This post hoc analysis of a randomized clinical trial examines factors associated with short-term relapse in patients with pemphigus treated with rituximab.
Key Points
Question
What are the factors associated with short-term relapse in patients with pemphigus who receive rituximab as first-line therapy?
Findings
This post hoc analysis of a randomized clinical trial that included 47 patients treated with rituximab as first-line therapy identified 2 factors associated with early relapse: a baseline Pemphigus Disease Area Index score of 45 or higher corresponding to severe pemphigus, and persistent anti–desmoglein 1 antibody enzyme-linked immunosorbent assay level greater than 20 IU/mL and/or anti–desmoglein 3 antibody enzyme-linked immunosorbent assay level greater than 130 IU/mL 3 months after rituximab treatment. These factors had a positive predictive value of 50% and a negative predictive value of 94% for the occurrence of early relapse.
Meaning
The findings suggest that these 2 factors might help differentiate a subgroup of patients with high risk of relapse who might benefit from maintenance rituximab infusion at month 6 from a subgroup of patients with low risk of relapse who do not need early maintenance therapy.
Abstract
Importance
Rituximab and short-term corticosteroid therapy are the criterion standard treatments for patients with newly diagnosed moderate to severe pemphigus.
Objective
To examine factors associated with short-term relapse in patients with pemphigus treated with rituximab.
Design, Setting, and Participants
This post hoc analysis of a randomized clinical trial (Comparison Between Rituximab Treatment and Oral Corticosteroid Treatment in Patients With Pemphigus [RITUX 3]) conducted from January 1, 2010, to December 31, 2015, included patients from 20 dermatology departments of tertiary care centers in France from the RITUX 3 trial and 3 newly diagnosed patients treated according to the trial protocol. Data analysis was performed from February 1 to June 30, 2019.
Exposure
Patients randomly assigned to the rituximab group in the RITUX 3 trial and the 3 additional patients were treated with 1000 mg of intravenous rituximab on days 0 and 14 and 500 mg at months 12 and 18 combined with a short-term prednisone regimen.
Main Outcomes and Measures
Baseline (pretreatment) clinical and biological characteristics (Pemphigus Disease Area Index [PDAI] score, ranging from 0-250 points, with higher values indicating more severe disease) and changes in anti–desmoglein (DSG) 1 and anti-DSG3 values as measured by enzyme-linked immunosorbent assay during the 3 months after rituximab treatment were compared between patients with disease relapse and those who maintained clinical remission during the first 12 months after treatment. The positive and negative predictive values of these factors were calculated.
Results
Among 47 patients (mean [SD] age, 54.3 [17.0] years; 17 [36%] male and 30 [64%] female) included in the study, the mean (SD) baseline PDAI score for patients with relapsing disease was higher than that of the patients with nonrelapsing disease (54 [33] vs 28 [24]; P = .03). At month 3, 7 of 11 patients with relapsing disease (64%) vs 7 of 36 patients with nonrelapsing disease (19%) had persistent anti-DSG1 antibody values of 20 IU/mL or higher and/or anti-DSG3 antibody values of 130 IU/mL or higher (P = .01). A PDAI score of 45 or higher defining severe pemphigus and/or persistent anti-DSG1 antibody values of 20 IU/mL or higher and/or anti-DSG3 antibody values of 130 IU/mL or higher at month 3 provided a positive predictive value of 50% (95% CI, 27%-73%) and a negative predictive value of 94% (95% CI, 73%-100%) for the occurrence of relapse after rituximab.
Conclusions and Relevance
The findings suggest that initial PDAI score and changes in anti-DSG antibody values after the initial cycle of rituximab might help differentiate a subgroup of patients with high risk of relapse who might benefit from maintenance rituximab infusion at month 6 from a subgroup of patients with low risk of relapse who do not need early maintenance therapy.
Trial Registration
Introduction
Pemphigus is a rare, life-threatening autoimmune blistering disease that involves the skin and mucosa in which pathogenic IgG antibodies are directed against desmosomal transmembrane glycoproteins: desmoglein (DSG) 3 and 1.1,2,3,4,5,6 Initial severity of skin and oral mucous membrane lesions is correlated with anti-DSG1 and anti-DSG3 antibody levels, respectively.7 High-dose oral corticosteroids that are sometimes associated with immunosuppressive drugs (azathioprine and mycophenolate mofetil) have been the mainstay of treatment for a long time.8,9,10,11,12,13,14,15
In the past decade, more than 1000 patients with severe recalcitrant or relapsing cases of pemphigus have been treated with rituximab, an anti-CD20 monoclonal antibody. The efficacy and safety of rituximab as first-line treatment in patients with moderate to severe forms of pemphigus have been recently demonstrated in a large randomized clinical trial (Comparison Between Rituximab Treatment and Oral Corticosteroid Treatment in Patients With Pemphigus [RITUX 3]) in which 90 patients were randomly assigned to receive a standard regimen of corticosteroids vs rituximab associated with a short-term corticosteroid regimen.16 In that trial, the 2-year rate of complete remission while not receiving therapy was 89% in the rituximab arm vs 34% in the standard corticosteroid arm. After 24 months of follow-up, 11 relapses (23%) occurred in the rituximab arm vs 20 relapses (46%) in the standard corticosteroid regimen arm. Relapses have been consistently reported in the literature in patients who received an initial cycle of rituximab without maintenance infusions during their disease.17,18,19,20,21 In the RITUX 3 trial, only 3 of the 11 relapses (27%) in the rituximab group occurred after the 2 maintenance infusions at months 12 and 18, whereas 8 of 11 relapses (73%) occurred between months 6 and 12, which might suggest a clinical benefit of a first maintenance infusion at month 6 in some patients.
The aims of the present study were to evaluate factors associated with short-term relapse of pemphigus and to identify a subgroup of patients who might benefit from an additional infusion of rituximab at month 6 after the initial cycle of rituximab. Because of the cost and immunosuppressive effect of rituximab, the identification of factors associated with early relapse may inform the risk and benefit considerations of an additional infusion of rituximab and its use only in patients with a high risk of relapse.
Methods
Study Design and Participants
This secondary analysis of a randomized clinical trial uses data obtained from patients from 20 dermatology departments of tertiary care centers in France in the rituximab group of the RITUX 3 trial, conducted from January 1, 2010, to December 31, 2015, and from 3 patients with newly diagnosed pemphigus who underwent first-line treatment with rituximab according to the same regimen outside the RITUX 3 trial. Patients with newly diagnosed pemphigus were treated with 1 infusion of 1000 mg of intravenous rituximab on days 0 and 14 and 1 infusion of 500 mg at months 12 and 18 combined with a short-term prednisone regimen (0.5 mg/kg daily for moderate pemphigus and 1 mg/kg daily for severe pemphigus). At the time of the study, classification of pemphigus severity was based on the criteria of Harman et al.7 Severe pemphigus was defined as skin involvement of more than 5% of the body surface area, significant mucosal involvement defined as more than 10 mucosal erosions, diffuse gingivitis or confluent large erosions, or involvement of 2 or more mucosal sites. The initial prednisone dose was maintained for 1 month and thereafter gradually reduced after achievement of disease control, with the aim to stop prednisone therapy after 3 months in patients with moderate pemphigus and after 6 months in patients with severe pemphigus. Data analysis was performed from February 1 to June 30, 2019. The CPP Nord-Ouest Ethics Committee approved the study. All patients provided written informed consent.
Clinical and Immunologic Settings
Clinical data (age, sex, body mass index [calculated as weight in kilograms divided by height in meters squared], and pemphigus type) and pemphigus severity were recorded from patients’ case report forms and patients’ medical files. Baseline pemphigus severity was assessed using the Pemphigus Disease Area Index (PDAI) score.22 This score was created by a panel of international experts to provide an objective measurement of pemphigus activity.23 It takes into account the number of skin and mucosal lesions, their extent, and the number of anatomical skin and mucosal areas involved. The PDAI activity score ranges from 0 to 250 points (120 points for skin, 10 for scalp, and 120 for mucosal activity). Cutoff values of 15 and 45 points have been proposed to define mild, moderate, and severe types of the disease.22 The PDAI score has high interrater reliability and is therefore a robust tool to accurately assess pemphigus activity.24 A relapse was defined as the reappearance of at least 3 new lesions in a month that did not heal spontaneously within 1 week.23 Short-term relapse was defined as a relapse that occurred during the first year after the initial cycle of rituximab.
Anti-DSG1 and anti-DSG3 antibody values were measured using a commercially available DSG enzyme-linked immunosorbent assay (ELISA) (EUROIMMUN Desmoglein test, Medizinische Labordiagnostika) according to the manufacturer’s instructions. Blood samples were collected on days 0, 30, 90, 180, 270, and 360. Peripheral blood mononuclear cells were isolated from whole blood by Ficoll-Paque gradients (GE Healthcare Lifescience) and cryopreserved. The phenotype of the peripheral blood mononuclear cells was determined by flow cytometry with monoclonal antibodies against CD19 phycoerythrin, CD4 electron-coupled dye, CD3 phycoerythrincyanin 5, and CD8 fluorescein isothiocyanate (Beckman Coulter) for T and B cells and CD4 peridinin chlorophyll protein, CD25 phycoerythrin, and CD127 fluorescein isothiocyanate (eBioscience, Becton Dickinson, and Beckman Coulter) for regulatory T cells. B cells were defined as CD19+, T cells were defined as CD3+CD4+, and regulatory T cells as CD4+CD25hiCD127low. Rituximab serum concentrations were measured using a commercially available ELISA (LISA-TRACKER Duo Rituximab, Theradiag) on days 30, 60, 90, and 180 after initial treatment with rituximab.
Statistical Analysis
To determine factors associated with short-term relapse after rituximab therapy, we compared baseline (pretreatment) clinical characteristics of age, sex, type of pemphigus (pemphigus vulgaris vs pemphigus foliaceus), type of involvement (skin, mucosal, or both), and PDAI severity score between patients who maintained complete remission for the first year of treatment and those who experienced disease relapse during the first year after the initial cycle of rituximab. Then we compared the evolution of anti-DSG1 and anti-DSG3 antibody values during the 6-month period after rituximab treatment between patients who maintained complete remission and those who experienced disease relapse during the first year.
To determine the sensitivity, specificity, and positive and negative predictive values of anti-DSG antibodies and PDAI score, we used previously published cutoff values. The cutoff value for the PDAI score differentiating severe from nonsevere pemphigus was determined from the study conducted by Boulard et al22 (severe pemphigus was defined by a PDAI score ≥45).22 According to the study by Abasq et al,25 cutoff values of 130 IU/mL for anti-DSG3 antibodies and 20 IU/mL for anti-DSG1 antibodies were used because they provide high positive predictive values (84% for anti-DSG3 and 79% for anti-DSG1) and negative predictive values (81% for anti-DSG3 and 84% for anti-DSG1) for the occurrence of mucosal and skin relapses.25 To evaluate these cutoff values from the literature on the present series of patients with pemphigus, a receiver operating characteristic curve was calculated. A cutoff value of 20 IU/mL for anti-DSG1 antibodies and 120 IU/mL for anti-DSG3 antibodies gave optimal sensitivity and specificity. These cutoff values were equal for anti-DSG1 antibodies (20 vs 20 IU/mL) and close for anti-DSG3 (120 vs 130 IU/mL) to those reported by Abasq et al.25 Positive and negative predictive values of factors associated with relapse were calculated based on the 23% rate of relapse observed in patients assigned to the rituximab arm in the RITUX 3 trial.
We also compared rituximab serum concentrations depending on time (from day 14 to day 180) between patients with relapsing and nonrelapsing disease (area under the concentration-time curve) by the trapezoidal method (Prism software, version 7; GraphPad Software Inc). We compared the proportions of B cells, CD4 T cells, and regulatory T cells between patients with relapsing and nonrelapsing disease from baseline to the month 6 evaluation using Prism software (2-way analysis of variance with Sidak posttest).
The Fisher exact test was used to compare qualitative variables, and the t test or Mann-Whitney test was used to compare quantitative variables. For all tests, 2-sided P < .05 was considered to be statistically significant. All analyses were performed with R statistical software, version 3.5.1 (R Foundation for Statistical Computing).
Results
Baseline Characteristics and Clinical Course
A total of 47 patients (mean [SD] age, 54.3 [17.0] years; 17 [36%] male and 30 [64%] female) were treated with rituximab plus short-term oral prednisone. Forty-four of these patients were included in the RITUX 3 trial, and the other 3 patients were treated with the same regimen of rituximab plus short-term oral prednisone after the end of the RITUX 3 study.
During the 12 months after the initial infusion of 2 g of rituximab, 11 of the 47 patients (23%; 95% CI, 13%-39%) experienced relapse, including 2 patients with pemphigus foliaceus and 9 patients with pemphigus vulgaris, after a median delay of 219 days (interquartile range, 154-277 days). All relapses occurred after month 6. There were 6 mucosal, 3 cutaneous, and 2 mucocutaneous disease relapses.
Mean (SD) age (50.10 [18.12] vs 55.58 [16.34] years; P = .30), sex (5 [45.5%] male and 6 [54.5%] female vs 12 [33.3%] male and 24 [66.7%] female; P = .49), mean (SD) body mass index (24.9 [3.9] vs 24.8 [3.7]; P = .37), type of pemphigus (9 [81.8%] vs 31 [86.1%] with pemphigus vulgaris vs 2 [18.2%] vs 5 [13.9%] with pemphigus foliaceus; P = .66), and median (range) delay between disease onset and first rituximab infusion (94 [35-563] days vs 112 [2-1604] days for cutaneous involvement, P = .47 vs 84 [27-324] days vs 123 [14-605] days for mucosal involvement, P = .75) were not significantly different between patients with disease relapse vs those without relapse (Table). Patients with disease relapse had an initially more severe pemphigus than those without relapse (mean [SD] baseline PDAI score, 54 [33] vs 28 [24]; P = .03) (Table). Accordingly, 6 of the 11 patients with disease relapse (55%) had severe pemphigus (baseline PDAI score ≥45) vs 5 of the 36 patients without relapse (14%) (P = .01).
Table. Baseline Clinical Characteristics of Patients With Relapsing and Nonrelapsing Diseasea.
| Characteristic | Relapsing Disease (N = 47) | P Value | |
|---|---|---|---|
| Yes | No | ||
| Patients | 11 (23.4) | 36 (76.6) | NA |
| Age, mean (SD), y | 50.10 (18.12) | 55.58 (16.34) | .30 |
| Sex | |||
| Male | 5 (45.5) | 12 (33.3) | .49 |
| Female | 6 (54.5) | 24 (66.7) | |
| BMI, mean (SD) | 24.9 (3.9) | 24.8 (3.7) | .37 |
| Type of pemphigus | |||
| Vulgaris | 9 (81.8) | 31 (86.1) | .66 |
| Foliaceus | 2 (18.2) | 5 (13.9) | |
| Initial presentation | |||
| Mucosal | 1 (9.1) | 8 (22.2) | .66 |
| Cutaneous | 2 (18.2) | 6 (16.7) | >.99 |
| Mucocutaneous | 8 (72.7) | 22 (61.1) | .72 |
| PDAI score, mean (SD) | 54.41 (33.3) | 28.5 (23.9) | .03 |
| Delay between disease onset and first rituximab infusion, median (range), d | |||
| Cutaneous involvement | 94 (35-563) | 112 (2-1604) | .47 |
| Mucosal involvement | 84 (27-324) | 123 (14-605) | .75 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; PDAI, Pemphigus Disease Area Index.
Data are presented as number (percentage) of patients unless otherwise indicated.
Anti-DSG Antibodies
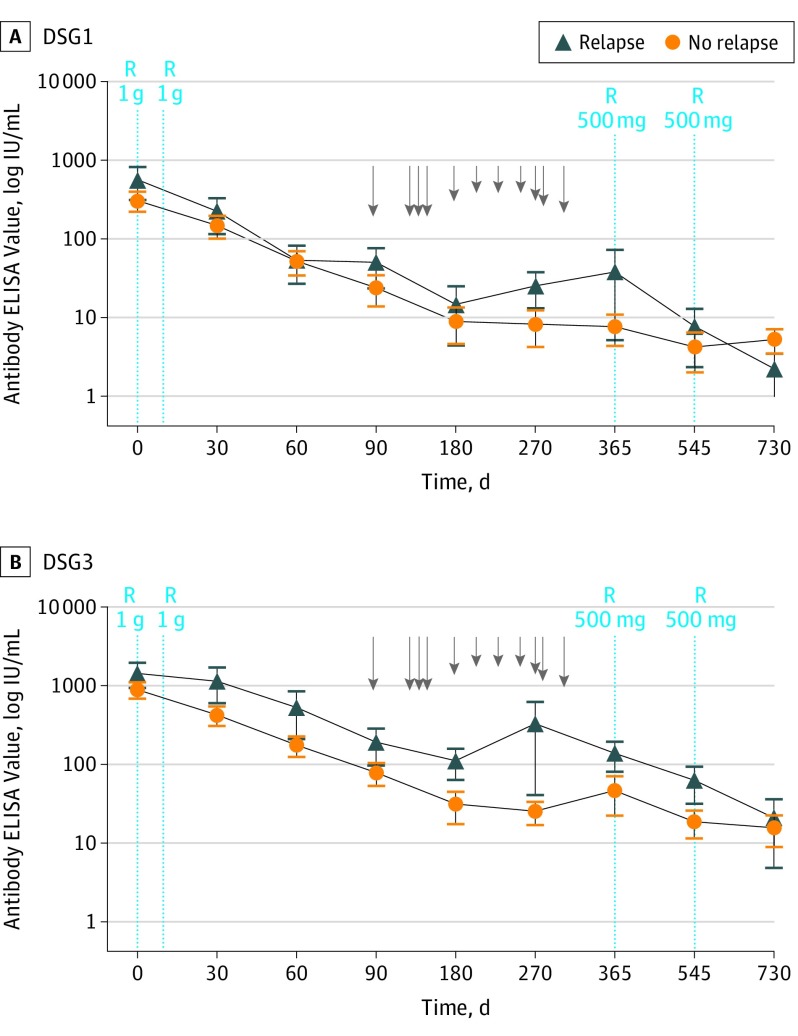
Mean anti-DSG1 values decreased from 257 IU/mL at baseline to 20 IU/mL at month 3 and 7 IU/mL at month 6, and mean anti-DSG3 values decreased from 850 IU/mL at baseline to 88 IU/mL at month 3 and 38 IU/mL at month 6 (Figure 1). To identify a subgroup of patients with a higher relapse risk who could benefit from a first maintenance infusion of rituximab at month 6, we compared the mean anti-DSG1 and anti-DSG3 antibody levels at baseline and at the month 3 evaluation between the subgroups of patients with relapsing and nonrelapsing disease.
Figure 1. Anti–Desmoglein (DSG) 1 and Anti-DSG3 Antibody Values During the 24-Month Period After Initial Treatment With Rituximab in Patients With Relapsing and Nonrelapsing Disease .
ELISA indicates enzyme-linked immunosorbent assay; R, rituximab infusions; arrows, relapses; and error bars, SDs.
Mean (SD) anti-DSG1 antibody values were not significantly higher in patients with relapsing disease compared with those with ongoing remission at baseline (520 [747] IU/mL vs 245 [280] IU/mL; P = .28) and month 3 (46 [76] IU/mL vs 16 [42] IU/mL; P = .28). Similar findings were observed for anti-DSG3 antibodies at baseline (1444 [1525] IU/mL vs 914 [1114] IU/mL; P = .35) and month 3 (193 [280] IU/mL vs 80 [133] IU/mL; P = .27).
B and T Cells
B Cells
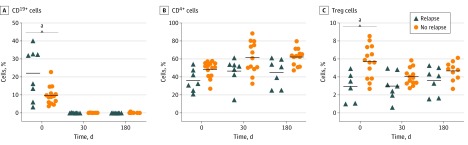
At baseline, patients who had disease relapse had a higher mean percentage of CD19+ B cells than did patients with nonrelapsing disease (22.11% vs 9.80%; P < .001) (Figure 2A). Complete B-cell depletion was observed in patients with relapsing and nonrelapsing disease at the month 1 evaluation after rituximab treatment. At month 6, the frequency of B cells was still close to 0 in patients with relapsing and nonrelapsing disease (0.07% vs 0.16%; P > . 99).
Figure 2. Frequencies of CD19+ B Cells, CD4+ T Cells, and Regulatory T (Treg) Cells in Peripheral Blood Lymphocytes During Follow-up.
aP < .001.
T Cells
The mean percentage of CD4+ T cells measured from baseline to month 6 was not significantly different between patients with relapsing and nonrelapsing disease (Figure 2B). The percentage of CD25hi CD127low regulatory T cells at baseline was higher in patients without disease relapse than in those with disease relapse (5.71% vs 2.91%, P < .001). However, the difference between the 2 subgroups was no longer statistically significant after rituximab treatment (month 6: 4.7% vs 3.6%, P = .35) (Figure 2C).
Rituximab Serum Concentrations
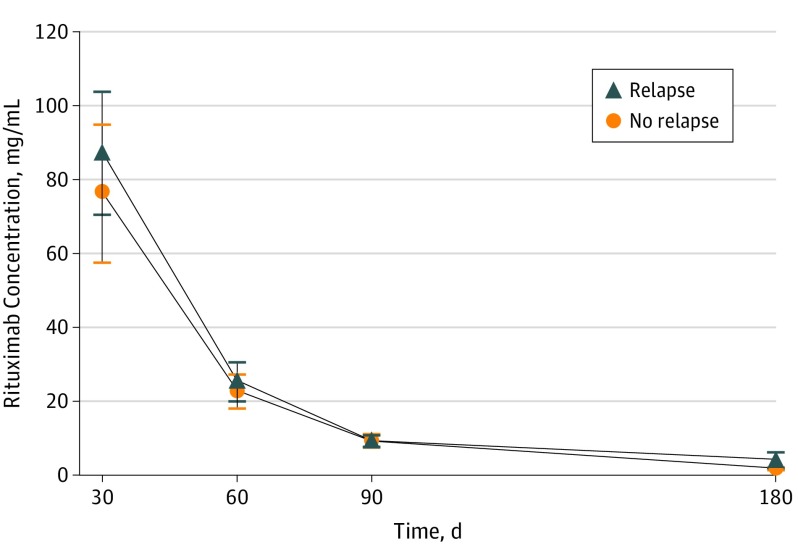
Rituximab serum concentrations in samples measured from day 30 to day 180 were not significantly different between patients with relapsing and nonrelapsing disease (area under the curve: 3056 [95% CI, 2261-3851] vs 3062 [95% CI, 1956-4168]) (Figure 3).
Figure 3. Comparison of Rituximab Serum Concentrations Between Patients With Relapsing and Nonrelapsing Disease.
Area under the curve of rituximab serum concentrations depending on time.
Factors Associated With Relapse
At baseline, a PDAI score of 45 or higher (corresponding to severe pemphigus) had a positive predictive value of 55% (95% CI, 23%-83%) for the occurrence of a relapse during the 12-month period after the initial cycle of rituximab treatment. Patients with a PDAI score less than 45 had a high negative predictive value (86%; 95% CI, 71%-95%) for the absence of relapse.
At month 3, according to the study by Abasq et al,25 cut-off values of 130 IU/mL for anti-DSG3 antibodies and 20 IU/mL for anti-DSG1 antibodies were used because they provide high positive predictive values (84% for anti-DSG3 and 79% for anti-DSG1) and negative predictive values (81% for anti-DSG3 and 84% for anti-DSG1) for the occurrence of mucosal and skin relapses. At month 3, 7 of the 11 patients with disease relapse (64%) had antibody values higher than 20 IU/mL for anti-DSG1 and/or 130 IU/mL for anti-DSG3 vs 7 of the 36 patients (19%) who had persistent clinical remission (P = .01).
This set of 2 criteria (ie, baseline PDAI score ≥45 and/or persistent anti-DSG1 antibody levels ≥20 IU/mL and/or anti-DSG3 antibody levels ≥130 IU/mL) 3 months after the initial cycle of rituximab provided a sensitivity of 91% (95% CI, 59%-100%), a specificity of 60% (95% CI, 39%-79%), a positive predictive value of 50% (95% CI, 27%-73%), and a negative predictive value of 94% (95% CI, 73%-100%) for the occurrence of a relapse after the initial cycle of rituximab treatment.
Discussion
This study identified 2 factors associated with short-term relapse after initial treatment with rituximab: the initial PDAI score, which reflects the severity of pemphigus, and the evolution of anti-DSG antibody values 3 months after the initial cycle of rituximab. These findings are important because rituximab has been recently approved as first-line treatment of moderate to severe pemphigus.26 The set of criteria identified in the present study provided a 50% positive predictive value. Assuming that a maintenance infusion of rituximab at month 6 would be consistently effective, our results suggest the possibility of treating 2 patients with a maintenance infusion of rituximab at month 6 to avoid relapse in 1 patient. Conversely, the 94% negative predictive value indicates that a maintenance infusion of rituximab could be avoided in approximately half of the patients treated with first-line therapy. Avoiding early relapse after an initial cycle of rituximab is also important because 20% of patients experienced relapse between months 6 and 12 in a clinical trial,16 whereas only a few relapses (3 of 11) occurred between month 12 and the end of the study at month 24.
The PDAI score was reported as an easy-to-use and robust tool to accurately assess pemphigus severity, with high interrater reliability and good correlation over time with other markers of pemphigus severity.22,24 Our findings suggest that initial pemphigus severity might be associated with early relapse after first-line treatment with rituximab because patients with disease relapse had a 2-fold higher baseline PDAI severity score compared with patients without disease relapse during the 12-month period after the initial cycle of rituximab. Patients with a PDAI score of 45 or higher had a positive predictive value of 55% for the occurrence of a relapse during the year after the initial infusion of rituximab, whereas those with a PDAI score less than 45 had an 86% negative predictive value for the absence of relapse after rituximab therapy.
A change in anti-DSG1 and, to a lesser degree, anti-DSG3 antibody levels has been reported to correlate with the course of pemphigus skin and mucosal lesions.7,25,27,28 Despite the fact that the number of circulating B lymphocytes was close to 0 from 3 to 6 months after the initial cycle of rituximab treatment in patients with and without disease relapse, we observed that patients with relapse had higher anti-DSG1 and anti-DSG3 antibody levels at the month 3 evaluation than did those without relapse after rituximab therapy. This time point was chosen because it is often used by practitioners to assess the epithelialization of skin and mucosal lesions after the delay of action of rituximab and because measurement of anti-DSG antibodies at month 3 allows a new, early infusion of rituximab to be scheduled if necessary.
Owing to the poor specificity of anti-DSG3 antibody level, we used cutoff values corresponding to 20 IU/mL for anti-DSG1 and 130 IU/mL for anti-DSG3, which have been reported to provide high positive and negative predictive values for the occurrence of skin and mucosal relapses, respectively.25 These cutoff values were close to those that we calculated using a receiver operating characteristic curve. The persistence of anti-DSG1 antibody values of 20 IU/mL or higher and/or anti-DSG3 antibody values of 130 IU/mL or higher at the month 3 evaluation provided a positive predictive value of 50% and a negative predictive value of 88%, which were close to those calculated from the initial PDAI score. Despite the difference between baseline anti-DSG1 and anti-DSG3 antibody values not reaching statistical significance, which was likely related to the low number of patients with relapse, the mean baseline anti-DSG1 and anti-DSG3 antibody values from patients with disease relapse (520 anti-DSG1 antibodies and 1444 anti-DSG3 antibodies) were almost twice as high as those observed in patients without relapse (245 anti-DSG1 antibodies and 914 anti-DSG3 antibodies). Additional studies are needed to validate whether baseline anti-DSG1 and anti-DSG3 antibody values are a significant indicator of relapse.
We assessed changes in peripheral blood B lymphocyte counts after treatment with rituximab because early B-cell recovery has been reported to precede the occurrence of a clinical relapse in some autoimmune diseases, in particular rheumatoid arthritis.29,30,31 We observed that at baseline, the mean percentage of peripheral blood CD19+ B cells was higher in patients with disease relapse than in those who maintained clinical remission. However, the significance of this finding is uncertain because we were not able to record patients’ blood cell counts and therefore were unable to determine the absolute number of blood B lymphocytes. Nevertheless, patients with relapsing and nonrelapsing disease had complete B-cell depletion during the first 3 months after rituximab treatment.
The CD4+ T-cell frequency was not different between patients with relapsing and nonrelapsing disease in our study, unlike the study by Albers et al,31 which found that relapses were associated with a lower number of circulating CD4+ T cells. However, in that study, T-cell counts were measured in patients previously treated with corticosteroids and or immunosuppressants, which induce T-cell lymphopenia; thus, one may hypothesize that the lower rate of CD4 T cells observed in patients with relapsing disease from their study was at least partly associated with the cumulative dose of corticosteroids and/or immunosuppressants previously received by these patients. Conversely, in the present study, a higher frequency of regulatory T cells was found in patients with nonrelapsing disease compared with those with relapsing disease, which is in accordance with regulatory mechanisms previously described in autoimmune diseases.32 Differences in serum concentrations of rituximab between patients with relapsing and nonrelapsing disease also were not found, which is in accordance with the absence of an association between the detection of rituximab human antichimeric antibodies and the occurrence of relapses reported in a recent study.33
The interest of using multiple infusions of rituximab during the initial phase of treatment34 or as maintenance therapy has been suggested in previous studies.35,36 Sanchez et al36 reported that maintenance infusions of rituximab (1 g every 6 months) for patients with clinical remission until negative serum anti-DSG antibody titers was associated with the maintenance of long-lasting complete remission, with good tolerance.36 Although there is increasingly more evidence that maintenance therapy with rituximab is useful to maintain complete remission, the exact modalities of this maintenance therapy remain to be determined. However, despite the short-term tolerance of these maintenance regimens using multiple infusions of rituximab appearing to be good, the use of repeated infusions of rituximab beyond 4 cycles may be costly and may be associated with prolonged B-cell depletion that is probably avoidable in some patients.
The rapid tapering of corticosteroid doses, which was planned in the protocol to demonstrate the interest of first-line treatment with rituximab, may have been associated with the occurrence of relapses after 6 months, which in addition corresponded to the time of B-cell recovery. Many clinical data are available from dermatologists to support the rapid tapering of corticosteroid doses without rituximab leading to a high rate of relapses, justifying the progressive tapering of corticosteroid doses (and consequently the high cumulative doses of corticosteroids); this treatment strategy was widely recommended in the treatment of pemphigus before the use of rituximab. Nevertheless, despite this rapid tapering of corticosteroid doses, the relapse rate in the rituximab arm was twice as low as that observed in the standard corticosteroid arm (24% vs 45%), in which patients were initially prescribed high corticosteroid doses that were then progressively tapered.
Limitations
The main limitation of our study was the low number of patients with relapsing disease. However, the present study was a post hoc analysis of a randomized clinical trial that was primarily designed to assess the rate of complete remission while not undergoing therapy, and the relapse rate was a secondary end point. Despite the number of patients with short-term relapse being low (n = 8), this number corresponds to almost 20% of patients receiving rituximab as first-line therapy. Thus, the potential prevention of these relapses is a major consideration to take into account in the treatment of patients with pemphigus. Nevertheless, we cannot exclude the possibility that the low number of patients with relapsing disease might not have led to the identification of other factors associated with relapse. This study focused on short-term relapses, which can be considered another limitation. We are currently studying the long-term outcomes in patients from the RITUX 3 trial and have observed that two-thirds of patients who experienced a long-term relapse previously had a short-term relapse within the first 12 months after the start of rituximab treatment, thus reinforcing interest in identifying those patients with the highest risk of short-term relapse to offer them a maintenance infusion of rituximab at month 6.
Conclusions
The presence of at least 1 of the 2 factors associated with relapse of pemphigus may help identify a group of patients with high risk for relapse who may benefit from a maintenance infusion of rituximab at month 6. Additional studies are needed to determine the optimal doses of maintenance therapy with rituximab.
References
- 1.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67(5):869-877. doi: 10.1016/0092-8674(91)90360-B [DOI] [PubMed] [Google Scholar]
- 2.Stanley JR. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody-mediated diseases. J Clin Invest. 1989;83(5):1443-1448. doi: 10.1172/JCI114036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306(20):1189-1196. doi: 10.1056/NEJM198205203062001 [DOI] [PubMed] [Google Scholar]
- 4.Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355(17):1800-1810. doi: 10.1056/NEJMra061111 [DOI] [PubMed] [Google Scholar]
- 6.Amagai M. Adhesion molecules, I: keratinocyte-keratinocyte interactions; cadherins and pemphigus. J Invest Dermatol. 1995;104(1):146-152. doi: 10.1111/1523-1747.ep12613668 [DOI] [PubMed] [Google Scholar]
- 7.Harman KE, Seed PT, Gratian MJ, Bhogal BS, Challacombe SJ, Black MM. The severity of cutaneous and oral pemphigus is related to desmoglein 1 and 3 antibody levels. Br J Dermatol. 2001;144(4):775-780. doi: 10.1046/j.1365-2133.2001.04132.x [DOI] [PubMed] [Google Scholar]
- 8.Zhao CY, Murrell DF. Pemphigus vulgaris: an evidence-based treatment update. Drugs. 2015;75(3):271-284. doi: 10.1007/s40265-015-0353-6 [DOI] [PubMed] [Google Scholar]
- 9.Martin LK, Werth V, Villanueva E, Segall J, Murrell DF. Interventions for pemphigus vulgaris and pemphigus foliaceus. Cochrane Database Syst Rev. 2009;(1):CD006263. doi: 10.1002/14651858.CD006263.pub2 [DOI] [PubMed] [Google Scholar]
- 10.Atzmony L, Hodak E, Leshem YA, et al. The role of adjuvant therapy in pemphigus: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(2):264-271. doi: 10.1016/j.jaad.2015.04.038 [DOI] [PubMed] [Google Scholar]
- 11.Hertl M, Jedlickova H, Karpati S, et al. Pemphigus: S2 guideline for diagnosis and treatment guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2015;29(3):405-414. doi: 10.1111/jdv.12772 [DOI] [PubMed] [Google Scholar]
- 12.Beissert S, Mimouni D, Kanwar AJ, Solomons N, Kalia V, Anhalt GJ. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130(8):2041-2048. doi: 10.1038/jid.2010.91 [DOI] [PubMed] [Google Scholar]
- 13.Ioannides D, Apalla Z, Lazaridou E, Rigopoulos D. Evaluation of mycophenolate mofetil as a steroid-sparing agent in pemphigus: a randomized, prospective study. J Eur Acad Dermatol Venereol. 2012;26(7):855-860. doi: 10.1111/j.1468-3083.2011.04170.x [DOI] [PubMed] [Google Scholar]
- 14.Ioannides D, Chrysomallis F, Bystryn JC. Ineffectiveness of cyclosporine as an adjuvant to corticosteroids in the treatment of pemphigus. Arch Dermatol. 2000;136(7):868-872. doi: 10.1001/archderm.136.7.868 [DOI] [PubMed] [Google Scholar]
- 15.Lever WF, Goldberg HS. Treatment of pemphigus vulgaris with methotrexate. Arch Dermatol. 1969;100(1):70-78. doi: 10.1001/archderm.1969.01610250076018 [DOI] [PubMed] [Google Scholar]
- 16.Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. ; French Study Group on Autoimmune Bullous Skin Diseases . First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031-2040. doi: 10.1016/S0140-6736(17)30070-3 [DOI] [PubMed] [Google Scholar]
- 17.Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5(175):175ra30. doi: 10.1126/scitranslmed.3005166 [DOI] [PubMed] [Google Scholar]
- 18.Joly P, Mouquet H, Roujeau J-C, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545-552. doi: 10.1056/NEJMoa067752 [DOI] [PubMed] [Google Scholar]
- 19.Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148(9):1031-1036. doi: 10.1001/archdermatol.2012.1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinay K, Cazzaniga S, Amber KT, Feldmeyer L, Naldi L, Borradori L. Rituximab as first-line adjuvant therapy for pemphigus: Retrospective analysis of long-term outcomes at a single center. J Am Acad Dermatol. 2018;78(4):806-808. doi: 10.1016/j.jaad.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 21.Baum S, Gilboa S, Greenberger S, Pavlotsky F, Trau H, Barzilai A. Adjuvant rituximab therapy in pemphigus: a single-center experience of 18 cases. J Dermatolog Treat. 2013;24(6):427-430. doi: 10.3109/09546634.2013.784391 [DOI] [PubMed] [Google Scholar]
- 22.Boulard C, Duvert Lehembre S, Picard-Dahan C, et al. Calculation of cut-off values based on the ABSIS and PDAI pemphigus scoring systems for defining moderate, significant and extensive types of pemphigus. Br J Dermatol. 2016;175(1):142-149. doi: 10.1111/bjd.14405 [DOI] [PubMed] [Google Scholar]
- 23.Murrell DF, Dick S, Ahmed AR, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58(6):1043-1046. doi: 10.1016/j.jaad.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hébert V, Boulard C, Houivet E, et al. ; French Study Group on Autoimmune Bullous Skin Diseases; Autoimmune Bullous Skin Disease Task Force of the European Academy of Dermatology and Venereology . Large international validation of ABSIS and PDAI Pemphigus Severity Scores. J Invest Dermatol. 2019;139(1):31-37. doi: 10.1016/j.jid.2018.04.042 [DOI] [PubMed] [Google Scholar]
- 25.Abasq C, Mouquet H, Gilbert D, et al. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus. Arch Dermatol. 2009;145(5):529-535. doi: 10.1001/archdermatol.2009.9 [DOI] [PubMed] [Google Scholar]
- 26.Jelti L, Prost-Squarcioni C, Ingen-Housz-Oro S, et al. ; Centre de référence des maladies bulleuses auto-immunes (MALIBUL); Groupe Bulles de la SFD et la Filière des maladies rares dermatologiques (FIMARAD) . Update of the French recommendations for the management of pemphigus [in French]. Ann Dermatol Venereol. 2019;146(4):279-286. doi: 10.1016/j.annder.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 27.Patsatsi A, Kyriakou A, Giannakou A, Pavlitou-Tsiontsi A, Lambropoulos A, Sotiriadis D. Clinical significance of anti-desmoglein-1 and -3 circulating autoantibodies in pemphigus patients measured by area index and intensity score. Acta Derm Venereol. 2014;94(2):203-206. doi: 10.2340/00015555-1666 [DOI] [PubMed] [Google Scholar]
- 28.Nakahara T, Takagi A, Yamagami J, et al. High anti–desmoglein 3 antibody ELISA index and negative indirect immunofluorescence result in a patient with pemphigus vulgaris in remission: evaluation of the antibody profile by newly developed methods. JAMA Dermatol. 2014;150(12):1327-1330. doi: 10.1001/jamadermatol.2014.411 [DOI] [PubMed] [Google Scholar]
- 29.Trouvin A-P, Jacquot S, Grigioni S, et al. Usefulness of monitoring of B cell depletion in rituximab-treated rheumatoid arthritis patients in order to predict clinical relapse: a prospective observational study. Clin Exp Immunol. 2015;180(1):11-18. doi: 10.1111/cei.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JCW. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(2):613-620. doi: 10.1002/art.21617 [DOI] [PubMed] [Google Scholar]
- 31.Albers LN, Liu Y, Bo N, Swerlick RA, Feldman RJ. Developing biomarkers for predicting clinical relapse in pemphigus patients treated with rituximab. J Am Acad Dermatol. 2017;77(6):1074-1082. doi: 10.1016/j.jaad.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 32.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123(1):66-73. doi: 10.1016/j.clim.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 33.Chen DM, Odueyungbo A, Csinady E, et al. ; French Study Group on Autoimmune Bullous Diseases . Rituximab is an effective treatment in patients with pemphigus vulgaris and demonstrates a steroid-sparing effect (published online September 5, 2019). Br J Dermatol. doi: 10.1111/bjd.18482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772-1779. doi: 10.1056/NEJMoa062930 [DOI] [PubMed] [Google Scholar]
- 35.Cianchini G, Lupi F, Masini C, Corona R, Puddu P, De Pità O. Therapy with rituximab for autoimmune pemphigus: results from a single-center observational study on 42 cases with long-term follow-up. J Am Acad Dermatol. 2012;67(4):617-622. doi: 10.1016/j.jaad.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez J, Ingen-Housz-Oro S, Chosidow O, Antonicelli F, Bernard P. Rituximab as single long-term maintenance therapy in patients with difficult-to-treat pemphigus. JAMA Dermatol. 2018;154(3):363-365. doi: 10.1001/jamadermatol.2017.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]