Abstract
Background:
Recurrent ischemic stroke (IS) increases the risk of cognitive decline. To lower the risk of recurrent IS, secondary prevention is essential.
Objective:
Our aim was to compare post-discharge secondary IS prevention and its maintenance up to 3 years after first IS in patients with and without Alzheimer’s disease and other dementia disorders.
Methods:
Prospective open-cohort study 2007–2014 from the Swedish national dementia registry (SveDem) and the Swedish national stroke registry (Riksstroke). Patients with dementia who experienced an IS (n = 1410; 332 [23.5%] with Alzheimer’s disease) were compared with matched non-dementia IS patients (n = 7150). We analyzed antiplatelet, anticoagulant, blood pressure lowering, and statin treatment as planned medication initiation at discharge and actual dispensation of medications at first, second, and third year post-stroke.
Results:
At discharge, planned initiation of medication was higher in patients with dementia compared to non-dementia patients for antiplatelets (OR with 95% CI for fully adjusted models 1.23 [1.02–1.48]) and lower for blood pressure lowering medication (BPLM; 0.57 [0.49–0.67]), statins (0.57 [0.50–0.66]), and anticoagulants (in patients with atrial fibrillation – AF; 0.41 [0.32–0.53]). When analysis for antiplatelets was stratified according to the presence of AF, ORs for receiving antiplatelets remained significant only in the presence of AF (in the presence of AF 1.56 [1.21–2.01], in patients without AF 0.99 [0.75–1.33]). Similar trends were observed in 1st, 2nd, and 3rd year post-stroke.
Conclusions:
Dementia was a predictor of lower statin and BPLM use. Patients with dementia and AF were more likely to be prescribed antiplatelets and less likely to receive anticoagulants.
Keywords: Alzheimer’s disease, anticoagulants, antihypertensive agents, cohort studies, dementia, hydroxymethylglutaryl-CoA reductase inhibitors, ischemic stroke, platelet aggregation inhibitors, secondary prevention
INTRODUCTION
Recurrent ischemic strokes account for approximately 25% of all strokes [1, 2], worsen prior stroke disability, and are more likely to cause dementia [3]. To reduce their burden, identification of stroke mechanisms and treatment of risk factors is crucial. The cornerstones of secondary prevention are antiplatelet therapy in non-embolic stroke, treatment of hypertension, diabetes, hyperlipidemia, anticoagulant treatment in patients with atrial fibrillation (AF), management of lifestyle risk factors (such as smoking and obesity), and early carotid revascularization in patients with ipsilateral severe carotid artery stenosis [4]. Although advances in carotid revascularization have contributed to the improvement in patient outcomes, the absolute effect on population level compared to a pharmacological secondary prevention is small [3]. Treatment of all major risk factors is estimated to reduce the risk of recurrent stroke by about 80% compared with no treatment [1, 3] and optimum control of risk factors through the regular use of cardiovascular medication also reduces risk of cognitive impairment and cardiovascular events [5, 6].
Depending on dementia severity, stroke is three to seven times more common in patients with dementia compared to dementia-free individuals [4]. Hypertension, diabetes, and hyperlipidemia predispose individuals not only to vascular and mixed dementia, but also to Alzheimer’s disease (AD). Overlap between these subtypes in clinical presentation and pathology is common [7]; moreover, cerebrovascular lesions may lower the threshold for clinical manifestation of AD [4]. Much of the research on secondary prevention has been performed in younger and healthier subjects, while the burden of stroke is highest in the oldest subset of the population [8]. Older age and cognitive impairment may hinder the institution of secondary prevention due to increased susceptibility to adverse drug events in older adults, ethical issues concerning prevention in patients with limited life expectancies [8], or the assumption that cognitively impaired patients have decreased adherence to drug regimens [5].
The influence of dementia status on initiation of secondary ischemic stroke (IS) prevention has not been addressed on a national scale. In studies to date, dementia was an independent predictor of nontreatment with aspirin or warfarin [9–11], while the initiation and/or maintenance of blood-pressure and lipid lowering therapies have been explored only as subanalyses in an underpowered number of dementia patients [12]. The aim of the study was to compare post-discharge secondary stroke prevention and its maintenance up to 3 years after first ischemic stroke in patients with and without dementia.
MATERIALS AND METHODS
We analyzed antiplatelet, anticoagulant, blood pressure lowering, and statin treatment at discharge as planned initiation of medications and their dispensation at first, second, and third year post-stroke.
Study population, registries, and variables
We performed an open-cohort study of patients diagnosed and registered with dementia who subsequently had a first IS between 2007 and 2014 in Sweden. At the time of dementia diagnosis, patients were registered in SveDem, the Swedish national dementia registry, together with information on dementia type [13]. Occurrence of IS was identified using Riksstroke, the Swedish national registry for acute stroke with coverage for IS > 90% [14]. Additional information on the registries is available at svedem.se and riksstroke.org.
We used data from additional registries; the Swedish Prescribed Drug Register (PDR) contains data on all prescription medication dispensed at Swedish pharmacies since 2005 with ∼100% coverage [15]. Data on comorbidities before stroke were collected from the Swedish National Inpatient Register (NIR) [16] and used to calculate the Charlson Comorbidity Index (CCI) [17]. All in-hospital and specialist clinic diagnoses were coded according to the ICD-10 and were available from 1998 [16]. Data on death in hospital was obtained from Riksstroke, while data on death after hospital discharge until August 2016 was obtained from the Swedish Cause of Death Register [18]. Data on medication was available until 31 December 2015, consequently, to ensure 3-year follow-up for all patients, patients with IS event after 31 December 2012 were excluded from 3-year and patients with IS after 31 December 2013 were excluded from 2- and 3-year follow-up analyses.
Out of 58,154 patients from SveDem diagnosed with dementia between 2007 and 2014, we identified 1410 patients with dementia and IS. These patients were compared with 7,150 non-dementia IS controls from Riksstroke matched by age (±3 years), sex, year of stroke, and geographic region. Patients with hemorrhagic stroke or prior IS were excluded. Included non-dementia controls never had a SveDem registration, were never diagnosed with dementia or confusional syndrome (ICD-10 codes F00-F09 or G30-G32), and had never been dispensed antidementia medication (donepezil, rivastigmine, galantamine, or memantine).
Variables
Information on dementia type and date was collected from SveDem. Data on subsequent stroke event, demographics, follow-up, and death in hospital was obtained from Riksstroke. Smoking was defined as more than one cigarette per day or if a patient quit less than 3 months ago. Data on medication prior to stroke and at 1st, 2nd, and 3rd year post-stroke was obtained from PDR according to following ATC codes: antiplatelets (platelet aggregation inhibitors – B01AC06, B01AC04 [acetylsalicylic acid, and clopidogrel]), anticoagulants (B01AA, B01AE, B01AF [vitamin K antagonists, direct thrombin, and factor Xa inhibitors]), blood pressure lowering medication – BPLM (C02, C03, C07, C08, C09A-D [diuretics, beta blocking agents, calcium-channel blockers, agents acting on the renin angiotensin system, other]), and statins (C10AA). Data on medication at discharge was obtained from Riksstroke. Similarly, medication at discharge was defined as antiplatelet, anticoagulant, statin therapy, and BPLM.
Medication at discharge is presented as planned initiation of medication (patient was receiving medication or its initiation was planned within 2 weeks of discharge), and for medication prior to stroke or at 1, 2, or 3-year follow-up after stroke, receiving therapy was registered if a patient collected at least one prescription from the pharmacy in the 6 months before the stroke event, 1, 2, or 3-year period after the stroke event. Anticoagulant use is presented only in patients with AF.
Statistical analysis
Data are presented as number of cases and percentages (categorical variables) and as mean (±standard deviation–SD) or median (±interquartile range–IQR) for continuous variables. For calculating significant differences, Chi-square test was used for categorical and Student t-test and Mann-Whitney U-test were used for continuous variables.
To assess the relationship between dementia status and receiving secondary prevention, multivariate logistic regression analyses were used. Initial regression models (Model 1) were adjusted for age and sex. Because of possible confounding, different covariates were added in a stepwise manner. In Model 2, we added nursing home placement after discharge as a surrogate of a worse functional status. Final model (Model 3) was adjusted for possible confounding comorbidities and medication. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are presented.
Tests with p value <0.05 (2-tailed) were considered significant. The IBM Statistical Package for Social Sciences (IBM SPSS) for Windows, version 22 (IBM Corporation, Armonk, NY, USA) was used.
Standard protocol approvals and patient consent
This study was approved by the regional ethical review board in Stockholm, Sweden (dnr 2015/743-31/4) and it complies with the Declaration of Helsinki. At the time of diagnosis, patients and relatives were informed of inclusion in SveDem and Riksstroke and could decline participation or withdraw consent. Data were de-identified before the analysis and no connection could be made to an individual.
RESULTS
Characteristics of patients with and without dementia and ischemic stroke at different time points are presented in Table 1. Before stroke, 430 (30.8%) dementia and 529 (7.4%) non-dementia patients were nursing home residents (p < 0.001). There were 60 (4.7%) dementia and 552 (8.5%) non-dementia smokers (p < 0.001). There were no differences in occurrence of heart failure (International Classification of Diseases 10 [ICD-10] I50; 294 [20.9%] in dementia and 1363 [19.1%] in non-dementia patients, p = 0.120) or ischemic heart disease (ICD-10 I20–I25, 394 [27.9%] in dementia and 1945 [27.2%] in non-dementia patients, p = 0.569) prior to stroke. Conversely, before stroke, more dementia patients had hypertension (799 [56.7%] dementia and 3624 [50.7%] non-dementia patients, p < 0.001).
Table 1.
Secondary stroke prevention at different time points in patients with and without dementia and first ischemic stroke
| Prior to Stroke | Initiation Planned at Discharge | 1-Year Post-Stroke | 2-Years Post-Stroke | 3-Years Post-Stroke | |||||||||||
| D | C | p | D | C | p | D | C | p | D | C | p | D | C | p | |
| n = 1410 | n = 7150 | n = 1241 | n = 6319 | n = 889 | n = 5224 | n = 651 | n = 4187 | n = 352 | n = 2619 | ||||||
| Age, mean (SD) | 83 (7) | 82 (7) | 0.043 | 82 (7) | 82 (7) | 0.025 | 82 (7) | 82 (7) | 0.108 | 83 (8) | 82 (7) | 0.043 | 83 (7) | 82 (7) | 0.025 |
| Female sex | 795 (56.4) | 4054 (56.7) | 0.827 | 693 (55.8) | 3543 (56.1) | 0.883 | 501 (56.4) | 2859 (54.7) | 0.367 | 300 (57.4) | 1858 (55.3) | 0.377 | 157 (58.8) | 1063 (54.4) | 0.173 |
| Antiplatelets | 674 (47.8) | 2641 (36.9) | <0.001 | 952 (77.5) | 4604 (73.1) | 0.001 | 621 (69.9) | 3039 (58.2) | <0.001 | 351 (67.1) | 1995 (59.4) | 0.001 | 173 (64.8) | 1106 (56.6) | 0.011 |
| in AF | 272 (56.9) | 874 (44.4) | <0.001 | 244 (56.5) | 788 (37.5) | <0.001 | 133 (49.3) | 376 (23.3) | <0.001 | 65 (44.8) | 234 (24.3) | <0.001 | 35 (52.2) | 119 (23.4) | <0.001 |
| in non-AF | 402 (43.1) | 1767 (34.1) | <0.001 | 706 (88.9) | 3794 (90.9) | 0.073 | 482 (78.8) | 2646 (73.9) | 0.011 | 283 (75.5) | 1750 (73.7) | 0.457 | 138 (69.0) | 978 (68.3) | 0.841 |
| Anticoagulants in AF | 54 (11.3) | 359 (18.2) | <0.001 | 132 (30.7) | 1188 (56.5) | <0.001 | 74 (27.4) | 762 (47.2) | <0.001 | 28 (19.3) | 429 (44.6) | <0.001 | 14 (20.9) | 228 (44.8) | <0.001 |
| BPLM | 930 (66.0) | 4792 (67.0) | 0.438 | 897 (73.1) | 5148 (81.8) | <0.001 | 612 (68.8) | 4035 (77.2) | <0.001 | 354 (67.7) | 2616 (77.9) | <0.001 | 173 (64.8) | 1490 (76.2) | <0.001 |
| Statins | 259 (18.4) | 1436 (20.1) | 0.140 | 540 (44.0) | 3980 (63.3) | <0.001 | 335 (37.7) | 2711 (51.9) | <0.001 | 168 (32.1) | 1679 (50.0) | <0.001 | 66 (24.7) | 953 (48.7) | <0.001 |
Data are represented as number of cases (n) and percentage proportion (%), if not stated otherwise. In variables where n (%) are reported, p-values were obtained by chi-square, whereas in variables where mean (SD) are reported, p-values were obtained by Student t-test. Patients who did not survive until the follow-up are excluded. Therapy was registered if a patient collected at least one prescription from the pharmacy in the 6-month period before the stroke, or 1st, 2nd, and 3rd year after stroke event. D, dementia; C, control; BPLM, blood pressure lowering medication; AF, atrial fibrillation. Missing values - medication at discharge: antiplatelets 33 (0.4), anticoagulants 7 (0.3), BPLM 36 (0.5), statins 42 (0.6).
In the dementia group, 332 (23.5%) had AD, 332 (23.5%) mixed, 325 (23.0%) vascular, 320 (22.7%) unspecified, and 101 (7.3%) other dementias (Parkinson’s disease dementia, dementia with Lewy bodies, frontotemporal dementia, other dementias). In patients with dementia, median Mini-Mental State Examination (MMSE) score was 22 (IQR 6), performed with a median 521 days (IQR 694 days) before IS.
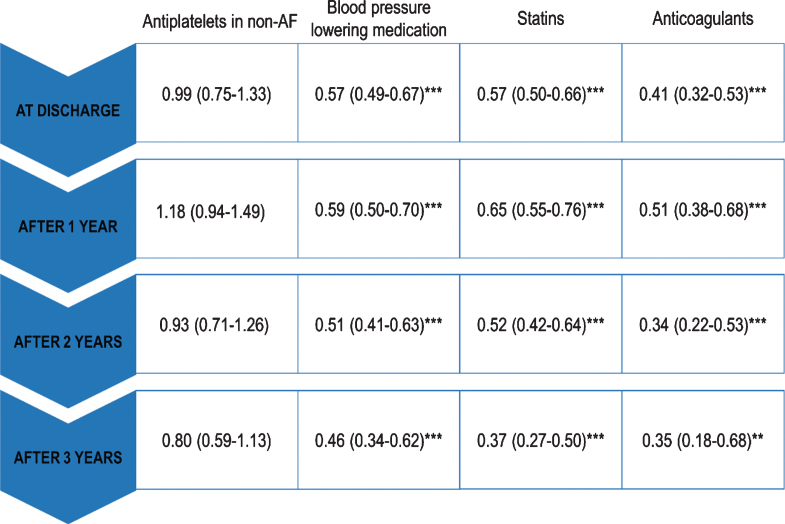
Regression models with ORs and 95% CI for receiving medication at different time points after IS are presented in Table 2. Patients with no pre-existing dementia were the reference category. Except for antiplatelets, the models remained statistically significant over 3-year post-stroke period. At discharge, planned initiation of medication was higher in patients with dementia for antiplatelets, (OR with 95% CI for fully adjusted models 1.23 [1.02–1.48]) and lower for BPLM, statins (0.57 [0.49–0.67] and 0.57 [0.50–0.66] respectively), and anticoagulants (AF patients only; 0.41 [0.32–0.53]). In the years after stroke, odds for receiving BPLM, statins, and anticoagulation remained lower in patients with dementia (at 3-years 0.46 [0.34–0.62], 0.37 [0.34–0.50], and 0.35 [0.18–0.68], respectively). For receiving antiplatelets, odds were increased the first year after stroke (1.23 [1.02–1.48]), while at 2 and 3 years after stroke, there were no significant differences between dementia and non-dementia patients in fully adjusted models (1.07 [0.86–1.34] and 1.03 [0.76–1.38], respectively). However, when patients where stratified according to the presence of AF, patients with dementia and AF were more likely to take antiplatelets (at 3 years 2.15 [1.15–4.00]), but these differences were not significant in the absence of AF (at 3 years 0.80 [0.59–1.13], Table 2). Fully adjusted regression models (Model 3) at different time-points after stroke are presented in Fig. 1.
Table 2.
Regression models for receiving medication at different time points after first ischemic stroke. Patients with no pre-existing dementia are the reference category
| Model 1 | Model 2 | Model 3 | ||
| Antiplatelets | 1.28 (1.11–1.48)*** | 1.41 (1.21–1.64)*** | 1.23 (1.02–1.48)* | |
| in AF | 2.22 (1.80–2.75)*** | 2.02 (1.62–2.51)*** | 1.56 (1.21–2.01)*** | |
| At discharge | in non-AF | 0.80 (0.63–1.03) | 1.07 (0.83–1.39) | 0.99 (0.75–1.33) |
| BPLM | 0.61 (0.53–0.70)*** | 0.66 (0.57–0.76)*** | 0.57 (0.49–0.67)*** | |
| Statins | 0.44 (0.39–0.50)*** | 0.57 (0.49–0.65)*** | 0.57 (0.50–0.66)*** | |
| Anticoagulants in AF | 0.39 (0.30–0.49)*** | 0.48 (0.37–0.62)*** | 0.41 (0.32–0.53)*** | |
| Antiplatelets | 1.67 (1.43–1.94)*** | 1.61 (1.38–1.89)*** | 1.48 (1.24–1.78)*** | |
| in AF | 3.34 (2.55–4.38)*** | 2.95 (2.24–3.89)*** | 2.43 (1.76–3.35)*** | |
| 1-year post-stroke | in non-AF | 1.30 (1.06–1.60)* | 1.23 (1.00–1.53) | 1.18 (0.94–1.49) |
| BPLM | 0.68 (0.57–0.80)*** | 0.71 (0.60–0.84)*** | 0.59 (0.50–0.70)*** | |
| Statins | 0.56 (0.48–0.65)*** | 0.65 (0.56–0.76)*** | 0.65 (0.55–0.76)*** | |
| Anticoagulants in AF | 0.42 (0.31–0.55)*** | 0.47 (0.35–0.62)*** | 0.51 (0.38–0.68)*** | |
| Antiplatelets | 1.40 (1.15–1.70)*** | 1.36 (1.11–1.66)** | 1.07 (0.86–1.34) | |
| in AF | 2.6 (1.81–3.75)*** | 2.21 (1.52–3.22)*** | 1.47 (0.97–2.23) | |
| 2-years post-stroke | in non-AF | 1.09 (0.85–1.40) | 1.07 (0.83–1.39) | 0.93 (0.71–1.26) |
| BPLM | 0.59 (0.49–0.72)*** | 0.61 (0.50–0.75)*** | 0.51 (0.41–0.63)*** | |
| Statins | 0.48 (0.39–0.59)*** | 0.53 (0.43–0.66)*** | 0.52 (0.42–0.64)*** | |
| Anticoagulants in AF | 0.29 (0.19–0.45)*** | 0.33 (0.21–0.51)*** | 0.34 (0.22–0.53)*** | |
| Antiplatelets | 1.40 (1.08–1.83)* | 1.31 (0.99–1.72)* | 1.03 (0.76–1.38) | |
| in AF | 3.73 (2.19–6.36)*** | 3.28 (1.89–5.66)*** | 2.15 (1.15–4.00)* | |
| 3-years post-stroke | in non-AF | 1.02 (0.74–1.41) | 0.94 (0.68–1.31) | 0.80 (0.59–1.13) |
| BPLM | 0.57 (0.43–0.75)*** | 0.57 (0.43–0.75)*** | 0.46 (0.34–0.62)*** | |
| Statins | 0.35 (0.26–0.47)*** | 0.39 (0.29–0.54)*** | 0.37 (0.27–0.50)*** | |
| Anticoagulants in AF | 0.32 (0.17–0.59)*** | 0.35 (0.19–0.67)*** | 0.35 (0.18–0.68)** |
Results are presented as odds ratio (ORs) with 95% CI. Only survivors are included in the analyses. AF, atrial fibrillation; BPLM, blood pressure lowering medication. For 2- and 3-years post-stroke: strokes after 31-Dec-2013 and 31-Dec-2012 respectively are excluded due to the lack of follow-up data. Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, nursing home placement at discharge. For antiplatelets, model 3 is adjusted for age, sex, nursing home placement at discharge, history of femur fracture, Charlson comorbidity index, and anticoagulants, for BPLM and statins, model 3 is adjusted for age, sex, nursing home placement at discharge, history of femur fracture, Charlson comorbidity index, and history of hemorrhagic stroke, while for anticoagulants, model 3 is adjusted for age, sex, nursing home placement at discharge, history of femur fracture, Charlson comorbidity index, and prior hemorrhagic stroke or history of any bleeding. *p < 0.05, **p < 0.01, ***p≤0.001.
Fig.1.
Fully adjusted regression models (Model 3) at different time-points. ORs with 95% CI are presented. Patients with no pre-existing dementia are the reference group. *p < 0.05, **p < 0.01, ***p≤0.001.
Subanalyses for dementia subtypes (AD, mixed, vascular, and other dementias) are presented in Supplementary Tables 1 and 2. Compared to patients with vascular dementia, patients with AD were less likely to receive blood pressure lowering medication and anticoagulation in AF. For antiplatelet use, significant differences disappeared after stratifying for AF. There were no differences in statin use between dementia types throughout the years after stroke.
DISCUSSION
The main findings of this study are: patients with dementia (1) are more likely to receive antiplatelet treatment at discharge, in the first year after stroke and in the presence of AF, (2) are less likely to receive blood pressure and lipid lowering therapies, and anticoagulation medication in the presence of AF, compared to non-dementia patients.
Anticoagulation
Oral anticoagulants (OACs) reduce the risk for stroke in patients with AF from 4.5% to 1.4% per year [19]. Long-term anticoagulation is warranted in patients with history of stroke and AF, as this places the patient at a high risk for systemic cardioembolism, with CHADS2 score of 2 or higher [20].
Our results are in line with previous studies, which reported underutilization of anticoagulation in patients with dementia and AF [9–11, 21]. In one study, patients with dementia were less likely to receive any antithrombotic medication (aspirin or warfarin) [9], while in another, dementia was a significant predictor of not receiving secondary prevention medication, including anticoagulants and statins, but only in a univariate and not in a multivariate analysis [12].
Multiple reasons exist why OACs are not initiated in patients with dementia. Patients with AF are undertreated with OACs; between 2005–2007 only 45% of patients with AF and IS or transitory ischemic attack received OACs [10]. This especially holds true for older patients, which have 1) a greater prevalence of amyloid-β deposition in the vasculature predisposing them to dementia and intracranial hemorrhage, 2) are taking more medication that may interact with warfarin, and 3) have a greater tendency to fall [8]. However, to offset the stroke reduction benefits of warfarin, it has been estimated that an older patient would need to fall 295 times in 1 year [22] and according to some studies benefit of oral anticoagulation might even increase with age [23, 24]. Older age, female sex, worsening disability status, and dementia, decreased the chances of receiving OACs [10]. Non-initiation of OACs during hospitalization despite recommendations was also associated with a lower adherence [25]. On the other hand, treatment in a specialized neurological department facilitates OAC initiation in secondary prevention [10].
Dementia is not a contraindication for OACs and clinicians should not withhold effective therapy in patients with cognitive decline if other contraindications are not present. Low utilization of OACs and high antiplatelet use in patients with AF could partly be due to our study’s time frame. Each year, OACs utilization in patients with AF increased substantially; e.g., between 2011–2015 in Stockholm, Sweden, OAC utilization climbed from 50% to 70%, which was accompanied by a concomitant decrease in use of aspirin in AF from 30% to 15% [26]. This could be partly explained by the introduction of non-vitamin K oral anticoagulants (NOACs), which were infrequent at the time of our study. In Sweden, the use of OAC in the AF-population overall has increased significantly after the introduction of NOAC [27, 28]. As newer anticoagulants require less monitoring, patients with dementia might benefit more from NOACs, and this might facilitate higher anticoagulant rates than those seen in our study—however, data on present anticoagulation rates in Sweden in patients with dementia is lacking.
Antiplatelets
Antiplatelets offer an absolute risk reduction of 2% in vascular events per year [2] and have probably a larger population impact on acute stroke treatment than thrombolysis [29]. To the best of the authors’ knowledge, there are no randomized placebo-controlled studies on the benefit of secondary prevention with antiplatelets versus increased bleeding risk specifically in patients with dementia. Thus, in all patients with a noncardioembolic ischemic stroke, antiplatelet agents are recommended to reduce the risk of recurrent stroke either as aspirin monotherapy, the combination of aspirin and extended-release dipyridamole, or clopidogrel monotherapy [19].
Patients with dementia were more likely to receive antiplatelet therapy at discharge and at first year after IS. In contrast to our findings, patients with dementia were less likely to receive aspirin or warfarin in an older study [9], while Eissa and colleagues did not find dementia was a significant predictor for receiving antiplatelets [12].
Even though antiplatelet therapy does not protect from embolic stroke, the increase in prescription of antiplatelets in patients with dementia was probably due to use of antiplatelet instead of OAC in patients with dementia and AF. This became apparent in a stratified analysis for AF. In the presence of AF, patients with dementia were more likely to receive antiplatelets, while in the non-AF group, there were no differences between dementia and nondementia patients. In fully adjusted models, this association disappeared or became less evident in the 2nd and 3rd year post-stroke. It seems other comorbidities play a more important role than dementia and AF when considering antiplatelet treatment in patients ≥2 years after stroke.
Blood pressure lowering medication (BPLM)
On a population level, targeting hypertension carries the highest benefit in reducing stroke burden. Newest guidelines define hypertension as blood pressure (BP) higher than 130/80 mmHg [30], but BPLM as stroke secondary prevention should not be reserved for only those with history of hypertension and should only be withdrawn in cases where hypotension could potentially be more harmful [2]. BP reduction with 10/5 mmHg reduces chances for recurrent stroke by about a third [1]; however, ACE inhibitors and diuretics reduce stroke risk regardless of BP levels and history of hypertension [8]. Moreover, not only the BP level, but also BP variability increases cerebrovascular risk, and diuretics and calcium antagonists have been shown to reduce BP fluctuations [31].
In our study, patients with dementia had a lower probability of receiving BPLM. No previous studies have addressed this topic. The reasons behind non-initiation and discontinuation could be poor adherence by the patient, the presence of potentially detrimental hypotension and greater prevalence of orthostatic hypotension, lack of guideline adherence by physicians or regular medical follow-up, or reduced need for lowering BP since it decreases after dementia diagnosis [32, 33].
Statins
Reduction in LDL cholesterol concentration has been shown to be associated with a 21% stroke risk reduction [3], as well as with reduced risk of cognitive impairment in stroke patients [6]. Even though statins are associated with numerous side effects [34], their withdrawal after stroke significantly worsens the outcome at 3 months [8]. The target LDL concentration is <2 mmol/L [1]. In our study, patients with dementia were half as likely to receive statins in years after stroke compared to patients without dementia. Reasons for non-initiation of lipid lowering therapies in patients with dementia could be shorter life expectancy.
Adherence and plausibility
The therapeutic benefit of secondary prevention medication is dependent on adherence. By 2 years, approximately 20% of patients discontinue aspirin or warfarin according to prior studies [35]. Adherence is even poorer in statins as only 43% of patients were taking them at 6 months [35]. The introduction of medication is time and place dependent; not being started on therapy in hospital or within 4 weeks of discharge were risk factors for poor adherence to therapy [11]. Evidence for the association between cognitive impairment and non-adherence is inconsistent. In one pooled analysis, there was no evidence of an association between cognitive impairment and non-adherence [5]. It may, however, be important to distinguish between degrees of cognitive impairment, as individuals with more severe impairments and dementia may rely on caregivers to administer medication, leading to increased adherence. The association between cognitive impairment and adherence, may in fact be U-shaped [5].
After IS, patients with dementia receive less specific diagnostic tests and assessments by the interdisciplinary stroke team [36] and have worse functional status [37, 38]. It is important to weigh possible therapeutic benefits after severe IS against a particular patient’s limited life expectancy. Most studies demonstrated a reduction in secondary events only after ≥2 years of therapy, implying some patients may not benefit from secondary prevention [8]. Biological age does not always match the chronological age and when planning treatment and rehabilitation plans, functional status, co-morbidities, and the patient’s goals of care should take precedence over age [8].
Limitations
Firstly, we used data on medication dispensation and not actual medication consumption, which may overestimate medication use. Whether there was a difference in primary non-compliance between dementia and non-dementia patients is outside the scope of this study. Because of the time period of the study, NOACs were not widely used and we were not able to assess their use in dementia and AF separately. Secondly, assessment of baseline functional status was limited to living situation and data on cognitive status (MMSE) were obtained with a median 1.5 years before the stroke at the time of dementia diagnosis. However, it is impossible to determine dementia severity at the time of stroke. Thirdly, the proportion between dementia and non-dementia patients at 3rd year post-stroke does not match the baseline ratio, since a greater proportion of dementia patients died. This may lead to some bias, as the two groups were matched at the time of stroke event only. Lastly, diabetes is an important comorbidity in stroke, but because of the absence of laboratory data which may influence the use and choice of antidiabetic medication, we did not include these in our study.
Despite these limitations, this study provides important insights into utilization of secondary stroke prevention in dementia.
Summary
Dementia was a predictor of lower anticoagulant, statin, and blood pressure lowering medication use for secondary stroke prevention; these represent key target areas for quality improvement initiatives.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank reporting units, coordinators, and steering committees in SveDem and Riksstroke and especially all participants and their caregivers. This project was conducted with support from the Swedish Order of Saint John/Johanniterorden, the Swedish Stroke Association, Stiftelsen Dementia, the Swedish Brain Foundation, the Swedish Associations of Local Authorities and Regions, FORTE Swedish Research Council for Health, Working Life and Welfare (2017-01646), the Swedish Society for Medical Research, Loo och Hans Ostermans stiftelse and KI foundation for Diseases of Aging. Winblad is supported by the Swedish Research Council, the Brain Foundation, Old Servants’ Foundation, Stohne’s Foundation and Margaretha af Ugglas Foundation. Eriksdotter was supported by the Swedish Research Council grants #2012-2291 and #2016-0231. Garcia-Ptacek holds a postdoctoral fellowship from the Swedish Society for Medical Research. Norrving was supported by a grant from Färs and Frosta Sparbank.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1011r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191011.
REFERENCES
- [1]. Hankey GJ (2014) Secondary stroke prevention. Lancet Neurol 13, 178–194. [DOI] [PubMed] [Google Scholar]
- [2]. Esenwa C, Gutierrez J (2015) Secondary stroke prevention: Challenges and solutions. Vasc Health Risk Manag 11, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Rothwell PM, Algra A, Amarenco P (2011) Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet 377, 1681–1692. [DOI] [PubMed] [Google Scholar]
- [4]. Subic A, Cermakova P, Norrving B, Winblad B, von Euler M, Kramberger MG, Eriksdotter M, Garcia-Ptacek S (2017) Management of acute ischaemic stroke in patients with dementia. J Intern Med 281, 348–364. [DOI] [PubMed] [Google Scholar]
- [5]. Rohde D, Merriman NA, Doyle F, Bennett K, Williams D, Hickey A (2017) Does cognitive impairment impact adherence? A systematic review and meta-analysis of the association between cognitive impairment and medication non-adherence in stroke. PLoS One 12, e0189339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Douiri A, McKevitt C, Emmett ES, Rudd AG, Wolfe CD (2013) Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation 128, 1341–1348. [DOI] [PubMed] [Google Scholar]
- [7]. Cermakova P, Johnell K, Fastbom J, Garcia-Ptacek S, Lund LH, Winblad B, Eriksdotter M, Religa D (2015) Cardiovascular diseases in∼ 30,000 patients in the Swedish Dementia Registry. J Alzheimers Dis 48, 949–958. [DOI] [PubMed] [Google Scholar]
- [8]. Bushnell CD, Colon-Emeric CS (2009) Secondary stroke prevention strategies for the oldest patients: Possibilities and challenges. Drugs Aging 26, 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Moroney JT, Tseng CL, Paik MC, Mohr J, Desmond DW (1999) Treatment for the secondary prevention of stroke in older patients: The influence of dementia status. J Am Geriatr Soc 47, 824–829. [DOI] [PubMed] [Google Scholar]
- [10]. Tanislav C, Milde S, Schwartzkopff S, Sieweke N, Kramer HH, Juenemann M, Misselwitz B, Kaps M (2014) Secondary stroke prevention in atrial fibrillation: A challenge in the clinical practice. BMC Neurol 14, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Shah R, Li S, Stamplecoski M, Kapral MK (2016) Low use of oral anticoagulant prescribing for secondary stroke prevention: Results from the Ontario Stroke Registry. Med Care 54, 907–912. [DOI] [PubMed] [Google Scholar]
- [12]. Eissa A, Krass I, Bajorek BV (2014) Use of medications for secondary prevention in stroke patients at hospital discharge in Australia. Int J Clin Pharm 36, 384–393. [DOI] [PubMed] [Google Scholar]
- [13]. Garcia-Ptacek S, Farahmand B, Kareholt I, Religa D, Cuadrado ML, Eriksdotter M (2014) Mortality risk after dementia diagnosis by dementia type and underlying factors: A cohort of 15,209 patients based on the Swedish Dementia Registry. J Alzheimers Dis 41, 467–477. [DOI] [PubMed] [Google Scholar]
- [14]. Asplund K, Sukhova M, Wester P, Stegmayr B (2015) Diagnostic procedures, treatments, and outcomes in stroke patients admitted to different types of hospitals. Stroke 46, 806–812. [DOI] [PubMed] [Google Scholar]
- [15]. Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundström A, Westerholm B, Rosén M (2007) The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16, 726–735. [DOI] [PubMed] [Google Scholar]
- [16]. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, Heurgren M, Olausson PO (2011) External review and validation of the Swedish national inpatient register. BMC Public Health 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40, 373–383. [DOI] [PubMed] [Google Scholar]
- [18]. Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R (2017) The Swedish cause of death register. Eur J Epidemiol 32, 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 2160–2236. [DOI] [PubMed] [Google Scholar]
- [20]. Barnes GD, Kaatz S, Winfield J, Gu X, Haymart B, Kline-Rogers E, Kozlowski J, Beasley D, Almany S, Leyden T (2014) Warfarin use in atrial fibrillation patients at low risk for stroke: Analysis of the Michigan Anticoagulation Quality Improvement Initiative (MAQI 2). J Thromb Thrombolysis 37, 171–176. [DOI] [PubMed] [Google Scholar]
- [21]. Subic A, Cermakova P, Religa D, Han S, von Euler M, Kareholt I, Johnell K, Fastbom J, Bognandi L, Winblad B, Kramberger MG, Eriksdotter M, Garcia-Ptacek S (2018) Treatment of atrial fibrillation in patients with dementia: A cohort study from the Swedish Dementia Registry. J Alzheimers Dis 61, 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Man-Son-Hing M, Nichol G, Lau A, Laupacis A (1999) Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 159, 677–685. [DOI] [PubMed] [Google Scholar]
- [23]. Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS (2009) The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 151, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Tsivgoulis G, Spengos K, Zakopoulos N, Manios E, Peppes V, Vemmos K (2005) Efficacy of anticoagulation for secondary stroke prevention in older people with non-valvular atrial fibrillation: A prospective case series study. Age Ageing 34, 35–40. [DOI] [PubMed] [Google Scholar]
- [25]. Gumbinger C, Holstein T, Stock C, Rizos T, Horstmann S, Veltkamp R (2015) Reasons underlying non-adherence to and discontinuation of anticoagulation in secondary stroke prevention among patients with atrial fibrillation. Eur Neurol 73, 184–191. [DOI] [PubMed] [Google Scholar]
- [26]. Loikas D, Forslund T, Wettermark B, Schenck-Gustafsson K, Hjemdahl P, von Euler M (2017) Sex and gender differences in thromboprophylactic treatment of patients with atrial fibrillation after the introduction of non-vitamin K oral anticoagulants. Am J Cardiol 120, 1302–1308. [DOI] [PubMed] [Google Scholar]
- [27]. Komen J, Forslund T, Hjemdahl P, Andersen M, Wettermark B (2017) Effects of policy interventions on the introduction of novel oral anticoagulants in Stockholm: An interrupted time series analysis. Br J Clin Pharmacol 83, 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Forslund T, Komen Joris J, Andersen M, Wettermark B, von Euler M, Mantel-Teeuwisse Aukje K, Braunschweig F, Hjemdahl P (2018) Improved stroke prevention in atrial fibrillation after the introduction of non–vitamin K antagonist oral anticoagulants. Stroke 49, 2122–2128. [DOI] [PubMed] [Google Scholar]
- [29]. Mair G, Wardlaw J (2014) Imaging of acute stroke prior to treatment: Current practice and evolving techniques. Br J Radiol 87, 20140216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483. [DOI] [PubMed] [Google Scholar]
- [31]. Niewada M, Czlonkowska A (2014) Prevention of ischemic stroke in clinical practice: A role of internists and general practitioners. Pol Arch Med Wewn 124, 540–548. [DOI] [PubMed] [Google Scholar]
- [32]. Pedersen RA, Petursson H, Hetlevik I (2018) Stroke follow-up in primary care: A prospective cohort study on guideline adherence. BMC Fam Pract 19, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Harrison JK, Van Der Wardt V, Conroy SP, Stott DJ, Dening T, Gordon AL, Logan P, Welsh TJ, Taggar J, Harwood R, Gladman JR (2016) New horizons: The management of hypertension in people with dementia. Age Ageing 45, 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Mancini GJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ (2016) Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group update (2016). Can J Cardiol 32, S35–S65. [DOI] [PubMed] [Google Scholar]
- [35]. Murphy SJ, Coughlan CA, Tobin O, Kinsella J, Lonergan R, Gutkin M, McCabe DJ (2016) Continuation and adherence rates on initially-prescribed intensive secondary prevention therapy after Rapid Access Stroke Prevention (RASP) service assessment. J Neurol Sci 361, 13–18. [DOI] [PubMed] [Google Scholar]
- [36]. Zupanic E, Kareholt I, Norrving B, Secnik J, von Euler M, Winblad B, Religa D, Kramberger MG, Johnell K, Eriksdotter M, Garcia-Ptacek S (2018) Acute stroke care in dementia: A cohort study from the Swedish Dementia and Stroke Registries. J Alzheimers Dis 66, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Zupanic E, von Euler M, Kåreholt I, Escamez BC, Fastbom J, Norrving B, Religa D, Kramberger MG, Winblad B, Johnell K (2017) Thrombolysis in acute ischemic stroke in patients with dementia A Swedish registry study. Neurology 89, 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Wakisaka Y, Matsuo R, Hata J, Kuroda J, Kitazono T, Kamouchi M, Ago T (2017) Adverse influence of pre-stroke dementia on short-term functional outcomes in patients with acute ischemic stroke: The Fukuoka Stroke Registry. Cerebrovasc Dis 43, 82–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.