology-based (EC). As first-line therapy, drugs with antidopaminergic (e.g., haloperidol) or antidopaminergic and other receptor affinity (e.g., metoclopramide) should be given (B/1-) (14, 16, 17). As second-line treatment options, other drug classes can be used: antipsychotics with a broad spectrum of action (e.g., levomepromazine) (B/3) (16, 18), antihistamines (e.g., dimenhydrinate) (0/4), and 5HT3-antagonists (0/1-) (19). Patients with brain metastases who have raised ICP should be given dexamethasone (B/1-) (20). Antiemetics with different receptor affinities should be combined if monotherapy is inadequate (B/4). Where vomiting is persistent, parenteral drug administration should be preferred (EC).
Malignant bowel obstruction
Malignant bowel obstruction (MBO) is the complete or partial obstruction of the bowel due to intra-abdominal tumor or intraperitoneal metastasis, diagnosed on the basis of clinical examination and imaging. Nonmechanical (paralytic) impairment of the passage of bowel contents is not covered in this section.
MBO-related symptoms such as nausea, vomiting, meteorism, and abdominal pain should be assessed together with any potentially treatable causes (EC). Further diagnostic investigations such as rectal digital palpation or computed tomography should be considered, depending among other things on the patient’s clinical condition and on other treatment options including surgery (EC).
Treatment
Parenteral nutrition should be viewed with caution in patients whose predicted survival time is limited to a few weeks (B/3); for patients with a predicted survival of several weeks or months, it should be considered (A/3) (21– 23).
The decision whether to place a stent or to operate to remove or bypass the obstruction should be made by an interdisciplinary team (EC). Placement of a PEG (percutaneous endoscopic gastrostomy) tube should be considered if nausea and vomiting can be effectively relieved by a nasogastric tube (EC).
The aim of symptomatic drug therapy of an incomplete MBO is to restore the passage of bowel contents; for this, prokinetic antiemetics such as metoclopramide are indicated (B/4), and glucocorticoids can be started to reduce edema over a few days (0/1+) (24). Softening laxatives may be given to treat constipation (EC). Enemas may be offered concomitantly (EC).
In patients with complete MBO, prokinetics should be avoided. For this reason, antipsychotics (B/3) (25) and/or antihistamines (B/4) are used for antiemesis; these may be combined with 5HT3-antagonists (0/4). Reducing intraluminal secretion can help to relieve nausea and vomiting, e.g., using an anticholinergic such as hyoscine butylbromide (0/3) and/or somatostatin analogs (0/1) (26) and ranitidine or a proton pump inhibitor (EC) (etable 3). Colic-type pain should be treated with butylscopolamine (EC).
eTABLE 3. Treatment for nausea and vomiting in patients with malignant bowel obstruction (MBO).
| Drug class | Medication | Dosage | Remarks |
| Prokinetics | Metoclopramide | 30–40* mg/day s.c.* | Drug of choice when incomplete obstruction or functional disorder is suspected. Because it promotes GI motility, pain and vomiting may increase. Caution: This drug should be stopped if complete obstruction is present |
| Antipsychotics | Haloperidol* | 2.5–10 mg/day s.c.* or i.v.* | Drug of choice when complete obstruction is present and no prokinetic antiemetic can be used |
| Levomepromazine* | 1–5 mg oral/s.c.* at night | Dose may be increased up to 25 mg/day, but this is rarely necessary | |
| Olanzapine* | 2.5 mg as initial dose, if needed, 5–10 mg oral/s.l. daily | Use with caution in older patients and those with dementia, due to increased half-life and increased mortality risk | |
| Antihistamines | Dimenhydrinate | 150 mg p.r. 62–400 mg/day s.c.* or i.v. | Has a sedative effect |
| Setrons | Ondansetron* | 8 mg oral or s. l./ s.c.*/ i.v. 2–3× daily | Increases constipation |
| Granisetron* | 1–2 mg p.o./s.c.*/ i.v. 1× daily | Increases constipation; dose may be increased up to 9 mg/day | |
| Anticholinergics | Butylscopolamine* | 40–80 mg/day s.c./i.v. dose may be increased up to 120 mg* | Used to reduce GI secretion; has no direct antiemetic effect. May increase oral dryness and thirst |
| Somatostatin analogs | Octreotide* | Initial dose 100 µg 12-hourly, dose may be increased up to 750 µg/day, further increase rarely has greater effect | Used to reduce GI secretion; second-line drug (because expensive) |
| Lanreotide* | 60 mg deep s.c. in upper outer quadrant of buttock every 3 months | If necessary, 120 mg every 4 weeks | |
| H2-blockers | Ranitidine* | 50 mg 2–4× daily, or continuously 100–200 mg/ 24 h i.v. | |
| Proton pump inhibitors | Omeprazole* | 40–80 mg/day i.v, s.c.* | |
| Corticosteroids | Dexamethasone* | 8–12 mg/day s.c.*/ i.v. | Used to reduce peritumoral edema (may restore passage of bowel contents) and for antiemesis; fewer mineralocorticoid adverse effects than methylprednisolone |
Malignant wounds
Malignant wounds are defined as wounds caused by a primary skin tumor, skin metastases, or breakthrough of a tumor in a deeper location. In addition to contributing factors, wound history-taking should cover impairment of the patient’s quality of life and the subjective lived experience of the patient and the patient’s relatives (EC) (etable 4).
eTable 4. Key elements of history taking in patients with malignant wounds.
| Key elements of wound-specific history |
| Disease history (primary disease, comorbidities) |
| Treatment so far for primary disease, wound diagnostic tests carried out, medications (e.g., cortisone, analgesics) and allergies |
| Patient’s and relatives‘ current information about/understanding of the cause of the wound, the condition of the wound, and the implementation of any special interventions (e.g., pressure relief/reduction, compression therapy) |
| The wound has existed since … (wound duration) |
| Wound care plan (wound care products used, frequency of dressing changes, who has been carrying these out so far, any restrictions caused by dressings) |
| Effects of the wound on patient’s quality of life |
| Motor/functional impairment caused by the wound (speech, swallowing, hearing, vision, protective posture, contractures) |
| Effects of the wound on everyday living (e.g., sleep disorders, activities of life, choice of clothing, financial strain) |
| Psychosocial and social consequences of the wound for the patient and the patient’s relatives (e.g., isolation, shame/embarrassment, revulsion, loss of control) |
| Social background and support in relation to wound care |
| Effects of the wound on the patient’s self-image/body image |
| Effects of the wound on the patient’s partner relationship, intimacy, sexuality, family relationships |
| Coping strategies used so far; capacity for self-management; external resources/support |
Alleviating psychosocial stress
Malignant wounds can have a powerful effect on quality of life. The change in the patient’s self-image and body image, and the social consequences of that change, should be touched upon empathetically (EC), and the patient’s self-management and sense of control should be supported (EC).
Pain relief
Before dressings are changed, analgesics should be given anticipatory to preempt any expected pain (EC). Treatment locally with a local anesthetic (0/4) or morphine gel (0/2-) (27) is recommended on the basis of indirect evidence relating to other types of wounds.
Odor control
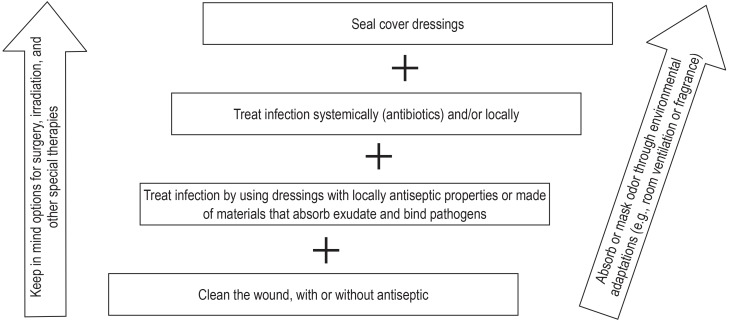
In addition to meticulous wound cleaning (EC), a variety of interventions are recommended (eFigure 3): the use of local antiseptics (0/3) (indirect evidence, e.g., [28]); wound dressing materials that have an antiseptic effect or absorb exudate and bind pathogens, e.g., those containing activated carbon (0/3) (29); metronidazole administered locally (0/1-) (29) or systemically (0/3) (30).
Prevention and management of bleeding
Slight bleeds require vasoconstrictive measures such as cooling or compression (EC). More copious bleeds should be treated with local or systemic antifibrinolytics such as tranexamic acid (B/2+) (31) or with local hemostatic agents (EC). The efficacy of topical tranexamic acid in comparison to placebo has been studied in a meta-analysis of mostly surgical patients (n = 6034, 67 RCTs) and is thus assessed as indirect evidence (weighted mean difference in mean blood loss: -276.6 mL; [-327.8; -225.4 mL]; p <0.0001) (31).
Anxiety
This section discusses subsyndromal and specific anxieties. Subsyndromal anxiety disorders are those that do not completely meet the ICD criteria for anxiety disorders (panic disorders, phobias, generalized anxiety disorders, adjustment disorders, and posttraumatic stress disorders).
Specific anxieties are triggered by the patient’s disease situation. They may relate to actual or feared symptoms, to treatment, to the end of life, or to relatives and loved ones, among others. Anxiety should be regularly recorded and assessed as necessary as to content and any need for treatment (EC).
Treatment
The care team needs to have an attitude and choose words that avoid triggering anxiety (EC). Uncontrolled symptoms that cause anxiety should be treated in the best way possible (EC).
If anxiety is burdensome or impairing, non-drug measures should be used (EC). These include psychological and/or psychotherapeutic techniques (0/1+).
A meta-analysis of RCTs with moderate risks of bias that studied palliative patients with anxiety showed a moderate, significant effect of cognitive behavioral therapy (Cohen’s d = 0.44; p <0.001), mindfulness-based stress reduction, and acceptance and commitment therapy (-0.67; p <0.001) (32). Other techniques using social-work– or spirituality-based approaches or art therapy are recommended by expert consensus (EC).
Drug therapy is offered when other techniques are insufficiently effective or cannot be implemented (EC) (table 1). The evidence base for the palliative setting is very weak. Based on clinical experience, short-acting benzodiazepines should be given to treat symptoms of acute anxiety (B/4). Alternatives that may be considered are antidepressants, antipsychotics, or other anxiolytics (B/1-) (33, 34).
Table 1. Dosage recommendations for acute and long-term treatment with anxiolytics in patients with incurable cancer.
| Acute treatment | ||||
| Indication | Drug class | Active ingredient | Single dose | |
| Acute anxiety state, panic attack | Benzodiazepines | Lorazepam Alprazolam Oxazepam Midazolam* |
1.0–2.5 mg 0.5–1.0 mg 10–40 mg 2.5–(5) 10 mg |
|
| Treatment in patients with a long-term disease | ||||
| Indication | Drug class | Active ingredient | Single dose/ initial dose | Max. daily dose |
| Recurrent anxiety or panic attacks | Antidepressants: SSRI SNRI |
Citalopram Escitalopram Sertralin Venlafaxin |
10–20 mg 5–10 mg 50 mg 37.5 mg |
40 mg 20 mg 200 mg 150 mg |
| History of anxiety or panic disorder, generalized anxiety disorder, and recurrent anxiety attacks in the present | Antidepressants: SSRI SNRI |
Escitalopram Venlafaxin |
5–10 mg 37.5 mg |
20 mg 150 mg |
| Anxiolytics | Opipramol | 50 mg | 300 mg | |
| Antipsychotics (augmentation if necessary) |
Quetiapine* Olanzapine* Risperidone* |
25 mg 5.0 mg 0.25 mg |
200 mg 15 mg 2 mg |
|
| Other | Pregabalin | 25 mg | 600 mg | |
* Off-label use. For licensing information in relation to specific anxiety disorders, please see the S3 clinical practice guideline for anxiety disorders (e1);
SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin-norepinephrine reuptake inhibitors;
source: „S3-Leitlinie Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung“ (2)
The desire to die
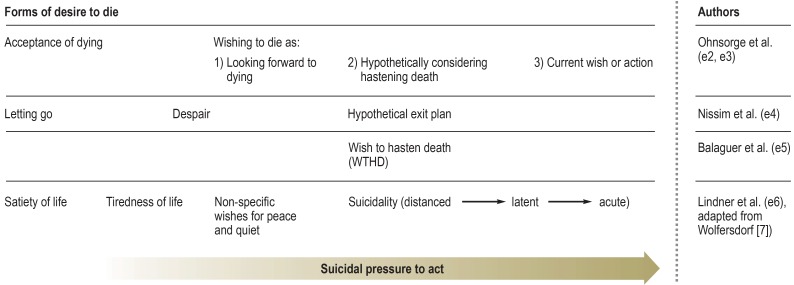
Based on the international literature, the Guideline refers to the desire to die as a complex phenomenon, affecting palliative care patients and ranging from the acceptance of death (“satiety of life”) to acute suicidality (figure 3). A desire to die can often exist alongside a will to live (35), and may be traced to a variety of causes: for example, physically or psychologically stressful symptoms, social problems such as isolation, worries about the future, and loss of autonomy (35). A desire to die may serve various different functions, such as opening up a way of escaping from an unbearable situation, having control over the end of one’s life, or as an expression of a sense of despair.
Figure 3.
Forms of desire to die, according to various authors
Proactiely addressing a desire to die
Patients with incurable cancer should be actively asked whether they feel a desire to die (B/4). There is no evidence that enquiring suicidal thoughts leads to their emergence or increase. In a meta-analysis of six studies, suicidal ideation was not significantly increased after participants were asked about suicidality or exposed to suicide-related content (overall effect, odds ratio = 0.973 [0.83; 1.15]; p = 0.749) (36).
Dealing with a desire to die
In conversations about a desire to die, the care team should maintain a basic attitude of openness and respect; this does not mean that they are assenting to the active ending of a life (EC). Based on various diagnostic and treatment considerations, strategies and techniques have been developed for use in dealing with a patient who desires to die; details are given in Table 2.
Table 2. Strategies and techniques in dealing with a (potential) desire to die.
| Strategy | Technique |
| 1. Ask how the patient feels psychologically and spiritually | Feelings of helplessness, hopelessness, meaninglessness, futility, loss of morale and loss of faith, need to be brought to light, as does the nature of the desire to die: how stable is it? Is there a sense that there is no alternative? This will make it possible to consider whether there might be any options for solutions. |
| 2. Investigate for depression or any other psychiatric disorder that can seriously impair judgment | If the patient is suffering from depression or any other severe psychiatric disorder, this should be investigated and its severity assessed. |
| 3. Notice your own feelings when talking with the patient | It is important to notice, define, and in the first instance merely “sit with” any feelings you may have of fear, distress, annoyance, tiredness, irritation, distraction, or suddenly wishing the patient were dead. Later, take time to assess whether they contain important clues to the patient‘s situation. |
| 4. If there are any hints that the patient harbors a desire to die, this should be proactively asked about. | A cautious approach to the subject can pay off: “Are you thinking about just giving up on yourself … chucking it all in?” “Would you rather be dead?” “Are you thinking of putting an end to your own life?” |
| 5. Involve relatives | If the patient agrees, it can be a good idea to talk with the relatives about the patient’s desire to die, and to get their view of the matter. |
| 6. Work out coping strategies that will help the patient to deal with the situation he or she is in | Evidence-based interventions are available: for example, existential techniques such as meaning- or value-centered therapy |
| 7. Explore and support the patient’s desire for increased personal influence and control | Support the patient in reflecting about the possibilities for being in control of the situation. If needed, concrete steps to improve the patient’s control should be initiated. |
| 8. Stay with suffering that cannot be prevented | If it is not possible to adequately relieve pain or suffering, it is important to continue to make oneself available to the patient, to continue to stay with the situation, talk about it, and look for solutions. Doing this is in itself the solution on offer. |
| 9. Change goals of care and decisions about life-prolonging interventions | Talking about these matters can in itself be a way for the patient to deal with the desire to die. |
| 10. Symptom control | Inadequately controlled symptoms such as pain, breathlessness, nausea, vomiting, anxiety, and depression play an important part in a patient‘s developing a desire to die. The best possible symptom control is part of caring for the patient. |
| 11. Consult colleagues and hold case conferences | In situations of tension and dilemma, consulting with colleagues or (ethical) case conferences are a proven means of finding new thoughts and options by bringing in a third party. |
| 12. Involve other professionals | When particular topics are at issue (more precise psychiatric diagnosis, religious confliction, etc.), psychiatric/psychotherapeutic expertise, social workers, religious pastoral workers, or other experts should be called upon. |
Source: Extracts from „S3-Leitlinie Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung“ (2)
Updates to the seven original topics
Since the landmark study by Temel et al. (37), several new, high-quality RCTs have shown the advantages of early integration of palliative care, such that two central recommendations, previous consensus-based, can now be formulated as evidence-based (A/1- and A/3, respectively) (38). A new addition is that every patient who has been diagnosed with incurable cancer should be offered a needs assessment by a specialist palliative care team.
In regard to the other recommendations on the original seven topics, few changes were required. Recent studies have indicated that steroids can relieve breathlessness even in patients without lymphangitis carcinomatosa or tumor-related airway obstruction (0/1+) (39).
In view of recent results about adverse effects, haloperidol should be used much more cautiously in a patient with delirium during the dying phase; if restlessness increases, it may be combined with a benzodiazepine (B/1-) (40).
Summary
The expanded and updated S3 Guideline formulates recommendations for clinical practice in 15 core topics of palliative medicine. These recommendations should guide the actions of all those working in general and specialist palliative care who are involved in the management of patients with incurable cancer. A representative and multiprofessional group of experts together with patient representatives developed and agreed these recommendations on the basis of current, up-to-date evidence, with the goal of providing the best possible palliative care for patients and their relatives.
Key messages.
Symptomatic treatment for fatigue should employ aerobic endurance and strength exercises and psychoeducative techniques. Psychostimulants (methylphenidate, modafinil) and/or corticosteroids can be given as part of any attempt at treatment.
Drug therapy for nausea and vomiting should start with drugs with antidopaminergic receptor affinity (e.g., haloperidol or metoclopramide). Second- and third-line drugs are antipsychotics with a broad spectrum of action (e.g., levomepromazine), antihistamines (e.g., dimenhydrinate), and 5HT3-antagonists.
Symptomatic treatment of malignant wounds includes relief of spontaneous or movement-related pain or pain associated with dressing changes; odor and exudate reduction; prevention and treatment of bleeding; and support with psychosocial stress.
In the palliative setting, a patient’s desire to die is a complex phenomenon, ranging from acceptance of death all the way to acute suicidality; not unusually, it can exist alongside a will to live. The care team should bring up the subject of the desire to die; the subjective reasons for it, how strong it is, and whether the patient has suicidal thoughts should be noted so that the patient can be offered appropriate support.
The effectiveness of early integration of palliative care with the goal of improving symptom control and maintaining quality of life has now been demonstrated in several high-quality studies.
eFigure 3.
Staged interventions for odor control
Acknowledgments
We are grateful to all the experts and elected representatives of medical specialist societies for their commitment and hard work in developing this Guideline. Grateful thanks are also due to Dr. Markus Follmann, Prof. Dr. Ina Kopp, Dr. Monika Nothacker, Thomas Langer, and Dr. Simone Wesselmann of the Cancer Guidelines Program for their advice on methods and constant support.
We are especially grateful to Verena Geffe, Dr. Susanne König, and Dr. Kerstin Kremeike of the Guideline Office. Finally, thanks go to the three supporting bodies of the Cancer Guidelines Program—the Association of Scientific Medical Societies in Germany (AWMF), the German Cancer Society (Deutsche Krebsgesellschaft), and German Cancer Aid (Deutsche Krebshilfe)—for creating the Guideline, and to German Cancer Aid for independent financial support.
Footnotes
Conflict of interest statement Professor Voltz has received lecture fees from Novocure and MSD Sharp & Dohme, and from the National Health Academy Ltd. (Nationale Gesundheits-Akademie NGA GmbH).
The other authors declare that they have no conflict of interest..
Clinical guidelines in the Deutsches Ärzteblatt, as in numerous other specialist journals, are not subject to a peer review procedure, since S3 guidelines represent texts that have already been evaluated, discussed, and broadly agreed upon multiple times by experts (peers).
References
- 1.Bausewein C, Simon ST, Pralong A, Radbruch L, Nauck F, Voltz R. Clinical practice guideline: palliative care of adult patients with cancer. Dtsch Arztebl Int. 2015;112:863–870. doi: 10.3238/arztebl.2015.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) S3-Leitlinie Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung, Langversion 2.0, 2019, AWMF-Registernummer: 128/001OL. www.leitlinienprogramm-onkologie.de/leitlinien/palliativmedizin/ (last accessed on 7 October 2019) [Google Scholar]
- 3.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132. doi: 10.1016/j.ypmed.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339 doi: 10.1136/bmj.b3410. b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poort H, Peters M, Bleijenberg G, et al. Psychosocial interventions for fatigue during cancer treatment with palliative intent. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD012030.pub2. CD012030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mücke M, Mochamat Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015;5 doi: 10.1002/14651858.CD006788.pub3. CD006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care. 2005;3:23–31. doi: 10.1017/s1478951505050042. [DOI] [PubMed] [Google Scholar]
- 8.Riemann D, Baum E, Cohrs S, et al. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen Kapitel „Insomnie bei Erwachsenen“ (AWMFRegisternummer 063-003), Update 2016. Somnologie. 2017;21:2–44. [Google Scholar]
- 9.Joffe H, Partridge A, Giobbie-Hurder A, et al. Augmentation of venlafaxine and selective serotonin reuptake inhibitors with zolpidem improves sleep and quality of life in breast cancer patients with hot flashes: a randomized, double-blind, placebo-controlled trial. Menopause. 2010;17:908–916. doi: 10.1097/gme.0b013e3181dbee1b. [DOI] [PubMed] [Google Scholar]
- 10.Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer. 2008;16:1291–1298. doi: 10.1007/s00520-008-0425-1. [DOI] [PubMed] [Google Scholar]
- 11.Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med. 2012;13:1184–1190. doi: 10.1016/j.sleep.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50:159–161. doi: 10.1176/appi.psy.50.2.159. [DOI] [PubMed] [Google Scholar]
- 13.Kurdi MS, Muthukalai SP. The efficacy of oral melatonin in improving sleep in cancer patients with insomnia: a randomized double-blind placebo-controlled study. Indian J. 2016;22:295–300. doi: 10.4103/0973-1075.185039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med. 2011;25:442–453. doi: 10.1177/0269216311404273. [DOI] [PubMed] [Google Scholar]
- 15.Sande TA, Laird BJA, Fallon MT. The management of opioid-induced nausea and vomiting in patients with cancer: a systematic review. J Palliat Med. 2018;21 doi: 10.1089/jpm.2018.0260. [DOI] [PubMed] [Google Scholar]
- 16.Benze G, Alt-Epping B, Geyer A, Nauck F. [Treatment of nausea and vomiting with prokinetics and neuroleptics in palliative care patients : a review] Schmerz. 2012a;26:500–514. doi: 10.1007/s00482-012-1216-7. [DOI] [PubMed] [Google Scholar]
- 17.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 18.Dietz I, Schmitz A, Lampey I, Schulz C. Evidence for the use of levomepromazine for symptom control in the palliative care setting: a systematic review. BMC Palliat Care. 2013;12 doi: 10.1186/1472-684X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benze G, Geyer A, Alt-Epping B, Nauck F. [Treatment of nausea and vomiting with 5HT3 receptor antagonists, steroids, antihistamines, anticholinergics, somatostatinantagonists, benzodiazepines and cannabinoids in palliative care patients: a systematic review] Schmerz. 2012;26:481–499. doi: 10.1007/s00482-012-1235-4. [DOI] [PubMed] [Google Scholar]
- 20.Vayne-Bossert P, Haywood A, Good P, Khan S, Rickett K, Hardy JR. Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemotherapy, radiotherapy, or surgery) Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD012002.pub2. CD012002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34:825–837. doi: 10.1016/j.clnu.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Sowerbutts AM, Lal S, Sremanakova J, et al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst Rev. 2018;8 doi: 10.1002/14651858.CD012812.pub2. CD012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD002764. CD001219. [DOI] [PubMed] [Google Scholar]
- 25.Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44:604–607. doi: 10.1016/j.jpainsymman.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52:901–919e1. doi: 10.1016/j.jpainsymman.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 27.LeBon B, Zeppetella G, Higginson IJ. Effectiveness of topical administration of opioids in palliative care: a systematic review. J Pain Symptom Manage. 2009;37:913–917. doi: 10.1016/j.jpainsymman.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Norman G, Dumville JC, Moore ZE, Tanner J, Christie J, Goto S. Antibiotics and antiseptics for pressure ulcers. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011586.pub2. CD011586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage. 2010;39:1065–1076. doi: 10.1016/j.jpainsymman.2009.11.319. [DOI] [PubMed] [Google Scholar]
- 30.Ramasubbu DA, Smith V, Hayden F, Cronin P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst Rev. 2017;8 doi: 10.1002/14651858.CD011609.pub2. CD011609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montroy J, Hutton B, Moodley P, et al. The efficacy and safety of topical tranexamic acid: a systematic review and meta-analysis. Transfus Med Rev. 2018 doi: 10.1016/j.tmrv.2018.02.003. pii: S0887-7963(17)30151-7]. [DOI] [PubMed] [Google Scholar]
- 32.Fulton JJ, Newins AR, Porter LS, Ramos K. Psychotherapy targeting depression and anxiety for use in palliative care: a meta-analysis. J Palliat Med. 2018;21:1024–1037. doi: 10.1089/jpm.2017.0576. [DOI] [PubMed] [Google Scholar]
- 33.Nubling G, Allmendinger S, Lorenzl S. [Drug therapy of anxiety and fear in palliative care patients with cancer or other illnesses: a systematic review] Schmerz. 2012;26:537–549. doi: 10.1007/s00482-012-1241-6. [DOI] [PubMed] [Google Scholar]
- 34.Stockler MR, O‘Connell R, Nowak AK, et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: a placebo-controlled double-blind randomised trial. Lancet Oncol. 2007;8:603–612. doi: 10.1016/S1470-2045(07)70148-1. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Prat A, Balaguer A, Booth A, Monforte-Royo C. Understanding patients‘ experiences of the wish to hasten death: an updated and expanded systematic review and meta-ethnography. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016659. e016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blades CA, Stritzke WGK, Page AC, Brown JD. The benefits and risks of asking research participants about suicide: a meta-analysis of the impact of exposure to suicide-related content. Clin Psychol Rev. 2018;64:1–12. doi: 10.1016/j.cpr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 38.Haun MW, Estel S, Rucker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD011129.pub2. CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haywood A, Duc J, Good P, et al. Systemic corticosteroids for the management of cancer-related breathlessness (dyspnoea) in adults. Cochrane Database Syst Rev. 2019;2 doi: 10.1002/14651858.CD012704.pub2. CD012704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177:34–42. doi: 10.1001/jamainternmed.2016.7491. [DOI] [PubMed] [Google Scholar]
- E1.Bandelow B, Lichte T, Rudolf S, Wiltink J, Beutel M. Clinical practice guideline: The diagnosis of and treatment recommendations for anxiety disorders. Dtsch Arztebl Int. 2014;111:473–480. doi: 10.3238/arztebl.2014.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Ohnsorge K, Gudat H, Rehmann-Sutter C. Intentions in wishes to die: analysis and a typology—a report of 30 qualitative case studies of terminally ill cancer patients in palliative care. Psychooncology. 2014;23:1021–1026. doi: 10.1002/pon.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Ohnsorge K, Gudat H, Rehmann-Sutter C. What a wish to die can mean: reasons, meanings and functions of wishes to die, reported from 30 qualitative case studies of terminally ill cancer patients in palliative care. BMC Palliative Care. 2014;13 doi: 10.1186/1472-684X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Nissim R, Gagliese L, Rodin G. The desire for hastened death in individuals with advanced cancer: a longitudinal qualitative study. Soc Sci Med. 2009;69:165–171. doi: 10.1016/j.socscimed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- E5.Balaguer A, Monforte-Royo C, Porta-Sales J, et al. An international consensus definition of the wish to hasten death and its related factors. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146184. e0146184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Lindner R. Suizidale Männer in der psychoanalytisch orientierten Psychotherapie Eine systematische qualitative Untersuchung. Gießen: Psychosozial-Verlag; 2006 [Google Scholar]
- E7.Wolfersdorf M, Etzersdorfer E. Kohlhammer. 1. Stuttgart: 2011. Suizid und Suizidprävention. [Google Scholar]
- E8.Bausewein C, Roller S, Voltz R. Urban & Fischer. 5. München: 2015. Leitfaden Palliative Care . [Google Scholar]
- E9.British Columbia Cancer Agency. Symptom Management Guidelines: Care of malignant wounds. www.bccancer.bc.ca/nursing-site/Documents/Bibliograpy%20-%20Master%20List.pdf (last accessed on 24 January 2020) [Google Scholar]
- E10.Deutsches Netzwerk für Qualitätsentwicklung in der Pflege. Expertenstandard - Pflege von Menschen mit chronischen Wunden. www.dnqp.de/fileadmin/HSOS/Homepages/DNQP/Dateien/Expertenstandards/Pflege_von_Menschen_mit_chronischen_Wunden/ChronWu_Akt_Auszug.pdf (last accessed on 24 January 2020) [Google Scholar]
- E11.Onkologiepflege Schweiz. Pflege und Behandlung der malignen Wunde - Konzept/Leitfaden. 2007 [Google Scholar]
- E12.Seaman S. Management of malignant fungating wounds in advanced cancer. Semin Oncol Nurs. 2006;22:185–193. doi: 10.1016/j.soncn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- E13.Uebach B. Wundversorgung in der Palliative Care (Teil 2) Zeitschrift für Palliativmedizin. 2012;13:280–283. [Google Scholar]
- E14.Uebach B, Kern M. PalliaMed Verlag; Bonn: 2010. Wunden sind nicht immer heilbar - Palliative Wundbehandlung exulzerierender Tumorwunden. [Google Scholar]
- E15.World Union of Wound Healing Societies (WUWHS) Principles of best practice: Wound exudate and the role of dressings. A consensus document. www.woundsinternational.com/media/issues/82/files/content_42.pdf (last accessed on 24 January 2020) [Google Scholar]