Abstract
Obesity predicts adverse microvasculature from childhood, potentially via inflammatory pathways. We investigated whether inflammation mediates associations between obesity and microvascular parameters. In 1054 children (mean age 11 years) and 1147 adults (44 years) from a cross-sectional study, we measured BMI (z-scores for children) and WHtR, Glycoprotein acetyls (GlycA), an inflammatory marker, and retinal arteriolar and venular calibre. Causal mediation analysis methods decomposed a “total effect” into “direct” and “indirect” components via a mediator, considering continuous and categorical measures and adjusting for potential confounders. Compared to normal-weight BMI children, those with overweight or obesity had narrower arteriolar calibre (total effects −0.21 to −0.12 standard deviation (SD)): direct (not mediated via GlycA) effects were similar. Children with overweight or obesity had 0.25 to 0.35 SD wider venular calibre, of which 19 to 25% was mediated via GlycA. In adults, those with obesity had 0.07 SD greater venular calibre, which was completely mediated by GlycA (indirect effect: 0.07 SD, 95% CI −0.01 to 0.16). Similar findings were obtained with other obesity measures. Inflammation mediated associations between obesity and retinal venules, but not arterioles from mid-childhood, with higher mediation effects observed in adults. Interventions targeting inflammatory pathways may help mitigate adverse impacts of obesity on the microvasculature.
Subject terms: Biomarkers, Chronic inflammation
Introduction
Obesity is a leading risk factor for cardiovascular disease (CVD), with adverse effects on the vasculature, including the microcirculation1,2. Obesity is associated with adverse microvascular parameters from early childhood, but underlying mechanisms remain unclear3,4. Inflammation is suggested to play a key role5.
Retinal microvasculature, which has been shown to mirror changes in other vascular beds, is a surrogate marker for systemic microvasculature6. Investigation of the retinal microvasculature is now feasible in population-based cohort studies by retinal imaging technique7, which is well-tolerated and reproducible from early childhood onwards8. Population-based studies have shown that increasing body mass index (BMI) and waist-to-height ratio (WHtR), two commonly used measures related to obesity, are associated with narrower retinal arterioles and wider venules in both children and adults5,9–11.
Inflammatory markers, such as high sensitivity C-reactive protein (hsCRP) and white blood cell count, have been associated with adverse retinal microvascular variation in adults, most consistently with venular rather than arteriolar calibre3,12,13. In children, acute phase reactants, such as hsCRP, are less informative about chronic inflammation. A novel biomarker, glycoprotein acetyls (GlycA), is suggested to be more indicative of chronic and cumulative inflammation in children and adults14,15. Few studies of the relationship between inflammation and the microvasculature have considered the role of obesity, a key driver of chronic inflammation12,13.
The question of whether the association between obesity and retinal microvasculature is explained by inflammatory pathways can be addressed within a causal mediation framework (Fig. 1a,b). Theoretically, this framework enables estimation of the extent to which the total causal effect of an exposure on an outcome occurs via an intermediate variable (“mediator”), by decomposing the total effect into an “indirect” effect through the mediator and a “direct” effect through other pathways.
Figure 1.
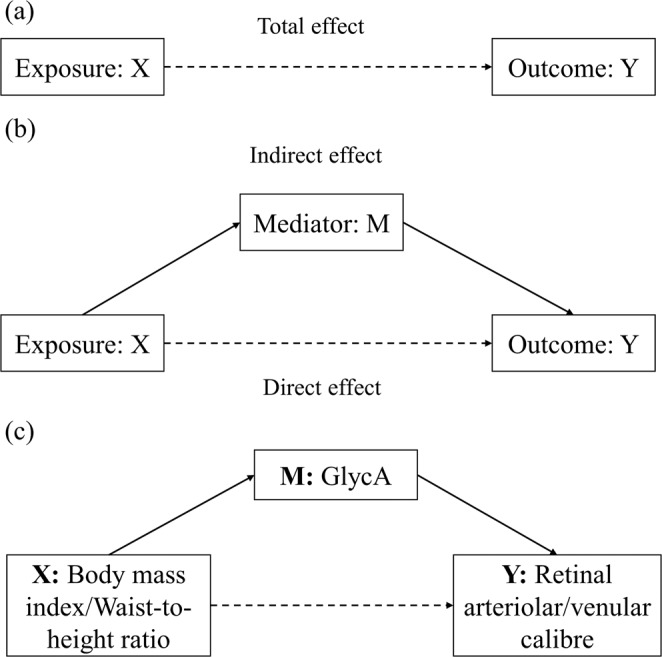
Causal mediation framework. (a) The effect of exposure X on outcome Y without taking into account mediator M is known as the “total effect”. (b) The effect of X on Y not via M is known as the “direct effect”, and the effect through M is the “indirect effect”. (c) X, M, Y are body mass index /waist-to-height ratio, glycoprotein acetyls (GlycA) and retinal arteriolar/venular calibre in the current study. Confounders included in the analysis were not shown in the figure.
While the “total (causal) effect” of obesity on any outcome is ill-defined, it is relevant to examine the extent to which obesity-related disparities in retinal microvascular parameters would be reduced, if the distribution of inflammatory markers in those with obesity were reduced to levels observed in the non-obese16, thereby representing potential “interventional effects”17,18. Using this approach, we examined whether the inflammatory biomarker GlycA mediates the association between measures relating to obesity (BMI and WHtR) and retinal microvasculature (Fig. 1c), in 11–12 year olds and in mid-life adults. The child and adult sample can provide information regarding associations across the life course. We hypothesise that the association between obesity and retinal vascular calibre and the mediation effect of inflammation will be stronger in adults than in children.
Results
Sample characteristics
The study flow from wave 1 of LSAC onwards is shown in the Supplementary Figure. Of the 1874 participating families, 1054 children (mean age 11.4 years) and 1147 adults (their parents, mean age 43.8 years) had data on all measures. Table 1 shows the characteristics of the analytic sample. Over a quarter of children were in the overweight (14.4%) and obese (8.6%) categories, and 8.6% had WHtR ≥0.5. In adults, 62.2% were in the overweight and obese categories, and 53.9% had WHtR ≥0.5.
Table 1.
Sample characteristics.
| Characteristics | Children (n = 1054) | Adults (n = 1147) |
|---|---|---|
| Means (SDa) or % | Means (SDa) or % | |
| Age (years) | 11.4 (0.5) | 43.8 (5.1) |
| Sex (% female) | 52.0 | 86.7 |
| Socioeconomic position | 0.3 (0.9) | 0.3 (1.0) |
| Body mass index z-scoreb | 0.3 (1.0) | |
| Body mass index (kg/m2) | 27.9 (6.1) | |
| Current BMI status (%) | ||
| Normal | 77.0 | 37.8 |
| Overweight | 14.4 | 32.9 |
| Obese | 8.6 | 29.3 |
| Waist-to-height ratio (WHtR) | 0.4 (0.1) | 0.5 (0.1) |
| Current WHtR status (%) | ||
| <0.5 | 91.4 | 46.1 |
| ≥0.5 | 8.6 | 53.9 |
| Retinal arteriolar calibre (µm) | 159.0 (11.9) | 151.1 (13.9) |
| Retinal venular calibre (µm) | 230.9 (16.5) | 219.0 (18.6) |
| Glycoprotein Acetyls (mmol/L) | 1.0 (0.1) | 1.0 (0.2) |
a.Standard deviation; bBody mass index was transformed to z-scores using the Centres for Disease Control and Prevention (US) growth charts.
Interrelationships between key variables
Table 2 shows results from multivariable linear regression models, adjusted for age, sex and SEP. BMI and WHtR were moderately associated with GlycA, with stronger evidence for associations in adults than children. For example, in children and adults, one SD greater in BMI were associated with 0.39 SD (95% CI 0.33 to 0.45) and 0.47 SD (95% CI 0.42 to 0.52) higher GlycA scores, respectively.
Table 2.
Inter-relationships of key variables, assessed via multivariable linear regression models, per SD unit higher in the exposure (adjusted for age, sex and SEP).
| Models (the exposure and outcome) | Children | Adults | ||
|---|---|---|---|---|
| Effect size (95% CI) | p | Effect size (95% CI) | p | |
| BMI/WHtR and proposed inflammatory mediator | ||||
| BMI and GlycA | 0.39 (0.33, 0.45) | <0.001 | 0.47 (0.42, 0.52) | <0.001 |
| WHtR and GlycA | 0.44 (0.38, 0.49) | <0.001 | 0.53 (0.48, 0.57) | <0.001 |
| BMI/WHtR and retinal vascular calibre outcomes | ||||
| BMI and arteriolar calibre | −0.12 (−0.19, −0.06) | <0.001 | −0.16 (−0.21, −0.08) | <0.001 |
| BMI and venular calibre | 0.03 (−0.04, 0.09) | 0.33 | 0.02 (−0.04, 0.07) | 0.58 |
| WHtR and arteriolar calibre | −0.10 (−0.16, −0.06) | <0.001 | −0.16 (−0.22, −0.10) | <0.001 |
| WHtR and venular calibre | 0.05 (−0.01, 0.12) | 0.08 | 0.03 (−0.03, 0.08) | 0.36 |
| Proposed inflammatory mediator and retinal vascular calibre outcomes | ||||
| GlycA and arteriolar calibre | −0.02 (−0.08, 0.04) | 0.52 | −0.09 (−0.15, −0.03) | <0.01 |
| GlycA and venular calibre | 0.09 (0.03, 0.15) | <0.01 | 0.06 (0.00, 0.12) | 0.03 |
| Proposed inflammatory mediator and retinal vascular calibre outcomes* | ||||
| GlycA and arteriolar calibre | 0.03 (−0.03, 0.09) | 0.41 | −0.02 (−0.08, 0.05) | 0.59 |
| GlycA and venular calibre | 0.09 (0.03, 0.16) | <0.01 | 0.07 (0.01, 0.14) | 0.03 |
Abbreviations: 95%CI, 95% confidence interval; BMI, body mass index; WHtR, waist-to-height ratio; GlycA, glycoprotein acetyls.
*The model estimates were further adjusted for body mass index as a confounding factor.
Higher BMI and WHtR showed small associations with narrower arteriolar calibre, with effects slightly larger in adults than children. In contrast, the associations of BMI with venular calibre were weak for both children and adults (0.03 (95% CI −0.04 to 0.09), 0.02 (95% CI −0.04 to 0.07), respectively). Similar findings were observed for WHtR and venular calibre.
Higher GlycA had small associations with narrower arteriolar calibre in adults but not children. In adults, one SD higher GlycA was associated with −0.09 SD (95% CI −0.15 to −0.03) narrower arteriolar calibre. Further adjustment for BMI fully attenuated this association in adults. The association between higher GlycA and wider venular calibre in children was less evident in adults, and BMI did not appear to confound this association.
Does GlycA mediate the association of obesity with retinal vascular calibre?
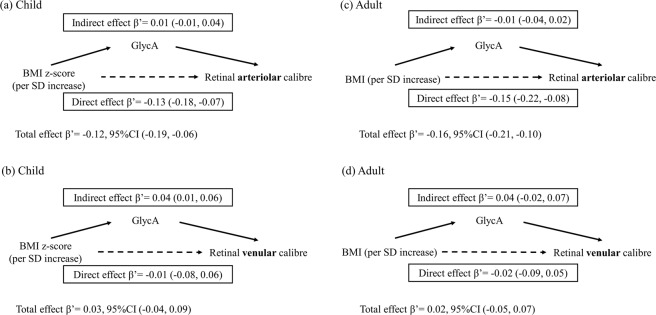
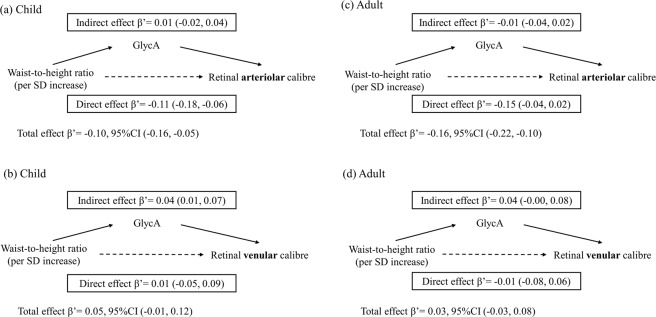
The results of the causal mediation analysis when exposures were considered as continuous variables are presented in Figs. 2 and 3. Although BMI had a small “total effect” on retinal arteriolar calibre (Fig. 2a,c), the direct effects, not mediated via GlycA, were similar to the total effects in children and adults. In contrast, BMI had a smaller effect on venular calibre for children and adults (Fig. 2b,d), with some evidence of mediation through GlycA in both groups. Direct effects, not involving GlycA, were in the opposite direction for children (−0.01 SD, 95% CI −0.08 to 0.06) and adults (−0.02 SD, 95% CI −0.01 to 0.05). The results were similar when the exposure was WHtR (Fig. 3).
Figure 2.
Mediation effects of GlycA on associations of body mass index (z-scores for children, standardised scores for adults) with standardised retinal venular and arteriolar calibre. Footnotes: β’ represents effect estimates for direct, indirect, and total effect derived from causal mediation analysis. SDs of retinal arteriolar and venular calibre in children are 11.9 µm and 16.5 µm, and SDs of BMI, retinal arteriolar and venular calibre in adults are 6.1 kg/m2, 13.9 µm and 18.6 µm. Abbreviations: 95%CI, 95% confidence interval; SD, standard deviation; BMI, body mass index; GlycA, glycoprotein acetyls.
Figure 3.
Mediation effects of GlycA on associations of standardised waist-to-height ratio with standardised retinal venular and arteriolar calibre. Footnotes: β’ represents effect estimates for direct, indirect, and total effect derived from causal mediation analysis. SDs of waist-to-height ratio, retinal arteriolar and venular calibre in children are 0.1, 11.9 µm and 16.5 µm, and SDs of waist-to-height ratio, retinal arteriolar and venular calibre in adults are 0.1, 13.9 µm and 18.6 µm. Abbreviations: 95%CI, 95% confidence interval; SD, standard deviation; WHtR, waist-to-height ratio; GlycA, glycoprotein acetyls.
When considering exposures as categorical variables, compared to those with normal weight, those with overweight or obesity - whether either measured by BMI or WHtR - had narrower arteriolar calibre, but again the direct effects not mediated via GlycA were similar to the total effects (Table 3).
Table 3.
Direct and indirect effects of overweight and obesity on retinal vascular calibre through GlycA; estimates adjusted for age, sex and SEP.
| Obesity category | Children | Adults | ||||||
|---|---|---|---|---|---|---|---|---|
| Retinal arteriolar calibre | Retinal venular calibre | Retinal arteriolar calibre | Retinal venular calibre | |||||
| β’ (95% CI) | p | β’ (95% CI) | p | β’ (95% CI) | p | β’ (95% CI) | p | |
| BMI: Overweight vs normal (ref) | ||||||||
| Indirect effect | 0.00 (−0.05, 0.05) | 0.93 | 0.05 (0.00, 0.10) | 0.05 | −0.01 (−0.04, 0.03) | 0.71 | 0.03 (−0.00, 0.06) | 0.08 |
| Direct effect | −0.11 (−0.32, 0.10) | 0.30 | 0.20 (0.01, 0.40) | 0.04 | −0.23 (−0.36, −0.08) | <0.01 | −0.01 (−0.17, 0.13) | 0.81 |
| Total effect | −0.11 (−0.32, 0.10) | 0.33 | 0.25 (0.06, 0.45) | 0.01 | −0.24 (−0.36, −0.10) | <0.01 | 0.02 (−0.13, 0.16) | 0.85 |
| Proportion mediated by GlycA | −0.01 (−1.98, 2.76) | 0.96 | 0.19 (−0.00, 0.78) | 0.06 | 0.03 (−0.13, 0.23) | 0.71 | 1.92 (−5.12, 7.21) | 0.88 |
| BMI: Obese vs normal (ref) | ||||||||
| Indirect effect | 0.00 (−0.08, 0.09) | 0.88 | 0.09 (0.01, 0.18) | 0.04 | −0.01 (−0.04, 0.03) | 0.71 | 0.07 (−0.01, 0.16) | 0.08 |
| Direct effect | −0.14 (−0.42, 0.14) | 0.32 | 0.26 (−0.04, 0.55) | 0.09 | −0.23 (−0.37, −0.09) | <0.01 | 0.00 (−0.18, 0.16) | 0.95 |
| Total effect | −0.13 (−0.41, 0.13) | 0.32 | 0.35 (0.07, 0.64) | 0.02 | −0.24 (−0.37, −0.10) | <0.01 | 0.07 (−0.08, 0.21) | 0.34 |
| Proportion mediated by GlycA | −0.02 (−2.40, 2.64) | 0.92 | 0.25 (−0.00, 1.25) | 0.06 | 0.03 (−0.13, 0.23) | 0.71 | 1.00 (−6.86, 10.23) | 0.39 |
| WHtR: ≥0.5 vs <0.5 (ref) | ||||||||
| Indirect effect | −0.00 (−0.09, 0.08) | 0.98 | 0.10 (0.02, 0.18) | 0.01 | −0.03 (−0.10, 0.03) | 0.27 | 0.05 (−0.02, 0.12) | 0.12 |
| Direct effect | −0.18 (−0.41, 0.03) | 0.11 | 0.13 (−0.08, 0.38) | 0.22 | −0.22 (−0.35, −0.09) | <0.001 | 0.02 (−0.12, 0.15) | 0.76 |
| Total effect | −0.18 (−0.41, 0.04) | 0.10 | 0.23 (0.03, 0.46) | 0.02 | −0.25 (−0.37, −0.14) | <0.001 | 0.07 (−0.04, 0.19) | 0.21 |
| Proportion mediated by GlycA | 0.01 (−1.29, 0.92) | 0.98 | 0.41 (0.04, 1.99) | 0.03 | 0.14 (−0.09, 0.46) | 0.27 | 0.69 (−5.69, 7.24) | 0.30 |
β’ represents effect estimates for direct, indirect, and total effect derived from causal mediation analysis, compared to the reference group.
The proportion of the total effect mediated by GlycA was calculated as the ratio of the indirect effect to the total effect.
Abbreviations: 95%CI, 95% confidence interval; BMI, body mass index; WHtR, waist-to-height ratio; GlycA, glycoprotein acetyls.
Children with overweight and obesity had 0.25 SD and 0.35 SD wider venular calibre (respectively) compared to those with normal-weight BMI, of which 19 to 25% was mediated via GlycA (indirect effect: overweight 0.05 SD, 95% CI 0.0 to 0.10; obese 0.09 SD, 95% CI 0.01 to 0.18). In adults, retinal venular calibre was similar for normal weight and overweight, but adults with obesity had 0.07 SD greater venular calibre and this effect was completely mediated by GlycA (indirect effect: 0.07 SD, 95% CI −0.01 to 0.16). Similar mediation effects were seen in children and adults with WHtR ≥0.5 compared with those WHtR <0.5, with 41% to 69% of the association with venular calibre mediated by GlycA, respectively.
Discussion
This is the first study to investigate whether the associations of obesity-related measures with microvascular parameters are mediated via inflammation. We showed that BMI and WHtR were associated both with GlycA and with adverse retinal vascular calibre (narrower arterioles and wider venules). Novel findings were that higher GlycA was associated with both wider venular calibre (in mid-childhood and mid-life adults) and narrower arteriolar calibre (in adults). Inflammation mediated associations between both measures related to obesity and retinal venular, but not arteriolar, calibre, with adults had higher mediation effects than children.
Our large, cross-generational cohort with standardised measurements using the same equipment and protocols may minimise potential measurement bias. The associations were investigated in children and adults, providing data from two ages in the life course. Similar results were observed for BMI and WHtR, which increases confidence in our findings, although these measures are highly correlated in children and adults (correlation coefficients 0.83 and 0.92 respectively). The use of GlycA, relatively novel in population-based studies19, may be advantageous given limitations of acute phase reactants in capturing chronic inflammation, especially in children15.
Potential limitations include the difficulty in interpreting mediated effects causally given the lack of a well-defined intervention on inflammation, so that our study can only suggest this as a potential intervention target. In addition, the cross-sectional and observational study design means there is potential for residual or unmeasured confounding. We assumed that there was no post-exposure confounding of mediator-outcome relationships that could arise through other potential mediating pathways. This assumption could be flawed; for example, higher LDL, another potential mediator, which may be increased in those with obesity and associate with narrower arteriolar and wider venular calibre directly20, or via the LDL-inflammation association to indirectly associate with poor retinal microvascular parameters21. However, in our additional analyses, LDL appears to confound the association with venular calibre only slightly in adults (Supplementary Table). Our participants were marginally more advantaged socioeconomically than the original nationally representative LSAC sample, so our conclusions may be less relevant to disadvantaged populations. Our analysis and findings were based on an existing cohort. Pre-specified analyses from future prospective studies are warranted to replicate the findings.
Retinal arteriolar and venular calibre showed different patterns of association with BMI and WHtR, in keeping with current evidence that they may be influenced by different factors22. Our study supports previous findings that inflammation is more consistently associated with retinal venular than arteriolar calibre in children and adults. The Generation R Study of children aged 6 years found that each SD higher CRP was associated with 0.10 SD (95% CI 0.06 to 0.14) wider venular calibre3. Similar findings are reported in mid-life and older adults and with other inflammatory markers, such as fibrinogen and white blood cells count23–27. GlycA is a novel composite inflammatory marker with greater stability and lower intra-individual variability than single acute phase inflammatory proteins, such as hsCRP28. In population studies, GlycA is a robust predictor of all-cause of mortality and CVD, even after adjusting for hsCRP29–31. In adolescents, GlycA is associated with obesity and prediabetes32. To the best of our knowledge, this is the first study to demonstrate that GlycA is associated with retinal venular calibre in children and adults and the magnitude of association is similar to previous findings.
We extended prior studies by exploring whether inflammation mediated the associations of obesity and retinal microvascular parameters. We found that the association of obesity with venular calibre was mediated by inflammation in both age groups. Data from both population-based studies and animal experiments support this interpretation. Yau et al. reported that in adolescents, low-grade inflammation partially mediates the relationship between cardiovascular fitness and more favourable retinal venular, but not arteriolar parameters33. In animal models, increased adiposity results in adipocyte dysfunction with increased pro-inflammatory cytokines and leukocyte activation, which may lead to the destruction of venular endothelium and consequently to wider retinal venular calibre34. In this study, the effect size in adults was small with wide confidence intervals, reflecting some lack of precision in our estimation of the degree of mediation by inflammation.
In contrast, although there is a small association between obesity and arteriolar calibre, the association between inflammation and arteriolar calibre was weak. Thus, the relationship of obesity and arteriolar calibre is unlikely to be mediated via inflammatory pathways, but by other non-inflammatory pathways. For example, obesity is a well-recognised risk factor for elevated blood pressure, a key determinant of retinal arteriolar calibre35. Structural adaptations of the endothelium and vessel wall in chronic hypertension may contribute to narrowing of the retinal arteriolar lumen36. Future studies could address the possible mediating role of blood pressure in this relationship.
Narrower retinal arterioles and wider retinal venules have been suggested as preclinical vascular phenotypes for CVD and in cohort studies have been associated with worse cardiovascular outcomes37. For example in older adults from the Atherosclerosis Risk in Communities study (n = 10470) with 16 years of follow-up, each SD increase in retinal venular and decrease in arteriolar calibre were associated with 18% and 14% higher risk of stroke, respectively (hazard ratio 1.18, 95% CI 1.07 to 1.31; 1.14, 95% CI 1.03 to 1.26)38. In our study, we observed a difference in retinal calibre in both children and adults with obesity: 0.35 SD and 0.07 SD wider venules, 0.13 SD and 0.24 SD narrower arterioles (respectively), compared to those with normal BMI. Obesity is a major risk factor of CVD, and children who have obesity are more likely to become adults with obesity39. Those with obesity have adverse microvascular parameters and may at risk of future CVD.
In addition, we found a large proportion of the association between obesity and retinal venular calibre was mediated via GlycA, 25–40% of the total effect in children and 70–100% in adults. This suggests that children have had less cumulative exposure to inflammation and that, with age, associations between obesity and adverse microvascular parameters may be increasingly mediated by inflammation. A large randomised control trial targeting interleukin-1ß (a prototypical inflammatory cytokine) has provided direct support for the ‘inflammatory hypothesis’ of CVD in humans40. Obesity is a major driver of inflammation41 and anti-inflammatory interventions may have a role in mitigating the adverse impacts of obesity on the systemic microvasculature. This may be a future therapeutic target given the recognition that the microvasculature is a key player in the obesity-associated CVD pathogenesis42. Currently, most interventions focus on nutritional intake and physical activity but do not result in sustained improvements in preventing or managing weight gain43. Addressing downstream adverse effects, such as inflammation, may be useful adjunctive interventions44. Further studies are required to understand the key therapeutic targets and the optimal age to intervene safely.
Our findings suggest that the association between obesity and wider retinal venules is mediated via inflammation, even at 11–12 years of age. Conversely, for retinal arterioles, non-inflammatory pathways appear more important.
Methods
Study design and participants
The Child Health CheckPoint (CheckPoint) is a cross-sectional population-based biophysical assessment substudy nested within the national Longitudinal Study of Australian Children (LSAC)45,46. Details of the initial study design and recruitment are outlined elsewhere47,48. Briefly, LSAC recruited a nationally representative birth cohort of 5107 infants using a 2-stage clustered design and has since collected data in biennial ‘waves’ for a decade. The initial response rate in 2004 was 57.2%, of whom 73.7% (n = 3764) were retained to wave 6 in 2014.
CheckPoint took place from February 2015 to March 2016, between LSAC waves 6 and 7 at child age 11–12 years. In total, 1874 children (53% of all wave 6 families) participated in this detailed cross-sectional biophysical assessment46. Most attended an assessment centre in seven cities around Australia. Families (n = 518) who had a more limited assessment in regional cities or home visits were not included as the equipment for retinal imaging could not be readily transported.
The CheckPoint study was approved by the Royal Children’s Hospital Melbourne Human Research Ethics Committee (33225D) and the Australian Institute of Family Studies Ethics Committee (14–26). The attending parents/caregivers provided written informed consent for themselves and their children to participate. All methods were performed in accordance with the relevant guidelines and regulations.
Measures relating to obesity
Height and weight (to the nearest 0.1 cm and 0.1 kg respectively) were measured up to three times by trained assessors, where the mean of all measurements was used. BMI was calculated as weight (kg)/height (m2). Children’s BMI was converted into age- and gender-specific z-scores using the US Centres for Disease Control (CDC) growth reference charts49, and also classified as normal (5 to 85th percentile), overweight (85th to <95th percentile) and obese (≥95th percentile)50. For adults, the BMI status was defined as normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), or obese (≥30 kg/m2)51. We did not include participants with underweight given the limited number in this study (child BMI z-score <5th percentile, 4.2%; adults BMI < 18.5 kg/m2, 0.3%).
Waist circumference (cm) was measured against bare skin around the navel. Two measurements were taken and the mean was used. For children and adults, continuous WHtR was calculated as waist (cm)/height (cm) and also classified into two categories (<0.5 and ≥0.5), where a ratio ≥0.5 indicates central obesity52,53.
Retinal vascular calibre
Two optic disc-centred digital photographs from each eye were taken by a fundus camera (EOS 60D SLR) in a darkened room without mydriasis. Experienced graders scored the images using the Integrative Vessel Analysis software program (IVAN, University of Wisconsin, Madison, USA). Details of scoring procedures are described elsewhere54. Briefly, right eye images were selected as the first choice for scoring. Retinal vessels were identified as arterioles or venules from a specific area (one-half to one-disc diameter from the optic disc margins) and a segment of each vessel within this area was selected for measurement. Diameters of all the selected segments were measured automatically using IVAN software. Summary estimates of the average retinal vascular calibre were calculated combining measurements of the six largest arterioles or venules55. Reproducibility of retinal vascular measurements has been reported, with inter- and intra-grader intraclass correlation coefficients of 0.76 to 0.9954.
Inflammatory marker
At the assessment centre, semi-fasting (median 4.2 hours postprandial) peripheral blood was processed within four hours of collection at an on-site processing laboratory, with serum aliquots frozen at −80 °C. Serum samples were shipped on dry ice for high-throughput proton NMR spectroscopy (Nightingale Ltd, Vantaa, Finland). Levels of GlycA were calculated using Nightingale 2017 quantification algorithms and were reported in mmol/L56.
Covariates
Age, sex and family socioeconomic position (SEP) were considered as potential confounders of the exposure-outcome, exposure-mediator and mediator-outcome associations22,57,58. SEP was measured in LSAC wave 6 (child age 10–11 years), approximately 12 months before the CheckPoint assessment. This SEP variable summarises parent-reported combined household income, current or most recent occupation of each parent, and highest educational qualification of each parent59. Each component was scaled and an unweighted average was calculated and standardised within the wave to have a mean of 0 and standard deviation (SD) of 1. A higher score indicates more advantaged socioeconomic position.
Statistical methods
Statistical analyses were performed in R software (version 3.5.2). Data from children and adults were analysed separately, using the records with complete data on all the variables. Scores on all continuous exposure and outcome measures were internally standardised ([observed value-mean]/SD).
Firstly, we fitted linear regression models to explore associations of each exposure (BMI and WHtR as continuous variables) with the proposed mediator (GlycA) and with the outcome (retinal vascular calibre), as well as the association of the mediator with the outcome. Linear regression models were adjusted for age, sex and SEP. In addition, when examining associations between GlycA and retinal vascular calibre, models were additionally adjusted for BMI, as it is a potential confounder.
We then investigated the possible mediating role of GlycA using the causal mediation framework, while considering BMI and WHtR continuously and categorically (Fig. 1c), adjusted for age, sex and SEP60. Specifically, we estimated the so-called “interventional” direct and indirect effects, which are estimable under relaxed assumptions compared to previous causal mediation approaches17,18. The interventional indirect effect through M is interpreted as the change in the risk of the outcome Y in the exposed if we were to shift the distribution of M to that in the unexposed, and the interventional direct effect is the effect of X on Y that would remain after that shift. We used the “mediation” R package61, and specified the X, Y, and M in the model as continuous and categorical BMI/WHtR, retinal arteriolar/venular calibre and GlycA (Fig. 1c). This utilises a g-computation approach with linear regression for the outcome and mediator given their antecedent variables as building blocks. The direct and indirect effect estimates indicate the magnitude of change (in SDs) in the outcome variable per SD change in the continuous exposure variable or compared to the categorical reference group (i.e. normal-weight BMI and <0.5 WHtR category).
Supplementary information
Acknowledgements
This study uses data from the Longitudinal Study of Australian Children (LSAC) and Child Health CheckPoint. We thank the LSAC and CheckPoint study participants and families. We also thank the CheckPoint team and the Murdoch Children’s Research Institute. LSAC is conducted in partnership between the Department of Social Services (DSS), the Australian Institute of Family Studies (AIFS) and the Australian Bureau of Statistics (ABS). The findings and views reported in this paper are those of the authors and should not be attributed to DSS, AIFS or the ABS. This work was supported by the Australian National Health and Medical Research Council (NHMRC) Project Grants [1041352, 1109355], The Royal Children’s Hospital Foundation [2014-241], Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia [100660] and Financial Markets Foundation for Children [2014-055, 2016-310]. M.L. is supported by a Melbourne Research Scholarship. KL is supported by Australian National Health & Medical Research Council (NHMRC) Early Career Fellowship [1091124] and National Heart Foundation Postdoctoral Fellowship [101239]. MMB is supported by an ARC DECRA Fellowship [DE190101326]. M.J. is supported by Juho Vainio Foundation and federal research grants to Turku University Hospital. M.W. was supported by NHMRC Senior Research Fellowship [1046518], Principal Research Fellowship [1160906] and Cure Kids New Zealand. D.P.B. is supported by NHMRC Senior Research Fellowship [1064629] and is an Honorary Future Leader Fellowship of the National Heart Foundation of Australia [100369]. Research at the Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Program. The funding bodies did not play any role in the study other than the generous provision of funds.
Author contributions
M.W. conceived and led the CheckPoint study with D.P.B., R.S., K.L., J.A.K., T.Y.W. and M.J. as key CheckPoint investigators. K.L. was M.L.’s primary student supervisor, along with D.P.B., M.W., T.Y.W. and M.H. and oversaw all aspects of the study and the manuscript preparation. M.H. and M.L. contributed to the acquisition of data. M.L. conducted the data analysis with the guidance of M.M.B. M.L. drafted the manuscript. M.M.B., T.Y.W., M.J., J.A.K., M.W. and R.S. advised on statistical issues and interpretation. All authors critically reviewed the manuscript and had final approval of the submitted and published version of this paper.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61801-w.
References
- 1.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 2.McCrindle BW. Cardiovascular consequences of childhood obesity. Can. J. Cardiol. 2015;31:124–130. doi: 10.1016/j.cjca.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Gishti O, et al. Body fat distribution, metabolic and inflammatory markers and retinal microvasculature in school-age children. The generation r study. Int. J. Obes. 2015;39:1482–1487. doi: 10.1038/ijo.2015.99. [DOI] [PubMed] [Google Scholar]
- 4.Gopinath B, et al. Influence of blood pressure and body mass index on retinal vascular caliber in preschool-aged children. J. Hum. Hypertens. 2013;27:523–528. doi: 10.1038/jhh.2013.15. [DOI] [PubMed] [Google Scholar]
- 5.Boillot A, et al. Obesity and the microvasculature: A systematic review and meta-analysis. PLoS ONE. 2013;8:e52708. doi: 10.1371/journal.pone.0052708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: A new tool in microvascular disease research. Circ. Cardiovasc. Imaging. 2008;1:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 7.Struijker-Boudier HA, Heijnen BF, Liu YP, Staessen JA. Phenotyping the microcirculation. Hypertension. 2012;60:523–527. doi: 10.1161/HYPERTENSIONAHA.111.188482. [DOI] [PubMed] [Google Scholar]
- 8.Li LJ, Ikram MK, Wong TY. Retinal vascular imaging in early life: Insights into processes and risk of cardiovascular disease. J. Physiol. 2016;594:2175–2203. doi: 10.1113/JP270947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochli S, et al. Obesity, blood pressure, and retinal vessels: A meta-analysis. Pediatrics. 2018;141:e20174090. doi: 10.1542/peds.2017-4090. [DOI] [PubMed] [Google Scholar]
- 10.Xiao W, et al. Association between body composition and retinal vascular caliber in children and adolescents. Invest. Ophthalmol. Vis. Sci. 2015;56:705–710. doi: 10.1167/iovs.14-14946. [DOI] [PubMed] [Google Scholar]
- 11.Li LJ, et al. The relationship of body fatness indices and retinal vascular caliber in children. Int. J. Pediatr. Obes. 2011;6:267–274. doi: 10.3109/17477166.2011.583657. [DOI] [PubMed] [Google Scholar]
- 12.Daien V, et al. Retinal vascular caliber is associated with cardiovascular biomarkers of oxidative stress and inflammation: The pola study. PLoS ONE. 2013;8:e71089. doi: 10.1371/journal.pone.0071089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yim-Lui Cheung C, et al. C-reactive protein and retinal microvascular caliber in a multiethnic asian population. Am. J. Epidemiol. 2010;171:206–213. doi: 10.1093/aje/kwp357. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie SC, et al. The biomarker glyca is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst. 2015;1:293–301. doi: 10.1016/j.cels.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Collier F, et al. Glycoprotein acetyls (glyca) at 12 months are associated with high-sensitivity c-reactive protein and early life inflammatory immune measures. Pediatr. Res. 2019;85:584–585. doi: 10.1038/s41390-019-0307-x. [DOI] [PubMed] [Google Scholar]
- 16.Micali N, Daniel RM, Ploubidis GB, De Stavola BL. Maternal prepregnancy weight status and adolescent eating disorder behaviors: A longitudinal study of risk pathways. Epidemiology. 2018;29:579–589. doi: 10.1097/EDE.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 17.Vanderweele TJ, Vansteelandt S, Robins JM. Effect decomposition in the presence of an exposure-induced mediator-outcome confounder. Epidemiology. 2014;25:300–306. doi: 10.1097/EDE.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno-Betancur M, Carlin JB. Understanding interventional effects: A more natural approach to mediation analysis? Epidemiology. 2018;29:614–617. doi: 10.1097/EDE.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 19.Otvos JD, et al. Glyca: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clin. Chem. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri S, et al. Systemic circulatory influences on retinal microvascular function in middle-age individuals with low to moderate cardiovascular risk. Acta Ophthalmol. 2015;93:e266–274. doi: 10.1111/aos.12594. [DOI] [PubMed] [Google Scholar]
- 21.Zhong, S. et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. S0891-5849(0819)30271-30270, 10.1016/j.freeradbiomed.2019.03.036 (2019). [DOI] [PubMed]
- 22.Ikram MK, Ong YT, Cheung CY, Wong TY. Retinal vascular caliber measurements: Clinical significance, current knowledge and future perspectives. Ophthalmologica. 2013;229:125–136. doi: 10.1159/000342158. [DOI] [PubMed] [Google Scholar]
- 23.De Jong FJ, et al. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann. Neurol. 2007;61:491–495. doi: 10.1002/ana.21129. [DOI] [PubMed] [Google Scholar]
- 24.Lammertyn L, et al. Retinal vessel calibres and haemostasis in black and white south africans: The sabpa study. J. Hypertens. 2015;33:2483–2490. doi: 10.1097/HJH.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 25.Liew G, et al. Complete blood count and retinal vessel calibers. PLoS ONE. 2014;9:e102230. doi: 10.1371/journal.pone.0102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liew G, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: The atherosclerosis risk in communities study. Arch. Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tien YW, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The multi-ethnic study of atherosclerosis (MESA) Invest. Ophthalmol. Vis. Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung CP, et al. GlycA, a novel marker of inflammation, is elevated in systemic lupus erythematosus. Lupus. 2016;25:296–300. doi: 10.1177/0961203315617842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duprez DA, et al. Comparison of the predictive value of glyca and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin. Chem. 2016;62:1020–1031. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 30.Muhlestein JB, et al. GlycA and hsCRP are independent and additive predictors of future cardiovascular events among patients undergoing angiography: The intermountain heart collaborative study. Am. Heart J. 2018;202:27–32. doi: 10.1016/j.ahj.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Lawler PR, et al. Circulating N-linked Glycoprotein Acetyls and longitudinal mortality risk. Circ. Res. 2016;118:1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson ML, et al. Decreased GlycA after lifestyle intervention among obese, prediabetic adolescent latinos. J. Clin. Lipidol. 2019;13:186–193. doi: 10.1016/j.jacl.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yau PL, et al. Insulin sensitivity and inflammation mediate the impact of fitness on cerebrovascular health in adolescents. Child. Obes. 2017;13:205–212. doi: 10.1089/chi.2016.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira MC, et al. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obes. 2013;21:E396–406. doi: 10.1002/oby.20230. [DOI] [PubMed] [Google Scholar]
- 35.Ding J, et al. Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J. Hypertens. 2014;32:207–215. doi: 10.1097/HJH.0b013e32836586f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegrist M, et al. Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int. J. Obes. 2014;38:1241–1247. doi: 10.1038/ijo.2013.226. [DOI] [PubMed] [Google Scholar]
- 37.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: Systemic, environmental, and genetic associations. Surv. Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Seidelmann SB, et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: The Atherosclerosis Risk in Communities Study. Circulation. 2016;134:1328–1338. doi: 10.1161/CIRCULATIONAHA.116.023425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the united states from adolescence to the third decade of life. Obes. 2010;18:1801–1804. doi: 10.1038/oby.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 41.Wurtz P, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorop O, et al. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 2017;113:1035–1045. doi: 10.1093/cvr/cvx093. [DOI] [PubMed] [Google Scholar]
- 43.Bleich SN, et al. Interventions to prevent global childhood overweight and obesity: A systematic review. Lancet Diabetes Endocrinol. 2018;6:332–346. doi: 10.1016/S2213-8587(17)30358-3. [DOI] [PubMed] [Google Scholar]
- 44.Truax AD, et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe. 2018;24:364–378 e366. doi: 10.1016/j.chom.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clifford SA, et al. Child health checkpoint: Cohort summary and methodology of a physical health and biospecimen module for the longitudinal study of australian children. BMJ Open. 2019;9:3–22. doi: 10.1136/bmjopen-2017-020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wake M, et al. Introducing Growing Up in Australia’s Child Health Checkpoint. Family Matters. 2014;94:15–23. [Google Scholar]
- 47.Edwards, B. Growing Up in Australia: The Longitudinal Study of Australian Children entering adolescence and becoming a young adults. Family Matters, 5–14 (2014).
- 48.Sanson, A. & Robert, J. Growing Up in Australia takes its first steps. Family Matters, 46–53 (2004).
- 49.Kuczmarski, R. J. et al. CDC growth charts: United states. Adv Data, 1–27 (2000). [PubMed]
- 50.Ogden, C. L. & Flegal, K. M. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report, 1–5 (2010). [PubMed]
- 51.WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000) . Obesity: Preventing and managing the global epidemic: Report of a WHO consultation. World Health Organ Tech Rep Ser. 894, i–xii, 1–253 (2000). [PubMed]
- 52.Bacopoulou F, et al. Waist circumference, waist-to-hip ratio and waist-to-height ratio reference percentiles for abdominal obesity among greek adolescents. BMC Pediatr. 2015;15:50. doi: 10.1186/s12887-015-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoo EG. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016;59:425–431. doi: 10.3345/kjp.2016.59.11.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dascalu J, et al. Retinal microvasculature: Population epidemiology and concordance in australian children aged 11-12 years and their parents. BMJ Open. 2019;9:44–52. doi: 10.1136/bmjopen-2018-022399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knudtson MD, et al. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 56.Wurtz P, et al. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: A primer on -omic technologies. Am. J. Epidemiol. 2017;186:1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varghese M, et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology. 2019;160:293–312. doi: 10.1210/en.2018-00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berger E, et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat. Commun. 2019;10:773. doi: 10.1038/s41467-019-08732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blakemore, T., Strazdins, L. & Gibbings, J. Measuring family socioeconomic position. Australian Social Policy, 121–168 (2009).
- 60.Hicks R, Tingley D. Causal mediation analysis. Stata Journal. 2011;11:605–619. doi: 10.1177/1536867X1201100407. [DOI] [Google Scholar]
- 61.Tingley D, et al. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014;59:38. doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.