Abstract
Miniaturization of electronic devices and systems enhances the complexity of inbuilt circuitry, thereby giving rise to electromagnetic interference (EMI). EMI is a serious cause of concern as it affects the performance of a device, transmission channel, or system. In a quest to find an effective solution to this problem, several materials, apart from the conventional metals, such as carbon derivatives, have been extensively explored recently. In addition to carbon derivatives, hybrid structures such as core–shell, conjugated systems, etc. have also been researched. However, semiconducting fillers have received less attention, especially in this application. Hence, this review article will primarily focus on the systematic understanding of the use of semiconductor-based polymer nanocomposites and how the band gap plays a crucial role in deciding the dielectric properties and subsequently the electromagnetic absorption behavior for shielding applications. Our primary aim is to highlight the mechanism of shielding involved in such nanocomposites in addition to discussing the synthesis and properties that lead to effective shielding. Such nanocomposites containing semiconductors can pave the way for alternate materials for EMI shielding applications that are lightweight, flexible, and easy to integrate.
1. Introduction
The growing demand for electronic and communication devices in various sectors of military, industry, and commercial applications has led to a new kind of challenge called electromagnetic (EM) pollution. To combat this problem, the scientific and research community has initiated the development of various materials for electromagnetic interference (EMI) shielding applications, which range from metals to polymer nanocomposites. Polymer nanocomposite-based shield materials are advantageous over the conventional metal-based shield due to its low density, corrosion resistivity, ease of processability, and other applications. Once we step into the field of polymer nanocomposites, it spans a vast range of materials starting from various conducting and semiconducting fillers to a few insulating fillers. The conducting fillers that are commercially used include metal nanoparticles, carbon-based fillers such as graphene, carbon nanotubes (CNTs), carbon fibers (CFs), etc.1 Mxene2 comprises a newer class of conducting filler being investigated for EMI shielding. Mxenes are two-dimensional inorganic compounds, consisting of few-atom-thick layers of transition metal carbides, nitrides, or carbonitrides. The high conductivity of Mxenes has compelled the researchers to explore it for applications in the EMI shielding domain. There are several reviews explaining conducting fillers and its commercial usage. Herein, we will focus on the semiconducting fillers that are recently being explored among the research groups working in this field. The main reason behind stepping from conducting fillers toward semiconducting fillers is the various losses involved during the interaction of EM waves with matter at the GHz frequency range. The losses in this frequency range are not only attributed to ohmic, conduction, or eddy current losses but also to polarization losses, which might be a possible reason that compelled the scientific community to investigate the semiconducting fillers. However, the exact mechanism of shielding and various losses involved in the GHz frequency range is not well-established and is still under investigation. This review article will highlight the EMI shielding properties of semiconducting filler-based polymer nanocomposites, and to the best of our knowledge, no such review article has discussed this topic in detail.
A semiconductor is a material whose electrical conductivity value falls between a conductor and an insulator. The conventional semiconductors have a band gap between 1 and 1.5 eV, whereas wide band gap semiconductors have a band gap between 2 and 4 eV. It is to be noted that the value of the band gap of a material is dependent on particle size3 and also the phase of the material obtained. In this review article, we will cover semiconducting filler-based polymer nanocomposites with varying band gaps, as demonstrated in Table 1. The various fillers in the table have been categorized as low, medium, and high band gap semiconductors for convenience. However, a few of the fillers mentioned in this list remain unexplored/less explored for EMI shielding applications. The primary aim of this work is to include literature that deals with a semiconducting filler in a polymer matrix, but due to lack of available data, a few papers that compared the shielding effectiveness data for the hybrid structures with control semiconducting fillers are also included in this review article. Semiconducting fillers such as BaTiO3 and ZnO are very well studied along with their hybrid structures for EMI shielding applications, and hence only a few representative papers have been highlighted here. In each subsection of this article, composites with only a semiconducting filler will be covered first, followed by composites with hybrid fillers. Moreover, the focus of this work is also on the nonmagnetic semiconducting material as the magnetic fillers have been explored for EMI shielding applications in the recent past. It is also to be noted that this work focuses on polymer nanocomposites, and hence, literature involving non-polymer-based nanocomposites for EMI shielding applications are excluded, as is evident from the title of this work.
Table 1. List of Semiconducting Materials That Can Be a Prospective Choice as Filler for EMI Shielding Applications.
2. Terminologies Used for EMI Shielding Measurements
EM shielding is defined as the material’s ability to attenuate EM radiations, and it is expressed either in terms of total shielding effectiveness (SET) or reflection loss/reflection coefficient (RL/RC).
SET is defined as the logarithm of the ratio of the incident power (PI) to the transmitted power (PT) and is calculated using eq 1.
| 1 |
where SEA, SER, and SEMR refer to the shielding via absorption, reflection, and multiple reflections, respectively.
SEA, SER, and SEMR can be represented by eqs 2, 3, and 4 respectively.
| 2 |
| 3 |
| 4 |
where σ represents the total conductivity, ω is the angular frequency (ω = 2πf), μr corresponds to the relative permeability of the shield material, d is the thickness of the shield, and ε0 represents the dielectric constant in free space.
However, for magnetic metallic materials with dielectric loss and magnetic loss properties (ε and μ), shielding is generally defined in terms of reflection loss or reflection coefficient (RL or RC) and can usually can be evaluated by eq 5. So, a minimum RL signifies that the losses are mainly via the absorption mechanism.
| 5 |
where Z0 is the impedance of free space and Zin is the input characteristic impedance and is defined as
| 6 |
where μr and εr are the relative permeability and relative permittivity of the shielding material, f is the frequency of incoming EM wave, d is the thickness of the shield material, and c is the velocity of light in a vacuum.
It is to be noted that few papers in the literature report the EMI shielding performance in terms of shielding effectiveness (SET, SEA, SER), whereas others report it in terms of reflection loss or reflection coefficient (RL or RC). The correlation between SE and RL is difficult to obtain without the values of the relevant input parameters (as per eqs 1–6). It would have been easier to compare data if the results were expressed in terms of either SE or RL; however, given that many papers did not include both RL and shielding effectiveness data in the same paper, we reported the available data as is.
3. Semiconducting Filler-Based Polymer Nanocomposites
3.1. Composites with Low Band Gap Fillers
3.1.1. MoS2-Based Composites
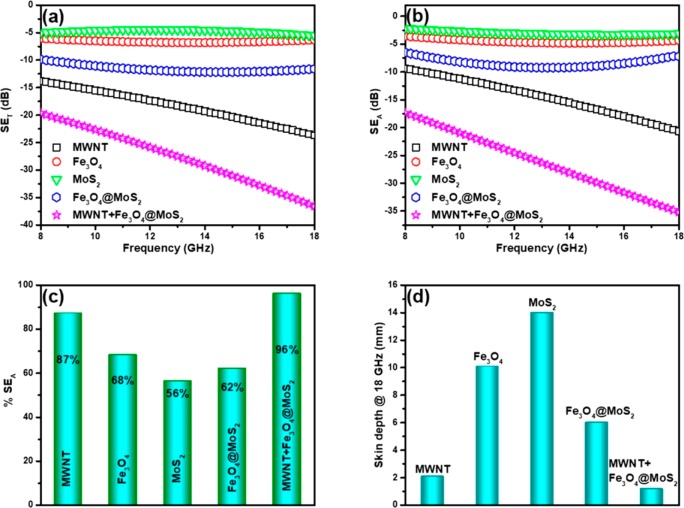
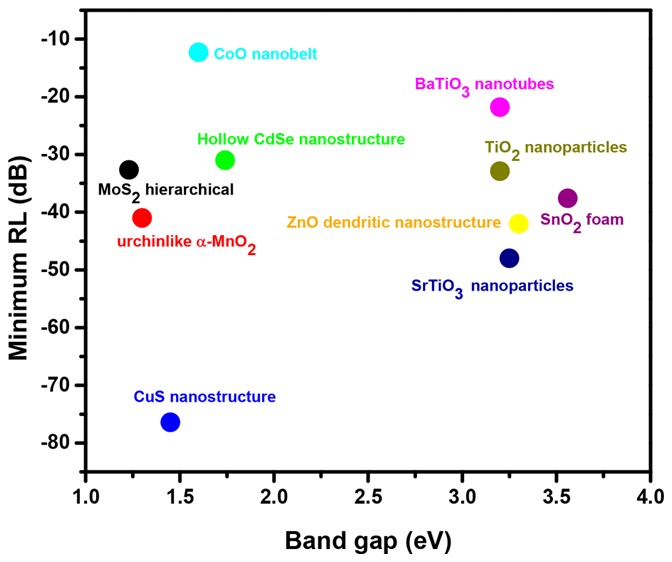
Transition metal dichalcogenides are 2D layered semiconductor materials of the type MX2, with M as the transition metal (Mo, W, etc.) and X as the chalcogen (S, Se, or Te). MoS2 is a well-known dichalcogenide used in photonics, photovoltaics, microelectronics, and hydrogen evolution applications. MoS2 is one of the dichalcogenides gaining attention for EMI shielding applications. Zhang et al.25 studied the MoS2/polyvinylidene fluoride (PVDF) system for EMI shielding performance. It is observed that the minimum RL value of MoS2/PVDF composites with a filler loading of 25 wt % reached −26.11 dB at 11.36 GHz for a thickness of 2.5 mm. In the 18–40 GHz frequency range, the minimum RL of −27.47 dB was observed at 18.47 GHz (for 30 wt % filler and 1.5 mm thickness) and of −32.67 dB was observed at 28.93 GHz (20 wt % filler and 3.5 mm thickness). Also, they observed that the MoS2 hierarchical nanostructure showed a minimum RL value of −26.11 dB (at 11.36 GHz), which is much higher than that of bulk MoS2/PVDF composites (−11.94 dB at ∼5.9 GHz) and micrometer-sized MoS2/PVDF composites (−12.27 dB at ∼13.5 GHz) for 25 wt % filler loading and for a thickness of 2.5 mm. The authors claim that the microwave absorption mechanisms include various polarization, destructive interference theory, and multiple reflections. Menon et al.26 measured the EMI shielding behavior (shown in Figure 1) of MoS2, Fe3O4, Fe3O4@MoS2, multiwalled carbon nanotube (MWCNT) and MWCNT/Fe3O4@MoS2 in a dopamine-functionalized polyurethane (PU) matrix. The concentration of MWCNTs and other nanoparticles was fixed at 3 and 5 wt %, respectively, and the composite sample was made 5 mm thick. The total shielding effectiveness for 5 wt % MoS2 is observed to be −5.5 dB, 5 wt % Fe3O4 is −6.3 dB, and 5 wt % Fe3O4@MoS2 is −11.6 dB at 18 GHz frequency. However, the total shielding effectiveness increases as frequency increases for composite samples with MWCNTs. At 18 GHz, SET for a composite with 3 wt % MWCNT is observed to be −23.6 dB, and for the composite with 3 wt % MWCNT/5 wt % Fe3O4@MoS2, it is observed to be −36.6 dB. The authors report shielding via absorption to be the primary cause of energy dissipation. They have attributed it to multiple internal reflections from MWCNTs, eddy current losses, interface polarization losses at the heterogeneous interfaces, and polarization losses from defect sites in the nanoparticles.
Figure 1.
(a) Total shielding effectiveness, (b) shielding effectiveness due to absorption, (c) % shielding effectiveness due to absorption, and (d) skin depth of the composites. Reprinted from ref (26). Copyright 2019 American Chemical Society.
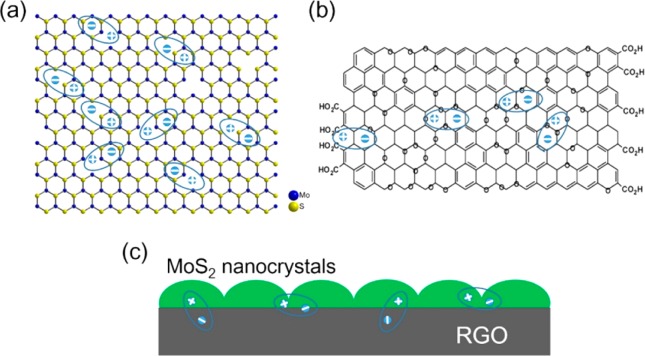
Wang et al.27 studied the hybrids of MoS2 and reduced graphene oxide (rGO) on microwave absorption performance. Both rGO and MoS2 are semiconducting; however, the degree of reduction decides the band gap of rGO. With 10 wt % loading of the MoS2/rGO in a wax matrix and for a sample thickness of 2.3 mm, the minimum RL of −50.9 dB was observed at 11.68 GHz frequency. They claimed that the microwave absorption is a result of three different mechanisms (shown in Figure 2): (a) the defect polarization of MoS2 nanocrystals, (b) the defect polarization of the oxygen-containing groups and the imperfect carbon structures in rGO, and (c) the multiple interfacial polarization in MoS2/rGO hybrids. Ding et al.28 synthesized rGO-MoS2 via hydrothermal synthesis and incorporated it in the paraffin matrix. They measured the EMI shielding performance of the composite and further compared it with pure MoS2 and a pure rGO-based composite. With 10 wt % filler loading of rGO-MoS2, the minimum RL value obtained was −41.53 dB at 11.36 GHz with a thickness of 3.0 mm. The proposed mechanism of shielding was similar to that mentioned by Wang et al.27 Ran et al.29 synthesized 3-methacryloxypropyltrimethoxysilane (KH570)-modified MoS2 (M/MoS2) and prepared a composite of the (M/MoS2)/rGO nanosheet by embedding it in a PVP matrix. For a thickness of 3 mm, they obtained a minimum RL of −49.7 dB at 13.68 GHz with 18 wt % (M/MoS2)/rGO. They have attributed the microwave absorption behavior to the defect dipole polarization as well as the multiple interfacial polarization.
Figure 2.
Schematic of dipole polarization in the MoS2/rGO hybrid: (a) defect dipole polarization of MoS2; (b) defect dipole polarization of rGO; (c) multiple interfacial polarizations in the MoS2/rGO hybrid. Reprinted from ref (27). Copyright 2015 American Chemical Society.
3.1.2. MnO2-Based Composites
Zhou et al.30 synthesized hollow urchin-like α-MnO2 nanostructures and studied the microwave absorption properties of α-MnO2 nanostructures by mixing it with paraffin wax in a mass ratio of 1:1. The minimum RL of −41 dB was obtained at 8.7 GHz with a thickness of 1.9 mm. It is to be noted that, in general, MnO2 nanostructures are antiferromagnetic materials, which implies that the magnetic loss is negligibly small and dielectric loss dominates the loss mechanism. The sample that exhibited minimum RL of −41 dB has lower tan δε but higher tan δμ as compared to the sample in which MnO2 was prepared under different experimental conditions. They further support their result by stating that it has been well-established in the literature that magnetic behavior can be disturbed by the dielectric behavior at a microwave frequency. Even with comparatively low tan δε compared to that of the other sample, it has proved to be a better EM shield, and thus they claim that both dielectric and magnetic losses play a crucial role in this scenario. Moreover, they suggest that the microcircuit model explains the dielectric loss due to the inner walls of the urchin-like nanostructures, which can be considered as microloops, whereas actinomorphic one-dimensional MnO2 can be considered as several antennas that convert electromagnetic waves into a vibrating microcurrent. This microcurrent can be produced in the microloops, leading to dielectric resonant peaks. Bora et al.31 synthesized a PANI-α-MnO2 nanorod composite and investigated the EMI shielding performance. With a thickness of 169 ± 2 μm, the composite showed most effective EMI SET of approximately −35 dB (EMI shielding due to absorption, SEA ∼ −24 dB and EMI shielding due to reflection SER ∼ −11 dB) in the X-band, whereas SET was found to be approximately −39 dB (SEA ∼ −29 dB and SER ∼ −10 dB) in the Ku-band. It is to be noted that α-MnO2 is reported to be a better microwave absorber compared to other crystalline states. The EM shielding property of PANI-MnO2 is attributed to the high dielectric loss incurred due to presence of MnO2 and high electrical loss (dissipation current) that takes place along the PANI-grafted MnO2 nanorods.
3.1.3. CdSe-Based Composites
Cao et al.32 reported a ligand-assisted solvothermal synthesis of CdSe hollow nanostructures (70 wt %) and incorporated it in paraffin for EMI shielding applications. They also compared it with solid CdSe nanoparticles (70 wt %) in the paraffin matrix. It is observed that with 3 mm thickness and at 11.7 GHz frequency, minimum RL is −14 dB for CdSe hollow nanostructures, whereas the reflection loss for the solid CdSe nanostructures is more than −3 dB for frequencies above ∼11–18 GHz. For 4 mm thickness, two minima are observed in the value of RL (−16 dB at 5.6 GHz and −31 dB at 17.8 GHz). The authors claim that this difference in RL between the hollow and solid CdSe nanostructures has a size dependence. They explain that the size of the building blocks of CdSe nanostructures (small or large) decides the properties of the nanostructure, which further has its role in shielding. Moreover, both the real and imaginary parts of the relative permittivity for the hollow sample are higher than those for the solid one. This also indicates that the hollow CdSe nanostructures have an electrical conductivity higher than that of the solid ones, enhancing the microwave absorption properties.
3.2. Composites with Medium Band Gap Fillers
3.2.1. CuS-Based Composites
He et al.33 synthesized CuS nanostructures by varying the ratio of the precursors and observed that change in nanostructure resulted in the change in EMI shielding performance. For a shield thickness of 3.5 mm and CuS loading of 20 wt %, the minimum RL value for the CuS–paraffin composite is observed to be −76.4 dB at 12.64 GHz. The authors attribute the microwave absorption behavior to the CuS dielectric losses and interfacial polarization due to the heterogeneous structure of the composite. The flower-like CuS with a band gap of 1.45 eV was prepared by Hu et al.34 using solvothermal synthesis. They prepared the EMI shielding samples by blending CuS with waterborne polyacrylate emulsion along with thickening agent and deionized water. Finally, this mixture was coated on a circular polytetrafluoroethylene (PTFE) plate to obtain a 0.6 mm thick EMI sample. SET was observed to be between −27 and −31 dB with CuS content as 28.6 wt % in the frequency of 300 kHz to 3 GHz. In another work by Hu et al.,35 they explored PANI/flower-like CuS for EMI shielding performance. Compared to the original CuS and pure PANI, PANI/flower-like CuS composites exhibited enhanced SET. The EMI shielding was measured after incorporating PANI/CuS in the paraffin matrix. The optimal EMI SET of the PANI–CuS (50 wt % CuS) composites in paraffin reached −45.2 dB at 2.78 GHz for 3 mm thickness. It is to be noted here that the authors first prepared the PANI/CuS composite (20 wt %) and mixed it in the paraffin matrix. Also, an improved shielding efficiency (or shielding effectiveness) below −18 dB was obtained over the entire frequency range from 300 kHz to 3 GHz. The authors explained that the mobile charge carriers in CuS interacted with the electromagnetic fields. However, the enhanced EMI SET of the PANI–CuS composites is mainly attributed to the coordination bonding and the strong π–π interactions between PANI and CuS. This may be useful for the charge transfer at the newly formed interface of PANI and CuS. Another reason that contributes to the SET is the flower-like structure CuS–PANI composites resulting in multiple scattering or reflections of incident EM radiations. Liu et al.36 investigated CuS nanoflakes which were vertically aligned on magnetically decorated (NiFe2O4) graphene for EMI shielding performance. With 20 wt % magnetically decorated graphene/CuS in paraffin and for a thickness of 2.5 mm, the minimum RL was obtained to be −54.5 dB at 11.4 GHz frequency. CuS powder being a low band gap semiconductor is often used as an additive coating on the polymer surface to increase its conductivity. The author explains the mechanism of shielding by citing the free-electron theory. It says that an increase in conductivity of a composite could result in strong dielectric loss, and hence, they concluded that magnetically decorated graphene@CuS shows a stronger dielectric loss. They further explain that the magnetic loss is probably caused by natural resonance and an eddy current effect. While delving deeper into the mechanism, they add that CuS nanoflakes can also act as a microwave receiver, as the EM waves can get trapped in the inner voids and can lead to multiple reflections and scattering. They also suggest that the addition of magnetic particles in graphene sheets@CuS increases the microwave absorption properties due to better impedance matching, interfacial polarization, dipole polarization, and ion polarization. Also, the residual defects and groups in graphene sheets tend to introduce defect polarization relaxation, electron polarization, and relaxation processes that favor microwave absorption.
3.2.2. CoO-Based Composites
Sun et al.37 studied the microwave absorption properties of CoO nanobelts and sub-micrometer CoO spheres. It was observed that the CoO nanobelts showed a strong broad peak at 14.2 GHz with a minimum RL of −12.3 dB, whereas the minimum RL reached −8.7 dB at 14.4 GHz for sub-micrometer spheres of CoO. It is to be noted that CoO nanostructures (70 wt %) were uniformly mixed in the paraffin matrix, and a shield of thickness 3 mm was obtained. CoO nanostructures are antiferromagnetic material, and thus there is negligible magnetic loss contribution. The major contribution to the microwave absorption is primarily due to the dielectric loss rather than the magnetic loss. The dielectric loss is known to originate from electronic polarization, ion polarization, and intrinsic electric dipole polarization, but it is the frequency range that decides which polarization is the dominant cause of the loss. The material crystal structure, size, and shape of nanomaterials influence the shielding properties as was observed in this paper,37 where the change in the shape of CoO resulted in a change in shielding properties. From the electron microscopy characterization results, the authors concluded that the microwave-absorbing performance is associated with the single-crystal structure, the crystallization, and the aggregation degree of the nanocrystal building blocks.
3.2.3. WS2-Based Composites
Zhang et al.38 explored the microwave absorption property of WS2-based composites. They synthesized WS2–rGO by varying the mass ratios of GO (3, 4, 5, and 6%) and denoted the samples as S3, S4, S5, and S6. These samples were incorporated in 60% wax, and a shield with a thickness of ∼1.5 mm was prepared. SET of over −20 dB is obtained in the frequency range of 2–18 GHz, with a minimum of −32 dB for sample S5. The EMI shielding mechanism for WS2–rGO architecture is explained in Figure 3. The dielectric loss and abundant multiscattering are the dominant reasons for energy dissipation. The dielectric loss comprises the conduction loss and relaxation loss. The difference in surface conductance between WS2 and rGO results in an additional loss via the interfacial polarization which can be modeled as a capacitor. The literature for WS2 is very limited, and dichalcogenides still remain an unexplored area for EMI shielding applications.
Figure 3.
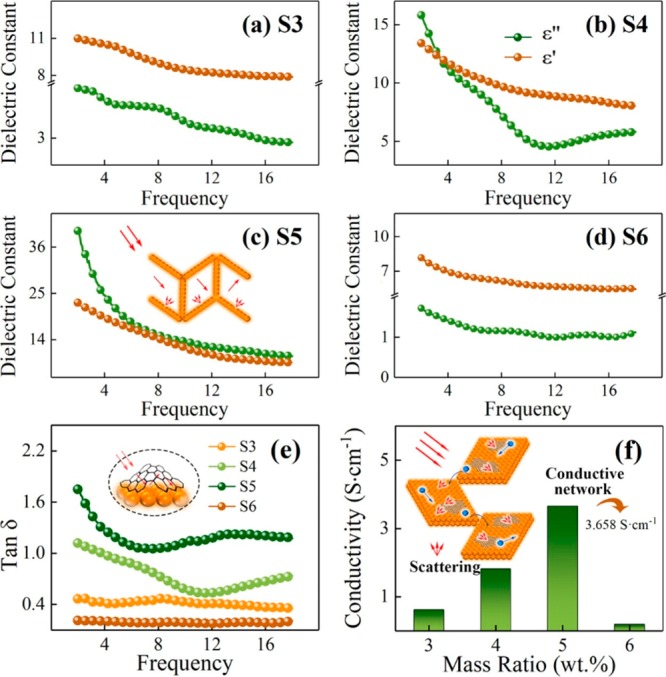
Real and imaginary permittivity plots of (a) S3 (mass ratio of GO/WS2: 3), (b) S4 (mass ratio of GO/WS2: 4), (c) S5 (mass ratio of GO/WS2: 5), and (d) S6 (mass ratio of GO/WS2: 6). (e) tan δ and (f) conductivity of WS2–rGO architecture incorporated in wax (60%). The insets of (e,f) are the schematic for the interfacial polarization and electron transport paths in the WS2–rGO architecture. Reprinted from ref (38). Copyright 2019 American Chemical Society.
3.2.4. CdS-Based Composites
Lu et al.39 studied the temperature-dependent microwave absorption properties of the CdS-MWCNT-based composite. They fabricated three kinds of structures, namely, MWCNTs coated with CdS nanocrystals (CdS-MWCNTs), coated with a thin CdS sheath (S-MWCNTs), and coated with a thick CdS sheath (TS-MWCNTs). For room-temperature tests, paraffin wax served the purpose of the matrix. The minimum RL of the wax matrix composite loading with CdS-MWCNTs is observed to be −25 dB (10 wt % filler loading and 3.5 mm thickness), which is 2.5 times higher than S-MWCNTs and 12 times higher than TS-MWCNTs. They attribute the strong absorption ability to the effective impedance matching in CdS-MWCNTs and dielectric loss. As per the Debye theory, ε″ denotes the dielectric loss, which comprises both polarizations and electrical conductivity originating from CdS-MWCNTs. The polarizations include the interfacial polarization due to abundant interfaces between CdS nanocrystals and MWCNTs and the dipolar polarization caused by surface functional groups, defects on/in acid-treated MWCNTs. Moreover, high aspect ratio and conductivity of MWCNTs and CdS-MWCNTs can build a conductive network in the composite for electron hopping and migration. In the case of S-MWCNTs and TS-MWCNTs, the CdS sheaths hinder the electron transfer between MWCNTs in the conductive network, which further decreases the dielectric loss.
3.2.5. WO3-Based Composites
Sastry et al.40 carried out an in situ polymerization of aniline monomer with camphor sulfonic acid (CSA) as dopant and ammonium persulfate as an oxidizing agent. They synthesized PANI-CSA tungsten oxide composites (PANI/CSA-WO3) with a chemical oxidation method. With 40 wt % filler content, the obtained SET ∼ −13.8 dB at 14.2 GHz frequency. They claim that WO3 acts as a lossy dielectric filler between the PANI/CSA chains, resulting in effective EM attenuation. Boujar Dolabi et al.41 synthesized WO3/MnFe2O3 nanoparticles (NPs core, 50/50 wt %) and via in situ polymerization obtained a core–shell structure of a WO3/MnFe2O3/PANI nanocomposite (50 wt % as the core). With a thickness of 1.5 mm, a minimum RL of −12 dB was observed at 8.8 and 11.6 GHz. They attribute the shielding to the dielectric and magnetic losses. They further claim that the interfacial losses due to the changing boundary condition at various interfaces also contributes to the loss mechanism. Wang et al.42 synthesized the hierarchical structure of graphene@Fe3O4@PANI@WO3 (graphene@Fe3O4/WO3 mass ratio = 2:1) and studied its microwave-absorbing performance after incorporating it in paraffin wax (70 wt % nanostructure). The EM wave absorption studies show that graphene@Fe3O4@PANI@WO3 exhibits high EM wave absorption properties, with a minimum RL of −46.7 dB at 9.3 GHz for a thickness of 4 mm, which is higher than that of graphene@Fe3O4 (minimum RL = −15.4 dB at 14.1 GHz for a thickness of 1.5 mm) and graphene@Fe3O4@PANI (minimum RL = −20.9 dB at 9.4 GHz with a coating of 4 mm). The enhanced EM wave absorption mechanism of graphene@Fe3O4@PANI@WO3 is ascribed to the improved impedance matching, enhanced interfacial polarization, and multiple scattering. Moreover, the presence of the PANI layer on the graphene sheet can lead to electron tunneling and enhanced electronic clouds, converting EM energy into heat. Herein, WO3 particles in the hierarchical structure were designed to decrease permittivity, leading to an improvement in impedance matching.
3.3. Composites with High Band Gap Fillers
3.3.1. MoO3-Based Composites
Lyu et al.43 synthesized MoO3/Mo4O11/MoO2 heterogeneous nanobelts (60 wt %) and incorporated them into the paraffin matrix. It showed a minimum RL of −59.2 dB at 10.8 GHz with a thickness of 4.6 mm. The EM absorption studies show that the high dielectric loss and enhancement in impedance matching due to the heterogeneous structure contribute to enhanced shielding. The heterogeneous interface leads to a formation of microcapacitance and leads to enhancement in multiple scattering. Li et al.44 synthesized 1D MoS2/MoO3 hybrids and incorporated them in the PVDF matrix. With a filler loading of 20 wt %, the minimum RL is found to be −38.5 dB at 8.7 GHz with a thickness of 1.9 mm. They attribute the shielding performance primarily to the dielectric losses. The uneven distribution of space charges between MoO3 cores and MoS2 shells leads to interfacial polarization, and the defects and residual groups in the low-crystalline MoS2 shells can accumulate enough bound charges (dipoles) to generate considerable orientation polarization.
3.3.2. BaTiO3-Based Composites
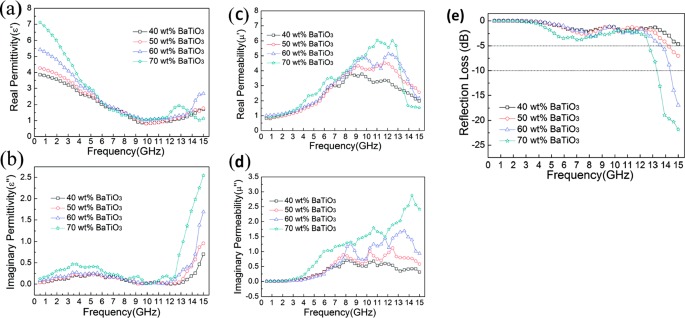
Zhu et al.45 synthesized BaTiO3 nanotubes via a wet chemical route. The dielectric loss at a lower frequency range (shown in Figure 4) is explained by the leak conductance, whereas relaxation polarization and electric conductance become dominant at a higher frequency range. The overall loss mechanisms in dielectric BaTiO3 can be explained by dielectric polarization, spontaneous polarization, and the associated relaxation phenomena. Here, it becomes important to understand the difference between dielectric polarization and spontaneous polarization (a characteristic of ferroelectric material). Dipolar/dielectric polarization arises from molecules with a permanent electric dipole moment that can change orientation in an applied electric field. If a molecule of a substance has a permanent dipole moment, that is, they have a moment even in the absence of an electric field, it is called dipolar molecule and substances containing such molecules are called dipolar substance (for example, H2O). It is to be noted that though the individual molecules in a dipolar substance have a permanent dipole moment, the net polarization disappears in the absence of an external electric field because the molecular moments are randomly oriented, resulting in complete cancellation of the polarization. However, BaTiO3 is a ferroelectric crystal (a special class of dielectric material), and ferroelectrics exhibit an electric dipole moment even in the absence of an external electric field. Ferroelectrics generally consist of regions called domains. Within each of these domains, the polarization is in the same direction, but in adjacent domains, the polarization is in different directions. The net polarization depends on the difference in the volumes of the upward- and downward-directed domains. In the ferroelectric state, the center of the positive charge of the crystal does not coincide with the center of negative charge. They exhibit a spontaneous nonzero polarization even when the applied electric field is zero. This spontaneous polarization can be reversed by a strong applied electric field in the opposite direction. The polarization is therefore dependent not only on the current electric field but also on its history, yielding a hysteresis loop. It is worthwhile to note that crystal in a normal dielectric state usually does not show significant hysteresis with the electric field, but ferroelectrics do exhibit hysteresis. Ferroelectricity usually disappears above a certain temperature called the transition temperature.
Figure 4.
(a) Real permittivity, (b) imaginary permittivity, (c) real permeability, (d) imaginary permeability of the mixture of BaTiO3 nanotubes and paraffin wax with different weight fraction, (e) RL for BaTiO3–paraffin composite sample vs frequency for a sample thickness of 2 mm. Reprinted from ref (45). Copyright 2012 American Chemical Society.
The paper also shows the plots of μ′ and μ″ (Figure 4c,d), which exhibits a resonance peak which may be related to natural resonance. However, as these values are higher than the ones reported in the literature, there could be a possibility of instrument error. In addition to this, the high interfacial area and hollow structure of nanotubes resulted in the minimum RL of −21.8 dB for BaTiO3 nanotubes/paraffin wax composite at 15 GHz for a shield thickness of 2 mm and weight fraction of 70% (Figure 4e). Chauhan et al.46 measured the EMI shielding performance of BaTiO3-filled polyetherketone (PEK) composites in the X-band. Total shielding effectiveness of −11 dB (∼92% attenuation) is observed for a shield thickness of 2 mm and filler content of 18 vol %. As the dielectric loss due to BaTiO3 is the major cause of EM attenuation, the author attributes the loss to the polarization effect. Abbas et al.47 studied the complex permittivity and RL of BaTiO3/polyaniline in the polyurethane matrix for X-band frequencies. Composite samples were prepared by dispersing BaTiO3/PANI particulates in a two-pack polyurethane matrix that consisted of polyol-8 and hexamethylene diisocyanate mixed in 50:50 ratios. A minimum RL of −15 dB was observed for a sample (BaTiO3/polyaniline = 1:3) with a thickness of less than 3.0 mm at 10 GHz frequency. BaTiO3 is a ferroelectric material exhibiting high resistivity. Ba2+ and O2– ions form a face-centered cubic lattice, with Ti4+ ions fitting in the octahedral interstices. At room temperature, the Ti4+ ions are possibly at minimum energy, which is off-centered and thus gives rise to permanent electric dipoles. When protonated by HCl acid solution, polyaniline also possesses permanent electric dipoles. Thus, dipolar polarization becomes one of the contributing factors in the loss mechanisms, owing to the dielectric nature of filler and matrix. Joule heating due to inherent conductivity of the polyaniline adds to the loss mechanism. Interfacial polarization is also expected due the heterogeneous structure of the composite.
Guo et al.48 also studied the BaTiO3-based composite for EMI shielding applications. The composite with 15 wt % rGO@BaTiO3 in the PVDF matrix showed the minimum RC of −45.3 dB at 5.44 GHz for a thickness of 4.5 mm. They also calculated the SET and found that 30 wt % filler demonstrates a minimum SET of up to −9.8 dB in the frequency range of 2–14 GHz.
3.3.3. TiO2- and ZnO-Based Composites
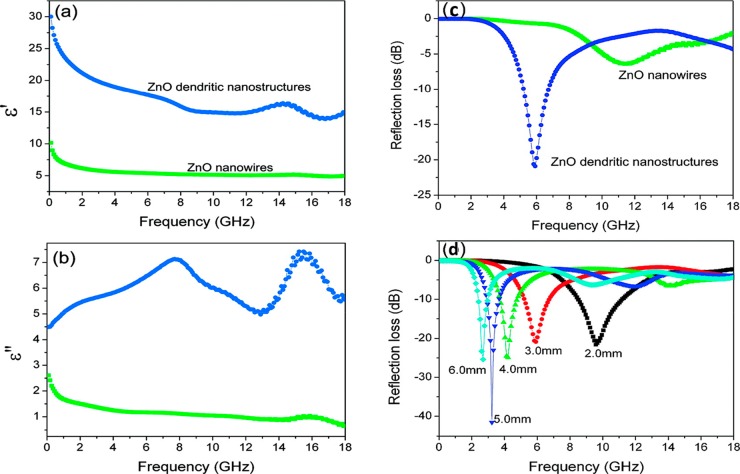
Zhuo et al.49 synthesized dendritic nanostructures by a two-step chemical vapor deposition process and compared the results of dielectric and microwave shielding properties with ZnO nanowires. Figure 5 shows the complex permittivity and RL plot of the primary ZnO nanowires composite versus the ZnO dendritic nanostructured composite. The filler content was 50 vol % in a paraffin matrix. The real permittivity of the ZnO nanowire composite is observed to be nearly 5.0, and the imaginary permittivity is about 1.0. However, the real part of relative permittivity showed a decrease from 30 to 15 as the frequency increases for ZnO dendritic nanostructures. It exhibits a peak in the frequency range of 13–17 GHz. The imaginary part of permittivity shows an increase from 4 to 7, and the curve exhibits two broad peaks in the frequency range of 0.1–13 and 13–17 GHz. This resonance behavior is expected for a highly conductive composite with significant skin effect. The response of ZnO nanostructures can be attributed to the electronic spin and charge polarization due to polarized centers. The value of the minimum RL for the ZnO dendritic nanostructure composite is −42 dB at 3.6 GHz with a thickness of 5.0 mm. Excellent dielectric and microwave shielding properties are observed for complicated interfaces of ZnO dendritic nanostructures compared to nanowires, and thus it can be concluded that morphology has a significant role in microwave absorption.
Figure 5.
(a) Real and (b) imaginary parts of relative complex permittivity for a ZnO nanowire composite vs a dendritic nanostructured composite. (c) RL of 50 vol % ZnO nanowires vs 50 vol % ZnO dendritic nanostructure composites with a thickness of 3.0 mm. (d) RL of 50 vol % ZnO dendritic nanostructures composite with different thicknesses. Reprinted from ref (49). Copyright 2008 American Chemical Society.
Singh et al.50 studied the EMI shielding behavior of nanocomposites based on transition metal oxides in poly(vinyl alcohol) (PVA). They studied different transition metal oxides such as Fe2O3, ZnO, SiO2, ZrO2, and TiO2 incorporated in the PVA matrix in the frequency range of 4–8 GHz (C-band) and 8–12 GHz (X-band). At 10 wt % concentration level and 0.5 mm thickness, the minimum reflection loss values for the composites containing Fe2O3, ZnO, SiO2, ZrO2, and TiO2 were found to be −38.85 (10.4 GHz), −33.65 (10.4 GHz), −41.90 (10.4 GHz), −24.90 (11.0 GHz), and −32.90 dB (9.76 GHz), respectively. PVA is not 100% transparent to EM radiations, and thus it also shows a minimum reflection loss of −21.50 dB at 10.4 GHz frequency. It is to be noted here that Fe2O3, ZnO, and TiO2 are the semiconducting oxides, whereas ZrO2 and SiO2 have a band gap greater than 5 eV. The authors have attributed the losses in SiO2-, ZnO-, ZrO2-, and TiO2-based nanocomposites to be dielectric in nature, whereas they claim that Fe2O3-based nanocomposites exhibit both dielectric and magnetic losses.
Phang et al.51 synthesized hexanoic acid (HA)-doped PANI nanocomposites containing TiO2. Pani/HA/TiO2 nanocomposite with the highest permittivity, heterogeneity, and loss tangent showed a minimum RL of −31 dB at 10 GHz. Dhawan et al.52 synthesized a conducting ferrimagnetic polyaniline nanocomposite with γ-Fe2O3 (9–12 nm) and TiO2 (70–90 nm) nanoparticles. A different formulation of polymer nanocomposite having an aniline/TiO2/γ-Fe2O3 weight ratio of 1:1:1 (PTF11) and 1:1:2 (PTF12) was chosen along with composite having aniline/TiO2 weight ratio of 1:1 and aniline/γ-Fe2O3 (PF12) weight ratio of 1:2 for comparison. The polyaniline–TiO2–γ-Fe2O3 nanocomposite showed enhanced shielding effectiveness (SEA ∼ −45 dB) compared to that of polyaniline−γ-Fe2O3 (SEA ∼ −8.8 dB) and polyaniline–TiO2 (SEA ∼ −22.4 dB) nanocomposite for the frequency range of 12.4–18 GHz (Ku-band). Losses due to TiO2 is a result of its high dielectric constant along with strong dipolar polarization and the associated relaxation phenomenon. γ-Fe2O3 and TiO2 in the polyaniline matrix leads to a significant increase in a real and imaginary part of complex permittivity. It can be attributed to the interfacial polarization due to the presence of insulating γ-Fe2O3 particles and high dielectric TiO2 particles. Absorption-based EMI shielding is mainly attributed to the combined effect of magnetic and dielectric losses incurred due to the incorporation of γ-Fe2O3 and TiO2. In another work by Akman et al.,53 they exploited the combined effect of BaFe12O19 and TiO2 on EMI shielding performance. They varied the weight fractions of BaFe12O19/TiO2 weight fractions and compared the nanocomposites with and without polypyrrole (Ppy) coating. The dielectric loss saw a significant jump in the samples with Ppy coating in the frequency range of 8–18 GHz. The sample having an equal amount of magnetic and dielectric fillers and with Ppy coating showed a minimum RL of −35 dB at 16.5 GHz. However, the minimum RL of −45 dB at 17.5 GHz was obtained for the sample with a higher amount of magnetic filler and without Ppy coating.
Kong et al.54 gave a good insight into the EM-absorbing material and its need to have a suitable permittivity and dielectric loss value. With the increase in dielectric loss, the absorption coefficient increases, but the problem arises because, as the dielectric loss is enhanced, the permittivity also increases and subsequently the EM reflection increases. This poses a problem as the aim of a good shield is to enhance the EM absorption capabilities. It is revealed that the materials having high EM absorption capability require a low real part of permittivity and an appropriately high electrical conductivity (around 1 S/m). Alternatively, when the permittivity meets the EM impedance match requirement, higher dielectric loss implies better EM absorption properties. It is to be noted that the electrical conductivity is related to the imaginary part of permittivity (ε″) according to eq 7.
| 7 |
Here, σ represents the electrical conductivity (S/m), ε0 is the permittivity of free space, and f is the frequency (Hz). From the above description, it is also evident that the value of 1 S/m required for good EM absorption ability lies in the semiconducting range. Moreover, they have also presented a chart, as shown in Figure 6, which relates the real and imaginary part of permittivity with RC/RL (calculated from eq 5). The minimum value of RC is a function of frequency and thickness (shown in Figure 6). The four-color lines in Figure 6 signify that the permittivity corresponding to a minimum RC decreases with the increase of thickness and frequency. The blue color line represents the minimum RC corresponding to 2 mm thickness. It is observed that the complex permittivity of the minimum RC is reduced from 20–5j to 10–3j when the frequency changes from 8.2 to 12.4 GHz. Orange, yellow, and red lines represent thicknesses of 2.86, 4, and 5 mm, respectively. Also, the paper claims that no material in literature satisfies the condition of optimum combination of the real and imaginary parts of permittivity to serve the dual purpose of RC and maximizing effective absorption bandwidth. Hence, to improve absorption properties and to obtain optimal permittivity, it is desired to design materials with special composition and microstructure. In their work, they have synthesized ZnO/ZnAl2O4 composite powders via sol–gel synthesis. Sub-micrometer-sized ZnO acts as an electrically lossy phase, and ZnAl2O4 nanograins act as a medium permittivity phase. ZnO/ZnAl2O4 (40 wt %), when incorporated in the paraffin matrix, exhibits appropriate permittivity and electrical conductivity, which can be attributed to the high carrier concentration and mobility at the interfaces. For a sample thickness of 2.86 mm, the minimum RC reaches −25 dB (whole X-band) for the sample with ZnO/ZnAl2O4 in the molar ratio of 88.9:11.1.
Figure 6.
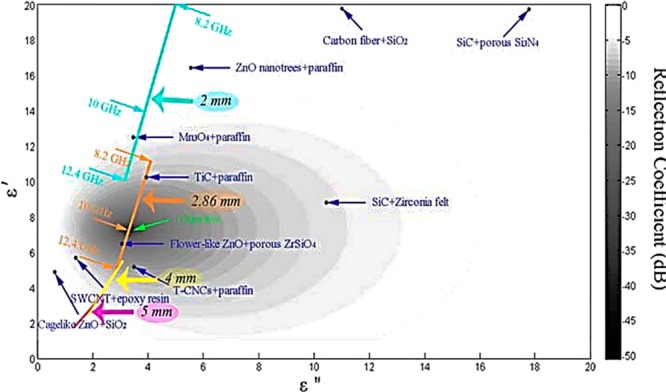
Chart relating different material with permittivity and reflection coefficient. Reprinted from re (54). Copyright 2013 American Chemical Society.
In another work by Song et al.,55 they decorated ZnO particles on MWCNTs and incorporated them in wax. With 40 wt % ZnO/MWCNT content in the wax, the minimum RL was obtained as −36 dB for a 5 mm thick shield between 5 and 6 GHz. They described shielding behavior using a resistor–capacitor model. They claim that the MWCNT/ZnO shows resonant absorption, which implies two different types of RL peaks caused by the quarter-wavelength attenuation and the MWCNT/ZnO interface. Han et al.56 studied C/ZnO nanoparticles mixed in paraffin wax for electromagnetic shielding performance. With 40 wt % nanofillers, the composite shows a minimum RC of −52 dB at 11 GHz with a sample thickness of 1.75 mm. Compared to pure carbon spheres and ZnO hollow spheres, the core/shell structure of C/ZnO composites showed promising results with high dielectric loss and moderate impedance match. In a work by Wu et al.,57 they synthesized 3D rGO/ZnO nanoparticles followed by subsequent UV irradiation to remove the remnant oxygen functional groups from rGO. They incorporated these nanoparticles in the paraffin matrix (10 wt %). It was observed that, for a sample thickness of 3 mm, the minimum RL is −25.31 dB at 11.52 GHz.
3.3.4. SrTiO3-Based Composites
Murugan et al.58 hydrothermally synthesized cubic SrTiO3 nanoparticles and made them into a composite with epoxy as a polymer matrix. With a filler content of 50 wt % and a shield thickness of 1 mm, the composite exhibited a minimum RL of −48 dB at 14.5 GHz frequency. SrTiO3 in an epoxy matrix was also studied by Choi et al.59 It is observed that the minimum RL for a 5 mm thick composite with 0.6 volume fraction of SrTiO3 is −30 dB at 9 GHz, and the composite containing a 0.3 volume fraction of SrTiO3 shows a minimum RL of −34 dB at 4.3 GHz. The authors have plotted the complex permittivity and permeability with an increase in frequency, and it is evident that the dielectric losses are one of the dominant factors resulting in EMI shielding performance.
3.3.5. SnO2-Based Composites
Feng et al.60 studied the effect of SnO2 nanowires (NWs) on the EMI shielding performance. The value of minimum RL for the composites with 20 vol % SnO2 NWs were found to be approximately −32.5 dB at 14 GHz with a thickness of 5.0 mm. They observe that microwave absorption exhibited by the composite is due to the dielectric loss rather than magnetic loss. The authors propose that dipolar polarization and the associated relaxation phenomena of SnO2 constitute the loss mechanisms. It has been studied that composite materials, in which semiconductor NWs are coated with a dielectric nanolayer, introduce additional interfaces and more polarization charges at the surface. The interfacial polarization and the associated relaxation in such cases add to the loss mechanism. Further, they have suggested that the molecular dipoles formed at the NWs’ surface interact with the microwave field, resulting in some absorption losses through heating. Zhao et al.61 synthesized honeycomb-like SnO2 foams via the polystyrene template method. The SnO2 foams were uniformly blended with paraffin wax at 30% weight fraction, and EMI shielding measurements were done for 2 mm thick samples. The minimum RL is reported to be −37.6 dB at 17.1 GHz, with RL below −10 dB at 5.6 GHz (12.4–18.0 GHz). The enhanced microwave absorption properties were ascribed to dipole polarization. The small SnO2 nanoparticles serve the purpose of dipoles in the SnO2 foam. Interfacial polarization, impedance match, multiple reflection, and diffuse scattering further adds to the mechanism of shielding. Liu et al.62 synthesized an Fe3O4@SnO2 yolk–double-shelled structure with a magnetic Fe3O4 core and a dielectric SnO2 as a double shell. The composite samples were prepared by mixing the Fe3O4@SnO2 and epoxy resin in a mass ratio of 1:5. The RL for the double-shelled Fe3O4@SnO2 yolk–shell microspheres reached a minimum of −36.5 dB at 7 GHz with a shield thickness of 2 mm. The authors suggest that the shielding is due to the absorption losses arising out of the interaction of EM waves with the shield material. It is not only the dielectric losses due to SnO2 and the magnetic losses due to the Fe3O4 playing role but also the synergistic effect of SnO2 and Fe3O4. The dielectric constant and loss increase due to effective interfaces between the dielectric and magnetic materials, thus resulting in loss via interfacial polarization. However, the authors suggest that the void spaces and high porosity of the yolk–shell microspheres might also act as active sites for internal scattering of EM waves.
4. Summary of Semiconducting Fillers Used in Polymeric Shields
Table 2 discusses the summary of the literature discussed in this article. It is quite difficult to compare different semiconducting materials as the parameter for measurement varies in each paper. So, we cannot exactly conclude the role of the band gap of filler material for EMI shielding applications. However, this table gives a preliminary idea as to how different semiconducting fillers perform in the area of microwave absorption via dielectric, interfacial, and scattering losses.
Table 2. List of Semiconducting Materials That Can Be a Prospective Choice as Filler for EMI Shielding Applications.
| Sl. no. | filler | matrix | SET/minimum RL | thickness (mm) | frequency | ref |
|---|---|---|---|---|---|---|
| 1 | (a) MoS2 hierarchical structure (25 wt %) | PVDF | RL= −26.11 dB | 2.5 | 11.36 GHz | (25) |
| (b) bulk MoS2 | RL= −11.94 dB | ∼5.9 GHz | ||||
| (c) micrometer-sized MoS2 | RL= −12.27 dB | ∼13.5 GHz | ||||
| 2 | MoS2 hierarchical structure (30 wt %) | PVDF | RL = −27.47 dB | 1.5 | 18.47 GHz | (25) |
| 3 | MoS2 hierarchical structure (20 wt %) | PVDF | RL = −32.67 dB | 3.5 | 28.93 GHz | (25) |
| 4 | MoS2 (5 wt %) | dopamine-functionalized PU | SET = −5.5 dB | 5 | 18 GHz | (26) |
| 5 | Fe3O4 (5 wt %) | dopamine-functionalized PU | SET = −6.3 dB | 5 | 18 GHz | (26) |
| 6 | Fe3O4@MoS2 (5 wt %) | dopamine-functionalized PU | SET = −11.6 dB | 5 | 18 GHz | (26) |
| 7 | 3 wt % MWCNT/5 wt % Fe3O4@MoS2 | dopamine-functionalized PU | SET = −36.6 dB | 5 | 18 GHz | (26) |
| 8 | MoS2/rGO (10 wt %) | wax | RL = −50.9 dB | 2.3 | 11.7 GHz | (27) |
| 9 | MoS2/rGO (10 wt %) | paraffin | RL = −41.53 dB | 3 | 11.7 GHz | (28) |
| 10 | (M/MoS2)/rGO (18 wt %) | PVP | RL = −49.7 dB | 3 | 13.7 GHz | (29) |
| 11 | α-MnO2 (α-MnO2/paraffin = 1:1 (mass ratio)) | paraffin | RL = −41 dB | 1.9 | 8.7 GHz | (30) |
| 12 | α-MnO2 | PANI | SET = −35–39 dB | ∼0.169 | 8.2–18 GHz | (31) |
| 13 | hollow CdSe nanostructure (70 wt %) | paraffin | RL = −31 dB | 4 | 17.8 GHz | (32) |
| 14 | CuS nanostructure (20 wt %) | paraffin | RL = −76.4 dB | 3.5 | 12.64 GHz | (33) |
| 15 | CuS (28.6 wt %) | PA/PTFE | SET = −27–31 dB | 0.6 | 300 kHz to 3 GHz | (34) |
| 16 | PANI/CuS (50 wt % CuS), 20 wt % PANI/CuS | paraffin | SET = −45.2 dB | 3 | 2.8 GHz | (35) |
| 17 | CuS/NiFe2O4/graphene (20 wt %) | paraffin | RL = −54.5 dB | 2.5 | 11.4 GHz | (36) |
| 18 | CoO nanobelt (70 wt %) | paraffin | RL = −12.3 dB | 3 | 14.2 GHz | (37) |
| 19 | CoO microsphere (70 wt %) | paraffin | RL = −8.7 dB | 3 | 14.4 GHz | (37) |
| 20 | WS2/rGO (40 wt %) | wax | SET = −20 to −32 dB | ∼1.5 | 2–18 GHz | (38) |
| 21 | Fe2O3 (10 wt %) | PVA | RL = −38.85 dB | 0.5 | 10.4 GHz | (50) |
| 22 | CdS-MWCNTs (10 wt %) | paraffin wax | RL = −25 dB | 3.5 | 8–9 GHz | (39) |
| 23 | WO3 (40 wt %) | PANI/CSA | SET ∼ −13.8 dB | 14.2 GHz | (40) | |
| 24 | WO3/MnFe2O3 (50/50 wt %) (50 wt % as core) | PANI (as shell) | RL = −12 dB | 1.5 | 8.8 and 11.6 GHz | (41) |
| 25 | graphene@Fe3O4@PANI@WO3 (70 wt %) (mass ratio of graphene@Fe3O4/WO3 = 2:1) | paraffin | RL = −46.7 dB | 4 | 9.3 GHz | (42) |
| 26 | MoO3/Mo4O11/MoO2 (60 wt %) | paraffin | RL = −59.2 dB | 4.6 | 10.8 GHz | (43) |
| 27 | 1D MoS2/MoO3 (20 wt %) | PVDF | RL = −38.5 dB | 1.9 | 8.7 GHz | (44) |
| 28 | BaTiO3 nanotubes (70 wt %) | paraffin | RL = −21.8 dB | 2 | 15 GHz | (45) |
| 29 | BaTiO3 | PEK | SET = −11 dB | 2 | 8.2–12.4 GHz | (46) |
| 30 | (BaTiO3/polyaniline = 1:3) | PU | RL = −15 dB | ∼ 3 | 10 GHz | (47) |
| 31 | rGO@BaTiO3 (wt % mentioned in RL/SET column) | PVDF | RL = −45.3 dB (15 wt %), SET = −9.8 dB (30 wt %) | 4.5 | 5.4 GHz (for RL), 10 GHz (for SET) | (48) |
| 32 | ZnO dendritic nanostructure (50 vol %) | paraffin | RL = −42 dB | 5 | 3.6 GHz | (49) |
| 33 | TiO2 (10 wt %) | PVA | RL = −32.90 dB | 0.5 | 9.8 GHz | (50) |
| 34 | ZnO (10 wt %) | PVA | RL = −33.65 dB | 0.5 | 10.4 GHz | (50) |
| 35 | TiO2 | Pani/HA | RL = −31 dB | 10 GHz | (51) | |
| 36 | TiO2/γ-Fe2O3 | Pani | SEA = −45 dB | ∼14.5 GHz | (52) | |
| 37 | BaFe12O19/TiO2 (1:1) | polypyrrole | RL = −45 dB | 2 | 17.5 GHz | (53) |
| 38 | ZnO/ZnAl2O4 (40 wt %) | paraffin | RL/RC = −25 dB | 2.86 | 9.7 GHz | (54) |
| 39 | ZnO/MWCNT (40 wt %) | wax | RL = −36 dB | 5 | 5–6 GHz | (55) |
| 40 | C/ZnO (40 wt %) | paraffin wax | RL/RC = −52 dB | 1.75 | 11 GHz | (56) |
| 41 | rGO/ZnO (10 wt %) | paraffin | RL = −25.31 dB | 3 | 11.5 GHz | (57) |
| 42 | SrTiO3 (50 wt %) | epoxy | RL = −48 dB | 1 | 14.5 GHz | (58) |
| 43 | SrTiO3 (0.3 volume fraction) | epoxy | RL = −34 dB | 5 | 4.3 GHz | (59) |
| 44 | SnO2 NWs (20 vol %) | paraffin | RL = −32.5 dB | 5 | 14 GHz | (60) |
| 45 | SnO2 foam (30 wt %) | paraffin wax | RL = −37.6 dB | 2 | 17.1 GHz | (61) |
| 46 | Fe3O4@SnO2 (Fe3O4@SnO2/epoxy = 1:5 by mass) | epoxy | RL = −36.5 dB | 2 | 7 GHz | (62) |
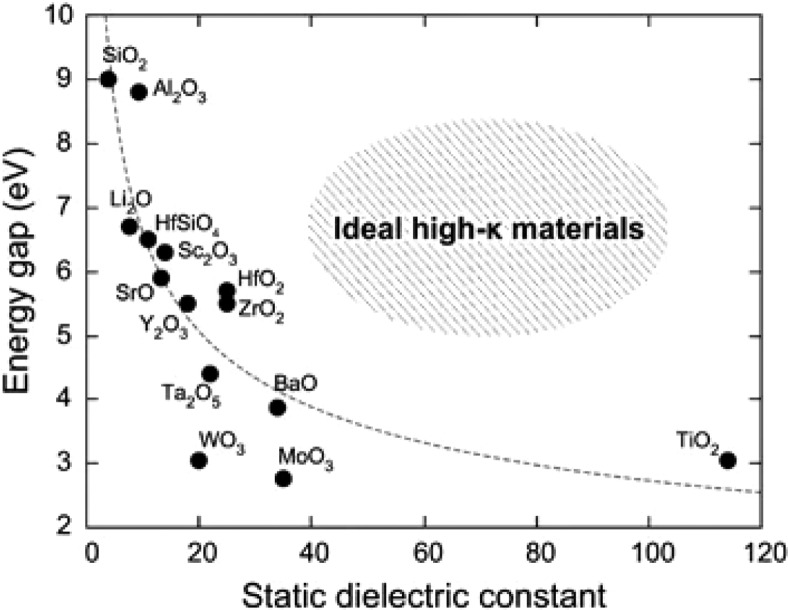
As most of the semiconducting fillers stated above exhibit dielectric losses as one of their primary mechanism of shielding, it is worthwhile to study the relation between semiconducting nature and dielectric behavior of a material. We carried out a literature survey to figure out if there exists any relationship between the band gap and dielectric properties. Yim et al.63 confirmed that, for most of the oxides, there is an inverse correlation between the band gap and the real part of permittivity. They compiled the result of well-known oxides in Figure 7. However, they also mentioned that there do exist certain materials that show large permittivity, despite their large band gaps.
Figure 7.
Experimental band gap and dielectric constant for well-known oxides. Reprinted with permission from ref (63). Copyright 2015 Springer Nature.
However, in EMI shielding applications, it is the trend of dielectric loss and tan δ that is a primary concern as our focus lies in lossy materials. Dielectric constant, dielectric loss, and tan δ of each material are all size-dependent and frequency-dependent functions. Thus, it becomes complicated to find a universal law that suits each material and for all frequencies. However, studies have been done to understand the underlying principles. Sun et al.64 studied the dielectric suppression and their dependence on band gap for nanometric semiconductors. The complex dielectric constant was correlated to the band gap, which has a dependence on the crystal field. The crystal field was further found to be a function of atomic separation and charge transportation. They have also plotted a graph showing energy dependence of the suppression of ε″ of the nanometric semiconductors with respect to the direct and indirect band gap transitions. It is observed that (Δεr″/εr′) increases as (ℏω/Eg) increases. Sati et al.65 studied the variation of dielectric constant and dielectric loss at 5 MHz frequency as a function of Hf doping in BaTiO3. They showed that with an increase in Hf doping in BaTiO3, the band gap increases and the value of the dielectric constant and that of dielectric loss/tan δ systematically decrease. They have also stated that it is well-studied that Hf doping at the Ti site reduces tetragonality, which probably leads to the decrease in the value of dielectric constant. They also mentioned that Penn theoretically derived the dielectric constant (ε′) of semiconductors in terms of plasma frequency (ωp) and optical band gap (Eg) as65
| 8 |
As per the Drude’s theory, ωp is defined as (ne2/meε0)0.5, where n is the number density of electrons, e is the electric charge, me is the effective mass of the electron, and ε0 is the permittivity of free space.66
For an insulating/semiconducting system, the dielectric loss, that is, leakage current, is related to band gap according to the following equation:65
| 9 |
Equation 9 implies that, with an increase in the band gap, the conductivity should decrease. In their work, they further proposed that increasing the value of Eg leads to a decrease in the tunneling probability of electron from the valence band to the conduction band, which resulted in the lowering of dielectric loss.
Hence, the general rule of thumb implies that, with the increase in the band gap, the dielectric constant decreases (as shown in Figure 7 and eq 8).54,63,65 The same trend is followed by dielectric loss. As the band gap increases, the conductivity decreases and subsequently the dielectric loss decreases (as shown in eqs 7 and 9).54,65 It is to be noted that this rule may not be universally applicable in all the cases as exceptions do exist.63 Moreover, unlike homogeneous material, these theories become even more complicated in the case of heterogeneous materials, such as polymer nanocomposites.
5. Do Semiconducting Fillers Have the Potential to Replace the Conventional Fillers?
Semiconducting fillers might stand as a potential candidate for EMI shielding applications. It depends primarily on electrical conductivity and the dielectric/magnetic properties of the material. It is observed that semiconductors with electrical conductivity near 1 S/m show reasonable EM absorption behavior. Many semiconductors such as ZnO, TiO2, MoS2, CoO, BaTiO3, SrTiO3, etc. are known to have good dielectric properties which play a crucial role in the losses, whereas there are a couple of semiconductors which have adequate magnetic properties (e.g., Fe2O3, Fe3O4, etc.), which aid in the EM absorption behavior. Hence, it is primarily the mechanism of shielding through the various types of losses (dipolar, interfacial, conduction, eddy current, magnetic) that decides whether a semiconducting material will prove to be a good shield for EM absorption. The mechanism of shielding is decided by the structure of each individual material/materials in the polymer matrix as well as their size and morphological features. Moreover, the mechanism is also governed by the interaction of these nanomaterials at various junctions, the state of aggregation, and the synergistic response of the polymer nanocomposite to the incoming EM radiations. Here, it is also worthwhile to note that there exist theories that correlate band gap to dielectric constant and dielectric loss. It is observed that with an increase in the band gap, both the dielectric constant and dielectric loss decrease. However, it may not be universally true for all semiconducting materials and at all frequencies. Moreover, for heterogeneous materials, such as polymer nanocomposites, these theories become even more complicated. To maximize the EM absorption properties, it is desirable to maximize tan δε using a material with low dielectric constant and high dielectric loss. The other aspect to be noted is that the characteristic impedance of the absorber material (z = (μ/ε)1/2, valid for ideal dielectric) should be as close to the characteristic impedance of free space (Z0 = (μ0/ε0)1/2 = 377 Ω) in order to maximize EM absorption. If permittivity meets the EM impedance match requirement, a higher dielectric loss implies better EM absorption properties.54
Our survey of the existing literature shows that semiconducting fillers meet the commercial requirement of EMI shielding value of −20 dB and above, only at high loading percentage. It is to be noted that as the loading percentage of filler is increased, the mechanical aspect of the shield material is compromised. However, to exploit the absorption losses incurred due to the presence of semiconducting filler, a hybrid composite serves as a better alternative solution. For example, composites consisting of metallic or semiconductor particles embedded in a dielectric matrix have been widely explored in the literature.60 The hybrid composite also enhances the number of interfaces and thus the dielectric mismatch which results in better shielding behavior.
Acknowledgments
The authors would like to acknowledge the funding support from DST-SERB.
Biographies
Kumari Sushmita is currently a Ph.D. Researcher at Indian Institute of Science (IISc), Bangalore, India. Prior to joining IISc, she did her M.Tech in Chemical Engineering from the Indian Institute of Technology (IIT), Gandhinagar. In her M.Tech, she primarily worked on nanoparticle synthesis with its application in catalysis. Her current research interests include synthesis of hybrid structures, polymer nanocomposites, polymer blends, and designing multilayer stack-based assembly for EMI shielding applications.
Giridhar Madras is currently a Professor in the interdisciplinary department for energy research at the Indian Institute of Science (IISc), Bangalore, India. He obtained his M.Tech from Indian Institute of Technology (IIT), Madras, and Ph.D. in chemical engineering from Texas A&M University, USA. Afterward, he worked as a researcher at the University of California at Davis, USA. He is a recipient of the Shanti Swarup Bhatnagar Prize in 2009. He has made fundamental contributions in the area of catalysis, reactions in supercritical fluids, and polymer nanocomposites for various applications such as water remediation and EMI shielding.
Suryasarathi Bose is currently an Associate Professor in the department of materials engineering at the Indian Institute of Science (IISc), Bangalore, India. He received his M.Tech degree from the Institute of Chemical Technology (erstwhile UDCT), Mumbai, and a Ph.D. degree from the Indian Institute of Technology (IIT), Bombay. He then worked as a research associate at IIT Bombay before he joined Prof. Paula Moldenaers’s group at Katholieke University of Leuven (Belgium) as a postdoctoral researcher. His research interests include polymer blends and nanocomposites, rheology, and structure–property correlations in homopolymers and blends, surface-initiated polymerization on nanoparticles and designing polymer-based materials for applications in EMI shielding and water filtration membranes.
The authors declare no competing financial interest.
References
- Sankaran S.; Deshmukh K.; Ahamed M. B.; Khadheer Pasha S. K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Composites, Part A 2018, 114, 49–71. 10.1016/j.compositesa.2018.08.006. [DOI] [Google Scholar]
- Shahzad F.; Alhabeb M.; Hatter C. B.; Anasori B.; Man Hong S.; Koo C. M.; Gogotsi Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353 (6304), 1137. 10.1126/science.aag2421. [DOI] [PubMed] [Google Scholar]
- Lin K.-F.; Cheng H.-M.; Hsu H.-C.; Lin L.-J.; Hsieh W.-F. Band gap variation of size-controlled ZnO quantum dots synthesized by sol–gel method. Chem. Phys. Lett. 2005, 409 (4), 208–211. 10.1016/j.cplett.2005.05.027. [DOI] [Google Scholar]
- Zhao J.-Y.; Zhang J.-M. Modulating the Band Gap of the FeS2 by O and Se Doping. J. Phys. Chem. C 2017, 121 (35), 19334–19340. 10.1021/acs.jpcc.7b04568. [DOI] [Google Scholar]
- Gorai S.; Ganguli D.; Chaudhuri S. Synthesis of 1D Cu2S with tailored morphology via single and mixed ionic surfactant templates. Mater. Chem. Phys. 2004, 88 (2), 383–387. 10.1016/j.matchemphys.2004.08.004. [DOI] [Google Scholar]
- Heinemann M.; Eifert B.; Heiliger C. Band structure and phase stability of the copper oxides Cu2O, CuO, and Cu4O3. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 87 (11), 115111. 10.1103/PhysRevB.87.115111. [DOI] [Google Scholar]
- Yun W. S.; Han S. W.; Hong S. C.; Kim I. G.; Lee J. D. Thickness and strain effects on electronic structures of transition metal dichalcogenides: 2H-MX2 semiconductors (M = Mo, W; X = S, Se, Te). Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85 (3), 033305. 10.1103/PhysRevB.85.033305. [DOI] [Google Scholar]
- Cockayne E.; Li L. First-principles DFT+U studies of the atomic, electronic, and magnetic structure of α-MnO2 (cryptomelane). Chem. Phys. Lett. 2012, 544, 53–58. 10.1016/j.cplett.2012.06.061. [DOI] [Google Scholar]
- Ninomiya S.; Adachi S. Optical properties of cubic and hexagonal CdSe. J. Appl. Phys. 1995, 78 (7), 4681–4689. 10.1063/1.359815. [DOI] [Google Scholar]
- Nemade K. R.; Waghuley S. A. Band gap engineering of CuS nanoparticles for artificial photosynthesis. Mater. Sci. Semicond. Process. 2015, 39, 781–785. 10.1016/j.mssp.2015.05.045. [DOI] [Google Scholar]
- Wang Y.; Ge H. X.; Chen Y. P.; Meng X. Y.; Ghanbaja J.; Horwat D.; Pierson J. F. Wurtzite CoO: a direct band gap oxide suitable for a photovoltaic absorber. Chem. Commun. 2018, 54 (99), 13949–13952. 10.1039/C8CC06777E. [DOI] [PubMed] [Google Scholar]
- Barakat N. A. M.; Khil M. S.; Sheikh F. A.; Kim H. Y. Synthesis and Optical Properties of Two Cobalt Oxides (CoO and Co3O4) Nanofibers Produced by Electrospinning Process. J. Phys. Chem. C 2008, 112 (32), 12225–12233. 10.1021/jp8027353. [DOI] [Google Scholar]
- Gusakova J.; Wang X.; Shiau L. L.; Krivosheeva A.; Shaposhnikov V.; Borisenko V.; Gusakov V.; Tay B. K. Electronic Properties of Bulk and Monolayer TMDs: Theoretical Study Within DFT Framework (GVJ-2e Method). Phys. Status Solidi A 2017, 214 (12), 1700218. 10.1002/pssa.201700218. [DOI] [Google Scholar]
- Valenzuela M. A.; Bosch P.; Jiménez-Becerrill J.; Quiroz O.; Páez A. I. Preparation, characterization and photocatalytic activity of ZnO, Fe2O3 and ZnFe2O4. J. Photochem. Photobiol., A 2002, 148 (1), 177–182. 10.1016/S1010-6030(02)00040-0. [DOI] [Google Scholar]
- Bagbi Y.; Sarswat A.; Mohan D.; Pandey A.; Solanki P. R. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7 (1), 7672. 10.1038/s41598-017-03380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya-Angel O.; Alvarado-Gil J. J.; Lozada-Morales R.; Vargas H.; Ferreira da Silva A. Band-gap shift in CdS semiconductor by photoacoustic spectroscopy: Evidence of a cubic to hexagonal lattice transition. Appl. Phys. Lett. 1994, 64 (3), 291–293. 10.1063/1.111184. [DOI] [Google Scholar]
- Bullett D. W. The energy band structure of V2O5: a simpler theoretical approach. J. Phys. C: Solid State Phys. 1980, 13 (23), L595–L599. 10.1088/0022-3719/13/23/006. [DOI] [Google Scholar]
- Wang F.; Di Valentin C.; Pacchioni G. Electronic and Structural Properties of WO3: A Systematic Hybrid DFT Study. J. Phys. Chem. C 2011, 115 (16), 8345–8353. 10.1021/jp201057m. [DOI] [Google Scholar]
- Chithambararaj A.; Bose A. C. Hydrothermal synthesis of hexagonal and orthorhombic MoO3 nanoparticles. J. Alloys Compd. 2011, 509 (31), 8105–8110. 10.1016/j.jallcom.2011.05.067. [DOI] [Google Scholar]
- Suzuki K.; Kijima K. Optical Band Gap of Barium Titanate Nanoparticles Prepared by RF-plasma Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2005, 44 (4A), 2081–2082. 10.1143/JJAP.44.2081. [DOI] [Google Scholar]
- Dette C.; Pérez-Osorio M. A.; Kley C. S.; Punke P.; Patrick C. E.; Jacobson P.; Giustino F.; Jung S. J.; Kern K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14 (11), 6533–6538. 10.1021/nl503131s. [DOI] [PubMed] [Google Scholar]
- van Benthem K.; Elsasser C.; French R. H. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90 (12), 6156–6164. 10.1063/1.1415766. [DOI] [Google Scholar]
- Cheng J.; Fan D.; Wang H.; Liu B.; Zhang Y.; Yan H. Chemical bath deposition of crystalline ZnS thin films. Semicond. Sci. Technol. 2003, 18 (7), 676–679. 10.1088/0268-1242/18/7/313. [DOI] [Google Scholar]
- Parthibavarman M.; Hariharan V.; Sekar C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng., C 2011, 31 (5), 840–844. 10.1016/j.msec.2011.01.002. [DOI] [Google Scholar]
- Zhang X.-J.; Li S.; Wang S.-W.; Yin Z.-J.; Zhu J.-Q.; Guo A.-P.; Wang G.-S.; Yin P.-G.; Guo L. Self-Supported Construction of Three-Dimensional MoS2 Hierarchical Nanospheres with Tunable High-Performance Microwave Absorption in Broadband. J. Phys. Chem. C 2016, 120 (38), 22019–22027. 10.1021/acs.jpcc.6b06661. [DOI] [Google Scholar]
- Menon A. V.; Madras G.; Bose S. Mussel-Inspired Self-Healing Polyurethane with “Flower-like” Magnetic MoS2 as Efficient Microwave Absorbers. ACS Appl. Polym. Mater. 2019, 1, 2417. 10.1021/acsapm.9b00538. [DOI] [Google Scholar]
- Wang Y.; Chen D.; Yin X.; Xu P.; Wu F.; He M. Hybrid of MoS2 and Reduced Graphene Oxide: A Lightweight and Broadband Electromagnetic Wave Absorber. ACS Appl. Mater. Interfaces 2015, 7 (47), 26226–26234. 10.1021/acsami.5b08410. [DOI] [PubMed] [Google Scholar]
- Ding X.; Huang Y.; Li S.; Zhang N.; Wang J. 3D architecture reduced graphene oxide-MoS2 composite: Preparation and excellent electromagnetic wave absorption performance. Composites, Part A 2016, 90, 424–432. 10.1016/j.compositesa.2016.08.006. [DOI] [Google Scholar]
- Ran J.; Shen L.; Zhong L.; Fu H. Synthesis of Silanized MoS2/Reduced Graphene Oxide for Strong Radar Wave Absorption. Ind. Eng. Chem. Res. 2017, 56 (38), 10667–10677. 10.1021/acs.iecr.7b02721. [DOI] [Google Scholar]
- Zhou M.; Zhang X.; Wei J.; Zhao S.; Wang L.; Feng B. Morphology-Controlled Synthesis and Novel Microwave Absorption Properties of Hollow Urchinlike α-MnO2 Nanostructures. J. Phys. Chem. C 2011, 115 (5), 1398–1402. 10.1021/jp106652x. [DOI] [Google Scholar]
- Bora P. J.; Vinoy K. J.; Ramamurthy P. C.; Madras G. Electromagnetic interference shielding efficiency of MnO2 nanorod doped polyaniline film. Mater. Res. Express 2017, 4 (2), 025013. 10.1088/2053-1591/aa59e3. [DOI] [Google Scholar]
- Cao M.; Lian H.; Hu C. Ligand-assisted fabrication of hollow CdSe nanospheres via Ostwald ripening and their microwave absorption properties. Nanoscale 2010, 2 (12), 2619–2623. 10.1039/c0nr00674b. [DOI] [PubMed] [Google Scholar]
- He S.; Wang G.-S.; Lu C.; Luo X.; Wen B.; Guo L.; Cao M.-S. Controllable Fabrication of CuS Hierarchical Nanostructures and Their Optical, Photocatalytic, and Wave Absorption Properties. ChemPlusChem 2013, 78 (3), 250–258. 10.1002/cplu.201200287. [DOI] [Google Scholar]
- Hu X.-S.; Shen Y.; Xu L.-H.; Wang L.-M.; Lu L.-s.; Zhang Y.-t. Preparation of flower-like CuS by solvothermal method for photocatalytic, UV protection and EMI shielding applications. Appl. Surf. Sci. 2016, 385, 162–170. 10.1016/j.apsusc.2016.05.089. [DOI] [Google Scholar]
- Hu X.-S.; Shen Y. Fabrication of novel polyaniline/flowerlike copper monosulfide composites with enhanced electromagnetic interference shielding effectiveness. J. Appl. Polym. Sci. 2017, 134 (34), 45232. 10.1002/app.45232. [DOI] [Google Scholar]
- Liu P.; Huang Y.; Yan J.; Yang Y.; Zhao Y. Construction of CuS Nanoflakes Vertically Aligned on Magnetically Decorated Graphene and Their Enhanced Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2016, 8 (8), 5536–5546. 10.1021/acsami.5b10511. [DOI] [PubMed] [Google Scholar]
- Sun G.; Zhang X.; Cao M.; Wei B.; Hu C. Facile Synthesis, Characterization, and Microwave Absorbability of CoO Nanobelts and Submicrometer Spheres. J. Phys. Chem. C 2009, 113 (17), 6948–6954. 10.1021/jp8092447. [DOI] [Google Scholar]
- Zhang D.-Q.; Liu T.-T.; Shu J.-C.; Liang S.; Wang X.-X.; Cheng J.-Y.; Wang H.; Cao M.-S. Self-Assembly Construction of WS2–rGO Architecture with Green EMI Shielding. ACS Appl. Mater. Interfaces 2019, 11 (30), 26807–26816. 10.1021/acsami.9b06509. [DOI] [PubMed] [Google Scholar]
- Lu M.; Wang X.; Cao W.; Yuan J.; Cao M. Carbon nanotube-CdS core-shell nanowires with tunable and high-efficiency microwave absorption at elevated temperature. Nanotechnology 2016, 27 (6), 065702. 10.1088/0957-4484/27/6/065702. [DOI] [PubMed] [Google Scholar]
- Sastry D. N.; Revanasiddappa M.; Suresh T.; Kiran Y. T. R.; Raghavendra S. C. Electromagnetic shielding effectiveness studies on polyaniline/CSA-WO3 composites at KU band frequencies. AIP Conf. Proc. 2017, 1953 (1), 090067. 10.1063/1.5032914. [DOI] [Google Scholar]
- Boujar Dolabi M.; Azimi A.; Hossein Hosseini S. Preparation of thermal infrared and microwave absorber using WO3/MnFe3O4/polyaniline nanocomposites. Mater. Res. Innovations 2019, 1–9. 10.1080/14328917.2019.1677078. [DOI] [Google Scholar]
- Wang Y.; Wu X.; Zhang W.; Luo C.; Li J.; Wang Q. 3D heterostructure of graphene@Fe3O4@WO3@PANI: Preparation and excellent microwave absorption performance. Synth. Met. 2017, 231, 7–14. 10.1016/j.synthmet.2017.06.013. [DOI] [Google Scholar]
- Lyu L.; Wang F.; Qiao J.; Ding X.; Zhang X.; Xu D.; Liu W.; Liu J. Novel synthesis of MoO3/Mo4O11/MoO2 heterogeneous nanobelts for wideband electromagnetic wave absorption. J. Alloys Compd. 2020, 817, 153309. 10.1016/j.jallcom.2019.153309. [DOI] [Google Scholar]
- Li C.-Q.; Shen X.; Ding R.-C.; Wang G.-S. Controllable Synthesis of One-Dimensional MoO3/MoS2 Hybrid Composites with their Enhanced Efficient Electromagnetic Wave Absorption Properties. ChemPlusChem 2019, 84 (2), 226–232. 10.1002/cplu.201800599. [DOI] [PubMed] [Google Scholar]
- Zhu Y.-F.; Zhang L.; Natsuki T.; Fu Y.-Q.; Ni Q.-Q. Facile Synthesis of BaTiO3 Nanotubes and Their Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2012, 4 (4), 2101–2106. 10.1021/am300069x. [DOI] [PubMed] [Google Scholar]
- Chauhan S. S.; Verma P.; Malik R. S.; Choudhary V. Thermomechanically stable dielectric composites based on poly(ether ketone) and BaTiO3 with improved electromagnetic shielding properties in X-band. J. Appl. Polym. Sci. 2018, 135 (26), 46413. 10.1002/app.46413. [DOI] [Google Scholar]
- Abbas S. M.; Dixit A. K.; Chatterjee R.; Goel T. C. Complex permittivity and microwave absorption properties of BaTiO3–polyaniline composite. Mater. Sci. Eng., B 2005, 123 (2), 167–171. 10.1016/j.mseb.2005.07.018. [DOI] [Google Scholar]
- Guo A.-P.; Zhang X.-J.; Qu J.-K.; Wang S.-W.; Zhu J.-Q.; Wang G.-S.; Guo L. Improved microwave absorption and electromagnetic interference shielding properties based on graphene–barium titanate and polyvinylidene fluoride with varying content. Mater. Chem. Front. 2017, 1 (12), 2519–2526. 10.1039/C7QM00204A. [DOI] [Google Scholar]
- Zhuo R. F.; Feng H. T.; Chen J. T.; Yan D.; Feng J. J.; Li H. J.; Geng B. S.; Cheng S.; Xu X. Y.; Yan P. X. Multistep Synthesis, Growth Mechanism, Optical, and Microwave Absorption Properties of ZnO Dendritic Nanostructures. J. Phys. Chem. C 2008, 112 (31), 11767–11775. 10.1021/jp804090q. [DOI] [Google Scholar]
- Singh R.; Kulkarni S. G. Nanocomposites based on transition metal oxides in polyvinyl alcohol for EMI shielding application. Polym. Bull. 2014, 71 (2), 497–513. 10.1007/s00289-013-1073-2. [DOI] [Google Scholar]
- Phang S. W.; Tadokoro M.; Watanabe J.; Kuramoto N. Microwave absorption behaviors of polyaniline nanocomposites containing TiO2 nanoparticles. Curr. Appl. Phys. 2008, 8 (3), 391–394. 10.1016/j.cap.2007.10.022. [DOI] [Google Scholar]
- Dhawan S. K.; Singh K.; Bakhshi A. K.; Ohlan A. Conducting polymer embedded with nanoferrite and titanium dioxide nanoparticles for microwave absorption. Synth. Met. 2009, 159 (21), 2259–2262. 10.1016/j.synthmet.2009.08.031. [DOI] [Google Scholar]
- Akman O.; Durmus Z.; Kavas H.; Aktas B.; Kurtan U.; Baykal A.; Sözeri H. Effect of conducting polymer layer on microwave absorption properties of BaFe12O19-TiO2 composite. Phys. Status Solidi A 2013, 210 (2), 395–402. 10.1002/pssa.201228498. [DOI] [Google Scholar]
- Kong L.; Yin X.; Ye F.; Li Q.; Zhang L.; Cheng L. Electromagnetic Wave Absorption Properties of ZnO-Based Materials Modified with ZnAl2O4 Nanograins. J. Phys. Chem. C 2013, 117 (5), 2135–2146. 10.1021/jp309984p. [DOI] [Google Scholar]
- Song W.-L.; Cao M.-S.; Wen B.; Hou Z.-L.; Cheng J.; Yuan J. Synthesis of zinc oxide particles coated multiwalled carbon nanotubes: Dielectric properties, electromagnetic interference shielding and microwave absorption. Mater. Res. Bull. 2012, 47 (7), 1747–1754. 10.1016/j.materresbull.2012.03.045. [DOI] [Google Scholar]
- Han M.; Yin X.; Ren S.; Duan W.; Zhang L.; Cheng L. Core/shell structured C/ZnO nanoparticles composites for effective electromagnetic wave absorption. RSC Adv. 2016, 6 (8), 6467–6474. 10.1039/C5RA25295D. [DOI] [Google Scholar]
- Wu F.; Xia Y.; Wang Y.; Wang M. Two-step reduction of self-assembed three-dimensional (3D) reduced graphene oxide (RGO)/zinc oxide (ZnO) nanocomposites for electromagnetic absorption. J. Mater. Chem. A 2014, 2 (47), 20307–20315. 10.1039/C4TA04959D. [DOI] [Google Scholar]
- Murugan M.; Bapat M. S.; Kokate V. K. Composites of nanosized strontium titanate epoxy resin as microwave absorber. Journal of Electromagnetic Waves and Applications 2016, 30 (3), 366–374. 10.1080/09205071.2015.1114907. [DOI] [Google Scholar]
- Choi H. D.; Shim H. W.; Cho K. Y.; Lee H. J.; Park C. S.; Yoon H. G. Electromagnetic and electromagnetic wave-absorbing properties of the SrTiO3–Epoxy composite. J. Appl. Polym. Sci. 1999, 72 (1), 75–83. . [DOI] [Google Scholar]
- Feng H.; Zhuo R.; Chen J.; Yan D.; Feng J.; Li H.; Cheng S.; Wu Z.; Wang J.; Yan P. Synthesis, Characterization, and Microwave Absorption Property of the SnO2 Nanowire/Paraffin Composites. Nanoscale Res. Lett. 2009, 4 (12), 1452. 10.1007/s11671-009-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B.; Fan B.; Xu Y.; Shao G.; Wang X.; Zhao W.; Zhang R. Preparation of Honeycomb SnO2 Foams and Configuration-Dependent Microwave Absorption Features. ACS Appl. Mater. Interfaces 2015, 7 (47), 26217–26225. 10.1021/acsami.5b08383. [DOI] [PubMed] [Google Scholar]
- Liu J.; Cheng J.; Che R.; Xu J.; Liu M.; Liu Z. Double-Shelled Yolk–Shell Microspheres with Fe3O4 Cores and SnO2 Double Shells as High-Performance Microwave Absorbers. J. Phys. Chem. C 2013, 117 (1), 489–495. 10.1021/jp310898z. [DOI] [Google Scholar]
- Yim K.; Yong Y.; Lee J.; Lee K.; Nahm H.-H.; Yoo J.; Lee C.; Seong Hwang C.; Han S. Novel high-κ dielectrics for next-generation electronic devices screened by automated ab initio calculations. NPG Asia Mater. 2015, 7 (6), e190–e190. 10.1038/am.2015.57. [DOI] [Google Scholar]
- Sun C. Q.; Sun X. W.; Tay B. K.; Lau S. P.; Huang H. T.; Li S. Dielectric suppression and its effect on photoabsorption of nanometric semiconductors. J. Phys. D: Appl. Phys. 2001, 34 (15), 2359–2362. 10.1088/0022-3727/34/15/316. [DOI] [Google Scholar]
- Sati A.; Kumar A.; Mishra V.; Warshi K.; Sagdeo A.; Anwar S.; Kumar R.; Sagdeo P. R. Direct correlation between the band gap and dielectric loss in Hf doped BaTiO3. J. Mater. Sci.: Mater. Electron. 2019, 30 (8), 8064–8070. 10.1007/s10854-019-01128-z. [DOI] [Google Scholar]
- Kittel C.Introduction to Solid State Physics, 8th ed.; Wiley, 1956; pp 393–401. [Google Scholar]