Abstract
Background
Withdrawal is a necessary step prior to drug‐free treatment or as the endpoint of long‐term substitution treatment.
Objectives
To assess the effectiveness of interventions involving the use of alpha2‐adrenergic agonists compared with placebo, reducing doses of methadone, symptomatic medications, or an alpha2‐adrenergic agonist regimen different to the experimental intervention, for the management of the acute phase of opioid withdrawal. Outcomes included the withdrawal syndrome experienced, duration of treatment, occurrence of adverse effects, and completion of treatment.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1946 to November week 2, 2015), EMBASE (January 1985 to November week 2, 2015), PsycINFO (1806 to November week 2, 2015), Web of Science, and reference lists of articles.
Selection criteria
Randomised controlled trials comparing alpha2‐adrenergic agonists (clonidine, lofexidine, guanfacine, tizanidine) with reducing doses of methadone, symptomatic medications or placebo, or comparing different alpha2‐adrenergic agonists to modify the signs and symptoms of withdrawal in participants who were opioid dependent.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included 26 randomised controlled trials involving 1728 participants. Six studies compared an alpha2‐adrenergic agonist with placebo, 12 with reducing doses of methadone, four with symptomatic medications, and five compared different alpha2‐adrenergic agonists. We assessed 10 studies as having a high risk of bias in at least one of the methodological domains that were considered.
We found moderate‐quality evidence that alpha2‐adrenergic agonists were more effective than placebo in ameliorating withdrawal in terms of the likelihood of severe withdrawal (risk ratio (RR) 0.32, 95% confidence interval (CI) 0.18 to 0.57; 3 studies; 148 participants). We found moderate‐quality evidence that completion of treatment was significantly more likely with alpha2‐adrenergic agonists compared with placebo (RR 1.95, 95% CI 1.34 to 2.84; 3 studies; 148 participants).
Peak withdrawal severity may be greater with alpha2‐adrenergic agonists than with reducing doses of methadone, as measured by the likelihood of severe withdrawal (RR 1.18, 95% CI 0.81 to 1.73; 5 studies; 340 participants; low quality), and peak withdrawal score (standardised mean difference (SMD) 0.22, 95% CI ‐0.02 to 0.46; 2 studies; 263 participants; moderate quality), but these differences were not significant and there is no significant difference in severity when considered over the entire duration of the withdrawal episode (SMD 0.13, 95% CI ‐0.24 to 0.49; 3 studies; 119 participants; moderate quality). The signs and symptoms of withdrawal occurred and resolved earlier with alpha2‐adrenergic agonists. The duration of treatment was significantly longer with reducing doses of methadone (SMD ‐1.07, 95% CI ‐1.31 to ‐0.83; 3 studies; 310 participants; low quality). Hypotensive or other adverse effects were significantly more likely with alpha2‐adrenergic agonists (RR 1.92, 95% CI 1.19 to 3.10; 6 studies; 464 participants; low quality), but there was no significant difference in rates of completion of withdrawal treatment (RR 0.85, 95% CI 0.69 to 1.05; 9 studies; 659 participants; low quality).
There were insufficient data for quantitative comparison of different alpha2‐adrenergic agonists. Available data suggest that lofexidine does not reduce blood pressure to the same extent as clonidine, but is otherwise similar to clonidine.
Authors' conclusions
Clonidine and lofexidine are more effective than placebo for the management of withdrawal from heroin or methadone. We detected no significant difference in efficacy between treatment regimens based on clonidine or lofexidine and those based on reducing doses of methadone over a period of around 10 days, but methadone was associated with fewer adverse effects than clonidine, and lofexidine has a better safety profile than clonidine.
Keywords: Humans, Acute Disease, Adrenergic alpha‐2 Receptor Agonists, Adrenergic alpha‐2 Receptor Agonists/therapeutic use, Clonidine, Clonidine/analogs & derivatives, Clonidine/therapeutic use, Controlled Clinical Trials as Topic, Methadone, Methadone/administration & dosage, Methadone/therapeutic use, Opiate Substitution Treatment, Opiate Substitution Treatment/methods, Opioid‐Related Disorders, Opioid‐Related Disorders/complications, Randomized Controlled Trials as Topic, Substance Withdrawal Syndrome, Substance Withdrawal Syndrome/rehabilitation
Plain language summary
Clonidine, lofexidine, and similar medications for the management of opioid withdrawal
Review question
We reviewed the evidence about the effect of alpha2‐adrenergic agonists (clonidine, lofexidine, guanfacine, and tizanidine) in managing withdrawal in people who are dependent on opioid drugs (for example heroin, methadone).
Background
Managed withdrawal, or detoxification, is a required first step for longer‐term treatments of opioid dependence. The combination of uncomfortable symptoms and intense craving makes completion of opioid withdrawal difficult for most people. For many years, the main approach to detoxification involved suppression of withdrawal with methadone and gradual reduction of the methadone dose. The use of methadone in this way has been limited by government restrictions on prescription of methadone and dislike of the drawn‐out nature of methadone withdrawal. Clonidine and similar medications (known as alpha2‐adrenergic agonists) offer an alternative approach. This review considered whether alpha2‐adrenergic agonists are more effective than reducing doses of methadone, and whether there are any differences in the effectiveness of different types of alpha2‐adrenergic agonist.
Search date
The evidence is current to November 2015.
Study characteristics
We identified 26 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups), involving 1728 opioid‐dependent participants. The studies were undertaken in 12 different countries and involved treatment with an alpha2‐adrenergic agonist (clonidine, lofexidine, guanfacine, and in one study, tizanidine) compared with reducing doses of methadone (12 studies), placebo (six studies), or symptomatic medications (four studies). Five studies compared different alpha2‐adrenergic agonists. Treatment was scheduled to last for one to two weeks in most studies; the shortest duration was three days, and the longest was 30 days.
Six studies received some financial support from a pharmaceutical company.
Key results
Opioid withdrawal was similar with alpha2‐adrenergic agonists and reducing doses of methadone, but the duration of treatment was longer and there were fewer adverse effects with methadone. Withdrawal signs and symptoms occurred earlier with alpha2‐adrenergic agonists, within a few days of cessation of the opioid drugs. The chances of completing withdrawal treatment were similar.
Clonidine and lofexidine were more effective than placebo in managing withdrawal from heroin or methadone, and were associated with higher chances of completing treatment.
Lofexidine had less effect on blood pressure than clonidine.
Quality of the evidence
For alpha2‐adrenergic agonists compared with placebo, the evidence was very low to moderate quality, indicating that further evidence would be likely to change the estimates of relative effect made in this review. However, the evidence is sufficient to indicate that alpha2‐adrenergic agonists are more effective than placebo, making further comparisons of this nature inappropriate on ethical grounds.
For the comparison of alpha2‐adrenergic agonists with reducing doses of methadone, the evidence was low to moderate quality. The key reasons for the low quality were small numbers of studies reporting some outcomes, low rates of occurrence of some events (for example drop‐out due to adverse effects), and variability between studies.
Summary of findings
Summary of findings for the main comparison. Alpha2‐adrenergic agonist versus methadone for the management of opioid withdrawal.
| Alpha2‐adrenergic agonist versus methadone for the management of opioid withdrawal | ||||||
| Patient or population: People undergoing managed opioid withdrawal Settings: Intervention: Alpha2‐adrenergic agonist versus methadone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Alpha2‐adrenergic agonist versus methadone | |||||
| Participants with severe withdrawal | Study population | RR 1.18 (0.81 to 1.73) | 340 (5 studies) | ⊕⊕⊝⊝ low1,2 | ‐ | |
| 205 per 1000 | 242 per 1000 (166 to 354) | |||||
| Moderate | ||||||
| 80 per 1000 | 94 per 1000 (65 to 138) | |||||
| Peak withdrawal score | ‐ | The mean peak withdrawal score in the intervention groups was 0.22 standard deviations higher (0.02 lower to 0.46 higher) | ‐ | 263 (2 studies) | ⊕⊕⊕⊝ moderate3 | SMD 0.22 (‐0.02 to 0.46) |
| Overall withdrawal severity | ‐ | The mean overall withdrawal severity in the intervention groups was 0.13 standard deviations higher (0.24 lower to 0.49 higher) | ‐ | 119 (3 studies) | ⊕⊕⊕⊝ moderate3 | SMD 0.13 (‐0.24 to 0.49) |
| Duration of treatment | ‐ | The mean duration of treatment in the intervention groups was 1.07 standard deviations lower (1.31 to 0.83 lower) | ‐ | 310 (3 studies) | ⊕⊕⊝⊝ low3,4 | SMD ‐1.07 (‐1.31 to ‐0.83) |
| Number experiencing hypotensive or other adverse effects | Study population | RR 1.92 (1.19 to 3.10) | 464 (6 studies) | ⊕⊕⊝⊝ low2,5 | ‐ | |
| 79 per 1000 | 151 per 1000 (93 to 243) | |||||
| Moderate | ||||||
| 33 per 1000 | 63 per 1000 (39 to 102) | |||||
| Drop‐out due to adverse effects | Study population | RR 3.62 (0.77 to 16.94) | 153 (4 studies) | ⊕⊕⊝⊝ low2 | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Completion of treatment | Study population | RR 0.85 (0.69 to 1.05) | 659 (9 studies) | ⊕⊕⊝⊝ low6,7 | ‐ | |
| 568 per 1000 | 483 per 1000 (392 to 597) | |||||
| Moderate | ||||||
| 750 per 1000 | 638 per 1000 (517 to 787) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One study at risk of selection bias, one at risk of performance and detection bias. 2Small number of events. 3Small number of participants. 4One study at risk of selection bias, one at risk of bias related to mandatory treatment. 5One study at risk of selection bias. 6Two studies at high risk of selection bias. 7Significant heterogeneity present.
Summary of findings 2. Alpha2‐adrenergic agonist versus placebo for the management of opioid withdrawal.
| Alpha2‐adrenergic agonist versus placebo for the management of opioid withdrawal | ||||||
| Patient or population: People undergoing managed opioid withdrawal Settings: Intervention: Alpha2‐adrenergic agonist versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Alpha2‐adrenergic agonist versus placebo | |||||
| Participants with severe withdrawal | Study population | RR 0.32 (0.18 to 0.57) | 148 (3 studies) | ⊕⊕⊕⊝ moderate1 | ‐ | |
| 589 per 1000 | 188 per 1000 (106 to 336) | |||||
| Moderate | ||||||
| 800 per 1000 | 256 per 1000 (144 to 456) | |||||
| Completion of treatment | Study population | RR 1.95 (1.34 to 2.84) | 148 (3 studies) | ⊕⊕⊕⊝ moderate1 | ‐ | |
| 288 per 1000 | 561 per 1000 (385 to 817) | |||||
| Moderate | ||||||
| 333 per 1000 | 649 per 1000 (446 to 946) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Small number of events.
Background
Description of the condition
Dependence on opioid drugs is a major health and social issue in most societies. Globally, it is estimated that around 0.2% of adults report unsanctioned use of opioid drugs (Gowing 2015). Despite this low prevalence of opioid use, unsanctioned use of opioid drugs contributes more to the burden of disease than other illicit psychoactive drugs. The burden to the individual user and the community of opioid dependence arises from premature mortality and disability associated with dependent use, with greatest impact in younger populations of drug users (Gowing 2015), transmission of human immunodeficiency virus (HIV) and hepatitis C, healthcare costs, crime and law enforcement costs, as well as the less tangible costs of family disruption and lost productivity (Mark 2001).
Treatment is central to the reduction of the harms incurred by individuals and the community from opioid dependence. Managed withdrawal, or detoxification, by itself is not an effective treatment for dependence (Lipton 1983; Mattick 1996). Rates of completion of withdrawal tend to be low, and rates of relapse to opioid use following detoxification are high (Broers 2000; Gossop 1989b; Vaillant 1988). However, withdrawal remains a required first step for many forms of longer‐term treatment such as residential rehabilitation and naltrexone maintenance (Kleber 1982). It may also represent the endpoint of an extensive period of substitution treatment such as methadone maintenance. As such, the availability of managed withdrawal is essential to an effective and comprehensive treatment system.
The signs and symptoms of the opioid withdrawal syndrome include irritability, anxiety, apprehension, muscular and abdominal pains, chills, nausea, diarrhoea, yawning, lacrimation, sweating, sneezing, rhinorrhoea, general weakness, and insomnia. Symptoms of the opioid withdrawal syndrome usually begin two to three half‐lives after the last opioid dose, that is six to 12 hours for short half‐life opioids such as heroin and morphine, and 36 to 48 hours for long half‐life opioids such as methadone. Following cessation of a short half‐life opioid, symptoms reach peak intensity within two to four days, with most of the obvious physical withdrawal signs no longer observable after seven to 14 days. As with the onset of withdrawal, the duration also varies with the half‐life of the opioid used, and the duration of regular use (Tetrault 2009). The opioid withdrawal syndrome is rarely life‐threatening or associated with significant aberrations of mental state (Farrell 1994), but the combination of uncomfortable symptoms and intense craving makes completion of withdrawal difficult for most people (Mattick 1996; Tetrault 2009).
Description of the intervention
For many years, routine procedures involved suppression of withdrawal with methadone and gradual reduction of the methadone dose (Kleber 1982). This approach derived from observations that the withdrawal syndrome from methadone was milder, though longer, than that from morphine. Methadone's high oral bioavailability, efficacy, and long duration of withdrawal relief (24 to 36 hours) were additional factors that have contributed to it being the main medication used in specialist withdrawal programmes since the 1980s.
Ambivalence to the use of a drug of dependence to treat opioid dependence, government restrictions on prescription of methadone, and consumer dislike of the protracted nature of methadone withdrawal have, to some extent, limited the use of methadone in this way (Farrell 1994). Discovery of the capacity of the alpha2‐adrenergic agonist clonidine to ameliorate some signs and symptoms of withdrawal led to widespread use of this drug as a non‐opioid alternative for managing withdrawal (Gossop 1988a). One mechanism underlying opioid withdrawal is noradrenergic hyperactivity (Gold 1989). The alpha2‐adrenergic agonists act centrally to moderate the symptoms of noradrenergic hyperactivity.
The use of clonidine in the management of opioid withdrawal has been hampered by side effects of sedation and hypotension. This, in turn, has led to the investigation of the effectiveness of other alpha2‐adrenergic agonists ‐ most commonly lofexidine and guanfacine ‐ in the management of opioid withdrawal, the aim being to find a drug that has clonidine's capacity to ameliorate the signs and symptoms of opioid withdrawal, but with fewer side effects.
How the intervention might work
The focus of this review is the effectiveness of alpha2‐adrenergic agonists (clonidine, lofexidine, guanfacine, tizanidine) in the management of opioid withdrawal, relative to other forms of treatment, placebo, and each other.
A complex range of variables can potentially influence the course and subjective severity of withdrawal, including the type of opioid used, dose taken, concomitant use of other drugs including alcohol, duration of use, general physical health, and psychological factors, such as the reasons for undertaking withdrawal and fear of withdrawal (Farrell 1994; Phillips 1986; Preston 1985). Outcomes of a withdrawal episode may also be influenced by a prior period of substitution treatment, since such treatment is likely to result in a degree of stabilisation in health and social functioning that may facilitate successful withdrawal. Where information was available, we have considered the influence of these variables.
The first, or acute, phase of withdrawal is followed by a period of about six months of a secondary or protracted withdrawal syndrome. This protracted syndrome is characterised by a general feeling of reduced well‐being, which is reflected in measurable abnormal physiological functioning. During this phase, strong cravings for opioids may be experienced periodically. The malaise associated with protracted abstinence is thought to be a major factor in relapse (Satel 1993). The protracted nature of withdrawal makes the period of recovery from dependence typically lengthy and influenced by a range of factors, both social and treatment related. The types of intervention offered following the acute phase of withdrawal to promote recovery and prevent relapse are substantially different to those offered in the management of withdrawal and may include psychological and lifestyle counselling, support groups, and pharmacological and medical treatment. We have excluded this long‐term aspect of treatment of opioid dependence from this review because of its substantially different nature.
Why it is important to do this review
This review is one of a series of Cochrane reviews relating to the management of opioid withdrawal. Other reviews consider the use of opioid antagonists with minimal sedation (Gowing 2009a), or under heavy sedation or anaesthesia (Gowing 2010), the use of buprenorphine (Gowing 2009b), the use of reducing doses of methadone (Amato 2013), inpatient versus other settings (Day 2005), detoxification treatments for adolescents (Minozzi 2014), and psychosocial and pharmacological treatments for opioid detoxification (Amato 2011).
Objectives
To assess the effectiveness of interventions involving the use of alpha2‐adrenergic agonists compared with placebo, reducing doses of methadone, symptomatic medications, or an alpha2‐adrenergic agonist regimen different to the experimental intervention, for the management of the acute phase of opioid withdrawal. Outcomes included the withdrawal syndrome experienced (encompassing intensity, time course and predominant signs and symptoms), duration of treatment, occurrence of adverse effects, and completion of treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials that provided detailed information on the type and dose of drugs used and the characteristics of participants treated. Studies were also required to provide information on the nature of withdrawal signs and symptoms experienced, the occurrence of adverse effects, or rates of completion of the withdrawal episode.
Types of participants
We included studies that involved participants who were primarily opioid dependent and underwent managed withdrawal.
Types of interventions
Experimental interventions involved the administration of an alpha2‐adrenergic agonist (clonidine, lofexidine, guanfacine, or tizanidine) as the principal medication to ameliorate the signs and symptoms of opioid withdrawal.
Comparison interventions involved the use of reducing doses of methadone, symptomatic medications, placebo, or an alpha2‐adrenergic agonist regimen different to the experimental intervention. For the purpose of this review, symptomatic medications are defined as benzodiazepines, antiemetics, antidiarrhoeals, antipsychotics, antispasmodics, muscle relaxants, or non‐opioid analgesics, administered in combination as needed or according to a defined regimen.
We excluded studies of interventions using alpha2‐adrenergic agonists in conjunction with opioid antagonists. The use of opioid antagonists to induce withdrawal with minimal sedation or under heavy sedation or anaesthesia is covered in separate Cochrane reviews (Gowing 2009a; Gowing 2010). We also excluded studies comparing interventions using alpha2‐adrenergic agonists with buprenorphine regimens. The use of buprenorphine to manage opioid withdrawal is covered by a separate Cochrane review (Gowing 2009b).
Types of outcome measures
Primary outcomes
We assessed the included studies on the basis of a number of measures:
withdrawal syndrome (intensity, time course, predominant signs and symptoms);
duration of treatment;
nature and incidence of adverse effects; and
completion of treatment.
Interventions aimed at the management of acute opioid withdrawal are typically of short duration. As a result, structured psychological therapies are generally not provided as adjuncts to interventions for managing withdrawal, but the episode of withdrawal management does provide the opportunity to inform people who are opioid dependent about the options for further treatment, and to encourage them to engage in treatment appropriate to their needs. The longer the duration of treatment, the more opportunities there are for interaction between treatment services and people who are opioid dependent. The relative time in treatment is also an indicator of the relative acceptability to participants of the interventions being compared. For these reasons, we considered duration of treatment in addition to rates of completion of treatment.
It is difficult to differentiate side effects of treatment from the signs and symptoms of opioid withdrawal. We have defined adverse effects as clinically significant signs and symptoms of opioid withdrawal (such as vomiting and diarrhoea) plus any incidents that are not typical components of the opioid withdrawal syndrome. Early experience with clonidine, which was developed as a hypotensive agent, was that low blood pressure is a common adverse effect of clonidine treatment. This review therefore considered the occurrence of hypotension or symptoms of hypotension, withholding doses of medication, and cessation of treatment because of adverse effects.
Secondary outcomes
We also sought to assess data on the number of participants engaged in further treatment following completion of the withdrawal intervention. As indicated in the Background, managed withdrawal by itself is not an effective treatment for dependence. Hence, we considered engagement in further treatment to be an outcome of interest. However, very few studies reported on this outcome.
Search methods for identification of studies
All searches included non‐English language literature. We assessed studies with English abstracts on the basis of the abstract. If we thought the study was likely to meet the inclusion criteria, we translated it sufficiently to extract study methods and results.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 11, 2015), MEDLINE (1946 to November week 2, 2015), EMBASE (January 1985 to November week 2, 2015), PsycINFO (1806 to November week 2, 2015), and Web of Science (as of 23 November 2015).
We developed a search strategy to retrieve references for all the Cochrane reviews relating to the management of opioid withdrawal in one operation. We adapted this strategy to each of the major databases and the supporting platform. See Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
Searching other resources
We also searched:
the reference lists of all relevant papers to identify further studies;
some of the main electronic sources of ongoing trials: National Research Register; Current Controlled Trials (www.controlled‐trials.com); ClinicalTrials.gov (clinicaltrials.gov/); Osservatorio Nazionale sulla Sperimentazione Clinica dei Medicinali (oss‐sper‐clin.agenziafarmaco.it); and Trialsjournal.com (www.trialsjournal.com/);
conference proceedings likely to contain trials relevant to the review.
We contacted investigators to seek information about unpublished or incomplete trials.
Data collection and analysis
Selection of studies
One review author (LG) assessed each potentially relevant study for inclusion according to the identified inclusion and exclusion criteria, using a form developed by the review authors. All four review authors confirmed the inclusion and exclusion decisions.
Data extraction and management
We developed a form for recording data on the outcomes of interest, taking into account the different ways that such data might be reported by studies. One review author (LG) extracted key information using this form, in consultation with the other review authors where there was any uncertainty. We summarised key findings of studies descriptively in the first instance and considered the capacity for quantitative meta‐analysis.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies according to the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This was based on the evaluation of seven specific methodological domains (namely, sequence generation, allocation concealment, blinding of participants and providers, blinding of outcome assessor, incomplete outcome data, selective outcome reporting, and other issues). For each study, we analysed the six domains, described them as reported in the study, and provided a final judgement on the likelihood of bias in terms of low, high, or unclear risk of bias. We based these judgements on the criteria indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and their applicability to the addiction field.
We considered blinding separately for subjective and objective outcomes. Lack of blinding is a source of serious risk of bias for subjective outcomes, but is less significant with objective outcomes, such as completion of treatment and duration of treatment. We only considered incomplete outcome data for intensity of withdrawal and nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently used primary outcome measures in addiction research. See Appendix 6 for detailed description.
Details of the assessments of risk of bias are included in the Characteristics of included studies.
Measures of treatment effect
For dichotomous data (for example number completing treatment), we calculated risk ratios, and for continuous data (for example withdrawal scores), we calculated standardised mean differences with the uncertainty in each result expressed with 95% confidence intervals.
Unit of analysis issues
Where there were trials with multiple arms relevant to meta‐analyses, we combined data from treatment arms involving different alpha2‐adrenergic agonists after checking that the outcomes for the groups to be combined were similar.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test and its P value, by visual inspection of the forest plots and the I2 statistic. A P value of the test lower than 0.10 or an I2 statistic of at least 50% indicated a significant statistical heterogeneity.
Data synthesis
We used Review Manager 5 for statistical analyses (RevMan 2014). In all analyses, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
This review also aimed to consider the following potential sources of heterogeneity through subgroup analyses, as this approach is considered to be associated with less risk of bias:
drug of dependence and severity of dependence (as indicated by duration and level of use);
polydrug use;
concurrent physical and psychiatric illness;
precipitants to the withdrawal episode;
the nature of the treatment setting; and
the nature of adjunct treatment, including other medications to manage symptoms.
The nature of the studies that met the inclusion criteria limited such analyses. Subgroup analysis was possible only for completion of withdrawal for adrenergic2‐agonists compared with methadone, looking at the effect of the type of opioid being used prior to withdrawal (heroin or methadone) and the setting in which withdrawal treatment occurred.
Sensitivity analysis
We did not use risk of bias as a criterion for inclusion in the review. However, we assessed the impact of risk of bias through sensitivity analysis. This involved considering the overall estimate of effect with studies with a high risk of bias, in at least one of the domains assessed, included or excluded. We undertook sensitivity analyses where there were at least three studies providing data on the outcome, and where at least two of these studies were assessed as having low or unclear risk of bias. The domains for which sensitivity analyses were undertaken were reporting bias (Analysis 1.2; Analysis 1.3), selection bias and attrition bias (Analysis 2.2; Analysis 2.5; Analysis 2.6), and performance and detection bias in subjective outcomes (Analysis 2.2; Analysis 2.5).
1.2. Analysis.
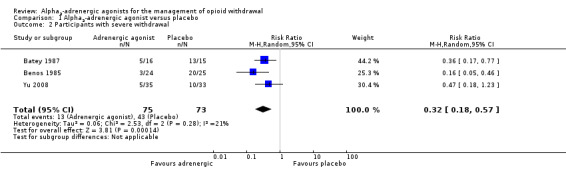
Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 2 Participants with severe withdrawal.
1.3. Analysis.
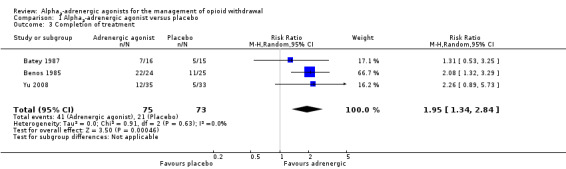
Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 3 Completion of treatment.
2.2. Analysis.
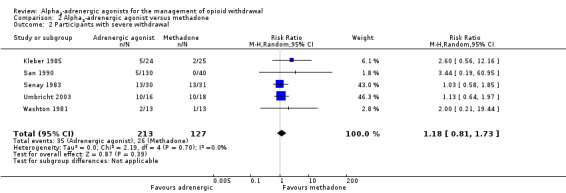
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 2 Participants with severe withdrawal.
2.5. Analysis.
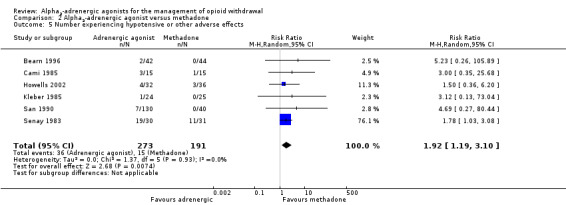
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 5 Number experiencing hypotensive or other adverse effects.
2.6. Analysis.
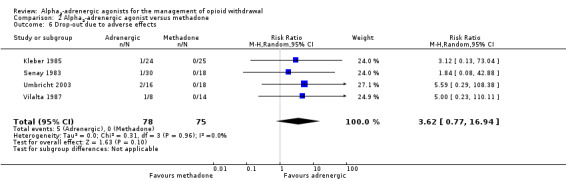
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 6 Drop‐out due to adverse effects.
Results
Description of studies
Results of the search
This is an update of a Cochrane review that was first published in 2001. The review was substantially updated in 2014 to incorporate a flow diagram of the search process, and with a modified search strategy. In the 2014 edition of this review, through the modified search strategy, we identified 2472 records after removing duplicates; we excluded 1969 records on first screening, and screened 503 records in more detail. Of these, 229 were excluded and the full text for 274 was obtained for more detailed assessment; 198 were excluded without listing, 42 records (32 studies) were excluded with reasons, and 34 records (25 studies) satisfied all the criteria to be included in the review.
In the present update, we again modified the search strategy, adding Web of Science as one of the databases searched. We also removed the term 'Metabolic Detoxication, Drug' from the MEDLINE and CENTRAL searches, as this now maps to 'metabolic inactivation', which is not relevant to this review. In this update, we identified 3377 records after removing duplicates (see Figure 1), of which 2764 were excluded on first screening and 613 were screened in more detail, with a further 282 being excluded on the basis of title and abstract. We assessed 331 full‐text articles, of which 252 were excluded without listing, 43 articles (34 studies) were excluded with reasons, and 36 articles (26 studies) satisfied all the criteria to be included in the review.
1.
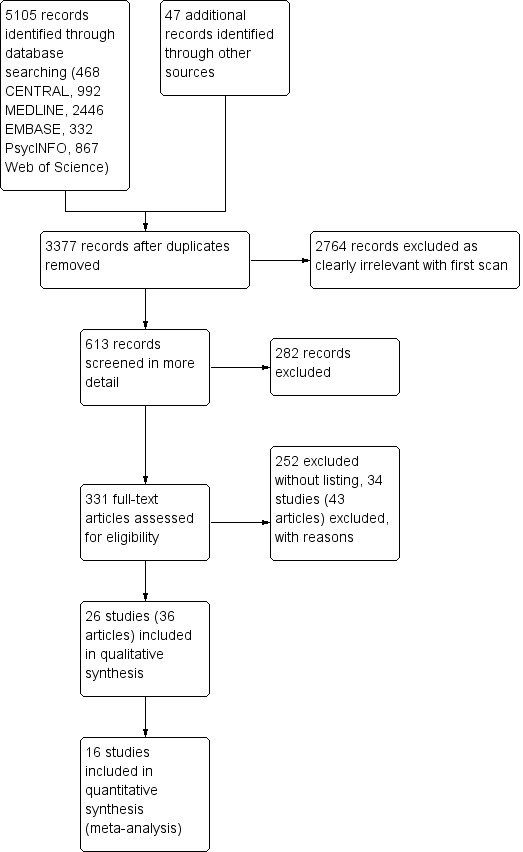
Study flow diagram.
Two studies were derived from a multicentre randomised controlled trial funded in part by Boehringer Ingelheim Pty Ltd (Kleber 1985; Senay 1983). A third study may also include participants from that trial, but we have been unable to confirm this (Washton 1981).
Included studies
Twenty‐six randomised controlled trials (35 reports) involving 1728 participants met the inclusion criteria for this review (see Characteristics of included studies). In total, 1012 participants were treated with an alpha2‐adrenergic agonist. Of these, 607 were treated with clonidine, 215 were treated with lofexidine, 174 were treated with guanfacine, and 16 were treated with tizanidine (a skeletal muscle relaxant with alpha2‐adrenergic agonist properties).
Six studies received some financial support from a pharmaceutical company. Six studies reported funding from sources other than industry, and in 14 studies, funding arrangements were unclear.
Major comparisons
The 26 studies that met the inclusion criteria involved five different comparisons:
an adrenergic agonist (clonidine, lofexidine, or guanfacine) versus reducing doses of methadone (12 studies);
an adrenergic agonist (clonidine or lofexidine) versus placebo (6 studies);
an adrenergic agonist (clonidine, lofexidine, or tizanidine) versus symptomatic medications (4 studies);
clonidine versus lofexidine (3 studies); and
clonidine versus guanfacine (2 studies).
One study compared clonidine and guanfacine with methadone (San 1990). In this review, we combined the clonidine and guanfacine groups for comparison with methadone to avoid double‐counting of participants.
In two studies that compared an adrenergic agonist with symptomatic medications, details of the symptomatic medications used were not reported (Li 2002; Sos 2000). In Bertschy 1997, the symptomatic medication regimen was based on carbamazepine plus mianserin, and in Gupta 1988, it was based on chlordiazepoxide plus chlorpromazine.
Five studies included comparison regimens that were not defined by the inclusion criteria for this review. Gerra 1995 and Gerra 2000 included groups treated with regimens based on opioid antagonists; this approach is covered by a separate Cochrane review (Gowing 2009a). Umbricht 2003 included a group treated with buprenorphine, which is also the subject of a separate Cochrane review (Gowing 2009b). Li 2002 included a group treated with Qigong, a traditional Chinese health practice. Nazari 2013 included a group treated with Hab‐o Shefa, a traditional Iranian medicine comprised of a combination of plant extracts. We excluded these groups from this review.
This review focuses on alpha2‐adrenergic agonists compared with placebo (6 studies), reducing doses of methadone (12 studies), and lofexidine compared with clonidine (3 studies), as these were the comparisons with sufficient suitable studies. We described the results of studies addressing the other comparisons narratively.
Treatment setting
In 16 of the 26 studies that met the inclusion criteria, treatment was provided on an inpatient basis. In one study, treatment occurred in the health centre of a prison (Howells 2002); in another study, participants were residents in a mandatory drug treatment centre (Li 2002); seven studies had an outpatient setting. In Gerra 1995, the duration of each treatment session effectively resulted in participants receiving day‐care during withdrawal. A similar approach was used for the clonidine group in Gerra 2000, but it is unclear whether the comparison methadone group received a similar amount of clinic time. In Carnwath 1998, withdrawal was home based with participants scheduled to receive four visits in the first week and three in the second (with the number of additional visits requested being one of the outcome indicators for the study).
Participant characteristics
In 19 of the 26 studies that met the inclusion criteria, all participants were withdrawing from heroin or other short‐acting opioids, while participants in five studies were either withdrawing from methadone, or were stabilised on methadone prior to, or as the first stage of, the withdrawal treatment. In Howells 2002, participants were withdrawing from either heroin or methadone, and in Nazari 2013 the majority of participants reported using opium.
Participants in three studies were notable for low levels of injecting use: all participants in Gupta 1988 used via the inhalation route; in Jiang 1993, 67% of participants treated with clonidine only used orally, 2% only used intravenously, while the remainder used both orally and intravenously; and in Nazari 2013, injecting use was reported by 20% of participants.
For participants withdrawing from methadone, doses of methadone at the commencement of treatment with an alpha2‐adrenergic agonist were 40 mg per day or less for four of the studies, and were not reported for one study (Kahn 1997). Only one study specified that all participants were withdrawing following a period of methadone maintenance treatment, with all participants stable on a dose of 20 mg per day at the commencement of withdrawal (Kleber 1985).
Opioid use was more common among men than women. Consistent with this, between two‐thirds and three‐quarters of participants in most of the 25 studies that met the inclusion criteria were men, while in five studies, all participants were men (Gupta 1988; Howells 2002; Li 2002; Nazari 2013; Senay 1983).
Twenty of the 26 studies reported the average age of participants. In 19 studies the average age was between 23 and 32 years. The average age of participants in Umbricht 2003 was 39.7 years, reflecting the recruitment of people admitted to hospital for the treatment of an acute acquired immunodeficiency syndrome (AIDS)‐related condition. None of the studies involved adolescents.
Countries
The countries were diverse: the USA (five studies); Spain (five studies); the UK (four studies); Italy (three studies); China (two studies); and one study in each of Australia, India, Switzerland, Taiwan, Germany, Hungary, and Iran. The cultural context of opioid use in India, China, and Taiwan could be a source of variability for those studies (Gupta 1988; Jiang 1993; Li 2002; Lin 1997). In Jiang 1993, only some of the participants entered treatment voluntarily; the majority were admitted for withdrawal under programmes of "mobilised rehabilitation". All participants in Li 2002 were in mandatory treatment.
Treatment regimens
In 15 of the 26 included studies, the scheduled duration of treatment reported was one to two weeks. In four studies (Batey 1987; Bruno 1979; Gerra 2000; Umbricht 2003), the period reported was less than one week. In five studies (Kleber 1985; Nazari 2013; San 1994; Senay 1983; Washton 1981), the scheduled duration was greater than two weeks. In Gerra 1995 and Gupta 1988, the scheduled duration was unclear.
Most studies did not specify the route of administration of the alpha2‐adrenergic agonists, but oral administration is most likely, except in Gerra 1995 and Gerra 2000. These two studies included antagonist‐induced withdrawal as comparison modalities. Participants allocated to treatment with alpha2‐adrenergic agonists or opioid antagonist regimens received four hours of intravenous therapy in the morning and three hours in the afternoon, with participants in the clonidine‐only groups receiving clonidine 0.15 mg in saline three or six times a day. Clonidine has high oral bioavailability, and hence these intravenous doses are approximately equivalent to oral doses. It is unclear whether this approach was common practice in the hospital concerned, or if it was specifically selected to suit the antagonist‐based treatment regimens in the studies.
For the studies that used clonidine orally, the maximum dose ranged from 0.9 mg per day to 1.35 mg per day. In most studies, clonidine was administered as three or four divided doses.
For the studies that used lofexidine, maximum doses ranged from 1.6 mg per day to 2.0 mg per day. In most studies, lofexidine was administered as two or three divided doses, but Lin 1997 administered four doses per day.
In summary, alpha2‐adrenergic agonists are typically administered orally as two to four doses per day, with the total dose adjusted daily according to withdrawal symptoms and side effects (particularly blood pressure). Clonidine is generally commenced at 0.1 mg per dose to 0.2 mg per dose, increasing to a maximum of around 1.0 mg per day, and lofexidine at 0.4 mg per dose to 0.6 mg per dose, increasing to a maximum of around 2 mg per day. Maximal doses are generally administered for only a few days around the time of maximal withdrawal, usually two to four days after cessation of opioids. Doses are then tapered, and ceased seven to 10 days after cessation of opioids. (There was insufficient information to determine typical doses of guanfacine or tizanidine, which were the only other alpha2‐adrenergic agonists used in the included studies.)
Excluded studies
We excluded 34 studies (43 reports) that we considered potentially relevant to the review and assessed in detail for the review (see Figure 1 and Characteristics of excluded studies). The reasons for exclusion were comparison of interventions other than those defined by the inclusion criteria (14 studies); methodology other than randomised controlled trials (15 studies); use of alpha2‐adrenergic agonists in combination with other medications and not as the primary treatment approach (5 studies); insufficient data reported on the outcomes of interest (5 studies); and the management of withdrawal was not the primary focus (2 studies). We excluded some studies for more than one reason.
Risk of bias in included studies
For summary results of the judged risk of bias across the included studies for each domain, see Figure 2 and Figure 3. The following sections summarise our judgements of the risk of bias for the included studies, grouped according to the interventions being compared.
2.
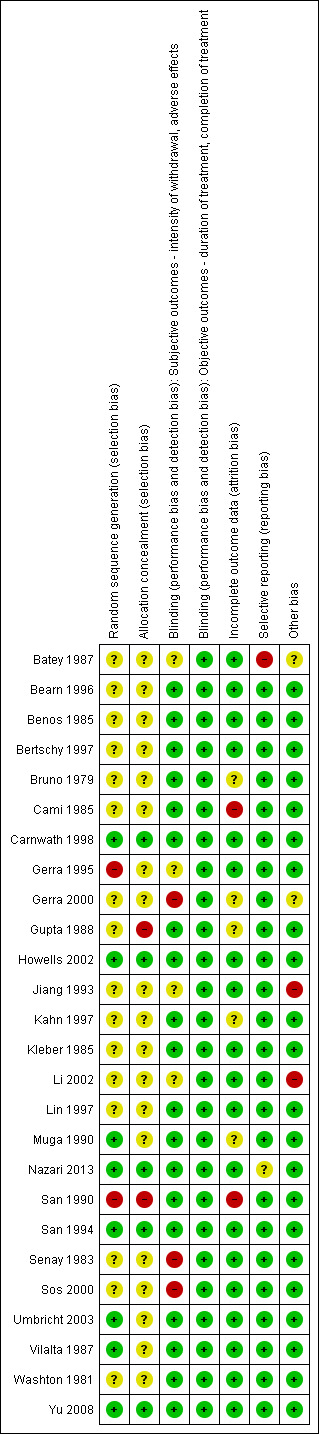
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
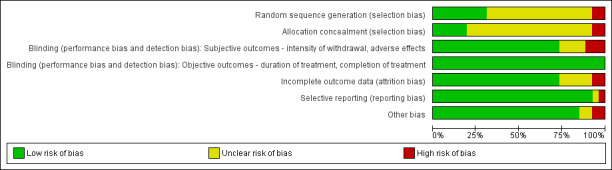
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Adrenergic agonist compared with placebo: we judged two studies to have a low risk of bias (Nazari 2013; Yu 2008), and one study, Gerra 1995, as being at high risk of selection bias due to sequence generation, and uncertain risk due to allocation concealment. For the other three studies, we judged the risk of selection bias as unclear.
Adrenergic agonist compared with methadone: we judged two studies to have a low risk of bias (Howells 2002; San 1994), and one study, San 1990, to have a high risk of bias due to both sequence generation and allocation concealment. For the other nine studies, the risk of bias was unclear for one or both dimensions of selection bias.
Adrenergic agonist compared with symptomatic medication: we judged one study to have a high risk of bias due to allocation concealment (Gupta 1988). For the other three studies, we judged the risk of selection bias as unclear.
Clonidine compared with lofexidine: we judged one study, Carnwath 1998, to have a low risk of bias. For the other two studies, the risk of selection bias was unclear.
Clonidine compared with guanfacine: we judged one study, San 1990, to have a high risk of bias, and the other study, Muga 1990, to have an unclear risk of bias due to allocation concealment.
Blinding
We considered the risk of performance and detection bias for objective outcomes (duration and completion of treatment) to be low for all studies, as these outcomes are unlikely to be affected by an awareness of group allocation. This section therefore focuses on the risk of assessment bias in relation to subjective outcomes (intensity of withdrawal, occurrence and severity of adverse effects).
Adrenergic agonist compared with placebo: we considered the risk of bias to be unclear for Batey 1987 and Gerra 1995, and low for the other four studies.
Adrenergic agonist compared with methadone: we judged two studies to have a high risk of assessment bias (Gerra 2000; Senay 1983), one study to have an unclear risk (Jiang 1993), and the other nine studies to have a low risk.
Adrenergic agonist compared with symptomatic medication: we judged one study to have a high risk of assessment bias (Sos 2000), one study to have an unclear risk (Li 2002), and the other two studies to have a low risk.
Clonidine compared with lofexidine: we judged all three studies to have a low risk of assessment bias.
Clonidine compared with guanfacine: we judged both studies to have a low risk of assessment bias.
Incomplete outcome data
We considered this domain only for the outcomes of intensity of withdrawal and adverse effects. Retention (duration of treatment) and completion of treatment are primary outcome measures for opioid withdrawal interventions.
Adrenergic agonist compared with placebo: we assessed the risk of bias as unclear for one study (Bruno 1979), and low for the other five studies.
Adrenergic agonist compared with methadone: we assessed two studies as having a high risk of outcome bias (Cami 1985;San 1990), in one study the risk was unclear (Gerra 2000), and the risk was low in the other nine studies.
Adrenergic agonist compared with symptomatic medication: in one study the risk was unclear (Gupta 1988), and in the other three studies the risk was low.
Clonidine compared with lofexidine: in one study the risk was unclear (Kahn 1997), and in the other two studies the risk of attrition bias was low.
Clonidine compared with guanfacine: we assessed one study as having a high risk of attrition bias (San 1990); in the other study the risk was unclear.
Selective reporting
Adrenergic agonist compared with placebo: we considered one study to be at high risk of reporting bias (Batey 1987), one study to be at unclear risk (Nazari 2013), and the other four studies to be at low risk.
Adrenergic agonist compared with methadone: we assessed all 12 studies as having a low risk of reporting bias.
Adrenergic agonist compared with symptomatic medication: we assessed all four studies as having a low risk of reporting bias.
Clonidine compared with lofexidine: we considered all three studies to be at low risk of bias.
Clonidine compared with guanfacine: we considered both studies to be at low risk of bias.
Other potential sources of bias
Adrenergic agonist compared with placebo: Batey 1987 refer to difficulties in recruiting participants for the trial. Willingness to participate in a trial could potentially result in selection bias, and impact on external validity of findings. However, 80% of people presenting for treatment during the study period were recruited, suggesting the perceived recruitment difficulties were unlikely to have a significant impact on findings. We assessed the risk of bias as unclear for this study; we considered the risk for the other five studies to be low.
Adrenergic agonist compared with methadone: Jiang 1993 was undertaken in a setting where at least some treatment episodes were mandatory. We did not use data on treatment duration and completion rates for this study, as they are confounded by the possibility of compulsion to complete withdrawal. In Gerra 2000, it was unclear whether all treatment arms received the same amount of clinic care; it is possible that the methadone group had less contact time. We considered the risk of other sources of bias for the other 10 studies to be low.
Adrenergic agonist compared with symptomatic medication: Li 2002 was undertaken in the context of mandatory treatment; we considered the other three studies to have low risk of other bias.
Clonidine compared with lofexidine: we considered all three studies to be at low risk of bias.
Clonidine compared with guanfacine: we considered both studies to be at low risk of bias.
Effects of interventions
We have presented the results in four sections according to the nature of comparison:
alpha2‐adrenergic agonists compared with placebo;
alpha2‐adrenergic agonists compared with reducing doses of methadone;
clonidine compared with other alpha2‐adrenergic agonists;
other comparisons.
Each of these sections is subdivided into parts addressing the types of outcome measures identified as being of interest: (a) withdrawal syndrome, (b) duration of treatment, (c) nature and incidence of adverse effects, and (d) completion of treatment.
1. Alpha2‐adrenergic agonists compared with placebo
(a) Withdrawal syndrome
Two studies involving 113 participants reported a peak withdrawal score (Analysis 1.1). Both studies indicate less severe withdrawal with adrenergic agonists compared with placebo, but due to the extreme degree of heterogeneity between the studies (I2 = 98%), an estimate of combined effect was not calculated. Some of the heterogeneity may have been due to the method of assessing withdrawal severity. In Yu 2008, withdrawal was assessed as significantly more severe in the placebo group when measured by the modified Himmelsbach and Objective Opioid Withdrawal Scales, but not the Subjective Opioid Withdrawal Scale. (The data in Analysis 1.1 is based on the modified Himmelsbach scores.)
1.1. Analysis.
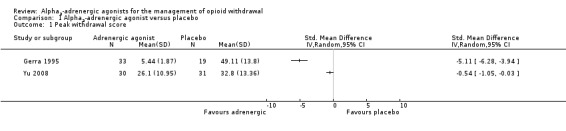
Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 1 Peak withdrawal score.
Three studies involving 148 participants reported the number of participants with severe withdrawal (Analysis 1.2), with this outcome significantly less likely with adrenergic agonist treatment compared with placebo (risk ratio (RR) 0.32, 95% confidence interval (CI) 0.18 to 0.57; 3 studies; 148 participants). The difference remained significant when the study with a high risk of bias, Batey 1987, was excluded (RR 0.28, 95% CI 0.09 to 0.83; 117 participants).
Benos 1985 reported that withdrawal scores were significantly lower in participants treated with clonidine compared with participants given placebo, from the time of the second dose on the first day of treatment. The significant difference was seen with both observer and participant ratings of withdrawal. On exit from treatment, 87.5% of participants in the clonidine group, compared with 20.8% in the placebo group, rated the efficacy of treatment as "good". In Yu 2008, 15 of 33 (45%) in the placebo group, compared with 6 of 35 (17%) in the lofexidine group, discontinued treatment because the study medication was "not working", suggesting less effective control of withdrawal in the placebo group.
(b) Duration of treatment
None of the studies reported the average time in treatment, but Gerra 1995 and Yu 2008 both reported that more participants receiving placebo dropped out early in treatment. In Gerra 1995, 2 of 33 (6%) participants treated with clonidine, compared with 5 of 19 (26%) participants receiving placebo, dropped out of treatment in the first week. In Yu 2008, there were significantly fewer early terminations in the lofexidine group: on day six (the third day of study treatment), 20 of 34 (59%) participants treated with lofexidine remained in treatment, compared with 8 of 33 (24%) participants receiving placebo.
(c) Adverse effects
Benos 1985 reported sedation and dry mouth to be approximately twice as common in participants treated with clonidine, compared with participants who received placebo. However, these adverse effects were rated as causing difficulty in only a small number of cases and were not considered a problem.
In Batey 1987, 7 of 16 participants treated with clonidine, compared with none of 15 participants receiving placebo, experienced drowsiness and dizziness. Participants in both groups frequently complained of dry mouth. None withdrew from treatment as a result of adverse effects.
In Yu 2008, blood pressure was significantly decreased in the lofexidine group on days four to seven of treatment. Asthenia, dizziness, hypotension (18% versus 0%) and insomnia (42% versus 9%) all occurred more frequently in the lofexidine group. Four of 35 participants in the lofexidine group, compared with none of 33 participants in the placebo group, terminated treatment due to adverse effects.
Nazari 2013 reported the average overall side effects score, with no significant differences between groups.
The other studies did not report any information about adverse effects.
(d) Completion of treatment
Based on three studies, completion of withdrawal treatment (Analysis 1.3) was significantly more likely with an adrenergic agonist (clonidine or lofexidine) compared with placebo (RR 1.95, 95% CI 1.34 to 2.84; 3 studies; 148 participants). The difference remained significant when the study with a high risk of bias, Batey 1987, was excluded (RR 2.12, 95% CI 1.40 to 3.19; 117 participants).
2. Alpha2‐adrenergic agonists compared with reducing doses of methadone
(a) Withdrawal syndrome
Intensity of withdrawal
Two studies reported data on peak withdrawal severity (Analysis 2.1). These data suggest somewhat lower severity with methadone (standardised mean difference (SMD) 0.22, 95% CI ‐0.02 to 0.46; 2 studies; 263 participants), but the difference was not statistically significant (P = 0.07).
2.1. Analysis.
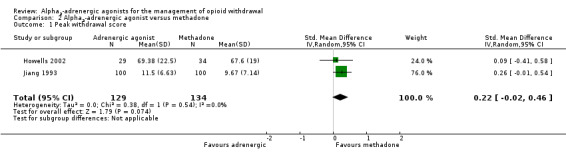
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 1 Peak withdrawal score.
Five studies reported the rate of occurrence of severe withdrawal (Analysis 2.2), which was defined by participants giving "intolerable withdrawal" as the reason for leaving treatment, or a score greater than five on the Objective Opiate Withdrawal Scale (Umbricht 2003). Severe withdrawal was somewhat more likely to occur in participants treated with an alpha2‐adrenergic agonist (RR 1.18, 95% CI 0.81 to 1.73; 5 studies; 340 participants), but the difference was not statistically significant (P = 0.39). Note that San 1990 compared clonidine, methadone, and guanfacine. In this analysis, we combined the clonidine and guanfacine groups to enable entry of both groups without double‐counting of the participants in the methadone group. Excluding the two studies at high risk of bias, San 1990 and Senay 1983, from this analysis made little difference to the result (RR 1.27, 95% CI 0.76 to 2.13; 109 participants).
Three studies reported data on overall withdrawal severity (Analysis 2.3). These data indicate no significant difference between alpha2‐adrenergic agonists and tapered methadone (SMD 0.13, 95% CI ‐0.24 to 0.49; 3 studies; 119 participants).
2.3. Analysis.
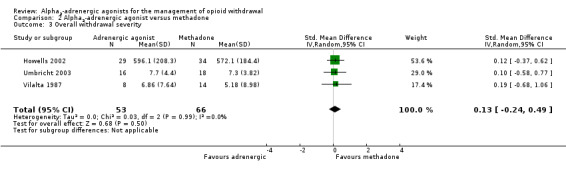
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 3 Overall withdrawal severity.
In addition to the quantitative data, Bearn 1996 reported overall withdrawal to be more severe on days three to seven and day 10 for the group receiving lofexidine, with both groups experiencing a similar gradual decline in symptoms over the next 14 days, and in Gerra 2000 withdrawal was assessed as more severe with methadone.
Taken together, these data suggest that peak withdrawal severity may be greater with alpha2‐adrenergic agonists, but there is no significant difference in severity when considered over the entire duration of the withdrawal episode.
Time course of withdrawal
Eight studies provided information on the time course of development and resolution of withdrawal signs and symptoms. This information was provided in terms of the time of drop‐out for participants giving unacceptable withdrawal as the reason, graphs of daily withdrawal scores and time of maximal withdrawal. In all studies, the signs and symptoms of withdrawal appeared earlier in withdrawal managed with an alpha2‐adrenergic agonist and resolved more quickly. In general, when withdrawal is managed with tapered methadone, peak withdrawal occurs at the end of the taper (Bearn 1996; Gerra 2000; Jiang 1993; Kleber 1985; Washton 1981).
Predominant signs and symptoms
Kleber 1985 reported higher levels of appetite loss, low energy, muscle pains, drowsiness, yawning, dry mouth, and sneezing among participants treated with clonidine compared with reducing doses of methadone. However, Washton 1981, using the same treatment regimen, reported that major symptomatic complaints (specifically lethargy, restlessness, and insomnia) were identical for both groups, and subjective ratings were indistinguishable. Cami 1985 reported sleep disturbances and "weeping" were more common in participants treated with clonidine, while muscular aching, flatulence, and drowsiness were more common in participants treated with methadone (tapered from 30 mg per day to 45 mg per day over 10 days). San 1990 reported sleeplessness, restlessness, muscular pain, and insomnia as the most frequent signs and symptoms in participants treated with methadone. In comparison, the most frequent signs and symptoms in the clonidine and guanfacine groups were mydriasis and sleeplessness, followed by chills, muscular pain, and insomnia in the clonidine group, or muscular pain and restlessness in the guanfacine group. Howells 2002 reported scores for feeling cold and aches/pains were higher for participants treated with lofexidine, whereas scores for drowsiness were higher for participants treated with methadone. However, the main items contributing to scores in both groups were sleep problems, anxiety/nervousness, irritability, lack of energy, aches/pains, and feeling cold. Gerra 2000 reported that observers noted only insomnia and slight anxiety in the group treated with clonidine, whereas they noted participants treated with methadone as experiencing anxiety, tachycardia, insomnia, rhinorrhoea, mydriasis, aching muscles, and irritability.
(b) Duration of treatment
The mean days in treatment was reported or was able to be calculated for three studies (Analysis 2.4). The mean duration of treatment was significantly longer for the group treated with reducing doses of methadone (SMD ‐1.07, 95% CI ‐1.31 to ‐0.83; 3 studies; 310 participants). The difference remained significant when we excluded the study with a high risk of bias, Jiang 1993 (SMD ‐1.04, 95% CI ‐1.44 to ‐0.64; 110 participants).
2.4. Analysis.
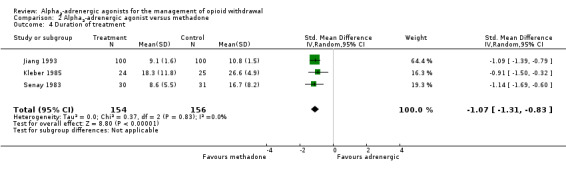
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 4 Duration of treatment.
Participants in Bearn 1996 who were treated with reducing doses of methadone remained in treatment for a mean 39.8 days, compared with 32 days for participants treated with lofexidine (P = 0.074). The time in treatment for this study extended beyond the period of acute withdrawal management, reducing the comparability with other studies, and since standard deviations were not reported, we were unable to include this study in the analysis.
In San 1990, there was no difference in overall mean duration of treatment, but a difference became apparent for participants identified as treatment "failures" (in treatment for less than 12 days). While the mean duration was similar for "successes" in each group (14.7 days for clonidine, 14.7 days for guanfacine, and 14.2 days for methadone), the mean duration for "failures" was greater in the group treated with reducing doses of methadone (5.7 days for clonidine, 4.9 days for guanfacine, and 7.3 days for methadone).
The other seven studies included in this comparison did not report data on average time in treatment.
(c) Nature and incidence of adverse effects
Six studies reported data on the incidence of hypotensive or other adverse effects (Analysis 2.5). Data for the clonidine and guanfacine groups in San 1990 were combined prior to entry. Overall, significantly more participants treated with an adrenergic agonist experienced adverse effects, compared with participants treated with reducing doses of methadone (RR 1.92, 95% CI 1.19 to 3.10; 6 studies; 464 participants). The strength of this finding is reduced by the low incidence of adverse effects and by the high risk of performance or attrition bias in three of the studies (Cami 1985; San 1990; Senay 1983). When we excluded these three studies from the analysis, the estimated effect size remained much the same, but the difference was no longer statistically significant (RR 2.02, 95% CI 0.62 to 6.64; 203 participants).
Jiang 1993 reported 89 out of 100 participants treated with clonidine experienced dizziness on standing, mostly in the second and third days of treatment when clonidine doses were at their highest (around 1 mg per day). Both systolic and diastolic blood pressure decreased in the clonidine group (by no more than 10 mmHg) during the first four days of treatment, whereas blood pressure was stable in the methadone group. San 1990 reported significantly lower mean blood pressure in participants treated with clonidine, compared with participants treated with methadone, on treatment days two, three, four, six, seven, and eight. Mean blood pressure in participants treated with guanfacine was also significantly lower compared with the methadone group on treatment days three, six, seven, and nine.
In a comparison between lofexidine and reducing doses of methadone, Bearn 1996 reported no significant difference in mean blood pressures. Howells 2002 also compared lofexidine with reducing doses of methadone; in this study, there was no appreciable change in either heart rate or blood pressure, and there was no significant difference in mean blood pressures for the two groups. San 1994 reported a greater decrease in blood pressure for the group treated with guanfacine 4 mg per day, compared with groups treated with guanfacine 3 mg per day or reducing doses of methadone. In addition, a bradycardic effect was seen in the guanfacine 4 mg per day group from day nine (the day on which guanfacine was substituted for methadone), whereas there was no difference in heart rate for the guanfacine 3 mg per day or methadone groups.
Four studies reported data on the number of participants who dropped out of treatment due to adverse effects (Analysis 2.6). While the risk of drop‐out due to adverse effects was somewhat higher for participants treated with an adrenergic agonist (clonidine for all four studies that reported data), the difference was not statistically significant (RR 3.62, 95% CI 0.77 to 16.94; 4 studies; 153 participants). The accuracy of this comparison is reduced by the small number of events and the absence of drop‐out due to adverse effects from the groups receiving methadone. Excluding the study at high risk of bias, Senay 1983, made little difference to the result (RR 4.48, 95% CI 0.76 to 26.34; 105 participants).
(d) Completion of treatment
We excluded data from three studies from the analysis of completion rates. In Howells 2002, completion was influenced by procedural issues related to the prison setting (participants did not return from court or were transferred to another prison) and not just medication. The majority of participants in Jiang 1993 entered treatment under programmes of "mobilised rehabilitation" (that is treatment was not voluntary). In this context, rates of completion of withdrawal are not meaningful. In Umbricht 2003, completion was influenced by the clinical status of the acute (AIDS‐related) condition that was the reason for hospital admission.
Four studies used naloxone challenge tests or urine screening to confirm the completion of withdrawal (Kleber 1985; Senay 1983; Vilalta 1987; Washton 1981). Three studies defined success as completion of the scheduled treatment programme (Bearn 1996; Cami 1985; San 1990). San 1994 reported the proportion of participants still in treatment on day 18 (when all treatment regimens were scheduled to have been completed), and Gerra 2000 reported the numbers of participants who accepted naltrexone treatment. These varying data have been taken as the number completing treatment (Analysis 2.7).
2.7. Analysis.
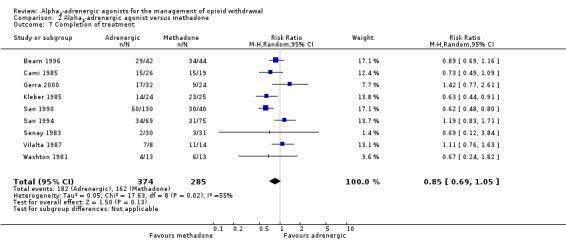
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 7 Completion of treatment.
Data for the two guanfacine groups in San 1994 and the clonidine and guanfacine groups in San 1990 were combined prior to entry. The analysis in Cami 1985 was based on 30 out of 45 participants entering the study who completed treatment, 15 with clonidine and 15 with methadone. Twelve participants dropped out of treatment, eight from the clonidine group and four from the methadone group, and three participants were stated to have completed treatment after transfer from clonidine to methadone. In line with principles of intention‐to‐treat analysis, we have included these three in the clonidine group, but did not count them as having completed withdrawal.
Overall, the combined result (Analysis 2.7) indicates no significant difference in rates of completion of withdrawal for alpha2‐adrenergic agonists compared with reducing doses of methadone (RR 0.85, 95% CI 0.69 to 1.05; 9 studies; 659 participants). We assessed one of the studies included in this analysis as having a high risk of selection bias (San 1990). When we excluded this study from the analysis, the combined result was similar (RR 0.91, 95% CI 0.75 to 1.11; 489 participants).
Effect of opioid used
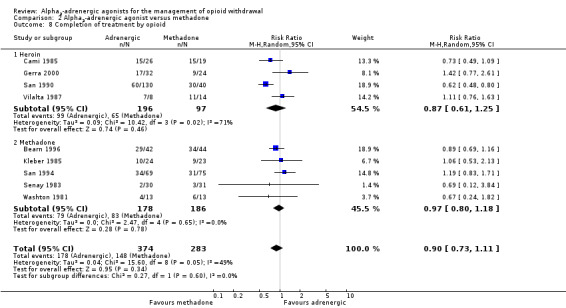
Participants in four of the nine studies that reported data on completion of withdrawal treatment were withdrawing from heroin, while participants in the other five studies were withdrawing from methadone (or were stabilised on methadone for a period prior to withdrawal). The relative rates of completion of withdrawal were similar for the two subgroups (Analysis 2.8): for participants withdrawing from heroin the RR was 0.87 (95% CI 0.61 to 1.25; 4 studies; 293 participants) and for participants withdrawing from methadone the RR was 0.97 (95% CI 0.80 to 1.18; 5 studies; 364 participants). The study assessed as having a high risk of bias relevant to this outcome involved participants withdrawing from heroin (San 1990). Excluding this study from the analysis made little difference to the estimated effect (RR 1.01, 95% CI 0.70 to 1.45; 123 participants).
2.8. Analysis.
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 8 Completion of treatment by opioid.
Effect of treatment setting
In five studies, withdrawal occurred in an inpatient setting, while the other four studies used an outpatient setting. The outcome was similar for the two subgroups (Analysis 2.9): for inpatient settings the RR was 0.87 (95% CI 0.68 to 1.12; 5 studies; 467 participants; I2 = 67%) and for outpatient settings the RR was 1.09 (95% CI 0.73 to 1.64; 4 studies; 190 participants; I2 = 0%). Excluding the study assessed as having a high risk of bias, San 1990, brought the group outcomes even closer together (inpatient: RR 0.96, 95% CI 0.79 to 1.18; 297 participants; I2 = 27%; outpatient: RR 1.09, 95% CI 0.73 to 1.64; 190 participants). This suggests that the effectiveness of alpha2‐adrenergic agonists relative to methadone is not influenced by the withdrawal setting, but the strength of this conclusion is reduced by significant heterogeneity in the inpatient data, and low precision of the data for the studies undertaken in outpatient settings.
2.9. Analysis.
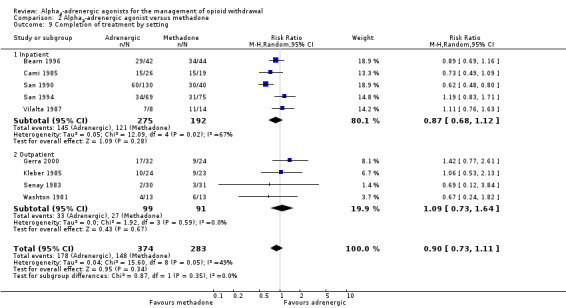
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 9 Completion of treatment by setting.
3. Clonidine compared to lofexidine or guanfacine
Three studies compared lofexidine with clonidine (Carnwath 1998; Kahn 1997; Lin 1997), and two studies compared guanfacine with clonidine (Muga 1990; San 1990). The data reported by these studies were both limited and diverse, preventing any quantitative analyses from being undertaken for these comparisons. This section summarises the findings narratively.
(a) Withdrawal syndrome
Carnwath 1998 reported no significant difference in any parameters for withdrawal managed with lofexidine compared with clonidine. A graph of mean daily symptom scores reported by Kahn 1997 for groups treated with clonidine or lofexidine showed an almost identical pattern of peak withdrawal on day seven (after cessation of methadone on day four), a plateau for three days, then a gradual reduction in the withdrawal score. No participants in either group experienced severe withdrawal, and no significant differences were detected. Lin 1997 also recorded almost identical patterns of withdrawal score for participants treated with lofexidine and participants treated with clonidine. In both groups, peak withdrawal occurred on the second day of treatment, and the score had largely subsided by the end of the fourth day.
Kahn 1997 reported almost identical use of lorazepam as adjunct medication by participants in the lofexidine and clonidine groups: 10 of 14 in each group used lorazepam on 72 (clonidine) and 71 (lofexidine) occasions.
Kahn 1997 stated the pattern of individual signs and symptoms of withdrawal to be similar with clonidine or lofexidine treatment, with bone pain and insomnia not responding to either drug. Lin 1997 also reported no difference between groups treated with clonidine or lofexidine in terms of predominant signs and symptoms of withdrawal. They reported six items were rated either moderate or severe during the period of peak withdrawal (days two and three) by more than 20% of participants. These items were irritability, agitation, back pain, muscular cramp, yawning, and lacrimation.
San 1990 reported no significant difference between clonidine and guanfacine in the time course of withdrawal, and found that mydriasis, sleeplessness, and muscular pain were common symptoms in both groups. Muga 1990 reported that scores for restlessness were significantly higher with clonidine, but there were no significant differences in any other items assessed.
(b) Duration of treatment
Only Lin 1997 reported data on time in treatment. They reported that participants treated with lofexidine remained on treatment for a median of five days (range one to nine days), while participants treated with clonidine remained on treatment for a median of four days (range one to eight days). This is in the context of treatment scheduled to last for around 10 days.
(d) Nature and incidence of adverse effects
Hypotension
Carnwath 1998 reported significantly more hypotension in the group treated with clonidine compared with participants treated with lofexidine between treatment days eight and 10. The incidence of hypotension in each group was not quantified.
Kahn 1997 reported significantly lower mean blood pressure for participants treated with clonidine compared with participants treated with lofexidine, from day five to day eight of treatment. Eight of 14 (57%) participants treated with lofexidine compared with 13 of 14 (93%) participants treated with clonidine experienced postural hypotension.
Lin 1997 reported omitting doses of medication due to low blood pressure on 25 of 615 (4.1%) possible occasions in 14 participants treated with lofexidine compared with 45 of 508 (8.9%) occasions in 16 participants treated with clonidine. Low blood pressure occurred on 19 of 188 (10.1%) patient‐days for the lofexidine group and 34 of 162 (21.0%) patient‐days for the clonidine group. The differences in the number of doses omitted and the number of patient‐days of low blood pressure were statistically significant. However, there was no clinically significant hypotension, with systolic and diastolic blood pressure being reduced by about 10 mmHg in both groups.
In San 1990, the mean blood pressure for the guanfacine group was largely between the levels for the clonidine and methadone groups, but was not significantly different from levels for the clonidine group.
Other adverse effects
In Carnwath 1998, lofexidine was compared with clonidine for the management of opioid withdrawal in a home setting. Significant differences were reported in three aspects relating to adverse effects: participants in the lofexidine group received a mean of 0.5 extra home visits compared with 1.3 for the clonidine group; the mean side effects score was 3.9 in the lofexidine group compared with 4.6 for the clonidine group; and the mean score for "feelings of control" was 4.2 for the lofexidine group compared with 3.7 for the clonidine group.
In Kahn 1997, there were 114 reports of adverse effects among participants treated with lofexidine compared with 226 reports among participants treated with clonidine. Drowsiness was experienced by 11 of 14 participants in the lofexidine group and 12 of 14 participants in the clonidine group. Two participants treated with lofexidine, compared with 12 participants treated with clonidine, felt "unwell" (anergy or weak/tired). Physicians assessed the adverse effects as interfering with functioning for four of the 14 participants treated with clonidine, but none of the participants treated with lofexidine.
(d) Completion of withdrawal
Of the three studies that compared lofexidine and clonidine, only Carnwath 1998 reported the number of participants who completed withdrawal. In this study, successful withdrawal was defined by an opiate‐free urine sample four weeks after the commencement of treatment. On this basis, 17 of 26 (65%) participants treated with lofexidine compared with 12 of 24 (50%) participants treated with clonidine successfully completed withdrawal.
4. Other comparisons
Due to the diverse nature of these comparisons, we undertook no meta‐analyses.
(a) Withdrawal syndrome
Bertschy 1997 compared clonidine with combination treatment using mianserin (antidepressant drug) and carbamazepine (antiepileptic drug). They reported no difference in withdrawal scores for the two groups, although "dry mouth" received lower ratings in the mianserin plus carbamazepine group and "runny nose" received lower ratings in the clonidine group. The groups were also similar in levels of use of adjunct medications. However, mean global satisfaction ratings (± standard deviations) by participants were 87.9 ± 7.6 for the clonidine group and 62.6 ± 21.2 for the mianserin‐carbamazepine group (assessed by visual analogue scale where 100 represented "my withdrawal could not have been better").
Gupta 1988 compared clonidine with chlordiazepoxide plus chlorpromazine combination treatment. They reported that rhinorrhoea, nausea, vomiting, diarrhoea, confusion, disorientation, and delirium occurred significantly less frequently in the clonidine group. It was also noted that insomnia occurred in most participants in both groups.
Li 2002 included a comparison of lofexidine and symptomatic medications. They reported that withdrawal scores were significantly lower for the lofexidine group compared with the group treated with symptomatic medications. Mean scores on day four of treatment were 9.9 for the lofexidine group and 44.4 for the symptomatic medications group.
Sos 2000 compared tizanidine, a skeletal muscle relaxant with alpha2‐adrenergic agonist properties, with symptomatic medications. Sweating, nervousness, tremor, diarrhoea, muscle pain, and craving were all reported as being significantly less in the group treated with tizanidine.
(b) Duration of treatment
None of the studies in this group reported data on the time in treatment.
(c) Nature and incidence of adverse effects
Bertschy 1997 reported significantly lower blood pressure in participants treated with clonidine compared with participants treated with mianserin plus carbamazepine combination. In the clonidine group, administration of medication was suppressed on a mean (± standard deviation) 1.3 ± 1.3 occasions, but not at all in the mianserin plus carbamazepine group. Gupta 1988 recorded severe hypotension (defined as systolic blood pressure less than 90 mmHg on two consecutive readings and clonidine withheld) in four of 60 participants treated with clonidine, but none of 60 participants treated with a chlordiazepoxide plus chlorpromazine combination. However, of the 60 participants treated with chlordiazepoxide plus chlorpromazine, 38 experienced extrapyramidal symptoms requiring benzhexol, and nine experienced severe dehydration requiring parenteral fluids. None of participants treated with clonidine experienced these adverse effects.
(d) Completion of withdrawal
Three studies reported comparative completion rates. Bertschy 1997 reported 10 of 16 (63%) participants treated with clonidine, and nine of 16 (56%) participants treated with a mianserin plus carbamazepine combination, completed treatment. Janiri 1994 reported 11 of 13 (85%) participants treated with clonidine, and 10 of 13 (77%) participants treated with lefetamine, completed the scheduled period of treatment. Sos 2000 reported that all participants completed withdrawal.
Discussion
We have reported the discussion in four sections according to the nature of comparison: (1) alpha2‐adrenergic agonists compared with placebo, (2) alpha2‐adrenergic agonists compared with reducing doses of methadone, (3) lofexidine and other alpha2‐adrenergic agonists compared with clonidine; and (4) other comparisons. Within each section, there is a brief summary of the findings from the Results, with comments and any additional relevant information from studies that did not meet the inclusion criteria for this review.
The final section of the discussion considers factors potentially influencing outcomes, specifically the nature of opioids used and the setting in which withdrawal occurs.
Summary of main results
1. Alpha2‐adrenergic agonists compared with placebo
Clonidine and lofexidine appear to be associated with more adverse effects than placebo when used to manage opioid withdrawal, but despite this, they are clearly more effective in ameliorating withdrawal and more acceptable to participants.
2. Alpha2‐adrenergic agonists compared with reducing doses of methadone
(a) Withdrawal syndrome experienced
The studies considering this comparison reported data on the severity of withdrawal in different ways. As a result, the quantitative analyses of withdrawal severity are not robust. However, the data point towards withdrawal associated with alpha2‐adrenergic agonist treatment being similar to, or somewhat more intense than, withdrawal associated with a regimen of reducing doses of methadone. In all studies, the signs and symptoms of withdrawal appeared earlier in withdrawal managed with alpha2‐adrenergic agonists and resolved more quickly. As a result, overall withdrawal severity was similar for the two approaches. The conclusion of a similar magnitude of withdrawal is supported by participant assessment of withdrawal tolerability and use of adjunct medication as well as comparative withdrawal score, suggesting that this is a reasonably robust conclusion.
There was considerable variability between studies in the signs and symptoms of withdrawal reported as being predominant during treatment. This prevents us from drawing any conclusions on relative effectiveness of alpha2‐adrenergic agonists and reducing doses of methadone in terms of the nature of residual signs and symptoms. However, it does appear that neither treatment suppresses fully the aches and pains, sleep disturbances, loss of energy, chills, or anxiety associated with opioid withdrawal. This indicates the need to use concomitant symptomatic medications to ameliorate residual symptoms of withdrawal.
(b) Duration of treatment
Interpretation of data on duration of treatment is limited by the small number of studies providing these data and is confounded by a number of factors. One factor is differences in the scheduled duration of administration of active medications being compared, as occurred in Senay 1983 and Kleber 1985. In these studies (components of a multicentre trial), participants allocated to methadone treatment had their methadone dose reduced from 20 mg per day to 0 mg per day over 20 days, then received placebo for 10 days before the outcome was assessed by a naloxone challenge on day 30. In comparison, participants in the clonidine groups received active medication for 10 days, then placebo for 20 days, with the aim of maintaining the double‐blind conditions. The substantial difference in time without administration of active medication is likely to bias results in favour of the methadone group. We have attempted to allow for this to some extent by comparing the proportion of participants retained in treatment to the end of the period of scheduled administration of active medication as well as the actual duration of treatment.
We conclude from the available data that when opioid withdrawal is managed with methadone, the duration of treatment is longer than when withdrawal is managed with clonidine.
Retention in treatment is a significant outcome with all forms of treatment for drug dependence. In methadone maintenance and residential drug‐free treatment, longer periods in treatment provide greater opportunity for delivery of psychosocial support, which might increase the likelihood of a successful outcome from either the current or future treatment episodes. Given the short‐term nature of detoxification and the physical stress of withdrawal, there is less opportunity during managed withdrawal for meaningful psychosocial support to be provided. Considering the high rates of relapse following detoxification, no matter how withdrawal is managed, engagement in follow‐up treatment is of greater clinical significance than the duration of the withdrawal episode.
(c) Adverse effects
Overall, management of withdrawal with clonidine is associated with more adverse effects than management by reducing doses of methadone. Adverse effects are most severe in the initial few days of peak withdrawal when maximal doses of clonidine are administered. Aside from hypotension, the adverse effects more commonly associated with clonidine than with reducing doses of methadone were drowsiness, fatigue, lethargy, and dry mouth. Clinically, hypotension is the most significant adverse effect. Dizziness, probably due to hypotension, occurred much more frequently in the studies than did cessation of treatment because of adverse effects. This suggests that hypotension can be adequately managed by withholding doses and reducing the dose of medication according to blood pressure changes. Hypotension can also be avoided by withholding doses of alpha2‐adrenergic agonists until withdrawal is manifested by a rise in blood pressure.
(d) Completion of withdrawal
The data indicate that the greater duration of treatment seen with reducing doses of methadone is not translated into a similar increase in rates of completion of withdrawal. Rather, the data indicate that there is no significant difference in completion rates for withdrawal managed with alpha2‐adrenergic agonists compared with reducing doses of methadone. The finding of no significant difference applies for all alpha2‐adrenergic agonists and is maintained when studies with high risk of bias are excluded.
3. Comparison of clonidine with lofexidine and guanfacine
Clonidine and lofexidine are the only alpha2‐adrenergic agonists for which there are sufficient studies to form a view on their relative effectiveness, and so this comparison is the focus of this section. However, there are some data comparing clonidine and guanfacine. San 1990 found no significant difference in withdrawal severity associated with clonidine compared with guanfacine treatment. Apart from a higher degree of restlessness among participants treated with clonidine, Muga 1990 also found no differences in the withdrawal syndromes associated with guanfacine or clonidine treatment. The findings of San 1990 and San 1994 suggest that guanfacine may be associated with fewer adverse effects than clonidine, but the influence of dose level remains uncertain.
(a) Withdrawal syndrome experienced
Limited data are available from studies directly comparing clonidine and lofexidine. However, the data that are available point towards these drugs having similar effectiveness in terms of overall intensity of withdrawal and the patterns of individual signs and symptoms. Separate comparisons of clonidine or lofexidine with reducing doses of methadone also point towards the drugs having similar efficacy.
(b) Duration of treatment
No data were reported on this aspect.
(c) Adverse effects
Hypotension occurs less frequently with lofexidine compared with clonidine treatment. This conclusion is supported by direct comparison of clonidine and lofexidine by Kahn 1997 and Lin 1997, but it is also supported indirectly with Bearn 1996 and Howells 2002 reporting no significant difference in mean blood pressure for participants treated with lofexidine compared with participants treated with reducing doses of methadone, whereas Jiang 1993 and San 1990 reported significantly lower mean blood pressures for participants treated with clonidine compared with participants treated with reducing doses of methadone. The findings of Carnwath 1998 and Kahn 1997 indicate greater incidence of adverse effects with clonidine compared with lofexidine, with the greater hypotensive effect of clonidine contributing to the difference. The studies comparing lofexidine and reducing doses of methadone focused on blood pressure changes. Consequently, information on overall adverse effects of lofexidine and reducing doses of methadone is lacking.
Some additional information on adverse effects associated with lofexidine was provided by Akhurst 1999 and Sheridan 1999. Akhurst 1999 (undertaken by the Britannia Pharmaceuticals) collected data retrospectively on 1074 detoxifications through a survey of treatment providers. Of the 1074 withdrawal episodes managed with lofexidine, 1.4% were ceased because of side effects. This was despite 8.5% experiencing dizziness, 7.5% hypotension, and 5.3% dry mouth. Spencer 1989 was a retrospective study of 214 detoxifications using lofexidine in an inpatient setting. This study identified 144 instances of hypotension, with 23 (16%) of these instances resulting in the dose of lofexidine being withheld or reduced. Similar post‐marketing survey data were not available for clonidine.
(d) Completion of withdrawal
Insufficient data were available to form a view as to the relative effectiveness of clonidine and lofexidine in terms of rates of completion of withdrawal. However, the findings of Carnwath 1998 suggest that the use of lofexidine may be associated with higher completion rates than clonidine.
4. Other comparisons
Consideration of the capacity of alpha2‐adrenergic agonists to ameliorate the signs and symptoms of withdrawal relative to other medications was limited by small numbers of studies and limitations of study design. Clonidine appears to be more effective than symptomatic medications in terms of achieving suppression of withdrawal signs and symptoms without adverse effects.
5. Factors potentially influencing outcomes
The subgroup analysis of completion of withdrawal treatment (Analysis 2.8) suggests that the nature of opioid used prior to withdrawal is not a significant factor influencing the effectiveness of alpha2‐adrenergic agonists relative to methadone. However, it should be noted that only Kleber 1985 selected participants from methadone maintenance treatment. In other studies involving withdrawal from methadone, participants were stabilised on methadone for a short period prior to withdrawal. Hence, the possibility remains that completion of withdrawal may be influenced by the duration of methadone treatment prior to detoxification.
There is currently a trend of increasing use of pharmaceutical opioid preparations for non‐medical purposes (World Drug Report 2013). Not only does this introduce diversity in the pharmacological properties of opioid drugs, but increasing use of pharmaceutical preparations may be associated with changes in the characteristics of users and patterns of use that have historically been associated with heroin. There is currently insufficient evidence available to determine the effect (if any) of the type of opioid, dose, route, and duration of use on withdrawal outcomes.
The second subgroup analysis (Analysis 2.9) found no difference between alpha2‐adrenergic agonists and reducing doses of methadone in rates of completion of withdrawal for treatment undertaken in inpatient or outpatient settings, but the strength of the finding is reduced by significant heterogeneity and low precision. One Cochrane review found only one study that directly compared inpatient and other settings for detoxification from opioid dependence (Day 2005). The authors of that review have subsequently undertaken a trial comparing inpatient and outpatient withdrawal from methadone managed with lofexidine, with no significant difference between settings other than in the duration of medication (Day 2011). The lack of evidence leaves the impact of setting a question to be answered.
Quality of the evidence
For the comparison of alpha2‐adrenergic agonists with reducing doses of methadone, we rated the quality of the evidence as low to moderate (see Table 1), meaning that further evidence is likely to result in some changes to the estimates of effect. The robustness of the quantitative analyses was reduced by the risk of bias in the studies included in this comparison, the small number of studies reporting some outcomes, significant heterogeneity between studies, and the small number of events that were reported for some outcomes.
For the comparison of alpha2‐adrenergic agonists with placebo, we rated the quality of evidence as very low to moderate (see Table 2), meaning that further evidence would be very likely to result in changes to the estimates of effect, particularly the peak withdrawal score. However, the evidence is sufficient to indicate that alpha2‐adrenergic agonists are more effective than placebo, making further comparisons of this nature inappropriate on ethical grounds. Comparisons with other active medications (methadone, buprenorphine, symptomatic medications) would be more appropriate.
Potential biases in the review process
This review was limited by small numbers of studies, small numbers of participants, and diversity in the interventions compared as well as the methods of assessing outcomes in the included studies. This reduced the capacity to assess with confidence the impact of methodological quality of the included studies and the possibility of publication bias.
The limitations were particularly marked in relation to assessing severity of withdrawal and the clinical significance of adverse effects. Differing approaches to assessing and reporting withdrawal signs and symptoms made it difficult to quantify accurately the effect of alpha2‐adrenergic agonists on withdrawal severity. Data on the occurrence of hypotensive and other adverse effects were of moderate quality, but the very low incidence of reported drop‐out from treatment due to adverse effects reduced the strength of conclusions regarding the clinical significance of these adverse effects.
It should be noted that the requirements of clinical trials, particularly the need to maintain double‐blind conditions, can limit the flexibility of dose regimens. The loss of flexibility is a potential source of bias, particularly for comparisons of alpha2‐adrenergic agonists and reducing doses of methadone. In clinical practice, flexible dosing with methadone for the purposes of detoxification would usually involve adjusting the rate of taper according to the person's withdrawal symptoms, craving, and general stability. Such flexibility is not provided in the clinical trials included in this review. Flexible dosing with alpha2‐adrenergic agonists usually involved withholding doses in response to lowered blood pressure; this is undertaken in both trial and clinical practice settings. The difference in capacity for flexibility would be expected to result in a negative bias against reducing doses of methadone.
Authors' conclusions
Implications for practice.
When used in the management of opioid withdrawal, alpha2‐adrenergic agonists are typically administered orally, in three or four doses per day, to a maximum of around 1.2 mg per day for clonidine and around 2 mg per day for lofexidine.
Compared to placebo, clonidine and lofexidine are associated with less severe withdrawal, longer time in treatment, and significantly higher rates of completion of treatment.
In comparison with reducing doses of methadone, the overall intensity of withdrawal associated with alpha2‐adrenergic agonist treatment appears similar to, or perhaps marginally greater than, that that associated with reducing doses of methadone. Withdrawal occurs at different stages of the treatment regimens. With alpha2‐adrenergic agonist treatment, the signs and symptoms of withdrawal occur earlier, within a few days of cessation of opioid drugs, whereas with reducing doses of methadone, the signs and symptoms of withdrawal do not become apparent until methadone doses approach zero. The signs and symptoms of withdrawal also resolve at an earlier stage when withdrawal is managed with alpha2‐adrenergic agonists.
Management of withdrawal with alpha2‐adrenergic agonists, compared with reducing doses of methadone, is associated with shorter duration of treatment but similar rates of completion of withdrawal.
Data are limited, but it appears that clonidine and lofexidine have similar capacity to ameliorate the signs and symptoms of opioid withdrawal. Lofexidine treatment is probably associated with rates of completion of withdrawal that are similar to rates associated with clonidine withdrawal.
Management of withdrawal with clonidine is associated with more adverse effects than management by reducing doses of methadone. Adverse effects were most severe during the few days of peak withdrawal when maximal doses of clonidine are administered. Hypotensive events associated with alpha2‐adrenergic treatment occurred much more frequently in the studies than did cessation of treatment because of those adverse events. This suggests that hypotension can be adequately managed by withholding doses and reducing the dose of medication according to blood pressure changes.
There is currently insufficient information available to compare lofexidine and methadone in terms of overall adverse effects, but what information is available indicates that blood pressure changes associated with lofexidine are not significantly greater than blood pressure changes associated with reducing doses of methadone. Direct comparison of lofexidine and clonidine indicates that lofexidine is associated with fewer adverse effects, particularly less frequent hypotensive events. On this basis, lofexidine should be preferred for withdrawal in an outpatient setting where monitoring of blood pressure and management of hypotension by withholding doses of medication is more difficult.
There are insufficient data available to support a conclusion on the efficacy of guanfacine, or other medications with alpha2‐adrenergic properties, such as tizanidine.
Overall, we detected no significant difference in efficacy of the alpha2‐adrenergic agonists clonidine and lofexidine for the management of withdrawal from heroin compared with reducing doses of methadone over a period of about 10 days.
Implications for research.
There remains limited information on the relative efficacy of clonidine, lofexidine, and particularly guanfacine. These three alpha2‐adrenergic agonists should be compared systematically in terms of amelioration of the signs and symptoms of withdrawal, time in treatment, capacity to support completion of withdrawal, and the occurrence of adverse effects including lethargy, fatigue, dry mouth, and hypotensive effects.
An alternative approach to improving the information available on comparative efficacy would be to undertake a post‐marketing survey of clonidine, similar to that undertaken with lofexidine. While it would not have the rigour of a randomised controlled trial, it would provide an indication of the performance of clonidine (a drug that is in widespread use for the management of opioid withdrawal) in routine clinical practice.
There remains uncertainty as to the nature of withdrawal signs and symptoms that are not significantly ameliorated by treatment with alpha2‐adrenergic agonists. It would be valuable to investigate adjunct medications that address the symptoms that are a problem for patients. These are likely to include sleep disturbances, anxiety, and aches and pains, which are suggested by studies included in this review to be incompletely suppressed by both alpha2‐adrenergic agonists and reducing doses of methadone.
It would be useful to further investigate the efficacy of the alpha2‐adrenergic agonists in managing withdrawal from different types of opioid drugs. In particular, it would be useful to determine the effect of the type of opioid drug, dose, route, and duration of use prior to withdrawal, including the effects of a prolonged period of opioid substitution treatment. Given that people who are dependent on opioid drugs are likely also to use other drugs, particularly benzodiazepines, cocaine, and alcohol, it would also be relevant to investigate the efficacy of alpha2‐adrenergic agonists when polydrug dependence is involved. There is a lack of evidence regarding the impact of setting on the effectiveness of alpha2‐adrenergic agonists for the management of opioid withdrawal. There is a trend globally towards withdrawal being managed on an outpatient or day‐care basis, making this an area of priority for research.
An aspect of considerable clinical relevance in considering the efficacy of approaches to the management of opioid withdrawal is the capacity to promote engagement in further treatment. We identified engagement in further treatment as an outcome of interest for this review, but was unable to assess it with the studies that met the criteria for inclusion in this review. The response to different approaches to the management of opioid withdrawal may vary with individual circumstance and the type of follow‐up treatment being considered. This suggests that approaches other than randomised controlled trials may be most appropriate to investigate this aspect of opioid withdrawal.
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2016 | New search has been performed | Search updated. |
| 7 January 2016 | New citation required but conclusions have not changed | 1 new study included. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 25 March 2014 | New citation required and conclusions have changed | No significant changes to conclusions. |
| 17 December 2013 | New search has been performed | New searches. New flow diagram of search. Minor changes to analyses. |
| 16 February 2009 | New search has been performed | Updated and conclusions changed. |
| 20 January 2009 | New citation required and conclusions have changed | New search, new trials, conclusions changed. |
| 17 September 2008 | Amended | New search, new assessment of included studies. |
| 17 September 2008 | New citation required and conclusions have changed | Substantive amendment. |
| 21 March 2008 | Amended | Converted to new review format. |
Acknowledgements
Boehringer Ingelheim Pty Ltd (Australia) provided information on trials involving clonidine, and Britannia Pharmaceuticals provided information on trials involving lofexidine. The assistance of Maria Chang (Boehringer Ingelheim) and Len Cretney and Jackie Akhurst (Britannia Pharmaceuticals) is gratefully acknowledged. We also acknowledge the assistance of Sharon Webb in the translation of one study published in German (Benos 1985).
Appendices
Appendix 1. CENTRAL search strategy
#1 (opiate or opioid or heroin or narcotic) near/2 (abuse or addiction or dependence):ti,ab,kw in Trials
#2 MeSH descriptor: [Opioid‐Related Disorders] explode all trees
#3 #1 or #2
#4 MeSH descriptor: [Substance Withdrawal Syndrome] explode all trees
#5 detoxification:ti,ab,kw in Trials
#6 #4 or #5
#7 #3 and #6
Appendix 2. MEDLINE search strategy via Ovid Online
exp Opioid‐Related Disorders/
((opiate$ or opioid$ or heroin$ of narcot$) adj2 (abus$ or depend$)).ti,ab
exp Substance Withdrawal Syndrome/
(detoxifi$ or desintoxi$ or disintoxi$ or disintossi$).ti,ab
1 or 2
3 or 4
5 and 6
exp clinical trial/ or exp comparative study/
random$.ti,ab
exp Double‐Blind Method/
(double adj2 blind).ti,ab
8 or 9 or 10 or 11
7 and 12
Limit 13 to humans
Appendix 3. EMBASE search strategy
#1 'opiate addiction'/exp
#2 'heroin dependence'/exp
#3 (opiate* or opioid* or heroin* or narcot*) NEAR/2 (abus* or depend*)
#4 'withdrawal syndrome'/exp
#5 detoxifi*.ab,ti or desintoxi*.ab,ti or disintoxi*.ab,ti or disintossi*.ab,ti
#6 'drug detoxification'/exp
#7 #1 or #2 or #3
#8 #4 or #5 or #6
#9 #7 and #8
#10 'clinical study'/exp
#11 random*.ab,ti
#12 'double blind procedure'
#13 double NEAR/1 blind
#14 #10 or #11 or #12 or #13
#15 #9 and #14 AND [humans]/lim
Appendix 4. PsycINFO search strategy via Ovid Online
exp drug dependency/
exp heroin addiction/
((opiate$ or opioid$ or heroin$ of narcot$) adj2 (abus$ or depend$)).ti,ab
exp drug withdrawal/ or exp detoxification/
(detoxifi$ or desintoxi$ or disintoxi$ or disintossi$).ti,ab
1 or 2 or 3
4 or 5
6 and 7
limit 8 to human
exp clinical trials/
random$.ti,ab
(double adj2 blind).ti,ab
10 or 11 or 12
9 and 13
Appendix 5. Web of Science search strategy
TS="opioid‐related disorders"
TS=opiate addiction
TS=opiate depend*
TS=heroin* depend*
TS=heroin* addict*
#5 or #4 or #3 or #2 or #!
TS="substance withdrawal syndrome"
TX=detox*
#8 or #7
#9 and #6
TS=clinical trial*
TS=random*
TS=double blind
#13 or #12 or #11
#14 and #10
Appendix 6. Criteria for 'Risk of bias' assessment
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants, providers, and outcome assessor (performance and detection bias) Objective outcomes |
Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Blinding of participants, providers, and outcome assessor and unlikely that the blinding could have been broken |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants, providers, and outcome assessor attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 4. Blinding of participants, providers, and outcome assessor (performance and detection bias) Subjective outcomes |
Low risk | Blinding of participants, providers, and outcome assessor and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants, providers, and outcome assessor attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 5. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop‐out |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods. All randomised participants are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. 'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group) | |
| 6. Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon) |
| High risk | Not all of the study's prespecified primary outcomes have been reported. One or more primary outcomes is reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified. One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 7. Other bias | Low risk | Potential confounding factors identified but evenly distributed between groups. Study ceased early but with no indications of selection bias. Interventions delivered consistently |
| High risk | Potential confounding factors unequally distributed between groups. Study ceased early with risk of selection bias. Differences in aspects of delivery of interventions. Mandatory treatment |
|
| Unclear risk | Confounding possible but not able to be assessed. Study ceased early and unable to determine possible bias. Unclear if delivery of interventions was equivalent |
Data and analyses
Comparison 1. Alpha2‐adrenergic agonist versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak withdrawal score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Participants with severe withdrawal | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.57] |
| 3 Completion of treatment | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.34, 2.84] |
Comparison 2. Alpha2‐adrenergic agonist versus methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak withdrawal score | 2 | 263 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 2 Participants with severe withdrawal | 5 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.81, 1.73] |
| 3 Overall withdrawal severity | 3 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.49] |
| 4 Duration of treatment | 3 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.31, ‐0.83] |
| 5 Number experiencing hypotensive or other adverse effects | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.19, 3.10] |
| 6 Drop‐out due to adverse effects | 4 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [0.77, 16.94] |
| 7 Completion of treatment | 9 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 8 Completion of treatment by opioid | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 8.1 Heroin | 4 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.61, 1.25] |
| 8.2 Methadone | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 9 Completion of treatment by setting | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 9.1 Inpatient | 5 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.12] |
| 9.2 Outpatient | 4 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.73, 1.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Batey 1987.
| Methods | Double‐blind, controlled study | |
| Participants | Setting: outpatient clinic, Australia. Participants: 31 heroin users seeking withdrawal assistance, with evidence of repeated iv drug use. Group sizes: (1) n = 16, (2) n = 15. Group characteristics not reported. Required to attend clinic with non‐drug‐using family member or partner |
|
| Interventions | (1) Clonidine, 15 μg/kg/day, 3 to 4 divided doses, tapered. (2) Placebo. Additional medication used but not reported. Scheduled duration 3 to 7 days |
|
| Outcomes | Number with signs or symptoms of withdrawal graded > 2 (on 0 to 4 scale); number successful (completed 3 to 7 days of treatment, no signs of withdrawal, negative supervised urine samples) | |
| Notes | Observers rated 4 signs (pupil diameter, sweating, rhinorrhoea, and lacrimation) and 3 symptoms (abdominal pain, leg cramps, diarrhoea) 0 to 4 each day. Participants rated management of withdrawal good, average, of no use, or terrible. Daily urine testing. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients ... who requested assistance for heroin withdrawal were advised of the placebo controlled trial being undertaken." Comment: Allocation may have been random, but this was not specifically reported. The participant characteristics were not reported, and there was no discussion of the similarity of the groups |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Quote: "The patients were seen on a daily basis by the medical officer and a series of observations were recorded on a standard flow sheet." "Sixteen patients were given clonidine and fifteen received the identical placebo tablets ..." Comment: Use of identical placebo suggests participants were blinded, but the treating medical officer, who was also the observer, may not have been |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Blinding was uncertain, but these outcomes were unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome of withdrawal episode (success/failure) reported for all participants. Withdrawal severity and adverse effects reported as dichotomous data (e.g. number with withdrawal graded > 2) and missing data unlikely to have clinically relevant impact on these data |
| Selective reporting (reporting bias) | High risk | Participant assessments of withdrawal management not reported. Use of additional medication not reported |
| Other bias | Unclear risk | Quote: "The study was closed after thirty‐one patients had been entered into the trial because of the difficulty in convincing patients that they should be willing to be treated with placebo for the sake of the study." Participants "represented 80% of those presenting during the study period." Comment: Insufficient information to determine the extent of risk of bias from participant's preference for active medication |
Bearn 1996.
| Methods | Randomised, controlled, double‐blind trial. Sample represented 34.7% of total patients admitted to unit during the study | |
| Participants | Setting: inpatient treatment in specialist drug and alcohol unit, London, UK. Participants: 86 opioid dependent by DSM‐IV, using heroin, methadone, or both. Group sizes: (1) n = 42, (2) n = 44. Groups similar on age, gender, body weight, and drug use history. Mean age: 31.7 years. 80% men. Mean duration of opioid use: 10.5 years. 43% also used benzodiazepines |
|
| Interventions | Stabilised on methadone (about 60 mg/day) for 3 days prior to detoxification with: (1) Lofexidine, initial dose 0.6 mg/day, increased by 0.4 mg/day until day 4, maintained at 2 mg/day for 3 days, then tapered over 3 days or (2) Methadone, starting dose variable, tapered over 10 days. Both drugs administered twice daily. Diazepam, 3 days of stabilisation then tapered over 21 days for those codependent on benzodiazepines. Scheduled duration of withdrawal treatment 10 days, inpatient stay 21 days |
|
| Outcomes | Mean daily withdrawal score (graph); length of stay; mean daily blood pressure (graph); number completing 20 days of treatment; number experiencing dizziness | |
| Notes | Short Opiate Withdrawal Scale (10 items, 0 to 4 severity) completed daily by participants. Study supported by funds from Britannia Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Quote: "If a patient left before completing the treatment programme, the treatment code was broken and the patient informed which treatment they were taking. Those who completed treatment remained blind to the treatment they had received ..." Comment: Participants at least were blind to treatment allocation, and withdrawal scores were rated by participants |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | The use of a treatment code suggests that personnel may also have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "... in the lofexidine group ... six patients dropped out within the first 10 days compared to only one of the methadone treated patients (P = 0.048)." Comment: Withdrawal severity was significantly greater in the lofexidine group in the first 10 days ‐ this difference cannot be attributed to the differential drop‐out. Indeed, data missing due to drop‐out might be expected to increase the difference |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | 37/86 participants simultaneously withdrawing from benzodiazepines; equally distributed between groups |
Benos 1985.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Polydrug dependence an exclusion criterion (1 excluded postrandomisation) | |
| Participants | Setting: inpatient treatment, Germany. Participants: 50 dependent heroin users. Group sizes: (1) n = 24, (2) n = 25. Groups similar on most characteristics, but at entry clonidine group had longer mean time since last heroin use. Mean age: 26 years. 78% men. 78% unemployed; around 5 previous withdrawal attempts |
|
| Interventions | Medication commenced with withdrawal symptoms. (1) Clonidine, 0.1 mg/tablet. (2) Placebo. Day 1, 1 or 2 tablets 3 times a day, increasing to 1 or 2 tablets 3 to 5 times a day depending on symptoms. Dose tapered over 10 days. Both groups given neuroleptics when necessary and counselling. Scheduled duration 10 days |
|
| Outcomes | Graph of withdrawal scores; global assessment of efficacy; side effects; number completing treatment | |
| Notes | Withdrawal rated by observers (22 items, 0 to 5 severity) and participants (38 items, "not there" to "hard"). Published in German. English translation obtained. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treatment was double‐blind randomised comparison." Comment: The method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated, and these outcomes unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion of treatment was the only outcome used in analyses for this review |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Bertschy 1997.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Dependence on methadone or other opioids, or polydrug use exclusion criteria | |
| Participants | Setting: inpatient treatment in non‐specialised unit of psychiatric hospital, Lausanne, Switzerland. Participants: 32 heroin users, dependent by DSM‐III‐R. Group sizes: 16 in each group. Groups similar except in frequency of heroin use: participants used heroin (mean ± SD) (1) 3.3 ± 2.1, (2) 4.8 ± 2.4 times a day. Mean age: about 24 years. 72% men. 66% used iv; remainder used by sniffing or smoking; 19% had previously been hospitalised for detoxification |
|
| Interventions | (1) Clonidine, max dose 0.6 mg/day, tapered over 7 days. (2) Carbamazepine, max dose 400 mg/day, plus mianserin (atypical antidepressant) to max 90 mg/day for 6 days. Adjunct medications as required, in both groups. Scheduled duration 7 days |
|
| Outcomes | Withdrawal scores (graphs); instances of comedication; global satisfaction score; retention to end of treatment; difference in blood pressure; instances medication withheld | |
| Notes | Opiate withdrawal questionnaire (30 items, rated 0 to 3) completed by participants. Intensity of global withdrawal by VAS. Observers rated withdrawal as "very difficult" to "very easy". Study "partially supported by a grant from AKZO‐Organon Switzerland." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized ... by groups of four." Comment: The method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomized and on double‐blind conditions allocated to one of the treatment groups." Comment: This information is insufficient to make a judgement on the adequacy of allocation concealment |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind, double‐dummy stated |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind, double‐dummy stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Missing data concerned (VAS) of one patient and exit laboratory tests one patient." Comment: Missing data not sufficient to have significant impact |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Bruno 1979.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: inpatient, Italy. Participants: 20 heroin addicts requesting detoxification. Group sizes: 10 in each group. Mean age: 26 years. 75% men |
|
| Interventions | (1) Clonidine, 5 μg/kg body weight in 2 doses/day. (2) Placebo. Both groups also received flunitrazepam and Laevosan (lactulose, a synthetic non‐digestible sugar used in the treatment of chronic constipation and hepatic encephalopathy). Scheduled duration of treatment unclear; outcomes reported for first 72 hours |
|
| Outcomes | Mean withdrawal score over 72 h, and at 24, 48, and 72 h (reported as mean score only, and with results of analysis of variance); mean scores of individual symptoms (graphs only) | |
| Notes | Details of scale for assessment of withdrawal not reported. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were divided randomly into two groups." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind (participants and observer) stated |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind (participants and observer) stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐out reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Cami 1985.
| Methods | Controlled, double‐blind trial. Detoxification preceded admission to drug‐free therapeutic community | |
| Participants | Setting: inpatient treatment, no telephone calls or visitors, Barcelona, Spain. Participants: 45 heroin users, dependent by DSM‐III‐R. Group sizes: (1) n = 26, (2) n = 19. Analysis based on 30/45 participants who completed 12 days of treatment. Of 30 who completed study. Mean age: 23.5 years. 80% men. Mean 4.2 years of heroin use, 1.8 previous supervised withdrawal attempts |
|
| Interventions | (1) Clonidine, 0.9 to 1.35 mg/day. (2) Methadone 30 to 45 mg/day. Initial dose based on participant's weight and heroin consumed in previous month. Both drugs given every 8 hours and tapered over 10 days. Flunitrazepam and acetylsalicylic acid (aspirin) as adjunct medications. Psychotherapeutic support given. Naloxone challenges (0.4 mg sc) on day of discharge. Scheduled duration 8 to 10 days |
|
| Outcomes | Number of participants with each of 4 withdrawal signs or symptoms (muscular aching, anxiety, weeping, sleep disorders) and each of 4 adverse effects (flatulence, daytime sleeping, asthenia, fatigue during walking); reported as graphs by day of detoxification; mean doses of drugs administered; mean duration for participants who completed treatment; number discharged drug‐free | |
| Notes | Withdrawal rated daily by nurses (19 withdrawal signs, 17 adverse effects rated present/absent). Participants completed State‐Trait Anxiety Inventory Questionnaire on days 1, 2, 3, 4, 7, and 10. Participants monitored by random urine screening. Source of funds research grants, with placebo clonidine provided by Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was conducted in double‐blind fashion ..." Comment: The characteristics of the groups were not compared. Information was insufficient to make a judgement on the adequacy of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated; placebos used |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated; placebos used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/26 (58%) participants taking clonidine and 15/19 (79%) participants taking methadone completed treatment. Data on withdrawal symptoms and adverse effects reported only for those who completed treatment. No information on characteristics of participants who dropped out |
| Selective reporting (reporting bias) | Low risk | All outcomes assessed appear to have been reported |
| Other bias | Low risk | None apparent |
Carnwath 1998.
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: home‐based, Manchester, UK. Participants: 50, opioid dependent by DSM‐IV, using methadone or other opiates. Group sizes: (1) n = 26, (2) n = 24. (1) 43%, (2) 71% used iv, otherwise groups similar. Mean age: 28 years. 70% men. Mean 6.9 years opiate use. 66% had previous detoxification experience; 17% employed, 63% supported by relative. |
|
| Interventions | All stabilised on methadone (40 mg/day or less) prior to study. (1) n = 26: lofexidine, 0.2 mg/capsule, or (2) n = 24: clonidine 0.1 mg/capsule. Both increased over 3 days to max 8 capsules/day, and tapered over last 3 days. Various adjunct medications available. Total duration of medication unclear. Home‐based treatment with participants visited at least 4 times in week 1, 3 times in week 2, and once in each of weeks 3 and 4. Treatment considered successful if participants opiate‐free by urine test at 4 weeks. Scheduled duration 12 days | |
| Outcomes | Number completing treatment; number with extra home visits; mean withdrawal scores; mean side effects score | |
| Notes | Participants completed Short Opiate Withdrawal Scale (10 items, 0 to 3 severity) during each visit by trial personnel. Financial support provided by Britannia Pharmaceuticals and the "North West Region Medical Innovation Scheme" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly ..." Comment: Although the method of sequence generation was not reported, the similarity of the groups and allocation by the separate pharmacy suggests the method was adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment courses were sent out to patients by Trafford pharmacy, which also conducted the treatment group assignment without knowledge of patient or drug team staff." |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Participants and treating staff blind to treatment. Drugs prepared in identical capsules |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Participants and treating staff blind to treatment. Drugs prepared in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Those [participants] stopping early experienced higher maximum SOWS scores." Comment: Differential drop‐out may have reduced mean daily SOWS score in clonidine group to a greater extent than the lofexidine group, but this outcome was not used in this review |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Gerra 1995.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: hospital outpatient clinic, Parma, Italy. Participants: 152, drug abuse disorder by DSM‐III‐R, heroin users. Group sizes: (1) n = 33, (2) n = 42, (3) n = 58, (4) n = 19. Similarity of groups not reported. Age: 18 to 32 years. 82% men |
|
| Interventions | (1) Clonidine, 0.15 mg iv 3 times a day. (2) Clonidine, 0.15 mg iv 3 times a day + naltrexone, 12.5 mg day 2 then 50 mg/day for 3 months. (3) Clonidine, 0.15 mg iv 3 times a day + naloxone, 0.2 mg iv day 2, 0.4 mg 2 times a day on days 3 and 4, then naltrexone 50 mg/day from day 5. (4) iv saline + oral placebo. Daily clinic attendance with 4 hours iv therapy in morning, 3 hours in afternoon. (Groups 2 and 3 not considered for this review.) Scheduled duration of treatment unclear |
|
| Outcomes | Mean total withdrawal score at 48 and 72 hours; bar graphs for days 1, 2, and 3 showing ratings of individual items of withdrawal scale; morphine metabolites in urine; Hamilton Rating Scale for Depression on day 1, day 8, and 6 months | |
| Notes | Withdrawal assessed by observer only using 9‐item scale, mainly of objective signs. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "All the patients were randomly divided into four groups ..." Comment: Group sizes differed, and similarity of the characteristics of the groups was not discussed. The adequacy of sequence generation is doubtful |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Double‐blind stated, but given the differences in group sizes, it is doubtful whether the blind was maintained for treating personnel, participants, and observers |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated. Although it is doubtful whether the blind was maintained, these outcomes are considered unlikely to be affected by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out in first week higher in placebo compared with other groups. Given the marked difference in withdrawal severity between clonidine and placebo groups, the differential drop‐out is unlikely to have a clinically significant impact on withdrawal scores (the main outcome reported) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Gerra 2000.
| Methods | Randomised, controlled trial. Heavy polydrug use, comorbid psychiatric or medical conditions were exclusion criteria | |
| Participants | Setting: outpatient clinic, Parma, Italy. Participants: 98 dependent by DSM‐IV, urine positive for morphine, withdrawing from heroin. Group sizes: (1) n = 32, (2) n = 32, (3) n = 34 (only groups 1 and 3 considered for this review). Groups similar in psychiatric and psychometric data. Age: 18 to 36 years. 72% men. Drug use: 2 to 6 years |
|
| Interventions | Heroin use continued until 12 hours before treatment. Withdrawal managed with: (1) Clonidine 0.15 mg/100 mL saline iv 6 times/day for 2 days, 0.15 mg 3 times/day for 3 days, additional 0.15 mg orally each evening. Total 5 days of treatment. (2) Clonidine + naloxone and naltrexone (not considered for this review). (3) Methadone, oral, 40 mg/day in single dose, tapered over 10 days. Treatment in outpatient clinic with those participants in groups (1) and (2) receiving 4 hours iv therapy morning and afternoon. Unclear whether the extent of clinic care was the same for group (3). All received counselling. Drug‐free programme postdetoxification with naltrexone. Naltrexone commenced (1) day 6, (2) during detox, (3) 5 days after taper. Scheduled duration (1) 5, (2) 3, (3) 10 days |
|
| Outcomes | Graphs of mean daily withdrawal scores; craving scores before and after detoxification; % of positive urine samples; number accepting naltrexone; % of participants in maintenance naltrexone treatment 3 months after detoxification | |
| Notes | Withdrawal rated by observer (9 items, 0 to 5 severity). Urine testing during detoxification and follow‐up period. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All the subjects were randomly divided into three groups." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | Blinding not discussed; the timing of naltrexone commencement in the treatment protocols differed. This suggests that there was probably no blinding of treatment personnel, and possibly not of participants either |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes are considered unlikely to be affected by knowledge of treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The 3 groups differed in the proportions who accepted and continued extended naltrexone maintenance treatment, but it is unclear how this difference might translate into missing data; it is also unclear whether differences in drop‐out may have influenced withdrawal scores. (This outcome was not used in this review.) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Unclear whether all 3 groups received same amount of clinic care |
Gupta 1988.
| Methods | Randomised, controlled, single‐blind trial | |
| Participants | Setting: inpatient treatment, New Delhi, India. Participants: 120 heroin dependent by ICD‐9. Group sizes: 60 per group. Groups comparable on demographic characteristics. Mean age: 26 years. 100% men. All using heroin by inhalation, mean: (1) 1.8 g/day, (2) 1.3 g/day. Duration of use: about 2.5 years. 51% married, 17% unemployed; male relative to accompany participants in hospital |
|
| Interventions | (1) Clonidine, 0.1 mg rising to 0.2 mg 3 times/day. (2) Chlordiazepoxide, 10 mg 3 times/day, plus chlorpromazine, 100 mg day 1, then 200 mg 3 times a day. Drugs tapered when withdrawal symptoms remitted. Additional symptomatic medications as needed. Scheduled duration of treatment not reported |
|
| Outcomes | Frequency of 17 withdrawal symptoms | |
| Notes | Participants interviewed for the presence of withdrawal symptoms each morning by person blind to treatment regimen. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two equal groups." Comment: Method not reported |
| Allocation concealment (selection bias) | High risk | Method not reported. Study stated as single‐blind (observer only), hence treating doctor and participants probably aware of allocation |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Observer rating withdrawal symptoms blind to group allocation |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | No objective outcomes reported, other than completion of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out not reported. Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Howells 2002.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Some drop‐out in both groups for logistic reasons associated with prison setting | |
| Participants | Setting: Prison healthcare centre, Winchester, UK. Participants: 68, opioid‐dependent by DSM‐IV, using heroin, methadone, or both. Group sizes: (1) n = 32, (2) n = 36. Groups similar on drug use, demographics, and severity of dependence. Mean age: 30 years. 100% men. Mean 9 years from first use of illicit heroin; some participants also dependent on benzodiazepines |
|
| Interventions | Most in custody 24 to 48 h before entering study. Withdrawal managed with: (1) Lofexidine, 0.6 mg/day, increased 0.4 mg/day to max 2 mg/day, tapered to 0 mg/day by day 11. (2) Methadone 30 mg/day, tapered to 0 mg/day over 10 days. Both drugs administered twice a day (supervised). Scheduled duration 10 days |
|
| Outcomes | Maximum, minimum withdrawal scores and time of occurrence; overall withdrawal score; use of adjunct medication; retention in treatment; completion of 10 days; reasons for withdrawal from study; incidence of adverse events | |
| Notes | Participants completed withdrawal problem scale (20 items) and Short Opiate Withdrawal Scale (8 items) daily ‐ reported as combined scores. Britannia Pharmaceuticals provided medication and support for independent trial monitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The pharmacist who made up the medication used a simple randomisation procedure to allocate each participant to one arm of the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "The independent pharmacy team at the prison oversaw the randomisation and blinding procedure." |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Quote: "Both the patient and health centre clinicians were blind to the assigned treatment group." |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Quote: "Both the patient and health centre clinicians were blind to the assigned treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Complete sets of withdrawal scale data were created from scores for 63 (92.6%) patients ..." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Jiang 1993.
| Methods | Randomised, controlled trial. Not all participants had entered treatment voluntarily. Concurrent medical condition, infectious diseases, mental illnesses exclusion criteria. Endpoint of naloxone challenge used for only 50% of participants | |
| Participants | Setting: inpatient treatment in 5 different rehabilitation centres, China. Participants: 200 opiate dependent by DSM‐III‐R, heroin users. Group sizes: 100 in each group. Methadone group had higher proportion using via oral route (80% compared with 67%). No other differences in demographics or drug use history. Mean age: 24.8 years. 78% men. 74% using orally, rest using iv or both orally and iv; mean duration of addiction: 15.5 months, at admission around 10 hours since last use; 71% had not previously received treatment |
|
| Interventions | (1) Clonidine, "sufficient" dose days 1 to 4, tapered days 5 to 8, ceased after day 11. Mean (± SD) max dose day 2: 1.05 ± 0.14 mg. (2) Methadone, max days 1 to 2 then tapered and ceased after day 12. Mean (± SD) max dose day 2: 21.6 ± 5.0 mg. For both drugs, initial dose dependent on body weight, physical condition, heroin intake previous week. Dose titrated against withdrawal and side effects. Scheduled duration 12 days |
|
| Outcomes | Mean daily withdrawal score; duration of treatment; side effects score | |
| Notes | Report in Chinese. English translation obtained. Symptoms and vital signs assessed daily using Himmelsbach scale as guide. 21 designated symptoms and vital signs also assessed. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly divided." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Insufficient information reported to determine whether there was any blinding |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes were not used in this review, and were unlikely to be affected by a lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | Rates of completion of withdrawal confounded as not all participants had entered treatment voluntarily, hence there was some compulsion to complete withdrawal |
Kahn 1997.
| Methods | Randomised, controlled, double‐blind trial. Alcohol dependence an exclusion criterion | |
| Participants | Setting: inpatient treatment, Birmingham, UK. Participants: 28 opiate dependent by history and urine screen, admitted for inpatient detoxification. Group sizes: 14 in each group. Characteristics of participants not reported, but groups stated to be well matched with regards to age, sex, and opiate use prior to trial. 68% men |
|
| Interventions | All stabilised on methadone for 3 to 4 days prior to study. Methadone stopped on day 3 by substitution with placebo; participants but not observer blind to cessation. Withdrawal managed with: (1) Clonidine, 0.2 mg rising to max 0.9 mg/day. (2) Lofexidine, 0.4 mg rising to max 1.8 mg/day. Methadone placebo stopped day 14. Clonidine or lofexidine tapered over following 4 days. Lorazepam as adjunct medication if needed. Any regular psychoactive medication maintained. Scheduled duration 16 days |
|
| Outcomes | Mean daily withdrawal score (graph); mean standing systolic blood pressure (graph); number of participants and number of occasions of use of lorazepam; number of participants reporting side effects and number of patient days of reported side effects | |
| Notes | Withdrawal rated by nurses (scale stated as similar to that used by Gold 1980c). Participants completed VAS. Study supported by Merrell Dow (lofexidine, placebo, technical assistance) and Wellcome (methadone, placebo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised double‐blind allocation." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated; medication prepared in identical capsules |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated; medication prepared in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out not reported. Unable to assess extent and impact of incomplete data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Kleber 1985.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Current alcohol abuse an exclusion criterion. Component of multicentre study ‐ see also Senay 1983 | |
| Participants | Setting: outpatient treatment, Connecticut, USA. Participants: 50 withdrawing from methadone maintenance treatment. Group sizes: 25 in each group. 1 in clonidine group did not commence treatment ‐ did not meet blood pressure criteria. Published report based on remaining 49 participants. Groups similar on age, sex, race, and length of addiction. Mean age: 29.5 years. 76% men. Mean length of addiction: 10 years |
|
| Interventions | Comfortable on methadone 20 mg/day for 2 weeks. (1) Clonidine (plus methadone placebo), initial dose 0.3 mg/day, 3 divided doses, tablets; gradual increase to max 1 mg/day by day 6, tapered by 20% to 25% per day from day 11. (2) Methadone (plus clonidine placebo), initial dose 20 mg/day, single daily dose as oral syrup, tapered by 1 mg/day. Chloral hydrate as adjunct medication. Scheduled duration of study 30 days |
|
| Outcomes | Retention in treatment; mean withdrawal scores at baseline, weeks 1 to 2 and 3 to 4; max ratings; number using sleep medications; number completing detoxification; incidence of side effects | |
| Notes | Withdrawal rated by nurses (24 items, 0 to 3 severity) and participants (31 items, 1 to 4 severity). Side effects rated by physicians and nurses. Supported by grants from National Institute on Drug Abuse and Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Participants and observers blind |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Participants and observers blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Most clonidine failures typically dropped out of treatment during the first week of the study, whereas the methadone failures tended to stay in the study until the third week" and withdrawal scores were higher for treatment failures compared to successes. Comment: The different timing of drop‐out potentially distorts mean withdrawal scores; this outcome not used in this review. The approach to analysis by study authors reduced the risk of bias |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Li 2002.
| Methods | Randomised controlled trial. Participants allocated at ratio of 1.5:1 for Qigong relative to other 2 groups. Blinding not able to be maintained, but each study group unaware of others. No dropouts; participants in mandatory treatment | |
| Participants | Setting: residential treatment, China. Participants: 86 heroin users, dependent by DSM‐III‐R, urine positive for morphine. Group sizes: (1) n = 26, (2) n = 34, (3) n = 26. No significant difference in baseline data of groups. Mean age: 32 years. 100% men. 79 using by injection, 7 by sniffing; mean 5.5 years of drug use; mean 27 hours between last use and entry to treatment centre |
|
| Interventions | (1) Symptomatic medications. (2) Qigong ‐ traditional Chinese health practice. (3) Lofexidine, 0.4 mg twice day 1, 0.6 mg 3 times/day for 3 days, then tapered to cease after day 10. Only groups (1) and (3) considered for this review. Scheduled duration about 10 days |
|
| Outcomes | Graph of daily withdrawal scores; Hamilton Anxiety Rating Scale scores days 0, 5, and 10; days to achieve morphine‐negative urine | |
| Notes | Withdrawal rated by observers, 5 levels, 23 symptoms. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned ..." (abstract) and "Qualified subjects were assigned into one of three groups according to the order in which they entered the treatment centre." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Study established with observers blind, but authors noted the blind was difficult to maintain. Blinding of participants was not possible, but participants were not aware of other treatment groups |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes unlikely to be affected by lack of blinding, but were confounded by treatment being mandatory. These outcomes not used for this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | Rates of completion of withdrawal confounded as participants were in mandatory treatment |
Lin 1997.
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient treatment in hospital detoxification ward, Taipei, Taiwan. Participants: 80 heroin users, opioid dependent by DSM‐IV. Group sizes: 40 in each group. Groups similar on demographics and drug use history. Mean age: 32 years. 81% men. All Chinese, 61 used iv, 9 im, 10 by smoking; 18% also used methamphetamine; mean duration of heroin use around 4 years; first detoxification attempt for 20% |
|
| Interventions | (1) Lofexidine, max dose 1.6 mg/day. (2) Clonidine, max dose 0.6 mg/day, in 4 divided doses. Total dosing period 6 days, with 10 days treatment |
|
| Outcomes | Symptom frequency on days 2 and 3; graph of mean scores; median duration of treatment (patient days used as denominator to adjust for different retention rates); number with 1 or more items rated moderate on day of discharge; number of doses omitted due to low blood pressure; retention rates (graph) | |
| Notes | Withdrawal rated by observers 3 times a day (15 items, 0 to 3 severity). Funding support from Britannia Pharmaceuticals and Taiwan Major Chem. & Pharm. Corp. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All study subjects were randomly assigned ..." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated. Medication prepared in identical capsules |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated. Medication prepared in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts excluded from analysis of withdrawal scores. Quote: "Significantly fewer subjects had self‐discharged from the lofexidine group than clonidine group at day four ... and at day five." Comment: Withdrawal severity similar for the 2 groups, so differential drop‐out unlikely to have clinically significant impact on outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Muga 1990.
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient, Spain. Participants: 88 heroin dependent by DSM‐III‐R, admitted for inpatient detoxification. Group sizes: (1) n = 43, (2) n = 45. Groups stated to be similar. Mean age: 25 years. 80% men. Mean 65 months of addiction. |
|
| Interventions | (1) Clonidine 0.9, 1.3 or 1.8 mg/day. (2) Guanfacine, 6, 12, or 18 mg/day. Doses of both medications determined by dose of heroin and body weight. Duration of treatment (9, 12, or 15 days) also dependent on heroin use and body weight. Alprazolam 2 mg/day as adjunct medication |
|
| Outcomes | Days of admission; days of treatment; mean scores for individual withdrawal and adverse effects signs and symptoms; changes in blood pressure and heart rate | |
| Notes | 17 withdrawal signs and symptoms and 19 adverse effects assessed. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assigned to either treatment group ... through a random computer table." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated, and these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Timing of drop‐out not reported. Unclear how withdrawal scores might be affected |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Nazari 2013.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: inpatient, treatment services operated by non‐government organisations, Tehran, Iran. Participants: 90 opioid dependent by DSM‐IV, using opium (93%), opium extract (42%), heroin (29%), and crack (74%). Use by injection reported by 20%. Group sizes: 30 in each. Group characteristics similar. Age: 25to 40 years All male. |
|
| Interventions | (1) Hab‐o Shefa, preparation of plant extracts used in traditional Iranian medicine, 3 g/day in 4 divided doses, tapered from day 8. (2) Clonidine, 0.2 to 0.4 mg days 1 to 2, 0.6 mg days 3 to 18, 0.4 to 0.2 mg days 20 to 21. (3) Placebo (sugar). Group (1) not considered for this review. All participants received an assisted self help intervention (behavioural therapy and the 12‐step principles). Scheduled duration of treatment 21 days. Naloxone challenge test on day 21 |
|
| Outcomes | Overall average scores for withdrawal, craving, depression, side effects and graphs of daily mean withdrawal scores | |
| Notes | Withdrawal assessed with Subjective (13 items, possible scores 0 to 13), Objective (16 items, possible scores 0 to 64), and Clinical (11 items, possible scores 0 to 48) Opiate Withdrawal Scales. Craving assessed by visual analogue scale. Depression assessed with Beck and Hamilton scales. Side effects rates by investigator as present or absent. Source of funds not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized random numbers" (Materials and Methods) |
| Allocation concealment (selection bias) | Low risk | Quote: Medication "in unit size capsules packed in the boxes that were encoded ... for each patient individually and were distributed by a third person who had no contact with ... the investigator [or] the patients". (Materials and Methods) |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated. Quote: "A physician ... who ... was blind to capsules content, performed all the clinical assessments". (Materials and Methods, Setting and Ethics) |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data replaced by last observation carried forward. Analysis of variance applied for two‐way comparisons |
| Selective reporting (reporting bias) | Unclear risk | Average side effects score reported but no details of nature of side effects experienced. Stated that all participants completed the study, but it is not specifically stated whether this meant that all stayed in treatment for 21 days |
| Other bias | Low risk | None apparent |
San 1990.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Participants and observers blind to medication. Polydrug use exclusion criterion. Analysis based on 90 (30 in each group) who completed 12 or more days of treatment | |
| Participants | Setting: inpatient treatment in general hospital detoxification unit, Barcelona, Spain. Participants: 170 heroin dependent by DSM‐III‐R. Group sizes: (1) n = 40, (2) n = 68, (3) n = 62. No differences in characteristics of groups. Mean age: about 24 years. 80% men. Mean duration of opioid use around 5 years. |
|
| Interventions | Initial dose of medication dependent on weight and heroin use in previous week. (1) Clonidine, mean (± SD) max dose 1.05 ± 0.1 mg/day. (2) Methadone, mean (± SD) max dose 37.3 ± 4.49 mg/day. (3) Guanfacine, mean (± SD) max dose 3.58 ± 0.41 mg/day. For all drugs, max dose given on days 2 and 3. Drug tapered over 11 days. Benzodiazepines as adjunct medication as needed. Scheduled duration 11 days |
|
| Outcomes | Time course (graphs) of withdrawal score, mydriasis, and side effects; mean max withdrawal score; time course of cardiovascular effects; mean duration of treatment for those who completed and those who did not complete treatment; number experiencing side effects; number completing detoxification | |
| Notes | Withdrawal and side effects rated by observers. Participants completed psychometric evaluations. Study supported by Boehringer Ingelheim and Sandoz SAE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were randomly assigned to ... one of ... three groups" but "In order to achieve 30 patients in each group a total of 170 (40 methadone, 68 clonidine, 62 guanfacine) had to be included." Comment: It is questionable whether a truly random sequence generation could achieve the different group sizes reported |
| Allocation concealment (selection bias) | High risk | Method of allocation not reported, but recruitment continued until 30 participants in each group had completed 12 or more days of treatment, suggesting inadequate concealment of allocation |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Research nurses (observers) and physician blind to treatment condition |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Research nurses (observers) and physician blind to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis based on participants who completed 12 or more days of treatment. No information on participants who dropped out |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
San 1994.
| Methods | Randomised, controlled, double‐blind study undertaken in 3 phases with third group (guanfacine 4 mg/day) introduced in second phase | |
| Participants | Setting: inpatient, Barcelona, Spain. Participants: 144 heroin dependent by DSM‐III‐R. Group sizes: (1) n = 75, (2) n = 43, (3) n = 26. Stated that there were no differences between groups. Mean age: 27.1 years. 71% men. Using heroin mean 656 mg/day, 52% HIV positive |
|
| Interventions | Stabilised on methadone, dose dependent on body weight and heroin consumption. Methadone tapered to 10% (methadone group) or 50% (guanfacine groups) of initial dose prior to detoxification. (1) Continued tapered methadone (3 divided doses/day). (2) Guanfacine 3 mg substituted for methadone on day 9. (3) Guanfacine 4 mg substituted for methadone on day 9. Benzodiazepines and hypnotics available as adjunct medication. Scheduled duration 18 days |
|
| Outcomes | Mean daily doses medication; retention rate (graph); mean daily withdrawal scores (graphs); mean dose diazepam | |
| Notes | Opiate withdrawal checklist completed by nurses. Opiate withdrawal scale completed by participants. Study supported by grants, but details of grant source unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The pharmaceutical unit of the hospital was responsible for the randomization ..." Comment: Although the method of sequence generation was not reported, the similarity of the groups and allocation by the separate unit suggests the method was adequate |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacy |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Nursing staff, treating doctor, and participants blind |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Nursing staff, treating doctor, and participants blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant difference between groups in length of stay. The similarity in withdrawal scores for the 3 groups indicates that drop‐out is unlikely to have a clinically significant impact on this outcome |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Senay 1983.
| Methods | Randomised, controlled, double‐blind trial. Codependence on other drugs or abuse of alcohol exclusion criteria. Component of multicentre study ‐ see also Kleber 1985 | |
| Participants | Setting: outpatient treatment, Chicago, USA. Participants: 61, stabilised on methadone 20 mg/day. Group sizes: (1) n = 30, (2) n = 31. 100% men. Mean duration of addiction: 11.4 years |
|
| Interventions | (1) Clonidine, 0.5 mg day 1, additional 0.1 to 0.3 mg/day as needed to max 1.0 mg/day, tapered after day 10. (2) Methadone decreased 1 mg/day. Diuretics and chloral hydrate only adjunct medications, both groups. Scheduled duration of study interventions 30 days |
|
| Outcomes | Retention in treatment; number completing detoxification; urine screening results; concomitant medications and illicit drugs; incidence of adverse experiences | |
| Notes | Withdrawal severity not assessed. Study partly supported by funds from Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized, double‐blind, parallel group, 30‐day study." Comment: Method of sequence generation not reported, and group similarities not assessed |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | Observers making symptom ratings blind, but those assessing adverse reactions and vital signs were aware of group allocations |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated, and these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention is an outcome. Other outcomes reported in such a way that is not influenced by drop‐out |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Sos 2000.
| Methods | Randomised controlled trial | |
| Participants | Setting: outpatient, Budapest, Hungary. Participants: 26 intravenous heroin users. Group sizes: (1) n = 16, (2) n = 10. Groups similar on demographics and drug use. Mean age: 24 years. 80.8% men. Mean duration of heroin use: 1.7 years. |
|
| Interventions | (1) Tizanidine 3 x 80 mg/day. (2) "Usual" treatment (symptomatic medication). (1) 13/16 and (2) 10/10 received tramadol (narcotic analgesic) with doses reduced by tapering. Various other adjunct medications as required. Scheduled duration 10 days |
|
| Outcomes | Comparison of withdrawal scores; graphs of subjective severity of tremor and diarrhoea; number completing withdrawal; number relapsing during follow‐up | |
| Notes | Participants rated withdrawal (7 items, each rated 0 to 5) daily. Published in Hungarian, information extracted with help of interpreter. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were divided into two groups." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | No blinding reported |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | No blinding, but these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts during withdrawal |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Umbricht 2003.
| Methods | Randomised controlled trial with stratification on withdrawal severity, pain, cocaine use, CD4 cell count. Groups similar except (1) more likely to have been admitted for fever/cellulitis | |
| Participants | Setting: inpatient treatment, AIDS service, Baltimore, USA. Participants: 55 HIV‐positive, opioid‐dependent by self report and physical examination, hospitalised for acute medical illness. Group sizes: (1) n = 21 (not considered for this review), (2) n = 16, (3) n = 18. (1) 71% (2) 69% (3) 44% men. Mean age (± SD): 39.7 ± 5.6. Duration of heroin use: about 18 years. Concurrent alcohol dependence, enrolment in methadone maintenance treatment both exclusion criteria. 95% to 100% African‐American |
|
| Interventions | All stabilised with morphine 10 mg im every 4 hours as needed up to 6 hours prior to enrolment in study. 3‐day taper with: (1) Buprenorphine 0.6 mg im every 4 hours day 1, every 6 hours day 2, every 8 hours day 3. (2) Clonidine, oral 0.2 mg loading dose, 0.1 mg every 4 hours day 1, every 6 hours day 2, every 8 hours day 3. (3) Methadone, oral 30 mg day 1, 20 mg day 2, 10 mg day 3. All received clonidine transdermal patch day 4. No adjunct treatment for withdrawal. Scheduled duration 3 days |
|
| Outcomes | Withdrawal severity; completion rate; adverse effects; use of supplemental morphine for pain | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale (participants) and Objective Opiate Withdrawal Scale (observers). Supported by National Institute on Drug Abuse Intramural Research Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomly assigned ..." "... patients were stratified on four characteristics ..." Comment: Method of sequence generation not specifically reported but with stratification on 4 characteristics is likely to be computer based |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Quote: "To maintain the blind, one active medication and two inactive medications were administered to all participants." |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated, placebos used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out was related to the acute medical condition that was the reason for hospital admission and was unlikely to introduce bias to outcome assessments. Statistical methods allowed for missing data and variation in time of assessment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Vilalta 1987.
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient, hospital, Barcelona, Spain. Participants: 32 heroin users, admitted for treatment of organic disease (mainly infectious disease related to consumption of drugs). Group sizes: (1) n = 14, (2) n = 8, (3) n = 10. Mean age: 23 years. 65% men. |
|
| Interventions | (1) Methadone, 30 mg/day. (2) Clonidine, 10 μg/kg/day. (3) Levomepromazine (neuroleptic) 75 mg/day. Doses of all drugs increased until stable, maintained 3 days, then tapered. Treatment scheduled for around 8 days |
|
| Outcomes | Mean opioid withdrawal score; mean score of secondary effects; mean score of adjustment to hospital setting; number completing treatment | |
| Notes | Ratings of withdrawal (24 items), side effects (19 items), attitudes and disruptive behaviour during hospitalisation (11 items) daily by single observer. Urine screening used. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Difference in drop‐out rates insufficient to distort reported outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Washton 1981.
| Methods | Randomised, placebo‐controlled, double‐blind trial. May include some participants from multicentre study ‐ see Senay 1983 | |
| Participants | Setting: outpatient, New York, USA. Participants: 26 withdrawing from methadone maintenance (n = 19) or heroin or methadone or both (n = 7). Group sizes: 13 in each group. Groups stated as similar. Mean age: 31 years. 85% men. Mean duration of addiction 10 years |
|
| Interventions | Stabilised for 3 weeks on methadone 15 to 30 mg/day, then: (1) Clonidine, dose titrated against symptoms and side effects to max 1.2 mg/day. (2) Methadone reduced by 1 mg/day. Clinic visits 3 to 5 times per week. Scheduled duration of study intervention 30 days |
|
| Outcomes | Number achieving 10 days opioid free; number initiating naltrexone maintenance treatment | |
| Notes | Ratings of withdrawal not reported. Partial support from National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned." Comment: Method of sequence generation not reported and insufficient information on group characteristics to make a judgement on adequacy |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Participants and investigators blind to medication. Investigators not informed of blood pressure measurements to avoid breaking blind |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Participants and investigators blind to medication. Investigators not informed of blood pressure measurements to avoid breaking blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion of treatment is the only outcome included in analyses for this review. Drop‐out was not clearly reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Yu 2008.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Use of long‐acting opioids (methadone, l‐alpha‐acetylmethadol, buprenorphine) an exclusion criterion | |
| Participants | Setting: inpatient, multiple sites, USA. Participants: 68 opioid‐dependent by DSM‐IV. Group sizes: (1) n = 35, (2) n = 33. Groups similar on demographics. Mean age: 41 years. 87% men. 67/68 heroin users, 1 using hydromorphone; 67.6% iv users; 17.5% married; 68% worked at least part time |
|
| Interventions | Stabilised on morphine sulphate 3 days (up to 100 mg/day in 4 doses sc). (1) Lofexidine 3.2 mg/day. (2) Placebo in 4 divided doses for 4 days. On day 8 (1) lofexidine 1.6 mg/day or (2) placebo. Placebo days 9 to 11. Scheduled duration of treatment 11 days |
|
| Outcomes | Mean withdrawal score; number retained in treatment; standing and sitting blood pressure | |
| Notes | Principal measure used was withdrawal assessed with Modified Himmelsbach Opiate Withdrawal Scale (completed by observer), but other scales also used including participant‐completed scales. Study terminated early due to significant findings. Study funded by research grants from National Institute on Drug Abuse. Britannia Pharmaceuticals provided medication and placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Coordinating Center ... generated a randomization sequence for each site separately, in blocks of four, using non‐sequential subject numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "(Coordinating Center) provided site with a randomization number which corresponded to a specific drug therapy kit that had previously been shipped to the site." |
| Blinding (performance bias and detection bias) Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Double‐blind stated, medications provided as identical tablets |
| Blinding (performance bias and detection bias) Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated, medications provided as identical tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Difference in drop‐out insufficient to distort outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | Trial stopped early due to significant findings |
DSM‐IIIR: Diagnostic and Statistical Manual of Mental Health ‐ Third Edition Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Health ‐ Fourth Edition; h: hour; HIV: human immunodeficiency virus; ICD: International Classification of Diseases; im: intramuscular; iv: intravenous; max: maximum; sc: subcutaneous; SD: standard deviation; SOWS: Short Opiate Withdrawal Scale; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhondzadeh 2000 | Comparison of clonidine and baclofen, which is not one of the modalities defined by the inclusion criteria (clonidine is the control intervention for the study) |
| Batey 1985 | Compares outcomes for 2 treatment regimens, 1 based on clonidine and 1 on clomethiazole (Heminevrin) plus symptomatic medications. Interventions not offered concurrently, limited information reported on medications and participant characteristics, and data collection probably retrospective |
| Bearn 1998 | Participants able to choose methadone or lofexidine as treatment approach, with 10‐day and 5‐day lofexidine regimens offered serially. Non‐random allocation introduces risk of bias. This version of review restricted to randomised controlled trials |
| Beswick 2003 | Randomised controlled trial comparing naloxone and placebo as adjuncts to lofexidine for management of opioid withdrawal. Regimens involving opioid antagonists are not one of the modalities defined by the inclusion criteria for this review |
| Casey 1988 | Randomised controlled trial comparing clonidine with reducing doses of methadone. Very little information on participant characteristics. Outcome data limited as no participants completed the study |
| Chattopadhyay 2010 | Study presented as randomised controlled trial, but allocation to groups was alternate, not random. Treatment does not appear to be voluntary ‐ participants arrested and subsequently taken to hospital outpatient department for treatment. Aim to assess effectiveness of tramadol; clonidine used as comparison intervention. Insufficient participant information and insufficient outcome data |
| Cheskin 1994 | Randomised, controlled, double‐blind study comparing clonidine and buprenorphine, which is not one of the modalities defined by the inclusion criteria |
| Chuang 1999 | Focus appears to be on assessment of withdrawal rather than clonidine as a modality for management of withdrawal. Unclear if participants were undergoing detoxification on a voluntary basis |
| Day 2011 | Comparison of inpatient and outpatient settings for opioid withdrawal managed with lofexidine |
| Gerra 2001 | Comparison of lofexidine and clonidine when administered in combination with opioid antagonist. Antagonist‐induced withdrawal is not one of the modalities defined by the inclusion criteria |
| Ghodse 1994 | Comparison of clonidine and placebo as adjuncts to tapered methadone for management of opioid withdrawal. Intervention not one defined by inclusion criteria |
| Gold 1978 | Effectively single‐group study (placebo controls and double‐blind method used only for first 2 doses of medication). Insufficient outcome data |
| Gold 1979 | Compares effect of clonidine on opioid withdrawal for group withdrawing from methadone and group withdrawing from heroin. Insufficient information on treatment and participant characteristics; non‐random allocation |
| Gold 1980a | Placebo controls and double‐blind methods used only for first 2 doses of medication and naloxone challenges at the end of withdrawal. 3 groups identified (non‐random allocation) on basis of methadone dose prior to clonidine. No treatment comparison |
| Gold 1980b | Reports outcomes of treatment with clonidine for 100 participants withdrawing from methadone. No treatment comparison |
| Gossop 1989 | Comparison of 3 cohorts of heroin‐dependent people undergoing inpatient detoxification. Not a controlled study ‐ medication regimens variable |
| Hartmann 1991 | Comparison of clonidine and Acetophen (enkephalinase inhibitor), which is not one of the modalities defined by the inclusion criteria. Limited data on treatment outcomes |
| Huertas 1995 | Compared guanfacine alone with a combination of guanfacine plus propoxyphene, which is not one of the modalities defined by the inclusion criteria |
| Janiri 1994 | Compared clonidine with lefetamine (an analgesic with partial opioid agonist activity) and buprenorphine, neither of which are modalities defined by the inclusion criteria |
| Jasinski 1985 | Comparison of clonidine, morphine, and placebo in opioid‐dependent participants. Not a complete withdrawal intervention. Morphine was withheld for 24 hours only for tests of the pharmacology of clonidine |
| Jimenez‐Lerma 2002 | Comparison of (1) a calcium channel blocking agent plus dextropropoxyphene, (2) dextropropoxyphene plus a benzodiazepine, and (3) guanfacine with increasing doses of naltrexone from day 4. The comparison modalities were not those defined by the inclusion criteria. Group allocation was sequential, not random |
| Kasvikis 1990 | No concurrent treatment comparison ‐ cohort treated with clonidine compared with other cohorts treated with reducing doses of methadone |
| Lerner 1995 | Randomised controlled trial comparing outcomes of opioid withdrawal managed with clonidine in home or outpatient setting. No treatment comparison as defined by inclusion criteria |
| Lin 2014 | Randomised controlled trial comparing dextromethorphan and placebo as adjuncts to clonidine for the management of opioid withdrawal. Comparison is not one defined for this review |
| Malhotra 1997 | Comparison of clonidine with meperidine (pethidine) for management of opioid withdrawal. Group allocation was alternate, not random; meperidine is not one of the modalities defined by the inclusion criteria; and insufficient outcome data were reported |
| McCambridge 2007 | Analysis of outcomes for participants randomly allocated to lofexidine‐naloxone or lofexidine‐placebo, and those ineligible for participation in randomised controlled trial or who refused random allocation and received reducing doses of methadone over 10 days. Significant differences in groups, indicating high risk of bias from allocation method for comparison of lofexidine and methadone. Insufficient outcome data relating to period of acute withdrawal (main outcomes were completion of withdrawal, retention in postdetoxification treatment, and abstinence at follow‐up postdetoxification) |
| O'Connor 1995 | Controlled trial comparing clonidine only with clonidine combined with naltrexone, which is not one of the modalities defined by the inclusion criteria. Group allocation by choice, not random |
| O'Connor 1997 | Randomised controlled trial comparing clonidine only with clonidine plus naltrexone, and buprenorphine followed by clonidine plus naltrexone. Neither of the comparisons are modalities defined by the inclusion criteria |
| Ockert 2011 | Retrospective study assessing effectiveness of adding stimulant to regimen of symptomatic medication (including clonidine) for management of opioid withdrawal |
| Pini 1991 | Comparison of dapiprazole, clonidine, and placebo for management of opioid withdrawal. Methadone administered in decreasing doses for 6 days while doses of study medications increased to maximum day 6. Insufficient outcome data. Unclear if group allocation was random |
| Rezaiyan 2014 | Randomised controlled trial comparing low‐dose naltrexone and placebo as adjuncts to clonidine for the management of opioid withdrawal. Comparison is not one defined by the inclusion criteria for this review |
| Strang 1997 | Randomised controlled trial comparing detoxification in specialist drug dependence unit (managed with reducing doses of methadone) and detoxification in general psychiatric ward of hospital (managed with clonidine). Primary purpose of study was to investigate effect of cue exposure on postwithdrawal outcomes; effect of setting was a secondary study (participants not randomly allocated to setting). Insufficient outcome data. Limited data on modification of signs and symptoms of withdrawal |
| Wilson 1993 | Comparison of methadone plus clonidine and clonidine alone for management of opioid withdrawal. Non‐concurrent cohort study. Insufficient outcome data |
| Wylie 1995 | Comparison of lofexidine and symptomatic medications for management of opioid withdrawal. Insufficient outcome data. No details of characteristics of participants. No concurrent treatment comparison |
Differences between protocol and review
In the present update, we again modified the search strategy, adding Web of Science as one of the databases searched. We also removed the term 'Metabolic Detoxication, Drug' from the MEDLINE and CENTRAL searches, as this now maps to 'metabolic inactivation', which is not relevant to this review.
Contributions of authors
Linda Gowing assessed each potentially relevant study according to identified inclusion and exclusion criteria.
All review authors confirmed inclusion and exclusion decisions.
Linda Gowing extracted key information from included studies and compiled a first draft of the review.
Mike Farrell, Robert Ali, and Jason White confirmed and commented on the review content.
Sources of support
Internal sources
-
Drug and Alcohol Services, South Australia, Australia.
Two authors (LG, RA) are employees of DASSA.
External sources
No sources of support supplied
Declarations of interest
Linda Gowing: None known.
Michael Farrell: None known.
Robert Ali: The review author's institution has received untied educational grants from Reckitt Benckiser to conduct scientific research and convene scientific meetings. No personal fees were paid to the review author.
Jason M White: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Batey 1987 {published data only}
- Batey R, Liddle C, Craig P. A placebo controlled trial of clonidine in the outpatient management of heroin withdrawal. Australian Drug and Alcohol Review 1987;6(1):11‐4. [Google Scholar]
Bearn 1996 {published data only}
- Bearn J, Gossop M, Strang J. Randomised double‐blind comparison of lofexidine and methadone in the in‐patient treatment of opiate withdrawal. Drug and Alcohol Dependence 1996;43(1‐2):87‐91. [DOI] [PubMed] [Google Scholar]
Benos 1985 {published data only}
- Benos VJ. Clonidine in opiate withdrawal syndrome [Clonidin beim opiatentzugssyndrom]. Fortschritte der Medizin 1985;103(42):991‐5. [PubMed] [Google Scholar]
Bertschy 1997 {published data only}
- Bertschy G, Bryois C, Bondolfi G, Velardi A, Budry P, Dascal D, et al. The association carbamazepine‐mianserin in opiate withdrawal: a double blind pilot study versus clonidine. Pharmacological Research 1997;35(5):451‐6. [DOI] [PubMed] [Google Scholar]
Bruno 1979 {published data only}
- Bruno F, Franceschini G. Treatment of opiate withdrawal syndrome with nonnarcotic drugs in addicts undergoing voluntary detoxication. I. Effect of clonidine [Il controllo della sindrome d'astinenza immediata da oppiacei con farmaci ad azione non narcotica in soggetti tossico‐dipendenti in disintossicazione volontaria. (1) Prime valutazioni cliniche sull'efficacia della clonidina]. Lavoro Neuropsichiatrico 1979;65(1‐2):1‐15. [Google Scholar]
Cami 1985 {published data only}
- Camí J, Torres S, San L, Solé À, Guerra D, Ugena B. Efficacy of clonidine and of methadone in the rapid detoxification of patients dependent on heroin. Clinical Pharmacology and Therapeutics 1985;38(3):336‐41. [DOI] [PubMed] [Google Scholar]
Carnwath 1998 {published data only}
- Carnwath T, Hardman J. Randomised double‐blind comparison of lofexidine and clonidine in the out‐patient treatment of opiate withdrawal. Drug and Alcohol Dependence 1998;50(3):251‐4. [DOI] [PubMed] [Google Scholar]
Gerra 1995 {published data only}
- Gerra G, Marcato A, Caccavari R, Fontanesi B, Delsignore R, Fertonani G, et al. Clonidine and opiate receptor antagonists in the treatment of heroin addiction. Journal of Substance Abuse Treatment 1995;12(1):35‐41. [PubMed] [Google Scholar]
Gerra 2000 {published data only}
- Gerra G, Zaimovic A, Rustichelli P, Fontanesi B, Zambelli U, Timpano M, et al. Rapid opiate detoxification in outpatient treatment: relationship with naltrexone compliance. Journal of Substance Abuse Treatment 2000;18(1):185‐91. [DOI] [PubMed] [Google Scholar]
Gupta 1988 {published data only}
- Gupta AK, Jha BK. Clonidine in heroin withdrawal syndrome: a controlled study in India. British Journal of Addiction 1988;83(9):1079‐84. [DOI] [PubMed] [Google Scholar]
Howells 2002 {published and unpublished data}
- Howells C, Allen S, Gupta J, Farrell M. A double blind study comparing the efficacy of methadone and lofexidine in the treatment of opiate detoxification in prisoners at HMP Winchester. Britannia Pharmaceuticals Ltd 1999.
- Howells C, Allen S, Gupta J, Stillwell G, Marsden J, Farrell M. Prison based detoxification for opioid dependence: a randomised double blind controlled trial of lofexidine and methadone. Drug and Alcohol Dependence 2002;67(2):169‐76. [DOI] [PubMed] [Google Scholar]
Jiang 1993 {published data only}
- Jiang Z. Rapid detoxification with clonidine for heroin addiction: a comparative study on its efficacy vs methadone. Chinese Journal of Neurology and Psychiatry 1993;26(1):10‐3. [Google Scholar]
Kahn 1997 {published data only}
- Kahn A, Mumford JP, Rogers GA, Beckford H. Double‐blind study of lofexidine and clonidine in the detoxification of opiate addicts in hospital. Drug and Alcohol Dependence 1997;44(1):57‐61. [DOI] [PubMed] [Google Scholar]
Kleber 1985 {published and unpublished data}
- Kleber HD, Riordan CE, Rounsaville B, Kosten T, Charney D, Gaspari J, et al. Clonidine in outpatient detoxification from methadone maintenance. Archives of General Psychiatry 1985;42(4):391‐4. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Comparison of clinician ratings to self reports of withdrawal during clonidine detoxification of opiate addicts. American Journal of Drug and Alcohol Abuse 1985;11(1‐2):1‐10. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Relationship of depression to clonidine detoxification of opiate addicts. Comprehensive Psychiatry 1984;25(5):503‐8. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten T, Kleber H. Success and failure at outpatient opioid detoxification: evaluating the process of clonidine and methadone assisted withdrawal. Journal of Nervous and Mental Disease 1985;173(2):103‐10. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li M, Chen K, Mo Z. Use of qigong therapy in the detoxification of heroin addicts. Alternative Therapies in Health and Medicine 2002;8(1):50‐9. [PubMed] [Google Scholar]
Lin 1997 {published data only}
- Lin S‐K, Strang J, Su L‐W, Tsai C‐J, Hu W‐H. Double‐blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug and Alcohol Dependence 1997;48(2):127‐33. [DOI] [PubMed] [Google Scholar]
Muga 1990 {published data only}
- Muga R, Tor J, Forteza‐Rei J, Jacas C, Altes J, Mestre L. Comparative effectiveness of alpha‐2 adrenergic agonists (clonidine and guanfacine) in the hospital detoxification of opiate addicts [Eficacia comparada de agonistas alpha2‐adrenergicos (clonidina‐guanfacina) en la desintoxicacion hospitalaria de adictos a opiaceos]. Medicina Clinica 1990;94(5):169‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Nazari 2013 {published data only}
- Nazari SM, Naseri M, Mokri A, Davati A, Kamalinejad M. Hab‐o Shefa (an Iranian traditional medicine compound) in withdrawal syndrome and its effects in acute detoxification of opiates addict: a randomized, double blind, clinical trials. Journal of Medicinal Plant Research 2013;7(22):1628‐35. [Google Scholar]
San 1990 {published data only}
- Cami J, Gilabert M, San L, Torre R. Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. British Journal of Addiction 1992;87(8):1145‐51. [DOI] [PubMed] [Google Scholar]
- San L, Camí J, Peri JM, Mata R, Porta M. Efficacy of clonidine, guanfacine and methadone in the rapid detoxification of heroin addicts: a controlled clinical trial. British Journal of Addiction 1990;85(1):141‐7. [DOI] [PubMed] [Google Scholar]
- San L, Peri JM, Mata R, Porta M. Success and failure at inpatient heroin detoxification. British Journal of Addiction 1989;84(1):81‐7. [DOI] [PubMed] [Google Scholar]
San 1994 {published data only}
- San L, Fernández T, Camí J, Gossop M. Efficacy of methadone versus methadone and guanfacine in the detoxification of heroin‐addicted patients. Journal of Substance Abuse Treatment 1994;11(5):463‐9. [DOI] [PubMed] [Google Scholar]
Senay 1983 {unpublished data only}
- Senay E, Tennant FS, Washton AM. [Boehringer Ingelheim GmbH report number U85‐0844]. Boehringer Ingelheim Pty Ltd 1983.
Sos 2000 {published data only}
- Sos I, Kiss N, Csorba J, Gerevich J. Tizanidine in the treatment of acute withdrawal symptoms in heroin dependent patients [A tizanidin hatekonysaga heroinfuggo betegek akut megvonasi tuneteinek kezeleseben]. Orvosi Hetilap 2000;141(15):783‐6. [PubMed] [Google Scholar]
Umbricht 2003 {published data only}
- Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin‐dependent patients with HIV infection. Drug and Alcohol Dependence 2003;69(3):263‐72. [DOI] [PubMed] [Google Scholar]
Vilalta 1987 {published data only}
- Vilalta J, Treserra J, Garcia‐Esteve L, Garcia‐Giralt M, Cirera E. Methadone, clonidine and levomepromazine in the treatment of opiate abstinence syndrome: double‐blind clinical trial in heroin‐addicted patients admitted to a general hospital for organic pathology [Metadona, clonidina y levomepromacina en el tratamiento del sindrome de abstinencia a opiaceos: ensayo clinico a doble ciego en pacientes heroinomanos ingresados por patologia organica en un hospital general]. Medicina Clinica 1987;88(17):674‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Washton 1981 {published data only}
- Washton AM, Resnick RB. Clonidine for opiate detoxification ‐ outpatient clinical trials. American Journal of Psychiatry 1980;137(9):1121‐2. [DOI] [PubMed] [Google Scholar]
- Washton AM, Resnick RB. Clonidine versus methadone for opiate detoxification. The Lancet 1980;2(8207):1297. [DOI] [PubMed] [Google Scholar]
- Washton AM, Resnick RB. Clonidine vs methadone for opiate detoxification: double‐blind outpatient trials. In: Harris LS editor(s). Problems of Drug Dependence, 1980. Proceedings of the 42nd Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc. Washington D.C.: Department of Health and Human Services, 1981. [Google Scholar]
- Washton AM, Resnick RB, Rawson RA. Clonidine hydrochloride: a nonopiate treatment for opiate withdrawal. NIDA Research Monograph 1979;27:233‐9. [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Yu E, Miotto K, Akerele E, Montgomery A, Elkashef A, Walsh R, et al. A Phase 3 placebo‐controlled, double‐blind, multi‐site trial of the alpha‐2‐adrenergic agonist, lofexidine, for opioid withdrawal. Drug and Alcohol Dependence 2008;97:158‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E, Miotto K, Akerele E, O'Brien CP, Ling W, Kleber H, et al. Clinical pharmacokinetics of lofexidine, the alpha 2‐adrenergic receptor agonist, in opiate addicts plasma using a highly sensitive liquid chromatography tandem mass spectrometric analysis. American Journal of Drug and Alcohol Abuse 2008;34(5):611‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhondzadeh 2000 {published data only}
- Ahmadi‐Abhari SA, Akhondzadeh S, Assadi SM, Shabestari OL, Farzanehgan ZM, Kamlipour A. Baclofen versus clonidine in the treatment of opiates withdrawal, side‐effects aspect: a double‐blind randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics 2001;26(1):67‐71. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Ahmadi‐Abhari SA, Assadi SM, Shabestari OL, Kashani AR, Farzanehgan ZM. Double‐blind randomised controlled trial of baclofen vs. clonidine in the treatment of opiates withdrawal. Journal of Clinical Pharmacy and Therapeutics 2000;25(5):347‐53. [DOI] [PubMed] [Google Scholar]
Batey 1985 {published data only}
- Batey R. Outpatient detoxification in heroin users ‐ a comparison of a hemineurin and a clonidine regime. Australian Alcohol/Drug Review 1985;4(1):53‐5. [Google Scholar]
Bearn 1998 {published data only}
- Bearn J, Gossop M, Strang J. Accelerated lofexidine treatment regimen compared with conventional lofexidine and methadone treatment for in‐patient opiate detoxification. Drug and Alcohol Dependence 1998;50:227‐32. [DOI] [PubMed] [Google Scholar]
Beswick 2003 {published data only}
- Beswick T, Best D, Bearn J, Gossop M, Rees S, Strang J. The effectiveness of combined naloxone/lofexidine in opiate detoxification: results from a double‐blind randomized and placebo‐controlled trial. American Journal on Addictions 2003;12(4):295‐305. [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addiction Biology 2003;8:49‐57. [DOI] [PubMed] [Google Scholar]
Casey 1988 {unpublished data only}
- Casey PR, Tomiak RHH. Clonidine in opiate withdrawal. Boehringer Ingelheim Ltd 1988.
Chattopadhyay 2010 {published data only}
- Chattopadhyay S, Singh OP, Bhattacharyya A, Sen S, Roy P, Debnath S. Tramadol versus clonidine in management of heroin withdrawal. Asian Journal of Psychiatry 2010;3(4):237‐9. [DOI] [PubMed] [Google Scholar]
Cheskin 1994 {published and unpublished data}
- Cheskin LJ, Fudala PJ, Johnson RE. A controlled comparison of buprenorphine and clonidine for acute detoxification from opioids. Drug and Alcohol Dependence 1994;36(2):115‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chuang 1999 {published data only}
- Chuang L, Jing L, Mingsheng H. The treatment of clonidine for opiate withdrawal symptoms. Chinese Journal of Psychiatry 1999;32(2):103‐5. [Google Scholar]
Day 2011 {published data only}
- Day E, Strang J. Outpatient versus inpatient opioid detoxification: a randomized controlled trial. Journal of Substance Abuse Treatment 2011;40(1):56‐66. [DOI] [PubMed] [Google Scholar]
Gerra 2001 {published data only}
- Gerra G, Zaimovic A, Giusti F, Gennaro C, Zambelli U, Gardini S, et al. Lofexidine versus clonidine in rapid opiate detoxification. Journal of Substance Abuse Treatment 2001;21(1):11‐7. [EMBASE: 2001291795] [DOI] [PubMed] [Google Scholar]
Ghodse 1994 {published data only}
- Ghodse H, Myles J, Smith SE. Clonidine is not a useful adjunct to methadone gradual detoxification in opioid addiction. British Journal of Psychiatry 1994;165(3):370‐4. [DOI] [PubMed] [Google Scholar]
Gold 1978 {published data only}
- Gold MS, Redmond DE, Kleber HD. Clonidine blocks acute opiate‐withdrawal symptoms. The Lancet 1978;2(8090):599‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 1979 {published data only}
- Gold MS, Redmond DE, Kleber HD. Noradrenergic hyperactivity in opiate withdrawal supported by clonidine reversal of opiate withdrawal. American Journal of Psychiatry 1979;136(1):100‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 1980a {published data only}
- Gold MS, Pottash ALC, Sweeney DR, Kleber HD. Effect of methadone dosage on clonidine detoxification efficacy. American Journal of Psychiatry 1980;137(3):375‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 1980b {published data only}
- Gold MS, Kleber HD. Clinical utility of clonidine in opiate withdrawal: a study of 100 patients. Progress in Clinical and Biological Research 1981;71:299‐306. [PubMed] [Google Scholar]
- Gold MS, Pottash AL, Sweeney DR, Davies RK, Kleber HD. Clonidine decreases opiate withdrawal‐related anxiety: possible opiate noradrenergic interaction in anxiety and panic. Substance and Alcohol Actions/Misuse 1980;1(2):239‐46. [PubMed] [Google Scholar]
Gossop 1989 {published data only}
- Gossop M. The detoxification of high dose heroin addicts in Pakistan. Drug and Alcohol Dependence 1989;24(2):143‐50. [DOI] [PubMed] [Google Scholar]
Hartmann 1991 {published data only}
- Hartmann F, Poirier M‐F, Bourdel M‐C, Loo H, LeComte J‐M, Schwartz J‐C. Comparison of Acetophen with clonidine for opiate withdrawal symptoms. American Journal of Psychiatry 1991;148(5):627‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huertas 1995 {published data only}
- Huertas D, Lopez‐Gomez I, Chamorro L, Martin M. A new combined treatment for opioid detoxification: the association between dextropropoxyphene and guanfacine [Nueva pauta combinada para la desintoxicacion de opiaceos: la linea DG]. Revista de Psiquiatria de la Facultad de Medicina de Barcelona 1995;22(5):109‐13. [PsycINFO 1996‐86268‐001] [Google Scholar]
Janiri 1994 {published data only}
- Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E. Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug and Alcohol Dependence 1994;36(2):139‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jasinski 1985 {published data only}
- Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal: differential effects on signs and symptoms. Archives of General Psychiatry 1985;42(11):1063‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jimenez‐Lerma 2002 {published data only}
- Jimenez‐Lerma JM, Landabaso M, Iraurgi I, Calle R, Sanz J, Gutierrez‐Fraile M. Nimodipine in opiate detoxification: a controlled trial. Addiction 2002;97(7):819‐24. [DOI] [PubMed] [Google Scholar]
Kasvikis 1990 {published data only}
- Kasvikis Y, Bradley B, Gossop M, Griffiths P, Marks I. Clonidine versus long and short term methadone aided withdrawal from opiates: an uncontrolled comparison. International Journal of the Addictions 1990;25(10):1169‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lerner 1995 {published data only}
- Lerner AG, Gelkopf M, Oyffe I, Sigal M. Home‐based inpatient treatment vs outpatient day clinic treatment: a preliminary report in opiate‐dependent patients. Journal of Nervous and Mental Disease 1995;183(11):715. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin S‐K, Pan C‐H, Chen C‐H. A double‐blind, placebo‐controlled trial of dextromethorphan combined with clonidine in the treatment of heroin withdrawal. Journal of Clinical Psychopharmacology 2014;34(4):508‐12. [DOI] [PubMed] [Google Scholar]
Malhotra 1997 {published data only}
- Malhotra A, Basu D, Chintalapudi M, Mattoo SK, Varma VK. Clonidine versus withdrawal using an opioid in in‐patient opioid detoxification. European Addiction Research 1997;3:146‐9. [Google Scholar]
McCambridge 2007 {published data only}
- McCambridge J, Gossop M, Beswick T, Best D, Bearn J, Rees S, et al. In‐patient detoxification procedures, treatment retention, and post‐treatment opiate use: comparison of lofexidine+naloxone, lofexidine+placebo, and methadone. Drug and Alcohol Dependence 2007;88(1):91‐5. [DOI] [PubMed] [Google Scholar]
O'Connor 1995 {published data only}
- O'Connor PG, Waugh ME, Carroll KM, Rounsaville BJ, Diakogiannis IA, Schottenfeld RS. Primary care‐based ambulatory opioid detoxification: the results of a clinical trial. Journal of General Internal Medicine 1995;10(5):255‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Connor PG, Waugh ME, Schottenfeld RS, Diakogiannis IA, Rounsaville BJ. Ambulatory opiate detoxification and primary care: a role for the primary care physician. Journal of General Internal Medicine 1992;7(5):532‐4. [DOI] [PubMed] [Google Scholar]
O'Connor 1997 {published data only}
- O'Connor PG, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ. Three methods of opioid detoxification in a primary care setting. A randomised trial. Annals of Internal Medicine 1997;127(7):526‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shi JM, O'Connor PG, Constantino JA, Carroll KM, Schottenfeld RS, Rounsaville BJ. Three methods of ambulatory opiate detoxification: preliminary results of a randomized clinical trial. NIDA Research Monograph Series 1993;132:309. [Google Scholar]
Ockert 2011 {published data only}
- Ockert DM, Volpicelli JR, Baier Jr AR, Coons EE, Fingesten A. A nonopioid procedure for outpatient opioid detoxification. Journal of Addiction Medicine 2011;5(2):110‐4. [DOI] [PubMed] [Google Scholar]
Pini 1991 {published data only}
- Pini LA, Sternieri E, Ferretti C. Dapiprazole compared with clonidine and a placebo in detoxification of opiate addicts. International Journal of Clinical Pharmacology Research 1991;11(2):99‐105. [MEDLINE: ] [PubMed] [Google Scholar]
- Pini LA, Sternieri E, Ferretti C. Dapiprazole versus clonidine and versus placebo in detoxification of opiate addicts. European Journal of Pharmacology 1990;183(2):209. [PubMed] [Google Scholar]
Rezaiyan 2014 {published data only}
- Rezaiyan MK, Moghadam HK, Khosrojerdi H, Afshari R. Very low‐dose naltrexone versus placebo in alleviating withdrawal manifestation. Clinical Toxicology 2014;52:368. [Google Scholar]
Strang 1997 {published data only}
- Dawe S, Gray JA. Craving and drug reward: a comparison of methadone and clonidine in detoxifying opiate addicts. Drug and Alcohol Dependence 1995;39(3):207‐12. [DOI] [PubMed] [Google Scholar]
- Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J, et al. Does post‐withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction 1993;88(9):1233‐45. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawe S, Richards D, Gossop M, Marks I, Strang J, et al. Can opiate addicts tell us about their relapse risk? Subjective predictors of clinical prognosis. Addictive Behaviors 1993;18(4):473‐90. [DOI] [PubMed] [Google Scholar]
- Strang J, Marks I, Dawe S, Powell J, Gossop M, Richards D, et al. Type of hospital setting and treatment outcome with heroin addicts. Results from a randomised trial. British Journal of Psychiatry 1997;171:335‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 1993 {published data only}
- Wilson RS, diGeorge WS. Methadone combined with clonidine versus clonidine alone in opiate detoxification. Journal of Substance Abuse Treatment 1993;10(6):529‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wylie 1995 {published data only}
- Wylie AS, Stewart AM. Lofexidine based regimen for opiate addicts. British Journal of General Practice 1995;45(393):217‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akhurst 1999
- Akhurst JS. The use of lofexidine by drug dependency units in the United Kingdom. European Addiction Research 1999;5:43‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amato 2011
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD005031.pub2] [DOI] [PubMed] [Google Scholar]
Amato 2013
- Amato L, Davoli M, Ferri M, Ali R. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003409.pub4] [DOI] [Google Scholar]
Broers 2000
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow‐up at 1 and 6 months. Drug and Alcohol Dependence 2000;58(1):85‐92. [DOI] [PubMed] [Google Scholar]
Day 2005
- Day E, Ison J, Strang J. Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004580.pub2] [DOI] [PubMed] [Google Scholar]
Farrell 1994
- Farrell M. Opiate withdrawal. Addiction 1994;89(11):1471‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 1980c
- Gold MS, Pottash AC, Sweeney DR, Kleber HD. Opiate withdrawal using clonidine. A safe, effective, and rapid nonopiate treatment. JAMA 1980;243(4):343‐6. [PubMed] [Google Scholar]
Gold 1989
- Gold MS, Pottash AC. The neurobiological implications of clonidine HCl. Annals of the New York Academy of Science 1989;362:191‐202. [DOI] [PubMed] [Google Scholar]
Gossop 1988a
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug and Alcohol Dependence 1988;21(3):253‐9. [DOI] [PubMed] [Google Scholar]
Gossop 1989b
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow‐up study. British Journal of Psychiatry 1989;154:348‐53. [DOI] [PubMed] [Google Scholar]
Gowing 2009a
- Gowing L, Ali R, White J. Opioid antagonists with minimal sedation for opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002021.pub3] [DOI] [PubMed] [Google Scholar]
Gowing 2009b
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002025.pub4] [DOI] [Google Scholar]
Gowing 2010
- Gowing L, Ali R, White J. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002022.pub3] [DOI] [Google Scholar]
Gowing 2015
- Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, et al. Global statistics on addictive behaviours: 2014 status report. Addiction 2015;110(6):904‐19. [DOI: 10.1111/add.12899] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 . Available from www.cochrane‐handbook.org.
Kleber 1982
- Kleber HD, Riordan CE. The treatment of narcotic withdrawal: a historical review. Journal of Clinical Psychiatry 1982;43(6):30‐4. [PubMed] [Google Scholar]
Lipton 1983
- Lipton D, Maranda M. Detoxification from heroin dependency: an overview of method and effectiveness. Advances in Alcohol and Substance Abuse 1983;2(1):31‐55. [Google Scholar]
Mark 2001
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug & Alcohol Dependence 2001;61(2):195‐206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mattick 1996
- Mattick RP, Hall W. Are detoxification programmes effective?. The Lancet 1996;347:97‐100. [DOI] [PubMed] [Google Scholar]
Minozzi 2014
- Minozzi S, Amato L, Davoli M. Detoxification treatments for opiate dependent adolescents. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD006749.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 1986
- Phillips GT, Gossop M, Bradley B. The influence of psychological factors on the opiate withdrawal syndrome. British Journal of Psychiatry 1986;149:235‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Preston 1985
- Preston KL, Bigelow GE. Pharmacological advances in addiction treatment. International Journal of the Addictions 1985;20(6&7):845‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Satel 1993
- Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM‐IV?. American Journal of Psychiatry 1993;150(5):695‐704. [DOI] [PubMed] [Google Scholar]
Sheridan 1999
- Sheridan J, Cook C, Strang J. Audit of the in‐patient management of opioid withdrawal using lofexidine hydrochloride. Journal of Substance Use 1999;4(1):29‐34. [CINAHL 1999061746] [Google Scholar]
Spencer 1989
- Spencer L, Gregory M. Clonidine transdermal patches for use in outpatient opiate withdrawal. Journal of Substance Abuse Treatment 1989;6:113‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tetrault 2009
- Tetrault JM, O'Connor PG. Management of opioid intoxication and withdrawal. In: Ries RK, Fiellin DA, Miller SC, Siatz R editor(s). Principles of Addiction Medicine. 4th Edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2009:589‐606. [Google Scholar]
Vaillant 1988
- Vaillant GE. What can long‐term follow‐up teach us about relapse and prevention of relapse in addiction?. British Journal of Addiction 1988;83(10):1147‐57. [DOI] [PubMed] [Google Scholar]
World Drug Report 2013
- United Nations Office on Drugs and Crime. World Drug Report 2013. www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf (accessed 18 March 2014).


