Abstract
Background
Policies and protocols vary widely for fetal surveillance in a pregnancy where the fetus is suspected to be large‐for‐gestational‐age (LGA). All ultimately culminate in decisions about the mode and timing of birth. LGA is known to be associated with increased risks to both the mother and baby. Interventions based on surveillance regimen findings may be associated with risks to the mother and baby.
Objectives
To assess the effectiveness or efficacy of different antenatal surveillance methods for the suspected LGA fetus on important health outcomes for the mother and baby.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 August 2015), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (21 August 2015).
Selection criteria
Published and unpublished randomised, quasi‐randomised and cluster‐randomised trials comparing the effects of described antenatal fetal surveillance regimens for women with suspected LGA infants.
Data collection and analysis
We identified no studies that met the inclusion criteria for this review.
Main results
There are no included trials.
Authors' conclusions
We found no randomised controlled trials that assessed the effect of antenatal fetal surveillance regimens of a suspected LGA fetus on important health outcomes for the mother and baby.
There has been a rise in the prevalence of LGA babies over the past few decades in many countries. Research is therefore required on regimens of antenatal surveillance of suspected LGA infants, in order to guide practice and improve the health outcomes for the mother and infant. In particular, randomised control trials to investigate whether serial antenatal clinic and ultrasound assessments of suspected LGA infants (including liquor volume and markers of fetal adiposity) would be useful, to assess whether surveillance methods improve health outcomes. In addition, as there are concerns that identifying suspected LGA fetuses may lead to unnecessary maternal anxiety, investigations and interventions, any such trial would need to assess the risks as well as benefits of regimens of fetal surveillance for suspected LGA fetuses.
Plain language summary
Use of different methods of detecting if a baby is large‐for‐gestational‐age, to improve health outcomes
What is the issue?
A baby may sometimes grow to be bigger than expected and be born with a high birthweight. When overgrowth of the baby is suspected during a pregnancy the mother can have extra scheduled antenatal visits and tests to assess her health and the health of her developing baby.
Why is this important?
Tests can detect if there are signs of any deterioration in the baby’s condition, or development of complications in the mother. The specified frequency and combinations of tests vary with local protocols and policies. Tests may include fetal movement counting, fetal heart rate assessment (cardiotocography), checking the mother's blood sugars or the use of ultrasound for fetal growth scans, Doppler ultrasound examination of fetal blood vessels and assessing the volume of fluid around the baby.
Large babies are associated with increased risks to both the mother and baby, including increased risk of intra‐uterine death and stillbirth. At birth the baby is at a higher risk of low oxygen levels, shoulder dystocia, nerve injuries, bone fracture, low blood sugar levels, and admission to the neonatal intensive care unit. Maternal complications include prolonged labour, operative births including caesarean section, perineal trauma, postpartum haemorrhage and uterine rupture.
Interventions that may slow growth acceleration and improve health outcomes for the mother and her baby include dietary advice, lifestyle modification, and in women with diabetes or gestational diabetes blood glucose monitoring and insulin therapy.
What evidence did we find?
We searched for studies on 10 August 2015 but did not find any randomised controlled trials looking at the effects of performing extra tests on health outcomes in pregnant women with overgrowth of the baby after 20 weeks gestation.
What does this mean?
There is a need for randomised controlled trials in this area in order to inform clinical practice when large babies are identified during a pregnancy, to assess if extra tests or surveillance can improve the health of these women and their babies. It is also important to identify any harms associated with extra tests and surveillance, as identifying women with suspected large babies may lead to unnecessary maternal anxiety with additional investigations and interventions, including induction of labour or caesarean section.
Background
Description of the condition
Large‐for‐gestational‐age (LGA) is used to describe fetuses or infants with a suspected disturbance of growth, where actual or estimated growth is more than expected for gestational age (Xu 2010).
There are various definitions used to describe growth of the baby during pregnancy. LGA is usually defined as an estimated fetal weight more than the 90th percentile (Pundir 2009). Macrosomia, a frequently‐used term, refers to growth beyond a specific threshold, usually defined as birthweight of 4000 g or more (Delpapa 1991), although some studies have suggested grading macrosomia depending on birthweight, into grade one (4000 to 4499 g), grade two (4500 to 4999 g) and grade three (5000 g or more) (Boulet 2003).
There is a wide variation in the prevalence of macrosomia in different countries. Amongst European countries, prevalence of grade one macrosomia varies between 8% and 21% (Bergmann 2003; Gyselaers 2012; Orland 2012). Macrosomia prevalence in Asian countries tends to be much lower, at 1% to 8% (Li 2014; Morikawa 2013). The worldwide prevalence of grade one macrosomia is approximately 9% and the prevalence of grade three macrosomia is approximately 0.1% (Chauhan 2005). LGA is associated with a number of maternal or fetal factors. The majority of cases of LGA infants are associated with maternal factors including maternal height, weight, body mass index (BMI), gestational weight gain, ethnicity, parity and maternal age (Bergmann 2003; Stotland 2004), as well as the presence of pre‐gestational or gestational diabetes (Ehrenberg 2004). Fetal factors contributing to LGA infants include fetal sex, post‐term birth, uncertain dates and, less commonly, fetal genetic abnormalities (Vora 2009).
LGA is known to be associated with increased risks to both the mother and baby. Fetal and neonatal implications include increased risk of intra‐uterine death and stillbirth, fetal hypoxia, shoulder dystocia, brachial plexus injuries, bone fracture, hypoglycaemia, and admission to the neonatal intensive care unit (Esakoff 2009; Ju 2009). Maternal complications include prolonged labour, operative births including caesarean section, perineal trauma, postpartum haemorrhage and uterine rupture (Ju 2009; King 2012; Stotland 2004).
It can be difficult to differentiate accurately between a constitutionally LGA infant, due to maternal height, weight and ethnicity, and an infant with abnormally elevated growth for gestation.
Description of the intervention
Management of suspected LGA fetuses requires several steps. Initially, those infants at risk of LGA are identified either by clinical assessment, maternal estimation, or knowledge of risk factors such as diabetes. Once a fetus is suspected of being LGA, further assessment to assist in the diagnosis is performed, such as an ultrasound scan. Following this, antenatal care and surveillance is ongoing and eventually decisions must be made regarding the mode and timing of birth.
Once a baby is suspected to be LGA, antenatal care providers may utilise a variety of methods, or regimens, to monitor the baby’s growth and well‐being. The type and frequency of monitoring techniques vary considerably, and the choice of methods may be affected by other factors such as the presence of pre‐gestational or gestational diabetes mellitus (Campaigne 2007).
Antenatal fetal surveillance methods aim to provide information about the health of the baby (Grivell 2012). The results of surveillance methods then guide decisions about ongoing surveillance and the mode and timing of delivery, which may in turn reduce the risks to both the baby and the mother associated with fetal overgrowth and suspected LGA fetuses.
There are many methods that may be used in clinical practice, alone or in combination, to monitor suspected LGA fetuses and to assess those at risk (Grivell 2012). These include abdominal palpation, measuring symphysial fundal height (SFH), ultrasound assessment of baby’s growth, fetal movement counting, fetal heart rate assessment (cardiotocography), ultrasound assessment of the baby’s well‐being by serial growth measurements or liquor volume, and assessment of maternal glycaemia (Aye 2010; Chauhan 2005; Grivell 2012).
When LGA is suspected in a fetus, either from clinical assessment or maternal estimation, ultrasound is often used to assess growth using various standard measurements. These may include: abdominal circumference, head circumference, bi‐parietal diameter and femur length. These measurements can then be used to estimate fetal weight. Many formulae have been proposed, with the most widely used being Hadlock's (Hadlock 1985). Additional parameters to identify LGA infants and assess fetal well‐being include subcutaneous fat measurements on ultrasound, amniotic fluid volume, three‐dimensional (3D) volumetric estimates, umbilical artery dopplers and fetal magnetic resonance imaging (MRI) (Aye 2010; Chauhan 2005; Orland 2012). Ultrasound measurements can then be plotted against gestation along known percentile lines on a growth curve, to assess severity and timing of increasing growth. Serial ultrasound measurements can then identify those infants who are suspected to be LGA as well as those with acceleration of growth over time.
Ultrasound surveillance has variable accuracy in identifying LGA fetuses. The post‐test probability of detecting a macrosomic fetus on ultrasound has been assessed in a systematic review (Chauhan 2005); in an uncomplicated pregnancy it is variable, ranging from 15% to 79%. In comparison, the post‐test probability of clinical estimate in predicting a macrosomic fetus is 40% to 52% (Chauhan 2005). The use of a single early third‐trimester fetal biometry for the prediction of birth weight deviations showed 90% sensitivity for detection of macrosomia (De Reu 2008).
Macrosomia detection in higher‐risk populations such as women with diabetes has more consistency. The above systematic review found sensitivities ranging from 33% to 69%, specificities from 77% to 98%, and post‐test probabilities from 44% to 81%, in detecting macrosomia in women with diabetes (Chauhan 2005).
3D ultrasound has been used to identify suspected LGA fetuses, in particular by measuring subcutaneous fetal fat. There is some evidence that this improves weight prediction to within 5% of the actual weight (Lee 2001). However, this technique is not widely used.
Fetal MRI may have better predictive value in fetal weight estimation compared with ultrasound (Uotila 2000). However, due to limited availability and high cost, it is not routinely used in the diagnosis of macrosomia.
Although accurate, ultrasound is expensive when used as a screening tool for abnormal growth detection. A low‐cost option for detecting abnormal fetal growth is SFH.
A Cochrane review found insufficient evidence to determine whether SFH measurement is effective in detecting abnormal fetal growth, but only one trial was eligible for inclusion (Robert Peter 2012).
There is concern that identifying suspected LGA fetuses may lead to unnecessary maternal anxiety, investigations and interventions. A prospective observational study found that after an ultrasound, women with a suspected macrosomic fetus were more likely to have induction of labour or caesarean section, compared with actual macrosomia in the comparison group of all other births (Sadeh‐Mestechkin 2008). A previous Cochrane review found that induction of labour is not recommended for suspected macrosomia, as it does not alter the risk of maternal or neonatal morbidity (Irion 1998). Our review will therefore assess the risks as well as benefits of regimens of fetal surveillance for suspected LGA fetuses.
The estimated fetal weight from an ultrasound, or a SFH measurement, can be plotted on a customised growth chart, which adjusts for factors such as the mother's weight and height, ethnicity and number of babies she has had. This may make assessment of fetal growth more precise, differentiating between constitutional LGA and fetal overgrowth, reducing unnecessary consultations for investigations, and parental anxiety (González 2013).
How the intervention might work
We anticipated that by increasing the frequency of monitoring of a suspected LGA fetus, or using different methods of surveillance in combination, it would be possible to identify babies that would either benefit from earlier birth, or benefit from intervention to slow growth acceleration. Interventions to slow growth acceleration include dietary advice, lifestyle modification, and in women with diabetes or gestational diabetes blood glucose monitoring and insulin therapy (Aye 2010; Campaigne 2007; Chauhan 2005). This in turn may be associated with improved health outcomes for the mother and baby.
Why it is important to do this review
There has been a rise in the prevalence of LGA babies over the past few decades in many countries (Bergmann 2003; Gyselaers 2012; Lahmann 2009). In high‐income countries, there has been an overall 15% to 25% increase in the proportion of women giving birth to large infants (Aye 2010). Data are not as easily available for low‐ to middle‐income countries, where the risks are even greater due to the reduced availability of operative delivery options (Koyanagi 2013).
There is evidence that LGA is associated with an increased risk of maternal and neonatal complications. However, policies and protocols for fetal surveillance where accelerated fetal growth is suspected vary widely, which has led to uncertainty among clinicians about appropriate surveillance.
This systematic review aimed to assess the benefits and harms associated with different combinations of surveillance methods for the fetus estimated to be LGA.
Objectives
To assess the effectiveness or efficacy of different antenatal surveillance methods for the suspected large‐for‐gestational‐age (LGA) fetus on important health outcomes for the mother and baby.
Methods
Criteria for considering studies for this review
Types of studies
We had planned to include published and unpublished randomised controlled trials (RCTs), quasi‐randomised trials and cluster‐randomised trials comparing alternative surveillance regimens for women with suspected LGA infants. We planned to include conference abstracts where a full publication was unavailable.
Cross‐over studies were not eligible for inclusion.
Types of participants
Pregnant women after 20 weeks of pregnancy, where the fetus is suspected to be LGA. The definition of LGA was to be determined by the individual trials.
Types of interventions
Routine antenatal care compared with various regimens that are put in place due to a suspicion of LGA. Routine care was to be described by individual trials. Regimens were to include one or more surveillance methods. Surveillance methods may have included: increased antenatal visits without ultrasound, ultrasound surveillance of fetal growth, measurement of liquor volume, biophysical profile, umbilical artery dopplers, diabetes screening, monitoring maternal glycaemia, HbA1c monitoring, fetal movement counting or other tests of fetal well‐being.
We also planned to include head‐to‐head analysis of different regimens compared directly.
Types of outcome measures
Primary outcomes
Primary neonatal outcomes
Perinatal mortality (death of a fetus after 24 weeks' gestation or death of an infant in the first seven days of life)
Composite of serious neonatal morbidity (as defined by the trial authors)
Primary maternal outcomes
Caesarean section
Shoulder dystocia
Secondary outcomes
Secondary neonatal outcomes
Gestational age at birth
Birthweight/birthweight Z score
LGA variously described by authors
Customised birthweight centile
Low Apgar defined as less than seven
Cord blood acidosis
Neonatal hypoglycaemia
Birth length and head circumference
Fetal/neonatal adiposity
Secondary maternal outcomes
Induction of labour
Operative vaginal birth
Perineal trauma
Postpartum haemorrhage
Breastfeeding
Women's views of care
Longer‐term outcomes for the baby as a child
Long‐term neurodevelopmental outcomes
Longer‐term metabolic outcomes
Obesity
Hypertension
Type 2 diabetes
Use of healthcare services
Admission to neonatal special care or intensive care unit
Costs of antenatal monitoring regimen
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 August 2015).
The Register is a database containing over 20,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1 (21 August 2015).
Searching other resources
We planned to search the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
In this review, we did not identify any studies for inclusion. Our methods of data collection and analysis to be used in future updates of this review (if more data become available) are listed in Appendix 2.
Results
Description of studies
Results of the search
See:Figure 1
1.
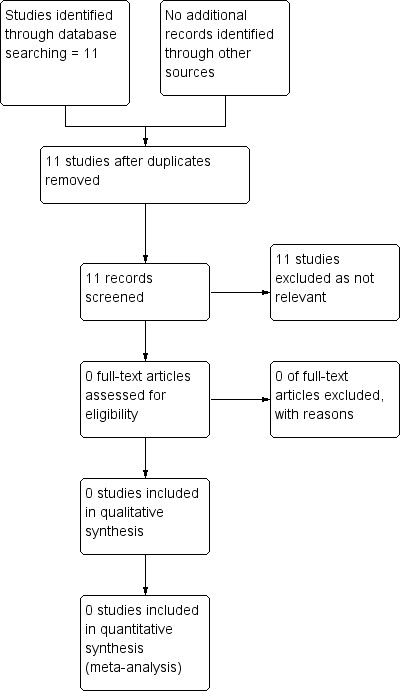
Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group’s Trials register did not retrieve any studies.
The search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) retrieved 11 reports; however, all included large‐for‐gestational‐age or macrosomia as an outcome only, and did not focus on interventions for suspected large‐for‐gestational‐age fetuses.
Risk of bias in included studies
No studies met the eligibility criteria for inclusion.
Effects of interventions
No studies met the eligibility criteria for inclusion.
Discussion
Large‐for‐gestational‐age (LGA) is known to be associated with increased risks to both the mother and baby, including increased risk of intra‐uterine death, fetal hypoxia, shoulder dystocia, brachial plexus injuries, bone fracture, hypoglycaemia, and admission to the neonatal intensive care unit (Esakoff 2009; Ju 2009). Maternal complications include prolonged labour, operative births including caesarean section, perineal trauma, postpartum haemorrhage and uterine rupture (Ju 2009; King 2012; Stotland 2004).
However, there is no evidence from randomised trials to guide which regimens of fetal surveillance should be used antenatally, once LGA is suspected, to impact these pregnancy outcomes. There are many different regimens which could be designed, but we found no evidence to support any individual regimen.
Potential biases in the review process
We conducted comprehensive searches of the Cochrane Pregnancy and Childbirth Trials Register and clinical trial registries. We did not identify any published, unpublished or ongoing studies that met the inclusion criteria for this review.
Agreements and disagreements with other studies or reviews
As we found no relevant eligible trials investigating the effect of antenatal surveillance regimens of suspected LGA fetuses, we are unable to draw any conclusions about the effectiveness of these regimens. Consequently, we are unable to identify any agreements/disagreements with other studies or reviews.
Authors' conclusions
Implications for practice.
There is no evidence from randomised controlled trials to evaluate regimens of fetal surveillance for suspected large‐for‐gestational‐age (LGA) fetuses to improve health outcomes.
Implications for research.
The absence of randomised controlled trials relating to regimens of fetal surveillance for suspected LGA fetuses to improve health outcomes reveals an area where research is needed.
There has been a rise in the prevalence of LGA babies over the past few decades in many countries (Aye 2010; Lahmann 2009), so research is required on regimens of antenatal surveillance of suspected LGA infants in order to improve the health outcomes for the mother and infant. In particular, randomised control trials to investigate if serial antenatal clinic and ultrasound assessment of suspected LGA infants (including liquor volume and markers of fetal adiposity) would be useful to assess if surveillance methods improve health outcomes. In addition, as there are concerns that identifying suspected LGA fetuses may lead to unnecessary maternal anxiety, investigations and interventions, any such trial would need to assess the risks as well as benefits of regimens of fetal surveillance for suspected LGA fetuses.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by an editor and members of the Pregnancy and Childbirth Group's international panel of consumers.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We acknowledge the support of the Australian and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by the National Medical and Health Research Council, Australia) and the support provided by the Liggins Institute, The University of Auckland, New Zealand.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
fetal AND growth AND surveillance
large AND gestational AND age
pregnancy AND surveillance AND regimens
macrosomia AND surveillance
fetal AND overgrowth
fetal AND adiposity
large for dates
Appendix 2. Methods of data collection and analysis to be used in future updates of this review
Data collection and analysis
In this review, we did not identify any studies for inclusion. Our methods of data collection and analysis to be used in future updates of this review (if more data become available) are listed here.
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third review author.
We will create a 'Study flow diagram' to map out the number of records identified, included and excluded.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third review author. We will enter data into Review Manager 5 software (RevMan 2014) and check for accuracy. When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessing the quality of the evidence using the GRADE approach
The quality of the evidence will be assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the comparison of regimens of fetal surveillance for suspected LGA fetuses compared with routine antenatal care:
Perinatal mortality (death of a fetus after 24 weeks' gestation or death of an infant in the first seven days of life)
Composite of serious neonatal morbidity
Neonatal hypoglycaemia
Admission to neonatal special care or intensive care unit
Induction of labour
Caesarean section
Shoulder dystocia
We will use the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We will produce a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as a summary risk ratio with a 95% confidence interval.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods. We will present data with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion.
Multiple arm studies
Where a trial has multiple intervention arms we will avoid 'double counting' of participants by combining groups to create a single pair‐wise comparison if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager 5 software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses,
Diabetic versus non‐diabetic
Gestational diabetes versus pre‐gestational diabetes
Primiparous versus multiparous
Normal body mass index (BMI) versus overweight and obese (i.e. BMI of 20 to 25 kg/m² versus BMI > 25 kg/m²)
We will use the following outcomes in subgroup analysis.
Perinatal mortality and composite of serious neonatal morbidity (as defined by the trial authors)
Caesarean section
Shoulder dystocia
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Where there is evidence of heterogeneity that cannot be explained by subgroup analysis, we will conduct sensitivity analysis by examining the quality of the included trials based on reporting of allocation concealment and blinding for primary outcomes of the review only.
Differences between protocol and review
Since publication of our protocol (Culliney 2015) we have edited our methods to include the use of GRADE and a summary of findings table.
Contributions of authors
Katherine Culliney, Julie Brown, Graham K Parry and Caroline A Crowther wrote the title registration. Katherine Culliney wrote the protocol and the systematic review with assistance, comments and feedback from Julie Brown, Graham K Parry and Caroline A Crowther.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Liggins Institute, University of Auckland, New Zealand.
Infrastructure support for Cochrane authors has been provided by the Liggins Institute, University of Auckland.
-
Cochrane Pregnancy and Childbirth Australia and New Zealand Satellite, Australia.
We acknowledge the infrastructure support of the Cochrane Pregnancy and Childbirth Australia and New Zealand Satellite (funded by NHMRC)
Declarations of interest
Katherine AT Culliney: is employed by Waitemata District Health board, North Shore Hospital and Taranaki District health board, Taranaki Base Hospital. Graham K Parry: none known. Julie Brown: none known. Caroline A Crowther: none known.
New
References
Additional references
Aye 2010
- Aye SS, Miller V, Saxena S, Farhan M. Review: management of large‐for gestational‐age pregnancy in non‐diabetic women. Obstetrician and Gynaecologist 2010;12:250‐6. [Google Scholar]
Bergmann 2003
- Bergmann RL, Richter R, Bergmann KE, Plagemann A, Brauer M, Dudenhausen JW. Secular trends in neonatal macrosomia in Berlin: influences of potential determinants. Paediatric and Perinatal Epidemiology 2003;17(3):244‐9. [PUBMED: 12839535] [DOI] [PubMed] [Google Scholar]
Boulet 2003
- Boulet SL, Alexander GR, Salihu HM, Pass MS. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. American Journal of Obstetrics and Gynecology 2003;188(5):1372. [PUBMED: 12748514] [DOI] [PubMed] [Google Scholar]
Campaigne 2007
- Campaigne A, Conway D. Dectection and prevention of macrosomia. Obstetrics and Gynecology Clinics of North America 2007;34(2):309‐22. [DOI] [PubMed] [Google Scholar]
Chauhan 2005
- Chauhan SP, Grobman WA, Gherman RA, Chauhan VB, Chang G, Magann EF, et al. Suspicion and treatment of the macrosomic fetus: a review. American Journal of Obstetrics and Gynecology 2005;193(2):332. [PUBMED: 16098852] [DOI] [PubMed] [Google Scholar]
De Reu 2008
- Reu PA, Smits LJ, Oosterbaan HP, Nijhuis JG. Value of a single early third trimester fetal biometry for the prediction of birth weight deviations in a low risk population. Journal of Perinatal Medicine 2008;36(4):324‐9. [DOI] [PubMed] [Google Scholar]
Delpapa 1991
- Delpapa EH, Mueller‐Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstetrics & Gynecology 1991;78(3 Pt 1):340‐3. [PubMed] [Google Scholar]
Ehrenberg 2004
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. American Journal of Obstetrics and Gynecology 2004;191(3):964‐8. [DOI] [PubMed] [Google Scholar]
Esakoff 2009
- Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2009;200(6):672‐6. [PUBMED: 19376489 ] [DOI] [PubMed] [Google Scholar]
González 2013
- González González NL, Plasencia W, González Dávila E, Padrón E, García Hernández JA, Renzo GC, et al. The effect of customised growth charts on the identification of large for gestational age newborns. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(1):62‐5. [DOI] [PubMed] [Google Scholar]
Grivell 2012
- Grivell RM, Wong L, Bhatia V. Regimens of fetal surveillance for impaired fetal growth. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD007113.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyselaers 2012
- Gyselaers W, Martens G. Increasing prevalence of macrosomia in Flanders, Belgium: an indicator of population health and a burden for the future. Facts, Views & Vision in OBGYN 2012;4(2):141‐3. [PMC free article] [PubMed] [Google Scholar]
Hadlock 1985
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements‐‐a prospective study. American Journal of Obstetrics and Gynaecology 1985;151(3):333‐7. [PUBMED: 3881966] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irion 1998
- Irion O, Boulvain M. Induction of labour for suspected fetal macrosomia. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000938] [DOI] [PubMed] [Google Scholar]
Ju 2009
- Ju H, Chadha Y, Donovan T, O'Rourke P. Fetal macrosomia and pregnancy outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(5):504‐9. [PUBMED: 19780734] [DOI] [PubMed] [Google Scholar]
King 2012
- King JR, Korst LM, Miller DA, Ouzounian JG. Increased composite maternal and neonatal morbidity associated with ultrasonographically suspected fetal macrosomia. Journal of Maternal‐fetal & Neonatal Medicine 2012;25(10):1953‐9. [PUBMED: 22439605] [DOI] [PubMed] [Google Scholar]
Koyanagi 2013
- Koyanagi A, Zhang J, Dagvadori A, Hirayama F, Shibuya K, Souza JP, et al. Macrosomia in 23 developing countries: an analysis of multicountry, facility‐based, cross‐sectional survey. Lancet 2013;381(9865):476‐83. [DOI] [PubMed] [Google Scholar]
Lahmann 2009
- Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatric and Perinatal Epidemiology 2009;23(6):533‐41. [DOI: 10.1111/j.1365-3016.2009.01075.x.] [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee W, Deter RL, Ebersole JD, Huang R, Blanckaert K, Romero R. Birthweight prediction by 3D ultrasonography: fractional limb volume. Journal of Ultrasound in Medicine 2001;20(12):1283–92. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li G, Kong L, Li Z, Zhang L, Fan L, Zou L, et al. Prevalence of macrosomia and its risk factors in China: a multivariate survey based on birth data involving 10,1723 singleton term infants. Paediatric and Perinatal Epidemiology 2014;28(4):345‐50. [DOI] [PubMed] [Google Scholar]
Morikawa 2013
- Morikawa M, Cho K, Yamada T, Yamada T, Sato S, Minakami H. Fetal macrosomia in Japanese women. Journal of Obstetrics and Gynaecology Research 2013;39(5):960‐5. [DOI] [PubMed] [Google Scholar]
Orland 2012
- Orland J. Prevalence and Predictors of Macrosomia Newborn: Norther‐Norwegian Mother‐and‐Child Study [Masters Thesis]. University of Tromso, 2012. [Google Scholar]
Pundir 2009
- Pundir J, Sinha P. Non‐diabetic macrosomia: an obstetric dilemma. Journal of Obstetrics and Gynaecology 2009;29(3):200‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robert Peter 2012
- Robert Peter J, Ho JJ, Valliapan J, Sivasangari S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008136.pub2] [DOI] [PubMed] [Google Scholar]
Sadeh‐Mestechkin 2008
- Sadeh‐Mestechkin D, Walfisch A, Shachar R, Shoham‐Vardi I, Vardi H, Hallak M. Suspected macrosomia? Better not tell. Archives of Gynecology and Obstetrics 2008;278(3):225‐30. [DOI] [PubMed] [Google Scholar]
Stotland 2004
- Stotland N, Caughey A, Breed E, Escobar G. Risk factors and obstetric complications associated with macrosomia. International Journal of Gynaecology and Obstetrics 2004;87(3):220‐6. [DOI] [PubMed] [Google Scholar]
Uotila 2000
- Uotila J, Dastidar P, Heinonen T, Ryymin P, Punnonen R, Laasonen E. Magnetic resonance imaging compared to ultrasonography in fetal weight and volume estimation in diabetic and normal pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2000;79(4):255–9. [PubMed] [Google Scholar]
Vora 2009
- Vora N, Bianchi DW. Genetic considerations in the prenatal diagnosis of overgrowth syndromes. Prenatal Diagnosis 2009;29(10):923‐9. [PUBMED: 19609940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2010
- Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut‐offs in defining small‐ or large‐for‐gestational‐age. Acta Paediatrica 2010;99(4):550‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Culliney 2015
- Culliney KAT, Parry GK, Brown J, Crowther CA. Regimens of fetal surveillance of suspected large‐for‐gestational‐age fetuses for improving health outcomes. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011739] [DOI] [PMC free article] [PubMed] [Google Scholar]


