Abstract
Bisphenol A (BPA) is one of the highest volume chemicals produced worldwide, which is used in many plastic industries. The present study aimed to evaluate the effect of BPA on cognitive functions and oxidative stress, and determine whether the naringin (NG) co-administration can modify the effect of this compound on cognitive functions and inhibit any possible oxidative stress in the brain tissue of rats. Adult male Wistar rats were divided into six groups. Group I: control, Group II: BPA-treated rats (50 mg/kg/day), Group III, IV, V: BPA+NG (40, 80, 160 mg/kg/day), Group VI: NG (160 mg/kg/day) alone. Cognitive functions were evaluated using step-down latency (SDL) on a passive avoidance apparatus, and transfer latency (TL) in elevated plus-maze. A significant decrease in SDL, prolongation of TL, noticeable oxidative impairment and increase in acetylcholinesterase activity were observed in the BPA-treated in comparison with the control group. Also, the co-administration of NG (160 mg/kg) antagonized the effect of BPA on SDL and TL, attenuated oxidative damage by lowering malondialdehyde and nitrite concentrations and restored superoxide dismutase, catalase, and glutathione S-transferase activities. On the other hand, acetylcholinesterase activity was reduced in the groups co-administred with NG (80 or 160 mg/kg) and BPA in comparison with the BPA alone-treated group. The present study highlighted the therapeutic potential of NG against BPA-induced cognitive impairment and oxidative damage.
Key Words: Bisphenol A, naringin, cognitive dysfunction, oxidative stress
Bisphenol A (2, 2-bis(4- hydroxyphenyl) propane; BPA), an estrogenic endocrine disrupting chemical, is used in several plastic consumer products, such as toys, water tubes, drinking containers, eyeglass lenses, sport equipment, dental monomers, and medical equipment. BPA can easily leach out from the polycarbonate plastics, either during heating, storage, brushing and dishwashing of cans or via contact with basic and acidic substances (1).
In humans, there is a relationship between BPA levels in blood and metabolic disorders, including type 2 diabetes, abnormal liver functions, prostate and breast cancers, endometrial hyperplasia, polycystic ovarian syndrome, and neurobehavioral problems (e.g., attention deficit and hyperactivity disorder) (2). In animal studies, there are reports on reduced motility and sperm count (3) and stimulation of prolactin hormone release due to BPA exposure (4). Moreover, some studies have demonstrated the effect of BPA on memory in experimental animals. Also, it was shown that prenatal and neonatal chronic exposure to BPA deteriorated the memory in mice due to dysfunction of hippocampus cholinergic system (5, 6).
Oxidative stress has long been associated with the pathogenesis of numerous diseases in humans, including Alzheimer’s disease, ischemic injury, Parkinson’s disease and Down syndrome, all of which exert impacts on cognitive functions (7). Oxidative stress occurs due to unregulated production of free radicals that may cause impairment of nucleic acid bases, lipids and proteins, leading to cell death. There is some evidence to suggest that BPA utilizes potent oxidant activity in several tissues, including kidney, liver, brain, and testis in rodents. According to the study of Bindhumol et al., BPA treatment induced oxidative stress in the liver of rats by diminishing antioxidant enzymes and increasing hydrogen peroxide and lipid peroxidation (8). Several compounds with antioxidative features have been evaluated to determine disease-associated oxidative stress.
Naringin (NG) is a flavone dispersed in citrus fruits, tomatoes, cherries, grapefruit, and cocoa (9). This flavonoid glycoside is recognized as a non-toxic natural product with many bioactive effects, including anti-cancer (10), anti-oxidative (11), anti-inflammatory (12), nephron-protective (13), and hepato-protective activities (14). The protective effect of NG against ischemic reperfusion cerebral damage has been reported (15). When administered orally, NG is metabolized to naringenin (40,5,7-trihydroxyflavanone), which has been shown to have good penetrability through the blood-brain barrier (16). Despite several studies on the positive effects of NG, its therapeutic potential as a neuro-protectant against cognitive dysfunction and free radical-mediated neurotoxicity has not been completely understood. Therefore, this study aimed to evaluate the neuro-protective effect of NG against BPA-induced cognitive impairment and oxidative stress in rats.
Materials and methods
Animals
In this experimental study, 36 healthy adult male Wistar rats weighing 160-180 g were purchased from the animal facility of Ahvaz Jundishapur University of Medical Sciences (AJUMS). Rats in this study were treated in accordance with AJUMS principles and guidelines for animal care as reviewed by an ethics committee (IR.AJUMS.REC.1395.141) and maintained at 20 ±4 °C with a 12 h light/dark cycle. Moreover, the samples were provided with a standard rat pellet diet and water ad libitum.
Experimental design
Animals were divided into six groups of eight rats for each experimental procedure of cognition.
Group I (control): animals received 1 ml of emulsion of olive oil orally for 30 days
Group II: animals received BPA (50 mg/kg) (Sigma-Aldrich Corp., St. Louis, MO, USA) in olive oil (1 ml) orally for 30 days.
Group III, IV and V: animals treated with BPA (50 mg/kg) and NG (40, 80 and 160 mg/kg, respectively) (Sigma-Aldrich Corp., St. Louis, MO, USA) orally for 30 days.
Group VI: animals received 160 mg/kg NG alone for 30 days orally.
The doses of BPA and NG were selected based on previous studies reported in literature(23, 24).
Assessment of cognition
Step down latency (SDL) in passive avoidance apparatus
The step-down passive avoidance task is used to evaluate state-dependent learning and memory. All groups experienced a behavioral process examination at 0, 3 and 4 weeks. The apparatus consisted of a box made of plexiglass with dimensions of 40 _ 30 _ 30 cm and a floor of steel bars. Each of the steel bars was 0.3 inches in diameter with spacing of 1 cm. A wooden platform with dimensions of 4_4_4 cm was provided in the center of floor. Electric shocks with a frequency of 1 Hz at 15 volts for 15 s using a stimulator connected to the floor bars transmitted a shock to the animals’ hands and feet. When the animal was placed on the podium, the natural tendency of the animal was to get down on the floor bars. However, animal’s behavior was avoided after receiving shock. The step-down latency was considered as memory retrieval during training and testing stages. In the training phase, animals were slowly placed on the wooden platform in the middle of the device, and the delay of coming down from the platform was recorded by a chronometer. When the rat stepped down from the platform and placed all its paws on the grid floor, intermittent electric shocks were delivered continuously for 15 s. Before ending of the shock, the animal was removed. The test phase was repeated after 1 h, and the time taken by the rat to step down was measured (acquisition of memory). The procedure was repeated after 24 h without the shock (retention of memory). A cut-off time of 180 s was taken, and SDL was taken as 180 s for the animal that did not step down during this period (17).
Transfer latency (TL) on elevated plus maze
The elevated plus-maze model was employed to evaluate learning and memory in rats, following the procedure described by Itoh et al. (18). Rats in the plus-maze escaped from the open arm to the enclosed arm because rats apparently dislike open and high spaces. The time taken for the rats to move from the open arm to the enclosed arm (transfer latency) was recorded. The technique, end point and criteria for testing learning and memory were as per the parameters described by the investigators in the area of neuro psychopharmacology (15-18). Briefly, the apparatus consisted of two open arms (16 x 5 cm) and two enclosed arms (16 x 5 x 12 cm). The arms were extended from a central platform (5 x 5 cm), and the maze was elevated to a height of 50 cm from the floor. On the first day, each rat was placed at the end of an open arm, facing away from the central platform. Transfer latency (TL) is the time taken by rat with all its four legs to move into one of the enclosed arms. TL was recorded on the first day. During the first time of screening, if the animal did not enter the closed arm within 180 s, it was not included in the experiment. To become acquainted with the maze, the animals were allowed to explore the maze for 20 s after reaching the closed arm, and then returned to their home cage. The learning was tested 1 h later on the same day, followed by retesting of the animals 24 h after the first training day to test their memory retention. A period of 180 s was considered as a cut-off, and animals not entering the closed arm during this period were assigned the TL of 180 s (19).
Evaluation of locomotor activity
The effect of BPA (50 mg/kg), NG (40, 80 and 160 mg/kg) or a combination of these compounds on the locomotor activity of the animals was evaluated using an open field test. The apparatus had a floor of 100×100 cm, divided by red lines into 25 squares of 20×20 cm with white walls (30 cm high). The total number of squares crossed with four paws of the animals (total locomotion activity) and the numbers of rearing and standing on two hind paws without touching the walls (increased exploratory behavior) were recorded for 10 min (20).
Biochemical analysis
Hippocampus tissue preparation
At the end of 30 days, the skulls of the rats were cut open to expose the brain from the dorsal side after cervical dislocation and decapitation of the rats. The whole brain was removed, cleaned and chilled in ice-cold normal saline. The hippocampus region was dissected away from the brain and a 10% (w/v) homogenate (0.03 M sodium phosphate buffer, pH 7.4), was prepared using an Ultra-Turrax-T25 homogenizer (USA) at a speed of 9500 rpm. The homogenized tissue preparation was used to measure acetylcholinesterase (AChE), reactive oxygen species (ROS), catalase, malondialdehyde (MDA), glutathione (GSH), and nitrite.
Estimation of total hippocampal protein
Total hippocampal protein was estimated according to the method of Lowry et al. (21) using BSA, as standard.
ROS level in tissues of hippocampus
The level of ROS in hippocampus tissue was measured using 2, 7-dichlorofluorescin diacetate (DCFDA) (Sigma-Aldrich Corp., St. Louis, MO, USA), which was converted into fluorescent DCF by cellular peroxides. The fluorescence was calculated using a fluorometer at 488 nm excitation and 525 nm emission wavelength (10, 22).
GSH in tissue of hippocampus
The method described by Thomas was used to measure the GSH content. The GSH reacts with (5,5'-dithio-bis-[2-nitrobenzoic acid]) (DTNB) (Merck, Germany) and forms a yellow-colored complex nitroblue tetrazolium (NBT). The absorbance was read at 412 nm, and the result was expressed as µmoles of GSH/mg protein (23, 24).
Thiobarbituric acid reactive substances (TBARS) in tissue of hippocampus
The level of lipid peroxidation was measured in terms of malondialdehyde (MDA) creation. The amount of TBARS was calculated using a molar extinction coefficient of ɛ= 1.56 × 105 / M/cm and was expressed as mol/mg protein (25).
Catalase activity in tissue of hippocampus
Catalase (CAT) activity was assayed according to the method used by Goth. The absorbance was read at 410 nm, and the result was expressed as U/mg protein (26).
Superoxide dismutase in tissue of hippocampus
The activity of superoxide dismutase (SOD) was determined using a xanthine/xanthine oxidase system for production of superoxide radical and subsequent measurement of cytochrome c as a scavenger of the radicals (Beyotime Institute of Biotechnology , Jiangsu, China). Furthermore, optical density was determined using a spectrometer at 550 nm (UV-1601, Shimadzu) (27).
Measurement of glutathione peroxidase activity
The activity of glutathione peroxidase (GPx)was determined using assay kits (Beyotime Institute of Biotechnology, Jiangsu, China). The GSH kit utilizes an enzymatic recycling method based on the reaction between GSH and DTNB that produces a yellow-colored compound (NBT) (28).
Measurement of acetylcholinesterase activity
AChE is a marker of loss of cholinergic neurons in the forebrain. In this study, the AChE activity was assessed via the Ellman method (29). Results were expressed as mmol of acetylthiocholine iodide hydrolyzed/min/mg protein.
Estimation of nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide, was determined by a colorimetric assay with Griess reagent (0.1% N-(1-napththyl) ethylene diamine dihydrochloride, 1% sulfanilamide, and 5% phosphoric acid) (Sigma-Aldrich Corp., St. Louis, MO, USA) according to the procedure of Green et al. (30).
Statistical analysis
All the results were statistically analyzed using Graph Pad Prism (version 5.04) as mean ± standard error of mean (SEM). Data distribution was normal. The behavioral assessments were analyzed by repeated-measures two-way analysis of variance (ANOVA). The biochemical estimations were analyzed by one way ANOVA. Post hoc comparisons between groups were made using Tukey’s test. P <0.05 was considered significant.
Results
Body and brain weights.
The final body weight and brain weight to body weight ratio of male rats in the control and treatment groups are given in Table 1. In the present study, no significant difference in mean body weight and brain weight to body weight ratio was observed in the treatment groups in comparison with the control group
Table 1.
Effect of BPA and NG on final body weight and brain weight to body weight ratio
| Parameters Treatment | Body weight (g) | Brain weight (mg) to body weight (g) ratio |
|---|---|---|
| Control | 230.14±3.12 | 7.11 ± 0.286 |
| BPA 50 mg/kg | 225.20±1.13 | 6.97 ± 0.241 |
| BPA 50 mg/kg + NG 40 mg/kg | 224±5.7 | 6.97 ± 0.161 |
| BPA 50 mg/kg + NG 80 mg/kg | 227±4.8 | 6.84 ± 0.161 |
| BPA 50 mg/kg + NG 160 mg/kg | 229.56±2.02 | 7.02 ± 0.212 |
| NG 160 mg/kg | 230.44±4.30 | 7.25 ± 0.245 |
Data are mean SD; n = 8. BPA: Bisphenol A; NG: Naringin. P values were from one-way ANOVA, followed by Tukey’s test for multiple comparisons
Effect of BPA and NG treatment on memory performance in passive avoidance paradigm
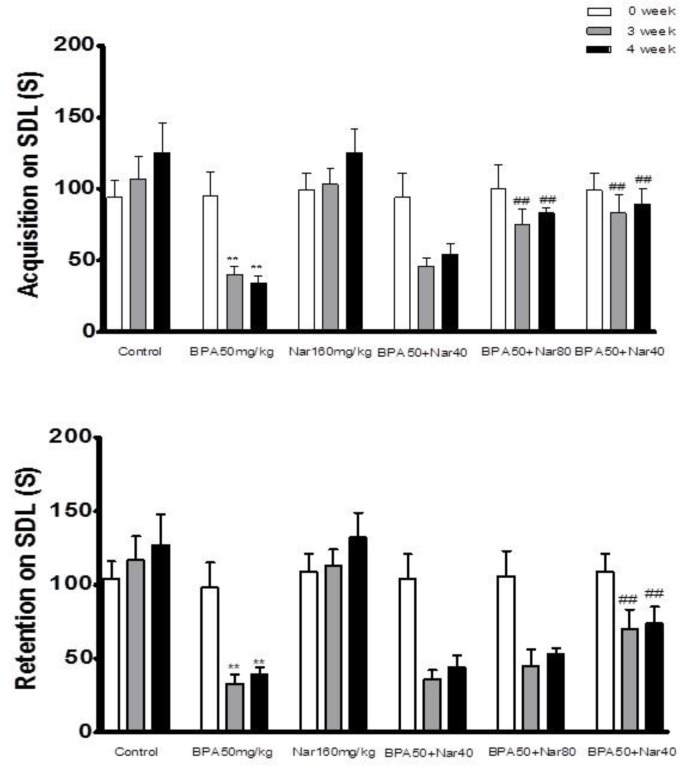
At the start of the experiment, no significant differences were found among the SDL values of all experimental groups. A significant reduction in both acquisition as well as retention in SDL paradigm was observed in the BPA 50 mg/kg treated group at weeks 3 and 4 when compared with SDL values of both control (P < 0.001) and day 0 of BPA -treated groups (P < 0.001) (Figure 1A, B). Co-administration of NG (80 and 160 mg/kg) with BPA 50 mg/kg significantly (P<0.01 and P<0.001, respectively) prolonged the acquisition SDL when compared with the BPA 50 mg/kg alone treated group. Co-treatment with NG (160 mg/kg) also significantly reversed the BPA-induced retention deficits. In almost all groups, there was no significant difference between the third and fourth week (Figure 1).
Fig. 1.
Effect of bisphenol A (BPA), and Naringin (NG) on step-down latency (SDL) acquisition and retention. Animals were trained on each day prior to assessment of cognition. Animals were tested after 60 min for acquisition and after 24 h for retention of memory. A: SDL acquisition; B: SDL retention. Each value was presented as mean ± SEM (n = 8). **: P <0 .001 when compared with normal control group; ##: P < 0 .001 as compared to BPA only group
Effect of BPA and NG on memory performance in the elevated plus maze paradigm
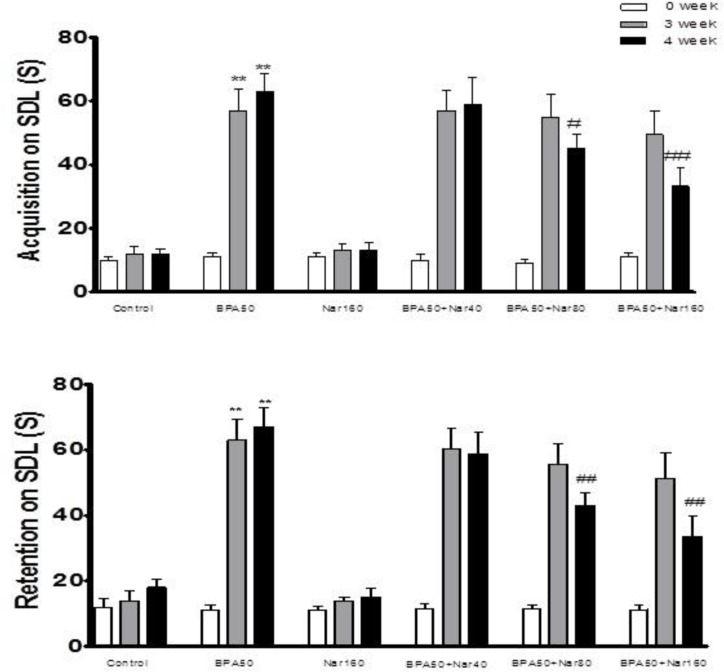
At day0, no significant difference was found among the TLs of all groups. In the BPA 50 mg/kg treated group, a significant (P <0.001) prolongation in both acquisition and retention was observed when compared with the control group (Figure 2A, B). In the present experiment, mean TL on 3 weeks for NG groups were relatively stable and showed no significant variation. There was a marked reduction in TL of BPA-NG (160 mg/kg) group (P <0.001) when compared with the BPA group at 4th week (Figure 2A, B).
Fig. 2.
Effect of bisphenol A (BPA), and Naringin (NG) on transfer latency (TL) acquisition and retention. Animals were trained on each day prior to assessment of cognition. Animals were tested after 60 min for acquisition and after 24 h for retention of memory. A: TL acquisition; B: TL retention. Each value was presented as mean ± SEM (n = 8). **: P < 0.001 when compared with normal control group; ##: P < 0.001 as compared to BPA only group
Effect of BPA and NG on the locomotor activity
Chronic administration of BPA (50 mg/kg),
NG (40, 80 and 160 mg/kg, p.o.) or their combination did not show any effect on the locomotor activity in comparison with the control rats at 3 and 4 weeks. Furthermore, NG treatment (40, 80 and 160 mg/kg, p.o.) did not cause any alteration in the locomotor activity in comparison with the BPA- treated rats at 3 and 4 weeks (Table 2).
Table 2.
The effect of BPA and NG on rearing and total locomotion of rats in open-field test
|
Parameters
Treatment |
Rearing
|
Total locomotion
|
||
|---|---|---|---|---|
| 3 weeks | 4 weeks | 3 weeks | 4 weeks | |
| Control | 13.1±3.7 12.8 ±2.3 | 230.6±14.3 228.6 ±7.3 | ||
| BPA 50 mg/kg | 12.8±5.7 11.9 ± 1.7 | 231.6±18.3 224.9 ± 3.7 | ||
| BPA 50 mg/kg + NG 40 mg/kg | 12.5±2.8 12.3 ± 1.6 | 228.5±10.3 227.5 ± 12.1 | ||
| BPA 50 mg/kg + NG 80 mg/kg | 11.9±.4 12.9 ± 2.7 | 226.4±4.9 230 ± 9.6 | ||
| BPA 50 mg/kg + NG 160 mg/kg | 13.3±2.7 13 ± 1.6 | 233.6±11.3 229.9 ± 8.6 | ||
| NG 160 mg/kg | 13.8±2.7 13.2 ± 3.1 | 231.6±12.4 230.7 ± 11.4 | ||
Control: emulsion of olive oil; Data are mean SEM; n = 8. BPA: Bisphenol A; NG: Naringin. P values were from one-way ANOVA, followed by Tukey’s test for multiple comparisons
Evaluation of enzymatic and non-enzymatic antioxidants
MDA and ROS levels have significantly (P < 0.05 and 0.001, respectively) increased after BPA (50 mg/kg) intoxication, in comparison with the normal control rats. The MDA and ROS levels significantly decreased when NG (160 mg/kg) was given to BPA-intoxicated rats (P < 0.05 and 0.001, respectively). No difference was observed in the hippocampus MDA and ROS levels of NG (160 mg/kg), and the control groups (Table 3).
Table 3.
Effect of BPA and NG on enzymatic and non-enzymatic antioxidants
|
Gropups
variables |
Control | BPA 50 mg/kg | BPA 50 mg/kg+NG 40 mg/kg | BPA 50 mg/kg+NG 80 mg/kg | BPA 50 mg/kg+NG 160 mg/kg | NG 160 mg/kg |
|---|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 1.16 ± 0.02 | 2.89 ± 0.04*** | 2.78 ± 0.06 | 2.80 ± 0.05 | 2.61 ± 0.04# | 1.2±0.81 |
| ROS (% of control) | 100 ±10.1 | 197.87±12.61*** | 166.65±11.76 | 141.76±11,6## | 117.64±14.7### | 94.89±17.1 |
| GSH (nmol/mg protein) | 100 ±2.6 | 25.48 ±5.2*** | 24.5 ±5.8 | 50.25 ±4.8## | 72.1 ±3.2### | 107.27 ±5.4** |
| Catalase (µmol/ mgprotein) | 50.22±1.34 | 22.48±3.71*** | 22.3±5.8 | 29.7±2.9# | 34.80±8.62## | 51.98±1.87 |
| SOD (U/mg protein) | 1.7±0.26 | 1.1±0.49** | 1.14±0.49 | 1.22±0.59 | 1.32±0.48# | 1.77±0.79 |
| GSH-Px (µmol/mg protein) | 1.3±0.47 | 0.9±0.17* | 0.9±0.05 | 1.03±0.07 | 1.14±0.45# | 1.35±0.99 |
Data are mean SD; n = 8. Difference between control and other groups is significant at P < 0.001(***), P < 0.01 (**) and P < 0.05 (*). Difference between BPA-treated and other groups is significant at P < 0.001(###), P < 0.01 (##) and P < 0.05 (#). BPA: bisphenol A; NG: naringin; MDA: malondialdehyde; ROS: reactive oxygen species; GSH: glutathione; SOD: superoxide dismutase; CAT: catalase; GSH-Px: glutathione peroxidase. P values were from one-way ANOVA, followed by Tukey’s test for multiple comparisons.
A significant (P <0.001) decrease was found in the hippocampus GSH levels of BPA (50 mg/kg) treated group when compared to the control group. A significant increase was noted for NG (80 and160 mg/kg) + BPA treated groups when compared with the BPA group (P <0.01 and 0.001, respectively). NG antagonized the effect of BPA on GSH. The difference between NG (160 mg/kg) alone and the control group was found to be statistically significant (P <0.01) (Table 3).
There was a marked and statistically significant decrease in the hippocampus CAT activity of te group treated with BPA (P <0.001). Treatment with NG attenuated the effect of BPA on CAT activity, the difference between BPA alone and NG (80 and160 mg/kg) + BPA was found to be significant (P < 0.05 and 0.001, respectively). No difference was observed in the hippocampus CAT activityof NG (160 mg/kg), and the control groups (Table 3).
As illustrated in Table 3, BPA administration induced a significant reduction in the hippocampus SOD activity in comparison with the control value (P<0.01). In contrast, NG (160 mg/kg) co-administered with BPA induced a significant elevation in the hippocampus SOD activity in comparison with the BPA treated group (P<0.05) (Table 3).
The enzyme GPx significantly decreased (P<0.05) after BPA (50 mg/kg) intoxication, in comparison with the normal control rats. However, the GPx activity significantly increased (P <0.05) in NG (160 mg/kg) BPA co-treatment group, in comparison with the BPA-intoxicated rats (Table3).
Effect of BPA and NG on hippocampus nitrite concentration
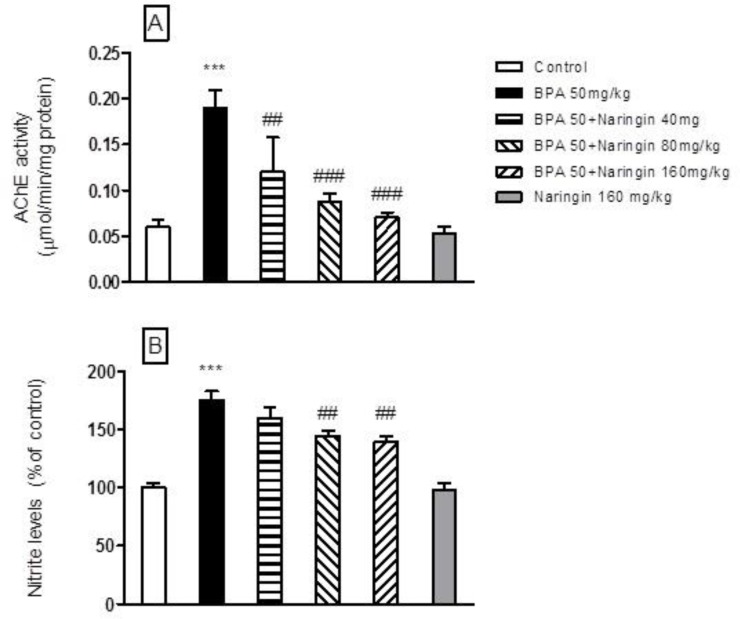
BPA (50 mg/kg) administration significantly increased nitrite concentration, but chronic NG (80 and 160 mg/kg, p.o.) administration significantly attenuated the rise in nitrite concentration (P<.001). However, NG (160 mg/kg, p.o) treatment alone did not produce any significant effect on this parameter in comparison with the control rats (Figure 3A).
Fig. 3.
Effect of bisphenol A (BPA), and Naringin (NG) on nitrite level and AChE activity. A: nitrite level; B: AChE activity. Each value was presented as mean ± SEM (n = 8). ***: P < 0.001 when compared with normal control group; ##: P < 0.01, and ###: P < 0.001 as compared to BPA only group. P values were from one-way ANOVA, followed by Tukey’s test for multiple comparisons
Effect of BPA and NG on hippocampus AChE activity
Oral BPA (50 mg/kg) administration significantly increased AChE activity in comparison with the control rats. However, chronic oral administration of NG (80 and 160 mg/kg, p.o.) treatment significantly attenuated the AChE activity in comparison with the BPA - treated animals
(P<.001) (Figure 3B).
Discussion
BPA is one of the most commonly known endocrine toxicant released by polycarbonate plastics, lining of food cans and dental sealants. Humans are repeatedly exposed to BPA owing to its extensive availability in the environment. It was demonstrated that BPA induces oxidative stress in several brain regions and seems to play an important role in many degenerative disorders (16). It is also known that BPA causes cognitive impairment in rodents. It has been described that exposure to BPA during the perinatal period affects behaviors in the offspring of experimental animals (31). Moreover, Miyagawa et al. (5) indicated that prenatal and neonatal BPA exposure produced memory damage in male pups and decreased acetylcholine production in the hippocampus. Furthermore, Xu et al. (6) found that perinatal exposure to BPA exerted impacts on the normal behavioral development in both spatial and avoidance memories, affecting the behavior of offsprings in adulthood.
The present study aimed to assess the effect of BPA (50 mg/kg /day) alone and in combination with NG on cognition. Research tools included step-down latency in passive avoidance apparatus and transfer latency on elevated plus maze. The results indicated that administration of BPA for 30 days significantly decreased the SDL in passive avoidance while increased TL prolonged on elevated plus maze, compared to the control group. Moreover, we also pointed out for the first time that the concomitant administration of NG (80 and 160 mg/kg) inhibited learning and memory weakening induced by BPA toxicity.
A number of reports suggested the involvement of oxidative stress in the pathophysiology of many chronic diseases, including cancer, diabetes, aging, and other degenerative disorders (32). The increased production of free radicals in the brain and other tissues is scavenged by antioxidants, such as superoxide dismutase, CAT, and GSH-dependent enzymes.
Oxidative damage is considered as a possible reason of brain dysfunction since the brain is supposed to be mainly susceptible to oxidative stress owing to a high rate of oxygen free radical generation without appropriate levels of antioxidant defenses, compared to other tissues. In particular, the brain contains large amounts of phospholipids composed of polyunsaturated fatty acid side chains with a tendency to peroxidation by oxygen free radicals. As described by Negishi et al. (33), BPA is capable of penetrating blood-brain barrier and damaging the central nervous system.
In the present study, administration of BPA altered the oxidative stress parameters as indicated by increasing MDA and ROS levels and decreasing GSH level, GPx, CAT and SOD activities in the hippocampus tissues of BPA-treated rats, in comparison with the control rats. This is in line with the data reported by previous studies, which demonstrated increased ROS generation in the brain and reduction of endogenous antioxidants in the liver and epididymal sperm of experimental animals after BPA exposure. Obata and Kubota (34) described that BPA increased hydroxyl radical formation in the striatum of the adult rat. Exposure of mice to BPA during embryonic/fetal life and during lactation by feeding their pregnant/lactating mothers has been established to increase thiobarbituric acid-reactive substance levels in the brain, kidney, and testis. However, no alteration in GSH level has been observed in this regard (8). Furthermore, Aydogan et al. (14) reported that administration of BPA decreased the level of GSH and increased the MDA, indicating oxidative damage in the brain of rats. In the present study, BPA-treated animals showed significant impairment of memory and increased oxidative stress, in comparison with the control group. Therefore, the increase in oxidative stress could be one of the possible mechanisms for the impairment of learning and memory observed by the administration of BPA.
Antioxidants are of great importance in terms of being therapeutic agents that inhibit disorders caused by oxidative stress. On the other hand, NG is a bioflavonoid derived from grapefruit and related citrus species. According to the literature, NG is a potent antioxidant and anti-inflammatory, and prevents nitric oxide production (35). Presently, significant attention is paid to the effectiveness of citrus fruits by researchers due to the fact that their intake appears to be associated with decreased risk of neurodegenerative diseases. In the current research, chronic administration of NG attenuated BPA-induced oxidative damage. However, the independent use of NG had no effect on the oxidative stress markers in the hippocampus of normal animals. Meanwhile, it significantly attenuated the rise in lipid peroxidation and nitrite levels, and restored the depletion of GSH levels and SOD, CAT, and GPx activities. This impact might be attributed to its strong antioxidant activity.
It has been reported that NG can block the lipid peroxidation induced by H2O2 (36). GSH is an endogenous antioxidant existing in the reduced form in the cells. This antioxidant reacts with free radicals and prevents the production of hydroxyl free radicals (30). The decreased GSH level and GPx activity in BPA-treated rats shows the enhanced production of free radicals and depleting of the activity of the glutathione system while opposing the oxidative stress. Treatment with NG led to the restoration of the depleted GSH level and GPx activity, suggesting the ability of NG in increasing oxidative defense by restoring the levels of these antioxidants. According to the literature, the antioxidant activity of NG itself is similar to that of GSH (36). At the cellular level, NG moderates the toxic effects of reactive oxygen species by directly stimulating the transcription of genes that code for several antioxidant enzymes, such as CAT, SOD, and GPx. This may clarify the fact that NG administration in BPA-treated rats produced a significant increase in the CAT and SOD activities (37, 38).
Administration of BPA also resulted in increased production of inducible nitric oxide synthase and nitric oxide in the brain. The produced nitric oxide can cause the creation of peroxynitrite free radicals by interacting with the superoxide anions. The produced nitric oxide also causes the nitrosylation of several enzymes, thus inhibiting glycolysis and producing extensive brain impairment (39). In addition, overproduction of nitric oxide is neurotoxic to cholinergic neurons, which demonstrates how administration of BPA causes a significant increase in the nitrite levels in the brain and how NG treatment is able to decline the nitrite levels (40). As a result, the observed beneficial effect of NG might be due to its inhibitory effect on the nitric oxide synthase enzyme and/or scavenging nitric oxide free radicals.
Levels of numerous neurotransmitters (e.g., dopamine, acetylcholine, and noradrenaline) change significantly through cognitive dysfunctions. However, the cholinergic system is certainly the most affected. It seems that the cholinergic system is responsible for storage and recovery of memory, and its destruction is associated with the level of cognitive deficit (41). Administration of BPA leads to the death of cerebellar granule cells, olfactory bulb neurons, and basal forebrain cholinergic neurons. Furthermore, BPA causes choline toxicity, resulting in decreased acetylcholine transferase activity. This demonstrates that administration of BPA decreases acetylcholine turnover, and causes cognitive dysfunction and oxidative stress. Chronic NG treatment also attenuated AChE activity following the administration of BPA. Therefore, it seems that NG might have its action on these pathways. However, the mechanism of the protective effect of NG on AChE activity remains uncertain. It has been observed that AChE activity increases in rat brain by free radicals generation, and acetylcholine which inhibits nitric oxide is synthesized in the nervous system (42). In the present study, BPA caused a significant increase in AChE activity, which probably led to cognitive deficits, suggesting the possible involvement of oxidative injury. In addition, NG was able to ameliorate the BPA-induced increase in the AChE activity. The present study suggested that the free radical scavenging and antioxidant effects of NG might have a critical role against the inhibition of AChE in the cholinergic system of cognitive dysfunction.
The limitation of this study is the lack of cellular and histochemical experiments to find out the molecular mechanism of bisphenol and NG and the role of oxidative stress, which is suggested to be investigated in future studies.
In conclusion, according to the results, NG reversed the BPA-induced cognitive impairment in the SDL and TL paradigms. In addition, it reversed the disorder in oxidative stress parameters, nitric oxide production, and AChE activity caused by BPA. The multitude effects of NG intensely supported its neuroprotective effects against BPA-induced cognitive dysfunction and oxidative damage. Further research regarding the use of NG as a favorable drug candidate for the treatment of cognitive dysfunctions and other neurodegenerative disorders is suggested.
Acknowledgments
This study was labeled Student Research Project No. 95S116, and was supported financially by the Student Research Committee of Ahvaz Jundishapur Medical Sciences University, Ahvaz, Iran.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Goodson A, Robin H, Summerfield W, et al. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit Contam. 2004;21:1015–26. doi: 10.1080/02652030400011387. [DOI] [PubMed] [Google Scholar]
- 2.vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitra KC, Latchoumycandane C, Mathur PP. Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch Toxicol. 2002;76:545–51. doi: 10.1007/s00204-002-0372-4. [DOI] [PubMed] [Google Scholar]
- 4.Steinmetz R, Brown NG, Allen DL, et al. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–6. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa K, Narita M, Narita M, et al. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci Lett. 2007;418:236–41. doi: 10.1016/j.neulet.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 6.Xu XH, Wang YM, Zhang J, et al. Perinatal exposure to bisphenol-A changes N-methyl-D-aspartate receptor expression in the hippocampus of male rat offspring. Environ Toxicol Chem. 2010;29:176–81. doi: 10.1002/etc.18. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Kumar CH, Suranagi UD, et al. Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem Toxicol. 2011;49:1404–9. doi: 10.1016/j.fct.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–24. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 9.Podder B, Song HY, Kim YS. Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J Microbiol Biotechnol. 2014;24:605–13. doi: 10.4014/jmb.1402.02001. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Yadav A, Bozhkov AI, et al. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74:607–14. doi: 10.1016/j.ecoenv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kannappan S, Palanisamy N, Anuradha CV. Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose-administered rats. Eur J Pharmacol. 2010;645:177–84. doi: 10.1016/j.ejphar.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Fang F, Tang Y, Gao Z, et al. A novel regulatory mechanism of naringenin through inhibition of T lymphocyte function in contact hypersensitivity suppression. Biochem Biophys Res Commun. 2010;397:163–9. doi: 10.1016/j.bbrc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 13.Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–34. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Aydogan M, Korkmaz A, Barlas N, et al. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology. 2008;249:35–9. doi: 10.1016/j.tox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Gaur V, Aggarwal A, Kumar A. Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol. 2009;616:147–54. doi: 10.1016/j.ejphar.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 16.Zbarsky V, Datla KP, Parkar S, et al. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–25. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 17.Hemmati AA, Alboghobeish S, Ahangarpour A. Effects of cinnamic acid on memory deficits and brain oxidative stress in streptozotocin-induced diabetic mice. Korean J Physiol Pharmacol. 2018;22:257–67. doi: 10.4196/kjpp.2018.22.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 1990;101:27–33. doi: 10.1007/BF02253713. [DOI] [PubMed] [Google Scholar]
- 19.Naidu PS, Singh A, Kulkarni SK. Reversal of reserpine-induced orofacial dyskinesia and cognitive dysfunction by quercetin. Pharmacology. 2004;70:59–67. doi: 10.1159/000074669. [DOI] [PubMed] [Google Scholar]
- 20.Alboghobeish S, Naghizadeh B, Kheirollah A, et al. Fluoxetine increases analgesic effects of morphine, prevents development of morphine tolerance and dependence through the modulation of L-type calcium channels expression in mice. Behav Brain Res. 2019;361:86–94. doi: 10.1016/j.bbr.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Ahangarpour A, Zeidooni L, Rezaei M, et al. Protective effect of metformin on toxicity of butyric acid and arsenic in isolated liver mitochondria and langerhans islets in male mice: an in vitro study. Iran J Basic Med Sci. 2017;20:1297–305. doi: 10.22038/IJBMS.2017.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahangarpour A, Alboghobeish S, Oroojan AA, et al. Effects of Combined Exposure to Chronic High-Fat Diet and Arsenic on Thyroid Function and Lipid Profile in Male Mouse. Biol Trace Elem Res. 2018;182:37–48. doi: 10.1007/s12011-017-1068-1. [DOI] [PubMed] [Google Scholar]
- 24.Ahangarpour A, Alboghobeish S, Oroojan AA, et al. Mice pancreatic islets protection from oxidative stress induced by single-walled carbon nanotubes through naringin. Hum Exp Toxicol . 2018:960327118769704. doi: 10.1177/0960327118769704. [DOI] [PubMed] [Google Scholar]
- 25.Ahangarpour A, Alboghobeish S, Rezaei M, et al. Evaluation of Diabetogenic Mechanism of High Fat Diet in Combination with Arsenic Exposure in Male Mice. Iran J Pharm Res. 2018;17:164–83. [PMC free article] [PubMed] [Google Scholar]
- 26.Ahangarpour A, Zeidooni L, Samimi A, et al. Chronic exposure to arsenic and high fat diet additively induced cardiotoxicity in male mice. Res Pharm Sci. 2018;13:47–56. doi: 10.4103/1735-5362.220967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmati AA, Alboghobeish S, Ahangarpour A. Chronic exposure to high fat diet exacerbates arsenic-induced lung damages in male mice: Possible role for oxidative stress. Monaldi Arch Chest Dis. 2018;88:903. doi: 10.4081/monaldi.2018.903. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver 1976. Biochem Biophys Res Commun. 2012;425:503–9. doi: 10.1016/j.bbrc.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Ellman GL, Courtney KD, Andres V Jr, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 30.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Itoh K, Sugimoto T, et al. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420:100–5. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 33.Negishi T, Ishii Y, Kyuwa S, et al. Inhibition of staurosporine-induced neuronal cell death by bisphenol A and nonylphenol in primary cultured rat hippocampal and cortical neurons. Neurosci Lett. 2003;353:99–102. doi: 10.1016/j.neulet.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Obata T, Kubota S. Formation of hydroxy radicals by environmental estrogen-like chemicals in rat striatum. Neurosci Lett. 2000;296:41–4. doi: 10.1016/s0304-3940(00)01619-0. [DOI] [PubMed] [Google Scholar]
- 35.Choi SY, Ko HC, Ko SY, et al. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol Pharm Bull. 2007;30:772–8. doi: 10.1248/bpb.30.772. [DOI] [PubMed] [Google Scholar]
- 36.Kanno S, Shouji A, Asou K, et al. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci. 2003;92:166–70. doi: 10.1254/jphs.92.166. [DOI] [PubMed] [Google Scholar]
- 37.Alboghobeish S, Mahdavinia M, Zeidooni L, et al. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran J Basic Med Sci. 2019;22:315–523. doi: 10.22038/ijbms.2019.29757.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khodayar MJ, Kalantari H, Mahdavinia M, et al. Protective effect of naringin against BPA-induced cardiotoxicity through prevention of oxidative stress in male Wistar rats. Drug Chem Toxicol . 2018:1–11. doi: 10.1080/01480545.2018.1504958. [DOI] [PubMed] [Google Scholar]
- 39.Wallis RA, Panizzon KL, Henry D, et al. Neuroprotection against nitric oxide injury with inhibitors of ADP-ribosylation. Neuroreport. 1993;5:245–8. [PubMed] [Google Scholar]
- 40.Harisa GI, Mariee AD, Abo-Salem OM, et al. Erythrocyte nitric oxide synthase as a surrogate marker for mercury-induced vascular damage: the modulatory effects of naringin. Environ Toxicol. 2014;29:1314–22. doi: 10.1002/tox.21862. [DOI] [PubMed] [Google Scholar]
- 41.Gasparini L, Racchi M, Binetti G, et al. Peripheral markers in testing pathophysiological hypotheses and diagnosing Alzheimer's disease. FASEB J. 1998;12:17–34. doi: 10.1096/fasebj.12.1.17. [DOI] [PubMed] [Google Scholar]
- 42.Roszer T, Jozsa T, Szentmiklosi AJ, et al. Acetylcholine inhibits nitric oxide (NO) synthesis in the gastropod nervous system. Cell Tissue Res. 2009;336:325–35. doi: 10.1007/s00441-009-0764-3. [DOI] [PubMed] [Google Scholar]