This study evaluates time spent outside, number of phototoxic reactions, disease-specific quality of life, usage of protective clothing, and adverse events in patients treated with afamelanotide for erythropoietic protoporphyria.
Key Points
Question
What is the association of afamelanotide treatment with outcomes for patients with erythropoietic protoporphyria in clinical practice?
Findings
This postauthorization safety and efficacy cohort study of 117 patients with erythropoietic protoporphyria showed an association with increased time spent outside (6.1 hours/week) and quality of life score (14%) during treatment with afamelanotide; phototoxic reactions were less painful. Minor and self-limiting adverse events were reported, such as nausea, fatigue, and headache.
Meaning
In routine clinical practice, afamelanotide treatment may be more effective in preventing phototoxic effects than previously estimated during placebo-controlled trials.
Abstract
Importance
The effectiveness of afamelanotide treatment in patients with erythropoietic protoporphyria (EPP) in clinical practice who experience pain after light exposure that substantially impairs quality of life is unknown.
Objective
To evaluate the association of afamelanotide treatment with outcomes in patients with EPP in regular practice during longer-term follow-up.
Design, Setting, and Participants
This single-center, prospective postauthorization safety and efficacy cohort study was directed and approved by the European Medicines Agency. Data were collected from patients with EPP treated with afamelanotide at Erasmus MC between June 2016 and September 2018. Analysis began October 2018.
Main Outcomes and Measures
Time spent outside during treatment, number of phototoxic reactions, disease-specific quality of life, usage of protective clothing, and adverse events.
Results
A total of 117 patients with EPP (59 women [50.4%]; mean [SD] age, 43.0 [15.5] years) were treated with afamelanotide. Nearly all patients continued treatment (115 [98%]) with a median (interquartile range) follow-up of 2.0 (1.3-2.1) years. Compared with baseline, mean time spent outside during treatment increased significantly by an added 6.1 hours per week (95% CI, 3.62-8.67; P < .001). Mean quality of life score improved significantly by 14.01% (95% CI, 4.53%-23.50%; P < .001). Phototoxic reactions were less painful (β, −0.85; 95% CI, −1.43 to −0.26; P < .001), but there was no significant difference in number or duration of reactions. Minor self-limiting adverse events occurred, such as nausea, fatigue, and headache.
Conclusions and Relevance
This cohort study found that afamelanotide treatment was associated with improved clinical outcomes and a good safety profile for patients with EPP. The treatment has clinically significant, sustained positive associations with quality of life, is associated with increased duration of sun exposure, and is associated with less severe phototoxic reactions.
Introduction
Erythropoietic protoporphyria (EPP) (OMIM 177000) is a rare inherited disorder of heme biosynthesis, causing painful photosensitivity.1 Erythropoietic protoporphyria is caused by FECH or ALAS2 gene mutations, leading to protoporphyrin IX accumulation in erythroid cells.2,3 It severely affects patients’ quality of life (QoL)4 by causing severe pain after light exposure, which lasts several days and is unresponsive to analgesics.2,5 This results in sunlight-avoiding behavior, limiting daily and social activities.4
Until recently, there was no effective therapy for EPP6,7; management consisted of strict avoidance of sunlight and wearing protective clothing. In 2016, afamelanotide became available after the European Medicines Agency recommended approval in the European Union with an obligatory postauthorization safety and efficacy study. Afamelanotide is a potent α–melanocyte-stimulating hormone analogue, which increases eumelanin and stimulates anti-inflammatory and antioxidative pathways.8 Placebo-controlled trials demonstrated statistically significant effects but a relatively small reduction in EPP symptoms.9,10 We report on the association of afamelanotide treatment with outcomes in EPP in regular practice during longer-term follow-up.
Methods
In this single-center, prospective postauthorization safety and efficacy cohort study of afamelanotide, all adult patients with a confirmed diagnosis of EPP attending the outpatient clinic of the Erasmus University Medical Center in Rotterdam, the Netherlands, were eligible for inclusion (eFigure in the Supplement). Afamelanotide was offered as a controlled-release 16-mg implant, administered subcutaneously with an interval of at least 60 days, and a maximum of 4 implants per year as recommended in the European Union. Contraindications are pregnancy, lactation, liver and kidney failure, and age younger than 18 years. Data were collected between June 2016 and September 2018. The study was approved by the Medical Ethics Review Board of the Erasmus MC Rotterdam. All patients gave written informed consent.
The methods are extensively described in eMethods 1 in the Supplement. In short, data collection occurred during routine visits to the outpatient clinic, according to our standard health care practice and to the postauthorization safety and efficacy study protocol (eAppendix in the Supplement). The following data were collected: demographic features, dermatological screening, routine laboratory tests (kidney function, liver function tests, blood cell count, and protoporphyrin IX levels in erythrocytes), adverse events, diaries (eMethods 2 in the Supplement), questionnaires on QoL and light exposure (eMethods 1, 3, 4, and 5 in the Supplement), and sunlight score (objective data on light intensity, as measured by The Royal Netherlands Meteorological Institute). Baseline data were defined as data collected before the first administration of afamelanotide.
Outcome measures are time spent outside; QoL; number, severity, and duration of phototoxic reactions; protective clothing score; and adverse events. Secondary end points are provided in eMethods 6 and eTable 1 in the Supplement. All data were entered in a database following the postauthorization safety and efficacy study protocol, programmed in OpenClinica open source software, version 2.1. Data were summarized using means, median, range, and SDs for continuous variables and using frequencies and percentages for categorical variables. Linear mixed modeling was used to estimate the association of afamelanotide with outcomes (eMethods 1 in the Supplement). Per parameter, a mixed model was used to determine the treatment effect of afamelanotide, besides other possible influencing factors (eg, age). Missing data (only the missing responses) and outliers (values outside of an SD of 4) were excluded from analysis for all variables. Statistical analyses were performed with SPSS, version 24.0 (SPSS Inc). All statistical tests were 2-sided with a significance level of .05. Analysis began October 2018.
Results
A total of 117 patients started receiving afamelanotide and 115 (98%) continued treatment. Baseline characteristics are summarized in Table 1. Time spent outside in week 1 increased 1.85 hours per week with afamelanotide (95% CI, −0.07 to 3.78 hours; P = .06), and increased 1.41 hours per week per year on treatment (95% CI, 0.04-2.77 hours; P = .04). There was a significant variation over the year, and time spent outside varied by 7.67 hours (95% CI, −14.63 to −0.71) between the month with the lowest (January) and highest (August) number of hours. Time spent outside in week 5 showed a significant increase of 6.14 hours per week (95% CI, 3.62-8.67 hours; P < .001), where time spent outside varied by 6.40 hours (95% CI, 0.60-12.19) between the month with the lowest (November) and highest (August) number of hours (Table 2).
Table 1. Baseline Patient Demographic and Clinical Characteristics.
| Demographic and clinical characteristics | Mean (SD) [range] |
|---|---|
| Patients treated, No. | 117 |
| Age, y | 43.0 (15.5) [18.3-79.0] |
| Women, No. (%) | 59 (50.4) |
| BMI | 25.2 (4.6) [18.3-41.2] |
| Total PPIX level, median (IQR), μmol/L in erythrocytes | 40.0 (7.9-278.0) |
| Total implants, No. | 852 |
| Mean implants per person, median (IQR) | 8.0 (5.5-10.0) |
| Continuation rate, % | 98.3 |
| Follow-up time, y, median (IQR) | 2.0 (1.3-2.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; PPIX, protoporphyrin IX.
Table 2. Linear Mixed Models for Outcomes .
| Outcomes | β (95% CI) | P value |
|---|---|---|
| Time outside week 1, h | ||
| Baseline, median (IQR) | 7.4 (3.6 to 15.6) | NA |
| Treatment effect | 1.85 (−0.07 to 3.78) | .06 |
| Sex | 3.09 (−0.34 to 6.51) | .08 |
| Age, y | −0.03 (−0.15 to 0.08) | .57 |
| BMI | −0.12 (−0.46 to 0.23) | .51 |
| Montha | NA | <.001 |
| January | −1.06 (−7.81 to 5.68) | .76 |
| February | −0.80 (−4.56 to 2.95) | .67 |
| March | 1.74 (−2.04 to 5.52) | .37 |
| April | 2.08 (−2.37 to 6.52) | .36 |
| May | 3.20 (−1.90 to 8.30) | .22 |
| June | 2.20 (−2.78 to 7.11) | .38 |
| July | 2.19 (−2.72 to 7.10) | .38 |
| August | 6.61 (2.03 to 11.19) | .01 |
| September | 3.76 (−0.32 to 7.83) | .07 |
| October | 3.18 (−0.97 to 7.32) | .13 |
| November | 2.72 (−1.93 to 7.37) | .25 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0.15 (−0.15 to 0.45) | .32 |
| Time since first treatment, y | 1.41 (0.04 to 2.77) | .04 |
| Time outside week 5, h | ||
| Baseline, median (IQR) | 7.4 (3.6 to 15.6) | NA |
| Treatment effect | 6.14 (3.62 to 8.67) | <.001 |
| Sex | 3.75 (0.02 to 7.48) | .05 |
| Age, y | −0.03 (−0.16 to 0.10) | .68 |
| BMI | −0.14 (−0.53 to 0.25) | .48 |
| Montha | NA | .001 |
| January | 0.97 (−7.83 to 9.76) | .83 |
| February | 1.60 (−3.44 to 6.64) | .53 |
| March | 0.42 (−4.99 to 5.82) | .88 |
| April | 2.13 (−4.48 to 8.74) | .53 |
| May | −0.12 ( −6.36 to 6.12) | .97 |
| June | 2.23 (−3.89 to 8.36) | .47 |
| July | 5.98 (0.30 to 11.66) | .04 |
| August | 6.22 (0.97 to 11.47) | .02 |
| September | 3.09 (−1.61 to 7.79) | .20 |
| October | 3.69 (−1.58 to 8.96) | .17 |
| November | −0.18 (−6.19 to 5.83) | .95 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0.36 (−0.03 to 0.76) | .07 |
| Time since first treatment, y | 0.38 (−1.31 to 2.07) | .66 |
| EPP QoL (range, 0%-100%) | ||
| Baseline, median (IQR) | 30.6 (18.8 to 45.1) | NA |
| Treatment effect | 14.01 (4.53 to 23.50) | <.001 |
| Sex | −0.79 (−5.74 to 4.16) | .75 |
| Age, y | −0.22 (−0.46 to 0.02) | .07 |
| BMI | 0.28 (−0.25 to 0.81) | .30 |
| Montha | NA | .04 |
| January | 6.53 (−5.64 to 18.70) | .29 |
| February | 0.34 (−7.22 to 7.89) | .93 |
| March | 1.97 (−5.24 to 9.18) | .59 |
| April | 0.33 (−9.08 to 9.74) | .94 |
| May | 2.77 (−9.21 to 14.75) | .65 |
| June | 3.91 (−11.58 to 19.40) | .62 |
| July | 9.81 (−6.34 to 25.95) | .23 |
| August | 7.89 (−7.59 to 23.36) | .32 |
| September | 7.04 (−6.30 to 20.38) | .30 |
| October | 11.55 (0.14 to 22.96) | .05 |
| November | 4.28 (−5.97 to 14.52) | .41 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | −0.22 (−0.37 to −0.07) | <.001 |
| Time since first treatment, y | −0.39 (−2.74 to 1.96) | .75 |
| Treatment × age | 0.38 (0.17 to 0.59) | <.001 |
| Sun protection score (range, 0-7) | ||
| Baseline, median (IQR) | 4.0 (3.0 to 5.0) | NA |
| Treatment effect | −1.33 (1.84 to 4.46) | <.001 |
| Sex | 0.17 (−0.26 to 0.60) | .42 |
| Age, y | 0 (−0.01 to 0.02) | .31 |
| BMI | −0.03 (−0.07 to 0.02) | .29 |
| Montha | NA | .03 |
| January | −1.28 (−2.33 to −0.22) | .02 |
| February | −0.51 (−1.16 to 0.14) | .12 |
| March | −0.81 (−1.44 to −0.17) | .01 |
| April | −0.02 (−0.85 to 0.81) | .96 |
| May | 0.12 (−0.95 to 1.18) | .83 |
| June | 0.29 (−1.09 to 1.67) | .68 |
| July | 0.35 (−1.08 to 1.78) | .63 |
| August | 0.13 (−1.24 to 1.51) | .85 |
| September | 0.48 (−0.71 to 1.66) | .43 |
| October | 0.07 (−0.97 to 1.10) | .90 |
| November | −0.05 (−0.99 to 0.89) | .92 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0.01 (−0.01 to 0.02) | .44 |
| Time since first treatment, y | 0.17 (−0.04 to 0.39) | .12 |
| Square root transformation No. of phototoxic reactions | ||
| Baseline, median (IQR) | 2.0 (1.0 to 3.0) | NA |
| Treatment effect | 0.62 (0.13 to 1.10) | .01 |
| Sex | −0.04 (−0.20 to 0.12) | .63 |
| Age, y | −0.01 (−0.01 to 0) | .06 |
| BMI | 0.01 (−0.03 to 0.01) | .10 |
| Montha | NA | .04 |
| January | −0.15 (−0.64 to 0.34) | .54 |
| February | −0.07 (−0.37 to 0.24) | .67 |
| March | −0.14 (−0.43 to 0.15) | .34 |
| April | 0.15 (−0.23 to 0.53) | .44 |
| May | 0 (−0.49 to 0.48) | .99 |
| June | −0.20 (−0.83 to 0.43) | .54 |
| July | −0.27 (−0.92 to 0.39) | .42 |
| August | −0.23 (−0.86 to 0.40) | .48 |
| September | −0.05 (−0.59 to 0.49) | .85 |
| October | −0.09 (−0.55 to 0.37) | .69 |
| November | −0.03 (−0.44 to 0.38) | .88 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0.02 (0.01 to 0.03) | <.001 |
| Time since first treatment, y | −0.16 (−0.25 to −0.06) | <.001 |
| Treatment × sunlight score | −0.01 (−0.01 to 0) | <.001 |
| Duration of phototoxic reaction, h | ||
| Baseline, median (IQR) | 56.0 (48.0 to 72.0) | NA |
| Treatment effect | −0.67 (−11.33 to 9.98) | .90 |
| Sex | −3.26 (−12.47 to 5.94) | .48 |
| Age, y | −0.34 (−0.68 to −0.01) | .04 |
| BMI | −0.17 (−1.22 to 0.89) | .76 |
| Montha | NA | .01 |
| January | −9.01 (−82.50 to 64.48) | .81 |
| February | 13.85 (−17.55 to 45.24) | .39 |
| March | 14.39 (−12.19 to 40.96) | .29 |
| April | 35.78 (6.31 to 65.25) | .02 |
| May | 35.25 (−0.57 to 71.08) | .05 |
| June | 30.54 (−14.20 to 75.27) | .18 |
| July | 36.73 (−9.38 to 82.83) | .12 |
| August | 29.84 (−14.53 to 74.21) | .19 |
| September | 23.06 (−15.84 to 61.95) | .24 |
| October | 5.86 (−28.66 to 40.38) | .74 |
| November | 31.41 (−1.60 to 64.42) | .06 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0.03 (−0.38 to 0.44) | .89 |
| Time since first treatment, y | −1.66 (−7.91 to 4.60) | .60 |
| Pain score (Likert scale range, 0-10) | ||
| Baseline, median (IQR) | 6.0 (3.0 to 7.0) | NA |
| Treatment effect | −0.85 (−1.43 to −0.26) | <.001 |
| Sex | 0.69 (0.15 to 1.23) | .01 |
| Age, y | −0.04 (−0.06 to −0.02) | <.001 |
| BMI | 0.05 (−0.01 to 0.11) | .13 |
| Montha | NA | .37 |
| January | −0.41 (−4.44 to 3.61) | .84 |
| February | 1.83 (0.21 to 3.44) | .03 |
| March | 0.78 (−0.63 to 2.20) | .28 |
| April | 1.58 (0.02 to 3.15) | .05 |
| May | 1.18 (−0.73 to 3.09) | .22 |
| June | 1.89 (−0.50 to 4.28) | .12 |
| July | 1.53 (−0.94 to 4.00) | .22 |
| August | 1.85 (−0.53 to 4.22) | .13 |
| September | 1.54 (−0.53 to 3.61) | .14 |
| October | 0.89 (−0.97 to 2.75) | .35 |
| November | 0.89 (−0.90 to 2.67) | .33 |
| December | 0 [Reference] | NA |
| Sunlight score, kJ/cm2 | 0 (−0.02 to 0.03) | .68 |
| Time since first treatment, y | −0.16 (−0.50 to 0.18) | .35 |
Abbreviations: BMI, body mass index; EPP, erythropoietic protoporphyria; IQR, interquartile range; NA, not applicable; QoL, quality of life.
December was set as reference as the last category because it is the month with the lowest hours of daylight. The specific months are compared with December, and all other variables are compared with baseline. P values are based on F test for any variation in coefficients (β) of the variable month.
Erythropoietic protoporphyria QoL (Table 2) increased significantly by 14.01% (95% CI, 4.53%-23.50%; P < .001), together with a positive interaction effect of age and treatment (size of the treatment effect increased with age) (β, 0.38; 95% CI, 0.17-0.59; P < .001). Quality of life varied over the year with a range of 11.55% (95% CI, 0.14-22.96) between the month with the lowest (December) and highest (October) QoL score and was lower on days with higher light intensity days (β, −0.22% per 1 kJ/cm2 increase in the sunlight score; 95% CI, −0.37 to −0.07; P < .001). The sun protection score varied by 1.75 (95% CI, 0.28-3.23) points between the month with the lowest (January) and highest (September) sun protection score (Table 2).
For the variable number of phototoxic reactions, a square root transformation was performed. As detailed in Table 2, the number of phototoxic reactions increased during treatment (95% CI, 0.13-1.10; P = .01), with a positive association with light intensity (95% CI, 0.01-0.03; P < .001) and a negative association of the duration of treatment with the number of reactions (95% CI, −0.25 to −0.06; P < .001). There was a negative interaction effect of sunlight score and treatment (95% CI, −0.01 to 0; P < .001), indicating that the higher the light intensity outside, the smaller the increase in the number of phototoxic reactions. There was no significant association of treatment with the duration of phototoxic reactions (β, −0.67; 95% CI, −11.33 to 9.98; P = .90), but there was seasonal variation resulting in longer phototoxic reactions during spring and summer, duration varied by 45.73 hours between the month with the lowest (January) and highest (July) number of hours (95% CI, −42.5 to 121.6). The pain score (Likert scale) significantly decreased by 0.85 points (95% CI, −1.43 to −0.26; P < .001). Women had on average 0.69 points higher pain scores than men (95% CI, 0.15-1.23; P = .01), and increasing age was associated with lower pain scores (β, −0.04; 95% CI, −0.06 to −0.02; P < .001; Table 2).
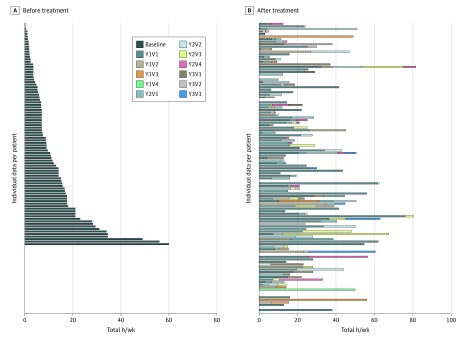
Time spent outside was also analyzed individually, looking at the maximum total time spent outside, in hours during a week taking afamelanotide treatment compared with baseline, without taking seasonal differences into account. Median total hours spent outside increased by 256% (interquartile range, 133%-393%) (Figure).
Figure. Individual Patient-Reported Time Outside.
Before (A) and after (B) treatment with afamelanotide, displayed as total hours spent outside during a week for their best treated period. In panel B, the total length of the bar represents the total hours in the week in which time spent outside was the longest (maximum). The colors provide information on how time spent outside increases over the subsequent visits, until it reaches its maximum. For patients with missing data, before or after treatment, the value 0 was filled in. Each line of data represents the same patient in panel A and panel B. Y indicates year; V, visit.
Adverse events occurred in 104 patients (88.9%) (eTable 2 in the Supplement). No abnormal nevi were detected, and regular laboratory test results showed no significant abnormalities. The most reported adverse events were nausea (60 [11.4%]), fatigue/malaise (51 [9.7%]), flushing (46 [8.8%]), and nausea with headache (44 [8.4%]). All were self-limiting with a mean duration of 1 to 2 days, occurring directly after implantation. There were no related serious adverse events (eTable 3 in the Supplement). Lack of effectiveness was reported 38 times (7.2%), indicating that the association of the implant with outcomes lasted less than 60 days. An overview of the results of the secondary end points are provided in eTables 4 to 6 in the Supplement.
Discussion
This study demonstrates that afamelanotide treatment for patients with EPP during routine clinical practice was associated with reduced phototoxicity, more than previously estimated during placebo-controlled trials.9 From a very young age, patients with EPP become conditioned to avoid direct sunlight owing to the painful attacks, and such conditioned behavior changes slowly over time if symptoms remain.11,12 The finding that afamelanotide treatment was associated with significantly increased time spent outside with an additional 6 hours per week, with less severe pain, and using less protective clothing is of great clinical relevance as is reflected by the high treatment continuation rate (115 [98%]). The association with outcomes became even larger as treatment duration increased. This study reproduced the finding of improved QoL during treatment, but the improvement was smaller than previously reported.9,10
The number of phototoxic reactions initially increased during treatment but thereafter decreased, demonstrating that patients tried to identify their new light exposure limit. Erythropoietic protoporphyria is not cured by afamelanotide, and despite the improvement, sunlight exposure restrictions remain.
Responses to therapy varied considerably between individual patients with EPP, irrespective of baseline tolerance, age, sex, or body mass index. It may be associated with disease-specific factors, skin type, individual pain perception, and geographical circumstances.13 Further research is needed. The current recommended dosing schedule of afamelanotide (4 per year and at least 60 days apart) was insufficient for several patients.
Strengths and Limitations
The strength of this study is the large number of treated patients with EPP with a relatively long duration of follow-up. The 98% continuation rate ensures minimal selection bias. Patients completed the questionnaires during clinic visits, concerning the previous weeks, possibly inducing recall bias.
Conclusions
This study demonstrates that afamelanotide may be effective at reducing EPP-related symptoms and phototoxic reactions in clinical practice. The treatment is associated with variable but sustained increase in quality of life and duration of light exposure and also is associated with less severe phototoxic reactions.
eFigure. Flowchart inclusion and exclusion of participants
eMethods 1. Overview of used questionnaires
eMethods 2. Daily diary
eMethods 3. EPP QoL questionnaire
eMethods 4. Phototoxic reaction questionnaire
eMethods 5. Sun protection questionnaire
eMethods 6. Secondary endpoints
eTable 1. Description of variables as secondary endpoint
eTable 2. Frequency of adverse events for afamelanotide
eTable 3. Serious adverse events (SAE) during afamelanotide treatment
eTable 4. Baseline values (before treatment) for all secondary endpoints
eTable 5. Mixed models for daily activity index, checking weather score and symptoms question
eTable 6. Mixed models for self-confidence score, influence of EPP on family question and effect afamelanotide on EPP
eAppendix. PASS protocol
References
- 1.Porter RM, Anstey A. Evidence and conjecture about mechanisms of cutaneous disease in photodermatology. Exp Dermatol. 2014;23(8):543-546. doi: 10.1111/exd.12467 [DOI] [PubMed] [Google Scholar]
- 2.Lecha M, Puy H, Deybach JC. Erythropoietic protoporphyria. Orphanet J Rare Dis. 2009;4:19. doi: 10.1186/1750-1172-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and heme metabolism and the porphyrias. Compr Physiol. 2013;3(1):365-401. doi: 10.1002/cphy.c120006 [DOI] [PubMed] [Google Scholar]
- 4.Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the UK: clinical features and effect on quality of life. Br J Dermatol. 2006;155(3):574-581. doi: 10.1111/j.1365-2133.2006.07472.x [DOI] [PubMed] [Google Scholar]
- 5.Todd DJ. Erythropoietic protoporphyria. Br J Dermatol. 1994;131(6):751-766. doi: 10.1111/j.1365-2133.1994.tb08577.x [DOI] [PubMed] [Google Scholar]
- 6.Tintle S, Alikhan A, Horner ME, Hand JL, Davis DMR. Cutaneous porphyrias part II: treatment strategies. Int J Dermatol. 2014;53(1):3-24. doi: 10.1111/ijd.12016 [DOI] [PubMed] [Google Scholar]
- 7.Minder EI, Schneider-Yin X, Steurer J, Bachmann LM. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand). 2009;55(1):84-97. [PubMed] [Google Scholar]
- 8.Luger TA, Brzoska T. α-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis. 2007;66(suppl 3):iii52-iii55. doi: 10.1136/ard.2007.079780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373(1):48-59. doi: 10.1056/NEJMoa1411481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider-Yin X, Minder EI. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br J Dermatol. 2015;172(6):1601-1612. doi: 10.1111/bjd.13598 [DOI] [PubMed] [Google Scholar]
- 11.Lynch PJ, Miedler LJ. Erythropoietic protoporphyria. Report of a family and a clinical review. Arch Dermatol. 1965;92(4):351-356. doi: 10.1001/archderm.1965.01600160007002 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt H, Snitker G, Thomsen K, Lintrup J. Erythropoietic protoporphyria: a clinical study based on 29 cases in 14 families. Arch Dermatol. 1974;110(1):58-64. doi: 10.1001/archderm.1974.01630070026004 [DOI] [PubMed] [Google Scholar]
- 13.Minder EI, Schneider-Yin X. Afamelanotide (CUV1647) in dermal phototoxicity of erythropoietic protoporphyria. Expert Rev Clin Pharmacol. 2015;8(1):43-53. doi: 10.1586/17512433.2014.956089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart inclusion and exclusion of participants
eMethods 1. Overview of used questionnaires
eMethods 2. Daily diary
eMethods 3. EPP QoL questionnaire
eMethods 4. Phototoxic reaction questionnaire
eMethods 5. Sun protection questionnaire
eMethods 6. Secondary endpoints
eTable 1. Description of variables as secondary endpoint
eTable 2. Frequency of adverse events for afamelanotide
eTable 3. Serious adverse events (SAE) during afamelanotide treatment
eTable 4. Baseline values (before treatment) for all secondary endpoints
eTable 5. Mixed models for daily activity index, checking weather score and symptoms question
eTable 6. Mixed models for self-confidence score, influence of EPP on family question and effect afamelanotide on EPP
eAppendix. PASS protocol