Abstract
Background
In Germany, 17–23% of the population suffers from chronic itching of the skin; in 5–10% of cases, the female genitalia are affected, specifically, the vulva. Vulvar pruritus is thus a common symptom that often markedly impairs the affected women’s quality of life.
Methods
This review is based on pertinent publications that were retrieved by a selective search in MEDLINE/PubMed for articles on the pathogenesis, diagnosis, and treatment of vulvar pruritus. The search terms were (in German and English) “vulvärer Juckreiz,” “pruritus vulvae,” and “genital itch,” alone and in combination with “Behandlung,” “Therapie,” or “treatment.”
Results
The most common cause of vulvar pruritus is vulvovaginal candidiasis followed by chronic dermatoses, such as lichen sclerosus and vulvar eczema. Especially in refractory cases, an invasive or preinvasive lesion such as squamous epithelial dysplasia (VIN, vulvar intraepithelial neoplasia) should be borne in mind in the differential diagnosis. Rarer causes include infection, atrophy, and vulvodynia. The essential elements of treatment are topical/oral antimycotic drugs and high-potency glucocorticoids, along with consistently applied, basic moisturizing care and the avoidance of potential triggering factors.
Conclusion
As vulvar pruritus has multiple causes, standardization of its diagnostic evaluation and treatment would be desirable, both to achieve optimal efficacy and to meet the diverse needs of women who suffer from this condition.
In Germany, 17% to 23% of the population suffer from chronic (≥ 6 weeks) pruritus of the skin (1, 2). In 5% to 10% of cases, the female genitalia—and in particular the vulva—are affected (3– 6). Thus, vulvar pruritus is a common symptom which often significantly impairs the patient‘s quality of life. The underlying pathophysiological mechanisms are not yet fully understood. In the past, itching was generally considered to be a subtype of pain sensations. Today, however, it is assumed that it is an independent sensory quality mediated by free nerve endings of unmyelinated C-fibers. These nerve endings respond to chemical, mechanical and thermal stimulation and are activated by specific mediators, such as kinins, prostaglandins, and neuropeptides (7, 8).
In patients experiencing genital itching, the medical history should be taken in a systematic manner (Figure 1), covering the following aspects:
Figure 1.
Differential diagnostic work-up and diagnosis of vulvar pruritus by morphology (nomenclature see Box); bact., bacterial; surg., surgical; ultrapot., ultrapotent; GC, glucocorticoids; Inf., infection; mechan., mechanical; NAAT, nucleic acid amplification test; p.o., per os; VIN, vulvar intraepithelial neoplasia
Symptom duration (acute/chronic)
Localization (local/generalized)
Intensity (scale 1–10)
Pre-existing systemic disorders (e.g. autoimmune disease/diabetes mellitus)
Ameliorating/aggravating modulators
Previous treatments.
Since the skin in the genital area is influenced by sex hormones, the question whether hormonal medications, such as contraceptives, are taken is also an important part of the clinical history.
The, at times, complex ties between the various causal factors involved in the pathophysiology of vulvar pruritus (figure 2) can often be satisfactorily evaluated by inspection, speculum examination and microscopy of vaginal discharge.
Figure 2.
Common differential diagnoses of vulvar pruritus
During the inspection of the external genitalia, attention should be paid to superficial skin changes, such as erosions or plaques. Besides discolorations and abnormalities of the skin markings, the margins and configurations of the lesions noted should also be taken into account. The classification of the International Society for the Study of Vulvovaginal Disease (www.issvd.org) is a tool to categorize the morphological (shape/form) features which has been shown to be particularly useful. Using this classification system, the range of potential differential diagnoses can be narrowed down.
In patients with refractory candidiasis/infection, microbiological swap tests, culture-based tests, and amplification techniques may be needed to confirm the diagnosis. For a more detailed assessment of the vulva, it is recommended to perform a vulvoscopy as this allows to exam the skin of the vulva under 7x to 30x magnification. 5% acetic acid is applied to aid visualization of suspicions areas. Findings suspicious of vulvar intraepithelial neoplasia (VIN)/invasive lesions as well as unclear or refractory vulvar skin changes are indications for a 4–6 mm punch biopsy which can usually be performed without difficulty under local anesthesia. In addition to the examination of the vulva, inspection of both groins, the anal fold and, in some cases, the entire integument is indicated as it may yield important clues about the underlying cause of the clinical signs and symptoms.
The aim of this article is to develop algorithms for the diagnosis and treatment of pruritus, on the basis of signs, symptoms and morphological features, which are suitable for use in clinical practice. In addition, clinically relevant parameters of the most common conditions associated with vulvar pruritus will be discussed.
Methods
A search of the literature was conducted in the MEDLINE/PubMed database without publication date limits. Publications on the etiology, diagnosis and treatment of valvular pruritis were included. The following search terms were used: “vulvärer Juckreiz“, “pruritus vulvae“, “genital itch“, alone and in combination with “Behandlung“, “Therapie“, “treatment“. The level (or quality) of evidence (LoE) is reported using the GRADE approach (9).
Common differential diagnoses of vulvar pruritus
The most important conditions associated with vulvar pruritus are presented in Figure 2. The respective clinical presentation, characteristic morphological features and the diagnostic approach, as well as the recommended specific treatment are shown in Figure 1 and the eFigure. The Table provides a detailed description of the most important conditions. In addition, three current topics of increasing importance in the future will be discussed in detail:
eFigure.
Differential diagnostic work-up and diagnosis of vulvar pruritus by symptom (according to Stockdale Obstet Gynecol 2018 [e46]); VIN, vulvar intraepithelial neoplasia
Table. Characteristic conditions*.
| Candidiasis | Lichen sclerosus | Lichen planus | VIN (vulvar intraepithelial neoplasia) | Eczema | Psoriasis | |
|
Clinical presentation |
 |
 |
 |
 |
 |
 |
| Frequency (prevalence) | 10–20% | 0.1–3% | 0.1–0.8% | Exact prevalence unknown, incidence Approx. 3/100 000 women in Germany | 0.5–10% | 2% |
| Morphological features | Red and white plaques, erosions, ulcers | – Early form: erythema, erosions – Late form: white, firm plaques, bleeding into skin, scarring, atrophy, fissures, exaggerated skin markings |
– Early form: charact. whitish reticulate (Wickham) striae – Late form/erosive form: erosive erythema, scarring, extragenital involvement (e.g. oral) |
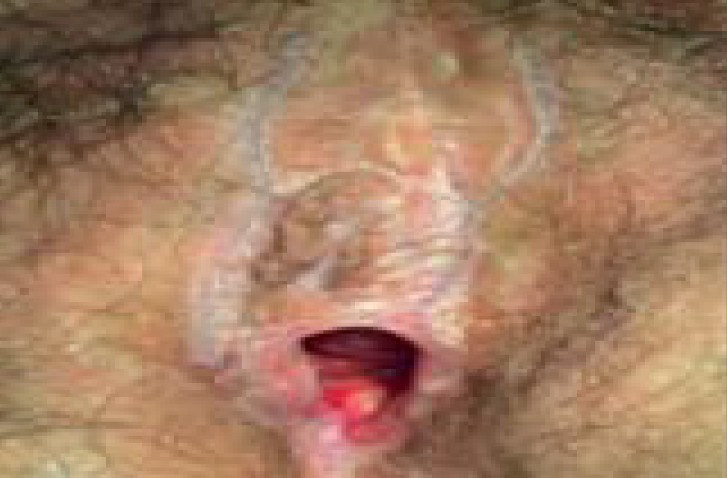
Skin-colored papules and nodules, white and red plaques, acetowhite lesions (after application of 5% acetic acid), erosions (especially with differentiated VIN) | Poorly marginated erythematous lesions on the labia majora and labia minora, edema (acute), lichenification (chronic) | Well-demarcated, slightly scaly, erythematous red plaques, accompanying fissures and rhagades |
| Signs & symptoms | Pruritus, erythema, increased vaginal discharge (white, curd-like, no odor) | Pruritus, burning, dyspareunia | – Early form: pruritus, burning, pain – Late form: dyspareunia, burning, vaginal stenosis |
Pruritus, burning, soreness, often asymptomatic | Pruritus, soreness | Pruritus |
| Diagnosis | pH value: normal (< 4.0) lactobacilli : yes leukocytes: increased clue cells: none microscopy: pseudomycelium & blastospores |
History, clinical examination, typical figure-of-8 pattern surrounding the vulva and anus; histological confirmation by biopsy, if required | History, clinical examination, characteristic Wickham striae, vaginal involvement, histological confirmation by biopsy | Vulvoscopy, histological confirmation by biopsy | History, clinical examination; if necessary, patch test | Typical involvement of labia majora, characteristic non-involvement of labia minora |
| Management | – Acute: Antifungal agent (e.g. oral fluconazole or topical clotrimazole preparations) – Chronic: Following the initial treatment, fluconazole 150 mg orally 1 x/week for at least 6 months after loading dose |
– Initially ultrapotent glucocorticoids (GC) (topical) – Life-long GC maintenance dose in reduced dose, lipid replenishment – Second-line therapy: calcineurin inhibitors (off-label) |
– Initially ultrapotent glucocorticoids (GC) (topical) – Life-long GC maintenance dose in reduced dose, lipid replenishment –Second-line therapy: calcineurin inhibitors (off-label) |
– Laser vaporization (standard with usual-type VIN) – Surgical excision rather with differentiated VIN (Warning: occult carcinoma) – Imiquimod 5%, topical for usual-type VIN (off-label) |
– Avoidance of trigger factors – Glucocorticoids (topical) |
– Avoidance of mechanical stress and trigger factors – Topical treatment as for extragenital psoriasis (typically GC/calcineurin inhibitors) |
Candida glabrata infections
The role of the vaginal microbiome
Genetic polymorphism in lichen sclerosus (LS).
Treatment of vulvar pruritus
As a rule, continuous basic care has a positive effect on the course of treatment in patients with vulvar itch. Basic care includes avoidance of potential trigger and provocation factors, such as fragrances, cleaning products with emulsifiers or antimicrobial agents (e.g. parabens), latex-containing condoms, as well as lubricants and preservatives. Apart from that, lipid-replenishing, breathable topical preparations should regularly be used to support the barrier function of the vulvovaginal skin/mucosa (10– 12). For this purpose, ointments with high fat content are especially useful and should be given preference over creams which contain the above mentioned additional ingredients.
Other recommendations include to refrain from wearing (too) tight clothing and shaving the pubic hair and to use silk or cotton underwear (13– 16). A study including 96 women with recurrent vulvovaginal candidiasis (VVC) (median age: 30 years) found that wearing silk underwear while continuing oral treatment with 150 mg fluconazole once weekly significantly reduced vulvar itching (p<0.0001) and burning (p<0.005) compared to the control group (16). A randomized trial including 42 women with lichen sclerosus (median age: 51.5 years) treated with clobetasol 0.05% (13) arrived at similar results: In addition to improvements in clinical symptoms, such as burning, skin irritation and pain (p<0.0001), faster relief of itching was observed in the group of patients wearing silk underwear compared to the control group (p<0.005).
Disease-specific treatment approaches are shown in Figure 1 and in the Table. Due to the, at times, complex pathogenesis of the condition, an interdisciplinary treatment strategy may be required to achieve favorable long-term results. Obtaining a biopsy is critical to ensure that in patients with refractory lesions possible (pre-) invasive changes are ruled out. These include, among others, (see Box):
BOX. Nomenclature of vulvar changes (morphology) according to the International Federation for Cervical Pathology and Colposcopy (IFCPC).
Definitions of primary lesion types
Macule = small (<1.5 cm) discolored, flat, non-palpable area
Patch = large (>1.5 cm) discolored, flat, non-palpable area
Papule = small (<1.5 cm) elevated and palpable lesion
Plaque = large (>1.5 cm) elevated, palpable and flat-topped lesion
Nodule = large papule (>1.5 cm), often hemispherical or poorly demarcated; on the surface; cystic or solid lesion
Vesicle = small (<0.5 cm) blister containing clear fluid
Bulla = large (>0.5 cm) blister containing clear fluid
Pustule = pus-filled blister; content is white or yellow
Definitions of secondary morphology presentation
(develops from the primary lesion by proliferative transformation, regression or healing)
Eczema = Inflammatory, non-infectious intolerance reaction characterized by the presence of itchy, poorly demarcated red plaques with minor evidence of microvasculation and/or (more frequently) subsequent surface disruption
Lichenification = Thickening of the tissue and increased prominence of skin markings, with or without scaling, bright red, dusky red, white or skin-colored.
Excoriation = Substance defect of the skin extending into the dermis (often scratch marks)
Purpura = Multiple smaller capillary hemorrhages in the skin, subcutis or mucosa
Scarring = Replacement tissue after wound healing; often white, fibrous, poorly vascularized
Erosion = A shallow defect in the skin/mucosa with absence of epidermis/epithelium; the dermis is intact
Fissure = A thin, linear erosion of the skin/mucosa
Ulcer = Deeper defect of the skin; absence of the epidermis and some, or all, of the dermis
Suspicion of neoplasia
Gross neoplasia, ulcer, necrosis, hemorrhage, exophytic lesion, hyperkeratosis with/without white, gray, reddish brown discoloration
Hyperkeratotic lesions which cannot be wiped off
Leukoplakia
Fissures
Erosions
Ulcers
This also applies to patients where the pathogenesis of the vulvar pruritus has remained unclear and a histopathological diagnosis could inform subsequent treatment. In both cases—presence of (pre-) invasive lesions and also inconclusive vulvar examination findings—these patients should be referred to a specialized outpatient clinic to ensure speedy initiation of an effective treatment.
In patients with persistent, idiopathic vulvar pruritus, as a rule the following treatment option should be considered:
Local cooling
Evening dosing of an antihistamine, e.g. hydroxyzine (10, 17)
Anticonvulsants, in the form of gabapentin (2)
Antidepressants, such as doxepin, sertraline, mirtazapine (2, 18, 19)
Opioid antagonists, e.g. naltrexone (20)
Transcutaneous electrical nerve stimulation (TENS) (21)
Acupuncture (22).
Furthermore, it is crucial to ask questions regarding potential psychosocial and psychosexual causes and, if any, to address these in collaboration with psychologists, psychotherapists and specialists in sexual medicine.
Vulvovaginal candidiasis
Genital candidiasis is a common infection and a primary cause of vulvar pruritus, accounting for 35% to 40% of cases (23). The most common pathogens are Candida albicans (>90%) and Candida glabrata (approx. 2%). 75% of women will have at least one episode of VVC during their lifetime and about 8% will have chronic VVC (at least 4 recurrences/year) (24– 29). Treatment recommendations for acute/chronic VVC are shown in Figure 1 and the Table. Below, individual aspects with relevance for clinical practice will be discussed in greater detail.
Compared to C. albicans-induced VVC, the clinical signs and symptoms of C. glabrata-induced VVC are generally milder. Other characteristic features of C. glabrata-induced VVC include absence of leukocytosis and pseudomycelium formation in the wet preparation as well as production of less vaginal discharge (30). The recommended treatment for C. glabrata-induced VVC is still an oral dose of 800 mg fluconazole for 2 to 3 weeks (level of evidence II; grade of recommendation: C) (25). Given the rapid development of resistance in recent years, which is primarily attributable to the genetic variability of the pathogen at doses of less than 800 mg fluconazole, satisfactory long-term treatment has increasingly become a challenge (31– 33). Today, fluconazole-sensitive strains are only occasionally encountered in clinical practice and the remaining strains cannot be treated with the triazole derivatives voriconazole or posaconazole either, because of additional cross-resistances. For this reason, it is necessary to use echinocandins for the systemic treatment of C. glabrata, a new class of antifungal agents which includes, besides caspofungin and anidulafungin, also micafungin, the currently preferred agent (30).
In a randomized controlled trial with 531 patients with invasive candidiasis, micafungin (100 mg/day or 2 mg/kg body weight [BW]/day) was as effective (89.6% success rate in both groups) as liposomal amphotericin B (L-AmB) (3 mg/kg BW), but caused fewer adverse events (34). While echinocandin class antifungal agents have been available for the treatment of esophageal and invasive candidiasis and other indications since the approval by the US Food and Drug Administration (FDA) in 2001 (35), no high-quality study data have yet become available to support their use in the treatment of chronic recurrent VVC. For this reason, micafungin, an expensive drug which is given intravenously, has not been approved for the treatment of vaginal mycosis, even though it has been shown to be highly effective against C. glabrata (36). First promising treatment concepts favor a systemic treatment approach consisting of a 15-day treatment with 150 mg micafungin intravenously/day in combination with ciclopirox olamine cream and nystatin vaginal ovules (30). Treatment strategies commonly recommended in other countries, such as boric acid vaginal suppositories (600 mg) or flucytosine 17%, are not available in Germany. However, because of their increased toxicity, their use is also not recommended (25, 37).
The importance of the human vaginal microbiome—i.e. the totality of all microorganisms present on the vaginal mucosa—in the pathogenesis of vulvar pruritus, especially if the itching is caused by chronic recurrent fungal infections, remains the subject of controversy in the current literature. Compared to the intestinal and oral microbiota, the vaginal microbiome shows less diversity, with 100 to 200 different species (38). The range of species changes several times over the course of a lifetime due to variations in hormone levels. The composition of the vaginal microbiome is influenced by genetic factors, among others, and shows ethnic differences. In more than 70% of cases, the natural colonization of the vagina is dominated by lactobacilli (primarily L. crispatus, L. gasseri, L. jensenii, and L. iners) (39, 40). By acidifying the vagina and producing antimicrobial substances, such as lactic acid and hydrogen peroxide, lactobacilli protect against the adhesion of facultative pathogens (e1).
Since 2008, research on the microorganisms inhabiting the human body has been carried out by the Human Microbiome Project and the Vaginal Microbiome Project of the National Institute of Health (NIH) (38). While more recent studies have demonstrated a causal relationship between changes in the composition of the vaginal microbiota and an increased risk of infection (e2– e5), no significant difference was found in a study comparing the dominant Lactobacillus species in women with VVC and without VVC (e6). Despite the lack of convincing long-term data regarding the therapeutic targets, it appears that the use of probiotics reduces the VVC recurrence rate (e1, e7– e9). For example, in first clinical studies women with chronic recurrent VVC were treated with 250g yoghurt containing L. acidophilus over a period of 6 months. The number of new vulvovaginal Candida infections was 2.5 in the control arm versus 0.38 in the treatment arm (p = 0.001) (e9, e10). Preparation of wet-mount slides continues to be the most important diagnostic test to differentiate between normal and abnormal composition of the vaginal microbiome. It is supplemented by a microbiological culture. In addition, modern sequencing techniques, such as next-generation sequencing (NGS), allow for a much more differentiated interpretation and have the potential to become the foundation of our future understanding of the genital tract’s colonization by a wide variety of species of microorganisms.
In Germany, patients with recurrent episodes of vaginitis and VVC are sometimes offered immunization with inactivated lactobacilli of various species. The aim of this immunization is to induce specific antibodies against aberrant strains in the Döderlein’s flora which do not contribute to the normal vaginal milieu. At the same time, this treatment aims to achieve regeneration of the affected vaginal flora and restoration of the normal vaginal pH and consequently a general enhancement of local vaginal immunity (e11). The recommended regimen consists of basic immunization with 3 doses at 2-week intervals and a booster dose after 6 to 12 months. The primary indication is bacterial vaginosis. The available studies showed a significant reduction in recurrence rate by up to 80% (e12– e14). Taking into account cross-protection mechanisms, therapeutic benefits are also expected in patients with VVC. However, based on the available data on the use of this approach in patients with VVC, the benefit of immunization treatment for this condition is still uncertain. Thus, it should only be used in addition to antifungal treatment.
Lichen sclerosus
LS is the most common dermatosis of the vulva associated with itching. It often takes a relapsing chronic course and is considered to be a facultative precancerous condition which can develop into squamous cell carcinoma (e15). The etiology of LS is still not fully understood. Currently, a multifactorial etiology is assumed, primarily driven by genetic and immunological factors. Evidence in support of genetic causes of LS include familial aggregation in up to 17% of cases (e16– e18), significant correlation of LS with Human Leucocyte Antigen (HLA)-A28 or HLA-B44 (e19) and the presence of HLA-DQ7 in 66% of the affected women (e20, e21).
In addition to the immunological aspects of LS pathogenesis, which appears to involve a local cell-mediated dermal response to an antigen which has not yet been identified with certainty (e22), a preponderance of cytotoxic CD8+ and CD57+ lymphocytes was found in the inflammatory cells present in lichenoid lesions—similar to the proliferation of CD8+ and CD57+ lymphocytes in chronic antigen-mediated inflammatory reactions (e23). Furthermore, 15% to 34% of patients with LS have T-cell-mediated autoimmune diseases, such as Hashimoto‘s thyroiditis (12%), alopecia areata (9%) and vitiligo (6%) (e24– e26).
In a study with 350 LS patients, 21.5% of patients had one or more autoimmune diseases, 21% had a family history of autoimmune disease and 42% had autoantibodies in titers of >1:20 (e26). The assumption of an autoimmune pathogenesis of LS is also supported by data from an English study which detected IgG autoantibodies to extracellular matrix protein-1 (ECM1) in the serum of 67% of patients compared to 7% in the control group (e27). Nevertheless, the existence of an LS-specific antibody cannot be concluded from these study results. The identified antibodies to extracellular matrix proteins are more likely a phenomenon secondary to tissue destruction (e28). Further studies found autoantibodies to the basal membrane proteins BP180 and BP320 (e29, e30) and thyroid autoantibodies in 30% to 80% and 9% of patients, respectively (e24). Even though the precise mechanisms underlying the synthesis of these antibodies are not fully understood, these results indicate that LS is caused by a humoral autoimmune reaction (e31).
In addition, infections with Borrelia burgdorferi (e32), human papillomavirus (HPV) (e33) and Epstein-Barr-Virus (e34), as well as local effects, such as Koebner phenomenon, are being discussed as possible causes of LS (e35).
Application of ultrapotent topical corticosteroids, such as clobetasol 0.05% ointment for initially 3 months, remains the first-line treatment for genital LS (LoE Ib, A) (e15, e36, e37). This should usually be followed by life-long reduced-dose maintenance therapy (LoE Ib, A). Both the therapeutic effectiveness and the cancer risk-reducing effect of corticosteroids have repeatedly demonstrated been in various studies (e38, e39). In refractory patients, an off-label treatment attempt with calcineurin inhibitors, such as 1% pimecrolimus and 0.03%/0.1% tacrolimus, can be made (LoE Ib, A) (e40).
Conclusion for clinical practice
Vulvar pruritus is a complex symptom of multifactorial origin, frequently affecting the patient’s quality of life. The treatment of patients with chronic pruritus (>6 weeks) is particularly challenging and often requires a multimodal strategy with an interdisciplinary approach. Besides the elimination of potential triggers and continuous lipid-replenishing basic care, antifungal agents and glucocorticoids are paramount. Medical professionals not specialized in the field should refer patients with refractory and/or suspicious lesions to an experienced gynecologist. A specimen should be obtained by punch biopsy for histological evaluation to rule out (pre-) malignant lesions.
It is central to the management of vulvar pruritus to offer a detailed discussion of the condition and treat patients with empathy. Their complaints should be taken seriously and patients should be informed about what could cause their symptoms and which treatment options are available for them.
Key messages.
Vulvar pruritus is an often complex symptom of multifactorial etiology. Its diagnosis and treatment requires an interdisciplinary approach, involving gynecologists, dermatologists, microbiologists, infectiologists, psychotherapists, physiotherapists, and, potentially, specialists in sexual medicine.
The most common cause of vulvar pruritus is vulvovaginal candidiasis followed by vulvar eczema and chronic dermatoses, such as lichen sclerosus.
75% of women will have at least one episode of vulvovaginal candidiasis during their lifetime and 6% to 8% will have chronic vulvovaginal candidiasis (at least 4 recurrences/year).
Vulvar skin changes, such as erosions, ulcers or hyperkeratotic lesions, which are refractory to treatment, require histological diagnosis, which is obtained by punch biopsy. Vulvar cytology has very low specificity and does not provide acceptably reliable results.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest
Prof. Woelber received authorship/co-authorship fees for a publication related to the topic from Springer Verlag and Thieme-Verlag. She received congress fees and reimbursement of travel expenses as well as lecture fees from med update GmbH, medac oncology, Jörg Eickeler, promedicis GmbH, AG-CPC, Roche, Tesaro, TEVA, OmniaMed, and AGO research GmbH. She received study support (third-party funding) from Greiner, Roche Diagnostics and medac oncology.
Dr. Prieske received reimbursement of travel expenses and lecture fees from medac oncology and Jörg Eickeler.
Prof. Mendling received congress fees and reimbursement of travel expenses as well as lecture fees from Dr. Kade GmbH.
Prof. Schmalfeldt received lecture fees from promedicis GmbH.
Prof. Tietz received reimbursement of travel expenses and lecture fees from Bayer Leverkusen.
Dr. Jaeger declares no conflict of interest.
References
- 1.Ständer S, Zeidler C, Augustin M, et al. S2k-Leitlinie zur Diagnostik und Therapie des chronischen Pruritus JDDG. J Dtsch Dermatol Ges. 2017;15:860–873. doi: 10.1111/ddg.13304_g. [DOI] [PubMed] [Google Scholar]
- 2.Yosipovitch G, Bernhard JD. Chronic pruritus. N Engl J Med. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 3.Petersen EE. Chronischer Juckreiz im Genital. Ars Medici. 2011;13+14:559–562. [Google Scholar]
- 4.Bohl T. Overview of vulvar pruritus through the life cycle. Clin Obstet Gynecol. 2005;48:786–807. doi: 10.1097/01.grf.0000179636.64663.e6. [DOI] [PubMed] [Google Scholar]
- 5.Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. Am J Obstet Gynecol. 2001;185:545–550. doi: 10.1067/mob.2001.116748. [DOI] [PubMed] [Google Scholar]
- 6.Lawrance N, Yell J. Bornstein J, editor. Vulvar rruritus Vulvar disease. Springer International Publishing AG, part of Springer Nature 57-64 [Google Scholar]
- 7.Schmelz M. A neural pathway for itch. Nat Neurosci. 2001;4:9–10. doi: 10.1038/82956. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17 doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meerpohl JJ, Langer G, Perleth M, Gartlehner G, Kaminski-Hartenthaler A, Schunemann H. [GRADE guidelines: 3 Rating the quality of evidence (confidence in the estimates of effect)] Z Evid Fortbild Qual Gesundhwes. 2012;106:449–456. doi: 10.1016/j.zefq.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 10.van der Meijden WI, Boffa MJ, Ter Harmsel WA, et al. European guideline for the management of vulval conditions 2016. J Eur Acad Dermatol Venereol. 2017;31:925–941. doi: 10.1111/jdv.14096. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank ME. Green top Guideline No58. The Management of vulval skin disorders. Royal College of Obstetricians and Gynaecoogists. http://mrcog.womanhospital.cn/ueditor/php/upload/file/20190821/1566377001.pdf (last accessed on 28 December 2019) 2011 [Google Scholar]
- 12.Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatologic Therapy. 2004;17:8–19. doi: 10.1111/j.1396-0296.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 13.D‘Antuono A, Bellavista S, Negosanti F, Zauli S, Baldi E, Patrizi A. Dermasilk briefs in vulvar lichen sclerosus: an adjuvant tool. J Low Genit Tract Dis. 2011;15:287–291. doi: 10.1097/LGT.0b013e31821380a0. [DOI] [PubMed] [Google Scholar]
- 14.Corazza M, Borghi A, Minghetti S, Toni G, Virgili A. Effectiveness of silk fabric underwear as an adjuvant tool in the management of vulvar lichen simplex chronicus: results of a double-blind randomized controlled trial. Menopause. 2015;22:850–856. doi: 10.1097/GME.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 15.Ansari P. Pruritus ani. Clin Colon Rectal Surg. 2016;29:38–42. doi: 10.1055/s-0035-1570391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Antuono A, Baldi E, Bellavista S, Banzola N, Zauli S, Patrizi A. Use of dermasilk briefs in recurrent vulvovaginal candidosis: safety and effectiveness. Mycoses. 2012;55:e85–e89. doi: 10.1111/j.1439-0507.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- 17.Swamiappan M. Anogenital pruritus—an overview. J Clin Diagn Res. 2016;10 doi: 10.7860/JCDR/2016/18440.7703. WE01-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. J Am Acad Dermatol. 2004;50:889–891. doi: 10.1016/j.jaad.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Tennyson H, Levine N. Neurotropic and psychotropic drugs in dermatology. Dermatol Clin. 2001;19:179–197. doi: 10.1016/s0733-8635(05)70239-4. [DOI] [PubMed] [Google Scholar]
- 20.Mattle V, Seeber B, Ruth-Egger S, Zervomanolakis I, Wildt L. Die Behandlung des therapierefraktären Pruritus vulvae mit Naltrexon, einem spezifischen Opiatantagonisten. Geburtshilfe Frauenheilkd. 2008;68 [Google Scholar]
- 21.Mohammad Ali BM, Hegab DS, El Saadany HM. Use of transcutaneous electrical nerve stimulation for chronic pruritus. Dermatol Ther. 2015;28:210–215. doi: 10.1111/dth.12242. [DOI] [PubMed] [Google Scholar]
- 22.Yu C, Zhang P, Lv Z-T, et al. Efficacy of acupuncture in itch: a systematic review and meta-analysis of clinical randomized controlled trials. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/208690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG), Arbeitsgemeinschaft für Infektionen und Infektionsimmunologie in der Gynäkologie und Geburtshilfe (AGII), Deutsche Dermatologische Gesellschaft (DDG), Deutschsprachige Mykologische Gesellschaft (DMyKG) Die Vulvovaginalkandidose AWMF 013/004 (S1+IDA)) 2010 [Google Scholar]
- 24.Foxman B. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. Low Genit Tract. 2012;17:1–6. doi: 10.1097/LGT.0b013e318273e8cf. [DOI] [PubMed] [Google Scholar]
- 25.Mendling W. Guideline: Vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis) Mycoses. 2015;58 S1:1–15. doi: 10.1111/myc.12292. [DOI] [PubMed] [Google Scholar]
- 26.Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS. 2018;29:1258–1272. doi: 10.1177/0956462418785451. [DOI] [PubMed] [Google Scholar]
- 27.Mendling W. Dyspareunie, Vulvodynie und Vestibulodynie: Schmerzen statt Lust. Gynäkologie und Geburtshilfe. 2019;24:24–27. [Google Scholar]
- 28.Mendling W. Gynäkologische Infektionen. Frauenheilkunde Up2 Date. 2019;13:1–19. [Google Scholar]
- 29.Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15–21. doi: 10.1016/j.ajog.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Tietz HJ. [Candida glabrata: pathogenicity and therapy update] Hautarzt. 2012;63:868–871. doi: 10.1007/s00105-012-2377-0. [DOI] [PubMed] [Google Scholar]
- 31.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the infectious diseases society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spellberg BJ, Filler SG, Edwards JE Jr. Current treatment strategies for disseminated candidiasis. Clin Infect Dis. 2006;42:244–251. doi: 10.1086/499057. [DOI] [PubMed] [Google Scholar]
- 33.Safdar A, van Rhee F, Henslee-Downey JP, Singhal S, Mehta J. Candida glabrata and candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplant. 2001;28:873–878. doi: 10.1038/sj.bmt.1703252. [DOI] [PubMed] [Google Scholar]
- 34.Kuse E-R, Chetchotisakd P, Cunha C, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–1527. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 35.Perlin DS. Current perspectives on echinocandin class drugs. Future Microbiol. 2011;6:441–457. doi: 10.2217/fmb.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira ER, Fothergill A, Kirkpatrick WR, Patterson TF, Redding SW. Antifungal susceptibility testing of micafungin against candida glabrata isolates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:457–459. doi: 10.1016/j.tripleo.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol. 2003;189:1297–1300. doi: 10.1067/s0002-9378(03)00726-9. [DOI] [PubMed] [Google Scholar]
- 38.Huttenhower C, Gevers D, Knight R, et al. The Human Microbiome Project (HMP) Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petricevic L. Das vaginale Mikrobiom. Der Gynäkologe. 2019;52:10–15. [Google Scholar]
- 40.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Steyer GE. Das vaginale Mikrobiom. EHK. 2017;66:44–50. [Google Scholar]
- E2.Wilson BA, Thomas SM, Ho M. The human vaginal microbiome Metagenomics of the Human Body. Springer Medizin. 2011:91–115. [Google Scholar]
- E3.Lamont RF, Sobel JD, Akins RA, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118:533–549. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Fettweis JM, Serrano MG, Girerd PH, Jefferson KK, Buck GA. A new era of the vaginal microbiome: advances using next-generation sequencing. Chem Biodivers. 2012;9:965–976. doi: 10.1002/cbdv.201100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.White BA, Creedon DJ, Nelson KE, Wilson BA. The vaginal microbiome in health and disease. Trends Endocrinol Metab. 2011;22:389–393. doi: 10.1016/j.tem.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol. 1996;34:2497–2499. doi: 10.1128/jcm.34.10.2497-2499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Ehrström S, Daroczy K, Rylander E, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes and Infection. 2010;12:691–699. doi: 10.1016/j.micinf.2010.04.010. [DOI] [PubMed] [Google Scholar]
- E8.Martinez RCR, Franceschini SA, Patta MC, et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol. 2009;48:269–274. doi: 10.1111/j.1472-765X.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- E9.Mendling W. Schwiertz A, editor. Vaginal microbiota Microbiota of the human body: implications in health and disease. Cham: Springer International Publishing. 2016:83–93. [Google Scholar]
- E10.Hilton E, Isenberg HD, Alperstein P, France K, Borenstein MT. Ingestion of yogurt containing lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116:353–357. doi: 10.7326/0003-4819-116-5-353. [DOI] [PubMed] [Google Scholar]
- E11.DIMDI. Fachinformation Gynatren. www.portaldimdide/amispb/doc/pei/Web/2602489-spcde-20100301pdf. 2010.(last accessed on 29 January 2020) [Google Scholar]
- E12.Siboulet A. Impfung gegen nichtspezifische bakterielle Vaginose Doppelblinduntersuchung von Gynatren. Gynakol Geburtshilfliche Rundsch. 1991;31:153–160. [PubMed] [Google Scholar]
- E13.Alderete JF. Does lactobacillus vaccine for trichomoniasis, Solco Trichovac, induce antibody reactive with Trichomonas vaginalis? Genitourin Med. 1988;64:118–123. doi: 10.1136/sti.64.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–1876. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- E15.Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) Lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:e1–e43. doi: 10.1111/jdv.13136. [DOI] [PubMed] [Google Scholar]
- E16.Thomas RHM, Kennedy CTC. The development of lichen sclerosus et atrophicus in monozygotic twin girls. Br J Dermatol. 1986;114:377–379. doi: 10.1111/j.1365-2133.1986.tb02831.x. [DOI] [PubMed] [Google Scholar]
- E17.Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803–806. doi: 10.1067/mjd.2001.113474. [DOI] [PubMed] [Google Scholar]
- E18.Sherman V, McPherson T, Baldo M, Salim A, Gao X, Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: an observational cohort study. J Am Acad Dermatol Venereol. 2010;24:1031–1034. doi: 10.1111/j.1468-3083.2010.03572.x. [DOI] [PubMed] [Google Scholar]
- E19.Purcell KG, Spencer LV, Simpson PM, Helman SW, Oldfather JW, Fowler JF Jr. HLA antigens in lichen sclerosus et atrophicus. Arch Dermatol. 1990;126:1043–1045. [PubMed] [Google Scholar]
- E20.Marren P, Jell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132:197–203. doi: 10.1111/j.1365-2133.1995.tb05013.x. [DOI] [PubMed] [Google Scholar]
- E21.Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142:481–484. doi: 10.1046/j.1365-2133.2000.03360.x. [DOI] [PubMed] [Google Scholar]
- E22.Gross T, Wagner A, Ugurel S, Tilgen W, Reinhold U. Identification of TIA-1+ and Granzyme B+ cytotoxic T cells in Lichen sclerosus et atrophicus. Dermatology. 2001;202:198–202. doi: 10.1159/000051636. [DOI] [PubMed] [Google Scholar]
- E23.Carlson JA, Grabowski R, Chichester P, Paunovich E, Malfetano J. Comparative immunophenotypic study of lichen sclerosus: Epidermotropic CD57+ lymphocytes are numerous—implications for pathogenesis. Am J Dermatopathol. 2000;22:7–16. doi: 10.1097/00000372-200002000-00002. [DOI] [PubMed] [Google Scholar]
- E24.Kreuter A, Kryvosheyeva Y, Terras S, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol. 2013;93:238–241. doi: 10.2340/00015555-1512. [DOI] [PubMed] [Google Scholar]
- E25.Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case-control study. Arch Dermatol. 2008;144:1432–1435. doi: 10.1001/archderm.144.11.1432. [DOI] [PubMed] [Google Scholar]
- E26.Thomas RHM, Ridley CM, Mcgibbon DH, Black MM. Lichen sclerosus et atrophicus and autoimmunity—a study of 350 women. Br J Dermatol. 1988;118:41–46. doi: 10.1111/j.1365-2133.1988.tb01748.x. [DOI] [PubMed] [Google Scholar]
- E27.Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. The Lancet. 2003;362:118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- E28.Gambichler T, Skrygan M, Tigges C, Kobus S, Gläser R, Kreuter A. Significant upregulation of antimicrobial peptides and proteins in lichen sclerosus. Br J Dermatol. 2009;161:1136–1142. doi: 10.1111/j.1365-2133.2009.09273.x. [DOI] [PubMed] [Google Scholar]
- E29.Howard A, Dean D, Cooper S, Kirtshig G, Wojnarowska F. Circulating basement membrane zone antibodies are found in lichen sclerosus of the vulva. Australas J Dermatol. 2004;45:12–15. doi: 10.1111/j.1440-0960.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- E30.Strittmatter HJ, Hengge UR, Blecken SR. Calcineurin antagonists in vulvar lichen sclerosus. Arch Gynecol Obstet. 2006;274:266–270. doi: 10.1007/s00404-006-0151-1. [DOI] [PubMed] [Google Scholar]
- E31.Chan I, Oyama N, Neill SM, Wojnarowska F, Black MM, McGrath JA. Characterization of IgG autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Clin Exp Dermatol. 2004;29:499–504. doi: 10.1111/j.1365-2230.2004.01573.x. [DOI] [PubMed] [Google Scholar]
- E32.Eisendle K, Grabner T, Kutzner H, Zelger B. Possible role of borreliaburgdorferi sensu lato infection in lichen sclerosus. Arch Dermatol. 2008;144:591–598. doi: 10.1001/archderm.144.5.591. [DOI] [PubMed] [Google Scholar]
- E33.Powell J, Strauss S, Gray J, Wojnarowska F. Genital carriage of human papilloma virus (HPV) DNA in prepubertal girls with and without vulval disease. Pediatr Dermatol. 2003;20:191–194. doi: 10.1046/j.1525-1470.2003.20301.x. [DOI] [PubMed] [Google Scholar]
- E34.Aidé S, Lattario FR, Almeida G, do Val IC, da Costa Carvalho M. Epstein-barr virus and human papillomavirus infection in vulvar lichen sclerosus. J Low Genit Tract Dis. 2010;14:319–322. doi: 10.1097/LGT.0b013e3181d734f1. [DOI] [PubMed] [Google Scholar]
- E35.Farrell AM, Dean D, Millard PR, Charnock FM, Wojnarowska F. Cytokine alterations in lichen sclerosus: an immunohistochemical study. Br J Dermatol. 2006;155:931–940. doi: 10.1111/j.1365-2133.2006.07414.x. [DOI] [PubMed] [Google Scholar]
- E36.Smith YR, Haefner HK. Vulvar lichen sclerosus: pathophysiology and treatment. Am J Clin Dermatol. 2004;5:105–125. doi: 10.2165/00128071-200405020-00005. [DOI] [PubMed] [Google Scholar]
- E37.Schwegler J, Schwarz J, Eulenburg C, et al. Health-related quality of life and patient-defined benefit of clobetasol 005% in women with chronic lichen sclerosus of the vulva. Dermatology. 2011;223:152–160. doi: 10.1159/000332831. [DOI] [PubMed] [Google Scholar]
- E38.Dalziel KL, Wojnarowska F. Long-term control of vulval lichen sclerosus after treatment with a potent topical steroid cream. J Reprod Med. 1993;38:25–27. [PubMed] [Google Scholar]
- E39.Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatology. 2015;151:1061–1067. doi: 10.1001/jamadermatol.2015.0643. [DOI] [PubMed] [Google Scholar]
- E40.Luesley D, Downey G. Topical tacrolimus in the management of lichen sclerosus. BJOG. 2006;113:832–834. doi: 10.1111/j.1471-0528.2006.00977.x. [DOI] [PubMed] [Google Scholar]
- E41.Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) Lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29 doi: 10.1111/jdv.13136. [DOI] [PubMed] [Google Scholar]
- E42.Eberz S, Regauer S. Häufigkeit von vaginalen Neoplasien bei anogenitalem Lichen planus. Geburtshilfe Frauenheilkd. 2009;69 A018. [Google Scholar]
- E43.AWMF. Vulvakarzinom und_Vorstufen. www.awmf.org/uploads/tx_szleitlinien/015-059l_S2k_Vulvakarzinom_und_Vorstufen_Diagnostik_Therapie_2016-10.pdf (last accessed on 23 January 2020) [Google Scholar]
- E44.Eberz B, Regauer S. Assoziation von anogenitalem Lichen sclerosus und Psoriasis bei erwachsenen Frauen. Geburtshilfe Frauenheilkd. 2008;68 [Google Scholar]
- E45.Gibbon K. Kirtschig G, Cooper S, editors. Atopic eczema, lichen simplex and seborrheic dermatitis. Gynecologic dermatology—symptoms, signs and clinical management. 2016:65–69. [Google Scholar]
- E46.Stockdale CK, Boardman L. Diagnosis and treatment of vulvar dermatoses. Obstet Gynecol. 2018;131:371–86. doi: 10.1097/AOG.0000000000002460. [DOI] [PubMed] [Google Scholar]





