Abstract
Importance
Less than half of US patients diagnosed with atrial fibrillation (AF) receive oral anticoagulation (OAC).
Objective
To identify patients with similar patterns of warfarin and direct oral anticoagulant (DOAC) adherence in the first year after AF diagnosis, and evaluate associations between patient characteristics and membership into latent classes of adherence.
Design
Retrospective cohort study
Setting and Participants
Using 2013–2016 Medicare claims data, we identified 7,491 and 9,478 patients newly diagnosed with AF in 2014–2015 who initiated warfarin and DOAC after AF diagnosis, respectively. Participants were followed for 12 months after AF diagnosis.
Exposure, Main Outcomes and Measures
The outcome was the proportion of days covered with warfarin and DOAC, measured in 30-day intervals after AF diagnosis. Independent variables included patient demographics, socioeconomic status, region of residence, and clinical characteristics. We used latent class mixed models to identify latent classes of warfarin and DOAC adherence, and polytomous logistic regression to assess the association between patient characteristics and membership into each latent class.
Results
The average age was 76 (SD=10.0) for warfarin users and 77 (SD=8.5) for DOAC users. We identified 4 latent classes of warfarin adherence: late initiators (n=980, 13%); early initiators who discontinued therapy at months 1–3 (n=1,297, 17%); or at months 5–10 (n=735, 10%); and continuously adherent patients (n=4,479, 60%); and 4 latent classes of DOAC adherence: patients who initiated DOAC in months 1–5 (n=1,368, 14%); or 6–11 (n=800, 8%); patients with suboptimal and decreasing adherence (n=2,267, 24%); and continuously adherent patients (n=5,043, 53%). Membership into latent classes of warfarin adherence was significantly associated with sex, eligibility for Medicaid and income subsidy, region of residence, CHAD2S2-VASc score, and HAS-BLED score. Membership into latent classes of DOAC adherence was significantly associated with race, region of residence, HAS-BLED score, and antiarrhythmic medication use.
Conclusion and Relevance
Among patients who initiated anticoagulation therapy, 40% of warfarin initiators and 47% of DOAC initiators did not continuously adhere to therapy in the first year after AF diagnosis. Identifying longitudinal patterns of warfarin and DOAC adherence and factors associated with them provide suggestion for design of targeted strategies to mitigate suboptimal OAC use.
Introduction
Atrial fibrillation (AF) affects 2.3 million individuals in the US and is the most common cardiac arrhythmia in older adults.1–4 AF is associated with a 5-fold increase in the risk of stroke, and this association between AF and stroke risk becomes more significant as age increases.5 Oral anticoagulant (OAC) therapy reduces the risk of stroke associated with AF by around 60%,6 but is associated with an increased risk of bleeding.7 Clinical guidelines recommend the use of OAC in patients with moderate and high risk of stroke, which is generally defined as having a CHA2DS2-VASc score≥2.6 In spite of the important role of OAC in stroke prevention, only 50%−60% of US patients with AF recommended for OAC actually receive these medications, and less than half of them adhere to them over time.8,9
Understanding the patterns of OAC use is important because prior research has shown that OAC underuse is not only due to lack of initiation of OAC therapy, but also to delayed initiation after diagnosis, therapy discontinuation, and suboptimal adherence while on therapy.10 Moreover, these patterns of OAC adherence have a large impact on stroke outcomes.11 However, it remains unknown whether patterns of adherence differ for patients who initiate OAC therapy with warfarin and with the new direct oral anticoagulants (DOACs). Comparing patterns of adherence to warfarin and DOACs is important because of the marked pharmacokinetic and pricing differences between them. It is possible, for instance, that patients who initiate DOAC are more likely to adhere to therapy because of the lack of requirement for warfarin monitoring. On the other hand, rates of discontinuation could be higher on DOACs because of higher costs and copayments.
To address this evidence gap, we applied latent class mixed models to a sample of patients newly-diagnosed with AF who initiated OAC therapy with warfarin or DOACs. We then assessed how patient characteristics affected membership into each latent class for each OAC type.
Methods
Data Source
We used 2013–2016 claims data from a 5% random sample of Medicare beneficiaries obtained from the Centers for Medicare and Medicaid Services (CMS). The datasets included Mater Beneficiary Summary File, Part D event file, plan/prescriber/pharmacy characteristics files, and Part A and B medical claims files, and contain information about demographics, zip code, Chronic Conditions Data Warehouse (CCW) indicators and dates of first diagnosis of CMS priority including AF, generic name, days of supply, and date of fill of pharmacy claims, and International Classification of Diseases (ICD) 9 and 10 diagnosis codes and dates of service for medical claims. This study was approved by the Institutional Review Board of the University of Pittsburgh as exempt.
Study Population
We identified 72,306 patients newly diagnosed with AF in 2014–2015, based on the CCW definition of AF as having one inpatient claim or two outpatient claims with ICD-9 diagnosis code 427.31.12 We excluded patients with valvular disease (n=1,453) or with CHA2DS2-VASc score < 2, because they are not recommended for OAC (n=3,457). Patients who died within 30 days of AF diagnosis (n=4,394) or who were not continuously enrolled in Medicare Part D Stand Alone Prescription Drug Plans during follow-up (n=28,848) were also excluded. We excluded patients who did not fill any prescription for warfarin or DOAC in the year after first AF diagnosis (n=15,065) or had missing values for any covariate included in the study (n=603). Finally, we excluded those who filled prescriptions for both warfarin and DOACs (n=1,517). The final study sample included 7,491 participants for warfarin users and 9,478 for DOAC users (Supplemental figure 1).
Independent Variables
We evaluated the association between time invariant covariates and membership into latent classes of adherence. Covariates included patient demographics, variables that capture socioeconomic status (SES), census division of residence, and clinical characteristics. Demographics included age, gender, and race. Socioeconomic variables included eligibility for Medicaid coverage and low-income subsidy (measured at the patient level), socioeconomic score (measured at the zip code level), and index of dissimilarity (measured at the Metropolitan Statistical Area level). We linked American Community Survey data obtained from the US Census Bureau and Medicare claims data using the zip code. We calculated the socioeconomic score using factor analysis. Specifically, factor analysis identified key census variables, which were then combined into a score representing SES using z- scores.13 The index of dissimilarity measured the fraction of blacks (or whites) who would have to move from their neighborhoods to other neighborhoods to achieve perfect integration, and was calculated using a previously defined formula.14,15 Clinical characteristics include CHAD2S2-VASc and HAS-BLED score,16,17 Alzheimer’s disease and dementia, use of nonsteroidal anti-inflammatory drugs (NSAIDs) and antiplatelet agents, as well as use of amiodarone, dronaderone, or verapamil. We used 12 months of claims data before AF diagnosis or CCW definitions to define clinical characteristics. The definitions of covariates have been previously published.10
Outcomes
The primary outcomes were adherence to warfarin use and adherence to DOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban. For each participant, we extracted prescriptions for warfarin or DOACs after AF diagnosis. Using the dates of fill and the days of supply, we calculated the proportion of days covered with warfarin or DOACs for each 30-day interval. We transformed the proportion of days covered outcome by taking the arcsine of the square root in order to stabilize the variance of outcome measures.18
Statistical Analysis
Latent Class Mixed Model Analysis
The latent class mixed model analysis was conducted in R software. Details of the model have been previously documented.19 We called the hlme estimation function using the lcmm R package to identify latent classes of warfarin and DOAC use over time. We included functions of time variable with up to 5th degree as the common fixed effects in the model, and an individual random effect on the intercept. We fitted a series of models from 2 through 6 latent classes. Models were estimated with the extended Marquardt algorithm in order to achieve maximum log-likelihood.20 Initial values were not specified. We assessed the posterior membership probabilities of each model, which were computed using the Bayes theorem.21 In addition, we compared the Bayesian information criterion (BIC) as well as the posterior classification rate across models to select the model with the best fit.
Evaluating the Association between Patient Characteristics and Latent Class Membership
We reported patient characteristics at the time of first AF diagnosis across latent classes identified by the selected models. Specifically, class-specific characteristics of warfarin and DOAC users were reported as means (SD) for continuous variables and counts (%) for categorical variables. We also compared the characteristics across classes using ANOVA analyses for continuous variables and chi-square tests for categorical variables and reported the p values. Polytomous logistic regression was conducted to assess the association between baseline characteristics and membership in latent classes among warfarin and DOAC users. The classes with the highest adherence to warfarin and DOAC were treated as the reference group. Odds ratios and 95% confident intervals (CIs) comparing the other classes with the reference group were computed. We applied Holm modified Bonferroni correction to account for multiple comparisons.22
Results
A total number of 16,969 participants were included in our study. Among them, 7,491 were warfarin users and 9,478 were DOAC users, both with the average follow-up of 11 months. The average age was 76 (SD=10.0) for warfarin users and 77 (SD=8.5) for DOAC users. The proportion of male participants was 42% for both warfarin (n=3,143) and DOAC users (n=3,982). The majority of participants were white: 87% (n=6,497) for warfarin users and 90% (n=8,506) for DOAC users.
Latent Class Mixed Model Selection
Supplemental Table 1 shows the comparison of performance across the models with different number of classes. For the modeling of both adherence to warfarin and DOACs, the BIC statistic decreased as the number of classes increased from 2 through 6, indicating a better fit of the model with greater number of classes. Although the models with 5 and 6 classes had a lower BIC, the high number of classes could represent overfitting and would decrease precision in the estimation of the association between patient characteristics and membership intro trajectory classes. We thus selected the models with 4 classes for the modeling of adherence to both warfarin and DOACs.
Adherence to Warfarin
Class-specific Patterns of Adherence
Our final model identified 4 latent classes of warfarin use: late initiators (class 1, n=980, 13%); early initiators who discontinued therapy at months 1–3 (class 2, n=1,297, 17%); early initiators who discontinued therapy at months 5–10 (class 3, n=735, 8%); and continuously adherent patients (class 4, n=4,479, 60%) (Figure 1).
Figure 1.
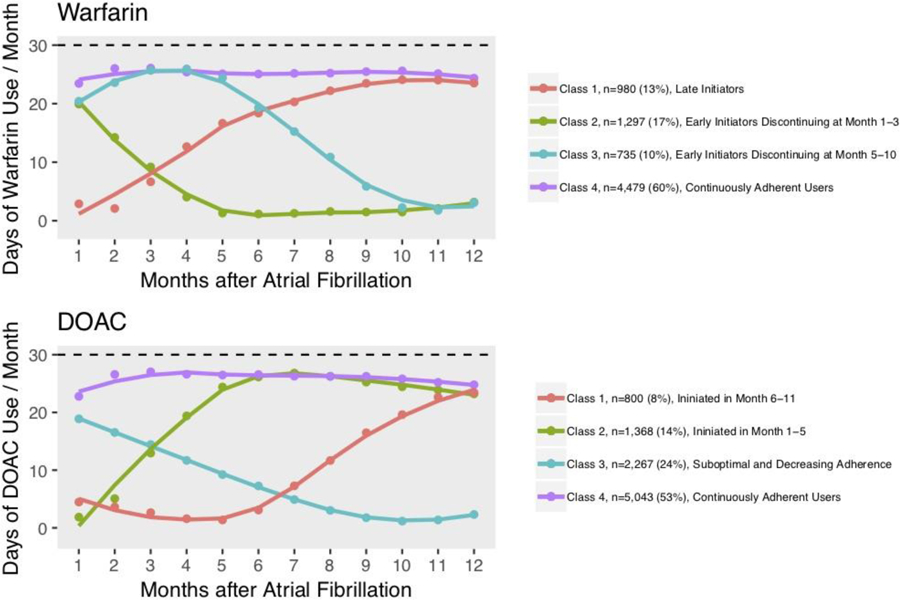
Class-Specific Mean Trajectories of Adherence to Warfarin and DOAC
Abbreviations: DOAC=Direct Oral Anticoagulant.
Baseline Patient Characteristics across Latent Classes
The average age of warfarin users was approximately 76 across the four classes (SD around 10.0) (Table 1). The proportion of male participants was slightly lower in the late initiators (n=357, 36%) than the other latent classes (43%, 43%, 43%). Patients eligible for Medicaid were underrepresented in the continuously adherent class (n=1,229, 27%). Eligibility for low-income subsidy was lower among initiators who discontinued therapy at months 5–10 (n=265, 37%) and continuously adherent users (n=1,763, 39%). The dissimilarity indexes were similar across classes. The continuously adherent users class had the lowest proportion of patients with a diagnosis of Alzheimer’s disease or other dementia (n=754, 17%) and with NSAID or antiplatelet use (n=1,048, 23%). CHAD2S2-VASc and HAS-BLED scores were similar across classes.
Table 1.
Class-specific Characteristics at the Time of Atrial Fibrillation Diagnosis for Warfarin Users
| Late initiators (Class 1) n=980 | Early initiators discontinuing at months 1–3 (Class 2) n=1,297 | Early initiators discontinuing at months 5–10 (Class 3) n=735 | Continuously adherent users (Class 4) n=4,479 | P-valuea | |
|---|---|---|---|---|---|
| Age, mean (SD), year | 77 (10.1) | 76 (10.1) | 76 (10.1) | 76 (9.8) | 0.32 |
| Male, No. (%) | 357 (36) | 558 (43) | 314 (43) | 1,913 (43) | 0.003 |
| Race, No. (%) | 0.006 | ||||
| White | 849 (87) | 1,084 (84) | 627 (85) | 3,937 (88) | |
| Black | 92 (9.4) | 142 (11.0) | 67 (9.1) | 378 (8.4) | |
| Other | 38 (3.9) | 71 (5.5) | 41 (5.6) | 164 (3.7) | |
| Medicaid, No. (%) | 333 (34) | 391 (30) | 222 (30) | 1,229 (27) | <0.001 |
| Income Subsidy, No. (%) | 455 (46) | 579 (45) | 265 (37) | 1,763 (39) | <0.001 |
| SES, mean (SD) | 0.12 (1.0) | 0.14 (1.0) | 0.16 (1.0) | 0.13 (1.0) | 0.80 |
| Dissimilarity, mean (SD) | 0.49 (0.17) | 0.49 (0.17) | 0.51 (0.17) | 0.49 (0.18) | 0.12 |
| Rural, No. (%) | 239 (24) | 332 (26) | 193 (26) | 1,246 (28) | 0.10 |
| Region, No. (%) | <0.001 | ||||
| Midwest | 311 (32) | 345 (27) | 232 (32) | 1,527 (34) | |
| Northeast | 248 (25) | 330 (25) | 178 (24) | 1,082 (24) | |
| Southeast | 237 (24) | 332 (26) | 167 (23) | 1,035 (23) | |
| Southwest | 67 (6.8) | 107 (8.3) | 48 (6.5) | 283 (6.3) | |
| West | 116 (12) | 183 (14) | 110 (15) | 552 (12) | |
| CHA2DS2-VASc, mean (SD)c | 5.2 (1.7) | 4.9 (1.7) | 5.1 (1.7) | 5.0 (1.7) | 0.001 |
| HAS-BLED, mean (SD)c | 3.2 (1.1) | 3.1 (1.1) | 3.1 (1.1) | 3.1 (1.1) | <0.001 |
| Alzheimer’s Disease or Dementia, No. (%)c | 209 (21) | 254 (20) | 132 (18) | 754 (17) | <0.001 |
| NSAID or Antiplatelet use, No. (%)c | 267 (27) | 334 (26) | 188 (26) | 1,048 (23) | 0.04 |
| Antiarrhythmic Medication Use, No. (%)b,c | 51 (5.2) | 118 (9.1) | 61 (8.3) | 331 (7.4) | 0.005 |
Abbreviations: SES=Social Economic Status; NSAID= Nonsteroidal Anti-inflammatory Drugs
p value: F test of ANOVA for continuous variables and chi-square test for categorical variables;
Antiarrhythmic medications included amiodarone, dronaderone, and verapamil;
Clinical characteristics were defined based on 12 months of claims data prior to AF diagnosis
Adjusted Results for the Association between Patient Characteristics and Class Membership
Table 3 shows the odds ratio (OR) for each covariate of belonging to a given latent class compared to belonging to the latent class characterized by continuous adherence (reference group). The reference class is often omitted in the text for the sake of simplicity.
Table 3.
Association between Patient Characteristics and Membership into Latent Classes of Adherence to Warfarin
| Late initiators (Class 1) n=980 | Early initiators discontinuing at months 1–3 (Class 2) n=1,297 | Early initiators discontinuing at months 5–10 (Class 3) n=735 | Continuously adherent users (Class 4) n=4,479 | P-valuea | |
|---|---|---|---|---|---|
| Age | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1 | 0.98 |
| Male | 0.75 (0.64, 0.88) | 0.91 (0.79, 1.05) | 1.04 (0.87, 1.24) | 1 | 0.002a |
| Race | 0.04 | ||||
| White | ref | ref | ref | ref | |
| Black | 0.93 (0.71, 1.20) | 1.28 (1.03, 1.61) | 1.08 (0.80, 1.46) | 1 | |
| Other | 0.94 (0.65, 1.36) | 1.42 (1.06, 1.92) | 1.45 (1.00, 2.09) | 1 | |
| Medicaid | 1.16 (0.92, 1.48) | 0.82 (0.66, 1.01) | 1.94 (1.38, 2.70) | 1 | <0.001a |
| Income Subsidy | 1.11 (0.89, 1.38) | 1.31 (1.09, 1.59) | 0.50 (0.37, 0.69) | 1 | <0.001a |
| SES | 1.00 (0.92, 1.08) | 1.05 (0.98, 1.12) | 1.04 (0.95, 1.13) | 1 | 0.56 |
| Dissimilarity | 1.17 (0.76, 1.81) | 1.43 (0.97, 2.11) | 2.03 (1.24, 3.30) | 1 | 0.02 |
| Rural | 0.87 (0.73, 1.03) | 0.98 (0.84, 1.14) | 0.98 (0.81, 1.19) | 1 | 0.44 |
| Region | 0.002a | ||||
| Midwest | 0.95 (0.78, 1.14) | 0.76 (0.64, 0.91) | 0.97 (0.78, 1.21) | 1 | |
| Northeast | ref | ref | ref | ||
| Southeast | 1.04 (0.84, 1.28) | 1.09 (0.90, 1.31) | 1.10 (0.87, 1.41) | 1 | |
| Southwest | 1.08 (0.79, 1.47) | 1.30 (1.00, 1.70) | 1.18 (0.83, 1.69) | 1 | |
| West | 0.98 (0.76, 1.27) | 1.15 (0.92, 1.43) | 1.36 (1.03, 1.79) | 1 | |
| CHA2DS2-VAScc | 0.95 (0.89, 1.01) | 0.92 (0.87, 0.97) | 1.06 (0.99, 1.14) | 1 | <0.001a |
| HAS-BLEDc | 1.21 (1.10, 1.33) | 1.10 (1.01, 1.20) | 0.98 (0.88, 1.10) | 1 | <0.001a |
| AD or Dementiac | 1.16 (0.97, 1.40) | 1.23 (1.04, 1.46) | 1.05 (0.84, 1.31) | 1 | 0.08 |
| NSAID or Antiplatelet Usec | 1.01 (0.84, 1.21) | 1.02 (0.87, 1.21) | 1.11 (0.90, 1.21) | 1 | 0.83 |
| Antiarrhythmic Medication Useb, c | 0.72 (0.53, 0.98) | 1.29 (1.03, 1.61) | 1.13 (0.85, 1.51) | 1 | 0.008 |
Abbreviations: SES: social economic status; AD: Alzheimer’s disease; NSAID, nonsteroidal anti-inflammatory drugs;
Results were statistically significant after the Holm modified Bonferroni correction;
Antiarrhythmic medications included amiodarone, dronaderone, and verapamil;
Clinical characteristics were defined based on 12 months of claims data prior to AF diagnosis.
Membership into latent classes of warfarin use was significantly associated with sex, eligibility for Medicaid coverage and low-income subsidy, region of residence, CHAD2S2-VASc score, and HAS-BLED score. Male participants were less likely to be late initiators (OR: 0.75, 95%CI: 0.64, 0.88). Patients who were eligible for Medicaid or for low-income subsidy were more likely to discontinue therapy in months 5–10 (OR: 1.94, 95%CI: 1.38, 2.70). Compared to patients living in the Northeast, patients living in Midwest were less likely to discontinue therapy in months 1–3 after diagnosis (OR: 0.76, 95%CI: 0.64, 0.91). Higher CHAD2S2-VASc scores were associated with lower odds of discontinuing therapy in months 1–3 after diagnosis (OR: 0.92, 95%CI: 0.87, 0.97). Higher HAS-BLED scores were associated with increased probability of late initiation (OR: 1.21, 95%CI: 1.10, 1.33) and discontinuation in months 1–3 after diagnosis (OR: 1.10, 95%CI: 1.01, 1.20).
Adherence to DOAC
Class-specific Patterns of Adherence
Our final model also identified 4 latent classes of DOAC use: patients who initiated DOACs in months 6–11 (class 1, n=800, 8%); patients who initiated DOACs in months 1–5 (class 2, n=1,368, 14%); patients with suboptimal and decreasing adherence (class 3, n=2,267, 24%); and continuously adherent patients (class 4, n=5,043, 53%) (Figure 1).
Baseline Patient Characteristics across Latent Classes
The average age of DOAC users was approximately 77 across the four classes (SD around 7.5) (Table 2). The proportion of male participants was slightly lower among patients who initiated DOAC in months 1–5 (n=528, 39%) and higher among patients with decreasing use (n=1,036, 46%). Patients eligible for Medicaid (n=952, 19%) and low-income subsidy (n=1,393, 28%) were less likely to belong to the continuously adherent latent class. Average SES score was the highest for continuously adherent users. The continuously adherent class had the lowest proportion of patients with a diagnosis of Alzheimer’s disease or other dementia (n=579, 11%) and with NSAID or antiplatelet use (n=1,283, 25%). CHAD2S2-VASc score was similar across classes. HAS-BLED score was slightly lower among continuously adherent users (2.8, SD=1.0) than in other classes (3.0, SD=1.1).
Table 2.
Class-specific Characteristics at the Time of Atrial Fibrillation Diagnosis for Direct Oral Anticoagulant Users
| Initiators in months 6–11 (Class 1) n=800 | Initiators in months 1–5 (Class 2) n=1,368 | Patients with suboptimal and decreasing adherence (Class 3) n=2,267 | Continuously adherent users (Class 4) n=5,043 | P valuea | |
|---|---|---|---|---|---|
| Age, mean (SD), year | 77 (8.7) | 78 (8.3) | 76 (8.8) | 77 (8.3) | <0.001 |
| Male, No. (%) | 324 (41) | 528 (39) | 1,036 (46) | 2,094 (42) | <0.001 |
| Race, No. (%) | <0.001 | ||||
| White | 702 (88) | 1,231 (90) | 1,997 (88) | 4,576 (91) | |
| Black | 45 (5.6) | 64 (4.7) | 164 (7.2) | 222 (4.4) | |
| Other | 53 (6.6) | 73 (5.3) | 106 (4.7) | 245 (4.9) | |
| Medicaid, No. (%) | 195 (24) | 294 (21) | 473 (21) | 952 (19) | 0.001 |
| Income Subsidy, No. (%) | 246 (31) | 417 (30) | 678 (30) | 1,393 (28) | 0.04 |
| SES, mean (SD) | 0.22 (1.1) | 0.28 (1.1) | 0.19 (1.1) | 0.33 (1.1) | <0.001 |
| Dissimilarity, mean (SD) | 0.48(0.17) | 0.49 (0.17) | 0.48 (0.17) | 0.49 (0.16) | 0.02 |
| Rural, No. (%) | 179 (22) | 305 (22) | 492 (22) | 1,043 (21) | 0.44 |
| Region, No. (%) | <0.001 | ||||
| Midwest | 166 (21) | 282 (21) | 562 (25) | 1,157 (23) | |
| Northeast | 181 (23) | 296 (22) | 457 (20) | 1,285 (25) | |
| Southeast | 246 (31) | 486 (36) | 710 (31) | 1,510 (30) | |
| Southwest | 99 (12.4) | 138 (10.1) | 230 (10.2) | 462 (9.2) | |
| West | 108 (14) | 166 (12) | 308 (14) | 629 (12) | |
| CHA2DS2-VASc, mean (SD)c | 4.8 (1.7) | 4.8 (1.7) | 4.7 (1.7) | 4.7 (1.7) | 0.002 |
| HAS-BLED, mean (SD)c | 3.0 (1.1) | 3.0 (1.1) | 3.0 (1.1) | 2.8 (1.0) | <0.001 |
| Alzheimer’s Disease or Dementia, No. (%)c | 120 (15) | 219 (16) | 283 (12) | 579 (11) | <0.001 |
| NSAID or Antiplatelet use, No. (%)c | 220 (28) | 382 (28) | 622 (27) | 1,283 (25) | 0.12 |
| Antiarrhythmic Medication Use, No. (%)b,c | 56 (7.0) | 81 (5.9) | 244 (10.8) | 401 (8.0) | <0.001 |
Abbreviations: SES=Social Economic Status; NSAID= Nonsteroidal Anti-inflammatory Drugs
p value: F test of ANOVA for continuous variables and chi-square test for categorical variables;
Antiarrhythmic medications included amiodarone, dronaderone, and verapamil;
Clinical characteristics were defined based on 12 months of claims data prior to AF diagnosis.
Adjusted Results for the Association between Patient Characteristics and Class Membership
Table 4 shows the odds ratio (OR) for each covariate of belonging to a given latent class compared to belonging to the continuously adherent latent class (reference group). The reference class is often omitted in the text for the sake of simplicity.
Table 4.
Association between Patient Characteristics and Membership into Latent Classes of Adherence to DOACs
| Initiators in months 6–11 (Class 1) n=800 | Initiators in months 1–5 (Class 2) n=1,368 | Patients with suboptimal and decreasing adherence (Class 3) n=2,267 | Continuously adherent users (Class 4) n=5,043 | P valuea | |
|---|---|---|---|---|---|
| Age | 1.00 (0.99, 1.01) | 1.01 (1.00, 1.02) | 1.00 (0.99, 1.00) | 1 | 0.20 |
| Male | 0.97 (0.82, 1.14) | 0.90 (0.79, 1.03) | 1.14 (1.02, 1.27) | 1 | 0.02 |
| Race | 0.003a | ||||
| White | ref | ref | ref | ref | |
| Black | 1.17 (0.83, 1.66) | 1.98 (0.73, 1.32) | 1.54 (1.23, 1.92) | 1 | |
| Other | 1.25 (0.90, 1.73) | 1.07 (0.80, 1.42) | 0.93 (0.73, 1.19) | 1 | |
| Medicaid | 1.59 (1.14, 2.22) | 1.09 (0.85, 1.40) | 1.01 (0.82, 1.24) | 1 | 0.05 |
| Income Subsidy | 0.73 (0.54, 0.99) | 1.00 (0.81, 1.24) | 1.02 (0.85, 1.21) | 1 | 0.21 |
| SES | 0.95 (0.88, 1.03) | 1.01 (0.94, 1.07) | 0.92 (0.87, 0.97) | 1 | 0.01 |
| Dissimilarity | 0.77 (0.47, 1.26) | 1.20 (0.81, 1.79) | 0.81 (0.58, 1.12) | 1 | 0.24 |
| Rural | 1.07 (0.88, 1.30) | 1.07 (0.91, 1.25) | 0.96 (0.84, 1.10) | 1 | 0.61 |
| Region | <0.001a | ||||
| Midwest | 1.01 (0.80, 1.27) | 1.08 (0.89, 1.30) | 1.31 (1.13, 1.52) | 1 | |
| Northeast | ref | ref | ref | ||
| Southeast | 1.07 (0.85, 1.33) | 1.43 (1.19, 1.70) | 1.17 (1.01, 1.36) | 1 | |
| Southwest | 1.41 (1.06, 1.87) | 1.35 (1.06, 1.72) | 1.27 (1.04, 1.55) | 1 | |
| West | 1.12 (0.85, 1.47) | 1.18 (0.94, 1.48) | 1.30 (1.08, 1.56) | 1 | |
| CHA2DS2-VAScc | 1.00 (0.93, 1.06) | 0.98 (0.93, 1.03) | 0.97 (0.92, 1.01) | 1 | 0.46 |
| HAS-BLEDc | 1.11 (1.00, 1.24) | 1.13 (1.04, 1.24) | 1.15 (1.07, 1.23) | 1 | <0.001a |
| AD or Dementiac | 1.26 (1.00, 1.58) | 1.30 (1.09, 1.56) | 1.09 (0.93, 1.28) | 1 | 0.02 |
| NSAID or Antiplatelet Usec | 0.94 (0.77, 1.16) | 0.96 (0.82, 1.14) | 0.92 (0.81, 1.06) | 1 | 0.69 |
| Antiarrhythmic Medication Useb, c | 0.84 (0.63, 1.13) | 0.72 (0.56, 0.92) | 1.33 (1.12, 1.57) | 1 | <0.001a |
Abbreviations: SES: social economic status; AD: Alzheimer’s disease; NSAID, nonsteroidal anti-inflammatory drugs;
Results were statistically significant after the Holm modified Bonferroni correction;
Antiarrhythmic medications included amiodarone, dronaderone, and verapamil;
Clinical characteristics were defined based on 12 months of claims data prior to AF diagnosis.
Membership into latent classes of DOAC use was significantly associated with race, region of residence, HAS-BLED score, and antiarrhythmic medication use. Male patients were more likely to belong to the latent class with suboptimal and decreasing adherence (OR: 1.14, 95%CI: 1.02, 1.27). Compared to white patients, black patients were more likely to initiate DOAC in months 1–5 after diagnosis (OR: 1.54, 95%CI: 1.23, 1.92). Compared to patients living in the Northeast, patients living in the other regions (Midwest, Southeast, Southwest and West) were more likely to belong to the class with suboptimal and decreasing adherence. Higher HAS-BLED scores and a diagnosis of Alzheimer’s disease or related dementia were associated with lower odds of belonging to the continuously adherent class.
Discussion
We applied latent class mixed models to a nationally representative sample of patients newly diagnosed with AF who initiated warfarin or DOAC. We identified four latent classes of adherence to warfarin and DOACs, with 60% of warfarin users and 53% of DOAC users belonging to the class characterized by continuous adherence. Membership into latent classes of warfarin adherence was significantly associated with sex, eligibility for Medicaid and income subsidy, region of residence, CHAD2S2-VASc score, and HAS-BLED score. Membership into latent classes of DOAC adherence was significantly associated with race, region of residence, HAS-BLED score, and antiarrhythmic medication use.
Our findings are consistent with prior evidence suggesting that patterns of use and adherence to OAC are not only affected by patient clinical characteristics, but also by demographics and region of residence.10 In a previous study, we used group-based trajectory models to study OAC use in patients who were diagnosed with AF,10 and found that black race, HAS-BLED score, and residence in the South decreased the likelihood of OAC initiation. Predictors that were associated with decreased odds of OAC initiation in our prior study were also associated with higher probability of belonging to the classes with suboptimal adherence in the current paper, which focused on patients who initiated OAC therapy.
Our study is an important contribution to the existing literature because we performed separate analyses for warfarin and DOACs. In doing so, we found that the proportion of continuously adherent patients was higher in the warfarin cohort than the DOAC cohort (60% vs. 53%). This difference was mostly driven by the higher proportion of patients initiating therapy late among the DOAC group. While this could represent the use of samples provided in physician offices, which would not be captured in our study, lower adherence to DOACs could also represent financial barriers to the use of DOACs, which are protected by patents and are considerably more expensive than warfarin. Rates of therapy discontinuation were relatively similar among two cohorts (27% for warfarin, 24% for DOACs), even when warfarin is considerably more inconvenient to use because it requires routine blood monitoring. However, patients discontinuing DOACs discontinued therapy sooner after AF diagnosis on average than those discontinuing warfarin, which once again could represent barriers to DOAC access rather than the occurrence of clinical events such as bleeds related to OAC use. Future analyses should leverage data sources with detailed clinical information to evaluate reasons behind late initiation and early discontinuation of each type of OAC therapy.
Our study also has important implications from a methodological perspective. It demonstrates how latent class mixed models can be applied to describe patterns of medication adherence over time. We tracked adherence of warfarin and DOAC longitudinally, and through the measurement of the proportion of days covered with therapy every 30 day interval, we evaluated the use of warfarin and DOAC in two-dimensions—adherence in each single unit and the longitudinal pattern composed of all the units. The extension of mixed models allowed more variability across individuals through the inclusion of random effects. In addition, the model allowed for variations in the number of measurements across participants, so that participants with missing data for the outcome could be included and selection bias could be mitigated. Regardless of these advantages, the application of these models presented certain challenges. First, the choice of number of classes is somewhat arbitrary. Models with a higher number of classes have a lower BIC in this case; however, this may led to overfitting the observed data. Second, because the analyses of clustering is data-driven, results might not be generalizable to other patient populations.23 Third, the global maximum likelihood was not guaranteed using the iteration algorithm from the model. In the future, multiple models with different initial set of values could be created to evaluate the consistency of results. Finally, our methods may not be sensitive enough to capture a single prescription or distinguish between patients with the same PDC but different use patterns in each time unit.
Limitations
Our study is subject to several additional limitations. First, claims data contain information on the filling of prescriptions, but not on the medications prescribed, or on whether patients take the medications they fill. As a result, it is not possible to distinguish whether suboptimal adherence was a product of prescriber decision making or of patient incompliance to the prescribed regimen. In addition, claims data do not contain prescriptions paid with cash, or information on the receipt of free samples, which could have led to an underestimation of adherence of warfarin and DOAC. Second, our methods did not allow for the estimation of how longitudinal patterns of adherence of warfarin and DOAC are altered by the occurrence of events during follow-up, such as stroke or bleeding events. Future analyses should assess longitudinal patterns of adherence of warfarin and DOAC by incorporating time-to-event measures for stroke and bleeding event. Third, our analyses did not evaluate patterns of switching across two types of OAC, because we excluded patients who had a prescription for both warfarin and DOACs in the first year after AF diagnosis.
Conclusions
Among newly diagnosed AF patients who initiated anticoagulation therapy, 40% of warfarin initiators and 47% of DOAC initiators did not continuously adhere to therapy in the first year after AF diagnosis. Adherence to warfarin and DOACs was not only associated with clinical characteristics, but also socioeconomic status and region of residence. Identifying longitudinal patterns of warfarin and DOAC adherence and factors associated with them could provide suggestions for the design of targeted strategies to mitigate suboptimal OAC use.
Supplementary Material
Key Points.
Question:
What are the longitudinal patterns of adherence to warfarin and direct oral anticoagulation use (DOAC) in atrial fibrillation (AF) patients who initiate anticoagulation therapy?
Findings:
In this population-based cohort study of 16,969 Medicare beneficiaries newly diagnosed with AF, only 60% of warfarin users and 53% of DOAC users belonged to the latent class characterized by continuous adherence. Adherence to warfarin and DOAC was associated with region of residence and HAS-BLED score, among other characteristics.
Meaning:
Among AF patients who initiated anticoagulation therapy, more than 40% did not continuously adhere to therapy in the first year after AF diagnosis.
Acknowledgment:
Dr. Hernandez and Ms. Chen had full access to all the data in the study and takes responsibility of the integrity of the data and the accuracy of the data analysis.
Sources of Funding: The project described was supported by the National Institutes of Health through Grant Number UL1TR001857. Hernandez is funded by the National Heart, Lung and Blood Institute (grant number K01HL142847). The sponsor played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: Hernandez has received consulting fees from Pfizer. There are no other conflict of interests.
Contributor Information
Nemin Chen, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, PA USA.
Maria M. Brooks, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, PA USA.
Inmaculada Hernandez, Department of Pharmacy and Therapeutics, School of Pharmacy, University of Pittsburgh, PA USA.
Reference
- 1.Go A, Hylek E, Phillips K, et al. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009;104(11):1534–1539. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2006;48(4):e149–e246. [DOI] [PubMed] [Google Scholar]
- 5.Wolf P, Abbott R, Kannel W. Atrial fibrillation a major contributor to stroke in the elderly the Framingham Study. Arch Intern Med 1987;147:1561–1564. [PubMed] [Google Scholar]
- 6.Hart Robert G. MLAP, MS; and Aguilar Maria I., MD. Meta analysis antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:11. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher AM, van Staa TP, Murray-Thomas T, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open 2014;4(1):e003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123(7):638–645 e634. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of Oral Anticoagulation Adherence Among Medicare Beneficiaries Newly Diagnosed with Atrial Fibrillation. J Am Heart Assn 2019;8(12):e011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of Oral Anticoagulation Adherence Among Medicare Beneficiaries Newly Diagnosed With Atrial Fibrillation. J Am Heart Assoc 2019;8(12):e011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez I, He M, Brooks MM, Saba S, Gellad WF. Adherence to Anticoagulation and Risk of Stroke Among Medicare Beneficiaries Newly Diagnosed with Atrial Fibrillation. Am J Cardiovasc Drugs. 2019. [DOI] [PMC free article] [PubMed]
- 12.CMS Chronic Conditions Data Warehouse (CCW)—CCW Condition Algorithms. In:2019.
- 13.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med 2003;349(14):1350–1359. [DOI] [PubMed] [Google Scholar]
- 14.Duncan O, Duncan B. A methodological analysis of segregation indexes. American Sociological Review. 1955;20(2):210–217. [Google Scholar]
- 15.Massey D, Denton N. The dimensions of residential segregation. Social Forces. 1988;67(2):281–315. [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 17.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138(5):1093–1100. [DOI] [PubMed] [Google Scholar]
- 18.Rohlf FJ, Sokal RR. Statistical tables. Macmillan; 1995. [Google Scholar]
- 19.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software. 2017;78(2). [Google Scholar]
- 20.Xu W, Hedeker D. A random-effects mixture model for classifying treatment response in longitudinal clinical trials. Journal of biopharmaceutical statistics. 2001;11(4):253–273. [PubMed] [Google Scholar]
- 21.Proust-Lima C, Séne M, Taylor JMG, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: A review. Statistical Methods in Medical Research. 2012;23(1):74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.S H. A simple sequentially rejective multiple test procedure. Scand J Statist 1979;6:65–70. [Google Scholar]
- 23.Frankfurt S, Frazier P, Syed M, Jung KR. Using Group-Based Trajectory and Growth Mixture Modeling to Identify Classes of Change Trajectories. The Counseling Psychologist 2016;44(5):622–660. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


