Abstract
BACKGROUND
Regorafenib is an oral small-molecule multikinase inhibitor approved in third or later line of treatment for patients with metastatic colorectal cancer (mCRC). Regorafenib has shown significant benefits in overall survival and progression free survival in two phase III trials compared to placebo in patients with mCRC who had progressed on previous therapy.
AIM
To identify an immune profile that might specifically correlate with the outcome in patients treated with regorafenib.
METHODS
Blood samples were collected from 17 patients before treatment with regorafenib and from 6 healthy volunteers. The proteins evaluated (TNF-α, TGF-β, VEGF, CCL-2, CCL-4, and CCL-5) were selected on the basis of their roles in angiogenesis and colorectal cancer pathogenesis.
RESULTS
We found that TNF-α basal level was significantly higher in mCRC patients compared to healthy individuals. Non Responder (NR) patients showing progression of disease (n = 12) had higher basal level of TGF-β, TNF-α, VEGF, CCL-2 and CCL-5 compared to Responder (R) patients (complete response CR, n = 1; partial response PR, n = 1; Stable Disease SD, n = 3). On the contrary, plasma basal level of CCL-4 was higher in R compared to NR patients. High values of TGF-β and TNF-α negatively correlated with progression free survival.
CONCLUSION
These results suggest a cytokine signature potentially able to discriminate between R and NR patients to treatment with regorafenib.
Keywords: Colorectal cancer, Multikinase inhibitor, Cytokines, Angiogenesis
Core tip: We analyzed levels of specific cytokines in plasma of metastatic colorectal cancer patients before treatment with regorafenib. Our aim was to identify biomarkers useful to select metastatic colorectal cancer responder patients and an immune profile potentially correlated with the outcome.
INTRODUCTION
Colorectal cancer (CRC) is one of the leading causes of cancer-related death and the third most commonly diagnosed cancer in humans in the world[1].
For many years treatment of metastatic CRC (mCRC) consisted mainly of single agent 5-fluorouracil but the addition of irinotecan and oxaliplatin to 5-fluorouracil increased the median progression free survival (PFS) to 8 mo[2], which further improved with the later addition of the vascular endothelial growth factor (VEGF) and EGFR inhibitors[3].
Regorafenib is an oral multikinase inhibitor that inhibits the activity of vascular endothelial growth factor receptor 1, 2, 3 (VEGFR-1,-2,-3), tyrosine kinase receptor with immunoglobulin-like and EGF-like domains 2 (TIE2), platelet-derived growth factor receptors, fibroblast growth factor receptors and oncogenic receptor tyrosine kinases (KIT, RET, RAF-1, BRAF, BRAFV600E)[4]. Regorafenib monotherapy significantly increased overall survival (OS) and PFS in the CORRECT and the CONCUR trials, which compared regorafenib treatment to placebo[5,6].
Cytokine levels could be potentially useful in monitoring disease progression and treatment outcome. Suenaga et al[7] demonstrated that baseline serum CCL-5 and VEGF-A levels may act as potential predictive markers for survival or treatment-specific toxicities in mCRC patients receiving regorafenib in salvage-line setting.
TGF-β is a multifunctional polypeptide promoting angiogenesis and expression of cell adhesion proteins, and inhibiting growth of epithelial and immune cells. High plasma levels of TGF-β were associated with progression of disease[8].
TNF-α might serve as a predictive marker for treatment efficacy and clinical outcome. Olsen et al[9] demonstrated that higher levels of TNF-α, as well as of other cytokines, are associated with a worse prognosis and mortality in CRC patients.
The expression of CCL-2, also known as monocyte chemotactic protein 1, in colorectal cancer cells is strictly related to advanced tumor stage, accumulation of tumor-associated macrophages, and negative prognosis[10,11].
Several reports demonstrated that the releasing of CCL-5 (also known as RANTES) promotes cancer cell invasiveness and decreases antitumor immunity by recruitment of C-C chemokine receptor type 5 (CCR-5)+ T-regulatory cells[12]. The interaction between CCL-5 and its receptor also participates in VEGF up-regulation in the osteosarcoma microenvironment[13].
CCL-4 is a chemokine released by a variety of immune and epithelial cells that interacts with CCR-5 to attract macrophages, T cells and, most important, immature dendritic cells (DCs) whose presence is essential for the activation of immune response. Unlike the two above mentioned chemokines, high serum levels of CCL-4 in CRC patients are associated with improved disease free survival and this might be related to increased recruitment of Th1 cells, which frequently express CCR-5[14].
In this “proof of concept” study we analyzed the level of all these soluble cytokines in the plasma of mCRC patients before treatment with regorafenib, with the aim to identify biomarkers potentially useful to select mCRC patients who could benefit from regorafenib therapy.
MATERIALS AND METHODS
Study design
This exploratory study was performed in a single centre. The aim of the study was to evaluate the role of specific biomarkers potentially involved in the clinical activity of regorafenib: TNF-α, TGF-β, VEGF, CCL-2, CCL-4 and CCL-5. The selected cytokines were measured at baseline as described below and the clinical outcome of each patient was correlated to the cytokines profile. Six healthy volunteers (2 men and 4 women) were also analyzed. Patients were treated following standard clinical practice and followed accordingly.
All enrolled patients signed an informed consent for the storage and analysis of their biological material approved by the local ethical committee (prot n° 24347; August 7, 2015).
Blood samples
We enrolled in the present study 17 mCRC patients treated at the Oncology Department, S. Croce and Carle Teaching Hospital in Cuneo from April 2016 to June 2018. All patients received 160 mg regorafenib once a day for 3 wk, followed by 1 wk treatment free.
Blood samples were collected into EDTA vacutainer tubes at baseline immediately before the first administration of regorafenib. Plasma samples were obtained through centrifugation step and stored in aliquots at −80 °C in the Biobank of the Oncology Department until use.
Patient characteristics
The mCRC cohort consisted of 53% males and 47% females. Median age was 63 (52 to 77 years). In 53% of the cases, the tumour was located in the colon (3 right colon and 6 left colon) and in 47% in the rectum.
The mCRC group included 82% of patients at third-line treatment, 12% of patients at fourth-line treatment, and 6% of patients at fifth-line treatment line. RAS mutations were detected in 76% of the cases. Patients’ main characteristics are reported in Table 1.
Table 1.
Patient characteristics (n = 17)
| Patient characteristics | n (%) |
| Sex (male/female) | 9/8 (53/47) |
| Median age (range) | 63 (52-77) |
| Primary tumor site (colon/rectum) | 9/8 (53/47) |
| Number of previous anticancer therapies (III/IV/V) | 14/2/1 (82/12/6) |
| Mutational RAS status (mutated/wild type) | 13/4 (76/24) |
Analysis method
Plasma levels of six cytokines were evaluated with ELISA kits from R and D Systems (TNF-α, TGF-β, VEGF, CCL-4 and CCL-5) and Invitrogen (CCL-2). For TGF-β analysis, the samples were incubated with 1 N HCL for 10 min followed by with 1.2 N NaOH/0.5 mol/L HEPES prior to perform the assay, in order to activate the latent TGF-β to the immunoreactive form. The ELISA assay employs the quantitative sandwich enzyme immunoassay technique. Analysis was performed according to the manufacturer's protocol. In brief, 100 μL of sample was used and incubated for two hours in a 96 well plate, coated with antibody against each cytokine. After washing, 200 μL of horseradish peroxidise-conjugated antibody was added to each well and incubated for one/two hours. After further washing, a substrate solution was added. Optical density was determined by reading the absorbance with a plate reader (Multiscan Ascent, Thermo fisher®) at 450 nm. Patient samples, standards and controls were assayed in triplicate, and the average values were recorded. The protein concentrations were expressed in pg/mL. CCL-2 was evaluated with uncoated ELISA using Corning Costar 9018 plates. Plates were incubated overnight at 4°C with the capture antibody anti-human CCL-2 overnight 4 °C. The assay was then carried out as described above.
Statistical analysis
Differences in the medians were tested by the Mann–Whitney U test. In order to find the optimal cut-off point at baseline, which might help in predicting survival, the receiver operating characteristic curve (ROC) analysis was performed. The cut-off was defined as the point on the ROC curve with the largest average sensitivity and specificity. Subgroups divided using the cut-off value were compared for PFS and OS. PFS was defined as the interval between the date of starting regorafenib treatment and the date of confirming disease progression, last follow-up or death. OS was calculated from the date of starting regorafenib treatment and the date of death or last follow-up.
PFS and OS were estimated by the Kaplan-Meier method and they were compared using the log-rank test, with predictive or prognostic factors being identified by univariate analysis. It was not possible to perform the Cox analysis due to the small number of patients.
Correlation analysis was used to describe the relationship between PFS and basal TGF-β and TNF-α levels and was performed using the Spearman test.
The statistical analyses were carried out using SPSS software version 24.0 (IBM Corporation, Armonk, NY, United States) and GraphPad Software 5.0 (San Diego, CA, United States). P < 0.05 was considered to indicate statistical significance.
RESULTS
Correlation between basal cytokines levels and response
Patients population was divided into two groups according to the best response to regorafenib treatment by instrumental evaluation every 3 mo. The 12 Non Responder (NR) patients showed disease progression. The 5 Responder (R) patients showed either complete response (CR, n = 1), partial response (PR, n = 1) or stable disease (SD, n = 3).
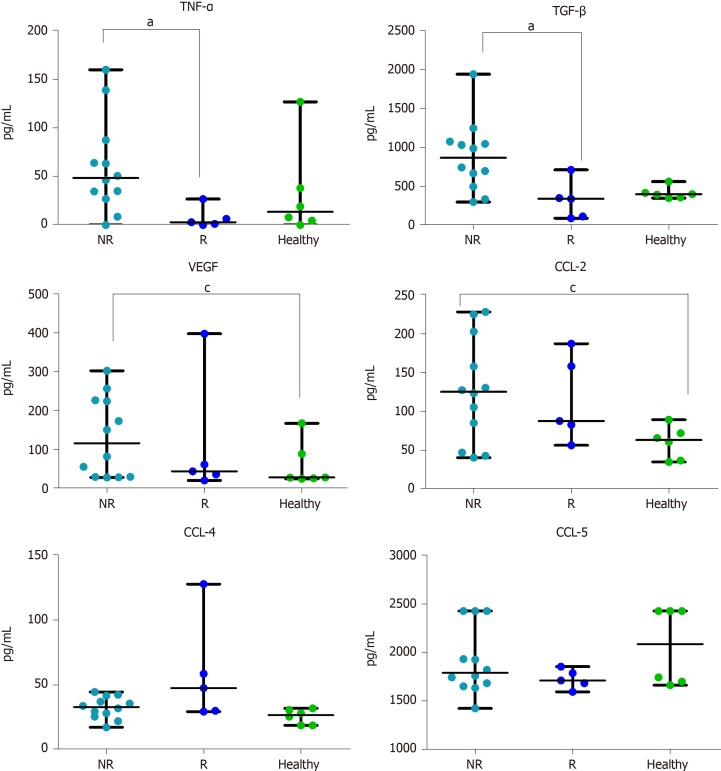
We measured cytokine levels in all the mCRC patients. We found that the plasma basal level of TNF-α was significantly higher in NR compared to R patients (P = 0.011).
Also TGF-β was significantly higher in NR compared to R patients (P = 0.031).
NR patients had a higher median level of VEGF with respect to R, but only the difference between NR and healthy controls was significant (P = 0.044).
The CCL-2 plasma level showed a trend similar to VEGF: Higher in NR vs R patients, but significantly higher only between NR and healthy controls (P = 0.035).
CCL-4 basal level, on the contrary, showed an opposite trend, in particular NR had lower median value than R.
The levels of CCL-5 did not show any difference among all the three groups (Figure 1).
Figure 1.
Plasma cytokines levels. Basal evaluation of 6 cytokines in non-responder patients (n = 12), Responder patients (n = 5) and in healthy volunteers (n = 6). Cytokine concentration is expressed in pg/mL. Data are shown as median with range. The difference in median values was computed using the non-parametric Mann Whitney U test. P < 0.05 was considered the statistical significance. aP < 0.05 NR vs R; cP < 0.05 NR vs Healthy. NR: Non-responder; R: Responder; TNF-α: Tumor necrosis alpha; TGF-β: Transforming growth factor alpha; VEGF: Vascular endothelial growth factor; CCL-2: Chemokine ligand 2; CCL-4: Chemokine ligand 4; CCL-5: Chemokine ligand 5.
Correlations between TNF-α, TGF-β levels and PFS
We further investigated the possible association between basal cytokine levels and PFS.
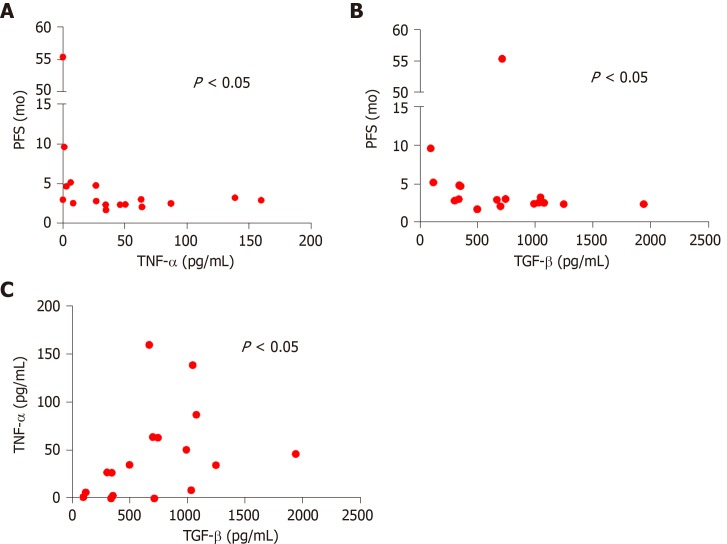
TNF-α (rs = -0.51, P = 0.033) and TGF-β (rs = -0.52, P = 0.038) negatively correlated with PFS in the patient cohort (Figure 2A and B).
Figure 2.
Correlation analysis. A: Correlation between tumor necrosis alpha and progression free survival in all metastatic colorectal cancer (mCRC) patients (n = 17) (rs = -0.52, P = 0.033); B: Correlation between transforming growth factor alpha and progression free survival in all mCRC patients (n = 17) (rs = -0.52, P = 0.038); C: Correlation between tumor necrosis alpha and transforming growth factor alpha in all mCRC patients (n = 17) (rs = 0.53, P = 0.028). P < 0.05 was considered the statistical significance. Each dot represents the value of one patients. Transforming growth factor alpha and tumor necrosis alpha are expressed as a concentration (pg/mL). Progression free survival is expressed in months. PFS: Progression free survival; TNF-α: Tumor necrosis alpha; TGF-β: Transforming growth factor alpha.
Furthermore there was a positive correlation between TNF-α and TGF-β basal values of all the mCRC patients (rs = 0.53, P = 0.028) (Figure 2C).
Instead there was no significant difference among the other cytokines at baseline and between them each of them and PFS.
ROC and Cox analysis, PFS and OS Kaplan-Meier curves
Median PFS was 2.97 mo [95% confidence interval (CI): 2.384–3.549 mo], median OS was 9.03 mo (95%CI: 6.704–11.356 mo). Median follow-up was 6.60 mo (95%CI: 5.285–7.915 mo).
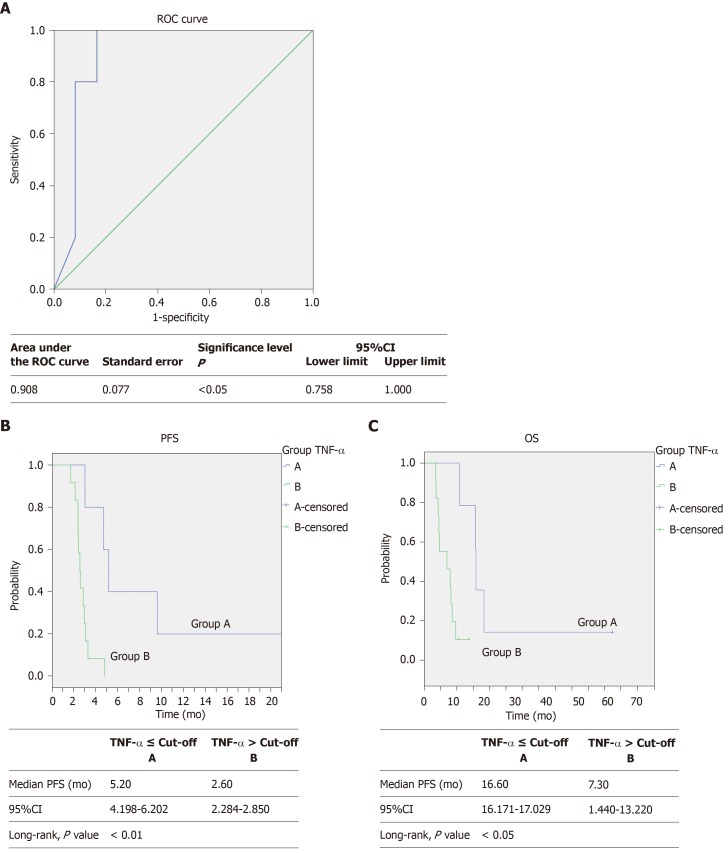
Using the ROC analysis (Figure 3A) we identified a cut-off value of 7.41 pg/mL for TNF-α basal level (AUC: 0.908, 95%CI: 0.758-1.000, P = 0.010). Then, using this cut-off value we clustered all patients into two groups, observing a higher PFS (5.20 mo, 95%CI: 4.198-6.202 vs 2.60 mo, 95%CI: 2.284-2.850, P = 0.005) in patients with baseline TNF-α below the cut-off point (Figure 3B).
Figure 3.
Receiver operating characteristic curve analysis, progression free survival and overall survival Kaplan-Meier curves. A: Receiver operating characteristic curve with area under the curve (0.908, 95CI: 0.758–1.000, P = 0.010) for predicting survival by plasma TNF-α basal levels in patients with metastatic colorectal cancer treated with regorafenib according to the baseline TNF-α levels ≤ (―, n = 5) or > (―, n = 12) the cut-off value (determined by receiver operating characteristic curve analysis); B: Progression free survival (5.2 vs 2.6 mo, Log-rank test, P = 0.005); one patient in Group A is not shown because of a graphic choice; C: Overall survival (16.6 vs 7.3 mo, Log-rank test, P = 0.010). PFS: Progression free survival; OS: Overall survival; ROC: Receiver operating characteristic; TNF-α: Tumor necrosis alpha; TGF-β: Transforming growth factor alpha.
We also observed in the same patients a better OS (16.60 mo 95%CI: 16.171-17.029 vs 7.30 mo, 95%CI: 1.440-13.220, P = 0.010) (Figure 3C).
There was no significant difference in PFS and OS according to the ROC analysis for the other cytokines (data not shown).
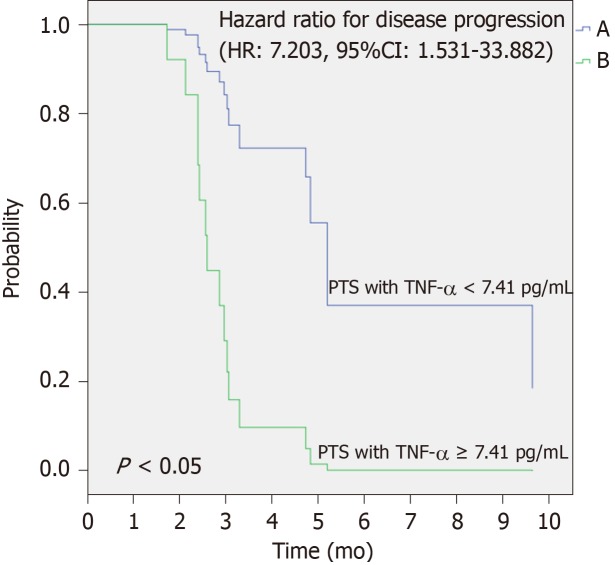
Moreover, using the univariate Cox analysis, we observed that patients with plasma basal levels of TNF-α ≥ 7.41 pg/mL had a significant increased risk to get progression disease compared to those with plasma basal levels of TNF-α < 7.41 pg/mL (HR: 7.203, 95%CI: 1.531–33.882, P = 0.012) (Figure 4).
Figure 4.
Univariate Cox analysis to predict risk of disease progression. Difference between the two survival curves was assessed by long rank test, the HR with 95%CI was calculated by the Cox regression model (HR = 7.203; 95%CI: 1.531–33.882; P = 0.012). PFS: Progression free survival; TNF-α: Tumor necrosis alpha.
DISCUSSION
Our data show that patients that do not benefit from regorafenib might be identified by basal values of TNF-α and TGF-β before treatment.
TNF-α promotes cancer invasion and angiogenesis associated with epithelial-mesenchymal transition through the involvement of canonical NF-κB signalling[15]. Moreover TNF-α is also expressed at higher levels in various pre-neoplastic and tumor tissues. Furthermore, the increased TNF-α expression level in pre-cancerous and tumor cells is associated with the progression of malignant diseases such as chronic lymphocytic leukemia, Barrett's adenocarcinoma, prostate cancer, breast cancer, and cervical carcinoma[16-18].
High plasma levels of TNF-α are associated to an increased risk of recurrence and mortality in CRC[9]. These findings indicate that TNF-α could be used as an indicator of cancer risk, prognosis and therapy response for cancer patients. Our results support its negative predictive role in regorafenib treatment.
High plasma levels of TGF-β are associated to mRNA over-expression in colon cancer tissues and related to disease progression[8]. Numerous studies also demonstrated that TGF-β production by tumour cells might promote tumor growth and immune escape and enhance angiogenesis[19-21]. Moreover, tumor development removes a cell growth inhibitory signal and increases the amount of TGF-β in the tumor microenvironment[22]. We found that the lower plasmatic levels of TGF-β are associated to longer PFS.
VEGF plays a key role in angiogenic process. Previous studies reported that colon cancer patients have high levels of VEGF compared to a healthy population[23] and that VEGF expression is associated with poor prognosis[24]. Also our results show a higher basal value of VEGF in NR compared to a healthy population.
CCL-2, CCL-4 and CCL-5 are small peptides structurally and functionally similar to growth factors which are able to induce leukocyte migration along a chemical gradient. The complex network of these chemokines and their receptors promotes carcinogenesis and metastasis[25].
The expression of CCL-2 correlates with lymph node metastasis and predicts the risk of liver metastasis[10]. Indeed, we found that CCL-2 is significantly higher in NR than in healthy controls.
CCL-4 is involved in the recruitment of CD103+-DCs. The failure of Batf3-dependent recruitment CD103+-DCs together with the activation of Wnt/β-catenin pathway is a cause of non-T cell-inflamed tumor development[26]. We found a difference between NR and R, even if no statistical significance was reached.
CCL-5 promotes angiogenesis of endothelial cells, chemotaxis and tumor angiogenesis by VEGF production in human cancer cells[12]. Our results do not show any difference in the distribution of this cytokine. Comparing our results with those of the Suenaga et al[7] study, the discrepancy in the levels of CCL-5 in the patients could be attributed to the cytokine analysis, which in our case was performed on serum samples instead of plasma samples which may contain platelet contamination.
We found that R mCRC patients are characterized by low basal values of TGF-β, TNF-α, VEGF, CCL-2 and high levels of CCL-4 compared to NR patients, even if the high CCL-4 median value in the R group is due to the very high value of one patient.
In general, the cytokine profile of R is similar to that of healthy volunteers.
This observation suggests that R have an active immune system and this aspect could be the real difference between R and NR. Of course, our study is hampered by important limitations.
First of all, the number of patients precludes any Cox analysis.
Another limitation is the lack of longitudinal analysis which precludes the possibility to distinguish between the prognostic or predictive role of this signature. However, since the purpose of our research work is to find predictive markers of treatment response, it is reasonable to take in consideration only basal cytokine values.
We are now leading a new study with the same experimental conditions but a different drug with the aim to verify the prognostic role, rather than predictive, of the proposed signature.
It would also be of interest to explore different tumor types to verify the hypothesis that the true value of our signature is to identify patients with a better prognosis due to a functional immune system.
On this basis, following a prospective study with a greater number of patients, we could identify a potential score, which might select a baseline cytokines profile able to identify NR patients who will not benefit from treatment with regorafenib. Also it might be a useful tool to drive decision-making process in daily clinical practice.
The main limitation of the study is the small population of mCRC patients analyzed. A validation in a larger patient population is strongly recommended.
In addition, the same signature should be evaluated also in patients receiving other treatments, and then the possibility that this could represent a prognostic tool should be considered.
In conclusion, taken together all these observations suggest that patients having high basal levels of TNF-α and TGF-β show a poor prognosis and, probably, will be less respondent to the regorafenib therapy. If our data is confirmed, it will be possible to identify NR mCRC patients in order to avoid ineffective treatments. We are aware that our population is small and data should be verified on larger and independent series of patients. It might also be of interest to extend analysis to other cytokine and cell populations not considered in our study.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer is one of the leading causes of cancer-related death and the third most commonly diagnosed cancer in humans in the world. For many years 5-fluorouracil was the only active drug for treatment of metastatic colorectal cancer (mCRC). The addition of irinotecan and oxaliplatin to 5-fluorouracil increased the median progression free survival (PFS), which further improved with the later addition of target therapies. Regorafenib is a multi-kinase inhibitor targeting VEGFR1-3, TIE2, fibroblast growth factor receptors 1 and platelet-derived growth factor receptors β, c-KIT, RET, c-RAF/RAF-1, BRAF V600E mutant. It can be used after failure of conventional treatment options.
Research motivation
In previous studies, regorafenib monotherapy showed the ability to improve PFS and overall survival in a subset of mCRC patients. However, no appropriate biomarkers are currently available. We analyzed the levels of many cytokines involved in angiogenesis and CRC pathogenesis, in plasma of mCRC patients before treatment with regorafenib. Our purpose was to identify potential biomarkers to select patients most likely to respond to regorafenib.
Research objectives
The aim of our study is to identify biomarkers useful to select mCRC patients for treatment with regorafenib and, possibly, an immune profile potentially correlated with the clinical outcome.
Research methods
We collected blood samples of mCRC patients before starting regorafenib therapy for the evaluation of circulating TNF-α, TGF-β, VEGF, CCL-2, CCL-4, and CCL-5. The cytokines were measured at baseline using ELISA tests and the clinical outcome of each patient was correlated to the cytokines profile. We also analyzed the same cytokines levels in six healthy volunteers.
Research results
We found higher basal levels of TNF-α, TGF-β, VEGF, CCL-2 and CCL-5 in non-responders (NR; patients showing progression of disease, n = 12) compared to those who respond to therapy (complete response CR, n = 1, partial response PR, n = 1, Stable Disease SD, n = 3), and a reversed trend for CCL-4. Moreover, we found that CCL-2 and VEGF basal levels were significantly higher in NR patients compared to healthy individuals. Furthermore, high values of TGF-β and TNF-α negatively correlated with PFS. We further investigated the possible association between basal cytokine levels and PFS and we found that TNF-α and TGF-β negatively correlated with PFS in the patient cohort. Both these basal cytokines positively correlated between them.
Research conclusions
We realized a cytokine signature which could potentially discriminate between responder and non-responder patients to Regorafenib therapy. If our data is confirmed, it will be possible to drive treatment with regorafenib to patients most likely respond to the drug.
Research perspectives
Our data should be verified on larger and independent series of patients. It might also be of interest to extend analysis to other cytokines and cells population not determined in our study.
ACKNOWLEDGEMENTS
We primarily thank the participating patients, their family members and all physicians and researchers involved in the study. We also thank the Prof G. Frumento and Dr L. Preston for their work review on the paper.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: November 14, 2019
First decision: November 18, 2019
Article in press: February 17, 2020
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF, Lin JM S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
Contributor Information
Vincenzo Ricci, Medical Oncology and Laboratory of Translational Oncology, Oncology Department, S. Croce and Carle Teaching Hospital Cuneo, Cuneo 12100, Italy. vincenzoricci22@libero.it.
Cristina Granetto, Medical Oncology and Laboratory of Translational Oncology, Oncology Department, S. Croce and Carle Teaching Hospital Cuneo, Cuneo 12100, Italy.
Antonella Falletta, Arco Cuneo Foundation, Cuneo 12100, Italy.
Matteo Paccagnella, Arco Cuneo Foundation, Cuneo 12100, Italy.
Andrea Abbona, Arco Cuneo Foundation, Cuneo 12100, Italy.
Elena Fea, Medical Oncology and Laboratory of Translational Oncology, Oncology Department, S. Croce and Carle Teaching Hospital Cuneo, Cuneo 12100, Italy.
Teresa Fabozzi, Medical Oncology, S. G. Bosco Hospital, Torino 10154, Italy.
Cristiana Lo Nigro, Laboratory, S. Croce and Carle Teaching Hospital Cuneo, Cuneo 12100, Italy.
Marco Carlo Merlano, Medical Oncology and Laboratory of Translational Oncology, Oncology Department, S. Croce and Carle Teaching Hospital Cuneo, Cuneo 12100, Italy; Arco Cuneo Foundation, Cuneo 12100, Italy.
Data sharing statement
No additional data are available.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016;27:1539–1546. doi: 10.1093/annonc/mdw206. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 7.Suenaga M, Mashima T, Kawata N, Wakatsuki T, Horiike Y, Matsusaka S, Dan S, Shinozaki E, Seimiya H, Mizunuma N, Yamaguchi K, Yamaguchi T. Serum VEGF-A and CCL5 levels as candidate biomarkers for efficacy and toxicity of regorafenib in patients with metastatic colorectal cancer. Oncotarget. 2016;7:34811–34823. doi: 10.18632/oncotarget.9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, Imai Y, Shimomukai H, Nomura Y, Matsuda Y, Matsuzawa Y. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–382. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 9.Olsen RS, Nijm J, Andersson RE, Dimberg J, Wågsäter D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23:6212–6219. doi: 10.3748/wjg.v23.i34.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P, Peck D, Darzi A. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24:121–130. doi: 10.1007/s10585-007-9060-3. [DOI] [PubMed] [Google Scholar]
- 11.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485–5493. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 12.Itatani Y, Kawada K, Inamoto S, Yamamoto T, Ogawa R, Taketo MM, Sakai Y. The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SW, Liu SC, Sun HL, Huang TY, Chan CH, Yang CY, Yeh HI, Huang YL, Chou WY, Lin YM, Tang CH. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis. 2015;36:104–114. doi: 10.1093/carcin/bgu218. [DOI] [PubMed] [Google Scholar]
- 14.Pfirschke C, Siwicki M, Liao HW, Pittet MJ. Tumor Microenvironment: No Effector T Cells without Dendritic Cells. Cancer Cell. 2017;31:614–615. doi: 10.1016/j.ccell.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, Wang Y, Lai YJ, LaBaff AM, Wu TJ, Lin BR, Yang MH, Hortobagyi GN, Hung MC. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl S, Lerner S, O'Brien S, Albitar M. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–1219. [PubMed] [Google Scholar]
- 17.Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F, Balkwill FR. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 18.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Väyrynen JP, Kantola T, Väyrynen SA, Klintrup K, Bloigu R, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, Mäkinen MJ. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016;139:112–121. doi: 10.1002/ijc.30040. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AB, Thompson NL, Heine U, Flanders C, Sporn MB. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988;57:594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 22.Merlano MC, Granetto C, Fea E, Ricci V, Garrone O. Heterogeneity of colon cancer: from bench to bedside. ESMO Open. 2017;2:e000218. doi: 10.1136/esmoopen-2017-000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar H, Heer K, Lee PW, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4:1279–1285. [PubMed] [Google Scholar]
- 24.Ferroni P, Palmirotta R, Spila A, Martini F, Formica V, Portarena I, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic value of carcinoembryonic antigen and vascular endothelial growth factor tumor tissue content in colorectal cancer. Oncology. 2006;71:176–184. doi: 10.1159/000106072. [DOI] [PubMed] [Google Scholar]
- 25.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 26.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell. 2017;31:711–723.e4. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.